Abstract
Aging is the single most important risk factor for AD (Alzheimer's disease). However, the molecular events that connect normal aging to AD are mostly unknown. The abnormal accumulation of Aβ (amyloid β-peptide) in the form of senile plaques is one of the main characteristics of AD. In the present study, we show that two members of the neurotrophin receptor superfamily, TrkA (tyrosine kinase receptor A) and p75NTR (p75 neurotrophin receptor), differentially regulate the processing of APP (amyloid precursor protein): TrkA reduces, whereas p75NTR activates, β-cleavage of APP. The p75NTR-dependent effect requires NGF (nerve growth factor) binding and activation of the second messenger ceramide. We also show that normal aging activates Aβ generation in the brain by ‘switching’ from the TrkA to the p75NTR receptor system. Such an effect is abolished in p75NTR ‘knockout’ animals, and can be blocked by both caloric restriction and inhibitors of nSMase (neutral sphingomyelinase). In contrast with caloric restriction, which prevents the age-associated up-regulation of p75NTR expression, nSMase inhibitors block the activation of ceramide. When taken together, these results indicate that the p75NTR–ceramide signalling pathway activates the rate of Aβ generation in an age-dependent fashion, and provide a new target for both the understanding and the prevention of late-onset AD.
Keywords: aging, Alzheimer's disease, amyloid precursor protein, ceramide, p75NTR (p75 neurotrophin receptor), neurotrophin
Abbreviations: AD, Alzheimer's disease; APP, amyloid precursor protein; APP-CTF, APP C-terminal fragment; Aβ, amyloid β-peptide; BACE1, β-site APP-cleaving enzyme-1; ESI–MS, electrospray ionization MS; NGF, nerve growth factor; SM, sphingomyelin; aSMase, acidic sphingomyelinase; nSMase, neutral sphingomyelinase; p75NTR, p75 neurotrophin receptor; PS1, presenilin 1; TNF, tumour necrosis factor; TACE, TNF-converting enzyme; TrkA, tyrosine kinase receptor A; WT, wild-type
INTRODUCTION
AD (Alzheimer's disease) is the most prevalent form of dementia in the world. Based on the onset of the symptoms, AD is normally divided into two groups: early- (<60 years) and late- (>60 years) onset. Late-onset AD is a complex and heterogeneous disease that accounts for 95–97% of all AD cases. The prevalence of late-onset AD increases during aging without reaching a plateau [1]. Because of the increasing life expectancy observed in many countries, it is estimated that in 2050 approx. 45 million individuals will be affected by AD worldwide. Even though aging is the single most important risk factor for late-onset AD, the molecular events that mediate the age-dependent effect on AD are still mostly unknown.
The abnormal accumulation of Aβ (amyloid β-peptide) in the form of senile (or amyloid) plaques and amyloid angiopathy is one of the main neuropathological hallmarks of this disorder. Additional features of postmortem-diagnosed AD are neurofibrillary tangles and diffuse loss of neurons and synapses in the neocortex, hippocampus and other subcortical regions of the brain [2,3]. Aβ is a 39–43 amino-acid-long peptide generated by sequential proteolysis of APP (amyloid precursor protein) at β- and γ-sites [2,3]. The β-site cleavage represents the rate-limiting step in Aβ generation and is catalysed by BACE1 (β-site APP-cleaving enzyme-1) [4]. Studies from normally aged rodents, primates and humans indicate that aging is accompanied by a progressive increase in BACE1 activity in the brain [5]. An additional increase in both activity and protein levels of BACE1 is observed in postmortem AD brains when compared with age-matched controls [6,7]. Studies from transgenic mice expressing human APP indicate that the abnormal accumulation of Aβ in the brain represents an important step for the development of the Alzheimer form of neurodegeneration [8]. In addition, BACE1 ‘knockout’ animals, which are not able to cleave APP at the β-site and generate Aβ, do not develop AD neuropathology [9,10].
We have shown recently [11] that the lipid ceramide regulates the rate of Aβ generation by affecting the molecular stability of BACE1. Ceramide is a second messenger involved in many biochemical and genetic events that occur during cellular senescence [12,13]. In addition, ceramide levels are increased by more than 3-fold in the brains of AD patients when compared with age-matched controls [14,15]. Endogenous active ceramide is mostly generated by de novo synthesis or hydrolysis of SM (sphingomyelin) at the cell surface, the latter being the most important source of the active pool of ceramide [16]. Hydrolysis of SM can be produced by either nSMase (neutral sphingomyelinase) or aSMase (acidic SMase). Only nSMase generates the signalling active ceramide; aSMase is involved in SM catabolism in the lysosomal compartment [16]. The generation of ceramide in neurons is mostly regulated by p75NTR (p75 neurotrophin receptor), which controls the activation of endogenous nSMase [17–19]. Oxidative and metabolic stresses have also been proposed as additional ways to activate nSMase, but final proof in vivo is lacking.
In the present study, we show that neurotrophin receptors TrkA (tyrosine kinase receptor A) and p75NTR differentially regulate APP processing: TrkA reduces, whereas p75NTR activates, β-cleavage of APP. The p75NTR-dependent effect requires NGF (nerve growth factor) binding and activation of the second messenger ceramide. More importantly, aging controls the rate of Aβ generation by ‘switching’ from TrkA to p75NTR. Such effect is abolished in p75NTR ‘knockout’ animals, and can be blocked by both caloric restriction and inhibitors of nSMase. In contrast with caloric restriction, which prevents the age-associated up-regulation of p75NTR expression, nSMase inhibitors block the activation of ceramide.
MATERIALS AND METHODS
Cell culture
Human neuroblastoma cells SK-N-BE, which do not express either p75NTR or Trk receptors [20], were stably transfected with p75NTR, TrkA or empty vectors, and were maintained in the presence of either hygromycin (150 μg/ml) or G418 (300 μg/ml; Calbiochem) as selection markers.
For neuronal cultures, hippocampi and frontal cortices were dissected from embryonic-day 16–18 (E16–18) mice and placed in DMEM (Dulbecco's modified Eagle's medium; Gibco BRL) [21]. The tissue was mechanically dissociated by pipetting, and neurons were plated on to poly(L-lysine)-coated six-well plates (Becton Dickinson Labware) for 2 h. The medium was then changed to Neurobasal medium containing 2% (v/v) B27 supplement (Gibco BRL) in the absence of serum or antibiotics. Cultures grown in serum-free media yielded approx. 99.5% neurons and 0.5% glia. Microscopically, glial cells were not apparent in cultures at the time (day 12) they were used for experimental analyses. However, some experiments were also performed in the presence of 10 μM cytosine β-D-arabinofuranoside hydrochloride (Sigma) in order to exclude any effect produced by possible proliferation of glial cells. Medium was changed every 3 days.
Animals and dietary manipulations
Male C57Bl6 mice were purchased from Harlan Sprague–Dawley and maintained under specific pathogen-free conditions until killed. Animal care was performed in accordance with Guidelines for the Ethical Care and Treatment of Animals from the Institutional Animal Care and Use Committee at the University of Wisconsin-Madison. To control caloric intake, mice were housed singly and fed less than ad libitum intakes [22]. The control group was fed 84 kcal/week (where 1 kcal≡4.184 kJ) of a modified formulation of AIN-76 semi-purified diet (Harlan Teklad), which is approx. 90% of the average ad libitum food intake of these mice. Mice on caloric restriction were restricted in their food intake from 6 weeks of age, being fed 63 kcal/week (a 32% reduction). The restricted diet was nearly isocaloric with the control diet, but enriched in proteins, vitamins and minerals to avoid malnutrition [22]. Mice were killed and brains were immediately removed; cortices and hippocampi were separated and rapidly frozen by immersion in liquid nitrogen.
For nSMase inhibition, slow-release pellets (Innovative Research of America), containing either manumycin A (3.5 mg/animal over a period of 2 months) or placebo, were implanted subcutaneously under isoflurane anaesthesia. No sign of infection, discomfort or distress was observed in association with implantation and treatment. During treatment, animals were allowed free access to food and water. No difference in food intake and body weight was observed between treated and control animals. Mice were killed and brains were rapidly removed for isolation of cortices and hippocampi.
p75NTR/ExonIII−/− mice [23] were obtained from The Jackson Laboratory (Bar Harbor, ME, U.S.A.). They have a targeted deletion of exon III and lack the extracellular domain responsible for neurotrophin binding. Animals are viable and fertile, but they exhibit minor deficits in the peripheral nervous system. To avoid injuries induced by reduced sensitivity, mice were housed singly and checked daily for injuries on their extremities.
Lipid labelling and extraction
Labelling of sphingolipids in SK-N-BE cells and primary neurons was performed using [9,10-3H(N)]palmitic acid (60 Ci/mmol; NEN Life Science) as described previously [11]. For lipid extraction, cells were washed twice in PBS, scraped and extracted in chloroform/methanol (2:1, v/v). The lipid phase was dried, resuspended in chloroform and applied, together with standards, to a Silica Gel-G (EM Science) TLC plate. Plates were developed as described previously [11]; spots were then scraped and counted in a liquid-scintillation counter.
For ceramide quantification in the brain, brain membrane extracts were analysed by both ESI–MS (electrospray ionization MS) and TLC. Identity and quantification of TLC spots was confirmed further by ESI–MS (performed at the Mass Spectrometry Facility of the University of Wisconsin Biotechnology Center). ESI–MS analysis of our samples did not reveal breakdown products of major glycosphingolipids in the membrane preparations, excluding any contamination from myelin structures/white matter (results not shown). Pixel densities of TLC spots were calculated from scanned images with Adobe Photoshop; densitometry was performed with the EpiChemi3 Darkroom™ (UVP Bioimaging Systems) using Labworks Image Acquisition and Analysis Software 4.5.
Antibodies and Western blot analysis
Western blot analysis was performed as described previously [11,21]. Polyclonal antibodies against the C-terminus of APP and BACE1 were from Chemicon International and Abcam respectively. A mouse antibody against the N-terminal domain of PS1 (presenilin 1) was from Santa Cruz Biotechnologies. Polyclonal antibodies against p75NTR and TrkA were either from Santa Cruz Biotechnologies or Promega. Polyclonal antibodies against BACE2 and TACE [TNF (tumour necrosis factor)-converting enzyme] were both from Abcam.
Pixel densities (for signal-area) of scanned images were calculated with Adobe Photoshop; densitometry (for signal-density) was analysed with the EpiChemi3 Darkroom™ (UVP Bioimaging Systems) using Labworks Image Acquisition and Analysis Software 4.5.
Aβ determination
For Aβ determinations, cortices and hippocampi were homogenized in GTIP buffer [100 mM Tris/HCl (pH 7.6), 20 mM EDTA and 1.5 M NaCl] containing protease inhibitors, 1.5% Triton X-100 and 0.25% NP-40 [21]. Detergent solubilization was sufficient to resolve more than 97% of total brain Aβ. Formic acid or guanidine extractions did not yield additional Aβ. In this regard, it is important to stress that rodent Aβ differs from human Aβ in three amino acids and does not aggregate in fibrils/plaques.
Total Aβ (1–40 plus 1–42) was quantified by standard sandwich ELISA using 9131 [for Aβ(1–40)] and 9134 [for Aβ(1–42)] as capture antibodies, and 9154 (specific for rodent Aβ) and 4G8 as biotinylated reporter antibodies (Signet Laboratories). For each sample, the levels of Aβ40, Aβ42 and Aβtotal were quantified in triplicate based upon standard curves run (on every ELISA plate) and then expressed as pmol of Aβ/mg of protein. The average values of endogenous Aβtotal obtained for 5-month-old animals was 7.8±1.1 pmol/mg of protein for cortex and 6.1±0.5 pmol/mg of protein for hippocampus. They are in the same range of values found previously [24] for endogenous Aβtotal using murine-specific reporter antibodies. Aβ42 was constantly found to be approx. 25–30% of total Aβ values.
β- and γ-Secretase activity in vitro
Tissue homogenates from hippocampi and cortices (prepared as described above) were assayed in vitro using the QTL Light-speed Assay (QTL Biosystems). The assay uses a specific substrate peptide in which the cleavage sites are flanked by biotin and a quencher. Cleavage of the peptide (at β- or γ-site respectively) separates the biotin-containing peptide fragment from the quencher fragment, which then becomes unable to bind the sensor or quench its fluorescence. The specific substrates were TEEISEVNL*DAEFK (for β-secretase cleavage) and GVV*IA*TVK (for γ-secretase cleavage; * indicates the specific cleavage site). The assay was performed as described by the manufacturer. Each sample was analysed in duplicate at two different concentrations. In addition to the controls suggested by the manufacturer, we also assayed the samples after boiling at 100 °C for 30 min prior to incubation with the substrate and sensor. Values were calculated over background (blank; no enzyme) and expressed as arbitrary units/g of protein.
Statistical analysis
The data were analysed by ANOVA and Student's t test comparison using GraphPad InStat3 software. Statistical significance was reached at P<0.05.
RESULTS
Neurotrophin receptors differentially regulate β-cleavage of APP
We initially analysed APP metabolism following NGF treatment in the human neuroblastoma SK-N-BE cell line expressing either p75NTR or TrkA. Before stable transfection with p75NTR or TrkA, the above cell line expressed neither of the receptors [20]. This is particularly relevant since NGF binding to TrkA inhibits the p75NTR-dependent activation of ceramide [25].
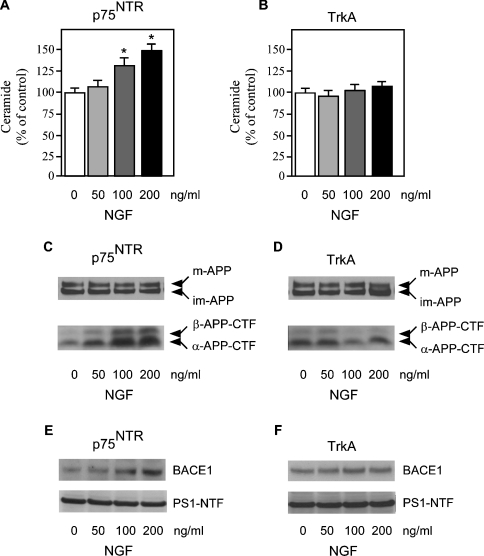
NGF treatment increased both ceramide levels and the steady-state levels of β-APP-CTF (APP C-terminal fragment) in p75NTR cells (Figures 1A and 1C). β-APP-CTF is the intermediate product of β-cleavage of APP and a direct indication of BACE1 activity. A parallel increase in α-APP-CTF, produced by α-cleavage of APP, was also evident (Figure 1C); however, α-cleavage of APP does not lead to the generation of Aβ. NGF treatment of p75NTR-expressing cells also increased the steady-state levels of BACE1 in the absence of any apparent effect on PS1 expression levels (Figure 1E). These results are consistent with our previous observation [11] that ceramide can regulate both β- and α-cleavage of APP in the absence of any effect on γ-secretase activity. The effect produced by NGF was dose-dependent and specific for p75NTR, since it was not observed in TrkA cells, which were unable to activate the ceramide signalling pathway (Figures 1B, 1D and 1F). It is worth noting that, prior to NGF treatment, the baseline levels of β-APP-CTF were already higher in cells expressing p75NTR than in those expressing TrkA alone (see Supplementary Figure 1B at http://www.BiochemJ.org/bj/391/bj3910059add.htm) and NGF produced a slight decrease in β-cleavage of APP in TrkA cells (Figure 1D, and see Supplementary Figure 1B at http://www.BiochemJ.org/bj/391/bj3910059add.htm). The effects produced by NGF treatment on p75NTR-expressing cells were limited to α- and β-APP-CTF; no additional modifications of APP processing were observed (see Supplementary Figure 1A at http://www.BiochemJ.org/bj/391/bj3910059add.htm). The liberation of the second messenger ceramide following NGF treatment did not affect cell viability, as assessed by LDH (lactate dehydrogenase) release into the media (see Supplementary Table 1 at http://www.BiochemJ.org/bj/391/bj3910059add.htm), confirming our previous conclusions [11] further that the regulation of APP processing downstream from ceramide is not secondary to the activation of the apoptotic cascade.
Figure 1. p75NTR and TrkA differentially regulate APP processing.
p75NTR and TrkA stably transfected SK-N-BE cells were treated with increasing concentrations of NGF for 48 h. (A and B) Endogenous ceramide was analysed as described in the Materials and methods section. *Significant difference from 0 ng/ml (no treatment). Error bars represent the S.D. for three different determinations. (C and D) Western blot analysis of APP-CTFs following NGF treatment. Total cell lysates were separated on 4–12% Bis-Tris SDS/PAGE gels, blotted on to a PVDF membrane, and probed with antibody AB5352 against the C-terminal domain of APP. The primary antibody was followed by a horseradish-peroxidase-conjugated monoclonal antibody and detected by chemiluminescence as described previously [11,21]. Mature (m-APP) and immature (im-APP) APP, together with β- and α-APP-CTFs, are indicated. (E and F) Western blot analysis of BACE1 and PS1-N-terminal-fragment (PS1-NTF) expression levels following NGF treatment. Total cell lysates were separated on 4–12% Bis-Tris SDS/PAGE gels, blotted on to a PVDF membrane, and probed with either antibody ab2077 (anti-BACE) or sc-8040 (anti-PS1). The primary antibody was followed by an horseradish-peroxidase-conjugated monoclonal antibody and was detected by chemiluminescence. BACE1 and PS1-NTF are indicated.
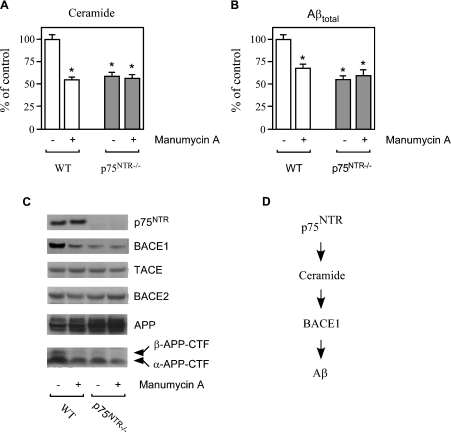
Next we analysed APP processing and Aβ generation in primary neurons from both WT (wild-type) and p75NTR−/− mice in which exon III of the p75NTR locus was targeted for deletion [23]. These mice produce a shorter isoform of p75NTR lacking the extracellular domain of the receptor required for neurotrophin binding [26]. Ceramide levels in p75NTR−/− neurons were found to be markedly reduced when compared with the WT counterpart (Figure 2A). We also observed a striking correlation between ceramide (Figure 2A) and Aβ levels in the conditioned media (Figure 2B) with an approx. 50% reduction in p75NTR−/− neurons. Manumycin A, a competitive and irreversible inhibitor of nSMase [27], reduced both ceramide levels and Aβ secretion in WT neurons (Figures 2A and 2B); no effect was observed when manumycin A was administered to p75NTR−/− neurons (Figures 2A and 2B), indicating that nSMase acts downstream from p75NTR (Figure 2D). Analysis of BACE1 and APP processing was consistent with the above results showing higher steady-state levels of both BACE1 and β-APP-CTF in WT compared with p75NTR−/− neurons (Figure 2C). Again, manumycin A reduced both BACE1 and β-APP-CTF levels in WT, but not in p75NTR−/−, neurons (Figure 2C). No overall effect was observed in the steady-state levels of either BACE2, a close homologue of BACE1, or TACE, a regulated form of α-secretase (Figure 2C).
Figure 2. Ceramide is required for the p75NTR-mediated regulation of Aβ generation.
(A–C) Primary neurons from WT and p75NTR−/− animals were prepared as described in the Materials and methods section, and were then incubated in the absence or presence of the nSMase inhibitor manumycin A (100 μM). (A) Endogenous ceramide was analysed by TLC following prelabelling with [9,10-3H(N)]palmitic acid, as described in the Materials and methods section. (B) Aβ levels in the conditioned media were determined by standard sandwich ELISA using an anti-rodent Aβ antibody, as described in the Materials and methods section. The average concentration of Aβtotal found in the conditioned media of WT neurons was 11984±995 pmol/mg of protein. No difference in the Aβ42/Aβtotal ratio was observed when comparing WT and p75NTR−/− neurons (results not shown). *Significant difference from WT neurons (no treatment). Error bars represent the S.D. for six different determinations. (C) Western blot analysis of WT and p75NTR−/− neurons maintained in the absence or presence of manumycin A. Total cell lysates were separated on 4–12% Bis-Tris SDS/PAGE gels, blotted on to a PVDF membrane, and probed with the appropriate antibodies (indicated in the Materials and methods section). (D) Schematic view to summarize the results observed with SK-N-BE (Figure 1) and primary neurons (Figure 2) showing that p75NTR acts upstream of ceramide in the regulation of BACE1 steady-state levels and Aβ generation.
When taken together, the above results obtained with human neuroblastoma cell lines and primary neurons indicate that p75NTR can activate Aβ generation through the liberation of the second messenger ceramide (Figure 2D), which is responsible for the molecular stabilization of BACE1 [11].
Aging regulates Aβ generation through the p75NTR receptor system
Neurotrophins bind to two different classes of receptors: p75NTR and the tyrosine kinase family of receptors (TrkA, TrkB and TrkC) [28]. Our results, obtained using a genetic approach, indicate that the p75NTR-, but not the TrkA-, dependent signalling pathway activates the biogenesis of Aβ. Previous studies with sensory and motor neurons [29,30] have shown that aging is accompanied by a progressive increase in p75NTR expression. The rise in p75NTR expression is also accompanied by a parallel decrease in the expression of Trk receptors, suggesting that the differential regulation of these two classes of neurotrophin receptors underlies a common age-associated mechanism [29,30]. Therefore our results would suggest that normal aging favours the p75NTR- compared with the TrkA-dependent signalling event.
In order to test this hypothesis, we analysed ceramide metabolism and APP processing in normally aged mice. These experiments were all performed with non-transgenic animals because AD transgenic mice develop a severe form of neuropathology early in life and do not allow for an accurate and definitive analysis of late-life events.
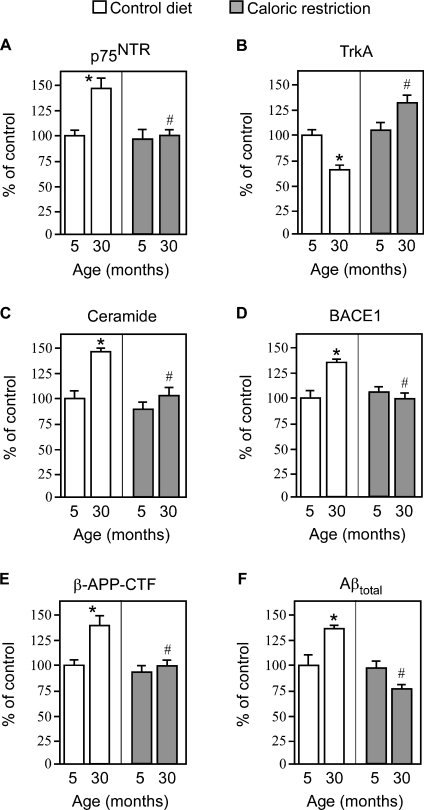
Figures 3(A) and 3(B) show that normal aging of the brain is accompanied by a shift in the relative expression of TrkA and p75NTR: p75NTR increases whereas TrkA decreases. This effect is accompanied by a parallel increase in the levels of ceramide, BACE1, β-APP-CTF and total Aβ (Figures 3C–3F). Analysis of cortex (Figure 3) and hippocampus (see Supplementary Figure 2 at http://www.BiochemJ.org/bj/391/bj3910059add.htm) yielded very similar results. The levels of activation of p75NTR, ceramide, BACE1 and Aβ biosynthesis detected in both the cortex and hippocampus (approx. 1.5-fold; 30 months compared with 5 months) are in the same range normally found with other age-related events using gene microarrays [31,32]. In this regard, it is important to remember that patients affected by Down's syndrome (trisomy 21), who have a 50% increase in gene dosage (extra copy of APP on chromosome 21) from birth, develop AD during their fourth decade of life. Therefore the 50–60% gradual activation of Aβ generation observed during the normal process of aging is consistent with the progression of late-onset AD, which normally strikes during or after the seventh decade of life.
Figure 3. Caloric restriction blocks the age-associated activation of Aβ generation by acting upstream of TrkA/p75NTR.
Analysis of the cerebral cortex from WT mice fed either a control (84 kcal/week) or caloric-restricted (63 kcal/week) diet. The expression levels of p75NTR (A), TrkA (B), BACE1 (D) and β-APP-CTF (E) were analysed by Western blot with the appropriate antibodies (as indicated in the Materials and methods section). Relative densities of images were calculated with the EpiChemi3 Darkroom™ (UVP Bioimaging Systems) using Labworks Image Acquisition and Analysis Software 4.5. Results are expressed as a percentage of the data for 5-month-old controls. (C) Ceramide was quantified by both ESI–MS and TLC as described in the Materials and methods section. (F) Aβ levels were determined by standard sandwich ELISA using an anti-rodent Aβ antibody, as described in the Materials and methods section. The average values of endogenous Aβtotal obtained for 5-month-old animals were 7.8±1.1 pmol/mg. Aβ42 was constantly found to be approx. 25–30% of total Aβ values. Error bars represent the S.D. for nine different determinations. Statistical significance compared with 5-month-old (*) or age-matched (#) control animals is indicated.
Caloric restriction is the only experimental intervention able to extend the maximum life span and to delay many biological changes that are associated with aging [33]. The ability of caloric restriction to delay or arrest the biochemical and physiological changes observed during aging has been confirmed continuously in many animals, suggesting common mechanisms that act to control the life span of all animals.
Therefore we decided to analyse whether the above events were truly under the general programming of aging by studying mice subjected to long-term caloric restriction. Our results indicated that caloric restriction was able to arrest the age-associated changes in the relative expression of neurotrophin receptors, ceramide production, BACE1 levels, APP processing and Aβ generation (Figure 3). No effect was observed on the steady-state levels of mature and immature APP (results not shown).
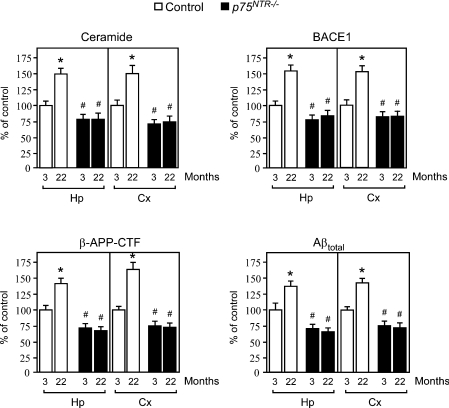
In order to confirm that the effect produced by aging on APP/Aβ metabolism required activation of the p75NTR signalling pathway, we analysed young and old p75NTR−/− mice. Figure 4 shows that p75NTR−/− animals were not able to raise ceramide levels in an age-dependent fashion, and did not show any increase in BACE1 levels, β-cleavage of APP and Aβ production. It is noteworthy that the levels of ceramide, BACE1, β-APP-CTF and Aβ in the knockout mice were always lower than those of their age-matched controls (Figure 4), suggesting that the constitutive expression of p75NTR is, at least in part, responsible for the control levels of APP/Aβ metabolism even before the additional activation produced by normal aging. This last conclusion is also supported by the results obtained with human neuroblastoma cells, where p75NTR expression increased β-cleavage of APP even in the absence of NGF treatment (see Supplementary Figure 1B at http://www.BiochemJ.org/bj/391/bj3910059add.htm), and with those obtained with primary neurons from p75NTR−/− animals, which showed levels of ceramide and Aβ below those of their WT counterpart (Figure 2).
Figure 4. Genetic disruption of p75NTR abolishes the age-associated activation of Aβ generation.
Analysis of both hippocampus (Hp) and cortex (Cx) from young and old p75NTR−/− mice. Analysis was performed as described in the legend to Figure 3. Results are expressed as percentage of the data for 3-month-old control animals. Error bars represent the S.D. for 12 different determinations. Statistical significance compared with 3-month-old (*) or age-matched (#) control animals is indicated.
Manumycin A prevents the age-associated activation of Aβ generation
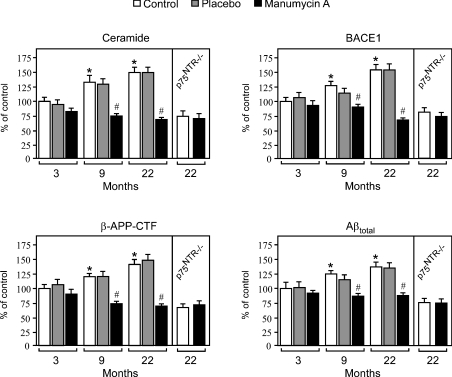
Finally, we decided to treat normal mice with the nSMase inhibitor manumycin A. In contrast with caloric restriction that acts by affecting the relative expression of neurotrophin receptors, nSMase inhibitors block the activation of the second messenger ceramide, therefore acting downstream of p75NTR. Manumycin A is predicted to pass the brain/blood barrier. Indeed, when administered through the diet for only 5 days, it reduced nSMase activity in brain homogenates by approx. 20% (from 47.8±5.0 to 37.4±2.7 nmol of ceramide formed·h−1·mg of protein−1). As expected, no effect was observed on the activity of aSMase (results not shown). Even though manumycin A was effective through the diet, we decided to deliver the inhibitor via implantable slow-release biopolymer pellets in order to obtain a continuous and controlled release of the active compound. Manumycin A was administered to 1-, 7- and 20-month-old mice at the concentration of 3.5 mg/animal over a period of 2 months, which has already been shown not to be toxic to rodents [34,35].
Analysis of both cortex (Figure 5) and hippocampus (results not shown) confirmed the age-associated increase in ceramide production, BACE1 levels, APP processing and Aβ generation. The results also revealed that manumycin A can successfully block the above events in an age-dependent fashion. It is worth stressing the fact that manumycin A was more effective in older mice, which show a more pronounced activation of nSMase (Figure 5). When administered to 20-month-old animals, manumycin A produced levels of APP processing and Aβ generation similar to p75NTR−/− mice, which were unable to activate the ceramide-dependent regulation of APP/Aβ metabolism (Figure 4; also see Figure 2). Finally, manumycin A did not produce any effect when administered to p75NTR−/− mice (Figure 5), confirming further that it acts downstream of p75NTR.
Figure 5. Manumycin A blocks the effects produced by normal aging.
Mice of 1, 7, and 20 months of age were implanted with slow-release pellets containing either manumycin A (3.5 mg/animal over a period of 2 months) or placebo. Animals were killed following 2 months of treatment (at 3, 9 and 22 months of age), and were analysed as described in the legend to Figure 3. Results are expressed as a percentage of the data for 3-month-old control animals. Error bars represent the S.D. for 12 different determinations. Statistical significance compared with 3-month-old (*) or age-matched (#) control animals is indicated.
In conclusion, the above results indicate that normal aging increases Aβ generation by acting through the p75NTR-mediated activation of the second messenger ceramide. They also suggest that nSMase inhibitors can provide an effective strategy to prevent the age-associated risk for AD.
Only β-secretase activity (and not γ-) is under the control of the p75NTR–ceramide pathway during aging
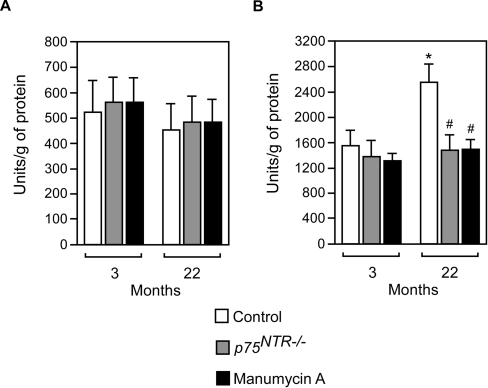
We have so far assumed that the increased levels of BACE1, with consequent activation of the β-cleavage of APP, are solely responsible for the effects produced by the p75NTR–ceramide signalling pathway on Aβ generation during aging. This assumption is supported by our previous studies [11] showing that ceramide regulates α- and β-, but not γ-, cleavage of APP and by the increased levels of β-APP-CTF, which were observed in the absence of any effect on APP levels. Indeed, if the increased levels of Aβ were a consequence of increased γ-cleavage, we would expect reduced β-APP-CTF levels. Instead, the levels of Aβ were always paralleled by similar changes in both β-APP-CTF and BACE1, indicating that the rate of β-cleavage is the primary event responsible for the levels of Aβ produced during aging.
However, we cannot rule out the possibility that aging is accompanied by increased activity of both β- and γ-secretase, which could be differentially regulated. In order to address this issue, we decided to analyse γ-secretase activity from brain homogenates in vitro. As a control, we also analysed β-secretase activity using a similar enzymatic approach. The results shown in Figure 6(A) indicate that γ-secretase activity was not significantly affected by aging, genetic disruption of p75NTR or nSMase inhibition. In contrast, β-secretase activity increased during aging (Figure 6B) by approx. 50% over a period of 19 months (from 3 to 22 months); such an effect was completely abolished by genetic disruption of the ligand-binding domain of p75NTR and manumycin A treatment (Figure 6B).
Figure 6. Enzymatic activity of β-secretase increases during aging and is under the control of the p75NTR–ceramide signalling pathway.
Brain homogenates from mice cortex were assayed in vitro for both γ- (A) and β- (B) secretase activity. Each determination was done in duplicate and at two different concentrations (50 μg and 100 μg). Values are expressed as arbitrary units/g of protein, and are calculated over background levels (blank). Boiled samples were always below background levels (results not shown). Error bars represent the S.D. of four different assays. Statistical significance compared with 3-month-old (*) or age-matched (#) control animals is indicated.
When taken together, these results confirm that increased steady-state levels and activity of BACE1, the rate-limiting enzyme in the biosynthesis of Aβ, cause the activation of Aβ generation observed during aging. They also confirm that the age-associated activation of β-secretase is under the control of the p75NTR–ceramide molecular pathway.
DISCUSSION
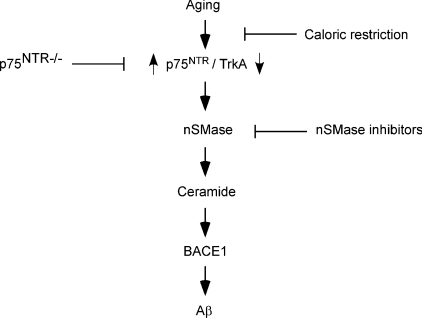
In the present study, we have identified the p75NTR system as a novel molecular link between normal aging of the brain and AD, providing a new target for both the understanding and prevention of the Alzheimer form of neurodegeneration. We have also shown that caloric restriction and nSMase inhibitors may provide efficient ways to reduce the risk associated with age. The former acts by preventing the ‘switch’ in the expression of neurotrophin receptors induced by aging, whereas the latter acts by blocking the activation of ceramide. In both cases the signalling pathway downstream p75NTR and ceramide is interrupted (Figure 7).
Figure 7. Schematic diagram summarizing the results of the present study showing the age-associated regulation of Aβ generation.
Aging regulates the rate of Aβ generation by controlling the relative expression of neurotrophin receptors p75NTR and TrkA. Upon binding to NGF, p75NTR activates nSMase and induces hydrolysis of sphingomyelin with consequent liberation of the second messenger ceramide. The ceramide-dependent signalling cascade increases the steady-state levels of BACE1, the rate-limiting enzyme in the biosynthesis of Aβ. Aβ then aggregates and precipitates in the brain in the form of senile (or amyloid) plaques. Even though the ceramide-dependent signalling cascade can also activates α-cleavage of APP (the present study and [11]), such an event does not lead to the generation of Aβ. Caloric restriction prevents the age-associated switch from the TrkA- to p75NTR-dependent signalling cascade. It is noteworthy that TrkA reduces APP processing and prevents Aβ generation. In contrast with caloric restriction, nSMase inhibitors block the generation of ceramide and prevent Aβ biosynthesis without affecting the expression of neurotrophin receptors.
While this manuscript was under preparation, Capsoni et al. [35a] have shown that deletion of p75NTR in AD11 anti-NGF transgenic mice completely abolished both amyloid load and Aβ accumulation, leading to a dramatic amelioration of AD-like neurodegeneration. Their results clearly indicate that the abnormal Aβ production and amyloid plaque accumulation in AD11 mice is produced solely by overstimulation of the p75NTR signalling pathway, and are consistent with our present results.
Recent studies have shown that pro-NGF, the uncleaved form of NGF, binds to p75NTR with higher affinity than mature NGF and that it prefers p75NTR to TrkA [36]. This might be particularly relevant considering that the levels of pro-NGF found in the brain of AD patients are higher than in age-matched controls [37], therefore elevating the risk already provided by normal aging. It is also worth noting that different studies have found a selective and marked reduction in the expression of cortical TrkA in early and late stages of AD when compared with age-matched controls [38,39]. Conversely, the expression of p75NTR has been found to be greatly increased in neurons of both cortex [40] and hippocampus [41] of AD patients. In addition, the latter study has also reported that the expression of p75NTR is increased highly in hippocampal neurons containing hyperphosphorylated tau. One study on cholinergic projection neurons of the forebrain nucleus basalis [39] found an inverse relationship between TrkA expression levels and AD progression, but failed to detect any change in p75NTR protein levels. However, the same neuronal population was also found to have increased p75NTR levels in AD patients at both the mRNA [42] and protein [43] levels.
p75NTR is part of the large family of TNF receptors and with them shares the ability to translate both death and survival signals [44]. This may either involve different affinities to the ligand(s) [28], in concert with co-receptors that would determine the exact message to be translated [45], or cytoplasmic interactors, which modulate the interaction of the receptor with the ‘active’ signalling molecule [44,46]. On this front, it is important to stress the fact that p75NTR is devoid of intrinsic catalytic activity and, therefore, its signalling abilities rely on intracellular interactors. Since the ability of p75NTR to liberate the second messenger ceramide requires activation of nSMase, the identification of the cytosolic interactors could provide additional molecular targets to use for the prevention of late-onset AD.
The ability of p75NTR to transduce both death and life signals has also been observed in the presence of Aβ. Indeed, although some studies have shown that extracellular Aβ can trigger cell toxicity/death through p75NTR [47,48], a recent study [49] has proposed a neuroprotective role for p75NTR against extracellular Aβ toxicity in human neurons. It is worth remembering that ceramide activation downstream of p75NTR has also been associated with neuronal survival [18,19]. The possible implications of the above conclusions, in view of the TrkA-to-p75NTR switch induced by aging, will need to be explored further using appropriate aging paradigms.
The identification of a TrkA-to-p75NTR switch as a possible molecular link between normal aging of the brain and AD provides novel insights in the understanding of this form of neuropathology. The fact that the TrkA-to-p75NTR transition is completely blocked by caloric restriction indicates that such an event is part of the general programming of aging. Therefore the molecular pathways that are involved in the regulation of life span and other basic age-related events will need to be targeted in order to fully understand how neurotrophin signalling is regulated during aging.
Online data
Acknowledgments
We thank Dr Dora M. Kovacs and Dr Rudy E. Tanzi (Genetics and Aging Research Unit, MassGeneral Institute for Neurodegenerative Disease, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA 02129, U.S.A.) for the cell lines mentioned in Supplementary Figure 1. This research was supported by the National Institute of Neurological Disorders and Stroke (grant NS045669 to L.P.), the Alzheimer's Association (to L.P.), the Department of Veteran's Affairs, and the Ministero Istruzione, Universita' e Ricerca, FIRB Projects (to G.D.V.). C.C. is enrolled in the Program of Molecular and Cellular Biology and Pathology (Department of Pathology, University of Verona) and is partially supported by a Fellowship from the University of Verona, Italy.
References
- 1.Morris J. C. Is Alzheimer's disease inevitable with age? Lessons from clinicopathologic studies of healthy aging and very mild alzheimer's disease. J. Clin. Invest. 1999;104:1171–11173. doi: 10.1172/JCI8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Strooper B., Annaert W. Proteolytic processing and cell biological functions of the amyloid precursor protein. J. Cell Sci. 2000;113:1857–1870. doi: 10.1242/jcs.113.11.1857. [DOI] [PubMed] [Google Scholar]
- 3.Puglielli L., Tanzi R. E., Kovacs D. M. Alzheimer's disease: the cholesterol connection. Nat. Neurosci. 2003;6:345–351. doi: 10.1038/nn0403-345. [DOI] [PubMed] [Google Scholar]
- 4.Skovronsky D. M., Lee V. M. β-Secretase revealed: starting gate for race to novel therapies for Alzheimer's disease. Trends Pharmacol. Sci. 2000;21:161–163. doi: 10.1016/s0165-6147(00)01467-x. [DOI] [PubMed] [Google Scholar]
- 5.Fukumoto H., Rosene D. L., Moss M. B., Raju S., Hyman B. T., Irizarry M. C. β-Secretase activity increases with aging in human, monkey, and mouse brain. Am. J. Pathol. 2004;164:719–725. doi: 10.1016/s0002-9440(10)63159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holsinger R. M., McLean C. A., Beyreuther K., Masters C. L., Evin G. Increased expression of the amyloid precursor β-secretase in Alzheimer's disease. Ann. Neurol. 2002;51:783–786. doi: 10.1002/ana.10208. [DOI] [PubMed] [Google Scholar]
- 7.Fukumoto H., Cheung B. S., Hyman B. T., Irizarry M. C. β-Secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch. Neurol. 2002;59:1381–1389. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- 8.Gotz J., Streffer J. R., David D., Schild A., Hoerndli F., Pennanen L., Kurosinski P., Chen F. Transgenic animal models of Alzheimer's disease and related disorders: histopathology, behavior and therapy. Mol. Psychiatry. 2004;9:664–683. doi: 10.1038/sj.mp.4001508. [DOI] [PubMed] [Google Scholar]
- 9.Cai H., Wang Y., McCarthy D., Wen H., Borchelt D. R., Price D. L., Wong P. C. BACE1 is the major β-secretase for generation of Aβ peptides by neurons. Nat. Neurosci. 2001;4:233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- 10.Luo Y., Bolon B., Kahn S., Bennett B. D., Babu-Khan S., Denis P., Fan W., Kha H., Zhang J., Gong Y., et al. Mice deficient in BACE1, the Alzheimer's β-secretase, have normal phenotype and abolished beta-amyloid generation. Nat. Neurosci. 2001;4:231–232. doi: 10.1038/85059. [DOI] [PubMed] [Google Scholar]
- 11.Puglielli L., Ellis B. C., Saunders A. J., Kovacs D. M. Ceramide stabilizes β-site amyloid precursor protein-cleaving enzyme 1 and promotes amyloid β-peptide biogenesis. J. Biol. Chem. 2003;278:19777–19783. doi: 10.1074/jbc.M300466200. [DOI] [PubMed] [Google Scholar]
- 12.Venable M. E., Obeid L. M. Phospholipase D in cellular senescence. Biochim. Biophys. Acta. 1999;1439:291–298. doi: 10.1016/s1388-1981(99)00101-8. [DOI] [PubMed] [Google Scholar]
- 13.Venable M. E., Lee J. Y., Smyth M. J., Bielawska A., Obeid L. M. Role of ceramide in cellular senescence. J. Biol. Chem. 1995;270:30701–30708. doi: 10.1074/jbc.270.51.30701. [DOI] [PubMed] [Google Scholar]
- 14.Han X., Holtzman D. M., McKeel D. W., Jr, Kelley J., Morris J. C. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer's disease: potential role in disease pathogenesis. J. Neurochem. 2002;82:809–818. doi: 10.1046/j.1471-4159.2002.00997.x. [DOI] [PubMed] [Google Scholar]
- 15.Cutler R. G., Kelly J., Storie K., Pedersen W. A., Tammara A., Hatanpaa K., Troncoso J. C., Mattson M. P. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proc. Natl. Acad. Sci. U.S.A. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandhoff K., van Echten G. Metabolism of gangliosides: topology, pathobiochemistry, and sphingolipid activator proteins. Current Topics in Membranes. In: Hoekstra D., editor. Cell Lipids. San Diego: Academic Press; 1994. pp. 75–91. [Google Scholar]
- 17.Dobrowsky R. T., Carter B. D. Coupling of the p75 neurotrophin receptor to sphingolipid signaling. Ann. NY Acad. Sci. 1998;845:32–45. doi: 10.1111/j.1749-6632.1998.tb09660.x. [DOI] [PubMed] [Google Scholar]
- 18.Brann A. B., Scott R., Neuberger Y., Abulafia D., Boldin S., Fainzilber M., Futerman A. H. Ceramide signaling downstream of the p75 neurotrophin receptor mediates the effects of nerve growth factor on outgrowth of cultured hippocampal neurons. J. Neurosci. 1999;19:8199–8206. doi: 10.1523/JNEUROSCI.19-19-08199.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeFreitas M. F., McQuillen P. S., Shatz C. J. A novel p75NTR signaling pathway promotes survival, not death, of immunopurified neocortical subplate neurons. J. Neurosci. 2001;21:5121–5129. doi: 10.1523/JNEUROSCI.21-14-05121.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bunone G., Mariotti A., Compagni A., Morandi E., Della Valle G. Induction of apoptosis by p75 neurotrophin receptor in human neuroblastoma cells. Oncogene. 1997;14:1463–1470. doi: 10.1038/sj.onc.1200972. [DOI] [PubMed] [Google Scholar]
- 21.Puglielli L., Konopka G., Pack-Chung E., Ingano L. A., Berezovska O., Hyman B. T., Chang T. Y., Tanzi R. E., Kovacs D. M. Acyl-coenzyme A:cholesterol acyltransferase modulates the generation of the amyloid β-peptide. Nat. Cell Biol. 2001;3:905–912. doi: 10.1038/ncb1001-905. [DOI] [PubMed] [Google Scholar]
- 22.Pugh T. D., Klopp R. G., Weindruch R. Controlling caloric consumption: protocols for rodents and rhesus monkeys. Neurobiol. Aging. 1999;20:157–165. doi: 10.1016/s0197-4580(99)00043-3. [DOI] [PubMed] [Google Scholar]
- 23.Lee K. F., Li E., Huber L. J., Landis S. C., Sharpe A. H., Chao M. V., Jaenisch R. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell. 1992;69:737–749. doi: 10.1016/0092-8674(92)90286-l. [DOI] [PubMed] [Google Scholar]
- 24.Bowen R. L., Verdile G., Liu T., Parlow A. F., Perry G., Smith M. A., Martins R. N., Atwood C. S. Luteinizing hormone, a reproductive regulator that modulates the processing of amyloid-β precursor protein and amyloid-β deposition. J. Biol. Chem. 2004;279:20539–20545. doi: 10.1074/jbc.M311993200. [DOI] [PubMed] [Google Scholar]
- 25.Plo I., Bono F., Bezombes C., Alam A., Bruno A., Laurent G. Nerve growth factor-induced protein kinase C stimulation contributes to TrkA-dependent inhibition of p75 neurotrophin receptor sphingolipid signaling. J. Neurosci. Res. 2004;77:465–474. doi: 10.1002/jnr.20189. [DOI] [PubMed] [Google Scholar]
- 26.von Schack D., Casademunt E., Schweigreiter R., Meyer M., Bibel M., Dechant G. Complete ablation of the neurotrophin receptor p75NTR causes defects both in the nervous and the vascular system. Nat. Neurosci. 2001;4:977–978. doi: 10.1038/nn730. [DOI] [PubMed] [Google Scholar]
- 27.Arenz C., Thutewohl M., Block O., Waldmann H., Altenbach H. J., Giannis A. Manumycin A and its analogues are irreversible inhibitors of neutral sphingomyelinase. Chembiochem. 2001;2:141–143. doi: 10.1002/1439-7633(20010202)2:2<141::AID-CBIC141>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 28.Ibanez C. F. Emerging themes in structural biology of neurotrophic factors. Trends Neurosci. 1998;21:438–444. doi: 10.1016/s0166-2236(98)01266-1. [DOI] [PubMed] [Google Scholar]
- 29.Bergman E., Fundin B. T., Ulfhake B. Effects of aging and axotomy on the expression of neurotrophin receptors in primary sensory neurons. J. Comp. Neurol. 1999;410:368–386. doi: 10.1002/(sici)1096-9861(19990802)410:3<368::aid-cne2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 30.Johnson H., Hokfelt T., Ulfhake B. Expression of p75NTR, trkB and trkC in nonmanipulated and axotomized motoneurons of aged rats. Brain Res. Mol. Brain Res. 1999;69:21–34. doi: 10.1016/s0169-328x(99)00068-6. [DOI] [PubMed] [Google Scholar]
- 31.Lee C. K., Klopp R. G., Weindruch R., Prolla T. A. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285:1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- 32.Lu T., Pan Y., Kao S. Y., Li C., Kohane I., Chan J., Yankner B. A. Gene regulation and DNA damage in the ageing human brain. Nature (London) 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- 33.Weindruch R., Sohal R. S. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. New Engl. J. Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeung S. C., Xu G., Pan J., Christgen M., Bamiagis A. Manumycin enhances the cytotoxic effect of paclitaxel on anaplastic thyroid carcinoma cells. Cancer Res. 2000;60:650–656. [PubMed] [Google Scholar]
- 35.Xu G., Pan J., Martin C., Yeung S. C. Angiogenesis inhibition in the in vivo antineoplastic effect of manumycin and paclitaxel against anaplastic thyroid carcinoma. J. Clin. Endocrinol. Metab. 2001;86:1769–1777. doi: 10.1210/jcem.86.4.7374. [DOI] [PubMed] [Google Scholar]
- 35a.Capsoni S., Margotti E., Stefanini B., Cattaneo A. 7th International Conference on AD/PD. Italy: Sorrento; 2005. The effects of knocking out of p75NTR on the neurodegenerative phenotype in AD11 anti-NGF mice. abstract 102. [Google Scholar]
- 36.Lee R., Kermani P., Teng K. K., Hempstead B. L. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- 37.Fahnestock M., Michalski B., Xu B., Coughlin M. D. The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer's disease. Mol. Cell. Neurosci. 2001;18:210–220. doi: 10.1006/mcne.2001.1016. [DOI] [PubMed] [Google Scholar]
- 38.Hock C., Heese K., Muller-Spahn F., Hulette C., Rosenberg C., Otten U. Decreased trkA neurotrophin receptor expression in the parietal cortex of patients with Alzheimer's disease. Neurosci. Lett. 1998;241:151–154. doi: 10.1016/s0304-3940(98)00019-6. [DOI] [PubMed] [Google Scholar]
- 39.Counts S. E., Nadeem M., Wuu J., Ginsberg S. D., Saragovi H. U., Mufson E. J. Reduction of cortical TrkA but not p75NTR protein in early-stage Alzheimer's disease. Ann. Neurol. 2004;56:520–531. doi: 10.1002/ana.20233. [DOI] [PubMed] [Google Scholar]
- 40.Mufson E. J., Kordower J. H. Cortical neurons express nerve growth factor receptors in advanced age and Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 1992;89:569–573. doi: 10.1073/pnas.89.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu X. Y., Zhang H. Y., Qin S., Xu H., Swaab D. F., Zhou J. N. Increased p75NTR expression in hippocampal neurons containing hyperphosphorylated tau in Alzheimer patients. Exp. Neurol. 2002;178:104–111. doi: 10.1006/exnr.2002.8018. [DOI] [PubMed] [Google Scholar]
- 42.Ernorf P., Lindefors N., Chan-Palay V., Persson H. Cholinergic neurons of the nucleus basalis express elevated levels of nerve growth factor receptor mRNA in senile dementia of the Alzheimer's type. Dementia. 1990;1:138–145. [Google Scholar]
- 43.Salehi A., Ocampo M., Verhaagen J., Swaab D. F. P75 neurotrophin receptor in the nucleus basalis of meynert in relation to age, sex, and Alzheimer's disease. Exp. Neurol. 2000;161:245–258. doi: 10.1006/exnr.1999.7252. [DOI] [PubMed] [Google Scholar]
- 44.Dechant G., Barde Y. A. The neurotrophin receptor p75NTR: novel functions and implications for diseases of the nervous system. Nat. Neurosci. 2002;5:1131–1136. doi: 10.1038/nn1102-1131. [DOI] [PubMed] [Google Scholar]
- 45.Bronfman F. C., Fainzilber M. Multi-tasking by the p75 neurotrophin receptor: sortilin things out? EMBO Rep. 2004;5:867–871. doi: 10.1038/sj.embor.7400219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zampieri N., Chao M. V. Structural biology. The p75 NGF receptor exposed. Science. 2004;304:833–834. doi: 10.1126/science.1098110. [DOI] [PubMed] [Google Scholar]
- 47.Rabizadeh S., Bitler C. M., Butcher L. L., Bredesen D. E. Expression of the low-affinity nerve growth factor receptor enhances β-amyloid toxicity. Proc. Natl. Acad. Sci. U.S.A. 1994;91:10703–10706. doi: 10.1073/pnas.91.22.10703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yaar M., Zhai S., Pilch P. F., Doyle S. M., Eisenhauer P. B., Fine R. E., Gilchrest B. A. Binding of β-amyloid to the p75 neurotrophin receptor induces apoptosis; a possible mechanism for Alzheimer's disease. J. Clin. Invest. 1997;100:2333–2340. doi: 10.1172/JCI119772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y., Hong Y., Bounhar Y., Blacker M., Roncou X., Tounekti O., Vereker E., Bowers W. J., Federoff H. J., Goodyer C. G., LeBlanc A. p75 neurotrophin receptor protects primary cultures of human neurons against extracellular amyloid β peptide cytotoxicity. J. Neurosci. 2003;23:7385–7394. doi: 10.1523/JNEUROSCI.23-19-07385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.