Abstract
The mammalian Rh (Rhesus) protein family belongs to the Amt/Mep (ammonia transporter/methylammonium permease)/Rh superfamily of ammonium transporters. Whereas RhCE, RhD and RhAG are erythroid specific, RhBG and RhCG are expressed in key organs associated with ammonium transport and metabolism. We have investigated the ammonium transport function of human RhBG and RhCG by comparing intracellular pH variation in wild-type and transfected HEK-293 (human embryonic kidney) cells and MDCK (Madin–Darby canine kidney) cells in the presence of ammonium (NH4+/NH3) gradients. Stopped-flow spectrofluorimetry analysis, using BCECF [2′,7′-bis-(2-carboxyethyl)-5(6)-carboxyfluorescein] as a pH-sensitive probe, revealed that all cells submitted to inwardly or outwardly directed ammonium gradients exhibited rapid alkalinization or acidification phases respectively, which account for ammonium movements in transfected and native cells. However, as compared with wild-type cells known to have high NH3 lipid permeability, RhBG- and RhCG-expressing cells exhibited ammonium transport characterized by: (i) a five to six times greater kinetic rate-constant; (ii) a weak temperature-dependence; and (iii) reversible inhibition by mercuric chloride (IC50: 52 μM). Similarly, when subjected to a methylammonium gradient, RhBG- and RhCG-expressing cells exhibited kinetic rate constants greater than those of native cells. However, these constants were five times higher for RhBG as compared with RhCG, suggesting a difference in substrate accessibility. These results, indicating that RhBG and RhCG facilitate rapid and low-energy-dependent bi-directional ammonium movement across the plasma membrane, favour the hypothesis that these Rh glycoproteins, together with their erythroid homologue RhAG [Ripoche, Bertrand, Gane, Birkenmeier, Colin and Cartron (2005) Proc. Natl. Acad. Sci. U.S.A. 101, 17222–17227] constitute a family of NH3 channels in mammalian cells.
Keywords: ammonium transport, kidney cell, NH3 channel, Rhesus family (Rh), stopped-flow fluorimetry
Abbreviations: Amt/Mep, ammonia/methylammonium permease transporter; BCECF, 2′,7′-bis-(2-carboxyethyl)-5(6)-carboxyfluorescein; DMEM, Dulbecco's modified Eagle's medium; Ea, activation energy; HEK-293, human embryonic kidney; MDCK, Madin–Darby canine kidney; pHi, intracellular pH; RBC, red blood cell; RPE, R-phycoerythrin; Rh, Rhesus; WT, wild type. The term ammonium is used where ammonium ions and ammonia are not distinguished. The chemical symbols for ammonia (NH3) and ammonium ions (NH4+) are used elsewhere to distinguish between the two forms of ammonium
INTRODUCTION
Despite the high biological relevance, in nearly all organisms, of ammonium transport across cellular membranes, specific ammonium transporters in mammals have not been characterized until recently. The possibility that Rh (Rhesus) proteins might represent the mammalian members of an Amt/Mep (ammonia transporter/methylammonium permease)/Rh superfamily of ammonium transporters has been raised owing to primary structure homology with Amt/Mep from bacteria, archaea, fungi and plants [1]. In humans, the Rh protein family is composed of five members, RhCE, RhD, RhAG, RhBG and RhCG [2]. In RBCs (red blood cells) RhCE, RhD and RhAG constitute the core of the Rh–membrane complex, the carrier of the Rh blood-group antigens [3,4]. Genetic complementation studies in Mep-deficient yeast provided the first experimental evidence for a direct role of RhAG in ammonium transport [5]. RhAG-dependent ammonium transport in a heterologous system was subsequently demonstrated in injected Xenopus oocytes [6]. Since RhAG expression is erythroid-restricted, this glycoprotein could not account for ammonium transport in the two organs which play a key role in ammonium metabolism and excretion: liver and kidney. However, two non-erythroid RhAG homologues, RhBG and RhCG, have been characterized and found to be expressed in various tissues including the kidney and the liver [5,7,8]. Interestingly, epithelial expression of RhBG and RhCG in the kidney is restricted to cells involved in ammonium secretion (connecting tubules and collecting ducts) and both proteins are expressed in the same cell types but with opposite polarity; RhBG is localized at the basolateral domain of the plasma membrane and RhCG at the apical domain [9–11]. In contrast, RhBG and RhCG are expressed in different cell types in the liver. RhBG is expressed at the basolateral membrane of perivenous hepatocytes, whereas RhCG is present in bile duct epithelial cells [12]. A recent study also identified RhBG and RhCG as having a cell specific, axially heterogeneous and polarized expression in the gastrointestinal tract [13]. As is the case for RhAG, ammonium transport activity of RhCG was first shown by complementation studies in yeast [5] and then confirmed by functional studies in Xenopus oocytes [14]. RhBG-mediated ammonium transport was also recently demonstrated in the Xenopus oocyte expression system [15,16]. Although providing strong evidence that all Rh glycoproteins may act as ammonium transporters in heterologous systems, these experiments failed to clearly determine the precise mechanism of ammonium transport mediated by these proteins. In particular, a clear-cut conclusion regarding which form of ammonium is transported, the NH4+ ion or the NH3 gas, was not obvious from these studies. From experiments carried out in yeast and Xenopus expression systems, RhAG was proposed to transport NH4+ by an H+ counter-transport mechanism [6,17], whereas independent electrophysiological studies using RhBG-expressing oocytes suggested a transport mechanism that was either electrogenic [16] or electroneutral, presumably mediated by NH4+/H+ exchange [15]. Similar studies in RhCG-expressing oocytes have concluded that RhCG could mediate electrogenic transport that is consistent with NH3 and NH4+ enhanced influx [14]. The discrepancies between these studies might be explained by the different methodologies used and by the existence of an ammonium-activated endogenous transport pathway in oocytes [18,19] that could hamper more precise analysis of an exogenous ammonium transporter. Furthermore, several of these studies were based on measurement of methylammonium uptake, although there is some concern about the validity of this analogue as a good substitute for ammonium [16].
Using stopped-flow fluorimetry, we have recently defined the ammonium transport characteristics of RhAG in RBCs from human and mouse genetic variants that exhibit quantitatively different expression of RhAG. The kinetic rates of pHi (intracellular pH) changes in resealed ghost cells submitted to inwardly directed methylammonium or ammonium gradients, and the ammonium permeability of the channel unit strongly suggested that RhAG facilitates NH3 movement across the RBC membrane [20]. Here, we used the stopped-flow based approach to analyse the characteristics of ammonium transport mediated by RhBG and RhCG in transfected kidney cells. Our results support the hypothesis that RhBG and RhCG, as well as RhAG, facilitate NH3 movement across the plasma membrane. In addition, we provide evidence for differences in substrate accessibility between RhBG and RhCG which may account for potentially different biological functions of these basolateral and apical epithelial transporters.
EXPERIMENTAL
Materials
Primers used in PCR and mutagenesis experiments were from MWG Biotech (Ebersberg, Germany). The QuikChange XL site-directed mutagenesis kit was from Stratagene (La Jolla, CA, U.S.A.). The pCEP4 vector was purchased from Invitrogen (Leek, The Netherlands). Rabbit polyclonal antisera raised against the cytoplasmic C-terminal of human RhBG (RhBG-Cter) and RhCG (RhCG-Cter) were described previously [9,11]. The LA18.18 anti-RhAG mouse monoclonal antibody was obtained from Dr A. E. Von dem Borne (Laboratory for Transfusion Science, Bloodbank Rotterdam, Rotterdam, the Netherlands). Alexa Fluor 488- or Alexa Fluor 568-conjugated goat anti-(mouse IgG) and goat anti-(rabbit IgG) used as secondary antibodies for confocal microscopy studies were purchased from Molecular Probes (Leiden, The Netherlands). RPE (R-phycoerythrin)-conjugated F(ab′)2 fragment of donkey anti-rabbit and goat anti-mouse immunoglobulins, used as secondary antibodies in flow cytometry analysis, were from Jackson Immunoresearch (West Grove, PA, U.S.A.) and Immunotech (Marseille, France) respectively. The BCECF-AM [2′,7′-bis-(2-carboxyethyl)-5(6)-carboxyfluorescein acetoxymethyl ester] fluorescent probe and the anti-BCECF antibody were obtained from Sigma–Aldrich and Molecular Probes respectively.
Construction of RhAG, RhBG and RhCG mammalian expression vectors and site-directed mutagenesis
The pCEP4-RhAG vector containing the full length cDNA for RhAG was described previously [21]. Full-length cDNAs encoding RhBG and RhCG were amplified by PCR from human kidney Marathon-Ready cDNA (Clontech) with the use of published data [7,8], both strands were sequenced using an ABI PRISM 310 Genetic Analyser (Applied Biosystems, Warrington, U.K.) and subcloned in to the pCEP4 expression vector. The mutated cDNAs were constructed by in vitro mutagenesis from pCEP4-RhBG and pCEP4-RhCG double-stranded recombinant DNA according to the supplier's instructions (Stratagene).
Cell culture and transfection
HEK-293 (human embryonic kidney) and MDCK (Madin–Darby canine kidney) cell lines were obtained from A.T.C.C. (Manassas, VA) and were grown at 37 °C, 5% CO2 in DMEM (Dulbecco's modified Eagle's medium)/Glutamax I (Invitrogen) supplemented with 10% (v/v) fetal calf serum. HEK-293 cells (5×106) and MDCK cells (5×106) were transfected with the relevant expression vectors by electroporation (Bio-Rad, 960 μF and 0,230 V) in 400 μl of PBS, 10 mM Hepes buffer. After selection for 2 weeks in culture medium supplemented with 0.3 mg/ml hygromycin (Invitrogen), pools of hygromycin-resistant cells were analysed for RhAG, RhBG and RhCG expression by flow cytometry and confocal microscopy.
Flow-cytometric analysis
Recombinant expression of RhBG and RhCG in HEK-293 cells and MDCK cells was detected using a FACSCalibur™ flow cytometer (BD Biosciences, Bedford, MA, U.S.A.), after permeabilization/fixation with 50% (v/v) ethanol in PBS and staining with the primary antibodies, rabbit polyclonal anti-RhBG-Cter (1:500), anti-RhCG-Cter (1:150) and secondary antibody RPE-conjugated anti-rabbit. Recombinant expression of RhAG was analysed on non-permeabilized cells using the anti-RhAG LA 18.18 (1:40) primary antibody and RPE-conjugated anti-mouse antibody secondary antibody. The cell-surface antigen expression of RhAG was quantified using as standards mouse-IgG-coated calibration beads (Qifikit; DAKO, Glostrup, Denmark) according to the manufacturer's instructions. The results were expressed as specific antibody-binding-capacity units that proved to be directly proportional to the number of molecules bound per cell.
Immunofluorescence confocal microscopy
Subconfluent monolayers of HEK-293 and MDCK transfectants were cultured on 12 mm diameter poly(L-lysine) coverslips (BD Biosciences) for 3 days before immunostaining. Cells were fixed in 4% (v/v) paraformaldehyde for 20 min and washed in PBS. Free aldehyde groups were blocked in 50 mM NH4Cl for 10 min, cells were then washed in PBS and permeabilized in 1% (w/v) SDS for 15 min. After two washes in PBS, cells were incubated with anti-RhAG, anti-RhBG-Cter or anti-RhCG-Cter antibodies diluted in background immunostaining reducing buffer (Dako Corp., Copenhagen, Denmark) for 1 h at room temperature. Cells were washed three times in PBS containing 0.5% BSA (v/v) and incubated for 1 h at room temperature with Alexa Fluor 568 goat anti-(rabbit IgG) diluted 1:200 in PBS/BSA. Samples were examined by wide-field microscopy using a Nikon Eclipse TE300 inverted confocal microscope equipped with a 60× oil-immersion objective, numerical aperture 1.4.
Stopped-flow analysis of pHi variation
All preparation steps were carried out at 4 °C and assays were performed at 15 °C on the same day, except for the activation-energy determination. For NH3 import analysis, 1×107 cells were washed twice in PBS and incubated for 20 min with gentle agitation at 30 °C in 1 ml of PBS containing 20 μM of the fluorescent probe BCECF-AM. After incubation, cells were centrifuged and washed twice with cold PBS, then equilibrated and resuspended in buffer A (130 mM NaCl, 5 mM KCl and 10 mM Hepes, adjusted to pH 7.0 by adding NaOH) to a concentration of 1×106 cells/ml. The cells were kept on ice before assays (no more than 3 h after the final centrifugation). Ammonium or methylammonium transport was measured in iso-osmotic conditions using a stopped-flow instrument (SFM3, Bio-Logic, Grenoble, France) equipped with a cuvette FC-15, 30 μl (dead-time 7.8 ms). The excitation wavelength was 485 nm and the emitted light was filtered with a 520 nm cut-on filter. Cells were rapidly mixed with an equal volume of methylammonium buffer (65 mM NaCl, 65 mM CH3NH3Cl, 5 mM KCl and 10 mM Hepes, adjusted to pH 7.0 with NaOH) or ammonium buffer (65 mM NaCl, 65 mM NH4Cl, 5 mM KCl and 10 mM Hepes, adjusted to pH 7.0) generating an inwardly directed methylammonium ion or NH4+ 32.5 mmol gradient. In some experiments, the same gradient was created using ethylamine, dimethylamine or trimethylamine. As a fluorescence increase corresponds to pH elevation and a fluorescence decrease to pH reduction the pH-dependent fluorescence changes to the BCECF probe were monitored and analysed [22]. In some experiments, fluorescence was also measured with excitation wavelengths of 505 nm and 439 nm and the same emission wavelengths. Analysis of the 505 nm/409 nm fluorescence ratio did not modify the rate constant of the fluorescence change. Preliminary experiments, where anti-BCECF antibody was added to quench extracellular fluorescence, revealed only a negligible amount of external fluorescence. The antibody was hence omitted in further experiments. Data from five to eight time-courses were averaged and fitted to a mono-exponential function using the simplex procedure of the Biokine software package (Bio-Logic). The equation is:
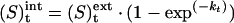 |
where (S)text is constant and (S)tint is the internal concentration of the substrate. k is the rate constant of the exponential. Over the pH range used (7.0–7.8), the relative fluorescence of the dye was proportional to the pH variation (R2=0.996), as determined by titration of cells incubated in 2 ml of buffer A containing 5 μM nigericin (Sigma) or 2 μM valinomycin and 2 μM carbonyl cyanid 4-(trifluoromethoxy phenylhydrazone) (Sigma). Titrations were performed with a classical spectrofluorimeter with a standard thermostatted cuvette with magnetic shaking. The pH changes were performed by step-wise additions of 2 μl KOH (1 M). Cell-buffer capacity (β) of the cell suspension was carried out by addition of 10 or 20 mM sodium acetate in one step. Calculating the pH change from the established standard curve, β was found to be in the same range for all HEK-293 cells tested at 23±7 mM/pH unit. Ammonium permeability was determined by rapid mixing of cells at pH 7.0 with ammonium buffer generating a final inwardly directed gradient of 32.5 mM NH4+. Under these conditions, more than 99% of the ammonium buffer is in the NH4+ form and less than 1% represents the NH3 form (pKa of ammonium is 9.56 at 15 °C). Ammonium influx was determined from the maximal pHi increase using the linear correlation between the relative fluorescence and pH. For ammonium efflux analysis, cells were incubated for 10 min in ammonium buffer, then washed and resuspended in the same buffer before analysis. In the stopped-flow instrument, cells were submitted to an outward gradient (49 mmol of NH4+), generated from the inside to the outside of the cells by rapid mixing of 1 vol. of cells with 3 vol. of buffer A. The acidification kinetics were recorded and the constant rates calculated as described below.
Ammonium and methylammonium permeability calculation
Apparent permeability (P′) was determined according to Priver et al. [23] using the following equation:
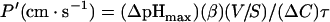 |
where ΔpHmax is the maximum pH change value, β is the cell-buffer capacity (mM/pH unit), ΔC is the difference in ammonium or methylammonium concentration (mM) and τ=1/k (s), which is the reciprocal of the exponential constant k. V/S (cm) is the cell volume/surface-area ratio. P′ does not take into account the unstirred layers, which presumably are in the same range for all cell samples tested. Based on the calculated V/S ratio given by Golchini and Kurtz [24], the V/S ratio of MDCK cells was equal to approx. 5.8×10−5 cm. In HEK-293 cells, the cell volume was calculated by assimilating the cell to a sphere with a diameter of 17.2±2.6 μm (S.D., n=70) estimated from photonic microscopy. The surface was calculated from the capacitance of these cells: 26.6 pF [25] (1 cm2=106 pF) giving the V/S ratio of HEK-293 cells as equal to 8.12×10−5 cm, close to the V/S MDCK cell ratio. Transfections did not significantly alter the volume parameters.
Treatment with mercurial reagents
RhCG or RhBG transfectants were resuspended in buffer A and incubated for 10 min in HgCl2 (10−5–10−4 M) before application of the ammonium gradient. In the SFM3 stopped-flow instrument, cells were submitted to a quick mixing with the ammonium or methylammonium buffer, supplemented with HgCl2 at the same concentration (10−4 M). The reversibility of the mercury effect was tested by adding 5 mM 2-mercaptoethanol to the cells incubated previously in HgCl2.
Temperature influence
The rate of fluorescence changes was also measured at different temperatures (8–30 °C) in order to determine the activation-energy [Ea (kcal/mol)] (1 kcal=4.184 kJ) deduced from the Arrhenius equation:
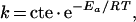 |
where k is the alkalinization kinetic-rate constant, T is temperature (K) and R is the perfect gas constant (1.98).
RESULTS
Stable expression of recombinant RhAG, RhBG and RhCG in HEK-293 cell transfectants
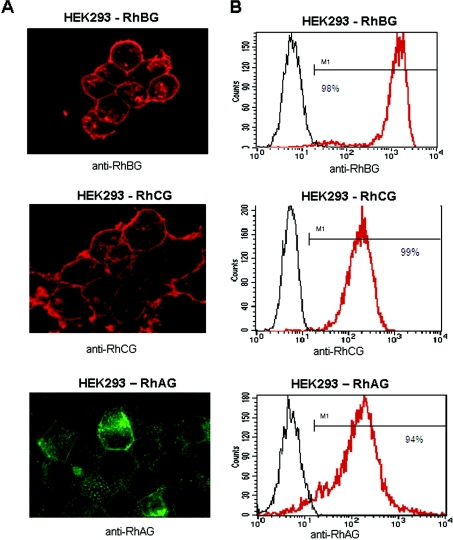
HEK-293 cells, although of renal origin, were previously shown to lack endogenous expression of RhBG and RhCG [9,11]. These cells were stably transfected by the pCEP4-RhAG, pCEP4-RhBG and pCEP4-RhCG vectors. Fluorescence microscopic analysis revealed plasma-membrane expression of all RhAG, RhBG and RhCG glycoproteins in transfected cells (Figure 1A), whereas no labelling could be detected in parental HEK-293 cells (results not shown). More than 90% of transfected cells expressed the Rh glycoproteins after 2 weeks of antibiotic selection, as determined by flow-cytometric analysis (Figure 1B). It was calculated, from the binding-capacity of the LA18.18 monoclonal antibody on unpermeabilized HEK-293-RhAG cells, that a mean of 9500 RhAG molecules were expressed at the cell surface in these recombinant cells. However, the number of RhBG and RhCG molecules expressed in the recombinant cells could not be determined by flow cytometry since labelling was performed on permeabilized cells and the antibodies used (rabbit polyclonal) were not suitable for specific antibody binding-capacity estimation.
Figure 1. Cell surface expression of the recombinant RhBG, RhCG and RhAG antigens in HEK-293 transfectants.
(A) Immunofluorescence microscopy analysis. HEK-293 transfectants cultured on poly(L-lysine) coverslips were fixed and permeabilized before adding the relevant anti-Rh primary antibodies. Alexa-Fluor labelled IgG were used as secondary antibodies. (B) Flow-cytometric analysis using LA18.18 monoclonal anti-RhAG, polyclonal anti-RhBG-Cter and anti-RhCG-Cter antibodies. The black lines represent the non-transfected cells and red lines represent transfected cells. Percentage of positive transfected cells is indicated.
pHi variation kinetics of WT (wild-type) and transfected HEK-293 cells in the presence of an inwardly directed ammonium gradient
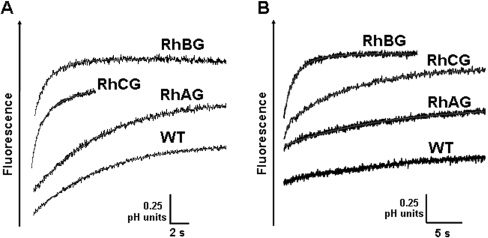
The NH4+/NH3 transport capacity of human RhAG, RhBG and RhCG proteins, stably expressed in HEK-293 cells, was investigated by stopped-flow spectrofluorometry using BCECF as an intracellular pH-sensitive probe. Figure 2 indicates the time-course of the fluorescence increase after exposure of HEK-293 cells to inwardly directed 32.5 mmol of NH4+ (Figure 2A) or CH3NH3+ gradients (Figure 2B), at pH 7.0. The fluorescence variation curves exhibited a rapid increment corresponding to intracellular alkalinization. This result may be accounted for, by NH3 or CH3NH2 entry into the cells and their protonation to form NH4+ or CH3NH3+, or by an NH4+/H+ exchange mechanism [26]. Although most experiments were performed using a 32.5 mM gradient, some were also carried out using 2.5–40 mM (pH 7.0) gradients and we found a linear relationship between ammonium concentration and fluorescence variation (results not shown). After this rapid alkalinization phase, pHi slowly returned to pH0 in the incubation medium (results not shown). As the acidification phase is more than 100 times slower than the alkalinization phase (as estimated by t1/2 values for CH3NH2 and NH3 respectively), we assume that it does not significantly interfere with the calculation of first-phase rate constants. This phase of slow acidification is a complex process as the cell membrane carries several transporters which could participate in pH regulation. Therefore, this was not investigated further. Whereas the alkalinization amplitudes between T0 and T equilibrium were similar in parental and all recombinant cells (ΔpH approx. 0.76±0.07 pH unit, n=6), the kinetic rates of alkalinization were different according to which Rh protein was expressed and the nature of the substrate used. In the presence of an inwardly directed ammonium gradient (32.4 mmol of NH4+ and 0.1 mM NH3) (Figure 2A), the alkalinization process was approx. 5–6 times faster in HEK-293 cells expressing RhBG and RhCG than in WT and RhAG-expressing HEK-293 cells (alkalinization rate constants were 1±0.15 and 0.88±0.07 compared with 0.18±0.01 and 0.15±0.04 s−1 respectively) (Table 1). To ascertain that these results are not cell-line-dependent and directly correlated to RhBG and RhCG expression, the same experiments were performed in MDCK cell lines. Similar results were obtained when RhBG and RhCG were expressed in transfected MDCK cells (Table 1). In the presence of an inwardly directed MA gradient (Figure 2B), the rate constants of alkalinization were much lower than those corresponding to ammonium entry for all cells investigated. Furthermore, as compared with WT HEK-293 cells, the increase in the alkalinization rate constant associated with expression of Rh glycoproteins in HEK-293 cells was much more drastic for RhBG than for RhCG [(0.029±0.01 s−1 (WT) compared with 0.53±0.06 (RhBG) and 0.11±0.023 s−1 (RhCG); Table 1)]. In MDCK cells, only RhBG expression was able to increase the rate constants of alkalinization as compared with WT MDCK cells (see Table 1). As observed with an NH4+ gradient, RhAG expression did not significantly modify the alkalinization rate of HEK-293 cells in the presence of an CH3NH3+ gradient (HEK-RhAG 0.04±0.01 s−1 compared with WT HEK-293 cells 0.029±0.01 s−1). Therefore, the effects of RhAG expression were not further explored. Finally, as compared with WT HEK-293 cells, only RhBG was able to increase the alkalinization rate of HEK-293 cells in the presence of an inwardly directed ethylammonium (C2H5NH3+) gradient (see Table 1). Neither of the two Rh glycoproteins were able to transport di- or trimethylammonium (results not shown).
Figure 2. Time course of fluorescence changes.
WT and RhBG-, RhCG- or RhAG-transfected HEK-293 cells were submitted to 32.5 mmol ammonium (A) or methylammonium (B) iso-osmotic inwardly-directed gradients (pH 7.0) at 15 °C. pHi changes were monitored through fluorescence change to the pH-sensitive dye BCECF in the stopped-flow spectrofluorimeter. A rapid initial alkalinization is observed. Smooth lines are exponential fits to the data.
Table 1. Alkalinization rate constants (s−1) of transfected HEK-293 and MDCK cells.
* P<0.001 compared with WT; † P<0.001 compared with RhBG; ‡ P<0.02 compared with RhBG; nd, not determined; n values in parentheses.
| Rate constant (s−1) | |||||||
|---|---|---|---|---|---|---|---|
| HEK-293 | MDCK | ||||||
| Substrate | WT | RhBG | RhCG | RhAG | WT | RhBG | RhCG |
| NH4+ | 0.18±0.011 (6) | 1±0.15* (5) | 0.88±0.07* (5) | 0.15±0.04 (3) | 0.18±0.009 (4) | 0.76±0.007* (4) | 0.45±0.02* (4) |
| CH3NH3+ | 0.029±0.01 (6) | 0.53±0.06* (4) | 0.11±0.023‡ (3) | 0.04±0.01 (3) | 0.042±0.02 (4) | 0.35±0.01* (4) | 0.045±0.012† (4) |
| C2H5NH3+ | 0.041 (2) | 0.11 (2) | 0.043 (2) | nd | nd | nd | nd |
pHi variation kinetics of WT and transfected HEK-293 cells in the presence of an outwardly directed ammonium gradient
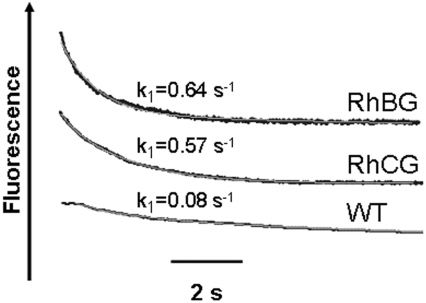
When an NH4+ outwardly directed gradient was applied to WT and RhBG- or RhCG-transfected HEK-293 cells, the recorded kinetics (Figure 3) showed a fluorescence decrease which corresponds to cell acidification. This result may be accounted for by NH3 loss from the cell and leaving an H+ ion behind or by H+/NH4+ exchange [26]. The rate constants of acidification were in the same range as those for alkalinization obtained in ammonium influx experiments. As is the case for ammonium influx, ammonium efflux was seven to eight times greater in RhBG- and RhCG-expressing HEK-293 cells than in WT HEK-293 cells (0.64 and 0.57 compared with 0.08 s−1).
Figure 3. Ammonium efflux from HEK-293 cells.
Time course of fluorescence changes in WT and RhBG- or RhCG-expressing HEK-293 cells submitted to an outwardly directed 49 mmol NH4+ gradient are shown. Kinetics of pHi changes were analysed as described in Figure 2. The calculated acidification kinetic rate constants are shown. Smooth lines are exponential fits to the data.
Ammonium and methylammonium permeability
Apparent ammonium and methylammonium cell permeability was determined at 15 °C, pH 7.0. Under these conditions, the apparent maximal ammonium or methylammonium gradient between the outside and inside of cells was 32.5 mmol. Priver's equation (see the Experimental section) allowed the calculation of permeability values from the time-constant rates. Calculated ammonium permeability values (10−4 cm·s−1) were 0.24, 1.5 and 1.31 for WT, RhBG- and RhCG-expressing HEK-293 cells respectively. Calculated methylammonium permeability values (10−5 cm·s−1) were 0.2, 6.5 and 1.03 for WT, RhBG- and RhCG-expressing HEK-293 cells respectively. Similar results were obtained in MDCK cells. It is noteworthy that the calculated value for WT MDCK cells is consistent with that calculated from the values published previously by others [24].
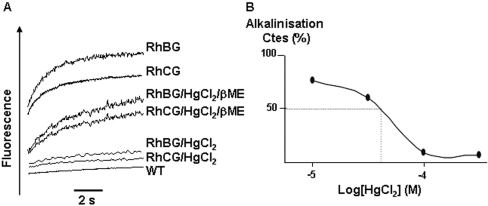
Effects of HgCl2 on ammonium transport of RhBG and RhCG HEK-293 transfectants
To further demonstrate that RhBG and RhCG proteins are directly involved in ammonium transport, we tested the effect of HgCl2 on the pHi variation rate constant. Figure 4(A) shows that pre-incubation of HEK-293-RhBG and HEK-293-RhCG transfectants with HgCl2 (10−4 M) reduced the rate of ammonium influx to that observed in WT HEK-293 cells submitted to a NH4+ gradient. Half-maximal inhibition occurred at 52 μM (Figure 4B). The uptake of ammonium was partially recovered when 5 mM 2-mercaptoethanol was added to HgCl2-treated RhBG- and RhCG-expressing cells (Figure 4A). Similarly, an HgCl2 inhibition of ammonium efflux was observed when cells were submitted to an outwardly directed NH4+ gradient (results not shown). Since HgCl2 interacts with thiol groups, we tested the effects of mutating the three cysteine residues, which are conserved between RhBG and RhCG [8], on the ammonium transport activity of transfected cells. Transfection with RhBG-C19A and RhCG-C15A mutant plasmids seemed to be toxic since no recombinant cells could be obtained in selective medium. Cells expressing the RhBG-C130A, RhCG-C131A and RhBG-C339A, RhCG-C338A mutant proteins exhibited alkalinization curves and HgCl2 inhibition similar to those observed in RhBG-WT- and RhCG-WT-expressing cells (results not shown).
Figure 4. HgCl2 sensitivity of RhBG and RhCG-mediated ammonium transport in HEK-293 transfectants.
(A) HEK-293 cells were incubated with 0.1 mM HgCl2 for 10 min before the time course of fluorescence changes in the presence of an inwardly directed ammonium gradient was determined, as described in Figure 2. The same samples were further analysed for pHi changes after treatment with 5 mM 2-mercaptoethanol. (B) Dose–response to mercury inhibition. RhBG transfectants were pre-incubated for 10 min with increasing concentrations of HgCl2 (20, 50, 100, 200 μM) before stopped-flow analysis was performed. Each point represents the mean value of 6 to 8 replicated experiments. IC50 was close to 52 μM.
Ea of ammonium uptake
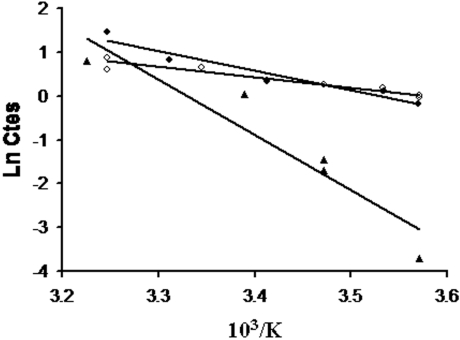
One criterion to discriminate between channel-mediated permeation of NH3 and active NH4+/H+ exchange is to demonstrate that ammonium uptake is either a weakly or a highly temperature-dependent process respectively. In the present experiments, ammonium uptake as deduced from the Ea determined from the Arrhenius equation (see the Experimental section), showed a much lower temperature-dependence in RhBG and RhCG transfectants than in WT HEK-293 cells (Ea=8.87 and 4.86 compared with 25.23 kcal/mol respectively) (Figure 5).
Figure 5. Arrhenius plot of temperature-dependent ammonium permeation in HEK-293 cells.
Temperature dependence of ammonium transport was analysed in WT (▲) and in RhBG- (◆) and RhCG- (◇) expressing HEK-293 cells. Arrhenius activation energies for ammonium permeation were 25.23, 8.87 and 4.86 kcal/mol in WT, RhBG- and RhCG-expressing HEK-293 cells respectively. Each point corresponds to individual experiments.
DISCUSSION
The present study indicates that RhBG and RhCG facilitate bi-directionnal ammonium transport across the plasma membrane of transfected kidney cells submitted to an inwardly or outwardly directed ammonium gradient. In the absence of a direct measurement of intracellular NH3 and NH4+, we attempted to determine which form of ammonium is transported by analysing the properties of pHi changes using a stopped-flow fluorimetry based approach. This method permits reliable measurement of rapid kinetics in a fraction-of-a-second range and is therefore particulary suitable if NH3 is the transported species. The intracellular alkalinization and acidification processes observed in the presence of inwardly and outwardly ammonium gradients, respectively, could be explained by NH4+/H+ exchange, as proposed by others [6,15,17] or by facilitated NH3 transport, as has been proposed for the erythroid Rh glycoprotein RhAG [20]. However, two arguments prompt us to favour the hypothesis that the uncharged molecule is transported: an influx of NH3 resulting in the intracellular captation of H+ to give NH4+, and an efflux of NH3 resulting from deprotonation of NH4+ leaving one intracellular H+ ion behind [26]. Firstly, the rapid kinetics of the pHi changes reported in the present study are in contrast with the much slower kinetics associated with antiport functions (i.e. Na+/H+ exchangers) [27]. Secondly, the significant decrease in Ea associated with RhBG and RhCG expression also points towards RhBG and RhCG as channels rather than as active exchangers whose function requires high-energy-dependent conformational changes [28,29]. The conclusion that RhBG and RhCG carry out NH3 transport fully correlates with our recent study demonstrating that the rate constant of NH3 movement in RBCs is strictly dependent on RhAG expression level [20]. These results, supported by crystallographic and functional studies demonstrating that AmtB, a bacterial member of the Amt/Mep/Rh superfamily, is a channel that conducts NH3 [30,31], first identified Rh glycoproteins as members of an NH3 channel family in mammals. In support of a physiological relevance for the capacity of RhBG and RhCG to transport NH3, it has been shown, at least in the kidney, that epithelial cells that exhibit the highest NH3 permeability, namely intercalated cells of the collecting tubules [32], are those which express RhBG and RhCG [9–11].
Since ammonium transport may play different physiological roles in different organisms and in different tissues within the same organism, the findings that bacterial AmtB and mammalian Rh glycoproteins function as a gas channel for NH3 should not rule out the possibility that other members of the Amt/Mep/Rh superfamily can function differently. For instance, the plant proteins LeAMT1;1 and LeAMT1;2 when expressed in Xenopus oocytes, have been proposed to be electrogenic NH4+ transporters [33,34]. Supporting the hypothesis that plant and mammalian Amts, or Rh-type Amts, might have distinct functional properties, recent paralleled electrophysiological measurements performed with injected Xenopus oocytes further demonstrate that LeAMT1;2 supports specific NH4+ currents while RhCG mediate electroneutral ammonium transport (U. Ludewig, personal communication). Likewise, it has been shown that AQP6, in contrast to the other members of the aquaporin family, exhibits low basal-water permeability accompanied by high anion conductance [35]. Moreover, the observation that ClC Cl− proteins function as chloride channels in eukaryotes but as H+/Cl− exchangers in prokaryotes led to the proposal that “the structural boundary separating channels and transporters is not as clear cut as generally thought” [36].
To further demonstrate that RhBG and RhCG proteins were directly involved in NH3 influx, we tested the effect of HgCl2 on the alkalinization rate constants of WT and transfected HEK-293 cells submitted to an NH4+ gradient. Together with the decreased Ea associated with RhBG and RhCG expression, the observation of an inhibitory effect of mercury on NH3 transport, that is partially reversible by 2-mercapthoethanol, supported the involvement of a protein dependent pathway in the accelerated transport of NH3. The high P′ NH3 mercury sensitivity of RhBG- and RhCG-expressing cells (IC50: 52 μM) is in agreement with results of previous studies on the postulated protein-mediated NH3 permeability of plant nodules [37]. Although mercury inhibition suggested involvement of one or more cysteine residues, a critical role for these residues could not be demonstrated by mutagenesis analysis. Indeed, among the three conserved cysteines that have been analysed so far, two did not seem to be crucial when individually mutated (Cys130/Cys131 and Cys338/Cys339), whereas mutations of the one other appeared to result in a toxic form of RhBG and RhCG (Cys19/Cys15).
Although the major conclusion from the present study is that NH3 is the ammonium form transported by both RhBG and RhCG, it is of interest that these two Rh glycoproteins are not equal in the transport of molecules larger than ammonium, such as methylammonium and ethylammonium. It is tempting to speculate that structural differences within the vestibule located at the top of the narrow channel of RhBG and RhCG, as hypothesized from the crystal structure of AmtB [30,31], should account for these different substrate accessibilities.
Although RhAG was shown to facilitate NH3 movement across the RBC membrane [20], the transport function of this Rh glycoprotein could not be assessed in the cellular model succesfully used for RhBG and RhCG. Of note, the expression level of RhAG in transfected HEK-293 cells (9500 copies of RhAG/cell) is lower than that observed in Rhnull RBCs of the amorph type (17000 copies of RhAG/RBC) which already exhibited much reduced alkalinization rate constant values, as compared with normal control RBCs (100000 copies of RhAG/cell) [20]. Accordingly, the alkalinization rate constant value of HEK-293 cells submitted to an inwardly directed ammonium gradient was not modified by the low expression level of RhAG which is presumably due to the absence in HEK-293 cells of the RhAG protein partners present in RBCs, i.e Rh (D and CE) [3], ankyrin R [38] and Band 3 [39], which are necessary for its full membrane expression.
From experimental evidence suggesting that AmtB and Mep proteins facilitate diffusion of NH3 across the cytoplasmic membrane of bacteria and fungi respectively [40,41], Soupene and co-workers have also speculated that mammalian Rh proteins might be gas channels. However, based on the relatively restricted organ and tissue distribution of Rh proteins and the observation that mutants of Chlamydomonas reinhardtii lacking the paralogue Rh1 protein grow very slowly under high CO2 conditions, these authors further suggested that mammalian Rh proteins might function as CO2 rather than as NH3 gas channels [42,43]. Although the narrowness of the channel in AmtB and the nature of the pore-lining amino acids [30,31] do not favour the hypothesis of a CO2 transporter, we cannot formally rule out that Rh glycoproteins are gas channels that might facilitate either NH3 or CO2 transport depending on which cell types they are expressed in. While there is increasing evidence for the existence of conserved NH3 gas channels in bacteria to mammals, the integrated biological role of Rh glycoproteins in mammal physiology remains unknown. We expect that animal models which are currently being generated will provide an answer to this important question in the near future.
Acknowledgments
N.Z.-Y. acknowledges la Société Française d'Hématologie for financial support. We are grateful to Dr Dominique Goossens, INSERM, Institut National de la Transfusion Sanguine, U665, Paris, France, and Dr Dominique Eladari, INSERM, U652, Paris, France, for critical reading of this manuscript. We thank A. Sentenac, Commissariat à l'Energie Atomique, Saclay, Gif sur Yvette, France, who made the stopped-flow equipment available to us.
References
- 1.Marini A. M., Urrestarazu A., Beauwens R., Andre B. The Rh (rhesus) blood group polypeptides are related to NH4+ transporters. Trends Biochem. Sci. 1997;22:460–461. doi: 10.1016/s0968-0004(97)01132-8. [DOI] [PubMed] [Google Scholar]
- 2.Huang C. H., Liu P. Z. New insights into the Rh superfamily of genes and proteins in erythroid cells and nonerythroid tissues. Blood Cells Mol. Dis. 2001;27:90–101. doi: 10.1006/bcmd.2000.0355. [DOI] [PubMed] [Google Scholar]
- 3.Cartron J. P. RH blood group system and molecular basis of Rh-deficiency. Baillieres Best Pract. Res. Clin. Haematol. 1999;12:655–689. doi: 10.1053/beha.1999.0047. [DOI] [PubMed] [Google Scholar]
- 4.Avent N. D., Reid M. E. The Rh blood group system: a review. Blood. 2000;95:375–387. [PubMed] [Google Scholar]
- 5.Marini A. M., Matassi G., Raynal V., Andre B., Cartron J. P., Cherif-Zahar B. The human Rhesus-associated RhAG protein and a kidney homologue promote ammonium transport in yeast. Nat. Genet. 2000;26:341–344. doi: 10.1038/81656. [DOI] [PubMed] [Google Scholar]
- 6.Westhoff C. M., Ferreri-Jacobia M., Mak D. O., Foskett J. K. Identification of the erythrocyte Rh blood group glycoprotein as a mammalian ammonium transporter. J. Biol. Chem. 2002;277:12499–12502. doi: 10.1074/jbc.C200060200. [DOI] [PubMed] [Google Scholar]
- 7.Liu Z., Chen Y., Mo R., Hui C., Cheng J. F., Mohandas N., Huang C. H. Characterization of human RhCG and mouse Rhcg as novel nonerythroid Rh glycoprotein homologues predominantly expressed in kidney and testis. J. Biol. Chem. 2000;275:25641–25651. doi: 10.1074/jbc.M003353200. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z., Peng J., Mo R., Hui C., Huang C. H. Rh type B glycoprotein is a new member of the Rh superfamily and a putative ammonia transporter in mammals. J. Biol. Chem. 2001;276:1424–1433. doi: 10.1074/jbc.M007528200. [DOI] [PubMed] [Google Scholar]
- 9.Eladari D., Cheval L., Quentin F., Bertrand O., Mouro I., Cherif-Zahar B., Cartron J. P., Paillard M., Doucet A., Chambrey R. Expression of RhCG, a new putative NH3/NH4+ transporter, along the rat nephron. J. Am. Soc. Nephrol. 2002;13:1999–2008. doi: 10.1097/01.asn.0000025280.02386.9d. [DOI] [PubMed] [Google Scholar]
- 10.Verlander J. W., Miller R. T., Frank A. E., Royaux I. E., Kim Y. H., Weiner I. D. Localization of the ammonium transporter proteins RhBG and RhCG in mouse kidney. Am. J. Physiol. Renal Physiol. 2003;284:F323–F337. doi: 10.1152/ajprenal.00050.2002. [DOI] [PubMed] [Google Scholar]
- 11.Quentin F., Eladari D., Cheval L., Lopez C., Goossens D., Colin Y., Cartron J. P., Paillard M., Chambrey R. RhBG and RhCG, the putative ammonia transporters, are expressed in the same cells in the distal nephron. J. Am. Soc. Nephrol. 2003;14:545–554. doi: 10.1097/01.asn.0000050413.43662.55. [DOI] [PubMed] [Google Scholar]
- 12.Weiner I. D., Miller R. T., Verlander J. W. Localization of the ammonium transporters, Rh B glycoprotein and Rh C glycoprotein, in the mouse liver. Gastroenterology. 2003;124:1432–1440. doi: 10.1016/s0016-5085(03)00277-4. [DOI] [PubMed] [Google Scholar]
- 13.Handlogten M. E., Hong S. P., Westhoff C. M., Weiner I. D. Basolateral ammonium transport by the mouse inner medullary collecting duct cell (mIMCD-3) Am. J. Physiol. Renal Physiol. 2004;287:F628–F638. doi: 10.1152/ajprenal.00363.2003. [DOI] [PubMed] [Google Scholar]
- 14.Bakouh N., Benjelloun F., Hulin P., Brouillard F., Edelman A., Cherif-Zahar B., Planelles G. NH3 is involved in the NH4+ transport induced by the functional expression of the human Rh C glycoprotein. J. Biol. Chem. 2004;279:15975–15983. doi: 10.1074/jbc.M308528200. [DOI] [PubMed] [Google Scholar]
- 15.Ludewig U. Electroneutral ammonium transport by basolateral rhesus B glycoprotein. J. Physiol. 2004;559:751–759. doi: 10.1113/jphysiol.2004.067728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakhoul N. L., Dejong H., Abdulnour-Nakhoul S. M., Boulpaep E. L., Hering-Smith K., Hamm L. L. Characteristics of renal Rhbg as an NH4+ transporter. Am. J. Physiol. Renal Physiol. 2005;288:F170–F181. doi: 10.1152/ajprenal.00419.2003. [DOI] [PubMed] [Google Scholar]
- 17.Westhoff C. M., Siegel D. L., Burd C. G., Foskett J. K. Mechanism of genetic complementation of ammonium transport in yeast by human erythrocyte Rh-associated glycoprotein. J. Biol. Chem. 2004;279:17443–17448. doi: 10.1074/jbc.M311853200. [DOI] [PubMed] [Google Scholar]
- 18.Burckhardt B. C., Burckhardt G. NH4+ conductance in Xenopus laevis oocytes. I. Basic observations. Pflugers Arch. 1997;434:306–312. doi: 10.1007/s004240050401. [DOI] [PubMed] [Google Scholar]
- 19.Cougnon M., Bouyer P., Hulin P., Anagnostopoulos T., Planelles G. Further investigation of ionic diffusive properties and of NH4+ pathways in Xenopus laevis oocyte cell membrane. Pflugers Arch. 1996;431:658–667. doi: 10.1007/BF02191917. [DOI] [PubMed] [Google Scholar]
- 20.Ripoche P., Bertrand O., Gane P., Birkenmeier C., Colin Y., Cartron J. P. Human Rhesus-associated glycoprotein mediates facilitated transport of NH3 into red blood cells. Proc. Natl. Acad. Sci. U.S.A. 2004;101:17222–17227. doi: 10.1073/pnas.0403704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mouro-Chanteloup I., D'Ambrosio A. M., Gane P., Le Van Kim C., Raynal V., Dhermy D., Cartron J. P., Colin Y. Cell-surface expression of RhD blood group polypeptide is posttranscriptionally regulated by the RhAG glycoprotein. Blood. 2002;100:1038–1047. [PubMed] [Google Scholar]
- 22.Rink T. J., Tsien R. Y., Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J. Cell Biol. 1982;95:189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Priver N. A., Rabon E. C., Zeidel M. L. Apical membrane of the gastric parietal cell: water, proton, and nonelectrolyte permeabilities. Biochemistry. 1993;32:2459–2468. doi: 10.1021/bi00061a002. [DOI] [PubMed] [Google Scholar]
- 24.Golchini K., Kurtz I. NH3 permeation through the apical membrane of MDCK cells is via a lipid pathway. Am. J. Physiol. 1988;255:F135–F141. doi: 10.1152/ajprenal.1988.255.1.F135. [DOI] [PubMed] [Google Scholar]
- 25.Yu X., Duan K. L., Shang C. F., Yu H. G., Zhou Z. Calcium influx through hyperpolarization-activated cation channels (Ih channels) contributes to activity-evoked neuronal secretion. Proc. Natl. Acad. Sci. U.S.A. 2004;101:1051–1056. doi: 10.1073/pnas.0305167101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boron W. F., De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J. Gen. Physiol. 1976;67:91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bierman A. J., Tertoolen L. G., de Laat S. W., Moolenaar W. H. The Na+/H+ exchanger is constitutively activated in P19 embryonal carcinoma cells, but not in a differentiated derivative. Responsiveness to growth factors and other stimuli. J. Biol. Chem. 1987;262:9621–9628. [PubMed] [Google Scholar]
- 28.Labotka R. J., Lundberg P., Kuchel P. W. Ammonia permeability of erythrocyte membrane studied by 14N and 15N saturation transfer NMR spectroscopy. Am. J. Physiol. 1995;268:C686–C699. doi: 10.1152/ajpcell.1995.268.3.C686. [DOI] [PubMed] [Google Scholar]
- 29.Smith B. L., Baumgarten R., Nielsen S., Raben D., Zeidel M. L., Agre P. Concurrent expression of erythroid and renal aquaporin CHIP and appearance of water channel activity in perinatal rats. J. Clin. Invest. 1993;92:2035–2041. doi: 10.1172/JCI116798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng L., Kostrewa D., Berneche S., Winkler F. K., Li X. D. The mechanism of ammonia transport based on the crystal structure of AmtB of Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 2004;101:17090–17095. doi: 10.1073/pnas.0406475101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khademi S., O'Connell J., 3rd, Remis J., Robles-Colmenares Y., Miercke L. J., Stroud R. M. Mechanism of ammonia transport by Amt/MEP/Rh: structure of AmtB at 1.35 Å. Science (Washington D.C.) 2004;305:1587–1594. doi: 10.1126/science.1101952. [DOI] [PubMed] [Google Scholar]
- 32.Knepper M. A., Packer R., Good D. W. Ammonium transport in the kidney. Physiol. Rev. 1989;69:179–249. doi: 10.1152/physrev.1989.69.1.179. [DOI] [PubMed] [Google Scholar]
- 33.Ludewig U., von Wiren N., Frommer W. B. Uniport of NH4+ by the root hair plasma membrane ammonium transporter LeAMT1;1. J. Biol. Chem. 2002;277:13548–13555. doi: 10.1074/jbc.M200739200. [DOI] [PubMed] [Google Scholar]
- 34.Ludewig U., Wilken S., Wu B., Jost W., Obrdlik P., El Bakkoury M., Marini A. M., Andre B., Hamacher T., Boles E., von Wiren N., Frommer W. B. Homo- and hetero-oligomerization of ammonium transporter-1 NH4 uniporters. J. Biol. Chem. 2003;278:45603–45610. doi: 10.1074/jbc.M307424200. [DOI] [PubMed] [Google Scholar]
- 35.Yasui M., Hazama A., Kwon T. H., Nielsen S., Guggino W. B., Agre P. Rapid gating and anion permeability of an intracellular aquaporin. Nature (London) 1999;402:184–187. doi: 10.1038/46045. [DOI] [PubMed] [Google Scholar]
- 36.Accardi A., Miller C. Secondary active transport mediated by a prokaryotic homologue of ClC Cl- channels. Nature (London) 2004;427:803–807. doi: 10.1038/nature02314. [DOI] [PubMed] [Google Scholar]
- 37.Niemietz C. M., Tyerman S. D. Channel-mediated permeation of ammonia gas through the peribacteroid membrane of soybean nodules. FEBS Lett. 2000;465:110–114. doi: 10.1016/s0014-5793(99)01729-9. [DOI] [PubMed] [Google Scholar]
- 38.Nicolas V., Le Van Kim C., Gane P., Birkenmeier C., Cartron J. P., Colin Y., Mouro-Chanteloup I. Rh-RhAG/ankyrin-R, a new interaction site between the membrane bilayer and the red cell skeleton, is impaired by Rhnull-associated mutation. J. Biol. Chem. 2003;278:25526–25533. doi: 10.1074/jbc.M302816200. [DOI] [PubMed] [Google Scholar]
- 39.Bruce L. J., Beckmann R., Ribeiro M. L., Peters L. L., Chasis J. A., Delaunay J., Mohandas N., Anstee D. J., Tanner M. J. A band 3-based macrocomplex of integral and peripheral proteins in the RBC membrane. Blood. 2003;101:4180–4188. doi: 10.1182/blood-2002-09-2824. [DOI] [PubMed] [Google Scholar]
- 40.Soupene E., Ramirez R. M., Kustu S. Evidence that fungal MEP proteins mediate diffusion of the uncharged species NH3 across the cytoplasmic membrane. Mol. Cell. Biol. 2001;21:5733–5741. doi: 10.1128/MCB.21.17.5733-5741.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soupene E., Lee H., Kustu S. Ammonium/methylammonium transport (Amt) proteins facilitate diffusion of NH3 bidirectionally. Proc. Natl. Acad. Sci. U.S.A. 2002;99:3926–3931. doi: 10.1073/pnas.062043799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soupene E., King N., Feild E., Liu P., Niyogi K. K., Huang C. H., Kustu S. Rhesus expression in a green alga is regulated by CO2. Proc. Natl. Acad. Sci. U.S.A. 2002;99:7769–7773. doi: 10.1073/pnas.112225599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soupene E., Inwood W., Kustu S. Lack of the Rhesus protein Rh1 impairs growth of the green alga Chlamydomonas reinhardtii at high CO2. Proc. Natl. Acad. Sci. U.S.A. 2004;101:7787–7792. doi: 10.1073/pnas.0401809101. [DOI] [PMC free article] [PubMed] [Google Scholar]