Abstract
The 5-methylcytosine (m5C) post-transcriptional modification has been linked with the development and progression of a variety of cancers. However, its specific functions and their underlying mechanisms are poorly understood in hepatocellular carcinoma (HCC). The present study showed abnormally increased levels of m5C modifications in HCC that were positively correlated with both HCC progression and worse patient prognosis. Landscape profiling of metabolic characteristics showed dysregulation of pyrimidine metabolism mediated by DNA methyltransferases 1 (DNMT1), and cyclin-dependent kinase 1 (CDK1) was identified as a downstream effector upregulated by DNMT1 in an m5C-dependent manner, with CDK1 promoting pyrimidine metabolism. Knockdown of DNMT1 or CDK1 was found to reduce the proliferation, invasion, and migration of HCC cells in vitro. Moreover, pharmacological targeting of the DNMT1/CDK1/pyrimidine metabolism axis with specific inhibitors effectively suppressed tumor progression in HCC model mice. These findings demonstrated the landscape profiles of m5C-related metabolic features in HCC, showing stabilization of CDK1 mRNA by DNMT1-mediated m5C modification, resulting in the promotion of pyrimidine metabolism, a crucial feature of HCC progression. These insights highlight the therapeutic potential of targeting the DNMT1/CDK1/pyrimidine metabolism axis as a strategy for combating HCC.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13148-025-01956-3.
Keywords: m5C modification, HCC, DNA-methyltransferase 1, Cyclin-dependent protein kinase 1, Pyrimidine metabolism
Introduction
Liver cancer ranks among the most prevalent cancers and is the third leading cause of cancer-related mortality [1]. Hepatocellular carcinoma (HCC), which makes up about 90% of all primary liver cancers, has a dismal 5-year survival rate of roughly 18% [2, 3]. Thus, clarification of oncogenic molecular mechanisms underlying HCC is urgently needed for the development of more therapeutic treatment options for HCC.
Recent evidence has elucidated the mechanisms involved in RNA modification, which is recognized as an important means of epigenetic regulation [4, 5]. RNA modifications influence a variety of biological processes, including RNA stability, localization, and metabolism, in turn affecting the cell cycle, cell metabolism, and differentiation [5–8]. Among various types of RNA modification, that of 5-methylcytosine (m5C), the most common reversible RNA modification, is particularly important due to its broad biological implications. Various proteins are involved in m5C modification, specifically, methyltransferases (the writers), binding proteins (the readers), and demethylases (the erasers) [5, 7, 9]. M5C modifications, commonly found on tRNA, rRNA, and mRNA [9] are primarily catalyzed by m5C writers such as NOP2/Sun RNA methyltransferases (NSUN1-7) and DNA methyltransferases (DNMT1-2) [10, 11]. M5C readers, including Aly/REF export factor (ALYREF) and Y-box binding protein 1 (YBX1), interact with the modified RNA to facilitate nuclear export and enhance its stability [5, 12, 13]. Lastly, the m5C erasers like ten-eleven translocation proteins (TET1-3) and AlkB homolog 1 (ALKBH1) oxidize m5C sites, terminating the binding of the m5C readers [14–16].
The liver is a key organ responsible for crucial metabolic functions in the human body. HCC development and progression are closely linked to metabolic dysregulation [5, 17–19]. For example, it was reported that reduced bile acid metabolism was correlated with malignant HCC progression [17]. Moreover, arginine accumulation has also been found to promote HCC tumorigenesis [18] despite suppression of arginine synthesis. In terms of the underlying mechanism, arginine binds specifically to RNA-binding motif protein 39 (RBM39), influencing the expression of metabolic genes associated with oncogenesis [18]. In addition, increased nucleotide metabolism has been shown to have a significant effect on tumorigenicity [20, 21]. Ubiquitin-conjugating enzyme E2T (UBE2T) activates the Akt/β-catenin cascade to stimulate de novo pyrimidine biosynthesis, thus promoting HCC progression [20]. However, the specific mechanisms underlying the ability of m5C modification to drive dysregulated metabolism in HCC are not fully understood [5, 22].
The objective of the present study was to undertake a comprehensive profiling of m5C modifications in HCC. This demonstrated that increased m5C levels were associated with poorer HCC survival. The findings identified a previously uncharacterized mechanism linking DNMT1-mediated m5C modifications to dysregulated pyrimidine metabolism in HCC. It was also found that DNMT1 regulated cyclin-dependent kinase 1 (CDK1) via m5C modification, followed by the promotion of pyrimidine metabolism by CDK1, accelerating HCC progression. In vivo experiments indicated that inhibition of DNMT1 and CDK1 utilizing GSK3685032 and Ro-3006 downregulated pyrimidine metabolism and suppressed HCC progression. These results contribute to a deeper insight into HCC onset and progression, and suggest potential treatment strategies.
Materials and methods
Data acquisition
We acquired transcriptomic data of HCC patients from The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov/), with a total of 374 samples of HCC tissues and 50 samples of normal tissues. The Gene Expression Omnibus (GEO) datasets: GSE76427 [23], GSE54236 [24], GSE104580 [25], and GSE149614 [26] were acquired from GEO. The Research Ethics Committee of Zhongshan Hospital approved the ethical use of human samples for this study. We acquired tumor tissue samples and corresponding peritumor tissue from HCC patients in Zhongshan Hospital, Fudan University, (Shanghai, China).
Cell lines culture
Human hepatocytes L02 and human HCC cell lines Huh7, MHCC97H, SMMC-7721, Bel-7402, HepG2, Hep3B were purchased from cell bank of Chinese Academy of Sciences. These cells were cultured in an incubator with 5% CO2 at 37 °C. The culture medium used was Dulbecco's Modified Eagle Medium (Gibco, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, USA) and 1% penicillin/streptomycin (Corning, USA).
Statistical analysis
Survival differences between groups were assessed using Kaplan–Meier curves and log-rank tests. Spearman correlation was used to calculate the correlation coefficients. The R value > 0.4 and P < 0.5 was the threshold for statistically positively relevant. For comparisons across multiple groups, one-way ANOVA and the Kruskal–Wallis test were applied for parametric and nonparametric data, respectively. All analyses were performed using R software and GraphPad Prism 9.0 software, with statistical significance defined as P < 0.05.
Results
Landscape analysis of m5C regulators highlights the significance of m5C modification in HCC
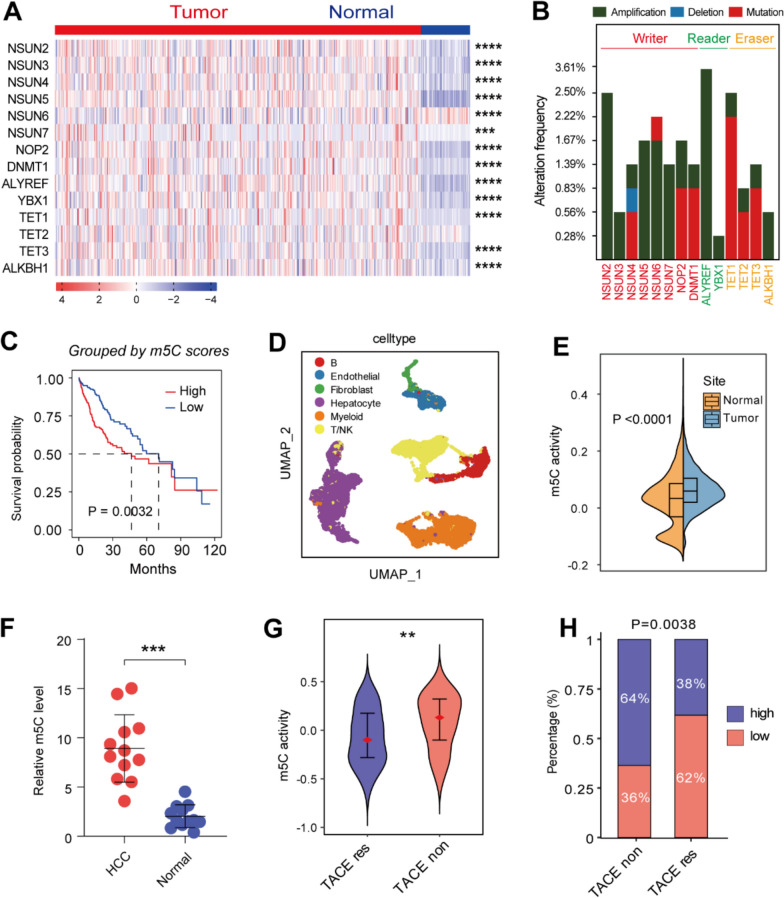
Fourteen m5C regulators were identified from the results of previous studies, including the m5C methyltransferases/writers NSUN2-7, NOP2, and DNMT1, the binding proteins/readers ALYREF and YBX1, and the demethylases/erasers TET1-3 and ALKBH1. The mRNA expression levels of these m5C regulators were then compared using transcriptomic data from the The Cancer Genome Atlas-Liver Hepatocellular Carcinoma (TCGA-LIHC) cohort. This showed marked differential expression of most of the regulators between HCC and normal tissues (Fig. 1A). The patterns of change in m5C regulator expression in HCC tissues were then analyzed using the online tool cBioPortal (Fig. 1B). Overall, the m5C regulators showed relatively low frequencies of altered expression, with changes falling into three categories, namely, amplification, deletion, and mutation. To explore the potential impact of m5C modifications on clinical prognosis, an analysis of overall survival (OS) in relation to the m5C regulators was performed using TCGA data (Supplementary Figure S1A–C). Kaplan–Meier survival curves showed significant associations between many of the m5C regulators and survival in patients with HCC. K–M curve based on overall m5C scores validated that patients with higher scores have shorter OS, respectively in TCGA and GSE54236 dataset (Fig. 1C; Supplementary Figure S1D). Moreover, single cell RNA sequencing (scRNA-seq) data from GSE149614 was analyzed to visualize overall m5C levels in tumor and normal sites (Fig. 1D; Supplementary Figure S1E), which manifested significantly higher levels of m5C in tumor tissues (Fig. 1E; Supplementary Figure S1F). We subsequently analyzed the correlation of m5C regulators and hallmark pathways, which revealed the potential participation of m5C modification in multiple significant signalling pathways (Supplementary Figure S2A). Samples from HCC patients validated our finding (Fig. 1F). These results confirmed that m5C modification was abnormally elevated in HCC and associated with worse prognosis.
Fig. 1.
Comprehensive profiling of the significance of m5C modification in HCC. A Heatmap showing a comparison of m5C regulator mRNA expression levels between HCC and normal tissues, based on TCGA data. B Frequencies of genetic alterations in m5C regulators. C K–M curve shows significant difference between m5Chigh and m5Clow groups. The threshold score for dividing high and low groups was − 0.559, which was the median score. D UMAP plot of scRNA-seq data from GSE149614. Total cell counts are 16,244. E Comparison of m5C scores between tumor and normal sites in scRNA-seq data. F Comparison of m5C levels in twelve pairs of HCC and normal samples. G Comparison of m5C scores between TACE responders and non-responders. H Stack plot shows proportion of m5Chigh versus m5Clow in responder and non-responder groups. (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001)
Next, to further investigate the roles of m5C modification in HCC, we utilized GSE104580 dataset to explore whether m5C modifications affect clinical treatment. We found that transcatheter arterial chemoembolization (TACE) treatment responders have overall lower m5C scores compared with non-responders (Fig. 1G, H). Moreover, the AUC area was 0.654, which represented well efficiency of m5C score for TACE response prediction. Together, our findings revealed the significant influence of m5C modification on HCC prognosis and clinical treatment.
Identification of three distinctive m5C modification clusters and their respective characteristics
Unsupervised clustering was then performed to analyze m5C modification patterns in HCC tissues using the “ConsensusClusterPlus” package in R. This showed that all samples were clustered into three clusters, represented as cluster 1 (n = 61), cluster 2 (n = 159), and cluster 3 (n = 154) (Fig. 2A–C). Principal component analysis (PCA) was then performed to verify the findings (Supplementary Figure S2A). The three m5C clusters were then analyzed using Kaplan–Meier curves. Consistent with the previous findings, cluster 1, which had the highest expression of most m5C regulators, was associated with the lowest OS rates, followed by cluster 2 (Fig. 2D). Also, the expression levels of m5C regulators were compared among the different clusters. This showed that the levels of most regulators were significantly upregulated in cluster 1, with intermediate levels in cluster 2, and the lowest levels in cluster 3 (Fig. 2E), suggesting that cluster 1 had the highest levels of m5C modification, followed by cluster 2, and then cluster 3. To examine differences in immune functions among the different clusters, the levels of immune cell infiltration (Supplementary Figure S1B)., stromal scores, immune scores, and ESTIMATE scores were evaluated (Supplementary Figure S1C–E). It was found that higher levels of m5C modification, were associated with greater infiltration of antitumor immune cells (Supplementary Figure S1B). However, the stromal, immune, and ESTIMATE scores did not differ significantly among the clusters. Genomic alterations associated with the three clusters were also analyzed in Supplementary Figure S2F which indicated that patients with different m5C modification activity might have different patterns of alterations. Evidence shows that m5C modifications influence cell metabolism and signal transduction [27, 28]. Also, differences in OS among the clusters were not specifically associated with immune differences, we thus assessed the Gene Set Variation Analysis (GSVA) scores of multiple metabolism pathways and classic pathways associated with oncogenic activities (Fig. 2F; Supplementary Figure S4A, B). As a result, all clusters exhibited completely different characteristics in metabolic perspective, and higher m5C activity aligned with higher oncogenic activities. In this part, our findings confirmed 3 clusters associated with m5C modifications, and comprehensively described their immune, genomic alterations and metabolic characteristics. These findings suggested that m5C modification could potentially activate oncogenic and metabolism pathways.
Fig. 2.
Identification of m5C modification-associated clusters by unsupervised consensus clustering. A Cumulative distribution function (CDF) displays the stability of clustering across k = 2–7. B Delta area plot displays the area under the CDF curve for different k-values, with k = 3 identified as the optimal k-value. C Consensus matrix (k = 3) heatmap demonstrating clustering consistency, with distinct borders indicating distinct and well-defined clustering. D Kaplan–Meier curves indicate distinct differences in OS among the m5C modification clusters. E Comparisons of m5C regulator expression levels in the three m5C clusters. P-values were determined by Kruskal–Wallis tests. F Heatmap showing the differences of multiple signaling pathways and dysregulated metabolism pathways across clusters. Data were analyzed by Kruskal–Wallis tests. (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001)
Metabolic landscape profiles of the m5C modification clusters show DNMT1-mediated dysregulated pyrimidine metabolism
Our previous work evaluated the GSVA scores of traditional pathways and metabolic characteristics of the three m5C clusters (Fig. 2F; Supplementary Figure S4A). This indicated significant activation of most traditional pathways in the highest m5C cluster (C1), together with relative suppression in the lowest cluster (C3) (Fig. 2F). Notably, it was observed that pyrimidine metabolism pathways (Fig. 3A) showed significantly different distribution patterns in the three m5C clusters, highest in cluster 1, intermediate in cluster 2, and lowest in cluster 3 (Fig. 3B). It was thus hypothesized that abnormally raised levels of m5C modification stimulate pyrimidine metabolism. To test this hypothesis, the expression of key enzymes associated with pyrimidine metabolism (Fig. 3C) was compared between the three clusters. As shown in the heatmap, the expression of these enzymes showed a positive correlation with m5C levels, aligning with previous findings.
Fig. 3.
Comprehensive profiling of the metabolic characteristics of the m5C clusters showed that DNMT1 mediated dysregulated pyrimidine metabolism. A The process of pyrimidine biosynthesis, from materials to DNA synthesis. Key enzymes are shown in red. B Comparisons of GSVA scores of pyrimidine metabolism. C Z-scores of mean expressions of key enzymes of pyrimidine biosynthesis. D Ranks of fold change of m5C regulators in cluster1. E Differences in m5C regulator expression levels in clinical samples of HCC and normal tissues. DNMT1 exhibited most distinct difference. F Relative expression of DNMT1 in six HCC cell lines. G, H DNMT1 expression levels after DNMT1 knockdown in MHCC97H and Huh7 cells, shown by RT-PCR (E) and western blotting (F). I Comparison of relative m5C levels between the control and DNMT1-knockdown groups. J, K Comparison of key pyrimidine metabolism-associated regulators expression between the control and DNMT1-knockdown groups, shown by RT-PCR (J) and WB (K). L Heatmap showing changes in metabolites of pyrimidine metabolism between control and DNMT1 knockdown group. M, N Colony formation assay and transwell assay results showing colony formation (K) in Huh7 and MHCC97H cells, as well as migration and invasion in MHCC97H cells (L) after DNMT1 knockdown. (*P < 0.05, ** P < 0.01, ***P < 0.001)
Next, we aimed to investigate whether an m5C methyltransferases changed the status of m5C modifications in cluster 1 to trigger the upregulated pyrimidine metabolism. We first conducted differentially expressed genes (DEG) analysis, which showed that DNMT1 was most elevated (Fig. 3D). The expression of m5C regulators in normal and HCC tissues reinforced this finding (Fig. 3E). Correlation analysis indicated that DNMT1 was closely associated with pyrimidine metabolism (Supplementary Figure S5A). Based on the results, DNMT1 was chosen as a representative m5C regulator. The relative expression levels of DNMT1 were examined in six cell lines, leading to the selection of the two with the highest DNMT1 expression, MHCC97H and Huh7 cell lines, for further experiments (Fig. 3F; Supplementary Figure S5B). To investigate potential mechanism of how DNMT1 regulates pyrimidine metabolism, DNMT1 was knocked down, with verification of the knockdown efficiency by RT-PCR and western blotting (Fig. 3G, H). Enzyme linked immunosorbent assay (ELISA) showed significant differences between the control and DNMT1-knockdown groups (Fig. 3I). It was thus concluded that DNMT1 plays a significant role in m5C modification. Furthermore, consistent with the previous hypothesis, knockdown of DNMT1 was found to reduce key enzymes expression in pyrimidine metabolism and the production of metabolites of pyrimidine metabolism (Fig. 3J–L; Supplementary Figure S5C, D). The cell densities of DNMT1-knockdown cells were markedly reduced compared with the controls (Supplementary Figure S5E, F). Furthermore, colony formation assay and transwell experiments showed that migration and invasion in both cell lines were significantly inhibited following DNMT1 knockdown (Fig. 3M, N; Supplementary Figure S5G). EdU experiment furthermore reinforced that knockdown of DNMT1 attenuates HCC proliferation (Supplementary Figure S5H). Together, these findings demonstrated that DNMT1 activated pyrimidine metabolism, thus promoting the migration and invasion of HCC cells.
Identification of five genes potentially associated with m5C modification by WGCNA analysis
To identify hub genes in the m5C clusters, Weighted Gene Co-expression Network Analysis (WGCNA) was performed (Fig. 4A–C; Supplementary Figure S6A–C, F). Hierarchical clustering of gene–gene modules (Fig. 4A) led to the identification of 19 distinct modules, shown by different colors in the figure (Fig. 4B, C). As shown in dendrogram and heatmap, the gray- and salmon-colored modules differed markedly from the others, and showed positive correlations with clusters cluster 2 and 3 (Fig. 4B, C), while the turquoise-colored module was primarily associated with cluster 1 (Fig. 4C). Fifty-three genes were identified in cluster 1 using a threshold of 0.8 for gene relativity. Furthermore, univariate Cox regression identified 50 genes that significantly affected survival. A protein–protein interaction (PPI) network of the m5C regulators and these 50 genes was then constructed using the STRING database (Fig. 4D). The PPI network showed that CDK1, RAD51, CCNB1, CENPA, and FOXM1 could potentially interact with DNMT1 (Fig. 4D). Analysis of differentially expressed genes, visualized by volcano and violin plots, showed that CDK1, RAD51, CCNB1, CENPA, and FOXM1 were upregulated in cluster 1, emphasizing the significance of these genes in cluster 1 (Supplementary Figure S6D, E). To visualize the significance of these 5 genes, scatter plots were used (Supplementary Figure S6F). Kaplan–Meier analysis of the effects of these genes on survival showed that higher expression was associated with poor prognosis (Fig. 4E). This finding might partially account for the poor survival outcomes observed in cluster 1. Given the previous demonstrations of the involvement of DNMT1 in both pyrimidine metabolism and m5C modification, its potential associations with key regulators of pyrimidine metabolism were investigated using Spearman correlations (Fig. 4F; Supplementary Figure S6G). The results indicated strong positive correlations between hub genes and pyrimidine metabolism. Together, these results indicate potential associations between DNMT1 and key genes involved in pyrimidine metabolism.
Fig. 4.
Discovery of fundamental genes related to clusters with highest m5C levels. A Hierarchical clustering of genes, with different gene modules represented by different colors. B Clustering of different gene modules. C Heatmap showing correlations between gene modules and m5C clusters. The turquoise module is associated predominantly with cluster 1. D Protein–protein interaction network illustrating the hub genes associated with m5C regulators and five genes strongly linked to cluster 1 (unrelated interactions are omitted). E Kaplan–Meier curves comparing the high- and low-expression groups, shown in red and blue, respectively. F Spearman correlation analysis indicating significant associations between the expression of the five genes and DNMT1
DNMT1 modulates CDK1 via m5C modification
Next, the downstream targets of DNMT1 were explored, using m5C-RNA-immunoprecipitation sequencing (RIP-seq) to identify the characteristics of m5C modifications of DNMT1. As shown in the pie plot, the m5C peaks were decreased in the CDS, intronic, and intergenic regions following DNMT1 knockdown (Fig. 5A). Motif enrichment showed that DNMT1 knockdown altered the m5C sequence motif (Fig. 5B). These results indicated that DNMT1 played a significant role in gene modulation. To identify the downstream effector or effectors of DNMT1, RNA-seq was performed on DNMT1-knockdown cells, and differential analysis was conducted, leading to the identification of 405 downregulated genes compared with controls. The m5C-RIP-seq results also revealed 503 genes with m5C enrichment peaks. Venn diagrams were used to visualize genes located in the intersection of the m5C m5C-RIP-seq, RNA-seq, and WGCNA results, resulting in the identification of CDK1, KIF4A, and TPX2 (Fig. 5C). However, only the levels of CDK1 were observed to decrease significantly after DNMT1 knockdown, as seen in both the mRNA and protein levels (Fig. 5D–G). To further determine whether DNMT1 modulates CDK1 expression via m5C modification, the m5C peaks of CDK1 were plotted (Fig. 5H), showing a marked reduction in the signal intensities following DNMT1 knockdown (Fig. 5H, I). Moreover, CDK1 showed higher expression in HCC tissues and was associated with poor prognosis (Fig. 4E; Fig. 5J, K). Moreover, the proportion of CDK1 remaining after DNMT1 knockdown was reduced after Actinomycin D treatment (Fig. 5L, M). As further confirmation of the results, Spearman correlation analysis was conducted in two GEO datasets, GSE76427 and GSE54236, resulting in significant positive correlations (Fig. 5N, O). Therefore, the results demonstrated that DNMT1 modified CDK1 expression via m5C modifications, promoting the expression of CDK1.
Fig. 5.
DNMT1 upregulates CDK1 via m5C modifications. A Comparison of the distribution of m5C modification sites in HCC cells between the control and DNMT1-knockdown groups. B m5C motifs on CDK1. C Venn diagram showing genes common to the m5C MeRIP-seq, RNA-seq, and WGCNA results. D, E Relative expression of CDK1, KIF4A, and TPX2 mRNA (D) and protein (E) levels after DNMT1 knockdown in two cell lines. F, G Western blot results (F) and RT-PCR measurements of the relative protein and mRNA expression, respectively, of CDK1 (G) showing that CDK1 levels were markedly reduced after DNMT1 knockdown. H m5C-IP results showing the m5C peaks in CDK1 mRNA. I Relative CDK1 fold enrichment after DNMT1 knockdown. J, K Comparison of CDK1 expression between tumor and normal tissues in the TCGA-LIHC cohort (J) and between normal and HCC tissues (K). L, M Analysis of CDK1 mRNA stability after DNMT1 knockdown and actinomycin D treatment. N, O Spearman correlations between DNMT1 and CDK1 expression in two GEO datasets GSE76427 (N) and GSE54236 (O). (*P < 0.05, ** P < 0.01, ***P < 0.001)
DNMT1 activates pyrimidine metabolism and promotes HCC progression by upregulating CDK1 in vitro
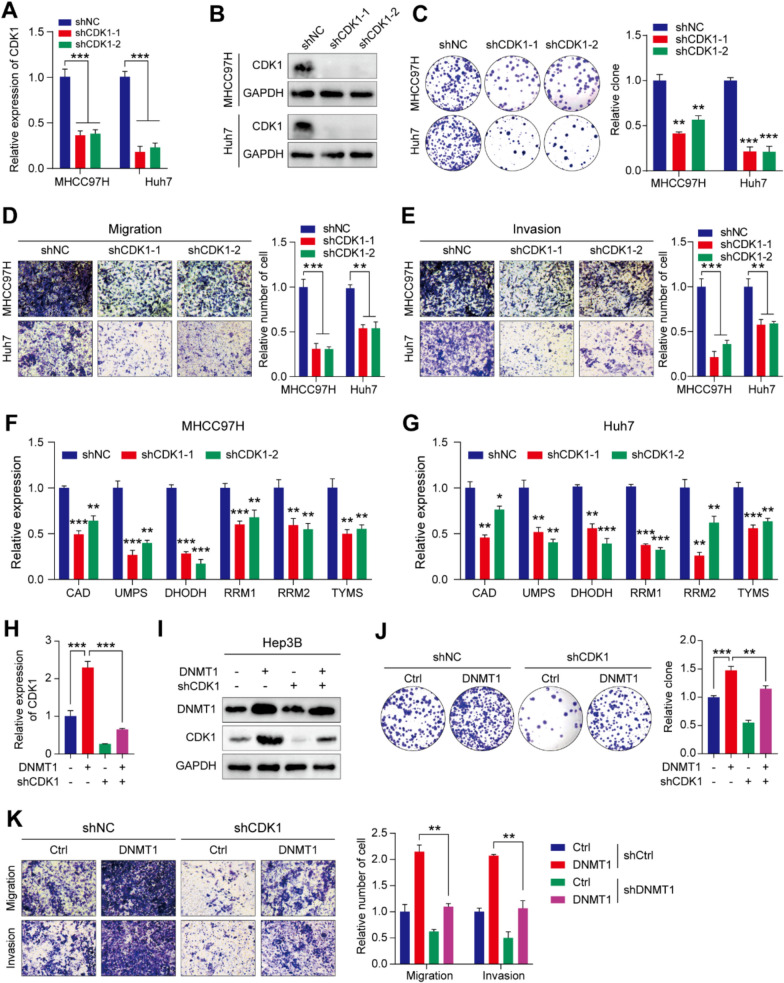
To investigate the role of CDK1 in HCC, the CDK1 gene was knocked down, resulting in significantly reduced colony formation on both the Huh7 and MHCC97H cell lines (Fig. 6A–C). In addition, migration and invasion of HCC cells were inhibited following CDK1 knockdown (Fig. 6D, E). Furthermore, EdU assays verified that CDK1 promoted HCC cell proliferation (Supplementary Figure S6A). To explore the association between CDK1 expression and survival outcomes, survival data from two GEO datasets, GSE76427 and GSE54236, were analyzed (Supplementary Figure S6B, C). Elevated CDK1 expression was linked to poorer clinical outcomes (Supplementary Figure S6B, C). The tumor doubling time was also significantly shorter in the high-CDK1 group compared with the low-CDK1 group (Supplementary Figure S6D). The result suggested that CDK1 may play a crucial role in tumor growth. Collectively, these findings suggest that CDK1 enhances HCC migration and invasion.
Fig. 6.
DNMT1 indirectly promotes pyrimidine metabolism via upregulation of CDK1 expression, promoting HCC progression. A mRNA expression of CDK1 following CDK1 knockdown in two HCC cell lines, shown by RT-PCR. B Protein levels of CDK1 following CDK1 knockdown, shown by Western blotting. C Colony formation in the CDK1-knockdown groups vs. the control group in two HCC cell lines. D, E Transwell assay results showing the migration and invasion abilities of HCC cell lines. F, G RT-PCR results showing reduced expression of pyrimidine metabolism-related genes after knockdown of CDK1. H Rescue experiment showing increased CDK1 expression levels following DNMT1 overexpression. I Western blotting showing protein expression of CDK1 under different treatments. J Colony formation in DNMT1-overexpressing cells. K Transwell assay results showing migration and invasion abilities of DNMT1-overexpressing cells. (*P < 0.05, ** P < 0.01, ***P < 0.001)
Given that pyrimidine biosynthesis is fundamental to DNA synthesis which was closely associated with cell cycle (Fig. 3A), it was speculated that CDK1 was associated with pyrimidine metabolism. To explore whether CDK1 enhanced HCC malignancy via upregulation of pyrimidine metabolism in HCC, correlations between the expression of CDK1 and key enzymes in pyrimidine metabolism, as well as the GSVA scores of pyrimidine metabolism, were investigated (Supplementary Figure S7E, F). A significant correlation indicated that CDK1 potentially contributed to the promotion of pyrimidine metabolism. The relative expression of key enzymes involved in pyrimidine metabolism following the knockdown of CDK1 was substantially decreased (Fig. 6F, G). These results suggested that CDK1 regulates pyrimidine metabolism, promoting the migration and invasion of HCC cells, as well as being associated with poorer clinical prognosis. As it was previously found that DNMT1 induces dysregulated pyrimidine metabolism, it was hypothesized that DNMT1 upregulates CDK1 leading to the potential promotion of pyrimidine metabolism by the latter. To examine this hypothesis, knockdown and rescue experiments were performed (Fig. 6H, I). The inhibition of CDK1 was observed to significantly suppress colony formation, while overexpression of DNMT1 reversed the inhibitory effects of CDK1 knockdown (Fig. 6J). Furthermore, DNMT1 overexpression reversed the inhibitory effect of CDK1 knockdown on the malignant phenotype, promoting the migration and invasion of HCC cells (Fig. 6K). Together, these results demonstrated that DNMT1 modified CDK1 via m5C modification to upregulate CDK1, while CDK1 contributed to the promotion of pyrimidine metabolism to stimulate HCC progression.
Inhibition of the DNMT1/CDK1/pyrimidine metabolism axis suppresses HCC progression in vivo
To evaluate the potential of targeting the DNMT1/CDK1/pyrimidine metabolism axis in alleviating HCC progression, GSK3685032, a specific DNMT1 inhibitor, was used to treat HCC cells at doses of 0.1 and 1 μM. It was found that GSK3685032 treatment led to reduced cell proliferation and colony formation (Fig. 7A–C; Supplementary Figure S7A). The inhibitory effect was dose-dependent, with greater inhibition seen at higher doses (Fig. 7A–C; Supplementary Figure S8A). Moreover, the tumorigenic behavior of HCC cells was suppressed by the inhibitor in a dose-dependent manner (Fig. 7D, E). The specific CDK1 inhibitor Ro-3306 was also used to treat HCC cells, after which the relative expression levels of key enzymes involved in pyrimidine metabolism were measured. This indicated significantly reduced expression of these enzymes in the presence of both GSK36 and Ro-3306 (Fig. 7F, G). Thus, treatment with GSK36 and Ro-3306 could downregulate pyrimidine metabolism in HCC cells. BAY2402234 is an inhibitor of pyrimidine metabolism. In vivo experiments in mice showed that the GSK36, Ro-3306, and BAY-treated groups showed significantly lower luminescence in tumors compared with the control group (Fig. 7H). Following the use of specific inhibitor of DNMT1, m5C levels were detected, and it was found that m5C reduced significantly after GSK36 treatment. Additionally, H&E and Ki-67 staining indicated that the GSK36, Ro-3306, and BAY24 treatment groups displayed reduced tumor cell densities relative to the control group (Fig. 7I). Together, these results demonstrated that targeting the DNMT1/CDK1/pyrimidine metabolism axis in HCC suppressed HCC progression.
Fig. 7.
Inhibition of DNMT1, CDK1, and pyrimidine metabolism reduces malignant progression of HCC. A, B Effects of the DNMT1 inhibitor (GSK3685032) on cell growth. Higher concentrations had a greater inhibitory impact on cell proliferation. C In vitro cell culture showing reduced colony formation after incubation with varying concentrations of the DNMT1 inhibitor in two cell lines. D, E Transwell assay results showing reduced migration and invasion after inhibition of DNMT1. F, G RT-PCR measurements of the relative expression of pyrimidine metabolism-related genes after inhibition of DNMT1 in two cell lines. H Treatment of MHCC97H mouse models with the DNMT1 inhibitor (GSK3685032), CDK1 inhibitor (Ro-3306), and pyrimidine metabolism inhibitor (BAY2402234). Luminescence intensities of tumors on days 7 and 35, indicating inhibitory effects on HCC. I H&E and Ki-67 staining of tumor tissues. Scale bar, 25 μm. (*P < 0.05, **P < 0.01, ***P < 0.001)
Discussion
5-Methylcytosine (m5C) posttranscriptional modifications of RNA have a wide range of biological implications [5, 9]. Abnormally increased RNA m5C modification has been reported to affect responses to liver injury and promote tumorigenicity and malignancy as well as tumor immune evasion [5, 29, 30]. Abnormal metabolic changes are also associated with malignancy [22, 31]. For example, the m5C methyltransferase NSUN2 is reported to bind glucose to maintain activation, which is essential for tumor cell malignancy. Furthermore, NSUN2 stabilizes TREX2 mRNA in an m5C-dependent manner and activates the cGAS-STING pathway to promote tumorigenicity and tumor resistance to immunotherapy [32]. Pyrimidine metabolism is an important metabolic pathway as it is linked to DNA synthesis and the cell cycle. However, the mechanisms underlying the association between m5C modification and dysregulated pyrimidine metabolism in HCC remains unknown.
The present study found abnormally elevated m5C modification levels in HCC cells. Identification of the transcriptomic characteristics of m5C regulators in HCC showed that levels of m5C modifications were in line with malignant HCC progression. Unsupervised clustering identified three distinct m5C modification clusters with patients in the high-m5C cluster showing the lowest OS rates, aligning with previous findings [33]. Moreover, the study showed for the first time that there was an association between pyrimidine metabolism and m5C modification in HCC. In terms of mechanism, it was found that DNMT1 played a significant role in HCC progression by modifying CDK1 in an m5C-dependent manner. CDK1 was further shown to upregulate pyrimidine metabolism, enhancing the HCC malignant phenotype. Inhibition of the DNMT1/CDK1/pyrimidine metabolism axis suppressed cell proliferation, migration, and invasion. These findings enrich the present understanding of the relationship between m5C modification and the regulation of metabolism.
Landscape profiles of the metabolic characteristics of the three clusters were constructed. It was observed that dysregulated pyrimidine metabolism was common in all three clusters. Analysis of the major m5C regulators responsible for modulating the dysregulated pyrimidine metabolism by assessing expression patterns in HCC tissues and correlation with regulators of pyrimidine metabolism identified DNMT1 as a key m5C regulator. DNMT1 is a DNA methyltransferase, and has been reported to function as an oncogene facilitating the onset and progression of various cancers [34, 35]. However, prior research has tended to focus on the role of DNMT1 as DNA methyltransferase [36]. Recently, emerging evidence has indicated that DNMT1 also interacts with multiple RNAs, playing an important role in m5C modification [5, 37], whereas current investigations have focused mainly on the function of NSUN2 in m5C modification. Therefore, the role of DNMT1 in RNA modification remains to be explored. In this study, it was found that abnormally elevated expression of DNMT1 in HCC was associated with unfavorable prognosis and malignant progression in HCC, further verifying its oncogenic function in HCC. Metabolic profiling of HCC demonstrated a correlation between DNMT1 expression and dysregulated pyrimidine metabolism. Knockdown of DNMT1 reduced the expression of key enzymes associated with pyrimidine metabolism, as well as inhibiting HCC cell proliferation, migration, and invasion. The combination of RNA-seq, RIP-seq, and WGCNA was used to show that DNMT1 upregulates CDK1 expression in an m5C-modification manner. Rescue experiments indicated that overexpression of DNMT1 restored HCC cell malignancy that had been reduced by CDK1 knockdown. Inhibition of DNMT1 by specific inhibitor GSK3685032 significantly suppressed tumor growth in HCC model mice and reduced HCC cell proliferation, migration, and invasion in a dose-dependent manner. However, further investigation is needed to determine whether DNMT1 can directly regulate key enzymes involved in pyrimidine metabolism. Although we identified DNMT1 as a key mediator in pyrimidine metabolism dysregulation, other m5C regulators may also directly or indirectly modulate pyrimidine metabolism. For example, YBX1 or ALYREF potentially recognized CDK1 mRNA and stabilized it. Other m5C methyltransferases (e.g., NSUN2, NOP2) and demethylases (e.g., TET1-3) could also regulate CDK1 expression via m5C modification. Functional validation of how these regulators affect pyrimidine metabolism was not conducted in this study. Future studies could profile their impact on dysregulation of pyrimidine metabolism. Nevertheless, these findings contribute to the current knowledge of the role of DNMT1 in m5C modification, providing crucial insights into targeting DNMT1 as a treatment option for HCC.
The study also identified CDK1 as a regulator for pyrimidine metabolism. CDK1 is a classic kinase that regulates the cell cycle, facilitating the G2/M transition [38]. Considering that cancer cells exhibit uncontrolled cell division, CDK1 plays a crucial role in cancer initiation and progression [39]. In the present study, the knockdown of CDK1 reduced both migration and invasion in HCC cells in vitro, consistent with previous findings. Moreover, the luminescence intensity of tumors in the HCC model mice was decreased compared with the control group. Although there are studies indicating that CDK1 is linked to metabolic activities [40], the association between CDK1 and pyrimidine metabolism has yet to be elucidated. Our in vitro and in vivo experiments discovered that key enzymes involved in pyrimidine metabolism decreased significantly after genetic knockdown or pharmacological inhibition of CDK1. While our work identified CDK1 as a potential regulator of pyrimidine metabolism in HCC, the detailed mechanisms underlying this association remain incompletely characterized. Previous literature has reported that CDK1 phosphorylated and stabilized transcriptional factors (TFs), for example, ISL1, to regulate cell growth and survival [40–42]. Thus, it was speculated that CDK1 may contribute to the promotion of pyrimidine metabolism by upregulating TFs levels. Also, CDK1 could potentially interact directly with pyrimidine metabolic enzymes and activate pyrimidine metabolism. Future investigations could include co-immunoprecipitation (Co-IP) assays and mass spectrometry to determine the potential interactions between CDK1 and TFs, or regulators of pyrimidine metabolism. Therefore, the findings of this study provide novel insight into the regulatory effects of the CDK1/pyrimidine metabolism pathway.
However, the study has several limitations. The RNA-binding protein that stabilizes CDK1 was not investigated, as accumulated evidence has fully analyzed the effects of m5C modifications [5, 12, 13, 16, 28]. Except DNMT1, other m5C regulators (e.g., NSUN2, NOP2) may also play significant roles in regulating pyrimidine metabolism, which were not explored in this study as described in previous context. Their roles might be synergistic or antagonistic to DNMT1, forming a sophisticated regulating network. Therefore, future research should systematically validate the impact of individual m5C regulator on pyrimidine metabolism and elucidate their potential coordination mechanisms with DNMT1 using co-depletion assays and multi-omics profiling. Moreover, how CDK1 regulates pyrimidine metabolism, whether through direct interaction with pyrimidine metabolic enzymes or via stabilization of transcriptional regulators, remains to be elucidated. Nevertheless, the findings demonstrate the association between m5C modification and pyrimidine metabolism, providing novel insights into targeting the DNMT1/CDK1/pyrimidine metabolism axis in HCC.
Conclusion
This study depicted landscape profiles of immune, genomic alterations and metabolic characteristics of m5C modifications in HCC, revealed the association between m5C modifications and prognosis as well as clinical treatment. Moreover, our findings demonstrated that DNMT1-mediated m5C modification of CDK1 upregulates the expression of the latter, while CDK1 facilitates pyrimidine metabolism which promotes HCC malignancy. In conclusion, this study provides new insights into investigation of patients with different m5C levels. Furthermore, it expands current knowledge of DNMT1 as an m5C methyltransferase, which lacks sufficient investigations. The findings provide novel insights into the potential targeting of the DNMT1/CDK1/pyrimidine metabolism axis as an appealing therapeutic strategy for combating HCC.
Supplementary Information
Acknowledgements
We thank Jin Wang (Soochow University) for generously sharing technical expertise and resources through his personal website (https://www.jingege.wang), which provided invaluable support for this study.
Abbreviations
- HCC
Hepatocellular carcinoma
- m5C
5-Methylcytosine
- DNMT1
DNA-methyltransferase 1
- CDK1
Cyclin-dependent protein kinase 1
- TCGA
The Cancer Genome Atlas
- LIHC
Liver hepatocellular carcinoma
- GEO
Gene Expression Omnibus
- OS
Overall survival
- ssGSEA
Single sample gene set enrichment analysis
- TACE
Transcatheter arterial chemoembolization
- GSVA
Gene set variation analysis
- GSEA
Gene set enrichment analysis
- RT-PCR
Reverse transcription polymerase chain reaction
- WGCNA
Weighted gene co-expression network analysis
- PPI
Protein–Protein interaction network
- CAD
Carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase
- UMPS
Uridine monophosphate synthetase
- DHODH
Dihydroorotate dehydrogenase
- RRM1
Ribonucleotide reductase subunit M1
- RRM2
Ribonucleotide reductase subunit M2
- TYMS
Thymidylate synthetase
- RIP-seq
RNA-immunoprecipitation sequencing
- AFP
Alpha-fetoprotein
- DAPI
4′,6-Diamidino-2-phenylindole
- GSK36
GSK3685032
- BAY
BAY2402234
- H&E
Hematoxylin & eosin
Author contribution
X. Z., and S. M.: Methodology, formal analysis, writing—original draft, visualization; Y. F. and J. C.: Investigation, formal analysis, visualization; L. B., J. L., J. W., J. G., and S. Y.: Validation, visualization; J. F., X. W., G. Z.: Data curation, investigation; Z. T., Y. S., J. Z., J. F.: Supervision, funding acquisition, resources; X. Z., J. J., and W. L.: Conceptualization, funding acquisition, resources, project administration, write—review and editing. All authors reviewed the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 82273386, 82273387, 82403408, 82403406 and 82403555). Shanghai Municipal Key Clinical Specialty. CAMS Innovation Fund for Medical Sciences (CIFMS) (2019-I2M-5-058). Youth fund of Zhongshan Hospital Affiliated to Fudan University (No. 2023ZSQN16).
Data availability
The data supporting the discovery of this study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval and consent to participate
The collection of specimens of clinical patients in this study was approved by the Research Ethics Committee of Zhongshan Hospital.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xuhui Zhao, Shengwei Mao, Yuan Fang, and Jiafeng Chen have contributed equally to this work.
Contributor Information
Xingxing Zhang, Email: simonzx1989@163.com.
Jinling Jiang, Email: jjl11872@rjh.com.cn.
Weiren Liu, Email: liu.weiren@zs-hospital.sh.cn.
References
- 1.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7: 6. [DOI] [PubMed] [Google Scholar]
- 3.Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400:1345–62. [DOI] [PubMed] [Google Scholar]
- 4.Zhao BS, Roundtree IA, He C. Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol. 2017;18:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berggren KA, Schwartz RE, Kleiner RE, Ploss A. The impact of epitranscriptomic modifications on liver disease. Trends Endocrinol Metab. 2024;35:331–46. [DOI] [PubMed] [Google Scholar]
- 6.Geula S, Moshitch-Moshkovitz S, Dominissini D, Mansour AA, Kol N, Salmon-Divon M, et al. Stem cells. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science. 2015;347:1002–6. [DOI] [PubMed] [Google Scholar]
- 7.Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:1187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fustin J-M, Doi M, Yamaguchi Y, Hida H, Nishimura S, Yoshida M, et al. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell. 2013;155:793–806. [DOI] [PubMed] [Google Scholar]
- 9.Nachtergaele S, He C. The emerging biology of RNA post-transcriptional modifications. RNA Biol. 2017;14:156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hussain S, Aleksic J, Blanco S, Dietmann S, Frye M. Characterizing 5-methylcytosine in the mammalian epitranscriptome. Genome Biol. 2013;14:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motorin Y, Lyko F, Helm M. 5-Methylcytosine in RNA: detection, enzymatic formation and biological functions. Nucleic Acids Res. 2010;38:1415–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X, Yang Y, Sun B-F, Chen Y-S, Xu J-W, Lai W-Y, et al. 5-methylcytosine promotes mRNA export—NSUN2 as the methyltransferase and ALYREF as an m5C reader. Cell Res. 2017;27:606–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Li A, Sun B-F, Yang Y, Han Y-N, Yuan X, et al. 5-methylcytosine promotes pathogenesis of bladder cancer through stabilizing mRNAs. Nat Cell Biol. 2019;21:978–90. [DOI] [PubMed] [Google Scholar]
- 14.Shen H, Ontiveros RJ, Owens MC, Liu MY, Ghanty U, Kohli RM, et al. TET-mediated 5-methylcytosine oxidation in tRNA promotes translation. J Biol Chem. 2021;296: 100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arguello AE, Li A, Sun X, Eggert TW, Mairhofer E, Kleiner RE. Reactivity-dependent profiling of RNA 5-methylcytidine dioxygenases. Nat Commun. 2022;13: 4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y-S, Yang W-L, Zhao Y-L, Yang Y-G. Dynamic transcriptomic m5C and its regulatory role in RNA processing. WIREs RNA. 2021;12: e1639. [DOI] [PubMed] [Google Scholar]
- 17.Zhang T, Gu J, Wang X, Luo J, Yan J, Cai K, et al. RNA methylation regulators contribute to poor prognosis of hepatocellular carcinoma associated with the suppression of bile acid metabolism: a multi-omics analysis. Am J Cancer Res. 2022;12:2989–3013. [PMC free article] [PubMed] [Google Scholar]
- 18.Mossmann D, Müller C, Park S, Ryback B, Colombi M, Ritter N, et al. Arginine reprograms metabolism in liver cancer via RBM39. Cell. 2023;186:5068-5083.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du D, Liu C, Qin M, Zhang X, Xi T, Yuan S, et al. Metabolic dysregulation and emerging therapeutical targets for hepatocellular carcinoma. Acta Pharm Sin B. 2022;12:558–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu Z, Cao C, Zhang D, Zhang Z, Liu L, Wu D, et al. UBE2T-mediated akt ubiquitination and akt/β-catenin activation promotes hepatocellular carcinoma development by increasing pyrimidine metabolism. Cell Death Dis. 2022;13:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Martino M, Rathmell JC, Galluzzi L, Vanpouille-Box C. Cancer cell metabolism and antitumour immunity. Nat Rev Immunol. 2024;24:654–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun L, Zhang H, Gao P. Metabolic reprogramming and epigenetic modifications on the path to cancer. Protein Cell. 2022;13:877–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grinchuk OV, Yenamandra SP, Iyer R, Singh M, Lee HK, Lim KH, et al. Tumor-adjacent tissue co-expression profile analysis reveals pro-oncogenic ribosomal gene signature for prognosis of resectable hepatocellular carcinoma. Mol Oncol. 2018;12:89–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villa E, Critelli R, Lei B, Marzocchi G, Cammà C, Giannelli G, et al. Neoangiogenesis-related genes are hallmarks of fast-growing hepatocellular carcinomas and worst survival. Results from a prospective study. Gut. 2016;65:861–9. [DOI] [PubMed] [Google Scholar]
- 25.He Q, Yang J, Jin Y. Development and validation of TACE refractoriness-related diagnostic and prognostic scores and characterization of tumor microenvironment infiltration in hepatocellular carcinoma. Front Immunol. 2022;13: 869993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu Y, Yang A, Quan C, Pan Y, Zhang H, Li Y, et al. A single-cell atlas of the multicellular ecosystem of primary and metastatic hepatocellular carcinoma. Nat Commun. 2022;13:4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cervantes-Villagrana RD, Albores-García D, Cervantes-Villagrana AR, García-Acevez SJ. Tumor-induced neurogenesis and immune evasion as targets of innovative anti-cancer therapies. Signal Transduct Target Ther. 2020;5:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Z, Zhou Y, Lv P, Zhou T, Liu H, Xie Y, et al. NSUN4 mediated RNA 5-methylcytosine promotes the malignant progression of glioma through improving the CDC42 mRNA stabilization. Cancer Lett. 2024;597: 217059. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Jin H, Li Q, Shi L, Mao Y, Zhao L. The role of RNA methylation in tumor immunity and its potential in immunotherapy. Mol Cancer. 2024;23:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui L, Ma R, Cai J, Guo C, Chen Z, Yao L, et al. RNA modifications: importance in immune cell biology and related diseases. Signal Transduct Target Ther. 2022;7:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng J, Li J, Wu L, Yu Q, Ji J, Wu J, et al. Emerging roles and the regulation of aerobic glycolysis in hepatocellular carcinoma. J Exp Clin Cancer Res. 2020;39:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen T, Xu Z-G, Luo J, Manne RK, Wang Z, Hsu C-C, et al. NSUN2 is a glucose sensor suppressing cGAS/STING to maintain tumorigenesis and immunotherapy resistance. Cell Metab. 2023;35:1782-1798.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li D, Li K, Zhang W, Yang K-W, Mu D-A, Jiang G-J, et al. The m6A/m5C/m1A regulated gene signature predicts the prognosis and correlates with the immune status of hepatocellular carcinoma. Front Immunol. 2022;13: 918140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu Y, Fu W, Xie Y, Jiang X, Wang H, Yang X. A review on recent advances in assays for DNMT1: a promising diagnostic biomarker for multiple human cancers. Analyst. 2024;149:1002–21. [DOI] [PubMed] [Google Scholar]
- 35.Fu Y, Zhang X, Liu X, Wang P, Chu W, Zhao W, et al. The DNMT1-PAS1-PH20 axis drives breast cancer growth and metastasis. Signal Transduct Target Ther. 2022;7:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu H, Song Y, Qiu H, Liu Y, Luo K, Yi Y, et al. Downregulation of FOXO3a by DNMT1 promotes breast cancer stem cell properties and tumorigenesis. Cell Death Differ. 2020;27:966–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang K, Li F-H, Zhou L-Y, Zhao X-M, Gao X-Q, Liu C-Y, et al. HNEAP regulates necroptosis of cardiomyocytes by suppressing the m5 C methylation of Atf7 mRNA. Adv Sci (Weinh). 2023;10: e2304329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14:130–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie B, Wang S, Jiang N, Li JJ. Cyclin B1/CDK1-regulated mitochondrial bioenergetics in cell cycle progression and tumor resistance. Cancer Lett. 2019;443:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ow JR, Caldez MJ, Zafer G, Foo JC, Li HY, Ghosh S, et al. Remodeling of whole-body lipid metabolism and a diabetic-like phenotype caused by loss of CDK1 and hepatocyte division. Elife. 2020;9: e63835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guan T, Li M, Song Y, Chen J, Tang J, Zhang C, et al. Phosphorylation of USP29 by CDK1 governs TWIST1 stability and oncogenic functions. Adv Sci (Weinh). 2023;10: e2205873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi Q, Ni X, Lei M, Xia Q, Dong Y, Zhang Q, et al. Phosphorylation of islet-1 serine 269 by CDK1 increases its transcriptional activity and promotes cell proliferation in gastric cancer. Mol Med. 2021;27:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the discovery of this study are available from the corresponding author on reasonable request.