Abstract
Efficient and biocompatible methods for synthesizing glycoconjugates are essential in chemical biology, as these molecules play pivotal roles in cellular recognition, signaling, and immune responses. Abnormal glycosylation is associated with diseases such as cancer, infections, and immune disorders, positioning glycoconjugates as promising candidates for therapeutic, diagnostic, and drug delivery applications. Traditional chemical approaches often lack biocompatibility and efficiency; however, the advent of metal-free click chemistry has revolutionized glycoconjugate synthesis by providing selective and versatile tools under mild conditions. This review highlights four remarkable metal-free click reactions: thiol–ene coupling (TEC), strain-promoted azide–alkyne cycloaddition (SPAAC), inverse electron-demand Diels–Alder (IEDDA) reaction, and sulfur fluoride exchange (SuFEx). TEC enables the regio- and stereoselective synthesis of glycoconjugates, including S-polysaccharides, glycopeptides, and glycoclusters, advancing vaccine development and carbohydrate-based therapeutics. SPAAC, a bioorthogonal and metal-free alternative, facilitates in vivo imaging, glycan monitoring, the synthesis of glycofullerenes and glycovaccines, and the development of targeted protein degradation systems such as lysosome-targeting chimeras (LYTACs). Additionally, the combination of SPAAC with biocatalysis offers a sustainable approach for preparing glycoconjugates with therapeutic potential. The IEDDA reaction, a highly efficient metal-free biorthogonal cycloaddition, plays a key role in metabolic glycoengineering for live-cell imaging and glycan-based therapies and also contributes to the creation of injectable hydrogels for drug delivery and tissue engineering. SuFEx, a more recent reaction, enables efficient sulfonamide and sulfonate bond formation, broadening the toolbox for glycoconjugate and protein functionalization. These methodologies are transforming glycochemistry and glycobiology, driving advancements in biomedicine, materials science, and pharmaceutical development.
1. Introduction
In the dynamic field of chemical biology, developing efficient, selective, and biocompatible strategies for synthesizing glycoconjugates is a central goal in glycochemistry and glycobiology. Glycoconjugates, such as glycoproteins, glycolipids, and proteoglycans, play crucial roles in cell recognition, signaling, adhesion and immune response. − They are also implicated in various pathological processes, including cancer, , infections, − inflammation, neurodegenerative disorders, and coagulation, making them valuable targets for therapeutic and diagnostic applications. ,
Traditional synthesis methods are often too harsh for biological systems. However, the introduction of click chemistry by Sharpless in 2001 revolutionized glycoconjugates synthesis. Click chemistry refers to a set of highly selective, efficient and versatile reactions that proceed under mild conditions, making it a valuable tool in carbohydrate chemistry. Among these, the Huisgen 1,3-dipolar cycloaddition between azides and alkynes has proven particularly significant in organic chemistry, offering broad utility across numerous scientific disciplines. − This reaction is catalyzed by copper ions, which also confer high regioselectivity, and it is therefore known as the copper-catalyzed Huisgen 1,3-dipolar cycloaddition (CuAAC). However, concerns about copper’s cytotoxicity and its interference with biological processes have limited its use in biological systems. , As a result, metal-free click reactions have gained growing interest. Some of these reactions are bioorthogonal, meaning that they can proceed inside living organisms without disrupting native biochemical processes. These features have made them essential tools for real-time biomolecule labeling, imaging, and modification. − Indeed, recent studies highlight metal-free click chemistry as a preferred approach in the design of biocompatible glycoconjugates. ,
This review focuses on four key metal-free click reactions (Table ): thiol–ene coupling (TEC), strain-promoted azide–alkyne cycloaddition (SPAAC), inverse electron-demand Diels–Alder (IEDDA) reaction, and sulfur fluoride exchange (SuFEx). Among them, SPAAC and IEDDA are bioorthogonal, allowing selective modification of biomolecules in living systems. In contrast, TEC and SuFEx though not bioorthogonal offer complementary advantages in reactivity and synthetic versatility (Table ).
1. Properties of the Metal-Free Click Reactions Covered in This Review.
TEC, also known as hydrothiolation, enables efficient formation of stable thioethers under mild, often aqueous, conditions. Driven by visible light via a radical mechanism, TEC is atom-economical and compatible with diverse functional groups, making it highly versatile in glycochemistry (Table ). −
SPAAC is a metal-free [3 + 2] cycloaddition between azides and strained cyclooctynes. Developed as a bio-orthogonal alternative to CuAAC, SPAAC proceeds efficiently under physiological conditions without toxic catalysts. It offers high chemoselectivity, biocompatibility, and fast kinetics. Despite limitations such as regioisomer formation and the synthetic complexity of cyclooctynes, SPAAC remains a valuable tool in carbohydrate chemistry (Table ). −
IEDDA reaction is a rapid and selective cycloaddition between electron-deficient dienes (e.g., tetrazines) and electron-rich dienophiles (e.g., strained alkenes). It is bioorthogonal and proceeds efficiently under mild, aqueous conditions, making it highly suitable for chemical biology applications (Table ). −
SuFEx is a newer click reaction that forms S–O and S–N bonds through reactions between sulfonyl fluorides or fluorosulfates and nucleophiles, such as silyl ethers or amines. It is driven by strong Si–F bond formation and proceeds under mild metal-free conditions. SuFEx offers high stability, chemoselectivity, and compatibility with biological environments (Table ). ,
Together, these metal-free reactions expand the toolbox for glycoconjugate synthesis, offering new possibilities for therapeutic and diagnostic innovation. This review highlights their mechanisms and applications, underscoring their potential to advance bioconjugation and glycochemistry in both biotechnology and pharmaceutical development.
2. Thiol–Ene Coupling (TEC)
2.1. Introduction
Hydrothiolation of terminal inactivated alkenes, also known as TEC, is a century-old reaction that has emerged as an effective and rapid metal-free click reaction between thiols and alkenes to create stable thioethers. This reaction proceeds with excellent yields and complete selectivity without the need for a metal catalyst, and it is compatible with aqueous conditions. Originally discovered by Posner in 1905, this reaction is driven by visible light, which supports a free radical mechanism. The process begins with the generation of a thiyl radical from a thiol, either by direct UV irradiation (at wavelengths between 254–405 nm) or in the presence of a radical photoinitiator, which induces homolytic cleavage of the sulfhydryl S–H bond. The thiyl radical then undergoes anti-Markovnikov addition to an alkene, forming an intermediate carbon center radical. This carbon radical subsequently abstracts a hydrogen atom from a second molecule of thiol affording the thioether product and a new thiyl radical, which perpetuates the radical cycle (Scheme A). ,
1. (A) Mechanism Pathway for Anti-Markovnikov Thiol–ene Reaction; (B) Thiol–Ene Reaction between Thioglycosides and Terminal Alkenes.
The high atom economy and the effectiveness of the TEC reaction significantly simplify the reaction workup and isolation of the final alkyl sulfide products. However, a primary limitation of this click reaction is the reversibility of the thiyl radical addition to the alkene step, conducting in some cases to the detection of a disulfide side product, coming from the thiyl radical homocoupling. The irreversibility of the reaction can be forced by carefully designing the structure of both reactants and optimizing reaction conditions, such as the alkene-to-thiol ratio, photoinitiator concentration, solvent and pH. ,,
The exceptional versatility of thiol–ene chemistry has expanded its applicability across various fields, including polymers and surface chemistry, ,, natural products and peptide synthesis. , Given the numerous benefits of TEC and the ubiquity of the carbon–sulfur bond in natural products and bioconjugate chemistry, TEC offers a useful and effective assembling platform for carbohydrate chemistry. , Specifically, thioglycosides play a significant role not only in biological function and drug design but also in synthetic chemistry as a key precursor for other glycosides, glycans, and glycoconjugates. The first thiol–ene reaction involving thioglycosides and alkenes was reported in 1988 by Pavia and co-workers. Alkyl 1-thioglycosides 2 were prepared in moderate to excellent yields by reacting peracetylated 1-thioglycoside 1 (mono- or disaccharide) with alkenes in acetonitrile at 80 °C using α-azobis(isobutyronitrile) (AIBN) as a radical initiator (Scheme B). Notably, this methodology allows the synthesis of alkylated thioglycosides containing different functional groups at the terminal position of the aglycon chain (R2), which are suitable for further conjugation with other biomolecules. Since this pioneering work, numerous articles have been published on the synthesis of thiosugars and sulfur containing glycoconjugates via TEC.
2.2. Applications
2.2.1. Synthesis of S-Polysaccharides via TEC
Glycomimetics are synthetic compounds designed to mimic the structure and function of carbohydrates, which play crucial roles in various biological processes such as cell recognition, signaling, and immune response. These compounds are useful for studying these processes and hold the potential for developing therapeutic agents that target carbohydrate-binding proteins involved in diseases such as cancer, inflammation, and infectious diseases. The application of TEC to the synthesis of S-linked oligosaccharides has attracted significant attention in the carbohydrate field because S-linkage is more resistant to acid hydrolysis or enzymatic degradation, offering enhanced stability, bioavailability and selectivity compared to O-glycosidic linkage. Dondoni and co-workers first applied this efficient methodology in 2009, performing the coupling between peracetylated thiol sugars 3a–b and exo-glycals 4 as a strategy for the synthesis of S-linked disaccharides 5 (Scheme ). The reaction mixture was irradiated with a UV–visible lamp (λmax = 420 nm) in the presence of 2,2-dimethoxy-2-phenylacetophenone (DPAP) as the photoinitiator. The 1,6-linked S-disaccharides were rapidly obtained in excellent yields with high diastereoselectivity, confirming the potential of TEC in glycochemistry. The same authors extended this methodology to evaluate the hydrothiolation reaction with the derived endo-glycals 6. The reaction was completely regioselective, with the thiyl radical attacking exclusively at the C-2 position of the glycal. However, the nature of the glycal moiety determined whether the coupling product was a single stereoisomer or as a mixture of stereoisomers 7–8 (Scheme ). Alternatively, Borbás and co-workers reported a similar study aimed at preparing new types of glycomimetic compounds with potential therapeutic applications. The addition of sugar thiol 3 to exo-galactal 9, which bears a double bound at the anomeric position, or 2,3-unsaturated glycoside 11, containing an endo-alkene, successfully afforded the corresponding compounds 10 and 12 respectively as single regioisomers. Remarkably, in this case, only one stereoisomer was detected (Scheme ).
2. Synthesis of S-Linked Disaccharides via TEC.

In 2017 Borbás and co-workers employed the same strategy for the synthesis of sugar-modified nucleosides. The therapeutic application of nucleosides and nucleic acids has promoted the development of nucleoside analogues with improved chemical and biological properties. However, achieving versatile and stereoselective alterations of the furanose residue in nucleosides to create new drug candidates remains a significant challenge for synthetic chemists. In this study, the authors demonstrated that the low temperature TEC reaction (−80 °C) between C3′-methylene derivatives of uridine 15a and ribothymidine 15b as ene-nucleosides provided an easy and efficient approach to prepare pyranose-modified nucleosides 16 (Scheme ). Remarkably, the addition of the thioglycoside 13a–b to the double bound proceeded with excellent level of d-xylo selectivity.
3. Synthesis of Sugar-Modified Nucleosides 16a–c and N-Linked and C-Linked Imino-disaccharides 18a–b via TEC .

a TBS = t-Butyldimethylsilyl.
Iminosugars are another glycomimetic compound that contain a nitrogen atom instead of an oxygen in the ring structure. They are known for their ability to inhibit glycosidase enzymes, making them valuable in various therapeutic applications, including the treatment of viral infections, diabetes, and lysosomal storage disorders. Recently, Marra et al. first reported the efficient synthesis of N-linked imino-disaccharides 18a or anomerically C-linked imino-disaccharides 18b via TEC from different sugar thiols 14a–d and the corresponding iminosugar alkenes 17a or 17b (Scheme ). The photoinduced radical addition reactions were performed under similar reaction conditions as previously described, with the addition of trifluoroacetic acid (TFA) to prevent the deprotonation of the sugar thiol by the iminosugar, thereby favoring the formation of a thiyl radical necessary for the coupling.
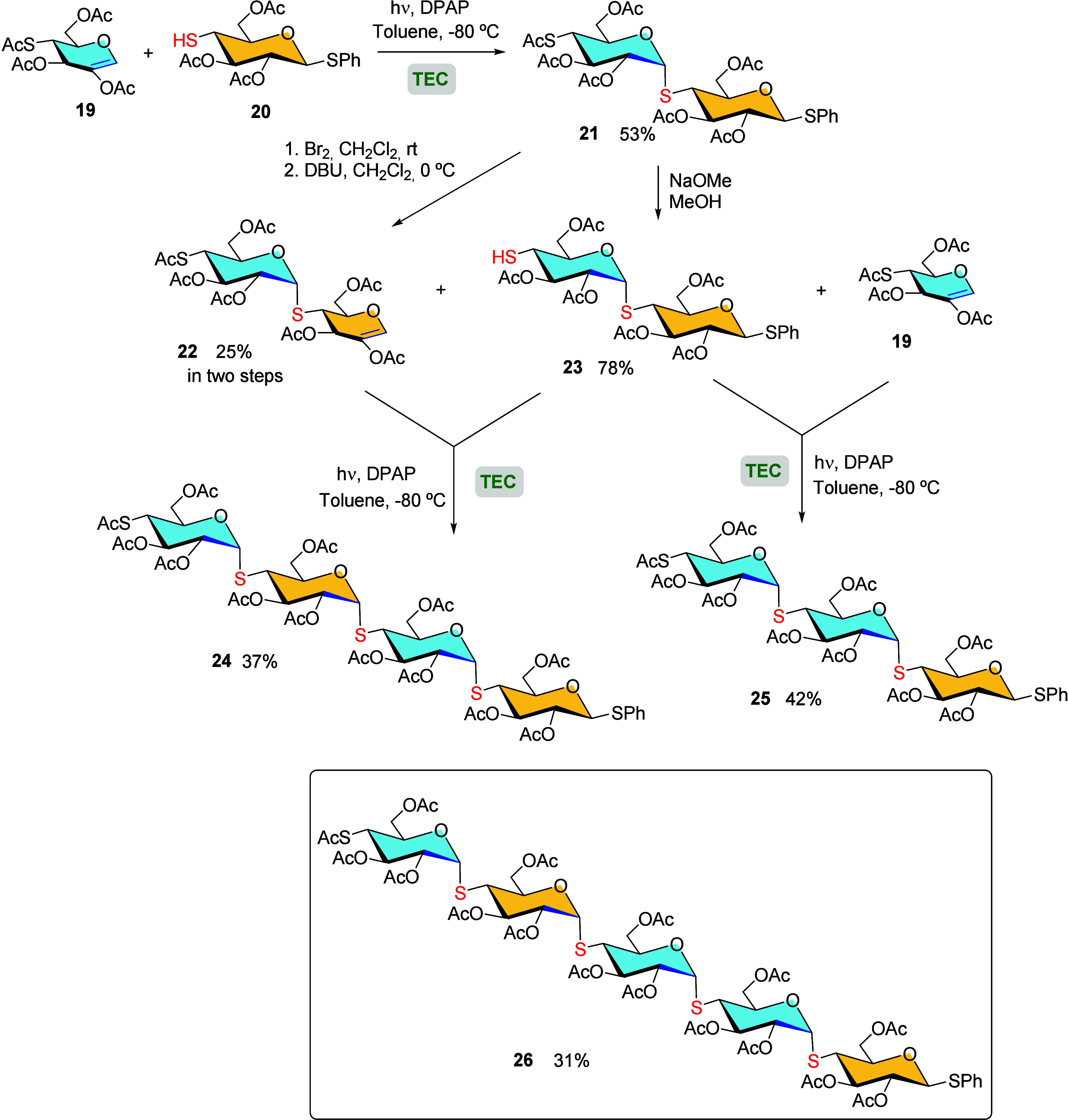
In a similar fashion, Lázár and co-workers reported the synthesis of more complex oligosaccharide homologues by photoinitiated TEC reaction with complete regio- and stereoselective control. The authors began preparing the α-S-linked disaccharide 21 through the free-radical addition of thiol 20 to 2-acetoxyglucal 19 in toluene at −80 °C, by irradiation at λmax = 365 nm in the presence of the photoinitiator DPAP (Scheme ). Subsequent, selective S-deacetylation of 21 to yield free thiol 23, followed by conjugation with another 2-acetoxyglucal 19 monosaccharide via the TEC reaction, allowed the effective creation of trisaccharide 25. Additionally, a tetrasaccharide structure was generated through the same TEC conditions to incorporate thiol disaccharide 23 into disaccharide 2-acetoxyglucal 22, which was prepared from 21 by regenerating the double bond under basic conditions. By following similar sequential reactions, we also achieved the rapid and efficient preparation of pentasaccharide 26 was also achieved.
4. Synthesis of Oligosaccharide Homologues by TEC.
2.2.2. Synthesis of Sugar-Peptides/Proteins via TEC
Glycopeptides play important roles in biology and medicine, serving as indispensable tools in fundamental biological processes, where the glycan and the peptidyl substructures often exhibit distinct and complex function, such as cellular recognition, adhesion, growth and differentiation. Moreover, aberrant glycosylation is associated with autoimmune and infectious diseases as well as cancer. The challenges in isolating glycopeptides or glycoproteins from natural sources have hampered efforts to elucidate the individual biological functions of glycoproteins, particularly when the precise structure of the glycan determines biological activity. Therefore, the development of new methods for linking sugars to peptides or proteins, with a well-defined structure, is an active area of research. Due to its mild conditions and high regioselectivity, the TEC reaction has become an effective synthetic approach for sugar-peptide or protein bioconjugation.
One of the first examples reported in the literature dates back to 2001, when Klaffke and co-workers described an elegant methodology for preparing N-linked neoglycopeptides 31a through a hydrotiolation reaction (Scheme A). The synthesis involved a photoinduced (254 nm) thiol–ene coupling of N-allyl glycosides 27a–c (α-d-Glc, β-d-Glc, β-d-GlcNAc and β-d-Gal) with cysteamine at room temperature, introducing a terminal amino-thioether spacer, followed by cross-linking of the corresponding amino group with Cbz-Gln-Gly dipeptide, catalyzed by microbial transglutaminase (TGase) to easily access to the desired neoglycopeptides.
5. (A) Synthesis of Neoglycopeptides 31a–b; (B) Synthesis of S-Glycopeptide 37 .

a Boc = t-butyloxycarbonyl. Cbz = (phenylmethoxy)carbonyl group. TGase = glutaminyl-peptide γ-glutamyl transferase.
b Fmoc = Fluorenylmethoxycarbonyl.
The versatility of the thiyl radical click reaction was also demonstrated by Scanlan and co-workers in a sequential combination of native chemical ligation and thiol–ene radical chemistry (NCL-TEC) as a novel methodology for rapidly accessing functionalized glycopeptides. The NCL reaction between Boc-protected alanine thioester and cysteine furnished dipeptide 30 in high yield. Then, the introduction of a galactose ring containing an anomeric terminal alkene 28 by the TEC reaction gave the desired thioether-linked compound 31b in 87% yield (Scheme A). In this instance, DPAP was employed as radical initiator, and the addition of 4-methoxyacetophenone (MAP) as photosensitizer significantly increased the reaction yield.
Dondoni et al. reported a complementary strategy to prepare S-glycopeptide 37 via TEC reaction, this time using a saccharide bearing a thiol group as the starting material. The coupling reaction between glycosyl thiols 3a–b and alkenyl glycine 32 by means of TEC, followed by the sequential incorporation of orthogonally protected amino acids into glycosylated TEC product 33, successfully afforded S-glycopeptide 37 (Scheme B).
O-Acetyl moieties in glycosyl thiols 3 were selected as protecting groups due to their compatibility with the photoreaction conditions. In addition, orthogonal protective groups in the amino acids were employed to obtain target S-glycosyl tripeptide 37, suitable for further elongation through peptide synthesis.
To simplify the synthesis process and reduce waste, Fairbanks and colleagues developed a method for preparing glycoconjugates that does not require protecting groups. Their approach is based on a combined two-click reaction process, which maintains selectivity and yield while eliminating the need for additional steps typically associated with protecting group strategies. First, the introduction of the alkene moiety into the sugar ring was achieved via CuAAC between the in situ formed glycosyl azide from aminosugar 38 and the allyl propargyl ether (Scheme ).
6. Synthesis of Glycopeptide 40 by Double-Click Approach .
a AMDP = 2-azido-1,3-dimethylimidazolinium hexafluorophosphate.
Then, the reaction of alkene-aminosugar 39 with glutathione afforded the corresponding glycopeptide 40 through thiol–ene click coupling in quantitative yield.
The scope of the TEC was expanded to more complex systems when Davis and co-workers first investigated the synthesis of S-linked glycoproteins in 2009, where site-specific ligation of glycosyl thiols to olefinic proteins was achieved. This approach utilized the incorporation of a non-natural amino acid, homoallylglycine (l-Hag), into a protein through gene sequence design, followed by a free-radical hydrothiolation reaction with β-GlcSH. This process produced glycoconjugate protein 41 in almost quantitative yields, while retaining the protein stability and full functionality (Scheme ). The reaction proceeded in aqueous solutions under irradiation at λmax = 365 nm with Vazo 044 as the initiator. The method’s versatility was demonstrated by using both protected and unprotected thiosugars, as well as a range of proteins with diverse structures. In addition, the authors extended the reaction conditions to self-assembled multimeric Qβ-(Hag 16) protein, confirming the complete glycoconjugation of all 180 olefins with excellent chemoselectivity. This effective and complete site-selective glycoconjugation offers significant potential for preparing glycoconjugate vaccines, where high levels of loading are desirable.
7. Protein S-Linked Glycoconjugation .
a Vazo 044 = 2,2′-azobis[2-(2-imidazolin-2-yl)propane] dihydrochloride.
Around the same time, Dondoni and co-workers reported a complementary strategy using the TEC to connect alkenyl C-glycoside to a peptide or protein containing SH-free cysteine groups. Initial studies demonstrated the accomplishment in TEC reaction between C-glycosides with an anomeric-allyl group and cysteine-containing peptides, such as the tripeptide glutathione (γ-Glu-Cys-Gly), using DPAP as radical initiator. The methodology was extended to couple C-galactoside 42 to thiol containing nonapeptide TALNCNDSL, and to globular bovine serum albumin protein (BSA), yielding glycoconjugates 43 and 44 respectively (Scheme ). Although native BSA is known to contain only one cysteine residue (Cys-34), spectrometric analysis of glycosylated protein 44 revealed the presence of three galactoside rings. The two additional sugar rings resulted from the TEC reaction between the alkene-sugar ring and sulfhydryl groups generated by disulfide bridge cleavage at positions 75–91.
8. (A) Preparation of TALNCNDSL Glycoconjugate 43; (B) Preparation of BSA Glycoconjugate 44 .

A few years later, Deming and co-workers described the synthesis of novel conformation-switchable glycopolypeptides 46, which undergo α-helix-to-coil transitions upon oxidation and exhibit exceptional water solubility in both conformational states. The preparation of glycopolypeptide 46 involved a polymerization process (Scheme ), where monomer fragments were designed to contain a stable thioether linkage introduced by TEC using C-linked glycosides and l-cysteine derivatives. The TEC reaction efficiently produced glycosylated amino acids 45 in high yield, containing the desired residues for subsequent polymer formation.
9. Synthesis of Glyco-C NCA Glycopolypeptides 46 Using TEC Reaction.

Another interesting application of TEC was the chemical neoglycosylation of collagen patches, which are promising materials for incorporating therapeutic strategies. Cipolla et al. described an efficient procedure for preparing glycan-functionalized collagen 48 via a thiol–ene approach between alkene-derived monosaccharides and the thiol-derived collagen 47. The reaction was carried out at room temperature in a MeOH:H2O (1:2) mixture under UV irradiation at 365 nm, with DPAP as the radical photoinitiator (Scheme ).
10. Thiol–Ene Reaction on Thiolated Collagen 47 .
A significant advancement was made by using the photochemical TEC for glycoconjugate vaccine preparation, as previously proposed by Davis’ group. In comparison to saccharide conjugation, coupling glycopeptide antigens to proteins is more challenging due to the variety of functional groups involved. However, Kunz et al. reported the orthogonal end efficient preparation of a cancer vaccine by combining a synthetic tumor-associated glycopeptide antigen with a carrier protein through TEC. Thiol functionalized glycopeptide 49 and alkyne-terminating BSA protein 50 were subjected to photoinduced thioether conditions, generating a thioether spacer in synthetic vaccine 51. The reaction proceeded in water at room temperature, and the Vazo 044 radical initiator significantly increased the reaction yield. An excess of thiol was necessary to provide the BSA conjugate vaccine 51, which contained an average of eight molecules per BSA molecule (Scheme ).
11. Photocatalyzed TEC-Ligation between a Thiolated Glycopeptide 49 and Allylated BSA Carrier 50 .

The nonimmunogenic thioether linker generated by this methodology offers a promising opportunity to create vaccines that stimulate the immune system against cancerous cells.
2.2.3. Synthesis of Glycodendrimers and Glycoclusters via TEC
Although protein–carbohydrate interactions are essential to many biological processes, individual interactions typically exhibit weak binding affinities. However, multiple interactions between multivalent ligands and receptors can significantly enhance the binding strength at the molecular scale. In this context, multivalent synthetic neoglycoconjugates with well-defined structures are potential inhibitors of natural oligosaccharide ligands and are useful tools for understanding such carbohydrate–protein interactions. The central core of a glycodendrimer serves as the focal point for the attachment of carbohydrate branches. This core can be composed of various materials, but it is typically a small organic molecule or a metal ion complex, which can influence the properties of glycodendrimers and functions.
Over the past decades, the photolytic thiol–ene reaction has emerged as a powerful method for synthesizing glycodendrimers. In 1997, Lindhorst and co-workers reported the first glycodendron synthesis using monosaccharides as multifunctional building blocks. The photoinduced TEC between perallylated α-d-glucopyranoside and cysteamine hydrochloride was carried out in MeOH, leading to a sugar-derived pentaamine core structure 52 in high yield. Further coupling with mannosyl isothiocyanate and subsequent O-acetyl deprotection afforded a thiourea-bridged pentaantennary glycocluster 53 (Scheme ). Years later, the same group employed a similar strategy, consisting of sequential thiol–ene/glycosylation/deprotection reactions, to prepare a new family of d-glucose-centered mannosyl clusters with valencies as high as 15 branches. Their affinities toward the mannose-specific lectin concavalin A (Con A) and their potential as inhibitors of type 1 fimbriae-mediated adhesion of to mannose polysaccharide was evaluated. It was concluded that a higher valency correlated with higher biological activity.
12. Synthesis of 5-Valent Glycocluster 53 via Sequential Thiol–Ene/Glycosylation Reactions.
Polyhedral oligosilsesquioxanes (POSS) hybrid scaffolds, a class of nanoscale cage-like structures composed of silicon and oxygen atoms, often with organic functional groups attached to the surface, offer a highly versatile platform for functionalization. These unique structures make them ideal candidates for synthesizing multivalent glycoclusters. Many efforts have been made to efficiently functionalize commercially available octavinyl POSS 54 with different saccharides via radical-photoinduced TEC. In 2004, Lee and co-workers successfully conjugated the thiol group of glycosyl γ-thiobutyramides 55 to cluster 54 in the presence of catalytic amounts of AIBN (Scheme ). The inhibitory effect of the prepared glycocluster 56 on human α1-acid glycoprotein by RCA120 (a β-galactose specific lectin) was evaluated, showing 200 times more activity than lactose, presumably due to the cluster effect of the binding of asialo-oligosaccharide. Dondoni and co-workers later studied the direct incorporation of 1-thio-β-d-glucose 58, without a suitable spacer, to the octavinyl-POSS 54 under standard TEC conditions with DPAP. Unfortunately, a mixture of partially glycosylated POSS products was detected, likely due to steric congestion around the octasilsesquioxane scaffold during the sequential attachment of thioglycoside residues. To address this limitation, the authors introduced a suitable arm between the saccharide and the thiol group. Thiopropyl α-d-C-glucopyranose or C-mannopyranose provided the corresponding glycocluster in high yields under similar TEC conditions. Alternatively, the authors proposed a procedure to space out the cluster backbone and the alkene groups consisting on: (i) HS-PEG fragment attachment to octavinyl-POSS 54 via photoinduced coupling, (ii) treatment of the octahydroxy functionalized POSS 57 to generate the corresponding alkene functionalities, (iii) treatment with PEG-linked octaene silsesquioxane with 1-thio-β-d-glucose 58 by means of TEC to successfully afford octavalent S-linked glycocluster 59 (Scheme ).
13. Synthesis of 8-Valent Glycocluster 56 and 59 Using POSS Platform and TEC Reaction.

A different central scaffold based on aromatic cores was described by Dondoni and Hawker. They described the regioselective introduction of thio-sugar residues to the fourth generation alkene functional dendrimer [G4]-ene48, based on the tris-alkene core 2,4,6-triallyloxy-1,3,5-triazineas as aromatic dendritic platform. The TEC reaction conducted in DMF under irradiation at λmax 365 nm in the presence of 2,2-dimethoxy-2-phenylacetophenone (DPAP), led to the quantitative conversion of all 48 alkene groups of the dendrimer within just 1 h. The resulting glycodendrimers 60, grafting 48 units of glucose, mannose, lactose or sialic acid, were isolated in excellent yields without the need of protecting groups (Figure ).
1.

Glycodendrimers 60 are based on an aromatic 48-valency dendrimer [G4]-ene48 central core.
Later, Dondoni and co-workers applied a similar methodology to produce globular glycodendrimers holding sugar fragments via flexible thioglycosidic linkages by photoinduced coupling of 2-acetamido-2-deoxy-1-thio-β-d-glucose (GlcNAc-SH) to alkene functional polyester-based dendrimers. ELLA-based bioassays of the prepared glycodendrimers demonstrated excellent binding properties toward wheat germ agglutinin (WGA) compared to the monosaccharidic GlcNAc used as monovalent reference.
An efficient protocol to prepare multivalent flexible trithiomannoside clusters 65, which have been shown enhanced antimicrobial activity against Gram-negative bacteria, was reported by Chan-Park et al. The methodology involved a 3-step process: (i) coupling of tri-O-allyl compound 61 and 2,3,4,6-tetra-O-acetyl-1-thio-α-d-mannopyranose under TEC conditions using dimethoxyphenyl acetophenone (DMPA) as radical initiator, followed by Boc and acetyl deprotections to afford free amine trithiomannoside cluster 62, (ii) amide coupling with the acid group in 63 using EDC·HCl and HOBt to obtain the tri-O-allyl terminated glycocluster 64, (iii) finally, the terminal olefins were subjected to thiol–ene click reaction with 2,3,4,6-tetra-O-acetyl-1-thio-α-d-mannopyranose to give, after deacetylation, bis-trithiomannoside cluster 65 (Scheme ).
14. Synthesis of 6-Armed Glycodendrimer 65 .

Cyclodextrins (CDs) are naturally occurring cyclic oligosaccharides that have been extensively studied due to their ability to form inclusion complexes with various guest molecules. Their unique structure, with a hydrophobic cavity and a hydrophilic exterior, allows them to encapsulate hydrophobic guest molecules, thereby improving their solubility, stability, and bioavailability. This property has been widely exploited in pharmaceutical drug formulations to enhance the delivery of poorly soluble drugs. However, the perfunctionalization of CDs is indeed a challenging task because of their complex structure. The TEC reaction offers advantageous properties for introducing new functionalities onto O-per-allylated cyclodextrins. In 1998, Roque and co-workers prepared polyanionic and polyzwitterionic cyclodextrins-based compounds as potential inhibitors of HIV transmission by radical addition of thiomalic acid or mercaptopropionic acid onto perallylated cyclodextrins (CDs) under UV irradiation with a catalytic amount of AIBN. Years later, Stoddart and co-workers used the TEC to decorate, either or both the primary and the secondary faces, of O-per-allylated cyclodextrins 66a with glycosyl thiols to efficiently access carbohydrate free clusters 68a in good yields after easy removal of protecting groups (Scheme ).
15. Preparation of Glycodendrimers 66a–b by TEC Reaction.

Calix[4]arenes have emerged as ideal central cores for preparing glycodendrimers due to their unique structural features. They are cyclic molecules composed of four aromatic rings connected by methylene bridges and provide a stable and well-defined platform. The first synthesis of calix[4]arene-based S-glycoclusters via photoinduced multiple TEC was reported by Dondoni and co-workers in 2009. Reaction of alkene-functionalized calix[4]arenes 66b with sugar thiols by irradiation at λmax = 365 nm in CH2Cl2 using DPAP as the sensitizer afforded the octavalent S-glycoside cluster 68b in good yield (Scheme ).
So far, the examples shown in this review refer to the synthesis of multivalent homogeneous glycoclusters via TEC reaction. However, the recognition process in nature often involves multiple types of sugar ligands. Therefore, the preparation of heteroglycodendrimers, where different types of sugar ligands are attached to the same dendritic scaffold, is crucial for understanding the roles of specific sugar moieties in biological recognition, and many efforts have recently been made in this context. García Fernándeźs group prepared heptavalent heteroglycoclusters 73 by subsequent photoinduced radical addition (Scheme ). Initially, two units of per-O-acetylated 1-thiosugars (yellow colored in scheme) were added using TEC reaction to the tri-O-allylated pentaerythritol derivative 69 by irradiation at 250 nm in MeOH. The relative quantities of the sugar moiety must be carefully regulated to achieve specific outcomes, leading to either mono- or di- derivatives. Then, divalent derivatives 70 were subjected to the same TEC conditions using a different thiosugar (blue colored in scheme), resulting in the effective formation of bifunctional ligands 71. The remaining primary hydroxy group was further converted into an isothiocyanate group, which was coupled to the face-selective functionalized per(C-6)-heptacysteaminyl-β-CD derivative through thiourea bridges. Subsequent deacetylation led to the heptavalent heteroglycoclusters 73 containing both α-Man β-Glc and α-Man β-Lac saccharides. Biological assays showed significantly higher binding affinity of heterocluster 73 to Con A compared to analogous homogeneous conjugates with the same number of mannose units. This higher binding affinity suggests that the presence of multiple sugar types in the heteroglycoclusters leads to more effective clustering effects, resulting in enhanced interactions with Con A on a mannose molar basis (up to 8-fold increase in affinity).
16. Preparation of Heteroglycoclusters 73 via TEC.

Later the authors extended their work on heteroclusters, and other research groups have also contributions to the development of efficient methodologies for preparing heteroclusters. ,
We cannot overlook the thiol–yne coupling (TYC) reaction, which has emerged as an important and widely utilized synthetic method. The reaction involves the coupling of one or two thiols across a C–C triple bond via free-radical chain mechanism (similar to TEC). Due to its compatibility, efficiency, and mild reaction conditions, TYC continues to be an active area of research with potential applications in bioconjugation chemistry.
In summary, the thiol–ene coupling reaction has gained significant attention due to its efficiency, selectivity, high yield, and fast reaction kinetics, making it a powerful tool for developing new glycoconjugates and biomaterials. Bioconjugation using this methodology has enabled the introduction of glycans to various sensitive biomolecules in the presence of a wide range of functional groups found in nature, confirming the reaction’s biocompatibility. Moreover, TEC is a biologically friendly coupling reaction that requires benign radical initiators, avoiding the use of toxic metal catalysts or reagents. However, the potential use of this methodology in vivo experiments is limited by the undesired reaction with thiol functions in cells, and the low tolerance of cells to high energy UV irradiation. Therefore, the bioorthogonality of the thiol–ene coupling is limited, and alternative strategies should be considered for bioconjugation in living organism.
3. Strain-Promoted Azide-Alkyne [3 + 2] Cycloaddition (SPAAC)
3.1. Introduction
In early 2002, Meldal and colleagues developed a catalytic version of the classical Huisgen 1,3-dipolar cycloaddition, , which requires copper(I) salts and terminal alkynes, producing stable 1,4-disubstituted 1,2,3-triazoles in a rapid and regioselective manner through a stepwise mechanism (Scheme A). This reaction, known as CuAAC, perfectly aligns with the criteria established by Sharpless, Kolb, and Finn in 2001 for a click-type reaction. Despite its widespread use and effectiveness demonstrated across various research fields, including chemical biology and drug design, the use of CuAAC for biological and medicinal applications is considerably restricted by the cytotoxicity of copper. In this sense, the development of a metal-free [3 + 2] cycloaddition that retains the advantageous properties of CuAAC while eliminating the necessity for metal catalysts is particularly essential. Bertozzi et al., − and later other authors, , developed a version of the [3 + 2] cycloaddition between organic azides and strained cyclooctynes as a bioorthogonal alternative to CuAAC (Scheme B). This reaction, named SPAAC, can be performed efficiently under physiological conditions and in living organisms, tolerating the presence of various functional groups commonly found in biological systems. Therefore, this approach meets the criteria for bioorthogonality, addressing the limitations that other click reactions, such as the TEC discussed earlier in this review. SPAAC offers high selectivity, versatility, biocompatibility, and fast reaction kinetics.
17. Comparison of the CuAAC and the SPAAC Reactions.
Despite SPAAC embodying the core principles of a click transformation, this approach has some drawbacks compared to Cu-catalyzed [3 + 2] cycloaddition: (i) the lack of regioselectivity, resulting in two possible regioisomers in similar ratios; (ii) the synthetic complexity of cyclooctynes, which shows a clear relationship between reactivity and synthetic difficulty; and (iii) the need to functionalize cyclooctynes for conjugation with other molecules or probes.
Cyclooctynes present a fine equilibrium between stability and reactivity, making them well-suited for SPAAC reactions. ,, A plethora of structurally diverse cyclooctyne scaffolds have been prepared and studied for cycloaddition with azides; however, discussing all the cyclooctyne variants that have been applied in SPAAC reactions goes beyond the scope of this review. ,
The desirable features mentioned above have led to increased interest in applying SPAAC in carbohydrate chemistry for the bioorthogonal functionalization of biomolecules with probes in biological systems and living organisms as well as for the efficient preparation of glycoconjugates and glycomimetics under mild conditions.
3.2. Applications
3.2.1. SPAAC Applications for in Vivo Cell Imaging
Bioorthogonal chemical reactions that enable rapid and selective biomolecule labeling in living organisms have become powerful tools for probing biological processes in vivo. Apart from Staudinger ligation, − there are very few examples of reactions that meet the bioorthogonality requirement, and among these, the SPAAC has recently emerged as the best candidate for the covalent direct conjugation of biomolecules with probes in biological systems and living organisms. In 2006, Bertozzi et al. first developed the bioorthogonal chemical reporter strategy to visualize cell-surface glycans, which cannot be easily visualized using standard molecular imaging tools, in a two-step procedure: (i) selected azide-functionalized monosaccharides are metabolized by cells and subsequently incorporated into cell-surface glycans, a process known as metabolic oligosaccharide engineering; and (ii) the azide-containing glycans are then reacted with an imaging probe-conjugated cyclooctyne through Cu-free click chemistry, enabling visualization of cell-surface azidosugars (Scheme ). A series of carbohydrates have been used as starting material to prepare azido-containing carbohydrates (ManNAz, SiaNAz, GalNAz, XylAz and FucAz) as metabolic precursors for cell labeling via the SPAAC reaction to image glycans in living systems.
18. Imaging Cell-Surface Azidosugars with Cyclooctyne Probes via SPAAC Reaction.

In this context, Bertozzi’s group applied the bioorthogonal [3 + 2] cycloaddition to rapid and selective label live cell surfaces glycans containing sialic acids, a family of negatively charged monosaccharides frequently expressed as external terminal residues on cell-surface. The metabolic incorporation of N-azidoacetyl sialic acid (SiaNAz) into cell-surface glycoproteins was achieved by treatment with Ac4ManNAz, and the resulting azide-labeled cells were then reacted with a series of fluorescent probe-conjugated cyclooctynes DIFO-R via a Cu-free click reaction (Scheme ).
19. Metabolic Labeling and Visualization of Cell-Surface Glycans Using Ac4ManNAz and Different Functionalized Cyclooctynes with Fluorescent Probes.

The DIFO (difluorinated cyclooctyne) reagent, containing two electron-withdrawing fluorine atoms, dramatically enhanced the selectivity and rate of the cycloaddition reaction compared to those of other cycloalkynes and other bioorthogonal ligations. Additionally, control experiments showed similar kinetic rates for the SPAAC reaction compared to the CuAAC version, performing effectively on live Jurkat cells. More interestingly, this nontoxic SPAAC approach enabled dynamic multicolor imaging of biochemical processes, and the internalization and trafficking of a population of labeled sialoglycoconjugates in live Chinese hamster ovary (CHO) cells were effectively monitored.
Around the same time, Boons et al. described a similar study to Bertozzi’s using 4-dibenzocyclooctynol (DIBO) as the crucial agent to which fluorescent probes were attached (Scheme ). The aromatic moieties in DIBO provide additional ring-strain, increasing its reactivity with azides via SPAAC reaction compared to nonaromatic cyclooctyne analogues. Cells were cultured in the presence of Ac4ManNAz, resulting in the metabolic incorporation of SiaNAz into their cell-surface glycoproteins. This was followed by the SPAAC reaction with biotin-based DIBO and subsequent treatment with avidin-Alexa Fluor 488. This two-step cell-surface labeling approach showed higher kinetic rates and fluorescence intensities than those observed for the Staudinger ligation in a comparative study enabling the monitorization in real time of the trafficking of glycoproteins in living CHO cells. Years later, the authors described two modified DIBO structures incorporating a ketone (74a) or oxime group (74b) and evidenced that the biotin-modified 74b and DIBO were useful probes to determine relative quantities of cell surface sialylation of wild-type and mutant cells (Scheme ). Successfully, the SPAAC reaction of biotinylated DIBO reagents with metabolically labeled azido-bearing monosaccharide not only allowed the determination of relative quantities of sialic acid of living cells but also, in combination with lectin staining, revealed defects in the glycan structures of glycoproteins (Lec CHO cells).
Van Delft and co-workers applied the bioorthogonal SPAAC labeling methodology to study the bioavailability and tolerability of imaging surface glycans on living human melanoma MV3 cells, a class of highly invasive and metastatic cells in which the abundant production of surface glycans has been reported to play a role in invasion processes. MV3 melanoma cells were incubated with Ac4ManNAz, labeled with BCN-biotin conjugate through a nontoxic SPAAC protocol, and stained with streptavidin-Alexa Fluor 488 to visualize the redistribution of glycans during invasive cell migration. It is noteworthy that the synthesis of bicyclo[6.1.0]nonyne (BCN) was straightforward and high yielding and exhibited relative stability. Moreover, the free metal cycloaddition reaction between BCN-biotin and glycan azides proceeded in excellent kinetic rates and led to a single regioisomer, due to the symmetric structure of BCN (Scheme ).
More recently Cheng et al. designed two novel derivatives of the above-mentioned Ac4ManNAz that incorporate fatty acid esters (C6 and C12) on the anomeric hydroxyl group. These derivatives were encapsulated in a liposome delivery system to improve the chemical stability and the cell labeling efficiency (Scheme ). Both Ac4ManNAz analogous 75a and 75b showed enhanced chemical stabilities and strong fluorescence intensity after forming the triazole rings using the azadibenzocyclooctyne DBCO-Cy5 fluorescent probe via the SPAAC reaction. However, the metabolic labeling efficiency on MDA-MB-231 cells was retained compared with Ac4ManNAz and appeared to be dependent on the length of the ester on the anomeric carbon, with compound 75b performing better.
20. Metabolic Cell Labeling and Imaging Using Liposomal Azido Mannosamine Lipids.

3.2.2. SPAAC Applications for Living Organism Imaging
Apart from living cells, other more complex organisms have been used to study the efficiency of bioorthogonal reactions. Bertozzi et al. explored the SPAAC protocol for noninvasive imaging of glycans in live zebrafish during embryogenesis using fluorophore-DIFO conjugates. Ac4GalNAz was used as a metabolic label precursor to selectively incorporate azide groups into cell-surface glycans of zebrafish embryos. The metabolically labeled mucin-type O-glycans were subsequently reacted with fluorescent probe-conjugated DIFO via Cu-free click chemistry, allowing for the visualization of glycans in vivo at subcellular resolution (Scheme ). This approach showed spatiotemporal changes in glycan distribution throughout zebrafish embryogenesis. Further investigations enabled the visualization of glycans dynamics in the enveloping layer during the early stages of embryogenesis, as early as 7 h post fertilization, using this methodology.
21. Noninvasive Imaging Strategy of Glycans in Live Developing Zebrafish.
Prompted by the involvement of fucosylation in many developmental processes in living organisms, Bertozzi’s group explored the monitoring of fucosylated glycans in developing zebrafish using a strategy previously mentioned. The administration of an unnatural azide-functionalized fucose derivative, such as FucAz or FucAz-1-P, as metabolic substrates to label glycoproteins was inefficient, despite the presence of fucose salvage pathway enzymes during zebrafish embryogenesis (Scheme ). This limitation was overcome by using the nucleotide sugar GDP-FucAz, a substrate for fucosyltransferase enzymes (FucTS), which allowed the incorporation of FucAz into glycoproteins in the Golgi lumen. These modified glycoproteins were then exported to the cell surface. To visualize FucAz incorporated into cell-surface glycans, the azide was reacted with the DIFO-488 fluorescent probe, allowing for the successful imaging of fucosylated glycans, which are difficult to monitor in vivo in the enveloping layer of zebrafish embryos during early development.
22. Metabolic Labeling of Fucosylated Glycans .

a FUK = fucose kinase, FucAz-1-P = FucAz-1-phosphate, FPGT = fucose-1-phosphate guanyltransferase, GDP = guanosine diphosphate.
Later, the same group again used a developing zebrafish as a model system to design a chain-terminator of glycosaminoglycan (GAG) biosynthesis, which represents the carbohydrate fraction of proteoglycans with crucial biological functions in animals (Scheme A). Taking into account that heparan sulfate (HS) and chondroitin sulfate (CS) glycosaminoglycans contain a conserved xylose residue (in blue in the scheme) that initiates the polysaccharide chain from the protein backbone, the authors metabolically replaced this monosaccharide with an unnatural azide-bearing xylose (4-XylAz) residue as a chemical chain-truncating analogue to probe GAG functions during zebrafish embryogenesis. Zebrafish embryos were exposed to UDP-4-azido-4-deoxyxylose (UDP-4-XylAz) to facilitate its incorporation into sites of GAG glycosylation, and the resulting embryos were allowed to develop (Scheme B).
23. (A) Heparan Sulfate and Chondroitin Sulfate Structures; (B) Visualization of the GAG Inhibition Site Using a Fluorescent Probe-Conjugated Cyclooctyne .
a UDP= uridine diphosphate.
The azide labeled zebrafish embryos were then reacted with difluorocyclooctyne DIFO-AlexaFluor 488 to enable the rapid, efficient, and selective visualization of the GAG inhibition site in vivo through the SPAAC reaction. The copper-free click chemistry approach supplements genetic strategies for studying GAG function in living systems.
To extend the scope of the biorthogonal SPAAC approach for imaging metabolically labeled glycoconjugates in living systems, Bertozzi’s group first applied this strategy in the context of human tissue cultured ex vivo in 2019. High levels of sialylated glycans have been found in many types of cancer cells; however, the specific glycoproteins involved in cell-surface sialylation are not well characterized in human disease tissue. Therefore, the authors monitored glycoproteins located at the surface of cancerous prostate tissues using the mentioned methodology. Ac4ManNAz was again used as a biosynthetic precursor of azidosialic acid to be metabolized and incorporated into cell surface as well as secreted sialoglycoproteins of both normal and cancerous prostate tissues. Biotinylation followed by mass spectrometry techniques allowed the identification of the cell surface and secreted glycoproteins. It was found that cancerous prostatic tissues contained exceptionally high levels of glycoproteins compared to normal tissues. These studies established the utility of the SPAAC reaction as an essential part of metabolic labeling strategies to address questions of biomedical relevance.
Similarly, the SPAAC approach has been applied to other more complex living systems such as mice. Ac4ManNAz was metabolically incorporated in live mice to label their cell-surface sialic acids with azides, followed by the reaction with various cyclooctyne-FLAG peptide probes (Scheme ). After the injection of cyclooctynes, labeled glycoconjugates were observed in various tissues, including the intestines, heart and liver, with no apparent toxicity. DIFO was identified as the cyclooctyne with the best intrinsic reactivity, although its bioavailability was a concern due to significant observed serum albumin binding. These studies establish SPAAC as an alternative bioorthogonal reaction to the Staudinger ligation that can be applied in live mice.
24. SPAAC in Mice .
a Mice were treated with Ac4ManNAz for metabolic labeling of glycans with SiaNAz. A series of cyclooctyne-FLAG conjugates were assayed for in vivo covalent labeling of azido glycans. R = FLAG peptide.
Although the mammalian brain is an organ rich in sialoglycans that play key roles in brain development, cognition, and disease progression, in vivo visualization of sialoglycan biosynthesis is a challenge due to the blood–brain barrier (BBB). A significant advance in this area was made by Chen et al., who designed a liposome-assisted bioorthogonal reporter (LABOR) strategy via SPAAC for metabolic labeling and visualization of brain sialoglycans in living mice. The authors demonstrated that liposomes encapsulating 9-azido sialic acid (9AzSia) can cross the BBB, delivering the azidosugar into the brain for the metabolic labeling of sialoglycoconjugates (Scheme ). The resulting 9AzSia-labeled glycoconjugates were subsequent reacted with azadibenzocyclooctyne-Cy5 conjugate (DBCO-Cy5) as a fluorescent probe through Cu-free click chemistry, leading to the fluorescence imaging of brain sialoglycans in living mice and brain sections. The LABOR strategy enabled in vivo visualization of brain sialoglycan turnover, which is spatially regulated in distinct brain regions.
25. Liposome-Assisted Bioorthogonal Reporter (LABOR) Strategy via SPAAC in Living Mice.

3.2.3. Synthesis of Glycofullerenes and Glycovaccines via SPAAC Reaction
The SPAAC reaction has also been applied to the preparation of multivalent systems based on hexakis-adducts of [60]fullerene bearing multiple carbohydrate units, which are crucial for biological recognition processes, where multivalent presentation is essential. Martín et al. were the first to describe the use of [60]fullerene to prepare orthogonally nonsymmetric click-adducts containing both amino acid and monosaccharide units of biological relevance through a thiol-maleimide conjugation-SPAAC sequence (Scheme ). This one-pot protocol allowed for the efficient preparation of mixed adducts that combined two different biomolecules.
26. One-Pot Thiol-Maleimide Conjugation-SPAAC Sequence to Prepare Amino Acid-Monosaccharide [60]Fullerene.

The same authors advanced this concept by designing a series of antivirals using the SPAAC approach, targeting the blockade of carbohydrate receptors as a novel strategy to inhibit the viral infection process. , They prepared a water-soluble tridecafullerene bearing 360 mannobioside units, which was tested to block DC-SIGN, a receptor involved in the entry of virus such as ZIKV and DENV into the cells. The results showed the best IC50 values reported to date for both viruses (67 pM for ZIKV and 35 pM for DENV). This strategy highlights the utility of the SPAAC reaction for the development of new antivirals against ZIKV, especially since there is currently no approved specific antiviral drug for treating of ZIKV infections.
Another interesting application of the SPAAC reaction in carbohydrate chemistry for therapeutic purposes is the preparation of carbohydrate-based vaccines. Adamo et al. reported a two-step protocol for the covalent conjugation of a polysaccharide antigen, functionalized with a cyclooctyne moiety, to a protein, which is conveniently modified with an azido-linker 76 (Scheme ). Initial studies reacted small-medium-sized glycans bearing a monofluorinated cyclooctyne (MFCO) arm with predetermined tyrosine residues of the CRM197 carrier protein 77, having an azide-linker via free-metal cycloaddition. The corresponding glycoconjugates vaccines were efficiently obtained with defined attachment points. Once the efficiency of this approach was proven, the same authors prepared a series of streptococcal polysaccharides bearing MFCO groups for the chemoselective conjugation to azide-containing Group B Streptococcus (GBS) pilus proteins as vaccine antigens, enabling the coupling of streptococcal polysaccharides. This technology provides an adequate strategy for selectively incorporating carbohydrates into proteins in the preparation of efficacious vaccines.
27. Tyrosine-Ligation SPAAC Reaction for the Synthesis of Glycoconjugates.

3.2.4. SPAAC Application for Developing Lysosome-Targeting Chimeras (LYTACs)
Targeted protein degradation (TPD) has emerged as a promising strategy for therapeutic development and a powerful tool in chemical biology. Among TPD approaches, proteolysis targeting chimeras (PROTACs) are notable for leveraging the ubiquitin-proteasome system (UPS) to selectively degrade intracellular proteins. This capability allows researchers to investigate biological pathways and cellular degradation mechanisms, particularly for cancer treatment. However, PROTACs are limited, because they cannot target extracellular proteins, restricting their therapeutic applications. Since extracellular and membrane proteins constitute about 40% of the proteome and play key roles in various diseases, lysosome-targeting chimeras (LYTACs) have been developed to address this gap. LYTACs, inspired by PROTACs, are cutting-edge bifunctional molecules that direct extracellular and membrane-bound proteins to lysosomes for degradation. They are composed of an antibody target protein binder and a lysosome-targeting receptor binder (Figure A). This strategy allows the selective breakdown of proteins that traditional UPS-based methods cannot reach, broadening the therapeutic potential of TPD to include diseases involving extracellular and membrane proteins.
2.
LYTACs use a glycopolypeptide ligand targeting CI-M6PR, conjugated to an antibody, to direct secreted and membrane-associated proteins to lysosomes.
Bertozzi’s group was the first to develop LYTACs using the SPAAC reaction, focusing on the cation-independent mannose-6-phosphate receptor (CI-M6PR). Their initial LYTACs linked a mannose-6-phosphate-based polyvalent ligand to an antibody that targets a specific protein for degradation. This complex then binds to CI-M6PR, resulting in the internalization of the complex and its delivery to the lysosome for degradation (Figure B).
To enhance the multivalent presentation, they synthesized a glycopolypeptide (Poly(M6Pn-co-Ala) with multiple serine-O-mannose-6-phosphonate (M6Pn) residues (Scheme ). This process began with the conversion of mannose pentaacetate into N-carboxyanhydride (NCA)-derived glycopolypeptides (M6Pn-NCA) through a 13-step synthesis. Subsequent copolymerization of M6Pn-NCA and alanine-NCA resulted in an M6Pn glycopolypeptide (Poly(M6Pn-co-Ala). To conjugate the Poly(M6Pn)-bearing glycopolypeptide to an antibody, the authors labeled Poly(M6Pn-co-Ala) with bicyclononyne to obtain Poly(N6Pn-co-Ala)-BCN and then coupled it to the antibody (previously functionalized with an azide group) through SPAAC (Scheme ). They first targeted EGFR protein, a known driver of cancer proliferation that functions beyond receptor tyrosine kinase activity inhibition. LYTACs were constructed using cetuximab (ctx), an FDA-approved EGFR-blocking antibody, and they were capable of effectively degrading EGFR protein.
28. Synthesis of LYTACs Using SPAAC Reaction.

Furthermore, the same group developed a second generation of LYTACs using the SPAAC reaction, targeting the liver-specific asialoglycoprotein receptor (ASGPR). ASGPR, a lectin expressed on liver cells, recognizes glycoproteins bearing N-acetylgalactosamine (GalNAc) or galactose ligands, internalizing them via endocytosis, followed by lysosomal degradation. Therefore, LYTACs using GalNAc ligands were developed to engage ASGPR, achieving higher internalization efficiency due to elevated expression of ASGPR in hepatocytes. Initially, triantennary GalNAc ligands (Tri-GalNAc-DBCO) were synthesized in 8 steps from peracetylated GalNAc and then conjugated to antibodies via SPAAC methodology (Scheme ), demonstrating effective degradation of EGFR in hepatocellular carcinoma cell lines.
This study underscores the potential of GalNAc-LYTACs for targeted protein degradation, particularly in treating diseases such as hepatocellular carcinoma with high specificity and minimal off-target effects. By utilizing liver-specific receptors like ASGPR, the therapeutic scope of LYTACs is expanded, paving the way for new treatments and insights into cellular biology.
3.2.5. SPAAC Combine with Biocatalysis for the Preparation of Glycoconjugates with Therapeutic Interest
The SPAAC reaction and biocatalysis have rapidly grown, driven by the development of robust biocatalysts and the widespread use of efficient click reactions. This convergence has given rise to “bioclick chemistry”, which combines biocatalytic enzyme activity with reliable click reactions for green and sustainable synthesis of high-value molecules. This section will highlight two examples of bioclick chemistry, focusing on applications in glycochemistry and sustainable synthesis.
In the first example van Delft et al. established a nongenetic technology termed GlycoConnect based on the conversion of native monoclonal antibodies (mAbs) into Antibody–Drug Conjugates (ADCs) through a combination of enzymatic synthesis with a SPAAC (Scheme ). This three-step protocol involves: (i) trimming a native mAb 78, which is a mixture of glycoforms at Asn-297, with an endoglycosidase to cleave the GlcNAc–GlcNAc linkage, resulting in structure 79; (ii) the catalytic attachment of an azide-modified GalNAc moiety using a glycosyl transferase to generate 80; and (iii) anchoring the bicyclononyne-modified toxic payload component (trastuzumab and maytansine) via the SPAAC reaction, yielding the corresponding ADC 81 with high stability and homogeneity. This GlycoConnect technology is very promising as a targeted therapy with a superior therapeutic index.
29. Development of GlycoConnect Based on Bioclick Chemistry Combining Biocatalytic Synthesis with SPAAC Reaction .
a UDP = uridine diphosphate.
Another example of bioclick chemistry was reported by Bojarová et al., who developed biocompatible glyconanomaterials based on N-(2-hydroxypropyl)methacrylamide (HPMA) copolymers for the specific targeting of galectin-3 (Gal-3). This protein is a promising target in cancer therapy because it is abundantly localized in tumor tissue and plays a crucial role in tumor development and proliferation. However, the clinical application of Gal-3-targeted inhibitors is often challenged by issues of insufficient selectivity and low biocompatibility. The authors envisioned HPMA-based nanocarriers pending Gal-3-targeted inhibitors as attractive glyconanomaterials for in vivo applications due to their good water solubility, low toxicity, and lack of immunogenicity. The enzymatic synthesis of a specific functionalized GalNAcβ1 → 4GlcNAc (LacdiNAc) epitope pending an azide group was accomplished by mutant β-N-acetylhexosaminidases (Scheme A). Then, the biocompatible HPMA copolymer decorated with cyclooctyne functionalities was combined with the Gal-3 specific epitope LacdiNAc by SPAAC to effectively target Gal-3 (Scheme B).
30. Development of Glycomaterials Based on Bioclick Chemistry.

In summary, the SPAAC, first explored by Bertozzi and later by other researchers, has emerged as the best candidate for the bioorthogonal functionalization of biomolecules with probes in biological systems and living organisms, due to its extreme selectivity, biocompatibility, and fast rate kinetics. The SPAAC reaction has established itself as a powerful tool for bioconjugation, enabling the in vivo visualization of glycan dynamics in various biological and pathological processes. Moreover, the remarkable properties of SPAAC have allowed its applications in fields such as biomedicine, material science, bioengineering, and nanotechnology, among others. For this reason, its potential is still being explored, promising further advancements and applications in all of these areas.
4. Inverse Electron-Demand Diels–Alder (IEDDA) Reaction
It is also worth mentioning the inverse electron-demand Diels–Alder (IEDDA) reaction as another prominent example of a biorthogonal ligation method. First introduced in 2008 by Blackman et al. has become widely recognized for its versatility across various fields of chemistry and chemical biology. This reaction, based on the classical Diels–Alder cycloaddition mechanism, has gained prominence due to its ability to selectively and efficiently couple functional groups in complex biological environments. Originally developed to overcome the limitations of traditional Diels–Alder reactions in biological systems, the IEDDA reaction has recently emerged as a powerful tool in carbohydrate modification. It involves a rapid and selective cycloaddition between an electron-deficient diene (such as a tetrazine) and an electron-rich dienophile (such as a strained alkene). This reaction proceeds under mild conditions with exceptional properties, making it well suited for applications in glycoscience (Table ).
One of the most relevant applications of the IEDDA reaction is in metabolic glycoengineering, which is a powerful approach for modifying cell surface glycans to study their biological functions, track glycan dynamics, and develop glycan-based therapeutic approaches. A major challenge in this field is the need for highly selective and rapid bioorthogonal reactions that enable the efficient labeling and functionalization of glycans in live cells. The IEDDA reaction meets these demands due to its exceptional kinetic efficiency, biocompatibility, and specificity. It proceeds without interference from native biomolecules, making it ideal for glycan labeling, live-cell imaging, and targeted drug delivery. The first example was reported in 2012 by the Prescher group, involving a sialic acid modified with a methyl-substituted cyclopropene (9-Cp-NeuAc). This derivative was metabolically incorporated into glycans on the surface of Jurkat cells and labeled through a two-step process using tetrazine-biotin, followed by an avidin-dye conjugate. Incorporation efficiency was assessed via flow cytometry (Scheme ). Additionally, some cyclopropene moieties were integrated into cell surface structures and subsequently detected by using covalent probes. Due to their small size and high selectivity, cyclopropenes hold great potential for tagging a wide range of biomolecules in vivo.
31. Cyclopropene-Modified Sialic Acid (9-Cp-NeuAc) Can Be Metabolically Incorporated onto Live Cell Surfaces Using IEDDA Reaction.

Another remarkable application of the IEDDA reaction is the development of injectable polysaccharide-based hydrogels. These materials have attracted significant attention in biomedical research because of their biocompatibility, biodegradability, and minimally invasive therapeutic interventions. Several polysaccharides, such as hyaluronic acid, alginate, chitosan, cellulose, and heparin, have been functionalized with the telechelic groups required for the IEDDA reaction. These modifications, which introduce dienes (primarily tetrazines) and dienophiles (such as norbornenes and trans-cyclooctenes) at low degrees of substitution, preserve the water solubility of the polysaccharides. When aqueous solutions of tetrazine-modified polysaccharides interact with dienophile-functionalized counterparts, rapid gelation occurs within minutes.
5. Sulfur(VI) Fluoride Exchange Reaction (SuFEx)
5.1. Introduction
Despite SPAAC and TEC being the main metal-free click reactions, this concept extends to other processes, including hetero-Diels–Alder cycloaddition, thiol-Michael addition and oxime ligation, among others. Recently, sulfur(VI) fluorine exchange (SuFEx) has emerged as a novel type of click reaction, although it was identified long ago. This reaction involves sulfonyl fluorides or fluorosulfates reacting with various nucleophiles to effectively form R-SO2–Y/RO–SO2–Y (Y = N, O) type linkages. Special focus has been given to sulfonamide group formation, as it is a crucial structural motif found in multiple therapeutic agents. ,
The formation of S–O bonds via SuFEx coupling is achieved from the corresponding sulfonyl fluorides or fluorosulfates using silyl ethers as nucleophiles in the presence of a non-nucleophilic base. The formation of the strong Si–F bond in the silyl fluoride byproduct drives the reaction. Similarly, the sulfonamide bond is successfully prepared by reacting sulfonyl fluoride with an excess of amine to neutralize the HF released during the reaction.
This novel reaction offers an alternative to the common nucleophilic addition of C-, N-, and O-nucleophiles to highly reactive sulfonyl chlorides, which are quite unstable under reductive and basic conditions. The success of SuFEx chemistry relies on the exceptional properties of sulfonyl fluorides, including: (i) ease of preparation, (ii) inertness to oxygen and water, (iii) hydrolytic stability under acidic and basic conditions, and (iv) high and selective-S reactivity toward C-, N- and O-nucleophiles.
Therefore, this reaction provides rapid and easy access to the S–O and S–N bonds under mild conditions in a highly effective manner. It has been demonstrated to be orthogonal to other click reactions, such as SPAAC and TEC. Moreover, the efficiency and the potential of this methodology have been validated in polymer chemistry and materials science. It also appears to be suitable for conjugation in biological systems, where other click reactions cannot be applied. Besides, the potential of this methodology has been recently expanded to carbohydrate chemistry.
5.2. Applications
The application of SuFEx in carbohydrates to connect a sugar ring to a scaffold via −SO2–N bond formation was first reported by Dondoni and co-workers in 2016. Their studies focused on the synthesis of carbohydrate sulfonamides to develop new molecules with potential clinical applications, including antibacterial diuretics, anticonvulsants, and HIV protease inhibitors. , Accordingly, C-glucosylsulfonyl fluoride 82a was reacted with various amines in DMF at 80 °C in the presence of an excess of base to neutralize the HF released during sulfonamide bond formation, affording C-glucosylsulfonyl amides 84a–e in good to excellent yields (Scheme ). Under these optimal conditions, glucosylsulfonyl fluoride 82a was reactive toward primary and secondary alkyl amines, but it was inert toward arylamines, even under forcing conditions. This chemoselectivity highlights SuFEx as a selective method for preparing N-alkyl sulfonyl amides.
32. Synthesis of Aliphatic Sulfonamides 84 .
The reaction scope was extended to obtain multivalent carbohydrate architectures, which are known to be involved in many biological recognition phenomena by interacting with proteins located on cell-membrane surface. Hence, Dondoni et al. successfully prepared the tetravalent glucosylsulfonamido calix[4]arene 85 by conjugation of calix[4]arene derivative 83f with glucosylsulfonyl fluoride 82b under previous conditions after an additional deprotection step (Scheme ). , The variation of the protecting group in the sugar sulfonylating agent from an O-acetyl to an O-benzyl group was due to the preferential transfer of the acetyl group from the sugar ring to the amino groups in cluster 83f under reaction conditions.
Encouraged by these results, the same group attempted to attach different sulfonyl fluoride-derived sugar rings to the central cluster core using this two-step methodology. Specifically, they tried to synthesize calix[4]arenes pending iminosugars motifs, but the corresponding iminosugar sulfonyl fluoride decomposed under reaction conditions. To overcome this limitation, the authors envisioned a complementary approach to achieve isomeric sulfonamide bioisosters. They accomplished the reaction between the amino group attached to the iminosugar ring and sulfonyl fluoride tethered to the cluster motif, yielding the sugar sulfonamide cluster in good overall yields after the debenzylation reaction.
In summary, while the SuFEx reaction is still in its early stages and requires further investigation, it seems to be an efficient, accessible, and complementary ligation tool that can complement other click reactions like TEC and strain promoted azide–alkyne cycloaddition. Furthermore, previous studies have shown that SuFEx is a viable metal-free method for the functionalization of proteins. This underscores their potential as a promising area for future research in the preparation of glycoconjugates in biological systems.
6. Conclusions and Perspectives
The development of efficient and biocompatible click chemistry reactions has significantly advanced the fields of glycochemistry and glycobiology. Metal-free click reactions, including TEC, SPAAC, IEDDA and SuFEx, have enabled the synthesis of complex glycoconjugates under mild conditions with exceptional regio- and stereoselectivity. These methodologies have opened new avenues in therapeutic design, diagnostics, materials science, and bioengineering by providing versatile, sustainable, and high-yielding approaches to functionalize biomolecules.
The TEC reaction represents a pivotal advancement in glycochemistry, providing an efficient, regio- and stereoselective approach for synthesizing complex glycoconjugates under mild conditions. TEC’s adaptability has facilitated its application in diverse areas, including the synthesis of S-polysaccharides with enhanced stability, sugar-modified nucleosides with improved therapeutic properties, and structurally defined glycopeptides and glycoproteins. Its integration into glycoconjugate vaccine development and the design of glycosylated biomaterials underscore its potential to address critical challenges in drug discovery, vaccine formulation, and biomaterial engineering. Future efforts should prioritize optimizing TEC for large-scale applications, exploring its compatibility with complementary bioconjugation techniques, and expanding its use in synthesizing novel glycomimetics and bioactive compounds.
The SPAAC has emerged as a transformative bioorthogonal reaction, providing a nontoxic, rapid, and selective tool for biomolecule labeling and imaging. Its ability to operate under physiological conditions makes it invaluable for studying glycosylation dynamics, metabolic pathways, and disease-related biomarkers in complex biological systems. Innovations such as structurally optimized cyclooctynes and advanced metabolic precursors have further enhanced its reactivity and versatility. The successful application of SPAAC in human tissues and live animals highlights its potential for translational research and diagnostic development. Future efforts should focus on overcoming existing challenges to fully harness SPAAC’s capabilities in advancing glycoscience and biomedicine.
SPAAC reaction has also revolutionized glycoconjugate synthesis, including the preparation of glycofullerenes for antiviral strategies, glycovaccines targeting infectious diseases, and lysosome-targeting chimeras (LYTACs) for selective protein degradation. Additionally, SPAAC’s integration with biocatalysis demonstrates its potential for sustainable synthesis, exemplified by the development of antibody–drug conjugates (ADCs) and glyconanomaterials for targeted cancer therapies. Beyond its biomedical impact, SPAAC has driven innovations in materials science and bioengineering, positioning itself as a cornerstone technology for therapeutic and diagnostic advancements in glycobiology.
The IEDDA reaction is a highly efficient and selective metal-free cycloaddition that has become a valuable tool in chemical biology due to its rapid kinetics, biocompatibility, and mild aqueous conditions. In glycoscience, it enables selective labeling of cell-surface glycans in living cells, making it ideal for live-cell imaging, glycan tracking, and therapeutic development. Its bioorthogonality allows for in vivo applications without disrupting native biomolecules. IEDDA has also been used to create injectable hydrogels by functionalizing natural polysaccharides (e.g., hyaluronic acid, alginate, and chitosan) with tetrazines and strained dienophiles, enabling rapid gelation for biomedical uses such as drug delivery and tissue engineering.
The SuFEx reaction has emerged as a powerful tool in metal-free click chemistry, offering a unique combination of efficiency, chemoselectivity, and stability under mild conditions. Its ability to form sulfonamide and sulfonate bonds with high specificity and compatibility has been demonstrated in diverse applications, including the synthesis of bioactive carbohydrate sulfonamides and multivalent glycosylated architectures. While still in its early stages, SuFEx complements existing click reactions, broadening the toolbox for glycoconjugate synthesis. The reaction’s orthogonality and utility in functionalizing proteins suggest potential applications in biological systems where other click reactions face limitations. Future research focused on expanding its substrate scope and optimizing conditions will further enhance its role as a transformative tool in chemical biology, therapeutic development, and materials science.
Acknowledgments
The authors gratefully acknowledge financial support provided by the Spanish Ministerio de Economía y Competitividad, Grants TED2021-130430B-C21, PDC2022-133817-I00 and PID2023-150195OB-I00.
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References
- Varki A.. Biological roles of glycans. Glycobiology. 2017;27(1):3–49. doi: 10.1093/glycob/cww086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard A. C., Boyce M.. Life is sweet: the cell biology of glycoconjugates. Mol. Biol. Cell. 2019;30(5):525–529. doi: 10.1091/mbc.E18-04-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhardt R. J., Toida T.. Role of glycosaminoglycans in cellular communication. Acc. Chem. Res. 2004;37(7):431–438. doi: 10.1021/ar030138x. [DOI] [PubMed] [Google Scholar]
- Arora K., Sherilraj P. M., Abutwaibe K. A., Dhruw B., Mudavath S. L.. Exploring glycans as vital biological macromolecules: A comprehensive review of advancements in biomedical frontiers. Int. J. Biol. Macromol. 2024;268:131511–131531. doi: 10.1016/j.ijbiomac.2024.131511. [DOI] [PubMed] [Google Scholar]
- Hakomori S.. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc. Natl. Acad. Sci. U.S.A. 2002;99(16):10231–10233. doi: 10.1073/pnas.172380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla L., Peri F., Airoldi C.. Glycoconjugates in cancer therapy. Anti-Cancer Agent. Me. 2008;8(1):92–121. doi: 10.2174/187152008783330815. [DOI] [PubMed] [Google Scholar]
- Hoyos P., Perona A., Juanes O., Rumbero A., Hernáiz M. J.. Synthesis of Glycodendrimers with Antiviral and Antibacterial Activity. Chem. Eur. J. 2021;27(28):7593–7624. doi: 10.1002/chem.202005065. [DOI] [PubMed] [Google Scholar]
- Zaslona M. E., Downey A. M., Seeberger P. H., Moscovitz O.. Review Article Semi- and fully synthetic carbohydrate vaccines against pathogenic bacteria: recent developments. Biochem. Soc. Trans. 2021;49(5):2411–2429. doi: 10.1042/BST20210766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi A., Jiménez-Barbero J., Casnati A., De Castro C., Darbre T., Fieschi F., Finne J., Funken H., Jaeger K. E., Lahmann M., Lindhorst T. K., Marradi M., Messner P., Molinaro A., Murphy P. V., Nativi C., Oscarson S., Penadés S., Peri F., Pieters R. J., Renaudet O., Reymond J. L., Richichi B., Rojo J., Sansone F., Schäffer C., Turnbull W. B., Velasco-Torrijos T., Vidal S., Vincent S., Wennekes T., Zuilhof H., Imberty A.. Multivalent glycoconjugates as anti-pathogenic agents. Chem. Soc. Rev. 2013;42(11):4709–4727. doi: 10.1039/C2CS35408J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astronomo R. D., Burton D. R.. Carbohydrate vaccines: developing sweet solutions to sticky situations? Nat. Rev. Drug Discovery. 2010;9(4):308–324. doi: 10.1038/nrd3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imberty A., Varrot A.. Microbial recognition of human cell surface glycoconjugates. Curr. Opin. Struct. Biol. 2008;18(5):567–576. doi: 10.1016/j.sbi.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Reily C., Stewart T. J., Renfrow M. B., Novak J.. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019;15(6):346–366. doi: 10.1038/s41581-019-0129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werz D. B., Seeberger P. H.. Carbohydrates as the next frontier in pharmaceutical research. Chem. Eur. J. 2005;11(11):3194–3206. doi: 10.1002/chem.200500025. [DOI] [PubMed] [Google Scholar]
- Bhavya P. V., Nayak M. R., Steiny R. P., Das T. M.. Sweet promise of glycochemistry in medicine. Trends Carbohydr. Res. 2021;12(3):56–76. [Google Scholar]
- Kolb H. C., Finn M. G., Sharpless K. B.. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001;40(11):2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Agrahari A. K., Bose P., Jaiswal M. K., Rajkhowa S., Singh A. S., Hotha S., Mishra N., Tiwari V. K.. Cu(I)-Catalyzed click chemistry in glycoscience and their diverse applications. Chem. Rev. 2021;121(13):7638–7955. doi: 10.1021/acs.chemrev.0c00920. [DOI] [PubMed] [Google Scholar]
- Hein C. D., Liu X. M., Wang D.. Click chemistry, a powerful tool for pharmaceutical sciences. Pharm. Res. 2008;25(10):2216–2230. doi: 10.1007/s11095-008-9616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder W. H., Kluger C.. Azide/alkyne-″click″ reactions: applications in material science and organic synthesis. Curr. Org. Chem. 2006;10(14):1791–1815. doi: 10.2174/138527206778249838. [DOI] [Google Scholar]
- Binder W. H., Sachsenhofer R.. ’Click’ chemistry in polymer and material science: An update. Macromol. Rapid Commun. 2008;29(12–13):952–981. doi: 10.1002/marc.200800089. [DOI] [Google Scholar]
- Breugst M., Reissig H. U.. The Huisgen reaction: milestones of the 1,3-dipolar cycloaddition. Angew. Chem. Int. Ed. 2020;59(30):12293–12307. doi: 10.1002/anie.202003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozorov K., Zhao J. Y., Aisa H. A.. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry:a recent overview. Bioorg. Med. Chem. 2019;27(16):3511–3531. doi: 10.1016/j.bmc.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amblard F., Cho J. H., Schinazi R. F.. Cu(I)-Catalyzed Huisgen azide-alkyne 1,3-dipolar cycloaddition reaction in nucleoside, nucleotide, and oligonucleotide chemistry. Chem. Rev. 2009;109(9):4207–4220. doi: 10.1021/cr9001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldal M.. Polymer ″Clicking″ by CuAAC reactions. Macromol. Rapid Commun. 2008;29(12–13):1016–1051. doi: 10.1002/marc.200800159. [DOI] [Google Scholar]
- Becer C. R., Hoogenboom R., Schubert U. S.. Click chemistry beyond metal-catalyzed cycloaddition. Angew. Chem. Int. Ed. 2009;48(27):4900–4908. doi: 10.1002/anie.200900755. [DOI] [PubMed] [Google Scholar]
- Yoon H. Y., Lee D., Lim D. K., Koo H., Kim K.. Copper-free click chemistry: applications in drug delivery, cell tracking, and tissue engineering. Adv. Mater. 2022 doi: 10.1002/adma.202107192. [DOI] [PubMed] [Google Scholar]
- Deb T., Tu J. L., Franzini R. M.. Mechanisms and substituent effects of metal-free bioorthogonal reactions. Chem. Rev. 2021;121(12):6850–6914. doi: 10.1021/acs.chemrev.0c01013. [DOI] [PubMed] [Google Scholar]
- Chaudhuri R., Bhattacharya S., Dash J.. Bioorthogonal chemistry in translational research: advances and opportunities. ChemBioChem. 2023 doi: 10.1002/cbic.202300474. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhang Y.. Applications of azide-based bioorthogonal click chemistry in glycobiology. Molecules. 2013;18(6):7145–7159. doi: 10.3390/molecules18067145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. R., Pratt M. R., Schumann B.. Celebrating the contributions of Carolyn Bertozzi to bioorthogonal chemistry and its application to glycoscience. Isr. J. Chem. 2023 doi: 10.1002/ijch.202300003. [DOI] [Google Scholar]
- Griesbaum K.. Problems and possibilities of the ree-radical addition of thiols to unsaturated compounds. Angew. Chem. Int. Ed. 1970;9(4):273–287. doi: 10.1002/anie.197002731. [DOI] [Google Scholar]
- Hoyle C. E., Bowman C. N.. Thiol-ene click chemistry. Angew. Chem. Int. Ed. 2010;49(9):1540–1573. doi: 10.1002/anie.200903924. [DOI] [PubMed] [Google Scholar]
- Dénès F., Pichowicz M., Povie G., Renaud P.. Thiyl radicals in organic synthesis. Chem. Rev. 2014;114(5):2587–2693. doi: 10.1021/cr400441m. [DOI] [PubMed] [Google Scholar]
- Dondoni A., Marra A.. Recent applications of thiol-ene coupling as a click process for glycoconjugation. Chem. Soc. Rev. 2012;41(2):573–586. doi: 10.1039/C1CS15157F. [DOI] [PubMed] [Google Scholar]
- Lian G., Zhang X., Yu B.. Thioglycosides in carbohydrate research. Carbohydr. Res. 2015;403:13–22. doi: 10.1016/j.carres.2014.06.009. [DOI] [PubMed] [Google Scholar]
- McSweeney L., Dénès F., Scanlan E. M.. Thiyl-radical reactions in carbo-hydrate chemistry: from thiosugars to glycoconjugate synthesis. Eur. J. Org. Chem. 2016;2016(12):2080–2095. doi: 10.1002/ejoc.201501543. [DOI] [Google Scholar]
- Sletten E. M., Bertozzi C. R.. Bioorthogonal chemistry: fishing for selectivity in a sea of functionality. Angew. Chem., Int. Ed. 2009;48(38):6974–98. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett J. C., Bertozzi C. R.. Cu-free click cycloaddition reactions in chemical biology. Chem. Soc. Rev. 2010;39(4):1272–1279. doi: 10.1039/b901970g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning X., Guo J., Wolfert M. A., Boons G.-J.. Visualizing metabolically labeled glycoconjugates of living cells by copper-free and fast Huisgen cycloadditions. Angew. Chem. Int. Ed. 2008;47(12):2253–2255. doi: 10.1002/anie.200705456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handula M., Chen K.-T., Seimbille Y.. IEDDA: An attractive bioorthogonal reaction for biomedical applications. Molecules. 2021;26(15):4640. doi: 10.3390/molecules26154640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman M. L., Royzen M., Fox J. M.. Tetrazine ligation: ast bioconjugation based on inverse-electron-demand Diels-Alder reactivity. J. Am. Chem. Soc. 2008;130(41):13518–13519. doi: 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira B. L., Guo Z., Bernardes G. J. L.. Inverse electron demand Diels-Alder reactions in chemical biology. Chem. Soc. Rev. 2017;46(16):4895–4950. doi: 10.1039/C7CS00184C. [DOI] [PubMed] [Google Scholar]
- Dong J., Krasnova L., Finn M. G., Sharpless K. B.. Sulfur(VI) Fluoride Exchange (SuFEx): another good reaction for click chemistry. Angew. Chem. Int. Ed. 2014;53(36):9430–9448. doi: 10.1002/anie.201309399. [DOI] [PubMed] [Google Scholar]
- Zelli R., Tommasone S., Dumy P., Marra A., Dondoni A.. A click ligation ased on SuFEx for the metal-free synthesis of sugar and iminosugar clusters. Eur. J. Org. Chem. 2016;2016(30):5102–5116. doi: 10.1002/ejoc.201600732. [DOI] [Google Scholar]
- Posner T.. Beiträge zur Kenntniss der ungesättigten Verbindungen. II. Ueber die Addition von Mercaptanen an ungesättigte Kohlenwasserstoffe. Berichte der deutschen chemischen Gesellschaft. 1905;38(1):646–657. doi: 10.1002/cber.190503801106. [DOI] [Google Scholar]
- Hoyle C. E., Lee T. Y., Roper T.. Thiol-enes: chemistry of the past with promise for the future. J. Polym. Sci., Part A: Polym. Chem. 2004;42(21):5301–5338. doi: 10.1002/pola.20366. [DOI] [Google Scholar]
- Machado T. O., Sayer C., Araujo P. H. H.. Thiol-ene polymerisation: a promising technique to obtain novel biomaterials. Eur. Polym. J. 2017;86:200–215. doi: 10.1016/j.eurpolymj.2016.02.025. [DOI] [Google Scholar]
- Cramer N. B., Scott J. P., Bowman C. N.. Photopolymerizations of thiol-ene polymers without photoinitiators. Macromolecules. 2002;35(14):5361–5365. doi: 10.1021/ma0200672. [DOI] [Google Scholar]
- Northrop B. H., Coffey R. N.. Thiol-ene click chemistry: computational and kinetic analysis of the influence of alkene functionality. J. Am. Chem. Soc. 2012;134(33):13804–13817. doi: 10.1021/ja305441d. [DOI] [PubMed] [Google Scholar]
- Nolan M. D., Scanlan E. M.. Applications of thiol-ene chemistry for peptide science. Front. Chem. 2020 doi: 10.3389/fchem.2020.583272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe J. M., Rakotomanomana N., Pavia A. A.. Free-radical addition of 1-thiosugars to alkenes a new general approach to the synthesis of 1-thioglycosides. Tetrahedron Lett. 1988;29(34):4293–4296. doi: 10.1016/S0040-4039(00)80478-5. [DOI] [Google Scholar]
- Wilson J. C., Kiefel M. J., Angus D. I., von Itzstein M.. Investigation of the stability of thiosialosides toward hydrolysis by sialidases using NMR spectroscopy. Org. Lett. 1999;1(3):443–6. doi: 10.1021/ol990652w. [DOI] [PubMed] [Google Scholar]
- Fiore M., Marra A., Dondoni A.. Photoinduced thiol-ene coupling as a click ligation tool for thiodisaccharide synthesis. J. Org. Chem. 2009;74(11):4422–5. doi: 10.1021/jo900514w. [DOI] [PubMed] [Google Scholar]
- Staderini S., Chambery A., Marra A., Dondoni A.. Free-radical hydrothiolation of glycals: a thiol-ene-based synthesis of S-disaccharides. Tetrahedron Lett. 2012;53(6):702–704. doi: 10.1016/j.tetlet.2011.11.140. [DOI] [Google Scholar]
- Lázár L., Csávás M., Herczeg M., Herczegh P., Borbás A.. Synthesis of S-linked glycoconjugates and S-disaccharides by thiol-ene coupling reaction of enoses. Org. Lett. 2012;14(17):4650–4653. doi: 10.1021/ol302098u. [DOI] [PubMed] [Google Scholar]
- Bege M., Bereczki I., Herczeg M., Kicsák M., Eszenyi D., Herczegh P., Borbás A.. A low-temperature, photoinduced thiol-ene click reaction: a mild and efficient method for the synthesis of sugar-modified nucleosides. Org Biomol. Chem. 2017;15(43):9226–9233. doi: 10.1039/C7OB02184D. [DOI] [PubMed] [Google Scholar]
- Jordheim L. P., Durantel D., Zoulim F., Dumontet C.. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat. Rev. Drug Discov. 2013;12(6):447–464. doi: 10.1038/nrd4010. [DOI] [PubMed] [Google Scholar]
- Conforti I., Marra A.. Iminosugars as glycosyltransferase inhibitors. Org. Biomol. Chem. 2021;19(25):5439–5475. doi: 10.1039/D1OB00382H. [DOI] [PubMed] [Google Scholar]
- Zelli R., Dumy P., Marra A.. Metal-free synthesis of imino-disaccharides and calix-iminosugars by photoinduced radical thiol-ene coupling (TEC) Org. Biomol. Chem. 2020;18(13):2392–2397. doi: 10.1039/D0OB00198H. [DOI] [PubMed] [Google Scholar]
- Lázár L., Borbás A., Somsák L.. Synthesis of thiomaltooligosaccharides by a thio-click approach. Carbohydr. Res. 2018;470:8–12. doi: 10.1016/j.carres.2018.09.005. [DOI] [PubMed] [Google Scholar]
- Williams D. H.. The glycopeptide story - how to kill the deadly ‘superbugs’. Nat. Prod. Rep. 1996;13(6):469–477. doi: 10.1039/NP9961300469. [DOI] [PubMed] [Google Scholar]
- Pratt M. R., Bertozzi C. R.. Synthetic glycopeptides and glycoproteins as tools for biology. Chem. Soc. Rev. 2005;34(1):58–68. doi: 10.1039/b400593g. [DOI] [PubMed] [Google Scholar]
- Ramos D., Rollin P., Klaffke W.. Chemoenzymatic synthesis of neoglycopeptides: application to an α-Gal-terminated neoglycopeptide. J. Org. Chem. 2001;66(9):2948–2956. doi: 10.1021/jo001439c. [DOI] [PubMed] [Google Scholar]
- Markey L., Giordani S., Scanlan E. M.. Native chemical ligation, thiol-ene click:a methodology for the synthesis of functionalized peptides. J. Org. Chem. 2013;78(9):4270–4277. doi: 10.1021/jo4001542. [DOI] [PubMed] [Google Scholar]
- Fiore M., Lo Conte M., Pacifico S., Marra A., Dondoni A.. Synthesis of S-glycosyl amino acids and S-glycopeptides via photoinduced click thiol-ene coupling. Tetrahedron Lett. 2011;52(3):444–447. doi: 10.1016/j.tetlet.2010.11.097. [DOI] [Google Scholar]
- Alexander S. R., Williams G. M., Brimble M. A., Fairbanks A. J.. A double-click approach to the protecting group free synthesis of glycoconjugates. Org. Biomol. Chem. 2018;16(8):1258–1262. doi: 10.1039/C8OB00072G. [DOI] [PubMed] [Google Scholar]
- Floyd N., Vijayakrishnan B., Koeppe J. R., Davis B. G.. Thiyl glycosylation of olefinic proteins: S-linked glycoconjugate synthesis. Angew. Chem. Int. Ed. 2009;48(42):7798–7802. doi: 10.1002/anie.200903135. [DOI] [PubMed] [Google Scholar]
- Dondoni A., Massi A., Nanni P., Roda A.. A New Ligation strategy for peptide and protein glycosylation: photoinduced thiol-ene coupling. Chem. Eur. J. 2009;15(43):11444–11449. doi: 10.1002/chem.200901746. [DOI] [PubMed] [Google Scholar]
- Kramer J. R., Deming T. J.. Glycopolypeptides with a redox-triggered helix-to-coil transition. J. Am. Chem. Soc. 2012;134(9):4112–4115. doi: 10.1021/ja3007484. [DOI] [PubMed] [Google Scholar]
- Russo L., Battocchio C., Secchi V., Magnano E., Nappini S., Taraballi F., Gabrielli L., Comelli F., Papagni A., Costa B., Polzonetti G., Nicotra F., Natalello A., Doglia S. M., Cipolla L.. Thiol-ene mediated neoglycosylation of collagen patches:a preliminary study. Langmuir. 2014;30(5):1336–1342. doi: 10.1021/la404310p. [DOI] [PubMed] [Google Scholar]
- Wittrock S., Becker T., Kunz H.. Synthetic vaccines of tumor-associated glycopeptide antigens by immune-compatible thioether linkage to bovine serum albumin. Angew. Chem. Int. Ed. 2007;46(27):5226–5230. doi: 10.1002/anie.200700964. [DOI] [PubMed] [Google Scholar]
- Gingras M., Chabre Y. M., Roy M., Roy R.. How do multivalent glycodendrimers benefit from sulfur chemistry? Chem. Soc. Rev. 2013;42(11):4823–4841. doi: 10.1039/c3cs60090d. [DOI] [PubMed] [Google Scholar]
- Kieburg C., Dubber M., Lindhorst T. K.. A new type of carbohydrate clustering: synthesis of a pentavalent glycocluster based on a carbohydrate core. Synlett. 1997;12(12):1447–1449. doi: 10.1055/s-2008-1067191. [DOI] [Google Scholar]
- Köhn M., Benito J. M., Ortiz Mellet C., Lindhorst T. K., García Fernández J. M.. Functional evaluation of carbohydrate-centred glycoclusters by enzyme-linked lectin assay: ligands for Concanavalin A. ChemBioChem. 2004;5(6):771–777. doi: 10.1002/cbic.200300807. [DOI] [PubMed] [Google Scholar]
- Heidecke C. D., Lindhorst T. K.. Iteratives synthesis of spacered glycodendrons as oligomannoside mimetics and evaluation of their antiadhesive properties. Chem. Eur. J. 2007;13(32):9056–9067. doi: 10.1002/chem.200700787. [DOI] [PubMed] [Google Scholar]
- Cordes D. B., Lickiss P. D., Rataboul F.. Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem. Rev. 2010;110(4):2081–2173. doi: 10.1021/cr900201r. [DOI] [PubMed] [Google Scholar]
- Gao Y., Eguchi A., Kakehi K., Lee Y. C.. Efficient preparation of glycoclusters from silsesquioxanes. Org. Lett. 2004;6(20):3457–3460. doi: 10.1021/ol040043a. [DOI] [PubMed] [Google Scholar]
- Lo Conte M., Staderini S., Chambery A., Berthet N., Dumy P., Renaudet O., Marra A., Dondoni A.. Glycoside and peptide clustering around the octasilsesquioxane scaffold via photoinduced free-radical thiol-ene coupling. The observation of a striking glycoside cluster effect. Org. Biomol. Chem. 2012;10(16):3269–3277. doi: 10.1039/c2ob07078b. [DOI] [PubMed] [Google Scholar]
- Conte M. L., Robb M. J., Hed Y., Marra A., Malkoch M., Hawker C. J., Dondoni A.. Exhaustive glycosylation, pegylation, and glutathionylation of a [G4]-ene48 dendrimer via photoinduced thiol-ene coupling. J. Polym. Sci., Part A: Polym. Chem. 2011;49(20):4468–4475. doi: 10.1002/pola.24888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirardello M., Öberg K., Staderini S., Renaudet O., Berthet N., Dumy P., Hed Y., Marra A., Malkoch M., Dondoni A.. Thiol-ene and thiol-yne-based synthesis of glycodendrimers as nanomolar inhibitors of wheat germ agglutinin. J. Polym. Sci., Part A: Polym. Chem. 2014;52(17):2422–2433. doi: 10.1002/pola.27262. [DOI] [Google Scholar]
- Mahadevegowda S. H., Ruan L., Zhang J., Hou S., Raju C., Chan-Park M. B.. Synthesis of dimeric and tetrameric trithiomannoside clusters through convenient photoinitiated thiol-ene click protocol for efficient inhibition of gram-negative bacteria. J. Carbohydr. Chem. 2021;40(1–3):83–96. doi: 10.1080/07328303.2021.1928154. [DOI] [Google Scholar]
- Leydet A., Moullet C., Roque J. P., Witvrouw M., Pannecouque C., Andrei G., Snoeck R., Neyts J., Schols D., De Clercq E.. Polyanion inhibitors of HIV and other viruses. 7. Polyanionic compounds and polyzwitterionic compounds derived from cyclodextrins as inhibitors of HIV transmission. J. Med. Chem. 1998;41(25):4927–4932. doi: 10.1021/jm970661f. [DOI] [PubMed] [Google Scholar]
- Fulton D. A., Stoddart J. F.. Synthesis of cyclodextrin-based carbohydrate clusters by photoaddition reactions. J. Org. Chem. 2001;66(25):8309–8319. doi: 10.1021/jo010705z. [DOI] [PubMed] [Google Scholar]
- Fiore M., Chambery A., Marra A., Dondoni A.. Single and dual glycoside clustering around calix[4]arene scaffolds via click thiol-ene coupling and azide-alkyne cycloaddition. Org. Biomol. Chem. 2009;7(19):3910–3913. doi: 10.1039/b912686d. [DOI] [PubMed] [Google Scholar]
- Mousavifar L., Roy R.. Design, synthetic strategies, and therapeutic applications of heterofunctional glycodendrimers. Molecules. 2021;26(9):2428. doi: 10.3390/molecules26092428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-García M., Benito J. M., Rodríguez-Lucena D., Yu J.-X., Chmurski K., Ortiz Mellet C., Gutiérrez Gallego R., Maestre A., Defaye J., García Fernández J. M.. Probing secondary carbohydrate-protein interactions with highly dense cyclodextrin-centered heteroglycoclusters: the heterocluster effect. J. Am. Chem. Soc. 2005;127(22):7970–7971. doi: 10.1021/ja050934t. [DOI] [PubMed] [Google Scholar]
- Gómez-García M., Benito J. M., Gutiérrez-Gallego R., Maestre A., Mellet C. O., Fernández J. M. G., Blanco J. L. J.. Comparative studies on lectin-carbohydrate interactions in low and high density homo- and heteroglycoclusters. Org. Biomol. Chem. 2010;8(8):1849–1860. doi: 10.1039/b920048g. [DOI] [PubMed] [Google Scholar]
- Daskhan G. C., Tran H.-T. T., Meloncelli P. J., Lowary T. L., West L. J., Cairo C. W.. Construction of multivalent homo- and heterofunctional ABO blood group glycoconjugates using a trifunctional linker strategy. Bioconjugate Chem. 2018;29(2):343–362. doi: 10.1021/acs.bioconjchem.7b00679. [DOI] [PubMed] [Google Scholar]
- Dondoni A., Marra A.. Metal-catalyzed and metal-free alkyne hydrothiolation: synthetic aspects and application trends. Eur. J. Org. Chem. 2014;2014(19):3955–3969. doi: 10.1002/ejoc.201301879. [DOI] [Google Scholar]
- Dondoni A.. The emergence of thiol-ene coupling as a click process for materials and bioorganic chemistry. Angew. Chem. Int. Ed. 2008;47(47):8995–8997. doi: 10.1002/anie.200802516. [DOI] [PubMed] [Google Scholar]
- Huisgen R.. 1.3-Dipolare cycloadditionen Ruckschau und ausblick. Angew. Chem. Int. Ed. 1963;75(13):604–637. doi: 10.1002/ange.19630751304. [DOI] [Google Scholar]
- Huisgen R.. Kinetik und mechanismus 1.3-dipolarer cycloadditionen. Angew. Chem. Int. Ed. 1963;75(16–7):742–754. doi: 10.1002/ange.19630751603. [DOI] [Google Scholar]
- Tornoe C. W., Christensen C., Meldal M.. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002;67(9):3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- Baskin J. M., Prescher J. A., Laughlin S. T., Agard N. J., Chang P. V., Miller I. A., Lo A., Codelli J. A., Bertozzi C. R.. Copper-free click chemistry for dynamic in vivo imaging. Proc. Natl. Acad. Sci. U. S. A. 2007;104(43):16793–7. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agard N. J., Prescher J. A., Bertozzi C. R.. A Strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 2004;126(46):15046–15047. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]
- Agard N. J., Baskin J. M., Prescher J. A., Lo A., Bertozzi C. R.. A comparative study of bioorthogonal reactions with azides. ACS Chem. Biol. 2006;1(10):644–648. doi: 10.1021/cb6003228. [DOI] [PubMed] [Google Scholar]
- Baskin J. M., Bertozzi C. R.. Bioorthogonal click chemistry: covalent labeling in living systems. QSAR Comb. Sci. 2007;26(11–12):1211–1219. doi: 10.1002/qsar.200740086. [DOI] [Google Scholar]
- Dommerholt J., Schmidt S., Temming R., Hendriks L. J., Rutjes F. P., van Hest J. C., Lefeber D. J., Friedl P., van Delft F. L.. Readily accessible bicyclononynes for bioorthogonal labeling and three-dimensional imaging of living cells. Angew. Chem. Int. Ed. 2010;49(49):9422–5. doi: 10.1002/anie.201003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R. B., Jarrett A. D., Goebel P., Mallon B. J.. Heats of hydrogenation. IX. Cyclic acetylenes and some miscellaneous olefins. J. Am. Chem. Soc. 1973;95(3):790–792. doi: 10.1021/ja00784a025. [DOI] [Google Scholar]
- Lutz J.-F.. Copper-free azide-alkyne cycloadditions: new insights and perspectives. Angew. Chem. Int. Ed. 2008;47(12):2182–2184. doi: 10.1002/anie.200705365. [DOI] [PubMed] [Google Scholar]
- Debets M. F., van der Doelen C. W. J., Rutjes F. P. J. T., van Delft F. L.. Azide: a unique dipole for metal-free bioorthogonal ligations. ChemBioChem. 2010;11(9):1168–1184. doi: 10.1002/cbic.201000064. [DOI] [PubMed] [Google Scholar]
- Saxon E., Bertozzi C. R.. Cell surface engineering by a modified Staudinger reaction. Science. 2000;287(5460):2007–10. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- Köhn M., Breinbauer R.. The Staudinger ligation: a gift to chemical biology. Angew. Chem. Int. Ed. 2004;43(24):3106–3116. doi: 10.1002/anie.200401744. [DOI] [PubMed] [Google Scholar]
- van Berkel S. S., van Eldijk M. B., van Hest J. C. M.. Staudinger ligation as a method for bioconjugation. Angew. Chem. Int. Ed. 2011;50(38):8806–8827. doi: 10.1002/anie.201008102. [DOI] [PubMed] [Google Scholar]
- Prescher J. A., Dube D. H., Bertozzi C. R.. Chemical remodelling of cell surfaces in living animals. Nature. 2004;430(7002):873–7. doi: 10.1038/nature02791. [DOI] [PubMed] [Google Scholar]
- Mbua N. E., Guo J., Wolfert M. A., Steet R., Boons G.-J.. Strain-Promoted Alkyne-Azide Cycloadditions (SPAAC) reveal new features of glycoconjugate biosynthesis. ChemBioChem. 2011;12(12):1912–1921. doi: 10.1002/cbic.201100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L., Cai K., Yu J., Cheng J.. Novel liposomal azido mannosamine lipids on metabolic cell labeling and imaging via Cu-free click chemistry. Bioconjugate Chem. 2019;30(9):2317–2322. doi: 10.1021/acs.bioconjchem.9b00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin S. T., Baskin J. M., Amacher S. L., Bertozzi C. R.. In Vivo imaging of membrane-associated glycans in developing Zebrafish. Science. 2008;320(5876):664–667. doi: 10.1126/science.1155106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin J. M., Dehnert K. W., Laughlin S. T., Amacher S. L., Bertozzi C. R.. Visualizing enveloping layer glycans during zebrafish early embryogenesis. Proc. Natl. Acad. Sci. U.S.A. 2010;107(23):10360–10365. doi: 10.1073/pnas.0912081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehnert K. W., Beahm B. J., Huynh T. T., Baskin J. M., Laughlin S. T., Wang W., Wu P., Amacher S. L., Bertozzi C. R.. Metabolic labeling of fucosylated glycans in developing Zebrafish. ACS Chem. Biol. 2011;6(6):547–552. doi: 10.1021/cb100284d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beahm B. J., Dehnert K. W., Derr N. L., Kuhn J., Eberhart J. K., Spillmann D., Amacher S. L., Bertozzi C. R.. A Visualizable chain-terminating inhibitor of glycosaminoglycan biosynthesis in developing Zebrafish. Angew. Chem. Int. Ed. 2014;53(13):3347–3352. doi: 10.1002/anie.201310569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiciarich D. R., Nolley R., Maund S. L., Purcell S. C., Herschel J., Iavarone A. T., Peehl D. M., Bertozzi C. R.. Bioorthogonal labeling of human prostate cancer tissue slice cultures for glycoproteomics. Angew. Chem. Int. Ed. 2017;56(31):8992–8997. doi: 10.1002/anie.201701424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P. V., Prescher J. A., Sletten E. M., Baskin J. M., Miller I. A., Agard N. J., Lo A., Bertozzi C. R.. Copper-free click chemistry in living animals. Proc. Natl. Acad. Sci. U.S.A. 2010;107(5):1821–1826. doi: 10.1073/pnas.0911116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie R., Dong L., Du Y., Zhu Y., Hua R., Zhang C., Chen X.. In vivo metabolic labeling of sialoglycans in the mouse brain by using a liposome-assisted bioorthogonal reporter strategy. Proc. Natl. Acad. Sci. USA. 2016;113(19):5173–5178. doi: 10.1073/pnas.1516524113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Soriano J., Reina J. J., Illescas B. M., Rojo J., Martín N.. Maleimide and cyclooctyne-based hexakis-adducts of fullerene: multivalent scaffolds for copper-free click chemistry on fullerenes. J. Org. Chem. 2018;83(4):1727–1736. doi: 10.1021/acs.joc.7b02402. [DOI] [PubMed] [Google Scholar]
- Ramos-Soriano J., Reina J. J., Illescas B. M., de la Cruz N., Rodríguez-Pérez L., Lasala F., Rojo J., Delgado R., Martín N.. Synthesis of highly efficient multivalent disaccharide/[60]fullerene nanoballs for emergent viruses. J. Am. Chem. Soc. 2019;141(38):15403–15412. doi: 10.1021/jacs.9b08003. [DOI] [PubMed] [Google Scholar]
- Ramos-Soriano J., Illescas B. M., Pérez-Sánchez A., Sánchez-Bento R., Lasala F., Rojo J., Delgado R., Martín N.. Topological and multivalent effects in glycofullerene oligomers as EBOLA virus inhibitors. Int. J. Mol. Sci. 2022;23(9):5083–5096. doi: 10.3390/ijms23095083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilo A., Allan M., Brogioni B., Proietti D., Cattaneo V., Crotti S., Sokup S., Zhai H., Margarit I., Berti F., Hu Q.-Y., Adamo R.. Tyrosine-directed conjugation of large glycans to proteins via copper-free click chemistry. Bioconjugate Chem. 2014;25(12):2105–2111. doi: 10.1021/bc500438h. [DOI] [PubMed] [Google Scholar]
- Luh L. M., Scheib U., Juenemann K., Wortmann L., Brands M., Cromm P. M.. Prey for the proteasome: targeted protein degradation: a medicinal chemist’s perspective. Angew. Chem. Int. Ed. 2020;59(36):15448–15466. doi: 10.1002/anie.202004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford K. A., Mcmanus K. J.. PROTACs: Current and future potential as a precision medicine Strategy to Ccombat cancer. Mol. Cancer Ther. 2024;23(4):454–463. doi: 10.1158/1535-7163.MCT-23-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalawansha D. A., Mangano K., den Besten W., Potts P. R.. TAC-tics for leveraging proximity biology in drug discovery. ChemBioChem. 2024;25(4):e202300712. doi: 10.1002/cbic.202300712. [DOI] [PubMed] [Google Scholar]
- Banik S. M., Pedram K., Wisnovsky S., Ahn G., Riley N. M., Bertozzi C. R.. Lysosome-targeting chimaeras for degradation of extracellular proteins. Nature. 2020;584(7820):291–297. doi: 10.1038/s41586-020-2545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P. X., Xu X. J., Wang L. Y., Zhu B. J., Wang X. D., Xia J. L.. The role of EGF-EGFR signalling pathway in hepatocellular carcinoma inflammatory microenvironment. J. Cell Mol. Med. 2014;18(2):218–230. doi: 10.1111/jcmm.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn G., Banik S. M., Miller C. L., Riley N. M., Cochran J. R., Bertozzi C. R.. LYTACs that engage the asialoglycoprotein receptor for targeted protein degradation. Nat. Chem. Biol. 2021;17(9):937–946. doi: 10.1038/s41589-021-00770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X. F., Hu J. J., Yoon J.. Cell membrane as a promising therapeutic target: from materials design to biomedical applications. Angew. Chem., Int. Ed. 2024;63(18):e202400249. doi: 10.1002/anie.202400249. [DOI] [PubMed] [Google Scholar]
- Rodríguez D. F., Moglie Y., Ramírez-Sarmiento C. A., Singh S. K., Dua K., Zacconi F. C.. Bio-click chemistry: a bridge between biocatalysis and click chemistry. Rsc. Adv. 2022;12(4):1932–1949. doi: 10.1039/D1RA08053A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijdeven M. A., van Geel R., Hoogenboom J. H., Verkade J. M. M., Janssen B. M. G., Hurkmans I., de Bever L., van Berkel S. S., van Delft F. L.. Enzymatic glycan remodeling-metal free click (GlycoConnect) provides homogenous antibody-drug conjugates with improved stability and therapeutic index without sequence engineering. MAbs. 2022;14(1):e2078466. doi: 10.1080/19420862.2022.2078466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojarová P., Tavares M. R., Laaf D., Bumba L., Petrásková L., Konefal R., Bláhová M., Pelantová H., Elling L., Etrych T., Chytil P., Kren V.. Biocompatible glyconanomaterials based on HPMA-copolymer for specific targeting of galectin-3. J. Nanobiotechnol. 2018 doi: 10.1186/s12951-018-0399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiber L. M., Kufleitner M., Wittmann V.. Application of the inverse-electron-demand Diels-Alder reaction for metabolic glycoengineering. Front. Chem. 2021;9:654932. doi: 10.3389/fchem.2021.654932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson D. M., Nazarova L. A., Xie B., Kamber D. N., Prescher J. A.. Functionalized cyclopropenes as bioorthogonal chemical reporters. J. Am. Chem. Soc. 2012;134(45):18638–18643. doi: 10.1021/ja3060436. [DOI] [PubMed] [Google Scholar]
- Yan L., Zhao Z., Liu Y., Hosseini S. H., Li C., Huang Y., Saeb M. R., Xiao H., Seidi F.. The inverse electron demand diels-alder (IEDDA): A facile bioorthogonal click reaction for development of injectable polysaccharide-based hydrogels for biomedical applications. Carbohydr. Polym. 2025;352:123142. doi: 10.1016/j.carbpol.2024.123142. [DOI] [PubMed] [Google Scholar]
- Escorihuela J., Marcelis A. T. M., Zuilhof H.. Metal-free click chemistry reactions on surfaces. Adv. Mater. Interface. 2015;2(13):1500135. doi: 10.1002/admi.201500135. [DOI] [Google Scholar]
- Apaydın S., Török M.. Sulfonamide derivatives as multi-target agents for complex diseases. Bioorg. Med. Chem. Lett. 2019;29(16):2042–2050. doi: 10.1016/j.bmcl.2019.06.041. [DOI] [PubMed] [Google Scholar]
- Ovung A., Bhattacharyya J.. Sulfonamide drugs: structure, antibacterial property, toxicity, and biophysical interactions. Biophys. Rev. 2021;13(2):259–272. doi: 10.1007/s12551-021-00795-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatvin J., Brooks K., Locklin J.. SuFEx on the surface: a flexible platform for postpolymerization modification of polymer brushes. Angew. Chem. Int. Ed. 2015;54(45):13370–13373. doi: 10.1002/anie.201506253. [DOI] [PubMed] [Google Scholar]
- Nakagawa, Y. ; Yukishige, I. , Chapter 1 - Molecular architecture and therapeutic potential of lectin mimics. In Adv. Carbohydr. Chem. Biochem.; Horton, D. , Ed.; Academic Press: 2012; Vol. 68, pp 1–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondoni A., Marra A.. SuFEx: a metal-free click ligation for multivalent biomolecules. Org. Biomol. Chem. 2017;15(7):1549–1553. doi: 10.1039/C6OB02458K. [DOI] [PubMed] [Google Scholar]

















