Abstract
Selective, and green spectrophotometric methods have been developed for the simultaneous analysis of remdesivir (RDV) and moxifloxacin hydrochloride (MFX), two active agents that are being used together in COVID-19 treatment. Because of the considerable spectral overlap, six mathematical spectrophotometric approaches were applied, including ratio derivative, ratio difference, mean centering of ratio spectra, area under the curve, Q-analysis, and bivariate calibration, to allow accurate interference-free determination without preliminary separation. The proposed methods were validated as per ICH guidelines, demonstrating excellent linearity over the concentration ranges of 1–15 µg/mL for RDV and 1–10 µg/mL for MFX, with correlation coefficients exceeding 0.999. They showed high sensitivity with LODs from 0.26 to 0.92 µg/mL and LOQs from 0.27 to 0.96 µg/mL for both drugs, enabling reliable trace analysis of the studied drugs in complex matrices. The methods also applied to dosage forms and spiked human plasma provided good recoveries with minimal matrix interference, demonstrating that the methods are robust and applicable. Eco-Scale, ComplexGAPI, and AGREE gave a high score on green profiles for environmental and practical sustainability. Further, the high whiteness and blueness scores indicated that the methods met the requirements of white analytical chemistry by RGB12 and BAGI tools. Thus, these methods offer a practical, eco-conscious solution for simultaneous determination of RDV and MFX in pharmaceutical and clinical settings, contributing to better therapeutic monitoring in COVID-19 management.
Keywords: COVID-19, Remdesivir, Moxifloxacin hydrochloride, Spectrophotometry
Introduction
As far as public health emergencies go, COVID-19 is regarded as the worst of the last century. COVID-19 is a respiratory infection that was spreading quickly [1] which prompted experts to seek therapy techniques to combat the rapidly progressing respiratory disease. The need for an efficient oral antiviral treatment is still a major worry due to insufficient vaccine uptake, even though many immunizations were offered to slow the transmission of the severe acute respiratory syndrome (SARS-COV-2) virus since its widespread dissemination in 2020 [2]. Employing FDA-approved drugs like remdesivir and favipiravir was the quickest and easiest option because approving a novel therapeutic for use in humans is a drawn-out process with multiple steps [3].
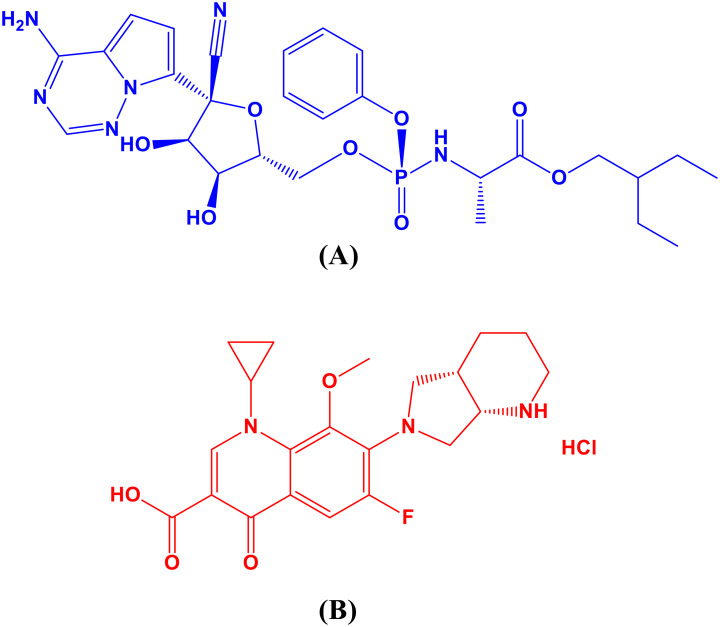
Remdesivir (RDV) Fig. 1 (a), is an adenosine triphosphate analogue that has wide antiviral action. Preliminary research suggests that RDV may hasten recovery for hospitalized patients with severe COVID-19. It was the first medication that the US Food and Drug Administration (FDA) had authorized for use in an emergency to treat hospitalized COVID-19 patients [4, 5]. Antivirals function by blocking RNA polymerase, hence stopping the propagation of coronaviruses. RDV measurement has been reported using a number of techniques, including chromatographic [6–9], spectrophotometric [10–15], electrochemical [16, 17] and spectrofluorimetric [18–23].
Fig. 1.
RDV (a) and MFX (b) chemical structures
Despite the fact that COVID-19 is a virus, there are concerns about the drug’s regular use in treating the disease. Viral respiratory infections can raise the likelihood of developing bacterial pneumonia, however medicines have no direct effect on SARS-CoV-2 [24]. A new in silico study suggests that fluoroquinolones, such moxifloxacin hydrochloride (MFX), Fig. 1(b), may prevent SARS-CoV-2 from replicating because of their high affinity for binding to the virus’s primary protease enzyme [25]. Many analytical techniques are listed in the literature review for MFX analysis, such as densitometric [26, 27], liquid chromatographic [28–33], spectrophotometric [34–36], and spectrofluorometric [37–40] approaches.
The simultaneous determination of remdesivir (RDV) and moxifloxacin (MFX) is crucial due to their frequent co-administration in COVID-19 treatment [41, 42]. Several spectrophotometric methods have been previously reported for resolving overlapping spectra of such drug combinations, including absorbance subtraction, extended ratio subtraction, and amplitude modulation [42]. These techniques have demonstrated successful applications, particularly in pharmaceutical dosage forms and even in spiked biological matrices. Nevertheless, their performance often depends on specific spectral characteristics, such as plateau regions or isoabsorptive points, and may require precise wavelength selection and mathematical manipulation.
To address these issues, the current study proposes a fully applicable spectrophotometric methodology that incorporates six mathematical techniques: ratio derivative (1DD), ratio difference (RD), mean centering of ratio spectra techniques (MC), area under the curve (AUC), Q-analysis, and bivariate calibration -all of which improve selectivity, sensitivity, and versatility by allowing quantification of both drugs without spectral interference. This proposed methodology is also more resilient than previously published methods because it uses less data processing and has a broader application, rather than being limited to specific wavelengths of each spectral point [43–47].
Scientific societies have recently pushed for the integration of green analytical chemistry (GAC) and white analytical chemistry (WAC) concepts into research workflows in response to the increased emphasis on comprehensive, sustainable, inventive, and validated analytical methodologies [48, 49]. Many techniques, including the ESA (Eco-Scale Assessment), GAPI (Green Analytical Procedure Index), and the AGREE (Analytical Greenness Metric) have recently been developed for evaluating “greenness” in accordance with the 12 GAC principles [50–52].
Furthermore, a variety of recently developed algorithms, including RGB12 and BAGI algorithms, have been developed for evaluating “whiteness” and “blueness” [53, 54].
Experimental
Instrumentation
A Shimadzu UV-Visible 1800 Spectrophotometer (Tokyo, Japan) with corresponding 10 mm quartz cells was used in the experiment. The apparatus was operated using Shimadzu’s UV-Probe personal spectroscopy software, version 2.21. Furthermore, the investigations made use of an analytical balance (Precisa125A, Switzerland).
Chemicals
Remdesivir (99.15%) and MFX (99.45%) powders were kindly given by EVA Pharma for Pharmaceuticals and Medical Appliances (Cairo, Egypt) according to the reference methods [15, 36]. EVA Pharma (Cairo, Egypt) produced Remdesivir Eva pharma® vials with 100 mg of RDV, which were purchased from the local market (batch numbers: 2009561, 2009563, 2009566, 2009567). Additionally, Moxiflox® tablets, produced by EVA Pharma (Cairo, Egypt) and containing 436.37 mg of MFX (400 mg moxifloxacin) per tablet, were bought locally (batch number 2212537). Zagazig University Hospital (Zagazig, Egypt) kindly provided plasma samples, which were kept frozen at -20 °C until assessment. Sigma-Aldrich in Germany supplied methanol.
Standard solutions
For RDV and MFX stock standards, it was necessary to dissolve 100 mg of each drug powder in 70 mL of methanol, sonicate for 15 min, and then complete to 100 mL with methanol. Then dilute the stock solutions with methanol to obtain working standard solutions (100 µg/mL).
Laboratory prepared mixtures
The standard solutions of RDV and MFX were accurately aliquoted into 10 mL volumetric flasks, and methanol was used to dilute the solution with various ratios.
Procedures
Calibration curves construction
Aliquots from RDV and MFX standard solutions (100 µg/mL) ranging from (10–150) µg for RDV and from (10–100) µg for MFX were transferred into two separate sets of 10-mL volumetric flasks and completed to the mark with methanol.
Ratio manipulating spectrophotometric methods (RD, 1DD and MC)
Optimization of experimental conditions
Several MFX concentrations (1, 2, 4, 5, 6, 7, 8, and 10 µg/ml) were tested using the general process under “3.1” for each method in order to determine the optimal divisor concentration for RDV.
Using the same general techniques, various RDV concentrations (1, 3, 5, 7, 8, 9, 11, 13, and 15 µg/ml) were tested for MFX.
The MFX spectrum (6.0 µg/ml) was split by the zero order absorption spectra (from 200 to 400 nm) for RDV. The RDV spectrum (8.0 µg/ml) was split by the zero order absorption spectra (from 200 to 400 nm) for MFX.
Ratio derivative method
Using Δλ = 4 nm and scaling factor = 10, the first derivative for each ratio spectrum was recorded. The calibration curve was created by plotting the amplitude values against each drug’s concentration after they were measured at 250 nm for RDV and 290 nm for MFX. Regression equations were computed.
Ratio difference method
The calibration curve was created by plotting the difference in peak amplitudes (ΔP) at the ratio spectra at 247 and 262 nm (ΔP247 − 262 nm) for RDV and at 299 and 313 nm (ΔP299 − 313 nm) for MFX against each drug’s concentration. Regression equations were computed.
Mean centering method
The ratio spectra (from 200 to 400 nm) were mean centered and the mean centered values were measured at 247 nm and 299 nm for RDV and MFX, respectively. The values were plotted against concentration of each drug to obtain the calibration curve. Equations for regression were calculated.
For area under the curve method
We applied the AUC method using two selected wavelength ranges. The AUC values were calculated from the overlain spectra of the binary mixture, and Cramer’s Rule in conjunction with the Matrix Method was used to perform the analysis. For the mixture of two component X and Y, the total AUC over the given range is equal to the sum of the individual AUCs of X and Y. This relationship can be defined by two equations which correspond to the intervals of selected wavelengths.
Then, the system of equations was reformulated in terms of values of absorptivity and concentration. Using Cramer’s Rule, the absolute concentrations of X and Y were determined with the equations:
 |
1 |
 |
2 |
In this work, the AUC values over the ranges 243–248 nm and 290–300 nm for remdesivir (RDV) and moxifloxacin (MFX) were also obtained. The values of absorptivity and AUC were incorporated in Eqs. (1) and (2) to compute the concentration of RDV and MFX respectively [55].
For Q-analysis method
As per the Q-analysis method, we assign the first drug as X and the second drug as Y. This method depends on measuring the absorbance at two wavelengths; the isoabsorptive point (λiso) and the λmax of one of the components. Using relationships given by ax1 = ay1 and a path length L = 1, two fundamental equations were formed. By dividing and rearranging, expressions for the fraction of each component were found. In order to determine the absolute concentrations of X and Y, further rearrangement lead to the equations below:
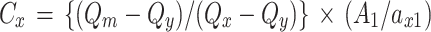 |
3 |
 |
4 |
Finally Eqs. (1) & (2) gives the absolute concentration value of drug X & Y.
Absorbance values of RDV and MFX were quantified at 229 nm (λiso) and 245 nm (λmax), and calibration graphs were generated. Equations (3) and (4) were employed to calculate the concentration of RDV and MFX by utilizing the absorptivity values of each component at the selected wavelengths [46, 56].
For bivariate method
Bivariate calibration involves measuring two components (X and Y) at specific wavelengths to generate equations. These equations allow for the evaluation of concentration (CX and CY) [45]. The Kaiser approach uses slope values from linear regression equations to build sensitivity matrices (K) and estimate optimal pair of wavelengths for binary mixture determination [57, 58]. The absorbance was measured at 245 and 295 nm, and the corresponding regression equations were computed for both drugs. The obtained slopes and intercepts were used to calculate RDV and MFX concentrations [57].
Methods applications
Laboratory prepared mixtures analysis
After preparing various ratios of laboratory-prepared mixtures, the spectra of these mixtures were measured and handled similarly to how the suggested procedures specify.
Pharmaceutical formulation analysis
RDV
Remdesivir-Eva Pharma® four vials (100 mg/ 20 mL vial) were mixed correctly. In a 100 mL volumetric flask, precisely 2 mL of remdesivir (10 mg) were added. A volume of about 70 mL of methanol was then added to the flask. The solution was shaken vigorously for 15 min, and then it was sonicated for 30 min. A concentration of 100 µg/mL was obtained by adding methanol to the fluid until it reached 100 mL.
MFX
Ten Moxiflox® tablets containing 400 mg of moxifloxacin hydrochloride (436.37 mg/tablet) were weighed and ground into a coarse powder. The powder, which is equivalent to 10 mg of MFX, was precisely weighed, then moved to a 100 mL volumetric flask, where methanol was added to bring the volume up to about 70 mL. The solution was sonicated for 30 min after being forcefully shaken for 15 min. After adding 100 mL of methanol, the fluid was filtered to achieve a concentration of 100 µg/mL.
Co-formulated RDV and MFX
A fixed-dose combination originated as RDV and MFX fixed-dose pills were not accessible in Egypt. Four finely powdered Moxiflox® tablets (400 mg/tablet) and four Remdesivir-Eva Phama® vials (100 mg/20 mL vial) were mixed together. After carefully adding two milliliters of this mixture (10 milligrams of RDV and 40 milligrams of MFX) to a 100 milliliter volumetric flask, the flask was filled with methanol to a capacity of about 70 milliliters. After 15 min of vigorous shaking, the mixture was subjected to a 30-minute sonication. Following the addition of 100 mL of methanol, the volume was filtered to achieve a concentration of 400 µg for MFX and 100 µg for RDV.
The reported method
A previously reported green UV spectrophotometric method-the first derivative of ratio spectra-was selected for the quantitative analysis of remdesivir [15].
UV spectrophotometric technique was described for quantifying MFX in various medicinal formulations. The MFX concentration was measured at 296 nm in 0.1 N hydrochloric acid (pH 1.2) [36].
Analysis of RDV and MFX in spiked human plasma
Various aliquots from the RDV and MFX standard solutions (10 µg mL− 1) were added to 15 mL centrifuge tubes that contained 1 mL of drug-free plasma. The protein was then denaturized by adding 3 mL of methanol. After two minutes of vortexing, the solutions in the centrifuge tubes were centrifuged for thirty minutes at 4000 rpm. Using a rotary evaporator, the protein-free supernatants were vacuum-evaporated until they were completely dry. The dry residue was dissolved in methanol, then placed in 10-mL volumetric flasks and completely filled with methanol [59, 60]. This method was performed with each medication at different concentrations within the working range. Both RDV and MFX contents have been evaluated using regression analysis.
Results and discussion
In the present study, six simple and sensitive spectrophotometric methods were suggested for the selective quantitative determination of RDV and MFX without previous separation.
Spectral characteristics
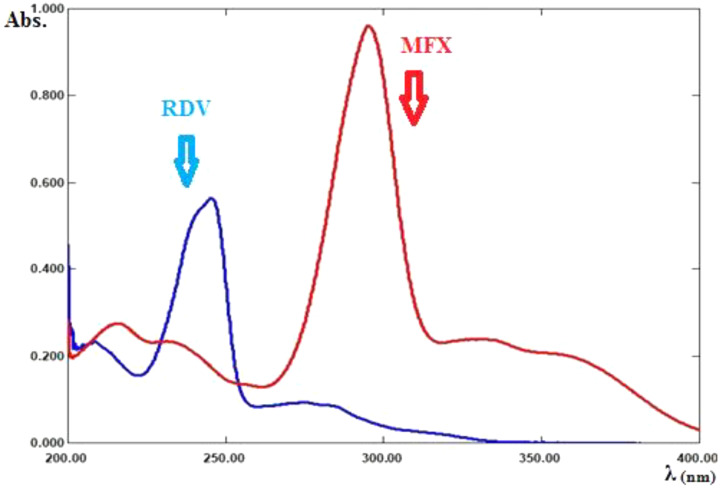
The zero-order absorption spectra of RDV and MFX show severe overlap, as shown in Fig. 2 which does not permit direct determination of RDV and MFX presence of each other.
Fig. 2.
Zero order absorption spectra of RDV (8 µg/mL), and MFX (8 µg/mL)
So, three proposed ratio methods are based on dividing the absorption spectra of each drug by the absorption spectrum of second drug, as a divisor, to get the ratio spectra, were used as shown in Figures (3, 4).
Fig. 3.
Ratio spectra of RDV at various concentrations (1–15 µg/ml) using 6 µg/ml of its MFX as a divisor
Fig. 4.
Ratio spectra of MFX at various concentrations (1–10 µg/ml) using 8 µg/ml of its RDV as a divisor
Ratio derivative method
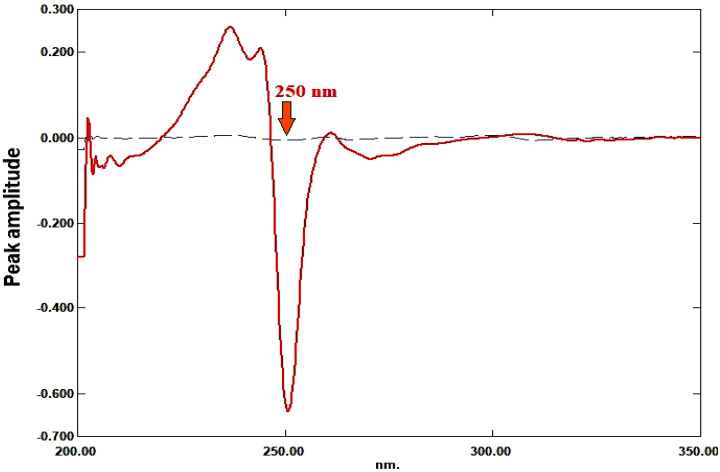
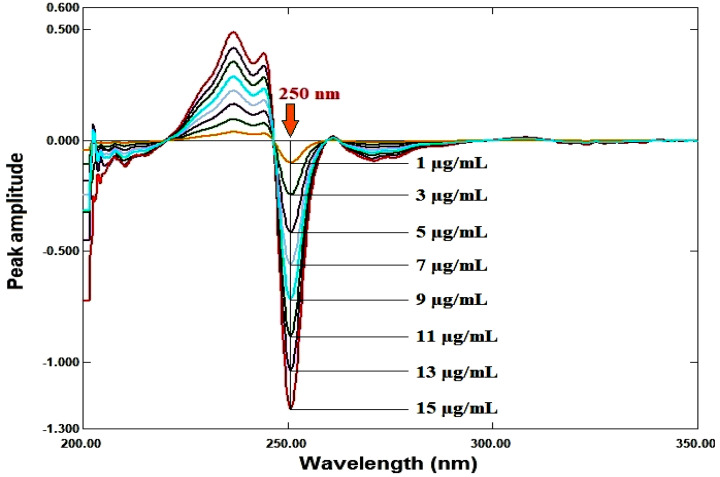
For RDV and MFX, respectively, the amplitudes of the first derivative of the ratio spectra at 250 nm and 290 nm are proportional to the concentrations of each medication independent of the other (divisor), as illustrated in Figures 5, 6, 7, 8.
Fig. 5.
First derivative of the ratio spectra of RDV, 8 µg/mL, (ــــــ) and MFX, 6 µg/mL, (ـــ ــــ) using 6 µg/mL of MFX as a divisor
Fig. 6.
First derivative of the ratio spectra of MFX, 8 µg/mL, (ــــــ) and RDV, 8 µg/mL, (ـــ ــــ) using 8 µg/mL of RDV as a divisor
Fig. 7.
First derivative of the ratio spectra of RDV at various concentrations (1–15 µg/mL) using 6 µg/mL of MFX as a divisor
Fig. 8.
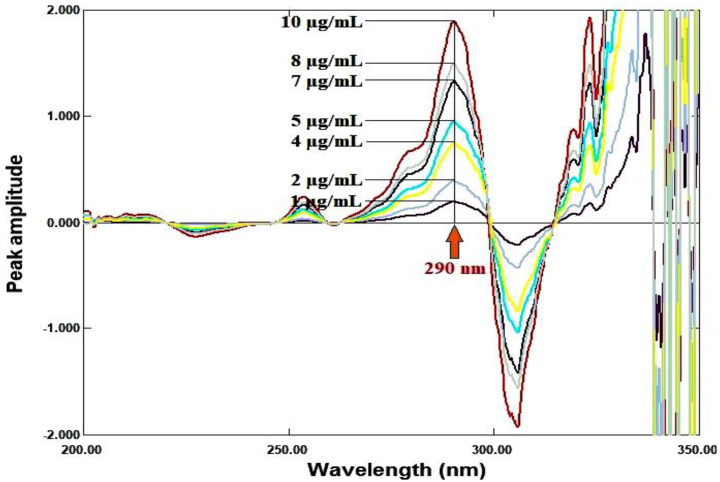
First derivative of the ratio spectra of MFX at various concentrations (1–10 µg/mL) using 8 µg/mL of RDV as a divisor
Ratio difference method
Without interference from the second medication (divisor), the ratio spectra’s peak amplitudes between (247–262) nm and (299–313) nm are proportionate to the RDV and MFX concentrations, respectively.
Mean centering method
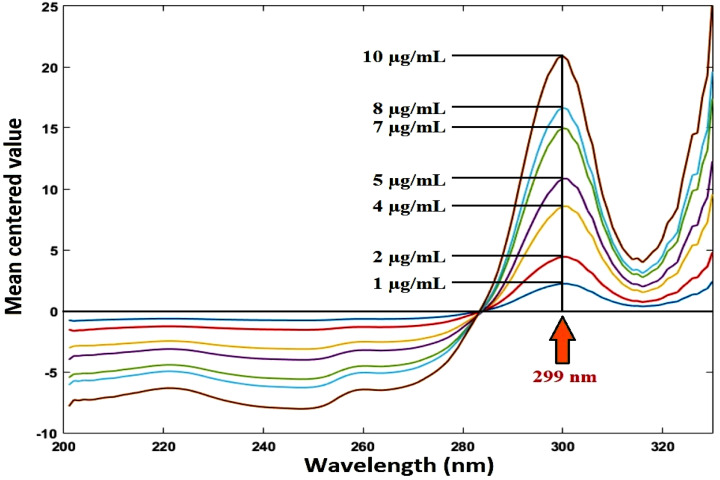
Mean-centered ratio spectra were obtained. Without the other drug interfering, the mean centered values at 247 nm and 299 nm are proportional to the concentrations of RDV and MFX, respectively, as illustrated in Figures 9, 10.
Fig. 9.
Mean centering of the ratio spectra of RDV at various concentrations (1–15 µg/ml) using 6 µg/mL of MFX as a divisor
Fig. 10.
Mean centering of the ratio spectra of MFX at various concentrations (1–10 µg/ml) using 8 µg/mL of RDV as a divisor
Other three methods were also used including:
Area under the curve method
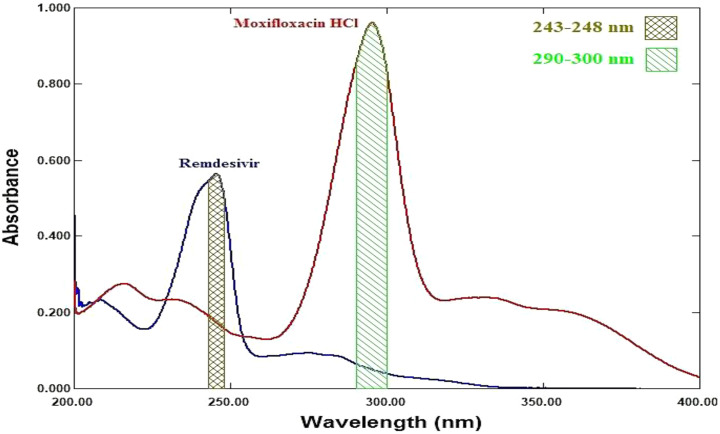
Figure 11 lists the area under the curves for RDV and MFX, respectively, over the ranges of (243–248) and (290–300) nm. We created the calibration graphs and calculated the regression equations that connect the measured areas under the curve to the concentration of each component in µg/ml. The concentrations of RDV and MFX were determined using Eqs. (1) and (2), respectively, based on the absorptivity values and areas under the curve for each component at the chosen wavelength ranges.
Fig. 11.
Zero order absorption spectrum of RDV (8 µg/mL) and MFX (8 µg/mL) showing area under the curve over the ranges (243–248) and (290–300) nm
Q-analysis method
The absorption spectra of RDV and MFX showed isoabsorptive point at 229 nm, as shown in Fig. 2. The spectra show also isoabsorptive point at 254 nm which was not involved in the method due to the low sensitivity of the drugs at this wavelength. The absorbance values were measured at 245 nm (λmax for RDV) and 229 nm (λiso) in the range of 1–15 µg/ml and 1–10 µg/ml for RDV and MFX, respectively. Absorptivity values were determined for both RDV and MFX. The values and the absorbance ratio were used to calculate the concentration of RDV and MFX in their binary mixture using Eqs. (3) and (4), respectively.
Bivariate method
To resolve RDV and MFX in their mixture using the bivariate approach, the absorbance of each component separately was measured at seven distinct wavelengths in the overlap region: 245, 255, 265, 275, 285, 295, and 305 nm. To make sure that there was a linear relationship between the absorbance and the appropriate concentration, the calibration curve equations and their accompanying linear regression coefficients were obtained. At the chosen wavelengths, every calibration curve displayed a reasonable linear determination coefficient (r2 > 0.9987). In accordance with the Kaiser approach, the sensitivity matrices (K) were computed using the slope values of the linear regression equations for both medications at the chosen wavelengths in order to determine the ideal pair of wavelengths at which the binary mixture was recorded. The slopes at 245 and 295 nm were selected for the analysis because they were found to provide the highest value of K (Table 1).
Table 1.
The absolute values of the sensitivity matrix determinates calculated according to Kaiser’s method (k x 105) for the mixture of RDV and MFX [57, 58]
| λ/λ | 245 | 255 | 265 | 275 | 285 | 295 | 305 |
|---|---|---|---|---|---|---|---|
| 245 | 0 | 948.57 | 1013.91 | 2186.31 | 5505.51 | 8509.11 | 4967.1 |
| 255 | 0 | 58.75 | 284.83 | 976.67 | 1642.28 | 952.33 | |
| 265 | 0 | 169.04 | 702.96 | 1228.39 | 710.29 | ||
| 275 | 0 | 597.92 | 1230.15 | 703.49 | |||
| 285 | 0 | 770.63 | 413.09 | ||||
| 295 | 0 | -56.81 | |||||
| 305 | 0 |
Optimization of experimental conditions
When using ratio-based techniques, it was crucial to carefully select the divisor concentration. Various drug concentrations were tested as divisors; the best ones were 6 µg/ml and 8 µg/ml for RDV and MFX, as they produced the least amount of noise and produced better results in terms of selectivity.
Method validation
The suggested techniques were successfully validated and produced satisfactory results in accordance with ICH criteria [61].
Linearity and range
The response of each drug for each method was plotted against the drug concentrations in µg/ml to create the calibration graphs for each drug for the three methods under the experimental circumstances indicated. The amplitudes of the first derivative of the ratio spectra at 250 nm and 290 nm for RDV and MFX, respectively, are the response for the ratio derivative method. The difference in peak amplitudes between the two chosen wavelengths (247–262) nm and (299–313) nm in the ratio spectra for RDV and MFX, respectively, is the response for the ratio difference method. The mean centered values of the ratio spectra at 247 nm and 299 nm for RDV and MFX, respectively, are the response for the mean centering approach. The area under the curve for the two chosen wavelength ranges, 243–248 nm (λ1-λ2) and 290–300 nm (λ3-λ4), is the response for the area under the curve method. Absorbance readings at 229 and 245 nm are the response for the Q-analysis approach. The absorbance readings at 245 and 295 nm provide the response for the bivariate technique. Table 2 displayed the regression data. The calibration graphs’ strong linearity was demonstrated by the coefficient of determination values.
Table 2.
Regression and validation data for determining RDV and MFX using the proposed methods
| Parameters | RDV | MFX | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1DD | RD | MC | AUC | Q-analysis | Bivariate | 1DD | RD | MC | AUC | Q-analysis | Bivariate | |||||||
| Wavelength (nm) | 250 | 247–262 | 247 | 243–284 | 290–300 | 229 | 245 | 245 | 295 | 290 | 299–313 | 299 | 243–284 | 290–300 | 229 | 245 | 245 | 295 |
| Linearity range (µg/mL) | 1–15 | 1–10 | ||||||||||||||||
| Slope | 0.0771 | 0.4736 | 0.4556 | 0.348 | 0.058 | 0.071 | 0.028 | 0.021 | 0.071 | 0.1868 | 1.6476 | 2.0704 | 1.150 | 0.120 | 0.029 | 0.021 | 0.021 | 0.122 |
| Intercept | 0.0093 | 0.0470 | 0.0487 | 0.037 | 0.006 | 0.009 | 0.005 | 0.005 | 0.009 | 0.0083 | 0.2893 | 0.2893 | 0.066 | 0.005 | 0.001 | 0.005 | 0.005 | 0.002 |
| LOD (µg/mL) | 0.264 | 0.311 | 0.302 | 0.312 | 0.316 | 0.301 | 0.911 | 0.278 | 0.314 | 0.242 | 0.294 | 0.262 | 0.307 | 0.305 | 0.278 | 0.844 | 0.287 | 0.259 |
| LOQ (µg/mL) | 0.8005 | 0.941 | 0.916 | 0.946 | 0.957 | 0.273 | 0.828 | 0.844 | 0.951 | 0.734 | 0.892 | 0.793 | 0.931 | 0.925 | 0.287 | 0.868 | 0.868 | 0.785 |
| Coefficient of determination (r2) | 0.9997 | 0.9996 | 0.9996 | 0.9997 | 0.9995 | 0.9997 | 0.9995 | 0.9997 | 0.9996 | 0.9997 | 0.9995 | 0.9995 | 0.9996 | 0.9995 | 0.9997 | 0.9996 | 0.9996 | 0.9996 |
| Accuracy (% R) a | 98.70 | 100.09 | 98.59 | 100.25 | 99.84 | 99.12 | 101.25 | 99.91 | 101.05 | 98.76 | 101.07 | 99.41 | ||||||
|
Precision (% RSD) b - Repeatability - Intermediate precision |
0.522 1.038 |
1.617 0.869 |
0.972 0.579 |
1.233 1.417 |
1.161 1.47 |
0.833 1.096 |
0.635 1.238 |
1.304 1.465 |
0.969 1.094 |
0.742 0.959 |
0.947 1.328 |
1.225 1.161 |
||||||
a Average of 9 determinations (3 concentrations repeated 3 times). b %RSD of 9 determinations (3 concentrations repeated 3 times)
Detection and quantitation limits
As shown in Table 2, the results of calculating LODs and LOQs values demonstrated the analytical sensitivity of the recommended techniques for the medications under investigation.
Accuracy and precision
The acquired good %R, as displayed in Table 2, indicated the accuracy of the suggested approaches. On the other hand, Table 2’s tiny percentage RSD values demonstrated the methodologies’ great precision.
Specificity
Different amounts of the drugs being studied were added to synthetic mixtures, which were then thoroughly mixed. Then, using the previously described basic approach of the process, the combinations were analyzed. Table 3 displays the excellent, satisfactory results that were obtained. As indicated in Table 4, the single dosage form of RDV and the co-formulated dosage form of MFX were identified in the laboratory using the recommended methodologies. Specificity was also evaluated by applying the traditional addition approach to pharmaceutical preparations that had previously been analyzed in order to determine the effect of the matrix on the identification of the two drugs. The data in Table 4 demonstrated that the recommended approach could assess the medication in a selected manner without influence from excipients.
Table 3.
Analysis of laboratory prepared mixture of RDV and MFX by the proposed methods
| Method | Laboratory prepared mixture (µg/mL) | % Recovery* | ||||
| RDV | MFX | RDV | MFX | |||
| Ratio derivative | 1 | 2 | 98.18 | 98.96 | ||
| 1 | 4 | 100.78 | 99.13 | |||
| 2 | 4 | 100.32 | 100.87 | |||
| 2 | 8 | 99.03 | 101.02 | |||
| 4 | 4 | 101.72 | 99.13 | |||
| Mean ± %RSD | 100.01 ± 1.406 | 99.82 ± 1.033 | ||||
| Ratio difference | 1 | 2 | 101.77 | 99.65 | ||
| 1 | 4 | 100.08 | 101.55 | |||
| 2 | 4 | 100.71 | 98.37 | |||
| 2 | 8 | 101.67 | 101.08 | |||
| 4 | 4 | 99.98 | 98.14 | |||
| Mean ± %RSD | 100.84 ± 0.842 | 99.76 ± 1.547 | ||||
| Mean centering | 1 | 2 | 101.38 | 100.79 | ||
| 1 | 4 | 99.36 | 101.86 | |||
| 2 | 4 | 100.42 | 98.72 | |||
| 2 | 8 | 101.50 | 100.98 | |||
| 4 | 4 | 99.48 | 98.12 | |||
| Mean ± %RSD | 100.43 ± 1.007 | 100.10 ± 1.593 | ||||
|
Area under curve |
1 | 2 | 98.55 | 101.51 | ||
| 1 | 4 | 100.36 | 98.18 | |||
| 2 | 4 | 99.88 | 98.50 | |||
| 2 | 8 | 100.06 | 99.02 | |||
| 4 | 4 | 98.27 | 100.76 | |||
| Mean ± %RSD | 99.42 ± 0.952 | 99.59 ± 1.468 | ||||
|
Q- analysis |
1 | 2 | 102.08 | 99.75 | ||
| 1 | 4 | 99.71 | 100.80 | |||
| 2 | 4 | 100.70 | 98.71 | |||
| 2 | 8 | 101.10 | 101.32 | |||
| 4 | 4 | 100.90 | 100.91 | |||
| Mean ± %RSD | 100.90 ± 0.844 | 100.30 ± 1.053 | ||||
| Bivariate | 1 | 2 | 98.48 | 98.76 | ||
| 1 | 4 | 98.87 | 101.88 | |||
| 2 | 4 | 101.63 | 101.30 | |||
| 2 | 8 | 98.76 | 100.92 | |||
| 4 | 4 | 99.72 | 100.22 | |||
| Mean ± %RSD | 99.29 ± 0.887 | 100.62 ± 1.191 | ||||
* Average of three determinations
Table 4.
Determination of RDV and MFX in pharmaceutical preparations and co-formulated dosage form by the proposed methods
| Method | Remdesivir-Eva 100 mg/vial | Moxiflox 400 mg/tablet | Co-formulated dosage form | |||||
|---|---|---|---|---|---|---|---|---|
| RDV | MFX | |||||||
| Conc. (µg/mL) | % R * | Conc. (µg/mL) | % R * | Conc. (µg/mL) | % R * | Conc. (µg/mL) | % R * | |
| Ratio derivative | 1 | 100.77 | 4 | 98.06 | 1 | 99.48 | 4 | 99.13 |
| 1.5 | 99.18 | 6 | 99.01 | 1.5 | 101.77 | 6 | 99.54 | |
| 2 | 98.38 | 8 | 98.41 | 2 | 100.97 | 8 | 100.62 | |
| 2.5 | 101.53 | 10 | 98.22 | 2.5 | 99.46 | 10 | 98.11 | |
| Mean | 99.97 | Mean | 98.43 | Mean | 100.42 | Mean | 99.35 | |
| %RSD | 1.144 | %RSD | 0.422 | %RSD | 1.142 | %RSD | 1.046 | |
| Ratio difference | 1 | 100.72 | 4 | 101.87 | 1 | 100.08 | 4 | 101.70 |
| 1.5 | 98.40 | 6 | 100.47 | 1.5 | 98.11 | 6 | 100.43 | |
| 2 | 100.30 | 8 | 101.16 | 2 | 99.66 | 8 | 99.73 | |
| 2.5 | 101.52 | 10 | 101.93 | 2.5 | 101.01 | 10 | 101.38 | |
| Mean | 100.23 | Mean | 101.36 | Mean | 99.72 | Mean | 100.81 | |
| %RSD | 1.323 | %RSD | 0.681 | %RSD | 1.213 | %RSD | 0.893 | |
| Mean centering | 1 | 99.93 | 4 | 99.29 | 1 | 98.18 | 4 | 100.28 |
| 1.5 | 98.67 | 6 | 100.25 | 1.5 | 99.25 | 6 | 98.18 | |
| 2 | 101.33 | 8 | 99.70 | 2 | 99.90 | 8 | 98.96 | |
| 2.5 | 100.82 | 10 | 99.13 | 2.5 | 98.45 | 10 | 99.32 | |
| Mean | 100.19 | Mean | 99.59 | Mean | 98.94 | Mean | 99.19 | |
| %RSD | 1.162 | %RSD | 0.501 | %RSD | 0.793 | %RSD | 0.877 | |
| Area under the curve | 1 | 101.80 | 4 | 98.14 | 1 | 101.57 | 4 | 98.10 |
| 1.5 | 98.76 | 6 | 98.47 | 1.5 | 99.60 | 6 | 98.26 | |
| 2 | 101.87 | 8 | 99.33 | 2 | 100.61 | 8 | 99.33 | |
| 2.5 | 98.16 | 10 | 98.29 | 2.5 | 98.89 | 10 | 98.16 | |
| Mean | 100.15 | Mean | 98.56 | Mean | 100.17 | Mean | 98.46 | |
| %RSD | 1.96 | %RSD | 0.539 | %RSD | 1.171 | %RSD | 0.59 | |
| Q-analysis | 1 | 98.91 | 4 | 99.26 | 1 | 98.91 | 4 | 99.26 |
| 1.5 | 98.65 | 6 | 100.19 | 1.5 | 98.92 | 6 | 100.71 | |
| 2 | 98.52 | 8 | 100.66 | 2 | 98.92 | 8 | 101.43 | |
| 2.5 | 100.49 | 10 | 99.73 | 2.5 | 99.86 | 10 | 100.24 | |
| Mean | 99.14 | Mean | 99.96 | Mean | 99.15 | Mean | 100.41 | |
| %RSD | 0.922 | %RSD | 0.601 | %RSD | 0.478 | %RSD | 0.904 | |
| Bivariate | 1 | 99.01 | 4 | 99.42 | 1 | 99.08 | 4 | 98.19 |
| 1.5 | 98.52 | 6 | 101.86 | 1.5 | 100.60 | 6 | 101.70 | |
| 2 | 99.81 | 8 | 101.21 | 2 | 100.49 | 8 | 101.82 | |
| 2.5 | 98.27 | 10 | 101.35 | 2.5 | 99.31 | 10 | 101.41 | |
| Mean | 98.90 | Mean | 100.96 | Mean | 99.87 | Mean | 100.78 | |
| %RSD | 0.680 | %RSD | 1.054 | %RSD | 0.788 | %RSD | 1.724 | |
Pharmaceutical applications
The recommended techniques were used to measure the concentration of RDV and MFX in a single dosage form or in a dosage form that was co-formulated in the laboratory. The label claim that there were no indications of additive or excipient interference was well supported by the results of the conventional addition technique, which are displayed in Table 5. The results were compared to those obtained using the previously indicated approaches using statistics [15, 36]. The student’s t-test and F-test revealed no significant differences at the 95% confidence level [62], demonstrating the accuracy and precision of the suggested approach for evaluating the tested drug in its pharmaceutical dose form, as shown in Table 6.
Table 5.
Application of standard addition technique using the proposed methods
| Method | Pharmaceutical (µg/mL) | Remdesivir | MFX | ||
| Pure added (µg/mL) | % R * | Pure added (µg/mL) | % R * | ||
| Ratio derivative |
RDV 1 (1.01)* MFX 4 (3.93)* |
1 | 98.18 | 3 | 99.34 |
| 2 | 99.03 | 4 | 101.27 | ||
| 3 | 98.01 | 5 | 100.07 | ||
| Mean | 98.41 | 100.23 | |||
| %RSD | 0.552 | 0.973 | |||
| Ratio difference |
RDV 1 (1.01)* MFX 4 (4.07)* |
1 | 100.92 | 3 | 99.05 |
| 2 | 99.98 | 4 | 100.57 | ||
| 3 | 100.65 | 5 | 98.48 | ||
| Mean | 100.52 | 99.36 | |||
| %RSD | 0.486 | 1.086 | |||
| Mean centering |
RDV 1 (0.99)* MFX 4 (3.97)* |
1 | 98.40 | 3 | 101.94 |
| 2 | 100.23 | 4 | 99.06 | ||
| 3 | 98.21 | 5 | 100.35 | ||
| Mean | 98.95 | 100.45 | |||
| %RSD | 1.129 | 1.436 | |||
| Area under curve method |
RDV 1 (1.02)* MFX 4 (3.92)* |
1 | 100.88 | 3 | 99.79 |
| 2 | 98.36 | 4 | 99.93 | ||
| 3 | 100.56 | 5 | 98.06 | ||
| Mean | 99.94 | 99.26 | |||
| %RSD | 1.373 | 1.052 | |||
| Q-analysis method |
RDV 1 (0.99)* MFX 4 (3.97)* |
1 | 100.31 | 3 | 101.80 |
| 2 | 100.11 | 4 | 100.74 | ||
| 3 | 98.86 | 5 | 101.51 | ||
| Mean | 99.76 | 101.35 | |||
| %RSD | 0.788 | 0.540 | |||
| Bivariate method |
RDV 1 (0.99)* MFX 4 (3.93)* |
1 | 101.65 | 3 | 98.33 |
| 2 | 98.95 | 4 | 101.75 | ||
| 3 | 98.81 | 5 | 98.52 | ||
| Mean | 98.80 | 99.53 | |||
| %RSD | 1.602 | 1.927 | |||
* Average of three determinations
Table 6.
Statistical study and determination of RDV and MFX in pharmaceutical formulations using the proposed spectrophotometric and previously described methods
| Parameters | RDV | MFX | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1DD | RD | MC | AUC | Q-analysis | Bivariate | Reported method [15] | 1DD | RD | MC | AUC | Q-analysis | Bivariate | Reported method [36] | |
| Number of measurements | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Mean % Recovery | 100.42 | 99.73 | 98.94 | 100.15 | 99.14 | 98.90 | 99.79 | 99.35 | 100.81 | 99.19 | 98.56 | 99.96 | 100.96 | 99.49 |
| % RSD | 1.142 | 1.213 | 0.792 | 1.958 | 0.922 | 0.688 | 1.197 | 1.046 | 0.893 | 0.877 | 0.539 | 0.601 | 1.054 | 1.496 |
| Variance | 1.316 | 1.463 | 0.616 | 3.845 | 1.427 | 0.463 | 1.427 | 0.172 | 0.811 | 0.756 | 0.282 | 0.361 | 1.132 | 2.216 |
| Student’s t-test * (2.447) | 0.761 | 0.084 | 1.183 | 0.311 | 0.861 | 1.292 | —— | 1.375 | 1.521 | 0.347 | 1.178 | 0.591 | 1.607 | —— |
| F-value* (9.277) | 1.085 | 1.024 | 2.320 | 2.693 | 1.708 | 3.087 | —— | 2.050 | 2.732 | 2.929 | 7.855 | 6.131 | 1.957 | —— |
* The values in parenthesis are tabulated values of “t ” and “F ” at (p = 0.05) [69]
Spiked human plasma analysis
The new method was successful in monitoring RDV, and MFX at therapeutic levels in spiked human plasma samples due to the high sensitivity of the proposed approach. The plasma Cmax for RDV, and MFX were 4420 ng/mL, and 3.56 mg/L, respectively, which exceed each drug’s LOD [63, 64]. Continuous monitoring of drug concentrations in plasma is crucial for optimizing therapeutic outcomes, ensuring drug efficacy, and avoiding toxicity—factors that ultimately contribute to lowering mortality rates. The newly developed techniques are appropriate for application in the study of the medications under research in human plasma due to their high sensitivity, as shown in Table 7.
Table 7.
Determination of RDV and MFX in spiked human plasma by the proposed methods
| Method | RDV | MFX | ||||
| Added (µg/mL) | Found* (µg/mL) | %Recovery | Added (µg/mL) | Found* (µg/mL) | %Recovery | |
| Ratio derivative | 1 | 0.956 | 95.59 | 1 | 0.925 | 92.45 |
| 1 | 0.943 | 94.29 | 4 | 3.740 | 93.50 | |
| 2 | 1.877 | 93.84 | 8 | 7.621 | 95.26 | |
| 3 | 2.772 | 92.39 | 4 | 3.714 | 92.83 | |
| 13 | 12.538 | 96.45 | 5 | 4.757 | 95.14 | |
| Mean ± %RSD | 94.51 ± 1.665 | Mean ± %RSD | 93.84 ± 1.389 | |||
| Ratio difference | 1 | 0.952 | 95.23 | 1 | 0.966 | 96.61 |
| 1 | 0.920 | 92.06 | 4 | 3.758 | 93.97 | |
| 2 | 1.891 | 94.59 | 8 | 7.579 | 94.75 | |
| 3 | 2.740 | 91.36 | 4 | 3.836 | 95.92 | |
| 13 | 12.426 | 95.59 | 5 | 4.697 | 93.95 | |
| Mean ± %RSD | 93.77 ± 2.055 | Mean ± %RSD | 95.04 ± 1.251 | |||
| Mean centering | 1 | 0.931 | 93.13 | 1 | 0.953 | 95.28 |
| 1 | 0.920 | 92.03 | 4 | 3.801 | 95.03 | |
| 2 | 1.827 | 91.34 | 8 | 7.598 | 94.98 | |
| 3 | 2.823 | 94.11 | 4 | 3.693 | 92.32 | |
| 13 | 12.141 | 93.39 | 5 | 4.665 | 93.29 | |
| Mean ± %RSD | 92.80 ± 1.192 | Mean ± %RSD | 94.18 ± 1.385 | |||
| Area under the curve | 1 | 0.933 | 93.31 | 1 | 0.961 | 94.66 |
| 1 | 0.946 | 94.63 | 4 | 3.771 | 93.98 | |
| 2 | 1.928 | 96.40 | 8 | 7.773 | 95.86 | |
| 3 | 2.854 | 95.13 | 4 | 2.809 | 91.52 | |
| 13 | 12.093 | 93.02 | 5 | 3.694 | 92.62 | |
| Mean ± %RSD | 94.50 ± 1.461 | Mean ± %RSD | 93.73 ± 1.816 | |||
| Q-analysis method | 1 | 0.948 | 94.75 | 1 | 0.923 | 92.33 |
| 1 | 0.961 | 96.09 | 4 | 3.756 | 93.89 | |
| 2 | 1.898 | 94.91 | 8 | 7.501 | 93.76 | |
| 3 | 2.856 | 95.21 | 4 | 3.728 | 93.20 | |
| 13 | 12.268 | 94.37 | 5 | 4.626 | 92.52 | |
| Mean ± %RSD | 95.07 ± 0.683 | Mean ± %RSD | 93.14 ± 0.759 | |||
| Bivariate method | 1 | 0.94 | 93.71 | 1 | 0.96 | 96.03 |
| 1 | 0.93 | 92.80 | 4 | 3.81 | 97.04 | |
| 2 | 1.93 | 94.07 | 8 | 7.53 | 96.40 | |
| 3 | 2.76 | 92.06 | 4 | 3.72 | 93.04 | |
| 13 | 12.39 | 95.31 | 5 | 4.86 | 97.26 | |
| Mean ± %RSD | 94.06 ± 1.897 | Mean ± %RSD | 95.12 ± 1.729 | |||
Method evaluation of greenness profiles
The analytical community’s foremost aim is to design procedures that address GC problems. GAC aims to promote a sustainable and eco-friendly approach to analytical chemistry, reducing the environmental effect while maintaining high accuracy and dependability. In recent years, various ways to evaluate greenness have emerged, including qualitative, semi quantitative, and quantitative methods. These approaches were created in line with the 12 GAC principles.
The provided approach’s environmental friendliness was assessed using the AGREE, ESA, and ComplexGAPI evaluation techniques. It’s interesting to note that every technique used to gauge greenness supported the idea that it is a very environmentally beneficial approach [65–67].
Assessment of greenness in compliance with ESA
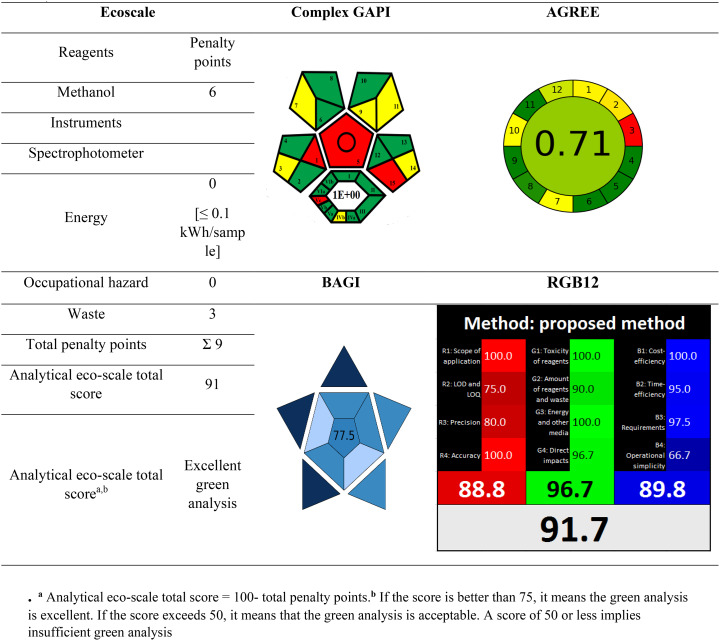
A newly developed, comprehensive, semi quantitative instrument for evaluating the greenness of the approach [50]. It relies on deducting points—also known as penalty points—for analytical process characteristics that don’t match the 12 GAC ideas. An analysis that is more environmentally friendly has a higher score (closer to 100). Table 8 displays the ESA scores for the recommended approaches. With a high ESA score of 91 points, the recommended approach’s greenness was instantly apparent.
Table 8.
Greenness, whiteness, and blueness assessment of the proposed spectrophotometric approach according to ESA, complexgapi, AGREE, BAGI and RGB12 tools
Assessment of greenness in compliance with complex GAPI
While ESA permits a basic assessment of greenness, Complex GAPI permits a more thorough semi-quantitative analysis [51]. Because it incorporates a hexagon-shaped region that depicts pre-analysis periods and stages, it is superior to the original GAPI measure. From sample collection and transportation to sample safety, storage, preparation, and analysis, this sophisticated tool manages every stage of the analytical process [51]. Simple pictogram software is provided by Complex GAPI. As demonstrated in Table 8, the suggested approach is more environmentally friendly because it demonstrated a lower E-factor (equal to 1), which suggests that the less waste produced, the greater and the more favorable the environmental impact.
Notwithstanding its advantages, ComplexGAPI’s focus is restricted to environmental standards, ignoring energy efficiency, waste reduction, and the integration of renewable resources. If ComplexGAPI is used excessively, it could lead to a limited and insufficient evaluation. This restriction can be addressed and a more thorough review can be obtained by combining ComplexGAPI analysis with quantitative evaluation techniques. In this integrated approach, ComplexGAPI and other supplementary tools will fill in research gaps and solve the limitations of utilizing a single evaluation method.
Assessment of greenness in compliance with AGREE
The most popular greenness assessment metric is AGREE [68]. All 12 of the GAC’s principles are included, making it comprehensive, adaptable (weighting is possible), easy to comprehend (a colored pictogram represents the end result), and simple to use (free software is accessible). The input parameters make reference to the 12 key principles and can be assigned different weights to offer some flexibility. The final score, which ranges from 0 to 1, is calculated using the 12 input parameters. A clock-like graph with a score and a color in the middle that represents the ultimate score is the end outcome. The range of possible scores is dusky green (= 1) to dusky red (= 0).
Assessment of the presented method’s whiteness
The RGB12 tool, developed by Paweł-Nowak and his colleagues in June 2021, is a simple and quantitative whiteness evaluation tool that computes the sustainability level in terms of whiteness assessment and provides an easy-to-understand assessment of the methods in accordance with the 12 WAC considerations [54]. The twelve distinct algorithms that make up the RGB 12 algorithm are arranged under the general headings of four red, four green, and four blue. The most important GAC criteria, such as toxicity, the quantity of reagents and waste, energy consumption, and the effects on people, animals, and/or genetic alterations (GMOs), are covered by the green group (G1–G4). The validation parameters, such as the scope of application, accuracy, LOD, precision, and LOQ, are covered by the second group, often known as the red group (R1–R4). The third group, known as the blue group (B1–B4), deals with practical/economic needs, time efficiency, and cost efficiency. By summing the marks the technique receives for each of the three areas/colors using the RGB algorithm, the final actual value of “whiteness,” which evaluates how well the approach adheres to the principles of WAC, is calculated. Table 8 demonstrates that the proposed method has a high whiteness score of 91.7, indicating its benefits in terms of greenness, whiteness, sustainability, analytical efficacy, and economic and practical aspects [65–67].
Assessment of the presented method’s blueness
The BAGI measure evaluates an analytical method’s “blueness” based on practical criteria, distinguishing it from greenness-focused tools [54]. BAGI evaluates an analytical process’s “blueness” by taking into account ten key factors, including the type of analysis, analytes, instrumentation, sample productivity, sample preparation requirements, number of samples examined per hour, reagents and materials, pre-concentration steps, automation level, and sample quantity. Each of the ten variables is scored from 1 (lowest) to 10 (highest). The final BAGI score is calculated as the geometric mean of all ten criteria. A higher BAGI score indicates that an analytical approach is more relevant, functional, and fits the intended goal. This study found that the proposed methods had high BAGI ratings of 71 as in Table 8, demonstrating their real-world applicability, high throughput, automation potential, and low operational costs. However, BAGI does not offer comprehensive sustainability quantification because it primarily concentrates on practical factors. As a result, we also used the RGB12 method to assess composite analytical sustainability while taking performance, usefulness, and greenness into account. The method’s value as a workable green chemistry substitute is confirmed by the consistently high tool ratings [65–67].
Importance of the multi-tool evaluation approach
The study illustrated the advantages of assessing the sustainability of analytical techniques across several dimensions utilizing a number of complimentary tools (ComplexGAPI, AGREE, ESA, BAGI, RGB12). Greenness (impact on the environment), blueness (practicality/cost-effectiveness), whiteness (total sustainability, including performance and safety), and other parameters were evaluated holistically by these instruments. According to criteria including decreased toxicity, waste reduction, material efficiency, high throughput, automation possibilities, cheap cost, and outstanding analytical performance, the devised analytical techniques demonstrated superior sustainability.
The approaches were validated as highly sustainable and implementable best practices by the combined qualitative and quantitative evaluation, which also showed that they are in line with the environmental, economic, and performance domains of green analytical chemistry.
Conclusion
This study proposes a complete set of six spectrophotometric methods developed for the simultaneous determination of RDV and MFX in difficult matrices such as pharmaceutical formulations and spiked plasma samples without any prior separation. The methods are based on solving the problem of spectral overlap using appropriate mathematical calculations, which proves that simple UV equipment, when used correctly, is as good as, if not better than, more sophisticated analytical equipment. The methods were rigorously validated according to ICH guidelines, confirming their accuracy, precision, specificity, and robustness, in addition to their analytical performance. Their sensitivity was adequate for the detection of RDV and MFX at clinically significant plasma concentrations, indicating their potential utility in therapeutic drug monitoring. One of the most important things about this work is that it uses advanced sustainability metrics. Using Eco-Scale, ComplexGAPI, AGREE, RGB12, and BAGI together made sure that the environmental impact, operational safety, and practical feasibility were all taken into account. The high scores for greenness, whiteness, and blueness show that the methods are in line with the ideas of Green and White Analytical Chemistry. These features make the methods particularly suited for use in routine quality control laboratories, especially in settings where access to sophisticated equipment is limited.
Author contributions
EAM: Conceptualization, Methodology, Investigation, Writing – original draft. AAE: Conceptualization, Writing – review & editing, Supervision. SME: Conceptualization, Writing – review & editing, Supervision. ASA: Conceptualization, Writing – review & editing, Supervision. All authors read and approved the final manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). There is no funding to declare.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards, and was approved by the Zagazig University Institutional Review Board (ZU-IRB #11330). The need for informed consent was waived by the ZU-IRB, as the human plasma was kindly provided by Zagazig University Hospitals for research purposes. All procedures were performed in accordance with relevant institutional and national guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kaul D. An overview of coronaviruses including the SARS-2 coronavirus–molecular biology, epidemiology and clinical implications. Curr Med Res Pract. 2020;10:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doerfler W. Adenoviral vector DNA-and SARS-CoV-2 mRNA-based Covid-19 vaccines: possible integration into the human genome-are adenoviral genes expressed in vector-based vaccines? Virus Res. 2021;302:198466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delang L, Neyts J. Medical treatment options for COVID-19. Eur Heart Journal: Acute Cardiovasc Care. 2020;9:209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eastman RT, Roth JS, Brimacombe KR, Simeonov A, Shen M, Patnaik S, Hall MD. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent Sci. 2020;6:672–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taha HR, Keewan N, Slati F, Al-Sawalha NA. Remdesivir: a closer look at its effect in COVID-19 pandemic. Pharmacology. 2021;106:462–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taha AM, Hassan WS, Elmasry MS, Sayed RA. A validated eco-friendly HPLC-FLD for analysis of the first approved antiviral remdesivir with other potential add-on therapies for COVID-19 in human plasma and pharmaceuticals. Anal Methods. 2023;15:6666–78. [DOI] [PubMed] [Google Scholar]
- 7.Bulduk I, Akbel E. A comparative study of HPLC and UV spectrophotometric methods for Remdesivir quantification in pharmaceutical formulations. J Taibah Univ Sci. 2021;15:507–13. [Google Scholar]
- 8.Ibrahim AE, Deeb SE, Abdelhalim EM, Al-Harrasi A, Sayed RA. Green stability indicating organic solvent-free HPLC determination of Remdesivir in substances and pharmaceutical dosage forms. Separations. 2021;8:243. [Google Scholar]
- 9.Hamdy MM, Abdel Moneim MM, Kamal MF. Accelerated stability study of the ester prodrug remdesivir: recently FDA-approved Covid‐19 antiviral using reversed‐phase‐HPLC with fluorimetric and diode array detection. Biomed Chromatogr. 2021;35:e5212. [DOI] [PubMed] [Google Scholar]
- 10.Darwish IA, Khalil NY, Darwish HW, Alzoman NZ, Al-Hossaini AM. Synthesis, spectroscopic and computational characterization of charge transfer complex of Remdesivir with chloranilic acid: application to development of novel 96-microwell spectrophotometric assay. J Mol Struct. 2022;1263:133104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdelazim AH, Ramzy S. Spectrophotometric quantitative analysis of Remdesivir using acid dye reagent selected by computational calculations. Spectrochim Acta Part A Mol Biomol Spectrosc. 2022;276:121188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batubara AS, Abdelazim AH, Almrasy AA, Gamal M, Ramzy S. Quantitative analysis of two COVID-19 antiviral agents, favipiravir and remdesivir, in spiked human plasma using spectrophotometric methods; greenness evaluation. BMC Chem. 2023;17:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imam MS, Abdelazim AH, Ramzy S, Batubara AS, Gamal M, Abdelhafiz S, Zeid AM. Adjusted green spectrophotometric determination of favipiravir and Remdesivir in pharmaceutical form and spiked human plasma sample using different chemometric supported models. BMC Chem. 2023;17:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elama HS, Zeid AM, Shalan SM, El-Shabrawy Y, Eid MI. Eco-friendly spectrophotometric methods for determination of Remdesivir and favipiravir; the recently approved antivirals for COVID-19 treatment. Spectrochim Acta Part A Mol Biomol Spectrosc. 2023;287:122070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Batubara AS, Abdelazim AH, Almrasy AA, Gamal M, Ramzy S. Quantitative analysis of two COVID-19 antiviral agents, favipiravir and remdesivir, in spiked human plasma using spectrophotometric methods; greenness evaluation. BMC Chem. 2023;17:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rizk M, Sultan MA, Tawfik BM, El-Eryan RT. Highly sensitive and selective sensing probe for determination of anti-Covid-19 remdesvir: application to pharmaceutical dosage form and biological fluids. J Electrochem Soc. 2022;169:026522. [Google Scholar]
- 17.El Azab NF, Ahmed N. Solid-state ion-selective electrodes for the first potentiometric determination of the anti-COVID 19 drug Remdesivir in human plasma; A comparative study. Microchem J. 2023;190:108658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elmansi H, Ibrahim AE, Mikhail IE, Belal F. Green and sensitive spectrofluorimetric determination of remdesivir, an FDA approved SARS-CoV-2 candidate antiviral; application in pharmaceutical dosage forms and spiked human plasma. Anal Methods. 2021;13:2596–602. [DOI] [PubMed] [Google Scholar]
- 19.Attia TZ, Boushra JM, Abdel Hakiem AF, Lashien AS, Noureldeen DA. Spectrofluorimetric determination of the anti-Covid 19 agent, remdesivir, in vials and spiked human plasma. Luminescence. 2022;37:1192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Sharkasy ME, Tolba MM, Belal F, Walash MI, Aboshabana R. Simultaneous spectrofluorimetic determination of Remdesivir and Simeprevir in human plasma. Sci Rep. 2022;12:21980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Awady M, Elmansi H, Belal F, Shabana RA. Insights on the quantitative concurrent fluorescence-based analysis of Anti-COVID-19 drugs Remdesivir and favipiravir. J Fluoresc. 2022;32:1941–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramzy S, Abdelazim AH, Osman AO, Hasan MA. Spectrofluorimetric quantitative analysis of favipiravir, Remdesivir and hydroxychloroquine in spiked human plasma. Spectrochim Acta Part A Mol Biomol Spectrosc. 2022;281:121625. [DOI] [PubMed] [Google Scholar]
- 23.El-Adl SM, El-Shanawani AA, Madbouly EA, Abdelkhalek AS. Green synchronous spectrofluorimetric analysis of remdesivir, the first approved antiviral, with Levodropropizine as add-on therapy for covid-19: application in their pharmaceutical dosage form, and spiked human plasma. BMC Chem. 2025;19:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beović B, Doušak M, Ferreira-Coimbra J, Nadrah K, Rubulotta F, Belliato M, Berger-Estilita J, Ayoade F, Rello J, Erdem H. Antibiotic use in patients with COVID-19: a ‘snapshot’infectious diseases international research initiative (ID-IRI) survey. J Antimicrob Chemother. 2020;75:3386–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marciniec K, Beberok A, Pęcak P, Boryczka S, Wrześniok D. Ciprofloxacin and Moxifloxacin could interact with SARS-CoV-2 protease: preliminary in Silico analysis. Pharmacol Rep. 2020;72:1553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhillon V, Chaudhary AK. A validated HPTLC method for Estimation of Moxifloxacin hydrochloride in tablets. Pharm Methods. 2010;1:54–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Motwani SK, Khar RK, Ahmad FJ, Chopra S, Kohli K, Talegaonkar S. Application of a validated stability-indicating densitometric thin-layer chromatographic method to stress degradation studies on Moxifloxacin. Anal Chim Acta. 2007;582:75–82. [DOI] [PubMed] [Google Scholar]
- 28.Kumar AH, Ramachandran G. Simple and rapid liquid chromatography method for determination of Moxifloxacin in plasma. J Chromatogr B. 2009;877:1205–8. [DOI] [PubMed] [Google Scholar]
- 29.Kumar AH, Sudha V, Srinivasan R, Ramachandran G. Simple and rapid liquid chromatography method for determination of Moxifloxacin in saliva. J Chromatogr B. 2011;879:3663–7. [DOI] [PubMed] [Google Scholar]
- 30.Pranger AD, Alffenaar J-WC, Mireille A, Wessels A, Greijdanus B, Uges DR. Determination of Moxifloxacin in human plasma, plasma ultrafiltrate, and cerebrospinal fluid by a rapid and simple liquid chromatography-tandem mass spectrometry method. J Anal Toxicol. 2010;34:135–41. [DOI] [PubMed] [Google Scholar]
- 31.Ulu ST. High-performance liquid chromatography assay for moxifloxacin: pharmacokinetics in human plasma. J Pharm Biomed Anal. 2007;43:320–4. [DOI] [PubMed] [Google Scholar]
- 32.Vishwanathan K, Bartlett MG, Stewart JT. Determination of Moxifloxacin in human plasma by liquid chromatography electrospray ionization tandem mass spectrometry. J Pharm Biomed Anal. 2002;30:961–8. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen HA, Grellet J, Ba BB, Quentin C, Saux M-C. Simultaneous determination of levofloxacin, Gatifloxacin and Moxifloxacin in serum by liquid chromatography with column switching. J Chromatogr B. 2004;810:77–83. [DOI] [PubMed] [Google Scholar]
- 34.Attimarad M, Al-Dhubiab BE, Alhaider IA, Nair AB. Simultaneous determination of Moxifloxacin and cefixime by first and ratio first derivative ultraviolet spectrophotometry. Chem Cent J. 2012;6:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elbashir AA, Ebraheem SA, Elwagee AH. H.Y. Aboul-Enein, new spectrophotometric methods for the determination of Moxifloxacin in pharmaceutical formulations. Acta Chimica Slovenica, 60 (2013). [PubMed]
- 36.Motwani SK, Chopra S, Ahmad FJ, Khar RK. Validated spectrophotometric methods for the Estimation of Moxifloxacin in bulk and pharmaceutical formulations. Spectrochim Acta Part A Mol Biomol Spectrosc. 2007;68:250–6. [DOI] [PubMed] [Google Scholar]
- 37.Ibrahim N, Elzanfaly ES, El Gendy AE, Hassan SA. Development, optimization, and validation of a green spectrofluorimetric method for the determination of Moxifloxacin using an experimental design approach. Res J Pharm Technol. 2021;14:1880–6. [Google Scholar]
- 38.Jasmin S, Jan MR, Khan M. Micellar-enhanced spectrofluorometric quantification of Moxifloxacin in pharmaceutical formulations, human urine and plasma samples. Afr J Pharm Pharmacol. 2011;5:616–24. [Google Scholar]
- 39.Shokoufi N, Vosough M, Rahimzadegan-Asl M, Abbasi-Ahd A, Khatibeghdami M. Fiberoptic-Coupled Spectrofluorometer with Array Detection as a Process Analytical Chemistry Tool for Continuous Flow Monitoring of Fluoroquinolone Antibiotics, International Journal of Analytical Chemistry, 2020 (2020). [DOI] [PMC free article] [PubMed]
- 40.Madbouly EA, El-Shanawani AA, Sobhy M, Abdelkhalek AS. Eco-friendly novel deconvoluted synchronous spectrofluorimetric approach for the determination of favipiravir, Levodropropizine and Moxifloxacin hydrochloride as an effective therapeutic combination for COVID-19; application in laboratory prepared mixtures and spiked human plasma. Spectrochim Acta Part A Mol Biomol Spectrosc. 2024;309:123823. [DOI] [PubMed] [Google Scholar]
- 41.Karampela I, Dalamaga M. Could respiratory fluoroquinolones, Levofloxacin and moxifloxacin, prove to be beneficial as an adjunct treatment in COVID-19? Arch Med Res. 2020;51:741–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madbouly EA, El-Shanawani AA, El-Adl SM, Abdelkhalek AS. Ecofriendly spectrophotometric methods for simultaneous determination of Remdesivir and Moxifloxacin hydrochloride as Co administered drugs in Corona virus treatment. Sci Rep. 2025;15:1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hajian R, Shams N, Kaedi I. Application of ratio derivative spectrophotometry for simultaneous determination of Naphazoline and antazoline in eye drops. J Chem. 2010;7:1530–8. [Google Scholar]
- 44.Lotfy HM, Saleh SS, Hassan NY, Elgizawy SM. A comparative study of the novel ratio difference method versus conventional spectrophotometric techniques for the analysis of binary mixture with overlapped spectra, (2012).
- 45.Darwish HW, Hassan SA, Salem MY, El-Zeany BA. Three different methods for determination of binary mixture of amlodipine and Atorvastatin using dual wavelength spectrophotometry. Spectrochim Acta Part A Mol Biomol Spectrosc. 2013;104:70–6. [DOI] [PubMed] [Google Scholar]
- 46.Abdelwahab NS. Spectrophotometric methods for simultaneous determination of carvedilol and Hydrochlorothiazide in combined dosage form. Arab J Chem. 2016;9:S355–60. [Google Scholar]
- 47.Salamaa FM, Nassar MW, El-Din MMS, Attia KA, Kaddah MY. Determination of Fenofibrate and the degradation product using simultaneous UV-derivative spectrometric method and HPLC. Am J Anal Chem. 2011;2:332–43. [Google Scholar]
- 48.Attia KA, El-Olemy A, Serag A, Abbas AEF, Eid SM. Environmentally sustainable DRS-FTIR probe assisted by chemometric tools for quality control analysis of Cinnarizine and Piracetam having diverged concentration ranges: validation, greenness, and whiteness studies. Spectrochim Acta Part A Mol Biomol Spectrosc. 2023;302:123161. [DOI] [PubMed] [Google Scholar]
- 49.Eid SM, Attia KA, El-Olemy A, Abbas AEF, Abdelshafi NA. An innovative nanoparticle-modified carbon paste sensor for ultrasensitive detection of Lignocaine and its extremely carcinogenic metabolite residues in bovine food samples: application of NEMI, ESA, AGREE, complexgapi, and RGB12 algorithms. Food Chem. 2023;426:136579. [DOI] [PubMed] [Google Scholar]
- 50.Gałuszka A, Migaszewski ZM, Konieczka P, Namieśnik J. Analytical Eco-Scale for assessing the greenness of analytical procedures. TRAC Trends Anal Chem. 2012;37:61–72. [Google Scholar]
- 51.Płotka-Wasylka J, Wojnowski W. Complementary green analytical procedure index (ComplexGAPI) and software. Green Chem. 2021;23:8657–65. [Google Scholar]
- 52.Pena-Pereira F, Wojnowski W, Tobiszewski M. AGREE—Analytical greenness metric approach and software. Anal Chem. 2020;92:10076–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nowak PM, Wietecha-Posłuszny R, Pawliszyn J. White analytical chemistry: an approach to reconcile the principles of green analytical chemistry and functionality. TRAC Trends Anal Chem. 2021;138:116223. [Google Scholar]
- 54.Manousi N, Wojnowski W, Płotka-Wasylka J, Samanidou V. Blue applicability grade index (BAGI) and software: a new tool for the evaluation of method practicality. Green Chem. 2023;25:7598–604. [Google Scholar]
- 55.Chaudhary J, Jain A, Saini V. Simultaneous Estimation of multicomponent formulations by UV-visible spectroscopy: an overview. Int Res J Pharm. 2011;2:81–3. [Google Scholar]
- 56.Pernarowski M, Knevel A, Christian J. Application of absorbancy ratios to the analysis of pharmaceuticals I: theory of the analysis of binary mixtures. J Pharm Sci. 1961;50:943–5. [DOI] [PubMed] [Google Scholar]
- 57.Massart D, Vandeginste B, Deming M, Michotte SN, Kaufman Y. L. Chemometrics: A Textbook, in, Elsevier, Amsterdam, 1988.
- 58.Mostafa NM, Abdel-Fattah L, Weshahy SA, Hassan NY, Boltia SA. Validated stability-indicating spectrophotometric methods for the determination of cefixime trihydrate in the presence of its acid and alkali degradation products. J AOAC Int. 2015;98:35–45. [DOI] [PubMed] [Google Scholar]
- 59.Madbouly EA, El-Shanawani AA, El-Adl SM, Abdelkhalek AS. Green chemometric-assisted UV-spectrophotometric methods for the determination of favipiravir, cefixime and Moxifloxacin hydrochloride as an effective therapeutic combination for COVID-19; application in pharmaceutical form and spiked human plasma. BMC Chem. 2024;18:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abdel-raoof AM, Madbouly EA, El-Shanawani AA, El-adl SM, Abdelkhalek AS. A lean six Sigma approach aided improvement for simultaneous electrochemical determination of antiviral drug favipiravir and Levodropropizine as an option in the treatment of COVID-19 using glassy carbon electrode modified NiFe2O4 nanocomposite in different matrices. Microchem J. 2024;207:111821. [Google Scholar]
- 61.Guideline IHT. Validation of analytical procedures: text and methodology, Q2 (R1), 1 (2005) 05.
- 62.Armitage P, Berry G, Matthews JNS. Statistical methods in medical research. Wiley; 2008.
- 63.Humeniuk R, Mathias A, Cao H, Osinusi A, Shen G, Chng E, Ling J, Vu A, German P. Safety, tolerability, and pharmacokinetics of remdesivir, an antiviral for treatment of COVID-19, in healthy subjects. Clin Transl Sci. 2020;13:896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moise P, Birmingham M, Schentag J. Pharmacokinetics and metabolism of moxifloxacin, Drugs of today (Barcelona, Spain: 1998), 36 (2000) 229–244. [DOI] [PubMed]
- 65.Abd-AlGhafar WN, Shabana RA, El-Shaheny R, Tolba MM. A fluorescence switch-off nanosensor for sensitive determination of the antiandrogen drug Flutamide in pharmaceutical and environmental samples. Analytical method greenness, blueness, and whiteness assessment. Microchem J. 2024;204:111078. [Google Scholar]
- 66.Abd-AlGhafar WN, Shabana RA, El-Shaheny R, Tolba MM. Integrating bio-based luminescent CQDs nanosensors and factorial-design-optimized sugaring-out homogenous liquid–liquid Microextraction for sensitive determination of two neurologic drugs. Microchem J, (2025) 113034.
- 67.Abd-AlGhafar WN, Shabana RA, El-Shaheny R, Tolba MM. Unlocking dual purposes of microwave radiation for ultrafast synthesis of carbon Dots and deep eutectic solvent: fluorescence switch-off nanosensor integrated with air-assisted liquid–liquid Microextraction for flavoxate analysis in plasma. Microchem J, (2025) 113777.
- 68.Gałuszka A, Migaszewski Z, Namieśnik J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TRAC Trends Anal Chem. 2013;50:78–84. [Google Scholar]
- 69.Dinç E, Onur F. Application of a new spectrophotometric method for the analysis of a ternary mixture containing metamizol, Paracetamol and caffeine in tablets. Anal Chim Acta. 1998;359:93–106. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.