Abstract
Objective
The atherogenic index of plasma (AIP) has emerged as a promising predictor for type 2 diabetes mellitus (T2DM), but population-specific patterns and underlying mechanisms remain poorly understood. This study investigated the association between AIP and T2DM risk in Chinese and Japanese populations, focusing on non-linear relationships, population-specific thresholds, and the mediating role of body mass index (BMI).
Methods
We conducted a retrospective cohort study using data from the China Rich Healthcare Group (n = 112,483) and the Japanese NAGALA database (n = 15,453). AIP was calculated as log10[triglyceride (TG)/high-density lipoprotein cholesterol (HDL-C)]. T2DM was defined as fasting plasma glucose (FPG) ≥ 7.0 mmol/L, hemoglobin A1c (HbA1c) ≥ 6.5%, or self-reported diabetes during follow-up. Cox proportional hazards models with restricted cubic splines were used to examine non-linear relationships. Two-piecewise regression models identified population-specific thresholds, and formal mediation analyses quantified BMI’s mediating effect.
Results
During a median follow-up of 3.0 years, 1,801 participants (1.41%) developed T2DM. AIP demonstrated a significant positive association with T2DM risk in both populations: hazard ratio (HR) per unit increase: Chinese 1.84, 95% confidence interval (CI) 1.54–2.21; Japanese 2.42, 95% CI 1.67–3.52) after comprehensive adjustment. We identified distinct population-specific non-linear relationships with different threshold effects: in Chinese participants, T2DM risk increased significantly until AIP reached 0.436, while in Japanese participants, significant risk elevation began at AIP values exceeding − 0.449. BMI mediated a considerably higher proportion of the total effect in Chinese (39.84%) compared to Japanese participants (27.11%), indicating differential pathophysiological mechanisms.
Conclusions
Our findings reveal substantial population-specific differences in the AIP-T2DM relationship, including population-specific thresholds and mediation pathways. These results underscore the importance of population-tailored screening strategies and suggest that interventions targeting lipid metabolism and BMI management may have varying efficacy across East Asian populations.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13098-025-01907-1.
Keywords: Atherogenic index of plasma, Type 2 diabetes mellitus, Hazard ratio, Body mass index, Population comparison, Mediation analysis, Threshold effect, East asian populations
Introduction
Diabetes mellitus (DM) has become one of the most prevalent chronic metabolic disorders globally, leading to significant health and economic burdens. The International Diabetes Federation reports that approximately 589 million adults (aged 20–79 years) currently live with diabetes, representing 11.1% of the adult population, with projections reaching 853 million by 2050. The global economic burden exceeds one trillion US dollars, highlighting the urgent need for improved prevention strategies [1]. In China, diabetes prevalence has reached approximately 140.9 million adults (13% prevalence), while Japan reports around 11 million cases (11.8% prevalence). Despite lower obesity rates than those in Western populations, both countries face significant diabetes challenges, indicating the involvement of other crucial risk factors[2, 3].
The Atherogenic Index of Plasma (AIP)—defined as the logarithm of the triglyceride to high-density lipoprotein cholesterol ratio (log [TG/HDL-c])—has emerged as an important biomarker for atherogenic dyslipidemia. Initially used for cardiovascular risk assessment, AIP also reflects the balance between atherogenic and protective lipoprotein particles [4]. Recent studies have established its utility beyond cardiovascular diseases, positioning AIP as a key indicator of metabolic health[5–10]. Recent high-quality meta-analyses have confirmed that AIP, as a comprehensive indicator of metabolic health, is associated with improvements in various cardiometabolic risk factors. For instance, in a systematic meta-analysis [11], researchers found that flaxseed supplementation effectively reduced anthropometric indices and triglyceride levels in individuals with type 2 diabetes, which could theoretically improve AIP values through its TG-lowering effect. Particularly, they observed that long-term (> 12 weeks) flaxseed supplementation significantly affected anthropometric measures in obese type 2 diabetes patients. These findings provide an important theoretical foundation for further exploring the relationship between AIP and diabetes risk.
The association between AIP and insulin resistance—central to type 2 diabetes mellitus (T2DM)—is gaining research attention[12]. Many studies show strong correlations between elevated AIP and insulin resistance, suggesting AIP could serve as a simple, cost-effective marker for diabetes risk [13, 14]. Research indicates non-linear relationships between AIP and T2DM, revealing demographic modifiers that complicate understanding this association [9].
In China, emerging evidence supports AIP’s predictive value for diabetes development, with several studies documenting significant non-linear associations that may indicate threshold effects for diabetes risk based on AIP levels[15, 16]. Similarly, studies in Japan report an independent positive association between elevated AIP and T2DM risk among individuals with normal fasting plasma glucose levels [17].
Several studies have demonstrated that elevated AIP levels correlate with increased adiposity and central obesity (measured by body mass index (BMI) and waist circumference (WC)), suggesting AIP may contribute to weight gain and altered body composition through disrupted lipid metabolism[18, 19]. Concurrently, obesity represents one of the strongest risk factors for T2DM development[20, 21]. A systematic review by Abdullah et al. [21] demonstrated that elevated BMI consistently predicts increased diabetes risk across diverse populations.
Given the distinct genetic backgrounds and lifestyle factors in Chinese and Japanese populations, this study aims to deepen the understanding of the AIP-T2DM relationship in these groups. We hypothesize that AIP’s diabetogenic effects are mediated by body mass index (BMI) and that population-specific variations exist in the threshold values of AIP related to diabetes risk. This research seeks to refine diabetes prediction models and inform targeted interventions specific to these populations.
Methods
Study design and data sources
This retrospective cohort study compared the association between the AIP and T2DM in two distinct East Asian populations. Data were obtained from two established databases: the China Rich Healthcare Group database and the Japanese NAGALA (NAfld in the Gifu Area, Longitudinal Analysis) database.
The Chinese cohort data originated from a comprehensive health examination database assembled by the Rich Healthcare Group, encompassing participants’ medical records from 32 locations across 11 major Chinese cities (including Beijing, Shanghai, Guangzhou, and Shenzhen). The database was constructed between 2010 and 2016, with participants recruited consecutively and non-selectively to minimize potential selection bias [22]. The raw data were obtained from the DATADRYAD open-access repository, specifically from a dataset provided by Chen, Ying et al. (2018), titled “Association of body mass index and age with incident diabetes in Chinese adults: a population-based cohort study” (DOI: 10.5061/dryad.ft8750v) [22].
The Japanese cohort data were sourced from the NAGALA database, a comprehensive medical records collection established by Murakami Memorial Hospital from 2004 to 2015. Participants were recruited consecutively from the hospital to ensure representative sampling, employing a methodology designed to minimize potential selection bias. The original dataset was freely accessible through the DATADRYAD open-access repository, originally published by Okamura, Takuro, et al. (2019) in their study on ectopic fat obesity and type 2 diabetes risk (DOI: 10.5061/dryad.8q0p192).[23].
Under Dryad’s open-access policy, the database is explicitly made available for secondary research analyses, ensuring researchers can utilize the data while fully respecting the original authors’ intellectual property rights.
Study population
Chinese cohort
From an initial population of 685,277 participants who underwent health examinations, we excluded individuals according to the following criteria: (1) those without available information on baseline fasting plasma glucose (FPG), weight, gender, or height (n = 135,317); (2) participants with insufficient follow-up duration (< 2 years) (n = 324,233); (3) those with outlier body mass index (BMI) values: (n = 152); (4) participants with unconfirmed diabetes status at follow-up (n = 6,630); (5) participants with pre-existing diabetes at baseline (n = 7,112); (6) participants with missing AIP values or components (n = 94,978); (5) those with fasting plasma glucose (FPG) over 6.l mmol/L at baseline (n = 4,372). Following these rigorous exclusion criteria, 112,483 participants were retained for the final analysis in the Chinese cohort.
Japanese cohort
The Japanese cohort initially included 20,944 participants. We excluded participants according to similar criteria: (1) participants diagnosed with T2DM (n = 323) or with FPG was over 6.1 mmol/L at baseline (n = 808); (2) participants with known liver disease, such as hepatitis B or C virus (n = 416); (3) anyone who took medication at baseline (n = 2,321); (4) participants with heavy drinking habits (more than 40 g per day for women and more than 60 g per day for men) (n = 739); (5) participants with a missed value of covariates, including abdominal ultrasonography, exercise, alcohol intake or laboratory variables (n = 863)[23]; (6) participants with incomplete high-density lipoprotein cholesterol (HDL-c) (n = 11). After applying these exclusion criteria, [15,453] participants in the Japanese cohort remained for analysis. Therefore, based on the Chinese and Japanese cohorts described above, 127,936 participants were ultimately included in the final analysis process (see Fig. 1 for a detailed flowchart).
Fig. 1.
Study flow diagram of participant selection. A total of 127,936 participants were included from two Asian cohorts. Of 685,277 Chinese adults, 112,483 were included after excluding those with missing data, extreme BMI values, diabetes at baseline, insufficient follow-up, and fasting plasma glucose > 6.1 mmol/L. Of 20,944 Japanese participants, 15,453 were included after excluding those with missing data, known liver disease, excessive alcohol consumption, medication use, diabetes at baseline, and elevated fasting glucose
Ethics statement
The Ethics Committee of the Rich Healthcare Group approved the study of the Chinese cohort. The Ethics Committee of Murakami Memorial Hospital approved the Japanese cohort study. Both studies were conducted following the Declaration of Helsinki, and informed consent was waived because of the retrospective nature of this study[24]. As it involved de-identified secondary data, additional ethical approval was not required for the present analysis.
Variables
Atherogenic index of plasma
AIP, the primary exposure variable, was calculated using the formula log10[TG (mmol/L)/ HDL-c (mmol/L)]. For participants with values originally reported in mg/dL, appropriate unit conversions were performed (dividing triglyceride (TG) by 88.5 and HDL-c by 38.7 to convert to mmol/L). AIP was analyzed as a continuous variable and categorized into quartiles based on the distribution in each population.
Outcome measures
Our interesting outcome variable was diabetes (dichotomous variable: 0 = non-diabetes, 1 = diabetes). The detailed process of defining prediabetes is described as follows: Diabetes was defined as FPG ≥ 7.0 mmol/L, hemoglobin A1c (HbA1c) ≥ 6.5%, or self-reported diabetes during follow-up[25]. We censored participants at the time of diagnosis of prediabetes or the last visit, whichever came first[26].
Data collection and covariates
In this study, we collected data from both Chinese and Japanese populations, focusing on shared variables such as demographic characteristics (age and gender), FPG, BMI, alanine aminotransferase (ALT), systolic blood pressures (SBP), TG, total cholesterol (TC), diastolic blood pressures (DBP), HDL-c, low-density lipoprotein cholesterol (LDL-c), aspartate aminotransferase (AST), smoking and drinking status.
The research employed a comprehensive standardized self-administered questionnaire to gather participants’ baseline clinical data. This detailed instrument captured critical information spanning medical history, personal health behaviors, and lifestyle characteristics. Participants were systematically categorized based on their health behaviors:
Smoking status: Classified as either “ever smokers” or “never smokers”.
Alcohol consumption: Grouped as “ever drinkers” or “never drinkers”.
These binary classifications were determined by participants’ current and historical patterns of tobacco and alcohol use.
All anthropometric measurements and blood specimens were collected by trained personnel following standardized protocols. Height and weight were measured with participants wearing light clothing and no shoes. BMI was calculated as weight in kilograms divided by the square of height in meters. Blood pressure was measured using calibrated sphygmomanometers after participants had rested for 5–10 min. Blood samples were collected after an overnight fast of at least 10 h and analyzed in standardized laboratories using automated analyzers.
Covariates were selected based on clinical relevance and previous literature[17, 27]. The following variables were included: age, gender, BMI, smoking status, drinking status, SBP, DBP, FPG, TC, LDL-c, ALT, and AST.
Missing data processing
The dataset contained varying proportions of missing values across different variables. Physiological parameters showed minimal missing data: blood pressure measurements (n = 16, 0.012%), TC (n = 1, 0.00078%), LDL-c (n = 184, 0.144%), and ALT (n = 397, 0.310%). More substantial missing data were observed for liver function (AST: n = 64807, 50.656%), and lifestyle factors (smoking and drinking status: n = 81136, 63.420%). To address potential bias and optimize data utilization, we implemented multiple imputation by chained equations[28]. This imputation included BMI, SBP, age, gender, DBP, ALT, TC, HDL-c, FPG, TG, LDL-c, AST, and drinking and smoking status. The missing data were analyzed based on the assumption that they were missing at random (MAR) [29].
Statistical analysis
Baseline characteristics were analyzed across AIP quartiles using comprehensive statistical methods. Continuous variables were presented as mean ± standard deviation for normally distributed data and median with interquartile range for skewed distributions, while categorical variables were reported as frequencies and percentages. Statistical comparisons employed one-way ANOVA or Kruskal-Wallis tests for continuous variables, and chi-square tests for categorical data. Survival analysis utilized the Kaplan-Meier method to estimate time-to-event variables, with the log-rank test applied to compare diabetes-free survival probabilities among different AIP groups, ensuring a rigorous and multifaceted approach to data interpretation.
Covariate collinearity was rigorously evaluated using the variance inflation factor (VIF)[30], calculated through the formula VIF = 1/(1-R²), where R² represents the coefficient of determination from a linear regression model. Each variable was systematically examined in this approach by regressing it against all other variables. A VIF exceeding 5 indicated significant multicollinearity, signaling that the variable would be excluded from subsequent multiple regression analyses to ensure statistical reliability and prevent potential bias in model interpretation (Table S1).
To analyze the association between AIP and the development of diabetes, we followed several analytical steps:
Step 1: univariate and multivariate Cox Proportional-hazards regression
The association between AIP and incident T2DM was examined using Cox proportional hazards regression models in the overall, Chinese, and Japanese populations. We constructed three models with progressive adjustment:
Model 1: Unadjusted.
Model 2: Adjusted for age, sex, BMI, SBP, DBP, smoking status, and drinking status.
Model 3: Additionally adjusted for FPG, TC, LDL-C, ALT, and AST.
Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated for each AIP quartile (using the lowest quartile as reference) and for AIP as a continuous variable (per 1 unit).
Covariate selection was guided by previous literature and collinearity assessment. TC was excluded from the multivariate analysis due to demonstrated collinearity (Table S1). The proportional hazards assumptions were validated using Schoenfeld residuals and log-minus-log plots. These models were designed to assess changes in effect estimates under different adjustment strategies and evaluate the results’ robustness.
Step 2: nonlinearity and Two-Piecewise regression analysis
To explore potential nonlinearity in the relationship between AIP and the risk of diabetes, a Cox proportional hazards regression model with cubic spline functions and smooth curve fitting (penalized spline method) was employed. If nonlinearity was detected, the inflection point was identified using a recursive algorithm, and a two-piecewise Cox proportional hazards regression model was constructed on either side of the inflection point. For sensitivity analysis, the standard linear regression model was compared to the two-piecewise Cox proportional hazards regression model, with the likelihood ratio test employed to determine the best fit for explaining the association between AIP and the risk of diabetes.
Step 3: subgroup analysis and sensitivity analysis
A stratified Cox proportional-hazards regression model was applied across subgroups (gender, BMI, age, SBP, DBP, drinking and smoking status, and country). Continuous variables were converted to categorical variables using specific clinical cut points: age (< 45, ≥ 45 to < 60, ≥60 years), BMI (< 18.5, ≥ 18.5 to < 24, ≥24 to 28, ≥ 28 kg/m2), SBP (< 140, ≥ 140mmHg), and DBP (< 90, ≥ 90mmHg) [27, 31]. We adjusted each stratification for all factors in addition to the stratification factor itself (SBP, gender, DBP, FPG, age, BMI, ALT, AST, LDL-c, drinking and smoking status). A likelihood ratio test comparing models with and without interaction terms was implemented to assess potential interactions between variables rigorously.
Multiple sensitivity analyses were conducted to validate the study’s findings. AIP was converted to a categorical variable based on quartiles, with trend analysis performed to examine potential non-linear relationships and confirm results obtained from continuous variable analysis. Additional sensitivity analyses were implemented to recognize the established links between lifestyle factors, metabolic conditions, and T2DM. These refined analyses systematically excluded participants with specific characteristics known to influence diabetes risk: those with histories of smoking and drinking[32], individuals with obesity (BMI ≥ 28 kg/m2), and participants presenting with hypertriglyceridemia (triglycerides ≥ 1.7 mmol/L)[33, 34]. In additional sensitivity analyses, we restricted the follow-up period to within 7 years to account for disparities in follow-up duration between the Chinese and Japanese cohorts. This methodical approach ensured the robustness and reliability of the study’s primary findings.
Step 4: predictive value of AIP for diabetes risk
The predictive ability of the AIP for incident T2DM was evaluated using receiver operating characteristic (ROC) curve analysis in the overall study population and population-specific subgroups (Chinese and Japanese). To ensure consistent follow-up periods between the two populations, we predicted the 5-year diabetes incidence risk.
The area under the curve (AUC) was calculated to assess the discriminative performance of AIP, with values of 0.5 indicating no discriminative ability and 1.0 indicating perfect discrimination. The Youden index (sensitivity + specificity − 1) was used to determine the optimal cut-off value of AIP for predicting T2DM. Sensitivity and specificity were calculated at this optimal threshold. Additionally, we performed sensitivity analyses by stratifying participants according to population-specific differences to evaluate the consistency of the predictive performance of AIP across different populations.
Step 5: mediation analysis of BMI in the AIP-diabetes association
To elucidate the potential pathways linking AIP to T2DM, we conducted formal mediation analyses to assess the potential mediating role of body mass index. The mediation analysis was performed using the method described by VanderWeele[35, 36], which decomposes the total effect of AIP on T2DM risk into direct effect (AIP→Diabetes) and indirect effect (AIP→BMI→Diabetes).
Cox proportional hazards models with bootstrapping procedures (5,000 replications) were employed to generate hazard ratios with 95% confidence intervals or direct, indirect, and total effects[37, 38]. The proportion mediated was calculated as the ratio of the natural indirect effect to the total impact, expressed as a percentage[39]. This represents the proportion of AIP’s effect on diabetes risk that operates through the BMI pathway. All mediation models were adjusted for potential confounders, including age, gender, SBP, DBP, FPG, LDL-c, ALT, AST, smoking, and drinking. Analyses were performed for the total study population and stratified by specific population (Chinese and Japanese subgroups) to assess potential population-specific mediation patterns. Recent research indicates that adult height significantly affects the utility of BMI as an estimate of fat mass and its association with type 2 diabetes risk[40]. Therefore, we conducted additional analyses to determine whether differences in adult height might explain our BMI-related results. In our mediation analyses, we adjusted for height as a potential confounder. Additionally, we performed a specific mediation analysis to evaluate whether height mediated the relationship between AIP and diabetes risk. This comprehensive approach allowed us to assess both the direct effect of AIP on diabetes risk and any indirect effects potentially mediated through adult height, providing insights into the complex pathways underlying these associations.
Given the notable difference in sample size between the Chinese (n = 112,483) and Japanese (n = 15,453) cohorts, we employed a stratified analytical approach rather than only data pooling. Each cohort was analyzed independently using identical statistical methods to ensure methodological consistency while preventing the larger Chinese cohort from dominating the findings. This separate analysis approach enabled valid within-population assessments while also allowing for between-population comparisons of effect patterns rather than direct statistical comparisons of raw values.
As an estimate of insulin resistance, the AIP may more strongly explain insulin resistance (IR) associated with hepatic steatosis rather than with visceral adiposity [41]. The authors calculate the fatty liver index (FLI) as an estimate of hepatic steatosis and the waist-to-height ratio (WHR), as an estimate of visceral adiposity and test whether differences in estimated hepatic steatosis and visceral adiposity may explain part of the observed findings. To further investigate the relationship between AIP and type 2 diabetes risk in a Japanese population, we conducted several statistical analyses. First, we used Pearson correlation coefficients to examine the correlations between AIP and measures of hepatic steatosis (FLI) and visceral adiposity (WHR). Cox proportional hazards regression models were constructed to assess the association between AIP and diabetes risk, with progressive adjustment for potential confounders. Model I included sex, age, drinking, BMI, ALT, SBP, DBP, FPG, smoking, AST, and LDL; Model II-a added WHR; Model II-b further adjusted for FLI; and Model II-c additionally controlled for FLI and WHR to determine whether these indices of hepatic steatosis and visceral adiposity attenuated the observed associations. To further elucidate potential effect modification, we performed stratified analyses by categorizing participants into tertiles of FLI (< 30, 30–60, ≥ 60) [42]and WHR (quartiles). Then we evaluated the association between AIP and diabetes risk within each stratum. Interaction terms were included to test for significant differences in AIP effects across these strata. Finally, we conducted formal mediation analyses to quantify the extent to which FLI and WHR independently mediated the relationship between AIP and diabetes risk.
Furthermore, considering the substantial number of participants with missing data for smoking status, alcohol consumption, and AST variables, which could potentially introduce bias through multiple imputation, we employed alternative missing data handling approaches. Participants with missing data were treated as a separate category for categorical variables with missing values. For continuous variables with missing values, we utilized dummy variable imputation. Following these alternative missing data handling procedures, we re-analyzed the relationship between AIP and diabetes risk using Cox regression analysis, threshold effect analysis, and mediation effect analysis to verify the robustness of our findings.
Statistical software
All statistical analyses were performed using R software (http://www.R-project.org, The R Foundation) and EmpowerStats software (X&Y Solutions, Inc; http://www.empowerstats.com). Statistical significance was defined as two-sided P < 0.05.
Results
Baseline characteristics of participants
The baseline characteristics of these included participants were listed in Table 1. The mean age was 43.67 ± 12.36 years, and 53.33% were male. The mean baseline AIP was − 0.099 ± 0.308. During a median follow-up time of 3.0 years, 1801(1.41%) people experienced diabetes.
Table 1.
The baseline characteristics of participants
| AIP group | Q1 (<-0.31) | Q2 (-0.31- -0.11) | Q3 (-0.11-0.10) | Q4 (≥ 0.10) | P-value |
|---|---|---|---|---|---|
| Participants | 31,980 | 31,988 | 31,983 | 31,985 | |
| Age (years) | 40.3 ± 10.7 | 42.7 ± 12.3 | 45.0 ± 12.9 | 46.6 ± 12.5 | < 0.001 |
| Gender, n (%) | < 0.001 | ||||
| Male | 8,761 (27.4) | 14,901 (46.6) | 19,810 (61.9) | 24,761 (77.4) | |
| Female | 23,219 (72.6) | 17,087 (53.4) | 12,173 (38.1) | 7,224 (22.6) | |
| Country, n (%) | < 0.001 | ||||
| Chinese | 24,616 (77.0) | 28,753 (89.9) | 29,376 (91.8) | 29,738 (93.0) | |
| Japanese | 7,364 (23.0) | 3,235 (10.1) | 2,607 (8.2) | 2,247 (7.0) | |
| Smoking status, n (%) | < 0.001 | ||||
| Non-smoker | 27,975 (87.5) | 26,048 (81.4) | 23,783 (74.4) | 20,781 (65.0) | |
| Smoker | 4,005 (12.5) | 5,940 (18.6) | 8,200 (25.6) | 11,204 (35.0) | |
| Drinking status, n (%) | < 0.001 | ||||
| Non-drinker | 24,756 (77.4) | 25,593 (80.0) | 24,780 (77.5) | 23,547 (73.6) | |
| Drinker | 7,224 (22.6) | 6,395 (20.0) | 7,203 (22.5) | 8,438 (26.4) | |
| BMI (kg/m²) | 21.1 ± 2.5 | 22.4 ± 2.9 | 23.7 ± 3.1 | 25.2 ± 3.0 | < 0.001 |
| SBP (mmHg) | 112.1 ± 14.5 | 116.7 ± 15.7 | 120.6 ± 16.3 | 124.4 ± 16.2 | < 0.001 |
| DBP (mmHg) | 69.6 ± 9.8 | 72.6 ± 10.3 | 75.1 ± 10.7 | 78.2 ± 10.9 | < 0.001 |
| FPG (mmol/L) | 4.8 ± 0.5 | 4.9 ± 0.5 | 4.9 ± 0.5 | 5.0 ± 0.6 | < 0.001 |
| TC (mmol/L) | 4.6 ± 0.8 | 4.7 ± 0.9 | 4.9 ± 0.9 | 5.1 ± 0.9 | < 0.001 |
| TG (mmol/L) | 0.6 (0.5–0.7) | 0.9 (0.8-1.0) | 1.3 (1.1–1.4) | 2.1 (1.7–2.7) | < 0.001 |
| HDL-C (mmol/L) | 1.6 ± 0.3 | 1.4 ± 0.2 | 1.3 ± 0.2 | 1.1 ± 0.2 | < 0.001 |
| LDL-C (mmol/L) | 2.6 ± 0.6 | 2.7 ± 0.7 | 2.9 ± 0.7 | 3.0 ± 0.8 | < 0.001 |
| ALT (U/L) | 14.0 (11.0-18.4) | 16.0 (12.0-22.4) | 19.0 (14.0-27.9) | 25.7 (18.0–38.0) | < 0.001 |
| AST (U/L) | 19.2 (15.6–24.0) | 20.9 (16.8–25.8) | 22.2 (18.0-27.9) | 24.8 (19.8–31.2) | < 0.001 |
| AIP | -0.5 ± 0.1 | -0.2 ± 0.1 | -0.0 ± 0.1 | 0.3 ± 0.2 | < 0.001 |
Values are n (%), mean ± SD or medians (quartiles)
BMI, body mass index; FPG, fasting plasma glucose; DBP, diastolic blood pressure; TC, total cholesterol; SBP, systolic blood pressure; TG, triglyceride; ALT, alanine aminotransferase; LDL-c, low-density lipid cholesterol; AST, aspartate aminotransferase; HDL-c, high-density lipoprotein cholesterol; AIP, atherogenic index of plasma
We assigned the adults into subgroups using AIP quartiles (<-0.31, -0.31 to -0.11, -0.11 to 0.10, ≥ 0.10). When compared with the Q1(<-0.31) group, the values or proportions of females, non-smokers, Japanese participants, and HDL-c decreased significantly in the Q4(AIP ≥ 0.10) group. In contrast, the opposite results were detected in covariates regarding age, BMI, SBP, DBP, FPG, TC, TG, LDL-C, ALT, AST, males, smokers, and drinkers. All comparisons demonstrated statistical significance (p < 0.001). Table S2 compares demographic and clinical characteristics between Chinese (n = 112,483) and Japanese (n = 15,453) populations, showing similar age distribution but significant differences in lifestyle factors, with Japanese having higher smoking (41.6% vs. 20.4%) and drinking rates (69.4% vs. 16.5%). Chinese participants demonstrated higher BMI (23.3 vs. 22.1 kg/m²) and blood pressure values. In contrast, Japanese participants had higher total cholesterol, LDL-c, and HDL-c levels, with all differences being statistically significant (p < 0.001).
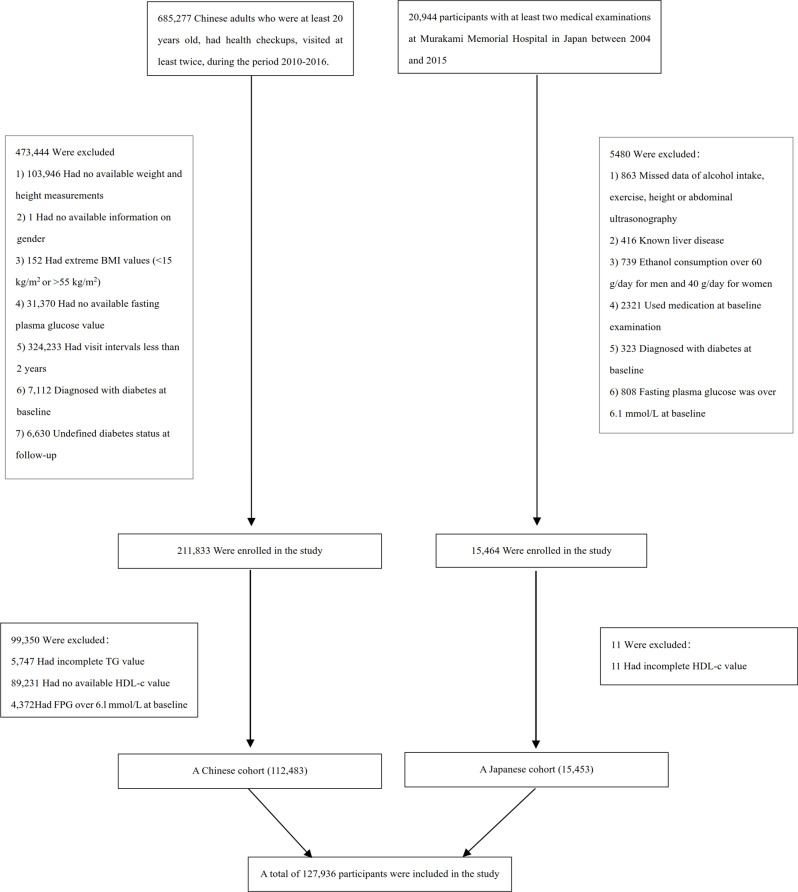
Figure 2 illustrates the distribution of AIP across three distinct populations. The histograms depict AIP values with normal distribution patterns across the overall population (panel A), the Chinese population (panel B), and the Japanese population (panel C). In the overall population, AIP values displayed a relatively normal distribution with slight right skewness. The Chinese population demonstrated a similar distribution pattern but with a slightly higher frequency in the mid-range AIP values, indicating a potential population-specific risk profile. The Japanese population exhibited a more concentrated distribution, with most individuals clustering around the lower to mid-range AIP values.
Fig. 2.
Distribution of Atherogenic Index of Plasma (AIP) across different populations. The histograms show the distribution of AIP values in (A) the overall study population (n = 127,936), (B) the Chinese subpopulation (n = 112,483), and (C) the Japanese subpopulation (n = 15,453). The x-axis represents AIP values, while the y-axis shows the count of individuals. The overall population showed a mean AIP of -0.099 (SD 0.308), while the Chinese and Japanese populations had mean AIP values of -0.075 (SD 0.295) and − 0.273 (SD 0.343), respectively
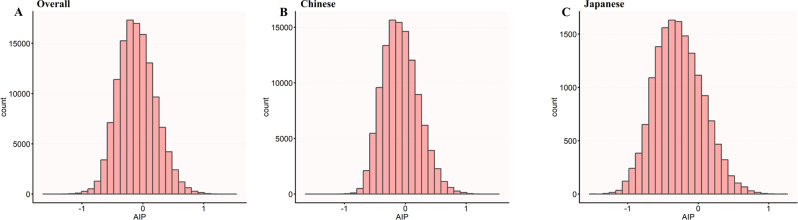
Figure 3 demonstrates distinct AIP distributions between diabetic and non-diabetic individuals across populations. The Japanese population (Panel C) notably exhibited the most pronounced separation between diabetic and non-diabetic groups, with minimal overlap between the two density curves. The diabetic Japanese subjects showed a clear rightward shift in AIP distribution compared to their non-diabetic counterparts. While the Chinese population (Panel B) also displayed differences between diabetic and non-diabetic groups, the separation was less distinct than in the Japanese cohort, with greater overlap between the curves.
Fig. 3.
AIP density distribution by diabetes status across populations. Kernel density plots of AIP by diabetes status (Group 0 = non-diabetic; Group 1 = diabetic) in (A) overall, (B) Chinese, and (C) Japanese populations. The Japanese population demonstrated the most pronounced separation between diabetic and non-diabetic groups, with diabetic individuals showing markedly higher AIP values. The minimal overlap between curves in the Japanese cohort suggests diabetes may strongly influence atherogenic risk in this population
The incidence rate of diabetes was analyzed according to age group, sex, and specific population. Figure 4 illustrates that the incidence rates progressively increased with advancing age across all demographic subgroups. A notable sex difference was observed, with males consistently exhibiting higher diabetes incidence than females across all age strata. When comparing population-specific groups, Japanese individuals demonstrated markedly higher incidence rates of diabetes compared to their Chinese counterparts in both sexes. This population-specific disparity was particularly pronounced in the middle and older age groups (40–70 years). The highest incidence rates were observed in Japanese males aged ≥ 70 years, approaching 7.14%. These findings suggest that age, male sex, and Japanese ethnicity are significant risk factors for diabetes development in Asian populations.
Fig. 4.
Age-specific incidence rates of diabetes stratified by sex and specific population. The figure depicts the incidence rates of diabetes (%) across different age groups (< 30, 30–40, 40–50, 50–60, 60–70, ≥ 70 years) in three population categories: overall (left panel), Chinese (middle panel), and Japanese (right panel), with further stratification by sex. Blue bars represent males, red bars represent females, and gray bars represent combined data (All). In all subgroups, incidence rates consistently increase with advancing age, with peak rates observed in the ≥ 70 age group. Males demonstrated higher incidence rates than females across all age groups and specific populations. Japanese populations exhibited substantially higher diabetes incidence compared to Chinese populations
The incidence rate of diabetes
Table 2 presents the incidence rates of diabetes across different AIP quartiles. Among 127,936 participants, 1,801 incident diabetes cases were identified during the follow-up period, with an overall incidence rate of 1.41% (95% CI: 1.34–1.47%) and a cumulative incidence of 4.07 per 1000 person-years.
Table 2.
Incidence rate of incident diabetes
| AIP | Participants(n) | Diabetes events(n) | Incidence rate (95% CI) (%) | Cumulative incidence (Per 1000 person-years) |
|---|---|---|---|---|
| All participants | ||||
| Total | 127,936 | 1801 | 1.41(1.34–1.47) | 4.07 |
| Q1(<-0.31) | 31,980 | 160 | 0.50(0.42–0.58) | 1.35 |
| Q2(-0.31- -0.11) | 31,988 | 255 | 0.80(0.70–0.89) | 2.35 |
| Q3(-0.11-0.10) | 31,983 | 482 | 1.51(1.37–1.64) | 4.49 |
| Q4(≥ 0.10) | 31,985 | 904 | 2.83(2.64–3.01) | 8.38 |
| P for trend | < 0.001 | |||
| Chinese participants | ||||
| Total | 112,483 | 1428 | 1.27(1.20–1.34) | 4.09 |
| Q1(<-0.31) | 24,616 | 98 | 0.40(0.32–0.48) | 1.28 |
| Q2(-0.31- -0.11) | 28,753 | 199 | 0.69(0.60–0.79) | 2.25 |
| Q3(-0.11-0.10) | 29,376 | 391 | 1.33(1.20–1.46) | 4.31 |
| Q4(≥ 0.10) | 29,738 | 740 | 2.49(2.31–2.67) | 7.91 |
| P for trend | < 0.001 | |||
| Japanese participants | ||||
| Total | 15,453 | 373 | 2.41(2.17–2.66) | 3.99 |
| Q1(<-0.31) | 7364 | 62 | 0.84(0.63–1.05) | 1.46 |
| Q2(-0.31- -0.11) | 3235 | 56 | 1.73(1.28–2.18) | 2.77 |
| Q3(-0.11-0.10) | 2607 | 91 | 3.49(2.79–4.20) | 5.52 |
| Q4(≥ 0.10) | 2247 | 164 | 7.30(6.22–8.37) | 11.48 |
| P for trend | < 0.001 |
AIP, atherogenic index of plasma
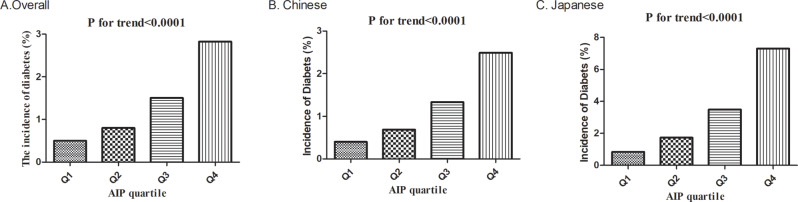
A significant dose-response relationship was observed between AIP quartiles and diabetes incidence (p for trend < 0.001). The incidence rate progressively increased from 0.50% (95% CI: 0.42–0.58%) in the lowest quartile (Q1: <-0.31) to 0.80% (95% CI: 0.70–0.89%) in Q2, 1.51% (95% CI: 1.37–1.64%) in Q3, and 2.83% (95% CI: 2.64–3.01%) in the highest quartile (Q4: ≥0.10). Similarly, the cumulative incidence increased substantially from 1.35 to 8.38 per 1000 person-years across the quartiles.
When stratified by specific population, Chinese participants (n = 112,483) exhibited an overall diabetes incidence of 1.27% (95% CI: 1.20–1.34%), with rates increasing from 0.40% in Q1 to 2.49% in Q4 (p for trend < 0.001). Among Japanese participants (n = 15,453), the overall incidence was higher at 2.41% (95% CI: 2.17–2.66%), with a steeper gradient across AIP quartiles, rising from 0.84% in Q1 to 7.30% in Q4 (p for trend < 0.001).
These findings indicate that elevated AIP values are associated with substantially increased risk of diabetes development, with potentially stronger associations observed in Japanese compared to Chinese participants (Fig. 5).
Fig. 5.
Diabetes incidence by AIP quartiles. Bar charts showing diabetes incidence (%) by AIP quartiles in (A) the overall population, (B) the Chinese population, and (C) the Japanese population. All populations demonstrated a significant positive association between AIP quartiles and diabetes incidence (P for trend < 0.0001 for all groups), with the Japanese population showing the most pronounced gradient
The results of univariate analyses using the Cox proportional-hazards regression model
Univariate Cox regression analysis (Table 3) identified multiple significant factors associated with incident diabetes. FPG (HR: 9.504, 95% CI: 8.525–10.596) and AIP (HR: 8.472, 95% CI: 7.426–9.666) showed substantial associations with diabetes risk. Demographic factors revealed that Japanese participants had a lower risk compared to Chinese (HR: 0.263), females demonstrated reduced risk relative to males (HR: 0.525), and increasing age was associated with diabetes development (HR: 1.069 per year). Among lifestyle factors, smoking status was positively associated with diabetes incidence (HR: 1.559), while alcohol consumption showed an inverse association (HR: 0.785). Clinical parameters, including BMI (HR: 1.237), blood pressure measures, and lipid profiles, all demonstrated significant associations with diabetes incidence (all p < 0.001).
Table 3.
Univariate analysis of incident diabetes risk factors among Chinese and Japanese populations
| Variable | Statistics | HR | 95% CI | P-value |
|---|---|---|---|---|
| Country | ||||
| Chinese | 112,483 (87.921%) | Ref. | ||
| Japanese | 15,453 (12.079%) | 0.263 | (0.222, 0.311) | < 0.001 |
| Age (years) | 43.669 ± 12.355 | 1.069 | (1.065, 1.072) | < 0.001 |
| BMI (kg/m²) | 23.125 ± 3.273 | 1.237 | (1.225, 1.250) | < 0.001 |
| SBP (mmHg) | 118.424 ± 16.343 | 1.039 | (1.036, 1.041) | < 0.001 |
| DBP (mmHg) | 73.892 ± 10.880 | 1.048 | (1.044, 1.052) | < 0.001 |
| FPG (mmol/L) | 4.922 ± 0.534 | 9.504 | (8.525, 10.596) | < 0.001 |
| TC (mmol/L) | 4.819 ± 0.896 | 1.286 | (1.225, 1.349) | < 0.001 |
| LDL (mmol/L) | 2.816 ± 0.706 | 1.220 | (1.149, 1.295) | < 0.001 |
| ALT (U/L) | 23.123 ± 20.935 | 1.004 | (1.004, 1.005) | < 0.001 |
| AST (U/L) | 23.301 ± 12.074 | 1.007 | (1.006, 1.008) | < 0.001 |
| Smoking Status | ||||
| Non-smoker | 98,587 (77.060%) | Ref. | ||
| Smoker | 29,349 (22.940%) | 1.559 | (1.416, 1.716) | < 0.001 |
| Drinking Status | ||||
| Non-drinker | 98,676 (77.129%) | Ref. | ||
| Drinker | 29,260 (22.871%) | 0.785 | (0.706, 0.873) | < 0.001 |
| AIP | -0.099 ± 0.308 | 8.472 | (7.426, 9.666) | < 0.001 |
| Gender | ||||
| Male | 68,233 (53.334%) | Ref. | ||
| Female | 59,703 (46.666%) | 0.525 | (0.475, 0.581) | < 0.001 |
BMI, body mass index; FPG, fasting plasma glucose; DBP, diastolic blood pressure; TC, total cholesterol; SBP, systolic blood pressure; ALT, alanine aminotransferase; LDL-c, low-density lipid cholesterol; AST, aspartate aminotransferase; AIP, atherogenic index of plasma
HR, Hazard ratios; CI: confidence interval, Ref: reference
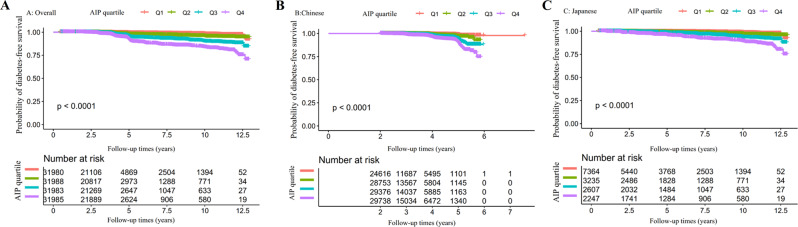
Kaplan-Meier survival analysis stratified by AIP quartiles demonstrated significant associations between AIP levels and diabetes-free survival (Fig. 6). In the overall cohort (Panel A), a stepwise decrease in diabetes-free survival probability was observed with increasing AIP quartiles (p < 0.0001), with the highest quartile (Q4) showing the poorest outcomes. This pattern was consistently maintained when analyzing population-specific subgroups separately.
Fig. 6.
Diabetes-free survival probability according to the atherogenic index of plasma quartiles in overall and specific populations. Kaplan-Meier curves showing diabetes-free survival probability stratified by AIP quartiles in the overall population (A), Chinese (B), and Japanese (C) cohorts. Higher AIP quartiles (Q4) consistently demonstrated significantly lower diabetes-free survival compared to lower quartiles (Q1) across all population groups (all p < 0.0001). Numbers at risk for each quartile are displayed below the graphs at specified follow-up time points. Follow-up ranged from 0 to 12.5 years in the overall and Japanese cohorts, and 0 to 7 years in the Chinese cohort. The stepwise separation of survival curves by AIP quartiles suggests a dose-response relationship between atherogenic lipid profiles and diabetes risk
In the Chinese population (Panel B), all four AIP quartiles showed distinct survival curves, with progressively worse diabetes-free survival from Q1 to Q4 (p < 0.0001). The Japanese cohort (Panel C) exhibited a similar trend with generally lower diabetes-free survival probabilities than their Chinese counterparts at equivalent AIP quartiles. Notably, the separation between survival curves was most pronounced between the extreme quartiles (Q1 vs. Q4) in both population-specific groups, suggesting that AIP may be a valuable predictor of diabetes development across diverse Asian populations.
Pearson correlation analysis revealed significant positive associations between AIP and metabolic parameters, with a moderate correlation observed for WHR (r = 0.444, 95% CI: 0.431–0.457, P < 0.001) and a strong correlation with FLI (r = 0.685, 95% CI: 0.677–0.693, P < 0.001) (Table S3).
Results from a multivariate Cox proportional-hazards regression model
Table 4 demonstrates the relationship between AIP and incident diabetes risk across three increasingly adjusted models, with analyses stratified by specific population.
Table 4.
Relationship between AIP and the incident diabetes in different models
| AIP Exposure | Overall (HR,95%CI, P) | Chinese (HR,95%CI, P) | Japanese (HR,95%CI, P) |
|---|---|---|---|
| Model I: | |||
| AIP | 8.472 (7.426, 9.666) P < 0.001 | 6.771 (5.814, 7.886) P < 0.001 | 10.260 (7.713, 13.648) P < 0.001 |
| AIP Quartiles | |||
| Q1 | Ref. | Ref. | Ref. |
| Q2 | 2.064 (1.693, 2.516) P < 0.001 | 1.839 (1.444, 2.342) P < 0.001 | 1.835 (1.279, 2.634) P = 0.001 |
| Q3 | 4.097 (3.424, 4.902) P < 0.001 | 3.540 (2.837, 4.418) P < 0.001 | 3.629 (2.628, 5.012) P < 0.001 |
| Q4 | 7.592 (6.411, 8.989) P < 0.001 | 6.199 (5.021, 7.653) P < 0.001 | 7.525 (5.617, 10.080) P < 0.001 |
| P for trend | P < 0.001 | P < 0.001 | P < 0.001 |
| Model II: | |||
| AIP | 3.032 (2.582, 3.560) P < 0.001 | 2.499 (2.081, 3.002) P < 0.001 | 4.119 (2.892, 5.866) P < 0.001 |
| AIP Quartiles | |||
| Q1 | Ref. | Ref. | Ref. |
| Q2 | 1.266 (1.036, 1.548) P = 0.021 | 1.180 (0.925, 1.507) P = 0.183 | 1.142 (0.787, 1.657) P = 0.485 |
| Q3 | 1.768 (1.465, 2.133) P < 0.001 | 1.567 (1.248, 1.969) P < 0.001 | 1.743 (1.229, 2.474) P = 0.002 |
| Q4 | 2.445 (2.033, 2.939) P < 0.001 | 2.046 (1.636, 2.559) P < 0.001 | 2.838 (2.020, 3.987) P < 0.001 |
| P for trend | P < 0.001 | P < 0.001 | P < 0.001 |
| Model III: | |||
| AIP | 2.390 (2.047, 2.792) P < 0.001 | 1.843 (1.540, 2.206) P < 0.001 | 2.420 (1.665, 3.516) P < 0.001 |
| AIP Quartiles | |||
| Q1 | Ref. | Ref. | Ref. |
| Q2 | 1.204 (0.984, 1.473) P = 0.071 | 1.099 (0.861, 1.403) P = 0.449 | 0.893 (0.612, 1.303) P = 0.557 |
| Q3 | 1.718 (1.423, 2.074) P < 0.001 | 1.432 (1.140, 1.800) P = 0.002 | 1.346 (0.944, 1.920) P = 0.101 |
| Q4 | 2.197 (1.825, 2.643) P < 0.001 | 1.704 (1.360, 2.134) P < 0.001 | 1.771 (1.237, 2.536) P = 0.002 |
| P for trend | P < 0.001 | P < 0.001 | P < 0.001 |
Model I: We did not adjust other covariates
Model II: We adjust age, gender, BMI, SBP, DBP, smoking, and drinking status
Model III: We adjust age, gender, BMI, SBP, DBP, FPG, LDL-c, ALT, AST, smoking, and drinking status
HR, Hazard ratios; CI: confidence, Ref: reference; AIP, atherogenic index of plasma
In the unadjusted model (Model I), AIP as a continuous variable was strongly associated with diabetes risk in the overall population (HR: 8.472, 95% CI: 7.426–9.666, P < 0.001), with a notably stronger association observed in Japanese (HR: 10.260, 95% CI: 7.713–13.648) compared to Chinese participants (HR: 6.771, 95% CI: 5.814–7.886). When analyzed by quartiles, a clear dose-response relationship was evident, with the highest quartile (Q4) showing substantially elevated risk compared to the lowest quartile (Q1) in the overall population (HR: 7.592, 95% CI: 6.411–8.989), Chinese (HR: 6.199, 95% CI: 5.021–7.653), and Japanese participants (HR: 7.525, 95% CI: 5.617–10.080) (all P for trend < 0.001).
After adjusting for demographic and lifestyle factors in Model II (age, gender, BMI, blood pressure, smoking, and drinking status), the associations remained significant. Still, they were attenuated, with AIP as a continuous variable showing HRs of 3.032 (95% CI: 2.582–3.560), 2.499 (95% CI: 2.081–3.002), and 4.119 (95% CI: 2.892–5.866) in the overall, Chinese, and Japanese populations, respectively. The Q4 versus Q1 comparison yielded HRs of 2.445 (95% CI: 2.033–2.939), 2.046 (95% CI: 1.636–2.559), and 2.838 (95% CI: 2.020–3.987) for the respective populations.
In the fully adjusted model (Model III), which additionally controlled for metabolic factors (FPG, LDL-c, ALT, and AST), AIP maintained significant associations with diabetes risk as a continuous variable (overall HR: 2.390, 95% CI: 2.047–2.792; Chinese HR: 1.843, 95% CI: 1.540–2.206; Japanese HR: 2.420, 95% CI: 1.665–3.516). Comparing extreme quartiles (Q4 vs. Q1), the risk remained significantly elevated in all populations (overall HR: 2.197, 95% CI: 1.825–2.643; Chinese HR: 1.704, 95% CI: 1.360–2.134; Japanese HR: 1.771, 95% CI: 1.237–2.536).
The significant positive trends across quartiles persisted in all three models (all P for trend < 0.001), indicating a robust dose-dependent relationship between AIP and diabetes risk, even after comprehensive adjustment for potential confounders.
Sensitivity analysis
Table 5 presents the sensitivity analyses examining the robustness of the association between AIP and incident diabetes across specific subgroups. Four separate models were constructed to address potential confounding factors, each targeting a distinct population while adjusting for comprehensive covariates.
Table 5.
Relationship between AIP and diabetes in different sensitivity analyses
| Exposure | Model I (HR,95%CI, P) | Model II (HR,95%CI, P) | Model III (HR,95%CI, P) | Model IV (HR,95%CI, P) |
|---|---|---|---|---|
| AIP | 2.507 (2.097, 2.996) < 0.001 | 2.104 (1.715, 2.582) < 0.001 | 2.355 (1.949, 2.846) < 0.001 | 2.236 (1.623, 3.080) < 0.0001 |
| AIP (Quartile) | ||||
| Q1 | Ref. | Ref. | Ref. | Ref. |
| Q2 | 1.206 (0.974, 1.492) 0.085 | 1.328 (1.047, 1.684) 0.020 | 1.245 (0.971, 1.596) 0.084 | 1.152 (0.940, 1.412) 0.173 |
| Q3 | 1.665 (1.360, 2.038) < 0.001 | 1.754 (1.399, 2.200) < 0.001 | 1.804 (1.430, 2.275) < 0.001 | 1.552 (1.278, 1.886) < 0.001 |
| Q4 | 2.228 (1.826, 2.719) < 0.001 | 2.074 (1.654, 2.600) < 0.001 | 2.243 (1.782, 2.824) < 0.001 | 1.534 (1.210, 1.946) < 0.001 |
| P for trend | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
Model I was a sensitivity analysis in participants without BMI ≥ 28 kg/ m2 (N = 118,405). We adjusted age, gender, BMI, SBP, DBP, FPG, LDL-c, ALT, AST, smoking, and drinking status
Model II was a sensitivity analysis performed on never-smoking participants (N = 98,587). We adjusted age, gender, BMI, SBP, DBP, FPG, LDL-c, ALT, AST, and drinking status
Model III was a sensitivity analysis performed on never-drinking participants (N = 98,676). We adjusted age, gender, BMI, SBP, DBP, FPG, LDL-c, ALT, AST, and smoking status
Model IV was a sensitivity analysis in participants without TG ≥ 1.7mmol/L (N = 101,364). We adjusted age, gender, BMI, SBP, DBP, FPG, LDL-c, ALT, AST, smoking, and drinking status
HR, Hazard ratios; CI: confidence, Ref: reference; AIP, atherogenic index of plasma
The association between AIP (as a continuous variable) and diabetes risk remained significant across all sensitivity analyses. In non-obese participants (BMI < 28 kg/m², Model I), AIP demonstrated a strong association with diabetes risk (HR: 2.507, 95% CI: 2.097–2.996, P < 0.001). This relationship persisted among never smokers (Model II: HR: 2.104, 95% CI: 1.715–2.582, P < 0.001), never drinkers (Model III: HR: 2.355, 95% CI: 1.949–2.846, P < 0.001), and notably, among participants with normal triglyceride levels (< 1.7mmol/L, Model IV: HR: 2.236, 95% CI: 1.623–3.080, P < 0.001).
When examined by quartiles, a consistent dose-response relationship was observed across all models (all P for trend < 0.001). The highest quartile (Q4) consistently demonstrated significantly elevated risk compared to the lowest quartile (Q1) in non-obese participants (HR: 2.228, 95% CI: 1.826–2.719), never smokers (HR: 2.074, 95% CI: 1.654-2.600), never drinkers (HR: 2.243, 95% CI: 1.782–2.824), and participants with normal triglycerides (HR: 1.534, 95% CI: 1.210–1.946) (all P < 0.001). In sensitivity analyses with follow-up time restricted to within 7 years, the positive association between AIP and diabetes risk remained consistent and statistically significant across all adjustment models (fully-adjusted HR = 2.406, 95% CI: 2.048–2.827, P < 0.001), with similar patterns observed in both Chinese and Japanese cohorts, confirming the robustness of our primary findings (Table S4). After applying alternative imputation methods for missing values, the strong positive association between AIP and incident diabetes remained consistent across all models in both Chinese and Japanese populations (fully-adjusted HR = 2.914, 95% CI: 2.483–3.421, P < 0.001), with a clear dose-response relationship observed across AIP quartiles, further confirming the robustness of our primary findings (Table S5). In the Japanese population, the positive association between AIP and diabetes risk remained significant and robust across all adjustment models, even after additional adjustment for potential mediators including WHR (HR = 3.286, 95% CI: 2.269–4.759, P < 0.001), FLI (HR = 2.344, 95% CI: 1.481–3.709, P < 0.001), or both WHR and FLI simultaneously (HR = 2.494, 95% CI: 1.568–3.967, P < 0.001), suggesting that the relationship between AIP and diabetes risk is independent of central obesity and fatty liver status (Table S6).
These findings indicate that the association between elevated AIP and increased diabetes risk is robust and independent of established risk factors, including obesity, smoking, alcohol consumption, and hypertriglyceridemia.
The non-linearity addressed by the Cox proportional hazards regression model with cubic spline functions
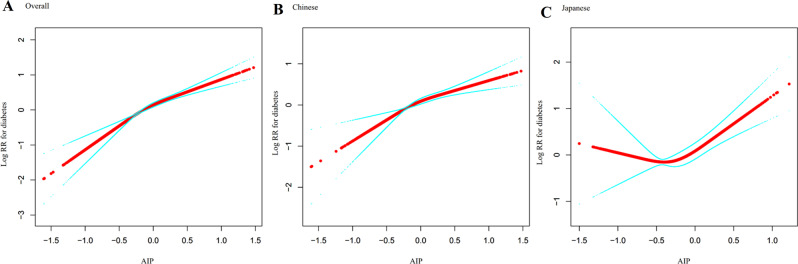
Figure 7 illustrates the complex, non-linear relationship between AIP and diabetes risk in Asian populations. The restricted cubic spline curves visually demonstrate the dose-response pattern between AIP and diabetes risk, while the two-piecewise Cox regression model (Table 6) quantifies this non-linear association with distinct inflection points across different population-specific groups.
Fig. 7.
Dose-response relationship between atherogenic index of plasma and diabetes risk with population-specific threshold effects. The figure shows restricted cubic spline curves depicting the dose-response relationship between AIP and the logarithm of relative risk (Log RR) for diabetes. Panel A presents data for the overall study population, while Panels B and C show population-stratified analyses for Chinese (left) and Japanese (right) cohorts. The solid lines represent the estimated Log RR values. All three analyses demonstrate positive associations between AIP and diabetes risk, with evidence of non-linearity
Table 6.
The result of the two-piecewise Cox regression model
| Incident diabetes | Overall (HR,95%CI, P) | Chinese (HR,95%CI, P ) | Japanese (HR,95%CI, P ) |
|---|---|---|---|
| Fitting the model by standard Cox regression | 2.390 (2.047, 2.792) < 0.0001 | 1.843 (1.540, 2.206) < 0.001 | 2.420 (1.665, 3.516) < 0.001 |
| Fitting model by two-piecewise Cox regression model | |||
| Inflection point of AIP | 0.432 | 0.436 | -0.449 |
| ≤Inflection point | 2.956 (2.408, 3.627) < 0.001 | 2.180 (1.717, 2.768) < 0.001 | 0.292 (0.051, 1.657) 0.165 |
| >Inflection point | 0.966 (0.528, 1.766) 0.910 | 0.951 (0.493, 1.834) 0.880 | 2.842 (1.915, 4.218) < 0.001 |
| P for log-likelihood ratio test | 0.001 | 0.034 | 0.027 |
We adjusted age, gender, BMI, SBP, DBP, FPG, LDL-c, ALT, AST, smoking, and drinking status
HR, Hazard ratios; CI: confidence, Ref: reference; AIP, atherogenic index of plasma
A significant non-linear association was observed in the overall population, with the two-piecewise Cox regression model identifying an inflection point at AIP = 0.432 (p for log-likelihood ratio test = 0.001). For AIP values below this threshold, each unit increase in AIP was associated with a nearly three-fold higher prediabetes risk (HR = 2.956, 95% CI: 2.408–3.627, p < 0.001), whereas for AIP values above this threshold, no significant association was detected (HR = 0.966, 95% CI: 0.528–1.766, p = 0.910).
Notable population-specific differences emerged in the analysis. The Chinese cohort exhibited a similar pattern to the overall population, with an inflection point at AIP = 0.436. The association was strong below this inflection point (HR = 2.180, 95% CI: 1.717–2.768, p < 0.001) but non-significant above it (HR = 0.951, 95% CI: 0.493–1.834, p = 0.880). In striking contrast, the Japanese cohort demonstrated a reversed pattern with an inflection point at AIP=-0.449. For this population, the association was non-significant below the threshold (HR = 0.292, 95% CI: 0.051–1.657, p = 0.165) but strongly significant above it (HR = 2.842, 95% CI: 1.915–4.218, p < 0.001).
Threshold effect analysis using alternative imputation methods confirmed non-linear relationships between AIP and diabetes risk across populations. Significant inflection points were identified in the overall (0.432), Chinese (0.433), and Japanese (-0.449) populations (all P < 0.05 for log-likelihood ratio tests). In the overall and Chinese cohorts, the risk increased dramatically below these thresholds but plateaued above them. In contrast, the Japanese cohort showed the opposite pattern with stronger associations above the threshold. These findings remained consistent with our primary analyses, further validating the robustness of the observed non-linear relationship between AIP and diabetes risk (Table S7).
The results of subgroup analyses
Table 7 illustrates the association between AIP and incident diabetes across various prespecified and exploratory subgroups. After comprehensive adjustment for potential confounders, significant effect modification was observed for age, BMI, and systolic blood pressure.
Table 7.
Effect size of AIP on incident diabetes in prespecified and exploratory subgroups
| Characteristic | No of participants | HR (95%CI) | P value | P for interaction |
|---|---|---|---|---|
| Age(years) | 0.0003 | |||
| 20 to <45 | 75,643 | 2.868 (2.125, 3.871) | <0.001 | |
| 45 to <60 | 36,278 | 2.789 (2.194, 3.546) | <0.001 | |
| ≥60 | 16,015 | 1.414 (1.071, 1.867) | <0.001 | |
| Gender | 0.7097 | |||
| Male | 68,233 | 2.265 (1.882, 2.726) | <0.001 | |
| Female | 59,703 | 2.416 (1.814, 3.219) | <0.001 | |
| BMI (kg/m2) | 0.0111 | |||
| <18.5 | 7561 | 2.322 (0.437, 12.334) | 0.323 | |
| ≥18.5, <24 | 72,305 | 3.416 (2.616, 4.462) | <0.001 | |
| ≥24, <28 | 38,156 | 2.474 (1.963, 3.117) | <0.001 | |
| ≥28 | 9914 | 1.678 (1.213, 2.322) | 0.002 | |
| Smoking status | 0.1639 | |||
| Non-smoker | 98,587 | 2.192 (1.799, 2.672) | <0.001 | |
| Smoker | 29,349 | 2.698 (2.143, 3.396) | <0.001 | |
| Drinking status | 0.1786 | |||
| Non-drinker | 98,676 | 2.234 (1.859, 2.686) | <0.001 | |
| Drinker | 29,260 | 2.747 (2.129, 3.544) | <0.001 | |
| SBP (mmHg) | 0.0401 | |||
| <140 | 115,579 | 2.601 (2.174, 3.111) | <0.001 | |
| ≥140 | 12,357 | 1.810 (1.340, 2.446) | <0.001 | |
| DBP (mmHg) | 0.7979 | |||
| <90 | 117,813 | 2.368 (1.996, 2.809) | <0.001 | |
| ≥90 | 10,123 | 2.492 (1.748, 3.553) | <0.001 | |
| Country | 0.1974 | |||
| Chinese | 112,483 | 1.862 (1.558, 2.227) | <0.001 | |
| Japanese | 15,453 | 2.423 (1.686, 3.481) | <0.001 |
Note 1: Above model was adjusted for age, gender, BMI, SBP, DBP, FPG, LDL-c, ALT, AST, smoking, and drinking status
Note 2: In each case, the model is not adjusted for the stratification variable
The strength of association between AIP and diabetes risk significantly decreased with advancing age (P for interaction = 0.0003). The association was most pronounced in younger adults aged 20–45 years (HR: 2.868, 95% CI: 2.125–3.871) and middle-aged adults aged 45–60 years (HR: 2.789, 95% CI: 2.194–3.546), while attenuated but still significant in participants aged ≥ 60 years (HR: 1.414, 95% CI: 1.071–1.867).
BMI significantly modified the AIP-diabetes relationship (P for interaction = 0.0111). The association was strongest among normal-weight individuals (BMI 18.5-<24 kg/m²; HR: 3.416, 95% CI: 2.616–4.462), followed by overweight individuals (BMI 24-<28 kg/m²; HR: 2.474, 95% CI: 1.963–3.117), and considerably weaker among obese participants (BMI ≥ 28 kg/m²; HR: 1.678, 95% CI: 1.213–2.322). No significant association was detected in underweight individuals (BMI < 18.5 kg/m²; HR: 2.322, 95% CI: 0.437–12.334, P = 0.323).
Similarly, systolic blood pressure significantly modified the association (P for interaction = 0.0401), with more potent effects observed in normotensive participants (SBP < 140 mmHg; HR: 2.601, 95% CI: 2.174–3.111) compared to hypertensive individuals (SBP ≥ 140 mmHg; HR: 1.810, 95% CI: 1.340–2.446).
No significant effect modification was observed for gender, smoking status, drinking status, diastolic blood pressure, or country of origin (all P for interaction > 0.05). Nevertheless, the association appeared numerically stronger among Japanese participants (HR: 2.423, 95% CI: 1.686–3.481) compared to Chinese participants (HR: 1.862, 95% CI: 1.558–2.227), though this difference was not statistically significant (P for interaction = 0.1974).
Subgroup analyses in the Japanese population demonstrated that the association between AIP and diabetes risk remained consistently significant across all FLI categories (< 30: HR = 2.733, P < 0.001; 30–60: HR = 2.371, P = 0.037; ≥60: HR = 3.650, P = 0.006) and all WHR quartiles (Q1-Q4: all P < 0.05), with no significant effect modification by either FLI (P for interaction = 0.692) or WHR (P for interaction = 0.176), suggesting that the relationship between AIP and diabetes risk is robust and independent of fatty liver status and central adiposity (Table S8).
Predictive performance of atherogenic index of plasma across populations
The AUC for the AIP demonstrated a moderate predictive ability for the overall cohort (AUC = 0.693), with similar performance observed among Chinese participants (AUC = 0.687) and a higher discriminative power in the Japanese subgroup (AUC = 0.735). These findings indicate that AIP may be a valuable marker for diabetes risk assessment, particularly within the Japanese population (Fig. 8).
Fig. 8.
Discriminative performance of the AIP for diabetes risk in overall, chinese, and Japanese populations. ROC curve illustrating the predictive value of AIP in the overall study cohort (AUC = 0.693) (A), in the Chinese subgroup (AUC = 0.687) (B), and the Japanese subgroup (AUC = 0.735) (C) sensitivity and 1-specificity values are plotted to assess the diagnostic performance of AIP in identifying individuals at increased risk of diabetes. AUC values were calculated to quantify the overall accuracy of AIP in each population. All analyses were conducted using standard ROC analysis methods. Higher AUC values are indicative of better discriminative capability
BMI partially mediates the association between AIP and incident diabetes
Table 8 presents the results of the mediation analysis examining the extent to which BMI mediates the relationship between AIP and incident diabetes. After adjusting for multiple confounding variables, we observed significant direct and indirect effects across all studied populations.
Table 8.
Mediation analysis of the effect of BMI on the AIP-diabetes relationship
| Effect | Total Population | Chinese Population | Japanese Population |
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Direct effect (AIP→Diabetes) | 2.35 (2.01–2.74) | 1.84 (1.54–2.21) | 2.42 (1.67–3.52) |
| Indirect effect (AIP→BMI→Diabetes) | 1.58 (1.50–1.67) | 1.45 (1.37–1.53) | 1.38 (1.26–1.50) |
| Total effect (AIP→Diabetes) | 3.43 (2.97–3.97) | 2.53 (2.13–2.99) | 3.28 (2.28–4.71) |
| Proportion mediated | 37.27% | 39.84% | 27.11% |
The model was adjusted for multiple variables, including age, gender, BMI, SBP, DBP, FPG, LDL-c, ALT, AST, smoking, and drinking status
AIP, Atherogenic Index of Plasma; BMI, Body Mass Index; HR, Hazard Ratio; CI, Confidence Interval
The proportion mediated refers to the percentage of the total effect of AIP on diabetes risk that is mediated through BMI. The analysis used bootstrap methods to derive confidence intervals for the mediation proportion
In the total population, AIP exhibited a substantial direct effect on diabetes risk (HR: 2.35, 95% CI: 2.01–2.74), while the indirect effect mediated through BMI was also significant (HR: 1.58, 95% CI: 1.50–1.67). The combined total effect yielded a hazard ratio of 3.43 (95% CI: 2.97–3.97), with BMI mediating 37.27% of this total effect.
We observed that the mediation pattern varied between populations when stratified by specific population. In the Chinese cohort, the direct effect of AIP on diabetes risk (HR: 1.84, 95% CI: 1.54–2.21) was complemented by a significant indirect effect through BMI (HR: 1.45, 95% CI: 1.37–1.53), with BMI mediating 39.84% of the total effect (HR: 2.53, 95% CI: 2.13–2.99). In contrast, the Japanese cohort demonstrated a stronger direct effect (HR: 2.42, 95% CI: 1.67–3.52) with a comparatively smaller proportion (27.11%) of the total effect (HR: 3.28, 95% CI: 2.28–4.71) being mediated through BMI (indirect effect HR: 1.38, 95% CI: 1.26–1.50).
Mediation analyses also demonstrated that BMI significantly mediated the association between AIP and diabetes risk across all populations even after adjustment for height, accounting for 34.67% of the total effect in the overall population (indirect effect HR = 1.54, 95% CI: 1.46–1.62), with similar patterns in Chinese (39.20%) and Japanese (27.00%) cohorts. In stark contrast, height showed virtually no mediation effect (proportion mediated: 0.18%, 0.03%, and − 0.50% in the total, Chinese, and Japanese populations, respectively), suggesting that adiposity, rather than body structure, plays a substantial role in the causal pathway between AIP and diabetes development (Table S9, S10).
In the Japanese population, mediation analyses revealed that fatty liver, as measured by FLI, played a substantially stronger role in mediating the association between AIP and diabetes risk compared to central adiposity (WHR). Specifically, FLI mediated 23.78% of the total effect (indirect effect HR = 1.24, 95% CI: 1.02–1.47), while WHR mediated only 7.30% (indirect effect HR = 1.07, 95% CI: 1.03–1.11), despite both mediators showing statistically significant indirect effects. These findings suggest that fatty liver development may be a more important pathophysiological pathway than central adiposity in linking atherogenic dyslipidemia to diabetes development in the Japanese population (Table S11).
Sensitivity analyses using alternative imputation methods confirmed the robustness of our primary findings on BMI’s mediating role in the AIP-diabetes relationship. The proportions of total effect mediated by BMI remained remarkably consistent with our main analysis: 35.7% in the overall population, 39.1% in the Chinese cohort, and 27.1% in the Japanese cohort. Similarly, the magnitudes of direct, indirect, and total effects were comparable between methods, demonstrating that our findings on adiposity as a significant mediator in the pathway from atherogenic dyslipidemia to diabetes development are not sensitive to the approach used for handling missing data (Table S12).
Discussion
Our study demonstrated a significant positive association between elevated AIP and incident diabetes in both Chinese and Japanese populations. This association remained robust after comprehensive adjustment for potential confounders, including demographic characteristics, lifestyle factors, and metabolic parameters. The relationship exhibited a dose-dependent pattern, with higher AIP quartiles consistently associated with greater diabetes risk. Furthermore, we observed non-linear relationships between AIP and diabetes risk, with identifiable threshold effects differing between Chinese and Japanese populations. Subgroup analyses revealed that the association was modified by age, BMI, and systolic blood pressure, while mediation analysis indicated that BMI partially mediated the effect of AIP on diabetes development.
In this study, we found that the overall diabetes incidence rate in the Chinese population was 1.27% (95% CI: 1.20–1.34%), while the Japanese population showed a significantly higher incidence of 2.41% (95% CI: 2.17–2.66%). This difference was consistent across all age groups, particularly pronounced in middle-aged and elderly populations between 40 and 70 years, with Japanese males over 70 years showing the highest diabetes incidence rate of nearly 7.14%. This difference in incidence rates may reflect variations in dietary patterns, lifestyle, and genetic factors between the two countries[43]. While rich in fish and vegetables, the traditional Japanese diet also contains a high proportion of refined carbohydrates such as white rice, which may lead to increased postprandial glucose fluctuations [44]. Additionally, Japanese populations generally face higher work stress and less physical activity, which have been established as independent risk factors for diabetes [45]. From a genetic perspective, studies have suggested that specific genetic variants in the Japanese population, such as polymorphisms in TCF7L2 and KCNQ1 gene loci, may increase susceptibility to diabetes[46]. Moreover, follow-up duration may also influence the difference in diabetes incidence rates between Chinese and Japanese populations. In this study, the maximum follow-up period for the Chinese cohort was approximately 7 years, while for the Japanese cohort it was around 12 years. Therefore, the incidence rate in the Japanese population might be relatively higher.
Moreover, we observed that the dose-effect relationship between AIP quartiles and diabetes incidence was steeper in the Japanese population, rising sharply from 0.84% in Q1 to 7.30% in Q4, compared to the Chinese population, which increased from 0.40% in Q1 to 2.49% in Q4. This suggests that elevated AIP levels may have a stronger impact on diabetes risk in the Japanese population, possibly related to their unique metabolic characteristics and lipid metabolism patterns[47].
After comprehensive adjustment for potential confounding factors, our study found a significant positive association between AIP and diabetes risk, consistent with previous research [15, 17]. In the fully adjusted model, each unit increase in AIP was associated with a 1.39-fold increase in diabetes risk in the overall population (HR: 2.390, 95% CI: 2.047–2.792), a 84% increase in the Chinese population (HR: 1.843, 95% CI: 1.540–2.206), and a 1.42-fold increase in the Japanese population (HR: 2.420, 95% CI: 1.665–3.516). These findings align with those of Yin et al. in the U.S. population, who reported that AIP was positively associated with insulin resistance and type 2 diabetes risk [13]. Notably, although the HR value for the Japanese population (2.42) appeared higher than that for the Chinese population (1.84) in the Cox regression analysis, our subgroup analysis showed that the interaction test by country did not reach statistical significance (P for interaction = 0.1974). This suggests that the strength of the association between AIP and diabetes may not substantially differ between the two populations, and the apparent difference might be attributed to imbalanced sample sizes and random variation. This finding enhances the robustness of our results, indicating that despite racial and cultural differences, the value of AIP as a marker of diabetes risk is consistent across different East Asian populations.
Sensitivity analyses further supported the robustness of the association between AIP and diabetes risk. In models excluding obese individuals, smokers, drinkers, and those with hypertriglyceridemia, the significant positive association between AIP and diabetes risk persisted. Particularly in individuals with normal triglyceride levels (< 1.7mmol/L), AIP remained significantly associated with diabetes risk (HR: 2.236, 95% CI: 1.623–3.080), suggesting that AIP may influence diabetes risk through mechanisms beyond merely reflecting elevated triglyceride levels [48].
Subgroup analyses revealed several essential effect modifiers. Age significantly influenced the association between AIP and diabetes risk (P for interaction = 0.0003), with the strongest associations observed in young (20–45 years) and middle-aged (45–60 years) populations, and weaker associations in the elderly (≥ 60 years). This is consistent with Sun et al.‘s findings that the association between AIP and type 2 diabetes weakened with increasing age (interaction P < 0.001) [16]. This age-dependent effect may reflect age-related changes in lipid metabolism and declining pancreatic function [49].
Using restricted cubic spline curves and piecewise Cox regression models, our study revealed a significant non-linear relationship between AIP and diabetes risk, with different inflection points identified in different populations. In the overall population, the inflection point was AIP = 0.432, below which each unit increase in AIP was associated with a nearly 2-fold increase in diabetes risk (HR = 2.956), while above this threshold, the association was no longer significant. The Chinese population exhibited a similar pattern to the overall population, with an inflection point at AIP = 0.436, below which the association was strong (HR = 2.180) and above which it was non-significant. This finding partially supports the study by Zhu et al. in non-obese Chinese adults, who identified a clinically significant threshold of -0.02 [15], but also differs from ours. This discrepancy may stem from differences in study population characteristics (such as including obese individuals in our study) and follow-up duration.
Interestingly, the Japanese population demonstrated an opposite pattern, with an inflection point at AIP=-0.449, below which the association was non-significant, while above this threshold it was strongly significant (HR = 2.842). This is similar to the J-shaped relationship observed by Zhou et al. in the Japanese population, who reported significantly increased type 2 diabetes risk when AIP exceeded − 0.268 [17].
The differences in non-linear relationship patterns between Chinese and Japanese populations may reflect multiple differences in genetic background and environmental exposure. First, the genetic basis of lipid metabolism may vary across different East Asian populations, with studies suggesting that specific gene polymorphisms can influence lipoprotein levels and insulin sensitivity[50]. Second, dietary pattern differences (such as higher seafood intake and specific fatty acid composition in the Japanese population) may influence lipid metabolism pathways and insulin resistance development[51]. Third, environmental factors such as physical activity patterns, occupational stress, and degrees of urbanization may collectively shape the population-specific manifestations of this non-linear relationship [52].
Our findings regarding the differential impact of AIP thresholds across different populations align with recent high-quality systematic reviews on cardiometabolic interventions. For example, in a meta-analysis on the effects of flaxseed supplementation in diabetic patients [11], researchers found that flaxseed effectively reduced anthropometric indices and triglyceride levels, particularly in long-term (> 12 weeks) interventions. However, similar to our findings on population-specific responses, they observed that baseline characteristics (including age and BMI) significantly moderated the intervention effects, supporting our argument for the necessity of individualized risk assessment.
Furthermore, the systematic review on the effects of purslane on glycemic indices[53] also supports the concept of population-specific responses to metabolic indicators. Their analysis showed that purslane supplementation significantly reduced fasting blood glucose across all populations, but its effects on insulin resistance markers differed significantly based on health status and demographic factors. This pattern of differential responses across different populations mirrors the variation in AIP thresholds we observed between different populations, highlighting the importance of considering population-specific differences when evaluating cardiometabolic risk factors.
Additionally, in their network meta-analysis on pharmacological interventions for youth obesity[54], researchers demonstrated that different medications had varying impacts on cardiometabolic risk factors, with some effectively improving anthropometric measures but not correspondingly improving lipid profiles and blood pressure. For instance, they found that high-dose phentermine/topiramate effectively reduced anthropometric measures, while orlistat better improved diastolic blood pressure, emphasizing the complex relationships between different cardiometabolic risk factors. This approach of comprehensively assessing multiple cardiometabolic risk factors aligns with our research methodology, where we focus on AIP as an indicator that integrates multiple lipid parameters and explores its relationship with diabetes risk.
Notably, the systematic review, meta-analysis, and GRADE assessment on the effect of berberis (Vulgaris and Integerrima) on cardiovascular risk factors in patients with type 2 diabetes mellitus [55]found that berberis supplementation effectively improved lipid profiles (triglycerides, total cholesterol, and low-density lipoprotein cholesterol) and glycemic indices. This study particularly emphasized the importance of evidence-based medicine approaches in evaluating the efficacy of metabolic interventions and provided strong evidence for berberis in improving AIP-related lipid parameters. These findings further support our conclusion that the impact of interventions on cardiometabolic risk factors is moderated by multiple factors that should be incorporated into personalized prevention and treatment strategies.
These comparative findings from recent high-quality systematic reviews and meta-analyses [11, 53–55] reinforce our conclusion that metabolic pathways and intervention responses differ across ethnic groups, necessitating population-specific approaches to cardiometabolic risk assessment and management. The differential threshold effects of AIP on diabetes risk that we observed between different populations may reflect fundamental differences in lipid metabolism, fat distribution, and insulin sensitivity pathways—differences that should inform personalized prevention strategies.
Our study revealed distinct AIP threshold values associated with increased T2DM risk in Chinese and Japanese populations. These differences should be interpreted within the context of substantial baseline characteristic variations between the cohorts. The Japanese cohort demonstrated significantly higher rates of smoking (41.6% vs. 20.4%) and alcohol consumption (69.4% vs. 16.5%), along with different metabolic profiles, including lipid parameters that directly influence AIP calculation. These differences reflect the distinct lifestyle patterns, dietary habits, and potentially environmental exposures characteristic of these populations.
Rather than undermining our findings, these population differences highlight the importance of establishing population-specific clinical thresholds that account for the unique constellation of risk factors and metabolic characteristics present in different groups. Despite these differences, the consistent association between AIP and T2DM risk across both populations strengthens the case for AIP as a robust indicator while simultaneously demonstrating the necessity of calibrating risk thresholds to specific population contexts.
The observed threshold differences likely result from complex interactions between multiple factors. Dietary patterns differ substantially between Chinese and Japanese populations, with variations in carbohydrate intake, types of dietary fats, and consumption of specific food groups like seafood and fermented products that may influence lipid metabolism. Physical activity patterns, body composition differences beyond BMI, and potentially genetic factors affecting lipid metabolism may further contribute to the observed threshold variations. Additionally, differences in healthcare systems and screening practices between countries may influence the timing of T2DM diagnosis.
This study evaluated AIP’s ability to predict diabetes risk, showing that AIP has good predictive value in both populations. However, we found important differences when comparing ROC curves between Chinese and Japanese populations. In the Japanese population, AIP’s ability to predict diabetes appeared superior to that in the Chinese population, which may reflect differences in the association between lipid metabolism abnormalities and diabetes development between the two populations. In the Japanese population, lipid metabolism abnormalities may be stronger predictors of diabetes development, consistent with the steeper dose-response relationship between AIP and diabetes risk observed in this population. Potential explanations for this difference in predictive performance include: First, the Japanese population may have unique lipid metabolism characteristics that make AIP a more sensitive predictor of diabetes risk [56]. Previous studies have suggested that despite lower BMI, Japanese individuals may face a higher risk of visceral fat accumulation, which is closely related to insulin resistance [57]. Second, dietary factors such as seafood consumption may influence lipid metabolism and insulin sensitivity [44]. Third, genetic factors may regulate lipid metabolism and glucose tolerance differently in the two populations [58].
Compared with previous studies, our AIP prediction model’s performance is generally consistent with Sun et al.‘s findings in the Chinese obese population[16], but slightly higher than the results reported by Zhou et al. in the Japanese population[17]. These differences may reflect methodological variations, including follow-up time, adjustment factors, and diagnostic criteria. Overall, our ROC analysis results not only support AIP as a valuable tool for predicting diabetes risk in East Asian populations but also emphasize the importance of population-specific reference values, which have important implications for clinical practice.
Mediation analysis revealed that BMI plays an important partial mediating role in the relationship between AIP and diabetes development. In the overall population, BMI mediated 37.27% of the total effect, suggesting that AIP influences diabetes risk through two pathways: an indirect pathway through BMI (AIP→BMI→diabetes) and a direct pathway independent of BMI (AIP→diabetes). Interestingly, we found that the mediation effect differed between the two populations. In the Chinese population, BMI mediated 39.84% of the total effect, while in the Japanese population, this proportion was only 27.11%. This suggests that in the Chinese population, AIP’s impact on diabetes risk is more mediated through obesity, while in the Japanese population, the direct effect is more pronounced.
Several factors may contribute to these observed variations regarding the differences in mediation proportions between Chinese (39.84%) and Japanese (27.11%) populations. These include biological, lifestyle, and methodological considerations that warrant careful interpretation.
From a biological perspective, research has documented ethnic variations in lipid metabolism and obesity-related diabetes risk among Asian populations. Yaghootkar et al.‘s research revealed that different ethnicities have genetic predispositions for differences in adipose tissue expansion capacity and fat storage efficiency [59]. Wulan et al.‘s review indicated that Asians have a higher body fat percentage, more pronounced abdominal obesity, and higher intramuscular lipid content than other ethnicities at the same BMI[60], which may influence the metabolic risk profile. Within Asian populations, Seah et al. compared type 2 diabetes risk differences among East Asian, South Asian, and Southeast Asian populations, finding that body fat distribution plays an important role in diabetes risk associations across different ethnicities [61]. They noted that adjusting for BMI explained many of the observed ethnic differences, suggesting that body composition measurements and their relationship to metabolic outcomes may vary among Asian populations. Lifestyle and dietary factors may also contribute to these differences. Le et al. compared cardiometabolic risk profiles between Chinese and Finnish adults with central obesity, finding significant differences in risk factors even when controlling for demographic variables [62]. They particularly emphasized that Chinese elderly individuals exhibit lipid metabolism dysfunction that differs from Finnish populations, indicating that dietary patterns and lifestyle factors play important roles in lipid metabolism across different populations.
From a methodological perspective, our observed differences may also reflect measurement variability, unmeasured confounding, or inherent baseline differences between cohorts. Mente et al.‘s study showed that Chinese populations have the lowest BMI and abdominal adiposity[63], and these baseline differences may affect the interpretation of mediation analysis results. While our findings suggest potentially population-specific biological pathways in the AIP-BMI-diabetes relationship, we cannot rule out that these differences may be influenced by methodological factors rather than genuine biological variation. This underscores the need for prospective studies to compare these relationships across well-characterized Asian populations.
From a physiological mechanism perspective, the indirect pathway through which AIP affects diabetes risk via BMI may involve the following processes: First, lipid metabolism disorders reflected by high AIP may promote adipose tissue expansion and ectopic fat deposition [4]; second, increased free fatty acids and inflammatory factors released from adipose tissue may impair insulin sensitivity [64]; finally, persistent insulin resistance ultimately leads to pancreatic β-cell dysfunction and diabetes onset[65]. The direct effect of AIP may reflect other mechanisms, such as increased small dense low-density lipoprotein particles leading to oxidative stress and vascular endothelial dysfunction [66], as well as lipid peroxidation and pancreatic β-cell apoptosis [67]. High levels of AIP are often associated with a state of chronic inflammation in the body. Chronic inflammation can impair pancreatic β-cell function, diminishing insulin secretion capacity and increasing diabetes risk[68]. Notably, inflammatory mediators such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) play a vital role in this process[69]. These findings highlight the potential value of AIP in diabetes risk assessment and suggest that regulating lipid metabolism may be an important strategy for diabetes prevention.
Based on our supplementary analyses in the Japanese cohort, we found a stronger correlation between AIP and FLI (r = 0.685) compared to AIP and WHR (r = 0.444), suggesting that AIP may be more closely linked to hepatic steatosis than visceral adiposity. This aligns with recent literature indicating that AIP reflects insulin resistance associated with fatty liver[41]. Our Cox proportional hazards models revealed that when adjusting for FLI (Adjust II-b model), the association between AIP and diabetes risk was attenuated from HR 3.520 (95% CI: 2.432–5.093) to 2.344 (95% CI: 1.481–3.709), representing a 33.4% reduction in effect size. In contrast, adjustment for WHR (Adjust II-a model) resulted in only a 6.6% reduction (HR: 3.286, 95% CI: 2.269–4.759). The mediation analysis further quantified these relationships, demonstrating that FLI mediated 23.78% of the AIP-diabetes association, while WHR mediated only 7.30%. This substantiates the hypothesis that hepatic steatosis plays a more prominent role than visceral adiposity in the pathway linking AIP to diabetes risk in Japanese adults. Interestingly, our subgroup analyses showed that the AIP-diabetes relationship remained consistent across all FLI categories (P for interaction = 0.692), suggesting that AIP predicts diabetes risk independently of baseline hepatic steatosis status. However, we observed a trend toward stronger associations in individuals with lower WHR quartiles (HR in Q1: 6.368 vs. HR in Q4: 2.830), though this interaction did not reach statistical significance (P = 0.176). These findings collectively suggest that the atherogenic lipid profile captured by AIP may contribute to diabetes risk partially through mechanisms related to hepatic fat accumulation, which aligns with the lipotoxicity theory of type 2 diabetes pathogenesis. The residual direct effect of AIP on diabetes risk after accounting for both FLI and WHR indicates that additional pathophysiological pathways likely exist beyond hepatic steatosis and visceral adiposity.
The results of this study have important clinical and public health implications. First, we confirm that AIP is a strong predictor of diabetes risk in East Asian populations, even after adjusting for traditional risk factors. As a simple, economical, and widely available biomarker, AIP can help clinicians more accurately identify high-risk individuals, especially in resource-limited healthcare settings[70]. Second, our findings of non-linear relationships and population-specific thresholds have important screening value. For the Chinese population, an AIP value of 0.436 may represent an important alert threshold, while the corresponding threshold for the Japanese population is -0.449. The differences in these thresholds emphasize the necessity of developing population-specific guidelines rather than adopting uniform global standards. Third, subgroup analysis results suggest that prevention strategies targeting specific high-risk populations (such as young to middle-aged adults, normal weight to mildly overweight individuals, and those with normal blood pressure) may be particularly effective. In these populations, the association between AIP and diabetes risk is strongest, suggesting that early regulation of lipid metabolism may yield the greatest preventive benefits[71]. Fourth, the BMI mediation analysis results indicate the importance of multi-target intervention strategies. In the Chinese population, weight management may be more critical, while in the Japanese population, interventions directly targeting lipid metabolism disorders (such as improving diet quality, increasing physical exercise, or appropriate pharmacological therapy) may be more important[72]. Finally, this study suggests that AIP may be a useful indicator for evaluating the effectiveness of diabetes prevention and lipid regulation interventions. Targeted reduction of AIP may be an important goal for future intervention studies, especially for high-risk individuals identified based on population-specific thresholds.
Our findings suggest that the AIP, as a key indicator of type 2 diabetes risk, can provide targeted prevention strategies for East Asian populations. Based on changes in AIP, the following targeted health interventions can be implemented:
(1) Personalized Dietary Interventions: Tailored dietary advice based on AIP and BMI levels for high-risk individuals could involve increasing the intake of foods rich in monounsaturated fatty acids (such as nuts and fish) to improve lipid metabolism and subsequently reduce diabetes risk.
(2) Lifestyle Modifications: Encouraging high-risk individuals to increase physical activity and improve lifestyle can significantly lower AIP levels and reduce the risk of developing diabetes. Regular health checks and health education should also be included in preventive strategies.
(3) Early Screening and Monitoring: Regular screening protocols for individuals with elevated AIP can be crucial in implementing early interventions to decrease the likelihood of diabetes onset, especially in high-risk populations.
This study has several important strengths. First, we employed a large prospective cohort design, covering two East Asian populations with different lifestyles and environmental exposures but similar genetic backgrounds, enhancing our findings’ external validity. Second, we implemented comprehensive data adjustment and analytical strategies, including multivariate Cox regression, sensitivity analyses, subgroup analyses, non-linear relationship exploration, and mediation analysis, providing a comprehensive perspective on the relationship between AIP and diabetes. Third, we used piecewise regression models to identify population-specific thresholds for the relationship between AIP and diabetes risk, providing concrete reference points for clinical practice. Fourth, our study is the first systematic comparison of the association patterns between AIP and T2DM risk across different East Asian populations, filling an important knowledge gap in this field. Fifth, we employed innovative statistical methods to assess the mediating role of BMI in the AIP-diabetes relationship, revealing potential pathogenic mechanisms that offer new insights for targeted interventions. Finally, our findings have clear clinical translation value, with the proposed population-specific AIP thresholds serving as reference standards for risk assessment and early intervention.
However, this study also has several limitations. First, there is a substantial difference in follow-up time and sample size between the Chinese (n = 112,483) and Japanese (n = 15,453) cohorts, necessitating separate analyses rather than direct statistical comparisons. While both cohorts maintained adequate statistical power for primary analyses, the smaller Japanese cohort may have limited power for detecting subtle effects in some subgroup analyses. Second, the baseline characteristics between cohorts demonstrated profound differences extending beyond expected demographic variations, including smoking rates (Chinese 20.4% vs. Japanese 41.6%), alcohol consumption patterns (Chinese 16.5% vs. Japanese 69.4%), and multiple metabolic parameters. These systematic differences indicate that these cohorts represent different populations with distinct lifestyle patterns and risk factor profiles. Consequently, the observed threshold differences should reflect the combined influence of multiple population-specific factors rather than isolated ethnic effects. Third, the inherent biases associated with retrospective studies may affect the interpretation of our results. While we adjusted for known confounders, residual confounding may persist due to unmeasured variables that differ between populations, such as detailed dietary composition, physical activity patterns, socioeconomic factors, and healthcare access. Additionally, differences in laboratory standards and diagnostic practices between cohorts may contribute to some observed variations despite our efforts to standardize definitions. Fourth, we relied only on baseline AIP values to predict diabetes risk, and we could not assess the impact of AIP changes over time on risk prediction. Fifth, the study includes different population-specific backgrounds and lifestyles in China and Japan, which could lead to physiological differences among populations. Therefore, generalizing our findings from the Chinese to the Japanese cohort should be done cautiously. Sixth, reliance on initially self-reported diabetes diagnosis introduces the possibility of ascertainment bias. Differences between Chinese and Japanese healthcare systems, including access to medical resources, routine screening practices, and application of diagnostic standards, may lead to systematic differences in diabetes detection rates that could be misinterpreted as population-specific risk patterns. While we employed standardized follow-up assessments and uniform diagnostic criteria (American Diabetes Association standards) and adjusted for socioeconomic and healthcare access factors in multivariate analyses, the influence of diagnostic practice variations cannot be entirely eliminated. In particular, Japan’s higher healthcare accessibility and routine health checks may result in higher early diabetes detection rates compared to China, which might partially explain the threshold effect differences we observed. Seventh, this study relies on secondary analysis of two independent cohorts with different data collection periods and potentially different laboratory standards. While standardized definitions and robust statistical methods were employed, we acknowledge the inherent limitations in cross-population comparisons using retrospectively collected data. Differences in laboratory assays, measurement protocols, and other unmeasured factors may influence the absolute values of the identified thresholds, though the consistent association pattern supports the biological relevance of AIP across both populations. Eighth, a primary limitation of our study is the substantial proportion of missing data, particularly for AST (50.656% missing) and lifestyle factors (63.420% missing). While multiple imputation is a recommended approach for handling missing data, its reliability may decrease with higher missing rates. To mitigate concerns, we conducted sensitivity analyses using alternative imputation approaches. The consistency of our findings across these different analytical strategies suggests a degree of robustness in our results. However, the high proportion of missing data introduces uncertainty that cannot be fully eliminated through statistical methods alone. Future studies should prioritize more complete data collection, particularly for important clinical parameters and lifestyle factors. Despite these limitations, we believe our findings provide valuable insights into population-specific relationships between AIP and T2D risk, though they should be interpreted with appropriate caution. Ninth, the differences in mediation proportions between Chinese (39.84%) and Japanese (27.11%) populations could be influenced by measurement variability, unmeasured confounding factors, or inherent baseline differences between cohorts, rather than representing genuine population-specific biological pathways. Without longitudinal data tracking changes in AIP, BMI, and diabetes development over time, our mediation analysis should be considered exploratory and hypothesis-generating rather than conclusive. Our findings highlight several important directions for future research. (1) Prospective cohort studies with longitudinal assessments of AIP, BMI, and glycemic parameters are needed to establish the temporal sequence necessary for confirming the proposed mediation pathways. Such studies should incorporate repeated measurements over time to track how changes in AIP relate to subsequent changes in BMI and diabetes risk. This longitudinal design can address the inherent bias of cross-sectional mediation analysis. (2). Studies specifically designed to compare these relationships across well-characterized Asian populations would help determine whether our observed population differences reflect genuine biological variation or methodological artifacts. Such research should include a comprehensive assessment of potential mediators and confounders, including detailed measures of body composition (beyond BMI), dietary patterns, physical activity, and genetic factors. Following Yaghootkar et al.‘s research methodology[59], these studies should explore how ethnic differences in adipose tissue expansion capacity and fat storage efficiency influence metabolic risk. (3) Time-lagged analyses that can better establish temporal precedence would be valuable for clarifying the direction of causality in the relationships between lipid profiles, adiposity, and diabetes risk. These analyses should include multiple potential mediators to identify the most relevant pathways. Tenth, the difference in follow-up periods between the Chinese (maximum 7 years) and Japanese (maximum 12 years) cohorts may introduce systematic bias. Although Cox proportional hazards models account for varying follow-up times, and we standardized risk measurements using incidence rates per 1000 person-years, this design discrepancy may affect direct comparisons between populations. Specifically, the longer follow-up in the Japanese cohort might increase the cumulative opportunity for diabetes detection, potentially influencing incidence estimates. To mitigate this concern, we conducted sensitivity analyses restricting both cohorts to a common 7-year maximum follow-up period, demonstrating that our primary findings remained robust. Eleventh, an important limitation of our study is the inconsistent availability of anthropometric and biochemical measures between the Chinese and Japanese cohorts. While the Japanese dataset contained comprehensive measurements allowing calculation of WHR and FLI, the Chinese cohort lacked waist circumference measurements and components necessary for FLI calculation. This disparity prevented us from conducting comparative analyses of the mediating roles of hepatic steatosis and visceral adiposity across both populations. Consequently, our mechanistic insights regarding the differential contribution of fatty liver and central obesity to the AIP-diabetes relationship are limited to the Japanese population only. Future studies should aim to standardize data collection across diverse Asian populations to enable more robust cross-ethnic comparisons of these pathophysiological pathways. Finally, the dramatically different threshold values identified for diabetes risk between Chinese (AIP = 0.436) and Japanese (AIP=-0.449) populations require careful interpretation. These population-specific thresholds represent exploratory findings rather than established clinical cutpoints. The marked threshold difference likely reflects a combination of genuine biological and environmental variations rather than merely statistical artifacts. These thresholds lack external validation in independent populations and have not been tested in clinical settings. The different association patterns observed above and below thresholds in the two populations may reflect complex non-linear relationships between AIP and diabetes risk, as suggested by recent studies identifying non-linear associations between AIP and insulin resistance[13, 16]. To address these limitations, future research should include: (1) external validation in independent Chinese and Japanese cohorts; (2) prospective studies specifically designed to test the predictive value of these thresholds; (3) mechanistic studies examining the biological basis for these ethnic differences; and (4) clinical utility assessments to determine whether population-specific AIP thresholds improve risk stratification and treatment outcomes compared to conventional approaches. Additionally, moving these exploratory findings toward clinical utility will require the development of standardized AIP measurement protocols, integration with established risk factors, and cost-effectiveness analyses of implementing AIP-based screening strategies. Until such validation is complete, these population-specific thresholds should be interpreted appropriately.
In summary, this study confirms a significant positive association between AIP and diabetes risk in both Chinese and Japanese populations, and this association remains robust after adjusting for multiple confounding factors. We identified population-specific non-linear relationships and threshold effects, indicating that in the Chinese population, diabetes risk no longer increases significantly when AIP > 0.436, while in the Japanese population, significant associations are observed only when AIP>-0.449. Age, BMI, and systolic blood pressure significantly modify the relationship between AIP and diabetes, suggesting the importance of risk assessment and intervention strategies targeted at specific subgroups. Mediation analysis revealed the partial mediating role of BMI in the relationship between AIP and diabetes, with a higher proportion of mediation in the Chinese population (39.84%) compared to the Japanese population (27.11%). Furthermore, in supplementary analyses of the Japanese cohort, we found that hepatic steatosis (FLI mediating 23.78%) plays a more important role than visceral adiposity (WHR mediating 7.30%) in mediating the AIP-diabetes relationship, supporting the critical role of fatty liver in diabetes pathogenesis. These findings emphasize the necessity of developing population-specific risk thresholds and intervention strategies for different East Asian populations, considering the significant differences between the two populations in lifestyle, dietary patterns, and metabolic characteristics. Future research should focus on standardizing data collection to enable more robust cross-population comparisons and validating the clinical utility of these thresholds through prospective designs.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We appreciate the contributors to the original data.
Abbreviations
- AIP
Atherogenic index of plasma
- T2DM
Type 2 diabetes mellitus
- BMI
Body mass index
- DM
Diabetes mellitus
- WC
Waist circumference
- IR
Insulin resistance
- FLI
Fatty liver index
- WHR
Waist-to-height ratio
- FPG
Fasting plasma glucose
- HDL-c
High-density lipoprotein cholesterol
- log[TG/HDL-c]
Logarithm of the ratio of triglycerides to high-density lipoprotein cholesterol
- TG
Triglyceride
- HbA1c
Hemoglobin A1c
- ALT
Alanine aminotransferase
- SBP
Systolic blood pressure
- TC
Total cholesterol
- DBP
Diastolic blood pressure
- LDL-c
Low-density lipid cholesterol
- AST
Aspartate aminotransferase
- MAR
Missing-at-random
- SD
Standard deviation
- VIF
Variance inflation factor
- HR
Hazard ratios
- Ref
Reference
- CI
Confidence intervals
- ROC
Receiver operating characteristic
- AUC
Area under the curve
Author contributions
Yuheng Liao, Yong Han and Changchun Cao contributed equally to this work as co-first authors. Yuheng Liao: Conceptualization, methodology, formal analysis, data curation, writing - original draft, and visualization. Conducted the statistical analyses, interpreted the results, and wrote the primary manuscript draft. Yong Han: Methodology, data curation, formal analysis, validation, and writing - original draft. Performed data processing, contributed to the statistical analyses, and participated in manuscript preparation. Changchun Cao: Investigation, formal analysis, validation, and writing - review & editing. Conducted literature review, assisted with data interpretation, and critically revised the manuscript. Haiying Song: Conceptualization, supervision, project administration, resources, and writing - review & editing. Designed the research framework, secured research resources, oversaw the overall study progress, and critically reviewed the manuscript. Haofei Hu: Conceptualization, supervision, methodology, funding acquisition, and writing - review & editing. Provided methodological guidance, supervised the analytical approach, critically reviewed and edited the manuscript, and was responsible for funding acquisition.Haiying Song and Haofei Hu contributed equally as corresponding authors. All authors have read and approved the final manuscript.
Funding
This study was supported by the Shenzhen Key Medical Discipline Construction Fund (SZXK009), the Sanming Project of Medicine in Shenzhen (SZSM202211013), the Medicine Engineering Interdisciplinary Research Foundation of Shenzhen University (2023YG033), and the Supply and Demand Matching Employment-Education Integration Project (2024012936000).
References.
Data availability
Data can be downloaded from the ‘DATADRYAD’ database (https://doi.org/www.Datadryad.org).
Declarations
Ethics approval and consent to participate
The Chinese and Japanese cohort studies in this study were approved by the ethics committees of Rich Healthcare Group and Murakami Memorial Hospital, respectively. Both studies were conducted in accordance with the Declaration of Helsinki, and informed consent was waived because of the retrospective nature of this study[24].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuheng Liao, Yong Han, and Changchun Cao contributed equally to this work as co-first authors.
Contributor Information
Haiying Song, Email: haiyingsong2024@126.com.
Haofei Hu, Email: huhaofei0319@126.com.
References
- 1.Atlas ID. Diabetes Facts and Figures. In.; 2025.
- 2.Worldwide trends in diabetes. Since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee M, Ohde S. PM(2.5) and diabetes in the Japanese population. INT J ENV RES PUB HE 2021, 18(12). [DOI] [PMC free article] [PubMed]
- 4.Dobiásová M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)). CLIN BIOCHEM. 2001;34(7):583–8. [DOI] [PubMed] [Google Scholar]
- 5.Assempoor R, Daneshvar MS, Taghvaei A, Abroy AS, Azimi A, Nelson JR, Hosseini K. Atherogenic index of plasma and coronary artery disease: a systematic review and meta-analysis of observational studies. Cardiovasc diabetol. 2025;24(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Q, Liu Z, Wei M, Huang Q, Feng J, Liu Z, Xia J. The atherogenic index of plasma and carotid atherosclerosis in a community population: a population-based cohort study in China. Cardiovasc diabetol. 2023;22(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.You F, Gao J, Gao Y, Li Z, Shen D, Zhong W, Yang J, Wang X, Song W, Yan H, et al. Association between atherogenic index of plasma and all-cause mortality and specific-mortality: a nationwide population–based cohort study. CARDIOVASC DIABETOL. 2024;23(1):276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ismaiel A, Ciobanu OS, Ismaiel M, Leucuta D, Popa S, David L, Ensar D, Al Srouji N, Dumitrascu DL. Atherogenic Index of Plasma in Non-Alcoholic Fatty Liver Disease: Systematic Review and Meta-Analysis. Biomedicines 2022, 10(9). [DOI] [PMC free article] [PubMed]
- 9.Wang B, Jiang C, Qu Y, Wang J, Yan C, Zhang X. Nonlinear association between atherogenic index of plasma and chronic kidney disease: a nationwide cross-sectional study. LIPIDS HEALTH DIS. 2024;23(1):312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lioy B, Webb RJ, Amirabdollahian F. The association between the atherogenic index of plasma and cardiometabolic risk factors: A review. Healthcare-basel 2023, 11(7). [DOI] [PMC free article] [PubMed]
- 11.Musazadeh V, Nezamoleslami S, Faghfouri AH, Shidfar F, Aryaeian N. The effect of flaxseed supplementation on anthropometric indices, blood pressure, and lipid profile in diabetic patients: A GRADE-assessed systematic review and meta-analysis of randomized controlled trials. DIABETES METAB SYND. 2025;19(5):103241. [DOI] [PubMed] [Google Scholar]
- 12.Czech MP. Insulin action and resistance in obesity and type 2 diabetes. NAT MED. 2017;23(7):804–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin B, Wu Z, Xia Y, Xiao S, Chen L, Li Y. Non-linear association of atherogenic index of plasma with insulin resistance and type 2 diabetes: a cross-sectional study. CARDIOVASC DIABETOL. 2023;22(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z, Huang Q, Sun L, Bao T, Dai Z. Atherogenic Index in Type 2 Diabetes and Its Relationship with Chronic Microvascular Complications. Int J Endocrinol 2018, 2018:1765835. [DOI] [PMC free article] [PubMed]
- 15.Cao J, Su Z, Yang J, Zhang B, Jiang R, Lu W, Huang Z, Xie Z. The atherogenic index of plasma is associated with an increased risk of diabetes in non-obese adults: a cohort study. FRONT ENDOCRINOL. 2024;15:1477419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y, Li F, Zhou Y, Liu A, Lin X, Zou Z, Lv X, Zhou J, Li Z, Wu X, et al. Nonlinear association between atherogenic index of plasma and type 2 diabetes mellitus in overweight and obesity patients: evidence from Chinese medical examination data. CARDIOVASC DIABETOL. 2024;23(1):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Q, Wu Y, Li M. Association between the atherogenic index of plasma and long-term risk of type 2 diabetes: a 12-year cohort study based on the Japanese population. CARDIOVASC DIABETOL. 2025;24(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q, Zheng D, Liu J, Fang L, Li Q. Atherogenic index of plasma is a novel predictor of non-alcoholic fatty liver disease in obese participants: a cross-sectional study. LIPIDS HEALTH DIS. 2018;17(1):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos HO, Earnest CP, Tinsley GM, Izidoro LFM, Macedo RCO. Small dense low-density lipoprotein-cholesterol (sdLDL-C): analysis, effects on cardiovascular endpoints and dietary strategies. PROG CARDIOVASC DIS. 2020;63(4):503–9. [DOI] [PubMed] [Google Scholar]
- 20.Eckel RH, Kahn SE, Ferrannini E, Goldfine AB, Nathan DM, Schwartz MW, Smith RJ, Smith SR. Obesity and type 2 diabetes: what can be unified and what needs to be individualized? Diabetes Care. 2011;34(6):1424–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdullah A, Peeters A, de Courten M, Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. DIABETES RES CLIN PR. 2010;89(3):309–19. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Zhang X, Yuan J, Cai B, Wang X, Wu X, Zhang Y, Zhang X, Yin T, Zhu X, et al. Association of body mass index and age with incident diabetes in Chinese adults: a population-based cohort study. BMJ OPEN. 2018;8(9):e21768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. Ectopic fat obesity presents the greatest risk for incident type 2 diabetes: a population-based longitudinal study. INT J Obes. 2019;43(1):139–48. [DOI] [PubMed] [Google Scholar]
- 24.Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, Labella A, Manson DK, Kubin C, Barr RG, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. NEW ENGL J MED. 2020;382(25):2411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.2. Diagnosis and classification of diabetes: standards of care in Diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S20–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harreiter J, Roden M. [Diabetes mellitus-Definition, classification, diagnosis, screening and prevention (Update 2019)]. WIEN KLIN WOCHENSCHR. 2019;131(Suppl 1):6–15. [DOI] [PubMed] [Google Scholar]
- 27.Chen Z, Hu H, Chen M, Luo X, Yao W, Liang Q, Yang F, Wang X. Association of triglyceride to high-density lipoprotein cholesterol ratio and incident of diabetes mellitus: a secondary retrospective analysis based on a Chinese cohort study. LIPIDS HEALTH DIS. 2020;19(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groenwold RHH, White IR, Donders ART, Carpenter JR, Altman DG, Moons KGM. Missing covariate data in clinical research: when and when not to use the missing-indicator method for analysis. CAN MED ASSOC J. 2012;184(11):1265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. STAT MED. 2011;30(4):377–99. [DOI] [PubMed] [Google Scholar]
- 30.Wax Y. Collinearity diagnosis for a relative risk regression analysis: an application to assessment of diet-cancer relationship in epidemiological studies. STAT MED. 1992;11(10):1273–87. [DOI] [PubMed] [Google Scholar]
- 31.He L, Zheng W, Li Z, Chen L, Kong W, Zeng T. J-shape relationship between normal fasting plasma glucose and risk of type 2 diabetes in the general population: results from two cohort studies. J TRANSL MED. 2023;21(1):175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee S. C Li 2021 Personality as a predictor of HbA1c level in patients with type 2 diabetes mellitus. Medicine 100 27 e26590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malone JI, Hansen BC. Does obesity cause type 2 diabetes mellitus (T2DM)? Or is it the opposite? PEDIATR DIABETES. 2019;20(1):5–9. [DOI] [PubMed] [Google Scholar]
- 34.Zhao J, Zhang Y, Wei F, Song J, Cao Z, Chen C, Zhang K, Feng S, Wang Y, Li W. Triglyceride is an independent predictor of type 2 diabetes among middle-aged and older adults: a prospective study with 8-year follow-ups in two cohorts. J TRANSL MED. 2019;17(1):403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Böhnke JR. Explanation in causal inference: methods for mediation and interaction. Q J EXP PSYCHOL. 2016;69(6):1243–4. [DOI] [PubMed] [Google Scholar]
- 36.VanderWeele TJ. Mediation analysis: A practitioner’s guide. ANNU REV PUBL HEALTH. 2016;37:17–32. [DOI] [PubMed] [Google Scholar]
- 37.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. PSYCHOL METHODS. 2013;18(2):137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richiardi L, Bellocco R, Zugna D. Mediation analysis in epidemiology: methods, interpretation and bias. INT J EPIDEMIOL. 2013;42(5):1511–9. [DOI] [PubMed] [Google Scholar]
- 39.Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. PSYCHOL METHODS. 2010;15(4):309–34. [DOI] [PubMed] [Google Scholar]
- 40.Stefan N, Schiborn C, Machann J, Birkenfeld AL, Schulze MB. Impact of higher BMI on cardiometabolic risk: does height matter? LANCET DIABETES ENDO. 2024;12(8):514–5. [DOI] [PubMed] [Google Scholar]
- 41.Stefan N, Schick F, Birkenfeld AL, Häring H, White MF. The role of hepatokines in NAFLD. CELL METAB. 2023;35(2):236–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC GASTROENTEROL. 2006;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan JCN, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon K, Hu FB. Diabetes in asia: epidemiology, risk factors, and pathophysiology. JAMA-J AM MED ASSOC. 2009;301(20):2129–40. [DOI] [PubMed] [Google Scholar]
- 44.Nanri A, Mizoue T, Noda M, Takahashi Y, Kato M, Inoue M, Tsugane S. Rice intake and type 2 diabetes in Japanese men and women: the Japan public health Center-based prospective study. AM J CLIN NUTR. 2010;92(6):1468–77. [DOI] [PubMed] [Google Scholar]
- 45.Heraclides A, Chandola T, Witte DR, Brunner EJ. Psychosocial stress at work doubles the risk of type 2 diabetes in middle-aged women: evidence from the Whitehall II study. Diabetes Care. 2009;32(12):2230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamauchi T, Hara K, Maeda S, Yasuda K, Takahashi A, Horikoshi M, Nakamura M, Fujita H, Grarup N, Cauchi S, et al. A genome-wide association study in the Japanese population identifies susceptibility loci for type 2 diabetes at UBE2E2 and C2CD4A-C2CD4B. NAT GENET. 2010;42(10):864–8. [DOI] [PubMed] [Google Scholar]
- 47.Fujihara K, Sugawara A, Heianza Y, Sairenchi T, Irie F, Iso H, Doi M, Shimano H, Watanabe H, Sone H, et al. Utility of the triglyceride level for predicting incident diabetes mellitus according to the fasting status and body mass index category: the Ibaraki prefectural health study. J Atheroscler thromb. 2014;21(11):1152–69. [DOI] [PubMed] [Google Scholar]
- 48.Lan Y, Chen G, Wu D, Ding X, Huang Z, Wang X, Balmer L, Li X, Song M, Wang W, et al. Temporal relationship between atherogenic dyslipidemia and inflammation and their joint cumulative effect on type 2 diabetes onset: a longitudinal cohort study. Bmc Med. 2023;21(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61(6):1315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yasuda K, Miyake K, Horikawa Y, Hara K, Osawa H, Furuta H, Hirota Y, Mori H, Jonsson A, Sato Y, et al. Variants in KCNQ1 are associated with susceptibility to type 2 diabetes mellitus. Nat Genet. 2008;40(9):1092–7. [DOI] [PubMed] [Google Scholar]
- 51.Nanri A, Mizoue T, Noda M, Takahashi Y, Matsushita Y, Poudel-Tandukar K, Kato M, Oba S, Inoue M, Tsugane S. Fish intake and type 2 diabetes in Japanese men and women: the Japan public health Center-based prospective study. Am J Clin Nutr. 2011;94(3):884–91. [DOI] [PubMed] [Google Scholar]
- 52.Ramachandran A, Snehalatha C, Shetty AS, Nanditha A. Trends in prevalence of diabetes in Asian countries. World J diabetes. 2012;3(6):110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abbasi S, Mashatan N, Farmani E, Khodashenas M, Musazadeh V, Ahrabi SS, Moridpour AH, Faghfouri AH. The effects of purslane (Portulaca oleracea) on glycemic indices: A GRADE-assessed systematic review and meta-analysis of randomized controlled trials. Phytother RES. 2023;37(12):5529–40. [DOI] [PubMed] [Google Scholar]
- 54.Faghfouri AH, Musazadeh V, Mokari-Yamchi A, Faramarzi F, Pam P, Shekari S, Mahmoudzadeh M, Gheibi S. Efficacy and safety of Pharmacological interventions in managing cardiometabolic risk factors in youth living with overweight and obesity: A systematic review and network Meta-Analysis. OBES REV 2025:e13946. [DOI] [PubMed]
- 55.Musazadeh V, Hamidi N, Noormohammadi M, Shidfar F. The effect of berberis (Vulgaris and Integerrima) on cardiovascular risk factors in patients with type 2 diabetes mellitus: A systematic review, Meta-Analysis, and GRADE assessment. CURR THER RES CLIN E. 2025;102:100788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kadowaki S, Tamura Y, Someya Y, Takeno K, Kaga H, Sugimoto D, Kakehi S, Funayama T, Furukawa Y, Suzuki R, et al. Fatty liver has stronger association with insulin resistance than visceral fat accumulation in Nonobese Japanese men. J ENDOCR SOC. 2019;3(7):1409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fujimoto WY, Bergstrom RW, Boyko EJ, Chen KW, Leonetti DL, Newell-Morris L, Shofer JB, Wahl PW. Visceral adiposity and incident coronary heart disease in Japanese-American men. The 10-year follow-up results of the Seattle Japanese-American community diabetes study. Diabetes Care. 1999;22(11):1808–12. [DOI] [PubMed] [Google Scholar]
- 58.Hara K, Fujita H, Johnson TA, Yamauchi T, Yasuda K, Horikoshi M, Peng C, Hu C, Ma RCW, Imamura M, et al. Genome-wide association study identifies three novel loci for type 2 diabetes. HUM MOL GENET. 2014;23(1):239–46. [DOI] [PubMed] [Google Scholar]
- 59.Yaghootkar H, Lotta LA, Tyrrell J, Smit RAJ, Jones SE, Donnelly L, Beaumont R, Campbell A, Tuke MA, Hayward C et al. Genetic Evidence for a Link Between Favorable Adiposity and Lower Risk of Type 2 Diabetes, Hypertension, and Heart Disease. DIABETES 2016, 65(8):2448–2460. [DOI] [PMC free article] [PubMed]
- 60.Wulan SN, Westerterp KR, Plasqui G. Ethnic differences in body composition and the associated metabolic profile: a comparative study between Asians and Caucasians. MATURITAS. 2010;65(4):315–9. [DOI] [PubMed]
- 61.Seah JYH, Sim X, Khoo CM, Tai ES, van Dam RM. Differences in type 2 diabetes risk between east, south, and Southeast Asians living in singapore: the multi-ethnic cohort. BMJ OPEN DIAB RES CA 2023, 11(4). [DOI] [PMC free article] [PubMed]
- 62.Le S, Zhang Y, Voutilainen A, Tan X, Laukkanen J, Wang C, Cheng S. Differences in cardiometabolic risk profiles between Chinese and Finnish older adults with glucose impairment and central obesity. J ENDOCRINOL INVEST. 2022;45(7):1427–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mente A, Razak F, Blankenberg S, Vuksan V, Davis AD, Miller R, Teo K, Gerstein H, Sharma AM, Yusuf S, et al. Ethnic variation in adiponectin and leptin levels and their association with adiposity and insulin resistance. Diabetes Care. 2010;33(7):1629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–28. [DOI] [PubMed] [Google Scholar]
- 65.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J CLIN INVEST. 2006;116(7):1802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lüscher TF, Landmesser U, von Eckardstein A, Fogelman AM. High-density lipoprotein: vascular protective effects, dysfunction, and potential as therapeutic target. CIRC RES. 2014;114(1):171–82. [DOI] [PubMed] [Google Scholar]
- 67.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. ENDOCR REV. 2008;29(3):351–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sahin GS, Lee H, Engin F. An accomplice more than a Mere victim: the impact of β-cell ER stress on type 1 diabetes pathogenesis. MOL METAB. 2021;54:101365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gomes BF, Accardo CDM. Immunoinflammatory mediators in the pathogenesis of diabetes mellitus. EINSTEIN-SAO PAULO. 2019;17(1):B4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Onat A, Can G, Kaya H, Hergenç G. Atherogenic index of plasma (log10 triglyceride/high-density lipoprotein-cholesterol) predicts high blood pressure, diabetes, and vascular events. J CLIN LIPIDOL. 2010;4(2):89–98. [DOI] [PubMed] [Google Scholar]
- 71.Abbasi F, Reaven GM. Comparison of two methods using plasma triglyceride concentration as a surrogate estimate of insulin action in nondiabetic subjects: triglycerides × glucose versus triglyceride/high-density lipoprotein cholesterol. METABOLISM. 2011;60(12):1673–6. [DOI] [PubMed] [Google Scholar]
- 72.Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart J, James WPT, Loria CM, Smith SCJ. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. CIRCULATION 2009, 120(16):1640–1645. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be downloaded from the ‘DATADRYAD’ database (https://doi.org/www.Datadryad.org).