Abstract
Background
In hemodynamically unstable patients on venoarterial extracorporeal membrane oxygenation (VA-ECMO), left ventricular unloading may be essential but technically challenging, especially after aortic valve replacement.
Case Summary
We report a case of a 64-year-old male patient with postcardiotomy cardiogenic shock after combined coronary and aortic valve surgery. VA-ECMO alone was insufficient, prompting the use of a microaxial flow pump via axillary access. Challenges included left ventricle wiring through the closed bioprosthetic aortic valve and navigating a severely stenotic subclavian artery. A combination of advanced imaging and vascular techniques enabled successful device placement.
Take-Home Messages
A transesophageal ultrasound-guided mother-and-child technique with steerable catheter allows precise control for atraumatic valve crossing in case of functionally closed aortic leaflets. Intravascular lithotripsy is a potent tool to facilitate large-bore access for mechanical circulatory support in patients with calcified peripheral artery disease.
Key words: aortic valve replacement, coronary revascularization, mechanical circulatory support, postcardiotomy cardiogenic shock
Graphical Abstract
History of Presentation
A 64-year-old man presented at our department after successful resuscitation from ventricular tachycardia. Echocardiography revealed markedly reduced left ventricular (LV) ejection fraction (28%) with moderate mitral valve insufficiently and classic low-flow low-gradient aortic valve stenosis (peak pressure gradient/mean pressure gradient: 20/10 mm Hg, aortic valve area: 1.0 cm2, peak aortic velocity: 2.0 m/s, stroke volume index: 20 mL/m2). Coronarography showed significant triple-vessel disease, including critical distal left main stenosis, chronic total occlusion of the left anterior descendant, severely diseased proximal left circumflex and ramus intermedius, as well as moderate stenosis of the right coronary artery.
Take-Home Messages
-
•
The transesophageal ultrasound-guided mother-and-child technique with steerable catheter allows precise control for atraumatic valve crossing in case of functionally closed aortic leaflets.
-
•
Intravascular lithotripsy is a potent tool to facilitate large-bore access for mechanical circulatory support in patients with calcified peripheral artery disease.
Based on the complex cardiac pathology and intermediate surgical risk (Euroscore II: 38%, Society of Thoracic Surgeons score: 39%) the multidisciplinary heart team decided on combined coronary artery bypass and aortic valve replacement during the same hospitalization.
Past Medical History
The patient had a medical history of hypertension, type 2 diabetes mellitus, severe peripheral artery disease, and markedly reduced LV systolic ejection fraction. He underwent cystectomy and chemotherapy owing to papillary urothelial carcinoma in 2023.
Investigations
Preoperatively, the patient had experienced a ventricular fibrillation, terminated by short resuscitation. Still, after that episode, he remained in hemodynamically unstable condition (Society for Cardiovascular Angiography and Interventions [SCAI] stage C cardiogenic shock) and required high dosages of catecholamines, indicating a need for an emergency surgery. The patient received 4 bypasses (left anterior descending, diagonal, left circumflex, and right coronary arteries), as well as a surgical bioprosthetic aortic valve (23-mm Inspiris Resilia, Edwards Lifesciences).
After the long and complex combined surgery, the patient was not eligible for weaning and required maintained support by venoarterial extracorporeal membrane oxygenation (VA-ECMO), cannulated through the right axillar artery and the right femoral vein owing to severe peripheral artery disease.
On postoperative day 1, the patient remained hemodynamically unstable (SCAI stage C) despite VA-ECMO support. Transthoracic echocardiography showed LV overload with scarce opening of the aortic prosthesis due to VA-ECMO flow.
Management
The interdisciplinary decision was mechanical support escalation to ECPELLA (ECMO therapy with Impella devices, Abiomed) with additional LV unloading by high-volume microaxial flow pump (Impella 5.5) inserted through the left subclavian artery access.
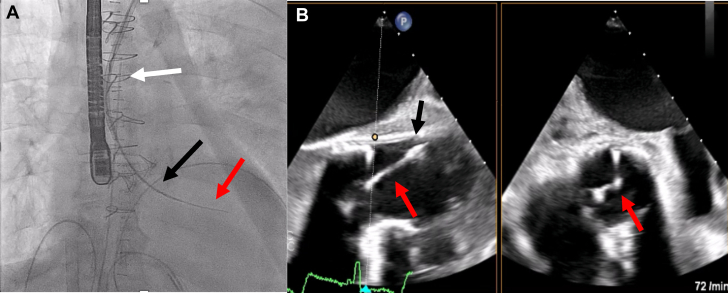
The first challenge was the crossing of functionally closed surgical aortic bioprosthesis. This was managed by three-dimensional navigation using the mother-and-child technique, with a multipurpose diagnostic catheter in a steerable guiding catheter (Agilis, Abbott) under transesophageal echocardiographic guidance. The latter provided clear visualization of the leaflet coaptation positions, allowing targeted, atraumatic crossing (Figure 1).
Figure 1.
Crossing of the Bioprosthetic Aortic Valve
(A) Fluoroscopic image showing the crossing with mother-and-child technique. The white arrow indicates the steerable sheath, the black arrow indicates the multipurpose catheter, and the red arrow indicates the wire. (B) Transesophageal echocardiogram showing the wire crossing. The black arrow indicates the multipurpose catheter, and the red arrow indicates the wire.
After the successful wiring of the LV, advancement of the pump was impossible despite surgical cutdown owing to the severely calcified left subclavian artery, which showed 80% stenosis. As plain balloon angioplasty was not sufficient, vessel preparation was escalated by using intravascular lithotripsy (Shockwave Medical) up to 6 mm diameter, after which stenosis severity was reduced to 30%. After that, the microaxial flow pump was successfully advanced into the LV, resulting in marked improvement in the patient's hemodynamic status.
Outcome and Follow-Up
The patient received several transfusions on postoperative days 2 and 4 because of ECMO-related access site bleeding, then treatment with broad-spectrum antibiotics on postoperative day 6 because of sepsis caused by Klebsiella pneumoniae; and these ultimately led to improved hemodynamic status. The extended duration of mechanical circulatory support was necessitated not only by the initial postcardiotomy cardiogenic shock but also by the development of severe septic and hemorrhagic shock. Additional factors contributing to prolonged support included persistent myocardial dysfunction with delayed recovery, transient right ventricular failure, and multiorgan instability, which made early weaning unfeasible.
After 20 days, de-escalation of the ECPELLA support was possible, and the VA-ECMO was removed. One week after the VA-ECMO removal, the patient developed seizures, and computer tomography showed signs of subarachnoid hemorrhage. Antithrombotic therapy was paused, but heparin administration was maintained owing to the ongoing microaxillary pump support; still, neurologic conditions did not progress. After 10 more days, the microaxillary pump was also successfully weaned and explanted. On day 40, the patient was discharged to a rehabilitation facility. At the 4-month follow-up, the patient was assessed as NYHA functional class I status, with no limiting symptoms. Echocardiography showed improved ejection fraction (34%) and good bioprosthesis function.
Discussion
With an increasing number of patients undergoing urgent cardiac surgery, the need for postoperative mechanical circulatory support devices for LV failure represents a daily clinical dilemma.1 VA-ECMO implanted either centrally or peripherally is still one of the most commonly used mechanical circulatory support devices in postcardiotomy cardiogenic shock. However, the overall survival is not more than 25% to 42% in such cases according to the literature.2 The lack of cardiac recovery in a sizable proportion of patients on VA-ECMO may be due to the increased cardiac afterload caused by VA-ECMO and the absence of active LV venting.
Microaxillary flow pump decreases wall tension of the ventricle, and therefore it reduces cardiac workload and myocardial oxygen consumption as well as attenuating LV afterload. Surgically implanted microaxillary flow pumps providing 5.5 L blood flow represent the possibility of combining full left-sided hemodynamic support with LV unloading, thus facilitating its recovery.3, 4, 5 Evidence of the beneficial effects of the combined use of mechanical support devices after cardiac surgery is limited. Still, data suggest lower mortality compared to VA-ECMO alone, with comparable incidence of neurologic, gastrointestinal, and limb-related complications.6, 7, 8, 9
In the case presented, such a combined mechanical support strategy was used in a patient with very complex cardiac and vascular pathology. Insertion of these large-bore devices requires meticulously performed vascular access to minimize potential vascular complications or to make it feasible at all. This includes the use of advanced interventional technologies such as intravascular lithotripsy, which modifies intimal and medial calcium, facilitating the introduction of large-bore devices without causing extensive dissections.10
Conclusions
In patients with postcardiotomy cardiogenic shock, combined circulatory support may be beneficial for providing sufficient organ perfusion and protecting the LV from overload stress. Still, its implantation can be challenging, requiring advanced interventional and imaging techniques for safe performance.
Visual Summary.
Timeline of the Case
| Timeline | Event |
|---|---|
| Day 1 | A 64-year-old man presented with ventricular tachycardia requiring advanced life support. Echocardiography showed severely reduced left ventricular ejection fraction with low-flow, low-grade aortic valve stenosis. Coronarography revealed triple-vessel disease with critical left main stenosis. Patient underwent combined CABG (4 bypasses) and AVR and required maintained support by venoarterial ECMO. |
| Day 2 | In the coronary care unit with progressive pulmonary edema. Echocardiography showed sign of left ventricular overload with scarce opening of the aortic prosthesis due to ECMO flow. Decision for mechanical support escalation to ECMO with Impella devices (ECPELLA, Abiomed). |
| Day 20 | ECMO removal |
| Day 22 | Tracheostoma |
| Day 26 | Subarachnoid hemorrhage |
| Day 30 | Impella 5.5 was removed. |
| Day 40 | Patient was discharged to rehabilitation facility |
AVR = aortic valve replacement; CABG = coronary artery bypass graft; ECMO = extracorporeal membrane oxygenation.
Funding Support and Author Disclosures
Dr Andreka was supported by the 2023 EAPCI Education and Training Grant, provided by Abbott Laboratories. Dr Toth has received consultancy fees from Abbott, Abiomed, Medtronic, Biotronik, Boston Scientific, and Terumo outside the present work. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Stretch R., Sauer C.M., Yuh D.D., Bonde P. National trends in the utilization of short-term mechanical circulatory support incidence, outcomes, and cost analysis. J Am Coll Cardiol. 2014;64:1407–1415. doi: 10.1016/j.jacc.2014.07.958. [DOI] [PubMed] [Google Scholar]
- 2.Lorusso R., Whitman G., Milojevic M., et al. 2020 EACTS/ELSO/STS/AATS expert consensus on post-cardiotomy extracorporeal life support in adult patients. Eur J Cardiothorac Surg. 2021;59:12–53. doi: 10.1093/ejcts/ezaa283. [DOI] [PubMed] [Google Scholar]
- 3.Van Diepen S., Katz J., Albert N., et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136:e232–e268. doi: 10.1161/CIR.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 4.Miller P.E., Solomon M.A., McAreavey D. Advanced percutaneous mechanical circulatory support devices for cardiogenic shock. Crit Care Med. 2017;45:1922–1929. doi: 10.1097/CCM.0000000000002676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H., Wang T., Wang J., et al. Different strategies in left ventricle unloading during venoarterial extracorporeal membrane oxygenation: a network meta-analysis. IJC Heart and Vasculature. 2024;54 doi: 10.1016/j.ijcha.2024.101506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sangani D., Patel P., Kunamalla A., Jolly N. Complex clinical cases a case of cardiogenic SHOCK following surgical valve replacements. JACC. 2024;83 [Google Scholar]
- 7.Chen S.W., Tsai F., Lin Y., et al. Long-term outcomes of extracorporeal membrane oxygenation support for postcardiotomy shock. J Thorac Cardiovasc Surg. 2017;154:469–477.e2. doi: 10.1016/j.jtcvs.2017.02.055. [DOI] [PubMed] [Google Scholar]
- 8.Siegenthaler M.P., Brehm K., Strecker T., et al. The Impella Recover microaxial left ventricular assist device reduces mortality for postcardiotomy failure: a three-center experience. J Thorac Cardiovasc Surg. 2004;127:812–822. doi: 10.1016/j.jtcvs.2003.09.055. [DOI] [PubMed] [Google Scholar]
- 9.Khorsandi M., Shaikhrezai K., Prasad S., et al. Advanced mechanical circulatory support for post-cardiotomy cardiogenic shock: a 20-year outcome analysis in a non-transplant unit. J Cardiothorac Surg. 2016;11:29. doi: 10.1186/s13019-016-0430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price L.Z., Safir S., Faries P., McKinsey J., Tang G., Tadros R. Shockwave lithotripsy facilitates large-bore vascular access through calcified arteries. J Vasc Surg Cases Innovative Tech. 2021;7:164–170. doi: 10.1016/j.jvscit.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]