Abstract
The effects of pressure on cultures of Lactobacillus plantarum were characterized by determination of the viability and activity of HorA, an ATP-binding cassette multidrug resistance transporter. Changes in the membrane composition of L. plantarum induced by different growth temperatures were determined. Furthermore, the effect of the growth temperature of a culture on pressure inactivation at 200 MPa was determined. Cells were characterized by plate counts on selective and nonselective agar after pressure treatment, and HorA activity was measured by ethidium bromide efflux. Fourier transform-infrared spectroscopy and Laurdan fluorescence spectroscopy provided information about the thermodynamic phase state of the cytoplasmic membrane during pressure treatment. A pressure-temperature diagram for cell membranes was established. Cells grown at 37°C and pressure treated at 15°C lost >99% of HorA activity and viable cell counts within 36 and 120 min, respectively. The membranes of these cells were in the gel phase region at ambient pressure. In contrast, cells grown at 15°C and pressure treated at 37°C lost >99% of HorA activity and viable cell counts within 4 and 8 min, respectively. The membranes of these cells were in the liquid crystalline phase region at ambient pressure. The kinetic analysis of inactivation of L. plantarum provided further evidence that inactivation of HorA is a crucial step during pressure-induced cell death. Comparison of the biological findings and the membrane state during pressure treatment led to the conclusion that the inactivation of cells and membrane enzymes strongly depends on the thermodynamic properties of the membrane. Pressure treatment of cells with a liquid crystalline membrane at 0.1 MPa resulted in HorA inactivation and cell death more rapid than those of cells with a gel phase membrane at 0.1 MPa.
High hydrostatic pressure is used in food applications to attenuate and inactivate microorganisms. The survival of microorganisms during high-pressure treatment is strongly dependent on environmental conditions, such as temperature, pH, and the presence of antimicrobial compounds. Therefore, knowledge of the mechanisms of pressure-induced cell death is paramount for the deliberate design of high-pressure processes in food technology (17, 19, 29, 44).
The mechanisms of pressure-induced cell death are under investigation. Transient permeabilization of the outer and inner membranes of Escherichia coli was observed by pressure treatment in the presence of nisin, propidium iodide, and N-phenylnaphthylamine, respectively (1, 16a, 43). Furthermore, lethal pressure treatment resulted in the loss of proteins from the outer membrane of Salmonella enterica serovar Typhimurium (38). Sublethal pressure treatment of Saccharomycopsis fibuligera led to loss of glycerol and ions through permeabilized membranes (36). The loss of acid resistance of E. coli upon sublethal pressure treatment suggested the inactivation of protective or repair functions relevant for pH homeostasis (24, 31). The factors involved in the loss of pH homeostasis of pressure-treated Lactobacillus plantarum were investigated by Wouters et al. (50). Sublethal pressure treatment of L. plantarum resulted in a loss of the cell's ability to maintain the internal pH, in decreased acid efflux, and in inactivation of the F0F1 ATPase, whereas the ability to generate ATP remained unaffected. The baroresistance of the same strain was also reported to be related to the phospholipid head group composition (43). Denaturation of ribosomes in vivo correlated with pressure-mediated cell death of E. coli. The authors suggested that ribosome denaturation was caused by leakage of Mg2+ from pressure-permeabilized membranes (30). Taken together, these results suggest that damage to membranes or membrane-bound transport systems is an important event in high-pressure-mediated cell death.
The activity and inactivation of membrane-bound enzymes depend on changes in protein structure, membrane fluidity, or both (7, 14). Lipids in biological membranes are in the fluid (liquid crystalline) phase, allowing fast lateral movement of molecules. Pressure upshift and/or temperature downshift in pure one-component phospholipid bilayers induces a phase transition from the liquid crystalline phase to a gel phase, characterized by an increased rigidity and reduced conformational degrees of freedom for the acyl chains. In natural membranes with a complex lipid composition, a more or less wide coexistence region of gel and liquid crystalline phases is observed at the phase transition. Studies of the pressure-induced denaturation of proteins in aqueous solution have led to the conclusion that pressure compresses cavities of proteins (21, 22). Furthermore, pressure-induced ionization of amino side chains and hydration of apolar amino acid side chains and concomitant loss of hydrophobic interactions destabilize the secondary structure of proteins, leading to protein denaturation (20, 28). As a general rule, oligomeric proteins are dissociated at relatively low pressure (<200 MPa), whereas the irreversible denaturation of enzymes and proteins in aqueous solution requires pressures higher than 300 MPa (21, 28). Few data are available on pressure-induced structural changes of proteins dissolved in membranes (52).
HorA is an ATP-dependent multidrug resistance (MDR) transporter of the ABC family conferring hop resistance on beer spoilage bacteria (40, 47). The observation that the fluorescent dye ethidium bromide (EB) is a substrate for HorA allows a straightforward determination of HorA activity. HorA of L. plantarum is therefore a suitable model system to investigate the effect of chemical and physical membrane properties on the pressure-mediated inactivation of membrane-bound enzymes and cell death. High-pressure inactivation of L. plantarum was previously found to involve HorA inactivation (45), and loss of HorA activity was correlated with the failure of pressure-treated cells to survive in the presence of hop bitter acids (16). HorA is homologous to mammalian MDR transporters and is a structural and functional homologue of the MDR transporter LmrA of Lactococcus lactis (39). The amino acid sequences of LmrA and HorA are 52% identical. LmrA possesses six hydrophobic membrane-spanning α-helical segments (5, 6, 47). The enzyme binds amphiphilic substrates in the inner leaflet of the membrane and catalyzes translocation to the outer leaflet of the bilayer (26, 46). The catalytic cycle of LmrA involves major rearrangements of the secondary structure induced by ATP binding and hydrolysis (48).
It was the objective of this work to determine membrane-triggered mechanisms resulting in pressure-mediated cell death and HorA inactivation of L. plantarum. Cultures were prepared by variation of the incubation temperature to achieve cells with different compositions of the cytoplasmic membrane. Temperature- and pressure-induced phase transitions of the cytoplasmic membrane were determined by Fourier transform-infrared (FT-IR) and Laurdan (see below) fluorescence spectroscopy, respectively. The consequences of changes in membrane properties for the resistance of cells to pressure as well as pressure-induced HorA inactivation were evaluated.
MATERIALS AND METHODS
Strains and culture conditions.
L. plantarum TMW 1.460, an organism previously isolated from spoiled beer, was cultivated using model beer (MB). Where appropriate, MB containing 5% ethanol was used. MB was prepared by fermentation of malt extract medium with Saccharomyces cerevisiae TMW 3.001 as previously described (45). L. plantarum was cultured in MB for 24 h and subcultured at 15, 30, and 37°C for 64, 24, and 20 h, respectively, to late stationary growth phase. The growth time in MB containing 5% ethanol at 30°C was 36 h.
High-pressure treatment.
An overnight culture of L. plantarum in MB was subcultured with 1% inoculum. Late-stationary-phase cells were harvested by centrifugation and resuspended in an equal volume of MB. This cell suspension was transferred to 2-ml Eppendorf reaction tubes, sealed with silicon stoppers, and stored on ice until pressurization. The high-pressure inactivation kinetics of L. plantarum were investigated in high-pressure autoclaves precooled to 15 or 37°C. The compression and decompression rates were 200 MPa min−1. Upon pressurization, samples were stored on ice until further analysis. For each high-pressure inactivation kinetics, untreated cultures and cultures sterilized by treatment at 800 MPa for 10 min were used for comparison. The sample nomenclature was chosen with respect to the growth temperature (TG) and the temperature of high-pressure treatment (TP). For example, a culture grown at 15°C and pressure treated at 37°C was labeled TG15/TP37.
Determination of plate counts.
Cell counts were determined on MRS agar (13) or MRS agar containing 4% NaCl (MRS-NaCl agar) for determination of viable and sublethally injured cells. Appropriate dilutions were plated using a spiral plater (IUL, Königswinter, Germany), and the plates were incubated at 30°C for 2 days under a controlled atmosphere (76% N2, 20% CO2, 4% O2). Cell counts of cultures of L. plantarum in MB were 4 × 108 ± 2 × 108 CFU ml−1 on either MRS or MRS-NaCl agar (mean, 20 determinations). The cell counts on MRS agar are referred to as viable cells, and the difference between cell counts on MRS and MRS-NaCl agars is referred to as sublethally injured cells.
Determination of HorA activity.
HorA activity was determined by using EB as a substrate according to the method of Ulmer et al. (45). EB stock solutions were prepared by dissolving 40 μmol of EB liter−1 in phosphate buffer (PB; 50 mM H2KPO4, 0.1 g of MgSO4 liter−1 · 7H2O, and 0.05 g of MnSO4 liter−1 · H2O, pH 6.5). After high-pressure treatment, the cells were harvested by centrifugation (10 min at 15°C; 6,000 × g) and resuspended in PB. Each sample received EB stock solution to obtain a staining EB concentration of 20 μmol liter−1. The samples were mixed and incubated at 30°C for 90 min in the dark to load the cells with EB in the absence of an energy source. After incubation, the cells were harvested and resuspended in PB. Two hundred microliters of this cell suspension was transferred to black microtiter plates. To determine the EB efflux kinetics, 2 μl of glucose stock solution (1 g of glucose ml−1) was added to the samples. Reenergized cells export EB, resulting in a lower fluorescence of the EB-nucleotide complex. Fluorescence was measured in a Spectrafluor microtiter plate reader (Tecan, Grödig, Austria) at 1-min intervals for 60 min at 30°C (excitation wavelength [λEx] = 485 nm; emission wavelength [λEm] = 595 nm). The initial rate of fluorescence reduction of every sample was calculated. EB efflux activity was normalized to the activity of untreated cells with each experiment. It was verified that L. plantarum carries a functional horA gene (45). EB transport in the presence of glucose was fully inhibited by 50 μM reserpine, an inhibitor of the HorA homologues human MDR1 and LmrA of L. lactis (47). This indicates that EB efflux in L. plantarum is mainly attributable to HorA activity.
Determination of membrane composition.
Cells were inoculated and grown as described above in 50 ml of MB for different periods and at different temperatures to get samples TG15, TG30, TG37, and TG30 + 5%. After lyophilization for 24 h, the samples were packed under an N2 atmosphere and sent to Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany). Membrane fatty acids were extracted, transesterified, and analyzed by gas chromatography.
Determination of temperature-dependent membrane phase state by FT-IR spectroscopy.
In order to avoid overlapping of acyl chain vibrational bands with IR absorption of H2O, the FT-IR measurements were performed in D2O as a solvent. One-milliliter cell suspensions of samples TG15, TG30, TG37, and TG30 + 5% were washed and incubated in 1 ml of D2O for 1 h at 15°C to exchange the remaining H2O for D2O. After incubation, the cells were harvested and resuspended in 100 μl of D2O, and 35 μl of cell suspension were poured into a 25-μm-thick infrared cell with CaF2 windows. The FT-IR spectra were recorded with a MAGNA 550 (ThermoNicolet, Madison, Wis.) spectrometer equipped with a liquid-nitrogen-cooled MCT (HgCdTe) detector. Each spectrum was obtained by co-adding 256 scans at a spectral resolution of 2 cm−1 and was apodized with a Happ-Genzel function. The sample chamber was purged with dry and carbon dioxide-free air. The infrared cell was controlled by an external water thermostat. To achieve thermal equilibrium conditions in the CaF2 cell, 20 min was allowed to elapse between measurements. Data were collected at temperatures of −4 to 45°C in steps of 2°C. Each sample was measured twice in two independent experiments.
The internal vibrational modes of the lipid acyl chains are assigned on the basis of the well-known studies of polymethylenes and polymethylene chain compounds (37, 42). In the 2,800- to 3,100-cm−1 region, there are infrared absorption bands due to symmetric and antisymmetric modes of the methylene chain at about 2,850 and 2,920 cm−1, respectively. The wave numbers of these bands are conformation sensitive and thus respond to temperature- and pressure-induced changes of the trans/gauche ratio in acyl chains. The vibrational mode (antisymmetric stretch) of the terminal CH3 group occurs at about 2,960 cm−1.
Determination of the pressure-dependent phase state of the membrane by fluorescence spectroscopy.
To study the polarity of the lipid interface and to detect phase changes of the lipid bilayer membrane, Laurdan fluorescence spectroscopy was used. Laurdan (6-dodecanoyl-2-dimethylaminonaphthalene) (Molecular Probes, Eugene, Oreg.) is an amphiphilic fluorescence probe which allows the determination of gel-to-fluid phase transitions in biological membranes (32, 33, 35). The incubations of cells were identical to those for FT-IR analyses. After incubation, 1-ml cell suspensions of samples TG15, TG30, TG30 + 5%, and TG37 were washed and incubated in 1 ml of D2O for 1 h at 15°C to exchange the remaining H2O for D2O. After incubation, cells were harvested and resuspended in 980 μl of D2O. After the addition of 20 μl of Laurdan stock solution in ethanol (2 mmol liter−1), an effective staining concentration of 40 μmol liter−1 was reached. The cells were stained for 30 min at 30°C in the dark. To measure the fluorescence spectra, cells were harvested, resuspended in 1 ml of D2O, and put in a glass vial. The vial was inserted into a high-pressure vessel with quartz windows, and the vessel was connected to a high-pressure pump. Laurdan emission spectra were obtained using the K2 multifrequency phase and modulation fluorometer (ISS Inc., Champaign, Ill.). The fluorometer uses a xenon arc lamp as a light source. The monochromator bandpass was 8 nm. The spectra were corrected only for lamp intensity variation. The excitation wavelength was 360 nm, and emission spectra were collected from 380 to 550 nm with steps of 1 nm. The steady-state fluorescence parameter known as generalized polarization (GP) was calculated as follows: GP = (I440 nm − I490 nm)/(I440 nm + I490 nm), where I is the relative fluorescence intensity at the respective wavelengths (34). The temperature was controlled to ±0.1°C by a circulating water bath. For the high-pressure fluorescence studies, the ISS high-pressure cell with quartz windows was used. Pressure was controlled with the APP automated pressure control system. The pressure steps were 20 MPa with a ramp of 10 MPa min−1. The time left for equilibration after each pressure step was 10 min.
RESULTS
Membrane composition of L. plantarum as a function of growth temperature.
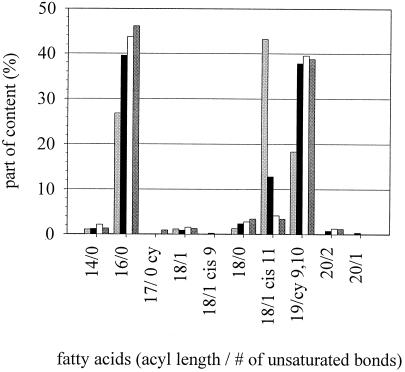
According to the theory of homeoviscous adaptation, cells change the composition of their membranes to achieve a state of optimal membrane functionality in response to nonambient conditions (2, 4, 8). Lactobacilli change membrane fatty acid composition to higher contents of unsaturated fatty acids if grown at low temperatures (15, 43). L. plantarum was grown at various growth temperatures (TG15, TG30, TG37, and TG30 + 5%), and the fatty acid composition of membranous phospholipids was determined. Figure 1 shows the most prominent fatty acids of L. plantarum. Hexadecanoic acid (16/0 [number of carbon atoms/number of double bonds]) is the most important of the saturated fatty acids. cis-11-Octadecenoic acid (18/1 cis 11) and cis-11,12-methyleneoctadecanoic acid (19/cy 9,10) are the most important representatives of the unsaturated fatty acids. cis-11-Octadecenoic acid and cis-11,12-methyleneoctadecanoic acid have equivalent effects on membrane fluidity. The proportion of saturated fatty acids was increased with increasing growth temperature, while the number of unsaturated fatty acids was decreased. The ratio of unsaturated to saturated fatty acids changed between 15 and 37°C from 70%:30% to 51%:49%. Harvesting temperature-adapted microorganisms for high-pressure experiments thus provides microorganisms with different membrane compositions.
FIG. 1.
Fatty acid composition of L. plantarum cytoplasmic membranes. The cells were grown at different temperatures (TG) (light shaded bars, 15°C; solid bars, 30°C; open bars, 30°C + 5% ethanol; dark shaded bars, 37°C).
Membrane phase state at different temperatures measured by FT-IR spectroscopy.
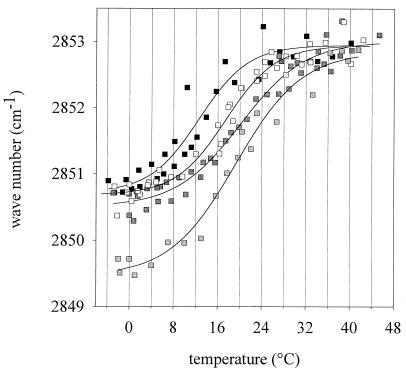
Using FT-IR spectroscopy, the effect of altered membrane composition on conformational and thermodynamic membrane properties was determined. In Fig. 2, the peak wave number of the CH2 stretching vibrations of the membrane lipids is shown. Wave numbers around 2,850.5 cm−1 represent a relatively rigid molecular packing of the fatty acyl chains, and the peak is shifted to higher wave numbers if the chains are “melting” and a high conformational disorder is reached at high temperatures in the liquid crystalline phase. The FT-IR melting curves were fitted to the following logistic function:
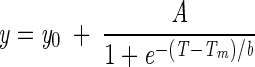 |
where y0 and A denote the lower and upper asymptotes, respectively, and b is a shape coefficient. The melting temperature of the membranes was defined as the turning point of the melting curve. All of the liquid crystalline or gel phase regions are characterized by a nearly horizontal slope. Figure 2 shows that the CH2 stretching vibrations of samples TG15, TG30, and TG37 occur at about 2,850.5 cm−1 at −4°C. The corresponding value for sample TG30 + 5% was 2,849.5 cm−1, pointing to a higher membrane rigidity at that temperature. The samples TG15, TG30, TG37, and TG30 + 5% exhibit mean chain melting temperatures of 12.6, 17.4, 20.1, and 19.3°C, respectively. A decreased incubation temperature thus induced a change in chain melting behavior which could be detected by FT-IR spectroscopy. Increasing amounts of unsaturated fatty acids shift the phase transition towards lower temperatures. However, the lowering of the melting points of the membranes is not proportional to the growth temperature changes. For example, a downshift of growth temperature by 15°C from 30 to 15°C induced a reduction of the membrane melting point by only 5°C.
FIG. 2.
Peak maxima of FT-IR spectroscopy measurements of L. plantarum cells grown at different temperatures (TG) (solid squares, 15°C; open squares, 30°C; light shaded squares, 30°C + 5% ethanol; dark shaded squares, 37°C). Shown are the wave numbers from two independent experiments. Every experiment was done by heating the cells from −4 to 45°C and cooling them from 45 to −4°C. The curves represent the best fit of the logistic function (r2 ≥ 0.98).
Membrane phase state at different temperatures and pressures as measured by Laurdan fluorescence spectroscopy.
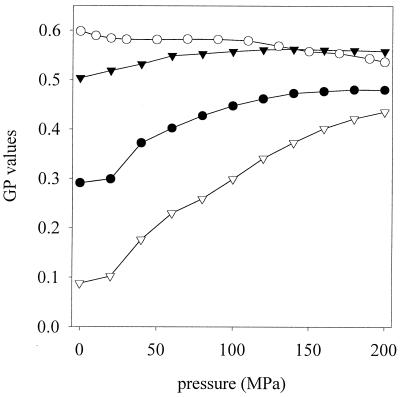
The FT-IR spectroscopy data provided information about the temperature-induced membrane phase transitions at ambient pressure. The pressure-dependent membrane phase behavior was determined by Laurdan fluorescence spectroscopy. The Laurdan fluorescence spectra depend on changes in environmental polarity induced by membrane phase transitions (3, 33). The determination of the pressure dependence of the GP values of cells grown (TG) and high-pressure treated (TP) at different temperatures are shown in Fig. 3. Data from samples TG15/TP15 and TG30 + 5%/TP15 were equivalent to those obtained with sample TG30/TP15 (data not shown). With increasing pressure, the polarity around the fluorophor in fluid membranes decreases, resulting in higher GP values. The change can be interpreted as pressure-induced phase transition from liquid crystalline to coexistence and gel phase regions. TG15/TP37 showed the greatest increase of GP with pressure. These cells possess a high content of unsaturated fatty acids. An increase of pressure at 37°C induces a marked phase transition indicated by a shift in the GP value from 0.09 to 0.42. TG37/TP37 showed a less pronounced phase transition with increasing pressure. On the other hand, the samples TG30/TP15, TG30 + 5%/TP15, and TG15/TP15 exhibited no significant changes of phase state with pressure. Their membranes stayed in an essentially rigid, gel-like state. The influence of ethanol during growth on membrane phase behavior with high pressure is negligible. The membrane in TG37/TP15 experiments was fully converted to a gel phase by cooling it from 37 to 15°C, and an additional change due to pressure was not detectable.
FIG. 3.
GP values of L. plantarum cells stained with Laurdan under pressure conditions. The cells were grown at temperature TG and were high pressure treated at temperature TP. •, TG37/TP37; ○, TG37/TP15; ▾, TG30/TP15; ▿, TG15/TP37. Data from samples TG15/TP15 and TG30 + 5% ethanol/TP15 were equivalent to those obtained with sample TG30/TP15 (data not shown).
Pressure inactivation of L. plantarum.
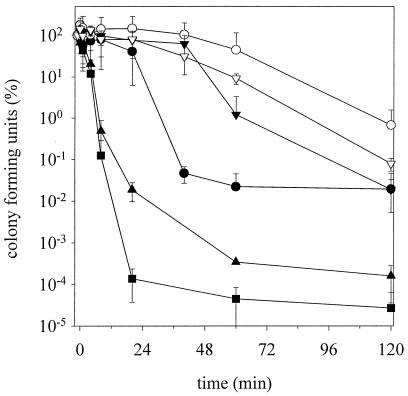
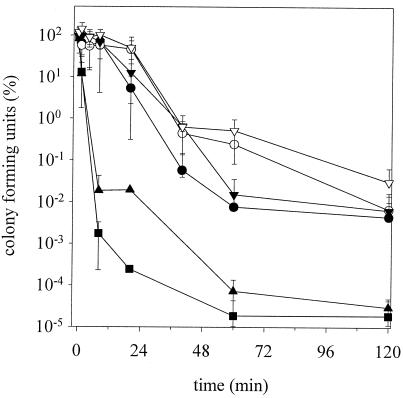
To evaluate the influence of altered chemical and physical properties on cytoplasmic membranes, cells were high pressure treated (200 MPa) and the kinetics of inactivation were determined by plate counts on selective and nonselective agar. Figure 4 shows the cell counts on MRS agar which was high pressure treated at 15 and 37°C. The sample TG15/TP37 showed the fastest inactivation, with more than 6 log cycles after 30 min. This speed of inactivation is equivalent to those seen in previous investigations of TG30/TP15 cells at 400 MPa (45). The inactivation velocity decreased in the order TG15/TP37 > TG37/TP37 > TG15/TP15 > TG30/TP15, TG37/TP15, and TG30 + 5%/TP15. The last cells (TG30 + 5%/TP15) showed no inactivation after 45 min. The incubation with 5% ethanol during growth apparently provided a baroprotection. The results on selective plates (MRS-NaCl agar) showed the same sequence of inactivation. In general, the rate of inactivation was threefold faster (Fig. 5).
FIG. 4.
High-pressure inactivation kinetics of L. plantarum at 200 MPa. The cells were grown at temperatures TG and were pressurized at temperatures TP. Shown are the means and standard deviations of viable cell counts of two experiments plated on MRS agar relative to initial cell counts. •, TG15/TP15; ○, TG30 + 5% ethanol/TP15; ▾, TG30/TP15; ▿, TG37/TP15; ▴, TG37/TP37; ▪, TG15/TP37.
FIG. 5.
High-pressure inactivation kinetics of L. plantarum at 200 MPa. The cells were grown at temperatures TG and were pressurized at temperatures TP. Shown are the means and standard deviations of viable cell counts from two experiments plated on MRS-NaCl agar relative to initial cell counts. •, TG15/TP15; ○, TG30 + 5% ethanol/TP15; ▾, TG30/TP15; ▿, TG37/TP15; ▴, TG37/TP37; ▪, TG15/TP37.
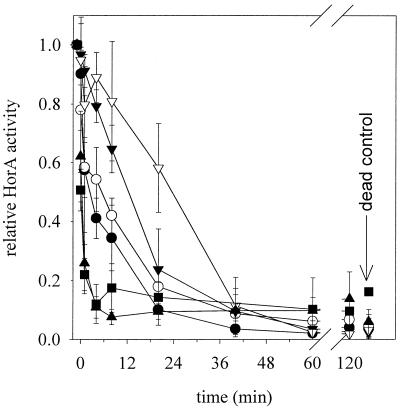
Pressure inactivation of HorA.
In addition to plate counts, high-pressure-treated cultures were also characterized with respect to HorA activity. The results of the fluorimetric assay for HorA activity are shown in Fig. 6. The EB transport activities of the samples TG15/TP15, TG30 + 5%/TP15, TG30/TP15, and TG37/TP15 were more pressure resistant than those of TG15/TP37 and TG37/TP37. After the first pressure application, the relative activity of the samples TG15/TP37 and TG37/TP37 decreased from 1.0 to 0.5 or 0.6, respectively. The HorA activity then decreased rapidly, and after 7 min of pressure treatment reached the level of control dead cells (treated at 800 MPa for 10 min). The inactivation of HorA in samples TG15/TP15, TG30 + 5%/TP15, TG30/TP15, and TG37/TP15 was slower and reached the level of control cells after between 36 and 60 min. No significant differences in HorA inactivation among samples TG30 + 5%/TP15, TG15/TP15, and TG30/TP15 were detected. Although the TG30 + 5%/TP15 membrane fatty acid composition is similar to that of cells grown at 37°C, their HorA pressure resistance is not increased. The most pressure-resistant sample was TG37/TP15, characterized by high levels of saturated fatty acids in its membrane. The inactivation of HorA is in general about three times faster than plate counts on MRS agar. A linear plot of HorA activity versus plate counts on MRS-NaCl agar shows that these data sets are highly correlated (r2 = 0.91). For example, after 40-min treatment of the sample TG30/TP15, HorA activity and MRS-NaCl and MRS agar cell counts were reduced by 80, 87, and 20%, respectively. Arranging the different samples in the order of decreasing speed of HorA inactivation shows the same pressure resistance of cells observed with viable cell counts and sublethal injury. However, TG30 + 5%/TP15 cells were about as resistant as TG37/TP15 cells, considering viable cell counts and sublethal injury, but were not different from TG15/TP15 cells with respect to HorA inactivation.
FIG. 6.
High-pressure inactivation kinetics of HorA at 200 MPa. The cells were grown at temperatures TG and were pressurized at temperatures TP. HorA activity was expressed relative to untreated cells. Shown are the means and standard deviations of three independent experiments. •, TG15/TP15; ○, TG30 + 5% ethanol/TP15; ▾, TG30/TP15; ▿, TG37/TP15; ▴, TG37/TP37; ▪, TG15/TP37. Control dead cells were prepared by pressure treatment at 800 MPa for 10 min.
DISCUSSION
Cells of L. plantarum differing in physical and chemical membrane properties and membrane phase state were high pressure treated. It could be demonstrated that membrane properties affect the rate of inactivation of viable cells as well as the inactivation of the membrane-bound ethidium transport activity. The kinetic analysis of cell physiology during high-pressure treatment provided further evidence that inactivation of MDR transport, and possibly other membrane-bound transport enzymes, is a crucial step during pressure-mediated elimination of microorganisms. Furthermore, HorA inactivation was shown to correlate with sublethal injury in L. plantarum.
In this study, energy-dependent ethidium efflux in whole cells of L. plantarum was determined, and the results were expressed as HorA activity based on the observation that a functional HorA is required for hop and ethidium resistance in beer spoilage lactobacilli (40, 41) and the finding that reserpine, an inhibitor of human MDR1 transporter and homologues, fully inhibited ethidium transport in L. plantarum TMW 1.460. In L. lactis, four different drug efflux systems have been characterized (51), and two of these transporters, ATP-dependent LmrA and proton motive force-dependent LmrP, mediate ethidium efflux. Therefore, it is possible that a minor contribution to ethidium efflux in energized L. plantarum stems from transport systems other than HorA.
An intact cytoplasmic membrane, as well as a source of ATP, is a prerequisite for ATP-dependent, MDR transporter-mediated ethidium efflux. Generally, pressure treatment of L. plantarum could affect membrane integrity, metabolic activity and hence energy generation, or HorA activity. It was previously shown that the glycolytic activity as well as the membrane integrity of L. plantarum TMW 1.460 were not irreversibly affected by pressure treatment as long as the cells were viable (45). This observation conforms to the results of Wouters et al. (50) showing that ATP generation in pressure-treated L. plantarum is not impaired as long as the cells remain viable. Therefore, a decreased ethidium efflux activity in pressure-treated L. plantarum is not caused by lack of ATP, or a compromised membrane, but is attributable to inactivation of HorA transporter.
In this study, pressure-mediated sublethal injury of L. plantarum was determined by plating on MRS-NaCl agar. HorA inactivation correlated with plate counts on MRS-NaCl agar rather than viable cell counts. This finding provides a rationale for the observation that pressure-treated, sublethally injured cells of L. plantarum TMW 1.460 specifically lost the ability to survive in hopped beer (16). Resistance of L. plantarum to 4% NaCl requires transport enzymes to balance osmotic pressure (18). Apparently, protective and repair functions required for growth under osmotic stress follow a pressure inactivation kinetics comparable to that of HorA.
The membrane of L. plantarum was altered in chemical composition by incubation at different temperatures without changing ethidium efflux activity. The physiological reaction to low temperature during growth is the incorporation of unsaturated acyl chains to maintain approximately constant fluidity of the membrane and to ensure the functionality of membranes and membrane-bound enzymes. Measurements using FT-IR spectroscopy provided information about the lipid bilayer phase transitions induced by temperature in L. plantarum. Under optimal growth conditions (30°C), the membrane of a microorganism is in the lamellar liquid crystalline, or Lα, phase, in which the phospholipid molecules are conformationally disordered. In natural membranes, there is a large compositional heterogeneity of constituent lipids. Hence, between a pure liquid crystalline state and a gel state, a more or less broad coexistence region, in which liquid crystalline and gel lipid domains coexist, is present. For comparison, in one-component lipid bilayers, such as dipalmitoylphosphatidylcholine, several different gel states occur in the pressure-temperature phase space (12). By lowering the temperature, the lipid bilayer is changed from the Lα to the Pβ" gel phase, whose chains are stretched and of all-trans conformation, and the surface of the membrane is undulated. A further decrease in temperature leads to the formation of the Lβ" gel phase, where the acyl chains are tilted with respect to the normal bilayer. Further pressure-induced gel phases, such as an interdigitated gel phase, are observed at high pressure (49). In L. plantarum membranes, no sharp liquid crystalline-gel or gel-gel phase transition was detected. The Laurdan fluorescence data also allowed us to study the pressure-induced phase behavior of the different samples. The membrane fluidity determined with Laurdan at ambient pressure was in agreement with FT-IR data.
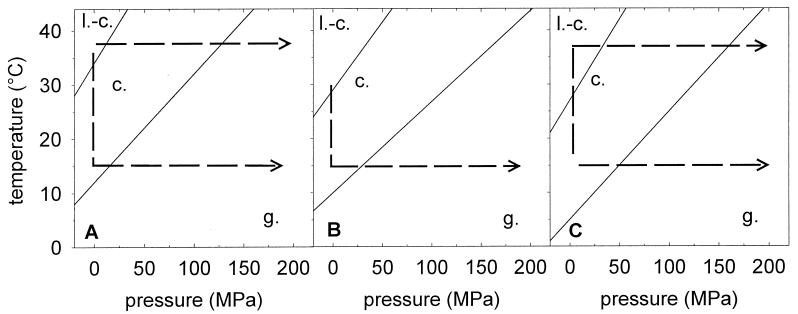
From these temperature- and pressure-dependent data, a tentative pressure-temperature phase diagram (p-T diagram) can be constructed for the L. plantarum membranes (Fig. 7). The temperature range of the coexistence phase is derived from FT-IR data. The pressure-induced phase transitions are deduced from the Laurdan spectra. This p-T diagram of cytoplasmic membranes of L. plantarum provides information about the membrane phase state before and during the high-pressure treatment. Changes of temperature and pressure during processing of the samples can be interpreted as horizontal and vertical movements in the p-T diagram, respectively. Cells pressurized at their growth temperature were at 0.1 MPa in the liquid crystalline or coexistence phase region, moving horizontally (isothermally) into the gel phase region at 200 MPa. Cells which are pressurized at temperatures differing from the growth temperature experience, by cooling or heating, a vertical movement (isobar at 0.1 MPa) in the p-T diagram before the application of pressure. All cell membranes are in the gel phase at 200 MPa.
FIG. 7.
p-T diagram of the cytoplasmic membrane of L. plantarum grown at 37 (A), 30 (B), and 15°C (C). The membrane phase state in the p-T diagram is based on the data presented in Fig. 2 and 3 (FT-IR measurements from −4 to 44°C at 0.1 MPa and determination of Laurdan fluorescence spectra from 0.1 to 200 MPa at 15 and 37°C). l.-c., liquid crystalline phase; c., coexistence phase; g., gel phase. The lines represent phase transitions interpolated from the raw data. The dashed lines indicate temperature and pressure shifts during pressure treatment of cells with various growth temperatures (TG) and pressurization temperatures (TP). Cooling induces an isobar (vertical) transit to the gel region, and pressurizing induces an isotherm (horizontal) transit to the gel region in the p-T diagram.
The p-T diagrams of the various cultures can be correlated with the respective kinetics of high-pressure inactivation. The phase transitions induced by variation of temperature were reversible and did not affect HorA activity or cell viability. Thus, the lipid environment does not seem to irreversibly influence HorA activity at ambient pressure over the whole temperature range covered. However, irreversible pressure denaturation of HorA differed in cells with different membrane properties. Those cells that were subjected to pressure at a temperature at which their membranes were in the liquid crystalline phase state and for which a gel phase of the membrane was induced only at pressures greater than 100 MPa were most sensitive with respect to both HorA inactivation and cell death. Those cells that were subjected to pressure at a temperature at which the membrane is in the gel phase region were most resistant to HorA activity and cell viability. These results are consistent with the hypothesis that irreversible pressure denaturation of HorA is faster if pressure is applied to protein embedded in a liquid crystalline or coexistence membrane compared to denaturation in a gel phase membrane. Furthermore, it is conceivable that pressure-induced membrane phases different from those observed at ambient pressure exert irreversible effects on HorA. Although few data are available on the effect of membrane properties on irreversible pressure denaturation of integral membrane proteins, it is well established that the structure and function of these proteins strongly depend on interactions with the lipid bilayers. The membrane phase is known to determine the activity of Na-K-ATPase activity at pressures ranging from 30 to 100 MPa (7, 25, 27). The refolding of bacteriorhodopsin in lipid bilayers is controlled by the physical pressure exerted by the membrane on the protein (11). The conformation of gramicidin, a low-molecular-weight peptide antibiotic, is modulated by phase transitions of the lipid matrix. Incorporation of the peptide leads to significant changes in the structure and pressure-temperature phase behavior of the lipid bilayer system (52).
The observation that HorA inactivation of ethanol-adapted cells was faster than that of 37°C-adapted cells with comparable membrane properties highlights the fact that factors other than membrane fatty acid composition and membrane fluidity affect the pressure denaturation of HorA, for example, the phospholipid head groups or chaperone proteins. The shock response of lactobacilli to lethal ethanol challenge involves the synthesis of ethanol shock proteins (23). However, the adaptation of lactobacilli to ethanol concentrations permitting growth did not involve protein synthesis but required adjustments of membrane composition (9, 10). The phospholipid head group composition of the membrane, which was not taken into account in this study, correlated with the barosensitivity of L. plantarum (43). Differential expression of chaperones, depending on the temperature of incubation, could contribute to the variation in barosensitivity of cells that were pressure treated at different temperatures. However, cells grown at 15°C and pressure treated at 37°C exhibited barosensitivities comparable to those of cells grown at 37°C and pressure treated at 37°C. These cells were found to have comparable membrane fluidities but cannot be expected to have comparable expression levels of either heat or cold shock proteins.
In conclusion, we have provided evidence that the composition and the phase behavior of cytoplasmic membranes affect the pressure denaturation of HorA. The inactivation of MDR transport activity by high pressure is measured before the loss of viability. These transport enzymes are important for the survival of microorganisms under adverse conditions encountered in food (16). Our results provide insight into mechanisms governing the loss of resistance of microorganisms through high-pressure processing and facilitate the application of high pressure in the context of a hurdle system for preservation of minimally processed food.
Acknowledgments
We thank Klaus Kilimann for his excellent assistance in the laboratory.
REFERENCES
- 1.Amparo, B., G. Ventoura, M. Casadei, T. Robinson, and B. Mackey. 1999. Variation in resistance of natural isolates of Escherichia coli O157 to high hydrostatic pressure, mild heat, and other stresses. Appl. Environ. Microbiol. 65:1564-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Annous, B. A., M. F. Kozempel, and M. J. Kurantz. 1999. Changes in membrane fatty acid composition of Pediococcus sp. strain NRRL B-2354 in response to growth conditions and its effect on thermal resistance. Appl. Environ. Microbiol. 65:2857-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagatolli, L. A., T. Parasassi, G. D. Fidelio, and E. Gratton. 1999. A model for the interaction of 6-lauroyl-2-(N,N-dimethylamino)naphthalene with lipid environments: implications for spectral properties. Photochem. Photobiol. 70:557-564. [PubMed] [Google Scholar]
- 4.Behan, M. K., A. G. Macdonald, G. R. Jones, and A. R. Cossins. 1992. Homeoviscous adaptation under pressure: the pressure dependence of membrane order in brain myelin membranes of deep-sea fish. Biochim. Biophys. Acta 1103:317-323. [DOI] [PubMed] [Google Scholar]
- 5.Bolhuis, H., D. Molenaar, G. Poelarends, H. W. van Veen, B. Poolman, A. J. M. Driessen, and W. N. Konings. 1994. Proton motive force-driven and ATP-dependent drug extrusion systems in multidrug resistant Lactococcus lactis. J. Bacteriol. 176:6957-6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolhuis, H., G. Poelarends, H. W. van Veen, B. Poolman, A. J. M. Driessen, and W. N. Konings. 1995. The lactococcal lmrP gene encodes a proton motive force dependent drug transporter. J. Biol. Chem. 270:26092-26098. [DOI] [PubMed] [Google Scholar]
- 7.Chong, P. L.-G., P. A. G. Fortes, and D. M. Jameson. 1985. Mechanisms of inhibition of (Na, K)-ATPase by hydrostatic pressure studied with fluorescent probes. J. Biol. Chem. 260:14484-14490. [PubMed] [Google Scholar]
- 8.Cossins, A. R., and A. G. Macdonald. 1989. The adaptation of biological membranes to temperature and pressure: fish from the deep and cold. J. Bioenerg. Biomembr. 21:115-135. [DOI] [PubMed] [Google Scholar]
- 9.Couto, J. A., N. Rozès, and T. Hogg. 1996. Ethanol-induced changes in the fatty acid composition of Lactobacillus hilgardii, its effects on plasma membrane fluidity and relationship with ethanol tolerance. J. Appl. Bacteriol. 81:126-132. [Google Scholar]
- 10.Couto, J. A., C. Pina, and T. Hogg. 1997. Enhancement of apparent resistance to ethanol in Lactobacillus hilgardii. Biotechnol. Lett. 19:487-490. [Google Scholar]
- 11.Curran, A. R., R. H. Templer, and P. J. Booth. 1999. Modulation of folding and assembly of the membrane protein bacteriorhodopsin by intermolecular forces within the lipid bilayer. Biochemistry 38:9328-9336. [DOI] [PubMed] [Google Scholar]
- 12.Czeslik, C., O. Reis, R. Winter, and G. Rapp. 1998. Effect of high pressure on the structure of dipalmitoylphosphatidylcholine bilayer membranes: a synchrotron-X-ray diffraction and FT-IR spectroscopy study using the diamond anvil technique. Chem. Phys. Lipids 91:135-144. [Google Scholar]
- 13.de Man, J. C., M. Rogosa, and M. E. Sharpe. 1960. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 23:130-135. [Google Scholar]
- 14.Dreyfus, G., H. Guimaraes-Motta, and J. L. Silva. 1988. Effect of hydrostatic pressure on the mitochondrial ATP synthase. Biochemistry 27:6704-6710. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez Murga, M. L., G. M. Cabrera, G. Font de Valdez, A. Disalvo, and A. M. Seldes. 2000. Influence of growth temperature on cryotolerance and lipid composition of Lactobacillus acidophilus. J. Appl. Microbiol. 88:342-348. [DOI] [PubMed] [Google Scholar]
- 16.Gänzle, M. G., H. M. Ulmer, and R. F. Vogel. 2001. Effects of hops and ethanol on high-pressure inactivation of Lactobacillus plantarum. J. Food Sci. 2001:1174-1181. [Google Scholar]
- 16a.Gänzle, M. G., and R. F. Vogel. On-line fluorescence determination of pressure-mediated outer membrane damage in Escherichia coli. Syst. Appl. Microbiol., in press. [DOI] [PubMed]
- 17.Garcia-Graells, C., K. J. A. Hauben, and C. W. Michiels. 1998. High-pressure inactivation and sublethal injury of pressure-resistant Escherichia coli mutants in fruit juices. Appl. Environ. Microbiol. 64:1566-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glaasker, E., F. S. B. Tjan, P. F. ter Steeg, W. N. Konings, and B. Poolman. 1998. Physiological response of Lactobacillus plantarum to salt and nonelectrolyte stress. J. Bacteriol. 180:4718-4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauben, K. J. A., K. Bernaerts, and C. W. Michiels. 1998. Protective effect of calcium on inactivation of Escherichia coli by high hydrostatic pressure. J. Appl. Microbiol. 85:678-684. [DOI] [PubMed] [Google Scholar]
- 20.Heremans, K., J. van Camp, and A. Huyghebaert. 1997. High-pressure effects on proteins, p. 473-502. In S. Damodaran (ed.), Food proteins and their applications. Dekker, New York, N.Y.
- 21.Heremans, K., and L. Smeller. 1998. Protein structure and dynamics at high pressure. Biochim. Biophys. Acta 1386:353-370. [DOI] [PubMed] [Google Scholar]
- 22.Hummer, G., S. Garde, A. E. Garcia, M. E. Paulaitis, and L. R. Pratt. 1998. The pressure dependence of hydrophobic interactions is consistent with the observed pressure denaturation of proteins. Proc. Natl. Acad. Sci. 95:1552-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laplace, J.-M., N. Sauvageot, A. Hartke, and Y. Auffray. 1999. Characterization of Lactobacilllus collinoides response to heat, acid and ethanol treatments. Appl. Microbiol. Biotechnol. 51:659-663. [Google Scholar]
- 24.Linton, M., J. M. J. McClements, and M. F. Patterson. 1999. Survival of Escherichia coli O157:H7 during storage in pressure-treated orange juice. J. Food Prot. 62:1038-1040. [DOI] [PubMed] [Google Scholar]
- 25.Macnaughtan, W., and A. G. Macdonald. 1982. Effects of pressure and pressure antagonists on the growth and membrane-bound ATP-ase of Acholeplasma laidlawii B. Comp. Biochem. Physiol. A 72:405-414. [DOI] [PubMed] [Google Scholar]
- 26.Margolles, A., M. Putman, H. W. van Veen, and W. N. Konings. 1999. The purified and functionally reconstituted multidrug transporter shamrock of Lactococcus lactis mediates the transbilayer movement of specific fluorescent phospholipids. Biochemistry 38:16298-16306. [DOI] [PubMed] [Google Scholar]
- 27.Marquis, R. E. 1984. Reversible actions of hydrostatic pressure and compressed gases on microorganisms, p. 273-302. In A. Hurst and A. Nasim (ed.), Repairable lesions in microorganisms. Academic Press Inc., New York, N.Y.
- 28.Masson, P. 1992. Pressure denaturation of proteins, p. 89-98. In C. Balny, R. Hayashi, K. Heremans, and P. Masson (ed.), High pressure and biotechnology. Libbey Eurotext Ltd., Montrouge, France.
- 29.Molina-Gutierrez, A., B. Rademacher, M. G. Gänzle, and R. F. Vogel. 2002. Effect of sucrose and sodium chloride on survival and metabolic activity of Lactococcus lactis under high pressure conditions, p. 295-302. In R. Hayashi (ed.), High pressure bioscience and biotechnology. Elsevier, Amsterdam, The Netherlands.
- 30.Niven, G. W., C. A. Miles, and B. M. Mackey. 1999. The effects of hydrostatic pressure on ribosome conformation in Escherichia coli: an in vivo study using differential scanning calorimetry. Microbiology 145:419-425. [DOI] [PubMed] [Google Scholar]
- 31.Pagán, R., S. Jordan, A. Benito, and B. Mackey. 2001. Enhanced acid sensitivity of pressure-damaged Escherichia coli O157 cells. Appl. Environ. Microbiol. 67:1983-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parasassi, T., E. K. Krasnowska, L. Bagatolli, and E. Gratton. 1998. Laurdan and Prodan as polarity-sensitive fluorescent membrane probes. J. Fluoresc. 8:365-373. [Google Scholar]
- 33.Parasassi, T., and E. Gratton. 1995. Membrane lipid domains and dynamics as detected by Laurdan fluorescence. J. Fluoresc. 5:59-68. [DOI] [PubMed] [Google Scholar]
- 34.Parasassi, T., G. de Stasio, A. d'Ubaldo, and E. Gratton. 1990. Phase fluctuation in phospholipid membranes revealed by Laurdan fluorescence. Biophys. J. 57:1179-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parasassi, T., G. de Stasio, G. Ravagnan, R. M. Rusch, and E. Gratton. 1991. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys. J. 60:179-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perrier-Cornet, J.-M., M. Hayert, and P. Gervais. 1999. Yeast cell mortality related to a high pressure shift: occurrence of cell membrane permeabilization. J. Appl. Microbiol. 87:1-7. [DOI] [PubMed] [Google Scholar]
- 37.Reis, O., R. Winter, and T. W. Zerda. 1996. The effect of high external pressure on DPPC-cholesterol multilamellar vesicles: a pressure-tuning Fourier transform infrared spectroscopy study. Biochim. Biophys. Acta 1279:5-16. [DOI] [PubMed] [Google Scholar]
- 38.Ritz, M., M. Freulet, N. Orange, and M. Federighi. 2000. Effects of high hydrostatic pressure on membrane proteins of Salmonella typhimurium. Int. J. Food Microbiol. 55:115-119. [DOI] [PubMed] [Google Scholar]
- 39.Sakamoto, K., A. Margolles, H. W. van Veen, and W. N. Konings. 2001. Hop resistance in the beer spoilage bacterium Lactobacillus brevis is mediated by the ATP-binding cassette multidrug transporter HorA. J. Bacteriol. 183:5371-5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sami, M., H. Yamashita, T. Hirono, H. Kadokura, K. Kitamoto, K. Yoda, and M. Yamasaki. 1997. Hop-resistant Lactobacillus brevis contains a novel plasmid harboring a multidrug resistance-like gene. J. Ferment. Bioeng. 84:1-6. [Google Scholar]
- 41.Sami, M., H. Yamashita, H. Kadokura, K. Kitamoto, K. Yoda, and M. Yamasaki. 1997. A new and rapid method for determination of beer spoilage ability of lactobacilli. J. Am. Soc. Brew. Chem. 55:137-140. [Google Scholar]
- 42.Snyder, G. R. 1961. Vibrational spectra of crystalline n-paraffins. J. Mol. Spectrosc. 7:116-144. [Google Scholar]
- 43.ter Steeg, P. F., J. C. Hellemons, and A. E. Kok. 1999. Synergistic actions of nisin, sublethal ultrahigh pressure, and reduced temperature on bacteria and yeast. Appl. Environ. Microbiol. 65:4148-4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tonello, C., S. Kesenne, C. Muterel, and F. Jolibert. 1997. Effect of high hydrostatic pressure treatments on shelf-life of different fruit products, p. 439-442. In K. Heremans (ed.), High pressure research in bioscience and biotechnology. Leuven University Press, Leuven, Belgium.
- 45.Ulmer, H. M., M. G. Gänzle, and R. F. Vogel. 2000. Effects of high pressure on survival and metabolic activity of Lactobacillus plantarum TMW 1.460. Appl. Environ. Microbiol. 66:3966-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Veen, H. W., A. Margolles, M. Müller, C. F. Higgins, and W. N. Konings. 2000. The homodimeric ATP-binding cassette transporter LmrA mediates multidrug transport by an alternating two-site (two-cylinder engine) mechanism. EMBO J. 19:2503-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Veen, H. W., K. Venema, H. Bolhuis, I. Oussenko, J. Kok, B. Poolman, A. J. M. Driessen, and W. N. Konings. 1996. Multidrug resistance mediated by a bacterial homolog of the human multidrug transporter MDR 1. Proc. Natl. Acad. Sci. 93:10668-10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vigano, C., A. Margolles, H. W. van Veen, W. N. Konings, and J. M. Ruysschaert. 2000. Secondary and tertiary structure changes of reconstituted LmrA induced by nucleotide binding or hydrolysis. J. Biol. Chem. 275:10962-10967. [DOI] [PubMed] [Google Scholar]
- 49.Winter, R. 1996. High pressure effects on the structure and mesophase behaviour of supramolecular lipid aggregates and model membrane systems, p. 21-28. In R. Hayashi and C. Balny (ed.), High pressure bioscience and biotechnology. Elsevier, Amsterdam, The Netherlands.
- 50.Wouters, P., E. Glaasker, and J. P. P. M. Smelt. 1998. Effects of high pressure on inactivation kinetics and events related to proton efflux in Lactobacillus plantarum. Appl. Environ. Microbiol. 64:509-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yokota, A., M. Veenstra, P. Kurdi, H. W. van Veen, and W. N. Konings. 2000. Cholate resistance in Lactococcus lactis is mediated by an ATP-dependent multispecific organic anion transporter. J. Bacteriol. 182:5196-5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zein, M., and R. Winter. 2000. Effect of temperature, pressure and lipid acyl chain length on the structure and phase behaviour of phospholipid-gramicidin bilayers. Phys. Chem. Chem. Phys. 2:4545-4551. [Google Scholar]