Abstract
Background
The relationship between clinical pregnancy rates and embryo transfer positioning remains a matter of considerable debate, with persistent inconsistencies reported across existing studies. A notable limitation of existing studies is their failure to systematically account for anatomical variations in the endometrial cavity dimensions. The purpose of the present study was to determine the ideal location for embryo transfer according to different endometrial cavity lengths.
Methods
This retrospective cohort study included 1237 patients who underwent frozen-thawed embryo transfer (FET). Patients were divided into two groups based on different endometrial cavity lengths (ECL): group 1 included 726 women with ECL < 32 mm, and group 2 included 581 women with ECL ≥ 32 mm. We then compared the clinical pregnancy rates according to the different air bubble-uterine fundus distances.
Results
In group 1, the optimal value of air bubble-uterine fundus distance (BFD) for the highest clinical pregnancy rate was 11 mm. In group 2, the ideal BFD for the highest clinical pregnancy rate was 15 mm.
Conclusions
Our study demonstrated a clear correlation between endometrial cavity length and the ideal embryo deposition site, highlighting the importance of measuring endometrial cavity length during embryo transfer. Moreover, the relative positioning methodology that determined the ideal embryo transfer distance from the fundal endometrium to the air bubble for the highest pregnancy rate based on individual endometrial cavity length was superior.
Keywords: Embryo transfer, Embryo transfer distance, Endometrial cavity length, Relative position
Background
Assisted reproductive techniques (ART) represent scientific advancements for infertile couples. It is widely acknowledged that in vitro fertilization and embryo transfer (IVF-ET) stands as one of the most pivotal components of assisted reproductive technology (ART). Various factors are related to the outcome of IVF-ET, such as the woman’s age, duration of infertility, main reason for infertility, ovarian reserve, embryo quality, endometrial receptivity, uterine cavity length, and embryo transfer technique. In recent years, as the methods of ovarian stimulation and oocyte retrieval have developed, the success rate has been significantly determined by the technique of embryo transfer [1].
In the procedure of embryo transfer, with the application of routine ultrasound technology, it was possible to visualize the embryo transfer depth position in a timely manner, achieved by ultrasonography-visible air bubbles loaded alongside the embryos in the transfer catheter [2]. The air bubble makes the location of embryo deposition easily visualized and has no adverse effect on the clinical pregnancy rate [2]. Thus, air bubbles can be considered markers of embryos in operation.
For the sake of a higher pregnancy rate, there are some debates regarding embryo transfer, especially about the ideal place for embryo transfer. Fıçıcıoğlu et al. placed the tip of the inner catheter 1.5–2 cm from the fundal endometrial surface and then measured the change in the embryo flash position 60 min after ET. Compared with the cervical and middle areas of the endometrial cavity, they found that the embryo transfer with the highest PR was in the fundal area. They also concluded that embryos located < 15 mm from the fundus can increase the pregnancy rate [3]. Frankfurter et al. compared the pregnancy and nonpregnancy cycles, then reported that embryos which were placed significantly closer to the middle of the endometrial cavity in pregnancy than in nonpregnancy cycles [4]. Oliveira et al. divided patients into three groups according to the distance between the catheter tip and fundal endometrium (group 1:10–15 mm, group 2:16–20 mm, and group 3: ≥ 21 mm) and found that pregnancy and implantation rates did not vary; however, they also concluded that pregnancy and implantation rates according to the relative position of the catheter tip in the endometrial cavity were higher when the catheter tip was positioned closer to the middle of the endometrial cavity (group 1: upper 40% ECL, group 2: upper 40% to midpoint ECL, group 3: from the midpoint to lower 40% ECL, and group 4: lower 40% ECL) [5].
We observed discrepancies in the relationship between pregnancy rate and embryo transfer position in transplant operation. Additionally, we found that while measuring the distance between the ultrasonography-visible air bubbles and the fundus accurately predicted embryo transfer position, it did not account for differences in endometrial cavity length (ECL). Hence, it would be optimal to research the relative position [6] of embryo transfer, which describes the transfer point of the uterus at a higher or lower position according to the endometrial cavity length. For example, it is the relative position that to research the air bubble in the fundal, middle, or cervical of the endometrial cavity, instead of only measuring the distance from the fundal endometrium to the air bubble (absolute position).
Bakas et al. measured the cervical length and uterine cavity length, then estimated the total length of placement and advancement of the inner catheter into the uterine cavity, aiming to reach the tip of the inner catheter to the middle of uterine cavity. They showed that the embryo distance from the middle of the cavity was related to endometrial cavity length and embryo distance from the fundus (calculated as follows: embryo transfer length = uterine cavity length/2 + cervical canal length) [1]. Therefore, it is important to study the impact of endometrial cavity length on ideal embryo transfer and measure the endometrial cavity length using ultrasound technology.
Although discrepancies exist regarding the pregnancy rate and the position of the embryo transfer in the operation, the middle point of the ECL is widely considered the reference point according to many previous studies on the absolute position. We believe that these studies, which measured absolute position, were limited by the fact that they did not measure the ECL or divide patients into different groups according to different endometrial cavity lengths. Rovei et al. reported a higher overall ongoing pregnancy rate in the group of patients whose embryos were released between 5 and 15 mm from the fundal endometrial surface [7]. We held the perspective that if the ECL was shorter, 5 mm might be the ideal distance from the fundal endometrial surface to the position of the embryo transfer, but if the ECL was longer, 5 mm might be unsuitable because for women with a long endometrial cavity, 5 mm from the fundal endometrial surface was no longer the middle of the endometrial cavity.
The purpose of the present study was to determine the ideal location for embryo transfer in different endometrial cavity lengths with the highest clinical pregnancy rate.
Materials and methods
Study design
The present retrospective cohort study included 1237 patients who underwent frozen-thawed embryo transfer (FET) ultrasound-guided embryo transfer at the reproductive center of SSL Central Hospital of Dongguan City, which is affiliated with Guangdong Medical University, China, between December 1, 2013, and June 30, 2023.
The women were divided into two groups based on different endometrial cavity lengths (ECL). Group 1 comprised 726 women with ECL measurements of < 32 mm. Group 2 included 581 women with ECL ≥ 32 mm. This study included all patients with infertility undergoing frozen-thawed cycle transfer of 2PN (two pronucleus) source of two usable embryos. The exclusion criteria were as follows: (i) women aged > 38 years, (ii) difficulty in ET, (iii) presence of hydrosalpinx, (iv) embryo flashes not visualized, (v) cycle cancelation, and (vi) endometrial thickness < 7 mm.
Ethical approvals
Institutional Review Board approval was not obtained in the present research for this study because it was a retrospective analysis of standard procedures included in assisted reproductive techniques.
Stimulation protocol and embryo cultivate
In vitro fertilization (IVF) cycles were performed according to routine protocols: Recombinant FSH (Gonal-F; Merck-Serono), hMG (Menopur; Ferring Pharmaceuticals), GnRH agonist suppression protocol, or a GnRH antagonist flexible protocol based on the ovarian reserve, which was used to fulfill the controlled ovarian stimulation. When there were more than two follicles beyond 18 mm, hCG at a dose of 5000–10,000 IU was used to induce final oocyte maturation. Transvaginal oocyte retrieval was performed 36 h later.
Under clinical indications, all embryos were scored according to cell number, morphology, and fragmentation 48 or 72 h after oocyte retrieval [8]. We froze 4 to 6 embryos, cultivated, and observed the excess embryos until the blastocyst stage was reached on day 6. Blastocysts were graded using the Gardner system [9], and embryos of grade 3BC or better that were suitable for vitrification on days 5 or 6 were selected.
Endometrial preparation
Endometrial preparation and FET are typically performed in natural, stimulation, or hormone therapy cycles. We performed FET in a natural cycle with regular menstrual cycles. From day 12, follicle growth was monitored using serum hormone levels and transabdominal ultrasound, and patients were regularly monitored every 2 days. When the dominant follicle diameter was > 16 mm, endometrial thickness was > 8 mm, estradiol (E2) > 150 pg/mL, and progesterone (P) < 1.0 ng/mL, we administered a bolus of urinary hCG (5000 IU) to induce ovulation. We then administered P (dydrogesterone 40 mg/d; Duphaston, Abbott Biologics, United States) after 2 and 3 days, and then transferred cleavage-stage embryo.
The preparation of the artificial endometrium included the sequential administration of E2 valerate and injection of progesterone. This meant that patients had to take 2 mg of E2 valerate at least twice daily for 14 to 16 days, and we adjusted the dose of E2 by measuring the endometrial thickness using vaginal ultrasound. If the endometrial thickness was ≥ 7 mm, treatment with progesterone injection was started. Progesterone was injected daily at a dose of 20 mg, and FET was performed 3 days later. Based on the embryo stage, embryo thawing and transfer were scheduled on the third day after P administration. In each FET cycle, two embryos were transferred to each patient. If the patient succeeds in becoming pregnant, luteal phase support is continued until 10 weeks of gestation.
Embryo transfer
Progesterone was injected daily at a dose of 20 mg, and FET was operated in 3 days later. On the basis of embryo stage, embryo thawing and transfer were scheduled on the third day after P administration. In each FET cycle, each patient was transferred two embryos. If patient succeed to pregnancy, luteal phase support continued until 10 weeks of gestation.
Embryo transfer
The thawing process was performed by vitrification. After thawed, the embryos were transferred into G-2 Plus culture medium and then cultured in a CO2 incubator for 1 to 2 h. When we observed that more than 50% of the blastomeres were survival, it was regarded as living embryos and then transferred. The distance between the air bubble (hyperechogenic spot) and the fundal endometrial surface (embryo-fundus distance, EFD) was measured by transvaginal US when the transfer catheter had been retracted completely for a few seconds.
In order to make an easier insertion of the embryo transfer catheter, patients retained their bladder moderately full [10]; then they were placed in the lithotomy position, and the cervix was exposed with a double valve speculum. We cleaned the mucus in the cervical canal with a sterile cotton swab immersed in the medium and then placed the embryos in the atraumatic Cook Sydney embryo transfer catheter (Cook Medical, IN, USA) using the “three-drop technique.” First, an air bubble was loaded into the catheter. Then, 20 μL of medium was aspirated into the catheter and the embryo was placed in the smallest possible medium. The second air bubble was loaded into the catheter. Finally, we aspirated sufficient medium to bring the total volume to 30 μL. The outer catheter was first inserted into the cervical canal of the patient. Once the guide was positioned before or after the internal os, the inner catheter was placed through it. The tip of the inner catheter was placed nearly in the middle of the endometrial cavity. The medium containing the embryos was gently released into the uterine cavity. The catheter was slowly withdrawn and examined by the same embryologist under a stereomicroscope to determine whether the embryos remained at the catheter tip. All patients were recommended to remain supine in bed for approximately 60 min.
Outcomes measure
The following factors were collected: age, BMI, AMH, type of infertility, duration of infertility, main reason of infertility, ART way, baseline serum FSH, baseline serum E2, endometrial thickness, distance between air bubble and fundal endometrial surface (BFD), embryo score, multinucleation blastocyst , No. of embryo transferred, protocol, and cycle outcome.
The outcome of the present study was the clinical pregnancy rate according to the different air bubble-uterine fundus distances. Clinical pregnancy is defined as a confirmed gestational status diagnosed through imaging modalities such as ultrasonography, which demonstrates direct intrauterine evidence of pregnancy, including the presence of a gestational sac, yolk sac, fetal pole, or fetal cardiac activity.
Statistical analysis
Continuous variables are presented as mean ± SD (normal distribution), and categorical variables are presented as N (%). One-way ANOVA (normal distribution), Kruskal–Wallis H (skewed distribution) test, and chi-square test (categorical variable) were used to determine the statistical differences in the mean and proportion between the two groups. We used a smoothing analysis curve to identify the nonlinear relationships between embryo transfer and clinical pregnancy rate. The method for using the smoothing analysis curve has been described in detail by Motulsky [11]. If a nonlinear correlation was observed, a segmented regression model, likelihood ratio test (LRT, to compare the difference between Models I and II), and bootstrap resampling method were used to calculate the threshold effect of BFD on clinical pregnancy rate. When the ratio between BFD and clinical pregnancy rate appeared in the smoothing analysis curve, the recursive method automatically calculated the inflection point, where the maximum model likelihood was used. The present study listed the multivariate-adjusted models adjusted for age, ART method, FET, type of infertility, duration of infertility, BMI, AMH, main reason for infertility, and endometrial thickness.
All of the analysis were performed with the statistical software EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA).
Result
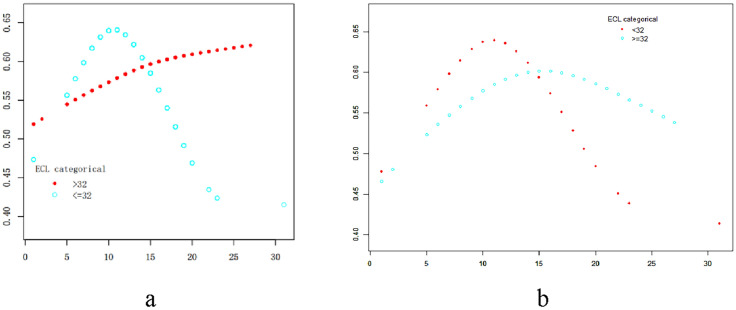
As shown in Table 1, we found that the results were similar in terms of the age of patients, BMI, AMH, number of embryos transferred, ART method, plan, and type of infertility. There were significant differences in endometrial thickness and the main cause of infertility between the two groups. As shown in Fig. 1b, we found that the relationship between distance from air bubble to uterine fundus (BFD) and pregnancy rate (PRs) approached a parabola (after adjusting for age, ART way, FET, type of infertility, duration of infertility, BMI, AMH, main reason for infertility, and endometrial thickness). The turning points of the parabola were obvious, indicating that the pregnancy rates were closely connected to the BFD. In group 1 (ECL < 32 mm), the turning point for BFD was at 11 mm, at which time the pregnancy rate was at its highest. When the BFD was < 11 mm, the pregnancy rates gradually increased as the BFD increased; when the BFD was > 11 mm, the pregnancy rates decreased as the BFD increased. It showed that if the embryo was placed––12 mm from the air bubble to the fundal endometrium, a higher pregnancy rate would be obtained when the endometrial cavity length was < 32 mm. In group 2 (ECL ≥ 32), the BFD relevant turning point was at 15 mm, and the pregnancy rate was at the highest level. The pregnancy rate increased with BFD when BFD was < 15 mm, but decreased with increasing BFD when BFD was > 15 mm. Furthermore, findings indicated that placing the embryo at a distance of 14–16 mm from the fundal endometrium in endometrial cavities > 32 mm in length resulted in a higher pregnancy rate.
Table 1.
Patients’ baseline characteristics
| Group 1: < 32 mm | Group 2: ≥ 32 mm | P value | |
|---|---|---|---|
| N | 692 | 554 | |
| Age | 31.491 ± 4.204 | 31.677 ± 3.951 | 0.427 |
| BMI | 21.580 ± 3.038 | 21.893 ± 3.009 | 0.070 |
| AMH | 4.639 ± 3.374 | 4.618 ± 3.071 | 0.907 |
| NO. of embryo transferred | 2.000 ± 0.000 | 2.000 ± 0.000 | 0.371 |
| Endometrial thickness | 9.472 ± 1.711 | 10.170 ± 2.067 | < 0.001 |
| ART way | 0.467 | ||
| IVF | 513 (74.133%) | 424 (76.534%) | |
| ICSI | 150 (21.676%) | 113 (20.397%) | |
| TESA | 29 (4.191%) | 17 (3.069%) | |
| Plan | 0.010 | ||
| Natural cycle | 508 (73.410%) | 365 (65.884%) | |
| Hormone therapy cycle | 177 (25.578%) | 178 (32.130%) | |
| Stimulation cycle | 7 (1.012%) | 11 (1.986%) | |
| Type of infertility | 0.064 | ||
| Primarily | 302 (43.642%) | 213 (38.448%) | |
| Secondly | 390 (56.358%) | 341 (61.552%) | |
| Main cause of infertility | 0.002 | ||
| Tubal factor | 397 (57.370%) | 377 (68.051%) | |
| Endometriosis | 14 (2.023%) | 12 (2.166%) | |
| Ovulation disorder | 40 (5.780%) | 24 (4.332%) | |
| Male factor | 219 (31.67%) | 122 (22.022%) | |
| Unexplained | 22 (3.179%) | 19 (3.430%) |
Fig.1.
Generalized additive models were used to visually assess functional relationships the pregnancy rates (PRs) in the y-axis and the distances from air bubble-uterine fundus distance (BFD) in the x-axis. Smooth curves between BFD and PRs stratified by endometrial cavity length (ECL). a was unadjusted. b was adjusted for age; ART way; FET; type of infertility; duration of infertility; BMI; AMH; main reason of infertility; endometrial thickness
In group 1, utilizing a segmented regression model, we determined that the optimal value of BFD for achieving the highest clinical pregnancy rate was 11 mm. The likelihood of pregnancy increased by 13.5% per unit increase of BFD when it was less than 11 mm (adjusted odds ratio [OR] = 1.135, 95% confidence interval [CI] 1.014, 1.270, P < 0.05). Conversely, the probability of pregnancy increased by 9.1% per unit decrease of BFD when it ranged from 11 to 35 mm (adjusted OR = 0.909, 95% CI 0.838, 0.987, P < 0.05). In group 2, we identified that the optimal BFD for achieving the highest clinical pregnancy rate was 15 mm. The clinical pregnancy rate increased by 3.99% per unit increase of BFD when it is less than 15 mm (adjusted OR = 1.0399, 95% CI 0.9655, 1.1201, P = 0.3). Conversely, the likelihood of pregnancy decreased by 7.55% per unit increase of BFD when it ranged from 15 to 35 mm (adjusted OR = 0.9245, 95% CI 0.8256, 1.0354, P = 0.2). (Table 2).
Table 2.
Threshold effect analysis of BFD on clinical pregnancy rates
| ECL | Group1: < 32 | Group2: > = 32 |
|---|---|---|
| Model I | ||
| One line effect | 0.991 (0.941, 1.042) 0.7172 | 0.9974 (0.9500, 1.0472) 0.9174 |
| Model II | ||
| Turning point(K) | 11 | 15 |
| < K effect 1 | 1.135 (1.014, 1.270) 0.0273 | 1.0399 (0.9655, 1.1201) 0.3016 |
| > K effect 2 | 0.909 (0.838, 0.987) 0.0223 | 0.9245 (0.8256, 1.0354) 0.1745 |
Results in table: β (95%CI) P value/OR (95%CI) P value
Outcome: clinical pregnancy rates
Exposure: BFD
Adjust for: age; ART way; FET; type of infertility; duration of infertility; BMI; AMH; main reason of infertility; endometrial thickness
Figure 1 Smoothing analysis curves for association between air bubble-uterine fundus distance and clinical pregnancy rates.
Discussion
Embryo transfer plays a crucial role in assisted reproduction techniques with numerous factors influencing the pregnancy rates. Several studies have investigated the optimal location within the endometrial cavity for transfering embryos to maximize the highest pregnancy rate.
Numerous studies have explored the relationship between the distance from an air bubble to the uterine fundus and pregnancy outcomes. A consensus has been reached indicating that the highest ongoing pregnancy rates are associated with embryo transfer at a distance ranging from 10 to 15 mm from the fundal endometrial surface. [12–14]. Our recent findings align closely with established literature. However, our study goes beyond traditional statistical comparisons by identifying uterine cavity length as a crucial factor in determining the optimal placement for embryo transfer. Therefore, the interpretation of statistical significance should extend beyond mere P values. The significance of our research lies in offering practical guidance for clinicians performing embryo transfer procedures, indicating that transfer positioning should be individualized based on the patient's uterine cavity length. Through the analysis of smoothing curves, we observed that for endometrial cavity lengths < 32 mm, a decrease in the distance between the air bubble and uterine fundus (BFD) by < 11 mm corresponded to a 13.5% increase in clinical pregnancy rate per unit increase in BFD. (95%CI 1.014, 1.270), and the clinical pregnancy rate increased by 9.1% per unit decrease of BFD when it from 11 to 35 mm (95% CI 0.838, 0.987). If the endometrial cavity length ≥ 32 mm, the clinical pregnancy rate increased by 3.99% per unit increase of BFD when it is < 15 mm (95% CI 0.9655, 1.1201), and clinical pregnancy rate decreased by 7.55% per unit increase of BFD when it from 15 to 35 mm (95% CI 0.8256, 1.0354). These findings underscored the significant impact of uterine cavity length on determining the optimal placement of embryo transfer. As the length of the patient's uterine cavity increased, the transfer point moved further from the fundus, with the optimal placement tending toward mid-cavity positioning. Conversely, in cases of a shorter uterine cavity, the transfer position shifted closer to the fundus, with the ideal placement leaning toward the middle to lower uterine segment.
The scientific discussion on the optimal location for embryo transfer has primarily emphasized the absolute position in relation to the distance from the fundal endometrium, overlooking a stratified analysis based on endometrial cavity length. Previous investigations into the preferred embryo transfer site have typically categorized it into two groups: the midpoint and the middle to lower uterine segment. Oliveira et al. [5] indicated that embryos were placed significantly closer to the middle of the endometrial cavity. While Frankfurter et al. [15] believed that middle to lower uterine segment improves implantation and pregnancy rates. The enduring debate in this field largely arises from the omission of endometrial cavity length stratification in the analysis of transfer distance, a crucial factor that significantly influences the ideal transfer placement. In contrast, Kwon H et al. [6] introduced a novel relative positioning methodology that determined the ideal embryo transfer distance from the fundal endometrium to air bubble for the highest pregnancy rate based on individual endometrial cavity length, thereby mitigating errors caused by neglecting the influence of different endometrial cavity length. In our operational protocol, we adopted a thorough measurement methodology akin to Bakas et al., employing a transvaginal probe to assess uterine cavity length. Cursors were positioned at the fundal region and the internal cervical os. Subsequently, utilizing prior data on cervical and uterine cavity lengths, we calculated the total distance for inserting and advancing the inner catheter within the uterine cavity (middle of uterine cavity = uterine cavity length/2 + cervical canal length) [1]. While our measurement technique focused on absolute positioning relative to the fundal endometrium, we concurrently performed stratified analysis based on endometrial cavity length. Frankfurter et al.[16]concluded that middle to lower uterine segment embryo transfer improves implantation and pregnancy rates compared with fundal embryo transfer. Our findings revealed that for endometrial cavities shorter than 32 mm, optimal transfer positions approached to the middle to lower uterine segment, aligning with the researches made by Frankfurter et al. [15]. Conversely, for endometrial cavities longer than 32 mm, mid-cavity placement yielded superior pregnancy rate, consistent with Oliveira et al. [5]. This dichotomy highlights the potential negative impact of rigidly adhering to either mid-cavity or mid-to-lower segment transfer protocols without considering individual variations in endometrial cavity lengths on pregnancy outcomes. While the absolute position remains a significant parameter, it should not be the sole criterion in evaluating embryo transfer protocols. Instead, incorporating the relative position based on patients'specific endometrial cavity lengths deserves increased attention and wider clinical adoption. In summary, our findings underscore the critical importance of assessing endometrial cavity measurements from previous transfers and determining individualized transfer positions to enhance pregnancy outcomes in assisted reproductive technology procedures, given the variability in women's endometrial cavity lengths.
Conclusions
In conclusion, our current study produced clinically significant findings regarding the relationship between endometrial cavity length and optimal embryo transfer position. The data demonstrated a clear correlation between endometrial cavity length and the ideal embryo deposition site, highlighting the previous measurement of endometrial cavity length in embryo transfer procedure. These findings underscored the clinical superiority of relative positioning strategies, which account for individual variations in endometrial cavity length, and it was superior to traditional absolute positioning methods based solely on fundal endometrium distance measurements. To enhance clinical pregnancy outcomes, it is crucial to implement an individualized embryo transfer strategy that integrates precise measurement of endometrial cavity length as a fundamental parameter in determining the optimal embryo deposition site.
Acknowledgements
The authors acknowledge the medical staff who assisted with this research in each of the reproductive center of SSL Central hospital of Dongguan City which is affiliated Guangdong medical university, including Departments of Obstetrics and Gynecology and Reproductive Medicine Center.
Author contributions
Shuman Li contributed to the articles writing and data collection. Qingyang Li and Li Zhao contributed to the Embryo operation. Xiaoyan Sun, Hongmei He contributed to the Clinical operation. Ruihua Pan. contributed to the follow-up visit. Bo Chen contributed to the article instruction. All of the authors contributed to the interpretation of data, preparation of the manuscript, and editing and review.
Funding
This research received funding from Traditional Chinese Medicine Scientific Research Projects of Guangdong Provincial Administration of Traditional Chinese Medicine. (NO: 20221416).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bakas P, Simopoulou M, Giner M, Tzanakaki D, Deligeoroglou E. Accuracy and efficacy of embryo transfer based on the previous measurement of cervical length and total uterine length. Arch Gynecol Obstet. 2019;299(2):565–70. 10.1007/s00404-018-4971-6. [DOI] [PubMed] [Google Scholar]
- 2.Tiras B, Korucuoglu U, Polat M, Saltik A, Zeyneloglu HB, Yarali H. Effect of air bubble localization after transfer on embryo transfer outcomes. Eur J Obstet Gynecol Reprod Biol. 2012;164(1):52–4. 10.1016/j.ejogrb.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 3.Fıçıcıoğlu C, Özcan P, Koçer MG, Yeşiladalı M, Alagöz O, Özkara G, Tayyar AT, Altunok Ç. Effect of air bubbles localization and migration after embryo transfer on assisted reproductive technology outcome. Fertil Steril. 2018;109(2):310-314.e1. 10.1016/j.fertnstert.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 4.Frankfurter D, Silva CP, Mota F, Trimarchi JB, Keefe DL. The transfer point is a novel measure of embryo placement. Fertil Steril. 2003;79(6):1416–21. 10.1016/s0015-0282(03)00263-2. [DOI] [PubMed] [Google Scholar]
- 5.Oliveira JB, Martins AM, Baruffi RL, Mauri AL, Petersen CG, Felipe V, Contart P, Pontes A, Franco Júnior JG. Increased implantation and pregnancy rates obtained by placing the tip of the transfer catheter in the central area of the endometrial cavity. Reprod Biomed Online. 2004;9(4):435–41. 10.1016/s1472-6483(10)61280-1. [DOI] [PubMed] [Google Scholar]
- 6.Kwon H, Choi DH, Kim EK. Absolute position versus relative position in embryo transfer: a randomized controlled trial. Reprod Biol Endocrinol. 2015;13:78. 10.1186/s12958-015-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rovei V, Dalmasso P, Gennarelli G, Lantieri T, Basso G, Benedetto C, Revelli A. IVF outcome is optimized when embryos are replaced between 5 and 15 mm from the fundal endometrial surface: a prospective analysis on 1184 IVF cycles. Reprod Biol Endocrinol. 2013;11:114. 10.1186/1477-7827-11-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinsden P. A textbook of in vitro fertilization and assisted reproduction: the bourn hall guide to clinical and laboratory practice. 3rd ed. Boca Raton: CRC Press; 2005. [Google Scholar]
- 9.Gardner DK. In vitro fertilization: a practical approach. 3rd ed. Boca Raton: CRC Press; 2007. [Google Scholar]
- 10.Lewin A, Schenker JG, Avrech O, Shapira S, Safran A, Friedler S. The role of uterine straightening by passive bladder distension before embryo transfer in IVF cycles. J Assist Reprod Genet. 1997;14(1):32–4. 10.1007/BF02765749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motulsky H, Christopoulos A. Fitting models to biological data using linear and nonlinear regression: a practical guide to curve fitting. Oxford: Oxford University Press; 2004. p. 12–47. [Google Scholar]
- 12.Saravelos SH, Wong AW, Chan CP, Kong GW, Cheung LP, Chung CH, Chung JP, Li TC. Assessment of the embryo flash position and migration with 3D ultrasound within 60 min of embryo transfer. Hum Reprod. 2016;31(3):591–6. 10.1093/humrep/dev343. [DOI] [PubMed] [Google Scholar]
- 13.Tiras B, Polat M, Korucuoğlu U, Zeyneloglu HB, Yarali H. Impact of embryo replacement depth on in vitro fertilization and embryo transfer outcomes. Fertil Steril. 2010;94(4):1341–5. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Zhu Y, Sun Y, Di W, Qiu M, Kuang Y, Shen H. Ideal embryo transfer position and endometrial thickness in IVF embryo transfer treatment. Int J Gynaecol Obstet. 2018;143(3):282–8. 10.1002/ijgo.12681. [DOI] [PubMed] [Google Scholar]
- 15.Frankfurter D, Trimarchi JB, Silva CP, Keefe DL. Middle to lower uterine segment embryo transfer improves implantation and pregnancy rates compared with fundal embryo transfer. Fertil Steril. 2004;81(5):1273–7. 10.1016/j.fertnstert.2003.11.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.