Abstract
Intrastriatal delivery of the tyrosine hydroxylase gene by viral vectors is being explored as a tool for local delivery of l-dopa in animals with lesions of the nigrostriatal pathway. The functional effects reported using this approach have been disappointing, probably because the striatal l-dopa levels attained have been too low. In the present study, we have defined a critical threshold level of l-dopa, 1.5 pmol/mg of tissue, that has to be reached to induce any significant functional effects. Using new generation high-titer recombinant adeno-associated virus vectors, we show that levels of striatal l-dopa production exceeding this threshold can be obtained provided that tyrosine hydroxylase is coexpressed with the cofactor synthetic enzyme, GTP-cyclohydrolase-1. After striatal transduction with this combination of vectors, substantial functional improvement in both drug-induced and spontaneous behavior was observed in rats with either complete or partial 6hydroxydopamine lesions of the nigrostriatal pathway. However, complete reversal of motor deficits occurred only in animals in which part of the striatal dopamine innervation was left intact. Spared nigrostriatal fibers thus may convert l-dopa to dopamine and store and release dopamine in a more physiologically relevant manner in the denervated striatum to mediate better striatal output-dependent motor function. We conclude that intrastriatal l-dopa delivery may be a viable strategy for treatment and control of adverse side effects associated with oral l-dopa therapy such as on-off fluctuations and drug-induced dyskinesias in patients with Parkinson's disease.
Restoration of striatal dopamine by peripheral administration of l-dopa can provide efficient symptomatic relief in patients with Parkinson's disease (PD). However, the therapeutic efficacy of l-dopa treatment diminishes over time, concomitant with the appearance of on-off fluctuations and drug-induced dyskinesia. These complications may be caused, at least in part, by the intermittent, pulsatile supply of l-dopa provided by the standard oral l-dopa medication. Indeed, there is considerable evidence that these side effects can be reduced greatly if l-dopa is administered at a constant level, and continuous l-dopa delivery may provide a more physiological stimulation of denervated striatal dopamine receptors (1, 2). One promising delivery method is via ex vivo or in vivo gene transfer of the l-dopa-synthesizing enzyme, tyrosine hydroxylase (TH). This approach has the additional advantage of local site-specific l-dopa delivery in striatum where the dopamine deficiency is most prominent.
Studies in rodent PD models have shown that this approach is feasible by either implantation of cells engineered to produce l-dopa (3–9) or direct injection of TH-expressing viral vectors into the denervated striatum (10–14). However, expression of TH alone may not be sufficient. l-Dopa synthesis by the transduced TH enzyme critically depends on the availability of its cofactor, tetrahydrobiopterin (BH4), and it has been shown that the level of l-dopa production in the transduced striatum is very low unless expression of TH is combined with exogenous administration of BH4 or coexpression of the primary rate-limiting synthetic enzyme for BH4, GTP-cyclohydrolase-1 (GCH1; refs. 9–11, 13, and 15).
For functional striatal l-dopa delivery, the critical synthetic enzymes must be expressed durably at stable levels. The most promising results have been obtained with recombinant adeno-associated viral (rAAV) vectors (10–12, 14). This vector allows stable transduction of the neurons in the adult striatum, in the absence of neurotoxic or inflammatory reactions, and expression of the TH transgene is maintained for at least 1 year (10, 11). Nevertheless, striatal l-dopa delivery in the rat PD model has yielded disappointing functional results to date. Indeed, the functional effects of TH gene delivery reported thus far have been limited to a variable reduction in apomorphine-induced rotation. This functional parameter, moreover, has to be interpreted with caution, because reductions in apomorphine-induced rotation may be caused not only by l-dopa but also by nonspecific effects of vector delivery (e.g., striatal damage caused by inflammatory or immunological reactions; see refs. 15–17).
Two major factors may explain this apparent inefficiency of striatal TH gene transfer: either the level of l-dopa production has been too low, or the handling of the newly formed l-dopa in the denervated striatum, i.e., dopamine synthesis, storage, or release or dopamine receptor stimulation has been functionally inefficient in the absence of endogenous dopaminergic terminals. In the present study, we sought to determine the level of striatal l-dopa production that is needed to produce long-lasting functional effects in the dopamine denervated striatum and to assess the functional efficacy of intrastriatal l-dopa delivery by combined rAAV-TH and rAAV-GCH1 gene transfer by using a wider range of behavioral tests. We report that long-lasting improvement of spontaneous and drug-induced motor behavior can be achieved in the rat PD model provided that vector-induced l-dopa production exceeds a critical threshold of ≈1.5 pmol/mg of tissue and the most pronounced effects are obtained in animals with partial nigrostriatal lesions.
Methods
Animals and Surgery.
Female Sprague–Dawley rats (B & K Universal, Sollentuna, Sweden) were housed under 12/12-h light-dark cycle with ad libitum food and water. All surgical procedures were performed under halothane anesthesia according to the rules set by the Ethical Committee for Use of Laboratory Animals at Lund University. 6-Hydroxydopamine (6-OHDA, Sigma) and vector suspension injections were injected stereotaxically using 5-μl Hamilton microsyringes fitted with pulled glass pipettes (OD, 60–80 μm). Eighty-two rats received 13.5 μg of 6-OHDA (calculated as free base dissolved in 0.05% ascorbate saline) into the medial forebrain bundle (MFB) at two sites (complete lesion), and the remaining 40 rats were injected with a total of 28 μg of 6-OHDA in four deposits in the right striatum (partial lesion) as described (18).
rAAV Vector.
The rAAV-CBA-TH and rAAV-CBA-GCH1 vectors were produced by using a double-transfection method with rAAV plasmids and a helper plasmid containing the necessary gene products normally provided by adenovirus (19, 20) and purified as described (21). The final titers were 5.2 × 1011 and 1.5 × 1011 infectious units per ml for the TH and GCH1 vectors, respectively, as determined by an infectious center assay (22). Both vectors express the transgene from a hybrid promoter consisting of an enhancer element from the cytomegalovirus promoter followed by the chicken β-actin promoter containing a rabbit β-globin intron termed CBA (23).
Experimental Design.
Experiment 1.
Twenty-four animals with partial 6-OHDA lesions were used for biochemical determination of the striatal tissue concentration of l-dopa that accumulated after a single i.p. injection of 6.25 mg/kg l-dopa methyl ester (Sigma). The animals were divided into four groups to receive (i) an i.p. injection of 15 mg/kg benserazide-HCl (benz, Hoffman–La Roche), a peripheral l-dopa decarboxylase inhibitor (n = 4); (ii) 15 mg/kg benz followed 15 min later with 6.25 mg/kg l-dopa (n = 5); (iii) 15 mg/kg benz + 6.25 mg/kg l-dopa followed 30 min later with 100 mg/kg NSD-1015 (aromatic amino acid decarboxylase inhibitor, Sigma; n = 5); or (iv) NSD-1015 alone (n = 5). The animals were killed by decapitation 30 min after NSD-1015 treatment (i.e., 75 min after benz and 60 min after l-dopa) for l-dopa, dopamine, and 3,4-dihydroxyphenylacetic acid assay (see below).
Experiment 2.
Sixty-six animals with complete 6-OHDA lesions were allocated into seven groups to receive 2- or 3-μl injections of the vectors encoding for TH (n = 10 at five sites, and n = 10 at two sites), GCH1 (n = 10 at five sites, and n = 10 at two sites), or the 1:1 mixture of the two vector suspensions (n = 11 at five sites, and n = 10 at two sites) or sham operations (n = 5). Three weeks postinjection five rats from each group were treated with NSD-1015 and killed 30 min later by decapitation for biochemical analysis; the remaining animals were processed for histological analysis (see below).
Experiment 3.
Rats with either complete (n = 16) or partial (n = 16) 6-OHDA lesions were allocated into two subgroups to receive either the GCH1 (n = 8 per group) or 1:1 mix of the TH and GCH1 vectors (n = 8 per group) at five sites as described above. After completion of the behavioral tests (see Fig. 5A for details) four animals per group were taken for biochemical assay, and four animals per group were taken for immunohistochemical analysis.
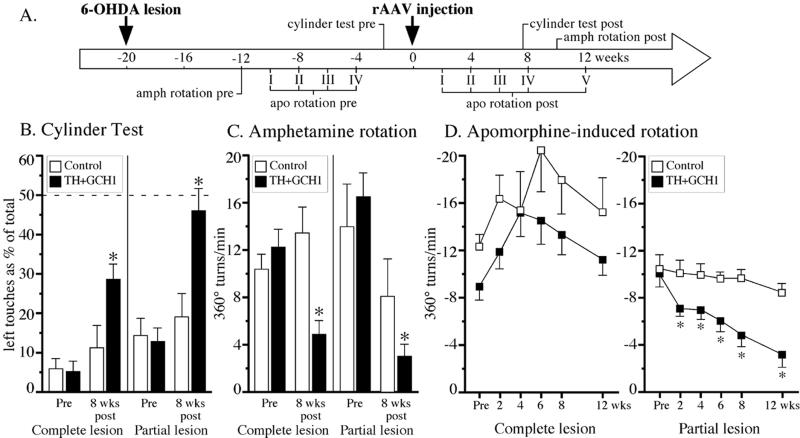
Figure 5.
Behavioral effects of l-dopa delivery. (A) Experimental time course. The 1:1 vector mix group showed clear improvements in the cylinder (B) and amphetamine-rotation (C) tests. Reduction in the apomorphine rotation (compared with the fourth pretest; Pre value in D) was observed only in the partially lesioned animals. *, significantly different from the control group and their baseline values before vector injection.
Behavioral Analysis.
Drug-induced rotation was assessed in automated rotometer bowls (24) after injection of apomorphine-HCl (Sigma; 0.25 mg/kg s.c., over 40 min) or d-amphetamine sulfate (Apoteksbolaget, Lund, Sweden; 2.5 mg/kg i.p., over 90 min). The data are expressed as net turns per min. The cylinder test (25) was performed as described (26) to quantify forelimb use. The animals were videotaped as they moved freely in a 20-cm-wide clear glass cylinder. Contacts made by each forepaw with the cylinder wall were scored from the videotapes by an observer blinded to the animals' identities. A total of 20 contacts were recorded for each animal and presented as impaired (left) forelimb contacts as a percentage of total.
Tissue Processing.
Forty-seven rats were anesthetized with pentobarbital and perfused through the ascending aorta with 50 ml of isotonic saline followed by 250 ml of 4% buffered paraformaldehyde. The tissue was sectioned on a freezing microtome at 40 μm and processed for immunohistochemical staining of TH (primary mouse anti-TH antibody, Chemicon) by using the avidin-biotin-peroxidase method (Vector Laboratories) as visualized using 3,3-diaminobenzidine as a chromogen as described (18).
The total numbers of TH+ cells in the transduced striatum were estimated by using the optical fractionator (27) with the Olympus CAST-Grid system (Olympus, Albertslund, Denmark). The coefficient of error was calculated according to ref. 28, and values ≤0.10 were accepted.
The remaining 75 rats were injected with NSD-1015 30 min before killing to measure the rate of l-dopa production in vivo (29). The striatum was dissected bilaterally, frozen, and stored at −80°C until processing for radioenzymatic determination of tissue l-dopa, dopamine, and 3,4-dihydroxyphenylacetic acid (30).
Statistical Analysis.
Group comparisons used factorial ANOVA or repeated-measures ANOVA. When an appropriate interaction term was significant (α-level < 0.05), post hoc analysis was performed by using either simple main effects with a Bonferroni correction for the P value or in other cases Student Newman Keuls test.
Results
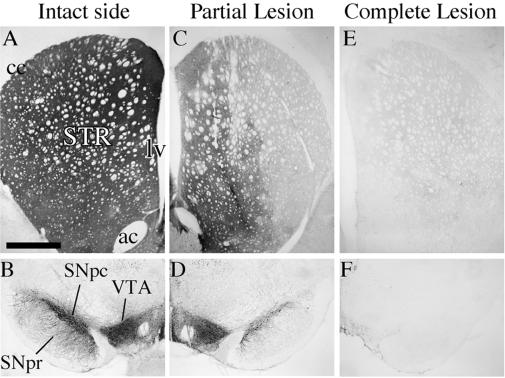
Two kinds of lesions were used: (i) complete nigrostriatal lesions induced by injection of 6-OHDA in the MFB, removing all ascending dopamine projections from the substantia nigra (SN) pars compacta and ventral tegmental area (VTA; Fig. 1 E and F) and (ii) partial lesions of the nigrostriatal dopamine projection induced by intrastriatal injections of 6-OHDA (Fig. 1 C and D). As illustrated in Fig. 1C, the intrastriatal 6-OHDA lesion lead to near complete degeneration of the fibers projecting to the central and lateral striatum, whereas the innervation of the medial and ventral parts were partially spared. At the level of SN, most of the laterally situated SN pars compacta cells were lost, whereas the TH+ cells in the VTA were preserved (Fig. 1E). In the complete MFB lesion, by contrast, the entire striatum as well as adjacent limbic areas (e.g., nucleus accumbens) were denervated (Fig. 1C), and the cell bodies in the SN pars compacta and the VTA were removed completely (Fig. 1F). In the partially lesioned animals, therefore, the residual striatal TH+ innervation provides an additional decarboxylation and storage site for the exogenously administered l-dopa, which is lacking in animals with complete lesions.
Figure 1.
TH immunohistochemistry from striatum (A, C, and E) and SN (B, D, and F). In partial lesions (C) striatal TH+ innervation is reduced largely in the lateral striatum, leaving some intact fibers medially, whereas TH+ fibers are abolished in the complete lesions (E) as compared with the intact side (A). Partial lesions destroyed dopamine neurons in the pars compacta (D), whereas the VTA cells were mostly spared (compare B and D). All TH+ cells were lost both in the SN and VTA in the complete lesions (F). ac, anterior commissure; cc, corpus callosum; lv, lateral ventricle; SNpc, SN pars compacta; SNpr, SN pars reticulata; STR, striatum. (The scale bar shown in A = 1 mm for A–C and 400 μm for D–F.)
Threshold Level of Striatal l-Dopa Accumulation.
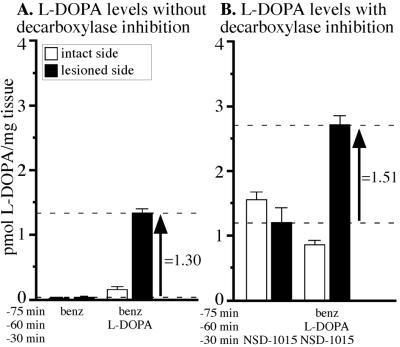
In the first experiment, we determined the level of l-dopa that accumulates in the striatum 60 min after a single i.p. injection of 6.25 mg/kg l-dopa + benz. From pilot experiments (C.W. and D.K., unpublished data) we determined that the dose used in this experiment represents the lowest effective dose of l-dopa (in combination with the peripheral aromatic amino acid decarboxylase inhibitor benz) that induces significant amelioration of motor impairments in rats with 6-OHDA lesions (conceptually comparable to a therapeutic dose in patients with PD). The time point chosen here (1 h) corresponds to the maximal behavioral effect induced by the l-dopa injection (cf. 31, 32). Under baseline conditions (benz alone group in Fig. 2A), l-dopa was barely detectable in the striatum on either the intact or lesioned sides, suggesting that newly synthesized l-dopa was converted efficiently to dopamine. The l-dopa injection resulted in a slight increase in the striatal l-dopa on the intact side, whereas on the lesioned side, there were ≈10-fold higher levels of l-dopa above that of the intact side. The increase in the striatal l-dopa induced by the peripheral injection under these conditions was ≈1.30 pmol/mg of tissue (see arrow in Fig. 2A). When the central aromatic amino acid decarboxylase enzyme was inhibited by NSD-1015, l-dopa accumulated in the lesioned as well as the intact striatum. The rate by which l-dopa accumulates after blockade of the decarboxylating enzyme provides a direct measure of the in vivo rate of tyrosine hydroxylation (33). The l-dopa synthesis rate thus could be estimated at ≈1.5 pmol/mg of tissue/30 min in the intact striatal dopamine afferents (open bar in NSD-1015 alone group in Fig. 2B). On the lesion side, the l-dopa production rate was maintained at a relatively high rate (1.20 pmol/mg of tissue) despite a strong lesion that led to ≈85% reduction in the dopamine content as compared with the intact side (data not shown), which is caused by a compensatory hyperactivity in the neurons spared by the lesion (34, 35). After administration of l-dopa in combination with NSD-1015, the striatal l-dopa level on the lesioned side was increased further to ≈2.71 pmol/mg of tissue, indicating that the level of l-dopa derived from the l-dopa injection was ≈1.51 pmol/mg of tissue (amount indicated by the arrow in Fig. 2B). We conclude, therefore, that for successful treatment by gene delivery, vector-induced l-dopa production in the striatum must be at least 1.5 pmol/mg.
Figure 2.
Striatal l-dopa levels after i.p. injection. (A) Two groups of partially lesioned animals received injections of benz or l-dopa. In the benz group, low levels of l-dopa were recovered on the lesion or the intact sides. Injection of l-dopa caused accumulation of 1.30 pmol/mg of l-dopa on the lesion side above that of benz alone (arrow). (B) When central decarboxylation is blocked for 30 min before killing, 1.2 pmol/mg of l-dopa was recovered on the lesion side (NSD-1015 alone group). l-Dopa injection increased striatal l-dopa to 2.75 pmol/mg. Thus, we estimated that accumulation of 1.51 pmol/mg of l-dopa was induced by the injection (arrow).
Cofactor Requirement for TH Transgene Activity.
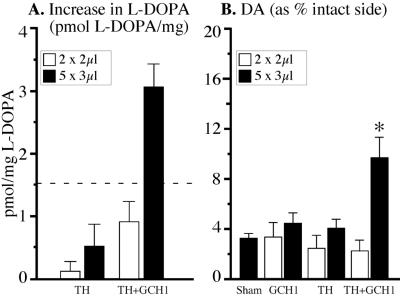
In the second experiment, we sought to assess the number of viral particles and the combination of vectors needed to reach this threshold l-dopa level. A total of 1.3 × 109 or 5.1 × 109 infectious particles (ratio of 1:3.75) were injected into two (2 × 2 μl) or five (5 × 3 μl) sites in the striatum in animals with complete MFB lesions. The levels of l-dopa (i.e., the rate of tyrosine hydroxylation in the transduced striatum) were 6–8-fold higher when TH and GCH1 were coexpressed (1:1 mix group) compared with the animals injected with the rAAV-TH vector alone (Fig. 3A). Thus, in animals receiving the rAAV-TH vector alone (open bars in Fig. 3) the vector-induced increase in striatal l-dopa levels was marginal (≈0.5 pmol/mg as determined by the difference from the control group given the rAAV-GCH1 vector alone), i.e., below the minimum required level determined by peripheral l-dopa injections (difference between two dashed lines in Fig. 3B), whereas the increase obtained in the five-site 1:1 mix group was well above this critical level. The two-site injection of the same vector mix, on the other hand, failed to produce sufficient levels of l-dopa (Fig. 3A). The increased l-dopa level in the five-site group was accompanied by an increase in striatal dopamine (to ≈10% of control side) as compared with ≈3% in the lesioned control animals (Fig. 3B). No increase in striatal dopamine above baseline was obtained in either the two-site injection group or the animals injected with the rAAV-TH vector alone.
Figure 3.
Striatal levels of l-dopa and dopamine in completely lesioned animals injected with rAAV vectors. (A) Striatal TH enzyme activity was estimated 30 min after NSD-1015 by measuring the accumulation of l-dopa. Increased rAAV-mediated striatal l-dopa in the five-site 1:1 mix group was above the threshold value obtained with peripheral injection (dashed line). In contrast, animals receiving the TH vector alone or the 1:1 mixed vectors in only two sites did not produce this threshold l-dopa level. (B) Dopamine (DA) levels were reduced greatly on the lesioned side in all groups. Striatal dopamine was increased only in the 5 × 3-μl mixed-vector group.
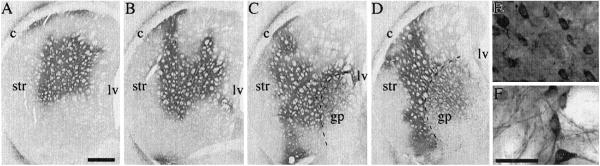
In the five-site injection group, the striatal area transduced by the rAAV-TH vector, as visualized by TH immunohistochemistry, covered large portions of the cross-sectional area in the head (Fig. 4 A and B) and the tail (Fig. 4 C and D) of the striatum. The numbers of TH+ cells in the 1:1 mix and TH alone groups at 3 weeks were 322,991 ± 36,580 and 349,528 ± 37,413, respectively (mean ± SEM). A significant number of cells was transduced also in the globus pallidus (Fig. 4 C and D). In the two-site injection group, by contrast, striatal transduction was more restricted; the total number of TH+ cells amounted to 69,165 ± 14,896 and 95,244 ± 11,986 in the AAV-TH alone and 1:1 mix groups, respectively. The vast majority of the transduced cells (>90%) appeared to be neurons in both striatum (Fig. 4E) and globus pallidus (Fig. 4F).
Figure 4.
TH immunohistochemistry in the striatum at 3 weeks after injection of the 1:1 rAAV-TH/rAAV-GCH1 vector mix. In large parts of the striatum (A–D) and globus pallidus (gp, C and D), cell bodies and fibers were TH+. Nearly all infected cells had neuron-like morphology both in striatum (E) and globus pallidus (F). ac, anterior commissure; cc, corpus callosum; lv, lateral ventricle; str, striatum. (The scale bar shown in A = 1 mm for A–D; the scale bars in E and F = 40 μm.)
Reversal of Motor Impairments.
To evaluate the effects of combined TH and GCH1 gene transfer on 6-OHDA-induced motor impairments, we applied the five-site injection protocol in rats that had received either complete or partial nigrostriatal lesions. Before vector treatment, all animals in both groups showed severe impairments including a high rate of turning in response to both apomorphine (Fig. 5D) and amphetamine (Fig. 5C) as well as impaired forelimb use in the cylinder test (Fig. 5B).
In the control groups receiving the rAAV-GCH1 vector alone, the animals remained impaired throughout the 12-week test period. In contrast, partially lesioned animals receiving the 1:1 vector mix displayed more pronounced recovery as compared with complete MFB-lesioned animals. In all three tests the animals with complete lesions remained partially impaired (Fig. 5 B and C), whereas the partially lesioned rats were restored to near normal performance (Fig. 5 B–D). Indeed, in the apomorphine test a gradual reduction in turning (70% reduction by 12 weeks) was observed only in the partially lesioned animals, whereas no change in apomorphine rotation was seen in the animals with complete MFB lesions (Fig. 5D). Because we used a simplified scoring system compared with the original designed by Schallert and Tillerson (25), it is conceivable that more detailed analysis (e.g., of sequential movements in the cylinder test) would reveal a remaining disability in the spontaneous use of the impaired paw. Indeed, this contention is supported by the incomplete recovery in the rotation tests.
In the 1:1 mix vector group, the increase in striatal l-dopa levels at 15 weeks posttransduction was 4.20 ± 0.53 and 7.75 ± 1.58 pmol/mg in the partial and completely lesioned animals, respectively (P < 0.02 compared with 3-week MFB lesion, compare to Fig. 3A), and the total number of TH-expressing striatal cells (288,346 ± 35,895 as assessed in the completely lesioned animals) remained stable over the 15-week period.
Discussion
The results highlight four critical factors that collectively determine the functional efficacy of intrastriatal l-dopa delivery by TH gene transfer to the dopamine-denervated striatum. First, striatal l-dopa delivery must reach a critical threshold to have any significant impact on motor impairments in the rat PD model. From the experiment using peripheral injections of l-dopa, we estimate that this level is ≈1.5 pmol/mg of tissue. Second, we show that this critical l-dopa level can be achieved by rAAV-mediated TH gene transfer but only if the TH enzyme is coexpressed with the primary cofactor-synthetic enzyme, GCH1. Third, we show that reversal of both spontaneous and drug-induced motor impairments can be obtained in 6-OHDA lesioned rats when this high level of l-dopa production is reached. Fourth, complete functional recovery, however, was seen only in animals with partial lesions of the nigrostriatal pathway, indicating that spared dopamine axons may contribute capacity for l-dopa decarboxylation and additional sites for functional storage and release of the newly synthesized dopamine.
Local l-dopa delivery to the striatum using either ex vivo or in vivo gene transfer techniques has been explored extensively in the rat PD model. In experiments using transplants of TH-expressing cells, TH transgene expression has been down-regulated within a few weeks after transplantation, and the functional effects have been limited to a partial reduction in apomorphine-induced rotation (4–7, 9, 36, 37). Similarly, initial attempts to use viral vectors for direct intrastriatal delivery of the TH enzyme have failed to achieve any significant functional effects beyond partial reductions in apomorphine rotation (12, 13, 38). Ushida et al. (6) and Bencsics et al. (9) were the first to show that expression of TH alone may not be sufficient, and codelivery of the BH4 cofactor may be necessary for l-dopa production in the transduced cells. Consistent with this result, Mandel et al. (11) could not detect any significant l-dopa synthesis when the striatum was transduced with the rAAV-TH vector alone. Significant increases in l-dopa production, however, were observed when the rAAV-TH vector was injected together with the rAAV-GCH1 vector or when exogenous BH4 was added to the dialysis perfusate, suggesting that the transduced striatal neurons contain insufficient endogenous levels of the BH4 cofactor. These observations suggest that the transgenic TH enzyme, when expressed in striatal neurons, is highly dependent on the presence of BH4 for its activity. Nevertheless, no vector-induced improvements in motor behavior were reported in any of these studies.
In agreement with previous findings (11, 39), our data show that the activity of the TH enzyme (as determined by the in vivo production rate of l-dopa after aromatic amino acid decarboxylase blockade) is very low when the rAAV-TH vector is injected alone. Coinjection of the rAAV-TH and rAAV-GCH1 vectors increased the in vivo activity of the TH enzyme 6–8-fold, resulting in a high level of l-dopa production and a significant 3-fold increase in striatal dopamine concentration. Indeed, the rate of tyrosine hydroxylation in the transduced striatum, 3.05 pmol/mg/30 min, was even higher than that measured in the normal, intact striatum (1.5 pmol/mg/30 min). The efficiency of the rAAV-CBA vectors used in the present study was considerably higher (at least 10-fold) compared with previous reports. This new rAAV vector, with much improved functional titer (1.5–5.2 × 1011 infectious units per ml) has several-fold higher striatal transduction capacity compared with earlier generation rAAV vectors (40). Using this new generation rAAV vector, the number of TH-expressing cells reached ≈95,000 in the animals receiving 4 μl (at two sites) and ≈350,000 after the injection of 15 μl (at five sites) of the mixed rAAV-TH/rAAV GCH1 vectors. These figures are 1–2 orders of magnitude greater than those reported in previous experiments [which range from 1,000 to 40,000 TH+ cells using earlier generation rAAV vectors (10–12)] and correspond to 3–12% of all neurons present in the adult rat striatum [2.79 × 106 cells (41)]. Moreover, our data suggest a linear relationship between number of intrastriatally injected infectious units and striatal l-dopa production. Thus, the difference between the infectious units injected in the two- and five-site groups (1:3.8 ratio) matches closely the difference in total TH-expressing cells (1:3.9 ratio), and a similar ratio (1:3.3) was obtained in striatal l-dopa levels in the two groups. In the 1:1 vector mix, the rAAV-TH vector concentration was diluted 2-fold; nevertheless, the number of TH+ striatal cells was similar to that obtained with the nondiluted vector in the rAAV-TH alone group, suggesting that not only the activity but also the stability of the TH protein may be increased by the presence of the cofactor, as suggested previously (37).
Consistent with the high level of l-dopa production, we observed marked improvements in both drug-induced and spontaneous motor behavior in the animals receiving combined injections of the rAAV-TH and rAAV-GCH1 vectors, thus providing unequivocal evidence that local delivery of l-dopa can be functional in the 6-OHDA-lesioned striatum. All previous studies on vector-mediated delivery of l-dopa have used apomorphine rotation as the sole behavioral indicator of the animals' recovery from motor impairments. However, reductions in apomorphine rotation may be misleading, because factors unrelated to l-dopa production or dopamine transmission (e.g., nonspecific striatal damage or local inflammatory reactions) can result in reduced turning rates in this test (15, 16). Therefore in the present study, we used, in addition to the apomorphine rotation test, the cylinder test as a measure of forelimb use and the amphetamine rotation test as a measure of dopamine release from neuronal storage sites to provide a more reliable assessment of motor function. That the magnitude of functional improvement observed here is critically dependent on the level of striatal l-dopa production is supported by a parallel experiment (D.K., A.B., and R.J.M., unpublished data) in which 12 μl of the earlier generation rAAV-MD-TH vector [mixed 1:1 with rAAV-MD-GCH1 (11)] was injected at four sites in the striatum in animals with partial 6-OHDA lesions. In these animals, the number of TH-expressing cells was ≈10-fold less than in the present experiment, and the vector-induced l-dopa production was ≈1 pmol/mg/30 min (i.e., ≈1/3 of that obtained in the present study and below the estimated threshold level). Indeed, no functional recovery on either drug-induced rotation or forelimb use was observed in these animals.
Another important finding in the present study was that functional recovery was more pronounced in animals with partial lesions of the nigrostriatal pathway, i.e., where part of the striatal dopamine innervation was left intact, suggesting that the spared nigrostriatal fibers may serve as an important site for functional storage and release of the newly synthesized dopamine. Residual dopaminergic fibers, in addition, may provide extra decarboxylating capacity and thus improve the conversion of l-dopa to dopamine, and dopamine released from spared nigrostriatal axons may allow for more physiological activation of striatal dopamine receptors at the appropriate synaptic target sites. In support of this interpretation we observed that amphetamine-induced rotation, which depends on release of endogenous dopamine from neuronal storage sites, was reversed almost completely in the partially lesioned animals.
In conclusion, the present results show that combined expression of the two enzymes, TH and GCH1, in striatal neurons can provide sustained local l-dopa delivery at a level sufficient to induce substantial functional recovery in the rat PD model. Although significant recovery in both spontaneous and drug-induced motor behavior was observed in animals with either complete or partial lesions of the nigrostriatal pathway, near complete reversal of motor impairments was obtained only in those animals in which a portion of the nigrostriatal dopamine projection was left intact. These data suggest that local intrastriatal l-dopa delivery may be a viable therapeutic strategy in PD not only for treatment of underlying motor deficits but also for control of adverse side effects associated with oral l-dopa therapy such as on-off fluctuations and drug-induced dyskinesias.
Acknowledgments
We thank K. Fogelström, B. Haraldsson, U. Jarl, B. Mattsson, and A. Oldén for technical assistance and the Powell Gene Therapy Center Vector Core for vector production (National Institutes of Health PO1 NS36302). This work was supported by Swedish MRC Grant K2000-99-XG-13285-02B, European Commission Grant QLK3-1999-00702, and National Institutes of Health Grant PO1 NS36302 (to N.M.).
Abbreviations
- PD
Parkinson's disease
- TH
tyrosine hydroxylase
- BH4
tetrahydrobiopterin
- GCH1
GTP-cyclohydrolase-1
- rAAV
recombinant adeno-associated virus
- 6-OHDA
6hydroxydopamine
- MFB
medial forebrain bundle
- benz
benserazide-HCl
- VTA
ventral tegmental area
- SN
substantia nigra
References
- 1.Mouradian M M, Chase T N. Exp Neurol. 1997;144:51–57. doi: 10.1006/exnr.1996.6388. [DOI] [PubMed] [Google Scholar]
- 2.Nutt J G, Obeso J A, Stocchi F. Trends Neurosci. 2000;23:S109–S115. doi: 10.1016/s1471-1931(00)00029-x. [DOI] [PubMed] [Google Scholar]
- 3.Horellou P, Brundin P, Kalen P, Mallet J, Björklund A. Neuron. 1990;5:393–402. doi: 10.1016/0896-6273(90)90078-t. [DOI] [PubMed] [Google Scholar]
- 4.Fisher L J, Jinnah H A, Kale L C, Higgins G A, Gage F H. Neuron. 1991;6:371–380. doi: 10.1016/0896-6273(91)90246-v. [DOI] [PubMed] [Google Scholar]
- 5.Wachtel S R, Bencsics C, Kang U J. J Neurochem. 1997;69:2055–2063. doi: 10.1046/j.1471-4159.1997.69052055.x. [DOI] [PubMed] [Google Scholar]
- 6.Uchida K, Tsuzaki N, Nagatsu T, Kohsaka S. Dev Neurosci (Basel) 1992;14:173–180. doi: 10.1159/000111661. [DOI] [PubMed] [Google Scholar]
- 7.Lundberg C, Horellou P, Mallet J, Björklund A. Exp Neurol. 1996;139:39–53. doi: 10.1006/exnr.1996.0079. [DOI] [PubMed] [Google Scholar]
- 8.Anton R, Kordower J H, Maidment N T, Manaster J S, Kane D J, Rabizadeh S, Schueller S B, Yang J, Edwards R H, Markham C H, et al. Exp Neurol. 1994;127:207–218. doi: 10.1006/exnr.1994.1097. [DOI] [PubMed] [Google Scholar]
- 9.Bencsics C, Wachtel S R, Milstien S, Hatakeyama K, Becker J B, Kang U J. J Neurosci. 1996;16:4449–4456. doi: 10.1523/JNEUROSCI.16-14-04449.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen Y, Muramatsu S I, Ikeguchi K, Fujimoto K I, Fan D S, Ogawa M, Mizukami H, Urabe M, Kume A, Nagatsu I, et al. Hum Gene Ther. 2000;11:1509–1519. doi: 10.1089/10430340050083243. [DOI] [PubMed] [Google Scholar]
- 11.Mandel R J, Rendahl K G, Spratt S K, Snyder R O, Cohen L K, Leff S E. J Neurosci. 1998;18:4271–4284. doi: 10.1523/JNEUROSCI.18-11-04271.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplitt M G, Leone P, Samulski R J, Xiao X, Pfaff D W, O'Malley K L, During M J. Nat Genet. 1994;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- 13.Horellou P, Vigne E, Castel M N, Barneoud P, Colin P, Perricaudet M, Delaere P, Mallet J. NeuroReport. 1994;6:49–53. doi: 10.1097/00001756-199412300-00014. [DOI] [PubMed] [Google Scholar]
- 14.Fan D S, Ogawa M, Fujimoto K I, Ikeguchi K, Ogasawara Y, Urabe M, Nishizawa M, Nakano I, Yoshida M, Nagatsu I, et al. Hum Gene Ther. 1998;9:2527–2535. doi: 10.1089/hum.1998.9.17-2527. [DOI] [PubMed] [Google Scholar]
- 15.Corti O, Sanchez-Capelo A, Colin P, Hanoun N, Hamon M, Mallet J. Proc Natl Acad Sci USA. 1999;96:12120–12125. doi: 10.1073/pnas.96.21.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isacson O. Science. 1995;269:856–857. doi: 10.1126/science.7638605. [DOI] [PubMed] [Google Scholar]
- 17.Mandel R J, Rendahl K G, Snyder R O, Leff S E. Exp Neurol. 1999;159:47–64. doi: 10.1006/exnr.1999.7159. [DOI] [PubMed] [Google Scholar]
- 18.Kirik D, Rosenblad C, Björklund A. Exp Neurol. 1998;152:259–277. doi: 10.1006/exnr.1998.6848. [DOI] [PubMed] [Google Scholar]
- 19.Hauswirth W W, Lewin A S, Zolotukhin S, Muzyczka N. Methods Enzymol. 2000;316:743–761. doi: 10.1016/s0076-6879(00)16760-6. [DOI] [PubMed] [Google Scholar]
- 20.Grimm D, Kern A, Rittner K, Kleinschmidt J A. Hum Gene Ther. 1998;9:2745–2760. doi: 10.1089/hum.1998.9.18-2745. [DOI] [PubMed] [Google Scholar]
- 21.Zolotukhin S, Byrne B J, Mason E, Zolotukhin I, Potter M, Chesnut K, Summerford C, Samulski R J, Muzyczka N. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin S K, Collis P, Hermonat P L, Muzyczka N. J Virol. 1988;62:1963–1973. doi: 10.1128/jvi.62.6.1963-1973.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu L, Daly T, Gao C, Flotte T R, Song S, Byrne B J, Sands M S, Parker Ponder K. Hum Gene Ther. 2001;12:563–573. doi: 10.1089/104303401300042500. [DOI] [PubMed] [Google Scholar]
- 24.Ungerstedt U, Arbuthnott G. Brain Res. 1970;24:485–493. doi: 10.1016/0006-8993(70)90187-3. [DOI] [PubMed] [Google Scholar]
- 25.Schallert T, Tillerson J L. Innovative Models of CNS Disease: From Molecule to Therapy. Clifton, NJ: Humana; 1999. [Google Scholar]
- 26.Kirik D, Rosenblad C, Björklund A, Mandel R J. J Neurosci. 2000;20:4686–4700. doi: 10.1523/JNEUROSCI.20-12-04686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.West M J. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- 28.Gundersen H J G, Jensen E B. J Microsc (Oxford) 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 29.Demarest K T, Moore K E. J Neural Transm. 1979;46:263–277. doi: 10.1007/BF01259333. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt R H, Ingvar M, Lindvall O, Stenevi U, Björklund A. J Neurochem. 1982;38:737–748. doi: 10.1111/j.1471-4159.1982.tb08693.x. [DOI] [PubMed] [Google Scholar]
- 31.Chang J W, Wachtel S R, Young D, Kang U J. Neuroscience. 1999;88:617–628. doi: 10.1016/s0306-4522(98)00217-6. [DOI] [PubMed] [Google Scholar]
- 32.Mandel R J. Exp Neurol. 2000;161:212–219. doi: 10.1006/exnr.1999.7245. [DOI] [PubMed] [Google Scholar]
- 33.Carlsson A, Davis J N, Kehr W, Lindqvist M, Atack C V. Naunyn Schmiedebergs Arch Pharmacol. 1972;275:153–168. doi: 10.1007/BF00508904. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt R H, Björklund A, Stenevi U, Dunnett S B, Gage F H. Acta Physiol Scand Suppl. 1983;522:19–28. [PubMed] [Google Scholar]
- 35.Agid Y, Javoy F, Glowinski J. Nat New Biol. 1973;245:150–151. doi: 10.1038/newbio245150a0. [DOI] [PubMed] [Google Scholar]
- 36.Wolff J A, Fisher L J, Xu L, Jinnah H A, Langlais P J, Iuvone P M, O'Malley K L, Rosenberg M B, Shimohama S, Friedmann T, et al. Proc Natl Acad Sci USA. 1989;86:9011–9014. doi: 10.1073/pnas.86.22.9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leff S E, Rendahl K G, Spratt S K, Kang U J, Mandel R J. Exp Neurol. 1998;151:249–264. doi: 10.1006/exnr.1998.6803. [DOI] [PubMed] [Google Scholar]
- 38.During M J, Naegele J R, O'Malley K L, Geller A I. Science. 1994;266:1399–1403. doi: 10.1126/science.266.5189.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szczypka M S, Mandel R J, Donahue B A, Snyder R O, Leff S E, Palmiter R D. Neuron. 1999;22:167–178. doi: 10.1016/s0896-6273(00)80688-1. [DOI] [PubMed] [Google Scholar]
- 40.Björklund A, Kirik D, Rosenblad C, Georgievska B, Lundberg C, Mandel R J. Brain Res. 2000;886:82–98. doi: 10.1016/s0006-8993(00)02915-2. [DOI] [PubMed] [Google Scholar]
- 41.Oorschot D E. Prog Neurobiol. 1994;44:233–247. doi: 10.1016/0301-0082(94)90040-x. [DOI] [PubMed] [Google Scholar]