Abstract
Background
Nonischemic cardiomyopathy (NICM) can be caused by single-gene mutations, including genes such as inorganic pyrophosphatase 2 (PPA2) with multisystem effects.
Case Summary
A 28-year-old woman presenting with respiratory symptoms was discharged with a diagnosis of decompensated idiopathic NICM. Her NICM progressively worsened, and the patient underwent a heart transplant at the age of 38 and again at the age of 42. At age 47, genetic testing confirmed 2 mutations in the PPA2 gene that had caused her NICM.
Discussion
This patient is to our knowledge the oldest published to date (48 years) presenting with cardiac symptoms who has PPA2 deficiency, a mitochondrial disease characterized by sudden cardiac death in infancy.
Take-Home Message
This case exemplifies the utility of employing genetic testing early in the diagnostic workup of NICM before applying the designation “idiopathic.”
Key words: cardiomyopathy, mitochondrial disease, monogenic, PPA2
Graphical Abstract
Visual Summary.
Timeline Summary of the Key Events in the Patient's Clinical Course
cMRI = cardiac magnetic resonance imaging; EF = ejection fraction; ICD = implantable cardioverter-defibrillator; LVAD = left ventricular assist device.
History of Presentation
A 28-year-old woman presented with 3 weeks of progressive exertional dyspnea after a suspected respiratory infection. Echocardiography showed a dilated hypokinetic left ventricle (LV) with severe LV impairment (LV ejection fraction [EF]: 20%) and moderate to severe mitral regurgitation. She was discharged on guideline-directed medical therapy for heart failure (HF) with reduced EF. She continued to exhibit NYHA functional class II symptoms after her initial presentation, and over the subsequent years, her course was complicated by ischemic strokes at ages 32 and 34.
Past Medical History
The patient had a history of migraines and borderline hypercholesterolemia, with no history of excessive alcohol or substance use. There was no family history of cardiomyopathy or sudden cardiac death (SCD).
Differential Diagnosis
The presumptive diagnosis since the time of her initial workup was idiopathic nonischemic cardiomyopathy (NICM), possibly due to remote viral myocarditis.
Investigations
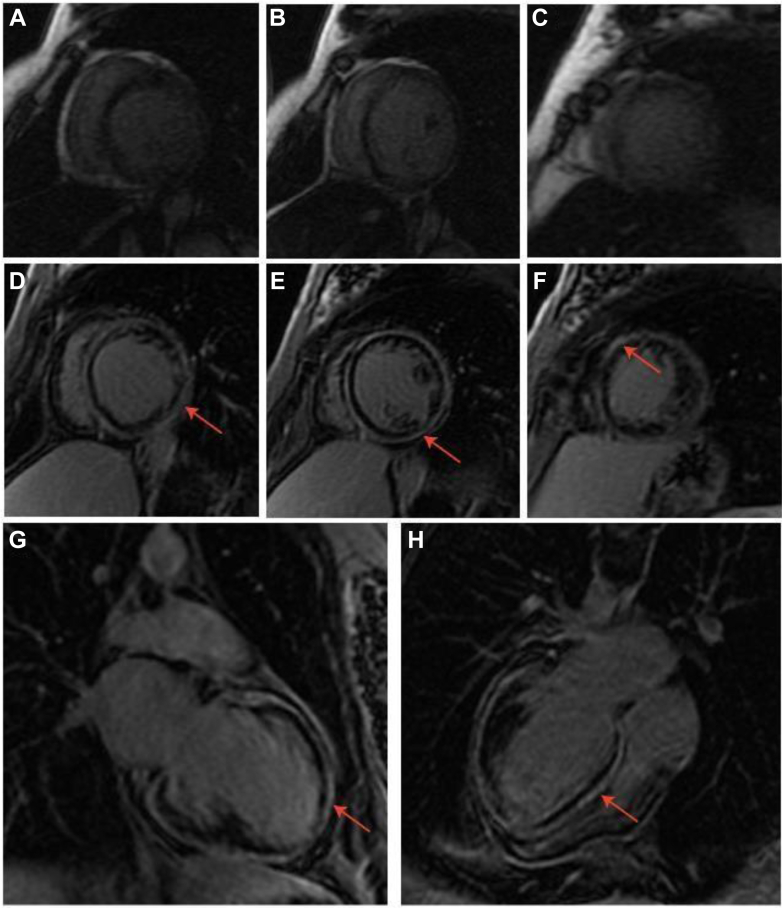
Laboratory investigations showed an elevated N-terminal pro–B-type natriuretic peptide of 708 pg/mL (normal: <180 pg/mL). An endomyocardial biopsy was performed at age 29, which revealed fibrosis and nonspecific histologic features of cardiomyopathy (Figure 1). Cardiac magnetic resonance imaging at age 29 demonstrated mild LV dilation, global LV hypokinesis with normal LV wall thickness, and no evidence of myocardial scarring (Figures 2A to 2C). Echocardiograms conducted between ages 29 to 31 after her initial presentation demonstrated stabilized LVEF (∼40%). Computed tomography of the brain after her stroke revealed left cerebellar infarction, coincident with LV thrombus on echocardiography. A repeat cardiac magnetic resonance imaging at age 32 revealed that her LVEF had dropped to 28%, and delayed enhancement images showed extensive circumferential and predominantly subepicardial myocardial fibrosis (Figures 2D to 2H). Upon increased system access to cardiogenetic testing, the Comprehensive Cardiology Panel (Blueprint Genetics) was arranged when the patient was age 47; test results identified 2 pathogenic missense variants in gene PPA2 (c.476C>T, p.(Thr159Met) and c.683C>T, p.(Pro228Leu)). Genetic epidemiologic data from the Genome Aggregation Database (gnomAD)1 indicate the variants are highly likely to be on opposite chromosomes.
Figure 1.
Right Ventricle Endomyocardial Biopsy (Age 29 Years)
Endomyocardial biopsy of the right ventricle (H&E stain; 100× magnification) shows focal interstitial fibrosis (asterisks) and hypertrophic cardiomyocytes with hyperchromatic and dysmorphic nuclei (arrows). Very little fat is present in the biopsy (not shown in this photomicrograph). There was no convincing histologic evidence of acute myocarditis, granulomatous inflammation, vasculitis, ischemia or thrombosis, glycogen storage disease, amyloid, or microorganisms/viral inclusions. A rare collection of mature adipocytes was identified; however, the extent of “marbling” was considered insufficient for a diagnosis of arrhythmogenic right ventricular cardiomyopathy. These findings are compatible with a cardiomyopathic process. H&E = hematoxylin and eosin.
Figure 2.
Delayed-Enhancement Cardiac Magnetic Resonance Imaging Scans
Delayed-enhancement cardiac magnetic resonance imaging scans from 2006 when the patient was age 29 (A to C) and 2010 when she was age 33 (D to H). The red arrows indicate late gadolinium enhancement. (A and D) Short-axis basal slice; (B and E) short-axis mid slice; (C and F) short-axis apical slice; (G) long-axis 2-chamber slice; (H) long-axis 4-chamber slice.
Management
Management included a single-chamber implantable cardioverter-defibrillator for primary prevention and mitral transcatheter edge-to-edge repair for severe functional mitral regurgitation. Despite the pharmacologic and interventional measures, the patient continued to decline from a functional and symptomatic perspective. Ultimately, a left ventricular assist device was implanted at the age of 38 as a bridge to transplant, and orthotopic heart transplantation followed shortly thereafter. Early after transplant, she developed severe cardiac allograft vasculopathy in the absence of any identifiable risk factors for early aggressive disease, and a second transplant was performed at age 42.
Based on the genetic findings, she was referred to a metabolic specialist for mitochondrial disease management. Her initially unrecognized mitochondrial disease was suspected of contributing to her longstanding myalgias, managed with pregabalin, and neuropathic-like pain, characterized as burning in her feet. Interestingly, since starting semaglutide (age 47), she noted significant improvements in both myalgias and fatigue, raising the possibility of an inflammatory component.2 Further management recommendations were primarily supportive given the lack of targeted PPA2 disease–modifying treatment, including avoidance of mitochondrial toxins such as ethanol, aminoglycosides, and tobacco smoking; avoidance of physical stressors such as extreme heat and sleep deprivation; and a trial of supplements (eg, coenzyme Q10, creatine, B vitamins, and antioxidants). A suggestion was made to avoid vinegars based on a previous anecdotal case report of acetic acid worsening myalgias in patients with PPA2-related mitochondrial disease.3
Outcome and Follow-Up
Between the second transplant and her present age of 48 years, the patient's allograft function has remained normal, with no rejection or recurrence of cardiac allograft vasculopathy. However, she has developed hypertension, neuropathic-like pain, myalgias, and premature cataracts during this period.
Discussion
Herein, we present a patient with PPA2-related mitochondrial disease who, to our knowledge, is the oldest reported individual with first-onset cardiac symptoms of HF at 28 years of age. She is also the oldest reported living affected person to date at age 48, after having received 2 heart transplants. Informed consent for publication was obtained. Ethics board review was not required for individual case report as per institutional policy.
Dilated NICM is a common structural heart disorder characterized by systolic dysfunction and an enlarged LV, with prevalence estimated between 1 per 2,500 and 1 per 250 people; it is complicated by HF, arrhythmia, and SCD.4 Monogenic causes of NICM are found in approximately 30% of patients, and it can be classified broadly as sarcomeric or nonsarcomeric in etiology.5
The inorganic pyrophosphatase 2 (PPA2) gene encodes a mitochondrial enzyme that hydrolyzes pyrophosphate (PPi) to inorganic phosphate, important for many biological processes such as adenosine triphosphate (ATP) synthesis. Presentations of PPA2-related mitochondrial disease include infant SCD (age <2 years) and SCD in teenagers triggered by variable alcohol intake or a viral infection.3
The 2 variants in this patient, T159M and P228L, have been functionally studied and described as “milder” variants compared with others reported, which may explain why our patient had later-onset NICM and HF than other affected individuals in the literature.3,6 Across the 62 other individuals reported to date, 16 (26%) have at least 1 copy of the P228L allele, and 3 (5%) have the T159M allele. Notably, T159M is the only PPA2 variant for which homozygosity has been observed in gnomAD,1 which aims to be a diverse reference genome database of people without severe pediatric disease. Observed homozygosity for T159M further supports the suggestion that it is a milder variant.
Table 1 summarizes the cardiac, myopathic, and neurologic phenotypic similarities between our patient and 5 other reported individuals with PPA2 deficiency who survived beyond 18 years of age (6/63, 9.5%). Four of the 5 other adult patients carry the P228L allele, similar to our patient. Notably, our patient is the only one who did not report extreme intolerance to alcohol during her teens and 20s, unlike other individuals with PPA2-related disease, who had adverse cardiac and/or neurologic symptoms and/or pain after ≤2 standard alcoholic beverages. However, she has since become aware in her 40s of alcohol sensitivity manifesting as acute myalgias after 1 to 2 servings of alcohol. The remainder of the phenotypic features were generally shared with the other affected individuals.
Table 1.
Characteristics of All Known Patients With PPA2-Mitochondrial Disease Who Have Lived Past Age 18 Years, Including Our Patient (6/63, 9.5%)
| Present Paper | Kennedy et al6 | Guimier et al3 | Manzanilla-Romero et al7 | |||
|---|---|---|---|---|---|---|
| Patient identifier | — | F1 II-2 | F1 II-4 | F4 II-4 | F17 II-1 | F1 II-1 |
| Sex | F | M | F | M | F | F |
| PPA2 pathogenic variants and protein change | c.476C>T, p.(Thr159Met) c.683C>T, (p.Pro228Le u) |
c.514G>A, p.(Glu172Lys) c.683C>T, p.(Pro228Leu) |
c.514G>A, p.(Glu172Lys) c.683C>T, p.(Pro228Leu) |
c.514G>A, p.(Glu172Lys) c.683C>T, p.(Pro228Leu) |
c.514G>A, p.(Glu172Lys) c.833T>C, p.(Leu278Ser) |
Homozygous c.683C>T (p.Pro228Leu) |
| Age at symptom onset (y) | 28 | 14 | 9 | 14 | 17 | 14 |
| Age at publication (y) | 47 | 38 | 33 | 19 | 40 | 21 |
| Enlarged left ventricle | Yes | No | No | No | Unk | Unk |
| Myocardial fibrosis | Yes | Yes | Yes | No | Yes | Yes |
| ICD implanted | Yes | Yes | Yes | No | No | Yes |
| Heart failure | Yes | No | No | No | Yes | Unk |
| Viral onset to symptoms | Yes | No | No | No | No | Yes |
| Sensitive to alcohol | No (20s); yes (40s) | Yes | Yes | Yes | Yes | Yes |
| Myalgias/evidence of extracardiac myopathy | Yes | No | Yes | No | No | No |
| Peripheral neuropathy | Yes | No | No | Yes | Yes | No |
ICD = implanted cardioverter defibrillator; PPA2 = inorganic pyrophosphatase 2; Unk = unknown.
Acute decompensation can be triggered by cellular stressors such as viral infection and alcohol, both well-known triggers in other mitochondrial diseases, which can cause cellular hyperthermia and increase metabolic demand.8 Viral infections and/or evidence of myocarditis have been noted in most (33/62, 53%) of the PPA2 case studies to date as potential agents affecting the disease course, particularly cardiac symptom onset (Supplemental Table 1). Guimier et al3 hypothesized that marked reduction in PPA2 activity in hyperthermic conditions may lead the cardiac muscle into an ATP deficit, and a secondary trigger may initiate acute symptom manifestations. This hypothesis is supported by previous observations of acute-onset lactic acidosis and cardiomyopathy in other PPA2 cases,6 as myocardial fibrosis could reflect a subclinical reduction in ATP availability in some myocardial cells. This has important implications for the potential genetic underpinning of cases of presumed myocarditis. In a study of 336 patients with acute myocarditis, Lota et al9 identified 8% of patients that harbored pathogenic variants for dilated NICM or arrhythmogenic cardiomyopathy. Hence, unconfirmed myocarditis may be a potential indication for genetic testing if HF symptoms are not resolved after viral clearance.
Alcohol has been associated with myalgias and/or extreme pain symptoms in almost all (13/14) individuals with PPA2-mitochondrial disease who lived beyond the age of 13 (Supplemental Table 1), with the exception of our patient, who did not report adverse reactions to consumption of 1 to 2 standard alcoholic beverages in her 20s. The buildup of PPi possibly tipping the muscle into an ATP deficit is a proposed mechanism for these symptoms.3,6 PPi is produced upon alcohol (and vinegar) metabolism into acetic acid and subsequent activation to acetyl-CoA. However, further functional studies are needed to characterize the precise mechanisms and conditions of these adverse effects of alcohol on cardiac phenotypes.
Mitochondrial diseases often have myopathic and neurologic presentations, with some affecting certain organs (ie, ocular, endocrine, heart) more than others; those with high energy demands are the most affected.10 Our patient's PPA2 mutations are suspected to be involved in the progression of her myalgias, premature cataracts, and migraines. Myopathic and neurologic features were frequent in other PPA2-affected individuals (16/62, 26%), with the most common findings being hypotonia, seizures, and myalgias (Supplemental Table 1). Therefore, specialists need to communicate and investigate for multisystemic consequences.
Conclusions
Genetic testing was key to the diagnosis of NICM in this young patient. Despite being conducted many years after initial presentation, the test result has affected therapeutic recommendations for management. Judicious use of genetic testing has the potential to improve clinical outcomes in patients with cardiomyopathy, especially as targeted therapies emerge. Diagnosis and interventions earlier in the disease course will likely have both physical and psychological benefits for patients and will also aid in early assessment of family members.2
Funding Support and Author Disclosures
Dr Roston has received honoraria from Cardurion Pharma and Solid Biosciences (advisory board). Dr Sedlak has received honoraria for speaking engagements and advisory boards from Eli-Lilly, Novo Nordisk, Novartis, KYE Pharmaceuticals, Amgen, Sanofi, Pfizer, Bayer, Pendopharm, CCRN, and CPD Network. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Take-Home Message
-
•
This case highlights the importance of conducting genetic testing early in the diagnostic workup of NICM, such as in patients with heart failure who are younger than 40 years.
-
•
Genetic testing has the opportunity to identify multisystem diseases with potential disease-specific therapies that would have not otherwise been recognized.
Acknowledgments
The authors sincerely thank our patient partner (J.C.) for her engagement and support of this publication, as well as other involved health care team members including genetic counsellors Ashley DeGraaf, clinical nurse specialist Wynne Chiu, and cardiologists Dr Elizabeth Swiggum and Dr Darwin Yeung.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental table, please see the online version of this paper.
Supplementary data
References
- 1.Karczewski K.J., Francioli L.C., Tiao G., et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581(7809):434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masson W., Lobo M., Nogueira J.P., Rodriguez-Granillo A.M., Barbagelata L.E., Siniawski D. Anti-inflammatory effect of semaglutide: updated systematic review and metaanalysis. Front Cardiovasc Med. 2024;11 doi: 10.3389/fcvm.2024.1379189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guimier A., Achleitner M.T., Moreau de Bellaing A., et al. PPA2-associated sudden cardiac death: extending the clinical and allelic spectrum in 20 new families. Genet Med. 2021;23(12):2415–2425. doi: 10.1038/s41436-021-01296-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merlo M., Cannatà A., Gobbo M., Stolfo D., Elliott P.M., Sinagra G. Evolving concepts in dilated cardiomyopathy. Eur J Heart Fail. 2018;20(2):228–239. doi: 10.1002/ejhf.1103. [DOI] [PubMed] [Google Scholar]
- 5.Arbelo E., Protonotarios A., Gimeno J.R., et al. 2023 ESC guidelines for the management of cardiomyopathies. Eur Heart J. 2023;44(37):3503–3626. doi: 10.1093/eurheartj/ehad194. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy H., Haack T.B., Hartill V., et al. Sudden cardiac death due to deficiency of the mitochondrial inorganic pyrophosphatase PPA2. Am J Hum Genet. 2016;99(3):674–682. doi: 10.1016/j.ajhg.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manzanilla-Romero H.H., Schermer E., Mayr A., Rudnik-Schöneborn S. Only one beer can be mortal: a case report of two sisters with cardiac arrest due to a homozygous mutation in PPA2 gene. Eur J Pediatr. 2023;182:3785–3788. doi: 10.1007/s00431-023-05034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanaford A., Johnson S.C. The immune system as a driver of mitochondrial disease pathogenesis: a review of evidence. Orphanet J Rare Dis. 2022;17(1):335. doi: 10.1186/s13023-022-02495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lota A.S., Hazebroek M.R., Theotokis P., et al. Genetic architecture of acute myocarditis and the overlap with inherited cardiomyopathy. Circulation. 2022;146(15):1123–1134. doi: 10.1161/CIRCULATIONAHA.121.058457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duran J., Martinez A., Adler E. Cardiovascular manifestations of mitochondrial disease. Biology (Basel) 2019;8(2):34. doi: 10.3390/biology8020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.