Dear editor,
We read the interesting work of Romero‑Garcia and colleagues on the association between partial pressure of O2 in arterial blood (PaO2) and neurological outcomes in a context of acute brain injury (ABI) [1]. This is a particularly relevant topic as the brain is highly vulnerable to excessive oxidative stress and reactive oxygen species (ROS) due to its huge dioxygen (O2) consumption and its high polyunsaturated fatty acid and iron content. The authors showed that high PaO2 (hyperoxemia) was associated with poor neurological outcomes and mortality and conclude that it is important to adjust oxygenation strategies in this population [1]. It gives us the opportunity to explore the physiological effect of O2, hyperoxemia and hyperoxia on cerebral blood flow and metabolism beyond the sole results of the present meta-analysis.
Hyperoxemia, hyperoxia and reactive oxygen species
Oxidative stress via the generation of reactive oxygen species (ROS) is one of the suggested mechanisms to explain the worsened outcomes associated with hyperoxemia [1, 2]. To properly understand how O2 affects the generation of ROS, it seems important to recall some fundamental of physiology. ROS are a normal product of aerobic metabolism and are generated inside the cells, mainly in the mitochondria [2, 3]. Their excessive production is observed in various conditions, but mostly when the O2 concentration reaching the cells abruptly changes as it happens during ischemia-reperfusion phenomenon (i.e., generation of superoxyde anion by the degradation of the accumulated hypoxanthine during ischemic phase by the xanthine oxidase following reestablishment of O2 supply) [4, 5].
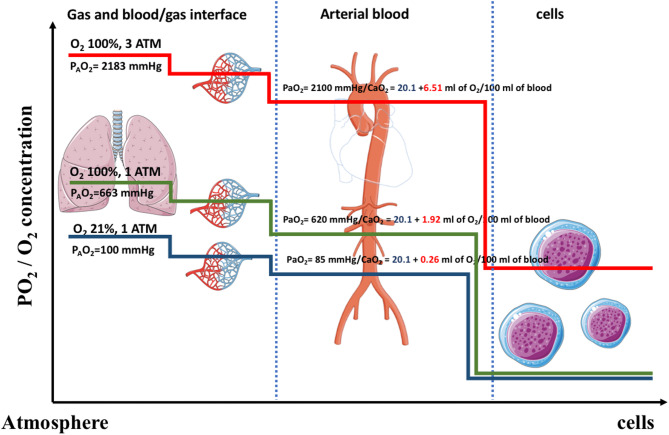
However, to hypothesize that hyperoxemia leads to increase ROS is an oversimplified statement [6]. Hyperoxemia refers by definition to a supranormal quantity of O2 in the blood (assuming that a normal quantity is observed in a healthy person breathing air at sea level). It usually refers to either the partial pressure of dissolved O2 (the PaO2 in clinical practice) or the arterial content in O2 (pooling both the dissolved O2 and the O2 carried by hemoglobin). Hyperoxia refers to a supranormal concentration of O2 reaching the intracellular compartment. In normobaric (and normothermic) conditions, the absolute quantity of O2 transported in its dissolved form is very low due to the low solubility coefficient of O2 in blood (Fig. 1). This is why a carrier system (the hemoglobin) is needed to supply the global demand in O2 [7]. However, only dissolved O2 molecules can diffuse through the tissue to reach their effect site [7].
Fig. 1.
O2 journey: from the airways to the mitochondria. Figure 1 represents a schematic representation of the concentration of free O2 molecules during their journey from the atmosphere to the cells in a healthy subject. Airway’s cells are directly exposed to O2 molecules whose partial pressure varies linearly with the fraction of inspired O2. Then O2 molecules diffuse into the capillaries blood where they are loaded by hemoglobin. In standard condition of pressure and temperature, the solubility of O2 in blood is very low limiting the concentration of free O2 in whole blood. Then in tissue’s capillaries, O2 diffuse into the cells depending on their metabolic activities and O2 consumption’s rate. Thus, the concentration of O2 in blood decreases, allowing the release of O2 by hemoglobin according to the metabolic demand and local factors (such as pH through the Bohr’s effect). All the purpose of the blood carrier system is to ensure sufficient O2 supply, while limiting its toxicity. One can easily see that except from the airways, other parts of the body, especially the cells are not exposed to high O2 concentration and that hyperoxemia is not a surrogate for hyperoxia. In hyperbaric condition, the dissolved O2 in blood is dramatically increased so it will diffuse to the cells and increased cellular O2 concentration beyond their needs although arteriolar vasoconstriction tends to limit it until a threshold. ATM=atmosphere; PaO2= Arterial blood O2 partial pressure; PAO2= Alveolar O2 partial pressure  ; O2= Dioxygen; CaO2=arterial content in O2 (
; O2= Dioxygen; CaO2=arterial content in O2 ( where SaO2 is the arterial blood O2 saturation).
where SaO2 is the arterial blood O2 saturation).
At tissue level, the hemoglobin unloads O2 molecules as the surrounding PaO2 decreases, in accordance to local consumption rate, phenomenon enhanced by local factors such as Bohr effect [7]. Consequently, in standard conditions of pressure and temperature, the quantity by time unit of supplemental O2 administered to the patient (i.e., fraction of inspired O2 (FIO2)) and time spent above a specific threshold (the equivalent of oxygen toxicity unit used by the divers) are the paramount variables. Indeed, the cells constitutive of the airways are directly exposed to O2 molecules and their potential toxicity, whereas other tissues are protected by the low O2 solubility, the O2 carrier system and the microcirculation response to excessive arterial dissolved O2 concentrations [8]. Previous studies demonstrated that the venoarterial difference in O2 which represents the actual quantity of O2 delivered to the tissue/cells was not increased -it even slightly decreased- during normobaric and hyperbaric exposure to 100% O2 compared to room air [9, 10]. More recently a study investigating the O2 consumption during deep hypothermic cardiopulmonary bypass during pulmonary endarterectomy surgery provides interesting insights. The authors showed that despite the increase in PaO2 (above 500 mmHg) during cooling (from 37 to 18 °C), the venoarterial difference in O2 and O2 extraction decreased (reflecting the metabolism slowdown related to hypothermia). It demonstrated that the cells used only the O2 that they need. It is a well illustration that hyperoxemia does not mean hyperoxia [11].Also, a pilot study compared the level of ROS, according to two level of FIO2 (40% and 70%) in mechanically ventilated patients in a context of traumatic brain injury. The blood level of ROS was similar between the two groups, suggesting that the relationship between hyperoxemia and excessive oxidative stress is not that obvious [12].
Effect of O2 on cerebral blood flow
The effect of hyperoxemia on cerebral blood flow has been extensively studied in animals and humans. In normobaric conditions, to breathe pure oxygen leads to a small non-significant decrease in cerebral blood flow (CBF) at constant level of arterial partial pressure of carbon dioxide (PaCO2) [2, 13, 14]. Furthermore, studying the own effect of O2 on CBF cannot be properly done focusing on PaO2 alone but should consider other conditions affecting the CBF (i.e., PaCO2, pH, preserved or altered cerebral autoregulation, spontaneous breathing or mechanical ventilation).
The response to change in O2 or CO2 partial pressures can be blunted or exacerbated following brain injury and evolves dynamically overtime according to clinical evolution [15]. Hyperoxemic vasoconstriction is a protective mechanism whose main purpose is to limit the quantity of O2 reaching the tissue and potentially the cells [16, 17]. This vasoconstriction is a local, microcirculatory response to the increased periarteriolar O2 concentration [17]. Although, the mechanism is incompletely understood, the role of prostaglandins on the hyperoxemic vasoconstriction pathway seems unlikely because of the lack of effect of cyclooxygenase inhibitors on hyperoxemic vasoconstriction [17, 18]. The role of direct vasoconstrictive substances such as leukotrienes seems more convincing to explain those findings [19].
PaO2 impact on CBF and metabolism is the cornerstone of the physiologic rationale justifying the use of hyperbaric oxygenation (HBO2) following traumatic brain injury, stroke or anoxic encephalopathy [18]. HBO2 have been associated with less reperfusion injuries (when applied early) possibly due to decreased endothelial neutrophils adhesion, decreased vascular permeability and vasogenic edema, decreased of intracranial pressure and the redistribution of flow from non-ischemic to ischemic area [20]. Conversely, the paradoxal hypoxia caused by the hyperoxemia-induced vasoconstriction has not been clinically demonstrated [16].
Is O2 harmful or beneficial in a context of acute brain injury in light of physiological knowledge?
Excessive oxidative stress may occur when flow and O2 supply are restored after a transitory ischemia/oligemia or in case of impaired O2 diffusion to the tissue (i.e., in case of cerebral edema). So, the timeline between the occurrence of brain injury and the quantity of O2 administered as well as the cause of the injury is essential to correctly identify if exposure to high level of O2 may be beneficial or harmful [20, 21]. From a physiological point of view, dramatically increasing PaO2 (above 225–300 mmHg) in normobaric (and normothermic) condition could be potentially harmful because of the low solubility of O2 in blood [21, 22].
However, in specific cases HBO2 could be an interesting therapeutic option. HBO2 increases O2 delivery even when flow is compromised and has been evaluated in various context of ABI [23]. Pre-clinical investigation showed that HBO2 administered within 6 h following different model of ABI was associated with reduced apoptosis, size and severity of the lesions by the maintenance of O2 delivery despite ischemia, as well as direct anti-inflammatory effect [20]. Neuroprotective effects through decreased blood brain barrier permeability and its dysfunction were also suggested [20].
Beyond lab models, HBO2 has also been evaluated in human patients. In a randomized trial, treatment by HBO2 (60 min each 8 h at 1.5 atmosphere for two weeks) implemented in the early phase of traumatic brain injury reduced mortality, particularly in the subgroup of patients with an intracranial pressure over 20 mmHg [23, 24]. Conversely, results were contrasted with HBO2 in the acute phase of stroke, as resumed by a Cochrane Review from 2014 which conclude that evidence is insufficient to recommend its routinely use [25].
In summary, the effects of supraphysiologic exposure to O2 on CBF and intracellular oxidative metabolism are complex and depend on the clinical context, the timing and duration of the exposure following brain injury. In our opinion, the question whether hyperoxemia is beneficial or deleterious will never find a definitive response, since it is not the right way to address it. We believed that the effect of supraphysiologic exposure to O2 cannot be addressed in a binary way, where it is safe below a specific threshold and harmful above. Individualizing the effect of O2 alone necessitate to closely monitor other covariables such as the cause of ABI, the timing of exposure to high O2 concentration, the PaCO2 among others.
We believe that the conclusion that hyperoxemia is associated with worse outcomes and higher mortality should be tempered and contextualized. We agreed that in normobaric condition, blindly increasing PaO2 could be harmful as it exposes the patient to O2 toxicity (particularly the lung) without increasing enough the dissolved O2 to have significant advantages. Evidences deriving from HBO2 studies and supported by physiological evidence suggest that hyperoxemia could be beneficial in several clinical context. The use of HBO2 in context of ABI is still to be evaluated by larger prospective studies as strong clinical evidence are lacking despite encouraging physiological rationale. Result from an ongoing Phase II randomized trial (NCT02407028) evaluating the optimal modality of HBO2 to treat acute traumatic brain injury should soon give us valuable insight.
Abbreviations
- ABI
Acute brain injury
- CBF
Cerebral blood flow
- FIO2
Fraction of inspired O2
- HBO2
Hyperbaric oxygenation
- O2
Dioxygen
- PaO2
Partial pressure of dioxygen
- PaCO2
Partial pressure of carbon dioxide
- ROS
Reactive oxygen species
Author contributions
SD and RM wrote the first draft of the manuscript. MA and HK reviewed the proof and made substantial correction. All authors reviewed the final version of the manuscript.
Funding
Financial/Non-financial disclosure: Support was provided solely from institutional and/or departmental sources.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
SysiPh group members
Roman Mounier, Sylvain Diop, Maxime Aparicio, Ariane Roujansky, Hatem Kallel.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
This comment refers to the article available online at https://doi.org/10.1186/s13054-025-05387-7
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Romero-Garcia Nekane, Robba C, Monleón B, Ruiz-Zarco A, Pascual-González M, Ruiz-Pacheco A, et al. Neurological outcomes and mortality following hyperoxemia in adult patients with acute brain injury: an updated meta-analysis and meta-regression. Crit Care. 2025;29(1): 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer M, Young PJ, Laffey JG, et al. Dangers of hyperoxia. Crit Care. 2021;25:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magder S. Reactive oxygen species: toxic molecules or spark of life? Crit Care. 2006;10:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolbarsht ML, Fridovich I. Hyperoxia during reperfusion is a factor in reperfusion injury. Free Radic Biol Med. 1989;6(1):61–2. [DOI] [PubMed] [Google Scholar]
- 5.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fridovich I. Oxygen is toxic! Bioscience. 1977;27:462–6. [Google Scholar]
- 7.Bucci E. Thermodynamic approach to oxygen delivery in vivo by natural and artificial oxygen carriers. Biophys Chem. 2009;142(1–3):1–6. [DOI] [PubMed] [Google Scholar]
- 8.Klein J. Normobaric pulmonary oxygen toxicity. Anesth Analg. 1990;70:195–207. [DOI] [PubMed] [Google Scholar]
- 9.Boerema I, Meyne NG, Brummelkamp WK, et al. Life without blood (a study of the influence of high atmospheric pressure and hypothermia on Dilution of the blood). J Cardiovasc Surg. 1959;13:133–46. [Google Scholar]
- 10.Reinhart K, Bloos F, König F, Bredle D, Hannemann L. Reversible decrease of oxygen consumption by hyperoxia. Chest. 1991;99(3):690–4. [DOI] [PubMed] [Google Scholar]
- 11.Diop S, Fadel E, Valentini P, Thepaut Alexandre, Genty T, Ion I, et al. Effect of deep hypothermia (18°C) on dioxygen metabolism during pulmonary thromboendarterectomy surgery. J Cardiothorac Vasc Anesth. 2024;38:2990–6. [DOI] [PubMed] [Google Scholar]
- 12.Lång M, Skrifvars MB, Siironen J, Tanskanen P, Ala-Peijari M, Koivisto T, et al. A pilot study of hyperoxemia on neurological injury, inflammation and oxidative stress. Acta Anaesthesiol Scand. 2018;62(6):801–10. [DOI] [PubMed] [Google Scholar]
- 13.Croal PL, Hall EL, Driver ID, Brookes MJ, Gowland PA, Francis ST. The effect of isocapnic hyperoxia on neurophysiology as measured with MRI and MEG. Neuroimage. 2014;105:323–31. [DOI] [PubMed] [Google Scholar]
- 14.Kolbitsch C, Lorenz IH, Hörmann C, Hinteregger M, Löckinger A, Moser PL, et al. The influence of hyperoxia on regional cerebral blood flow (rCBF), regional cerebral blood volume (rCBV) and cerebral blood flow velocity in the middle cerebral artery (CBFVMCA) in human volunteers. Magn Reson Imaging. 2002;20(7):535–41. [DOI] [PubMed] [Google Scholar]
- 15.Torbati D, Carey ME, orbati D. Effect of Normobaric hyperoxia on regional cerebral blood flow before and, after brain missile wounding in anesthetised cats. Undersea Biomedical Res. 1989;16:78–9. [Google Scholar]
- 16.Bean JW. Cerebral O2 in exposures to O2 at atmospheric and higher pressure, and influence of CO2. Am J Physiol-Legacy Content. 1961;201(6):1192–8. [DOI] [PubMed] [Google Scholar]
- 17.Jackson WF. Arteriolar oxygen reactivity: where is the sensor and what is the mechanism of action? J Physiol. 2016;594(18):5055–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson WF. Prostaglandins do not mediate arteriolar oxygen reactivity. AJP Heart Circ Physiol. 1986;250(6):H1102-8. [DOI] [PubMed] [Google Scholar]
- 19.Jackson WF. Arteriolar oxygen reactivity is inhibited by leukotriene antagonists. Am J Physiol. 1989;257(5):H1565-72. [DOI] [PubMed] [Google Scholar]
- 20.Rockswold SB, Rockswold GL, Zaun DA, Liu J. A prospective, randomized phase II clinical trial to evaluate the effect of combined hyperbaric and normobaric hyperoxia on cerebral metabolism, intracranial pressure, oxygen toxicity, and clinical outcome in severe traumatic brain injury. J Neurosurg. 2013;118(6):1317–28. [DOI] [PubMed] [Google Scholar]
- 21.Eastwood G, Bellomo R, Bailey M, Taori G, Pilcher D, Young P, et al. Arterial oxygen tension and mortality in mechanically ventilated patients. Intensive Care Med. 2011;38(1):91–8. [DOI] [PubMed] [Google Scholar]
- 22.Brugniaux JV, Coombs GB, Barak OF, Dujic Z, Sekhon MS, Ainslie PN. Highs and lows of hyperoxia: physiological, performance, and clinical aspects. Am J Physiology-Regulatory Integr Comp Physiol. 2018;315(1):R1–27. [DOI] [PubMed] [Google Scholar]
- 23.Bennett MH, Trytko B, Jonker B. Hyperbaric oxygen therapy for the adjunctive treatment of traumatic brain injury. Cochrane Database Syst Rev. 2012.12. 10.1002/14651858.CD004609.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rockswold GL, Ford SE, Anderson DC, Bergman TA, Sherman RE. Results of a prospective randomized trial for treatment of severely brain-injured patients with hyperbaric oxygen. J Neurosurg. 1992;76(6):929–34. [DOI] [PubMed] [Google Scholar]
- 25.Bennett MH, Weibel S, Wasiak J, Schnabel A, French C, Kranke P. Hyperbaric oxygen therapy for acute ischaemic stroke. Cochrane Database Syst Rev. 2014. 11. 10.1002/14651858.CD004954.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.