Abstract
We have shown previously that LPPs (lipid phosphate phosphatases) reduce the stimulation of the p42/p44 MAPK (p42/p44 mitogen-activated protein kinase) pathway by the GPCR (G-protein-coupled receptor) agonists S1P (sphingosine 1-phosphate) and LPA (lysophosphatidic acid) in serum-deprived HEK-293 cells [Alderton, Darroch, Sambi, McKie, Ahmed, N. J. Pyne and S. Pyne (2001) J. Biol. Chem. 276, 13452–13460]. In the present study, we now show that this can be blocked by pretreating HEK-293 cells with the caspase 3/7 inhibitor, Ac-DEVD-CHO [N-acetyl-Asp-Glu-Val-Asp-CHO (aldehyde)]. Therefore LPP2 and LPP3 appear to regulate the apoptotic status of serum-deprived HEK-293 cells. This was supported further by: (i) caspase 3/7-catalysed cleavage of PARP [poly(ADP-ribose) polymerase] was increased in serum-deprived LPP2-overexpressing compared with vector-transfected HEK-293 cells; and (ii) serum-deprived LPP2- and LPP3-overexpressing cells exhibited limited intranucleosomal DNA laddering, which was absent in vector-transfected cells. Moreover, LPP2 reduced basal intracellular phosphatidic acid levels, whereas LPP3 decreased intracellular S1P in serum-deprived HEK-293 cells. LPP2 and LPP3 are constitutively co-localized with SK1 (sphingosine kinase 1) in cytoplasmic vesicles in HEK-293 cells. Moreover, LPP2 but not LPP3 prevents SK1 from being recruited to a perinuclear compartment upon induction of PLD1 (phospholipase D1) in CHO (Chinese-hamster ovary) cells. Taken together, these data are consistent with an important role for LPP2 and LPP3 in regulating an intracellular pool of PA and S1P respectively, that may govern the apoptotic status of the cell upon serum deprivation.
Keywords: apoptosis, lipid phosphate phosphatase (LPP), lysophosphatidic acid, mitogen-activated protein kinase (MAPK), phosphatidic acid, sphingosine 1-phosphate (S1P)
Abbreviations: Ac-DEVD-CHO, N-acetyl-Asp-Glu-Val-Asp-CHO (aldehyde); CHO, Chinese-hamster ovary; C1P, ceramide 1-phosphate; DG, diacylglycerol; FCS, foetal calf serum; GFP, green fluorescent protein; GPCR, G-protein-coupled receptor; LPA, lysophosphatidic acid; LPP, lipid phosphate phosphatase; MAPK, mitogen-activated protein kinase; OMPT, 1-O-oleoyl-2-O-methyl-glyceryl-3-phosphothionate; PA, phosphatidic acid; PARP, poly(ADP-ribose) polymerase; PLD, phospholipase D; S1P, sphingosine 1-phosphate; SK, sphingosine kinase; TRITC, tetramethylrhodamine β-isothiocyanate
INTRODUCTION
LPPs (lipid phosphate phosphatases) are integral membrane proteins that display broad substrate specificity in vitro catalysing the dephosphorylation of lipid phosphates [e.g. S1P (sphingosine 1-phosphate), LPA (lysophosphatidic acid), PA (phosphatidic acid) and C1P (ceramide 1-phosphate)] in a Mg2+-independent and N-ethylmaleimide-insensitive manner [1]. Three mammalian LPP isoforms have been cloned, termed LPP1 (and a spliced variant form, LPP1a), LPP2 and LPP3 [2–6], with the last corresponding to the previously identified endoplasmic reticulum protein Dri42 that is up-regulated during differentiation of intestinal epithelial cells [7]. More recently, a novel member of the family has been suggested, PRG-1 (plasticity-related gene-1), which is similar to other LPPs with the exception that it has a long C-terminal hydrophilic domain of approx. 400 amino acids [8].
The LPPs are predicted to have six transmembrane domains with the active site facing the extracellular side of the plasma membrane or the luminal side of intracellular membranes [9]. The plasma membrane location of LPP1, LPP2 and LPP3 is supported by the detection of endogenously and ectopically expressed proteins with isoform-selective antibodies [2,10–12] and epitope-tagged antibodies [13,14]. LPP1 and LPP3 have also been identified in caveolae [11,12]. In addition, LPP2 and LPP3 are expressed within intracellular organelles [10,11].
LPPs have the potential to influence physiological processes that are regulated by the GPCR (G-protein-coupled receptor) agonists LPA and S1P, including proliferation/survival, apoptosis, differentiation and cell migration. This may involve dephosphorylation of extracellular S1P and LPA via an ecto-LPP activity, which may limit the bioavailability of these agonists at their GPCR, S1P1–5 and LPA1–3 [4,10,14,15], and thereby reduce their biological effects. Recent studies have shown that the overexpression of LPP1 reduces both acute and chronic LPA-stimulated responses, including activation of p42/p44 MAPK (mitogen-activated protein kinase), PLD (phospholipase D), DNA synthesis and cell division, but without affecting either LPA receptor function or expression [10,16]. Similarly, overexpression of LPP3 in ovarian cancer cells reduces colony formation and reduces tumour growth in vitro and in vivo [17]. In addition, overexpression of LPP1, LPP1a or LPP2 reduces the S1P- and LPA-stimulated activation of p42/p44 MAPK via an unidentified mechanism in HEK-293 cells [10]. In this respect, only a small proportion of total cellular LPP activity can be measured as ecto-LPP activity in HEK-293 cells [10]. Moreover, the bulk concentrations of extracellular LPA and S1P are not significantly reduced under these conditions.
In addition, LPPs may provide a point of cellular regulation, since they have the potential to both remove and produce intracellular bioactive lipids of differing function, i.e. removal of PA, LPA, S1P and C1P and production of DG (diacylglycerol), sphingosine and ceramide. For example, PA is required, in part, for Raf translocation to the plasma membrane [18], activation of mTOR (mammalian target of rapamycin) [19], cytoskeletal structure [20] and stimulation of several enzymes in vitro, including PKC (protein kinase C)-ζ, phosphatidylinositol 4-phosphate 5-kinase, PLCγ (phospholipase Cγ) and monoacylglycerol acetyltransferase [21], whereas intracellular S1P can mobilize intracellular stores of Ca2+ [22] and may also influence the activities of intracellular enzymes to promote cell survival [23]. Relative amounts of PA and DG were changed when LPP1 was overexpressed in endothelial cells [6], but not in embryonic fibroblasts derived from LPP1 transgenic mice [24]. Conversely, DG levels were reduced and PA levels increased in embryonic fibroblasts derived from LPP3 knockout mice [25].
In the present study, we provide evidence that is consistent with an important role for LPP2 and LPP3 in regulating intracellular pools of PA and S1P respectively, which may determine the apoptotic status of cells that have been subjected to cellular stress (serum deprivation). This might, in turn, regulate the ability of exogenous LPA/S1P to activate the p42/p44 MAPK pathway, which is critical for cell survival.
EXPERIMENTAL
Materials
All biochemicals, including oleoyl-LPA and TRITC (tetramethylrhodamine β-isothiocyanate)-conjugated secondary antibody, were from Sigma; S1P was from Avanti, and Ac-DEVD-CHO [N-acetyl-Asp-Glu-Val-Asp-CHO (aldehyde)] was from Merck Biosciences. Cell culture supplies and Lipofectamine™ 2000 were from Invitrogen. Anti-(phospho-p42/p44 MAPK) (polyclonal) and anti-(p42 MAPK) antibodies were from New England Biolabs. Affinity-purified anti-LPP2 and anti-LPP3 antibodies were prepared as described previously [10]. Anti-FLAG epitope-tag antibody was from Stratagene. OMPT (1-O-oleoyl-2-O-methyl-glyceryl-3-phosphothionate) was generously provided by Dr Glenn Prestwich (Department of Medicinal Chemistry, University of Utah, Salt Lake City, UT, U.S.A.). [3H]Palmitate, [35S]methionine and [γ-32P]ATP were purchased from GE Healthcare, and [3H]sphingosine was from Tocris Cookson. [32P]Dioleoylphosphatidic acid and [32P]S1P were prepared as described previously [10]. Whatman silica gel G60 LK6D TLC plates were purchased from VWR. Human genome U133A genechips were purchased from Affymetrix.
Cell culture
HEK-293 cells that separately stably overexpress either LPP2 or LPP3 [10] and PLD1-inducible CHO (Chinese-hamster ovary) cells [26] were maintained in MEM (minimum essential medium) and Ham's F12 medium respectively, supplemented with 10% (v/v) FCS (foetal calf serum) and penicillin/streptomycin. In all cases, cells were deprived of serum for 24 h prior to experimentation.
Transient transfections
Cells were transiently transfected with plasmid constructs as required. Cells at 75–95% confluence were placed in medium containing 1% (v/v) FCS and transfected with 1 μg of plasmid construct following complex formation with Lipofectamine™ 2000, according to the manufacturer's instructions. The cDNA-containing media was removed after incubation for 24 h at 37 °C, and the cells were incubated for a further 18 h prior to agonist additions.
SDS/PAGE and Western blotting
Cell lysates were prepared using sample buffer containing 62 mM Tris/HCl (pH 6.7), 1.25% (w/v) SDS, 10% (v/v) glycerol, 3.75% (v/v) mercaptoethanol and 0.05% (w/v) Bromophenol Blue, and proteins were resolved by SDS/PAGE. Western blotting with specific antibodies was used to identify proteins of interest [10]. For example, the phosphorylated forms of p42/p44 MAPK were detected by Western blotting cell lysates with anti-phospho-specific antibodies. Anti-(p42 MAPK) antibodies were also used for Western blotting to establish equal loading of protein in each sample. Immunoreactive proteins were visualized using enhanced chemiluminesence detection.
Caspase 3/7 assays and DNA fragmentation
Caspase 3/7 activity assays using 35S-labelled PARP [poly(ADP-ribose) polymerase] and DNA fragmentation assays were performed as described previously [27].
S1P measurements
Cells (1.2×106 cells/well) were grown to confluency and equilibrated in 1 ml of DMEM (Dulbecco's modified Eagle's medium) containing fatty-acid-free BSA (2 mg/ml) at 37 °C for 1 h. [3H]Sphingosine (222000 d.p.m./well; final concentration, 5–10 nM) was resuspended in PBS containing fatty-acid-free BSA (2 mg/ml) by sonication and was added for 3 min. The medium was removed and 3H-labelled lipids were extracted in chloroform/methanol before resolution of [3H]S1P on silica gel G60 LK6D TLC plates using chloroform/methanol/acetic acid/water (25:10:1:2, by vol.) in parallel with a [32P]S1P standard. [3H]S1P was quantified by liquid-scintillation counting [22].
PA measurements
Cells were preincubated with [3H]palmitate (1 μCi/ml) for 18 h before extraction of lipids using chloroform/methanol/10 mM HCl (15:30:2, by vol.) and resolution of PA on silica G150 TLC plates using the upper phase of ethyl acetate/2,2,4-trimethylpentane/water/acetic acid (13:3:10:2, by vol.) in parallel with a standard. Radioactivity was quantified by excision of silica corresponding to PA and scintillation counting [28].
SK (sphingosine kinase) assay
Cells (approx. 107) were homogenized in SK assay buffer [26], and soluble and particulate fractions were prepared by centrifugation at 14000 g for 15 min at 4 °C. Membranes were resuspended in SK assay buffer. Aliquots of each (approx. 100 μg of protein/incubation) were incubated with sphingosine in the presence of Triton X-100 and [γ-32P]ATP for 15 min at 37 °C. The resulting [32P]S1P was extracted using butanol, which was washed twice using 2 M KCl before quantification of radioactivity and calculation of SK activity [26].
Immunofluorescence
Cells were grown on 12 mm glass coverslips to 60–90% confluence and transfected as described above. PLD1 expression was induced in the PLD1-inducible CHO cells, as required, by the addition of doxycycline (1 μg/ml) for 18 h. Cells were fixed in 3.7% (v/v) formaldehyde in PBS for 10 min, then permeabilized in 0.1% Triton X100 in PBS for 1 min. Non-specific binding was reduced by preincubating cells in blocking solution containing 5% (v/v) FCS and 1% (w/v) BSA in PBS for 1 h. Cells were incubated in primary antibodies (1:100 dilution in blocking solution) for 1 h at room temperature (or overnight at 4 °C), and then incubated with the appropriate TRITC-conjugated secondary antibodies (1:100) for 1 h. Cells were mounted on glass slides using Vectashield mounting medium and visualized using a Nikon E600 epi-fluorescence microscope.
RESULTS
Overexpression of LPP2 or LPP3 reduces the activation of p42/p44 MAPK by S1P, LPA and OMPT
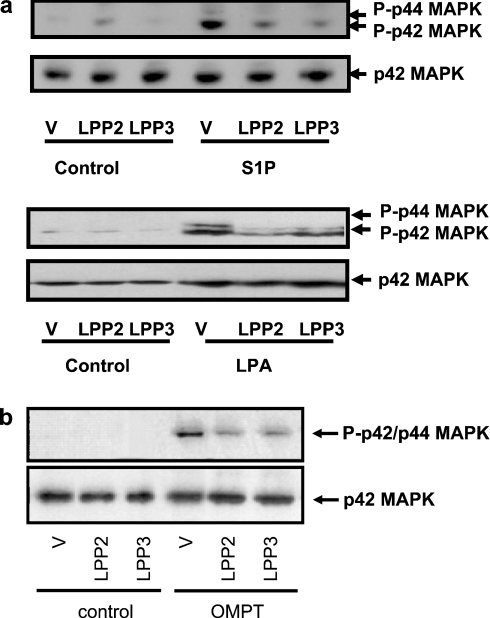
We have shown previously [10] that S1P and LPA stimulate activation of p42/p44 MAPK in serum-deprived HEK-293 cells. This is mediated by S1P and LPA receptors respectively, since activation of p42/p44 MAPK by LPA or S1P is markedly reduced by pretreating HEK-293 cells with pertussis toxin [10], which functions to uncouple Gi from LPA and S1P receptors. Moreover, we have shown previously [29] that transient overexpression of recombinant S1P1 receptor in HEK-293 cells increases the S1P-dependent activation of p42/p44 MAPK. The stable overexpression of LPP2 and LPP3 in HEK-293 cells, which migrate as 32–36 kDa proteins on SDS/PAGE respectively, leads to the dephosphorylation of [32P]PA as assessed in cell membrane pellets in vitro [10]. Overexpression of LPP2 or LPP3 reduced the activation of p42/p44 MAPK by S1P and LPA in serum-deprived HEK-293 cells (Figure 1a). A number of studies have proposed that ecto-LPP activity may account for the ability of these enzymes to reduce LPA-dependent activation of p42/p44 MAPK, calcium mobilization and PLD in other cell types. However, overexpression of LPP2 and LPP3 also reduced the activation of p42/p44 MAPK in response to the dephosphorylation-resistant thio-LPA analogue 2S-OMPT (Figure 1b). The latter has been shown [30,31] to act at LPA receptors and is resistant to LPP-catalysed dephosphorylation. Therefore these findings support an intracellular action of overexpressed LPP2 and LPP3 in the reduction of the LPA-dependent stimulation of p42/p44 MAPK in serum-deprived HEK-293 cells.
Figure 1. Overexpression of LPP2 or LPP3 reduces the activation of p42/p44 MAPK by LPA, S1P and OMPT.
Western blots showing that activation of p42/p44 MAPK by (a) 5 μM S1P or 1 μM LPA for 10 min, or (b) 5 μM OMPT for 10 min, is reduced in HEK-293 cells stably overexpressing either LPP2 or LPP3. HEK-293 cell lysates were Western blotted and probed with an anti-(phospho- p42/p44 MAPK) antibody. Blots were stripped and re-probed with anti-(p42 MAPK) antibody (to confirm equal protein loading). V, vector.
PA and S1P
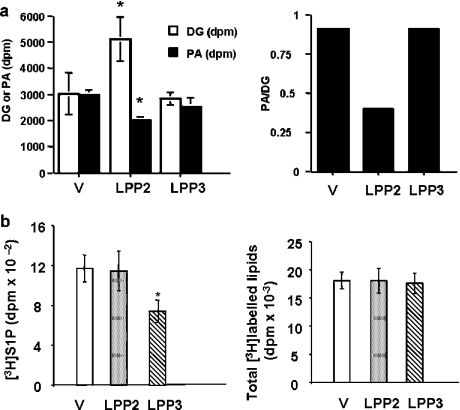
Recent evidence presented by us [26] indicates that the cell survival protein SK1, which catalyses the formation of intracellular S1P, is recruited to PLD1-derived PA in a discrete intracellular compartment in CHO cells upon induction of PLD1 expression. In this regard, SK1 contains a PA binding domain in the C-terminal region of the protein [26]. These findings are significant as intracellular S1P, and therefore SK1, has been shown to protect cells from apoptosis [23]. We therefore reasoned that the overexpression of LPPs might enable close proximity localization of these enzymes with PLD1 and/or SK1, where they may function to attenuate the action of PA and S1P. To evaluate this possibility, we examined the effect of LPP2 and LPP3 on the steady-state levels of PA and S1P. In this regard, LPP2, but not LPP3, reduced basal intracellular PA and increased DG (Figure 2a).
Figure 2. LPP2- and LPP3-dependent changes in intracellular PA and S1P levels.
Intracellular PA and S1P were measured as described in the Experimental section. The histograms show the effect of (a) overexpression of LPP2 and LPP3 on basal intracellular PA and DG levels; and (b) overexpression of LPP2 and LPP3 on intracellular S1P produced from exogenous sphingosine and total [3H]sphingosine-labelled lipids. *P<0.05 compared with stable vector-transfected HEK-293 cells. V, vector.
LPP3, but not LPP2, reduced intracellular S1P produced from exogenous sphingosine in serum-deprived HEK-293 cells (Figure 2b). The net amount of S1P in cells reflects the balance between its synthesis and removal. In this regard, a decrease in SK activity might result in a net reduction in S1P. However, SK activity was increased by approx. 2-fold in the soluble fraction of cells overexpressing LPP3 (specific activities: vector-transfected, particulate, 4.08±0.22 pmol·min−1·mg−1 of protein; soluble, 5.7±0.12 pmol·min−1·mg−1 of protein; LPP3-overexpressing, particulate 3.78±0.54 pmol·min−1·mg−1 of protein; soluble, 9.11±1.74 pmol·min−1·mg−1 of protein; n=3). There was no change in particulate SK activity, indicating that the increase in soluble SK activity was not due to redistribution of SK from the particulate to the soluble fraction. Therefore the net reduction in S1P may be due to increased dephosphorylation of S1P by LPP3. Neither LPP isoform had an effect on sphingosine uptake into cells (Figure 2b). These data are also in line with in vitro LPP2 and LPP3 activity measurements in HEK-293 cell membranes. In this regard, LPP2 preferentially catalyses dephosphorylation of dioleoyl PA compared with LPP3 (LPP2: Km=122 μM, Vmax=492 nmol·min−1·mg−1; LPP3: Km=193 μM, Vmax=60 nmol·min−1·g−1; n=3) [10].
We therefore surmized that the reduction of intracellular PA or S1P by LPP2 and LPP3 respectively might reduce the protective effect of PLD1 and SK1 on cell survival. By inducing an apoptotic state in HEK-293 cells, LPP2 and LPP3 might then affect the ability of exogenous LPA or S1P to activate the p42/p44 MAPK pathway, which is critical for cell survival.
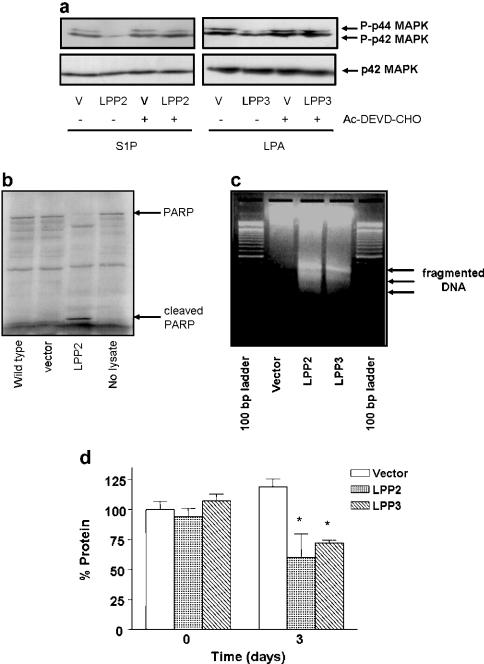
Caspases 3 and 7 play a key role in initiating apoptosis. We therefore assessed whether LPPs modulate caspase 3/7 activity and whether this enzymatic system is linked to the attenuating effect of LPPs on p42/p44 MAPK signalling. This possibility was supported by data showing that the pretreatment of serum-deprived HEK-293 cells with the caspase 3/7 inhibitor Ac-DEVD-CHO completely abrogated the ability of LPP2 and LPP3 to reduce the S1P- and LPA-dependent activation of p42/p44 MAPK respectively (Figure 3a). Moreover, the apoptotic state of serum-deprived HEK-293 cells overexpressing LPP2 and LPP3 was verified by the finding that caspase 3/7 activity was increased in LPP2-overexpressing cells compared with vector-transfected cells, as assessed by the ability of caspase 3/7 in cell lysates to proteolyse 35S-labelled PARP (molecular mass=120 kDa) into 85 and 30 kDa fragments (Figure 3b). In addition, LPP2- and LPP3-overexpressing HEK-293 cells exhibited some limited internucleosomal DNA fragmentation when serum-deprived, suggesting the onset of early-stage apoptotic events, whereas no significant DNA laddering was evident in serum-deprived vector-transfected cells (Figure 3c). Therefore the reduction in the ability of LPA or S1P to induce activation of p42/p44 MAPK can be correlated with the onset of an apoptotic state of LPP2- and LPP3-overexpressing HEK-293 cells, where cells are still viable but are compromised in their biochemical responsiveness to GPCR agonists.
Figure 3. LPP2 and LPP3 attenuate p42/p44 MAPK activation via a caspase 3/7-dependent mechanism dependent on the apoptotic state of the cells.
Stable vector or LPP2- or LPP3-transfected HEK-293 cells were treated without or with Ac-DEVD-CHO (100 μM; 18 h) prior to stimulation with 1 μM LPA or 5 μM S1P for 10 min. (a) Western blot showing that Ac-DEVD-CHO blocks the ability of LPP2 and LPP3 to reduce the activation of p42/p44 MAPK by S1P and LPA respectively; (b) autoradiograph showing the cleavage of 35S-labelled PARP in lysates from vector and LPP2-overexpressing cells; (c) agarose gel showing the intranucleosomal DNA laddering in LPP2- and LPP3- overexpressing cells compared with vector-transfected cells; (d) protein determination (Bradford assay) in vector and LPP2- and LPP3-overexpressing cells after serum deprivation. Results are presented as the percentage of protein in vector-transfected cells before serum deprivation. *P<0.05 for vector-transfected versus LPP2- and LPP3-transfected cells (n=3 experiments).
The action of LPPs appears to be dependent upon serum deprivation, as non-confluent LPP2- or LPP3-overexpressing HEK-293 cells grown in serum are viable, actively proliferate and do not undergo apoptosis. The cellular stress appears to be serum deprivation, and this is computed into an apoptotic response by LPP2 and LPP3. To provide additional evidence, we assessed the growth (indicated by protein content) of vector compared with LPP2- and LPP3-overexpressing cells after serum deprivation. These data clearly demonstrated that vector-transfected cells were still capable of growth after serum deprivation, but that growth was arrested in LPP2- and LPP3-overexpressing cells where protein content was actually reduced 3 days after serum removal, indicative of impaired cell survival and consistent with cell death (Figure 3d).
In addition to the above, we found that the expression of a number of genes directly involved in cell proliferation and survival were modulated by LPP2 or LPP3. Affymetrix array analysis using whole-genome chips indicated a reduced expression level of fibronectin 1 (50%) and EGR3 (80%; early growth response 3) in LPP2-overexpressing cells compared with vector-transfected cells (see Supplementary Figure 1a at http://www.BiochemJ.org/bj/391/bj3910025add.htm). Fibronectin 1 is linked to integrin signalling and cell matrix regulation of cell proliferation, whereas EGR3 is involved in regulating F-actin cytoskeleton rearrangement and cell survival. The expression levels of several immediate early genes are down-regulated in LPP3-transfected cells, including Jun B, ETR101, Fos B, vFosFBJ and EGR3 (see Supplementary Figure 1b at http://www.BiochemJ.org/bj/391/bj3910025add.htm). On the other hand, inositol polyphosphate 5-phosphatase is up-regulated in LPP2-overexpressing cells (see Supplementary Figure 1a at http://www.BiochemJ.org/bj/391/bj3910025add.htm), whereas several death-promoting factors are significantly up-regulated in LPP3-overexpressing cells, including FADD (Fas-associated death domain), DAP4 (death-associated protein 4) and catalase (see Supplementary Figure 1b at http://www.BiochemJ.org/bj/391/bj3910025add.htm).
LPP–SK1 co-localization
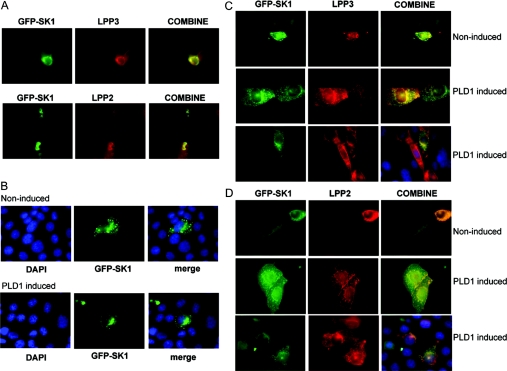
To investigate further the role of LPPs in regulating lipid phosphates in the cells, we determined the subcellular distribution of LPP2, LPP3 and SK1. LPP3- or LPP2-overexpressing HEK-293 cells were transiently transfected with a plasmid construct encoding GFP (green fluorescent protein)-tagged SK1 (green fluorescence). Overexpressed LPP2 and LPP3 were detected with anti-LPP2 and anti-LPP3 antibodies respectively (red fluorescence). These antibodies are highly specific for LPP2 or LPP3 respectively [10]. In these experiments, we found that GFP-tagged SK1 constitutively co-localized with LPP2 and LPP3 in HEK-293 cells, as indicated by the yellow fluorescence in the merged panels (Figure 4A). We conclude that LPP2 co-localizes with SK1, yet has no effect on intracellular S1P levels. In contrast, LPP3 co-localizes with SK1 and this is associated with a reduction in intracellular S1P levels.
Figure 4. LPP–SK1 co-localization.
(A) HEK-293 cells stably expressing LPP3 or LPP2 were transiently transfected with plasmid construct encoding GFP-tagged SK1. LPP3 or LPP2 expression was detected using anti-LPP3 or anti-LPP2 antibodies respectively. The panel shows constitutive co-localization of LPP3 or LPP2 and SK1 (yellow fluorescence) in HEK-293 cells. (B–D) CHO cells were transiently transfected with vector or plasmid constructs encoding GFP-tagged SK1 (green) and FLAG-tagged LPP3 or FLAG-tagged LPP2 for 24 h prior to treatment without or with doxycycline to induce PLD1 expression. Epitope-tagged LPPs were visualized using anti-FLAG primary and TRITC-coupled secondary antibodies (red). (B) SK1 (green) moves to a perinuclear compartment under conditions of PLD1 induction. Cells were co-stained with DAPI (4,6-diamidino-2-phenylindole) to identify the nucleus. In this case, cells were not co-transfected with LPP plasmid constructs, but instead were co-transfected with corresponding empty vector. (C) In cells co-transfected with FLAG–LPP3 and GFP–SK1 plasmid constructs, LPP3 and SK1 are co-localized (yellow fluorescence) in control cells and move from a vesicular localization in the cytoplasm to a perinuclear compartment upon induction of PLD1. (D) In cells co-transfected with FLAG–LPP2 and GFP–SK1 plasmid constructs, LPP2 and SK1 are co-localized in cytoplasmic vesicles (yellow fluorescence), but fail to move to the perinuclear compartment upon induction of PLD1. In (C) and (D), additional panels are presented to show co-staining with DAPI (blue), GFP–SK1 and anti-FLAG antibody in PLD1-induced cells.
We were also interested in the potential link between PA (produced by PLD1), SK1, LPP2 and LPP3. This was established using a PLD1-inducible CHO cell line. CHO cells were transiently transfected with plasmid constructs encoding GFP-tagged SK1 (green fluorescence) and FLAG-tagged LPP2 or FLAG-tagged LPP3 (red fluorescence detected with an anti-FLAG antibody) under conditions where PLD1 expression was induced by the addition of doxycycline. Indeed, we have shown previously [26] that GFP–SK1, but not GFP, is recruited to PLD1-derived PA in the perinuclear compartment and that the induced PLD1 is co-localized with SK1 in this compartment. Moreover, SK1 uses a PA binding domain in its C-terminal region to interact with PA [26]. In addition, SK1 was shown to co-localize with the Golgi marker mannosidase 2, and partially with the early endosomal marker EEA1, in the perinuclear compartment [26]. The results concerning SK1 localization in the perinuclear compartment of PLD1-induced CHO cells were confirmed in the present study, where we show that GFP–SK1 relocalized from the cytoplasm to the perinuclear compartment in cells treated with doxycycline (Figure 4B).
SK1 was constitutively co-localized with LPP3 in cytoplasmic vesicles in CHO cells that have not been treated with doxycycline (Figure 4C). Upon induction of PLD1 with doxycycline, SK1 and LPP3 remained co-localized, but were now concentrated in the perinuclear compartment of the cell. These findings suggest recruitment of both LPP3 and SK1 to the perinuclear compartment upon induction of PLD1 and PA formation. LPP2 was also constitutively co-localized with SK1 in cytoplasmic vesicles in CHO cells, but did not move to the perinuclear region upon induction of PLD1. Instead, SK1 and LPP2 appeared to remain dispersed in discrete vesicle structures throughout the cytoplasm of the cell (Figure 4D).
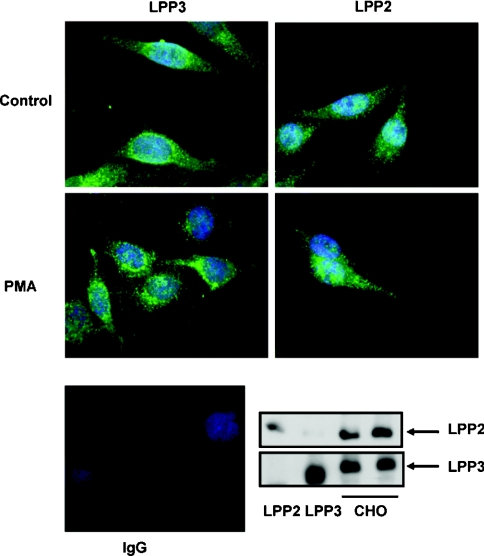
To directly address the question of the intracellular localization of LPP2 and LPP3, we looked at the subcellular distribution of the endogenous forms of LPP2 and LPP3 in CHO cells. This employed antibodies against the endogenous forms of LPP2 and LPP3, and which are highly specific [10]. CHO cells were treated without and with PMA, which has been shown previously [32] to activate PLD in these cells. This increased PA levels and was therefore comparable with a similar effect of PLD1 induction by doxycycline. Endogenous LPP2 and LPP3 were expressed in CHO cells, as determined by Western blot analysis. Immunoflourescence imaging with these antibodies in CHO cells demonstrated that endogenous LPP3 was predominantly localized in cytoplasmic vesicles that were distributed throughout the cell with some accumulation in the perinuclear compartment (Figure 5). The treatment of these cells with PMA induced increased accumulation of LPP3 in the perinuclear compartment, consistent with the observed redistribution of recombinant LPP3 in response to PLD1 induction by doxycycline (Figure 4C). Endogenous LPP2 was distributed in cytoplasmic vesicles throughout the cell and did not accumulate in the perinuclear compartment in either control or PMA-treated cells (Figure 5). These data are entirely consistent with the distribution of recombinant LPP2 in these cells and the lack of effect of PLD1 induction by doxycycline upon the subcellular localization of LPP2 (Figure 4D).
Figure 5. Subcellular distribution of endogenous LPP2 and LPP3 in CHO cells.
Serum-deprived CHO cells were stimulated without and with PMA (1 μM; 10 min). LPP3 or LPP2 expression was detected using anti-LPP3 or anti-LPP2 antibodies (green) respectively. The photograph shows re-localization of endogenous LPP3, but not LPP2, to the perinuclear compartment in response to PMA. The nuclei were stained with DAPI (4,6-diamidino-2-phenylindole) (blue). Also shown are Western blots probed with anti-LPP3 and -LPP2 antibodies to demonstrate expression of endogenous LPP3 and LPP2 in cell lysates respectively. Recombinant LPP2 and LPP3 in detergent extracts of cell membranes from stably transfected HEK-293 cells are shown as positive controls. Secondary antibody alone (Ig) was used in immunofluorescent staining experiments to show the specificity of the LPP2 and LPP3 immunoreactivity with the respective anti-LPP2 and -LPP3 antibodies.
DISCUSSION
A number of studies have proposed that the ecto-activity of LPPs may account for the ability of these enzymes to reduce the LPA-dependent activation of p42/p44 MAPK in mammalian cells. However, we demonstrate in the present study that the dephosphorylation-resistant thio-LPA analogue OMPT stimulated an activation of p42/p44 MAPK that was reduced in serum-deprived LPP2- or LPP3-overexpressing cells. Therefore our data support an intracellular action of LPP2 and LPP3 that appears to interrupt signal transmission from LPA or S1P receptors to p42/p44 MAPK. These data are in line with our previous findings [10] concerning a lack of correlation between ecto-LPP activity and attenuation of S1P/LPA-stimulated activation of p42/p44 MAPK in HEK-293 cells. The data are also supported by recent studies from Zhao et al. [33], who showed that a related LPP, LPP1, attenuates LPA-induced NF-κB (nuclear factor κB) activation and IL8 (interleukin 8) production, in part, via a mechanism that does not require its ecto-activity.
These findings have led us to define possible intracellular actions of LPPs, with the specific focus on the regulation of intracellular S1P and PA. We therefore considered whether the ability of LPP2 and LPP3 to reduce the activation of p42/p44 MAPK by exogenous S1P or LPA might be related to the apoptotic status of the cell. In this regard, the ability of LPP2 and LPP3 to attenuate S1P- and LPA-dependent activation of p42/p44 MAPK respectively, was blocked by pretreating serum-deprived cells with the caspase 3/7 inhibitor Ac-DEVD-CHO. This is consistent with other findings showing that the activation of the apoptotic executioner protease, caspase 3, leads to proteolytic inactivation of Raf, an upstream regulator of p42/p44 MAPK [34]. The putative action of caspase 3 on a common signalling intermediate (such as Raf) in the p42/p44 MAPK cascade is consistent with the heterologous effect of LPP2 and LPP3 with respect to receptor stimulation, as demonstrated by their effect on LPA, S1P and also thrombin signalling [10]. Changes in the activation status of p42/p44 MAPK in response to LPA, S1P and OMPT in LPP2- and LPP3-overexpressing cells is not a consequence of reduced recovery of cell protein, as total p42 MAPK loading is equal between samples. On the contrary, such changes in p42/p44 MAPK activation may reflect deregulation of this kinase pathway during the early onset of the apoptotic process itself that precedes eventual cell death.
Evidence from the present study indicates that LPP2 and LPP3 can reduce intracellular PA and S1P produced from exogenous sphingosine respectively, in serum-deprived HEK-293 cells. In this regard, intracellular S1P and PA have been shown to protect cells from apoptosis [23,35]. Therefore removal of intracellular S1P by LPP3 and intracellular PA by LPP2 is consistent with a pro-apoptotic action of the enzymes that appears to be dependent on serum withdrawal. This is also evident from data showing that caspase 3/7 activity and limited intranucleosomal DNA fragmentation are increased in cells overexpressing LPP2 or LPP3. The apoptotic action of LPP2 and LPP3 is supported by the fact that the products of LPP-dependent dephosphorylation of PA and S1P, DG and sphingosine respectively, have been implicated in inducing apoptosis. For instance, PKC, which is activated by DG, is involved in the promotion of apoptosis in certain cell types, whereas sphingosine activates PKCδ to promote apoptosis [36]. We therefore suggest that LPP2 and LPP3 might act on specific small intracellular pool(s) of PA and S1P that regulate the apoptotic status of the cell. These pools of PA and S1P appear to be accessed by LPPs under conditions of cellular stress (e.g. serum deprivation), which may lead to the attenuation of p42/p44 MAPK signalling in response to exogenous LPA, S1P or OMPT. Results showing that LPP3 reduces S1P are consistent with the finding that LPP3 is co-localized with SK1 in HEK-293 cells.
Evidence was also obtained to demonstrate a link between PA (derived from PLD1), LPP3, LPP2 and SK1 in CHO cells. In this regard, we demonstrated previously [26] that SK1 is recruited to a discrete intracellular perinuclear compartment upon increased PLD1 expression in CHO cells, and confirm these findings in the present study. There is a specific interaction between PLD1-derived PA and SK1 that is required in order for SK1 to move to the perinuclear compartment [26]. In the present study, we show that LPP3 and SK1 are co-localized in cytoplasmic vesicles, and that both proteins move to the perinuclear compartment upon induction of PLD1 with doxycycline in CHO cells. The data are compatible with a model in which the recruitment of SK1 to PLD1-derived PA to the perinuclear compartment might not be regulated by LPP3. In contrast, LPP2 may function to prevent recruitment of SK1 to the perinuclear compartment in PLD1-induced CHO cells. This is evident from the finding that LPP2 and SK1 remain co-localized in vesicles in the cytoplasm of PLD1-induced CHO cells. This is consistent with a model in which LPP2 might remove PLD1-derived PA, thereby preventing recruitment of SK1 to the perinuclear compartment. Additionally, our data illustrate the intracellular localization of endogenous LPP2 and LPP3 and recruitment of the latter to the perinuclear location upon PMA stimulation. In this regard, a perinuclear/endoplasmic reticulum localization of endogenous LPP3 has also been reported by others in HEK-293 cells and platelets [11,37].
In conclusion, the findings of the present study suggest that the LPP2 and LPP3 might act to dephosphorylate PA and S1P produced by PLD1 and SK1 respectively, to regulate cell survival. In the experimental systems studied in the present study, LPP2 and LPP3 are constitutively co-localized with SK1 in both HEK-293 and CHO cells. In this context, the overexpression of LPP2 or LPP3 may mask a potential agonist-dependent regulation of the co-localization of these enzymes with SK1. Therefore further studies are required to formally test whether, for instance, death-inducing agents promote co-localization of LPP2 or LPP3 with SK1.
Online data
Acknowledgments
We thank Pawel Herzyk and Giorgia Riboldi-Tunnicliffe at the Sir Henry Wellcome Functional Genomics Unit, University of Glasgow, for Affymetrix analysis. These studies were funded by BBSRC (Biotechnology and Biological Sciences Research Council) and The Wellcome Trust.
References
- 1.Brindley D. N., Waggoner D. W. Mammalian lipid phosphate phosphohydrolases. J. Biol. Chem. 1998;273:24281–24284. doi: 10.1074/jbc.273.38.24281. [DOI] [PubMed] [Google Scholar]
- 2.Kai M., Wada I., Imai S., Sakane F., Kanoh H. Identification and cDNA cloning of 35-kDa phosphatidic acid phosphatase (type 2) bound to plasma membranes. Polymerase chain reaction amplification of mouse H2O2-inducible hic53 clone yielded the cDNA encoding phosphatidic acid phosphatase. J. Biol. Chem. 1996;271:18931–18938. doi: 10.1074/jbc.271.31.18931. [DOI] [PubMed] [Google Scholar]
- 3.Kai M., Wada I., Imai S., Sakane F., Kanoh H. Cloning and characterization of two human isozymes of Mg2+-independent phosphatidic acid phosphatase. J. Biol. Chem. 1997;272:24572–24578. doi: 10.1074/jbc.272.39.24572. [DOI] [PubMed] [Google Scholar]
- 4.Roberts R., Sciorra V. A., Morris A. J. Human type 2 phosphatidic acid phosphohydrolases. Substrate specificity of the type 2a, 2b, and 2c enzymes and cell surface activity of the 2a isoform. J. Biol. Chem. 1998;273:22059–22067. doi: 10.1074/jbc.273.34.22059. [DOI] [PubMed] [Google Scholar]
- 5.Leung D. W., Tompkins C. K., White T. Molecular cloning of two alternatively spliced forms of human phosphatidic acid phosphatase cDNAs that are differentially expressed in normal and tumor cells. DNA Cell. Biol. 1998;17:377–385. doi: 10.1089/dna.1998.17.377. [DOI] [PubMed] [Google Scholar]
- 6.Tate R., Tolan D., Pyne S. Molecular cloning of magnesium-independent type 2 phosphatidic acid phosphatases from airway smooth muscle. Cell. Signalling. 1998;11:515–522. doi: 10.1016/s0898-6568(99)00028-5. [DOI] [PubMed] [Google Scholar]
- 7.Barilá D., Plateroti M., Nobili F., Onetti Muda A., Xie T., Morimoto G., Perozzi G. The Dri 42 gene, whose expression is up-regulated during epithelial differentiation, encodes a novel endoplasmic reticulum resident transmembrane protein. J. Biol. Chem. 1996;271:29928–29936. doi: 10.1074/jbc.271.47.29928. [DOI] [PubMed] [Google Scholar]
- 8.Bräuer A. U., Savaskan N. E., Kühn H., Prehn S., Ninnemann O., Nitsch R. A new phospholipid phosphatase, PRG-1, is involved in axon growth and regenerative sprouting. Nat. Neurosci. 2003;6:572–578. doi: 10.1038/nn1052. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Q. X., Pilquil C. S., Dewald J., Berthiaume L. G., Brindley D. N. Identification of structurally important domains of lipid phosphate phosphatase-1: implications for its sites of action. Biochem. J. 2000;345:181–184. [PMC free article] [PubMed] [Google Scholar]
- 10.Alderton F. A., Darroch P., Sambi B., McKie A., Ahmed I. S., Pyne N. J., Pyne S. G-protein-coupled receptor stimulation of the p42/p44 mitogen-activated protein kinase pathway is attenuated by lipid phosphate phosphatases 1, 1a, and 2 in human embryonic kidney 293 cells. J. Biol. Chem. 2001;276:13452–13460. doi: 10.1074/jbc.M006582200. [DOI] [PubMed] [Google Scholar]
- 11.Sciorra V. A., Morris A. J. Sequential actions of phospholipase D and phosphatidic acid phosphohydrolase 2b generate diglyceride in mammalian cells. Mol. Cell. Biol. 1999;10:3863–3876. doi: 10.1091/mbc.10.11.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nanjundan M., Possmayer F. Pulmonary lipid phosphate phosphohydrolase in plasma membrane signalling platforms. Biochem. J. 2001;358:637–646. doi: 10.1042/0264-6021:3580637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jasinska R., Zhang Q. X., Pilquil C. S., Singh I., Xu J., Dewald J., Dillon D. A., Bertiaume L. G., Carman G. M., Waggoner D. W., Brindley D. N. Lipid phosphate phosphohydrolase-1 degrades exogenous glycerolipid and sphingolipid phosphate esters. Biochem. J. 1999;340:677–686. [PMC free article] [PubMed] [Google Scholar]
- 14.Ishikawa T., Kai M., Wada I., Kanoh H. Cell surface activities of the human type 2b phosphatidic acid phosphatase. J. Biochem. (Tokyo) 2000;127:645–651. doi: 10.1093/oxfordjournals.jbchem.a022652. [DOI] [PubMed] [Google Scholar]
- 15.Jia Y-J., Kai M., Wada I., Sakane F., Kanoh H. Differential localization of lipid phosphate phosphatases 1 and 3 to cell surface subdomains in polarized MDCK cells. FEBS Lett. 2003;552:240–246. doi: 10.1016/s0014-5793(03)00931-1. [DOI] [PubMed] [Google Scholar]
- 16.Hooks S. B., Santos W. L., Im D.-S., Heise C. E., Macdonald T. L., Lynch K. R. Lysophosphatidic acid-induced mitogenesis is regulated by lipid phosphate phosphatases and is Edg-receptor independent. J. Biol. Chem. 2001;276:4611–4621. doi: 10.1074/jbc.M007782200. [DOI] [PubMed] [Google Scholar]
- 17.Tanyi J. L., Morris A. J., Wolf J. K., Fang X., Hasegawa Y., Lapushin R., Auersperg N., Sigal Y. J., Newman R. A., Felix E. A., et al. The human lipid phosphate phosphatase-3 decreases the growth, survival, and tumorigenesis of ovarian cancer cells: validation of the lysophosphatidic acid signalling cascade as a target for therapy in ovarian cancer. Cancer Res. 2003;63:1073–1082. [PubMed] [Google Scholar]
- 18.Rizzo M. A., Shome K., Vasudevan C., Stolz D. B., Sung T. C., Frohman M. A., Watkins S. C., Romero G. Phospholipase D and its product, phosphatidic acid, mediate agonist-dependent raf-1 translocation to the plasma membrane and the activation of the mitogen-activated protein kinase pathway. J. Biol. Chem. 1999;274:1131–1139. doi: 10.1074/jbc.274.2.1131. [DOI] [PubMed] [Google Scholar]
- 19.Fang Y., Vilella-Bach M., Bachmann R., Flanigan A., Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signalling. Science. 2001;294:1942–1945. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- 20.Exton J. H. Phospholipase D: enzymology, mechanisms of regulation, and function. Physiol. Rev. 1997;77:303–320. doi: 10.1152/physrev.1997.77.2.303. [DOI] [PubMed] [Google Scholar]
- 21.Brindley D. N., English D., Pilquil C., Buri K., Ling Z.-C. Lipid phosphate phosphatases regulate signal transduction through glycerolipids and sphingolipids. Biochim. Biophys. Acta. 2002;1582:33–44. doi: 10.1016/s1388-1981(02)00135-x. [DOI] [PubMed] [Google Scholar]
- 22.Meyer zu Heringdorf D., Liliom K., Schaefer M., Danneberg K., Jaggar J. H., Tigyi G., Jakobs J. H. Photolysis of intracellular caged sphingosine-1-phosphate causes Ca2+ mobilization independently of G-protein-coupled receptors. FEBS Lett. 2003;554:443–449. doi: 10.1016/s0014-5793(03)01219-5. [DOI] [PubMed] [Google Scholar]
- 23.Spiegel S., Milstein S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell. Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 24.Yue J., Yokoyama K., Balazs L., Baker D. L., Smalley D., Pilquil C., Brindley D. N., Tigyi G. Mice with transgenic overexpression of lipid phosphate phosphatase-1 display multiple organotypic deficits without alteration in circulating lysophosphatidate level. Cell. Signalling. 2004;16:385–399. doi: 10.1016/j.cellsig.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Escalante-Alcalde D., Hernandez L., Le Stunff H., Maeda R., Lee H.-S., Cheng G., Jr, Sciorra V. A., Daar I., Spiegel S., Morris A. J., Stewart C. L. The lipid phosphatase LPP3 regulates extra-embryonic vasculogenesis and axis patterning. Development. 2003;130:4623–4637. doi: 10.1242/dev.00635. [DOI] [PubMed] [Google Scholar]
- 26.Delon C., Manifava M., Wood E., Thompson D., Krugmann S., Pyne S., Ktistakis N. T. Sphingosine kinase 1 is an intracellular effector of phosphatidic acid. J. Biol. Chem. 2004;279:44763–44774. doi: 10.1074/jbc.M405771200. [DOI] [PubMed] [Google Scholar]
- 27.Frame M., Tate R., Adams D. R., Morgan K. M., Houslay M. D., Vandenabeele P., Pyne N. J. Interaction of caspase-3 with the cyclic GMP binding cyclic GMP specific phosphodiesterase (PDE5a1) Eur J. Biochem. 2001;270:962–970. doi: 10.1046/j.1432-1033.2003.03464.x. [DOI] [PubMed] [Google Scholar]
- 28.Tolan D., Conway A. M., Pyne N. J., Pyne S. Sphingosine prevents diacylglycerol signalling to mitogen-activated protein kinase in airway smooth muscle. Am. J. Physiol. 1997;273:C928–C936. doi: 10.1152/ajpcell.1997.273.3.C928. [DOI] [PubMed] [Google Scholar]
- 29.Alderton F., Rakhit S., Kong K. C., Palmer T., Sambi B., Pyne S., Pyne N. J. Tethering of the platelet-derived growth factor β receptor to G-protein-coupled receptors. A novel platform for integrative signalling by these receptor classes in mammalian cells. J. Biol. Chem. 2001;276:28578–28585. doi: 10.1074/jbc.M102771200. [DOI] [PubMed] [Google Scholar]
- 30.Qian L., Xu Y., Hasegawa Y., Aoki J., Mills G. B., Prestwich G. D. Enantioselective responses to a phosphorothioate analogue of lysophosphatidic acid with LPA3 receptor-selective agonist activity. J. Med. Chem. 2003;46:5575–5578. doi: 10.1021/jm034207p. [DOI] [PubMed] [Google Scholar]
- 31.Hasegawa Y., Erickson J. R., Goddard G. J., Yu S., Liu S., Cheng K. W., Eder A., Bandoh K., Aoki J., Jarosz R., et al. Identification of a phosphothionate analogue of lysophosphatidic acid (LPA) as a selective agonist of the LPA3 receptor. J. Biol. Chem. 2003;278:11962–11969. doi: 10.1074/jbc.M209168200. [DOI] [PubMed] [Google Scholar]
- 32.Bosch R. R., Smeets R. L., Sleutels F., Patel A. M., Emst-de Vries S. E., Joep J., de Pont H. H., Willems P. H. Concerted action of cytosolic Ca2+ and protein kinase C in receptor-mediated phospholipase D activation in Chinese hamster ovary cells expressing the cholecystokinin-A receptor. Biochem. J. 1999;337:263–268. [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y., Usatyuk P. V., Cummings R., Saatian B., He D., Watkins T., Morris A., Spannhake E. W., Brindley D. N., Natarajan N. Lipid phosphate phosphatase-1 regulates lysophosphatidic acid-induced calcium release, NF-κB activation and interleukin-8 secretion in human bronchial epithelial cells. Biochem. J. 2005;385:493–502. doi: 10.1042/BJ20041160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim S., Kang J., Hu W., Evers B. M., Chung D. H. Geldanamycin decreases Raf-1 and Akt levels and induces apoptosis in neuroblastomas. Int. J. Cancer. 2003;103:352–359. doi: 10.1002/ijc.10820. [DOI] [PubMed] [Google Scholar]
- 35.Athenstaedt K., Daum G. Phosphatidic acid, a key intermediate in lipid metabolism. Eur. J. Biochem. 1999;266:1–16. doi: 10.1046/j.1432-1327.1999.00822.x. [DOI] [PubMed] [Google Scholar]
- 36.Megidish T., Cooper J., Zhang L., Fu H., Hakomori S. A novel sphingosine-dependent protein kinase (SDK1) specifically phosphorylates certain isoforms of 14-13-3 protein. J. Biol. Chem. 1998;273:21834–21845. doi: 10.1074/jbc.273.34.21834. [DOI] [PubMed] [Google Scholar]
- 37.Smyth S. S., Sciorra V. A., Sigal Y. J., Pamuklar Z., Wang Z., Xu Y., Prestwich G. D., Morris A. J. Lipid phosphate phosphatases regulate lysophosphatidic acid production and signaling in platelets:studies using chemical inhibitors of lipid phosphate phosphatase activity. J. Biol. Chem. 2003;278:43214–43223. doi: 10.1074/jbc.M306709200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.