Abstract
Communication between wireless field receivers and biological sensors remains a key constraint in the development of wireless electronic devices for minimally invasive medical monitoring and biomedical applications involving gene and cell therapies. Here we describe a nanoparticle–cell interface that enables electromagnetic programming of wireless expression regulation (EMPOWER) of transgenes via the generation of cellular reactive oxygen species (ROS) at a biosafe level. Multiferroic nanoparticles coated with chitosan to improve biocompatibility generate ROS in the cytoplasm of cells in response to a low-frequency (1-kHz) magnetic field. Overexpressed ROS-responsive KEAP1/NRF2 biosensors detect the generated ROS which is rewired to synthetic ROS-responsive promoters to drive transgene expression. In a proof-of-concept study, subcutaneously implanted alginate-microencapsulated cells stably expressing an EMPOWER-controlled insulin expression system normalized blood-glucose levels in a mouse model of type 1 diabetes in response to a weak magnetic field.
Subject terms: Biomaterials, Biosensors, Bionanoelectronics, Nanoparticles
Wireless magnetic control of gene expression in mammalian cells has been developed based on intracellular nanointerface and ROS-mediated signalling. The approach allows remotely tunable insulin release and regulates blood glucose in diabetic mice.
Main
Synthetic biology has revolutionized cell engineering for alleviating numerous diseases1,2, including chronic pain3, obesity4, diabetes5, cancer6 and muscle atrophy7, and for investigating neural circuits8 and bioelectronics interfaces9,10. In particular, physical stimuli of gene circuits, such as light11, sound12, electrical signals13,14 and magnetic fields15,16, have been intensively explored for spatiotemporal control of therapeutic outputs. To circumvent the challenge of wireless signal propagation, electromagnetic fields (EMFs) of varying strength, frequency, duration and location have been exploited in conjunction with the mechanical17–19 or thermal properties8,20,21 of magnetic nanoparticles to enable coupling with ion channels on cell membranes. EMFs with an amplitude of <50 mT and a frequency of <1 MHz minimize energy dissipation in living tissues22, and therefore are suitable for remotely programmable switches to stimulate cellular functions with minimal influence on native systems. However, legacy technology pioneering the electromagnetic programming of cellular behaviour was based on cell-specific in vivo coordination of inorganic nanoparticles to channels or receptors of native or engineered cells using antibodies23,24 or tags16,18,25, which may elicit off-target effects of conjugated nanoparticles26,27, promote liver toxicity20,28 or limit robustness due to intracellular trafficking of channels and receptors29, resulting in limited tunability22 and biosafety30. We have therefore designed and tested a versatile and robust genetic interface enabling tunable remote control of therapeutic transgene expression by microencapsulated designer cells using low-power EMF.
Multiferroic materials that harmonize magnetostrictive and piezoelectric effects can exploit magnetic fields to generate electricity for biological applications, such as remote brain activity detection, deep neural stimulation31, bone defect repair32 and degradation of Alzheimer’s β-amyloid aggregates33. These effects occur as aqueous solvents and solutes transfer charge carriers from multiferroic material surfaces to produce electrophiles, mostly reactive oxygen species (ROS)34,35. Thus, a.c. millitesla EMFs in the low-frequency range (0.1–1 kHz) hold promise as a biological portal via ROS.
ROS act as native cytoplasmic signals in living systems, and human cells contain components that can sense and respond to them36,37. When exposed to elevated ROS, Kelch-like ECH-associated protein 1 (KEAP1), which contains ROS-sensitive cysteine residues, releases nuclear factor erythroid 2 p45-related factor 2 (NRF2), allowing NRF2 to translocate and bind to intranuclear antioxidant-response elements (AREs), resulting in transcriptional antioxidant responses38. Here we utilized magnetoresponsive ROS-generating multiferroic (CoFe2O4@BiFeO3@chitosan, CBCFO) nanoparticles to communicate with cells sensitized to ROS by overexpressing KEAP1/NRF2 and rewired NRF2 to synthetic ARE-containing promoters, thereby constructing a system that we term electromagnetic programming of wireless expression regulation (EMPOWER). In this system, the embedded CBCFO nanoparticles serve as nanoreceivers of an external electromagnetic field, providing electromagnetic tunability of ROS generation to drive transgene expression of the target protein by the host cells. For proof of concept and as an example, we chose to validate the EMPOWER system for blood-glucose management in experimental type 1 diabetes (T1D) because diabetes is a dynamically highly challenging medical condition with dramatically increasing prevalence39–41. Therefore, we implanted transgenic human cells with the EMPOWER system enclosed in coherent, clinically licensed alginate microcapsules into T1D mice and exposed them to an EMF to control insulin release. Low-frequency EMF (1 kHz) stimulation of 21 mT for 3 min per day effectively induced insulin secretion from the subcutaneously implanted EMPOWER-controlled designer cells and restored normoglycaemia in T1D mice over the entire 4-week experimental period.
Results
Characterization of chitosan-multiferroic nanoparticles
To sense the magnetic field for ROS-mediated transgene expression control, we synthesized core–shell CoFe2O4@BiFeO3 (BCFO) multiferroic nanoparticles consisting of magnetostrictive CoFe2O4 (CFO) nanoparticle cores and piezoelectric BiFeO3 (BFO) shells, with the chitosan outer layer to form the CBCFO nanoparticles, as illustrated in Fig. 1a. Scanning transmission electron microscopy (STEM) imaging and corresponding energy-dispersive X-ray spectroscopy (EDX) mapping (Fig. 1b) confirmed the structure of the CBCFO nanoparticles, as demonstrated by the distributions of cobalt, bismuth and nitrogen in the CFO, BFO and chitosan. The line profile of the CBCFO nanoparticle shows the representative spatial distributions of cobalt, bismuth and nitrogen, quantitatively confirming the structure. The EDX spectrum indicated similar atom contents of cobalt and bismuth in the CBCFO nanoparticles (Supplementary Fig. 1), in contrast with CFO (Supplementary Fig. 2a) and BCFO (Supplementary Fig. 2b) nanoparticles. The CBCFO nanoparticles were 35.5 ± 10.3 nm in diameter, while the diameters of CFO and BCFO nanoparticles were 25.4 ± 6.1 nm and 32.9 ± 8.5 nm, respectively, according to the transmission electron microscopy (TEM) results (n = 50 particles). At the physiological pH of 7.4, the hydrodynamic diameter of CBCFO nanoparticles is 36.3 ± 4.8 nm and their polydispersity index reaches 0.190 (Supplementary Fig. 3). The X-ray diffraction (XRD) patterns revealed the cubic spinel structure of CFO with an Fd3m space group, and the rhombohedral perovskite structure of BFO with an R3c space group (Fig. 1c)33,42.
Fig. 1. Construction and characterization of CBCFO nanoparticles.
a, Illustration of the synthesis of CBCFO nanoparticles. b, STEM bright-field (BF) images and corresponding EDX results with colocalized elemental mapping of cobalt, bismuth and nitrogen are consistent with a core–shell structure of CBCFO nanoparticles. Scale bar, 50 nm. c, XRD pattern displaying the crystallinity of BCFO. Black, BCFO; B, peaks from BiFeO3; C, peaks from CoFe2O4. d, Attenuated total reflectance infrared spectra of BCFO, chitosan and CBCFO. e, Zeta potential of BCFO and CBCFO, before and after coating with the chitosan layer. f, Cell viability upon exposure to CBCFO nanoparticles is dependent on nanoparticle mass per million cells. Data are presented as mean ± s.d., n = 3 (e) or n = 4 (f) independent experiments. P values in f were calculated versus the corresponding non-induced control by a two-sided unpaired t-test.
In attenuated total reflectance infrared analysis, the region between 800 and 1,200 cm−1 shows characteristic absorption of chitosan saccharide structure (Fig. 1d)43. The chitosan protonation and hydration processes during coating of CBCFO nanoparticles are reflected in changes in the asymmetric –NH band between 1,300 and 1,700 cm−1 compared with chitosan powder. The resulting ammonium groups within the chitosan layer of CBCFO nanoparticles contribute to the positive surface charge of 31.6 ± 4.6 mV compared with the negative charge (−22.5 ± 5.5 mV) of BCFO nanoparticles (Fig. 1e and Supplementary Fig. 4). Cells containing up to 50 μg BCFO or 100 μg CBCFO per 106 human embryonic kidney cells (HEK-293) retained more than 95% viability after 48 h (Fig. 1f). These results guided our choice of concentration range for the following in vitro evaluation. The decrease in cell viability at higher CBCFO concentrations was directly correlated with cytosolic accumulation, and presumably resulted from excessive changes in mitochondrial membrane potential which triggered apoptosis-associated release of cytochrome C (Extended Data Fig. 1).
Extended Data Fig. 1. Impact of CBCFO nanoparticle concentration and EMFS on mitochondrial membrane potential (MMP) and cytochrome C release.
(a) Relationship between MMP and CBCFO nanoparticle concentration 24 h after addition. (b) CBCFO nanoparticle concentration-dependent MMP 24 h after EMF stimulation (21 mT, 1 kHz, 3 min). (c) EMFS time-dependent MMP by CBCFO nanoparticles (50 µg/106 cells) 24 h after EMFS (21 mT, 1 kHz, 0–5 min). (d) Western blot-based analysis of cytochrome C release from mitochondria 24 h after addition of different concentrations of CBCFO nanoparticles (0–500 µg/106 cells). Vinculin was used as a loading control. All data are presented as means ± s.d.; n = 6 independent experiments. Statistical significance was analysed by one-way ANOVA with Tukey’s multiple comparisons test in (a) and two-way ANOVA with Dunnett’s multiple comparisons test in (b, c).
Electromagnetically induced ROS production in vitro
Under a.c. electromagnetic field stimulation (EMFS), multiferroic BCFO generates electric polarization due to the interfacial lattice strain between BFO and CFO44,45. Charge separation of BCFO affords excited charge carriers on the surface of CBCFO nanoparticles, leading to local production of ROS such as superoxide radical (O2−·) and hydroxyl radical (OH·) in an aqueous environment33 (Fig. 2a). CBCFO nanoparticles exhibit magnetic hysteresis loops (EMF range, −30 to 30 kOe) under ambient conditions (Fig. 2b), with a saturation magnetization (Ms) and remnant magnetization (Mr) of 77.8 and 45.2 emu g−1, respectively, signifying room-temperature ferromagnetism. The decreased ferromagnetism of CBCFO derived from BCFO nanoparticles (Ms = 96.8 emu g−1, Mr = 57.2 emu g−1) is attributable to the content of chitosan. For the following experiments, we employed EMFs of 1 kHz frequency and up to 21 mT field strength to avoid any adverse thermal effect22 in living systems and to maintain effective coupling of the BFO–CFO interface44. A Helmholtz-coil-based device was assembled to generate a uniform a.c. EMF of 9–21 mT in multiwell plates (Supplementary Fig. 5). The induced electrical potential of CBCFO powder in an open-circuit-voltage (OCV) set-up (Supplementary Fig. 6a) was measured with an EMF of 1 kHz and 21 mT and reached 0.11 V (Fig. 2c), in contrast with the device bias control of 0.016 V (Supplementary Fig. 6b). The relative charge separation was detected by a terephthalic acid (TA) assay depending on the EMFS strength (Extended Data Fig. 2a,b). The capability of the charge carriers to induce ROS was evaluated by measuring the non-specific ROS-mediated decolorization of methylene blue (MB assay, Extended Data Fig. 2c,d). The degradation rate of 39% with CBCFO nanoparticles (5 mg ml−1) after 1-h EMFS (1 kHz, 21 mT) indicates a significant ROS production from CBCFO with EMFS, compared with bare CBCFO and EMFS-alone control groups.
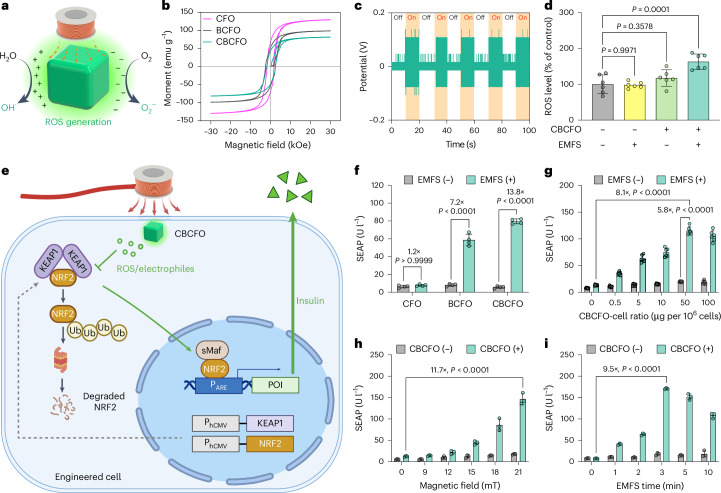
Fig. 2. Electromagnetically stimulated ROS production and transgene expression in transiently transfected cells.
a, Schematic illustration of the magnetoelectric effect of CBCFO nanoparticles and ROS production. b, Magnetic hysteresis curves of CFO, BCFO and CBCFO at r.t. with magnetic field strengths ranging from −30 kOe to +30 kOe. c, The on/off behaviour of the OCV induced by CBCFO nanoparticles depends on the applied a.c. magnetic field (21 mT, 1 kHz). d, Fluorescence-based quantification of cellular ROS levels after EMF stimulation (21 mT, 1 kHz, 3 min). e, Scheme of the proposed mechanism of electromagnetically induced gene expression in engineered responsive cells transfected with pJH1003, pJH1004 and pJH1005. The ROS generated by EMF-stimulated CBCFO disrupts the interaction between KEAP1 and NRF2, thereby inhibiting ubiquitination by the KEAP1-associated ubiquitin (Ub) ligase complex. Consequently, NRF2 translocates to the nucleus, where it binds to small Maf proteins (sMaf) and to the antioxidant-response elements (ARE) in the regulatory regions of its target genes. f, SEAP production by transiently transfected ROS-responsive cells containing CFO, BCFO and CBCFO nanoparticles. g, SEAP expression is dependent on the CBCFO–cell ratio (magnetic field, 21 mT; 3 min). h, Electromagnetic-field-dependent gene expression (stimulation time, 3 min). SEAP expression was maximum at 21 mT, reaching peak levels that compare to SEAP levels of isogenic cells in which SEAP is driven by a strong constitutive promoter (149.3 ± 8.7 U l−1). i, Stimulation-time-dependent gene expression (magnetic field, 21 mT). Data are presented as mean ± s.d., n = 6 (d,g), n = 4 (f) or n = 3 (h,i) independent experiments. P values in d–i were calculated versus the corresponding non-stimulated control. Statistical significance was analysed by one-way ANOVA with Dunnett’s multiple-comparisons test (d), two-way ANOVA with Bonferroni’s multiple comparisons test (f) and two-way ANOVA with Dunnett’s multiple comparisons test (g,h,i). Mechanism schematics created with BioRender.com.
Extended Data Fig. 2. Quantification of charge carriers and ROS.
(a) The mechanism of TA assay to detect charge carriers. (b) The mean fluorescence intensities (MFI) show the magnetic-field-dependent generation of 2-hydroxyterephthalic acid. (c) The mechanism of MB assay to detect ROS production in the aqua-based environment. (d) Decrease of MB induced by CBCFO and EMFS, measured in terms of the absorbance at 664 nm. All data are presented as means ± s.d.; (b, d) n = 3 independent experiments. Statistical significance was analysed by one-way ANOVA with Tukey’s multiple comparisons test in (b, d).
In vitro quantification showed that intracellular ROS production was accelerated with CBCFO in contrast to control groups immediately after 3 min EMFS (1 kHz, 21 mT) (Fig. 2d). The acceleration occurred mostly within the first 30 min (Extended Data Fig. 3a) and declined in the following 3–6 h (Extended Data Fig. 3b). We confirmed no significant difference in cell viability among stimulated and non-stimulated groups due to this accelerated ROS production (Extended Data Fig. 3c), and cell viability started to decrease with only EMFS of 5 min or longer (Extended Data Fig. 3d).
Extended Data Fig. 3. In vitro ROS production stimulated by the magnetic field.
(a) Significantly increased production of ROS over 30 min in HEK-293 cells with CBCFO after EMFS (1 kHz, 21 mT, 3 min). (b) Accumulated ROS in the cells reached a maximum at about 6 h after stimulation in (a). All groups show similar increases in ROS production at 12 h after stimulation. Percent mean fluorescence intensity (MFI %) represents the mean fluorescence intensity normalized to blank assay fluorescence. (c) Cell viability 72 h after EMF stimulation. (d) EMF stimulation-time-dependent cell viability (21 mT, 1 kHz, 0–10 min). All data are presented as means ± s.d.; n = 4 independent experiments. Statistical significance was analysed by one-way ANOVA with Dunnett’s multiple comparisons test in (c, d).
Electromagnetically controlled transient gene expression
To utilize EMF for gene expression, we cotransfected HEK-293 cells with constitutive KEAP1 (pJH1004, PhCMV-KEAP1-pA) and NRF2 (pJH1003, PhCMV-NRF2-pA) expression plasmids to construct a ROS-biosensing system, together with the reporter pJH1005 (PDART-SEAP-pA; PDART, OARE-PhCMVmin) encoding the model human glycoprotein SEAP (human placental secreted alkaline phosphatase) for quantification of the expression level (Supplementary Table 1). In these cells, electromagnetically induced cellular ROS production via CBCFO nanoparticles interferes with the NRF2–KEAP1 interaction, leading to release and translocation of NRF2 to the nucleus, which results in expression of the protein of interest (POI) from the NRF2-specific ARE-containing PDART promoter (Fig. 2e). In comparison with CFO-embedded engineered cells, electromagnetically stimulated SEAP expression was significantly elevated (13.8-fold) compared with the non-stimulated control (Fig. 2f). The CBCFO group afforded lower leakiness and a higher expression level than the BCFO group, in accordance with the higher cellular uptake and improved endosome-escape capability as judged from time-lapse microscopy images (Extended Data Fig. 4a,b), fluorescence colocalization (Extended Data Fig. 4c–e) and flow cytometry (Extended Data Fig. 4f,g). These results can be attributed to the proton sponge effect of chitosan modification46. Cellular uptake of CBCFO nanoparticles occurs via classical clathrin-mediated endocytosis (Extended Data Fig. 5a) and no cellular nanoparticle extrusion occurred beyond 3 days after cellular uptake (Extended Data Fig. 5b). Leakage was not observed from implant preparations in 4 weeks, confirming the integrity of the alginate-based microcapsules (Extended Data Fig. 5c). The transgene expression level increased with increasing concentration of CBCFO, peaking at a CBCFO concentration of 50 μg per 106 cells under an EMF of 21 mT and 1 kHz for 3 min (Fig. 2g), corresponding to 1.5 × 104 J s m−3 in volume-averaged energy density. At the CBCFO concentration of 50 μg per 106 cells, the SEAP level increased along with EMF strength (0–21 mT; Fig. 2h). The expression level of SEAP could be precisely adjusted by varying the EMFS time at 21 mT (Fig. 2i). The EMPOWER is characterized in HEK-293 cells, known for their convenience in engineering and their use in biopharmaceutical manufacturing7,41,47, but it also works in a variety of mammalian cells (Extended Data Fig. 6).
Extended Data Fig. 4. Endosome escape behaviour.
Representative fluorescence microscopy images of HEK-293 cells incubated with RITC (Ex/Em: 531/593, red)-labelled (a) CBCFO and (b) BCFO nanoparticles for 12, 24 and 48 h. For co-localization analysis, lysosomes (Lyso tracker green, Ex/Em: 466/495, green) and nuclei (Hochst-33342, Ex/Em: 387/409, blue) were labelled. The region of interest (ROI, white) was derived from the bright-field images. Pearson’s correlation coefficient (PCC) was calculated from the co-localization results between lysosomes and (c) CBCFO, (d) BCFO nanoparticles. Statistical quantification (e) shows enhanced endosome escape of CBCFO nanoparticles from the lysosomes. Flow-cytometric analysis of cells incubated with (f) CBCFO and (g) BCFO nanoparticles confirmed the endosome escape behaviour. In all these experiments, CBCFO nanoparticles are applied as 50 μg/106 cells. All data are presented as means ± s.d.; n = 5 independent experiments.
Extended Data Fig. 5. Endocytosis and cellular extrusion of CBCFO nanoparticles.
(a) Impact of CBCFO nanoparticle endocytosis inhibition on EMF-stimulated SEAP expression. Inhibition of classical clathrin-mediated endocytosis by chlorpromazine decreases endocytosis-mediated uptake of CBCFO nanoparticles, reduces EMF-triggered ROS-production and attenuates ROS-induced SEAP expression. (b, c) Fluorescence-based extrusion of rhodamine B isothiocyanate-labelled CBCFO nanoparticles (50 mg/106 cell) from native (b) and microencapsulated (c) HEKEMPOWER cells. Fluorescence intensity (I) (b, c) was normalized to DMEM fluorescence (I0). Data are presented as means ± s.d.; n = 5 (a); n = 3 (b, c) independent experiments. Statistical significance was analysed by two-way ANOVA with Dunnett’s test.
Extended Data Fig. 6. EMF-controlled SEAP expression in various mammalian cell types.
104 hMSC-TERT (human), HepG2 (human), CHO-K1(hamster), BHK-21 (hamster), and AtT-20 (mouse) cells were co-transfected with EMPOWER vectors pJH1003, pJH1004 and pJH1005, loaded with CBCFO nanoparticles (50 μg/106 cells) and optionally stimulated by EMF (21 mT, 1 kHz, 3 min). All data are presented as means ± s.d.; n = 6 independent experiments. Statistical significance was calculated by two-way ANOVA with Dunnett’s multiple comparisons test.
Stimulated insulin release in encapsulated HEKEMPOWER cells
To construct the EMPOWER system for EMF-controlled insulin production and release in human cells, we first established stable HEK-293 cell lines engineered for constitutive expression of KEAP1 (ITR-PhCMV-KEAP1-P2A-BlastR-pA-ITR, pJH1054), NRF2 (ITR-PhCMV-NRF2-pA:PRPBSA-ECFP-P2A-PuroR-pA-ITR, pJH1101) and NRF2-dependent expression of insulin (ITR-PDART4-NLuc-P2A-mINS-pA:PmPGK-ZeoR-pA-ITR, pJH1196; PDART4, OARE4-PhCMVmin). Nanoluciferase (NLuc) was used as a bioluminescent reporter for screening. The best-in-class monoclonal cell line, HEKEMPOWER, exhibited ectopic KEAP1 and NRF2 expression and showed the highest NLuc fold induction (Extended Data Fig. 7). To customize HEKEMPOWER cells for implantation, they were mixed with CBCFO nanoparticles and enclosed in clinically licensed alginate microcapsules48 to shield the engineered cells from the host immune system while enabling diffusion of nutrients and the release of biopharmaceuticals. The performance of the HEKEMPOWER-containing implants was validated under a Helmholtz-coil-based uniform EMF. The magnetic field strength (Fig. 3a) and stimulation time dependence (Fig. 3b) of insulin production were evaluated. The highest insulin level of 2.76 ± 0.45 μg l−1 was obtained under an EMFS of 21 mT and 3 min, which is consistent with the results for NLuc expression (Extended Data Fig. 8). A kinetic study revealed that stimulated insulin production from the HEKEMPOWER cells reached a significant level in the culture supernatant within 3 h and was maintained for over 24 h (Fig. 3c). We also confirmed the excellent reversibility in on/off stimulation patterns at 24-h intervals over 5 days (Fig. 3d and Extended Data Fig. 8d). Under standard stimulation conditions (1 kHz, 21 mT, 3 min), transgene expression compared favourably with reported levels of ROS-triggered gene expression49, and daily EMF exposure (21 mT, 1 kHz, 3 min per day) had no impact on cell viability during the experimental period of 4 weeks (Fig. 3e), suggesting that the EMPOWER system was operating at near-optimal performance (Figs. 2 and 3).
Extended Data Fig. 7. Screening of monoclonal HEK-293 cell lines based on NLuc expression in response to electromagnetic stimulation.
(a) The EMPOWER system is based on the constitutive expression of KEAP1 (ITR-PhCMV-KEAP1-P2A-BlastR-pA-ITR, pJH1054) and NRF2 (ITR-PhCMV-NRF2-pA: PRPBSA-ECFP-P2A-PuroR-pA-ITR, pJH1101), and four-tandem ARE (DART4)-controlled NLuc reporter followed by mouse insulin (mINS) (ITR-PDART4-NLuc-P2A-mINS: PmPGK-ZeoR-pA-ITR, pJH1196). All the constructs are flanked by inverted terminal repeats (ITR) for SB100X-based Sleep Beauty transposase recognition. CBCFO concentration was fixed at 50 μg/106 cells. Electromagnetic stimulation: 1 kHz, 21 mT, 3 min. All data are presented as means ± s.d.; n = 2 independent experiments. Western blot analysis of KEAP1 (b) and NRF2 (c) levels in parental (HEK-293) and HEKEMPOWER cells. Vinculin was used as a loading control.
Fig. 3. Electromagnetically stimulated gene expression in microencapsulated HEKEMPOWER cells.
a, Magnetic-field-dependent insulin expression (stimulation time, 3 min). b, Stimulation-time-dependent insulin expression (magnetic field, 21 mT, 1 kHz). c, Time-dependent insulin production during 36 h after an EMFS stimulation of 21 mT, 1 kHz, 3 min. Profiling was started immediately after EMF stimulation. d, Reversibility of insulin production. The cells were alternatively stimulated with an EMFS of 21 mT for 3 min (on) or unstimulated (off) at 24-h intervals. The cell culture medium was renewed each time the EMF stimulation was switched from on-to-off or from off-to-on. e, Viability of HEKEMPOWER following daily 3-min EMF stimulation for 4 weeks (21 mT, 1 kHz, 3 min per day), compared with unstimulated HEKEMPOWER cells. All data are presented as mean ± s.d.; n = 6 (a,b) and n = 3 (c,d) independent experiments. P values in a–c were calculated versus the corresponding non-stimulated control. The induction factors were calculated between non-stimulated (EMFS (−)) and stimulated (EMFS (+)) groups. Statistical significance was analysed by two-way ANOVA with Tukey’s test (a,b) and one-way ANOVA with Dunnett’s multiple comparisons test (c,d).
Extended Data Fig. 8. NLuc expression of encapsulated HEKEMPOWER cells.
(a) Field-strength-dependent NLuc expression (1 kHz, 3 min). (b) Stimulation-time-dependent NLuc expression (1 kHz, 21 mT). (c) Time-dependent expression over 36 h (1 kHz, 21 mT, 3 min). (d) Reversible expression (1 kHz, 21 mT, 3 min). The CBCFO concentration was fixed at 50 μg/106 cells. P values and fold changes in (a, b, c) were calculated versus the corresponding non-stimulated control. All data are presented as means ± s.d.; (a and b) n = 6; (c) n = 3; (d) n = 4 independent experiments. Statistical significances were calculated via two-way ANOVA Dunnett’s multiple comparison tests.
Electromagnetically powered glucose homeostasis in T1D
For in vivo validation, we designed a single-coil EMF generator with an E-shaped iron core (Fig. 4a). The EMF from this single-coil device reached 20–22 mT at a plane 3–5 mm from the coil surface, which matches the depth of subcutaneous implantation in mice. This device generates a magnetic field gradient rather than the uniform field from the Helmholtz-coil device. The device was able to stimulate transgene expression from the encapsulated cells in vitro, and no significant difference was observed compared with the Helmholtz-coil device (Supplementary Fig. 7). For in vivo single-coil EMFS, five devices were fitted into a 3D-printed holder to facilitate parallel stimulation of mice (Fig. 4b and Supplementary Fig. 8).
Fig. 4. In vivo evaluation of HEKEMPOWER cells for wireless-controlled treatment of T1D.
a, Scheme illustrating the magnetic field stimulation of encapsulated HEKEMPOWER cells implanted in the dorsoventral side of mice, using a centimetre-sized single-coil device. b, Scheme showing the simultaneous stimulation of an experimental group of mice with a parallel assembly of single-coil devices. c, Stimulation-time-dependent tunability of insulin secretion (21 mT, 1 kHz, 0–3 min). d,e, Reversibility of EMF-controlled insulin (d) and blood-glucose (e) levels. Microencapsulated subcutaneous HEKEMPOWER implants were exposed to alternating on-to-off and off-to-on EMF stimulation every 3 days (on: 21 mT, 1 kHz, 3 min; off: unstimulated). f,g, Fasting blood-insulin (f) and blood-glucose (g) levels were recorded before implantation (week 0) and for up to 4 consecutive weeks after implantation of HEKEMPOWER cells in T1D mice stimulated for 3 min (21 mT, 1 kHz): EMFS (+) group. T1D and WT mice groups with non-stimulated HEKEMPOWER cell implants (EMFS (−)), and without implants (untreated) were used as controls. Over the entire treatment period of 4 weeks fed WT mice maintained average blood-insulin levels of 2.1 ± 0.8 µg l−1. h, Intraperitoneal GTT was performed on mice 3 days after implantation of microencapsulated cells and after fasting for 8 h. Data are presented as mean ± s.d., n = 5 (c–g) and n = 10 (h) biological replicates. P values in d,e were calculated between the indicated data and the initial (day 0) unstimulated T1D control. P values in g,h were calculated versus the corresponding non-stimulated control (black, bottom) and WT control (green, top). Statistical significance in d–h was analysed with two-way ANOVA Dunnett’s multiple comparison tests. Mouse schematic illustrations created with BioRender.com.
The encapsulated HEKEMPOWER cells implanted in T1D mice were subjected to a 3-min magnetic field stimulation (EMFS (+) T1D group) using the single-coil devices. Insulin secretion kinetics matched those found for other transcription-control modalities and the insulin levels were consistent with those in previous studies using experimental T1D as a proof-of-concept model (Extended Data Fig. 9)7,12,41,47,50,51. The insulin secretion levels of HEKEMPOWER could be adjusted by varying the EMF stimulation time (Fig. 4c) and the glycaemic control was fully reversible; switching the EMFS from off-to-on or from on-to-off every 3 days resulted in corresponding changes in insulin (Fig. 4d) and blood-glucose levels (Fig. 4e). The EMFS-driven secretion of insulin (Fig. 4f) from the HEKEMPOWER cells attenuated blood-glucose levels and subsequently maintained normoglycaemia in the T1D mice (Fig. 4g). Furthermore, EMFS-triggered insulin production by the HEKEMPOWER cells ameliorated postprandial glycaemic excursions in glucose tolerance tests (GTTs) and restored normoglycaemic levels (Fig. 4h). Real-time glycaemic measurements confirmed that daily stimulation of the HEKEMPOWER cells for 3 min could restore normoglycaemic levels in T1D mice and maintain glucose homeostasis for at least 4 weeks without any hypoglycaemic excursion. No significant difference in blood glucose or insulin levels was observed in non-stimulated wild-type (WT) mice implanted with HEKEMPOWER cells (EMFS (−) WT group) compared with non-treated WT mice. This confirms non-leakiness of the EMPOWER system and is consistent with the absence of hypoglycaemic episodes. At the end of the treatment period, the animals showed no sign of macroscopic (Extended Data Fig. 10a) or systemic inflammation (Extended Data Fig. 10b–d), and histological analyses of the implantation site indicated that the EMPOWER capsules remained in place, intact and unaffected by EMF stimulation (Extended Data Fig. 10e,f). The body weight gain (1.5 ± 0.6 g per mouse), daily food intake (6.5 ± 0.8 g per mouse) and water consumption (7.7 ± 1.6 ml per mouse) of EMF-stimulated T1D mice were identical to those of WT mice in the terminal phase of the 4-week treatment period.
Extended Data Fig. 9. EMF-stimulated blood-insulin levels in type-1 diabetic mice.
Type-1-diabetic mice implanted with microencapsulated HEKEMPOWER cells were stimulated with EMF (21 mT, 1 kHz, 3 min). Blood insulin reached wild-type levels within 12 h. Data are presented as mean ± s.d., n = 5 biological replicates. Statistical significance of differences between EMF-stimulated and unstimulated control groups was analysed by two-way ANOVA with Dunnett’s multiple comparisons test.
Extended Data Fig. 10. Impact of subcutaneous EMPOWER implants in mice.
(a) Pictures of the implantation site of a representative T1D mouse subcutaneously implanted with the EMPOWER system and treated for four weeks with daily EMF stimulation (21 mT, 1 kHz, 3 min). The implant site is encircled and does not show any macroscopic signs of inflammation. (b, c) Histological analyses of tissue sections around the implant site of EMF-stimulated (b) and unstimulated (c) T1D mice. Microcapsules containing HEKEMPOWER cells are indicated with arrows. Scale bar, 100 μm. (d-f) Profiling of key inflammatory cytokines, IL-6 (d), TNF-α (e) and IFN-γ (f), in the bloodstream of T1D mice four weeks after implantation of the EMPOWER system and daily EMF stimulation (21 mT, 1 kHz, 3 min). All data are presented as means ± s.d.; n = 5 biological replicates. Statistical significance was analysed by two-way ANOVA with Dunnett’s multiple comparisons test.
Conclusions
EMFs represent promising, minimally invasive control modalities for next-generation gene- and cell-based therapies. First-in-class magnetic stimulation methodologies reported so far mostly use membrane channels or receptors conjugated to inorganic nanoparticles activated by thermal or mechanical coupling8,15,31. However, challenges still remain associated with receptor and channel functionalization and intracellular trafficking as well as off-target effects and toxicities, limited robustness, tunability and clinical translation of these methods22,27,29,30. Instead, our work utilizes modified multiferroic nanoparticles to communicate with cytoplasmic ROS sensors KEAP1/NRF2, affording a nanoparticle–cell interface to drive transgene expression via synthetic promoters for wireless electromagnetic cell therapy. To test this approach, we focused on T1D, one of the dynamically most challenging chronic diseases, requiring meticulous blood-glucose control and daily insulin administration. In a T1D mouse model, daily EMFS (21 mT, 1 kHz, 3 min.) of subcutaneously implanted, microencapsulated HEKEMPOWER cells was sufficient to drive transgene expression of insulin at a level sufficient to produce sustained normoglycaemia. Our proof-of-concept study successfully restored normoglycaemia in a mouse model of experimental T1D throughout the 4-week experimental period, demonstrating dynamically robust, reversible and tunable in vivo control. The EMPOWER system compared favourably in performance with established cell-based therapeutic modalities using chemical7,41,49,52 and physical stimuli12,13 with identical cell-encapsulation technology, which has been validated for longevity53 and in human clinical trials48.
The CBCFO nanoparticles used here exhibit efficient coupling between magnetostrictive and piezoelectric composites45, while the bio-originated, positively charged polymer chitosan improves biocompatibility and cell adhesion54. In addition to shielding the bare ferric oxides from the cellular environment, chitosan also enables the short-lived ROS generated by the CBCFO nanoparticles to escape from the endosomes into the cytoplasm via the proton sponge effect46. Indeed, such multiferroic nanoparticles have been directly injected into the brain or blood circulation for deep neuron stimulation26, guided central nervous delivery55 and dissociation of Alzheimer’s β-amyloid aggregates33. A key advantage of our system is that cellular stimulation can be triggered at a much lower dose of nanoparticles (50 μg per 106 cells, over 20 times lower than in the aforementioned applications)56. The alginate-microencapsulated implants also minimize the risk of liver damage53 associated with the direct administration of nanoparticles27. In addition, cellular ROS levels increased immediately after stimulation and then declined within 3–6 h, and the KEAP1/NRF2 system recognizes this ROS peak, not a gradual accumulation of ROS14, as typically observed in ROS-signalling systems57. Such kinetics limit the adverse effect of ROS on HEKEMPOWER cells, as evidenced by the reversibility of the stimulation of therapeutic protein expression.
A low-frequency EMF of 1 kHz imposes a negligible magnetothermal effect or mechanical force on the cells22. More importantly, because even chemical ROS inducers producing systemic ROS surges have no apparent impact on cell physiology or metabolism14, EMF-triggered ROS induction confined to the vicinity of intracellular CBCFO nanoparticles should bear little risk of potential side effects. Additionally, our work highlights the use of weak EMFs (up to 21 mT), much weaker than those used in MRI scanners (in the tesla range), promising safety in clinical use. This level of EMFS can be achieved by a single induction coil with a fixed coil structure and input parameters (akin to wireless phone chargers), and tuned by adjusting a single parameter, stimulation time, avoiding the need for complex software or electronic implants. We believe that this kind of interface between programmable electronic devices and genetic therapies has the potential to dramatically streamline the treatment regimen for patients with chronic diseases.
Methods
Fabrication of CBCFO
CFO nanoparticles were synthesized according to the literature with modifications33. To prepare CFO nanoparticles, iron(III) chloride hexahydrate (0.995 g) and cobalt(II) chloride (0.239 g) were mixed in deionized water (35 ml) containing hexadecyltrimethylammonium bromide (2.041 g). Sodium hydroxide solution (6 M) was then added dropwise to the mixture under continuous stirring to achieve a final pH of 11.0. After ultrasound stimulation for 30 min, additional hydrothermal treatment was applied to the mixture at 180 °C for 24 h in a 50-ml Teflon-lined stainless-steel autoclave. The resulting black precipitates were washed with deionized water and ethanol several times after cooling to room temperature.
To synthesize BCFO magnetoelectric nanoparticles, a sol–gel treatment was applied to the as-prepared CFO nanoparticles33. Briefly, CFO nanoparticles (50 mg) were dispersed into 30 ml ethylene glycol (catalogue number 324588, Sigma-Aldrich) containing bismuth(III) nitrate pentahydrate (0.160 g) and iron(III) nitrate nonahydrate (0.121 g). After 2 h of sonication, the sol mixture was moved to a vacuum oven and dried for 24 h. Next, the resulting gel-state mixture was preheated at 400 °C for 30 min to eliminate organic compounds and successively calcined at 500 °C for 90 min. The resulting BCFO nanoparticles were washed several times with deionized water and ethanol on a nylon membrane and collected with a neodymium permanent magnet after ultrasound treatment.
Chitosan (catalogue number 448877-50 G, Sigma-Aldrich) was first dissolved in 0.1-M NaCl to form a 0.1% solution after acidification with 1% acetic acid. Rhodamine B isothiocyanate (RITC)-labelled chitosan was prepared by dissolving RITC (40 µM, catalogue number CAY20653-100 mg, Cayman) in methanol and mixing it 1:1 with a 10 mg ml−1 chitosan solution under nitrogen protection, followed by dialysis against 0.1-M NaCl. The prepared BCFO nanoparticles were then dispersed and mixed in the chitosan solution (5 mg ml−1) by sonication for 1 h. The CBCFO nanoparticles were collected by centrifugation and washed with water three times. RITC-CBCFO nanoparticles were fabricated by mixing BCFO nanoparticles with RITC-labelled chitosan.
For cellular uptake, all nanoparticles were sonicated at 35 kHz for 30 min (Bandelin Electronic, RK100H) and filtered through a 0.22-µm filter (catalogue number P668.1, Carl Roth).
Characterization of CBCFO
The morphology of the obtained CFO, BCFO and CBCFO nanoparticles was examined by TEM (FEI F30) and STEM (JEM-F200). The distribution of elements along the nanoparticles was studied by STEM EDX mapping (JEM-F200). The crystallographic structure of the nanostructures was analysed by XRD on a Bruker AXS D8 Advance 1 X-ray diffractometer, equipped with a copper target at a wavelength of 1.542 Å. The magnetic properties were evaluated by scanning probe microscopy (Bruker Dimension ICON) according to the magnetic force model. The zeta potential and the hydrodynamic size of samples were measured by a dynamic light scattering Zetasizer (Malvern, ZEN3600) in DPBS (0.01 M, pH 7.4). Relative charge separation and ROS induction from nanoparticles were evaluated by TA assay (3 mM, λex/λem = 310/430 nm) and MB assay (5 mM, λabs = 664 nm), respectively, using a plate reader (Tecan, Spark Reader). For TA and MB assays, an aqueous solution (400 μl) containing different nanoparticles was exposed to a magnetic field under constant agitation, and 100-μl aliquots of the supernatant were transferred to 96-well plates for colorimetric or fluorometric measurement.
Magnetic field stimulation
Electromagnet-containing 3D-printed holders (Supplementary Figs. 5a and 8c) were designed to minimize the thermal effect on biological systems. Samples were exposed to a uniform EMF by placing them in the central area (5.8 cm × 5.8 cm) of a Helmholtz-coil-based device. The circuits (Supplementary Fig. 5c) for magnetic field stimulation were powered by custom-designed electrical drivers. The field strength generated by the Helmholtz-coil device was 9–21 mT and that generated by the single-coil device was 20–22 mT at a plane of 0.3–0.5 cm from the coil, with the frequency fixed at 1 kHz (sinusoidal). The amplitude of the applied alternating magnetic field was confirmed by a gaussmeter.
Cell culture and engineering
Cell culture
Human embryonic kidney cells (HEK-293, ATCC, CRL-11268), human telomerase-immortalized mesenchymal stem cells (hMSC-TERT, RRID: CVCL_Z015), human liver cancer cell line (HepG2, ATCC, CRL-11997), Chinese hamster ovary cells (CHO-K1, ATCC, CCL-61), baby hamster kidney cells (BHK-21, ATCC, CCL-10) and mouse pituitary tumour cells (AtT-20, ATCC, CCL-89), were cultivated in Dulbecco’s modified Eagle’s medium (DMEM, catalogue number 52100-39, Thermo Fisher Scientific) supplemented with 100 mM proline (CHO-K1 only), 10% fetal bovine serum (FBS, catalogue number F7524, Sigma-Aldrich) and 1% (v/v) streptomycin/penicillin (catalogue number L0022, Biowest) at 37 °C in a humidified atmosphere containing 5% CO2.
Cell transfection
For transfection, 104 cells (CellDrop BF Brightfield Cell Counter, DeNovix) were seeded per well in a 96-well plate (catalogue number 3599, Corning Life Sciences) 24 h before transfection by addition of 20 µl of a mixture containing 0.3 µg polyethyleneimine (PEI MAX, mol. wt 40,000, 1 μg μl−1 in double-distilled H2O, catalogue number 24765-2, Polysciences) and 0.1 µg plasmid DNA (equimolar concentrations for plasmid mixtures) per well. After 8 h, the mixture was replaced with a standard cultivation medium or nanoparticle medium suspension (100 µl) for further characterization.
Monoclonal cell line construction
HEK-293 cells (1.5 × 105) were cotransfected with pJH1101 (ITR-PhCMV-NRF2-pA: PRPBSA-ECFP-P2A-PuroR-pA-ITR) (200 ng), pJH1054 (ITR-PhCMV-KEAP1-P2A-BlastR-pA-ITR) (550 ng), pJH1096 (ITR-PARE-NLuc-P2A-mINS:PmPGK-ZeoR-pA-ITR) (400 ng) and pJH42 (PhCMV-SB100X-pA) encoding constitutive expression of a hyperactive Sleeping Beauty (SB) transposase (200 ng)58. After selection for two passages in culture medium supplemented with 2.5 μg ml−1 puromycin, 300 μg ml−1 blasticidin and 300 μg ml−1 zeocin, the resistant polyclonal population was divided by ECFP-based FACS-mediated single-cell sorting into 48 monoclonal cell lines. Twelve monoclonal cell lines with the highest ECFP-based fluorescence intensity were loaded with CBCFO nanoparticles (50 μg per 106 cells) and stimulated by EMF (1 kHz, 21 mT, 3 min). HEKEMPOWER (clone number 3), showing best-in-class EMF-stimulated transgene-fold induction, was chosen for further studies (Extended Data Fig. 7a).
Microencapsulation and implantation of HEKEMPOWER cells
To protect HEKEMPOWER cells from the mouse immune system while permitting the exchange of nutrients and release of therapeutic proteins, we used a clinical trial-validated alginate-based encapsulation technology48. HEKEMPOWER cells were encapsulated in alginate/poly(l-lysine)/alginate microcapsules with a diameter of 400 µm by treating a mixture of 9.0 × 107 cells with 18 ml alginate (w/v, 1.6%; Na-alginate, catalogue number 71238, Sigma-Aldrich) in an encapsulator (Inotech Encapsulator IE-50R, EncapBiosystems) equipped with a 200-μm nozzle. A 20-ml syringe was operated at a flow rate of 20 ml min−1 with a vibration frequency of 1.2 kHz and 1.2 kV voltage for bead dispersion. A 100-ml poly(l-lysine) 2000 (w/v, 0.05%; catalogue number 25988-63-0, Alamanda Polymers) solution and a 100-ml 0.03% alginate solution were sequentially used to form the microcapsules. For delivery, 2.5 × 106 encapsulated cells in 0.5 ml serum-free DMEM were subcutaneously implanted through a 3-ml syringe (catalogue number 9400038, Becton Dickinson) with a 0.7-mm × 30-mm needle (catalogue number 30382903009009, Becton Dickinson).
Animal experiments
Preparation of experimental mouse models
C57BL/6JRJ mice were kept and monitored in groups (n = 5) in an environment controlled at 21 ± 2 °C and 55 ± 10% humidity and maintained under a 12-h reverse light–dark cycle, with free access to standard diet and water. All procedures were performed in compliance with Swiss animal welfare regulations, approved by the Veterinary Office of the Canton Basel-Stadt, Switzerland (license number 2996_34477), the French Republic (project number DR2018-40v5 and APAFIS number 16753) and the People’s Republic of China (Institutional Animal Care and Use Committee of Westlake University, protocol ID20-009-XMQ). The experiments were conducted by P.G.R. (license number LTK 5507), G. Charpin-El Hamri (number 69266309; University of Lyon, Institut Universitaire de Technologie) or by S. Xue (Westlake University). Two groups of mice were utilized: WT and experimentally induced T1D mice. To induce the T1D condition, male WT mice (8–9 weeks old, 18–23 g) were intraperitoneally injected with streptozotocin (STZ; 75 mg kg−1, 0.2 M citrate buffer, pH 4.2; Sigma-Aldrich, catalogue number S0130) for 4 consecutive days following a 6-h fasting period59. Control WT mice from Janvier Labs (18–23 g) received identical injections without STZ. At 10 days after the final injection of STZ, fasting blood-glucose levels were measured using ContourNext test strips and a ContourNext ONE reader (Ascensia Diabetes Care; catalogue numbers 84191451 and 85659367) to confirm persistent hyperglycaemia and T1D status in the STZ-treated group.
Experimental procedure
Microencapsulated HEKEMPOWER cells with CBCFO nanoparticles (50 μg per 106 cells) were subcutaneously implanted in the experimental and control groups. The hair on the dorsoventral side of the mice was completely shaved, and the animals were anaesthetized with 4% isoflurane and maintained under 2% isoflurane during surgery. Microencapsulated HEKEMPOWER cells were injected subcutaneously (0.5 ml DMEM, 5 × 106 cells) on the dorsoventral side using a 5-ml syringe with a 21-gauge needle to reduce the risk of aseptic loosening. After a 24-h stabilization period, the HEKEMPOWER cells were wirelessly stimulated using a portable (single-coil-based) device (Fig. 4b) for 3 min once every 24 h in the EMFS (+) group. For the rest of each day, treated animals were not restrained. The single-coil devices (n = 5) were fitted into a 3D-printed holder (Supplementary Fig. 8d) and a rectangular tunnel (with five parallel holes, Supplementary Fig. 8b) was used to maximize efficiency and facilitate parallel experiments. The animals were fasted for 6 h before measuring blood-glucose and insulin levels. For the GTT experiment, treated animals were intraperitoneally injected with 1.5 g kg−1 glucose and glycaemia was recorded at regular intervals over 2 h. Real-time blood-glucose monitoring was performed at regular time points over a period of 4 weeks after a fasting period of 6 h. Alongside glycaemic levels, the corresponding blood insulin levels were also measured and compared with those of untreated WT and T1D groups.
Blood collection
The level of blood glucose was monitored periodically using ContourNext test strips and a ContourNext ONE reader (catalogue numbers 84191451 and 85659367, Ascensia Diabetes Care)60. Blood insulin levels were assessed in serum samples collected in Microtainer serum separator tubes (centrifuged at 6,000g for 10 min at 4 °C; catalogue number 365967, Becton Dickinson) with an ultrasensitive ELISA assay (catalogue number 10-1247-01, Mercordia).
Histology
Microencapsulated HEKEMPOWER and surrounding tissue were explanted from EMF-stimulated and unstimulated mice and fixed overnight in 10% buffered formalin (100 ml 40% formalin, 900 ml double-distilled H2O, 4 g l−1 NaH2PO4, 6.5 g l−1 Na2HPO4, pH 7). The tissue samples were trimmed, dehydrated in increasing concentrations of ethanol, cleared with xylene, embedded in paraffin wax, processed into 5-µm slices using an EXAKT 300 CP system (EXAKT Technologies) and stained with haematoxylin and eosin. The tissue sections were analysed by light microscopy (Olympus CKX53) and images were acquired with an Olympus DP75 camera.
Statistics and reproducibility
The data presentation, sample size of biological replicates (n), statistical analysis and significance of differences are shown in the figure legends. All in vitro experiments were repeated at least twice unless otherwise stated. For the mouse experiments, biological replicates (n = 5 mice per group) were randomly assigned to different experimental groups. The details are described in each figure legend. To determine the statistical significance of differences in the case of multiple comparisons we used GraphPad Prism 10 (v.10.1.0, GraphPad Software) and a two-tailed, unpaired, Student’s t-test and one-way or two-way analysis of variance (ANOVA). No statistical methods were used to prespecify sample sizes, but our sample sizes are the same as previously reported12,14. Data distribution was assumed to be normal, but was not formally tested. All investigators involved in this study were blinded to group allocation during data collection and analysis. No animals or data points were excluded from the analyses for any reason.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41565-025-01929-w.
Supplementary information
Supplementary Figures 1–8 and Supplementary Table 1
Statistic source data for Supplementary Figures.
Source data
Statistical source data for Fig. 1.
Statistical source data for Fig. 2.
Statistical source data for Fig. 3.
Statistical source data for Fig. 4.
Statistical source data for Extended Data Fig. 1.
Unprocessed western blots for Extended Data Fig. 1d
Statistical source data for Extended Data Fig. 2.
Statistical source data for Extended Data Fig. 3.
Statistical source data for Extended Data Fig. 4.
Statistical source data for Extended Data Fig. 5.
Statistical source data for Extended Data Fig. 6.
Statistical source data for Extended Data Fig. 7.
Unprocessed western blots for Extended Data Fig. 7b
Statistical source data for Extended Data Fig. 8.
Statistical source data for Extended Data Fig. 9.
Statistical source data for Extended Data Fig. 10.
Acknowledgements
This work was financially supported through a European Research Council advanced grant (ElectroGene, number 785800) and in part by the Swiss National Centre of Competence in Research (NCCR) for Molecular Systems Engineering. We are grateful to G. Charpin-El-Hamri, M. Xie and S. Xue for generous advice and support in animal experimentation.
Extended data
Author contributions
Z.L. and M.F. designed the project. Z.L. and P.B. conducted device fabrication and electrical characterization and the in vitro experiments. Z.L., P.G.R., J.H. and M.F. designed the experiments and analysed the results. P.G.R. performed the animal experiments. Z.L., P.G.R., J.H. and M.F. wrote the paper.
Peer review
Peer review information
Nature Nanotechnology thanks Victoria Cogger and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Funding
Open access funding provided by Swiss Federal Institute of Technology Zurich.
Data availability
All data supporting the findings of this study are presented in the paper and the Supplementary Information. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
is available for this paper at 10.1038/s41565-025-01929-w.
Supplementary information
The online version contains supplementary material available at 10.1038/s41565-025-01929-w.
References
- 1.Cubillos-Ruiz, A. et al. Engineering living therapeutics with synthetic biology. Nat. Rev. Drug Discov.20, 941–960 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Xie, M. & Fussenegger, M. Designing cell function: assembly of synthetic gene circuits for cell biology applications. Nat. Rev. Mol. Cell Biol.19, 507–525 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Wang, H., Xie, M., Charpin-El Hamri, G., Ye, H. & Fussenegger, M. Treatment of chronic pain by designer cells controlled by spearmint aromatherapy. Nat. Biomed. Eng.2, 114–123 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Wang, C.-H. et al. CRISPR-engineered human brown-like adipocytes prevent diet-induced obesity and ameliorate metabolic syndrome in mice. Sci. Transl. Med.12, eaaz8664 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao, H., Xue, S., Hussherr, M.-D., Teixeira, A. P. & Fussenegger, M. Autonomous push button controlled rapid insulin release from a piezoelectrically activated subcutaneous cell implant. Sci. Adv.8, eabm4389 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu, Y. et al. Optogenetic-controlled immunotherapeutic designer cells for post-surgical cancer immunotherapy. Nat. Commun.13, 6357 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai, P. et al. A fully human transgene switch to regulate therapeutic protein production by cooling sensation. Nat. Med.25, 1266–1273 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Sebesta, C. et al. Subsecond multichannel magnetic control of select neural circuits in freely moving flies. Nat. Mater.21, 951–958 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maity, D., Guha Ray, P., Buchmann, P., Mansouri, M. & Fussenegger, M. Blood-glucose-powered metabolic fuel cell for self-sufficient bioelectronics. Adv. Mater.35, 2300890 (2023). [DOI] [PubMed] [Google Scholar]
- 10.Holt, B. A. & Kwong, G. A. Protease circuits for processing biological information. Nat. Commun.11, 5021 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuwasaki, Y. et al. A red light-responsive photoswitch for deep tissue optogenetics. Nat. Biotechnol.40, 1672–1679 (2022). [DOI] [PubMed] [Google Scholar]
- 12.Zhao, H. et al. Tuning of cellular insulin release by music for real-time diabetes control. Lancet Diabetes Endocrinol.11, 637–640 (2023). [DOI] [PubMed] [Google Scholar]
- 13.Krawczyk, K. et al. Electrogenetic cellular insulin release for real-time glycemic control in type 1 diabetic mice. Science368, 993–1001 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Huang, J., Xue, S., Buchmann, P., Teixeira, A. P. & Fussenegger, M. An electrogenetic interface to program mammalian gene expression by direct current. Nat. Metab.5, 1395–1407 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdel Fattah, A. R. et al. Targeted mechanical stimulation via magnetic nanoparticles guides in vitro tissue development. Nat. Commun.14, 5281 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, J.-U. et al. Non-contact long-range magnetic stimulation of mechanosensitive ion channels in freely moving animals. Nat. Mater.20, 1029–1036 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Choi, S.-H. et al. In vivo magnetogenetics for cell-type-specific targeting and modulation of brain circuits. Nat. Nanotech.19, 1333–1343 (2024). [DOI] [PubMed] [Google Scholar]
- 18.Kim, J.-W. et al. Single-cell mechanogenetics using monovalent magnetoplasmonic nanoparticles. Nat. Protoc.12, 1871–1889 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, J.-H. et al. Magnetic nanoparticles for ultrafast mechanical control of inner ear hair cells. ACS Nano8, 6590–6598 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Tay, Z. W. et al. Magnetic particle imaging-guided heating in vivo using gradient fields for arbitrary localization of magnetic hyperthermia therapy. ACS Nano12, 3699–3713 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, H., Delikanli, S., Zeng, H., Ferkey, D. M. & Pralle, A. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nat. Nanotech.5, 602–606 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Romero, G., Park, J., Koehler, F., Pralle, A. & Anikeeva, P. Modulating cell signalling in vivo with magnetic nanotransducers. Nat. Rev. Methods Primers2, 92 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perica, K. et al. Magnetic field-induced T cell receptor clustering by nanoparticles enhances T cell activation and stimulates antitumor activity. ACS Nano8, 2252–2260 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng, K. et al. Magnetic antibody-linked nanomatchmakers for therapeutic cell targeting. Nat. Commun.5, 4880 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu, S. et al. Genetically magnetic control of neural system via TRPV4 activation with magnetic nanoparticles. Nano Today39, 101187 (2021). [Google Scholar]
- 26.Kozielski, K. L. et al. Nonresonant powering of injectable nanoelectrodes enables wireless deep brain stimulation in freely moving mice. Sci. Adv.7, eabc4189 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boey, A. & Ho, H. K. All roads lead to the liver: metal nanoparticles and their implications for liver health. Small16, 2000153 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Gao, C. et al. Biomedical micro-/nanomotors: from overcoming biological barriers to in vivo imaging. Adv. Mater.33, 2000512 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Rennick, J. J., Johnston, A. P. R. & Parton, R. G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotech.16, 266–276 (2021). [DOI] [PubMed] [Google Scholar]
- 30.Stueber, D. D., Villanova, J., Aponte, I., Xiao, Z. & Colvin, V. L. Magnetic nanoparticles in biology and medicine: past, present, and future trends. Pharmaceutics13, 943 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen, J. C. et al. Self-rectifying magnetoelectric metamaterials for remote neural stimulation and motor function restoration. Nat. Mater.23, 139–146 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu, W. et al. In situ activation of flexible magnetoelectric membrane enhances bone defect repair. Nat. Commun.14, 4091 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jang, J. & Park, C. B. Magnetoelectric dissociation of Alzheimer’s β-amyloid aggregates. Sci. Adv.8, eabn1675 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vensaus, P., Liang, Y., Ansermet, J.-P., Soler-Illia, G. J. A. A. & Lingenfelder, M. Enhancement of electrocatalysis through magnetic field effects on mass transport. Nat. Commun.15, 2867 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim, D. et al. Magnetoelectric effect in hydrogen harvesting: magnetic field as a trigger of catalytic reactions. Adv. Mater.34, 2110612 (2022). [DOI] [PubMed] [Google Scholar]
- 36.Balaban, R. S., Nemoto, S. & Finkel, T. Mitochondria, oxidants, and aging. Cell120, 483–495 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Lennicke, C. & Cochemé, H. M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell81, 3691–3707 (2021). [DOI] [PubMed] [Google Scholar]
- 38.Baird, L. & Yamamoto, M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell. Biol.40, e00099–20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang, J. et al. Week-long normoglycaemia in diabetic mice and minipigs via a subcutaneous dose of a glucose-responsive insulin complex. Nat. Biomed. Eng.8, 1214–1225 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen, Z. et al. Synthetic beta cells for fusion-mediated dynamic insulin secretion. Nat. Chem. Biol.14, 86–93 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie, M. et al. Β-Cell–mimetic designer cells provide closed-loop glycemic control. Science354, 1296–1301 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Bae, I.-T. et al. Elucidation of crystal and electronic structures within highly strained BiFeO3 by transmission electron microscopy and first-principles simulation. Sci. Rep.7, 46498 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Branca, C. et al. Role of the OH and NH vibrational groups in polysaccharide–nanocomposite interactions: a FTIR-ATR study on chitosan and chitosan/clay films. Polymer99, 614–622 (2016). [Google Scholar]
- 44.Huang, Z. et al. Interface engineering and emergent phenomena in oxide heterostructures. Adv. Mater.30, 1802439 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Li, Y. et al. Magnetoelectric quasi-(0–3) nanocomposite heterostructures. Nat. Commun.6, 6680 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Richard, I., Thibault, M., De Crescenzo, G., Buschmann, M. D. & Lavertu, M. Ionization behavior of chitosan and chitosan–DNA polyplexes indicate that chitosan has a similar capability to induce a proton-sponge effect as PEI. Biomacromolecules14, 1732–1740 (2013). [DOI] [PubMed] [Google Scholar]
- 47.Mansouri, M. et al. Smart-watch-programmed green-light-operated percutaneous control of therapeutic transgenes. Nat. Commun.12, 3388 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacobs-Tulleneers-Thevissen, D. et al. Sustained function of alginate-encapsulated human islet cell implants in the peritoneal cavity of mice leading to a pilot study in a type 1 diabetic patient. Diabetologia56, 1605–1614 (2013). [DOI] [PubMed] [Google Scholar]
- 49.Huang, J., Xue, S., Teixeira, A. P. & Fussenegger, M. A gene-switch platform interfacing with reactive oxygen species enables transcription fine-tuning by soluble and volatile pharmacologics and food additives. Adv. Sci.11, 2306333 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bertschi, A. et al. Controlling therapeutic protein expression via inhalation of a butter flavor molecule. Nucleic Acids Res.51, e28–e28 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ye, H., Baba, M. D.-E., Peng, R.-W. & Fussenegger, M. A synthetic optogenetic transcription device enhances blood-glucose homeostasis in mice. Science332, 1565–1568 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Shao, J. et al. Engineered poly(A)-surrogates for translational regulation and therapeutic biocomputation in mammalian cells. Cell Res.34, 31–46 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lai, J. et al. Alginate-based encapsulation fabrication technique for drug delivery: an updated review of particle type, formulation technique, pharmaceutical ingredient, and targeted delivery system. Pharmaceutics16, 370 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kou, S., Peters, L. M. & Mucalo, M. R. Chitosan: a review of sources and preparation methods. Int. J. Biol. Macromol.169, 85–94 (2021). [DOI] [PubMed] [Google Scholar]
- 55.Kaushik, A. et al. Magnetically guided central nervous system delivery and toxicity evaluation of magneto-electric nanocarriers. Sci. Rep.6, 25309 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feliu, N. et al. Nanoparticle dosage—a nontrivial task of utmost importance for quantitative nanosafety research. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol.8, 479–492 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Murphy, M. P. et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab.4, 651–662 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mátés, L. et al. Molecular evolution of a novel hyperactive Sleeping Beauty transposase enables robust stable gene transfer in vertebrates. Nat. Genet.41, 753–761 (2009). [DOI] [PubMed] [Google Scholar]
- 59.Furman, B. L. Streptozotocin-Induced diabetic models in mice and rats. Curr. Protoc.1, e78 (2021). [DOI] [PubMed] [Google Scholar]
- 60.Christiansen, M. et al. A new, wireless-enabled blood glucose monitoring system that links to a smart mobile device: accuracy and user performance evaluation. J. Diabetes Sci. Technol.11, 567–573 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1–8 and Supplementary Table 1
Statistic source data for Supplementary Figures.
Statistical source data for Fig. 1.
Statistical source data for Fig. 2.
Statistical source data for Fig. 3.
Statistical source data for Fig. 4.
Statistical source data for Extended Data Fig. 1.
Unprocessed western blots for Extended Data Fig. 1d
Statistical source data for Extended Data Fig. 2.
Statistical source data for Extended Data Fig. 3.
Statistical source data for Extended Data Fig. 4.
Statistical source data for Extended Data Fig. 5.
Statistical source data for Extended Data Fig. 6.
Statistical source data for Extended Data Fig. 7.
Unprocessed western blots for Extended Data Fig. 7b
Statistical source data for Extended Data Fig. 8.
Statistical source data for Extended Data Fig. 9.
Statistical source data for Extended Data Fig. 10.
Data Availability Statement
All data supporting the findings of this study are presented in the paper and the Supplementary Information. Source data are provided with this paper.