Abstract
Obesity increases the risk of many cancers and impairs the anti-tumour immune response. However, little is known about whether the source or composition of dietary fat affects tumour growth or anti-tumour immunity in obesity. Here, we show that high-fat diets (HFDs) derived from lard, beef tallow or butter accelerate tumour growth in a syngeneic model of melanoma, but HFDs based on coconut oil, palm oil or olive oil do not, despite equivalent obesity. Using butter-based and palm oil-based HFDs as examples, we find that these dietary fat sources differentially regulate natural killer and CD8 T cell infiltration and function within the tumour microenvironment, governed by distinct effects on the plasma metabolome and intracellular metabolism. We identify diet-related lipid intermediates, namely long-chain acylcarnitine species, as immunosuppressive metabolites enriched in mice fed butter compared to palm oil HFD. Together, these results highlight the significance of diet in maintaining a healthy immune system and suggest that modifying dietary fat may improve cancer outcomes in obesity.
Subject terms: Immunosurveillance, Obesity, Metabolism
This study shows that animal-based high-fat diets accelerate tumour growth and impair anti-tumour response to melanoma in obese mice, whereas plant-based high-fat diets do not.
Main
An estimated one billion people worldwide with obesity1 are at risk for a variety of obesity-related diseases, including cardiovascular disease and cancer. Obesity increases the risk for at least 13 types of cancer, including breast, liver and colorectal cancers2, and increases cancer-related mortality3. Even as rates of other cancers have declined, the incidence of obesity-related cancers has increased in recent years4. Therefore, there is an urgent need to better understand the pathology of obesity-related cancers and develop novel strategies for their treatment.
The systemic changes associated with obesity, such as hyperinsulinaemia5, hyperglycaemia6, hyperlipidaemia7 and chronic inflammation8, have been shown to directly promote tumour cell transformation, growth and metastasis9. Recently, we10,11 and others12,13 have demonstrated that obesity also causes changes in the immune system that contribute to tumour progression by suppressing anti-tumour immunity, primarily through impairment of cytotoxic CD8 T cell and natural killer (NK) cell responses.
The type of fat consumed in the diet can have profound effects on health, as has been shown in heart disease, whereby substitution of dietary saturated fatty acids with monounsaturated or polyunsaturated fatty acids reduces cholesterol and attenuates the risk of coronary heart disease14,15. The link between dietary fat and cancer risk was first described in 1930, when researchers showed that a diet with added butter increased the incidence of carcinogen-induced tumours in mice16. Since then, the role of dietary fat in cancer development and progression and as a potential cancer treatment has remained a topic of research17–19. It has been shown that specific commonly consumed dietary fatty acids, such as palmitic acid (C16:0) and oleic acid (C18:1), have differential effects on the proliferation and migration of tumour cells in vitro20,21 and in vivo22. However, few studies have examined how different dietary fats impact tumour growth in obesity23 and whether diet composition in this context alters anti-tumour immunity24.
In this study, we examined how different sources of dietary fat influence tumour growth and anti-tumour immunity in the context of obesity. We demonstrate that the source of dietary fat in a diet-induced obesity (DIO) model influences subsequent tumour growth independent of the level of obesity. A HFD based primarily on butter accelerates the growth of syngeneic tumours and impairs NK and CD8 T cell function in the murine tumour microenvironment, while a palm oil-based HFD protects from obesity-accelerated tumour growth and immune dysfunction, despite exhibiting similar severity of obesity and gross systemic metabolic dysfunction. Mechanistically, we found that a palm oil-based HFD prevented NK cell metabolic paralysis, probably through the sustained activation of c-Myc. Moreover, the accumulation of immunosuppressive acylcarnitines in mice fed a butter-based HFD impairs CD8 T cell anti-tumour immunity by inducing mitochondrial dysfunction, resulting in loss of interferon-γ (IFNγ) and impaired cytotoxicity. Together, these findings demonstrate that the dietary fat source has a significant influence on tumour growth in obesity and emphasize the potential of dietary intervention as a therapeutic strategy in treating obesity-related cancers.
Results
Dietary fat source modifies tumour growth in obesity
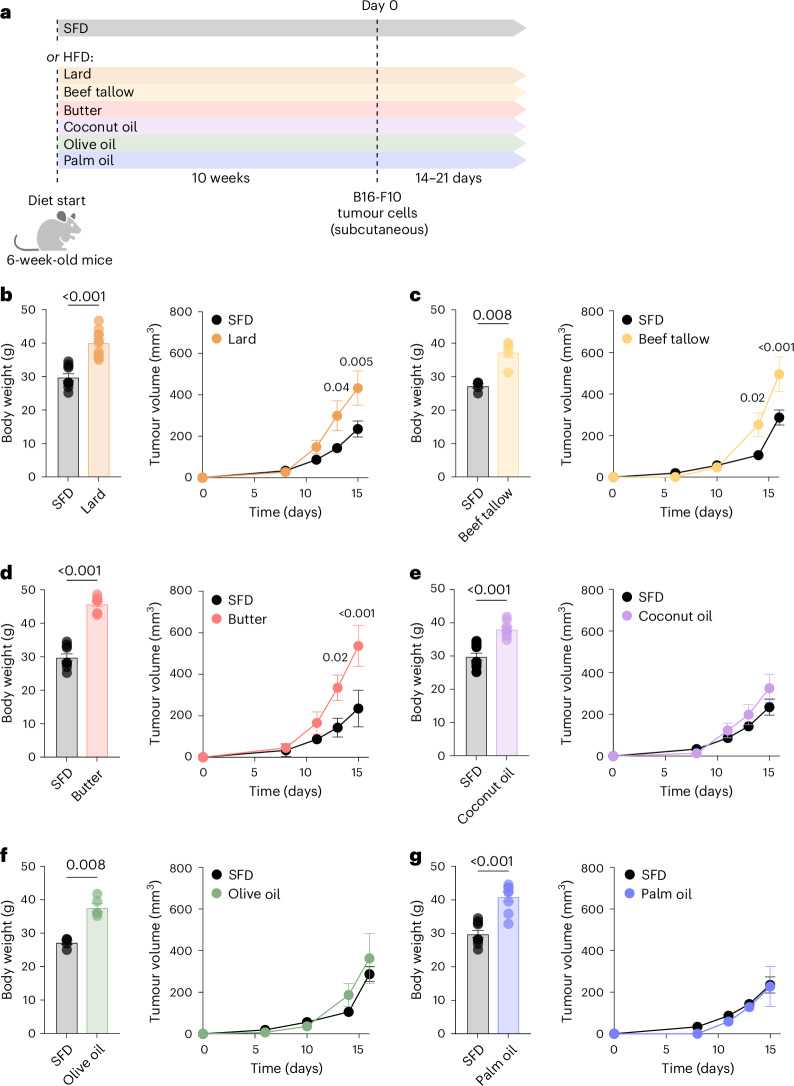
To study the impact of dietary lipids on tumour growth in obesity, we used a mouse model of DIO in which mice were fed a custom HFD (45% kcal from fat; Supplementary Table 1) based on a variety of fat sources. C57BL/6J mice (6 weeks old) were fed a standard rodent diet (SFD, 13% kcal from fat) or a HFD primarily derived from lard, beef tallow, butter, coconut oil, olive oil or palm oil (Fig. 1a). The various HFDs were isocaloric and contained identical base ingredients, including fibre, carbohydrates, protein, vitamins and minerals (Supplementary Table 2). Regardless of fat source, mice fed a HFD gained significantly more weight than SFD-fed controls (Fig. 1b–g, left). After 10 weeks, the mice were injected subcutaneously with B16-F10 melanoma cells (B16), and the assigned diets were continued until the study endpoint. As described previously11, B16 tumours grew more rapidly in mice fed lard-based HFD than in those fed a SFD (Fig. 1b, right), but surprisingly, tumour growth dynamics in HFD-fed mice differed significantly with the source of dietary fat (Fig. 1b–f, right). Mice fed beef tallow-based or butter-based HFD exhibited accelerated tumour growth compared to those fed a SFD, whereas tumour growth in mice fed coconut oil-based, olive oil-based or palm oil-based HFD was not different from SFD. Taken together, these results indicate that the source of dietary fat influences tumour growth in obesity and can uncouple tumour growth rate from adiposity.
Fig. 1. Dietary fat source influences growth of B16 melanoma tumours in obesity.
a, Schematic of experimental design. b–g, Body weight (left) and tumour volumes (right) of subcutaneous B16-F10 melanoma tumours in mice fed a SFD (13% fat) or HFD (45% fat) from various plant and animal fat sources for 12 weeks. HFDs were derived mostly from lard (b), beef tallow (c), butter (d), coconut oil (e), olive oil (f) or palm oil (g); n = 5, representative of three (b), two (c), four (d), three (e), two (f) and four (g) independent experiments. SFD control groups are the same in (b, d, e, g) and (c, f). In bar graphs, bar height represents the mean; error bars, s.e.m. In line graphs, each data point represents the mean; error bars, s.e.m. Comparisons were made using the two-tailed Mann–Whitney test (b–g, left) or two-way repeated-measures ANOVA followed by Bonferroni’s multiple comparisons test (b–g, right). Created in BioRender.com.
To better understand how dietary fat influences tumour growth, we focused on the HFDs that had the most differential effects on B16 tumour growth: butter-based HFD (butter-HFD), which resulted in the development of the largest B16 tumours, and palm oil-based HFD (palm oil-HFD), which exhibited no obesity-associated acceleration of tumour growth.
Mice with DIO from the butter-HFD consistently developed B16 tumours that were significantly larger than those in both palm oil-HFD DIO mice and mice fed a SFD (Fig. 2a,b and Extended Data Fig. 1a). We also examined additional C57BL/6J syngeneic tumour models, including E0771 breast adenocarcinoma, LSL-Kras(G12D); Trp53fl/fl; Ad-Cre-derived lung adenocarcinoma (LGSP)22, Pan02 pancreatic ductal adenocarcinoma, Yumm1.7 melanoma and MC38 colorectal adenocarcinoma. Orthotopic E0771 and subcutaneous LGSP tumours in mice fed butter-HFD grew significantly faster than those in mice fed palm oil-HFD or SFD (Fig. 2c,d), but there were no significant differences in subcutaneous Pan02 or Yumm1.7 tumours (Extended Data Fig. 1b,c). Consistent with previous studies11,12, subcutaneous MC38 tumours grew faster in butter-HFD mice, but tumour growth in palm oil-HFD mice was not significantly different from butter-HFD or SFD mice (Extended Data Fig. 1d). This suggests that the impact of dietary fat source on tumour growth is influenced by tumour type and may not affect all tumour types to the same degree. Importantly, when mice were fed a HFD for only 1 week before tumour injection, and therefore did not develop obesity (Extended Data Fig. 1e), there were no differences in B16 tumour growth between mice fed a butter-HFD, palm oil-HFD or SFD (Extended Data Fig. 1f,g), indicating that long-term HFD feeding or development of obesity is necessary for the dietary fat source to impact tumour growth.
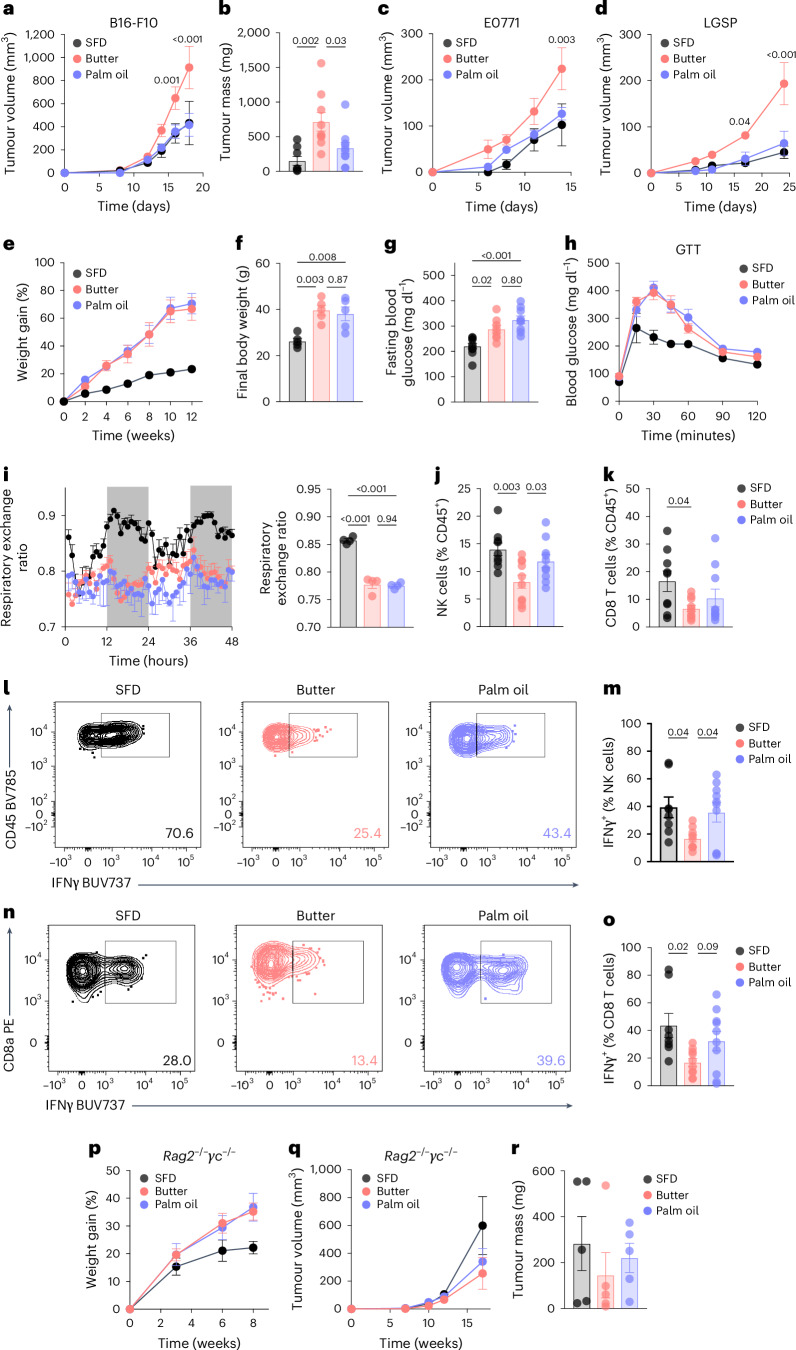
Fig. 2. Anti-tumour immunity in obesity is altered by the source of dietary fat.
a,b, Tumour volumes (a) and endpoint tumour weights (b) of B16-F10 tumours in mice fed HFD derived from butter or palm oil, or a SFD. c,d, Volumes of E0771 (c) and LGSP (d) tumours in mice fed SFD, butter or palm oil. Female mice were used for E0771 and one cohort of B16-F10. e,f, Weight gain (e) and endpoint weight (f) of male mice fed the assigned diet. g,h, Fasting blood glucose (g) and glucose tolerance test (GTT) (h) of mice fed the assigned diet for 10 weeks. i, Hourly (left) and daily average (right) respiratory exchange ratio of mice fed the assigned diet for one week. j–o, Frequency (j,k) and IFNγ expression (l–o) of tumour-infiltrating NK and CD8 T cells in B16-F10 tumours in mice fed SFD, butter or palm oil. p–r, Weight gain on the assigned diet (p), tumour volumes (q) and endpoint tumour weights (r) of B16-F10 tumours in Rag2−/−γc−/− mice. Sample sizes are as follows: in a, SFD n = 18, butter n = 18, palm oil n = 20, pooled from four independent experiments, including experiment in Fig. 1d,g; in b, SFD n = 8, butter n = 9, palm oil n = 10, pooled from two independent experiments; in c, SFD n = 4, butter n = 5, palm oil n = 5; in d, SFD n = 5, butter n = 4, palm oil n = 4; in e and f, n = 5, representative of six independent experiments; in g, SFD n = 10, butter n = 8, palm oil n = 9, combined from two independent experiments; in h, n = 5; in i, n = 4, combined from two independent experiments; in j and k, SFD n = 9, butter n = 10, palm oil n = 10, pooled from two independent experiments; in m and o, SFD n = 8, butter n = 9, palm oil n = 10, pooled from two independent experiments; in p–r, n = 5. In bar graphs, bar height represents the mean; error bars, s.e.m. In line graphs, each data point represents the mean; error bars, s.e.m. Comparisons were made using two-way repeated-measures ANOVA followed by Bonferroni’s multiple comparisons test, or one-way ANOVA followed by Sidak’s multiple comparisons test.
Extended Data Fig. 1. Additional characterization of tumour growth in mice fed butter- or palm oil-based high fat diet.
a, Individual tumour growth curves of subcutaneous B16-F10 melanoma tumours in mice fed a HFD derived from Butter, Palm Oil, or SFD for 12 weeks. b-d, Volume of subcutaneous Pan02 (b), Yumm1.7 (c), and MC38 (d) tumours in mice fed SFD, butter HFD, or palm oil HFD for 12 weeks. p = 0.04 for comparison of butter-HFD and SFD. Male mice were used for Pan02 and Yumm1.7 cohorts and female mice for MC38 cohorts. e-g, Male mice were fed a HFD derived from Butter, Palm Oil, or SFD for one week prior to subcutaneous B16-F10 tumour initiation and maintained on the assigned diet until tumour endpoint. (e) Body weight of mice after one week of butter-HFD, palm oil-HFD, or SFD feeding. Tumour volumes (f) and endpoint tumour weights (g). (a) SFD n = 18, Butter n = 18, Palm Oil n = 20, data pooled from 4 independent experiments; (b) n = 4-5; (c) n = 3-4; (d) n = 5-7; (e-g) n = 10, data pooled from two independent experiments. In bar graphs, bar height represents mean with error bars ± SEM. In line graphs, each data point represents the mean with error bars ± SEM. Comparisons were made using two-way repeated measures ANOVA followed by Bonferroni’s multiple comparisons test (b-d, f) and one-way ANOVA followed by Tukey’s (e, g) multiple comparisons test.
Differences in systemic metabolism, such as hyperglycaemia and hyperinsulinaemia, have been previously implicated in obesity-associated tumour cell growth5,25–29; therefore, we characterized the whole-body metabolic changes in DIO mice fed butter-HFD and palm oil-HFD. Mice fed butter-HFD and palm oil-HFD gained significantly more weight than mice fed SFD throughout the study (Fig. 2e), and final body weight (Fig. 2f) and mass of major adipose tissue depots (Extended Data Fig. 2a,b) were comparable between the two HFD-fed groups. There were no significant differences between any of the diets in daily calorie consumption (Extended Data Fig. 2c) or nutrient absorption based on faecal bomb calorimetry (Extended Data Fig. 2d). Compared to SFD-fed mice, mice fed both butter-HFD and palm oil-HFD developed the characteristic metabolic changes associated with obesity, including hyperglycaemia (Fig. 2g), glucose intolerance (Fig. 2h and Extended Data Fig. 2e), hyperinsulinaemia (Extended Data Fig. 2f), insulin intolerance (Extended Data Fig. 2g) and hepatic steatosis (Extended Data Fig. 2h), with no significant differences between the HFDs. A detailed metabolic assessment by indirect calorimetry revealed a similar increase in lipid metabolism compared to SFD, indicated by a decrease in respiratory exchange ratio, in mice fed butter-HFD or palm oil-HFD (Fig. 2i). There were no differences between mice fed butter-HFD and those fed palm oil-HFD in any of the measured characteristics that could impact metabolic health, including locomotor activity and energy expenditure (Extended Data Fig. 2i,j). Taken together, these results suggest that the differences in tumour growth are not a result of gross differences in systemic metabolism.
Extended Data Fig. 2. High fat diet causes obesity and impairs systemic metabolism, regardless of dietary fat source.
a-h, Male mice were fed HFD derived from Butter, Palm Oil, or SFD for 12 weeks. Weight of visceral (a) and subcutaneous (b) adipose tissue at study endpoint. (c) Average daily food consumption measured 6 weeks after diet initiation. (d) Bomb calorimetry of faeces collected after 10 weeks on diet. (e) Area under the curve of glucose tolerance test, performed 10 weeks after diet initiation. Fasting serum insulin (f) and insulin tolerance test (g) performed 10 weeks after diet initiation. (h) Representative oil red O staining (left) and summary data (right) of livers from HFD-fed mice. i, j, Mice were fed assigned diet for one week and singly housed for indirect calorimetry. Hourly (i, left) and average daily (i, right) locomotor activity. Hourly (j, left) and average daily (j, right) energy expenditure. (a, b) n = 10; (c) SFD n = 4, Butter n = 7, Palm Oil n = 7, data pooled from 2 independent experiments; (d) n = 7, (e) n = 5; (f) n = 4-5; (g, left) n = 10, combined from 2 independent experiments; (g, right) n = 5, representative of 2 independent experiments; (h) SFD n = 3, Butter n = 5, Palm Oil n = 5; (i-j) n = 4, representative of 2 independent experiments. In bar graphs, bar height represents mean with error bars ± SEM. In line graphs, each data point represents the mean with error bars ± SEM. Comparisons were made using one-way ANOVA followed by Tukey’s multiple comparisons test (a-j) or two-way repeated measures ANOVA followed by Bonferroni’s multiple comparisons test (g, left).
Dietary fat regulates tumour-infiltrating immune cells
We10,11 and others12,30 have shown that obesity impairs tumour infiltration of NK and CD8 T cells, which contributes to accelerated tumour growth in mice. Therefore, we used flow cytometry to examine the immune infiltration of B16 tumours from DIO mice fed butter-HFD or palm oil-HFD (Fig. 2j–o and Extended Data Fig. 3). In butter-HFD tumours, we observed significant changes in lymphocyte populations. Infiltration of NK cells, CD8 T cells and FoxP3+CD4+ T cells (Treg cells) was significantly reduced compared to SFD (Fig. 2j,k and Extended Data Fig. 3b), and the percentage of all CD4 T cells also trended lower (P = 0.0848; Extended Data Fig. 3c). By contrast, there were significantly more NK cells present in tumours from mice fed palm oil-HFD compared to those from butter-HFD, reaching a level intermediate between SFD and butter-HFD tumours (Fig. 2j). However, CD8 T cell, CD4 T cell and Treg cell infiltration in palm oil-HFD were not significantly different than either SFD or butter-HFD (Fig. 2k and Extended Data Fig. 3b,c).
Extended Data Fig. 3. Additional analysis of tumour-infiltrating immune cells in mice fed butter- or palm oil-based high fat diet.
a, Gating strategy used to identify tumour-infiltrating NK and CD8 T cells. b-i, Characterization of tumour-infiltrating immune cells of B16-F10 tumours in mice fed butter-HFD, palm oil-HFD, or SFD for 12 weeks. Frequency of FoxP3 + CD4+ Tregs (b), CD4 T cells (c), CD11b+ myeloid cells (d), F4/80 CD64+ tumour-associated macrophages (e), CD11c+ MHC-II+ dendritic cells (f), and CD11b + Ly6G+ neutrophil-like cells (g). (h) IFN-γ expression by tumour-infiltrating CD4 T cells. (i) GzmB expression by tumour-infiltrating CD8 T cells. (b) n = 5; (c) n = 9-10, data pooled from 2 independent experiments; (d, e, g) n = 11-14, data pooled from 3 independent experiments; (f, h, i) n = 8-10, data combined from 2 independent experiments. In bar graphs, bar height represents mean with error bars ± SEM. Comparisons were made using one-way ANOVA followed by Sidak’s (b-i) multiple comparisons test.
Among myeloid cells in the tumour microenvironment, total CD11b+ myeloid cells, CD11b+F4/80+ tumour-associated macrophages and CD11c+MHCII+ dendritic cells, which present antigen to T cells, were not different between the three diets (Extended Data Fig. 3d–f). CD11b+Ly6G+ cells, which often exert immunosuppressive effects in the tumour microenvironment, were significantly increased in butter-HFD compared to SFD but were highly variable in palm oil-HFD (Extended Data Fig. 1g). These findings suggest that dietary fat primarily regulates the frequency of lymphocytes in the tumour immune microenvironment.
To understand how lymphocyte function may differ between HFDs, we measured production of IFNγ, an essential cytokine that promotes tumour cytotoxicity and activates immune cells31, and granzyme B (GzmB), which promotes target cell apoptosis32, in tumour-infiltrating cells. Consistent with previous studies of immune function in obesity11,30, production of IFNγ was reduced in both tumour-infiltrating NK and CD8 T cells in mice fed butter-HFD (Fig. 2l–o). However, IFNγ was significantly higher in NK cells and trended higher in CD8 T cells from mice fed palm oil-HFD compared to butter-HFD (Fig. 2l–o). A similar pattern was observed in CD4 T cells, but without statistical significance (Extended Data Fig. 1k). By contrast, CD8 T cells from butter-HFD and palm oil-HFD produced similar amounts of GzmB (Extended Data Fig. 1l), indicating that palm oil-HFD protects from some, but not all, obesity-related functional defects in tumour-infiltrating lymphocytes.
We next sought to determine whether the increased tumour growth in butter-HFD was a result of these defects in tumour-infiltrating lymphocytes. To test this idea, Rag2−/−γc−/− mice, which lack both NK cells and T cells33, were fed butter-HFD or palm oil-HFD for 8 weeks and subsequently challenged with B16 tumours. Although mice fed both types of HFDs gained comparable weight throughout the study, there were no significant differences in B16 tumour growth rate or size when mice lacked NK cells and T cells (Fig. 2p–r). Taken together, these results suggest that the source of dietary fat impacts tumour growth in obesity by regulating NK and T lymphocyte subsets and indicate that immune dysfunction is a major component of tumour progression in obesity. Therefore, we next sought to uncover why palm oil-HFD protected against these obesity-related functional defects and determine the mechanisms of NK and T cell dysfunction in mice fed a butter-HFD.
Dietary fat regulates NK cell metabolism and function
We have previously described significant metabolic and functional changes in NK cells in obesity. We found that NK cells from humans with obesity accumulate intracellular lipids that interfere with cellular metabolism and impair functional responses10, and similar findings have been reported in splenic NK cells from mice fed 60% HFD34 and dysfunctional NK cells in human lymphoma35. Therefore, we examined whether these changes were present in NK cells in DIO mice fed butter-HFD and palm oil-HFD. Consistent with our previous studies10, NK cells isolated from the spleens of mice fed butter-HFD exhibited impaired Ifng expression (Extended Data Fig. 4a), whereas those from mice fed palm oil-HFD did not. Moreover, neutral lipid staining revealed accumulation of lipid droplets in NK cells in mice fed butter-HFD, but NK cells from mice fed palm oil-HFD had significantly less neutral lipid content (Fig. 3a,b), suggesting potential differences in intracellular metabolism in NK cells from mice fed butter-HFD and palm oil-HFD.
Extended Data Fig. 4. Butter- and palm oil-based high fat diets differentially regulate NK cell protein expression.
a-f, NK cells were isolated from the spleens of naïve mice fed HFD derived from Butter, Palm Oil, or SFD for 12 weeks and stimulated with IL-2, IL-12, and IL-15 for 20 hr. (a) Relative Ifng gene expression. (b) Heatmap of 50 most differentially expressed proteins detected by mass spectrometry. Abundance of NKG2D (c), ARL8B (d), STAT5B (e), and mTOR (f) detected by mass spectrometry. g, h, Representative histogram (g, left) and summary data (g, right) of LipidTOX MFI of circulating CD8 T cells from mice fed SFD (gray), butter-HFD (pink), or palm oil-HFD (blue). (a) n = 2; (b-f) SFD n = 4, Butter n = 4, Palm Oil n = 5; (g) n = 5. In bar graphs, bar height represents mean with error bars ± SEM. Comparisons were made using one-way ANOVA followed by Sidak’s multiple comparisons test.
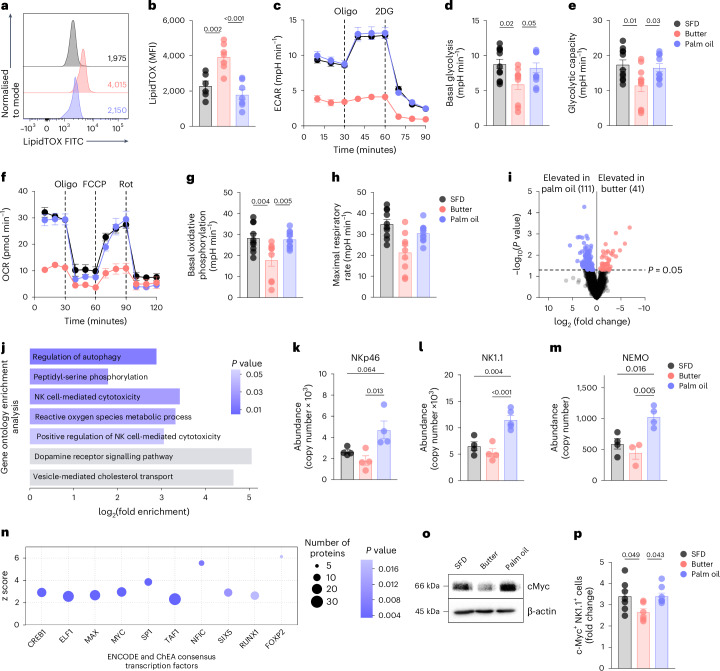
Fig. 3. Butter-HFD and palm oil-based HFD differentially regulate NK cell metabolism and function in obesity.
a,b, Representative histograms (a) and summary data (b) of LipidTOX in NK cells from mice fed HFD derived from butter (pink), palm oil (blue) or SFD (grey). MFI, mean fluorescence intensity. c–h, Seahorse real-time metabolic flux assays of splenic NK cells from mice fed SFD, butter or palm oil for 12 weeks and stimulated with IL-2, IL-12 and IL-15. Extracellular acidification rate (ECAR) (c) of purified NK cells was measured to assess basal glycolysis (d) and glycolytic capacity (e). Oxygen consumption rate (OCR) (f) of purified NK cells was measured to determine basal oxidative phosphorylation (g) and maximal respiratory rate (h). i–n, Proteomic analysis of splenic NK cells stimulated with IL-2, IL-12 and IL-15: volcano plot representing proteins differentially expressed between mice fed butter-HFD and palm oil-HFD (i); KEGG pathway analysis of proteins upregulated in NK cells from mice fed palm oil relative to mice fed butter (j); expression of NK cell activation-related proteins NKp46 (k), NK1.1 (l) and NEMO (m) and ChIP-X enrichment analysis (ChEA) of proteins significantly increased in NK cells from mice fed palm oil relative to those fed butter (n). o, Western blot analysis of c-Myc expression in NK cells pooled from five mice per diet. p, c-Myc expression in splenic NK cells stimulated with IL-2, IL-12 and IL-15 for 4 h, measured by flow cytometry. Sample sizes are as follows: in a and b, n = 7, data combined from two independent experiments; in c and f, n = 3, representative of three independent experiments; in d and e, SFD n = 11, butter n = 10, palm oil n = 11, combined from three independent experiments; in g and h, SFD n = 10, butter n = 9, palm oil n = 11, combined from three independent experiments; in i–n, SFD n = 4, butter n = 4, palm oil n = 5; in p, SFD n = 7, butter n = 6, palm oil n = 8. In bar graphs, bar height represents the mean; error bars, s.e.m. In line graphs, each data point represents the mean; error bars, s.e.m. Comparisons were made using one-way ANOVA followed by Tukey’s multiple comparisons test. Oligo, oligomycin; 2DG, 2-deoxyglucose; Rot, rotenone plus antimycin-A.
Given that lipid accumulation induces metabolic ‘paralysis’ of NK cells10 and intracellular metabolism has a critical role in NK cell activation and function36–38, we used real-time extracellular metabolic flux assays to examine the metabolic function of NK cells from mice fed butter-HFD and palm oil-HFD. We found that NK cells from butter-HFD mice exhibited a significantly impaired metabolic response to cytokine stimulation—characterized by reduced extracellular acidification rate and oxygen consumption rate, corresponding to impaired glycolysis and reduced oxidative phosphorylation—compared to SFD controls (Fig. 3c–h). This metabolic paralysis is comparable to our previous observations in NK cells from obese humans and mice10. However, palm oil-HFD NK cells exhibited significantly higher rates of glycolysis and oxidative phosphorylation in response to cytokine stimulation as well as increased glycolytic capacity and maximal respiratory rate compared to NK cells from butter-HFD mice (Fig. 3c–h), indicating that palm oil-HFD NK cells are protected from the metabolic paralysis that is typical in obesity. The increases in both basal glycolysis and glycolytic capacity are particularly important because NK cells require increased glycolytic flux to achieve full effector function37,38. This suggests that palm oil-HFD NK cells are metabolically better poised for full effector function in response to activation than butter-HFD NK cells.
To investigate the mechanism by which palm oil-HFD NK cells are protected from metabolic paralysis, we performed proteomic analysis of NK cells isolated from the spleens of mice fed butter-HFD or palm oil-HFD. Regardless of the source of fat, both HFDs significantly altered the NK cell proteome (Extended Data Fig. 4b). However, nearly 150 proteins were differentially expressed between NK cells from mice fed butter-HFD or palm oil-HFD (Fig. 3i). KEGG pathway analysis of proteomic data showed that signatures related to NK cell activation and cytotoxicity were among the most highly enriched in palm oil-HFD NK cells compared to butter-HFD, consistent with our in vivo data (Fig. 3j). Several of these proteins were also significantly higher than in SFD NK cells, including killer cell lectin-like receptor subfamily B member 1C (NK1.1), NF-κB essential modulator (NEMO), and natural cytotoxicity triggering receptor 1 (NKp46), all of which promote NK cell activation and cytotoxic function39,40 (Fig. 3k–m). NKG2D, which promotes the production of cytolytic molecules and inflammatory cytokines41, ADP-ribosylation factor-like 8b (ARL8B), a protein required for lytic granule polarization and formation of the microtubule organizing centre42 and activating transcription factor STAT5b43 also trended to increase in palm oil-HFD NK cells compared to butter-HFD, although these did not reach statistical significance (Extended Data Fig. 4c–e). Consistent with the increase in glycolysis observed in NK cells from mice fed palm oil-HFD38, mTOR protein expression also trended up in palm oil-HFD NK cells compared to butter-HFD (Extended Data Fig. 4f).
ChIP-X enrichment analysis44 of the proteomic data identified several transcription factors whose products were significantly overrepresented in palm oil-HFD NK cells compared to butter-HFD NK cells. One of the most enriched was Myc (Fig. 3n), which is critical for NK cell metabolic reprogramming in response to cytokine stimulation34,37. c-Myc regulates the expression of glycolytic genes, promotes glucose uptake and increases glycolytic flux45,46, modulates mitochondrial function and oxidative phosphorylation47 and inhibits lipid droplet accumulation48. NK cells lacking c-Myc exhibit impaired metabolism37, IFNγ and GzmB expression37,49 and decreased ability to kill tumour cells and control B16 melanoma metastasis49. Although ChIP-X enrichment analysis is not always a robust method to identify transcription factors like Myc that have well-known translation-dependent mechanisms, western blotting of NK cells isolated from mice fed butter-HFD or palm-HFD and stimulated with IL-2 and IL-12 confirmed that c-Myc protein levels were reduced in NK cells from mice fed butter-HFD and upregulated in NK cells from mice fed palm oil-HFD (Fig. 3o). A similar result was obtained by flow cytometry, in which the induction of c-Myc in response to cytokine stimulation was blunted in NK cells isolated from butter-HFD but not palm oil-HFD (Fig. 3p). Taken together, these data demonstrate that NK cells from mice fed palm oil-HFD are resistant to lipid accumulation and maintain robust metabolic and functional responses, possibly because of sustained c-Myc activity.
Butter-based HFD increases abundance of stearoyl-carnitine
We next examined whether CD8 T cells also exhibit differences in lipid accumulation between butter-HFD and palm oil-HFD. However, we found no clear differences in LipidTOX staining from mice fed a SFD or any HFD (Extended Data Fig. 4g,h), suggesting that CD8 T cell dysfunction is regulated by a different mechanism.
It has been shown that accumulation of long-chain fatty acids (LCFAs) in the tumour microenvironment leads to CD8 T cell functional impairment by suppressing intracellular metabolism30,50–52. Previous studies have focused on lipid species within the tumour interstitial fluid, whereas we compared the fatty acid constituents of butter-HFD and palm oil-HFD to identify potentially harmful lipids present in the diet. Fatty acid profiling using fatty acid methyl ester (FAME) analysis showed that butter-HFD and palm oil-HFD differed significantly in fatty acid composition. Butter-HFD comprised 63.1% saturated fatty acids while palm oil-HFD contained only 43.5% saturated fatty acids, and palm oil-HFD contained a greater proportion of both monounsaturated and polyunsaturated fatty acids (39.9% and 16.5%, respectively; Extended Data Fig. 5a). Butter-HFD contained more short-chain and medium-chain fatty acids than palm oil-HFD (Extended Data Fig. 5b), and the LCFAs myristic acid (C14:0) and stearic acid (C18:0) were also significantly higher in butter-HFD. Palm oil-HFD was enriched in the LCFAs palmitic acid (C16:0), oleic acid (C18:1) and linoleic acid (C18:2) (Fig. 4a).
Extended Data Fig. 5. Analysis of dietary fatty acids and metabolome of mice fed butter- and palm oil-based high fat diet.
a, b, Analysis of fatty acid composition of butter- and palm oil-derived HFD: (a) fatty acid saturation proportion and (b) abundance of short- and medium- chain fatty acids. c, IFN-γ expression by splenic CD8 T cells cultured with stearic acid and stimulated with anti-CD3, CD28, and IL-2 for 48 hr. d-i, Plasma metabolome of mice fed HFD derived from Butter, Palm Oil, or SFD for one week analysed by mass spectrometry. (d) PCA plot of plasma metabolome of mice fed SFD, Butter, or Palm Oil. (e) Heatmap of 50 most differentially abundant metabolites in plasma of mice fed SFD, Butter, or Palm Oil. Volcano plots comparing metabolites upregulated in Butter (f) and Palm Oil (g) mice compared to SFD. Relative abundance of myristoyl-carnitine (CAR14:0) and palmitoyl-carnitine (CAR16:0) in plasma. j, k, Relative abundance of CAR14:0 (j) and CAR16:0 (k) in serum of B16-F10 tumour-bearing mice fed HFD derived from Butter, Palm Oil, or SFD for 12 weeks. l, m, Tracing of [U-13C]-stearate administered to mice fed HFD derived from Butter, Palm Oil, or SFD for one week. (l) Relative abundance of 13C-labeled stearoyl-carnitine (CAR18:0) in liver. (m) Relative abundance of 13C-labeled TCA cycle intermediates fumarate (m, left) and malate (m, right) in liver. (b) n = 4; (c) n = 4-5; pooled from 4 independent experiments (d-i) SFD n = 5, Butter n = 5, Palm Oil n = 4; (j, k) n = 5; (l, m) n = 6-8. In bar graphs, bar height represents mean with error bars ± SEM. Comparisons were made using one-way ANOVA followed by Tukey’s multiple comparisons test.
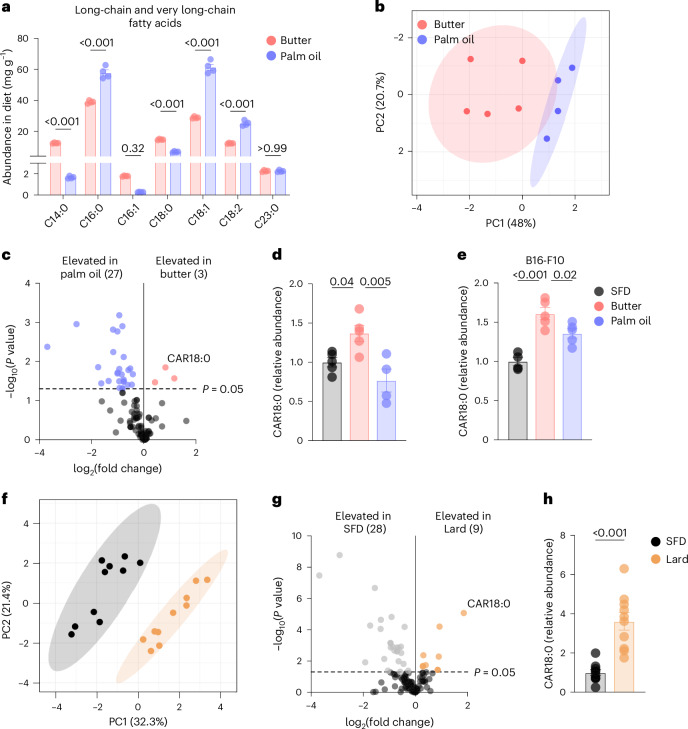
Fig. 4. Dietary fat modulates acylcarnitine levels in plasma of DIO mice.
a, Long-chain and very long-chain fatty acid composition of HFDs derived from butter and palm oil. b–d, Plasma metabolome of mice fed HFD derived from butter, palm oil or SFD for 1 week, analysed by mass spectrometry: PCA plot of plasma metabolomes of mice fed butter-HFD and palm oil-HFD (b); volcano plot of differentially expressed metabolites in mice fed butter-HFD (pink) and palm oil-HFD (blue); and relative abundance of CAR18:0 (d). e, Relative abundance of CAR18:0 in serum of B16-F10 tumour-bearing mice fed butter, palm oil or SFD for 12 weeks. f–h, Plasma metabolome of mice fed HFD based on lard (60% fat) or SFD for 8 weeks, analysed by mass spectrometry: PCA plot of plasma metabolomes of lard-fed (orange) and SFD-fed (grey) mice (f); volcano plot of metabolites differentially expressed in lard-fed (orange) and SFD-fed (grey) mice (g); and relative abundance of CAR18:0 in lard and SFD (h). Sample sizes are as follows: in a, n = 4; in b–d, SFD n = 5, butter n = 5, palm oil n = 4; in e, SFD n = 4, butter n = 5, palm oil n = 5; in f–h, n = 10. In bar graphs, bar height represents the mean; error bars, s.e.m. Comparisons were made using one-way ANOVA followed by Tukey’s multiple comparisons test or the Mann–Whitney test.
To examine whether differentially present LCFAs contributed to the CD8 T cell dysfunction observed in butter-HFD tumours, we cultured CD8 T cells with C18:0. Although IFNγ production by these cells trended lower with increasing C18:0 concentration, the changes did not reach statistical significance at concentrations that did not affect viability, suggesting that C18:0 was not the key cause of CD8 T cell dysfunction in butter-fed mice (Extended Data Fig. 5c). Therefore, we performed metabolomic analysis of the blood plasma after butter-HFD or palm oil-HFD feeding to identify more physiologically relevant metabolic mediators.
Metabolomic analysis of mice fed butter-HFD, palm oil-HFD or SFD showed that the metabolome of mice fed a HFD is distinct from SFD-fed mice, regardless of the source of fat (Extended Data Fig. 5d–g). Several metabolites were reduced in the plasma of mice fed butter-HFD and palm oil-HFD compared to SFD, including aminobenzoic acid, which is synthesised by the microbiome53, and hippurate, which is also associated with microbial degradation of dietary components54 (Extended Data Fig. 5f,g). These data demonstrate that microbiome dysbiosis, a characteristic feature of obesity and metabolic syndrome55–57, occurs irrespective of the source of dietary fat.
PCA clusters of the metabolomes of mice fed butter-HFD and palm oil-HFD were also distinct (Fig. 4b). We identified 30 differentially expressed metabolites, three of which were significantly increased in butter-HFD relative to palm oil-HFD (Fig. 4c). These included stearoyl-carnitine (CAR18:0) and myristoyl-carnitine (CAR14:0), which are long-chain acylcarnitines (LCACs) produced to facilitate fatty acid oxidation of the LCFAs C18:0 and C14:0, respectively (Fig. 4d and Extended Data Fig. 5h). Both acylcarnitine species were also significantly increased in butter-HFD relative to SFD. Interestingly, the chain length of the acylcarnitines elevated in butter-HFD mice corresponded to the LCFAs enriched in the butter-based diet itself. However, the acylcarnitines (such as palmitoyl-carnitine, CAR16:0) corresponding to those enriched in palm oil-HFD (C16:0) were not similarly elevated in the plasma of palm oil-HFD mice (Extended Data Fig. 5i). The same pattern of LCAC enrichment was observed in the serum of B16 tumour-bearing DIO mice that were fed butter-HFD or palm oil-HFD for 12 weeks: CAR18:0 and CAR14:0 were increased in butter-HFD compared to both palm oil-HFD and SFD, while CAR16:0 was not different between butter-HFD and palm oil-HFD (Fig. 4e and Extended Data Fig. 5j,k). Reanalysis of metabolomic data from mice fed a lard-based 60% HFD11 revealed that CAR18:0 was the most enriched metabolite in lard-HFD compared to SFD, suggesting that CAR18:0 may be a particularly important metabolite in DIO models based on animal-derived HFDs (Fig. 4f–h).
Plasma LCACs are known indicators of lipid metabolic flux58, and accumulation of LCACs in the plasma suggests the export of intracellular acylcarnitines owing to incomplete fatty acid oxidation59. Therefore, we compared fatty acid oxidation in the livers of mice fed butter-HFD and palm oil-HFD. Following intravenous administration, [U-13C]-stearate was taken up by the liver and oxidized (Extended Data Fig. 5l). Fractional labelling of TCA cycle intermediates fumarate and malate were significantly decreased in both butter-HFD and palm oil-HFD compared to SFD (Extended Data Fig. 5m), indicating a decrease in complete fatty acid oxidation in both HFDs60 and consistent with previous reports on incomplete fatty acid oxidation in obesity61–63. Notably, there were no significant differences in stearate metabolism to TCA intermediates between butter-HFD and palm oil-HFD livers, indicating that the differential accumulation of plasma CAR18:0 and CAR14:0 is not a result of differences in fatty acid oxidation rate between the HFDs and may instead be related to the amount of the precursor fatty acids present in the diet (Fig. 4a).
CAR18:0 inhibits CD8 T cell mitochondria and function
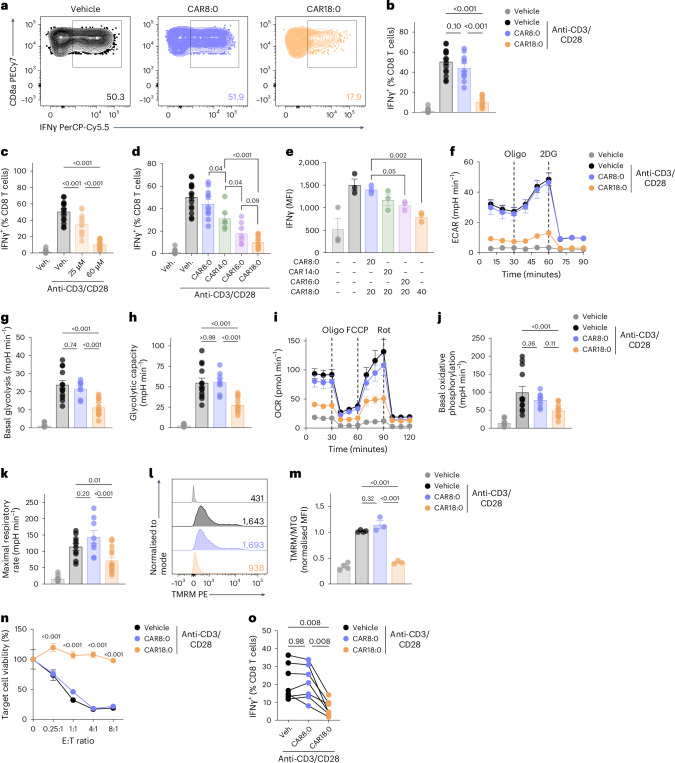
We next investigated whether CAR18:0 altered CD8 T cell function and explained the impaired IFNγ production and anti-tumour immunity observed in mice fed butter-HFD but not palm oil-HFD. Splenic CD8 T cells were cultured with CAR18:0 and stimulated with anti-CD3/CD28. After 48 h, cells cultured with CAR18:0 produced significantly less IFNγ than cells cultured with the medium-chain acylcarnitine octanoyl-carnitine (CAR8:0) or vehicle control (Fig. 5a,b), without any changes in expression of the activation marker CD69 (Extended Data Fig. 6a). This inhibition of IFNγ expression by CAR18:0 exhibited concentration dependence (Fig. 5c) without causing significant cytotoxicity (Extended Data Fig. 6b,c). When a range of acylcarnitines were tested, IFNγ production was increasingly impaired with increasing acylcarnitine chain length, as CD8 T cells treated with CAR14:0 expressed less IFNγ than those treated with CAR8:0, and treatment with CAR16:0 or CAR18:0 further decreased IFNγ expression (Fig. 5d). Treatment with multiple acylcarnitines at once resulted in an additive impact on IFNγ expression (Fig. 5e). Notably, acylcarnitine treatment had no significant impact on NK cell IFNγ production or c-Myc expression in response to cytokine stimulation (Extended Data Fig. 6d,e), further supporting the idea that NK and CD8 T cell dysfunction in obesity are regulated by distinct mechanisms.
Fig. 5. LCACs impair CD8 T cell metabolism and function.
a–m, Analysis of splenocytes cultured with the specified LCACs or vehicle and stimulated with anti-CD3, anti-CD28 and IL-2 for 48 h. a,b, IFNγ expression by CD8 T cells treated with 60 µM CAR8:0 or CAR18:0. c, IFNγ expression by CD8 T cells cultured in 25 µM or 60 µM CAR18:0. d, IFNγ expression by CD8 T cells treated with 60 µM CAR8:0, CAR14:0, CAR16:0 or CAR18:0. e, MFI of IFNγ in CD8 T cells cultured in combinations of LCAC (20 µM) or 40 µM CAR18:0 alone. f–k, Seahorse real-time metabolic flux assays of purified CD8 T cells cultured with 60 µM CAR8:0 or CAR18:0: ECAR (f), measured basal glycolysis (g) and glycolytic capacity (h); OCR (i), measured basal oxidative phosphorylation (j) and maximal respiratory rate (k). l,m, TMRM staining of CD8 T cells treated with 60 µM CAR8:0 or CAR18:0; summary data normalized to mitochondrial mass by MitoTracker Green (MTG) MFI. n, Viability of B16-OVA cells after co-culture with OT-I CD8 T cells pre-treated with 60 µM CAR8:0, CAR18:0 or vehicle. o, IFNγ expression in human CD8 T cells treated with 30 µM CAR8:0, CAR18:0 or vehicle and stimulated with anti-CD3, anti-CD28 and IL-2. Sample sizes are as follows: in a–d, Veh. n = 15, Veh. + Stim. n = 15, CAR8:0 n = 11, CAR14:0 n = 6, CAR16:0 n = 7, CAR18:0 n = 13, combined from three independent experiments; In e, n = 3; in f and i, n = 2–4, representative of three independent experiments; in g, h, j and k, n = 8–14, combined from three independent experiments; in l and m, n = 3–4, combined from three independent experiments; in n, n = 5; in o, n = 7 donors, four independent experiments. In bar graphs, bar height represents the mean; error bars, s.e.m. In line graphs, each data point represents the mean; error bars, s.e.m. Comparisons were made using one-way ANOVA followed by Sidak’s multiple comparisons test, two-way repeated-measures ANOVA followed by Bonferroni’s multiple comparisons test or repeated-measures one-way ANOVA followed by Tukey’s multiple comparisons test.
Extended Data Fig. 6. Impact of acylcarnitines on immune cell function.
Splenocytes from naïve mice were cultured with the specified acylcarnitines or vehicle and stimulated with anti-CD3, anti-CD28, and IL-2 for 48 hr. (a) CD69 expression on CD8 T cells cultured in 60 µM CAR8:0, CAR18:0, or vehicle. Viability of lymphocytes cultured with CAR8:0 (b), CAR18:0 (c), or vehicle. d, e, Splenocytes were cultured with the specified acylcarnitines or vehicle and stimulated with IL-2, IL-12, and IL-15. (d) IFN-γ expression by NK cells cultured in 60 µM CAR8:0, CAR18:0, or vehicle for 20 hr. (e) c-Myc expression by NK cells cultured in 60 µM CAR8:0, CAR18:0, or vehicle for 4 hr. f-h, Splenocytes were cultured with the specified acylcarnitines or vehicle and stimulated with anti-CD3, anti-CD28, and IL-2 for 48 hr. (f) MitoTracker Green (MTG) MFI of CD8 T cells cultured with 60 µM CAR8:0, CAR18:0, or vehicle, normalised to correct for intra-assay variability. Representative histogram (g) and summary data (h) of MitoSOX staining for mitochondrial reactive oxygen species in CD8 T cells cultured with 60 µM CAR8:0 (blue), CAR18:0 (orange), or vehicle (dark gray) and stimulated with anti-CD3, anti-CD28, and IL-2, or vehicle alone (light gray). i, GzmB expression by CD8 T cells treated with 30 µM CAR8:0, CAR18:0, or vehicle and stimulated with anti-CD3, CD28, and IL-2 for 48 hr. (a) n = 6; (b, c) n = 14-20, data combined from 4 independent experiments; (d) n = 5-6, data pooled from 3 independent experiments; (e) n = 8, data pooled from 2 independent experiments; (f) n = 3-4; (g,h) n = 5-6; data pooled from 4 independent experiments; (i) n = 7 human donors, 4 independent experiments. In bar graphs, bar height represents mean with error bars ± SEM. Comparisons were made using one-way ANOVA followed by Tukey’s multiple comparisons test (a-f, h) or repeated measures one-way ANOVA followed by Tukey’s multiple comparisons test (i).
Given that CD8 T cell IFNγ production is intrinsically linked to cellular metabolism64, we next examined the effects of CAR18:0 on cell metabolism and mitochondrial energetics. CAR8:0 had no impact on CD8 T cell metabolism, while CAR18:0 significantly reduced the rates of glycolysis and oxidative phosphorylation compared to CAR8:0 and vehicle control (Fig. 5f–k), mirroring the functional effects of CAR8:0 and CAR18:0. Although there were no differences in mitochondrial mass (Extended Data Fig. 6f), CAR18:0 caused significant mitochondrial depolarization, as measured by the mitochondrial membrane potential-sensitive dye tetramethylrhodamine (TMRM) (Fig. 5l,m). Low mitochondrial membrane potential has been shown to impair IFNγ production and promote expression of the exhaustion markers PD-1 and LAG3 in tumour-infiltrating CD8 T cells65. CAR18:0 treatment also induced a modest increase in mitochondrial reactive oxygen species compared to vehicle control (Extended Data Fig. 6g,h), an indicator of mitochondrial damage that can lead to T cell exhaustion and decreased IFNγ production64. Functionally, CAR18:0 treatment of tumour-specific OT-I CD8 T cells significantly inhibited killing of B16-OVA tumour cells in a co-culture assay (Fig. 5n), demonstrating that the impaired mitochondrial function and IFNγ expression caused by CAR18:0 blunts CD8 T cell anti-tumour immunity.
These findings with CAR18:0 may be translatable to human obesity and type 2 diabetes, which have been shown to increase concentrations of LCACs, including CAR18:0 (refs. 58,66). When human CD8 T cells were treated with acylcarnitines and stimulated by anti-CD3/CD28, we found that CAR18:0 inhibited production of IFNγ and GzmB compared to CAR8:0 and vehicle control (Fig. 5o and Extended Data Fig. 6i). Together, these data show that the LCACs, particularly CAR18:0, present in the serum of mice fed butter-HFD but not palm oil-HFD induce mitochondrial dysfunction and impaired IFNγ and GzmB production in CD8 T cells, leading to impaired anti-tumour immunity.
Discussion
Obesity is an established risk factor for cancer development, owing to a combination of sequelae that promote tumour proliferation and metastasis and impair anti-tumour activity by cytotoxic lymphocytes2. Using DIO models, typically established with a HFD based on lard, we and others have reported that obesity promotes tumour growth by disrupting NK and CD8 T cell bioenergetics, which precludes an effective anti-tumour immune response6–8. Here, our findings demonstrate that tumour growth and cytotoxic immune cell functions in obesity are also dependent on the source of dietary fat. In particular, we have shown that butter-based HFD accelerates tumour growth and impairs intra-tumoral NK and CD8 T cell function in a manner similar to the lard-based HFDs published previously. By contrast, anti-tumour immune functions are preserved in mice fed palm oil-HFDs, owing to reduced lipid accumulation in NK cells and the lack of a potent lipid inhibitor of CD8 T cell function, resulting in protection against obesity-associated tumour growth.
In this study, the mechanism of NK cell metabolic and functional impairment was consistent with our previous findings10. NK cells from mice fed a butter-HFD accumulated intracellular lipids, which impaired their metabolism and decreased production of IFNγ. Intriguingly, we found that in mice fed palm oil-HFD, NK cells were protected from this lipid accumulation and metabolic paralysis. This is possibly caused by increased expression of c-Myc, which is essential for metabolic reprogramming and for maintaining anti-tumour functions of NK cells in response to stimulation. This result is consistent with previous studies demonstrating that c-Myc is increased by particular fatty acids67,68, including C18:1 (refs. 69,70), which is enriched in palm oil-HFD. These results suggest that NK cells are sensitive to lipids in the circulation or tumour microenvironment but may be protected from effector dysfunction through activation of key metabolic regulators. Diets rich in fats present in palm oil may confer metabolic protection in NK cells by activating c-Myc to maintain efficient cytotoxicity, thus enabling optimal anti-tumour responses.
There were no differences in total intracellular lipid accumulation in CD8 T cells from mice fed butter-HFD or palm oil-HFD, so we turned to metabolomics to understand the differential impact the diets had on CD8 T cell function. Diet composition controls the abundance of nutrients and metabolites in the blood, which can, in turn, indicate disease risk in lean and obese individuals14,17,71. Indeed, a recent study found that the plasma metabolome is a better indicator of metabolic health than body mass index and can predict an individual’s risk of cardiac events or developing insulin resistance much better than body weight71. Additionally, evidence is now emerging that metabolic markers and plasma metabolome signatures may indicate those at higher risk of developing certain cancers, including breast cancer72. In this study, we have shown that DIO established with a HFD based on butter or lard is associated with increased LCACs in the plasma, particularly CAR18:0. We also demonstrate that this is a result of obesity alone but is dependent on the source of dietary fat, as obese mice fed a palm oil-based HFD exhibited no elevation of CAR18:0. CAR18:0 is primarily derived from C18:0, which is enriched in butter compared to palm oil, supporting the idea that diet composition directly dictates certain metabolite abundance in the plasma. This result is consistent with multiple studies examining the impact of diet composition on the metabolome in humans, which have shown increased CAR18:0 in the plasma of people who consume red meat73–75, particularly when compared to a plant-based diet, in a randomized controlled trial73.
Our finding that CAR18:0 has a negative impact on CD8 T cell anti-tumour function is consistent with previous studies of hepatocellular carcinoma, in which CAR18:0 is significantly increased76,77. A recent publication77 showed that LCACs, including CAR18:0, impair lipid metabolism and promote senescence of invariant NK T cells in hepatocellular carcinoma, contributing to disease progression. LCACs have also been associated with increased risk of type 2 diabetes78 and cardiovascular disease79, increased mortality in patients with heart failure80 and are consistently increased in obesity81,82. Notably, it has been shown that elevated CAR18:0 and obesity combine to have an additive impact on disease risk79.
Obesity is associated with an immune paradox of both increased inflammation, which promotes metabolic dysfunction, and immunosuppression, which increases the risk of cancer and infection. Acylcarnitines have been shown to promote sterile inflammation in obesity by enhancing IL-17F production by TH17 cells83 and pro-inflammatory signalling pathways, including NF-κB, in multiple cell types84. Here, we show that LCACs, particularly CAR18:0, also suppress CD8 T cell anti-tumour responses, resulting in impaired mitochondrial function and reduced IFNγ production in vitro, and may have consequences for CD8 T cell responses in other contexts, including infection and immunization. These diverging immunomodulatory properties of acylcarnitines may partially explain the immune paradox observed in obesity.
Recent work has shown that diet composition has an important role in human immunity24. Our results demonstrate that some dietary fats are more harmful to anti-tumour immunity and promote tumour growth in obesity. Interestingly, a recent paper examining cancer incidence in the animal kingdom demonstrated that cancer mortality is associated with diet composition, and that carnivorous animals had the highest cancer-related mortality among the species studied85. This is consistent with our work, in which animal-derived HFDs, including lard, beef tallow and butter, accelerated tumour growth compared to several plant-derived HFDs, including palm oil, coconut oil and olive oil. Moreover, recent reports demonstrate that plant-fat-based diets prolong the survival of patients with pancreatic cancer more so than diets rich in animal fats22. Modifying the source of dietary fat, and thus the related metabolite milieu, may be an easy and affordable way to promote an effective anti-tumour immune response in some obesity-related cancers, potentially without the requirement for weight loss. Precision nutrition approaches that leverage dietary substitutions to reconfigure cell metabolism alongside traditional cancer therapies could prove beneficial for patients with cancer, given the multi-factorial effects of diet on the immune system.
Our study provides an important proof of principle that dietary fat can regulate immune function in obesity-related cancers, a significant conceptual development in the field of immuno-oncology. However, some limitations must be considered when interpreting this work.
The HFDs used in this study were derived from single fat sources, which does not reflect the diversity of the typical human diet. Therefore, we cannot draw conclusions about how changes in human consumption of a particular fat, such as palm oil, will impact immune function or tumour growth. Also, we emphasize that the impact of HFD was not the same for all tumour types tested, and the diets used were not exhaustive. Therefore, it remains unknown whether other plant-derived and animal-derived diets cause changes in the immune system and tumour growth comparable to those observed in this study.
Additionally, although we have identified CAR18:0 as an immune-modulating metabolite enriched in butter-based HFD, we were unable to precisely determine the specific dietary component or metabolic process responsible for its enrichment in mice fed a butter-HFD or demonstrate that it was solely responsible for the dysfunction of CD8 T cells in vivo. Indeed, based on our study and work done by others12,71,86, it is likely that many metabolic factors are regulated in concert by obesity and diet, and these may have combinatorial effects on immune function and tumour growth.
There are also some technical limitations to our study. For example, although our tumour study in Rag2−/−γc−/− mice, which lack both T cells and NK cells, demonstrates that loss of these cytotoxic lymphocytes prevents the diet-induced differences in tumour growth, it does not determine the precise contributions of each cell subset to the phenotype. Additional studies focused on each cell type individually would be required to determine how much the observed dysfunction in NK or CD8 T cells contributes to accelerated tumour growth. In addition, the C57BL/6J mice used for several of our in vivo studies of immune function harbour a spontaneous mutation within the nicotinamide nucleotide transhydrogenase (Nnt) gene, which encodes an enzyme that regulates mitochondrial redox homoeostasis. Although we do not believe the NNT mutation in these mice accounts for the phenotypes observed in this study, it may impact the translational relevance of the changes in mitochondrial function we observed.
With these caveats, our study clearly demonstrates that diet has a role in determining anti-tumour immunity in obesity. Future investigation must focus on how modulation of diet in humans alters the metabolic milieu, immune function and tumour outcomes to evaluate the potential for diet-based interventions to support successful cancer treatment.
Methods
Ethics statement
All experiments were performed with ethical approval from the Trinity College Dublin University Ethics Committee, the Animal Research Ethics Committee from the Health Products Regulatory Authority (HPRA) and the Institutional Animal Care and Use Committee (IACUC) of Brigham and Women’s Hospital.
Animals
C57BL/6J mice were bred in-house or purchased from the Jackson Laboratory. C;129S4-Rag2tm1.1FlvIl2rgtm1.1Flv/J (Rag2−/−γc−/−, strain no. 014593) and C57BL/6-Tg(TcraTcrb)1100Mjb/J (OT-I, strain no. 003831) mice were purchased from the Jackson Laboratory. Mice were maintained in accordance with Irish and European Union regulations and the recommendations of the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences). Specifically, mice were housed in specific-pathogen-free conditions with a 12 h light–dark cycle. Temperature was maintained at 20–25 °C and humidity at 40–60%; food and water were provided ad libitum.
The mice (6–8 weeks old) were fed a HFD (45% calories from fat; Research Diets) based on different fat sources (see Supplementary Tables 1 and 2) or a SFD (13% calories from fat; PicoLab Rodent Diet 20, LabDiet) for 1–12 weeks, as specified in figure legends. Within each experimental cohort, age-matched and sex-matched littermates were used, as specified in the procedures below and figure legends.
Tumour models
After 10 weeks of HFD or SFD feeding, mice were anaesthetized and injected on the right flank with 2 × 105 (B16-F10, Yumm1.7) or 5 × 105 (LGSP, Pan02, MC38) cells or in the mammary gland with 1 × 106 E0771 cells suspended in PBS. For B16-F10 tumours, cohorts of both male and female mice were used; male mice were used for LGSP, Pan02 and Yumm1.7 tumours and female mice were used for E0771 and MC38 tumours. HFD or SFD feeding continued until the study endpoint. Once tumours were palpable, tumour size was measured with callipers every 2–3 days, and tumour volume was calculated using the formula for ellipsoid volume (0.5 × D × d2, where D is the long diameter and d is the short diameter). Mice were killed at humane endpoints, typically 14–18 days post injection. The maximal tumour burden permitted by the IACUC of Brigham and Women’s Hospital (2,000 mm3 or 20 mm diameter in any direction) and the HPRA (15 mm diameter in any direction) was not exceeded in this study.
Male mice were preferred for in vivo experiments because male C57BL/6J mice more consistently develop metabolic disease on 45% HFD. No sex-based differences were detected, so data are reported in aggregate.
Tumour-infiltrating leucocyte isolation and stimulation
At humane endpoints, tumours were excised and mechanically dissociated, then digested in RPMI 1640 containing collagenase D (1 mg ml−1, Roche) and DNAse I (20 U ml−1, Sigma-Aldrich) for 1 h at 37 °C with gentle shaking. Tumour solutions were vortexed for 60 s before digestion, 30 min into digestion and at the end of the digestion period. Cells were filtered through 100 µm nylon mesh, and erythrocytes were lysed by resuspending the cells in RBC lysis buffer (BioLegend) for 2 min. Cell were then plated in RPMI 1640 supplemented with 10% FBS, 1% penicillin–streptomycin and 1% l-glutamine (complete RPMI) and treated with Cell Activation Cocktail (BioLegend) containing phorbol 12-myristate-13-acetate (10 ng ml−1) and ionomycin (1 µg ml−1) and brefeldin A (5 µg ml−1, Sigma-Aldrich) for 4 h at 37 °C before staining for flow cytometry.
Metabolic phenotyping
Fresh faecal specimens were collected from mice fed butter-HFD, palm oil-HFD or SFD for 10 weeks and stored at −80 °C. Specimens were dried at 60 °C for 48 h to remove water, and then bomb calorimetry was performed using a Parr 6725EA Semimicro Calorimeter and a 1109 Oxygen Bomb.
Fasting blood glucose concentration was measured by tail vein in mice fasted for 6 h using an AlphaTrak2 glucose meter. For glucose tolerance testing, glucose (2 g kg−1) was injected intraperitoneally, and blood glucose concentration was measured 10, 20, 45, 60, 90 and 120 min after injection.
Blood insulin concentration was measured in blood collected by cardiac puncture from mice fasted overnight, using an insulin ELISA kit (CrystalChem). For insulin tolerance testing, insulin (0.75 U kg−1) was injected intraperitoneally, and blood glucose concentration was measured 15, 30, 60, 90 and 120 min after injection.
To estimate lipid accumulation in the liver, frozen liver tissue was sectioned to 5 µm, fixed in 10% formalin and stained with oil red O solution. Stained tissue was imaged using an Olympus BX51 upright microscope, and images were analysed using ImageJ software, as previously described87.
Promethion rodent metabolic cages (Sable Systems International) for indirect calorimetry were used to assess the systemic metabolism and behaviour of mice. Mice were singly housed for 48 h, and O2 consumption, CO2 production, body weight, food and water intake and locomotor activity were monitored throughout. Data were analysed using the CalR online software (v.1.3; https://calrapp.org).
Flow cytometry
Single-cell suspensions were washed in 1 ml PBS and incubated with the fixable viability stain Zombie Aqua or NIR (BioLegend, diluted 1:1,000) and TruStain FcX anti-mouse CD16/32 antibody (BioLegend, diluted 1:200) for 20 min at room temperature (18–23 °C). For surface staining, cells were treated with a fluorochrome-labelled antibody cocktail in FACs buffer (2% FBS, 1 mM EDTA and 0.1% sodium azide in PBS) for 30 min at 4 °C. For intracellular staining, cells were fixed with IC fixation buffer (Fisher Scientific), permeabilized and incubated in the dark with a fluorochrome-labelled antibody cocktail in 1× permeabilization buffer (Fisher Scientific) for 30 min at room temperature. All antibodies used for flow cytometry and working dilutions are listed in Supplementary Table 3.
For analysis of total lipid levels, cells were stained with LipidTOX Green Neutral Lipid Stain (Fisher Scientific, diluted 1:1,000) in PBS for 30 min at 4 °C. For quantification of mitochondrial mass and membrane potential, cells were co-stained with MitoTracker Green FM (1 µM) and TMRM (100 nM) dyes (Invitrogen) in warm RPMI 1640 for 30 min at 37 °C. To quantify the production of mitochondrial reactive oxygen species, cells were stained with MitoSOX dye (Invitrogen) in warm RPMI 1640 for 15 min at 37°C. Cells stained with TMRM, MitoTracker Green or MitoSOX were not fixed before analysis by flow cytometry. Flow cytometry was performed using FACS Canto or LSRFortessa X-20 (BD Biosciences) and BD FACSDiva software (v.9.0). Data were analysed with FlowJo software (v.10.9).
Mouse NK and CD8 T cell isolation and stimulation
Murine spleens were removed from male or female mice, and splenocytes were isolated by gentle mashing through 70 µm filters into complete RPMI. Erythrocytes were lysed by resuspending the cells in RBC lysis buffer (BioLegend) for 2 min. NK and CD8 T cells were purified by immunomagnetic selection using the EasySep Mouse NK Cell Isolation Kit (Stem Cell) and the EasySep Mouse CD8a Positive Selection Kit 2 (Stem Cell), respectively. NK cells were resuspended and plated in complete RPMI at a concentration of 2 × 106 cells per ml and activated with IL-2 (10 ng ml−1), IL-12 (20 ng ml−1) and IL-15 (5 ng ml−1) for 20 h. CD8 T cells were plated in complete RPMI at a concentration of 1 × 106 cells per ml and cultured with plate-bound anti-CD3 (1 µg ml−1), soluble anti-CD28 (3 µg ml−1) and IL-2 (5 ng ml−1) for 48 h.
Seahorse metabolic flux analysis
A Seahorse XFe-96 Analyzer was used to measure real-time metabolic flux of purified NK or CD8 T cells. Following stimulation, cells were collected, and 0.2 × 106 cells in XF RPMI Seahorse medium were added to the wells of a CellTak (Corning)-coated 96-well XF96 cell culture microplate (Agilent Technologies). Real-time measurements of extracellular acidification and oxygen consumption rates were acquired following the addition of the inhibitors oligomycin (2 µM), FCCP (0.5 µM), rotenone (100 nM) plus antimycin-A (4 µM) and 2-deoxyglucose (30 mM). Basal glycolysis, glycolytic capacity, basal mitochondrial respiration and maximal mitochondrial respiration were calculated according to Agilent instructions.
Proteomic analysis
Following stimulation with IL-2 (10 ng ml−1), IL-12 (20 ng ml−1) and IL-15 (5 ng ml−1) for 20 h, live NK cells were collected by FACS, snap-frozen and stored at −80 °C before sample preparation for proteomic analysis. Sample preparation was done using the SP3 method88. Cell pellets were lysed in 400 µl SDS lysis buffer (4% SDS, 10 mM TCEP and 50 mM TEAB), with samples processed as previously described89. Samples were digested overnight at 37 °C with LysC in a 1:100 ratio of LysC to protein, then digestion was repeated with trypsin in a 1:100 ratio of trypsin to protein. Peptide clean-up was performed as previously described89.
For fractionation, samples were adjusted to 5% formic acid and separated into 16 fractions using a high pH reverse phase method on an UltiMate 3000 HPLC system on a 2.1 × 150 mm XBridge Peptide BEH C18 column with 3.5 µm particles (Waters). Samples were separated over a 25 min elution gradient between buffer A (10 mM ammonium formate, 2% acetonitrile, pH 9.0) and buffer B (10 mM ammonium formate, 80% acetonitrile, pH 9.0) with a flow rate of 0.3 ml min−1 as follows: 0 min, 10% B; 11 min, 50% B; 12–17 min, 100% B; 18–25 min, 10% B. Fractions were combined to form eight fractions and dried down in a GeneVac EZ-2 evaporator.
Mass spectrometry analysis was performed by the Fingerprints Proteomics Facility, University of Dundee. Samples were resuspended in 5% formic acid. Every eighth sample was processed with the HiPPR Detergent Removal Column kit (Thermo Scientific) before analysis. For analysis, 15 µl of each sample was injected onto an UltiMate 3000 RSLCnano system (Thermo Scientific) HPLC system. Peptides were trapped on an Acclaim PepMap 100 column (C18, 100 µm × 2 cm) with 0.1% TFA loading buffer and separated on an Easy-Spray PepMap RSLC C18 column (75 µm × 50 cm, Thermo Scientific). The following buffers were used during separation: buffer A (0.1% formic acid) and buffer B (0.1% formic acid, 80% acetonitrile), at a flow rate of 0.3 µl min−1. The gradient was as follows: 0–5 min, 2% B; 5–130 min, 5–35% B; 130–132 min, 35–98% B; 132–152 min, 98% B; 153–170 min, 2% B. Following separation, peptides were electrosprayed by an Easy-Spray source set at 50 °C and source voltage 2.0 kV to a Q-Exactive Plus Mass Spectrometer (Thermo Scientific), which was operated in positive ion mode. Data were acquired in a data-dependent acquisition mode using a top15 method with a 150 min runtime. The mass scan range was set to 350–1,600 m/z, and resolution was set to 70,000.
Raw data files were searched using MaxQuant (v.1.6.2.6). Files were searched against the reviewed mouse proteome database (downloaded August 2017) and the MaxQuant contaminant file. A reverse library was used to identify false matches, and the peptide and protein FDR was set to 1%. Minimum peptide length was set to six. Cysteine carbamidomethylation was included as a fixed modification, and methionine oxidation, protein amino-terminal acetylation, deamination of asparagine and glutamine and N-terminal conversion of glutamine to pyro-glutamic acid were included as variable modifications. Match between runs was deselected. The proteingroups.txt output file was loaded into the Perseus programme, and reverse hits, potential contaminants and proteins identified by site were removed before the calculation of estimated protein copy numbers and cell weights using the proteomic ruler function90. Differentially expressed proteins were identified using Perseus. Gene ontology and transcription factor enrichment analyses were performed using Enrichr91.
Western blotting
After stimulation with IL-2 (10 ng ml−1), IL-12 (20 ng ml−1) and IL-15 (5 ng ml−1) for 20 h, 10 × 106 splenic NK cells were lysed in 1 ml sample loading buffer containing 20 mM dithiothreitol and phosphatase and protease inhibitors (Sigma-Aldrich). Cell lysates were sonicated for 30 s to shear DNA and boiled at 99 °C for 5 min to denature proteins. Proteins were resolved on 10% polyacrylamide gels and transferred onto PVDF membranes (Merck) in transfer buffer containing 10% methanol. Membranes were blocked in 5% milk in 1× Tris-buffered saline (TBST) for 1 h at room temperature with gentle rocking and then incubated with c-Myc or β-actin primary antibodies (diluted 1:1,000 and 1:10,000, respectively, in 5% BSA in 1× TBST) at 4 °C overnight (Cell Signaling Technologies). Membranes were washed and then incubated with HRP-conjugated secondary antibodies (Sigma-Aldrich) diluted 1:1,000 in 5% BSA in 1× TBST for 1 h at room temperature. Membranes were then incubated in ECL substrate (Merck) for 2 min and visualized with a Bio-Rad Gel Doc imaging system. Western blot antibodies used, including dilutions, are listed in Supplementary Table 4.
Fatty acid analysis
FAMEs were prepared using the MARS 6 express 40-position microwave reaction system and quantified by gas chromatography as previously described92. In brief, diet pellets were crushed into a fine powder, and 0.5 g of sample was added to an Xpress vessel tube containing a 10 mm stir bar. Next, 10 ml of 2.5% KOH in methanol and 100 µl internal standard (ISTD) (10 mg ml−1 C23:0–methyl ester in heptane) were added to each tube. Samples were loaded into the microwave reaction system for saponification. The reaction vessels were heated to 130 °C over 4 min and held at 130 °C for an additional 4 min. Samples were then removed and cooled on ice for 5 min; 15 ml of 5% acetyl chloride in methanol was then added to each tube. Samples were loaded into the microwave reaction system for esterification. The reaction vessels were heated to 120 °C over 4 min and held at 120 °C for a further 2 min. Samples were removed and cooled on ice. To extract FAMEs, 10 ml of pentane was added to each reaction vessel and gently mixed. A total of 20 ml of saturated NaCl solution in dH2O was added to each tube and gently mixed, allowing the aqueous (bottom) and organic (top, pentane) layers to separate. Finally, 1 ml of the top, pentane layer was transferred into a gas chromatography vial containing 0.2 g of anhydrous Na2SO4.
Quantification of FAMEs was performed using a PerkinElmer Clarus 580 gas chromatograph fitted with a flame ionization detector. Analytes were separated using a CP-Sil 88 capillary with a 100 m × 0.25 mm internal diameter × 0.2 µm film thickness column. The oven was heated to 220 °C from 80 °C at 6.2 °C min−1, held for 3.2 min and heated to 240 °C at 6.3 °C min−1, where it was held for 6.5 min. The injector temperature was 270 °C, and 0.5 µl of sample was injected with the split set to 10:1. The carrier gas used was hydrogen with a constant flow rate of 1.25 ml min−1. The temperature of the flame ionization detector was maintained at 270 °C. Compounds were identified by comparing their retention times with FAME Supelco standards. Peak area analysis was conducted using TotalChrom 6.3.2 software. The content of each fatty acid was calculated according to the following equation: content (mg g−1 diet pellet) = (peak area (FAME) / peak area (ISTD)) × (weight ISTD / weight sample) × (ISTD purity) × 10.
Metabolomic analysis
For analysis of the metabolome of non-tumour-bearing mice, blood plasma was collected in lithium heparin-lined microtainer tubes and centrifuged at 16,000g for 10 min. As previously described93, metabolites were extracted from plasma by adding cold extraction buffer (80% methanol containing inosine-15N4, thymine-d4 and glycocholate-d4; Cambridge Isotope Laboratories) in a 1:4 ratio. Samples were then centrifuged twice at 16,000g for 5 min at 4 °C, and supernatants were collected.
Metabolite extracts were subjected to liquid chromatography–mass spectrometry using a Luna-HILIC column (Phenomenex), an UltiMate 3000 TPLRS liquid chromatography system and a Q-Exactive HF-X mass spectrometer (Thermo) as previously described93. Targeted processing of a subset of known metabolites was conducted using TraceFinder software (v.4.1; Thermo Fisher Scientific). Compound identities were confirmed using reference standards. Metabolite abundance was normalized using internal standards, and relative changes were assessed by comparison with metabolites extracted from the same sample type. Metabolomics data were analysed using MetaboAnalyst 5.0 software94, and PCA plots generated with ggplot2.
For the analysis of the metabolome of mice with B16-F10 tumours, serum was collected in SST Microtainer Blood Collection Tubes (BD) and centrifuged at >6,000g for 2 min. Samples were analysed as previously described95 with a Kinetex evo C18 column (2.6 μm, 150 mm × 2.0 mm internal diameter; Phenomenex) coupled to an Agilent 6546 LC/Q-TOF system with an ESI source operated in positive mode. The identity of the metabolite was confirmed by matching retention time and tandem mass spectrometry fragmentation data to standard compounds and/or a database.
13Carbon stearate tracing
[U-13C]-stearate (10 mg kg−1, Cambridge Isotope Laboratories) was conjugated to BSA and administered as a bolus by tail vein injection. Mice were housed individually for 1 min, and then liver tissue was dissected and snap-frozen in liquid nitrogen and stored at −80 °C. Samples were prepared as above, and liquid chromatography–mass spectrometry analysis was performed as previously described93.
Lipid preparation for cell culture
Unconjugated stearic acid (Cayman Chemical) was resuspended in ethanol (100 mM). Working stock was prepared in glass vials as previously described10: stearic acid was dissolved in complete RPMI with 0.5 mM l-carnitine and 3% fatty-acid-free BSA and then heated and sonicated for 2 min using a water bath sonicator. Acylcarnitines (Sigma-Aldrich) were resuspended in methanol (50–200 mM) and diluted in complete RPMI.
Cytotoxicity assay
B16-OVA cells were plated at 7,000 cells per well in a 96-well plate in DMEM supplemented with 10% FBS and 1% penicillin–streptomycin and rested overnight. Purified and activated CD8 T cells were plated in complete RPMI with IL-2 (5 ng ml−1) in the presence of 60 μM CAR(8:0) or 60 μM CAR(18:0) for 48 h with a range of B16-OVA cancer cell to effector cell ratios. The medium and non-adherent cells were then removed, and the wells were carefully washed with 100 μl PBS. MTT was prepared at 4.5 ng ml−1 in PBS, and 100 μl of MTT mix was added to each well. Plates were incubated at 37 °C in a non-CO2 incubator for 4–6 h. Then, 100–150 μl of acidified isopropanol was used to dissolve MTT crystals post incubation. Absorbance was measured at 560 nM, and the proportion of live cells was calculated according to the formula: absorbance560nm of sample − absorbance560nm of blank (MTT only, no cells) / absorbance560nm of control (no T cells).
Human PBMC isolation and stimulation
Peripheral blood mononuclear cells were collected from healthy blood donors attending the Kraft Family Blood Donor Center at Brigham and Women’s Hospital as previously described10. Cells were cultured in complete RPMI and stimulated with anti-CD3 (1 µg ml−1), CD28 (3 µg ml−1) and IL-2 (100 ng ml−1) for 48 h.
Data collection and statistical analyses
No statistical methods were used to pre-determine sample sizes, but our sample sizes are similar to those reported in previous publications11,12. All experimental groups for animal studies were age-matched and sex-matched, then randomly assigned to different dietary conditions.
Data collection and analysis were not performed blind to dietary conditions of the experiments because group allocation was readily visible to investigators during data collection based on animal size and discolouration of faeces, which reflected the colour of the assigned diet.
Unless otherwise described, data were analysed and visualized with Prism 10 (GraphPad) and are presented as mean ± s.e.m. A P value of <0.05 was considered statistically significant. Data were tested for normality and variance before statistical testing was applied. For experiments with two groups, an unpaired two-tailed Mann–Whitney test was used. In experiments with more than two groups, one-way ANOVA was performed, followed by Tukey’s or Sidak’s multiple comparisons test, depending on whether all groups or a subset of groups were compared. When comparing matched data with two variables, a repeated-measures two-way ANOVA followed by Bonferroni’s multiple comparisons test was performed. For experiments with multiple matched groups, repeated-measures one-way ANOVA followed by Tukey’s multiple comparisons test was performed. Statistical testing for each experiment is detailed in the figure legends. Data were excluded from the analyses only if identified as a statistically significant outlier based on Grubb’s test, with α = 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Supplementary Tables 1–4.
Source data
Statistical Source Data.
Statistical Source Data.
Statistical Source Data.
Statistical Source Data.
Statistical Source Data.
Statistical Source Data.
Statistical Source Data.
Statistical Source Data.
Statistical Source Data.
Statistical Source Data.
Statistical Source Data.
Unprocessed western blots.
Acknowledgements
This work was funded by the Mark Foundation Emerging Leader Award (L.L.), the Nutrition and Obesity Research Center at Harvard Pilot Award (L.L.), National Institutes of Health (NIH) grant 1R01AI134861 (L.L.), the Science Foundation Ireland Future Research Leaders Programme (L.L.), the European Research Council Grant StG_6791 (L.L.) and the Ludwig Cancer Research Institute. B.K. was supported by NIH training grants T32DK007260 and T32DK007529. H.P. was supported by the Irish Research Council grant GOIPG/2018/1945. C.M. was supported by the Cancer Research Institute Irvington Postdoctoral Fellowship. A.T. was supported by the Landry Cancer Biology Consortium Fellowship at Harvard Medical School. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Extended data
Author contributions
B.K., H.P., C.M., R.M.L. and L.L. conceptualized the study. B.K., H.P., C.M., A.T., R.M.L., C.Y., L.D., E.L.M. and L.L. designed experiments and developed methodology. B.K., H.P., C.M., A.T., R.M.L., C.Y., L.D., L.V.S., A.D., G.P., K.A.J.M., C.H., M.R., R.W., H.K., J.K., K.L.O., M.B., F.S. and B.S. performed experiments. B.K., H.P., C.M., A.T., R.M.L., C.Y., L.D., L.V.S., A.D., G.P., C.H. and E.L.M. analysed data. H.M.K., D.K.F., D.A.C., E.T.C., S.K., E.L.M. and M.H. assisted in data interpretation. B.K. and H.P. visualized the data. B.K., H.P. and L.L. wrote the manuscript with input from all authors. B.K., H.P., C.M., A.T., L.D., L.V.S., H.K., H.M.R., E.L.M. and L.L. reviewed and edited the manuscript. L.V.S., C.R., H.M.R., E.C.L. and L.L. provided and managed resources. L.L. supervised and coordinated the study. D.A.C., E.T.C., E.L.M., M.H. and L.L. were responsible for funding.
Peer review
Peer review information
Nature Metabolism thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Jean Nakhle, in collaboration with the Nature Metabolism team.
Data availability
The mass spectrometry dataset generated for proteomic analysis of NK cells is available from the PRIDE Archive (https://www.ebi.ac.uk/pride), project accession number PXD062745. The metabolomic dataset is available from the corresponding author on reasonable request. Source data are provided with this paper.
Competing interests
L.L. is a member of the scientific advisory board for MiNK Therapeutics, a consultant for Bayer and a member of the Scientific Advisory Board of Faeth Therapeutics and Deciduous Therapeutics. The interests of L.L. were reviewed and managed by the Brigham and Women’s Hospital in accordance with their conflict-of-interest policies. M.H. is a member of the Scientific Advisory Board of the MD Anderson Allison Institute. The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Britta Kunkemoeller, Hannah Prendeville.
Extended data
is available for this paper at 10.1038/s42255-025-01330-w.
Supplementary information
The online version contains supplementary material available at 10.1038/s42255-025-01330-w.
References
- 1.World obesity day 2022—accelerating action to stop obesityhttps://www.who.int/news/item/04-03-2022-world-obesity-day-2022-accelerating-action-to-stop-obesity (2022).
- 2.Lauby-Secretan, B. et al. Body fatness and cancer—viewpoint of the IARC Working Group. N. Engl. J. Med.375, 794–798 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrelli, F. et al. Association of obesity with survival outcomes in patients with cancer: a systematic review and meta-analysis. JAMA Netw. Open4, e213520 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steele, C. B. et al. Vital signs: trends in incidence of cancers associated with overweight and obesity—United States, 2005–2014. MMWR Morb. Mortal. Wkly Rep.66, 1052–1058 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nasiri, A. R., Rodrigues, M. R., Li, Z., Leitner, B. P. & Perry, R. J. SGLT2 inhibition slows tumor growth in mice by reversing hyperinsulinemia. Cancer Metab.7, 10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park, J., Sarode, V. R., Euhus, D., Kittler, R. & Scherer, P. E. Neuregulin 1-HER axis as a key mediator of hyperglycemic memory effects in breast cancer. Proc. Natl Acad. Sci. USA109, 21058–21063 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beyaz, S. et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature531, 53–58 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quail, D. F. et al. Obesity alters the lung myeloid cell landscape to enhance breast cancer metastasis through IL5 and GM-CSF. Nat. Cell Biol.19, 974–987 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park, J., Morley, T. S., Kim, M., Clegg, D. J. & Scherer, P. E. Obesity and cancer—mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol.10, 455–465 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michelet, X. et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol.19, 1330–1340 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Dyck, L. et al. Suppressive effects of the obese tumor microenvironment on CD8 T cell infiltration and effector function. J. Exp. Med.219, e20210042 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ringel, A. E. et al. Obesity shapes metabolism in the tumor microenvironment to suppress anti-tumor immunity. Cell183, 1848–1866.e26 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dyck, L. & Lynch, L. Cancer, obesity and immunometabolism—connecting the dots. Cancer Lett.417, 11–20 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Wu, J. H. Y., Micha, R. & Mozaffarian, D. Dietary fats and cardiometabolic disease: mechanisms and effects on risk factors and outcomes. Nat. Rev. Cardiol.16, 581–601 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Shannon, C. E. et al. Precision nutrition for targeting pathophysiology of cardiometabolic phenotypes. Rev. Endocr. Metab. Disord.24, 921–936 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watson, A. F. & Mellanby, E. Tar cancer in mice. II. The condition of the skin, when modified by external treatment or diet, as a factor in influencing the cancerous reaction. Br. J. Exp. Pathol.11, 311–322 (1930). [Google Scholar]
- 17.Kanarek, N., Petrova, B. & Sabatini, D. M. Dietary modifications for enhanced cancer therapy. Nature579, 507–517 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Rose, D. P. Dietary fatty acids and cancer. Am. J. Clin. Nutr.66, 998s–1003s (1997). [DOI] [PubMed] [Google Scholar]
- 19.Soldati, L. et al. The influence of diet on anti-cancer immune responsiveness. J. Transl. Med.16, 75 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fatima, S. et al. High-fat diet feeding and palmitic acid increase CRC growth in β2AR-dependent manner. Cell Death Dis.10, 711 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giulitti, F. et al. Anti-tumor effect of oleic acid in hepatocellular carcinoma cell lines via autophagy reduction. Front. Cell Dev. Biol.9, 629182 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lien, E. C. et al. Low glycaemic diets alter lipid metabolism to influence tumour growth. Nature599, 302–307 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, L. et al. Consumption of the fish oil high-fat diet uncouples obesity and mammary tumor growth through induction of reactive oxygen species in protumor macrophages. Cancer Res.80, 2564–2574 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Link, V. M. et al. Differential peripheral immune signatures elicited by vegan versus ketogenic diets in humans. Nat. Med.30, 560–572 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stattin, P. R. et al. Prospective study of hyperglycemia and cancer risk. Diabetes Care30, 561–567 (2007). [DOI] [PubMed] [Google Scholar]
- 26.de Candia, P. et al. The pleiotropic roles of leptin in metabolism, immunity, and cancer. J. Exp. Med.218, e20191593 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leitner, B. P. & Perry, R. J. The impact of obesity on tumor glucose uptake in breast and lung cancer. JNCI Cancer Spectr.4, pkaa007 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasconcelos-dos-Santos, A. et al. Hyperglycemia exacerbates colon cancer malignancy through hexosamine biosynthetic pathway. Oncogenesis6, e306 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, Y. et al. Uncoupling hepatic oxidative phosphorylation reduces tumor growth in two murine models of colon cancer. Cell Rep.24, 47–55 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang, C. et al. STAT3 activation-induced fatty acid oxidation in CD8+ T effector cells is critical for obesity-promoted breast tumor growth. Cell Metab.31, 148–161.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tau, G. Z., Cowan, S. N., Weisburg, J., Braunstein, N. S. & Rothman, P. B. Regulation of IFN-γ signaling is essential for the cytotoxic activity of CD8+ T cells. J. Immunol.167, 5574–5582 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trapani, J. A. & Smyth, M. J. Functional significance of the perforin/granzyme cell death pathway. Nat. Rev. Immunol.2, 735–747 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Mazurier, F. et al. A novel immunodeficient mouse model—RAG2 × common cytokine receptor γ chain double mutants—requiring exogenous cytokine administration for human hematopoietic stem cell engraftment. J. Interferon Cytokine Res.19, 533–541 (1999). [DOI] [PubMed] [Google Scholar]
- 34.Jiao, D. et al. Lipid accumulation-mediated histone hypoacetylation drives persistent NK cell dysfunction in anti-tumor immunity. Cell Rep.42, 113211 (2023). [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi, T. et al. Increased lipid metabolism impairs NK cell function and mediates adaptation to the lymphoma environment. Blood136, 3004–3017 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Terrén, I., Orrantia, A., Vitallé, J., Zenarruzabeitia, O. & Borrego, F. NK cell metabolism and tumor microenvironment. Front. Immunol.10, 2278 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loftus, R. M. et al. Amino acid-dependent cMyc expression is essential for NK cell metabolic and functional responses in mice. Nat. Commun.9, 2341 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donnelly, R. P. et al. mTORC1-dependent metabolic reprogramming is a prerequisite for NK cell effector function. J. Immunol.193, 4477–4484 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orange, J. S. et al. Deficient natural killer cell cytotoxicity in patients with IKK-γ/NEMO mutations. J. Clin. Invest.109, 1501–1509 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spielmann, J. et al. Effects of obesity on NK cells in a mouse model of postmenopausal breast cancer. Sci. Rep.10, 20606 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wensveen, F. M., Jelencic, V. & Polic, B. NKG2D: a master regulator of immune cell responsiveness. Front. Immunol.9, 441 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuli, A. et al. Arf-like GTPase Arl8b regulates lytic granule polarization and natural killer cell-mediated cytotoxicity. Mol. Biol. Cell24, 3721–3735 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vargas-Hernández, A. et al. Human signal transducer and activator of transcription 5b (STAT5b) mutation causes dysregulated human natural killer cell maturation and impaired lytic function. J. Allergy Clin. Immunol.145, 345–357.e9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lachmann, A. et al. ChEA: transcription factor regulation inferred from integrating genome-wide ChIP-X experiments. Bioinformatics26, 2438–2444 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim, J. W. et al. Evaluation of myc E-box phylogenetic footprints in glycolytic genes by chromatin immunoprecipitation assays. Mol. Cell. Biol.24, 5923–5936 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Osthus, R. C. et al. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J. Biol. Chem.275, 21797–21800 (2000). [DOI] [PubMed] [Google Scholar]
- 47.Li, F. et al. Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol. Cell. Biol.25, 6225–6234 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zirath, H. et al. MYC inhibition induces metabolic changes leading to accumulation of lipid droplets in tumor cells. Proc. Natl Acad. Sci. USA110, 10258–10263 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khameneh, H. J. et al. Myc controls NK cell development, IL-15-driven expansion, and translational machinery. Life Sci Alliance6, e202302069 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manzo, T. et al. Accumulation of long-chain fatty acids in the tumor microenvironment drives dysfunction in intrapancreatic CD8+ T cells. J. Exp. Med.217, e20191920 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gudgeon, N. et al. Uptake of long-chain fatty acids from the bone marrow suppresses CD8+ T-cell metabolism and function in multiple myeloma. Blood Adv.7, 6035–6047 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu, S. et al. Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8+ T cells in tumors. Immunity54, 1561–1577.e7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Engevik, M. A. et al. Microbial metabolic capacity for intestinal folate production and modulation of host folate receptors. Front. Microbiol.10, 2305 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lees, H. J., Swann, J. R., Wilson, I. D., Nicholson, J. K. & Holmes, E. Hippurate: the natural history of a mammalian–microbial cometabolite. J. Proteome Res.12, 1527–1546 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Guzman, J. R., Conlin, V. S. & Jobin, C. Diet, microbiome, and the intestinal epithelium: An essential triumvirate? BioMed. Res. Int.2013, 425146 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pallister, T. et al. Hippurate as a metabolomic marker of gut microbiome diversity: modulation by diet and relationship to metabolic syndrome. Sci. Rep.7, 13670 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Le Chatelier, E. et al. Richness of human gut microbiome correlates with metabolic markers. Nature500, 541–546 (2013). [DOI] [PubMed] [Google Scholar]
- 58.Schooneman, M. G., Vaz, F. M., Houten, S. M. & Soeters, M. R. Acylcarnitines: Reflecting or inflicting insulin resistance? Diabetes62, 1–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rinaldo, P., Cowan, T. M. & Matern, D. Acylcarnitine profile analysis. Genet. Med.10, 151–156 (2008). [DOI] [PubMed] [Google Scholar]
- 60.Gao, X. et al. Serine availability influences mitochondrial dynamics and function through lipid metabolism. Cell Rep.22, 3507–3520 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Houmard, J. A. Intramuscular lipid oxidation and obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol.294, R1111–R1116 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koves, T. R. et al. Peroxisome proliferator-activated receptor-γ co-activator 1α-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J. Biol. Chem.280, 33588–33598 (2005). [DOI] [PubMed] [Google Scholar]
- 63.Bell, J. A. et al. Lipid partitioning, incomplete fatty acid oxidation, and insulin signal transduction in primary human muscle cells: effects of severe obesity, fatty acid incubation, and fatty acid translocase/CD36 overexpression. J. Clin. Endocrinol. Metab.95, 3400–3410 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reina-Campos, M., Scharping, N. E. & Goldrath, A. W. CD8+ T cell metabolism in infection and cancer. Nat. Rev. Immunol.21, 718–738 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu, Y.-R. et al. Disturbed mitochondrial dynamics in CD8+ TILs reinforce T cell exhaustion. Nat. Immunol.21, 1540–1551 (2020). [DOI] [PubMed] [Google Scholar]
- 66.Mihalik, S. J. et al. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity (Silver Spring)18, 1695–1700 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rao, G. N., Alexander, R. W. & Runge, M. S. Linoleic acid and its metabolites, hydroperoxyoctadecadienoic acids, stimulate c-Fos, c-Jun, and c-Myc mRNA expression, mitogen-activated protein kinase activation, and growth in rat aortic smooth muscle cells. J. Clin. Invest.96, 842–847 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang, Q. et al. Reprogramming of palmitic acid induced by dephosphorylation of ACOX1 promotes β-catenin palmitoylation to drive colorectal cancer progression. Cell Discov.9, 26 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang, Y. et al. Oleic acid and insulin as key characteristics of T2D promote colorectal cancer deterioration in xenograft mice revealed by functional metabolomics. Front. Oncol.11, 685059 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kulathunga, N. et al. Peripubertal high-fat diet promotes c-Myc stabilization in mammary gland epithelium. Cancer Sci.111, 2336–2348 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cirulli, E. T. et al. Profound perturbation of the metabolome in obesity is associated with health risk. Cell Metab.29, 488–500.e2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fan, Y. et al. Effects of diets on risks of cancer and the mediating role of metabolites. Nat. Commun.15, 5903 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li, C. et al. Development and validation of a metabolite score for red meat intake: an observational cohort study and randomized controlled dietary intervention. Am. J. Clin. Nutr.116, 511–522 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xie, Y. et al. Plant-based meat analogues aggravated lipid accumulation by regulating lipid metabolism homeostasis in mice. Food Sci. Hum. Wellness13, 946–960 (2024). [Google Scholar]
- 75.Wittenbecher, C. et al. Amino acids, lipid metabolites, and ferritin as potential mediators linking red meat consumption to type 2 diabetes. Am. J. Clin. Nutr.101, 1241–1250 (2015). [DOI] [PubMed] [Google Scholar]
- 76.Lu, X. et al. Metabolic profiling analysis upon acylcarnitines in tissues of hepatocellular carcinoma revealed the inhibited carnitine shuttle system caused by the downregulated carnitine palmitoyltransferase 2. Mol. Carcinog.58, 749–759 (2019). [DOI] [PubMed] [Google Scholar]
- 77.Cheng, X. et al. Long-chain acylcarnitines induce senescence of invariant natural killer T cells in hepatocellular carcinoma. Cancer Res.83, 582–594 (2023). [DOI] [PubMed] [Google Scholar]
- 78.Guasch-Ferré, M. et al. Plasma acylcarnitines and risk of type 2 diabetes in a Mediterranean population at high cardiovascular risk. J. Clin. Endocrinol. Metab.104, 1508–1519 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ruiz-Canela, M. et al. Plasma acylcarnitines and risk of incident heart failure and atrial fibrillation: the Prevención con dieta mediterránea study. Rev. Esp. Cardiol. (Engl. Ed.)75, 649–658 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ahmad, T. et al. Prognostic implications of long-chain acylcarnitines in heart failure and reversibility with mechanical circulatory support. J. Am. Coll. Cardiol.67, 291–299 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McCoin, C. S., Knotts, T. A. & Adams, S. H. Acylcarnitines—old actors auditioning for new roles in metabolic physiology. Nat. Rev. Endocrinol.11, 617–625 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sánchez-Pintos, P. et al. Similarities between acylcarnitine profiles in large for gestational age newborns and obesity. Sci. Rep.7, 16267 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nicholas, D. A. et al. Fatty acid metabolites combine with reduced β oxidation to activate Th17 inflammation in human type 2 diabetes. Cell Metab.30, 447–461.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rutkowsky, J. M. et al. Acylcarnitines activate proinflammatory signaling pathways. Am. J. Physiol. Endocrinol. Metab.306, E1378–E1387 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vincze, O. et al. Cancer risk across mammals. Nature601, 263–267 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hirschberger, S. et al. Very‐low‐carbohydrate diet enhances human T‐cell immunity through immunometabolic reprogramming. EMBO Mol. Med.13, e14323 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mehlem, A., Hagberg, C. E., Muhl, L., Eriksson, U. & Falkevall, A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat. Protoc.8, 1149–1154 (2013). [DOI] [PubMed] [Google Scholar]
- 88.Hughes, C. S. et al. Ultrasensitive proteome analysis using paramagnetic bead technology. Mol. Syst. Biol.10, 757 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marchingo, J. M., Sinclair, L. V., Howden, A. J. M. & Cantrell, D. A. Quantitative analysis of how Myc controls T cell proteomes and metabolic pathways during T cell activation. eLife9, e53725 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wiśniewski, J. R., Hein, M. Y., Cox, J. & Mann, M. A “proteomic ruler” for protein copy number and concentration estimation without spike-in standards. Mol. Cell Proteom.13, 3497–3506 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xie, Z. et al. Gene set knowledge discovery with Enrichr. Curr. Protoc.1, e90 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nian, Y., Allen, P., Harrison, S. M. & Kerry, J. P. Effect of castration and carcass suspension method on the quality and fatty acid profile of beef from male dairy cattle. J. Sci. Food Agric.98, 4339–4350 (2018). [DOI] [PubMed] [Google Scholar]
- 93.Mills, E. L. et al. UCP1 governs liver extracellular succinate and inflammatory pathogenesis. Nat. Metab.3, 604–617 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pang, Z. et al. MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res.49, W388–W396 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yao, C. H. et al. Uncoupled glycerol-3-phosphate shuttle in kidney cancer reveals that cytosolic GPD is essential to support lipid synthesis. Mol. Cell83, 1340–1349.e7 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables 1–4.
Statistical Source Data.
Statistical Source Data.
Statistical Source Data.
Statistical Source Data.
Statistical Source Data.
Statistical Source Data.
Statistical Source Data.
Statistical Source Data.
Statistical Source Data.
Statistical Source Data.
Statistical Source Data.
Unprocessed western blots.
Data Availability Statement
The mass spectrometry dataset generated for proteomic analysis of NK cells is available from the PRIDE Archive (https://www.ebi.ac.uk/pride), project accession number PXD062745. The metabolomic dataset is available from the corresponding author on reasonable request. Source data are provided with this paper.