Abstract
Three members of Gab family docking proteins, Gab1, Gab2 and Gab3, have been identified in humans. Previous studies have found that the hepatocyte growth factor preferentially utilizes Gab1 for signalling, whereas Bcr-Abl selectively signals through Gab2. Gab1–SHP2 interaction has been shown to mediate ERK (extracellular-signal-regulated kinase) activation by EGF (epidermal growth factor). However, it was unclear whether EGF selectively utilizes Gab1 for signalling to ERK and whether Gab2 is dispensable in cells where Gab1 and Gab2 are co-expressed. Using T47D and MCF-7 human breast carcinoma cells that express endogenous Gab1 and Gab2, we examined the role of these docking proteins in EGF-induced ERK activation. It was found that EGF induced a similar amount of SHP2–Gab1 and SHP2–Gab2 complexes. Expression of either SHP2-binding defective Gab1 or Gab2 mutant blocked EGF-induced ERK activation. Down-regulation of either Gab1 or Gab2 by siRNAs (small interfering RNAs) effectively inhibited the EGF-stimulated ERK activation pathway and cell migration. Interestingly, the inhibitory effect of Gab1 siRNA could be rescued not only by expression of an exogenous mouse Gab1 but also by an exogenous human Gab2 and vice versa, but not by IRS1 (insulin receptor substrate 1). These results reveal that Gab2 plays a pivotal role in the EGF-induced ERK activation pathway and that it can complement the function of Gab1 in the EGF signalling pathway. Furthermore, Gab1 and Gab2 are critical signalling threshold proteins for ERK activation by EGF.
Keywords: carcinoma cell, epidermal growth factor (EGF), extracellular-signal-regulated kinase (ERK), Gab, SHP2, small-interfering RNA (siRNA)
Abbreviations: DMEM, Dulbecco's modified Eagle's medium; EGF, epidermal growth factor; ER, oestrogen receptor; ERK, extracellular-signal-regulated kinase; FBS, fetal bovine serum; HA, haemagglutinin; IRS, insulin receptor substrate; MAPK, mitogen-activated protein kinase; MBP, myelin basic protein; MBS, c-Met-binding site; MOI, multiplicity of infection; PKB, protein kinase B; siRNA, small interfering RNA
INTRODUCTION
It is now recognized that two signal transduction pathways mediate activation of ERK1 (extracellular-signal-regulated kinase 1) and ERK2/MAPKs (mitogen-activated protein kinases) by growth factors, cytokines and certain oncoproteins. One is recruitment of the Ras guanine nucleotide-exchange factor Sos to the plasma membrane, the other is activation of the SHP2 protein tyrosine phosphatase [1]. SHP2 is activated by binding to docking proteins such as Gab, IRS (insulin receptor substrate) and FRS2 in growth factor-stimulated cells [2–4]. We and others previously found that SHP2 bound to Gab1 in EGF (epidermal growth factor)-stimulated cells to mediate ERK activation [5–7].
Besides the widely expressed Gab1, two other members of Gab family docking proteins, Gab2 and Gab3, have been identified in humans [1,8]. Studies on Gab2 and Gab3 have mostly been focused on their roles in cytokine signalling in haematopoietic cells where they are preferentially expressed [9–16]. However, while Gab2 and Gab3 have more restricted expression patterns, Gab2 was found to be co-expressed with Gab1 in certain epithelial cells, such as breast cancer cells [17,18].
The Gab family of docking proteins are characterized by a conserved pleckstrin homology domain, five tyrosine-based activation motifs for binding SHP2 and PI3K (phosphoinositide 3-kinase), and proline-rich sequences that bind Grb2 [6,19–22]. In addition to these common features, Gab1 has a unique MBS (c-Met-binding site) that mediates the interaction between c-Met and Gab1 [23]. Gab2 is associated with Akt/PKB (where PKB stands for protein kinase B) and is phosphorylated by Akt/PKB on Ser-159 [24]. Phosphorylation of Ser-159 on Gab2 by Akt/PKB appears to negatively regulate Gab2 tyrosine phosphorylation by the ErbB [avian erythroblastic leukaemia viral (v-erb-b) oncogene homologue] receptor tyrosine kinases, although the underlying mechanism has not been solved [24].
The existence of different Gab proteins in a single cell raises the question about whether a specific growth factor or protein tyrosine kinase selectively utilizes a particular Gab protein for signalling. It was observed that the hepatocyte growth factor preferentially induces tyrosine phosphorylation of Gab1 in MDCK cells (Madin–Darby canine kidney cells) that express endogenous Gab1 and Gab2 [17]. This effect is partly due to the presence of MBS in Gab1. Conversely, the Bcr-Abl oncoprotein selectively utilizes Gab2 for signalling, probably because Gab2 is a better substrate of the Bcr-Abl tyrosine kinase compared with Gab1 [9,14].
Both Gab1 and Gab2 are phosphorylated in EGF- or heregulin-stimulated cells [18]. However, a direct comparison of these two docking proteins in EGF-stimulated ERK activation in cells that co-express these molecules has not been reported. The observation that Gab2 may be tightly regulated by Akt/PKB raises the speculation that Gab2 may not be able to effectively mediate the EGF-stimulated ERK activation pathway. As such, Gab2 may play a dispensable role in breast carcinoma cells that co-express Gab1 and Gab2 [18]. In fact, in breast carcinoma cells that co-express Gab1 and Gab2, overexpression of Gab1 mutants could inhibit EGF-induced ERK activation [25,26]. In Gab1−/− mouse embryonic fibroblasts that contain endogenous Gab2, EGF-induced ERK activation and ErbB2-mediated cell transformation were impaired [7]. These observations seemingly support a predominant role of Gab1 over Gab2 in EGF-induced ERK activation in cells that co-express these two docking proteins.
In the present study, we developed Gab1- and Gab2-specific siRNAs (small interfering RNAs) to knock down Gab1 and Gab2 respectively in human cells. Using these siRNAs, we demonstrate that both Gab1 and Gab2 mediate the EGF-stimulated ERK activation pathway and cell migration in breast carcinoma cells that co-express these two docking proteins. Furthermore, rescue experiments indicate that Gab1 and Gab2 are functionally equivalent in the EGF-stimulated ERK activation pathway. Thus the requirement of both docking proteins is due to a threshold effect of signalling molecules. These results clarify the functionality of Gab2 in the EGF-induced ERK activation pathway.
EXPERIMENTAL
Antibodies
Antibodies to SHP2 and phosphotyrosine (RC20H) were from Pharmingen (San Diego, CA, U.S.A.). Anti-Gab1 antibodies were from Upstate Biotechnology (Lake Placid, NY, U.S.A.) and Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). The antibody to FLAG tag (M2) and β-actin were from Sigma. The anti-HA (haemagglutinin)-tagged antibody was from Covance Research Products (Berkeley, CA, U.S.A.) and the anti-Mek1 antibody was from Santa Cruz Biotechnology. The anti-Gab2 antibody was prepared as reported previously [27].
Plasmids
The wild-type and SHP2-binding defective (Gab1FF) human Gab1 have been reported [5,6]. The mouse Gab1 cDNA (Mus musculus growth factor receptor bound protein 2-associated protein 1; GenBank® accession no. BC007483) was amplified by PCR using pfuUltra high-fidelity DNA polymerase (Stratagene, La Jolla, CA, U.S.A.). The PCR fragment was subcloned into the pcDNA3.1-FLAG vector [5] between XhoI and XbaI sites to generate the expression plasmid for a FLAG-tagged mouse Gab1.
The wild-type and SHP2-binding defective (Gab2F) mouse Gab2 have been described previously [14]. For preparation of an expression vector for human Gab2, we first prepared the N-terminal 136 bp coding region by nested PCR using human K562 leukaemia cDNA as the template. Nucleotides 112–2028 (the last coding nucleotide) of the human Gab2 coding sequence was amplified by PCR from the KIAA0571 plasmid (Homo sapiens mRNA for KIAA0571 protein; GenBank® accession no. AB011143) kindly provided by Dr T. Nagase (Kazusa DNA Research Institute, Kisarazu, Chiba, Japan). The two overlapping PCR fragments covering the complete human Gab2 coding region were annealed and used as the template for PCR to produce a complete Gab2 coding cDNA. The PCR fragment was digested with KpnI and XbaI and then subcloned into pcDNA3.1-FLAG.
An expression vector for FLAG-tagged IRS1 was generated by PCR cloning of the human IRS1 coding sequence from BC053895 (from Open Biosystems, Huntsville, AL, U.S.A.) into pcDNA3.1-FLAG. The cloning sites were HindIII and XbaI. DNA sequences were verified by sequence analysis. PCR primer sequence information will be provided upon request.
Preparation of plasmid-based siRNAs
The target sequence for human Gab2 is 1652-GGGCCAACCACACCTTCAACT (the number indicates the location of the first nucleotide of the target sequence in the human Gab2 coding region). Oligonucleotides 5′-AACCACACCTTCAACTAAGCTTAGTTGAAGGTGTGGTTGGCCCTTTTTG and 5′-AATTCAAAAAGGGCCAACCACACCTTCAACTAAGCTTAGTTGAAGGTGTGGTTGGCC containing the siRNA coding sequence and an AAGCTT loop were annealed and cloned between the ApaI and EcoRI sites of plasmid pBS/U6 [28] to generate pGab2siRNA-1652. To generate a plasmid (pGab2siRNA) containing two copies of the Gab2 siRNA transcription units, the siRNA transcription unit was excised from pGab2siRNA-1652 by BamHI digestion. The DNA fragment was made bluntended by treatment with the Klenow fragment of DNA polymerase, and inserted into the SmaI site of pGab2siRNA-1652. An siRNA plasmid targeting human Gab1 sequence 976-GGAACCTTGGGACAGACAT and containing an ACA loop was also constructed in pBS/U6.
To facilitate the preparation of other siRNAs, we made a pBSU6-BbsI vector. Plasmid pBS/U6 [28] was digested with ApaI and the 3′-protruding ends were converted into blunt-ends with the Klenow fragment of DNA polymerase I. The plasmid was then digested with EcoRI. Oligonucleotides 5′-pTGGTCTTCGTCG (top) and 5′-pAATTCGACGAAGACCA (bottom, BbsI recognition sequence underlined) were annealed and inserted between the blunt-end and the EcoRI site to make the pBSU6-BbsI vector.
siRNA plasmids targeting human Gab1 siRNA sequences 874-GGGACATCGAGTGTAGAGACT and 1135-GGGATGTCGCCTTCACGTAGT were prepared in pBSU6-BbsI. The loop for both of these siRNAs was AAGCTT. A plasmid containing triple siRNA transcription units (pGab1siRNA) for Gab1-874, Gab1-1135 and Gab1-976 was constructed by sequential insertion of blunt-ended BamHI fragments containing the siRNA transcription units into the SmaI site of the plasmid containing siRNA for Gab1-874. A plasmid containing siRNA for both human Gab1 and Gab2 (pGab1/pGab2siRNA) was generated by subcloning the blunt-ended BamHI fragment containing pGab2siRNA-1652 into pGab1siRNA. Again, oligonucleotide sequence information for the construction of these siRNA vectors is available upon request.
Preparation of adenovirus-based siRNA and adenovirus encoding Gab1
The AdEasy XL adenoviral vector system (Stratagene) was used to generate adenovirus-based siRNAs for human Gab1 and Gab2. The three Gab1 siRNA transcription units, two Gab2 siRNA transcription units, and the U6 promoter transcription unit were excised from pGab1siRNA, pGab2siRNA and pBS/U6 respectively by XbaI digestion. These DNA fragments were subcloned into the XbaI site of pShuttle. Recombinant adenoviral plasmids were isolated using BJ5183-AD-1 cells according to the manufacturer's instructions. Recombinant adenoviral plasmids were amplified in XL10-Gold cells and used to transfect AD-293 cells for the preparation of recombinant adenoviruses for AdGab1siRNA (containing Gab1-874, Gab1-1135 and Gab1-976 siRNA transcription units), AdGab2siRNA (containing two copies of Gab2-1652 siRNA transcription units), and the AdU6Vector control. Primary viral stocks were amplified for three rounds in AD-293 cells. Titres of viral stocks were determined by the end-point dilution assay according to the manufacturer's instructions. The titres of recombinant adenoviruses used in the present study were in the range of 2.5–5×1010 pfu (plaque-forming units)/ml.
The human Gab1 coding sequence [5] was excised from pcDNA3.1 by HindIII and PmeI digestion and subcloned into HindIII and EcoRV sites of pShuttle. Recombinant adenoviral plasmids and adenovirus encoding Gab1 were generated as above for adenoviral siRNAs.
Cell culture and transfection
T47D, MCF-7 and COS-7 cells were grown in DMEM (Dulbecco's modified Eagle's medium) containing 10% (v/v) FBS (fetal bovine serum). For transfection, cells were plated in 60 mm plates 24 h before transfection to yield a 70% confluent culture on the day of transfection. Transfection was performed using Lipofectamine™ (Invitrogen) for COS-7 cells and Lipofectamine™ 2000 for T47D cells according to the manufacturer's instructions. Briefly, COS-7 cells were incubated with 2 μg of DNA (total) and 12 μl of Lipofectamine™ complex in serum-free medium for 5–7 h. After transfection, the medium was removed. The cells were either directly incubated with serum-free DMEM (for dominant negative construct experiments) or incubated with complete growth medium for 24 h, followed by serum starvation in DMEM for 20 h and then used for experiments. For transfection of T47D cells, the DNA–lipid complex was prepared with 4 μg of DNA (total) and 10 μl of Lipofectamine™ 2000 per transfection.
Immunoprecipitation, immunoblotting and kinase assays
Cells were lysed in buffer A (50 mM Tris/HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 25 mM NaF, 5 mM NaP2O7, 1% Triton X-100, 1 mM Na3VO4, 20 mM p-nitrophenylphosphate, 2 μg/ml leupeptin, 2 μg/ml aprotinin and 100 μg/ml PMSF). Precleared cell lysate supernatants were incubated with specific antibodies indicated in the Figure legends. Immunocomplexes were collected with protein G–agarose or protein A–agarose. Immunoblotting was performed as described previously [5,6,29]. ERK2 immunocomplex kinase assay was performed using MBP (myelin basic protein) as substrate [6]. Mek1 immunocomplex kinase assay was performed by phosphorylation of a kinase-defective ERK2 (KR) [30]. After kinase reaction, the reaction mixtures were separated on SDS/polyacrylamide gels and then transferred on to nitrocellulose membranes. The phosphorylation was quantified with a phosphoimager, after which nitrocellulose membranes were used for immunoblot analysis of ERK2 or Mek1 in immunoprecipitates.
Cell-migration assay
Transwell cell culture insert polycarbonate membranes (6.5 mm, 8.0 μm pore size; Costar, Acton, MA, U.S.A.) were coated underside with rat-tail type I collagen as described in [25,26]. T47D cells were incubated with recombinant adenoviruses [MOI (multiplicity of infections) 250] for 16 h and the medium was changed after the infection period. Infected cells were cultured in fresh DMEM/10% FBS for another 48 h and then serum-starved in DMEM/0.1% BSA for 20 h. Cells were detached from plates by trypsin digestion, washed and resuspended in DMEM/0.1% BSA at 5×105 cells/ml. Cells (1×105) were placed in the upper chamber. The lower chamber contains 0.6 ml DMEM/0.1% BSA with or without EGF. After incubation for 8 h at 37 °C in 5% CO2, cells on the upper membrane surface were mechanically removed with a cotton swab. Cells that had been migrated to the lower side of the membrane were fixed and stained with HEMA3 reagents (Fisher Scientific, Pittsburgh, PA, U.S.A.) and then counted under a microscope (using 10×20 lenses) in five randomly chosen fields. Statistical analysis was performed using the Wilcoxon rank sum test.
RESULTS
EGF induces SHP2 binding to Gab1 and Gab2
We and others have shown previously that binding of SHP2 to Gab1 or Gab2 mediates ERK activation by growth factors, cytokines and oncoproteins [5,9,15,23,31,32]. However, little is known about whether a particular growth factor, such as EGF, selectively utilized one of the two Gab proteins for activating the ERK/MAPK pathway when both of these Gab proteins were expressed in the same cells. T47D human breast carcinoma cells contain Gab1 and Gab2 [18]. To begin analysing whether one or both Gab1 and Gab2 are involved in the EGF-induced ERK activation pathway, we determined Gab1 and Gab2 tyrosine phosphorylation and their association with SHP2 in response to EGF. Near-confluent T47D cells were serum-starved and stimulated with EGF. Gab1 and Gab2 were immunoprecipitated. Immunoprecipitates were analysed by immunoblotting for Gab1 and Gab2 tyrosine phosphorylation and co-immunoprecipitation of SHP2. Under serum-starved conditions, tyrosine phosphorylation of Gab1 and Gab2 was minimal. Upon stimulation with EGF, tyrosine phosphorylation of Gab1 and Gab2 was markedly increased (Figure 1). This resulted in binding of SHP2 to Gab1 and Gab2. The extent of Gab1 and Gab2 tyrosine phosphorylation and the amount of SHP2 co-immunoprecipitated with Gab1 and Gab2 were similar. Thus EGF induces binding of SHP2 to both Gab1 and Gab2 when these two docking proteins are co-expressed in the same cells.
Figure 1. Formation of Gab1–SHP2 and Gab2–SHP2 complexes in T47D cells.
Near-confluent T47D cells in 100 mm plates were serum-starved and stimulated with EGF or mock-treated with BSA for 10 min. Gab1 and Gab2 were immunoprecipitated with antibodies to Gab1 and Gab2. Immunoprecipitates were analysed by immunoblotting with antibodies to phosphotyrosine, SHP2, and Gab1 or Gab2. IP, immunoprecipitation; IB, immunoblotting; α, antibody.
Expression of SHP2-binding defective Gab1 and Gab2 inhibits EGF-stimulated ERK2 activation
We previously identified Tyr-627 and Tyr-659 in human Gab1 as essential residues that constitute a bisphosphoryl tyrosine-based activation motif (BTAM) for binding and activation of SHP2 [6]. Mutations at either Tyr-627 or Tyr-659 or both (Gab1FF) are sufficient to disrupt Gab1–SHP2 interaction, and expression of these Gab1 mutants inhibits ERK activation by EGF [6]. The residue corresponding to Gab1 Tyr-627 in mouse Gab2 is Tyr-604. Y604F (Tyr-604→Phe) mutant Gab2 (Gab2F) is unable to bind SHP2 [14]. Expression of Gab2F is sufficient to block the Bcr-Abl-induced ERK signalling pathway in K562 leukaemia cells [14].
To assess further the involvement of Gab1 and Gab2 in EGF-induced ERK activation, we compared the effect of expressing SHP2-binding defective Gab1 (Gab1FF) and Gab2 (Gab2F) in T47D cells. T47D cells were transfected with HA-ERK2 expression plasmid together with Gab1FF, Gab2F or an empty vector, and EGF-induced ERK2 activation in transfected cells was measured. As shown in Figure 2(A), EGF activated ERK2 approx. 23-fold. Expression of Gab1FF and Gab2F resulted in 81 and 76% inhibition of EGF-induced ERK2 activation respectively. This result indicates that expression of either an SHP2-binding defective Gab1 or an SHP2-binding defective Gab2 is sufficient to block EGF-induced ERK activation in T47D cells. A simple interpretation of the results is that each of these Gab1 and Gab2 dominant mutants specifically inhibited endogenous Gab1 and Gab2 in T47D cells and thus both Gab1 and Gab2 are necessary for EGF-induced ERK activation in T47D cells. An alternative interpretation of the results is that Gab2F could cross-inhibit both endogenous Gab2 and Gab1 or vice versa and thus only one of the two Gabs is involved in EGF-induced ERK activation.
Figure 2. Effects of SHP2-binding defective Gab1 and Gab2 mutants on ERK activation.
(A) T47D cells in 60 mm plates were co-transfected with HA–ERK2 (0.4 μg) and Gab1FF, Gab2F or an empty vector (3.6 μg). Transfected cells were serum-starved and stimulated with EGF (5 ng/ml, 5 min) or mock-treated. An aliquot of each cell lysate supernatant (10 μg) was analysed for expression of Gab1FF and Gab2F by immunoblotting with an anti-FLAG antibody (lower panel). HA–ERK2 kinase activity was determined by an immunocomplex kinase assay using MBP as substrate. Reaction mixtures were separated by SDS/polyacrylamide gel and transferred on to a nitrocellulose membrane. After detection of MBP phosphorylation, the membrane was used for immunoblot analysis to determine the relative amount of HA–ERK2 protein in each sample. (B) COS-7 cells in 60 mm plates were co-transfected with HA–ERK2 (0.2 μg) and Gab1FF, Gab2F or an empty vector (1.8 μg) as indicated. Transfected cells were serum-starved and stimulated with EGF (0.5 ng/ml, 5 min) or mock-treated. Gab1FF and Gab2F expression was examined and ERK2 kinase activities were determined as above. Graphs represent averages and ranges of the ERK2 kinase activity from two independent experiments.
To test whether Gab2F could cross-inhibit Gab1, we compared the effect of Gab1FF and Gab2F on EGF-induced ERK activation in COS-7 cells, which express only endogenous Gab1. Similar previous observations [6], Gab1FF inhibited EGF-induced ERK activation (Figure 2B). Remarkably, although there was no endogenous Gab2 in COS-7 cells, Gab2F was just as effective as Gab1FF in inhibiting EGF-induced ERK activation in these cells. This result illustrates that an SHP2-binding-defective Gab2 mutant is able to cross-inhibit the function of endogenous Gab1. Therefore experiments using dominant negative constructs are inadequate to assess the involvement of individual Gab docking proteins in the ERK activation pathway.
Development of human Gab1 and Gab2 siRNAs
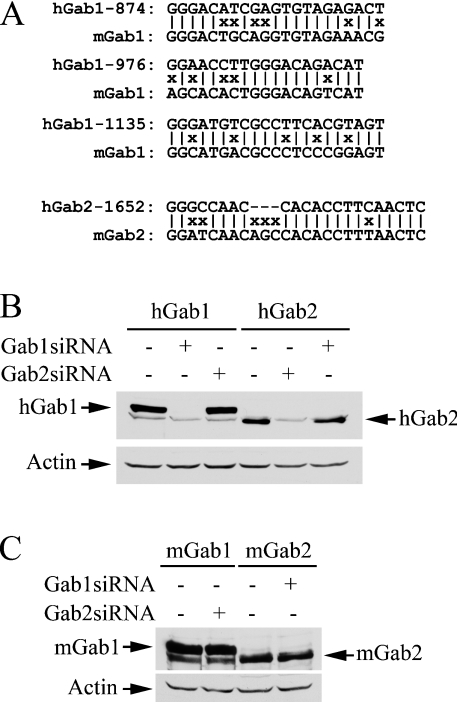
We then developed siRNAs for Gab1 and Gab2 in order to explore the possibility of using the RNAi knock-down approach to assess the role of these docking proteins in EGF signalling. We chose to target human specific Gab1 and Gab2 sequences (Figure 3A). This allows us to use mouse cDNA to determine if an effect on ERK activation is specifically attributed to the down-regulation of the intended siRNA target. We initially prepared two siRNAs targeting human Gab1-874 and Gab1-1637 (results not shown). Gab1-1637 was ineffective. While Gab1-874 was able to knock down Gab1, Gab1 was still detectable in immunoblots. We reckoned that if we targeted several Gab1 sequences simultaneously, this might increase the effectiveness of siRNA on knocking down Gab1. Consequently, we prepared a plasmid (pGab1siRNA) containing three siRNA transcription units that target Gab1-874, Gab1-976 and Gab1-1135. As shown in Figures 3(B) and 3(C), Gab1siRNA was very effective in knocking down human Gab1, but did not affect human Gab2 or mouse Gab1.
Figure 3. Specificities of Gab1 and Gab2 siRNAs.
(A) Alignment of human Gab1 and Gab2 siRNA targeting nucleotide sequences with the corresponding sequences in mouse Gab1 and Gab2. (B, C) COS-7 cells were co-transfected with pGab1siRNA, pGab2siRNA or empty vector (1.8 μg) and FLAG-tagged human Gab1 (hGab1), human Gab2 (hGab2), mouse Gab1 (mGab1) or mouse Gab2 (mGab2; 0.2 μg) as indicated. Cell lysates (30 μg) were subjected to immunoblot analyses with antibodies to the FLAG-tag and β-actin after 48 h transfection.
The first Gab2 siRNA that we made (Gab2-1652) appeared to completely suppress human Gab2 expression in co-transfection experiments. Consequently, we prepared a plasmid (pGab2siRNA) that contains two copies of the same Gab2 siRNA transcription unit to further increase the dose effect. Figures 3(B) and 3(C) show that Gab2siRNA specifically knocked down human Gab2, but had no effect on human Gab1 or mouse Gab2.
Inhibition of EGF-induced ERK2 activation by Gab1 and Gab2 siRNAs
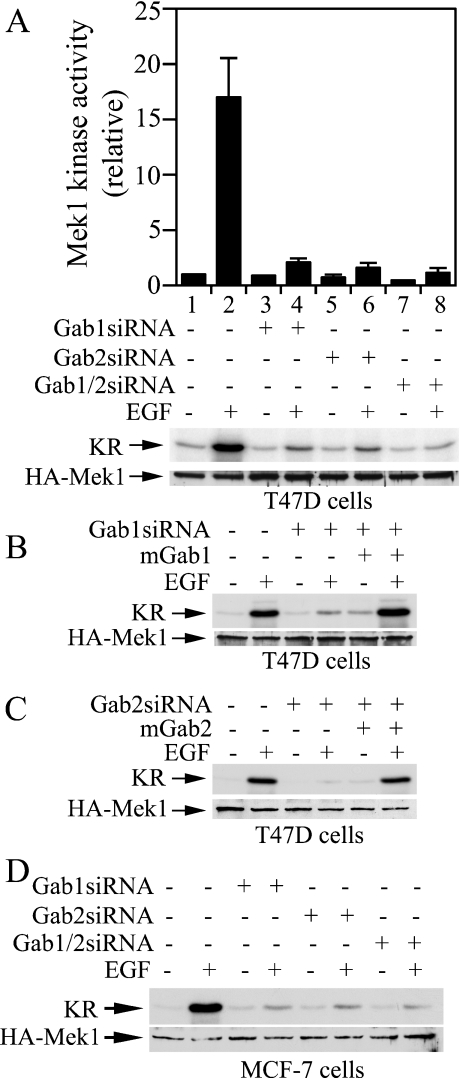
To determine if knocking down Gab1 and Gab2 in T47D cells affects the EGF-induced ERK activation pathway, T47D cells were co-transfected with pGab1siRNA or pGab2siRNA together with a HA–Mek1 expression vector. We measured the Mek1 activity in these experiments because ERK expression was self-regulated. Transfected cells would need to be incubated in serum-free medium immediately after the transfection procedure in order to keep the ERK protein level constant if the construct affected ERK activity [30]. Mek1 expression level, on the other hand, did not appear to be affected by ERK activity, and Mek1 activation correlated with ERK2 activation in the cells [30]. Thus Mek1 was more suitable for these siRNA experiments in which the effect was measured 2–3 days post-transfection.
Figure 4(A) shows that Gab1siRNA, Gab2siRNA and Gab1/Gab2siRNA inhibited EGF-induced Mek1 activation by 88, 90 and 93% respectively. Therefore knocking down either Gab1 or Gab2 prevented EGF-induced activation of the ERK/MAPK pathway in T47D cells. The inhibitory effect of Gab1siRNA could be rescued by co-expression of mouse Gab1, whereas the inhibitory effect of Gab2siRNA could be rescued by co-expression of mouse Gab2 (Figures 4B and 4C). These results suggest that the inhibitory effect of Gab1 and Gab2 siRNAs were specifically due to down-regulation of the endogenous Gab1 and Gab2 respectively. To determine if the effect of Gab1 and Gab2 siRNAs on EGF-induced Mek1 activation is only restricted to T47D cells, we performed the siRNA experiment in another breast carcinoma cell line (MCF-7) that also co-expresses Gab1 and Gab2 [18]. Similar to results obtained in T47D cells, Gab1si-RNA, Gab2siRNA and Gab1/Gab2siRNA effectively blocked EGF-induced Mek1 activation in MCF-7 cells (Figure 4D).
Figure 4. Inhibition of EGF-stimulated Mek1 activation in T47D and MCF-7 cells by Gab1siRNA and Gab2siRNA.
(A, D) T47D cells or MCF-7 cells in 60 mm plates were co-transfected with HA–Mek1 (0.4 μg) and pGab1siRNA, pGab2siRNA, pGab1/pGab2siRNA, or the empty vector (3.6 μg). Cells were serum-starved for 20 h after 24 h transfection, and then stimulated with EGF (5 ng/ml, 5 min) or mock-treated with BSA. HA–Mek1 was immunoprecipitated and Mek1 kinase activity was determined by phosphorylation of a kinase-defective ERK2 (KR) in the presence of [γ-32P]ATP. After autoradiography and phosphoimaging analysis, membranes were subjected to immunoblotting analysis of HA–Mek1. The histogram in (A) represents the averages and ranges from two independent experiments. (B, C) T47D cells in 60 mm plates were transfected with HA–Mek1 (0.4 μg)+siRNA and mouse Gab expression plasmids (1.8 μg/each) or control vector (−) as indicated. EGF-induced HA–Mek1 activation was measured as in (A and D).
Cross-substitution of Gab1 and Gab2 for their role in EGF-induced Mek1 activation
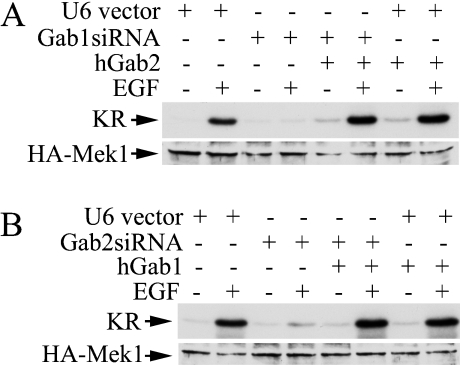
Results presented in Figure 4 suggest that both Gab1 and Gab2 are necessary for EGF-induced Mek1 activation in T47D and MCF-7 cells. One possible explanation is that these two docking proteins have unique signalling functions, both of which are required for the activation of the ERK/MAPK pathway. Another possibility is that these two docking proteins perform essentially the same function in the EGF-induced ERK activation pathway, but the amount of these two proteins is limited in these breast carcinoma cells. As such, down-regulation of either Gab1 or Gab2 prevented Mek1 activation because it lowered the total amount of Gab proteins below the threshold necessary for the propagation of the EGF signal.
To distinguish between these two mechanisms, we tested if the inhibitory effect caused by Gab1siRNA could be rescued by overexpression of human Gab2. T47D cells were transfected with pGab1siRNA together with or without a human Gab2 expression plasmid and the EGF-stimulated Mek1 activation was measured. Figure 5(A) shows that Gab1siRNA blocked EGF-induced Mek1 activation. Co-expression of human Gab2 fully restored the ability of EGF to activate Mek1 (Figure 5A). Conversely, overexpression of exogenous human Gab1 with Gab2siRNA completely rescued the inhibitory effect of Gab2siRNA on EGF-induced Mek1 activation in T47D cells (Figure 5B). Similarly, in COS-7 cells that contain only endogenous Gab1, inhibition of EGF-induced Mek1 activation by Gab1siRNA could be completely rescued by co-expression of human Gab2 with the Gab1siRNA (results not shown). These results illustrate that Gab1 can substitute for Gab2 and vice versa in the EGF-stimulated ERK/MAPK pathway. Thus the reason the knocking down of either Gab1 or Gab2 in T47D cells blocked activation of the ERK/MAPK pathway by EGF is a threshold effect of Gab1 and Gab2 proteins in these cells.
Figure 5. Gab1 and Gab2 are functionally equivalent in the EGF-induced ERK activation pathway.
T47D cells in 60 mm plates were co-transfected with HA–Mek1 (0.4 μg)+pGab1siRNA, pGab2siRNA, human Gab1 or Gab2 expression vector, or a control plasmid as indicated. The ratio of siRNA to Gab expression plasmid was 1:1 (1.8 μg/each). Transfected cells were processed for the determination of EGF-induced HA–Mek1 activation as described in the legend to Figure 4. (A) Co-expression of human Gab2 (hGab2) with Gab1siRNA. (B) Co-expression of human Gab1 with Gab2siRNA.
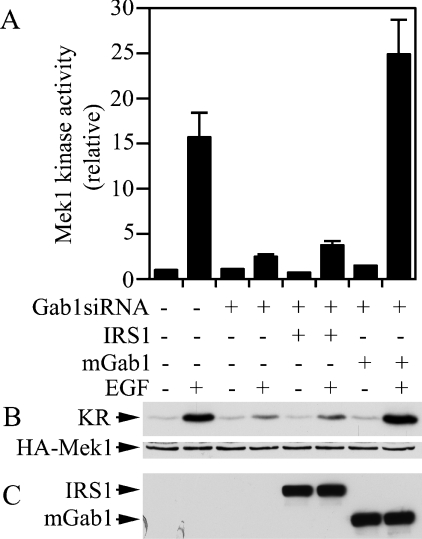
Our finding that Gab1 and Gab2 could cross-substitute for each other in the EGF-induced ERK activation pathway raises the question about whether other SHP2 docking proteins could also complement the requirement for Gab proteins. To address this issue, we co-expressed Gab1siRNA with exogenous mouse Gab1 or human IRS1 in T47D cells and determined EGF-induced Mek1 activation. As observed in Figure 4, co-expression of the exogenous mouse Gab1 rescued the EGF-stimulated Mek1 activation in T47D cells transfected with Gab1siRNA (Figure 6). However, while the exogenous human IRS1 was expressed at a level similar to that of the mouse Gab1, it did not have any effect on rescuing the inhibition by Gab1siRNA (Figure 6). Thus IRS1 was unable to compensate for the requirement of Gab docking proteins in EGF signalling.
Figure 6. IRS1 cannot complement Gab1 in the EGF-induced ERK activation pathway.
T47D cells were co-transfected with HA–Mek1+pGab1siRNA and expression vectors for human IRS1 or mouse Gab1 as indicated. EGF-induced HA–Mek1 activation was determined as in the legend to Figure 4. The averages and ranges of Mek1 activity from two independent experiments are shown (A). (B) Autoradiograph (upper panel) and immunoblot (lower panel) of a representative experiment. (C) Cell lysates were analysed by immunoblotting with an anti-FLAG antibody for expression of IRS1 and mGab1.
Both Gab1 and Gab2 are involved in EGF-induced cell migration
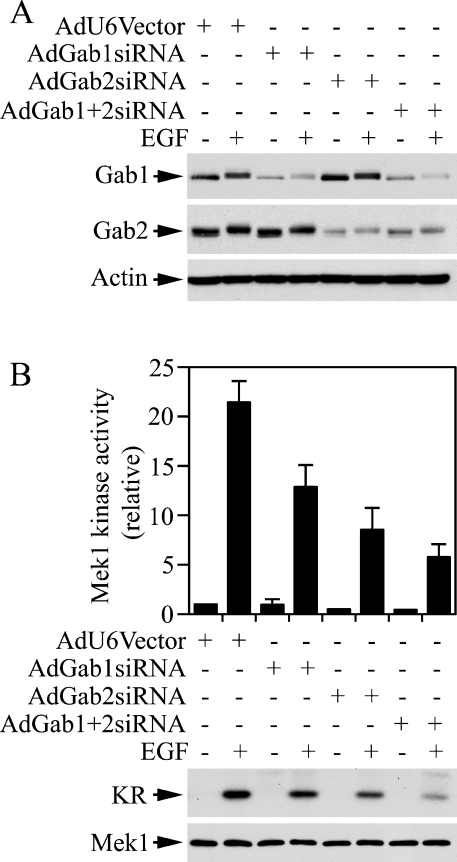
The ERK/MAPK pathway regulates migration of breast carcinoma cells [25,33]. To determine if both Gab1 and Gab2 are involved in EGF-induced migration of breast carcinoma cells, we developed adenovirus-based Gab1 and Gab2 siRNAs. Recombinant adenoviruses have higher efficiencies of introducing cargo transcription units into cells. This would allow us to express a specific siRNA in most of the cells under study. Figure 7(A) shows that we were able to knock down Gab1 by 72% in T47D cells with recombinant adenoviruses harbouring the three Gab1 siRNA transcription units (AdGab1siRNA). Infection of T47D cells with AdGab1siRNA had no apparent effect on Gab2 (Figure 7A). Similarly, Gab2 was knocked down by 82% in T47D cells with AdGab2siRNA, but Gab1 was not affected by AdGab2siRNA (Figure 7A). Attempts to generate a recombinant adenovirus containing both Gab1siRNA and Gab2siRNA were not successful. However, we were able to knock down Gab1 and Gab2 simultaneously by co-infection of T47D cells with AdGab1siRNA and AdGab2siRNA (Figure 7A).
Figure 7. Effects of adenoviral-based siRNAs on endogenous Gab protein levels and Mek1 activity.
T47D cells were incubated with recombinant adenoviruses as indicated for 16 h and then with fresh medium for 48 h followed by serum starvation and EGF stimulation. AdGab1+2siRNA indicates a 1:1 mixture of AdGab1siRNA and AdGab2siRNA viruses (MOI 125). Aliquots of cell lysates were analysed by immunoblotting with antibodies to Gab1, Gab2 and β-actin (A). Mek1 was immunoprecipitated from cell lysates and Mek1 kinase activity was determined (B). The histogram represents data from two independent experiments performed in duplicates (n=4).
As illustrated in Figure 7(B), EGF-induced activation of the endogenous Mek1 was inhibited by 42, 58 and 78% in T47D cells infected with AdGab1siRNA, AdGab2siRNA and AdGab1si-RNA+AdGab2siRNA respectively. We next performed a Transwell cell-migration assay using EGF as the chemoattractant. A 2.6-fold increase in cell migration was induced by EGF. Down-regulation of Gab1 and Gab2 had no apparent effect on the basal activity of cell migration, whereas the EGF-induced cell-migration activity was inhibited by 60, 70 and 82% in T47D cells infected with AdGab1siRNA, AdGab2siRNA and AdGab1si-RNA+AdGab2siRNA (Figure 8). These results demonstrate that both Gab1 and Gab2 are utilized by EGF to regulate ERK/MAPK activation and cell migration in T47D breast carcinoma cells.
Figure 8. Effects of down-regulation of Gab1 and Gab2 on EGF-stimulated cell migration.
T47D cells were infected with recombinant adenoviruses and serum-starved as mentioned in the legend to Figure 7 and used for the Transwell cell-migration assay in DMEM with EGF as the chemoattractant in the lower chamber. Migrated cells on the lower membrane surface were enumerated under a microscope (10×20 lens) in five randomly chosen fields and the average numbers of cells per field are shown. The data were from four independent experiments (n=6).
The total amount of Gab proteins in the rescue experiment cells is comparable with that of endogenous Gab proteins in control cells
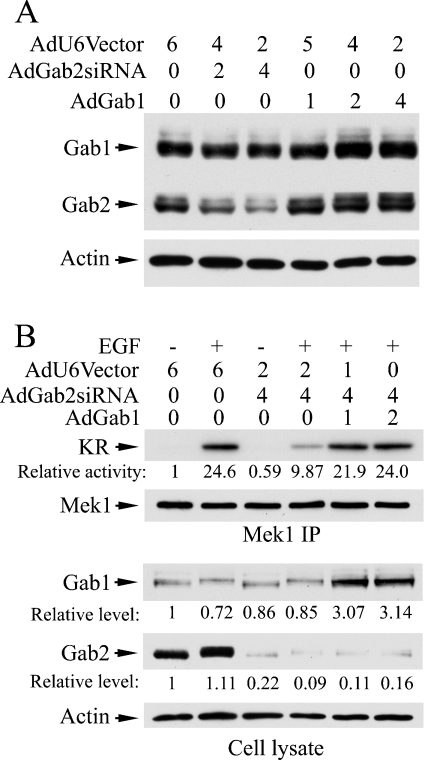
To compare the total amount of Gab1 and Gab2 proteins in the rescue experiment cells with that of endogenous Gab1 and Gab2 proteins in control cells, we co-infected T47D cells with AdGab2siRNA and adenovirus encoding human Gab1 (AdGab1). Following a preliminary experiment (Figure 9A), we co-infected T47D with 4:1 and 4:2 ratios of AdGab2siRNA and AdGab1 (1 portion=42 MOI) and then analysed EGF-stimulated Mek1 activation and the relative levels of Gab1 and Gab2 proteins in the cells (Figure 9B). Consistently, the exogenous Gab1 rescued the inhibition of ERK activation caused by the Gab2 siRNA. The Gab2 protein level was reduced to an average of 14.5%, whereas the Gab1 protein level was increased approx. 3-fold. Since endogenous Gab1 and Gab2 protein levels appear to be similar in T47 cells (Figure 1), data presented in Figure 9 suggest that the total amount of Gab proteins was comparable in control cells and cells infected with AdGab2siRNA and AdGab1. Thus our results argue against the possibility that the rescue effect of Gab1 is due to gross overexpression of Gab1.
Figure 9. Comparison of total Gab proteins in adenovirus-based rescue experiment.
T47D cells were incubated with recombinant adenoviruses as indicated (1=42 MOI) for 18 h. After the infection period, cells were cultured in fresh DMEM/10% FBS for another 10 h before serum-starvation in DMEM/0.1% BSA for 18 h, and then stimulated with EGF (5 ng/ml, 5 min) or mock-treated. Portions of cell lysates were subjected to immunoprecipitation with an anti-Mek1 antibody followed by the immunocomplex kinase assay. One half of each reaction mixture was used for autoradiography/phosphoimaging analysis and the other half for immunoblotting analysis of Mek1 after electrophoresis on SDS/polyacrylamide gels (B, upper panels). Aliquots of cell lysates (20 μg/each) were also used for immunoblottting analyses of Gab1, Gab2 or β-actin (A and lower panels of B). Relative activity was obtained from phosphoimager data. Relative levels of immunoreactive band intensities were measured from scanned images of X-ray films using the ImageQuant program.
DISCUSSION
Both Gab1 and Gab2 are expressed in most breast carcinoma cells and in ER (oestrogen receptor)-positive breast cancer specimens [18]. However, it was unclear if Gab2 could effectively mediate EGF signalling in breast carcinoma cells because Gab2 was believed to be tightly regulated by Akt/PKB-mediated serine phosphorylation [24]. Furthermore, expression of Gab1FF was sufficient to inhibit EGF-induced ERK activation in breast carcinoma cells that contain both Gab1 and Gab2 [25]. Using siRNA-mediated gene knockdown and rescue approaches, we demonstrate here that Gab2 plays a pivotal role in the EGF-stimulated ERK activation pathway.
Gab proteins mediate ERK activation through phosphotyrosine-dependent binding and activation of SHP2. Although it has been reported that S159A mutation in Gab2 could increase Gab2 tyrosine phosphorylation, we observed a similar level of Gab1 and Gab2 tyrosine phosphorylation in EGF-stimulated T47D cells. Gab docking proteins contain multiple potential tyrosine phosphorylation sites and it may be possible that different tyrosine residues were phosphorylated to different levels between Gab1 and Gab2 in response to EGF. However, the amount of SHP2 co-immunoprecipitated with Gab1 and Gab2 in EGF-stimulated T47D cells was similar, suggesting that these two docking proteins contributed to a similar extent in mediating SHP2 activation in EGF-stimulated T47D cells.
Down-regulation of either Gab1 or Gab2 protein was sufficient to inhibit the EGF-induced ERK activation pathway in T47D and MCF-7 cells. Since the inhibitory effect of down-regulating Gab1 on Mek1 activation could be rescued by overexpression of exogenous Gab2 and vice versa, it appears unlikely that the inhibitory effect was due to a requirement for specific functions of these two Gab proteins. Rather, these results suggest that Gab1 and Gab2 are both required in these cells because Gab1 and Gab2 are signalling threshold proteins that limit the flow of signal from activated EGF receptor to the ERK/MAPK. Knocking down Gab1 or Gab2 with siRNA produces a Gab-insufficiency condition and thus impairs the EGF-stimulated ERK activation. While Daly et al. [18] reported that the Gab2 protein level appeared to be elevated in ER-positive breast cancer cells, re-examination of their data (see Figure 1 in [18]) indicates that the Gab1 protein level in these cells appears to be lower than that in normal breast epithelial cells. The total amount of Gab1 and Gab2 proteins appears similar in most breast carcinoma cell lines. These results are in agreement with the notion that there is no excess Gab protein in most breast carcinoma cells and thus both Gab1 and Gab2 are required because of a threshold effect of these signalling molecules.
Besides IRS, insulin and insulin-like growth factor are known to also use Gab1 for cell signalling [34–38]. In fact, Gab1 was originally identified as a signalling molecule not only in response to EGF but also in response to insulin [34]. We found here that IRS1 was unable to substitute for Gab1 in mediating the EGF-induced ERK activation pathway. This is probably because EGF cannot induce IRS1 tyrosine phosphorylation [39]. Therefore Gab1 can complement the function of IRS1 in insulin signalling, but IRS1 cannot substitute for Gab1 in EGF signalling.
Our experiment using the SHP2-binding-defective Gab2 mutant indicates that the Gab2 mutant could cross-inhibit endogenous Gab1. This cross-inhibitory activity is not limited to the Gab2 mutant because overexpression of an SHP2-binding defective Gab3 mutant in COS-7 cells also had a dominant negative effect over the endogenous Gab1 (results not shown). Therefore it is likely that an SHP2-binding defective Gab1 mutant could not only inhibit endogenous Gab1, but also would cross-inhibit Gab2 or Gab3 if these docking proteins are present in the cells. Therefore caution should be exercised when interpreting experimental data obtained from dominant negative Gab constructs.
In gene knockout studies, Gab1−/− mouse embryos die in utero [40,41]. Consequently, the functions of Gab1 in adult animals and if Gab2 can compensate for these functions in adult animals are unclear. Furthermore, no results were available previously about whether overexpression of Gab2 could overcome the deficiency in growth factor signalling in Gab1−/− mouse embryo fibroblasts or whether Gab2 could complement the requirement for Gab1 in any human cells. In this regard, our results provide a new insight into the ability of Gab2 to complement Gab1 function in the EGF-regulated ERK/MAPK pathway.
Acknowledgments
We thank Dr Y. Shi (Harvard Medical School, Boston, MA, U.S.A.) for the pBS/U6 siRNA vector, and Dr T. Nagase for the KIAA0571 plasmid. This work was supported by the National Institutes of Health grant CA77467 and in part by American Heart Association grant 0455429B. We also acknowledge the Moffitt Cancer Center Core Facilities.
References
- 1.Nishida K., Hirano T. The role of Gab family scaffolding adapter proteins in the signal transduction of cytokine and growth factor receptors. Cancer Sci. 2003;94:1029–1033. doi: 10.1111/j.1349-7006.2003.tb01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neel B. G., Gu H., Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 3.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell (Cambridge, Mass.) 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 4.Feng G. S. Shp-2 tyrosine phosphatase: signaling one cell or many. Exp. Cell Res. 1999;253:47–54. doi: 10.1006/excr.1999.4668. [DOI] [PubMed] [Google Scholar]
- 5.Cunnick J. M., Dorsey J. F., Munoz-Antonia T., Mei L., Wu J. Requirement of SHP2 binding to Grb2-associated binder-1 for mitogen-activated protein kinase activation in response to lysophosphatidic acid and epidermal growth factor. J. Biol. Chem. 2000;275:13842–13848. doi: 10.1074/jbc.275.18.13842. [DOI] [PubMed] [Google Scholar]
- 6.Cunnick J. M., Mei L., Doupnik C. A., Wu J. Phosphotyrosines 627 and 659 of Gab1 constitute a bisphosphoryl tyrosine-based activation motif (BTAM) conferring binding and activation of SHP2. J. Biol. Chem. 2001;276:24380–24387. doi: 10.1074/jbc.M010275200. [DOI] [PubMed] [Google Scholar]
- 7.Yamasaki S., Nishida K., Yoshida Y., Itoh M., Hibi M., Hirano T. Gab1 is required for EGF receptor signaling and the transformation by activated ErbB2. Oncogene. 2003;22:1546–1556. doi: 10.1038/sj.onc.1206284. [DOI] [PubMed] [Google Scholar]
- 8.Gu H., Neel B. G. The ‘Gab’ in signal transduction. Trends Cell Biol. 2003;13:122–130. doi: 10.1016/s0962-8924(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 9.Gu H., Pratt J. C., Burakoff S. J., Neel B. G. Cloning of p97/Gab2, the major SHP2-binding protein in hematopoietic cells, reveals a novel pathway for cytokine-induced gene activation. Mol. Cell. 1998;2:729–740. doi: 10.1016/s1097-2765(00)80288-9. [DOI] [PubMed] [Google Scholar]
- 10.Nishida K., Yoshida Y., Itoh M., Fukada T., Ohtani T., Shirogane T., Atsumi T., Takahashi-Tezuka M., Ishihara K., Hibi M., et al. Gab-family adapter proteins act downstream of cytokine and growth factor receptors and T- and B-cell antigen receptors. Blood. 1999;93:1809–1816. [PubMed] [Google Scholar]
- 11.Miyakawa Y., Rojnuckarin P., Habib T., Kaushansky K. Thrombopoietin induces phosphoinositol 3-kinase activation through SHP2, Gab, and insulin receptor substrate proteins in BAF3 cells and primary murine megakaryocytes. J. Biol. Chem. 2001;276:2494–2502. doi: 10.1074/jbc.M002633200. [DOI] [PubMed] [Google Scholar]
- 12.Gu H., Maeda H., Moon J. J., Lord J. D., Yoakim M., Nelson B. H., Neel B. G. New role for Shc in activation of the phosphatidylinositol 3-kinase/Akt pathway. Mol. Cell. Biol. 2000;20:7109–7120. doi: 10.1128/mcb.20.19.7109-7120.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu H., Botelho R. J., Yu M., Grinstein S., Neel B. G. Critical role for scaffolding adapter Gab2 in Fc gamma R-mediated phagocytosis. J. Cell Biol. 2003;161:1151–1161. doi: 10.1083/jcb.200212158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorsey J. F., Cunnick J. M., Mane S. M., Wu J. Regulation of the Erk2-Elk1 signaling pathway and megakaryocytic differentiation of Bcr-Abl+ K562 leukemic cells by Gab2. Blood. 2002;99:1388–1397. doi: 10.1182/blood.v99.4.1388. [DOI] [PubMed] [Google Scholar]
- 15.Wolf I., Jenkins B. J., Liu Y., Seiffert M., Custodio J. M., Young P., Rohrschneider L. R. Gab3, a new DOS/Gab family member, facilitates macrophage differentiation. Mol. Cell. Biol. 2002;22:231–244. doi: 10.1128/MCB.22.1.231-244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bourgin C., Bourette R. P., Arnaud S., Liu Y., Rohrschneider L. R., Mouchiroud G. Induced expression and association of the Mona/Gads adapter and Gab3 scaffolding protein during monocyte/macrophage differentiation. Mol. Cell. Biol. 2002;22:3744–3756. doi: 10.1128/MCB.22.11.3744-3756.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lock L. S., Maroun C. R., Naujokas M. A., Park M. Distinct recruitment and function of Gab1 and Gab2 in Met receptor-mediated epithelial morphogenesis. Mol. Biol. Cell. 2002;13:2132–2146. doi: 10.1091/mbc.02-02-0031.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daly R. J., Gu H., Parmar J., Malaney S., Lyons R. J., Kairouz R., Head D. R., Henshall S. M., Neel B. G., Sutherland R. L. The docking protein Gab2 is overexpressed and estrogen regulated in human breast cancer. Oncogene. 2002;21:5175–5181. doi: 10.1038/sj.onc.1205522. [DOI] [PubMed] [Google Scholar]
- 19.Maroun C. R., Moscatello D. K., Naujokas M. A., Holgado-Madruga M., Wong A. J., Park M. A conserved inositol phospholipid binding site within the pleckstrin homology domain of the Gab1 docking protein is required for epithelial morphogenesis. J. Biol. Chem. 1999;274:31719–31726. doi: 10.1074/jbc.274.44.31719. [DOI] [PubMed] [Google Scholar]
- 20.Lock L. S., Royal I., Naujokas M. A., Park M. Identification of an atypical Grb2 carboxyl-terminal SH3 domain binding site in Gab docking proteins reveals Grb2-dependent and -independent recruitment of Gab1 to receptor tyrosine kinases. J. Biol. Chem. 2000;275:31536–31545. doi: 10.1074/jbc.M003597200. [DOI] [PubMed] [Google Scholar]
- 21.Lewitzky M., Kardinal C., Gehring N. H., Schmidt E. K., Konkol B., Eulitz M., Birchmeier W., Schaeper U., Feller S. M. The C-terminal SH3 domain of the adapter protein Grb2 binds with high affinity to sequences in Gab1 and SLP-76 which lack the SH3-typical P-x-x-P core motif. Oncogene. 2001;20:1052–1062. doi: 10.1038/sj.onc.1204202. [DOI] [PubMed] [Google Scholar]
- 22.Rodrigues G. A., Falasca M., Zhang Z., Ong S. H., Schlessinger J. A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol. Cell. Biol. 2000;20:1448–1459. doi: 10.1128/mcb.20.4.1448-1459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaeper U., Gehring N. H., Fuchs K. P., Sachs M., Kempkes B., Birchmeier W. Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J. Cell. Biol. 2000;149:1419–1432. doi: 10.1083/jcb.149.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lynch D. K., Daly R. J. PKB-mediated negative feedback tightly regulates mitogenic signalling via Gab2. EMBO J. 2002;21:72–82. doi: 10.1093/emboj/21.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren Y., Meng S., Mei L., Zhao Z. J., Jove R., Wu J. Roles of Gab1 and SHP2 in paxillin tyrosine dephosphorylation and Src activation in response to epidermal growth factor. J. Biol. Chem. 2004;279:8497–8505. doi: 10.1074/jbc.M312575200. [DOI] [PubMed] [Google Scholar]
- 26.Ren Y., Wu J. Simultaneous suppression of Erk and Akt/PKB activation by a Gab1 pleckstrin homology (PH) domain decoy. Anticancer Res. 2003;23:3231–3236. [PubMed] [Google Scholar]
- 27.Dorsey J. F., Jove R., Kraker A. J., Wu J. The pyrido[2,3-d]pyrimidine derivative PD180970 inhibits p210Bcr-Abl tyrosine kinase and induces apoptosis of K562 leukemic cells. Cancer Res. 2000;60:3127–3131. [PubMed] [Google Scholar]
- 28.Sui G., Soohoo C., Affar el B., Gay F., Shi Y., Forrester W. C. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 2002;99:5515–5520. doi: 10.1073/pnas.082117599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cunnick J. M., Dorsey J. F., Standley T., Turkson J., Kraker A. J., Fry D. W., Jove R., Wu J. Role of tyrosine kinase activity of epidermal growth factor receptor in the lysophosphatidic acid-stimulated mitogen-activated protein kinase pathway. J. Biol. Chem. 1998;273:14468–14475. doi: 10.1074/jbc.273.23.14468. [DOI] [PubMed] [Google Scholar]
- 30.Cunnick J. M., Meng S., Ren Y., Desponts C., Wang H. G., Djeu J. Y., Wu J. Regulation of the mitogen-activated protein kinase signaling pathway by SHP2. J. Biol. Chem. 2002;277:9498–9504. doi: 10.1074/jbc.M110547200. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi-Tezuka M., Yoshida Y., Fukada T., Ohtani T., Yamanaka Y., Nishida K., Nakajima K., Hibi M., Hirano T. Gab1 acts as an adapter molecule linking the cytokine receptor gp130 to ERK mitogen-activated protein kinase. Mol. Cell. Biol. 1998;18:4109–4117. doi: 10.1128/mcb.18.7.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi Z. Q., Yu D. H., Park M., Marshall M., Feng G. S. Molecular mechanism for the Shp-2 tyrosine phosphatase function in promoting growth factor stimulation of Erk activity. Mol. Cell. Biol. 2000;20:1526–1536. doi: 10.1128/mcb.20.5.1526-1536.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Badache A., Hynes N. E. Interleukin 6 inhibits proliferation and, in cooperation with an epidermal growth factor receptor autocrine loop, increases migration of T47D breast cancer cells. Cancer Res. 2001;61:383–391. [PubMed] [Google Scholar]
- 34.Holgado-Madruga M., Emlet D. R., Moscatello D. K., Godwin A. K., Wong A. J. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature (London) 1996;379:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- 35.Winnay J. N., Bruning J. C., Burks D. J., Kahn C. R. Gab-1-mediated IGF-1 signaling in IRS-1-deficient 3T3 fibroblasts. J. Biol. Chem. 2000;275:10545–10550. doi: 10.1074/jbc.275.14.10545. [DOI] [PubMed] [Google Scholar]
- 36.Rocchi S., Tartare-Deckert S., Murdaca J., Holgado-Madruga M., Wong A. J., Van Obberghen E. Determination of Gab1 (Grb2-associated binder-1) interaction with insulin receptor-signaling molecules. Mol. Endocrinol. 1998;12:914–923. doi: 10.1210/mend.12.7.0141. [DOI] [PubMed] [Google Scholar]
- 37.Lehr S., Kotzka J., Herkner A., Sikmann A., Meyer H. E., Krone W., Muller-Wieland D. Identification of major tyrosine phosphorylation sites in the human insulin receptor substrate Gab-1 by insulin receptor kinase in vitro. Biochemistry. 2000;39:10898–10907. doi: 10.1021/bi000982k. [DOI] [PubMed] [Google Scholar]
- 38.Harada S., Esch G. L., Holgado-Madruga M., Wong A. J. Grb-2-associated binder-1 is involved in insulin-induced egr-1 gene expression through its phosphatidylinositol 3′-kinase binding site. DNA Cell Biol. 2001;20:223–229. doi: 10.1089/104454901750219107. [DOI] [PubMed] [Google Scholar]
- 39.Kadowaki T., Koyasu S., Nishida E., Tobe K., Izumi T., Takaku F., Sakai H., Yahara I., Kasuga M. Tyrosine phosphorylation of common and specific sets of cellular proteins rapidly induced by insulin, insulin-like growth factor I, and epidermal growth factor in an intact cell. J. Biol. Chem. 1987;262:7342–7350. [PubMed] [Google Scholar]
- 40.Itoh M., Yoshida Y., Nishida K., Narimatsu M., Hibi M., Hirano T. Role of Gab1 in heart, placenta, and skin development and growth factor- and cytokine-induced extracellular signal-regulated kinase mitogen-activated protein kinase activation. Mol. Cell. Biol. 2000;20:3695–3704. doi: 10.1128/mcb.20.10.3695-3704.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sachs M., Brohmann H., Zechner D., Muller T., Hulsken J., Walther I., Schaeper U., Birchmeier C., Birchmeier W. Essential role of Gab1 for signaling by the c-Met receptor in vivo. J. Cell Biol. 2000;150:1375–1384. doi: 10.1083/jcb.150.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]