Abstract
Background
Iatrogenic ventricular septal defects (VSDs) are rare but important complications of transcatheter aortic valve replacement (TAVR). In patients who develop conduction abnormalities post-TAVR, the presence of a VSD can complicate pacing strategies.
Case Summary
We report 2 cases of perimembranous VSDs after TAVR, both complicated by conduction disease and managed with left bundle branch pacing (LBBP). The first patient underwent a redo valve procedure that was complicated by a Gerbode-type defect and pulmonary hypertension, culminating in a palliative approach. The second patient developed a restrictive VSD diagnosed on follow-up, with symptom improvement on medical therapy. In both cases, successful LBBP was achieved using septal drilling and precise lead placement despite septal disruption.
Discussion
Post-TAVR VSDs are uncommon and poorly characterized. These cases underscore the feasibility of LBBP in this complex setting and the need for individualized procedural planning.
Take-Home Messages
Post-TAVR VSDs warrant high clinical suspicion. LBBP is a viable pacing strategy when tailored to altered septal anatomy.
Key words: aortic valve, bradycardia, cardiac pacemaker, complication, echocardiography, valve replacement, ventricular septal defect
Graphical Abstract

Transcatheter aortic valve replacement (TAVR) is a widely adopted, minimally invasive procedure that has revolutionized interventions for aortic valve disease. Despite its benefits, postprocedural complications including arrhythmias are common, such as new-onset atrial fibrillation, high-degree atrioventricular (AV) block, and bundle branch blocks, and they may require permanent pacemaker implantation.1 Another rarer but significant complication after TAVR is the development of ventricular septal defects (VSDs), which can affect both hemodynamics and conduction owing to proximity to the aortic annulus and ventricular septum. We present 2 patients with post-TAVR VSDs requiring pacemakers, highlighting preventative strategies for both complications as well as their electrophysiologic implications and management.
Take-Home Messages
-
•
Iatrogenic VSDs are a rare but important complication after TAVR that can affect hemodynamics and conduction.
-
•
Left bundle branch pacing can be safely and effectively performed in the presence of a post-TAVR VSD with careful lead positioning and septal drilling.
-
•
Multimodality imaging and hemodynamic assessment are critical for diagnosing VSDs and guiding individualized pacing or surgical strategies.
Patient 1
Presentation
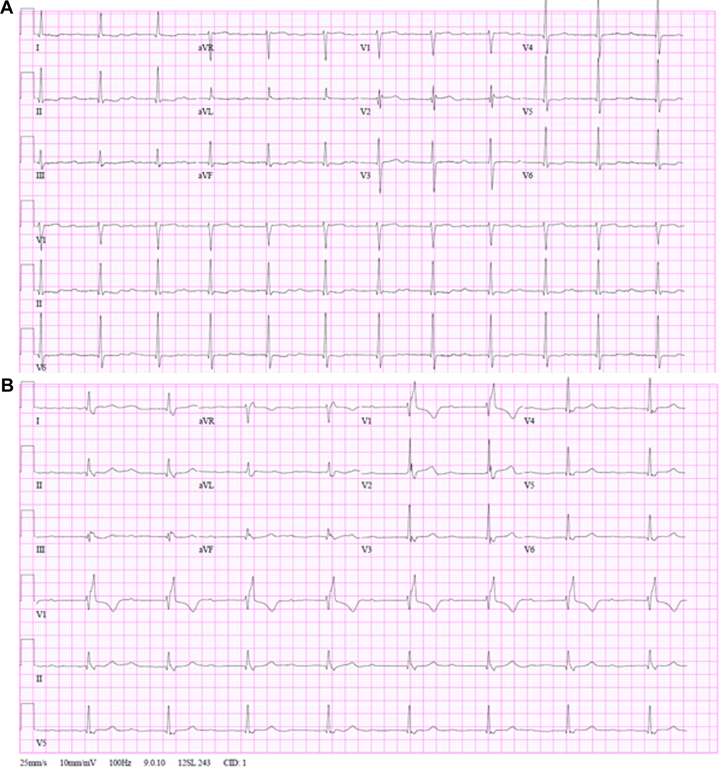
An 84-year-old woman with a history of paroxysmal atrial fibrillation, chronic heart failure with preserved ejection fraction, and aortic stenosis status post transfemoral aortic valve replacement presented with severe prosthetic aortic valve stenosis. Her electrocardiogram demonstrated sinus bradycardia at 52 beats/min with first-degree AV block and a PR interval of 216 milliseconds (Figure 1A).
Figure 1.
ECGs Before and After TAVR in Patient 1
Electrocardiograms for patient 1 (A) pre-TAVR and (B) post-TAVR. TAVR = transcatheter aortic valve replacement.
Diagnostic workup and intervention
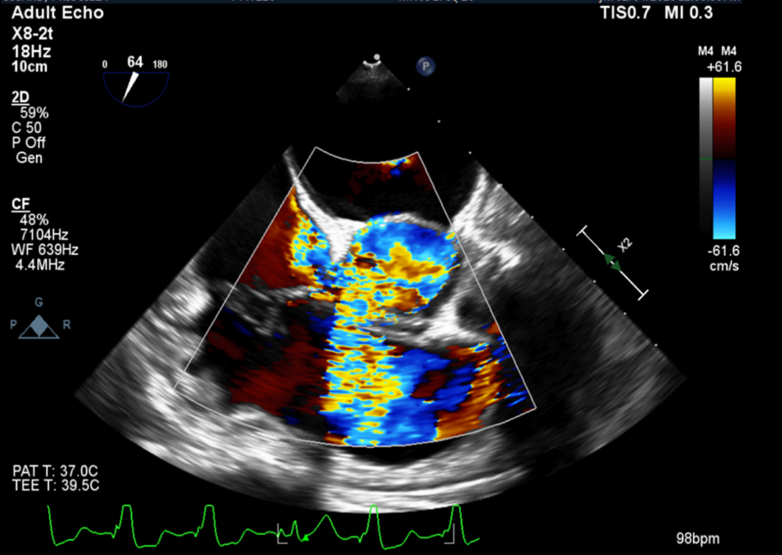
The patient underwent a transcatheter redo TAVR procedure during which a 23-mm Sapien 3 valve (Edwards Lifesciences) was implanted inside her preexisting 23-mm Evolut valve (Medtronic). The procedure was complicated by complete heart block (Figure 1B) and the development of a perimembranous VSD with a restrictive Gerbode-type shunt, with communication between the left ventricle and both the right ventricle and right atrium (Figure 2). The peak gradient across the VSD was measured at 71 mm Hg, and the pulmonary-to-systemic flow (Qp:Qs) ratio was calculated to be 1.4. After multidisciplinary discussions with the cardiac surgery team, the patient declined surgical septal repair. A dual-chamber pacemaker with left-bundle pacing configuration was implanted, and she was discharged home the next day.
Figure 2.
Perimembranous VSD Seen on Transesophageal Echocardiogram for Patient 1
End-systolic midesophageal short-axis view with color Doppler demonstrating flow from the left ventricle to the right atrium and right ventricle. VSD = ventricular septal defect.
Follow-up and outcome
At her 1-month follow-up, the patient returned with symptoms of fatigue, lower extremity edema, and abdominal distention. She was noted to be hypoxic. A right heart catheterization revealed a Qp:Qs ratio of 1.42, elevated biventricular filling pressures, and postcapillary pulmonary hypertension, with a right atrial pressure of 19 mm Hg, a pulmonary artery mean pressure of 47 mm Hg, and a pulmonary capillary wedge pressure of 23 mm Hg. Device interrogation demonstrated normal pacemaker function and no new arrhythmias. She was admitted to the cardiovascular intensive care unit for further diuresis. Despite renewed recommendation for surgical repair, the patient declined and instead chose to transition to comfort-focused care.
Patient 2
Presentation
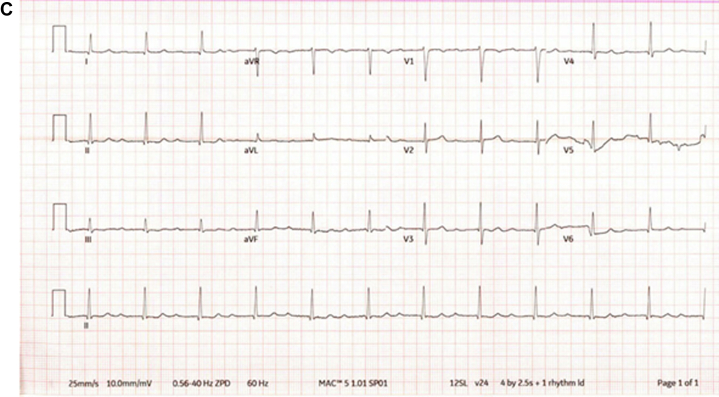
A 79-year-old man with a history of hypertension, first-degree AV block, and severe aortic stenosis underwent a transfemoral TAVR with postdilatation. His preprocedural electrocardiogram (ECG) demonstrated first-degree AV block and an incomplete right bundle branch block (RBBB) (Figure 3A).
Figure 3.
ECG Progression in Patient 2
Electrocardiograms for patient 2 (A) pre-TAVR, (B) post-TAVR, and (C) at the 9-month follow-up. TAVR = transcatheter aortic valve replacement.
Diagnostic workup and intervention
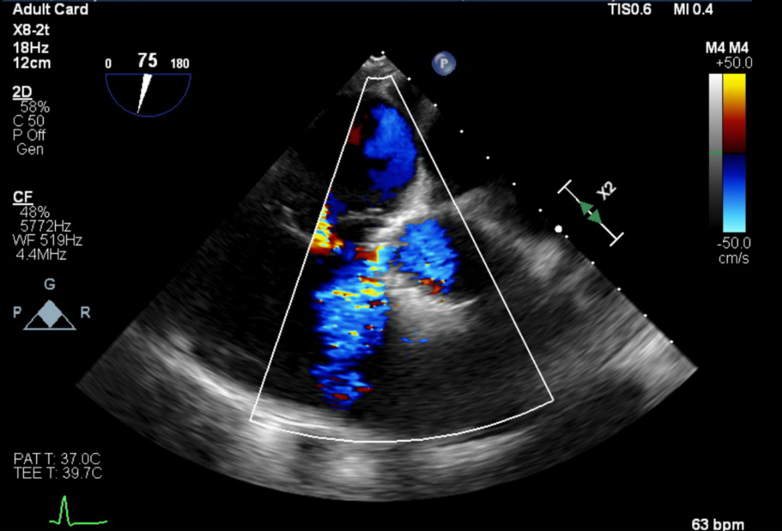
After the procedure, the patient’s ECG revealed a new complete RBBB, worsening first-degree AV block, and episodes of intermittent third-degree heart block (Figure 3B). Owing to these conduction abnormalities, a dual-chamber pacemaker with left-bundle pacing configuration was implanted. A postprocedural transthoracic echocardiogram demonstrated normal prosthetic valve function and was otherwise unremarkable. The patient was discharged in stable condition. At follow-up, a repeat transthoracic echocardiogram revealed a small perimembranous VSD, prompting further evaluation with a transesophageal echocardiogram, which confirmed a 0.5-cm perimembranous VSD located between the left ventricular outflow tract (LVOT) pole of the TAVR cage and the right ventricular inlet (Figure 4). Continuous left-to-right shunting was observed throughout systole, with an estimated Qp:Qs ratio of 1.34. Owing to new symptoms of dyspnea and lower extremity swelling, a right heart catheterization was performed. It revealed a Qp:Qs of 1.2, normal pulmonary arterial pressures, and preserved cardiac output—all consistent with a small restrictive VSD. No surgical intervention was planned, and the patient’s symptoms improved with adjustments to his diuretic regimen.
Figure 4.
Echocardiographic Views of VSD and Pacing Lead in Patient 2
Transesophageal echocardiogram for patient 2 showing VSD on midesophageal short-axis view (top) and the left bundle pacing lead anatomically distant from the VSD near the LVOT seen on a deep gastric view (bottom). LVOT = left ventricular outflow tract; VSD = ventricular septal defect.
Follow-up and outcome
At approximately 9 months post-TAVR, his most recent ECG showed resolution of the previously observed RBBB but with persistent first-degree AV block (Figure 3C).
Discussion
To the best of our knowledge, this is the first published case series demonstrating successful LBBP in patients who required pacemakers after TAVRs complicated by VSD formation. Firstly, we describe the incidence of this iatrogenic complication, risk factors associated with it, and preventative measures. We then explore the association of VSDs with conduction abnormalities and the electrophysiologic implications.
Post-TAVR VSDs
The incidence of VSDs after TAVR is rare. One large retrospective study reported VSDs in 7 of 1,908 patients (0.37%).2 However, underdiagnosis is suspected because of low clinical suspicion and limited postprocedural septal imaging.3 These iatrogenic VSDs are typically perimembranous and located near the aortic annulus.4 Reported risk factors include valve oversizing, heavy calcification, low implantation depth, and balloon postdilatation.3,5 Zeniou et al described heavy annular or LVOT calcification as an element leading to increased mechanical stress on the membranous septum, particularly in anatomies with short septal length or asymmetrical calcium.3 Together, they may result in septal disruption or delayed VSD formation.
Additional contributing mechanisms may include septal trauma from the TAVR delivery system or postprocedural inflammation.2,5 Use of self-expanding valves, such as the Evolut seen in our first case, has been associated with delayed VSD formation.3 Though some reports describe VSDs after valve-in-valve procedures, the extent of additional risk remains unclear.6
In both of our patients, postdilatation, balloon valvuloplasty, and valve-in-valve complexity were likely contributing factors.
Prevention and Surveillance
Preventive strategies include comprehensive preprocedural imaging to assess calcification and septal anatomy, appropriate valve sizing, and cautious use of balloon dilatation.2 However, overly conservative deployment may increase the risk of paravalvular leak, requiring a careful balance.7
VSD diagnosis timing also varies. In one of our patients, transesophageal echocardiogram detected the defect early postoperatively; in the other, the VSD was only noted at 1-month follow-up. Some VSDs may be diagnosed as late as 1 year after TAVR.4
Management depends on symptom severity and hemodynamic impact. A systematic review reported 12 symptomatic patients out of 20 VSD cases, with 6 undergoing surgical repair.4 Symptoms such as dyspnea or lower extremity edema should prompt echocardiographic evaluation. In our first patient, worsening pulmonary hypertension and heart failure symptoms highlighted the significance of the shunt. When surgical repair is deferred, ongoing imaging and device follow-up become essential.
Interplay Between VSDs and Conduction Abnormalities
Perimembranous VSDs are anatomically close to the His bundle and left bundle branch, increasing the risk of conduction disturbances.5 Prior studies have reported bradyarrhythmias after both surgical and transcatheter closure of congenital perimembranous VSDs.8 In our cases, TAVR likely triggered the arrhythmias, but it is notable that pacemaker lead trauma can also contribute to iatrogenic VSDs.9
In our second patient, resolution of RBBB after pacemaker implantation suggests intact conduction system pathways, with improved synchronization from LBBP supporting recovery.1 This finding indicates that perimembranous VSDs do not always preclude physiologic conduction recovery.
Procedure Considerations and Lead Placement
In patients with VSDs requiring pacemakers, lead placement must avoid the defect to prevent dislodgement or further septal injury. Fluoroscopic and echocardiographic guidance is critical. If a VSD is known before implantation, procedural planning should emphasize avoiding its vicinity.
In our first case, septal drilling, a technique involving the controlled advancement and rotation of the pacing lead into the interventricular septum to achieve capture of the left bundle branch, was performed more distally to avoid the defect. LBBP was used in both patients and may be preferred over His-bundle pacing because of its more distal septal location. Gradual drilling and shallow penetration are important in already compromised septal tissue. In patients with baseline conduction disease (eg, RBBB, left anterior fascicular block), a backup right ventricular lead may help ensure stability in case of further deterioration.
Conduction system pacing such as LBBP is preferred because of its benefits in reducing pacemaker-induced cardiomyopathy, heart failure hospitalizations, and all-cause mortality compared with right ventricular pacing.10 A careful discussion regarding the risks and benefits of both approaches should be undertaken if VSDs are present. Factors to consider should include the size of the defect and the hemodynamic status and indications for pacing, and an individualized approach is paramount.
Conclusions
Our case series highlights a rare complication of perimembranous VSDs after TAVR and its implications on the management of conduction abnormalities. Preventive strategies, such as thorough preprocedural imaging and careful valve selection, are key in reducing this risk. We demonstrate that LBBP, when combined with proper planning and technique, can safely manage these cases. Symptom monitoring, routine echocardiograms, and device checks are essential, particularly when the VSD is not repaired. Further studies are needed that look into long-term outcomes in patients who do not undergo repair, predictive factors and strategies to avoid VSD formation, and the impact of these defects on electrophysiologic interventions.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Siontis G.C.M., Praz F., Lanz J., et al. New-onset arrhythmias following transcatheter aortic valve implantation: a systematic review and meta-analysis. Heart. 2018;104(14):1208–1215. doi: 10.1136/heartjnl-2017-312310. [DOI] [PubMed] [Google Scholar]
- 2.Nona P., Mahmood S., Lemor A., et al. Incidence of acquired ventricular septal defect after transcatheter aortic valve replacement: a large single-center experience. Catheter Cardiovasc Interv. 2021;98(5):975–980. doi: 10.1002/ccd.29897. [DOI] [PubMed] [Google Scholar]
- 3.Zeniou V., Chen S., Gilon D., et al. Ventricular septal defect as a complication of TAVI: mechanism and incidence. Struct Heart. 2018;2(3):235–239. [Google Scholar]
- 4.Ando T., Holmes A.A., Taub C.C., et al. Iatrogenic ventricular septal defect following transcatheter aortic valve replacement: a systematic review. Heart Lung Circ. 2016;25(10):968–974. doi: 10.1016/j.hlc.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Auffret V., Puri R., Urena M., et al. Conduction disturbances after transcatheter aortic valve replacement: current status and future perspectives. Circulation. 2017;136(11):1049–1069. doi: 10.1161/CIRCULATIONAHA.117.028352. [DOI] [PubMed] [Google Scholar]
- 6.Molina A.A., Quero M.F., Haldón J.E.L., et al. An unusual complication after transcatheter aortic valve implantation: a case report. Eur Heart J Case Rep. 2024;8(2) doi: 10.1093/ehjcr/ytae045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilczek K., Bujak K., Reguła R., et al. Risk factors for paravalvular leak after transcatheter aortic valve implantation. Kardiochir Torakochirurgia Pol. 2015;12(2):89–94. doi: 10.5114/kitp.2015.52848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandal K.D., Su D., Pang Y. Long-term outcome of transcatheter device closure of perimembranous ventricular septal defects. Front Pediatr. 2018;6 doi: 10.3389/fped.2018.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pizzino F., Vizzari G., Bajwa T., et al. Iatrogenic ventricular septal defect after dual-chamber pacemaker implantation. Eur Heart J Cardiovasc Imaging. 2015;16(1):46. doi: 10.1093/ehjci/jeu161. [DOI] [PubMed] [Google Scholar]
- 10.Leventopoulos G., Alabed S., Akhtar Z., et al. Safety and efficacy of left bundle branch area pacing compared with right ventricular pacing in patients with bradyarrhythmia: a systematic review and meta-analysis. Int J Cardiol. 2023;384:1–10. doi: 10.1016/j.ijcard.2023.131230. [DOI] [PubMed] [Google Scholar]