Abstract
The cysteine-rich domain of the haemorrhagic metalloproteinase atrolysin A was shown to inhibit collagen-stimulated platelet aggregation and to interact with MG-63 osteosarcoma cells via integrin α2β1 to inhibit adhesion to collagen I. In addition, we demonstrate by solid-phase binding assays that atrolysin A binds to collagen I and to vWF (von Willebrand factor) via exosites in the cysteine-rich domain. Interestingly, the binding site of the cysteine-rich domain on collagen I is distinct from the cell adhesion site, since the incubation of collagen-I-coated plates with the cysteine-rich domain did not prevent the adhesion of MG-63 cells to collagen. Finally, we show by surface plasmon resonance (BIAcore™) analyses that the cysteine-rich domain can block vWF binding to collagen I as well as the binding of collagen I to vWF. Taken together, these results indicate that this domain may function as a cell-surface-receptor-binding site and/or a substrate recognition exosite and may thus play a role in the pathologies associated with atrolysin A.
Keywords: atrolysin A, collagen I, cysteine-rich domain, platelet aggregation, snake venom metalloproteinase, von Willebrand factor
Abbreviations: A/C, recombinant cysteine-rich domain of atrolysin A produced in Sf9 cells; A/C-e, recombinant cysteine-rich domain of atrolysin A produced in Escherichia coli; A/DC, recombinant disintegrin-like/cysteine-rich domain of atrolysin A produced in Sf9 cells; ADAM, a disintegrin and metalloproteinase; ADAMTS, ADAM with thrombospondin motifs; HRP, horseradish peroxidase; SVMP, snake venom metalloproteinase; vWF, von Willebrand factor
INTRODUCTION
The distinctive feature of Viperidae snake envenomation is the pronounced local and, in severe cases, systemic haemorrhage resulting from the synergistic action of several toxins in the venom [1–3]. Viperidae venoms are a rich source of haemorrhagic toxins characterized as zinc metalloproteinases, which are members of the reprolysin subfamily of the M12 family of metalloproteinases [1]. SVMPs (snake venom metalloproteinases) are secreted as preproenzymes and contain additional regulatory modules that are involved in their interactions with the extracellular matrix and integrins [4]. Structurally, they are classified as the following: P-I (SVMPs having a metalloproteinase domain only), P-II (SVMPs being synthesized with a metalloproteinase domain and a disintegrin domain), P-III (SVMPs being synthesized with a metalloproteinase domain, a disintegrin-like domain and a cysteine-rich domain) and P-IV (SVMPs having the P-III domain structure plus lectin-like domains connected by disulphide bonds) [5]. Similar domain structures are found in the members of the ADAM (a disintegrin and metalloproteinase) protein subfamily, which additionally contain an epidermal growth factor-like domain, a transmembrane region and a cytoplasmic tail [6,7]. The proteinase domain of all classes of the haemorrhagic toxins primarily functions by degrading capillary basement membranes and surrounding stroma to allow the escape of blood from the capillary [8,9]. The enzymes also degrade blood coagulation proteins, such as fibrinogen, fibrin, and vWF (von Willebrand factor) [10,11]. Native, as well as recombinant, disintegrin-like/cysteine-rich domains of haemorrhagic toxins have been shown to be potent inhibitors of collagen-induced platelet aggregation by blocking collagen binding to integrin α2β1 on platelets [12–14]. Therefore the haemorrhage caused by SVMPs is believed to be the result of the synergistic effect of proteolytic degradation of capillary basement membranes and plasma proteins, and of inhibition of platelet aggregation [3].
Collagens, in particular types I, III and VI, are important platelet activators in the vascular subendothelium and vessel wall. Several collagen-binding proteins are expressed on the platelet surface that may mediate collagen-induced platelet activation and/or platelet adhesion under flow, including integrin α2β1 and glycoprotein VI [15]. Plasma vWF is a multimeric protein that mediates adhesion of platelets to sites of vascular injury and thrombus formation. It functions by linking subendothelial collagen with glycoproteins Ib–IX–V receptor complex on platelets [16]. The A3 domain of vWF contains the major binding site for collagens I and III [17,18]. Structural studies on the vWF A3 domain showed that it assumes the same fold as the α2-I domain, but binding of A3 to collagen does not require a metal ion [18–20]. Owing to the multidomain composition of the P-III haemorrhagic toxins and the different mechanisms of platelet aggregation, and hence inhibition, it is conceivable that these toxins could have more than one site of interaction with the platelets by which inhibition could occur. The multidomain venom toxins, P-II and P-III, are typically one or two orders of magnitude more potent than P-I toxins, suggesting a role for the disintegrin-like and cysteine-rich domains in haemorrhage production. It is considered that the non-proteinase domains would have an anticoagulant effect, resulting in a synergism of haemorrhage production with the degradation of capillary basement membranes. One of the mechanisms by which P-III toxins may act in promoting haemorrhage is by inhibiting platelet aggregation. It was first shown by Zhou et al. [21] that catrocollastatin, a P-III metalloproteinase from the venom of Crotalus atrox, was able to inhibit platelet aggregation and adhesion to collagen. In addition, crovidisin, another P-III toxin from the venom of Crotalus viridis, was shown to be able to prevent platelet–collagen interaction [22]. At the same time, the cleavage and consequent disruption of vWF structure by P-III toxins such as jararhagin and kaouthiagin could also contribute to the bleeding observed upon envenomation [3,11]. However, it is not yet known which of the non-proteinase domains is responsible for directing the P-III toxins to their plasma and cell-surface targets.
Until recently, little consideration had been given to a functional role for the highly conserved cysteine-rich domain of the P-III haemorrhagic toxins. As for the ADAMs, not much is known about the function of the cysteine-rich domain, and, in general, it is believed that the cysteine-rich domain complements the binding capacity of the disintegrin domain, and perhaps imparts specificity to disintegrin-domain-mediated interactions [23]. Previously, we have shown that the recombinant cysteine-rich domain of atrolysin A, a P-III metalloproteinase from C. atrox venom, can function as a potent inhibitor of collagen- but not ADP-stimulated platelet aggregation and that it was able to inhibit the osteosarcoma cell line MG-63 adhesion to collagen I [24]. A recent study supported these observations by the demonstration of two peptide sequences derived from the homologous cysteine-rich domains of atrolysin A and jararhagin that interfere with interaction of platelets and other cells with collagen [25]. In order to define further the role of the cysteine-rich domain as an exosite of the multidomain metalloproteinases that is involved in the interaction of these toxins with their specific targets, we have demonstrated the ability of atrolysin A, and its recombinant disintegrin-like/cysteine-rich and cysteine-rich domains, to bind directly to collagen I and to vWF and thereby disrupt various functional aspects of those proteins.
EXPERIMENTAL
Binding proteins
Atrolysin A [26], atrolysin C [27], catrocollastatin C [13], jararhagin [28] (a gift from Dr A. M. Moura-da-Silva), A/DC (recombinant disintegrin-like/cysteine-rich domain of atrolysin A produced in Sf9 cells) [12] and A/C (recombinant cysteine-rich domain of atrolysin A produced in Sf9 cells) [24] were obtained as described previously.
Construction of A/C-e (recombinant cysteine-rich domain of atrolysin A produced in Escherichia coli) expression vector (pET102/D-A/C-e)
A cDNA fragment coding for residues 301–413 of atrolysin A was amplified by PCR from the plasmid pMbacA/C [24] and was subcloned into the pET102/D-TOPO® bacterial expression vector (Invitrogen). The two oligonucleotides used for PCR were 5′-CACCCTACACAACTTCGGTTAC-3′ (forward primer) and 5′-GGTTGATTTGTAGGCTG-3′ (reverse primer). The forward primer included a CACC sequence that was necessary for directional cloning into pET102/D-TOPO® expression vector into which the 343-bp PCR product was directly subcloned. One Shot® TOP10 E. coli competent cells were transformed with the resulting plasmid (pET102/D-A/C-e), and colonies were restricted with PstI. Plasmids were sequenced on both strands to ensure that the coding sequence was correct.
Production of A/C-e in E. coli
BL21 Star™ (DE3) E. coli cells (Invitrogen) were transformed by heat shock with the plasmid pET102/D-A/C-e, according to the manufacturer's instructions and were grown overnight at 37 °C. Then bacterial cells were inoculated in LB (Luria–Bertani) medium containing 100 μg·ml−1 ampicillin and grown at 37 °C to a cell density (D600) of 0.5–0.8. The expression of recombinant proteins was induced by adding 1 mM IPTG (isopropyl β-D-thiogalactoside), and incubation was continued for 3 h at 37 °C. The cell pellet was lysed by sonication in lysis buffer [50 mM potassium phosphate, pH 7.8, 400 mM NaCl, 100 mM KCl, 10% (v/v) glycerol, 0.5% (v/v) Triton X-100, 0.1 mg/ml lysozyme, 10 mM imidazole and 1 mM PMSF], and the resulting suspension was centrifuged at 10000 g for 30 min at 4 °C. The supernatant from the centrifugation step was dialysed against binding buffer (0.05 M Tris/HCl, pH 8.0, and 0.15 M NaCl) followed by a final centrifugation step of 10000 g for 30 min. The soluble fraction was then applied on to a Talon® metal affinity column (Clontech), 1 cm×10 cm, equilibrated with binding buffer. The column was washed exhaustively with binding buffer, and then bound proteins were eluted with binding buffer containing 0.15 M imidazole. Fractions from the chelating chromatography were submitted to SDS/PAGE and Western blot analysis.
Reduction and alkylation of A/C-e protein
A/C-e (0.4 nmol) was incubated in 0.25 M Tris/HCl buffer, pH 8.5, 8 M guanidinium chloride, 5 mM EDTA and 7 mM dithiothreitol at 37 °C for 90 min. Iodoacetamide was added to the mixture to a final concentration of 20 mM and the incubation was continued for 20 min in the dark [29]. After adding dithiothreitol to a final concentration of 13 nM, alkylated A/C-e was desalted by HPLC using a Zorbax SB300-C3 (2.1 mm×150 mm) column at 55 °C. Elution was performed with 20% acetonitrile in 0.1% trifluoroacetic acid for 5 min, followed by a gradient of 20–90% acetonitrile in 0.1% trifluoroacetic acid over 45 min at a flow rate of 0.2 ml/min. The effluent was monitored at 215 nm.
Solid-phase binding assays
Assays with one ligand adsorbed to plastic microtitre wells were carried out according to established ELISA-type protocols. Microtitre plates (96-well) were coated with 10 μg/ml rat skin collagen I (a gift from Dr Gary Balian, University of Virginia) or human vWF (a gift from Dr Adrian Gear, University of Virginia) in carbonate buffer, pH 9.6, for 16 h and then blocked with PBS/Tween 20 containing 2% (w/v) BSA. The plates were incubated further with atrolysin A, or A/DC or A/C, for 2 h at room temperature (25 °C). Primary antibody [1:1000 anti-(atrolysin A) from mouse polyascites fluid] was added to the plates and incubated for 90 min, followed by incubation with the second antibody for 1 h [1:4000 anti-(mouse IgG)–HRP (horseradish peroxidase)]. After washing with PBS/Tween 20, HRP was determined with ABTS [2,2′-azinobis-(3-ethylbenzothiazoline-6-sulphonic acid)] as a substrate and the A414 was measured using a microtitre plate reader.
For the competition assay by A/C for atrolysin A binding to collagen I plates were coated with collagen I (10 μg/ml) and incubated with increasing concentrations of A/C protein for 1.5 h at room temperature. The plates were washed three times with PBS and then incubated with 1.0 μg of atrolysin A/well for 1 h followed by five washes with PBS. The primary antibody used was polyclonal mouse anti-(atrolysin E) (1:3000), which recognizes the proteinase domain of atrolysin A, but not the cysteine-rich domain [30]. The goat anti-mouse IgG–HRP conjugate was used as the secondary antibody for colour development.
Cell adhesion assays
Microtitre plates (96-well) were coated with 100 μl/well of 10 μg/ml rat skin collagen I at 4 °C overnight. The coated wells were washed with PBS and then blocked with PBS containing 1% (w/v) BSA at room temperature for 60 min. The plates were washed three times with PBS and then incubated with increasing concentrations of A/C for 2 h at room temperature. The wells were washed five times with PBS and then incubated with 105 MG-63 cells/well for 1 h. As a positive control for β1 integrin-directed adhesion, cells were also incubated with the anti-β1 subunit function blocking monoclonal antibody mAb13 (Becton Dickinson) and added to collagen-I-coated wells that were incubated with the A/C protein. The wells were washed five times with PBS, and the adherent cells were fixed with 10% (w/v) formaldehyde in PBS. The bound cells were stained with 2% (w/v) Crystal Violet for 30 min [31]. The unbound dye was washed from the plates, and the stained cells were lysed with 1% (w/v) SDS for 60 min, and the A595 of the wells was measured using a microtitre plate reader.
Surface plasmon resonance assays
Interactions were studied by surface plasmon resonance using the BIAcore™ 3000 system, at 25 °C. Native calf skin type I collagen (IBFB, Leipzig, Germany) was covalently immobilized on the BIAcore™ CM-5 sensorchip (carboxylated dextran matrix) according to the manufacturer's instructions. Briefly, the CM-5 chip was activated with a 1:1 mixture of 75 mg/ml EDC [1-ethyl-3-(3-dimethylaminopropyl)carbodi-imide] and 11.5 mg/ml NHS (N-hydroxysuccinimide) for 7 min. Type I collagen (200 μg/ml in 10 mM sodium citrate, pH 4.0) was injected over the CM-5 chip for 7 min at a flow rate of 10 μl/min, at 4 °C. Remaining active groups on the matrix were blocked with 1 M ethanolamine/HCl, pH 8.5. Immobilization of type I collagen on a CM-5 sensorchip resulted in a surface concentration of 3.9 ng/mm2. vWF was immobilized by injecting the protein (10 μg/ml in 10 mM sodium citrate, pH 4.5) over the CM-5 chip for 7 min at a flow rate of 5 μl/min, at 4 °C, resulting in a surface concentration of 12.5 ng/mm2. Protein solutions of atrolysin A, atrolysin C, catrocollastatin C and A/C-e (0.25–5.0 μM) and jararhagin (0.0375–0.3 μM) were prepared in HBS-EP buffer [10 mM Hepes, pH 7.4, 150 mM NaCl, 3.4 mM EDTA and 0.005% (v/v) surfactant P20; BIAcore™] and were injected at a flow rate of 50 μl/min. The non-linear fitting of association and dissociation curves according to a 1:1 model was used for the calculation of kinetic constants (BIAevaluation software, version 3.1). Individual experiments were performed six times.
Analytical procedures
SDS/PAGE (12% polyacrylamide) was performed according to Laemmli [32]. Western blot analysis was performed according to the method of Burnette [33]. The primary antibody was a polyclonal antibody generated in mice against native atrolysin A as described previously [12]. The secondary antibody was goat anti-mouse IgG conjugated with HRP (Promega). DNA sequence analysis was performed on a PerkinElmer Prizm DNA sequencer in the University of Virginia Biomolecular Research Facility following the manufacturer's instructions.
RESULTS
Recombinant proteins
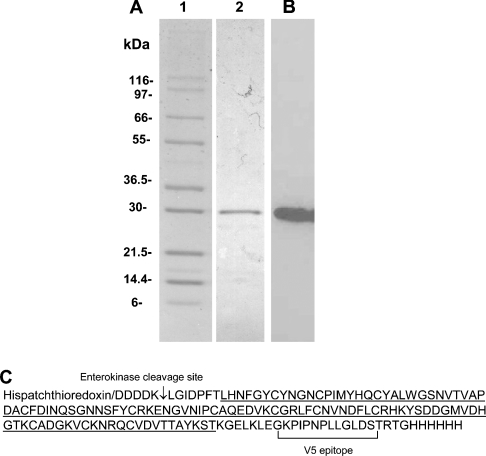
In the present study, we used A/DC [12] and A/C [24] for the cell adhesion and solid-phase binding assays. For those studies which required significant amounts of recombinant protein, an additional heterologous expression system was developed using E. coli (A/C-e). The amino acid sequence of A/C-e protein in fusion with His-patch thioredoxin is shown in Figure 1. The chromatography (not shown) of 500 ml of culture from pET102/D-A/C-e-transformed BL21 Star™ (DE3) E. coli cells on Talon® metal affinity column typically yielded 2 mg of (His-patch thioredoxin)–A/C-e protein. SDS/PAGE and Western blot analysis of affinity-purified fusion protein showed that the (His-patch thioredoxin)–cysteinerich protein was essentially homogeneous (Figure 1). This protein was used in the kinetic measurements by surface plasmon resonance.
Figure 1. Expression of recombinant (His-patch thioredoxin)–A/C-e.
(A) SDS/PAGE of molecular-mass markers (lane 1), Talon® metal-affinity-column-purified fusion protein A/C-e (lane 2); (B) Western blot of purified A/C-e fusion protein identified with anti-(atrolysin A) antibody. (C) Protein sequence of recombinant fusion protein A/C-e. Underlined is the amino acid sequence of the cysteine-rich domain of atrolysin A.
Solid-phase binding assays to examine the interaction of atrolysin A domains with collagen I and vWF
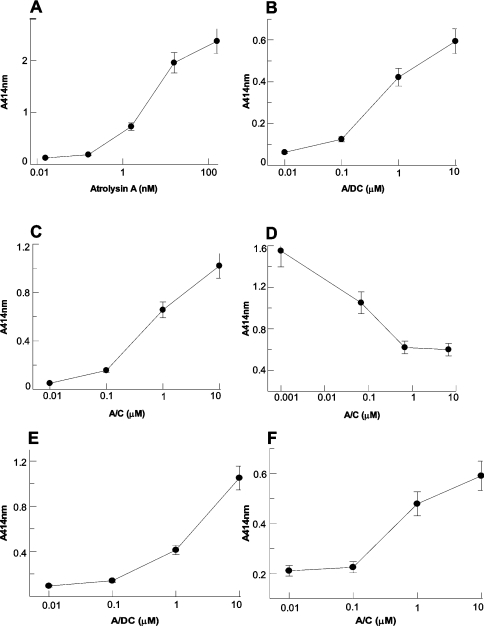
Our aim with these studies was to investigate further the role of the cysteine-rich domain in the interaction of P-III haemorrhagic toxins with adhesion proteins. To analyse the interaction between atrolysin A domains and adhesion proteins collagen I and vWF in a cell-free system, solid-phase binding assays were performed. As seen in Figures 2(A)–2(C), the proteins bound to immobilized collagen I in a concentration-dependent fashion: atrolysin A binding was at nanomolar concentrations, whereas, for A/DC and A/C, micromolar concentrations were used. These data indicate that the cysteine-rich domain alone is capable of interacting with collagen I. To demonstrate that the binding of atrolysin A to collagen I is through its cysteine-rich domain, and not the proteinase or disintegrin-like domains, a competition assay by A/C-protein for atrolysin A binding to collagen-I-coated wells was performed. From Figure 2(D), it can be seen that pre-binding of A/C-protein to collagen I blocks subsequent binding of atrolysin A, indicating that atrolysin A binds collagen solely through its cysteine-rich domain.
Figure 2. Binding of atrolysin A, A/DC and A/C proteins to collagen I and to vWF by solid-phase binding assay.
Microtitre plates (96-well) were coated with 10 μg/ml rat skin collagen I (A–D) or vWF (E, F) followed by incubation with atrolysin A (A), A/DC (B, E) or A/C (C, F) proteins. (D) Competition of atrolysin A binding to immobilized collagen I by A/C protein. Collagen-I-coated wells were incubated with A/C protein, followed by incubation with 1.0 μg/well atrolysin A. Bound protein was detected as described in the Experimental section. Data are the means±S.D. of four determinations.
We also investigated the ability of A/DC and A/C to bind to vWF. In Figures 2(E) and 2(F), it can be seen that the binding of A/DC and A/C to vWF at micromolar concentrations occurred in a dose-dependent fashion. These results corroborate the fact that the cysteine-rich domain alone is able to bind to adhesion proteins.
Cell adhesion assay
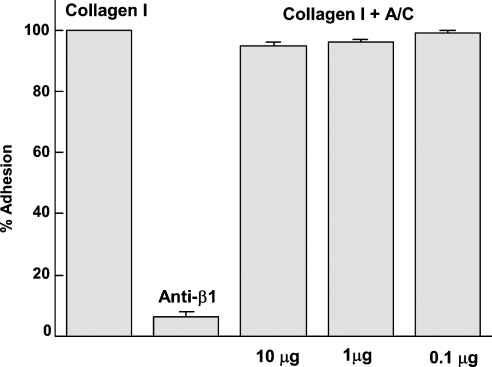
Previous studies have shown a concentration-dependent inhibition of cell adhesion to collagen I when the recombinant cysteine-rich domain of atrolysin A was pre-incubated with MG-63 cells before their incubation with immobilized collagen I. This is indicative of an interaction of A/C with integrin α2β1 on the cells [24]. Considering that the cysteine-rich domain is able to bind to collagen I, we decided to determine whether the presence of the cysteine-rich domain bound to collagen I could block the binding of the osteosarcoma cell line MG-63. In this study, we incubated collagen-I-coated plates with A/C before adding the MG-63 cells and verified that the presence of the cysteine-rich domain bound to collagen I did not prevent the adhesion of MG-63 cells (Figure 3). These results suggest that there are distinct sites on collagen I which support the binding of the cysteine-rich domain and MG-63 cells respectively.
Figure 3. Adhesion of MG-63 cells to collagen I pre-incubated with A/C.
The bar labelled Collagen I represents the control for MG-63 cell adhesion to collagen-coated wells. The bar labelled anti-β1 represents the adhesion to collagen-I-coated wells of MG-63 cells following treatment with anti-β1 antibody. Bars labelled Collagen I+A/C represent adhesion of MG-63 cells to collagen-coated wells pre-incubated with increasing amounts of A/C.
Interactions studied by surface plasmon resonance
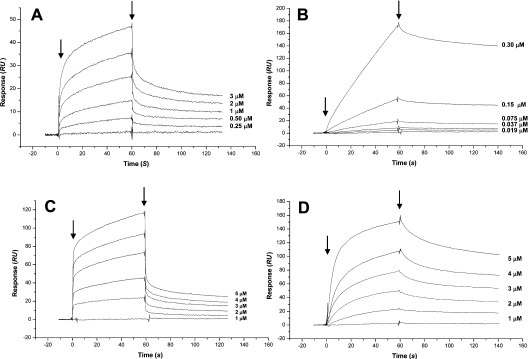
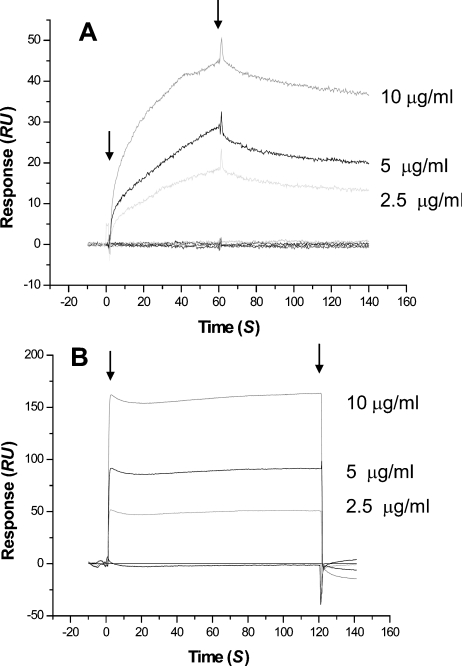
We next examined the collagen-I-binding kinetics of SVMPs by surface plasmon resonance using a sensor chip on which native calf skin type I collagen had been immobilized. In these experiments, we used A/C-e in fusion with His-patch thioredoxin. Kinetic evaluation of the interaction of full-length atrolysin A, and jararhagin, a P-III metalloproteinase isolated from Bothrops jararaca venom, with collagen I according to a 1:1 model (Figures 4A and 4B respectively) showed a high-affinity binding, as indicated by the high association rates and the low dissociation rates, which gave equilibrium dissociation constants (Kd) of 117 and 21.6 nM respectively (Table 1). The A/C-e protein showed a similar dissociation constant (Kd) of 72.6 nM (Figure 4D and Table 1), while, for catrocollastatin C, a disintegrin-like/cysteine-rich protein isolated from C. atrox venom, a Kd value of 777 nM was obtained (Figure 4C and Table 1). Injections of atrolysin C, a P-I metalloproteinase isolated from the venom of C. atrox, on the collagen I sensor chip gave a very low signal, from which a Kd value of 509 μM was calculated (Table 1). His-patch thioredoxin expressed and purified under the same conditions as A/C-e did not bind to the collagen I sensor chip (results not shown).
Figure 4. Interaction of venom proteins with collagen I using the BIAcore™ 3000 system.
Atrolysin A (A), jararhagin (B), catrocollastatin C (C) and A/C-e (D) at different concentrations were injected over immobilized collagen I at a flow rate of 50 μl/min. The arrows indicate the beginning and the end of injections. RU, response units.
Table 1. Kinetic evaluation of the interaction of venom proteins with collagen I immobilized on CM-5 sensor chip in the BIAcore™ 3000 system.
Evaluation was performed according to a 1:1 binding model. Results are the means±S.D. for six different analyte concentrations. n.d., not determined.
| Kd (μM) | kd (×10−3 s−1) | ka (×103 M−1·s−1) | |
|---|---|---|---|
| Jararhagin | 0.0216 | 1.22±0.0084 | 56.4±1.3 |
| Atrolysin A | 0.117 | 4.71±0.0374 | 40.2±3.77 |
| Catrocollastatin C | 0.777 | 4.23±0.0258 | 5.45±0.105 |
| A/C-e | 0.0726 | 1.93±0.014 | 26.5±0.271 |
| Atrolysin C (Ht-C) | 509 | n.d. | n.d. |
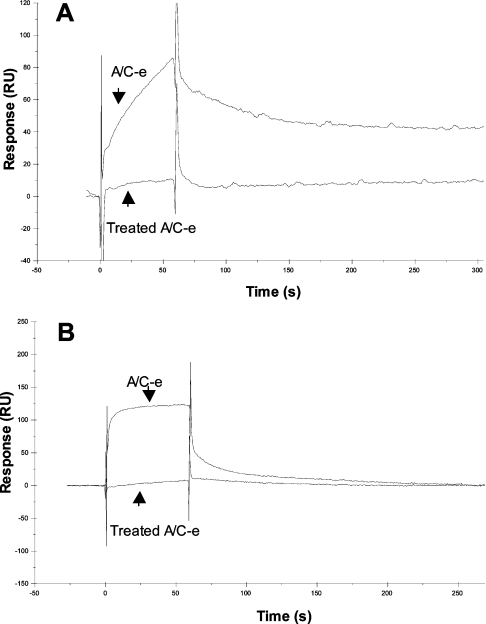
We next examined the specificity of binding of the cysteine-rich domain to both collagen I and vWF immobilized on sensor chips. Figure 5 shows the interaction of the cysteine-rich domain at 33 nM (A/C-e) to collagen I (Figure 5A) and to vWF (Figure 5B) in the BIAcore™ 3000 system. Reduction and alkylation of the disulphide bonds of A/C-e (treated A/C-e) abolished the binding both to collagen I and to vWF, showing that the interaction of the cysteine-rich domain with adhesion proteins is specific and dependent on its tertiary structure.
Figure 5. Interaction of A/C-e with collagen I and vWF using the BIAcore™ 3000 system.
A/C-e (at 33 nM) or reduced and alkylated A/C-e (treated A/C-e, at 33 nM) was injected over immobilized collagen I (A) or vWF (B) at a flow rate of 50 μl/min. RU, response units.
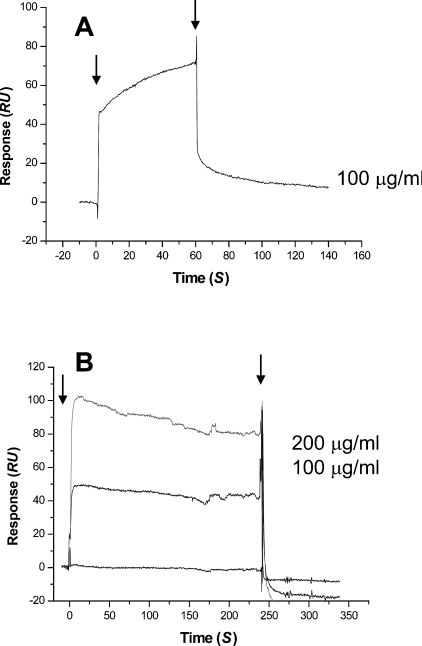
We also analysed the direct interaction of vWF to immobilized collagen I via surface plasmon resonance, from which a Kd value of 2.74×10−10 M was calculated (results not shown). To test whether the cysteine-rich domain could interfere with the binding between vWF and collagen I, we saturated both proteins with A/C-e (450 nM) and verified that they no longer bound to each other, indicating that the cysteine-rich domain occupies site(s) on collagen I and vWF that are important for the interaction of these proteins (Figures 6 and 7).
Figure 6. Inhibition of binding of vWF to collagen I by A/C-e using the BIAcore™ 3000 system.
(A) vWF at different concentrations was injected over immobilized collagen I. (B) vWF at different concentrations and saturated with A/C-e (450 nM) was injected over immobilized collagen I saturated with A/C-e (450 nM). The injection flow rate was 50 μl/min. The arrows indicate the beginning and the end of injections. RU, response units.
Figure 7. Inhibition of binding of collagen I to vWF by A/C-e using the BIAcore™ 3000 system.
(A) Collagen at 0.1 mg/ml was injected over immobilized vWF. (B) Collagen at different concentrations and saturated with A/C-e (450 nM) was injected over immobilized vWF saturated with A/C-e (450 nM). The injection flow rate was 50 μl/min. The arrows indicate the beginning and the end of injections. RU, response units.
DISCUSSION
Platelet adhesion to damaged vessel walls is the first step in the formation of an occluding platelet plug during normal haemostasis. The role of vWF in blocking haemorrhage is centred on its ability to function as an adhesive molecule that brings together platelets and components of the extracellular matrix or other platelets [34]. Collagens I and III act as binding sites for vWF in the perivascular connective tissue and collagen IV in the subendothelial matrix [19]. Integrin α2β1 is the major human collagen adhesion receptor, which is expressed on a variety of cell types [35]. Platelet activation and aggregation by collagen depend on the co-operative action of α2β1 and glycoprotein VI [36]. Peptides containing both disintegrin-like and cysteine-rich domains of P-III metalloproteinases, purified from the venoms of B. jararaca and C. atrox or expressed as recombinant proteins, were shown to inhibit collagen-induced platelet aggregation by interacting with integrin α2β1, thereby blocking collagen binding and its subsequent cell-surface-receptor-mediated signaling [12,13,37]. On the other hand, Zhou et al. [38] have shown that catrocollastatin exerts its effect on platelet–collagen adhesion by binding to collagen apparently via its disintegrin-like domain. Moreover, recently, the recombinant disintegrin-like domain of a metalloproteinase from Gloydius halys was shown to inhibit platelet aggregation by suppressing platelet adhesion to collagen [39].
We have shown that the cysteine-rich domain of atrolysin A is able to inhibit collagen-induced platelet aggregation [24]. This suggests that the cysteine-rich domain may serve to localize the haemorrhagic toxin to the sub-endothelial matrix by interacting with integrin α2β1 and/or collagen. Several lines of evidence from the present study indicate that the cysteine-rich domain alone is able to interact with integrin α2β1, collagen I and vWF. We have demonstrated using a solid-phase binding assay and using surface plasmon resonance that different proteins containing the cysteine-rich domain, e.g. the full-length P-III haemorrhagic toxins atrolysin A and jararhagin, as well as the recombinant protein corresponding to the disintegrin-like/cysteine-rich domains of atrolysin A (A/DC) and the recombinant cysteine-rich domain of atrolysin A (A/C and A/C-e), bind to collagen I in a dose-dependent fashion (Figures 2 and 4). Moreover, the binding of the cysteine-rich domain to collagen I was shown to be specific, since its pre-binding to collagen I blocked subsequent binding of atrolysin A (Figure 2). The binding of the recombinant proteins A/DC and A/C to vWF confirmed the ability of the cysteine-rich domain of atrolysin A to interact with adhesion proteins that are important for platelet function (Figure 2). It is interesting to note that the A3 domain of vWF, which contains the major collagen-binding site, resembles the α2 I-domain [40].
In a previous study, we demonstrated that the cysteine-rich domain is able to prevent the adhesion of MG-63 osteosarcoma cells to collagen I via α2β1 [24]. Our present results showing that these cells are able to bind to collagen-I coated plates that were incubated previously with the recombinant cysteine-rich domain indicate that distinct sites on collagen I support the binding of both the cysteine-rich domain and MG-63 cells via α2β1 (Figure 3).
The cysteine-rich domain specifically bound to collagen I and to vWF, since reduction and alkylation of the protein abolished its ability to interact with these proteins (Figure 5). The cysteine-rich domain not only bound directly to vWF and collagen I, but also blocked the collagen–vWF interaction (Figures 6 and 7). The conclusion is that either the cysteine-rich domain binding to vWF blocks the collagen site on vWF or the binding of the cysteine-rich domain to collagen blocks the site on collagen for binding vWF. Functionally, this would be expected to prevent the activation of vWF and hence further inhibit platelet aggregation. The participation of the cysteine-rich domain in the binding of kaouthiagin, a P-III metalloproteinase from the venom of Naja kaouthia, to vWF has been demonstrated [41]. These authors showed that the treatment of the metalloproteinase with o-phenanthroline did not interfere with the binding of kaouthiagin to vWF, while incubation with CNBr, which cleaved the cysteine-rich domain at two sites, completely abolished the binding ability of kaouthiagin, indicating a role for this domain in the interaction of kaouthiagin with vWF.
Our results clearly show the ability of the cysteine-rich domain of atrolysin A to interact with collagen, integrin α2β1 and vWF. Therefore we hypothesize that the cysteine-rich domain has distinct sites for the interaction with collagen I and α2β1/vWF. Likewise, collagen I contains distinct sites that support the binding of the cysteine-rich domain and the cell-surface integrin α2β1 or vWF. According to this hypothesis, it is suggested that the cysteine-rich domain and collagen I have a common site to bind α2β1 or vWF. This implies that the cysteine-rich domain could mimic collagen I in binding to integrin α2β1. In fact, it has been recently shown that jararhagin, a P-III haemorrhagic toxin containing metalloproteinase, disintegrin-like and cysteine-rich domains, functions as a collagen-mimetic substrate that binds to and activates integrins in fibroblasts [42]. Moreover, inactivation of the metalloproteinase domain had no effect on this property of jararhagin, therefore it is likely that the proteinase domain does not play a significant role in this activity. The cysteine-rich domain of members of the ADAM and ADAMTS (ADAM with thrombospondin motifs) families have been demonstrated to bind to various receptors [23]. For example, the cysteine-rich (and perhaps also the disintegrin) domain of ADAM12 promotes the adhesion of fibroblasts and myoblasts [43]. Also, the disintegrin and cysteine-rich domains of ADAM13 bind both to fibronectin and to β1-containing integrin receptors, and binding can be inhibited with antibodies against the cysteine-rich domain [44]. In another case, the cysteine-rich/spacer domains of ADAMTS-13 were shown to be essential for its vWF cleaving activity [45]. In the present study, we show that the cysteine-rich domain of atrolysin A is able to block vWF binding to collagen I.
Taken together, these results underscore additional mechanisms by which the cysteine-rich domain of P-III haemorrhagic toxins may interfere with adhesion of platelets at the site of envenoming, and they indicate a multifunctional role for the cysteine-rich domains of class P-III haemorrhagic toxins in the coagulopathies associated with crotalid and viperid envenoming.
Acknowledgments
We thank Dr Bojan P. Dragulev, University of Virginia, and Dr Vincenzo Politi, Polifarma, Rome, Italy, for their assistance in this work.
References
- 1.Bjarnason J. B., Fox J. W. Snake venom metalloendopeptidases: reprolysins. Methods Enzymol. 1995;248:345–368. doi: 10.1016/0076-6879(95)48023-4. [DOI] [PubMed] [Google Scholar]
- 2.Rucavado A., Lomonte B., Ovadia M., Gutierrez J. M. Local tissue damage induced by BaP1, a metalloproteinase isolated from Bothrops asper (Terciopelo) snake venom. Exp. Mol. Pathol. 1995;63:186–199. doi: 10.1006/exmp.1995.1042. [DOI] [PubMed] [Google Scholar]
- 3.Kamiguti A. S., Hay C. R., Theakston R. D., Zuzel M. Insights into the mechanism of haemorrhage caused by snake venom metalloproteinases. Toxicon. 1996;34:627–642. doi: 10.1016/0041-0101(96)00017-7. [DOI] [PubMed] [Google Scholar]
- 4.Fox J. W., Long C. The ADAMs/MDC family of proteins and their relationships to the snake venom metalloproteinases. In: Bailey G., editor. Snake Venom Enzymes. Fort Collins: Alaken Press; 1998. pp. 151–178. [Google Scholar]
- 5.Fox J. W., Serrano S. M. T. Structural considerations of the snake venom metalloproteinases, key members of the M12 reprolysin family of metalloproteinases. Toxicon. 2005 doi: 10.1016/j.toxicon.2005.02.012. in the press. [DOI] [PubMed] [Google Scholar]
- 6.Black R. A., White J. M. ADAMs: focus on the protease domain. Curr. Opin. Cell Biol. 1998;10:654–659. doi: 10.1016/s0955-0674(98)80042-2. [DOI] [PubMed] [Google Scholar]
- 7.White J. M. ADAMs: modulators of cell–cell and cell–matrix interactions. Curr. Opin. Cell Biol. 2003;15:598–606. doi: 10.1016/j.ceb.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Shannon J. D., Baramova E. N., Bjarnason J. B., Fox J. W. Amino acid sequence of a Crotalus atrox venom metalloproteinase which cleaves type IV collagen and gelatin. J. Biol. Chem. 1989;264:11575–11583. [PubMed] [Google Scholar]
- 9.Baramova E. N., Shannon J. D., Bjarnason J. B., Fox J. W. Degradation of extracellular matrix proteins by hemorrhagic metalloproteinases. Arch. Biochem. Biophys. 1989;275:63–71. doi: 10.1016/0003-9861(89)90350-0. [DOI] [PubMed] [Google Scholar]
- 10.Kamiguti A. S., Slupsky J. R., Zuzel M., Hay C. R. Properties of fibrinogen cleaved by jararhagin, a metalloproteinase from the venom of Bothrops jararaca. Thromb. Haemostasis. 1994;72:244–249. [PubMed] [Google Scholar]
- 11.Hamako J., Matsui T., Nishida S., Nomura S., Fujimura Y., Ito M., Ozeki Y., Titani K. Purification and characterization of kaouthiagin, a von Willebrand factor-binding and -cleaving metalloproteinase from Naja kaouthia cobra venom. Thromb. Haemostasis. 1998;80:499–505. [PubMed] [Google Scholar]
- 12.Jia L. G., Wang X. M., Shannon J. D., Bjarnason J. B., Fox J. W. Function of disintegrin-like/cysteine-rich domains of atrolysin A: inhibition of platelet aggregation by recombinant protein and peptide antagonists. J. Biol. Chem. 1997;272:13094–13102. doi: 10.1074/jbc.272.20.13094. [DOI] [PubMed] [Google Scholar]
- 13.Shimokawa K.-I., Shannon J. D., Jia L. G., Fox J. W. Sequence and biological activity of catrocollastatin-C: a disintegrin-like/cysteine-rich two-domain protein from Crotalus atrox venom. Arch. Biochem. Biophys. 1997;343:35–43. doi: 10.1006/abbi.1997.0133. [DOI] [PubMed] [Google Scholar]
- 14.Souza D. H., Iemma M. R., Ferreira L. L., Faria J. P., Oliva M. L., Zingali R. B., Niewiarowski S., Selistre-de-Araujo H. S. The disintegrin-like domain of the snake venom metalloprotease alternagin inhibits α2β1 integrin-mediated cell adhesion. Arch. Biochem. Biophys. 2000;384:341–350. doi: 10.1006/abbi.2000.2120. [DOI] [PubMed] [Google Scholar]
- 15.Ruggeri Z. M. Platelets in atherothrombosis. Nat. Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 16.Ruggeri Z. M. von Willebrand factor. J. Clin. Invest. 1997;99:559–564. doi: 10.1172/JCI119195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lankhof H., van Hoeij M., Schiphorst M. E., Bracke M., Wu Y. P., Ijsseldijk M. J., Vink T., de Groot P. G., Sixma J. J. A3 domain is essential for interaction of von Willebrand factor with collagen type III. Thromb. Haemostasis. 1996;75:950–958. [PubMed] [Google Scholar]
- 18.Romijn R. A., Bouma B., Wuyster W., Gros P., Kroon J., Sixma J. J., Huizinga E. G. Identification of the collagen-binding site of the von Willebrand factor A3-domain. J. Biol. Chem. 2001;276:9985–9991. doi: 10.1074/jbc.M006548200. [DOI] [PubMed] [Google Scholar]
- 19.Huizinga E. G., van der Plas R. M., Kroon J., Sixma J. J., Gros P. Crystal structure of the A3 domain of human von Willebrand factor: implications for collagen binding. Structure. 1997;5:1147–1156. doi: 10.1016/s0969-2126(97)00266-9. [DOI] [PubMed] [Google Scholar]
- 20.Whittaker C. A., Hynes R. O. Distribution and evolution of von Willebrand/integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere. Mol. Biol. Cell. 2002;13:3369–3387. doi: 10.1091/mbc.E02-05-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Q., Smith J. B., Grossman M. H. Molecular cloning and expression of catrocollastatin, a snake-venom protein from Crotalus atrox (western diamondback rattlesnake) which inhibits platelet adhesion to collagen. Biochem. J. 1995;307:411–417. doi: 10.1042/bj3070411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu C.-Z., Tuang T.-F. Crovidisin, a collagen-binding protein isolated from snake venom of Crotalus viridis, prevents platelet-collagen interaction. Arch. Biochem. Biophys. 1997;337:291–299. doi: 10.1006/abbi.1996.9787. [DOI] [PubMed] [Google Scholar]
- 23.Seals D. F., Courtneidge S. A. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 2003;17:7–30. doi: 10.1101/gad.1039703. [DOI] [PubMed] [Google Scholar]
- 24.Jia L. G., Wang X. M., Shannon J. D., Bjarnason J. B., Fox J. W. Inhibition of platelet aggregation by the recombinant cysteine-rich domain of the hemorrhagic snake venom metalloproteinase, atrolysin A. Arch. Biochem. Biophys. 2000;373:281–286. doi: 10.1006/abbi.1999.1517. [DOI] [PubMed] [Google Scholar]
- 25.Kamiguti A. S., Gallagher P., Marcinkiewicz C., Theakston R. D. G., Zuzel M., Fox J. W. Identification of sites in the cysteine-rich domain of the class P-III snake venom metalloproteinases responsible for inhibition of platelet function. FEBS Lett. 2003;27511:1–6. doi: 10.1016/s0014-5793(03)00799-3. [DOI] [PubMed] [Google Scholar]
- 26.Bjarnason J. B., Hamilton D., Fox J. W. Studies on the mechanism of hemorrhage production by five proteolytic hemorrhagic toxins from Crotalus atrox venom. Biol. Chem. Hoppe Seyler. 1988;369:121–129. [PubMed] [Google Scholar]
- 27.Bjarnason J. B., Fox J. W. Characterization of two hemorrhagic zinc proteinases, toxin c and toxin d, from western diamondback rattlesnake (Crotalus atrox) venom. Biochim. Biophys. Acta. 1987;911:356–363. doi: 10.1016/0167-4838(87)90077-x. [DOI] [PubMed] [Google Scholar]
- 28.Paine M. J. I., Desmond H. P., Theakston R. D. G., Crampton J. M. Purification, cloning, and molecular characterization of a high molecular weight hemorrhagic metalloproteinase, jararhagin, from Bothrops jararaca venom. J. Biol. Chem. 1992;267:22869–22876. [PubMed] [Google Scholar]
- 29.Henzel W. J., Grimley C., Bourell J. H., Billeci T. M., Wong S. C., Stults J. T. Analysis of two-dimensional gel proteins by mass spectrometry and microsequencing. Methods: A Companion to Methods in Enzymology. 1994;6:239–247. [Google Scholar]
- 30.Shimokawa K., Jia L.-G., Wang X.-M., Fox J. W. Expression, activation and processing of recombinant snake venom metalloproteinase, pro-atrolysin E. Arch. Biochem. Biophys. 1996;335:283–294. doi: 10.1006/abbi.1996.0509. [DOI] [PubMed] [Google Scholar]
- 31.Aumailley M., Wiedemann H., Mann K., Timpl R. Binding of nidogen and laminin–nidogen complex to basement membrane collagen type IV. Eur. J. Biochem. 1989;184:241–248. doi: 10.1111/j.1432-1033.1989.tb15013.x. [DOI] [PubMed] [Google Scholar]
- 32.Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 33.Burnette W. N. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 34.Varughese K. I., Celikel R., Ruggeri Z. M. Structure and function of the von Willebrand factor. Curr. Protein Pept. Sci. 2002;3:301–312. doi: 10.2174/1389203023380620. [DOI] [PubMed] [Google Scholar]
- 35.Santoro S. A., Zutter M. M. The α2β1 integrin: a collagen receptor on platelets and other cells. Thromb. Haemostasis. 1995;74:813–821. [PubMed] [Google Scholar]
- 36.Kamiguti A. S., Theakston R. D., Watson S. P., Bon C., Laing G. D., Zuzel M. Distinct contributions of glycoprotein VI and α2β1 integrin to the induction of platelet protein tyrosine phosphorylation and aggregation. Arch. Biochem. Biophys. 2000;374:356–362. doi: 10.1006/abbi.1999.1627. [DOI] [PubMed] [Google Scholar]
- 37.Moura-da-Silva A. M., Linica A., Della-Casa M. S., Kamiguti A. S., Ho P. L., Crampton J. M., Theakston R. D. Jararhagin ECD-containing disintegrin domain: expression in Escherichia coli and inhibition of the platelet-collagen interaction. Arch. Biochem. Biophys. 1999;369:295–301. doi: 10.1006/abbi.1999.1372. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Q., Dangelmaier C., Smith J. B. The hemorrhagin catrocollastatin inhibits collagen-induced platelet aggregation by binding to collagen via its disintegrin-like domain. Biochem. Biophys. Res. Commun. 1996;219:720–726. doi: 10.1006/bbrc.1996.0301. [DOI] [PubMed] [Google Scholar]
- 39.You W. K., Jang Y. J., Chung K. H., Kim D. S. A novel disintegrin-like domain of a high molecular weight metalloprotease inhibits platelet aggregation. Biochem. Biophys. Res. Commun. 2003;309:637–642. doi: 10.1016/j.bbrc.2003.08.049. [DOI] [PubMed] [Google Scholar]
- 40.Emsley J., Cruz M., Handin R., Liddington R. Crystal structure of the von Willebrand Factor A1 domain and implications for the binding of platelet glycoprotein Ib. J. Biol. Chem. 1998;273:10396–10401. doi: 10.1074/jbc.273.17.10396. [DOI] [PubMed] [Google Scholar]
- 41.Ito M., Hamako J., Sakurai Y., Matsumoto M., Fujimura Y., Suzuki M., Hashimoto K., Titani K., Matsui T. Complete amino acid sequence of kaouthiagin, a novel cobra venom metalloproteinase with two disintegrin-like sequences. Biochemistry. 2001;40:4503–4511. doi: 10.1021/bi0022700. [DOI] [PubMed] [Google Scholar]
- 42.Zigrino P., Kamiguti A. S., Eble J., Drescher C., Nischt R., Fox J. W., Mauch C. The reprolysin jararhagin, a snake venom metalloproteinase, functions as a fibrillar collagen agonist involved in fibroblast cell adhesion and signaling. J. Biol. Chem. 2002;277:40528–40535. doi: 10.1074/jbc.M202049200. [DOI] [PubMed] [Google Scholar]
- 43.Zolkiewska A. Disintegrin-like/cysteine-rich region of ADAM 12 is an active cell adhesion domain. Exp. Cell Res. 1999;252:423–431. doi: 10.1006/excr.1999.4632. [DOI] [PubMed] [Google Scholar]
- 44.Gaultier A., Cousin H., Darribere T., Alfandari D. ADAM13 disintegrin and cysteine-rich domains bind to the second heparin-binding domain of fibronectin. J. Biol. Chem. 2002;277:23336–23344. doi: 10.1074/jbc.M201792200. [DOI] [PubMed] [Google Scholar]
- 45.Soejima K., Matsumoto M., Kokame K., Yagi H., Ishizashi H., Maeda H., Nozaki C., Miyata T., Fujimura Y., Nakagaki T. ADAMTS-13 cysteine-rich/spacer domains are functionally essential for von Willebrand factor cleavage. Blood. 2003;102:3232–3237. doi: 10.1182/blood-2003-03-0908. [DOI] [PubMed] [Google Scholar]