Abstract
Gut microbiomes play critical roles in host-environment interactions, reflecting habitat and foraging niches. North American bison (Bison bison) subspecies—plains bison (B. bison bison) and wood bison (B. bison athabascae)—exhibit limited genetic variation from historic population bottleneck events, potentially undermining their evolutionary potential. Understanding variation in gut microbiota composition between subspecies may shed light on genetic, phenotypic, and ecological divergence relevant to their adaptive capacities. Using 16S rRNA metabarcoding of fecal samples, we characterized the gut microbiota of both subspecies in the sympatric environment of Elk Island National Park, providing insight into potential phylogenetic gut microbiome divergence. Like other ruminants, the gut microbial community of both subspecies consists primarily of the bacterial phyla Firmicutes and Bacteroidetes. Subspecific classification explained no significant differences in alpha diversity (p > 0.05) in the overall dataset, but has a potentially significant effect on beta diversity (p < 0.05, R2 = 0.04). Gut microbiota divergence between subspecies may be driven by differential abundance of specific taxa and associated functional pathways, likely influenced by dietary preferences, ancestral phenotypes, and historical ranges. Our findings support further investigation into diet-microbiome relationships between subspecies in sympatric environments and metagenomic approaches to explore functional differences in the gut microbiome.
Supplementary Information
The online version contains supplementary material available at 10.1186/s42523-025-00451-7.
Keywords: Gut microbiome, North American bison, Subspecific variation, Phylogenetic divergence
Background
Comprised of a diverse community of bacteria, archaea, fungi, protozoa, viruses, and their associated genomes [1], the gut microbiome is imperative for development, metabolism, digestion, and immunity, and essential to animal health and fitness in local environments [2, 3]. The gut microbiome also plays a critical role in host-environment interactions, including regulation of heat tolerance [4, 5], disease resistance [6], and response to environmental factors (e.g., diet, geography, changing climates) [7]. Climate factors, such as precipitation, are shifting polewards and Northern hemisphere temperatures are expected to rise upwards of 4 °C by the end of the 21st century [8]. Shifts in these environmental regimes increase physiological stress in mammalian species, elevating thermoregulation requirements and altering forage composition, quality, and quantity, thereby impacting energy acquisition [9, 10]. Plasticity in the gut microbiome may allow the host to adapt to change on shorter time scales than genetic adaptation [11], where gut microbiota amplify environmental and developmental signals allowing hosts to rapidly adjust their ecological and dietary niches [12]. The adaptive plasticity of the gut microbiome is likely imperative in promoting the persistence of genetically depauperate species with long generation times in the face of accelerated environmental change [13].
While the gut microbiome is crucial for all host species, it is especially important in ruminants, as they do not produce enzymes necessary for cellulose digestion, despite its prevalence in their diets [14]. By anaerobically fermenting cellulose into short chain fatty acids (e.g., butyrate, propionate, and acetate), gut microbiota produce nearly 70% of the total energy used by ruminant hosts to grow, develop, and reproduce [15–18]. The bacterial component of ruminant gut microbiota constitute over 50% of total microbial biomass [16, 19], a reflection of their critical functional role; demonstrated by significantly reduced ruminal fermentation when bacteria are absent [20]. The ruminant gut microbial community consists primarily of taxa within Bacteroidetes and Firmicutes phyla, both known for cellulolytic enzyme production [14]. Studies of wild ruminant gut microbiomes have revealed distinct signatures of seasonal [21–23], geographic [24, 25], and dietary [26, 27] changes, where the microbial community shifts to adapt to local conditions reflective of environmental variation. Management practices, including supplemental feeding [26, 28, 29] and levels of captivity [28, 30, 31], also influence gut microbiota composition and diversity, as these practices diverge from diets and habitats wild species have adapted to.
North American bison (Bison bison) are an ecologically important ruminant species of immense cultural significance to Indigenous peoples of North America [32]. Bison have been subclassified into two distinct subspecies, wood bison (B. b. athabascae) and plains bison (B. b. bison), based on morphological differences and distinct historic ranges. Wood and plains bison can be distinguished by body and horn size, pelage colour and density, angle and position of the shoulder hump, and demarcation of the woolly cape [33–35]. The historic range of plains bison extended throughout the Great Plains of the United States and into the southern Canadian prairies; the historic range of wood bison met the northern edge of the plains bison range and extended into Northern Canada and Alaska [36–39]. The two subspecies are thought to have diverged approximately 5,000 years ago [40], however hybridization events between subspecies and with cattle have occurred in more recent history [36, 41, 42]. Extreme overhunting and loss of habitat due to agricultural practices in the 18th and 19th centuries resulted in the decimation of bison populations across North America, extirpating plains bison from the wild in Canada and diminishing wood bison populations to less than 300 individuals [36, 37]. Current threats to this species include accelerated habitat loss, limited genetic variation, and diseases such as brucellosis (Brucella abortus), bovine tuberculosis (Mycobacterium bovis), and anthrax (Bacillus anthracis) [36].
Elk Island National Park (Alberta, Canada; EINP) represents a unique opportunity to study wood and plains bison as it is the only contemporary location where both subspecies co-exist in the wild [43]. Wood and plains bison in EINP are separated only by fencing, which surrounds the entire park, and the Trans Canada Highway. The 159km2 northern block of EINP houses plains bison, whereas, the 59km2 southern block of EINP houses wood bison [44]. Forested regions within EINP consist primarily of trembling aspen (Populus tremuloides), and woody shrubs including prickly rose (Rosa acicularis), raspberry (Rubus idaeus), and beaked hazelnut (Corylus cornuta), with wetter regions containing abundant horsetail (Equisetum spp.) [44]. Three primary types of wetlands can be found throughout EINP; wet meadows with scattered conifers and abundant Labrador tea (Rhododendron groenlandicum), fens high in grasses, sedges (Carex spp.), and willows (Salix spp.), and marshes dominated by sedges [44]. Grassland regions in EINP consist primarily of species within Poa and Bromus genera [44]. While proportions of land cover types differ between North and South blocks of EINP, both subspecies presumably have similar access to habitat and forage [44]. Population sizes of wood and plains bison within the park are maintained between 300 and 400 and 500–700 individuals, respectively [45]. Furthermore, the fenced-in nature of EINP has prevented cattle introgression and maintained the ‘disease free’ status of these populations regarding brucellosis, bovine tuberculosis, and anthrax.
Bison conservation goals include: maintaining and increasing the genetic diversity of managed populations, limiting cattle and subspecific introgression, reintroducing bison to their historic habitats, and restoring populations as a part of reconciliation with Indigenous peoples [36, 41, 46–49]. Federal, provincial, and territorial agencies currently manage wood and plains bison separately given their subspecific classification. A key factor missing from current conservation considerations in North America, however, is the influence of the gut microbiome on host health and fitness in local environments in the context of increased droughts, fire season pressure, and disease. Previous gut microbiome analysis of plains bison in the United States found diet diversity positively correlated with gut microbial community diversity, where foraging behaviour increases diet and gut microbiota diversity compared to grain-based diets [50, 51]. Further, variation between spring and summer seasons has been observed in the plains bison gut microbiota, likely correlated with seasonal shifts in diet composition, increasing their relative fitness in local environments and accommodating environmental change [52]. In Canada, bison gut microbiome analysis is limited to a single study of wood bison in EINP that uncovered potential enterotypes—distinct, population level classifications of microbial communities that achieve the same function—independent of sex, age, and housing, within this population [53].
Non-invasive fecal sampling has been used as a pragmatic, representative method to sample the gut microbiota of elusive, wild ungulates [54]. It is not always possible to distinguish between fecal samples of individuals within the same spatially located group, potentially biasing results towards the profile of a single individual. As such, individualizing samples using host genetic signatures presents an additional consideration not often applied to such studies [54]. Furthermore, genotyping samples can provide metadata relevant to microbiome studies, such as sex identification or repeated sampling of individuals over time [54–56].
The present study compares the gut microbial community composition and diversity of wood and plains bison in EINP via passive fecal sampling. Given the potential for individual replication in the dataset, we set out to individualize each fecal sample prior to gut microbiome analysis to ensure a balanced representation of individuals and sexes. Comparing both subspecies in a sympatric environment provides crucial insights into how preferential habitat use and phylogenetic differences influence gut microbiome composition and function. We hypothesized gut microbial communities of wood and plains bison within EINP would be similar, given their recent divergence, overlapping dietary niches, and habitat similarities. By limiting environmental factors that could confound gut microbiome divergence due to subspecific differences, this study has the potential to enhance our understanding of subspecific interaction with local environments and elucidate taxa that are indicative of response to changing forage, historic disease status, and subspecific genetic differences, serving as a baseline for the gut microbial community of the two subspecies.
Materials and methods
Sample design and collection
We passively collected fecal samples in Elk Island National Park (Alberta, Canada; EINP) between October 21–23, 2023. Plains bison fecal patties (n = 100) were sampled in the North Block of the park by surveying the roadside and the parkland East of and surrounding the Bison Loop Road (Fig. 1). Wood bison fecal patties (n = 100) were sampled in the South Block of the park by surveying the Wood Bison Trail and parkland surrounding Flyingshot Lake (Fig. 1). Sampling was performed under Parks Canada Research and Collection Permit EINP-2023-45630.
Fig. 1.
Map of Elk Island National Park (EINP) with collection areas indicated. Adapted from “Elk Island National Park Site Map” [57]. Wood bison (n = 100) and plains bison (n = 100) fecal samples were collected between October 21–23, 2023
Fecal samples were collected if they appeared to have been deposited within 24 h prior to collection based on visual assessment of moisture content, as the fecal microbiome is influenced by environmental variables [58]. We collected two swabs of the mucosal coating on each fecal patty for individualization and sex identification of samples via short-tandem repeat (STR) genotyping. Where possible, we separated the folds of the fecal patty and used a twirling motion to pick up visible mucus onto the swab. For extremely fresh samples with high moisture content, where no visible mucus was present, we gently rolled the swab across the surface of the feces. Swabs were stored in 2 mL screwcap tubes with 500 uL nucleic acid preservation buffer (4 M Urea, 0.2 M NaCl, 0.5% Sarkosyl, 10mM EDTA, 0.1 M Tris pH 8). We collected two subsamples from each fecal patty using sterile spatulas, collecting only the inner fecal portion to mitigate environmental contamination. Field negatives were collected by adding 500 uL of lysis buffer to unused tubes during collection. Fecal swabs, samples, and negatives were stored in 2 mL screwcap tubes at ambient conditions during collection (mean ambient temperature − 5.4 °C to 5.2 °C) and 4 °C post-collection day, and shipped frozen to Trent University.
Short tandem repeat (STR) genotyping
We used STRs to individualize fecal samples prior to microbiome analysis to avoid replication in the dataset as suggested by Combrink et al. [54]. Fecal swabs were prepared for DNA isolation through Proteinase K digestion (20 uL) and incubation at 56 °C for 1 h and 37 °C until completely lysed. Total DNA was extracted using the DNeasy Blood and Tissue Kit (QIAGEN), as per the manufacturer’s recommendations, with buffer and ethanol volumes adjusted to 500 uL to account for increased lysis buffer volume. We performed two consecutive DNA elution steps of 50 uL to maximize DNA yield, as recommended by the manufacturer. Isolated DNA was amplified using polymerase chain reaction (PCR) with 21 loci [59], including a sex determining marker (Supplementary Table 1). Genetic profiles were generated using capillary electrophoresis on an automated ABI 3730 DNA Analyzer and identified using Gene Marker v.3.0.1 software (SoftGenetics). Samples with greater than 40% missing data were excluded from further analysis. Individuals were identified using GENECAP [60], where profiles with zero to two allele differences were considered to have originated from the same individual. Both negative and positive controls were used during DNA isolation and amplification to assess contamination and sample processing efficiencies to maintain data integrity.
High-throughput sequencing for microbiome analysis
DNA extraction
Total genomic DNA was isolated using the QIAamp PowerFecal DNA Kit (QIAGEN) per manufacturer recommendations, extracting approximately 250 mg of feces. We performed two consecutive DNA elution steps of 50 uL to maximize DNA yield. We extracted multiple negative controls to evaluate contamination and used the ZymoBIOMICS Microbial Community Standard (Zymo Research), which provides known ratios of specific microbial taxa, as a positive control.
Amplification of 16 S rRNA V4 region
Each sample was amplified in triplicate via PCR using the 16S rRNA primer pair 515F-GTGYCAGCMGCCGCGGTAA and 806R-GGACTACHVGGGTWTCTAAT [61], with the NEBNext Illumina adaptor sequence (Forward overhang: 5’ ACACTCTTTCCCTACACGACGCTCTTCCGATCT; Reverse overhang: 5’ GACTGGAGTTCAGACGTGTGCTCTTCCGATCT). Reaction conditions were as follows: 6.25 uL KAPA HiFi HotStart ReadyMix (Roche Scientific), 0.25 uL primer 515 F, 0.25 uL primer 806R, 2.5 uL stock DNA, and 3.25 uL nuclease-free water for a total reaction volume of 12.5 uL. PCRs were performed on a SimpliAmp™ Thermal Cycler (Applied Biosystems) using an initial denaturation step of 95 °C for 3 min, followed by 25 cycles of 95 °C for 30 s (denaturation), 55 °C for 30 s (annealing), and 72 °C for 30 s (extension), with a final extension step of 72 °C for 5 min. Amplification success and amplicon size were confirmed via gel electrophoresis on a 2% agarose gel. Triplicate amplifications were pooled equally to a total volume of 18 uL and cleaned using the size selective AMPure XP (Beckman Coulter) bead clean-up protocol to remove unwanted DNA sequences at a bead to DNA ratio of 80%.
Library preparation and sequencing
Pooled, cleaned amplification products were indexed for multiplexing with NEBNext® Multiplex Oligos for Illumina® Set A and Set B (New England Biolabs) per manufacturer recommendations via PCR. Reaction conditions were as follows: 12.5 uL KAPA HiFi HotStart ReadyMix (Roche Scientific); 2.5 uL i5 index; 2.5 uL i7 index; 2.5 uL amplified, pooled DNA; and 5 uL nuclease-free water for a total reaction volume of 25 uL. PCRs were performed in an identical manner to the amplicon PCR, with cycles reduced from 25 to 8. Indexed libraries were cleaned a second time using size selective AMPure XP (Beckman Coulter) beads, at a bead to DNA ratio of 80%. Concentration of sequencing competent DNA molecules within indexed libraries was determined prior to normalization, using the NEBNext® Library Quant Kit for Illumina® (New England Biolabs) per manufacturer recommendations, using half-reaction volumes. Individual libraries were normalized to 10nM and pooled into the final multiplexed library at equimolar concentrations. Final pooled library concentration was determined using the NEBNext® Library Quant Kit for Illumina® (New England Biolabs) and the size of the final library was confirmed on an Agilent 2200 TapeStation system using the D1000 High-Sensitivity kit. The final library was denatured and diluted per the Illumina 16 S Metagenomic Sequencing Library Preparation Guide [62] and sequenced on an Illumina MiSeq instrument using the MiSeq Reagent Kit v3 (Illumina), with a 20% PhiX spike as an internal sequencing control, accounting for low library diversity.
Bioinformatic analysis
Sequence filtering, classification, and rarefaction
DNA sequences obtained from the Illumina MiSeq were processed using the microbial pipeline of Quantitative Insights Into Molecular Ecology 2 (QIIME2) v 2022.11 software [63, 64]. Raw sequences were trimmed of primers and adaptors using the cutadapt tool [65] and denoised into Amplicon Sequence Variants (ASVs) using the DADA2 algorithm [66]. We assigned taxonomy to ASVs by training a SILVA 138.1 SSU Ref NR99 full-length database [67] using the “feature-classifier classify-sklearn” QIIME2 plugin [68]. Denoised reads were rarefied to an equal sequencing depth of 6924 reads, based on the lowest depth sample, for beta diversity, relative abundance, and differential abundance analyses. Non-rarefied data was used for alpha diversity, co-occurrence network, and predictive functional analyses, as well as random forest classification. Alpha rarefactions curves were generated in QIIME2 to assess the diversity maintained after rarefaction (Supplementary Fig. 1).
Gut microbial community composition and differential abundance between subspecies
Rarefied and non-rarefied data were imported into R [69] and analyzed using the phyloseq package [70]. Relative abundance of gut microbial community members was plotted at family level classification using the fantaxtic package in R [71]. Differentially abundant taxa were identified independently by three packages in R: ALDEx2 [72], ANCOMBC [73, 74], and MaAsLin2 [75]. Taxa identified as differentially abundant by ALDEx2 and at least one other package were considered to be truly differentially abundant taxa. Typically, an ALDEx2 effect size of 1 is suggested as an arbitrary cutoff to limit false positives [72], however, effect sizes determined here were less than this cutoff across all identified taxa. Instead, in an attempt to mitigate false positives, we analyzed taxa identified as differentially abundant by multiple differential abundance analysis methods [76]. Differentially abundant taxa were filtered to remove results with a corrected p-value above 0.05 and are reported based on their effect size, calculated by ALDEx2.
To assess whether microbial composition could reliably distinguish between plains and wood bison, we conducted a supervised machine learning analysis using a Random Forest classifier. Non-rarefied ASV tables were transformed to relative abundances to normalize for sequencing depth, following recommendations against rarefaction [77]. Data were randomly split into a 70:30 training and test set using stratified sampling to preserve class proportions using the R package caret [78, 79]. A Random Forest classifier (ntree = 1000) was trained on the training set with ASVs as predictors and subspecies as response variables using R package randomForest [80]. Feature importance was calculated using the mean decrease in Gini index, which quantifies how much each taxon contributes to reducing classification error across trees in the random forest model [81], where taxa with higher values are more informative in distinguishing between subspecies. To assess model robustness and tuning, we implemented repeated 10-fold cross-validation (3 repeats) using the “rf” method from the caret package in R [78].
Microbial co-occurrence network analysis and functional prediction via PiCRUSt2
Microbial co-occurrence networks were constructed separately for wood and plains bison to investigate patterns of microbial association within each subspecies. To exclude rare taxa, non-rarefied ASVs were filtered to remove those not present in at least 10% of samples [82]. Taxa correlations were determined using Spearman’s rank correlation via the Hmisc package in R [83]. Pairwise associations with correlation coefficients greater than 0.6 and adjusted p-values below 0.05 were retained, excluding self-correlations and duplicates. We then constructed undirected, weighted networks and calculated network-level statistics using the igraph package in R [84], where nodes represent ASVs and edges represent significant co-occurrence relationships; edges were colored based on positive or negative association. Community structure within each network was detected using the Louvain algorithm for modularity-based clustering. We visualized taxonomy within clusters at the phylum level using igraph [84] and ggplot [85] packages in R.
Subspecific functional differences were inferred using Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PiCRUSt2) [86–91]. Predicted Kyoto Encyclopedia of Genes and Genomes (KEGG) Ortholog (KO) abundances were imported into R and analyzed using the ggpicrust package [92]. Differential pathway abundances were assessed using ALDEx2, with statistical significance evaluated using Wilcoxon’s rank-sum test. Functional categories associated with ‘Human Disease’, ‘Brite Hierarchies’, and ‘Organismal Systems’ were excluded to focus on ecologically relevant pathways. Visualization of differential pathway abundances and log2fold changes was performed using the ggpicrust package [92].
Gut microbial community diversity
Gut microbiome diversity was measured by two metrics: alpha (within-sample) diversity and beta (between-sample) diversity [93]. We assessed alpha diversity between subspecies and sexes through observed ASV richness counts as a measure of richness [94], the Shannon Index as a measure of combined richness and evenness [95], Simpson’s Index as a measure of dominance [96], and Faith’s PD as a measure of phylogenetic diversity [97]. Wilcoxon’s rank-sum test was performed to test the significance of subspecies and sex on alpha diversity. A Principal Coordinate Analysis (PCoA) of the Bray-Curtis dissimilarity was generated to assess beta diversity between subspecies and sexes, with 95% confidence interval ellipses calculated via the ggplot R package [85]. Significant differences in beta diversity between groups was tested via PERMANOVA (9999 permutations); to assess if the assumption of homogeneity of dispersions was met for PERMANOVA, we conducted a PERMDISP (Permutational Analysis of Multivariate Dispersions) using the R package vegan [98].
Results
Individual identification
Genetic profiles with less than 40% missing data were obtained for 70% and 52% of plains and wood bison samples, respectively, identifying 58 plains bison and 44 wood bison as individuals (Supplementary Table 2). Replication in sample collection was uncovered, as multiple samples could not be excluded as originating from the same individual (Supplementary Table 2). For samples originating from the same individual, one was randomly chosen to be included for downstream microbiome analysis.
Sequencing and library results
We obtained a total of 14,102,062 paired reads from our total sequenced library of 183 samples (n = 89 wood bison, n = 85 plains bison, n = 9 Controls), representing individuals identified by genotyping and replicate samples from a random selection of individuals, ranging from 37,430 to 142,520 reads per individual library. 2,734,918 paired end reads (19.4%) were retained post-denoising, ranging from 6,924 to 28,036 reads per individual library (mean reads per sample = 17,768). The low error rates and strict filtering within DADA2 [99] result in high certainty for retained reads, and this level of read loss is typical [25, 100]. Denoising also identified a total of 5,307 Amplicon Sequence Variants (ASVs), ranging from 128 to 581 ASVs per individual library. Data was rarefied to a minimum of 6,924 reads, based on the lowest depth sample. 11 of 12 negative controls retained less than 18 reads per sample post denoising. One PCR negative control had 28,019 reads, however all reads from this control were classified into only 3 ASVs belonging to the microbial genus Escherichia-Shigella, compared to the minimum of 128 ASVs found in fecal samples. Thus, reads within this negative control were likely due to cross-contamination or index-jumping [101], and were removed for downstream analysis. The ZymoBIOMICS Microbial Community Standard was assessed to ensure sequencing accuracy and results were within the expected range for this positive control. Filtering and removal of individual replication resulted in the retention of 54 plains bison individuals (n = 22 males, n = 21 females, n = 11 unknown sex) and 41 wood bison individuals (n = 17 males, n = 18 females, n = 6 unknown sex).
Bison gut microbial community composition
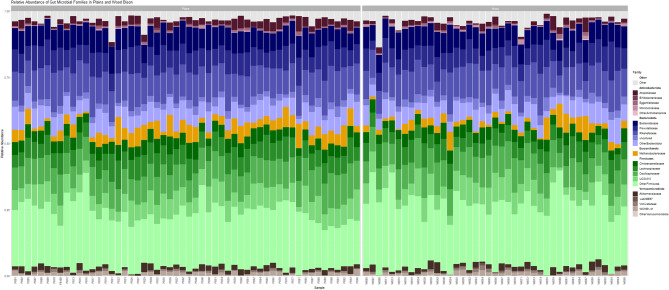
Community composition analysis revealed a high degree of similarity in the gut microbial profiles of plains and wood bison. The gut microbial community of plains and wood bison were dominated by the phyla Firmicutes (51.00% and 51.96%, respectively), followed by Bacteroidetes (35.38% and 34.65%, respectively; Fig. 2). Within the Firmicutes phylum, the most dominant families were Oscillospiraceae, UCG-010, Lachnospiraceae, and Christensenellaceae. Within the Bacteroidetes phylum, highly abundant families were Rikenellaceae, Bacteroidaceae, and Prevotellaceae. Other taxa contributing to greater than 1% of the gut microbial profile included the families Methanobacteriaceae (phylum Euryarchaeota), Atophobiaceae (phylum Actinobacteriota), and Akkermansiaceae (phylum Verrucomicrobiota) (Fig. 2).
Fig. 2.
Relative abundance bar plot of taxa classified by QIIME2 at the family level for plains and wood bison for the top five most abundant phyla. Up to the top 5 taxonomic families within each of the top 5 phyla are represented here, with all others grouped into the category “Other”. Each bar represents the gut microbial profile of an individual bison, with colour corresponding to microbial phylum and shade corresponding to families within their respective phylum
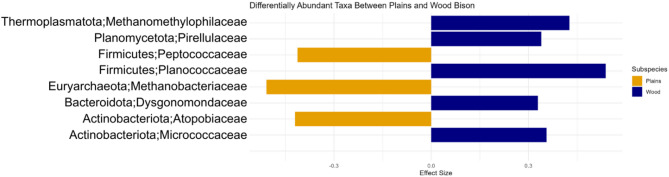
A total of 79 families were identified as being differentially abundant between plains and wood bison gut microbial communities by ALDEx2 and at least one other differential abundance analysis method. All significant taxa were identified by both ALDEx2 and MaAsLin, whereas ANCOM-BC only identified the family Dysgonomondaceae as being significantly different between subspecies. Eight identified families, representing six phyla, were significantly different (p < 0.05, Fig. 3). Families Peptococcaceae (phylum Firmicutes), Methanobacteriaceae (phylum Euryarchaeota), and Atophobiaceae (phylum Actinobacteriota) were increased in plains bison, whereas Methanomethylophilaceae (phylum Thermoplasmatota), Planococcaceae (phylum Firmicutes), Pirellulaceae (phylum Planomycetota), Dysgonomondaceae (phylum Bacteroidetes), and Micrococcacea (phylum Actinobacteriota) were increased in wood bison, based on the effect size reported by ALDEx2 (Fig. 3).
Fig. 3.
Effect size of statistically significant, differentially abundant families within plains and wood bison gut microbial community profiles, as determined by ALDEx2 analysis. A negative effect size indicates an increased abundance in plains bison (yellow), whereas a positive effect size indicates an increased abundance in wood bison (blue). Significance was determined within ALDEx2 by a Benjamini-Hochberg corrected, Wilcoxon rank-sum test p-value of less than 0.05
The Random Forest classifier trained on microbiome relative abundances was able to accurately distinguish between wood and plains bison. The model trained on 70% of the data (n = 29 wood bison, n = 38 plains bison) achieved an overall classification accuracy of 85.7% (95% CI: 67.3–96.0%), with a Kappa of 0.70, indicating substantial agreement beyond chance (Supplementary Table 3). The model demonstrated high accuracy for plains bison (93.8%) and reasonable accuracy for wood bison (75.0%), yielding a balanced accuracy of 84.4% (Supplementary Table 3). Repeated 10-fold cross-validation on the 70% training set further confirmed the optimal model’s (mtry = 87) predictive ability, with a cross-validated accuracy of 93.7% and a Kappa of 0.87 across 30 resampling iterations (Supplementary Table 3). Feature importance analysis identified multiple ASVs as strong predictors of subspecies identity, based on mean decrease in Gini index. The top 20 predictive ASVs were retained for further taxonomic annotation and interpretation (Supplementary Fig. 3).
To investigate subtle differences in gut microbial community structure between wood and plains bison, we performed co-occurrence network analyses on each subspecies separately, using Spearman correlation coefficients to infer significant associations between ASVs (rs > 0.6, p < 0.05; Supplementary Fig. 4). The wood bison network was substantially larger than that of plains bison, including 404 ASVs with 486 significant pairwise associations, whereas the plains bison network included only 173 ASVs with 191 associations (Supplementary Table 4). ASVs engaging in significant associations represented 44.8% and 19.2% of the total input dataset for wood and plains bison co-occurrence networks, respectively. Analysis of unique network members revealed 302 ASVs exclusive to the wood bison network, and 71 ASVs unique to the plains bison network. The plains bison network exhibited slightly higher modularity (0.86 vs. 0.83) and transitivity (0.32 vs. 0.25) than the wood bison gut microbial network (Supplementary Table 4). The largest co-occurrence clusters included 19 ASVs in plains bison (cluster 2) and 40 ASVs in wood bison (cluster 9). Taxonomic profiling of co-occurrence clusters indicated consistent representation of Firmicutes and Bacteroidetes, and to a lesser degree Verrucomicrobiota and Actinobacteriota for both subspecies (Supplementary Fig. 4). The number of co-occurring phyla within each cluster ranged from 1 to 6 for plains bison, and 1 to 8 for wood bison (Supplementary Fig. 4).
Predictive differential functional analysis of plains and wood bison gut microbiota
From our 16S rRNA dataset, 246 KEGG pathways were identified and annotated. At KEGG pathway level 2, the most abundant groups of pathways were associated with metabolism (59.47% and 59.27% for plains and wood bison, respectively), followed by pathways associated with genetic information processing, environmental information processing, and finally cellular processes (Supplementary Table 3). After filtering non-biologically relevant pathways, a total of 27 pathways were significantly different between subspecies (p < 0.05), as determined by ALDEx2 (Fig. 4). Overall, pathways related to cell transport and catabolism, protein processing, translation, carbohydrate metabolism, methane metabolism, glycan production, bile acid production, and terpenoid and polyketide production were significantly enriched in plains bison (Fig. 4). Conversely, pathways associated with cell motility, secondary metabolite metabolism, steroid production, and xenobiotics degradation were enriched in wood bison (Fig. 4).
Fig. 4.
PICRUSt2 predicted KEGG pathways significantly different (p < 0.05) between plains and wood bison. Differential abundance was determined via ALDEx2, with significance tested by a Benjamini-Hochberg corrected, Wilcoxon rank-sum test. Negative log2fold changes indicate enrichment in the wood bison gut microbial community, whereas positive log2fold changes indicate enrichment in the plains bison gut microbial community
Diversity in the plains and wood bison gut microbial communities
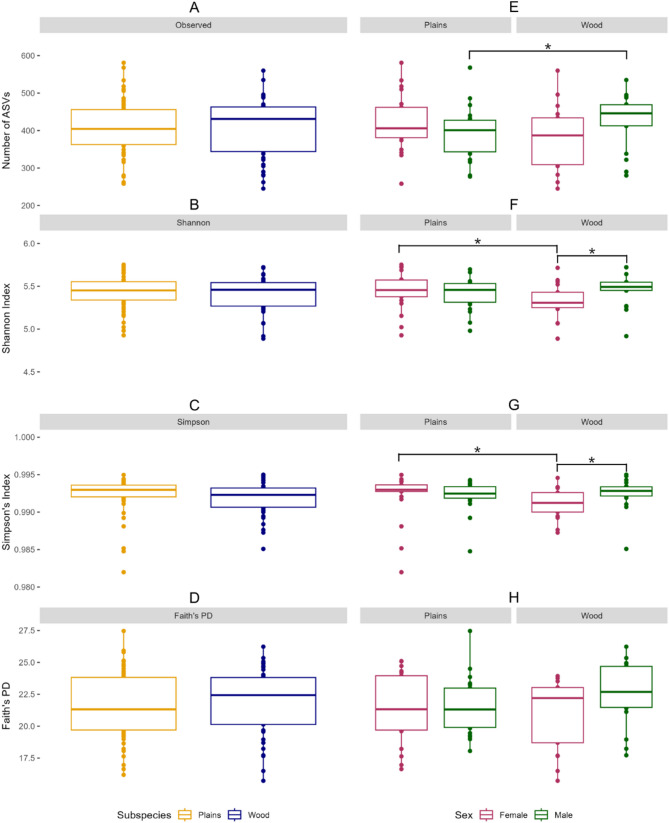
Alpha diversity assessed using observed ASV richness, the Shannon index, Simpson’s index, and Faith’s PD, did not differ significantly between subspecies when all individuals were analyzed together (p > 0.05; Fig. 5A-D). When stratified by sex, significant subspecific differences in observed ASV richness were found between male plains and wood bison (p < 0.05, Fig. 5E), while the Shannon index, Simpson’s index, and Faith’s PD remained non-significant (p > 0.05, Fig. 5F-H). Among females, the Shannon and Simpson’s indices differed significantly between subspecies (p < 0.05, Fig. 5F & G), whereas observed ASV richness and Faith’s PD did not (p > 0.05, Fig. 5E & H). Within wood bison, males and females differed significantly in the Shannon and Simpson’s indices (p < 0.05, Fig. 5C-D), but not in observed ASV richness or Faith’s PD (p > 0.05, Fig. 5E & H). No significant sex-based differences were observed among plains bison across any alpha diversity metric (p > 0.05, Fig. 5E-H).
Fig. 5.
Alpha diversity assessment of the gut microbial community of plains and wood bison in EINP. (A) Observed ASV richness for plains and wood bison, (B) Shannon Diversity Index for plains and wood bison, (C) Simpson’s Diversity Index for plains and wood bison, (D) Faith’s PD for plains and wood bison, (E) Observed ASV richness for female and male bison of both subspecies, (F) Shannon Diversity Index for female and male bison of both subspecies, (G) Simpson’s Diversity Index for female and male bison of both subspecies, (H) Faith’s PD for female and male bison of both subspecies. Boxplots A-D were generated using the complete dataset of males, females, and individuals of unknown sex for both subspecies. Boxplots E-H were generated using a subset of the data, with individuals of unknown sex removed from the analysis; boxplots have been visually split to highlight differences between subspecies. Asterisks above boxplots indicate statistically significant differences (p < 0.05) as determined by the Wilcoxon rank-sum test. Lines connecting boxplots denote the specific comparisons where significance was observed
The PCoA of the Bray-Curtis distance, with group centroids and 95% confidence ellipses, showed strong overlap in clustering between subspecies, with plains bison clustering more tightly than wood bison (Fig. 6). A PERMANOVA test revealed that the effect of subspecies on the gut microbial community was significant (p = 0.0001). However, the effect of subspecies classification explains only a small portion of variation (R2 = 0.04185). A PERMDISP test indicated that dispersions differed significantly between subspecies (F = 8.35, p = 0.005), suggesting a violation of the PERMANOVA assumption of homogeneity of dispersions. Visual inspection of boxplots measuring dispersion within each subspecies confirmed dispersion within the gut microbial community of wood bison was greater than plains bison (Supplementary Fig. 2). While the PERMANOVA revealed significant differences in microbial community composition between subspecies, significant PERMDISP results indicate that these differences may be influenced, at least in part, by differences in within-group variability rather than true shifts in microbial communities. Sex was found to have a statistically significant effect on gut microbiota beta diversity for plains bison (R2 = 0.03, p < 0.05), but not for wood bison (R2 = 0.002, p > 0.05). PERMDISP analysis indicated that dispersions did not differ significantly between sexes for either subspecies (plains F = 1.0368, p = 0.3188; wood F: 0.002, p = 0.9755), thus differences between sexes are unlikely due to differences in dispersion.
Fig. 6.
Beta diversity assessment of the gut microbial communities of plains and wood bison in EINP. (A) Bray-Curtis Dissimilarity comparing plains and wood bison, (B) Bray-Curtis Dissimilarity comparing male and female individuals of both subspecies, visually split to highlight differences between subspecies. Ellipses represent 95% confidence intervals. Significance was tested via PERMANOVA, with assumptions checked via PERMDISP. Subspecies was found to have a significant effect on beta diversity (R2 = 0.04, p < 0.05), however the PERMDISP was also significant (F = 8.35, p 0.05); perceived differences between subspecies could be due to within subspecies dispersion as opposed to compositional differences in the gut microbial community. The PERMDISP results were not significant between sexes of either subspecies (p > 0.05); sex differences differed significantly between male and female plains bison (R2 = 0.03, p < 0.05), but not between male and female wood bison (R2 = 0.002, p > 0.05)
Discussion
The gut microbiome demonstrates phylosymbiosis, where host phylogeny accounts for upwards of 30% of the total variation in associated gut microbial communities [102]. Gut microbiota also shift in accordance with environmental change, producing key physiological signals that allow hosts to exploit altered ecological niches and persist in different environments [12]. Thus, overlapping diets and niches can result in gut microbiome convergence [103, 104], whereas differing life histories and genetics between species can result in divergence [105]. Comparing the gut microbiota of closely-related, sympatric organisms provides an opportunity to understand the combined influence of host genetics and environment on host-microbiome interactions. Herein, we compare the gut microbiota of both North American bison subspecies—plains bison and wood bison—occupying the sympatric environment of Elk Island National Park (EINP). Gut microbiota of both subspecies was found to be typical of a healthy ruminant [14, 27]. In agreement with our initial hypothesis, we found limited differences in overall diversity and composition of gut microbiota between subspecies, however, we observed significant differences in differential abundance of specific microbial taxa and functional pathways. It should be noted that this study represents a temporal snapshot of gut microbial community composition and diversity for both subspecies, where differences between subspecies may be more or less pronounced in different seasons or years.
Passive fecal collection results in individual replication
In the present study, we generated individual host genetic profiles from fecal mucosal swabs. We identified replication of individuals among fecal samples, with one individual representing 5% samples within subspecies, highlighting the importance of individualizing samples prior to fecal microbiome analysis. Unaccounted replication of an individual (e.g., comprising 5% of samples) could skew population-level gut microbial profiles. That said, a considerable proportion of the fecal swabs analyzed in this study produced insufficient DNA from mucosal swabs to obtain genetic profiles for individualization, and thus were not considered for subsequent fecal microbiome analysis. Herein lies the trade-off regarding individual identification from passive fecal collections: genotyping mitigates potential replication in the dataset and allows for repeated sampling of the same individual over time, but may reduce the number of samples available for subsequent microbiome analysis if no genetic profile can be obtained. There is no clear answer in the literature regarding whether it is best to replicate individuals, given the potentially high levels of individual variation in the gut microbiome, or put resources towards including more individuals in a given study [106]. It should be noted that unaccounted overrepresentation of a single individual in a dataset is inherently different than the inclusion of technical replicates in a microbiome study, which can aid in mitigating DNA extraction, PCR, and sequencing biases [107, 108]. Previous studies have either not considered the possibility of individual replication in the dataset (e.g., [22, 27, 109]) or have attempted to individualize samples through other methods, such as direct observation (e.g., [100]) or direct animal handling (e.g., [26, 53, 110, 111]), with only a minor proportion utilizing STR genotyping from fecal samples (e.g., [21, 112, 113]). That said, the addition of genotyping to the project represents a significant economic consideration, given the time and materials that go into this additional set of analyses [54]. Further optimization of swab collections from samples with and without obvious mucosal coat across seasons is likely to enhance profiling success. Future studies should consider how unaccounted replication of individuals in a microbiome dataset alters the community level microbiome profile to determine if genotyping prior to microbiome analysis should be standard practice.
Limited differences exist in overall gut microbiome composition and diversity between wood and plains bison, regardless of sex
The core ruminant gut microbiome is dominated by families Prevotellaceae and Bacteroidales, of phylum Bacteroidetes, and families Butyrivibrionaceae, Ruminococcus, Lachnospiraceae, Ruminococcaceae, and Clostridiales of phylum Firmicutes [14]. Like other ruminants, we found that the most abundant phyla in plains and wood bison gut microbial communities were Firmicutes (51.00% and 51.96% abundance, respectively) and Bacteroidetes (35.38% and 34.65% abundance, respectively). These results showed a slight decrease in the Firmicutes to Bacteroidetes ratio for plains bison, with similar findings to Bergmann et al. [52], where gut microbial communities of plains bison in Kansas, United States, during the growing season (April-September) were dominated by Firmicutes and Bacteroidetes phyla, representing 53% and 33% of the gut microbial community, respectively. Our finding of prevalent Bacteroidetes within the wood bison gut microbial community, however, contrasts with Weese et al. [53], who analyzed gut microbiota of 40 wood bison in EINP and found phyla Firmicutes, Proteobacteria, and Actinobacteria most abundant, with Bacteroidetes representing only a small proportion of the gut microbial community (< 1% overall). Weese et al.’s [53] finding somewhat contradicts the observed prevalence of Bacteroidetes in other ruminants, including browsers such as North American moose [114] and grazers such as cattle [115], combined with the known importance of Bacteroidetes in cellulose and protein metabolism [116]. Further, Weese et al. [53] found two enterotypes independent of sex, age, and housing, within the EINP wood bison population, a finding that was not replicated herein.
While compositional analyses revealed limited differences in gut microbiota of wood and plains bison, Random Forest classification identified a subset of microbial taxa that consistently differentiate bison subspecies. A classifier trained on Amplicon Sequence Variant (ASV) level profiles achieved 93% cross-validated accuracy with strong agreement beyond chance (Kappa = 0.87), highlighting the discriminatory power of specific taxa despite overall community similarity. This is consistent with growing evidence that host-associated microbial communities may harbour fine-scale taxonomic signals not readily captured by conventional analyses, such as diversity metrics [117]. In wildlife systems, subtle microbial shifts can reflect underlying host ecology, behavior, genetics, or environmental conditions [118]. While predictive performance of the model is strong, we acknowledge that functional roles of identified taxa remain to be investigated and future metagenomics studies are necessary to elucidate whether taxonomic differences reflect ecological adaptation or dietary differences between subspecies.
Although overall microbial composition appeared broadly similar between wood and plains bison, co-occurrence network analysis revealed differences in microbial interaction structure. The wood bison network was larger and clusters were more taxonomically diverse, with over four times as many unique ASVs as the plains bison network. Moreover, the clustering coefficient was moderately higher in plains bison compared to wood bison; taken together these results could indicate that wood bison gut microbiota are more resilient to disturbances [119]. However, modularity of networks was similar between subspecies and the average number of associations between co-occurring ASVs was only slightly higher in wood bison, indicating a similar level of partitioning within gut microbiota of both subspecies [119]. Taxonomic analysis of clusters revealed that core phyla Firmicutes and Bacteroidetes consistently co-occurred in both subspecies, both of which play key roles in dietary energy acquisition [14]. Actinobacteria and Verrucomicrobia, known for their important roles in gut homeostasis despite general low abundance compared to other major phyla [120, 121], also co-occurred to a lesser degree.
We found no significant differences in the overall richness, evenness, dominance, or phylogenetic breadth of the gut microbial community alpha diversity between wood and plains bison when all individuals were analyzed together. However, when sex was considered, significant differences arose dependent on the alpha diversity metric employed. Among males, observed ASV richness was significantly higher in wood bison compared to plains bison, suggesting a greater number of unique, low abundance taxa in wood bison gut microbial communities. In contrast, no significant subspecific differences were observed between males with Shannon’s index, Simpson’s index, or Faith’s PD, indicating similar community structure, dominance patterns, and evolutionary relatedness, suggesting that differences in observed richness may not be biologically meaningful. Among females, observed ASV richness and Faith’s PD did not differ significantly between subspecies, indicating a similar number and phylogenetic breadth of taxa. However, female plains bison exhibited significantly higher Shannon’s and Simpson’s indices, suggesting a more even distribution of microbial abundances dominated by fewer taxa compared to female wood bison. Thus, alpha diversity differences between females of both subspecies could be due to restructuring of gut microbial communities, rather than addition or loss of taxa. Environmental differences between subspecies influence gut microbiota composition and diversity, potentially confounding subspecies level gut microbial divergence [122, 123]. Similar environmental conditions are known drive alpha diversity convergence even among distantly related species [124, 125]. The gut microbiome of sympatric ruminants is expected to be more similar than that of allopatric ruminants, due to shared available forage and environmental conditions [126, 127]. Along these lines, the EINP system provides opportunity to explore phylogenetic differences while controlling for environmental variation between subspecies. In the absence of environmental differences, it is likely that dietary differences between subspecies [44] play a role in the subtle gut microbial community diversity differences observed herein.
Subspecies classification appeared to be a significant factor influencing beta diversity, as samples clustered by subspecies in the PCoA of Bray-Curtis dissimilarity. However, it should be noted that subspecies classification contributed only a minor proportion of variance, reflected by a low R2 value. Furthermore, a PERMDISP test revealed significant differences in dispersion between subspecies, suggesting within-subspecies heterogeneity may have influenced PERMANOVA results. While there may be an evolutionary influence on overall patterns of gut microbial community diversity, other factors beyond subspecies classification could contribute to subspecific divergence, and caution is warranted in interpreting this effect as purely due to differences in community composition. Similar experiments in other subspecies systems, such as Mus musculus musculus and M. m. domesticus have found subspecific classifications are a significant contributing factor regarding gut microbial community composition [128]. That said, the two subspecies of house mouse diverged allopatrically approximately 500,000 years ago, whereas bison have only recently diverged approximately 5,000 years ago [40], so it is possible that insufficient evolutionary time has passed for gut microbial divergence to occur between the two subspecies of bison [129]. On the other hand, bison have also experienced over a century of translocations, cattle introgression, and hybridization [36, 48], likely undermining historical gut microbiome differences between subspecies.
We did not find significant differences in alpha or beta diversity between sexes in overall bison gut microbial communities when both subspecies were considered together (p > 0.05). Our sample collection does not coincide with rutting or calving seasons, periods during which the gut microbiome might be particularly divergent between sexes, potentially contributing to the lack of variation observed. That said, when gut microbial community profiles were separated by subspecies or sex classification, significant patterns emerged dependent on the diversity metric used. No significant differences between plains bison males and females were found with any diversity metric employed herein, indicating that both sexes harbour a similar number and abundances of species within their gut microbial communities. Conversely, both the Shannon and Simpson’s indices were significantly different between male and female wood bison, suggesting sexes harbour similar phylogenetic diversity, however their gut microbial community compositions are structured differently. Inconsistencies regarding the significance of sex depending on the alpha diversity metric used has also been observed in other species, such as red deer [113]. A similar trend was uncovered for beta diversity, where sex was a significantly contributing factor for plains bison (R2 = 0.03, p < 0.05), but not for wood bison (R2 = 0.002, p > 0.05). While sex is generally considered a significant contributor to gut microbiota composition and diversity, largely due to specific sex hormones at different life stages and during temporal cycles [130], the influence of sex on the gut microbial profile of ruminants in the literature shows contrasting results. For example, the Firmicutes to Bacteroidetes ratio in gut microbiota of cattle may be higher in females compared to males, leading to increased female body fat [131]. Conversely, the Firmicutes to Bacteroidetes ratio is increased in adult Tibetan sheep males, as compared to females at the end of the rutting season [132]. In some species, including Tibetan goats [133], sex has no significant impact on alpha diversity. Given this, the influence of sex on gut microbial communities of bison subspecies warrants further investigation. Future metagenomic and metabolomic studies during various seasons may provide insights into the effect of sex hormones and sex specific physiological processes on the gut microbiota.
Gut microbiome divergence between wood and plains bison results from differential abundance of specific taxa
While alpha and beta diversity metrics revealed limited differences in gut microbial communities of plains and wood bison, several taxa were differentially abundant. Reduced abundance of both Peptococcaceae and Pirellulaceae has been linked to increased dietary starch content in dairy cows [134]. Consequently, observed increases in Peptococcaceae abundance in plains bison and Pirellulaceae in wood bison may reflect changes in starch intake for both subspecies during seasonal transitions from fall to winter, although direct dietary starch content data are lacking. Although Peptococcaceae is more abundant in plains bison and Pirellulaceae in wood bison, these taxa may share overlapping roles in dietary carbohydrate metabolism, potentially contributing to microbial functional redundancy that supports microbiome stability during ecological disturbances [135]. Our sample collection period coincides with the end of the growing season and cusp of the non-growing season; bison in EINP are known to undergo dietary shifts reflective of available forage during this time, as forbs and browse species die back, leaving graminoids as the only available biomass [44]. Furthermore, increased abundance of Peptococcaceae has been associated with improved food and energy utilization in ruminants, particularly in the production of butyrate from cellulose [136], and has been positively linked to fatty acid transport and energy production [137]. The family Atopobiaceae, recognized as producers of vital short-chain fatty acids including lactate, acetate, and butyrate, and as a potential probiotic [138], was also significantly increased in the plains bison gut microbiome. Along these lines, PiCRUSt analysis revealed that glycan biosynthesis, carbohydrate metabolism, and polyketide biosynthesis pathways were enriched in plains bison gut microbial communities. While causality cannot be inferred from these observations, increased abundance of taxa associated with efficient energy production, alongside enriched metabolic pathways, in plains bison gut microbiota may reflect differences in microbial functional potential that are better suited to seasonal dietary landscapes of EINP. In contrast, wood bison may be best adapted to their more northern historic range, including to winter foraging conditions and behaviours, as evidenced by increased abundance of Micrococcaceae, described as tolerant to cold climates [22].
Given the influence of the gut microbiota on dietary energy acquisition and potential divergent local adaptation between subspecies, it is possible that differences in gut microbial community composition are due to dietary preferences between subspecies related to their respective historic ranges. Previous studies have found that allopatric plains and wood bison populations have different diets, where wood bison consume more browse than plains bison [139]. However, these studies have examined bison diet across a wide geographic range, where dietary divergence could be due to differing available forage or other disparate environmental factors. Hecker et al. (2025) have explored dietary differences between subspecies within EINP, and found that wood and plains bison employ different, seasonally dependent diet strategies, likely associated with their evolved ranges. While diets between subspecies were more similar in fall and winter, compared to summer and spring, long-term ecological divergence may contribute to distinct foraging behaviors that persist even in shared environments [44]. Future metagenomic and metatranscriptomic studies should investigate differentially expressed genes within gut microbiota of both wood and plains bison to determine whether these subspecies exhibit similar functional responses to environmental conditions and dietary changes.
Plains bison shared much of their historic range with cattle, extending across the Great Plains of the United States and southern Canadian prairies. Overlapping habitats presumably exposed plains bison and cattle to similar forage, as compared to wood bison populations farther north. Cattle and bison have previously been shown to produce similar levels of methane when fed the same diet [140], likely resulting from historic close contact and ecological similarities between these species. We found significantly increased Methanobacteriaceae within gut microbiota of plains bison compared to wood bison, in fact an influential ASV distinguishing the two subspecies by random forest was classified as Methanobacteriaceae (Supplementary Fig. 3), alluding to differential methane production between subspecies. Predictive functional analysis supports this interpretation, as methane metabolism pathways were significantly enriched in plains bison gut microbial communities. Furthermore, wood bison seem to exhibit reduced methane production compared to plains bison, due to significantly increased abundance of Methanomethylophilaceae, a common rumen archaea linked to reduced methane emissions [141, 142]. Methane production has been associated with a 12% energy loss from forage in cattle [143], and thus is energetically taxing on the host organism. Decreased methane production in wood bison could reflect historical environmental pressures given their historic northern range, where energy loss due to methane production could adversely impact fitness in colder seasons. However, differential methane production and associated fitness advantages between subspecies remains speculative until future studies can quantify methane production for both subspecies in context of diet and gut microbiomes.
Increased abundance of the families Planococcaceae and Dysgonomonadaceae in wood bison gut microbial communities may reflect a legacy of historical disease exposure or microbiome disruption. Elevated levels of Planococcaceae have been associated with Mycobacterium avium paratuberculosis infection in cattle [144–146], while members of Dysgonomonadaceae family, including Dysgonomonas, have been recognized as opportunistic pathogens [147]. Wood Buffalo National Park wood bison—the vaccinated offspring of which served as the founders of the EINP wood bison population [40]—are endemically infected with bovine tuberculosis and brucellosis [36] and are susceptible to anthrax infection [148]. Thus, it is plausible that the increased abundance of Planococcaceae and Dysgonomonas may reflect a history of host-pathogen interactions. Notably, a key ASV identified by random forest as distinguishing the two subspecies was classified within Dysgonomonadaceae (Supplementary Fig. 3), further supporting the relevance of this taxon. While long-term impacts of disease events on bison gut microbiota remain unknown, observed microbial abundances could represent a lingering ecological imprint of earlier dysbiosis. Future studies might compare gut microbiota of EINP wood and plains bison to other populations, specifically to their ancestral population in Wood Buffalo National Park, to determine whether active disease alters the gut microbial profile. An alternative, but not mutually exclusive, explanation is that increased abundance of Planococcaceae and Dysgonomonadaceae may contribute to xenobiotic degradation. Notably, members of these families, including Psychrobacillus (Planococcaceae) and Dysgonomonas (Dysgonomonadaceae), have previously been associated with degradation of various xenobiotic compounds [149, 150]. Although overall relative abundance of xenobiotic degradation pathways was low, several were significantly enriched in wood bison gut microbial communities as compared to plains bison. Enrichment may be linked to increased prevalence of these taxa and could represent a microbial response to environmental exposure. The Trans-Canada Highway—a heavily trafficked four lane roadway—bisects the northern and southern blocks of EINP, where both subspecies are frequently observed foraging along the park-highway boundary. Due to the relatively smaller North-South range of wood bison, a greater proportion of their habitat is adjacent to this highway compared to that of plains bison. This increased proximity raises the possibility of greater environmental exposure to anthropogenic pollutants, such as polycyclic aromatic hydrocarbons and ethylbenzene, both of which are associated with vehicular emissions [151] and have corresponding degradation pathways enriched in the wood bison gut microbiota. As these hypotheses remain speculative in the absence of direct environmental exposure data, future metagenomic and metatranscriptomic analyses are needed to validate the functional potential of wood bison gut microbiota to understand how anthropogenic pressures on bison habitat shape gut microbial communities.
Conclusions
The present study provides valuable insights into gut microbiome analysis of wood and plains bison, emphasizing the challenges and significance of understanding gut microbial divergence between subspecies. We highlight the importance of individual identification to prevent overrepresentation of single individuals and accurately capture microbial diversity, and recommend individual identification prior to gut microbiome analysis for studies employing passive fecal collection. Overall, we found limited differences between subspecies in terms of gut microbial community composition and diversity, with some variation in alpha diversity arising between subspecies and sexes, depending on the metric employed. Beta diversity analysis revealed that while samples cluster closely within subspecies classification, the degree of divergence remains minimal, and may be due to dispersion within subspecies. Most notably, differential abundance of specific taxa and functional pathways may provide insight into the physiological and behavioral processes unique to each subspecies. Furthermore, our random forest model accurately classified subspecies based on ASV-level gut microbial community profiles, suggesting signatures of evolutionary divergence. Our findings provide a baseline for gut microbiota of both subspecies, and will help to inform conservation and management decisions around reintroducing bison to their historic habitats by informing founder selection. Future research should compare the gut microbial profile of bison populations across Canada to better understand environmental influences on gut microbiota for these subspecies. Future metagenomics studies to investigate differentially expressed genes between subspecies and populations may help to better comprehend evolutionary divergence in behavioural preferences and host-environment interactions; questions beyond the capabilities of predictive functional analyses employed herein. Such investigations, building on the baseline provided here, will deepen our understanding of the gut microbiome’s role in the health and ecology of bison and contribute to conservation and management efforts in the face of changing ecosystems.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to acknowledge that Elk Island National Park is located on the traditional territory of the Treaty 6 First Nations and the Métis Nation. We would like to thank Ashley Friedenberger for her assistance in sample collection, and Clayton Szafron and Jonathan DeMoor for their assistance in navigating the park and coordinating our research permit.
Author contributions
MLG: conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing – original draft, writing – review and editing, visualization. RMP: Supervision, software, writing – review and editing, visualization, funding acquisition. TMB: Investigation, writing – review and editing. GAW: Resources, writing – editing and review. CJK: conceptualization, resources, writing – review and editing, supervision, project administration, funding acquisition.
Funding
This work was supported by a Natural Sciences and Engineering Research Council discovery grant (NSERC; RGPIN-2023-04503 to C.J.K) and the Government of Canada through the Genomics Research and Development Initiative – Genomic Adaption and Resilience to Climate Change (GenARCC) project (2022-2027).
Data availability
The data that support the findings of this study have been submitted to the NCBI Sequence Read Archive (SRA) under BioProject number PRJNA1217016.
Declarations
Ethics approval and consent to participate
The authors of this study confirm that we have adhered to all prevailing local, national and international regulations and conventions, and have followed normal ethical scientific practices throughout the study. Sampling was performed under Parks Canada Research and Collection Permit EINP-2023-45630.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The Human Microbiome Project. Nat. 2007;449(7164):804–10. 10.1038/nature06244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McFall-Ngai M, Hadfield MG, Bosch TCG, Carey HV, Domazet-Lošo T, Douglas AE, et al. Animals in a bacterial world, a new imperative for the life sciences. Proc Natl Acad Sci. 2013;110(9):3229–36. 10.1073/pnas.1218525110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shreiner AB, Kao JY, Young VB. The gut microbiome in health and in disease. Curr Opin Gastroenterol. 2015;31(1):69–75. 10.1097/MOG.0000000000000139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cholewińska P, Górniak W, Wojnarowski K. Impact of selected environmental factors on microbiome of the digestive tract of ruminants. BMC Vet Res. 2021;17(1):25. 10.1186/s12917-021-02742-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He Y, Maltecca C, Tiezzi F. Potential use of gut microbiota composition as a biomarker of heat stress in monogastric species: a review. Animals. 2021;11(6):1833. 10.3390/ani11061833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rupasinghe R, Chomel BB, Martínez-López B. Climate change and zoonoses: a review of the current status, knowledge gaps, and future trends. Acta Trop. 2022;226:106225. 10.1016/j.actatropica.2021.106225 [DOI] [PubMed] [Google Scholar]
- 7.Candela M, Biagi E, Maccaferri S, Turroni S, Brigidi P. Intestinal microbiota is a plastic factor responding to environmental changes. Trends Microbiol. 2012;20(8):385–91. 10.1016/j.tim.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 8.Intergovernmental Panel On Climate Change (Ipcc). Climate change 2021 – the physical science basis: working group I contribution to the sixth assessment report of the intergovernmental panel on climate change. 1st ed. Cambridge University Press; 2023.
- 9.Martin JM, Mead JI, Barboza PS. Bison body size and climate change. Ecol Evol. 2018;8(9):4564–74. 10.1002/ece3.4019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMillan NA, Fuhlendorf SD, Luttbeg B, Goodman LE, Davis CA, Allred BW, et al. Bison movements change with weather: implications for their continued conservation in the anthropocene. Ecol Evol. 2022;12(12):e9586. 10.1002/ece3.9586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voolstra CR, Ziegler M. Adapting with microbial help: microbiome flexibility facilitates rapid responses to environmental change. BioEssays. 2020;42(7):2000004. 10.1002/bies.202000004 [DOI] [PubMed] [Google Scholar]
- 12.Moeller AH, Sanders JG. Roles of the gut microbiota in the adaptive evolution of mammalian species. Philos Trans R Soc B Biol Sci. 2020;375(1808):20190597. 10.1098/rstb.2019.0597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frankham R. Conservation genetics. Encyclopedia of ecology. Elsevier; 2019. pp. 382–90.
- 14.Henderson G, Cox F, Ganesh S, Jonker A, Young W, Global Rumen Census Collaborators. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci Rep. 2015;5(1):14567. 10.1038/srep14567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andoh A. Physiological role of gut microbiota for maintaining human health. Digestion. 2016;93(3):176–81. 10.1159/000444066 [DOI] [PubMed] [Google Scholar]
- 16.Clemmons BA, Voy BH, Myer PR. Altering the gut microbiome of cattle: considerations of host-microbiome interactions for persistent microbiome manipulation. Microb Ecol. 2019;77(2):523–36. 10.1007/s00248-018-1234-9 [DOI] [PubMed] [Google Scholar]
- 17.Hills R, Pontefract B, Mishcon H, Black C, Sutton S, Theberge C. Gut microbiome: profound implications for diet and disease. Nutrients. 2019;11(7):1613. 10.3390/nu11071613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ricci S, Pacífico C, Castillo-Lopez E, Rivera-Chacon R, Schwartz-Zimmermann HE, Reisinger N, et al. Progressive microbial adaptation of the bovine rumen and hindgut in response to a step-wise increase in dietary starch and the influence of phytogenic supplementation. Front Microbiol. 2022;13:920427. 10.3389/fmicb.2022.920427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Creevey CJ, Kelly WJ, Henderson G, Leahy SC. Determining the culturability of the rumen bacterial microbiome. Microb Biotechnol. 2014;7(5):467–79. 10.1111/1751-7915.12141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mobashar M, Hummel J, Blank R, Südekum K. Contribution of different rumen microbial groups to gas, short-chain fatty acid and ammonium production from different diets—an approach in an in vitro fermentation system. J Anim Physiol Anim Nutr. 2019;103(1):17–28. 10.1111/jpn.12996 [DOI] [PubMed] [Google Scholar]
- 21.Bird S, Prewer E, Kutz S, Leclerc L, Vilaça ST, Kyle CJ. Geography, seasonality, and host-associated population structure influence the fecal microbiome of a genetically depauparate Arctic mammal. Ecol Evol. 2019;9(23):13202–17. 10.1002/ece3.5768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guan T-P, Teng JLL, Fong JYH, Lau SKP, Woo PCY. Seasonal shift in gut microbiome diversity in wild Sichuan takin (Budorcas tibetanus) and environmental adaptation. Comput Struct Biotechnol J. 2023;21:1283–91. 10.1016/j.csbj.2022.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu Z, Sun Y, Zhu F, Liu Z, Pan R, Teng L, et al. Seasonal variation and sexual dimorphism of the microbiota in wild blue sheep (Pseudois nayaur). Front Microbiol. 2020;11:1260. 10.3389/fmicb.2020.01260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu W, Wang Q, Song J, Xin J, Zhang S, Lei Y, et al. Comparison of gut microbiota of yaks from different geographical regions. Front Microbiol. 2021;12:666940. 10.3389/fmicb.2021.666940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf JF, Kriss KD, MacAulay KM, Munro K, Patterson BR, Shafer ABA. Gut microbiome composition predicts summer core range size in two divergent ungulates. FEMS Microbiol Ecol. 2021;97(5):fiab048. 10.1093/femsec/fiab048 [DOI] [PubMed] [Google Scholar]
- 26.Couch CE, Stagaman K, Spaan RS, Combrink HJ, Sharpton TJ, Beechler BR, et al. Diet and gut microbiome enterotype are associated at the population level in African Buffalo. Nat Commun. 2021;12(1):2267. 10.1038/s41467-021-22510-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Jonge N, Carlsen B, Christensen MH, Pertoldi C, Nielsen JL. The gut microbiome of 54 mammalian species. Front Microbiol. 2022;13:886252. 10.3389/fmicb.2022.886252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Víquez-R L, Henrich M, Riegel V, Bader M, Wilhelm K, Heurich M, et al. A taste of wilderness: supplementary feeding of red deer (Cervus elaphus) increases individual bacterial microbiota diversity but lowers abundance of important gut symbionts. Anim Microbiome. 2024;6(1):28. 10.1186/s42523-024-00315-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng P, Gao W, Cong S, Leng L, Wang T, Shi L. High-energy supplemental feeding shifts gut microbiota composition and function in red deer (Cervus elaphus). Animals. 2024;14(10):1428. 10.3390/ani14101428 [DOI] [PMC free article] [PubMed]
- 30.McKenzie VJ, Song SJ, Delsuc F, Prest TL, Oliverio AM, Korpita TM, et al. The effects of captivity on the mammalian gut microbiome. Integr Comp Biol. 2017;57(4):690–704. 10.1093/icb/icx090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metcalf JL, Song SJ, Morton JT, Weiss S, Seguin-Orlando A, Joly F, et al. Evaluating the impact of domestication and captivity on the horse gut microbiome. Sci Rep. 2017;7(1):15497. 10.1038/s41598-017-15375-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shamon H, Cosby OG, Andersen CL, Augare H, BearCub Stiffarm J, Bresnan CE, et al. The potential of bison restoration as an ecological approach to future tribal food sovereignty on the Northern great plains. Front Ecol Evol. 2022;10:826282. 10.3389/fevo.2022.826282 [Google Scholar]
- 33.Bork AM, Strobeck CM, Yeh FC, Hudson RJ, Salmon RK. Genetic relationship of wood and plains bison based on restriction fragment length polymorphisms. Can J Zool. 1991;69(1):43–8. 10.1139/z91-007 [Google Scholar]
- 34.Peden DG, Kraay GJ. Comparison of blood characteristics in plains bison, wood bison, and their hybrids. Can J Zool. 1979;57(9):1778–84. 10.1139/z79-231 [DOI] [PubMed] [Google Scholar]
- 35.Van Zyll CG, Gates C, Reynolds H, Olson W. Phenotypic variation in remnant populations of North American bison. J Mammal. 1995;76(2):391–405. 10.2307/1382350 [Google Scholar]
- 36.Ball MC, Fulton TL, Wilson GA. Genetic analyses of wild bison in Alberta, Canada: implications for recovery and disease management. J Mammal. 2016;97(6):1525–34. 10.1093/jmammal/gyw110 [Google Scholar]
- 37.Freese CH, Aune KE, Boyd DP, Derr JN, Forrest SC, Cormack Gates C, et al. Second chance for the plains bison. Biol Conserv. 2007;136(2):175–84. 10.1016/j.biocon.2006.11.019 [Google Scholar]
- 38.McNew LB, Dahlgren DK, Beck JL, editors. Rangeland wildlife ecology and conservation. Cham: Springer International Publishing; 2023. [Google Scholar]
- 39.Soper JD. History, range, and home life of the Northern bison. Ecol Monogr. 1941;11(4):347–412. 10.2307/1943298 [Google Scholar]
- 40.COSEWIC. COSEWIC assessment and status report on the plains bison (Bison Bison Bison) and the wood bison (Bison Bison athabascae) in Canada. Ottawa: Canadian Wildlife Service = Service canadien de la faune; 2013. [Google Scholar]
- 41.Stroupe S, Forgacs D, Harris A, Derr JN, Davis BW. Genomic evaluation of hybridization in historic and modern North American bison (Bison bison). Sci Rep. 2022;12(1):6397. 10.1038/s41598-022-09828-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson GA, Strobeck C. Genetic variation within and relatedness among wood and plains bison populations. Genome. 1999;42(3):483–96. 10.1139/g98-147 [PubMed] [Google Scholar]
- 43.Markewicz L. Like distant thunder. Gatineau, QC, CA: Parks Canada; 2017. [Google Scholar]
- 44.Hecker LJ, DeMoor J, Found R. Comparison of concurrent plains and wood bison diets at Elk Island National Park. J Wildl Manag. 2025;89(4):e70015. 10.1002/jwmg.70015 [Google Scholar]
- 45.Found R, DeMoor J. Development of Bison handling practices in elk Island National park. Stock J. 2021;7:80–97. [Google Scholar]
- 46.Cherry SG, Merkle JA, Sigaud M, Fortin D, Wilson GA. Managing genetic diversity and extinction risk for a rare plains bison (Bison Bison Bison) population. Environ Manage. 2019;64(5):553–63. 10.1007/s00267-019-01206-2 [DOI] [PubMed] [Google Scholar]
- 47.Cronin MA, MacNeil MD, Vu N, Leesburg V, Blackburn HD, Derr JN. Genetic variation and differentiation of bison (Bison bison) subspecies and cattle (Bos taurus) breeds and subspecies. J Hered. 2013;104(4):500–9. 10.1093/jhered/est030 [DOI] [PubMed] [Google Scholar]
- 48.Wilson GA, Fulton TL, Heuer K. When theory meets practice: balancing genetic diversity and behaviour when choosing founders for a recently reintroduced bison (Bison bison) herd in Banff National park. Can Divers. 2023;15(3):366. 10.3390/d15030366 [Google Scholar]
- 49.Yang T, Miller M, Forgacs D, Derr J, Stothard P. Development of SNP-based genomic tools for the Canadian bison industry: parentage verification and subspecies composition. Front Genet. 2020;11:585999. 10.3389/fgene.2020.585999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bergmann GT. Microbial community composition along the digestive tract in forage- and grain-fed bison. BMC Vet Res. 2017;13(1):253. 10.1186/s12917-017-1161-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fresno Rueda A, Griffith JE, Kruse C, St-Pierre B. Effects of grain-based diets on the rumen and fecal bacterial communities of the North American bison (Bison Bison). Front Microbiol. 2023;14:1163423. 10.3389/fmicb.2023.1163423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bergmann GT, Craine JM, Robeson MS, Fierer N. Seasonal shifts in diet and gut microbiota of the American bison (Bison bison). PLoS ONE. 2015;10(11):e0142409. 10.1371/journal.pone.0142409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weese J, Shury T, Jelinski MD. The fecal microbiota of semi-free-ranging wood bison (Bison Bison athabascae). BMC Vet Res. 2014;10(1):120. 10.1186/1746-6148-10-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Combrink L, Humphreys IR, Washburn Q, Arnold HK, Stagaman K, Kasschau KD, et al. Best practice for wildlife gut microbiome research: a comprehensive review of methodology for 16S rRNA gene investigations. Front Microbiol. 2023;14:1092216. 10.3389/fmicb.2023.1092216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Björk JR, Dasari MR, Roche K, Grieneisen L, Gould TJ, Grenier J-C, et al. Synchrony and idiosyncrasy in the gut Microbiome of wild baboons. Nat Ecol Evol. 2022;6(7):955–64. 10.1038/s41559-022-01773-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murillo T, Schneider D, Heistermann M, Daniel R, Fichtel C. Assessing the drivers of gut microbiome composition in wild redfronted lemurs via longitudinal metacommunity analysis. Sci Rep. 2022;12(1):21462. 10.1038/s41598-022-25733-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parks Canada. Elk Island National Park Site Map. 2024.
- 58.Jones J, Reinke SN, Ali A, Palmer DJ, Christophersen CT. Fecal sample collection methods and time of day impact microbiome composition and short chain fatty acid concentrations. Sci Rep. 2021;11(1):13964. 10.1038/s41598-021-93031-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hartway C, Hardy A, Jones L, Moynahan B, Traylor-Holzer K, McCann B, et al. Long-term viability of department of the interior bison under current management and potential metapopulation management strategies. Colorado: Fort Collins; 2022. [Google Scholar]
- 60.Wilberg MJ, Dreher BP. Genecap : a program for analysis of multilocus genotype data for non-invasive sampling and capture‐recapture population estimation. Mol Ecol Notes. 2004;4(4):783–5. 10.1111/j.1471-8286.2004.00797.x [Google Scholar]
- 61.Wasimuddin SK, Ronchi F, Leib SL, Erb M, Ramette A. Evaluation of primer pairs for microbiome profiling from soils to humans within the one health framework. Mol Ecol Resour. 2020;20(6):1558–71. 10.1111/1755-0998.13215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Illumina. 16S Metagenomic Sequencing Library Preparation. Preparing 16S ribosomal RNA gene amplicons for the illumina miseq system. San Diego, CA: Illumina, Inc.; 2013. [Google Scholar]
- 63.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(8):852–7. 10.1038/s41587-019-0209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Estaki M, Jiang L, Bokulich NA, McDonald D, González A, Kosciolek T, et al. QIIME 2 enables comprehensive end-to‐end analysis of diverse microbiome data and comparative studies with publicly available data. Curr Protoc Bioinforma. 2020;70(1):e100. 10.1002/cpbi.100 [DOI] [PMC free article] [PubMed]
- 65.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17(1):10. 10.14806/ej.17.1.200 [Google Scholar]
- 66.Nowinski B, Smith CB, Thomas CM, Esson K, Marin R, Preston CM, et al. Microbial metagenomes and metatranscriptomes during a coastal phytoplankton bloom. Sci Data. 2019;6(1):129. 10.1038/s41597-019-0132-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 2012;41(D1):D590–6. 10.1093/nar/gks1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bokulich NA, Kaehler BD, Rideout JR, Dillon M, Bolyen E, Knight R, et al. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome. 2018;6(1):90. 10.1186/s40168-018-0470-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.R Core Team. R: a language and environment for statistical computing. 2022.
- 70.McMurdie PJ, Holmes S. Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE. 2013;8(4):e61217. 10.1371/journal.pone.0061217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Teunisse GM. Fantaxtic - nested bar plots for phyloseq data. 2022.
- 72.Fernandes AD, Macklaim JM, Linn TG, Reid G, Gloor GB. ANOVA-Like differential expression (ALDEx) analysis for mixed population RNA-Seq. PLoS ONE. 2013;8(7):e67019. 10.1371/journal.pone.0067019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin H, Eggesbø M, Peddada SD. Linear and nonlinear correlation estimators unveil undescribed taxa interactions in microbiome data. Nat Commun. 2022;13(1):4946. 10.1038/s41467-022-32243-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin H, Peddada SD. Analysis of compositions of microbiomes with bias correction. Nat Commun. 2020;11(1):3514. 10.1038/s41467-020-17041-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mallick H, Rahnavard A, McIver LJ, Ma S, Zhang Y, Nguyen LH, et al. Multivariable association discovery in population-scale meta-omics studies. PLOS Comput Biol. 2021;17(11):e1009442. 10.1371/journal.pcbi.1009442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nearing JT, Douglas GM, Hayes MG, MacDonald J, Desai DK, Allward N, et al. Microbiome differential abundance methods produce different results across 38 datasets. Nat Commun. 2022;13(1):342. 10.1038/s41467-022-28034-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McMurdie PJ, Holmes S. Waste not, want not: why rarefying microbiome data is inadmissible. PLoS Comput Biol. 2014;10(4):e1003531. 10.1371/journal.pcbi.1003531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuhn M. caret: classification and regression training. 2007.
- 79.Kuhn M. Building predictive models in R using the caret package. J Stat Softw. 2008;28(5). 10.18637/jss.v028.i05
- 80.Breiman L, Cutler A, Liaw A, Wiener M. randomForest: Breiman and Cutlers random forests for classification and regression. 2002.
- 81.Han H, Guo X, Hua Yu. Variable selection using mean decrease accuracy and, mean decrease gini based on random forest, Science S. (ICSESS). Beijing, China: IEEE, 2016;219–24.
- 82.Wang M, Tu Q. Effective data filtering is prerequisite for robust microbial association network construction. Front Microbiol. 2022;13:1016947. 10.3389/fmicb.2022.1016947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harrell FE Jr. Hmisc: Harrell miscellaneous. 2003.
- 84.Csárdi G, Nepusz T, Müller K, Horvát S, Traag V, Zanini F et al. igraph for R: R interface of the igraph library for graph theory and network analysis. 2025.
- 85.Wickham H, Chang W, Henry L, Pedersen TL, Takahashi K, Wilke C et al. ggplot2: create elegant data visualisations using the grammar of graphics. 2007.
- 86.Douglas GM, Maffei VJ, Zaneveld JR, Yurgel SN, Brown JR, Taylor CM, et al. PICRUSt2 for prediction of metagenome functions. Nat Biotechnol. 2020;38(6):685–8. 10.1038/s41587-020-0548-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mirarab S, Nguyen N, Warnow T, SEPP. SATé-Enabled phylogenetic placement. Biocomputing 2012. Kohala Coast. Hawaii, USA: WORLD SCIENTIFIC; 2011. pp. 247–58. [DOI] [PubMed] [Google Scholar]
- 88.Barbera P, Kozlov AM, Czech L, Morel B, Darriba D, Flouri T, et al. EPA-ng: massively parallel evolutionary placement of genetic sequences. Syst Biol. 2019;68(2):365–9. 10.1093/sysbio/syy054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Czech L, Barbera P, Stamatakis A. Genesis and gappa: processing, analyzing and visualizing phylogenetic (placement) data. Bioinformatics. 2020;36(10):3263–5. 10.1093/bioinformatics/btaa070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Louca S, Doebeli M. Efficient comparative phylogenetics on large trees. Bioinformatics. 2018;34(6):1053–5. 10.1093/bioinformatics/btx701 [DOI] [PubMed] [Google Scholar]
- 91.Ye Y, Doak TG. A parsimony approach to biological pathway reconstruction/inference for genomes and metagenomes. PLoS Comput Biol. 2009;5(8):e1000465. 10.1371/journal.pcbi.1000465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang C, Mai J, Cao X, Burberry A, Cominelli F, Zhang L. ggpicrust2: an R package for PICRUSt2 predicted functional profile analysis and visualization. Bioinformatics. 2023;39(8):btad470. 10.1093/bioinformatics/btad470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kers JG, Saccenti E. The power of microbiome studies: some considerations on which alpha and beta metrics to use and how to report results. Front Microbiol. 2022;12:796025. 10.3389/fmicb.2021.796025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Callahan BJ, McMurdie PJ, Holmes SP. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 2017;11(12):2639–43. 10.1038/ismej.2017.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shannon CE. A mathematical theory of communication. Bell Syst Tech J. 1948;27(3):379–423. 10.1002/j.1538-7305.1948.tb01338.x [Google Scholar]
- 96.Simpson EH. Measurement of diversity. Nature. 1949;163(4148):688–688. 10.1038/163688a0 [Google Scholar]
- 97.Faith DP. Conservation evaluation and phylogenetic diversity. Biol Conserv. 1992;61(1):1–10. 10.1016/0006-3207(92)91201-3 [Google Scholar]
- 98.Oksanen J, Simpson GL, Blanchet FG, Kindt R, Legendre P, Minchin PR et al. vegan: community ecology package. 2001.
- 99.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: high-resolution sample inference from illumina amplicon data. Nat Methods. 2016;13(7):581–3. 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haworth SE, White KS, Côté SD, Shafer ABA. Space, time and captivity: quantifying the factors influencing the fecal microbiome of an alpine ungulate. FEMS Microbiol Ecol. 2019;95(7):fiz095. 10.1093/femsec/fiz095 [DOI] [PubMed] [Google Scholar]
- 101.Hornung BVH, Zwittink RD, Kuijper EJ. Issues and current standards of controls in microbiome research. FEMS Microbiol Ecol. 2019;95(5). 10.1093/femsec/fiz045 [DOI] [PMC free article] [PubMed]
- 102.Rojas CA, Ramírez-Barahona S, Holekamp KE, Theis KR. Host phylogeny and host ecology structure the mammalian gut microbiota at different taxonomic scales. Anim Microbiome. 2021;3(1):33. 10.1186/s42523-021-00094-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Härer A, Rennison DJ. The effects of host ecology and phylogeny on gut microbiota (non)parallelism across birds and mammals. mSphere. 2023;8(6):e00442-23. 10.1128/msphere.00442-23 [DOI] [PMC free article] [PubMed]
- 104.Sottas C, Schmiedová L, Kreisinger J, Albrecht T, Reif J, Osiejuk TS, et al. Gut microbiota in two recently diverged passerine species: evaluating the effects of species identity, habitat use and geographic distance. BMC Ecol Evol. 2021;21(1):41. 10.1186/s12862-021-01773-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang Z, Zhang C, Li G, Yi X. The influence of species identity and geographic locations on gut microbiota of small rodents. Front Microbiol. 2022;13:983660. 10.3389/fmicb.2022.983660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goodrich JK, Di Rienzi SC, Poole AC, Koren O, Walters WA, Caporaso JG, et al. Conducting microbiome study. Cell. 2014;158(2):250–62. 10.1016/j.cell.2014.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci. 2011;108(supplement1):4516–22. 10.1073/pnas.1000080107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nearing JT, Comeau AM, Langille MGI. Identifying biases and their potential solutions in human microbiome studies. Microbiome. 2021;9(1):113. 10.1186/s40168-021-01059-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guo N, Wu Q, Shi F, Niu J, Zhang T, Degen AA, et al. Seasonal dynamics of diet–gut microbiota interaction in adaptation of yaks to life at high altitude. Npj Biofilms Microbiomes. 2021;7(1):38. 10.1038/s41522-021-00207-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Andrade BGN, Bressani FA, Cuadrat RRC, Cardoso TF, Malheiros JM, De Oliveira PSN, et al. Stool and ruminal microbiome components associated with methane emission and feed efficiency in Nelore beef cattle. Front Genet. 2022;13:812828. 10.3389/fgene.2022.812828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Casto-Rebollo C, Argente MJ, García ML, Pena RN, Blasco A, Ibáñez-Escriche N. Selection for environmental variance shifted the gut microbiome composition driving animal resilience. Microbiome. 2023;11(1):147. 10.1186/s40168-023-01580-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Prewer E, Vilaça ST, Bird S, Kutz S, Leclerc L, Kyle CJ. Metabarcoding of fecal pellets in wild muskox populations reveals negative relationships between microbiome and diet alpha diversity. Ecol Evol. 2023;13(6):e10192. 10.1002/ece3.10192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun Y, Yu Y, Guo J, Zhong L, Zhang M. Alterations in fecal microbiota linked to environment and sex in red deer (Cervus elaphus). Animals. 2023;13(5):929. 10.3390/ani13050929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fountain-Jones NM, Clark NJ, Kinsley AC, Carstensen M, Forester J, Johnson TJ, et al. Microbial associations and spatial proximity predict North American moose (Alces alces) gastrointestinal community composition. J Anim Ecol. 2020;89(3):817–28. 10.1111/1365-2656.13154 [DOI] [PubMed] [Google Scholar]
- 115.Azad E, Derakhshani H, Forster RJ, Gruninger RJ, Acharya S, McAllister TA, et al. Characterization of the rumen and fecal microbiome in bloated and non-bloated cattle grazing alfalfa pastures and subjected to bloat prevention strategies. Sci Rep. 2019;9(1):4272. 10.1038/s41598-019-41017-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang YK, Zhang XX, Li FD, Li C, Li GZ, Zhang DY, et al. Characterization of the rumen microbiota and its relationship with residual feed intake in sheep. Animal. 2021;15(3):100161. 10.1016/j.animal.2020.100161 [DOI] [PubMed] [Google Scholar]
- 117.Topçuoğlu BD, Lesniak NA, Ruffin MT, Wiens J, Schloss PD. A framework for effective application of machine learning to microbiome-based classification problems. mBio. 2020;11(3):e00434–20. 10.1128/mBio.00434-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Suzuki TA. Links between natural variation in the microbiome and host fitness in wild mammals. Integr Comp Biol. 2017;57(4):756–69. 10.1093/icb/icx104 [DOI] [PubMed] [Google Scholar]
- 119.Kajihara KT, Hynson NA. Networks as tools for defining emergent properties of microbiomes and their stability. Microbiome. 2024;12(1):184. 10.1186/s40168-024-01868-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.De Vos WM. Microbe profile: Akkermansia muciniphila: a conserved intestinal symbiont that acts as the gatekeeper of our mucosa: this article is part of the microbe profiles collection. Microbiology. 2017;163(5):646–8. 10.1099/mic.0.000444 [DOI] [PubMed] [Google Scholar]
- 121.Binda C, Lopetuso LR, Rizzatti G, Gibiino G, Cennamo V, Gasbarrini A, Actinobacteria. A relevant minority for the maintenance of gut homeostasis. Dig Liver Dis. 2018;50(5):421–8. 10.1016/j.dld.2018.02.012 [DOI] [PubMed] [Google Scholar]
- 122.Kreisinger J, Čížková D, Vohánka J, Piálek J. Gastrointestinal microbiota of wild and inbred individuals of two house mouse subspecies assessed using high-throughput parallel pyrosequencing. Mol Ecol. 2014;23(20):5048–60. 10.1111/mec.12909 [DOI] [PubMed] [Google Scholar]
- 123.Muhammad R, Klomkliew P, Chanchaem P, Sawaswong V, Kaikaew T, Payungporn S, et al. Comparative analysis of gut microbiota between common (Macaca F.scicularis F.scicularis) and Burmese (M. F. aurea) long-tailed macaques in different habitats. Sci Rep. 2023;13(1):14950. 10.1038/s41598-023-42220-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang X, Wu X, Shang Y, Gao Y, Li Y, Wei Q, et al. High-altitude drives the convergent evolution of alpha diversity and indicator microbiota in the gut microbiomes of ungulates. Front Microbiol. 2022;13:953234. 10.3389/fmicb.2022.953234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhou Z, Tang L, Yan L, Jia H, Xiong Y, Shang J, et al. Wild and captive environments drive the convergence of gut microbiota and impact health in threatened equids. Front Microbiol. 2022;13:832410. 10.3389/fmicb.2022.832410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fu H, Zhang L, Fan C, Li W, Liu C, Zhang H, et al. Sympatric yaks and plateau Pikas promote microbial diversity and similarity by the mutual utilization of gut microbiota. Microorganisms. 2021;9(9):1890. 10.3390/microorganisms9091890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu R, Shi J, Shultz S, Guo D, Liu D. Fecal bacterial community of allopatric przewalski’s gazelles and their sympatric relatives. Front Microbiol. 2021;12:737042. 10.3389/fmicb.2021.737042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bendová B, Mikula O, Vošlajerová Bímová B, Čížková D, Daniszová K, Ďureje Ľ, et al. Divergent gut microbiota in two closely related house mouse subspecies under common garden conditions. FEMS Microbiol Ecol. 2022;98(8):fiac078. 10.1093/femsec/fiac078 [DOI] [PubMed] [Google Scholar]
- 129.Nishida AH, Ochman H. Rates of gut microbiome divergence in mammals. Mol Ecol. 2018;27(8):1884–97. 10.1111/mec.14473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Valeri F, Endres K. How biological sex of the host shapes its gut microbiota. Front Neuroendocrinol. 2021;61:100912. 10.1016/j.yfrne.2021.100912 [DOI] [PubMed] [Google Scholar]
- 131.He Y, Wang H, Yu Z, Niu W, Qiu Q, Su H, et al. Effects of the gender differences in cattle rumen fermentation on anaerobic fermentation of wheat straw. J Clean Prod. 2018;205:845–53. 10.1016/j.jclepro.2018.09.156 [Google Scholar]
- 132.Han X, Liu H, Hu L, Ma L, Xu S, Xu T, et al. Impact of sex and age on the bacterial composition in rumen of Tibetan sheep in Qinghai China. Livest Sci. 2020;238:104030. 10.1016/j.livsci.2020.104030 [Google Scholar]
- 133.Guo X, Sha Y, Lv W, Pu X, Liu X, Luo Y, et al. Sex differences in rumen fermentation and microbiota of Tibetan goat. Microb Cell Factories. 2022;21(1):55. 10.1186/s12934-022-01783-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Plaizier JC, Li S, Danscher AM, Derakshani H, Andersen PH, Khafipour E. Changes in microbiota in rumen digesta and feces due to a grain-based subacute ruminal acidosis (SARA) challenge. Microb Ecol. 2017;74(2):485–95. 10.1007/s00248-017-0940-z [DOI] [PubMed] [Google Scholar]
- 135.Tian L, Wang X-W, Wu A-K, Fan Y, Friedman J, Dahlin A, et al. Deciphering functional redundancy in the human microbiome. Nat Commun. 2020;11(1):6217. 10.1038/s41467-020-19940-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Qin W, Song P, Lin G, Huang Y, Wang L, Zhou X, et al. Gut microbiota plasticity influences the adaptability of wild and domestic animals in co-inhabited areas. Front Microbiol. 2020;11:125. 10.3389/fmicb.2020.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhen J, Yuan X, Tao L, Zhang H, Ren Y, Xie S, et al. Intestinal ecology changes in diarrheic Père david’s deer revealed by gut microbiota and fecal metabolites analysis. Animals. 2022;12(23):3366. 10.3390/ani12233366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Morinaga K, Kusada H, Tamaki H. Bile salt hydrolases with extended substrate specificity confer a high level of resistance to bile toxicity on Atopobiaceae bacteria. Int J Mol Sci. 2022;23(18):10980. 10.3390/ijms231810980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hecker LJ, Coogan SCP, Nielsen SE, Edwards MA. Latitudinal and seasonal plasticity in American bison Bison Bison diets. Mammal Rev. 2021;51(2):193–206. 10.1111/mam.12229 [Google Scholar]
- 140.Malik PK, Trivedi S, Mohapatra A, Kolte AP, Sejian V, Bhatta R, et al. Comparison of enteric methane yield and diversity of ruminal methanogens in cattle and buffaloes fed on the same diet. PLoS ONE. 2021;16(8):e0256048. 10.1371/journal.pone.0256048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Anderson CJ, Koester LR, Schmitz-Esser S. Rumen epithelial communities share a core bacterial microbiota: a meta-analysis of 16S rRNA gene illumina miseq sequencing datasets. Front Microbiol. 2021;12:625400. 10.3389/fmicb.2021.625400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Poulsen M, Schwab C, Borg Jensen B, Engberg RM, Spang A, Canibe N, et al. Methylotrophic methanogenic thermoplasmata implicated in reduced methane emissions from bovine rumen. Nat Commun. 2013;4(1):1428. 10.1038/ncomms2432 [DOI] [PubMed] [Google Scholar]
- 143.Thacharodi A, Hassan S, Ahmed ZHT, Singh P, Maqbool M, Meenatchi R, et al. The ruminant gut microbiome vs enteric methane emission: the essential microbes may help to mitigate the global methane crisis. Environ Res. 2024;261:119661. 10.1016/j.envres.2024.119661 [DOI] [PubMed] [Google Scholar]
- 144.Derakhshani H, De Buck J, Mortier R, Barkema HW, Krause DO, Khafipour E. The features of fecal and ileal mucosa-associated microbiota in dairy calves during early infection with Mycobacterium avium subspecies paratuberculosis. Front Microbiol. 2016;7. 10.3389/fmicb.2016.00426 [DOI] [PMC free article] [PubMed]
- 145.Elmagzoub WA, Idris SM, Elnaiem MHE, Mukhtar ME, Eltayeb E, Bakhiet SM, et al. Faecal microbial diversity in a cattle herd infected by Mycobacterium avium subsp. Paratuberculosis: a possible effect of production status. World J Microbiol Biotechnol. 2024;40(9):276. 10.1007/s11274-024-04080-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Khalil A, Batool A, Arif S. Healthy cattle microbiome and dysbiosis in diseased phenotypes. Ruminants. 2022;2(1):134–56. 10.3390/ruminants2010009 [Google Scholar]
- 147.Bridges CM, Gage DJ. Development and application of aerobic, chemically defined media for dysgonomonas. Anaerobe. 2021;67:102302. 10.1016/j.anaerobe.2020.102302 [DOI] [PubMed] [Google Scholar]
- 148.Salb A, Stephen C, Ribble C, Elkin B, Descriptive epidemiology of detected anthrax outbreaks. In wild wood bison (Bison Bison Athabascae) in Northern Canada, 1962–2008. J Wildl Dis. 2014;50(3):459–68. 10.7589/2013-04-095 [DOI] [PubMed] [Google Scholar]
- 149.Pham VHT, Jeong S-W, Kim J. Psychrobacillus soli sp. nov., capable of degrading oil, isolated from oil-contaminated soil. Int J Syst Evol Microbiol. 2015;65(Pt9):3046–52. 10.1099/ijs.0.000375 [DOI] [PubMed] [Google Scholar]
- 150.Zhang S, Hou R, Wang Y, Huang Q, Lin L, Li H, et al. Xenobiotic metabolism activity of gut microbiota from six marine species: combined taxonomic, metagenomic, and in vitro transformation analysis. J Hazard Mater. 2024;480:136152. 10.1016/j.jhazmat.2024.136152 [DOI] [PubMed] [Google Scholar]
- 151.Mallah MA, Changxing L, Mallah MA, Noreen S, Liu Y, Saeed M, et al. Polycyclic aromatic hydrocarbon and its effects on human health: an overeview. Chemosphere. 2022;296:133948. 10.1016/j.chemosphere.2022.133948 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Estaki M, Jiang L, Bokulich NA, McDonald D, González A, Kosciolek T, et al. QIIME 2 enables comprehensive end-to‐end analysis of diverse microbiome data and comparative studies with publicly available data. Curr Protoc Bioinforma. 2020;70(1):e100. 10.1002/cpbi.100 [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data that support the findings of this study have been submitted to the NCBI Sequence Read Archive (SRA) under BioProject number PRJNA1217016.