Abstract
As a key bacterial actin-like protein, MreB plays crucial roles in maintaining cell shape, regulating peptidoglycan synthesis, and coordinating chromosome segregation, making it a promising target for novel antibiotics. This review comprehensively explores MreB’s molecular architecture, its assembly into antiparallel protofilaments, and its pivotal roles in bacterial cell morphology and division. We also delve into how MreB interacts with membrane-associated proteins such as RodZ and MreC/D to coordinate cell wall synthesis and respond to environmental signals like ion gradients and temperature changes. Furthermore, we highlight the cooperation and functional divergence between MreB and FtsZ, underscoring the evolutionary adaptability of bacterial cytoskeletal structures. The structural and functional parallels between MreB and eukaryotic cytoskeletal proteins are also examined, offering new insights into the evolution of cytoskeletal systems. By integrating insights from structural biology, synthetic biology, and microbial ecology, this review aims to provide a deeper understanding of MreB’s role in bacterial biology, its dynamic responses to environmental cues, and its implications for therapeutic innovation. This comprehensive analysis not only enhances our knowledge of bacterial self-organization mechanisms but also paves the way for the development of innovative antimicrobial strategies to address the growing challenge of antibiotic resistance.
Keywords: MreB, Bacterial cytoskeleton, Cell wall synthesis, Morphogenesis, Cell polarity
Introduction
The cytoskeleton, a dynamic protein network orchestrating cellular organization and mechanics, has long been considered a hallmark of eukaryotic systems. Among the components, actin is distinguished by its function in activities like motility and cytokinesis, facilitated by ATP-driven polymerization into polarized filaments called F-actin [1, 2]. However, the discovery of MreB in 2001 revealed that bacteria also possess organized structural frameworks [3, 4]. As a bacterial actin homolog, MreB shares structural homology with eukaryotic actin, including conserved ATP-binding motifs and protofilament assembly, while it has evolved distinct functional adaptations. The sequence and structural similarity between MreB and eukaryotic actin were first predicted based on bioinformatics analysis [5]. Unlike actin’s polar filaments, MreB forms antiparallel double-stranded polymers that generate mechanical forces on bacterial membranes, enabling functions such as cell shape maintenance, peptidoglycan synthase positioning, and chromosome segregation [6, 7]. The functional diversification of MreB in bacterial taxa underscores its evolutionary plasticity. In Escherichia coli (E. coli), MreB depletion causes cells to lose their rod shape and form spheres with division defects [8]. In contrast, Bacillus cereus (B. cereus) has three MreB paralogs (MreB, Mbl (Metallo-β-lactamase-like), and MreBH) that share functions in cell wall synthesis. Deletion of all three leads to cell rounding and lysis [9]. Spiroplasma species utilize multiple MreB homologs, like MreB5, to drive helical movement by creating twists, independent of cell wall synthesis [10, 11]. Pathogens like Chlamydia further repurpose MreB to coordinate division in the absence of FtsZ, highlighting its adaptability [11].
FtsZ, a tubulin homolog, orchestrates bacterial division by polymerizing into dynamic filaments analogous to microtubules, while MreB, as an actin-like protein, maintains cell shape. Despite two decades of research, critical gaps remain in our understanding of how MreB integrates mechanical, metabolic, and environmental signals to regulate bacterial physiology. For instance, the mechanism by which MreB filamentous structures sense membrane curvature or dynamically respond to stress remains unresolved. This review synthesizes the current knowledge of the molecular architecture, functional networks, and environmental adaptability of MreB. It aims to elucidate the role of MreB as a multifunctional scaffold in bacterial biology, explore its potential as a target for novel antimicrobial strategies, and identify key unanswered questions that merit future investigation.
Molecular structure and dynamic assembly
Phylogenetic conservation and divergence of MreB
To summarize the evolutionary dynamics of the MreB protein family, we constructed a phylogenetic tree covering diverse bacterial taxa, including Enterobacteriaceae, Bacillaceae, Spiroplasmataceae, and key pathogens like Mycobacterium and Pseudomonas. Our analysis reveals a “conserved core – divergent periphery” pattern. The core regions, including ATP-binding motifs and polymerization interfaces, are highly conserved to maintain essential cytoskeletal functions such as cell shape. In contrast, significant diversification occurs through C-terminal modifications, gene duplication, and horizontal gene transfer.
In the evolutionary history of bacteria, the MreB protein has played a crucial role, with its functional and structural evolution closely tied to bacterial adaptation to various environments. Initially, MreB was identified as a protein responsible for maintaining cell shape in bacteria [12]. Over time, MreB genes underwent duplication events, leading to functional diversification. For example, in some bacteria, MreB proteins became involved in cell wall synthesis and chromosome segregation [4, 13]. Horizontal gene transfer further contributed to the acquisition of new functions, such as antibiotic resistance [11]. In cell wall-less bacteria like spiroplasmas, MreB evolved to drive helical cell movement, facilitating effective locomotion in their specific environments [10, 14] (Fig. 1A).
Fig. 1.
Discovery History and Evolutionary Tree of MreB Protein Functions. A The timeline, progressing from left to right, begins with the emergence of the MreB protein. Subsequent gene duplication events drove the diversification of MreB’s functions. Horizontal gene transfer then enabled MreB to contribute to the development of antibiotic resistance. Ultimately, MreB adapted to cell wall-less environments and evolved the capacity for helical movement. B Maximum-likelihood tree of MreB and MreB-like protein sequences from diverse bacterial species generated using MEGA 11. Branches are color-coded to highlight major functional categories: blue signifies proteins involved in maintaining cell shape; red identifies proteins associated with antibiotic resistance; and green denotes specialized cytoskeletal variants. Bootstrap values ≥ 60% are labeled for major nodes
Three major clades were identified. The first includes classic MreB homologs in Gram-negative rods like E. coli, with high sequence conservation (bootstrap ≥ 94). The second is exemplified by Spiroplasma culicicola, which has seven MreB paralogs (MreB1–MreB7) forming a robust cluster (bootstrap 100). These paralogs show pronounced functional divergence through subtype specialization: MreB3 lacks the catalytic glutamate and threonine residues required for ATP hydrolysis, likely relegating it to auxiliary functions, whereas MreB5 possesses an extended C-terminal domain enabling lateral interactions. These adaptations collectively facilitate helical cell shape maintenance through antiparallel filament bundling in wall-less bacteria [10, 15–17]. The third clade comprises Metallo-β-lactamase-like (Mbl) proteins, distinct from canonical MreB (bootstrap 97), with minimal sequence homology but strong evidence of horizontal gene transfer across diverse genera, including Klebsiella pneumoniae, Mycobacterium tuberculosis, and Staphylococcus aureus (S. aureus), indicating dissemination of antibiotic resistance (Fig. 1B).
Lineage-specific adaptations are also observed, such as the co-clustering of Bacillus subtilis (B. subtilis) MreBH and Listeria monocytogenes Mbl (bootstrap 89) and the independent branching of S. aureus MreB, suggesting unique pathogenic adaptations. Overall, MreB’s plasticity enables bacteria to adapt to diverse environments, from maintaining cell shape to driving motility and conferring drug resistance.
Molecular architecture of MreB: secondary and tertiary structure features
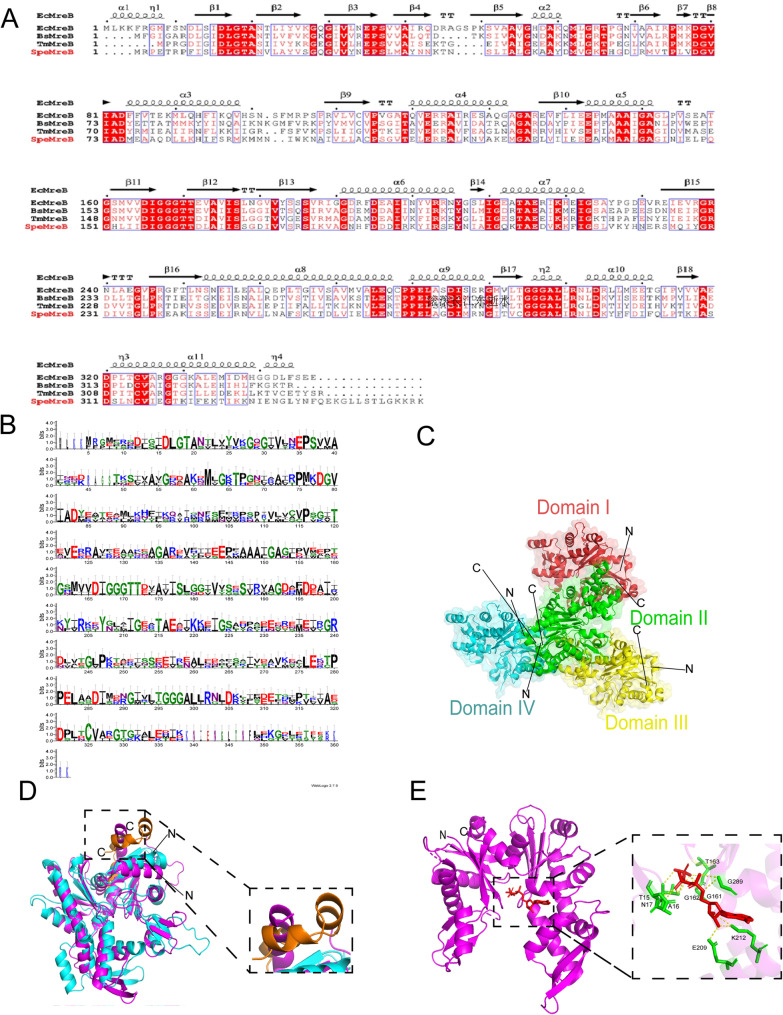
Computational analyses across diverse bacterial lineages delineate conserved and divergent structural features of MreB, providing insights into its functional underpinnings. Secondary structure predictions for MreB orthologs from B. subtilis (Gram-positive), E. coli (Gram-negative), Thermotoga maritima (T. maritima, extremophile), and Spiroplasma citri (S. citri, wall-less) using ESPrint 3.0 reveal consistent core elements, including α-helices, β-strands, and notably, conserved N-terminal DLGTA/GXGXG motifs characteristic of ATP-binding domains in actin-like proteins (Fig. 2A). This conservation strongly suggests a fundamental role for nucleotide-dependent regulation in MreB function across evolutionarily distant bacteria.
Fig. 2.
Structural features of MreB and actin. A Predicted secondary structures of MreB orthologs from four key bacterial groups: B. subtilis (Gram-positive), E. coli (Gram-negative), T. maritima (extremophile) and S. citri (wall-less), as generated by ESPrint 3. 0. B Sequence conservation analysis of MreB from the four bacterial groups listed was performed using WebLogo, highlighting the positions of highly conserved amino acid residues. C The domain architecture of B. taurus actin, which includes domains I through IV, is presented for structural comparison with other proteins. D An interaction model between B. taurus actin (blue) and G. stearothermophilus MreB (purple) was generated using AlphaFold3. Regions of significant structural misalignment are highlighted in orange. E The ATP-binding pocket in G. stearothermophilus MreB is visualized using PyMOL, with key residues (T15, A16, N17, G161, G162, T163, E209, K212, G289) labeled
WebLogo analysis of MreB sequences from these four groups further identifies positions of high amino acid conservation (Fig. 2B). The pronounced conservation of glycine and threonine residues within the N-terminal region aligns precisely with established ATP binding and hydrolysis sites in related proteins. Central hydrophobic patches likely represent interfaces for monomer interaction and polymerization, while conserved C-terminal motifs potentially facilitate the recruitment of partner proteins, such as cell wall synthesis machinery. Importantly, these functional interpretations are based on sequence motif conservation and analogy to actin; definitive roles await experimental confirmation.
For comparative context, the canonical four-domain architecture of eukaryotic actin (Bos taurus, B. taurus) serves as a structural reference point (Fig. 2C). Computational modeling via AlphaFold3 predicts significant local conformational differences at the interaction interface between B. taurus actin and Geobacillus stearothermophilus (G. stearothermophilus) MreB, highlighted by misaligned regions (Fig. 2D). These structural distinctions plausibly underlie the specialized functions of MreB, such as its propensity to form antiparallel polymers generating circumferential forces for morphogenesis, contrasting with actin’s polarized filaments driving motility.
Critical examination of the G. stearothermophilus MreB structure identifies key residues (T15, A16, N17, G161, G162, T163, E209, K212, G289) spatially arranged to form the ATP-binding pocket (Fig. 2E). The location and composition of this pocket, corroborated by evidence from the literature, robustly support its essential function in nucleotide-driven polymerization dynamics, a core mechanism underpinning MreB’s role in bacterial cellular organization [18].
Collectively, these analyses characterize the structural landscape of MreB. The conserved ATP-binding motifs and pocket architecture (Fig. 2A, B, E), combined with predicted polymerization interfaces (Fig. 2B), align with and reinforce the established model of MreB utilizing ATP-dependent filament assembly to orchestrate essential processes like cell shape maintenance. The observed structural divergence from actin (Fig. 2D) correlates well with MreB’s unique functional adaptations, particularly its capacity for antiparallel filament formation and circumferential force generation within the bacterial cell envelope.
Structural and functional parallels with actin
Evolutionary conservation and divergence
MreB and actin exhibit striking structural similarities, including conserved ATP-binding motifs and polymerization dynamics [3]. Functionally, however, their architectures drive distinct mechanical outputs. MreB generates circumferential forces along bacterial membranes to direct cell wall synthesis and morphogenesis, whereas actin’s stably polarized filaments enable eukaryotic motility, vesicle trafficking, and cytokinesis [2, 6]. Notably, MreB’s role in maintaining membrane tension and rigidity exhibits functional convergence with cortical actin’s regulation of lipid raft dynamics in eukaryotes [19]. Further parallels exist in unexplored interactions: MreB coordinates chromosome segregation through RNA polymerase (RNAP) coupling, mirroring nuclear actin’s transcriptional regulatory roles [13].
Functional specialization across domains
While both proteins polymerize in an ATP-dependent manner, their roles diverge sharply. In walled bacteria, MreB directs peptidoglycan synthase localization and cell wall synthesis, whereas actin orchestrates cytoskeletal remodeling in eukaryotes. Notably, MreB typically forms antiparallel protofilaments without intrinsic unidirectional polarity unlike eukaryotic actin, recent evidence suggests that under specific conditions, such as at membrane concavities, MreB can exhibit treadmilling behavior with directional polarity [20]. In the absence of stable polarity, MreB compensates through interactions with membrane proteins like RodZ. RodZ anchors peptidoglycan (PG) synthases by forming a bridge complex, for example, with MreC/D, thereby linking the cytoskeleton to cell wall synthesis machinery [7, 21–23]. This functional adaptation exhibits convergence with cortical actin: both regulate membrane rigidity through lipid domain organization [24]. Furthermore, MreB’s coordination of chromosome segregation via RNAP coupling parallels nuclear actin’s involvement in transcriptional regulation, suggesting conserved mechanotransduction pathways across domains [13].
Structural features
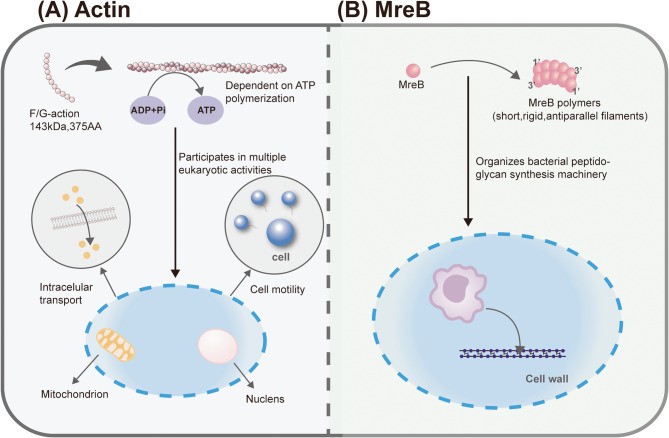
The eukaryotic actin monomers (G-actin) are 43 kDa globular proteins composed of 375 amino acids, which polymerize via ATP-dependent processes into polarized, double-helical filaments (F-actin), with dynamic assembly regulated by ATP hydrolysis [25–27]. Although both MreB and actin exhibit ATP-binding capacity and dynamic polymerization, key differences distinguish them. Structurally, MreB forms shorter, rigid anti-parallel filaments, whereas actin microfilaments adopt unidirectional helical polarity [4, 6, 28]. Functionally, MreB specializes in spatially organizing bacterial peptidoglycan synthesis machinery, while actin participates in diverse eukaryotic processes such as cell motility, intracellular transport, and cytoskeletal remodeling [2, 29] (Fig. 3). These divergences in structural dimensions and functional specialization underscore the evolutionary divergence between the cytoskeletal systems of prokaryotes and eukaryotes.
Fig. 3.
Structural and functional comparison between MreB and eukaryotic actin. A Eukaryotic Actin System: G-actin monomers polymerize ATP-dependently into polarized double-helical F-actin filaments, undergoing dynamic cycles regulated by ATP hydrolysis. Actin drives cell motility, cytokinesis, vesicle trafficking, and intracellular transport. B Bacterial MreB System: MreB monomers assemble via ATP binding into short, rigid, single-stranded helical filaments. MreB maintains bacterial shape by coordinating peptidoglycan synthesis enzymes during cell wall elongation
Functional redundancy and specialization of MreB paralogs
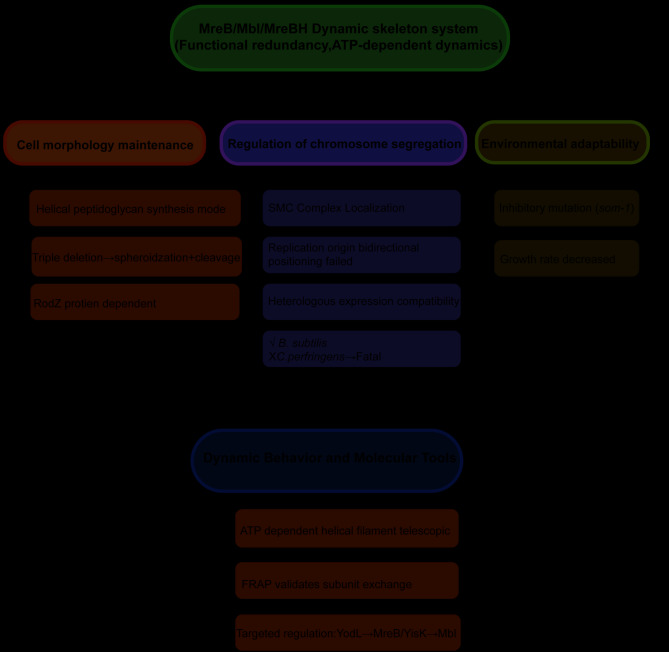
In B. subtilis, MreB and its homologous proteins Mbl and MreBH constitute a dynamic cytoskeletal system, which is crucial for maintaining cell morphology and chromosome segregation. Studies have revealed that the protein encoded by the Mbl gene exhibits 53% sequence similarity with E. coli MreB (EcMreB) and shares 86% sequence with B. cereus MreB, suggesting that its function is conserved [30]. Although the inactivation of Mbl does not directly affect cell viability or sporulation, it leads to a reduced growth rate, morphological distortion, and the emergence of intergenic suppressor mutations (such as som-1) to compensate for the defects. Further studies indicate that MreB and Mbl regulate chromosome segregation by forming dynamic submembrane helical filaments. Their absence disrupts the subcellular localization of SMC (structural maintenance of chromosomes) complexes, leading to the failure of bipolar localization at the replication origin and even unidirectional movement [30, 31]. In B. subtilis, the proteins MreB, Mbl, and MreBH all help control the helical pattern of peptidoglycan synthesis. Even if one of them is mutated, the cell can still maintain rod shape. But if MreB is depleted in an Mbl mutant, or if all three proteins are depleted, the cell wall’s peptidoglycan synthesis is disrupted, causing the cell to become spherical and lyse. This redundancy extends to heterologous expression systems. MreB from Bacillus licheniformis can partially substitute for its homolog in B. subtilis. Conversely, MreB from Clostridium perfringens can cause cell death in B. subtilis by disrupting the endogenous cytoskeleton [32]. Dynamic behavior studies have shown that these three proteins form ATP-dependent spiral filaments capable of extension and contraction within seconds; their subunit turnover properties have been confirmed by Fluorescence Recovery after Photobleaching (FRAP) [33]. Specifically, MreB can induce membrane protrusions in Drosophila Schneider 2 cells, suggesting that it possesses mechanical functions similar to those of eukaryotic actin [34]. Additionally, the localization of membrane proteins such as RodZ depends on the scaffold effect of MreB/Mbl, confirming that this cytoskeletal system has the ability to recruit proteins [34]. Functional specificity studies have revealed that YodL and YisK can target MreB and Mbl, providing tools to reveal their molecular mechanisms [35]. These findings collectively reveal that B. subtilis constructs a robust regulatory network through the dynamic coordination of multiple actin-like proteins, which plays a critical role in maintaining morphogenesis and chromosome organization (Fig. 4).
Fig. 4.
Integrated description of the MreB/Mbl/MreBH cytoskeletal system. The MreB/Mbl/MreBH cytoskeletal system orchestrates critical bacterial functions through functional redundancy and ATP-dependent dynamic behaviors, enabling multidimensional regulation. Central to cell morphogenesis, this system coordinates helical peptidoglycan synthesis and interacts with RodZ; its triple deletion (MreB/Mbl/MreBH) disrupts cell shape maintenance, leading to spherical morphology and lysis. Concurrently, it regulates chromosome segregation by positioning the SMC complex, ensuring bidirectional origin localization during replication, and exhibiting compatibility with heterologous expression systems. Environmental adaptability is mediated via stress-responsive mechanisms, including suppressor mutations such as som-1, and growth rate modulation under adverse conditions. Dynamic ATP-driven filament extension/contraction underpins its mechanical plasticity, validated by FRAP-based subunit exchange assays. Targeted regulation is achieved through dedicated pathways, such as YodL regulating MreB and YisK regulating Mbl, highlighting its integration into broader cellular networks
The polymerization characteristics of MreB
MreB can polymerize to form filamentous structures, which are essential for maintaining cell shape, synthesizing the cell wall, organizing chromosomes and establishing cell polarity [36]. Although S. citri MreB5 (ScMreB5) can form filaments in different nucleotide states, efficient polymerization and filament organization require the ATP-bound state. Conversely, ATP hydrolysis (metabolism) regulates filament dynamics and triggers disassembly. The impaired hydrolysis in the E134A mutant, which locks the protein in the ATP-state, dramatically reduces polymerization efficiency, demonstrating this dual dependence [27]. Studies on B. subtilis MreB (BsMreB) further revealed that in vitro polymerization requires millimolar concentrations of divalent cations such as Mg²⁺ and Ca²⁺, and acidic pH value, while being inhibited by low temperatures or high concentrations of monovalent ions such as K⁺ and Na⁺. Intriguingly, although BsMreB exhibits ATP/GTP binding and hydrolytic activity, its critical concentration for polymerization (~ 900 nM) remains unaffected by the type of nucleotide or binding state [37]. This contrasts sharply with Marine thermophile (Thermotoga maritima) MreB1, which strictly requires ATP for polymerization and is highly temperature-sensitive [38]. Such interspecies divergence suggests that MreB dynamics in vivo likely depend on cofactors such as cytoskeletal regulators and microenvironmental cues such as membrane potential and metabolite gradients, which may fine-tune its activity [37].
It is noteworthy that the antiparallel polymerization mode of MreB protofilaments is a key feature distinguishing it from eukaryotic actin. Crystallographic studies have shown that the Caulobacter MreB protofilament dimer assembles in an antiparallel arrangement and maintains cell shape through tight binding to the membrane. The antibiotics A22 and MP265 inhibit MreB function by binding to regions adjacent to the nucleotide-binding site of MreB, preventing ATP hydrolysis and destabilizing the protofilament dimer [39]. Critical studies reveal that A22 exhibits MreB-independent cytotoxicity and growth inhibition influenced by drug concentration, medium conditions, and bacterial species. In contrast, the structural analog MP265 maintains equivalent MreB disruption efficacy while significantly reducing off-target toxicity. This enhanced specificity allows MP265 to serve as a superior probe for reversible MreB inhibition studies, as demonstrated in Caulobacter crescentus (C. crescentus) models where its withdrawal restores rod morphology via peptidoglycan remodeling [40].
Membrane-Binding properties of MreB
The MreB protein has the ability of membrane-binding, and this ability is crucial for its biological functions. The analysis of MreB’s membrane binding characteristics shows that its nucleotide state regulates membrane interactions, and the key catalytic sites also regulate membrane interactions. Studies have shown that the membrane-binding ability of ScMreB5 is regulated by the ATP binding state and allosterically regulated by the ATP hydrolysis state. ScMreB5WT can form filamentous structures under nucleotide-independent conditions. The ATPase-deficient mutant ScMreB5E134A can also form filaments, but the mutant shows significant defects in lateral interaction, which leads to the disorder of filamentous structure organization. Catalytic glutamate residues (Glu134) have been proven to have dual functions. On the one hand, it senses the ATP-bound filament assembly state in this way; on the other hand, it may trigger filamentous depolymerization by promoting ATP hydrolysis. It is worth noting that the mutation at the Glu134 site leads to differential changes in the liposome binding ability, and the type of nucleotide bound can also cause such changes. This indicates that this site regulates the interaction between MreB and the membrane through allosteric mechanisms. These findings suggest that MreB retains the conserved ATP-dependent polymerization mechanism shared with the actin family. However, it was reprogrammed to dynamically regulate the assembly of the filamentous network on the membrane surface through nucleotide state transitions. It also regulates the organization of the filament network on the membrane surface [41].
Effect of MreB on cell structure and function
Cell wall and morphological maintenance
MreB coordinates peptidoglycan synthesis and cell shape determination
MreB is the central orchestrator of bacterial morphogenesis by spatially organizing the PG synthesis machinery, establishing the structural framework governing cell shape. In rod-shaped bacteria such as E. coli and B. subtilis, MreB assembles into short, membrane-associated filaments that adopt circumferential orientations perpendicular to the long cell axis [22, 31]. Super-resolution microscopy reveals that these filaments undergo rotational dynamics along the inner membrane, with their migration speed inversely correlating to cell width (∼85 nm/s in wild-type vs. ∼45 nm/s in ΔRodZ mutants) [32, 38, 39]. This mechanical feedback regulates diameter homeostasis [42]. At the molecular level, MreB anchors PG synthesis enzymes (such as penicillin-binding protein 2, PBP2) through direct interaction with RodZ, forming discrete PG assembly centers that coordinate lateral wall synthesis [7, 8, 22]. Notably, this system exhibits significant evolutionary plasticity. While C. crescentus MreB drives de novo rod-shape establishment, MreB depletion in spherical bacteria like S. aureus disrupts cell wall synthesis and enlarges cell size but does not alter spherical morphology, reflecting its role in cell expansion rather than shape determination.
While some interesting phenomena are presented in the Mollicutes class, which encompasses four phylogenetic categories—Spiroplasma, Hominis, Pneumoniae, and Acholeplasma/Anaeroplasma/Phytoplasma [43, 44]. These organisms impact the health of both animals [45–50] and plants [51]. For instance, Mycoplasma pneumoniae is a prevalent atypical pneumonia pathogen [52–55]. Additionally, Mycoplasm hominis [56], Mycoplasm genitalium [57, 58], and Ureaplasma urealyticum [59] cause urogenital infections and are associated with various pregnancy complications [60]. Having evolved from Gram-positive bacterial ancestors, Mycoplasma have dispensed with their peptidoglycan layer and undergone genome reduction, resulting in a distinctive wall-less structure [61, 62]. Meantime, Mycoplasma exhibit gliding motility despite the absence of MreB in their genome. Spiroplasma, a member of Mollicutes, characterized by the absence of a cell wall and the presence of a mere membrane bilayer delineating its internal and external environments, represents one of the simplest and smallest prokaryotic organisms identified to date. Spiroplasma relies on MreB5 to transform from a rod-shaped form to a spiral form. The depletion of MreB5 results in the loss of helicity and motility, while its complementation restores kink propagation and cell elongation [10, 40, 63]. This system operates through MreB5-fibril interactions, in which antiparallel protofilaments generate mechanical forces to alter cellular chirality—a mechanism that underscores the species-specific regulatory network [10, 64, 65]. They are prime examples of the minimalistic adaptation of cytoskeletal systems in wall-less environments [44, 61]. The heterologous expression of Spiroplasma MreB5 in Mycoplasm capricolum bestows a helical morphology and kink propagation, emulating Spiroplasma’s motility-related deformations [66]. However, these recombinant cells failed to achieve directional swimming in liquid media, even when co-expressed with fibril [66]. Cryo-electron microscopy confirmed MreB5 filaments anchored to the plasma membrane through direct lipid interactions [17, 65]. However, the lack of coordinated movement implies that extra Spiroplasma-specific elements, such as polar-anchored structures or regulatory proteins, are needed to change local membrane deformations into translational movement [66]. This modular reconstitution underscores MreB5’s capacity to independently apply helicity and membrane dynamics, while emphasizing the necessity of complementary factors for full motility, reflecting the evolutionary adaptations of the cytoskeletal systems in the bacterial lineages. Furthermore, the physical coupling between MreB and the division protein FtsZ achieves spatiotemporal decoupling of the prolonging and division phases, providing a dual guarantee for the fidelity of morphogenesis [67].
Multilayered regulatory networks governing cell wall homeostasis
The MreB system integrates mechanochemical signals with metabolic regulation to maintain the dynamic balance of the cell wall. In B. subtilis, the three MreB paralogs (MreB, Mbl, and MreBH) exhibit functional redundancy in PG synthesis. However, their combined deletion disrupts lateral cell wall assembly, revealing their cooperative roles in environmental adaptation [9]. The biophysical properties of the membrane regulate MreB dynamics through multiple mechanisms. Flotillin-mediated lipid raft formation enhances the fluidity of MreB filaments, while anionic phospholipids restrict their spatial distribution via electrostatic repulsion [68, 69]. Single-molecule tracking reveals distinct kinetic behaviors: MreB exhibits sustained rotational movement guiding cell wall synthesis, whereas PG enzymes transiently bind to insertion sites for glycan chain incorporation [70]. This dynamic coupling operates through mechanochemical feedback: the MreB-RodZ complex senses membrane curvature changes induced by PG synthesis and adaptively adjusts filament trajectories [7, 71]. Post-translational modifications add regulatory complexity: acetylation reduces the size of the PG synthesis zone to fine-tune cell diameter, while phosphorylation may reprogram MreB activity under stress conditions [72].
MreB-Driven morphological diversification in bacterial evolution
The modular architecture of the MreB system enables remarkable morphological diversification across bacteria. In Streptomyces coelicolor MreB, originally utilized for vegetative hyphae, was repurposed during the developmental transition period to mediate spore-specific cell wall remodeling, demonstrating functional plasticity during morphogenesis [73]. C. crescentus exemplifies localized mechanical adaptation by redistributing divisome components to stalk synthesis regions [74]. Pathogenic Chlamydia represents an extreme case: despite lacking canonical FtsZ, it has evolved an alternative division mechanism via MreB-lipid II synthase interactions to adapt to intracellular parasitic life [11]. Similarly, Spiroplasma employs MreB isoforms (MreB1-5) to coordinate the shape and movement of helical cells. Heterologous expression of MreB4-MreB5 or MreB1-MreB5 in the minimal synthetic bacteria (JCVI-syn3B) recapitulated the helical morphology and swimming, demonstrating MreB’s role as a minimal motility module [75]. Additionally, MreB5 interacted with host Rab7 GTPase during Spiroplasma eriocheiris (S. eriocheiris) infection, enhancing phagosome fusion and limiting intracellular replication, revealing its dual roles in pathogenicity and cytoskeletal regulation [76].
The functional plasticity of MreB is further exemplified in S. eriocheiris, a wall-less bacterium. Studies have shown that MreB5 isoforms (e.g., MreB4-MreB5 and MreB1-MreB5) are essential for maintaining helical morphology and generating kink propagation, which drives motility in viscous environments [77]. This modular reconstitution underscores MreB5’s capacity to independently apply helicity and membrane dynamics, while emphasizing the necessity of complementary factors for full motility, reflecting the evolutionary adaptations of the cytoskeletal systems in the bacterial lineages. Intriguingly, MreB loss creates evolutionary trade-offs. Its absence hinders growth efficiency and disrupts β-lactam antibiotic targeting by compromising cell wall integrity, providing new insights into the evolution of antibiotic resistance [78].
Cell membranes and dynamic regulation
Membrane anchoring of MreB: structural insights and species-specific adaptations
The MreB cytoskeleton orchestrates bacterial morphogenesis through dynamic membrane interactions. Structural studies reveal species-specific membrane-binding strategies: T. maritima MreB (TmMreB) employs membrane-insertion loops for anchoring, whereas EcMreB utilizes an N-terminal amphiphilic helix [6]. Despite mechanistic divergence, functional conservation is evident.
In B. subtilis, the heterologously expressed MreB autonomously assembles into membrane-associated filaments [34]. Super-resolution microscopy demonstrates that these filaments adopt dynamic helical geometries, measuring ~ 3.4 μm in length and oriented at 40° relative to the cell circumference. ATPase activity drives the filament to extend at 85 nm/s [79]. Notably, MreB forms antiparallel double filaments on lipid membranes, inducing curvature that facilitates mechanical force propagation [6]. These membrane-bound filaments act as scaffolds to recruit effector molecules, including elongation factor Tu (EF-Tu) and phage protein p16.7, thereby positioning MreB as a central organizer of membrane-associated processes [19, 80].
MreB-Mediated regulation of membrane dynamics and lipid microdomain organization
MreB not only maintains cellular morphology but also modulates membrane biophysical properties, including the formation of specific membrane regions with increased fluidity (RIFs) [24]. The filament of MreB enhances the local membrane fluidity. The depletion of MreB disrupts lipid organization and causes mislocalization of membrane proteins, a functional convergence with the role of cortical actin underlying the eukaryotic cell membrane, which similarly regulates lipid raft dynamics, suggesting an evolutionarily conserved membrane-cytoskeleton crosstalk mechanism [4, 24]. Spatially, MreB is confined to the cell poles through interactions with anionic phospholipids such as phosphatidylglycerol and cardiolipin, where electrostatic repulsion governs its distribution [81]. The membrane curvature further recruits MreB, establishing a geometric feedback loop. Under stress conditions, this system activates in sequence: initially, PspA oligomers gather in cardiolipin-rich polar areas, then move laterally depending on MreB/RodZ, indicating a mechanism for sensing membrane tension [82]. Stress adaptation is tightly coupled with this network. The bacterial flotillin-like protein YqiK notably enhanced membrane fluidity, which in turn increased the mobility of MreB. This lipid-protein synergy directly impacts cytoskeletal dynamics, demonstrating how the membrane physical states modulate MreB functionality [68].
Metabolic integration of MreB in membrane-wall homeostasis
The MreB network tightly connects membrane dynamics with cell wall biosynthesis as a mechanochemical integrator. In Pseudomonas mendocina NK-01, MreB overexpression induces cellular elongation and enhances alginate oligosaccharides production by 5.86-fold compared to wild-type strains [83]; this enhancement correlates with MreB’s role in spatially organizing peptidoglycan synthases. Confocal imaging located MreB-GFP in the periplasmic space, suggesting the mechanochemical coordination between extracellular wall synthesis and intracellular metabolic flux. The evolutionary expansion of MreB’s membrane remodeling function is evidenced by its homologues directing thylakoid biogenesis in cyanobacteria and plants [82, 83]. Pathogens like Chlamydia employ MreB to guide polar peptidoglycan ring assembly, exemplifying its functional plasticity in replacing FtsZ-dependent division. Mechanistically, MreB interacts with the lipid II synthase MurF to enhance the polymerization efficiency, forming a feedback loop to ensure local synthesis at division sites [33, 84].
MreB orchestrates chromosome segregation and division site positioning
MreB coordinates chromosome segregation through direct and indirect mechanisms. In B. subtilis, MreB anchors replisomes in mid-cell to ensure symmetric chromosome partitioning, with its depletion causing replisome mislocalization and segregation defects [85]. Although EcMreB lacks direct DNA binding, it modulates the chromosomal topological structure via topoisomerase IV regulation [86, 87]. Species-specific differences may reflect distinct chromosome organization strategies. The transcription-replication coupling via MreB-RNAP interactions facilitates the directional movement of the bacterial replication origins, while the A22-induced MreB inactivation confirmed its topological role through segregation failure [13, 38]. In Spiroplasma, A22 treatment reduces pathogenicity by disrupting MreB-dependent cell shape, while actin stabilizers (phalloidin) stabilize the MreB filamentous form, enhancing host cells’ invasion and cytotoxicity [88]. Notably, Spiroplasma MreB5 exhibits nucleotide state-dependent filament organization, with ATP hydrolysis regulating membrane binding and disassembly, offering a target for species-specific inhibitors. In cyanobacteria with multiple chromosome copies, MreB enhances the segregation fidelity through restricted diffusion [41, 89].
Pathogen adaptation through functional modularity of the MreB network
The obligate intracellular pathogens exemplify MreB’s evolutionary plasticity. Chlamydia achieves FtsZ-independent division via MreB-guided polar peptidoglycan synthesis, requiring cardiolipin-driven MreB oligomerization [90–92]. The MreB-RodZ complex likely determines the segmentation plane through membrane curvature sensing [93]. Its ability to recruit FtsK demonstrates functional conservation across species, enabling MreB to maintain rod-shaped morphology while coordinating spherical cell division via the MurF-PBP2-FtsK network [11, 94]. Notably, the depletion of MreB induces cell lysis, underscoring its essential role in synchronizing peripheral peptidoglycan synthesis with septation. This property positions MreB as a promising antimicrobial target [92]. Additionally, in the pathogenic Shigella, MreB drives the formation of actin tails by mediating the polar localization of the virulence protein IcsA, thereby facilitating intercellular spread. When MreB polymerization is inhibited by MP265 or A22, the polar distribution of IcsA is disrupted, and bacterial motility is significantly reduced [95]. This suggests that targeting MreB can block the host-invasion mechanisms of pathogens, offering a new strategy for anti-infective therapy [95].
MreB-Associated regulatory complexes: RodZ, MreC/D and functional partnerships
RodZ interaction and function with MreB
RodZ interacts with MreB through the cytoplasmic and periplasmic domains, making it one of the few proteins capable of direct MreB binding [96]. This interaction enhances MreB’s circumferential rotation and polymer stability while modulating its curvature preference [97]. In E. coli, RodZ maintains MreB’s helical assembly pattern, ensuring the occurrence of a uniform cylindrical morphology. Deletion of RodZ causes MreB misassembly into non-helical structures, leading to severe cell shape defects, establishing RodZ as a key regulator of MreB dynamics [98].
Functionally, RodZ bridges the cytoskeleton and cell wall synthesis by coupling MreB to synthases through direct/indirect interactions, thereby coordinating synthase positioning and activity [7]. This coupling mediates MreB’s rotational motion and ensures cell wall synthesis rates match growth demands, promoting uniform cell elongation [99]. For instance, the depletion of RodZ can disrupt MreB-directed wall synthesis, uncoupling growth-division coordination [98]. RodZ further regulates MreB functionality through the geometric localization of polymers along the cell axis [99]. Its interaction with anionic lipids and MreB modulates the spatiotemporal distribution of the membrane stress sensor PspA. This multifactorial synergy enables RodZ to maintain MreB integrity and mediate mechanical stress adaptation. RodZ deletion induces MreB mislocalization, compromising membrane stress regulation and exacerbating morphological defects, ultimately positioning RodZ as the central hub linking MreB function to environmental adaptation [82].
MreB-FtsZ functional relationship
In E. coli, MreB and FtsZ were found to have direct physical binding. This interaction is crucial for the contraction of the Z-ring (the constricting structure formed by FtsZ), and it also helps the cell wall synthesis enzymes transfer from the cell sidewall to the division septum to ensure the normal formation of the septum [67]. This means that the FtsZ-driven division process requires the assistance of MreB to coordinate the cell wall reconstruction, closely coupling cell elongation (the classic function of MreB) with the division event (the core role of FtsZ). For example, when the interaction between the two is disrupted, the division septum cannot be synthesized correctly, resulting in cell division failure. Chlamydia and other spherical bacteria lack FtsZ but retain MreB, revealing the functional plasticity of MreB under extreme conditions. Studies have found that Chlamydia MreB not only directly binds to key enzymes in lipid II biosynthesis such as MurF and MraY, but also can recruit other division proteins such as FtsK by forming a scaffold at the division site and replace FtsZ to become the “general commander” of division [11, 84, 94]. This functional substitution is analogous to a substitute player stepping in, whereby MreB maintains Chlamydia viability in the absence of FtsZ by coordinating peptidoglycan synthesis and divisome assembly.
Although the two cooperate closely, their functions also have obvious differences. FtsZ is mainly responsible for forming and contracting the fission body, while MreB usually maintains the elongation and lateral wall morphology of cells [100]. For example, in B. subtilis, the absence of MreB leads to cell swelling and lysis, while the defect of FtsZ directly blocks division [101]. However, certain toxins such as YeeU-YeeV (CbtA) can target both at the same time and independently inhibit cell elongation and division, indicating that they are in different branches of the same regulatory network [100]. In addition, the dynamic nature of MreB filaments is affected by FtsZ. Mutations in FtsZ alter the localization pattern of MreB, resulting in smaller circumferential bands. This suggests coordination between the two proteins through spatial positioning [92].
In some bacteria such as Rhizobia, abnormal interactions between MreB and FtsZ may lead to abnormal division. For example, when MreB binds to variants of FtsZ, it can impede symbiotic differentiation. This suggests that the cooperative relationship between these two proteins may need to maintain a balance in the course of evolution. Either excessive dependence or interference might result in reduced adaptability [102]. This dynamic balance is also seen in bacteria like cyanobacteria that have multiple chromosomes. MreB can assist FtsZ in completing the division by randomly distributing chromosome copies [89].
The relationship between FtsZ and MreB is akin to that of an “architect” and a “construction team”. FtsZ is like an architect who designs the blueprint for cell division, while MreB acts as the construction team, supplying building materials (peptidoglycan) and adjusting the construction direction. In the classical model, the two have a clear division of labor. However, under evolutionary pressures such as genome reduction or morphological specialization, MreB can “take on important responsibility in times of crisis” and take over part of the functions of FtsZ. This collaborative and standby relationship highlights the flexibility and robustness of bacteria in the division mechanism.
The synergistic mechanism of the MreB/C/D complex and bacterial cell shape maintenance
MreB, MreC and MreD form the core complex that determines the bacterial cell morphology. As an actin-like protein skeleton, MreB directly binds to membrane proteins MreC and MreD, and regulates the peptidoglycan synthesis pathway through interaction with cell wall synthesis-related proteins, such as RodA, MurG and MraY [22]. MreC and MreD have clear divisions of labour during this process. MreC is responsible for the spatial arrangement of peptidoglycan synthases, such as penicillin-binding proteins and transglycosylases, in the periplasmic space, while MreD, as a transmembrane protein, is directly involved in the regulation of lateral peptidoglycan precursor synthesis and combines with precursor synthases such as MurG and MraY. These three proteins ensure the spatial coordination of cell wall synthesis through physical binding and functional complementarity [21, 22].
Not only does MreD participate in the synthesis of peptidoglycan precursors, but it also indirectly affects cell morphology by regulating the localization of MreB [21]. This interdependent localization relationship indicates that the spatial distribution of MreB requires the assistance of MreD, while the function implementation of MreD depends on the skeleton support of MreB. For example, if MreD is missing, MreB may not be able to be correctly anchored in specific regions of the cell membrane, resulting in disordered spatial organization of peptidoglycan synthesis complexes such as MurG-MraY, abnormal cell morphology [21].
MreC and MreB are essential players in the geometric regulation of cell wall synthesis. MreC ensures that newly synthesized peptidoglycan chains are incorporated into the cell wall in a specific pattern by spatially orienting peptidoglycan synthases, such as cleavage transglycosylases, in the periplasmic space [22]. Concurrently, MreB directs cell wall synthesis complexes such as RodA to the designated locations on the membrane through the dynamic cytoplasmic skeletal network. The synergistic interaction between these two proteins facilitates “spatial coordination” and enhances the “efficiency” of peptidoglycan synthesis [22]. Experimental evidence indicates that depletion of MreC or MreB can result in cells adopting a rounded morphology due to their inability to maintain structural rigidity, thereby underscoring the crucial role of these two proteins in maintaining the geometry of cells [103]. While Mbl, a homolog of MreB, is involved in chromosomal segregation, MreC, together with MreD and MreBH (found in B. subtilis), primarily concentrates on preserving cellular shape [103]. The functional specificity associated with complexes containing MreB is illustrated by distinct morphological changes observed upon their depletion; for instance, the loss of both MreC and MreD leads to a transition from rod-shaped to globular forms, while the absence of MreBH results in a Vibrio-like curved phenotype [103]. Through mechanisms independent of chromosome segregation processes, the cooperative action between the MreC/MreD/MreB complex regulates three fundamental aspects: cell wall biogenesis, division site localization, and maintenance of shape homeostasis across spatiotemporal dimensions. This diverse function includes roles such as shape - determining coordinators (defining cell geometry), synthetic frameworks (directing peptidoglycan biosynthesis), and molecular integrators (building interaction networks), thereby ensuring bacterial survival adaptation and their responsiveness to environmental stimuli (Fig. 5).
Fig. 5.
The fundamental mechanism underlying bacterial cell wall synthesis and morphological maintenance involves the interaction and function of key proteins. Green (PspA): A membrane protein regulating mechanical stress via interactions with RodZ and MreB. Red (MreB): A cytoskeletal protein coordinating cell wall synthesis, division site localization, and morphological homeostasis, collaborating with FtsZ. Dark Blue (RodZ): The main regulatory factor for MreB, connecting MreB with cell wall synthetases. Pink (FtsZ): A splitting ring protein driving division ring contraction, relying on MreB for cell wall remodeling. Orange (RodA): A peptidoglycan synthetase inserting and extending peptidoglycan chains, regulated by MreB and RodZ. Purple (MurG): A peptidoglycan precursor synthetase working with MreD. Blue (PBP2): Penicillin-binding proteins in cell wall synthesis, regulated by MreC. Yellow (CbtA): A toxin disrupting membrane pressure sensing and PspA function. Light Gray (FtsZ ring): The division ring facilitating contractile processes. Gray (MreC-MreD complex): A complex organizing peptidoglycan synthetases and regulating precursor synthesis
Environmental modulation of MreB: connecting polymerization dynamics to pathogenic adaptation
Temperature and ionic cues modulate MreB polymerization dynamics
MreB’s polymerization behavior is strongly influenced by environmental signals, especially temperature and ion concentration. Studies indicate that the ultrastructure and polymerization of MreB filaments are heavily dependent on these factors [104]. For example, TmMreB polymerization rate increases significantly with rising temperature, its critical concentration decreases, and it assembles over a wide temperature range. This is distinct from the conclusion of the traditional view that high temperature inhibits polymerization [28]. However, temperature does not act in isolation: monovalent salts such as K⁺ and Na⁺ can inhibit polymerization, while divalent cations such as Mg²⁺ and Ca²⁺ promote nucleation and elongation through a staged mechanism [28, 37]. Interestingly, although BsMreB can be assembled without nucleotides, its polymerization is still inhibited by pH value and monovalent salts, suggesting that different bacterial strains may have evolved differentiated environmental response strategies [37].
Anionic phospholipids, such as phosphatidylglycerol and cardiolipin, act as ‘gatekeepers’ of spatial localization, indirectly controlling cell morphology by regulating the subcellular localization of MreB. In E. coli, these lipids are enriched in the cell poles and preferentially bind to monomeric MreB, thereby excluding the polymerized MreB outside the polar region and maintaining the polar growth of rod-shaped cells [81, 105]. If anionic phospholipids are absent, MreB will be abnormally localizes at the cell poles, leading to the formation of Y-shaped cells during cell division [105]. Further research has found that anionic phospholipids may affect the movement speed of MreB-related complexes, such as the Rod complex by changing membrane fluidity. Moreover, changes in membrane fluidity, such as high temperature or fatty acid synthesis defect can directly interfere with the function of these complexes, leading to abnormal cell morphology [106]. This lipid-protein interaction highlights the close coupling between the physical properties of the membrane and the dynamics of the cytoskeleton.
The synergistic effect between nucleotides and cations
The nucleotide-binding state and cation concentration jointly shape the assembly mode of MreB. For instance, TmMreB requires binding to purine nucleotides (ATP/GTP) to polymerize, for polymerization, yet following assembly, it rapidly hydrolyzes ATP to ADP. Consequently, most MreB filaments within the cell exist in an ADP-bound state [28]. This dynamic hydrolysis may endow the filaments with reversible structural changes. When ADP or GDP is present, MreB tends to form parallel linear protofilaments, while ATP may promote the formation of sheet or bundle structures by regulating electrostatic interactions [16, 107]. Additionally, cation concentrations, particularly those of K⁺ and Na⁺, influence the polymerization rate and determine the morphology of the filament, specifically, bundle structures form when ion concentrations are low, while sheet structures form at high concentrations [16]. This ion dependence has even been developed into a tool for measuring the concentration of cations within prokaryotic cells [108].
Niche-specific adaptations of MreB signaling networks
MreB exhibits marked functional diversity in environmental signals across different bacterial species, reflecting its complex regulatory roles. For instance, when polymerizing independently of nucleotides, BsMreB requires micromolar concentrations of divalent cations, with a critical concentration of ~ 900 nM [37]. In contrast, TmMreB strictly depends on nucleotides for polymerization, operating at a significantly lower critical concentration [28]. Streptococcus pneumoniae (S. pneumoniae) MreB leverages a positively charged C-terminal domain to mediate lateral interactions, and assembly requires specific pH values and Mg²⁺ conditions [16]. In S. eriocheiris, the assembly of MreB5 depends on pH value and ions: under acidic conditions, it inhibits lamellar formation but promotes Mg²⁺-dependent aggregation, while its disordered C-terminal region enhances lateral interactions [16]. Such environmental sensitivity aligns with MreB’s role in adapting to host niches, as evidenced by the reduced pathogenicity of B. subtilis following treatment with actin stabilizers or the MreB-specific inhibitor A22 [88]. These cross-species variations likely represent evolutionary adaptations to ecological niches, underscoring the limitations of relying on a single in vitro model to replicate native in vivo dynamics [109] (Fig. 6).
Fig. 6.
Dynamic regulation network of MreB polymerization and species heterogeneity. MreB polymerization is regulated through temperature-dependent feedback mechanisms, such as the reduction of critical concentration at high temperatures by T. maritima to facilitate stable assembly. It is also influenced by ionic gradient modulation, where monovalent ions inhibit nucleation while divalent ions promote elongation, with ion concentrations determining the microstructure morphology. Additionally, MreB polymerization involves coupling with nucleotide metabolism: ATP and GTP drive the initiation process, ADP helps maintain equilibrium, and the type of nucleotide dictates structural transitions. Different species exhibit heterogeneity: Bacillus depends on high divalent ion concentrations and pH sensitivity, S. pneumoniae forms pH-Mg²⁺-dependent lateral interactions via its positively charged C-terminal domain, and T. maritima relies on nucleotide cycling for low critical concentration adaptation across temperatures. These findings highlight the evolutionary plasticity and environmentally adaptive molecular design in prokaryotic morphogenesis networks
Pharmacological targeting of MreB: from inhibitors to therapeutic strategies
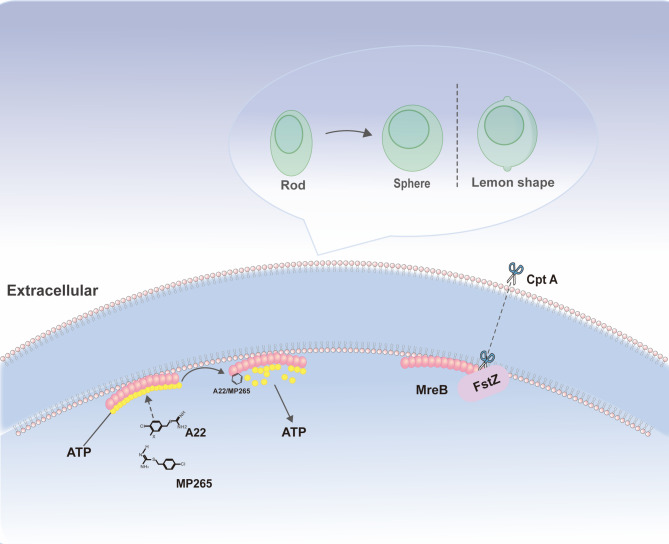
A22 was the first widely studied small molecule inhibitor of MreB. Its mechanism of action revealed the dynamic regulation of environmental signals on the bacterial cytoskeleton. This S-benzyl isothiourea compound does not directly kill bacteria. Instead, it competitively inhibits the binding of ATP to MreB (similar to the ADP-bound state), forcing the rapid depolymerization of MreB filaments in vivo [38]. Interestingly, this “signal interference” does not completely block the synthesis of the cell wall, but instead leads to the transformation of E. coli from rod-shaped to spherical, suggesting that environmental pressure may reshape the bacterial morphology through conformational changes of MreB [110]. The particularity of A22 lies in providing a non-lethal intervention model and opening up the path for the developing of new anti-infective drugs targeting the cytoskeleton [38].
Compound A found in Pseudomonas aeruginosa (P. aeruginosa) further verified the universality of this regulatory strategy. Although it is highly similar in structure to A22, the resistance screening of compound A showed that its activity is unaffected by efflux pumps, which is particularly important for clinical drug resistance problems. The morphological transition from rod-shaped to spherical, induced by environmental cues, may diminish the bacteria’s capacity to invade host cells and decrease endotoxin release. This implies a profound connection between the mechanical signals conveyed by MreB and bacterial pathogenicity. More interestingly, analogues such as 9 and 10, even exceed the ATPase inhibitory potency of the classic inhibitor CBR-4830 (with an IC50 as low as 6 ± 2 µM) for EcMreB, indicating that chemical modification can finely regulate the action strength of such environmental signaling molecules [111, 112].
The regulation of MreB by environmental signals is not limited to exogenous compounds; bacteria have also evolved endogenous regulatory systems. The newly discovered membrane-binding toxin CptA (YgfX) directly blocks the polymerization of MreB and FtsZ through its cytoplasmic domain [113]. This “suicidal” regulation leads to cell swelling and a lemon-shaped appearance. As the first membrane-associated TA system toxin, the transmembrane segment of CptA may sense physical signals such as membrane tension and then transmit them to the cytoskeletal network through MreB [113]. This cross-regulatory network of internal and external signals suggests that the interfering with the bacteria’s stress response mechanisms should be considered in the design of drugs targeting MreB.
Current research reveals that both exogenous compounds (A22/Compound A) and endogenous toxins (CptA) achieve regulation by changing the nucleotide-binding state of MreB. This “signal hijacking” strategy shows potential in combined therapy. The synergistic effect of A22 with clinical antibiotics, or the derivatives obtained through structural optimization with anti-efflux pump properties all provide new ideas for addressing the crisis of drug resistance [112, 114]. The molecular mechanism of A22 involves its competitive binding to a site adjacent to MreB’s nucleotide-binding pocket, effectively inhibiting ATP hydrolysis and destabilizing the antiparallel protofilament dimer structure [38, 39]. This precise targeting of a functionally critical site provides a foundation for rational drug design aimed at disrupting MreB dynamics. The discovery of MreB inhibitors often uses strategies similar to those for FtsZ-targeting compounds. These include phenotypic screening for morphological changes like rod-to-sphere transition, biochemical assays for ATPase inhibition or polymerization disruption, and structure-based virtual screening against the conserved nucleotide-binding pocket to identify novel scaffolds.
Future research requires in-depth analysis of how environmental signals couple cell morphology and metabolic pathways through changes in the ATPase activity of MreB, which will promote the development of more precise “signal-skeleton” regulatory drugs [115] (Fig. 7).
Fig. 7.
Pharmacological Targeting of MreB and Resulting Cellular Morphological Changes. A22 and Mp265 competitively inhibit the binding of ATP to MreB, causing rapid depolymerization of MreB filaments in vivo and leading to a morphological shift of E. coli from rod-shaped to spherical. In contrast, CptA (YgfX) directly blocks the polymerization of MreB and FtsZ, resulting in cell swelling and a lemon-shaped appearance in E. coli
The diversity of MreB-targeting compounds underscores its potential as a therapeutic target. We summarize the key pharmacological agents, their specific targets, mechanisms of action, and resultant antibacterial activities (Table 1). These inhibitors exploit distinct vulnerabilities, competitive ATP binding inhibition (A22, Compound A), ATP hydrolysis blockade and filament destabilization (MP265, CBR-4830), dual inhibition of MreB and FtsZ polymerization (CbtA/CptA), or filament stabilization (Phalloidin). This spectrum of mechanisms leads to characteristic morphological defects such as rod-to-sphere transition, lemon-shaped deformation and attenuation of virulence, highlighting the critical role of a functional MreB cytoskeleton in bacterial survival and pathogenicity.
Table 1.
Antimicrobial molecules targeting MreB and their mechanisms of action
| Molecule Name | Target Specificity | Mechanism of Action | Antibacterial Activity |
|---|---|---|---|
| MP265 (A22 analog) | Directly targets MreB | Binds adjacent to MreB’s nucleotide-binding site, inhibits ATP hydrolysis, and destabilizes antiparallel protofilament dimers | Blocks polar localization of virulence protein IcsA in Shigella, significantly reducing motility and host invasion |
| A22 (S-benzyl isothiourea) | Directly targets MreB | Competitively inhibits ATP binding to MreB, mimics ADP-bound state, and induces filament depolymerization | Induces rod-to-sphere transition in E. coli and Shigella, attenuating virulence |
| Compound A (A22 analog) | Directly targets MreB | ATP-competitive inhibition mechanism similar to A22; evades efflux pump-mediated resistance | Effective against P. aeruginosa; induces morphological defects |
| CbtA/CptA | Dually targets MreB & FtsZ | Membrane-bound toxin whose cytoplasmic domain directly inhibits polymerization of both MreB and FtsZ | Induces cell swelling and lemon-shaped deformation, leading to lysis |
| CBR-4830 | Directly targets MreB | ATPase inhibitor (IC₅₀ = 6 ± 2 µM); disrupts dynamic assembly | Effective against Gram-negative bacteria |
| Phalloidin | Stabilizes MreB filaments | Binds and stabilizes MreB polymers (particularly in Spiroplasma) | Enhances Spiroplasma pathogenicity; low selectivity for bacterial cells |
Conclusion
MreB is a key part of bacterial cellular organization, joining cytoskeletal dynamics with cell wall synthesis, membrane remodeling, and chromosome segregation. Its structural homology with actin belies unique functional adaptations: anti-parallel protofilament assembly, nucleotide-state-dependent membrane interactions, and species-specific roles, such as guiding lipid II synthases in Chlamydia or driving helical motility in Spiroplasma. Environmental plasticity enables MreB to rapidly adapt to ionic gradients, temperature shifts, and membrane composition changes, positioning it as a central hub for stress survival. Pharmacologically, small-molecule inhibitors like A22 target the nucleotide-binding dynamics of MreB, inducing morphological aberrations and attenuating virulence in pathogens like Shigella flexneri. Yet, its evolutionary versatility is most striking: from replacing FtsZ for division to enabling synthetic motility systems, MreB demonstrates how conserved modules can be repurposed across biological contexts. We integrate this functional diversity and emphasized how MreB compensates for the absence of certain cellular mechanisms in evolutionarily distinct bacteria (Table 2).
Table 2.
Functional diversification of MreB paralogs in bacteria
| Category | Typical Species | Protein | Classical Function | Acquired Function | Driving Deficiency | Evolutionary Driver | |
|---|---|---|---|---|---|---|---|
| Rod-shaped bacteria | E. coli, B. subtilis | MreB, Mbl, MreBH | Cell shape maintenance and peptidoglycan synthesis coordination | Chromosome segregation via RNA polymerase (RNAP) coupling and replisome anchoring | None (ancestral function) | Optimization of cytoskeletal multitasking | |
| Wall-less bacteria | Spiroplasma | MreB1–MreB7 (such as MreB5) | Helical motility, kink propagation, and membrane deformation | Absence of cell wall | Loss of rigid wall enables mechanical force-driven motility | ||
| FtsZ-deficient pathogens | Chlamydia | MreB | Peptidoglycan ring assembly and lipid II synthase recruitment | FtsZ-independent division and divisome scaffolding | Absence of FtsZ | Genome reduction in obligate intracellular pathogens | |
| Morphologically plastic bacteria | S. coelicolor | MreB | Vegetative hyphae shape | Spore-specific wall remodeling | Developmental reprogramming | Lifecycle adaptation (hyphae to spores) | |
| Morphologically plastic bacteria | C. crescentus | MreB | Cell elongation | Stalk synthesis | Localized divisome redistribution | Niche adaptation (polar growth) | |
| Pathogen virulence | S. flexneri | MreB | Cell shape maintenance | Actin tail formation (via IcsA) | Host invasion mechanism | Pathoadaptation for intercellular spread | |
To synthesize these multifaceted roles and interactions, we propose a conceptual framework of the MreB system, encompassing its core network, functional outputs, environmental responsiveness, and pharmacological targeting (Fig. 8). MreB serves as the central scaffold of a complex network involving key partners including RodZ, MreC, and FtsZ. This network coordinates fundamental cellular processes, including morphogenesis, peptidoglycan synthesis, chromosome segregation, and division site positioning. Critically, MreB’s function is modulated by environmental cues such as ions, temperature, lipids and can be disrupted by specific inhibitors, such as A22, MP265, Compound A, CptA, CBR-4830 and Phalloidin, leading to loss of shape control and virulence.
Fig. 8.
MreB interaction network and functional outcomes. MreB’s role in core network formation, antibacterial targeting, environmental responses, and function outputs, detailing specific interactions and outcomes
This review consolidates two decades of research, highlighting MreB’s dual identity as a structural scaffold and a regulatory nexus. Furter investigations, resolving its mechanobiological principles and ecological interactions will be essential to unlock its full therapeutic and biotechnological potential.
Future perspectives
Bacterial actin homolog MreB exemplifies the evolutionary adaptability of conserved molecular scaffolds. While its roles in morphogenesis, peptidoglycan synthesis and chromosome segregation are well established, critical gaps remain in understanding its mechanochemical regulation [11, 116]. For instance, how the MreB-RodZ complex converts membrane curvature into localized peptidoglycan synthesis remains unresolved [7, 97]. Advanced techniques, such as molecular dynamics simulations combined with optical tweezers, can map force-dependent conformational changes, while cryo-electron tomography may resolve native-state filament architectures across different species [6, 109]. Addressing these challenges will require integrating advanced imaging techniques such as cryo-electron tomography, with synthetic biology approaches. This integration will enable a detailed dissection of MreB’s mechanochemical signaling networks [109]. The structural differences of MreB homologs, like nucleotide-dependent polymerization in T. maritima and nucleotide-independence in B. subtilis, show the need to conduct comparative studies to develop species-specific antimicrobials [28, 37]. Recent work on Chlamydia MreB, which compensates for FtsZ absence by coordinating lipid II synthase activity, underscores its potential as a therapeutic target [11, 91]. However, challenges persist: MreB-depleted E. coli develops β-lactam tolerance via efflux pumps, necessitating combinatorial therapies [101, 115]. Emerging tools like optogenetics and synthetic biology approaches could further harness MreB’s modularity for biotechnological applications, such as programmable morphogenesis or enhanced metabolite production [66, 75, 83]. Future efforts must also address ecological and evolutionary dimensions. Laboratory evolution under antibiotic stress and metagenomic analyses of environmental niches may reveal conserved stress responsive motifs [78]. These insights will refine phylogenetic modeling and resistance prediction. Furthermore, MreB potentially assumes critical functions in bacteria without a cell wall or lacking most proteins from the divisome complex. Future studies could be directed towards investigating this area. This comprehensive overview establishes the framework for future research on the structure and construction of microorganisms.
Acknowledgements
Not applicable.
Authors' contributions
YQW: Writing – original draft, Project administration. ZXS and YLJ: Software and Visualization. CBZ and YJT: Revision. JFP and PL: Funding, Conception and Supervision.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) [32370209]; Natural Science Foundation of Hunan Province, China [2023JJ30503]; The Scientific Research Foundation of Hunan Provincial Education Department, China [22A0297]; Guidance Plan Project of Hengyang City, China [2024017529].
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jiaofeng Peng, Email: 382278527@qq.com.
Peng Liu, Email: pengliu@live.cn.
References
- 1.Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326(5957):1208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature. 2010;463(7280):485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Ent F, Amos LA, Lowe J. Prokaryotic origin of the actin cytoskeleton. Nature. 2001;413(6851):39–44. [DOI] [PubMed] [Google Scholar]
- 4.Jones LJ, Carballido-López R, Errington J. Control of cell shape in bacteria: helical, actin-like filaments in Bacillus subtilis. Cell. 2001;104(6):913–22. [DOI] [PubMed] [Google Scholar]
- 5.Bork P, Sander C, Valencia A. An ATPase domain common to prokaryotic cell cycle proteins, sugar kinases, actin, and hsp70 heat shock proteins. Proc Natl Acad Sci U S A. 1992;89(16):7290–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salje J, van den Ent F, de Boer P, Löwe J. Direct membrane binding by bacterial actin MreB. Mol Cell. 2011;43(3):478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgenstein RM, Bratton BP, Nguyen JP, Ouzounov N, Shaevitz JW, Gitai Z. RodZ links MreB to cell wall synthesis to mediate MreB rotation and robust morphogenesis. Proc Natl Acad Sci U S A. 2015;112(40):12510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varma A, Young KD. In Escherichia coli, MreB and FtsZ direct the synthesis of lateral cell wall via independent pathways that require PBP 2. J Bacteriol. 2009;191(11):3526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawai Y, Asai K, Errington J. Partial functional redundancy of MreB isoforms, mreb, Mbl and mrebh, in cell morphogenesis of Bacillus subtilis. Mol Microbiol. 2009;73(4):719–31. [DOI] [PubMed] [Google Scholar]
- 10.Harne S, Duret S, Pande V, Bapat M, Béven L, Gayathri P. MreB5 is a determinant of Rod-to-Helical transition in the Cell-Wall-less bacterium Spiroplasma. Curr Biol. 2020;30(23):4753–e47624757. [DOI] [PubMed] [Google Scholar]
- 11.Gaballah A, Kloeckner A, Otten C, Sahl HG, Henrichfreise B. Functional analysis of the cytoskeleton protein MreB from Chlamydophila pneumoniae. PLoS ONE. 2011;6(10):e25129. [DOI] [PMC free article] [PubMed]
- 12.van den Ent F, Amos LA, Löwe J. Prokaryotic origin of the actin cytoskeleton. Nature. 2001;413(6851):39–44. [DOI] [PubMed]
- 13.Kruse T, Blagoev B, Løbner-Olesen A, Wachi M, Sasaki K, Iwai N, Mann M, Gerdes K. Actin homolog MreB and RNA polymerase interact and are both required for chromosome segregation in Escherichia coli. Genes Dev. 2006;20(1):113–24. [DOI] [PMC free article] [PubMed]
- 14.Takahashi D, Miyata M. Sequence analyses of a lipoprotein conserved with bacterial actins responsible for swimming motility of wall-less helical Spiroplasma. MicroPubl Biol 2023, 2023. [DOI] [PMC free article] [PubMed]
- 15.Takahashi D, Fujiwara I, Miyata M. Phylogenetic origin and sequence features of MreB from the wall-less swimming bacteria Spiroplasma. Biochem Biophys Res Commun. 2020;533(4):638–44. [DOI] [PubMed]
- 16.Takahashi D, Miyata M, Fujiwara I. Assembly properties of bacterial actin MreB involved in Spiroplasma swimming motility. J Biol Chem. 2023;299(6):104793. [DOI] [PMC free article] [PubMed]
- 17.Takahashi D, Fujiwara I, Sasajima Y, Narita A, Imada K, Miyata M. ATP-dependent polymerization dynamics of bacterial actin proteins involved in Spiroplasma swimming. Open Biol. 2022;12(10):220083. [DOI] [PMC free article] [PubMed]
- 18.Mao W, Renner LD, Cornilleau C, Li de la Sierra-Gallay I, Afensiss S, Benlamara S, Ah-Seng Y, Van Tilbeurgh H, Nessler S, Bertin A et al. On the role of nucleotides and lipids in the polymerization of the actin homolog MreB from a Gram-positive bacterium. Elife 2023, 12. [DOI] [PMC free article] [PubMed]
- 19.Muñoz-Espín D, Daniel R, Kawai Y, Carballido-López R, Castilla-Llorente V, Errington J, Meijer WJ, Salas M. The actin-like MreB cytoskeleton organizes viral DNA replication in bacteria. Proc Natl Acad Sci U S A. 2009;106(32):13347–52. [DOI] [PMC free article] [PubMed]
- 20.Baranova N, Radler P, Hernández-Rocamora VM, Alfonso C, López-Pelegrín M, Rivas G, Vollmer W, Loose M. Diffusion and capture permits dynamic coupling between treadmilling FtsZ filaments and cell division proteins. Nat Microbiol. 2020;5(3):407–17. [DOI] [PMC free article] [PubMed]
- 21.White CL, Kitich A, Gober JW. Positioning cell wall synthetic complexes by the bacterial morphogenetic proteins MreB and MreD. Mol Microbiol. 2010;76(3):616–33. [DOI] [PubMed] [Google Scholar]
- 22.Divakaruni AV, Baida C, White CL, Gober JW. The cell shape proteins MreB and MreC control cell morphogenesis by positioning cell wall synthetic complexes. Mol Microbiol. 2007;66(1):174–88. [DOI] [PubMed] [Google Scholar]
- 23.Figge RM, Divakaruni AV, Gober JW. MreB, the cell shape-determining bacterial actin homologue, co-ordinates cell wall morphogenesis in caulobacter crescentus. Mol Microbiol. 2004;51(5):1321–32. [DOI] [PubMed] [Google Scholar]
- 24.Strahl H, Bürmann F, Hamoen LW. The actin homologue MreB organizes the bacterial cell membrane. Nat Commun. 2014;5:3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dominguez R, Holmes KC. Actin structure and function. Annu Rev Biophys. 2011;40:169–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holmes KC, Popp D, Gebhard W, Kabsch W. Atomic model of the actin filament. Nature. 1990;347(6288):44–9. [DOI] [PubMed] [Google Scholar]
- 27.Pollard TD, Cooper JA. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. [DOI] [PubMed] [Google Scholar]
- 28.Bean GJ, Amann KJ. Polymerization properties of the thermotoga maritima actin mreb: roles of temperature, nucleotides, and ions. Biochemistry. 2008;47(2):826–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gitai Z. The new bacterial cell biology: moving parts and subcellular architecture. Cell. 2005;120(5):577–86. [DOI] [PubMed] [Google Scholar]
- 30.Abhayawardhane Y, Stewart GC. Bacillus subtilis possesses a second determinant with extensive sequence similarity to the Escherichia coli MreB morphogene. J Bacteriol. 1995;177(3):765–73. [DOI] [PMC free article] [PubMed]
- 31.Kruse T, Møller-Jensen J, Løbner-Olesen A, Gerdes K. Dysfunctional MreB inhibits chromosome segregation in Escherichia coli. Embo J. 2003;22(19):5283–92. [DOI] [PMC free article] [PubMed]
- 32.Schirner K, Errington J. Influence of heterologous MreB proteins on cell morphology of Bacillus subtilis. Microbiol (Reading). 2009;155(Pt 11):3611–21. [DOI] [PubMed]
- 33.Soufo HJ, Graumann PL. Bacillus subtilis MreB paralogues have different filament architectures and lead to shape remodelling of a heterologous cell system. Mol Microbiol. 2010;78(5):1145–58. [DOI] [PubMed]
- 34.Dempwolff F, Reimold C, Reth M, Graumann PL. Bacillus subtilis MreB orthologs self-organize into filamentous structures underneath the cell membrane in a heterologous cell system. PLoS ONE. 2011;6(11):e27035. [DOI] [PMC free article] [PubMed]
- 35.Duan Y, Sperber AM, Herman JK. YodL and YisK possess Shape-Modifying activities that are suppressed by mutations in Bacillus subtilis MreB and Mbl. J Bacteriol. 2016;198(15):2074–88. [DOI] [PMC free article] [PubMed]
- 36.Mao W, Renner LD, Cornilleau C, Li de la Sierra-Gallay I, Benlamara S, Ah-Seng Y, Van Tilbeurgh H, Nessler S, Bertin A, Chastanet A et al. Polymerization cycle of actin homolog MreB from a Gram-positive bacterium. bioRxiv 2022:2022.2010.2019.512861. [DOI] [PMC free article] [PubMed]
- 37.Mayer JA, Amann KJ. Assembly properties of the Bacillus subtilis actin, MreB. Cell Motil Cytoskeleton. 2009;66(2):109–18. [DOI] [PubMed]
- 38.Bean GJ, Flickinger ST, Westler WM, McCully ME, Sept D, Weibel DB, Amann KJ. A22 disrupts the bacterial actin cytoskeleton by directly binding and inducing a low-affinity state in MreB. Biochemistry. 2009;48(22):4852–7. [DOI] [PMC free article] [PubMed]
- 39.van den Ent F, Izoré T, Bharat TA, Johnson CM, Löwe J. Bacterial actin MreB forms antiparallel double filaments. Elife. 2014;3:e02634. [DOI] [PMC free article] [PubMed]
- 40.Takacs CN, Poggio S, Charbon G, Pucheault M, Vollmer W, Jacobs-Wagner C. MreB drives de Novo rod morphogenesis in caulobacter crescentus via remodeling of the cell wall. J Bacteriol. 2010;192(6):1671–84. [DOI] [PMC free article] [PubMed]
- 41.Pande V, Mitra N, Bagde SR, Srinivasan R, Gayathri P. Filament organization of the bacterial actin MreB is dependent on the nucleotide state. J Cell Biol 2022, 221(5). [DOI] [PMC free article] [PubMed]
- 42.Kurita K, Shin R, Tabei T, Shiomi D. Relation between rotation of MreB actin and cell width of Escherichia coli. Genes Cells. 2019;24(3):259–65. [DOI] [PubMed]
- 43.Zhang S, Chen J, Jiang Y, Li Y, Zhu C, Tang Y, Wang Y, Huang H, Zhong K, Xiong Y, et al. Unraveling Spiroplasma eriocheiris: morphological motility, virulence mechanisms, and host defense strategies in crustacean pathogenesis. Aquaculture. 2025;606:742580.
- 44.Miyata M, Hamaguchi T. Integrated information and prospects for gliding mechanism of the pathogenic bacterium Mycoplasma pneumoniae. Front Microbiol. 2016;7:960. [DOI] [PMC free article] [PubMed]
- 45.Luo H, He J, Qin L, Chen Y, Chen L, Li R, Zeng Y, Zhu C, You X, Wu Y. Mycoplasma pneumoniae lipids license TLR-4 for activation of NLRP3 inflammasome and autophagy to evoke a Proinflammatory response. Clin Exp Immunol. 2021;203(1):66–79. [DOI] [PMC free article] [PubMed]
- 46.Yu Y, Wang J, Han R, Wang L, Zhang L, Zhang AY, Xin J, Li S, Zeng Y, Shao G, et al. Mycoplasma hyopneumoniae evades complement activation by binding to factor H via elongation factor thermo unstable (EF-Tu). Virulence. 2020;11(1):1059–74. [DOI] [PMC free article] [PubMed]
- 47.He J, Wang S, Zeng Y, You X, Ma X, Wu N, Wu Y. Binding of CD14 to Mycoplasma genitalium-derived lipid-associated membrane proteins upregulates TNF-α. Inflammation. 2014;37(2):322–30. [DOI] [PubMed]
- 48.Huang J, Zhu H, Wang J, Guo Y, Zhi Y, Wei H, Li H, Guo A, Liu D, Chen X. Fructose-1,6-bisphosphate aldolase is involved in Mycoplasma Bovis colonization as a fibronectin-binding adhesin. Res Vet Sci. 2019;124:70–8. [DOI] [PubMed]
- 49.He J, Xiu F, Chen Y, Yang Y, Liu H, Xi Y, Liu L, Li X, Wu Y, Luo H, et al. Aerobic Glycolysis of bronchial epithelial cells rewires Mycoplasma pneumoniae pneumonia and promotes bacterial elimination. Infect Immun. 2024;92(2):e0024823. [DOI] [PMC free article] [PubMed]
- 50.Hu J, Chen C, Ou G, You X, Tan T, Hu X, Zeng Y, Yu M, Zhu C. Nrf2 regulates the inflammatory response, including Heme oxygenase-1 induction, by Mycoplasma pneumoniae lipid-associated membrane proteins in THP-1 cells. Pathog Dis 2017, 75(4). [DOI] [PubMed]
- 51.You Y, Xiao J, Chen J, Li Y, Li R, Zhang S, Jiang Q, Liu P. Integrated information for pathogenicity and treatment of Spiroplasma. Curr Microbiol. 2024;81(8):252. [DOI] [PubMed]
- 52.Qin L, Liu L, Wu Y, Chen Y, Wu Y, Luo H, Xi Y, Xiu F, Hu J, Chen L, et al. Mycoplasma pneumoniae downregulates RECK to promote matrix metalloproteinase-9 secretion by bronchial epithelial cells. Virulence. 2022;13(1):1270–84. [DOI] [PMC free article] [PubMed]
- 53.Luo Y, Li C, Zhou Z, Gong Z, Zhu C, Lei A. Biological functions of IL-17-producing cells in Mycoplasma respiratory infection. Immunology. 2021;164(2):223–30. [DOI] [PMC free article] [PubMed]
- 54.Chen Y, Wu Y, Qin L, Yu L, Luo H, Li Y, Wang K, Chen L, Zhu C, He J, et al. T-B cell epitope peptides induce protective immunity against Mycoplasma pneumoniae respiratory tract infection in balb/c mice. Immunobiology. 2021;226(3):152077. [DOI] [PubMed]
- 55.Hu X, Zeng W, You X, Ding W, Liu P, Chen L, Zeng Y, Zhu C. Exogenous hydrogen sulfide regulates Mycoplasma pneumoniae Lipid-Associated membrane proteins to induce expression of Heme Oxygenase-1 and Proinflammatory cytokines. Inflammation. 2020;43(3):847–56. [DOI] [PubMed]
- 56.Zeng T, Wu Y, Yang Z, Luo M, Xu C, Liu Z, Ouyang J, Liu L, Zhang X. Clinical and Microbiological characterization of bloodstream infections caused by Mycoplasma hominis: an overlooked pathogen. Infect Dis Ther. 2022;11(3):1003–17. [DOI] [PMC free article] [PubMed]
- 57.Deng X, Dai P, Yu M, Chen L, Zhu C, You X, Li L, Zeng Y. Cyclophilin A is the potential receptor of the Mycoplasma genitalium adhesion protein. Int J Med Microbiol. 2018;308(3):405–12. [DOI] [PubMed]
- 58.Wang X, Hu Y, Li X, Huang L, Yang Y, Liu C, Deng Q, Yang P, Li Y, Zhou Y, et al. Mycoplasma genitalium membrane lipoprotein induces GAPDH malonylation in urethral epithelial cells to regulate cytokine response. Microb Pathog. 2024;195:106872. [DOI] [PubMed]
- 59.Tang Y, Guo F, Lei A, Xiang J, Liu P, Ten W, Dai G, Li R. GrpE immunization protects against Ureaplasma urealyticum infection in BALB/C mice. Front Immunol. 2020;11:1495. [DOI] [PMC free article] [PubMed]
- 60.Qiu Y, Mao S, Li X, Chen Y, Chen W, Wen Y, Liu P. Chinese advances in Understanding and managing genitourinary tract infections caused by Mycoplasma genitalium, Mycoplasma hominis, and Ureaplasma urealyticum. Arch Microbiol. 2024;207(1):5. [DOI] [PubMed]
- 61.Miyata M, Ogaki H. Cytoskeleton of mollicutes. J Mol Microbiol Biotechnol. 2006;11(3–5):256–64. [DOI] [PubMed]
- 62.Hu J, Ye Y, Chen X, Xiong L, Xie W, Liu P. Insight into the pathogenic mechanism of Mycoplasma pneumoniae. Curr Microbiol. 2022;80(1):14. [DOI] [PMC free article] [PubMed]
- 63.Yepes A, Koch G, Waldvogel A, Garcia-Betancur JC, Lopez D. Reconstruction of MreB expression in Staphylococcus aureus via a collection of new integrative plasmids. Appl Environ Microbiol. 2014;80(13):3868–78. [DOI] [PMC free article] [PubMed]
- 64.Sasajima Y, Kato T, Miyata T, Kawamoto A, Namba K, Miyata M. Isolation and structure of the fibril protein, a major component of the internal ribbon for Spiroplasma swimming. Front Microbiol. 2022;13:1004601. [DOI] [PMC free article] [PubMed]
- 65.Sasajima Y, Miyata M. Prospects for the mechanism of Spiroplasma swimming. Front Microbiol. 2021;12:706426. [DOI] [PMC free article] [PubMed]
- 66.Lartigue C, Lambert B, Rideau F, Dahan Y, Decossas M, Hillion M, Douliez JP, Hardouin J, Lambert O, Blanchard A, et al. Cytoskeletal components can turn wall-less spherical bacteria into kinking helices. Nat Commun. 2022;13(1):6930. [DOI] [PMC free article] [PubMed]
- 67.Fenton AK, Gerdes K. Direct interaction of FtsZ and MreB is required for septum synthesis and cell division in Escherichia coli. Embo J. 2013;32(13):1953–65. [DOI] [PMC free article] [PubMed]
- 68.Zielińska A, Savietto A, de Sousa Borges A, Martinez D, Berbon M, Roelofsen JR, Hartman AM, de Boer R, Van der Klei IJ, Hirsch AK et al. Flotillin-mediated membrane fluidity controls peptidoglycan synthesis and MreB movement. Elife 2020, 9. [DOI] [PMC free article] [PubMed]
- 69.van Teeffelen S, Gitai Z. Rotate into shape: MreB and bacterial morphogenesis. Embo J. 2011;30(24):4856–7. [DOI] [PMC free article] [PubMed]
- 70.Dersch S, Mehl J, Stuckenschneider L, Mayer B, Roth J, Rohrbach A, Graumann PL. Super-Resolution Microscopy and Single-Molecule Tracking Reveal Distinct Adaptive Dynamics of MreB and of Cell Wall-Synthesis Enzymes. Front Microbiol 2020, 11:1946. [DOI] [PMC free article] [PubMed]
- 71.Ouzounov N, Nguyen JP, Bratton BP, Jacobowitz D, Gitai Z, Shaevitz JW. MreB orientation correlates with cell diameter in Escherichia coli. Biophys J. 2016;111(5):1035–43. [DOI] [PMC free article] [PubMed]
- 72.Carabetta VJ, Greco TM, Tanner AW, Cristea IM, Dubnau D. Temporal regulation of the Bacillus subtilis acetylome and evidence for a role of MreB acetylation in cell wall growth. mSystems 2016, 1(3). [DOI] [PMC free article] [PubMed]
- 73.Mazza P, Noens EE, Schirner K, Grantcharova N, Mommaas AM, Koerten HK, Muth G, Flärdh K, van Wezel GP, Wohlleben W. MreB of streptomyces coelicolor is not essential for vegetative growth but is required for the integrity of aerial hyphae and spores. Mol Microbiol. 2006;60(4):838–52. [DOI] [PubMed]
- 74.Billini M, Biboy J, Kühn J, Vollmer W, Thanbichler M. A specialized MreB-dependent cell wall biosynthetic complex mediates the formation of stalk-specific peptidoglycan in caulobacter crescentus. PLoS Genet. 2019;15(2):e1007897. [DOI] [PMC free article] [PubMed]
- 75.Kiyama H, Kakizawa S, Sasajima Y, Tahara YO, Miyata M. Reconstitution of a minimal motility system based on Spiroplasma swimming by two bacterial actins in a synthetic minimal bacterium. Sci Adv. 2022;8(48):eabo7490. [DOI] [PMC free article] [PubMed]
- 76.Ma Y, Zhou Z, Luo T, Meng Q, Wang H, Li X, Gu W, Zhou J, Meng Q. Rab7 gtpase, a direct target of miR-131-3p, limits intracellular Spiroplasma eriocheiris infection by modulating phagocytosis. Fish Shellfish Immunol. 2024;154:109879. [DOI] [PubMed]
- 77.Zhang S, Chen J, Jiang Y, Li Y, Zhu C, Tang Y, Wang Y, Huang H, Zhong K, Xiong Y et al. Unraveling Spiroplasma eriocheiris: morphological motility, virulence mechanisms, and host defense strategies in crustacean pathogenesis. Aquaculture 2025, 606.
- 78.Barton B, Grinnell A, Morgenstein RM. Disruption of the MreB elongasome is overcome by mutations in the Tricarboxylic acid cycle. Front Microbiol. 2021;12:664281. [DOI] [PMC free article] [PubMed]
- 79.Reimold C, Defeu Soufo HJ, Dempwolff F, Graumann PL. Motion of variable-length MreB filaments at the bacterial cell membrane influences cell morphology. Mol Biol Cell. 2013;24(15):2340–9. [DOI] [PMC free article] [PubMed]
- 80.Defeu Soufo HJ, Reimold C, Linne U, Knust T, Gescher J, Graumann PL. Bacterial translation elongation factor EF-Tu interacts and colocalizes with actin-like MreB protein. Proc Natl Acad Sci U S A. 2010;107(7):3163–8. [DOI] [PMC free article] [PubMed]
- 81.Shiomi D. Polar localization of MreB actin is inhibited by anionic phospholipids in the rod-shaped bacterium Escherichia coli. Curr Genet. 2017;63(5):845–8. [DOI] [PubMed]
- 82.Jovanovic G, Mehta P, Ying L, Buck M. Anionic lipids and the cytoskeletal proteins MreB and RodZ define the spatio-temporal distribution and function of membrane stress controller PspA in Escherichia coli. Microbiol (Reading). 2014;160(Pt 11):2374–86. [DOI] [PubMed]
- 83.Fan X, Gong T, Wu Y, Zhao F, Qiao M, Wang S, Yang C. Enhanced synthesis of alginate oligosaccharides in Pseudomonas mendocina NK-01 by overexpressing MreB. 3 Biotech. 2019;9(9):344. [DOI] [PMC free article] [PubMed]
- 84.Lee J, Cox JV, Ouellette SP. Critical role for the extended N terminus of chlamydial MreB in directing its membrane association and potential interaction with divisome proteins. J Bacteriol 2020, 202(9). [DOI] [PMC free article] [PubMed]
- 85.Defeu Soufo HJ, Graumann PL. Bacillus subtilis actin-like protein MreB influences the positioning of the replication machinery and requires membrane proteins mrec/d and other actin-like proteins for proper localization. BMC Cell Biol. 2005;6(1):10. [DOI] [PMC free article] [PubMed]
- 86.Karczmarek A, Martínez-Arteaga R, Alexeeva S, Hansen FG, Vicente M, Nanninga N, den Blaauwen T. DNA and origin region segregation are not affected by the transition from rod to sphere after Inhibition of Escherichia coli MreB by A22. Mol Microbiol. 2007;65(1):51–63. [DOI] [PubMed]
- 87.Madabhushi R, Marians KJ. Actin homolog MreB affects chromosome segregation by regulating topoisomerase IV in Escherichia coli. Mol Cell. 2009;33(2):171–80. [DOI] [PMC free article] [PubMed]
- 88.Li R, Cao X, Chen J, He T, Zhang Y, Wang W, Wang Y, Wang Y, Qiu Y, Xie M, et al. Deciphering the impact of MreB on the morphology and pathogenicity of the aquatic pathogen Spiroplasma eriocheiris. Biol Direct. 2024;19(1):98. [DOI] [PMC free article] [PubMed]
- 89.Hu B, Yang G, Zhao W, Zhang Y, Zhao J. MreB is important for cell shape but not for chromosome segregation of the filamentous Cyanobacterium Anabaena sp. PCC 7120. Mol Microbiol. 2007;63(6):1640–52. [DOI] [PubMed]
- 90.Liechti G, Kuru E, Packiam M, Hsu YP, Tekkam S, Hall E, Rittichier JT, VanNieuwenhze M, Brun YV, Maurelli AT. Pathogenic chlamydia lack a classical sacculus but synthesize a narrow, Mid-cell peptidoglycan ring, regulated by mreb, for cell division. PLoS Pathog. 2016;12(5):e1005590. [DOI] [PMC free article] [PubMed]
- 91.Ouellette SP, Fisher-Marvin LA, Harpring M, Lee J, Rucks EA, Cox JV. Localized Cardiolipin synthesis is required for the assembly of MreB during the polarized cell division of chlamydia trachomatis. PLoS Pathog. 2022;18(9):e1010836. [DOI] [PMC free article] [PubMed]
- 92.Velázquez-Suárez C, Luque I, Herrero A. The role of mreb, MreC and MreD in the morphology of the diazotrophic filament of Anabaena sp. PCC 7120. Life (Basel) 2022, 12(9). [DOI] [PMC free article] [PubMed]
- 93.Ranjit DK, Liechti GW, Maurelli AT. Chlamydial MreB directs cell division and peptidoglycan synthesis in Escherichia coli in the absence of FtsZ activity. mBio 2020, 11(1). [DOI] [PMC free article] [PubMed]
- 94.Ouellette SP, Karimova G, Subtil A, Ladant D. Chlamydia co-opts the rod shape-determining proteins MreB and Pbp2 for cell division. Mol Microbiol. 2012;85(1):164–78. [DOI] [PubMed] [Google Scholar]
- 95.Krokowski S, Atwal S, Lobato-Márquez D, Chastanet A, Carballido-López R, Salje J, Mostowy S. Shigella MreB promotes Polar IcsA positioning for actin tail formation. J Cell Sci 2019, 132(9). [DOI] [PMC free article] [PubMed]
- 96.van den Ent F, Johnson CM, Persons L, de Boer P, Löwe J. Bacterial actin MreB assembles in complex with cell shape protein RodZ. Embo J. 2010;29(6):1081–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Colavin A, Shi H, Huang KC. RodZ modulates geometric localization of the bacterial actin MreB to regulate cell shape. Nat Commun. 2018;9(1):1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bendezú FO, Hale CA, Bernhardt TG, de Boer PA. RodZ (YfgA) is required for proper assembly of the MreB actin cytoskeleton and cell shape in E. coli. Embo J. 2009;28(3):193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bratton BP, Shaevitz JW, Gitai Z, Morgenstein RM. MreB polymers and curvature localization are enhanced by RodZ and predict E. coli’s cylindrical uniformity. Nat Commun. 2018;9(1):2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heller DM, Tavag M, Hochschild A. CbtA toxin of Escherichia coli inhibits cell division and cell elongation via direct and independent interactions with FtsZ and MreB. PLoS Genet. 2017;13(9):e1007007. [DOI] [PMC free article] [PubMed]
- 101.Formstone A, Errington J. A magnesium-dependent MreB null mutant: implications for the role of MreB in Bacillus subtilis. Mol Microbiol. 2005;55(6):1646–57. [DOI] [PubMed]
- 102.Zhao W, Zhu H, Wei F, Zhou D, Li Y, Zhang XX. Investigating the involvement of cytoskeletal proteins MreB and FtsZ in the origin of Legume-Rhizobial symbiosis. Mol Plant Microbe Interact. 2021;34(5):547–59. [DOI] [PubMed]
- 103.Soufo HJ, Graumann PL. Actin-like proteins MreB and Mbl from Bacillus subtilis are required for bipolar positioning of replication origins. Curr Biol. 2003;13(21):1916–20. [DOI] [PubMed]
- 104.Esue O, Cordero M, Wirtz D, Tseng Y. The assembly of mreb, a prokaryotic homolog of actin. J Biol Chem. 2005;280(4):2628–35. [DOI] [PubMed]
- 105.Kawazura T, Matsumoto K, Kojima K, Kato F, Kanai T, Niki H, Shiomi D. Exclusion of assembled MreB by anionic phospholipids at cell Poles confers cell Polarity for bidirectional growth. Mol Microbiol. 2017;104(3):472–86. [DOI] [PubMed]
- 106.Kurita K, Kato F, Shiomi D. Alteration of membrane fluidity or phospholipid composition perturbs rotation of MreB complexes in Escherichia coli. Front Mol Biosci. 2020;7:582660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Popp D, Narita A, Maeda K, Fujisawa T, Ghoshdastider U, Iwasa M, Maéda Y, Robinson RC. Filament structure, organization, and dynamics in MreB sheets. J Biol Chem. 2010;285(21):15858–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barkó S, Szatmári D, Bódis E, Türmer K, Ujfalusi Z, Popp D, Robinson RC, Nyitrai M. Large-scale purification and in vitro characterization of the assembly of MreB from leptospira interrogans. Biochim Biophys Acta. 2016;1860(9):1942–52. [DOI] [PubMed] [Google Scholar]
- 109.Tesson B, Dajkovic A, Keary R, Marlière C, Dupont-Gillain CC, Carballido-López R. Magnesium rescues the morphology of Bacillus subtilis MreB mutants through its inhibitory effect on peptidoglycan hydrolases. Sci Rep. 2022;12(1):1137. [DOI] [PMC free article] [PubMed]
- 110.Noguchi N, Yanagimoto K, Nakaminami H, Wakabayashi M, Iwai N, Wachi M, Sasatsu M. Anti-infectious effect of S-benzylisothiourea compound A22, which inhibits the actin-like protein, mreb, in Shigella flexneri. Biol Pharm Bull. 2008;31(7):1327–32. [DOI] [PubMed] [Google Scholar]
- 111.Yamachika S, Sugihara C, Tsuji H, Muramatsu Y, Kamai Y, Yamashita M. Anti-Pseudomonas aeruginosa compound, 1,2,3,4-tetrahydro-1,3,5-triazine derivative, exerts its action by primarily targeting MreB. Biol Pharm Bull. 2012;35(10):1740–4. [DOI] [PubMed]
- 112.Sagong HY, Rosado-Lugo JD, Bryan EJ, Ferrer-González E, Wang Y, Cao Y, Parhi AK, Pilch DS, LaVoie EJ. Novel MreB inhibitors with antibacterial activity against gram (-) bacteria. Med Chem Res. 2022;31(10):1679–704. [DOI] [PMC free article] [PubMed]
- 113.Masuda H, Tan Q, Awano N, Yamaguchi Y, Inouye M. A novel membrane-bound toxin for cell division, CptA (YgfX), inhibits polymerization of cytoskeleton proteins, FtsZ and mreb, in Escherichia coli. FEMS Microbiol Lett. 2012;328(2):174–81. [DOI] [PMC free article] [PubMed]
- 114.Awuni E, Mu Y. Effect of A22 on the conformation of bacterial actin MreB. Int J Mol Sci 2019, 20(6). [DOI] [PMC free article] [PubMed]
- 115.Kotzialampou A, Protonotariou E, Skoura L, Sivropoulou A. Synergistic antibacterial and antibiofilm activity of the MreB inhibitor A22 hydrochloride in combination with conventional antibiotics against Pseudomonas aeruginosa and Escherichia coli clinical isolates. Int J Microbiol. 2021;2021:3057754. [DOI] [PMC free article] [PubMed]
- 116.Typas A, Banzhaf M, Gross CA, Vollmer W. From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nat Rev Microbiol. 2011;10(2):123–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.