Summary
Jasmonates (JAs) have traditionally been studied for their defensive roles against wounding and detrimental organisms, but they are also crucial hormones for plant–microbe beneficial interactions. Here, we review the most recent advances in this overlooked field. We cover the evolutionary divergences of JA biosynthesis and signalling across various plant lineages and present the molecular mechanisms of action through which beneficial microbes interact with the host JA signalling pathway as well as environmental integration. Special emphasis is given to the cutting‐edge tools to study the spatial compartmentalization and cell and tissue specialization of JA signalling. This review underscores the role of the JA signalling pathway, with the MYELOCYTOMATOSIS2 transcription factor as a potential integrator of biotic and environmental cues, and highlights its significance in mutualistic interactions.
Keywords: defence, environmental integration, evolution, jasmonates, mutualism, plant–microbe interactions, rhizobiome, spatial compartmentalization
I. Introduction
Jasmonic acid (JA) is a lipid‐derived plant hormone that plays a pivotal role in regulating plant development and responses to biotic and abiotic stresses. Its biosynthesis involves a series of enzymatic reactions occurring in plastids and peroxisomes. In Arabidopsis thaliana, the process begins with alpha‐linoleic acid (α‐LeA) and proceeds through reduction in oxo‐phytodienoic acid (OPDA) by oxo‐phytodienoic acid reductase 3 (OPR3), followed by three rounds of β‐oxidation and conjugation of JA to isoleucine via Jasmonate‐Resistant 1 (JAR1), producing JA‐Ile. JA and JA‐Ile can be further converted to other JA derivatives – collectively termed jasmonates (JAs), including methyl jasmonate (MeJA) and cis‐jasmone (CJ). The canonical JA signalling pathway involves the interaction between the SCFCOI1 complex and JASMONATE ZIM DOMAIN (JAZ) proteins, which regulate the transcription of JA‐responsive genes, primarily through the basic Helix–Loop–Helix (bHLH) transcription factors (TFs). Among these TFs, MYELOCYTOMATOSIS2 (MYC2) is considered the master regulator. In the absence of stimuli, JAZ proteins repress the activity of MYC2. However, when extracellular stimuli or developmental processes trigger JA biosynthesis, the bioactive form JA‐Ile mediates the interaction between JAZ proteins and SCFCOI1. This interaction leads to the ubiquitination and degradation of JAZ proteins through the 26S proteasome, thereby releasing the repression of MYC2. Consequently, MYC2 binds to DNA motifs (G‐box) in the promoters of JA‐responsive genes to activate their transcription (for reviews about JA biosynthesis, perception and signalling, check Wasternack & Hause, 2013 and, more recently, Ghorbel et al., 2021). In A. thaliana, there are up to 13 different JAZ proteins, most of which are functionally redundant, although spatial‐specific expression patterns of individual JAZ proteins also suggest diversification of function (Howe et al., 2018). The subfunctionalization of AtJAZ‐AtMYC regulons in the control of tryptophan metabolism and defence pathways (Johnson et al., 2023), or the specific expression of AtJAZ2 in guard cells to modulate stomatal dynamics during Pseudomonas invasion (Gimenez‐Ibanez et al., 2017), serve as compelling examples of this diversification.
Although JAs play key roles in many different processes, including growth and development (Huang et al., 2017), reproduction (L. Zhao et al., 2022) or abiotic stress response (Raza et al., 2021), they have been historically studied for their crucial importance in defence against biotic factors (Zhu et al., 2013; Fan et al., 2015; Gao et al., 2025; Zhao et al., 2025). JA signalling is also essential for regulating plant–microbe mutualistic interactions, which support plant growth and development (Pozo et al., 2004). This review highlights recent developments in the role of JA in regulating interactions between plant roots, mutualistic microbes and their surrounding environments. It underscores the necessity to broaden the research to nonmodel plants (from bryophytes to angiosperms) and concludes with comments on how spatiotemporal approaches applied to JA‐signalling might help to better appreciate the nuanced roles of JA in plant–microbe interactions and its broader implications for plant health and ecology.
II. Evolutionary divergence of JA biosynthesis and signalling pathway
Comparative genomics and functional studies in bryophytes reveal considerable divergence in the JA pathway. While key components such as CORONATINE INSENSITIVE 1 (COI1) and JAZ are conserved, upstream JA biosynthesis differs (Fig. 1a). For example, the hornwort Marchantia polymorpha and the moss Physcomitrium patens lack OPR3 and JAR1 (Bowman et al., 2017), which prevents the synthesis of JA‐Ile. Instead, these plants rely on 12‐oxo‐phytodienoic acid (12‐OPDA) and shorter derivatives such as dinor‐12‐oxo‐phytodienoic acid (dn‐OPDA) for signalling (Monte et al., 2018; Delaux & Schornack, 2021). Interestingly, a single amino acid substitution in M. polymorpha COI1 alters its ligand specificity to that of Arabidopsis COI1, highlighting the molecular basis for ligand divergence between early‐ and late‐diverging plant lineages. This brings into question how variation in JA ligand chemistry may influence the regulation of immune responses and the nature of plant–microbe interactions across diverse taxa. This divergence in ligand specificity suggests that while the COI1‐JAZ signalling module is ancestral, it may be subject to lineage‐specific adaptation. In angiosperms, including trees, legumes and grasses, expansions of MYC and JAZ gene families may enable specialized functions across tissues and microbial contexts (Howe et al., 2018). Thus, the evolutionary trajectory of JA signalling cascade may reflect adaptation to increasingly complex plant–environment–microbe interfaces (Delaux & Schornack, 2021).
Fig. 1.

Jasmonate signalling as an integrator of environmental cues and plant–microbe interactions in roots. Diagram summarizing the novel insights about the role of jasmonates (JAs) as integrators of root–microbe–environment interactions. A schematic representation of a plant is shown at the centre of the image. Red arrows indicate the crosstalk between JAs' signalling with diverse stimuli and plant processes. Yellow lightings represent environmental stresses. Simplified schemes of JAs biosynthesis and signalling are shown in green boxes. (a) While key components of JA signalling are strongly conserved across species, divergence in JA biosynthesis and the ligand specificity of COI1 reflect evolutionary adaptations of the JA signalling pathway, which may influence how plants perceive and respond to their dynamic surroundings. 12‐OPDA, 12‐oxo‐phytodienoic acid; dn‐OPDAs, dinor‐oxo‐phytodienoic acids; α‐LeA, alpha linoleic acid. (b) Different microbes from diverse guilds present evolutionarily divergent effectors that converge in a parallel mechanism of action that stabilizes JASMONATE ZIM DOMAIN proteins and repress JAs‐responsive genes to ensure microbial colonization. (c) MYELOCYTOMATOSIS2 (MYC2) is the master regulator of JA‐responsive genes, controlling defence and mutualism genes while it also promotes certain mutualistic interactions through distinct mechanisms in root nodule symbiosis (RNS) and ectomycorrhiza (ECM). Most representative genes involved in MYC2's defence/mutualism coordination in RNS are depicted with blue circles, while those described in ECM are in red. CHI, chitinase; DNF1, defective in nitrogen fixation 1; IPD3, interacting protein of DMI3, also known as CYCLOPS; NCR, nodule‐specific cysteine‐rich peptide; NIN, nodule inception; NRT, nitrogen transporter; PCWDE, plant cell wall degrading enzymes; PDF1.2, plant defensin 1.2; PI, protease inhibitor; PR, pathogenesis related; TPS, terpene synthase. (d) Finally, MYC2 also promotes mycorrhiza‐induced resistance in arbuscular mycorrhiza. (e) Microbiomes isolated from warm environments enhance the synthesis of 12‐OPDA and dn‐OPDAs, which are related to enhanced thermotolerance in Bryophytes in a CORONATINE INSENSITIVE 1‐independent manner. (f) MYC2 transcription factor integrates environmental cues, such as light or nutrients, which are capable of reshaping JA signalling and affecting growth‐defence trade‐offs in response to microbial interaction.
III. Microbial effectors targeting the JA pathway: convergence across biotic interactions
To successfully interact with their hosts, microbes and microfauna (including pathogens, parasitic nematodes or beneficial symbionts) secrete effectors to control plant immunity. These effectors convergently target key JA signalling nodes (Fig. 1b). This is exemplified by diverse effectors, such as HARP1 from leaf‐feeding caterpillars (Chen et al., 2019), MiISE23 from Meloidogyne incognita (Shi et al., 2025), Avh94 from Phytophthora sojae (Y. Zhao et al., 2022) and MiSSP7 from the ectomycorrhizal (ECM) fungus Laccaria bicolor (Plett et al., 2011, 2014; Daguerre et al., 2020). All these effectors suppress JA signalling by interacting and stabilizing JAZ proteins. Despite converging on the same core pathway, the mentioned effectors differ in their target specificity. For instance, L. bicolor MiSSP7 has been shown to bind poplar JAZ6, thereby suppressing the expression of terpene synthases (TPSs), which are under direct or indirect control of PtMYC2, and are crucial for defence mechanisms (Marqués‐Gálvez et al., 2024). The monoterpenes α‐/β‐pinene, camphene and γ‐terpinene produced by PtTPS16 and PtTPS21 have been shown to inhibit fungal and oomycete colonization in poplar (Lackus et al., 2018; Marqués‐Gálvez et al., 2024). Conversely, other effectors, such as MiISE23 and Avh94, target different JAZ proteins: JAZ1/2/5/6 or JAZ1/2, respectively (Y. Zhao et al., 2022; Shi et al., 2025), suggesting that JAZ diversity may underpin tissue or species‐specific defence modulation.
IV. The broad role of JA‐signalling across mutualistic relationships: MYC2 as a coordinator between immunity and mutualism
While traditionally associated with defence, JA signalling can also facilitate mutualistic interactions (Fig. 1c). In Medicago truncatula, MYC2 acts as a molecular switch coordinating defence suppression and symbiotic gene activation to allow root nodule symbiosis (RNS). MtMYC2 forms regulatory modules with the transcriptional activator MtCYCLOPS/IPD3 and the transcriptional regulator Nodule Inception (MtNIN) on the one hand, and with a nodule‐specific signal peptidase complex (MtDNF1) and rhizobial differentiation‐mediating peptides (MtNCRs), on the other hand. These interactions ultimately promote nodule development. In parallel, MtMYC2 participates in the suppression of immune genes via a putative phosphatidylinositol phospholipase C‐like protein (MtDNF2), an endoplasmic reticulum transmembrane protein (MtNAD1), and the symbiotic cysteine‐rich kinase (MtSymCRK) (Guo et al., 2024). Interestingly, the G‐box MYC2‐binding site in the promoter region of MtCYCLOPS/IPD3 – a core gene in the common symbiosis pathway – is observed only in legumes (Guo et al., 2024), raising questions about the relevance of this regulatory mechanism in other beneficial interactions governed by the same signalling pathway, such as arbuscular mycorrhiza (AM), in nonleguminous species. How rhizobia are capable of directly or indirectly interacting with the host SCFCOI1‐JAZ‐MYC2 module to promote symbiosis is still unknown. Whereas in RNS, MYC2 promotes symbiosis and suppresses certain genes related to defence; in ECM, a similar output is achieved, but through the suppression of MYC2, via MiSSP7‐JAZ6 stabilization (as discussed earlier) (Daguerre et al., 2020). In the interaction between poplar and L. bicolor, unless repressed, PtMYC2 activates JA‐responsive genes and negatively regulates cell‐wall remodelling and nitrate transporter genes (Marqués‐Gálvez et al., 2024), known to be involved in successful colonization and functional mycorrhiza. The suppression of the JA‐signalling pathway is also associated with the beneficial interaction between pines and Suillus luteus (Wen et al., 2025), while the ability to modulate the activation of JA‐responsive genes over time seems to contribute to the mycorrhizal compatibility between poplar and different ECM partners (Marqués‐Gálvez et al., 2025), although the underlying mechanisms remain to be elucidated. Additionally, JAs also participate in the hormonal control of mycorrhiza‐induced resistance (Fig. 1d), extensively described in AM symbiosis (for a review, see Fiorilli et al., 2024). Altogether, these findings indicate a role of JA signalling, especially through the master regulator of defences MYC2, coordinating immunity and mutualism. The distinct mechanisms observed in RNS, ECM and AM symbioses underscore JAs' broad role across mutualistic relationships, which deserve more attention in future research.
V. Environmental integration: light, heat, nutrients and microbes
Environmental cues such as nutrient availability, light intensity or heat can reshape JA signalling, especially its role in balancing growth‐defence trade‐offs. Under phosphate starvation, PHR1 interacts with JAZ proteins and MYC2 to induce JA responses (He et al., 2023). This crosstalk between JA and phosphate starvation signalling suggests that JA signalling does not operate in isolation but adapts to nutrient availability and other stress signals. Likewise, under suboptimal light, MYC2 protein mediates a root–shoot circuit that prioritizes growth over defence in the presence of a supportive root microbiome in Arabidopsis plants (Hou et al., 2021). These studies identify MYC2 as a key integrator of environmental and microbial signals into whole‐plant adaptive responses (Fig. 1e).
In the bryophyte Sphagnum, microbiomes from warm habitats induce allene oxide cyclase (AOC) expression, a key enzyme in the synthesis of 12‐OPDA, a central signalling intermediate in the JA pathway and enhance thermotolerance (Carrell et al., 2022). Similarly, elevated 12‐OPDA and its derivative dn‐OPDA levels in M. polymorpha have been shown to enhance thermotolerance and activate heat shock proteins and antioxidants independently of the canonical COI1 receptor (Monte et al., 2018). Phenylalanine ammonia lyase (PAL), a JA‐responsive gene linked to phenolic compounds synthesis, is induced by warm‐microbiome inoculations with Sphagnum (Carrell et al., 2022), and by abiotic and biotic stress across multiple species (De León & Montesano, 2017; Rizwan et al., 2025; Fig. 1f). JA signalling is tightly integrated with abiotic stress responses, such as drought and salinity, through extensive crosstalk with abscisic acid (ABA), ethylene and reactive oxygen species (ROS) signalling pathways (Raza et al., 2021). This integration is further modulated by symbiotic relationships with AM fungi and fungal endophytes, which influence JA pathway components and promote enhanced stress resilience (Fiorilli et al., 2024). These findings underscore how JA, coordinated largely through MYC2, integrates abiotic and microbial cues to mediate ecological strategies.
VI. Multilayered and spatial regulation of JA signalling
Recent multi‐omic studies in model plants have revealed how JA signalling is regulated by multiple layers and at the spatial level (Fig. 2a–d). In rice, Chen et al. (2024) described three regulatory sectors: a MYC2‐dependent core (activation of JA‐responsive responses), tissue‐specific branches (specialized defence responses in individual tissues) and a feedback module that restrains overactivation of defence (MYC2‐regulated repressors). Similarly, Zander et al. (2020) identified C2H2‐type zinc‐finger transcription factor ZAT10 (STZ/ZAT10) as a repressor prioritizing JA responses over other hormone pathways. They also highlighted the dynamic nature of JA signalling and its coordination of different regulatory mechanisms such as gene expression, post‐translational modifications (PTMs) through phosphorylation, and chromatin landscape remodelling across time and cellular contexts. JAZ protein ubiquitination leads to protein degradation, while phosphorylation, SUMOylation or arginine methylation have been reported to increase its stability (Xu et al., 2024). Orosa et al. (2018) showed that SUMOylation suppresses the activity of COI1. MYC2 SUMOylation participates in root development (Srivastava et al., 2022), while targeted MYC2 stabilization suppresses ubiquitination and improves bacterial resistance in citrus trees (Zhao et al., 2025). Nonetheless, the putative role of these PTMs in the interplay between JAs and mutualistic interactions is still unexplored. JAs are able to profoundly influence the methylation landscape of plants, with an important role of H3K4me3 (trimethylation of lysine 4 on histone H3) and the histone variant H2A.Z domains (Zander et al., 2020). The other way around, DNA methylation also modulates JA pathway gene expression. In poplar, both host and fungal DNA methylation are crucial for ECM formation and regulate JA signalling (Vigneaud et al., 2023). These multilayered controls (Fig. 2a–c) likely help plants fine‐tune JA responses in fluctuating environments.
Fig. 2.
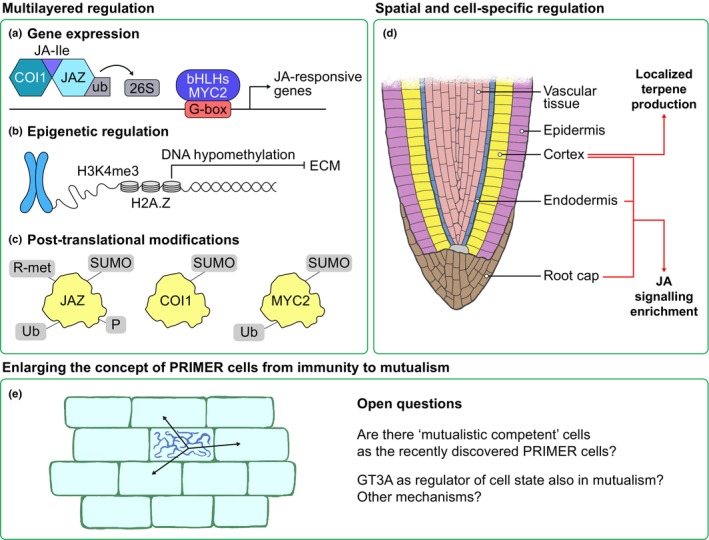
Current and future perspectives on jasmonate (JA) signalling multilayered regulation, spatial compartmentalization and PRIMER cell state concept in mutualistic interactions. Multiple layers of gene and protein regulation may act to fine‐tune JA responses in fluctuating environments. (a) JA core signalling hub COI1‐JAZ‐MYC2 regulates JA‐responsive genes through JA‐Ile‐mediated ubiquitination (Ub) of JAZ corepressor and activation of MYC2 transcription factor. (b) JA alters H3K4me3 and H2A.Z methylation landscape, whereas host hypomethylation impairs ectomycorrhizal interaction. (c) Post‐translational modifications, such as ubiquitination (Ub), phosphorylation (P), SUMOylation (SUMO) and arginine methylation (R‐met), have been reported to influence key JA signalling proteins. (d) Spatial compartmentalization of JA signalling may allow plants to mount strong but localized defence responses while maintaining overall growth. (e) A novel ‘PRIMER’ cell state initiating defence responses has been recently described in plants. Future studies could investigate whether this novel state also occurs in ‘mutualistic competent’ cells and whether it involves shared or distinct molecular mechanisms. COI1, CORONATINE INSENSITIVE 1; JAZ, JASMONATE ZIM DOMAIN; MYC2, MYELOCYTOMATOSIS2.
At the spatial level, Nguyen et al. (2023) showed that triterpene biosynthesis in Arabidopsis roots is activated in outer layers by bHLH coactivators and repressed in inner tissues by DNA‐binding with one finger (DOF) factors such as DOF AFFECTING GERMINATION 1 (DAG1). This fine‐tuned spatial regulation ensures that costly metabolite production is limited to where it is most effective. In Lotus japonicus, single‐cell RNA‐seq (sc‐RNA‐seq) (Frank et al., 2023; Sun et al., 2023) uncovered cell‐type‐specific expression patterns linked to phytohormones, root development and nodule symbiosis. Most genes potentially involved in JA signalling were enriched in the root cap, endodermis and cortex, suggesting that these zones are involved in initiating symbiosis and defence responses. These datasets provide a powerful foundation for mapping JA‐responsive genes in root tissues, in the presence of mutualistic microbes (Fig. 2d). Notably, Nobori et al. (2025) identified a rare cell state called ‘PRIMER’ cells that initiate immune responses via a unique TF GT‐3A, which then spreads to neighbouring ‘bystander’ cells, which amplify and relay immune signals systematically across tissues. This spatial organization of immune activation, akin to immune hotspots, suggests a new paradigm in plant immunity with cellular heterogeneity and local specialization. Comparative genomics and data mining of sc‐RNA‐seq would provide experimental support for a possible conserved GT‐3A‐mediated regulatory mechanism, reinforcing its evolutionary significance across the plant kingdom. It would open the question of whether this spatial organization may also apply to ‘mutualistic competent’ root cells (Fig. 2e). These studies emphasize that JA responses are compartmentalized, allowing plants to balance systemic regulation with tissue‐specific specialization. This compartmentalization of JA‐mediated responses likely allows plants to mount strong but localized defence responses while maintaining overall growth.
VII. Conclusion and future perspectives
Jasmonate signalling pathway exemplifies evolutionary ingenuity: conserved yet adaptable, modular yet context specific. From bryophytes to angiosperms, from root exudates to cell‐specific expression, JA shapes plant–microbe interactions at multiple levels. This review highlights the shift in the perspective of JA biology from a uniform systemic signal to a highly localized, dynamic and integrative network. Central TFs such as MYC2 lie at the core of this system, regulating not only defence gene expression but also feedback control, tissue specificity and crosstalk with environmental and microbial signals. Future research should explore how MYC2‐JAZ diversification correlates with ecological strategy, identify ‘mutualistic competent’ root cells and integrate spatial omics to resolve tissue‐specific signalling modules. Comparative synthetic biology could provide powerful tools to dissect and re‐engineer these networks for improved plant resilience and microbial compatibility.
Future efforts should focus on: (1) functional dissection of tissue‐ and cell‐specific modules in different plant species; (2) cross‐species comparisons to explore conserved vs specialized regulatory networks, to highlight how perennial plants (such as trees) integrate signals to maintain plant–microbes interactions in a long‐term manner; (3) how lineage‐specific differences in JA influence the balance between immune responsiveness and growth; and (4) how to resolve how JA network complexity covaries with growth strategy, ecological niche and microbiome compatibility – using single‐cell and spatial transcriptomics, motif discovery and comparative synthetic biology to reconstruct and test modular network components across diverse plant systems.
Competing interests
None declared.
Disclaimer
The New Phytologist Foundation remains neutral with regard to jurisdictional claims in maps and in any institutional affiliations.
Acknowledgements
This research was sponsored, in part, by the Genomic Science Program, US Department of Energy, Office of Science, Biological and Environmental Research, as part of the Plant‐Microbe Interfaces Scientific Focus Area at ORNL (http://pmi.ornl.gov). Oak Ridge National Laboratory, managed by UT‐Battelle, LLC, for the US Department of Energy under contract no. DE‐AC05–00OR22725. We thank Francis Martin for the nomination to write the article and the New Phytologist team, editors and reviewers for their time and work.
References
- Bowman JL, Kohchi T, Yamato KT, Jenkins J, Shu S, Ishizaki K, Yamaoka S, Nishihama R, Nakamura Y, Berger F et al. 2017. Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell 171: 287–304. [DOI] [PubMed] [Google Scholar]
- Carrell AA, Lawrence TJ, Cabugao KGM, Carper DL, Pelletier DA, Lee JH. 2022. Habitat‐adapted microbial communities mediate Sphagnum peatmoss resilience to warming. New Phytologist 234: 2111–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Liu YQ, Song WM, Chen DY, Chen FY, Chen XY, Chen ZW, Ge SX, Wang CZ, Zhan S et al. 2019. An effector from cotton bollworm oral secretion impairs host plant defense signaling. Proceedings of the National Academy of Sciences, USA 116: 14331–14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Jin G, Liu M, Wang L, Lou Y, Baldwin I, Li R. 2024. Multiomic analyses reveal key sectors of jasmonate‐mediated defense responses in rice. Plant Cell 36: 3362–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daguerre Y, Basso V, Hartmann‐Wittulski S, Schellenberger R, Meyer L, Bailly J, Kohler A, Plett JM, Martin F, Veneault‐Fourrey C. 2020. The mutualism effector MiSSP7 of Laccaria bicolor alters the interactions between the poplar JAZ6 protein and its associated proteins. Scientific Reports 10: 20362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De León IP, Montesano M. 2017. Adaptation mechanisms in the evolution of moss defenses to microbes. Frontiers in Plant Science 8: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaux PM, Schornack S. 2021. Plant evolution driven by interactions with symbiotic and pathogenic microbes. Science 371: eaba6605. [DOI] [PubMed] [Google Scholar]
- Fan JW, Hu CL, Zhang LN, Li ZL, Zhao FK, Wang SH. 2015. Jasmonic acid mediates tomato's response to root knot nematodes. Journal of Plant Growth Regulation 34: 196–205. [Google Scholar]
- Fiorilli V, Martínez‐Medina A, Pozo MJ, Lanfranco L. 2024. Plant immunity modulation in arbuscular mycorrhizal symbiosis and its impact on pathogens and pests. Annual Review of Phytopathology 62: 127–156. [DOI] [PubMed] [Google Scholar]
- Frank M, Fechete LI, Tedeschi F, Nadzieja M, Nørgaard MMM, Montiel J, Andersen KR, Schierup MH, Reid D, Andersen SU. 2023. Single‐cell analysis identifies genes facilitating rhizobium infection in Lotus japonicus . Nature Communications 14: 7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Jin N, Shen Z, Guo J, Lu H, Han S, Xiao W, Lu J, Lou Y. 2025. Both jasmonic acid‐ and abscisic acid‐mediated signalling pathways regulate the ovicidal defence of plants against phloem‐feeding insects. Plant, Cell & Environment 48: 1–4491. [DOI] [PubMed] [Google Scholar]
- Ghorbel M, Brini F, Sharma A, Landi M. 2021. Role of jasmonic acid in plants: the molecular point of view. Plant Cell Reports 40: 1471–1494. [DOI] [PubMed] [Google Scholar]
- Gimenez‐Ibanez S, Boter M, Ortigosa A, García‐Casado G, Chini A, Lewsey MG, Ecker JR, Ntoukakis V, Solano R. 2017. JAZ 2 controls stomata dynamics during bacterial invasion. New Phytologist 213: 1378–1392. [DOI] [PubMed] [Google Scholar]
- Guo D, Li J, Liu P, Wang Y, Cao N, Fang X, Wang T, Dong J. 2024. The jasmonate pathway promotes nodule symbiosis and suppresses host plant defense in Medicago truncatula . Molecular Plant 17: 1183–1203. [DOI] [PubMed] [Google Scholar]
- He K, Du J, Han X, Li H, Kui M, Zhang J, Huang Z, Fu Q, Jiang Y, Hu Y. 2023. PHOSPHATE STARVATION RESPONSE1 (PHR1) interacts with JASMONATE ZIM‐DOMAIN (JAZ) and MYC2 to modulate phosphate deficiency‐induced jasmonate signaling in Arabidopsis. Plant Cell 35: 2132–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S, Thiergart T, Vannier N, Mesny F, Ziegler J, Pickel B, Hacquard S. 2021. A microbiota–root–shoot circuit favours Arabidopsis growth over defence under suboptimal light. Nature Plants 7: 1078–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe GA, Major IT, Koo AJ. 2018. Modularity in jasmonate signaling for multistress resilience. Annual Review of Plant Biology 69: 387–415. [DOI] [PubMed] [Google Scholar]
- Huang H, Liu B, Liu L, Song S. 2017. Jasmonate action in plant growth and development. Journal of Experimental Botany 68: 1349–1359. [DOI] [PubMed] [Google Scholar]
- Johnson LY, Major IT, Chen Y, Yang C, Vanegas‐Cano LJ, Howe GA. 2023. Diversification of JAZ‐MYC signaling function in immune metabolism. New Phytologist 239: 2277–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackus ND, Lackner S, Gershenzon J, Unsicker SB, Köllner TG. 2018. The occurrence and formation of monoterpenes in herbivore‐damaged poplar roots. Scientific Reports 8: 17936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marqués‐Gálvez JE, de Freitas Pereira M, Nehls U, Ruytinx J, Barry K, Peter M, Martin F, Grigoriev IV, Veneault‐Fourrey C, Kohler A. 2025. Comparative transcriptomics uncovers poplar and fungal genetic determinants of ectomycorrhizal compatibility. The Plant Journal 123: e70352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marqués‐Gálvez JE, Pandharikar G, Basso V, Kohler A, Lackus ND, Barry K, Keymanesh K, Johnson J, Singan V, Grigoriev IV et al. 2024. Populus MYC2 orchestrates root transcriptional reprogramming of defence pathway to impair Laccaria bicolor ectomycorrhizal development. New Phytologist 242: 658–674. [DOI] [PubMed] [Google Scholar]
- Monte I, Ishida S, Zamarreño AM, Hamberg M, Franco‐Zorrilla J, García‐Casado G, Gouhier‐Darimont C, Reymond P, Takahashi K, García‐Mina JM et al. 2018. Ligand‐receptor co‐evolution shaped the jasmonate pathway in land plants. Nature Chemical Biology 14: 480–488. [DOI] [PubMed] [Google Scholar]
- Nguyen TH, Thiers L, Van Moerkercke A, Bai Y, Fernández‐Calvo P, Minnie M, Depuydt T, Colinas M, Verstaen K, Van Isterdael G et al. 2023. A redundant transcription factor network steers spatiotemporal Arabidopsis triterpene synthesis. Nature Plants 9: 926–937. [DOI] [PubMed] [Google Scholar]
- Nobori T, Monell A, Lee TA, Sakata Y, Shirahama S, Zhou J, Nery JR, Mine A, Ecker JR. 2025. A rare PRIMER cell state in plant immunity. Nature 638: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orosa B, Yates G, Verma V, Srivastava AK, Srivastava M, Campanaro A, de Vega D, Fernandes A, Zhang C, Lee J et al. 2018. SUMO conjugation to the pattern recognition receptor FLS2 triggers intracellular signalling in plant innate immunity. Nature Communications 9: 5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plett JM, Daguerre Y, Wittulsky S, Vayssières A, Deveau A, Melton SJ, Kohler A, Morrell‐Falvey JL, Brun A, Veneault‐Fourrey C et al. 2014. Effector MiSSP7 of the mutualistic fungus Laccaria bicolor stabilizes the Populus JAZ6 protein and represses jasmonic acid (JA) responsive genes. Proceedings of the National Academy of Sciences, USA 111: 8299–8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plett JM, Kemppainen M, Kale SD, Kohler A, Legué V, Brun A, Tyler BM, Pardo AG, Martin F. 2011. A secreted effector protein of Laccaria bicolor is required for symbiosis development. Current Biology 21: 1197–1203. [DOI] [PubMed] [Google Scholar]
- Pozo MJ, Van Loon LC, Pieterse CM. 2004. Jasmonates‐signals in plant–microbe interactions. Journal of Plant Growth Regulation 23: 211–222. [Google Scholar]
- Raza A, Charagh S, Zahid Z, Mubarik MS, Javed R, Siddiqui MH, Hasanuzzaman M. 2021. Jasmonic acid: a key frontier in conferring abiotic stress tolerance in plants. Plant Cell Reports 40: 1513–1541. [DOI] [PubMed] [Google Scholar]
- Rizwan HM, He J, Arshad MB, Wang M. 2025. Characterization of phenylalanine ammonia‐lyase genes in soybean: genomic insights and expression analysis under abiotic stress tolerance. Plant Stress 16: 100896. [Google Scholar]
- Shi Q, Liu R, Jiang L, Gao S, Ma J, Tian X, Jiang C, Liang C, Zhao H, Song W et al. 2025. The nuclear effector MiISE23 from Meloidogyne incognita targets JAZ proteins and suppresses jasmonate signalling, increasing host susceptibility. Plant, Cell & Environment 48: 4611–4624. [DOI] [PubMed] [Google Scholar]
- Srivastava M, Srivastava AK, Roy D, Mansi M, Gough C, Bhagat PK, Zhang C, Sadanandom A. 2022. The conjugation of SUMO to the transcription factor MYC2 functions in blue light‐mediated seedling development in Arabidopsis. Plant Cell 34: 2892–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Jiang S, Wang D, Li L, Liu B, Ran Q, Hu L, Xiong J, Tang Y, Gu X et al. 2023. Single‐cell RNA‐seq of Lotus japonicus provide insights into identification and function of root cell types of legume. Journal of Integrative Plant Biology 65: 1147–1152. [DOI] [PubMed] [Google Scholar]
- Vigneaud J, Kohler A, Sow MD, Delaunay A, Fauchery L, Guinet F, Daviaud C, Barry KW, Keymanesh K, Johnson J et al. 2023. DNA hypomethylation of the host tree impairs interaction with mutualistic ectomycorrhizal fungus. New Phytologist 238: 2561–2577. [DOI] [PubMed] [Google Scholar]
- Wasternack C, Hause B. 2013. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Annals of Botany 111: 1021–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Manninen MJ, Asiegbu FO. 2025. Beneficial mutualistic fungus Suillus luteus provided excellent buffering insurance in Scots pine defense responses under pathogen challenge at transcriptome level. BMC Plant Biology 25: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Hu J, Yuan Z. 2024. Stabilization or degradation? Post‐translational modifications of JAZ proteins in plants. Molecular Plant 17: 1002–1004. [DOI] [PubMed] [Google Scholar]
- Zander M, Lewsey MG, Clark NM, Yin L, Bartlett A, Saldierna Guzmán JP. 2020. Integrated multi‐omics framework of the plant response to jasmonic acid. Nature Plants 6: 290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Li X, Chen W, Xu Z, Chen M, Wang H, Yu D. 2022. The emerging role of jasmonate in the control of flowering time. Journal of Experimental Botany 73: 11–21. [DOI] [PubMed] [Google Scholar]
- Zhao P, Yang H, Sun Y, Zhang J, Gao K, Wu J, Zhu C, Yin C, Chen X, Liu Q et al. 2025. Targeted MYC2 stabilization confers citrus Huanglongbing resistance. Science 388: 191–198. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Yang B, Xu H, Wu J, Xu Z, Wang Y. 2022. The Phytophthora effector Avh94 manipulates host jasmonic acid signaling to promote infection. Journal of Integrative Plant Biology 64: 2199–2210. [DOI] [PubMed] [Google Scholar]
- Zhu W, Wei W, Fu Y, Cheng J, Xie J, Li G, Yi X, Kang Z, Dickman MB, Jiang D. 2013. A secretory protein of necrotrophic fungus Sclerotinia sclerotiorum that suppresses host resistance. PLoS ONE 8: e53901. [DOI] [PMC free article] [PubMed] [Google Scholar]


