ABSTRACT
Background
Feline panleukopenia virus (FPV) is a highly contagious and often fatal disease affecting domestic and wild felines. Accurate diagnosis and understanding of circulating strains are essential for effective control.
Objectives
This study aimed to evaluate the diagnostic accuracy of a rapid immunochromatographic (IC) antigen test compared to PCR for FPV detection in clinically suspected pet cats in Bangladesh. It also aimed to investigate the genetic and evolutionary characteristics of circulating FPV strains.
Methods
Faecal or rectal swab samples from suspected cats were tested using both IC strip tests and PCR. Sensitivity and specificity of the IC test were analysed using PCR as the reference. Partial sequencing of the VP2 gene was performed on four PCR‐positive samples for phylogenetic and mutational analysis. Structural modelling of VP2 proteins was conducted to predict conformational changes.
Results
The IC test detected FPV in 84% of cases, whereas PCR confirmed only 60%, indicating a 24% false‐positive rate. PCR showed higher diagnostic reliability. FPV prevalence was 92% among unvaccinated cats. Phylogenetic analysis of VP2 sequences revealed close genetic similarity with Chinese and Portuguese strains, suggesting possible cross‐border transmission. Mutations such as A756G, A896G, E299G and T236I were consistently observed. Structural modelling indicated minor conformational changes in VP2.
Conclusion and clinical significance
PCR offers superior accuracy over IC testing for FPV diagnosis. Mutational changes may impact antigenicity and diagnostic performance. Improved diagnostic accuracy, molecular surveillance and updated vaccination strategies are essential to control FPV outbreaks in feline populations.
Keywords: Feline panleukopenia virus, IC strip test, PCR, phylogenetic analysis, VP2 gene mutations
PCR analysis of rectal swab samples from cats suspected of Feline panleukopenia virus (FPV) infection demonstrated higher diagnostic reliability compared to the immunochromatographic (IC) strip test. Epidemiological and demographic data revealed a high prevalence of FPV among unvaccinated, male and local‐breed cats. Foul‐smelling faeces and diarrhoea were identified as key clinical indicators. Phylogenetic analysis of VP2 gene sequences showed close genetic similarity to strains from China and Portugal. Several amino acid mutations were detected in the VP2 proteins, which may cause minor conformational changes in the protein structure.
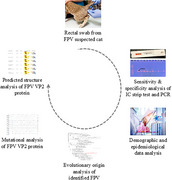
1. Introduction
Feline panleukopenia virus (FPV) is a highly contagious and significant viral pathogen affecting domestic cats, often leading to fatal outcomes in severe cases (Barrs 2019; Kruse et al. 2010). It infects all felids and some related species, including domestic and wild cats, raccoons and minks (Barrs 2019). FPV primarily causes high mortality in kittens with underdeveloped immune systems (Cave et al. 2002; Raheena et al. 2017). Transmission occurs mainly through direct contact with infected cats, respiratory droplets and contaminated fomites (Truyen et al. 2009). The severity of FPV infection is closely related to clinical signs and symptoms (Porporato et al. 2018), which vary based on age, immune status, vaccination history and concurrent infections (Foley et al. 1999; Kruse et al. 2010). Clinical manifestations range from mild illness to severe disease, which can lead to rapid death (Kruse et al. 2010). Infected cats commonly exhibit symptoms such as depression, anorexia, vomiting, diarrhoea and severe dehydration (Sykes 2013). The disease progresses through four clinical forms: subacute, peracute, acute and perinatal. Moreover, the morbidity and mortality rates are influenced by the pathogenicity of the viral strain (Tuzio 2021).
FPV is a small, non‐enveloped, single‐stranded DNA virus belonging to the Parvoviridae family. It shares genetic, structural and antigenic similarities with canine parvovirus (CPV), which can also infect cats (CPV strains 2a, 2b and 2c) (Holzworth 1987; Porporato et al. 2018). The most widely accepted theory suggests that FPV is the ancestral virus of CPV, emerging due to spontaneous genetic mutations (Horiuchi et al. 1998). Feline parvoviruses within the genus Parvovirus include FPV, CPV and mink enteritis virus (MEV), which are closely related antigenically (Carmichael et al. 1980; Horiuchi et al. 1998; Johnson and Spradbrow 1979; Siegl et al. 1985).
The FPV genome is approximately 5.2 kb long and contains four open reading frames (ORFs) encoding two structural proteins (VP1, VP2) and two non‐structural proteins (NS1, NS2) (Balboni et al. 2018; Yang et al. 2022). VP2, encoded by the fourth ORF, constitutes about 90% of the viral capsid. It plays a crucial role in determining host range and contains several B‐cell epitopes responsible for inducing protective antibodies with neutralizing capacity (Chang et al. 2020). Due to its genetic and epidemiological significance, the ORF encoding VP2 has been extensively studied, particularly as an antigen for subunit vaccine development (Chang et al. 2020; Su et al. 2009).
Meta‐analysis data indicate a high global prevalence of FPV infection in domestic cats (Alessa et al. 2026). Several diagnostic methods are available for detecting FPV, including immunochromatographic (IC) strip tests, haemagglutination (HA) tests, serum neutralization (SN) tests, haemagglutination inhibition (HI) assays, SNAP ELISA and PCR (Brower et al. 2004; Jacobson et al. 2021; Raheena et al. 2017). Although FPV does not directly impact the economy, it can cause significant financial losses for breeders and pet owners.
In Bangladesh, a large number of cats die each year due to FPV infection (Kabir et al. 2023). False‐negative or false‐positive test results can occur, making early and accurate diagnosis essential for saving lives. Additionally, significant global variations in FPV infection rates suggest that current preventive measures may be inadequate. Findings emphasize the need for stricter control strategies and a greater focus on risk factors (Alessa et al. 2026). Furthermore, the potential genomic relationship between viral antigenicity and diagnostic test failures should be confirmed using PCR (Jacobson et al. 2021).
As no molecular data on circulating FPV strains have been reported to date from districts of Bangladesh with relatively high pet populations—such as Narayanganj, Narsingdi and Cumilla—this study aims to detect FPV infection in suspected cats using a commercially available rapid antigen detection kit and PCR, followed by sensitivity and specificity analyses. Additionally, the study seeks to characterize the partial VP2 gene from these underrepresented regions to explore their evolutionary origins. Furthermore, amino acid (aa) mutations and structural alterations in VP2 proteins were examined.
2. Methods
2.1. Questionnaire Preparation
A structured questionnaire was developed to document the clinico‐epidemiological data of suspected pet cats. It included qualitative data such as age, sex and vaccination history, along with clinical data, including anorexia, vomiting, diarrhoea, foul‐smelling faeces, fever and other symptoms. As the primary aim of this study was the molecular detection and genetic characterization of FPV, information on treatment was not collected during clinical data collection. The questionnaire was designed on the basis of literature reviews relevant to FPV infection. Informed consent was obtained from all cat owners before data and sample collection.
2.2. Sample Collection
The samples were collected from the Teaching and Training Pet Hospital and Research Centre (TTPHRC), Purbachal, a major healthcare facility for pet cats in Dhaka, Bangladesh. The hospital was selected due to its high case volume, providing a representative sample of suspected cases. A convenience sampling approach was used, and pet cats were selected on the basis of their clinical presentation and suspected illness. A total of 50 faecal or rectal swab samples were collected under aseptic conditions. Samples were obtained from four districts: Dhaka, Narayanganj, Narsingdi and Cumilla (Figure 1). The collected samples were transported to the Virology Laboratory at the Department of Microbiology and Hygiene, Bangladesh Agricultural University, and stored at −80°C until further analysis. However, the survival outcomes of cats after diagnosis could not be recorded due to the lack of systematic follow‐up and limited access to hospital records. Similarly, treatment modalities administered to the infected cats were not documented, as they were beyond the scope of the study.
FIGURE 1.

Geographical representation of the locations included in this FPV study. The map was created using QGIS software.
2.3. IC Strip Test
The Rapid FPV Antigen Test Kit (Testsealabs F. Panleukopenia Antigen FPV Ag Test, Hangzhou Testsea Biotechnology Co. Ltd.) was used for the preliminary detection of FPV infection. A sufficient amount of the faecal swab sample was mixed with the sample dilution buffer and stirred to disperse the sample. Then, 3–4 drops of the diluted sample were added drop by drop into the test device's sample well. The test was performed following the manufacturer's instructions, and results were interpreted visually on the basis of the presence of coloured lines in the control (C) and test (T) regions. The outcomes were recorded as either positive or negative (Figure 2A).
FIGURE 2.

Identification of FPV. (A) Preliminary detection using the IC test. Coloured lines in both the test (T) and control (C) regions indicate the presence of the target analyte in the sample, confirming a positive result. (B) Identification by PCR. Gel electrophoresis showing the VP2 gene amplicons of FPV (698 bp). Lanes: M—100 bp DNA ladder (Thermo Fisher, USA), N—Negative control, C—Positive control, Lanes 1–9—Representative FPV‐positive samples showing bands at approximately 698 bp. FPV, Feline panleukopenia virus.
2.4. Extraction of Viral DNA
An adequate amount of PBS was added to the samples and vortexed thoroughly. The samples were then centrifuged at 3000 rpm for 3 min. The clear supernatant was collected and used for DNA extraction. Viral DNA was extracted using the boiling snap‐chilling method, as described previously (Parthiban et al. 2012). Briefly, approximately 200 µL of the prepared sample was boiled for 10 min, followed by rapid cooling on ice for another 10 min. The chilled samples were centrifuged at 10,000 rpm for 10 min, and the clear supernatant was collected as extracted DNA, which was subsequently stored at −80°C for further analysis.
2.5. Molecular Detection
A VP2 gene‐specific primer set (FM‐F: 5′‐GCTTTAGATGATACTCATGT‐3′ and FM‐R: 5′‐GTAGCTTCAGTAATATAGTC‐3′) was used to amplify a 698 bp fragment (Mochizuki et al. 1993; Yang et al. 2010). PCR amplification was performed under the following conditions: initial denaturation at 94°C for 30 s, followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 2 min, extension at 72°C for 2 min and a final extension at 72°C for 7 min. The reactions were carried out using GoTaq G2 Green Master Mix (Promega Fisher Scientific, USA). The PCR‐amplified products were separated on a 1.5% agarose gel in Tris‐acetate EDTA buffer, stained with ethidium bromide and electrophoresed for 25 min at 100 V. The amplified products were visualized using a gel documentation system (GelDoc Go, BioRad, USA).
2.6. Sensitivity and Specificity Analysis
The sensitivity and specificity of the rapid antigen test (IC strip) and PCR were calculated and expressed as percentages using the following formulas, × 100 and × 100 as described previously (Baratloo et al. 2015).
2.7. Statistical Analysis of Demographic and Epidemiological Data
All collected data were initially organized using Microsoft Excel 2016 and then exported to SPSS for descriptive statistical analysis. Demographic variables such as age, sex, vaccination status and breed were analysed using Pearson's Chi‐square test to determine potential risk factors for FPV infection (Awad et al. 2018). Additionally, multivariable logistic regression analysis (MLRA) was conducted to examine the relationship between clinical signs and FPV infection (Table S1) (Rehme et al. 2022).
2.8. Sequencing and Evolutionary Origin Analysis
Four representative PCR‐amplified partial VP2 gene sequences were selected for sequencing; three FPV‐positive samples containing a 698 bp VP2 gene fragment, one FPV‐positive sample containing a 1073 bp VP2 gene fragment. The sequencing was conducted at the National Institute of Bangladesh (https://nib.gov.bd/) and the University of São Paulo, Brazil (Table 1). The raw sequences were edited, annotated and analysed using CLC Sequence Viewer version 8.0 (http://www.clcbio.com) and compared with the reference sequence (MZ712026.1). Additional reference FPV VP2 gene sequences were retrieved from GenBank for evolutionary analysis. A phylogenetic tree was constructed using MEGA11 software. Nucleotide sequences were aligned using the ClustalW technique. The Tamura–Nei model and Neighbour‐Joining method were used to infer evolutionary relationships (Tamura and Nei 1993). The proportion of trees where associated taxa clustered together is shown below the branches. Initial trees for heuristic searches were automatically generated using the Neighbour‐Joining and BioNJ algorithms on the basis of a pairwise distance matrix computed with the Tamura–Nei model. The topology with the highest log‐likelihood value was selected (Tamura et al. 2021).
TABLE 1.
List of primers used for sequencing the partial VP2 gene.
| Primer names | Sequence (5′–3′) |
|---|---|
| FM‐F | GCTTTAGATGATACTCATGT |
| FM‐R | GTAGCTTCAGTAATATAGTC |
| FPV_5_F | CCAACCATACCAACTCCATGG |
| FPV_5a_F | GTTCAACAAGATAAAAGACGTGG |
| FPV_5_R | CATTAATACTCATTTGTTGAATTGG |
| FPV_6_F | ATTGCTACCAACAGATCCAATTGGAG |
| FPV_6a_F | CAAAATATTAACTTTAACCTTCC |
| FPV_6_R | ACTATGATCTAAATGTTCTTCTAT |
| FPV_7_F | ATAGAAGAACATTTAGATCATAGT |
Abbreviation: FPV, Feline panleukopenia virus.
2.9. Mutational Analysis and Structural Modelling of VP2 Protein
The identified partial VP2 gene sequences were compared against the reference sequence (MZ712026.1) and the Indian FPV strain (PP035817.1) for mutational analysis. Using CLC Sequence Viewer version 8.0, the nucleotide sequences were translated into aa sequences and aligned with the reference sequences. For structural analysis, the secondary structures of VP2 proteins were predicted using the SOPMA online server with default parameters. Tertiary structures of the mutant proteins were modelled using SWISS‐MODEL (https://swissmodel.expasy.org/) (Waterhouse et al. 2018). The protein sequence was submitted to the server for template search, and models were selected on the basis of GMQE (Global Model Quality Estimation) scores, query coverage and identity values. The final 3D structural models were visualized using PyMOL software. To validate the modelled structures, Ramachandran plot analysis and Z‐score calculations were performed using Procheck tools from SAVESv6.0 (https://saves.mbi.ucla.edu/) (Laskowski et al. 1993) and ProSA‐web (https://prosa.services.came.sbg.ac.at/prosa.php) (Wiederstein and Sippl 2007).
3. Results
3.1. Comparison of Rapid Antigen Test and PCR for FPV Detection
Initially, FPV was detected using a rapid antigen detection kit from faecal or rectal swab samples of suspected cats, followed by conventional PCR. The results indicated that 84% of the samples (42/50) tested positive using the IC strip test kit (Figure 2A). PCR is considered the gold standard for FPV detection (Abdelbaky et al. 2024). In this study, conventional PCR targeting the VP2 gene revealed that 60% (30/50) of the samples were positive for FPV (Figure 2B and Table S1). These 30 PCR‐positive samples also tested positive using the IC strip test and were considered true positives. Conversely, 24% (12/50) of the IC test‐positive samples tested negative using PCR and were classified as false positives. These findings suggest that PCR is highly specific compared to the IC strip test for FPV detection.
3.2. Epidemiology and Susceptibility Patterns of FPV‐Infected Cats
Most affected cats exhibited common clinical symptoms, including diarrhoea, vomiting, anorexia, foul‐smelling faeces, weakness and elevated body temperature (Table S1 and Figure 3). The ages of the suspected cats ranged from 1 to 84 months, with the majority being male (72%) and a smaller proportion female (28%). A total of 92% (46/50) of the suspected cats were unvaccinated, whereas 8% (4/50) were vaccinated (Table S1 and Figure 4B,C). According to Pearson's Chi‐Square test analysis of positive samples identified by both IC strip and PCR, approximately 93.33% of FPV‐infected cats exhibited diarrhoea, vomiting and anorexia. Additionally, the percentages of FPV‐infected cats displaying fever, weakness and foul‐smelling faeces were 26.67%, 70% and 63.33%, respectively (Figure 3). The study revealed that the susceptibility of kittens (≤5 months) and older cats (>5 months) was similar, with infection rates of 60.71% and 59.09%, respectively (Figure 4A). Furthermore, male cats were found to be more susceptible (73.3%) than female cats (66.7%) (Figure 4B). Among the infected cats, 60.9% were unvaccinated, whereas 50% of the vaccinated cats were also infected (Figure 4C). The highest number of positive cases (76.92%) were found in local breeds, followed by 50% in mixed breeds and 40.9% in Persian breeds (Figure 4D). The study identified diarrhoea and foul‐smelling faeces as diagnostic symptoms of FPV infection. An MLRA showed significant correlations with FPV infection (p < 0.05) among suspected cats (Table 2). According to the analysis, cats with diarrhoea (OR = 8.2) and foul‐smelling faeces (OR = 23.1) had 8.2 and 23.1 times higher chances of testing positive for FPV infection, respectively, compared to cats without these symptoms.
FIGURE 3.

Distribution of the cat population based on clinical signs and symptoms. The clustered column bar diagrams illustrate the number of FPV‐infected cats exhibiting clinical symptoms such as diarrhoea, fever, weakness, vomiting, anorexia and foul‐smelling faeces.
FIGURE 4.

Distribution of FPV‐infected cats by age, sex and vaccination status. (A) Age distribution: cats aged ≤5 months versus >5 months. (B) Sex distribution: male versus female cats. (C) Vaccination status: vaccinated versus unvaccinated cats. (D) Breed distribution: Persian, local and mixed breeds.
TABLE 2.
Summary of multivariate logistic regression analysis.
| Multivariate | ||
|---|---|---|
| Variable | OR | p value |
| Diarrhoea | 8.2 | 0.03114 |
| Bad odour in faeces | 23.1 | 0.00536 |
3.3. Sequencing and Evolutionary Origin Analysis
Four representative samples were selected on the basis of PCR band intensity for further genome sequence analysis. The partial VP2 genes were successfully sequenced using VP2 gene‐specific primer sets (Table 1). The lengths of the partially sequenced VP2 genes were 331–969, 325–969, 344–967 and 682–1755 bp, respectively. We obtained accession numbers from GenBank for the identified isolates: PV211206 (FPV‐VP2/Dhaka‐1/MGH‐BD), PV211207 (FPV‐VP2/Dhaka‐2/MGH‐BD), PV211208 (FPV‐VP2/Narayanganj/MGH‐BD) and PV211209 (FPV‐VP2/Cumilla/MGH‐BD). Phylogenetic tree analysis showed that three strains, FPV‐VP2/Dhaka‐1/MGH‐BD, FPV‐VP2/Dhaka‐2/MGH‐BD and FPV‐VP2/Narayanganj/MGH‐BD formed an independent branch and were evolutionarily related to FPV isolates from China. Meanwhile, one strain, FPV‐VP2/Cumilla/MGH‐BD, showed close relation to isolates from both China and Portugal (Figure 5).
FIGURE 5.

Phylogenetic tree of FPV isolates identified in this study. (A) Neighbour‐Joining tree was constructed using 79 partial VP2 gene sequences retrieved from GenBank. Evolutionary distances were calculated using the Tamura–Nei method. Sequence alignment and evolutionary analyses were performed using MEGA11 software and ClustalW. Sequences obtained in this study are highlighted in blue.
3.4. Genomic Characteristics of the Identified FPV VP2 Sequences
The VP2 protein plays a crucial role in determining the host range of the virus and is considered the major capsid protein of parvovirus (Brindhalakshmi et al. 2016). Variations among different parvovirus strains result from mutations in specific aas of the VP2 capsid protein (Chowdhury et al. 2021). Nucleotide substitution analysis, compared to two reference sequences, REF_FPV_VP2 (MZ712026.1) and FPV_VP2_India (PP035817.1), revealed several nucleotide substitutions in all four identified and sequenced isolates (Figure 6). Notably, an A756G substitution was found in all identified strains compared to both reference sequences. Additionally, an A896G substitution was observed in all isolates compared to REF_FPV_VP2, whereas T871C and C942T mutations were reported in all isolates compared to FPV_VP2_India (Figure 6).
FIGURE 6.

Nucleotide substitution analysis of the VP2 gene in identified FPV isolates. Mutational analysis was conducted by aligning VP2 gene sequences from this study with reference sequences using CLC Sequence Viewer 8.0. Dots represent identical residues, whereas letters indicate nucleotide substitutions.
3.5. AA Mutations and Structural Alterations in VP2 Proteins
The impact of nucleotide substitutions on aa mutations in VP2 proteins was analysed. All VP2 proteins of the identified FPV isolates exhibited an E299G mutation compared to REF_FPV_VP2 (Figure 7). The S122N and T179S mutations were found in three isolates, FPV‐VP2/Dhaka‐1/MGH‐BD, FPV‐VP2/Dhaka‐2/MGH‐BD and FPV‐VP2/Narayanganj/MGH‐BD compared to FPV_VP2_India. Moreover, FPV‐VP2/Cumilla/MGH‐BD displayed two aa mutations at positions T236I and D237E compared to both reference sequences, as well as another substitution at V401I compared to FPV_VP2_India (Figure 7). Additionally, an L111W mutation was reported only in FPV‐VP2/Dhaka‐2/MGH‐BD compared to REF_FPV_VP2 (Figure 7). Secondary structure analysis of VP2 proteins from all identified FPV isolates revealed slight variations in the structures of beta‐turns, random coils, alpha‐helices and extended strands compared to the reference sequences (Figure 8 and Table 3). Despite minor structural inconsistencies, the tertiary structures of the identified FPV isolates were of high quality, as confirmed by the Ramachandran plot and Z‐score analysis (Figure 9). Tertiary structural analysis of VP2 proteins from the four identified FPV isolates, in comparison with reference sequences, revealed alterations in their physicochemical characteristics, including molecular weight, theoretical pI, aliphatic index, instability index and GRAVY value (Figure 10). The FPV‐VP2/Dhaka‐1/MGH‐BD, FPV‐VP2/Narayanganj/MGH‐BD and FPV‐VP2/Cumilla/MGH‐BD exhibited similar most‐favoured regions (85%), generously allowed regions (1.50%) and additional allowed regions (12.60%) compared to FPV_VP2_India. However, a slight increase in most‐favoured regions (85.3%) and additional allowed regions (12.8%) was observed in FPV‐VP2/Dhaka‐2/MGH‐BD, with a decrease in generously allowed regions (1.1%) (Table 4).
FIGURE 7.

Amino acid variations in the VP2 protein of identified FPV isolates. Amino acid sequences of VP2 proteins were aligned and compared with reference strains. Dots indicate identical residues, whereas letters denote substitutions. Analysis was performed using CLC Sequence Viewer 8.0.
FIGURE 8.

Secondary structure analysis of the VP2 protein in identified FPV isolates and reference strains. Secondary structures were predicted and analysed using the SOPMA online server with default parameters.
TABLE 3.
Secondary structure analysis of VP2 protein compared with reference proteins.
| Protein name | Alpha helix | Extended strand | Random coil |
|---|---|---|---|
| REF_FPV_VP2 | 3.77 | 18.66 | 77.57 |
| FPV_VP2_India | 4.45 | 18.49 | 77.06 |
| FPV‐VP2/Dhaka‐1/MGH‐BD | 3.94 | 19.35 | 76.71 |
| FPV‐VP2/Dhaka‐2/MGH‐BD | 3.94 | 19.35 | 76.71 |
| FPV‐VP2/Narayanganj/MGH‐BD | 3.60 | 18.66 | 77.74 |
| FPV‐VP2/Cumilla/MGH‐BD | 4.45 | 17.81 | 77.74 |
Abbreviation: FPV, Feline panleukopenia virus.
FIGURE 9.

Prediction and analysis of the tertiary structure of the VP2 protein. The hydrophobicity of the predicted VP2 protein structures was analysed using SWISS‐MODEL and compared with reference proteins. Identity, Z‐score, Ramachandran plots and hydrophobic surface regions (orange patches) are depicted.
FIGURE 10.

Physicochemical properties of the VP2 protein compared with reference proteins. Clustered column bar diagrams depict various properties. (A) Molecular weight and aliphatic index. (B) Theoretical pI and instability index. (C) GRAVY value of the VP2 capsid protein.
TABLE 4.
Protein structure validation.
| Statistics of Ramachandran plot | |||||
|---|---|---|---|---|---|
| Protein name | Most favoured regions (%) | Additional allowed regions (%) | Generously allowed regions (%) | Disallowed regions (%) | Z‐score |
| REF_FPV_VP2 | 84.2 | 13.4 | 1.5 | 0.9 | −6.16 |
| FPV_VP2_India | 85 | 12.6 | 1.5 | 0.9 | −6.10 |
| FPV‐VP2/Dhaka‐1/MGH‐BD | 85 | 12.6 | 1.5 | 0.9 | −6.15 |
| FPV‐VP2/Dhaka‐2/MGH‐BD | 85.3 | 12.8 | 1.1 | 0.9 | −6.16 |
| FPV‐VP2/Narayanganj/MGH‐BD | 85 | 12.6 | 1.5 | 0.9 | −6.14 |
| FPV‐VP2/Cumilla/MGH‐BD | 85 | 12.6 | 1.5 | 0.9 | −6.12 |
Abbreviation: FPV, Feline panleukopenia virus.
4. Discussion
F. panleukopenia is one of the most contagious and fatal diseases affecting the global feline population. Despite routine vaccination practices, the incidence of FPV infection is rising among cats in Bangladesh. FPV detection in suspected cases is commonly performed using rapid antigen test kits; however, these tests may yield false results (Gans et al. 2022; Herbert et al. 2024). Moreover, genomic analyses have revealed unique variations in the antigenicity of FPV field and vaccine strains (Raja et al. 2024). In this study, we conducted a comparative analysis of the sensitivity and specificity of rapid antigen test kits versus PCR for FPV detection. Additionally, we investigated the evolutionary origin of FPV circulating in Bangladesh by analysing VP2 gene sequences, identifying mutations and predicting structural changes in VP2 proteins of the identified strains.
Detection of FPV using rapid antigen testing and PCR revealed significant differences in sensitivity and specificity. The IC strip test detected FPV in 84% of samples, whereas PCR confirmed only 60% positivity. This discrepancy likely arises due to differences in detection mechanisms. The IC test identifies viral antigens in faecal samples, whereas PCR amplifies viral DNA, ensuring higher specificity (Raheena et al. 2017). False positives in the IC test may result from antigen persistence or cross‐reactivity with closely related viruses, such as CPV (Sykes 2013). Additionally, IC test based diagnostic performance may be compromised by an operational factor, including result variability due to user handling (Miller and Sikes 2015). Hence, the implementation of IC test kits from reputable manufacturers should be accompanied by proper user training, strict adherence to standardized protocols and local validation efforts to ensure reliable detection of FPV in resource‐constrained settings. As PCR targets the VP2 gene, which encodes the viral capsid protein, it offers greater accuracy due to its conserved nature (Kabir et al. 2023; Xue et al. 2023).
FPV primarily targets rapidly dividing cells, including those in the intestinal crypts and bone marrow, leading to severe immunosuppression and enteritis (Litster and Benjanirut 2014). The clinical symptoms observed in this study, including diarrhoea, vomiting and anorexia, result from the destruction of intestinal epithelial cells, leading to malabsorption and increased gut permeability (Ramadhani et al. 2024). The higher susceptibility observed in male cats aligns with previous studies; however, the underlying cause remains unclear, as this study did not investigate behavioural factors that may influence exposure risk (Rehme et al. 2022). Additionally, the similar infection rates among kittens (≤5 months) and older cats (>5 months) indicate that unvaccinated individuals remain highly vulnerable regardless of age (Rehme et al. 2022). The findings highlight the diagnostic significance of clinical signs such as diarrhoea and foul‐smelling faeces in case of FPV. Diarrhoea results from enterocyte depletion, whereas malodorous faeces may be attributed to dysbiosis and bacterial overgrowth in the gut (Sykes 2013). These findings reinforce the critical role of vaccination, as 92% of infected cats in this study were unvaccinated, confirming its importance in controlling FPV spread. However, although a few vaccinated cats were found positive for FPV, the sample size was too limited to draw any reliable statistical comparison regarding severity between vaccinated and unvaccinated cats. Furthermore, the study was conducted in specific districts which were previously unrepresented in FPV surveillance. These regional data help fill critical epidemiological gaps, thereby strengthening the overall national surveillance framework. By revealing local strain diversity and identifying high‐risk, unvaccinated populations, our findings offer valuable guidance for improving disease control measures and optimizing vaccination programmes across Bangladesh.
Phylogenetic analysis of VP2 gene sequences revealed that three FPV isolates formed an independent branch closely related to Chinese strains, whereas FPV‐VP2/Cumilla/MGH‐BD shared similarities with strains from both China and Portugal. Although the partial VP2 gene sequences showed phylogenetic similarity with strains from other countries, the limited number of analysed samples prevents drawing a definitive conclusion about possible cross‐border transmission of FPV into Bangladesh—likely facilitated by international pet trade and animal migration—highlighting the need for more comprehensive regional disease surveillance (Chowdhury et al. 2021). Further investigations should include full‐length VP2 or whole‐genome sequencing from a larger and more diverse sample pool, encompassing a wider range of geographic regions and clinical cases, to enhance the accuracy of phylogeographic mapping and viral evolutionary analysis. The VP2 gene, crucial for viral infectivity and host adaptation, exhibited multiple mutations across the identified FPV strains (Li et al. 2024). The A756G and A896G substitutions, found in all isolates, suggest ongoing viral evolution and potential adaptation to local feline populations (Wen et al. 2024; Zhang et al. 2024). Mutations such as E299G and S122N may impact capsid stability and receptor‐binding affinity, potentially influencing virulence (Yi et al. 2021). Structural alterations in the VP2 protein, including T236I and D237E mutations, may contribute to changes in antigenicity and immune evasion mechanisms (Leal et al. 2020; Li et al. 2017; Li et al. 2024). Secondary structure analysis revealed subtle shifts in beta‐turns, random coils and alpha‐helices of the VP2 protein, which may affect capsid assembly and stability (Wen et al. 2024; Zhang et al. 2024). Tertiary structural modelling suggested that all identified FPV strains retained overall structural integrity, despite minor physicochemical variations. Changes in molecular weight, theoretical pI and aliphatic index suggest potential implications for host‐virus interactions (Li et al. 2024). However, these interpretations are stated on the basis of previously published literature and still remain speculative, as no functional assays were conducted in this study to confirm such effects.
In conclusion, this study provides critical insights into FPV detection accuracy, epidemiological patterns and genetic evolution in previously unreported regions, thereby expanding the geographic coverage of FPV monitoring in Bangladesh and strengthening the national molecular surveillance framework. The higher specificity of PCR over the IC test highlights the necessity of molecular confirmation in diagnostic workflows. The VP2 mutations and structural variations indicate ongoing potential viral adaptation, necessitating continuous genomic surveillance. Given the high prevalence of FPV in unvaccinated cats, improved vaccination coverage and targeted regional monitoring are essential to mitigate future outbreaks. However, whole‐genome sequencing of both field and vaccine strains is required to better understand the genetic relationship between naturally circulating strains and the vaccine strain.
Author Contributions
Md. Golzar Hossain designed the study. Nurejunnati Jeba, Md. Masum Billah Tarafder, Mohammad Bayazid Bostami, S. M. Nazmul Hasan and Abdul Mannan collected the samples. Nurejunnati Jeba, Roni Mia, S. M. Nazmul Hasan conducted the laboratory experiments. A. K. M. Anisur Rahman, Nurejunnati Jeba and Roni Mia analysed data. Roni Mia, Raduyan Farazi and Anandha Mozumder analysed the protein structures. Nurejunnati Jeba, Roni Mia and Md. Golzar Hossain wrote the draft version of the manuscript. Sharmin Akter, Sukumar Saha, Tofazzal Islam, Antonio Charlys da Costa, Elcio Leal and Md. Golzar Hossain reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Ethics Statement
All the protocols in this investigation have been performed in accordance with the guidelines of the Animal Welfare and Experimentation Ethics Committee (AWEEC) of Bangladesh Agricultural University.
Conflicts of Interest
The authors declare no conflicts of interest.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.70594.
Supporting information
Table S1. Clinical and diagnostic data of study subjects based on sample collection locations.
Acknowledgements
The authors would like to acknowledge the pet owners and staffs of the Teaching and Training Pet Hospital and Research Center, Chattogram Veterinary and Animal Sciences University, Chattogram 4225, Bangladesh.
Jeba, N. , Mia R., Tarafder M. M. B., et al. 2025. “Comparative Detection and Genetic Characterization of Feline Panleukopenia Virus in Bangladesh.” Veterinary Medicine and Science 11, no. 5: 11, e70594. 10.1002/vms3.70594
Funding: This study was supported by a research grant from the Ministry of Science and Technology, Government of the People's Republic of Bangladesh via Project SRG‐231080.
Data Availability Statement
The data generated in this study are available in the supplementary table and within the manuscript.
References
- Abdelbaky, M. M. , El‐Khabaz K. A., and Hamed M. I.. 2024. “Diagnostic Performance of a Rapid in‐Clinic Test for the Detection of Feline Parvovirus.” Assiut Veterinary Medical Journal 70, no. 182: 253–262. [Google Scholar]
- Alessa, M. , Marzok M., Elnafarawy H., et al. 2026. “Prevalence of Feline panleukopenia (FPL) in Domestic Cats: A Systematic Meta‐Analysis.” Veterinary Integrative Sciences 24, no. 1: 1–17. 10.12982/VIS.2026.001. [DOI] [Google Scholar]
- Awad, R. A. , Khalil W. K. B., and Attallah A. G.. 2018. “Epidemiology and Diagnosis of Feline panleukopenia Virus in Egypt: Clinical and Molecular Diagnosis in Cats.” Veterinary World 11, no. 5: 578–584. 10.14202/vetworld.2018.578-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboni, A. , Bassi F., De Arcangeli S., et al. 2018. “Molecular Analysis of Carnivore Protoparvovirus Detected in White Blood Cells of Naturally Infected Cats.” BMC Veterinary Research 14, no. 1: 41. 10.1186/s12917-018-1356-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratloo, A. , Hosseini M., Negida A., and El Ashal G.. 2015. “Part 1: Simple Definition and Calculation of Accuracy, Sensitivity and Specificity.” Emergency 3, no. 2: 48–49. [PMC free article] [PubMed] [Google Scholar]
- Barrs, V. R. 2019. “ Feline panleukopenia: A Re‐Emergent Disease.” Veterinary Clinics of North America: Small Animal Practice 49, no. 4: 651–670. 10.1016/j.cvsm.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Brindhalakshmi, B. , Mukhopadhyay H., Antony P., Thanislass J., Vijayalakshmi P., and Mangadevi N.. 2016. “Isolation and Molecular Characterization of Canine and Feline Parvovirus Strains‐An Updated Review.” Journal of Dairy, Veterinary & Animal Research 3, no. 5: 164–169. [Google Scholar]
- Brower, A. I. , Radi C., Krueger D., and Toohey‐Kurth K.. 2004. “ Feline panleukopenia: A Diagnostic Laboratory's Perspective.” Veterinary Medicine 99, no. 8: 714–720. [Google Scholar]
- Carmichael, L. E. , Joubert J. C., and Pollock R. V.. 1980. “Hemagglutination by Canine Parvovirus: Serologic Studies and Diagnostic Applications.” American Journal of Veterinary Research 41, no. 5: 784–791. [PubMed] [Google Scholar]
- Cave, T. A. , Thompson H., Reid S. W., Hodgson D. R., and Addie D. D.. 2002. “Kitten Mortality in the United Kingdom: A Retrospective Analysis of 274 Histopathological Examinations (1986 to 2000).” Veterinary Record 151, no. 17: 497–501. 10.1136/vr.151.17.497. [DOI] [PubMed] [Google Scholar]
- Chang, D. , Liu Y., Chen Y., et al. 2020. “Study of the Immunogenicity of the VP2 Protein of Canine Parvovirus Produced Using an Improved Baculovirus Expression System.” BMC Veterinary Research 16, no. 1: 202. 10.1186/s12917-020-02422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury, Q. , Alam S., Chowdhury M. S. R., et al. 2021. “First Molecular Characterization and Phylogenetic Analysis of the VP2 Gene of Feline panleukopenia Virus in Bangladesh.” Archives of Virology 166, no. 8: 2273–2278. 10.1007/s00705-021-05113-y. [DOI] [PubMed] [Google Scholar]
- Foley, J. E. , Orgad U., Hirsh D. C., Poland A., and Pedersen N. C.. 1999. “Outbreak of Fatal Salmonellosis in Cats Following Use of a High‐Titer Modified‐Live Panleukopenia Virus Vaccine.” Journal of the American Veterinary Medical Association 214, no. 1: 67–70. [PubMed] [Google Scholar]
- Gans, J. S. , Goldfarb A., Agrawal A. K., Sennik S., Stein J., and Rosella L.. 2022. “False‐Positive Results in Rapid Antigen Tests for SARS‐CoV‐2.” Jama 327, no. 5: 485–486. 10.1001/jama.2021.24355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert, C. , McManus D. D., and Soni A.. 2024. “Persistent False Positive Covid‐19 Rapid Antigen Tests.” New England Journal of Medicine 390, no. 8: 764–765. 10.1056/NEJMc2313517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzworth, J. 1987. Diseases of the Cat: Medicine & Surgery. Saunders. https://books.google.com.bd/books?id=JQyu5FcHV6gC. [Google Scholar]
- Horiuchi, M. , Yamaguchi Y., Gojobori T., et al. 1998. “Differences in the Evolutionary Pattern of Feline panleukopenia Virus and Canine Parvovirus.” Virology 249, no. 2: 440–452. 10.1006/viro.1998.9335. [DOI] [PubMed] [Google Scholar]
- Jacobson, L. S. , Janke K. J., Giacinti J., and Weese J. S.. 2021. “Diagnostic Testing for Feline panleukopenia in a Shelter Setting: A Prospective, Observational Study.” Journal of Feline Medicine and Surgery 23, no. 12: 1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, R. , and Spradbrow P.. 1979. “Isolation From Dogs With Severe Enteritis of a Parvovirus Related to Feline panleukopenia Virus.” Australian Veterinary Journal 55: 151–152. [Google Scholar]
- Kabir, A. , Habib T., Chouhan C. S., Hassan J., Rahman A., and Nazir K.. 2023. “Epidemiology and Molecular Characterization of Feline panleukopenia Virus From Suspected Domestic Cats in Selected Bangladesh Regions.” PLoS ONE 18, no. 10: e0282559. 10.1371/journal.pone.0282559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse, B. D. , Unterer S., Horlacher K., Sauter‐Louis C., and Hartmann K.. 2010. “Prognostic Factors in Cats with Feline panleukopenia .” Journal of Veterinary Internal Medicine 24, no. 6: 1271–1276. 10.1111/j.1939-1676.2010.0604.x. [DOI] [PubMed] [Google Scholar]
- Laskowski, R. A. , MacArthur M. W., Moss D. S., and Thornton J. M.. 1993. “PROCHECK: A Program to Check the Stereochemical Quality of Protein Structures.” Applied Crystallography 26, no. 2: 283–291. [Google Scholar]
- Leal, É. , Liang R., Liu Q., et al. 2020. “Regional Adaptations and Parallel Mutations in Feline panleukopenia Virus Strains from China Revealed by Nearly‐Full Length Genome Analysis.” PLoS ONE 15, no. 1: e0227705. 10.1371/journal.pone.0227705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, G. , Ji S., Zhai X., et al. 2017. “Evolutionary and Genetic Analysis of the VP2 Gene of Canine Parvovirus.” BMC Genomics 18, no. 1: 534. 10.1186/s12864-017-3935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Peng J., Zeng Y., et al. 2024. “Isolation of a Feline‐Derived Feline panleukopenia Virus With an A300P Substitution in the VP2 Protein and Confirmation of Its Pathogenicity in Dogs.” Animal Diseases 4, no. 1: 5. [Google Scholar]
- Litster, A. , and Benjanirut C.. 2014. “Case Series of Feline panleukopenia Virus in an Animal Shelter.” Journal of Feline Medicine and Surgery 16, no. 4: 346–353. 10.1177/1098612X13497738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, E. , and Sikes H. D.. 2015. “Addressing Barriers to the Development and Adoption of Rapid Diagnostic Tests in Global Health.” Nanobiomedicine (Rij) 2: 6. 10.5772/61114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki, M. , San Gabriel M., Nakatani H., Yoshida M., and Harasawa R.. 1993. “Comparison of Polymerase Chain Reaction With Virus Isolation and Haemagglutination Assays for the Detection of Canine Parvoviruses in Faecal Specimens.” Research in Veterinary Science 55, no. 1: 60–63. [DOI] [PubMed] [Google Scholar]
- Parthiban, M. , Saranya R., Divya K. C., and Kumanan K.. 2012. “Detection of Antigenic Variation of Canine Parvovirus Strains of Tamil Nadu Using Differential PCR.” Indian Journal of Animal Sciences 82, no. 3: 237–239. [Google Scholar]
- Porporato, F. , Horzinek M. C., Hofmann‐Lehmann R., et al. 2018. “Survival Estimates and Outcome Predictors for Shelter Cats with Feline panleukopenia Virus Infection.” Journal of the American Veterinary Medical Association 253, no. 2: 188–195. 10.2460/javma.253.2.188. [DOI] [PubMed] [Google Scholar]
- Raheena, K. P. , Priya P., Mani B. K., Mini M., and Pillai U. N.. 2017. “Comparison of Different Diagnostic Test to Detect Feline panleukopenia Virus Among Cats in Kerala, India.” Indian Journal of Animal Research 51, no. 2: 347–349. [Google Scholar]
- Raja, P. , Mallika K. S., Viva V. Y., et al. 2024. “Complete Genome Sequence and Phylogenetic Analysis of Feline panleukopenia Virus From India.” Virusdisease 35, no. 1: 34–40. 10.1007/s13337-023-00854-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadhani, M. E. , Indarjulianto S., Yanuartono Y., et al. 2024. “Diagnosis of Feline panleukopenia Based on Clinical Signs and Polymerase Chain Reaction in Various Ages of Cats.” Jurnal Sain Veteriner 42, no. 1: 121–128. [Google Scholar]
- Rehme, T. , Hartmann K., Truyen U., Zablotski Y., and Bergmann M.. 2022. “ Feline panleukopenia Outbreaks and Risk Factors in Cats in Animal Shelters.” Viruses 14, no. 6: 1248. 10.3390/v14061248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl, G. , Bates R. C., Berns K. I., et al. 1985. “Characteristics and Taxonomy of Parvoviridae.” Intervirology 23, no. 2: 61–73. 10.1159/000149587. [DOI] [PubMed] [Google Scholar]
- Su, W. J. , Shen W. D., Li B., Wu Y., Gao G., and Wang W. B.. 2009. “A Novel Way to Purify Recombinant Baculoviruses by Using bacmid.” Bioscience Reports 29, no. 2: 71–75. 10.1042/bsr20080074. [DOI] [PubMed] [Google Scholar]
- Sykes, J. E. 2013. “ Feline panleukopenia Virus Infection and Other Viral Enteritides.” Canine and Feline Infectious Diseases 19: 187–194. [Google Scholar]
- Tamura, K. , and Nei M.. 1993. “Estimation of the Number of Nucleotide Substitutions in the Control Region of Mitochondrial DNA in Humans and Chimpanzees.” Molecular Biology and Evolution 10, no. 3: 512–526. 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- Tamura, K. , Stecher G., and Kumar S.. 2021. “MEGA11: Molecular Evolutionary Genetics Analysis Version 11.” Molecular Biology and Evolution 38, no. 7: 3022–3027. 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truyen, U. , Addie D., Belák S., et al. 2009. “ Feline panleukopenia. ABCD Guidelines on Prevention and Management.” Journal of Feline Medicine and Surgery 11, no. 7: 538–546. 10.1016/j.jfms.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuzio, H. 2021. “ Feline panleukopenia .” In Infectious Disease Management in Animal Shelters, 337–366. Wiley. [Google Scholar]
- Waterhouse, A. , Bertoni M., Bienert S., et al. 2018. “SWISS‐MODEL: Homology Modelling of Protein Structures and Complexes.” Nucleic Acids Research 46, no. W1: W296–W303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, Y. , Tang Z., Wang K., et al. 2024. “Epidemiological and Molecular Investigation of Feline panleukopenia Virus Infection in China.” Viruses 16, no. 12: 1967. 10.3390/v16121967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederstein, M. , and Sippl M. J.. 2007. “ProSA‐Web: Interactive Web Service for the Recognition of Errors in Three‐Dimensional Structures of Proteins.” Nucleic Acids Research 35, no. S2: W407–W410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, H. , Liang Y., Gao X., et al. 2023. “Development and Application of nanoPCR Method for Detection of Feline panleukopenia Virus.” Veterinary Sciences 10, no. 7: 440. 10.3390/vetsci10070440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, D.‐K. , Park Y.‐R., Park Y., et al. 2022. “Isolation and Molecular Characterization of Feline panleukopenia Viruses From Korean Cats.” Korean Journal of Veterinary Research 62, no. 1: e10. [Google Scholar]
- Yang, S. , Wang S., Feng H., et al. 2010. “Isolation and Characterization of Feline panleukopenia Virus From a Diarrheic Monkey.” Veterinary Microbiology 143, no. 2–4: 155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, S. , Liu S., Meng X., et al. 2021. “ Feline panleukopenia Virus With G299E Substitution in the VP2 Protein First Identified From a Captive Giant Panda in China.” Frontiers in Cellular and Infection Microbiology 11: 820144. 10.3389/fcimb.2021.820144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Zhang W., Pan Y., et al. 2024. “Evolutionary Dynamics and Pathogenicity Analysis of Feline panleukopenia Virus in Xinjiang, China.” Microorganisms 12, no. 11: 2205. 10.3390/microorganisms12112205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical and diagnostic data of study subjects based on sample collection locations.
Data Availability Statement
The data generated in this study are available in the supplementary table and within the manuscript.


