Abstract
Introduction
A common complication of interstitial lung disease (ILD) is pulmonary hypertension (PH), which is associated with increased morbidity and mortality and worsened quality of life. In ILD, evaluating for PH is recommended prior to lung transplantation. However, this is not standardized or routinely performed in earlier stages of ILD, and guidelines lack an evidence-based approach for PH screening in this population. Furthermore, right-heart catheterization (RHC) access can be limited in many settings. The objective of PHINDER (Pulmonary Hypertension Screening in Patients with Interstitial Lung Disease for Earlier Detection) is to prospectively develop screening strategies for PH in patients with ILD.
Methods
PHINDER is a prospective, non-interventional study that will enroll approximately 200 patients with ILD treated in a variety of settings in the United States (community centers, academic institutions, etc.). Patients must be diagnosed with ILD by high-resolution computed tomography (HRCT) and must not have a previously reported mean pulmonary arterial pressure (mPAP) > 20 mmHg. To enrich the population for PH, patients must meet additional criteria on Pulmonary Function Tests, HRCT, signs/symptoms, 6-min walk test, or echocardiography. Patients will undergo a variety of routine ILD clinical assessments. Lastly, patients receive a RHC to assess for PH, defined as mPAP > 20 mmHg with pulmonary arterial wedge pressure ≤ 15 mmHg and a pulmonary vascular resistance > 2 Wood Units. All treatment decisions are at the discretion of the provider and not influenced by study participation.
Planned Outcomes
Following study completion, statistical tools will be used to derive a practical model for a screening algorithm using the variables identified in the study as most predictive of PH in patients with ILD.
Conclusions
Using a previously developed list of clinical assessments from PH and ILD experts, the PHINDER study aims to be the first prospectively enrolled study to evaluate prognostic screening strategies that can be used to develop an algorithm to predict the risk of PH in patients with ILD.
Trail Registration
Supplementary Information
The online version contains supplementary material available at 10.1007/s41030-025-00307-0.
Keywords: Algorithm, Interstitial lung disease, Pulmonary hypertension, Pulmonary hemodynamics, Right heart catheterization, Risk score, Screening
Key Summary Points
| There remains a need for a standardized, evidence-based approach to diagnose PH earlier in patients with ILD. |
| The objective of the Pulmonary Hypertension Screening in Patients with Interstitial Lung Disease for Earlier Detection (PHINDER) study is to prospectively evaluate screening strategies for pulmonary hypertension in patients with ILD in an effort to promote awareness and encourage screening for PH in this patient population. |
| Results from this study will be used to identify and weigh specific clinical parameters based on their prognostic significance for right heart catheterization (RHC)-confirmed PH in patients with ILD. |
| Upon study completion, statistical and epidemiological analyses will be conducted to build a practical screening algorithm using the most predictive variables identified during the study. |
Introduction
Interstitial lung disease complicated by pulmonary hypertension (PH-ILD) has been classified as group 3 pulmonary hypertension (PH) by the World Symposium on Pulmonary Hypertension [1, 2]. Interstitial lung disease (ILD) encompasses a heterogeneous group of parenchymal lung diseases that are characterized by significant scarring or fibrosis within the lung interstitium [3, 4]. One subtype of ILD, idiopathic pulmonary fibrosis (IPF), has targeted therapies approved for use, which include pirfenidone and nintedanib. Nintedanib is also approved for progressive fibrotic ILD [5, 6]. PH-ILD emerges as a common comorbidity of ILD, with increased fibrotic tissue in the lungs contributing to elevated pulmonary vascular resistance and pulmonary arterial pressure. The fibrotic changes hinder oxygenation and impede free gas exchange between the pulmonary capillaries and alveolar sacs, exacerbating the pulmonary vascular remodeling and leading to further deterioration of cardiopulmonary function [3, 4]. Connective tissue disease (CTD)-associated ILD is one of the leading causes of morbidity and mortality of CTD, and one of the principal pathological features of CTD are chronic inflammation of blood vessels and connect tissues which can lead to systematic damage [5]. Notably, PH exacerbates the already severe health outcomes of ILD. The incidence of PH in ILD has been reported in up to 86% of patients and is associated with reduced exercise capacity, greater need for supplemental oxygen, decreased quality of life, and earlier death (Nathan, 2020) [6–8]. With the recent approval of Tyvaso® (inhaled treprostinil) by the FDA in 2021, supported by the INCREASE trial results, there is a renewed focus on early detection and management strategies for PH-ILD [9].
Historically, physicians have employed several clinical assessments to identify patients at risk of PH (Table 1). However, no single clinical variable in isolation is adequate to screen for PH. Several tools and studies utilizing combinations of patient characteristics and clinical tests have contributed to the understanding of PH screening and detection in patients with ILD. Zisman et al. proposed a method to predict PH in advanced idiopathic pulmonary fibrosis and validated this PH predictor in an external population, laying the groundwork for subsequent research [10, 11]. The DETECT study introduced a risk calculator for PH for patients with systemic sclerosis, providing valuable insights into early detection [12]. More recently, Parikh et al. and Nathan et al. developed early detection tools for PH-ILD, which were both externally validated [13–15]. Another contribution was a Delphi study which serves as an expert consensus on factors to consider when screening for PH in this patient population [16]. While consensus was reached on parameters such as diffusing capacity of the lungs for carbon monoxide (DLCO) percent predicted < 40% as a trigger for suspicion of PH, a specific 6-min walk distance (6MWD) threshold or other exercise test did not reach consensus. The Pulmonary Hypertension Screening in Patients with Interstitial Lung Disease for Earlier Detection (PHINDER) study aims to prospectively establish a more definitive set of parameters that will allow us to predict PH in patients with ILD. These prior studies are limited by biases associated with Delphi surveys, incomplete patient assessments, and retrospective evaluations that are lacking in non-PH diagnoses, as in the case with the risk calculators mentioned. Additionally, the published screening tools were derived from patient populations using previous hemodynamic definitions of PH; in recent years, the PH thresholds for the mean pulmonary artery pressure and pulmonary vascular resistance have been lowered [17].
Table 1.
Features suggestive of pulmonary hypertension in patients with interstitial lung disease
| Clinical assessment | Findings suggestive of pulmonary hypertension [2, 13, 14, 22–25] |
| Symptoms | Symptoms (cough, dyspnea, fatigue, exercise intolerance, dizziness, syncope, edema, and palpitations) not explained by ILD severity |
| Physical exam |
Jugular venous distension Loud P2 or S2 heart sound Parasternal heave Hepatomegaly or ascites Peripheral edema |
| Chest computed tomography |
RV enlargement PA enlargement or PA/aorta ratio > 1 Enlarged pulmonary arteries in the lung periphery |
| Echocardiography |
Right atrial enlargement Elevated RVSP RV dilation or other RV abnormalities Septal flattening Decreased TAPSE |
| Pulmonary function testing |
Low DLCO Worsening FVC/DLCO ratio FVC%/DLCO% > 1.6 |
| 6-min walking test |
Low 6MWD New or worsening desaturation during 6MWT |
| Blood biomarkers | Elevated BNP or NT-proBNP |
6MWD six-minute walk distance, 6MWT six-minute walk test, BNP b-type natriuretic peptide, DLCO diffusing capacity of the lungs for carbon monoxide, FVC forced vital capacity, ILD interstitial lung disease, NT-proBNP N-terminal pro B-type natriuretic peptide, PA pulmonary artery, RV right ventricle, RVSP right ventricular systolic pressure, TAPSE tricuspid annular plane systolic excursion
Without evidence-based guidelines for screening patients with ILD for PH, there is a need for prospective evaluation of prognostic screening strategies. Because no universally accepted screening or detection tool currently exists for this patient population, healthcare providers have little guidance on when or how to screen for PH in patients with ILD. In fact, a recent survey of ILD specialists reflects the variability in screening strategies and the timing of screening for PH in patients with ILD [18]. Therefore, the primary objective of the PHINDER study is to prospectively evaluate screening strategies for PH in patients with ILD, aiming to identify specific clinical parameters with diagnostic utility for prediction of right heart catheterization-confirmed PH based on the current hemodynamic definition of PH. Additionally, exploratory objectives will be performed to identify and weigh specific parameters based on their prognostic significance for PH in this patient population, with the goal of developing an algorithm to help detect PH in patients with ILD.
Methods
Study Design
The PHINDER study is a multicenter, open-label, non-randomized prospective study enrolling approximately 200 patients diagnosed with ILD across academic and community healthcare settings in the US (NCT05776225). Under a recent protocol amendment, the sample has been increased to 300–350 patients to ensure representation of potential subgroups. Over two study visits, eligible patients will undergo a series of assessments identified as useful for PH screening in ILD by the recent Delphi consensus [16], followed by a right heart catheterization (RHC), the “gold standard” to assess for PH [17]. The study-collected high-resolution computed tomography (HRCT), echocardiography, and RHC tracings will be centrally reviewed. In addition to the central reviewer, acquisition guidelines for RHC, echocardiography, HRCT, and 6MWD were added to the protocol to ensure assessments were as standardized as possible. Data to be collected from HRCT include but are not limited to enlarged pulmonary artery (PA), PA:aorta ratio, and right ventricle (RV):left ventricle (LV) ratio. Data to be collected from the echocardiography include but are not limited to right atrial area, RV fractional area change, Tricuspid regurgitant velocity, strain, and myocardial performance index. The ECHO images themselves will also be collected to allow for additional assessment in the future. Lastly, data to be collected from RHC include but are not limited to mean pulmonary arterial pressure (mPAP), pulmonary arterial wedge pressure (PAWP), pulmonary vascular resistance (PVR), systolic pulmonary arterial pressure, diastolic pulmonary arterial pressure, right atrial pressure, cardiac output, cardiac index, and stroke volume index. The study began enrollment in August 2023 and is anticipated to be completed in the first half of 2025. The study protocol has been approved by the institutional review board at each participating site. This study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments. All subjects provided informed consent to participate in the study.
For this study, Advarra is the central IRB/main center, but some sites utilize local IRB in addition to or instead of Advarra (see supplementary material for details) and the study was approved by all institutions.
Sample Selection
The study will enroll approximately 200 patients with clinically diagnosed ILD across various healthcare settings (e.g., community, academic institutions). The diagnosis of ILD was left to the discretion of the Investigators. Physical exam and Pulmonary function tests were collected as part of study enrolment. Computed tomography was required for confirming a specific diagnosis of ILD. No diagnostic laboratory work, tissue diagnosis or confirmation by a multidisciplinary discussion was mandated, emulating real-world diagnostic patterns. To enrich the population for PH (i.e., increase the likelihood of observing PH as compared with the general ILD population in order to facilitate the study), patients must meet additional criteria based on the results of previous investigations and a clinical assessment (Category 1: Pulmonary Function Test (PFT) and HRCT; Category 2: signs and symptoms; Category 3: echocardiography) (Table 2). The patient population included only those etiologies that have been formerly studied for treatment intervention with inhaled treprostinil, which does include patients with idiopathic interstitial pneumonia (including pulmonary fibrosis), connective tissue disease-associated ILD with Forced Vital Capacity (FVC) < 70%, hypersensitivity pneumonitis, scleroderma-related ILD, autoimmune ILD, nonspecific interstitial pneumonia, occupational lung disease, and combined pulmonary fibrosis and emphysema (CPFE) (Fig. 1). Patients with CPFE have been enrolled based on Investigator discretion, but an ongoing minor protocol amendment will add further clarification stating that CPFE patients are included if they have mild emphysema on lung imaging as determined by the Investigator. Patients with sarcoidosis secondary to ILD are eligible for the study; however, those with sarcoidosis as a primary diagnosis are excluded from the study.
Table 2.
PH enrichment criteria
| Category 1: PFT and HRCT | Category 2: signs and symptoms | Category 3: echocardiography |
|---|---|---|
|
DLCO < 40% DLCO decline ≥ 15% on two most recent assessments DLCO decline > 10% and FVC decline < 5% on two most recent assessments PA enlargementa PA to Aorta ratio > 1 RV to LV ratio > 1 Ventricular septal flattening Enlarged peripheral PAsb |
Symptoms disproportionate to ILD severity or progression, in the opinion of the investigator Desaturation during 6MWT disproportionate to ILD severity, in the opinion of the investigator Worsening desaturation BNP > 200 pg/ml or NT-proBNP > 395 pg/ml ≥ 1 of the following on physical exam: syncope, JVD, loud S2 heart sound, peripheral edema, ascites, or hepatomegaly |
TAPSE < 2 cm RSVP > 35 mmHg Any dilation, enlargement, or other abnormality of the RV |
6MWT six-minute walk test, BNP B-type natriuretic peptide, DLCO diffusing capacity of the lungs for carbon monoxide, FVC forced vital capacity, HRCT high-resolution computed tomography, ILD interstitial lung disease, JVD jugular vein distention, LV left ventricle, NT-proBNP N-terminal pro B-type natriuretic peptide, PA pulmonary artery, PFT pulmonary function test, RV right ventricle, RVSP right ventricular systolic pressure, TAPSE tricuspid annular plane systolic excursion
aDetermination of PA enlargement is based on a composite evaluation of central pulmonary arteries integrating trunk diameter > 30–32 mm, PA/Aorta ratio > 1, and subjective enlargement of the main and interlobular arteries
bPeripheral PA enlargement is based on non-tapering vessels, vessels extending to pleural surface, and acute peripheral arterial dilation after visually excluding any areas with fibrosis
Fig. 1.

Interstitial lung disease subtypes. The figure illustrates the specific ILD subtypes eligible for participation. ILD interstitial lung disease
Measurements and Data Collection
Primary and secondary endpoints align with established guidelines for PH diagnosis. The primary endpoint of this study is the percentage of patients with PH as defined by the 2022 European Society of Cardiology/European Respiratory Society (ESC/ERS) Guidelines for PH; mPAP > 20 mmHg, PAWP ≤ 15 mmHg, and PVR > 2 Wood Units (WU) (Humbert 2022). The secondary endpoint is the percentage of patients with severe PH, as defined by the 2022 ESC/ERS Guidelines for PH; mPAP > 20 mmHg, PAWP ≤ 15 mmHg, and PVR > 5 WU [17]. The exploratory endpoint of the study is to develop a PH screening algorithm using clinical parameters found to be closely associated with pulmonary hypertension.
The study has two visits: the Screening Visit and Study Visit 1, with assessments potentially spanning up to 14 days. After providing informed consent, patients meeting study eligibility criteria will undergo a comprehensive series of assessments outlined in Fig. 2. Blood samples will be drawn for clinical labs, B-type natriuretic peptide (BNP), and N-terminal pro-B-type natriuretic peptide (NT-proBNP). Subsequently, all participants will undergo RHC to assess for PH. Any PH treatment decisions are to be made at the discretion of the patient’s healthcare provider and not influenced by study participation. Statistical methodology will be used to assess the agreement between clinical parameters and the presence of PH.
Fig. 2.
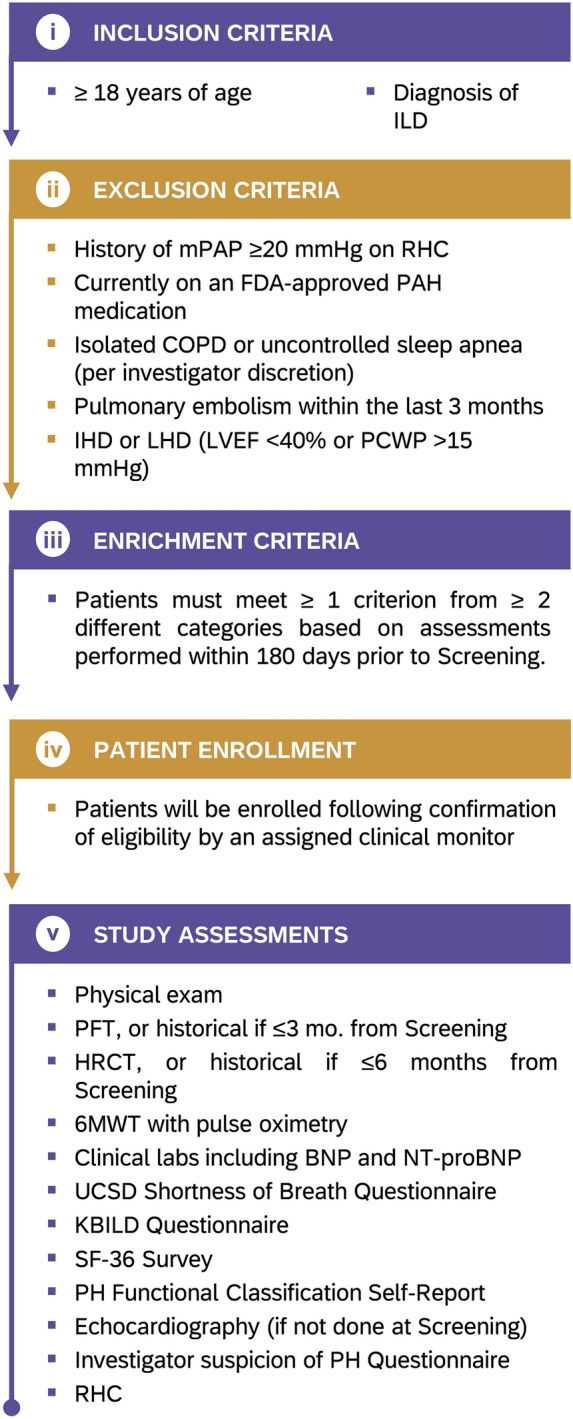
Study design. The figure shows the key inclusion and exclusion criteria for the study participants, as well as the overall schema of the PHINDER study after meeting PH enrichment criteria. PH pulmonary hypertension, PHINDER Pulmonary Hypertension Screening in Patients with Interstitial Lung Disease for Earlier Detection
Assessments for PH screening in patients with ILD were identified through a Delphi consensus of pulmonologists [13]. In this Delphi, experts in ILD and PH independently rated a comprehensive list of assessments based on clinical relevance and diagnostic accuracy, resulting in a consensus on the most significant parameters to be considered and which were adapted for inclusion in the study protocol. As this study is not blinded, interim analyses may be performed to ensure a sufficient rate of diagnosed PH for development of a model to predict for PH.
Data Analysis
A sample size calculation was performed pertaining to the exploratory objective of the study (i.e., development of a PH screening algorithm). Assuming PH prevalence rate changes over time, 14% in earlier stages, 32–50% in advanced stages, and more than 80% in end-stage disease, a sample size of 200 patients allows for an estimation of 90% sensitivity with a precision (i.e., margin of error) of ± 7.5%, where sensitivity refers to the probability of the algorithm correctly identifying PH in a patient with ILD, as verified by RHC [19]. Descriptive statistics will be used to evaluate baseline demographics. For the interim analyses, correlations with PH-ILD will be assessed using point-biserial correlation (r ≥ 0.7, strong correlation) and Cramer’s V (V ≥ 0.3, strong correlation) for continuous and categorical variables, respectively. For the final analysis upon completion of the study, correlations to PH-ILD will be assessed using univariate logistic regression analyses. Risk prediction models will be developed based on regression and decision tree models, with physician and investigator input on candidate variables. The sensitivity and specificity of these models will be analyzed using receiver operating characteristic curves to select a final risk calculator deemed most useful to clinicians.
Discussion
Despite the severe outcomes associated with PH-ILD, formal evidence-based guidance on screening and early detection of PH in patients with ILD is lacking, and PH-ILD often remains undiagnosed. The PHINDER study aims to fill this gap by identifying and weighing specific clinical parameters for RHC-confirmed PH based on prospectively collected data.
While all patients with ILD should be closely monitored for PH, overlapping symptoms of PH with the underlying ILD and other comorbidities makes it impossible to rely solely on the patients’ symptoms. At the same time, it is not cost-effective or unjustifiably invasive for all patients with ILD to undergo a RHC as part of a diagnostic process for PH. Findings from PHINDER could prospectively identify the key symptomatic, clinical, and imaging parameters that have diagnostic utility for the identification of PH. This multimodal approach could revolutionize screening strategies for PH-ILD, leading to earlier diagnosis and treatment, a more efficient use of diagnostic procedures, and consequently improving outcomes for patients with ILD.
Strengths and Limitations
In contrast to previous research related to the diagnosis of PH in patients with ILD, PHINDER is novel in its prospective design, potentially offering robust diagnostic predictions utilizing both RHC assessments from patients with and without PH. To date, consensus statements made by the Pulmonary Hypertension Association/Pulmonary Fibrosis Foundation and Pulmonary Vascular Research Institute rely on expert consensus and synthesis of existing evidence to provide recommendations for clinical practice [20, 21]. The FORD score and Parikh et al.’s early detection tool are based on statistical models developed from retrospective data [13–15]. Zisman et al.’s cross-sectional study proposed a method to predict PH, validated this PH predictor in an external population, and laid the groundwork for subsequent research; however, this study only included patients with idiopathic pulmonary fibrosis [10]. The PHINDER study will build upon the previous research by providing prospective evidence to guide screening strategies within this patient population. Findings of this study will be evaluated in individual subgroups, including (CPFE), to determine the usability of this detection tool in this patient population. Varying degrees of fibrosis and emphysema across severity of disease will also be evaluated.
While the prospective nature of this study is a strength, limitations still exist. Due to the additional eligibility criteria employed to enrich the study population for PH, there is a risk of detection bias. If there is disproportionate enrollment of patients with suspected PH, this could overestimate the prevalence of PH within the ILD population and improperly weigh different patient parameters for the diagnosis of PH. This bias could affect the generalizability of study findings and limit the applicability to patients with ILD with more severe disease; however, this will be addressed by validating in a general ILD population. To mitigate this bias, interim analyses are allowed by the study protocol to evaluate the incidence of PH and assess the suitability of the study’s eligibility criteria. Additionally, imperfect screening and early detection measures might be present as the components were chosen from a Delphi consensus study [16]. For purposes of the study, HRCTs could either be collected historically if available within 180 days of screening or performed at Study Visit 1, leading to a limitation where not all scans were obtained at baseline. Despite this, their predictive value will be assessed by categorizing them based on time intervals relative to baseline. Based on prior screening tools, many parameters have been determined to be associated with PH, but for purposes of this study and to reduce operational burden, we have focused on those identified in the Delphi. Next, while it is exciting for a PH detection tool to be developed, such a tool will need to be validated in an external population to confirm its sensitivity and specificity. Lastly, questions remain regarding what clinical parameters may predict hemodynamic worsening over time so longitudinal follow-up (e.g., in a patient registry) is of great interest to the PH-ILD community.
Conclusions
In conclusion, the PHINDER study will prospectively evaluate screening strategies for PH-ILD, addressing a critical gap in the clinical assessment of patients with ILD. The study’s goal is to allow for earlier and more consistent detection of the disease in order to improve outcomes in patients with this serious condition.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contributions
The authors confirm contribution to the paper as follows: study conception and design: David Zisman, Oksana Shlobin, Mary Beth Scholand, Eric Shen, Kevin Maher, Meredith Broderick. Data acquisition: Draft manuscript preparation: Tejaswini Kulkarni, David Zisman, David Kiely, Oksana Shlobin, Maral DerSarkissian, Eric Shen, Kevin Maher, Meredith Broderick, Mary Beth Scholand. All authors reviewed the manuscript and approved the final version for submission. Eric Shen, Meredith Broderick, and Mary Beth Scholand are the guarantors of this manuscript. The PHINDER Study Team thanks all PHINDER patients, research staff, and investigators for their participation in this study.
Funding
This study is sponsored by United Therapeutics Corporation, a public benefit corporation. The journal’s Rapid Service Fee was funded by United Therapeutics Corporation.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Conflict of Interest
Tejaswini Kulkarni reports consultation fees from United Therapeutics and Aileron, speaker/ consultation fees from Boehringer Ingelheim Inc. and Veracyte, consultation/advisory board fees from Avalyn and PureTech Health. David Zisman is on the speaker’s bureau for, and received consultation fees from United Therapeutics Corp. Also, he declares he is partly funded by a generous gift from William and Jean Soman. Zisman was a previous author on the Delphi (Rahaghi, et al.) that was used to create the enrichment criteria for this study. David Kiely reports support from the Sheffield Biomedical Research Centre, consulting fees and other payments from Jansen Pharmaceuticals, Ferrer, Altavant, MSD and United Therapeutics. Oksana Shlobin reports consultancy fees from United Therapeutics, Merck, Janssen and Aerami, payment or honoraria for lectures, presentations, manuscript writing or educational events from Ferrera and United Therapeutics, participation on a data safety monitoring board or advisory board with Janssen, and leadership roles with ACCP/CHEST and World Symposium on Pulmonary Hypertension task force. Shlobin was a previous author on the Delphi (Rahaghi, et al.) that was used to create the enrichment criteria for this study. Maral DerSarkissian is an employee of Analysis Group, Inc. which received research funds for her participation in this study. Eric Shen, Kevin Maher, and Meredith Broderick are employees of United Therapeutics. Mary Beth Scholand reports consultation fees from Boehringer Ingelheim, Genentech, Veracyte, Imvaria, and United Therapeutics. Scholand was a previous author on the Delphi (Rahaghi, et al.) that was used to create the enrichment criteria for this study.
Ethical Approval
This study protocol has been approved by the institutional review board at each participating site. This study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments. All subjects provided informed consent to participate in the study. For this study, Advarra is the central IRB/main center, but some sites utilize local IRB in addition to or instead of Advarra (see supplementary material for details) and the study was approved by all institutions.
References
- 1.Kovacs G, Bartolome S, Denton CP, et al. Definition, classification and diagnosis of pulmonary hypertension. Eur Respir J. 2024. 10.1183/13993003.01324-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shlobin OA, Adir Y, Barbera JA, et al. Pulmonary hypertension associated with lung diseases. Eur Respir J. 2024. 10.1183/13993003.01200-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seeger W, Adir Y, Barbera JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25 Suppl):D109–16. 10.1016/j.jacc.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 4.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–48. 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shao T, Shi X, Yang S, et al. Interstitial lung disease in connective tissue disease: a common lesion with heterogeneous mechanisms and treatment considerations. Front Immunol. 2021;12: 684699. 10.3389/fimmu.2021.684699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathan SD. Pulmonary hypertension in interstitial lung disease. Int J Clin Pract Suppl. 2008;160:21–8. 10.1111/j.1742-1241.2008.01624.x. [DOI] [PubMed] [Google Scholar]
- 7.Nathan SD, Hassoun PM. Pulmonary hypertension due to lung disease and/or hypoxia. Clin Chest Med. 2013;34(4):695–705. 10.1016/j.ccm.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 8.King CS, Shlobin OA. The trouble with group 3 pulmonary hypertension in interstitial lung disease: dilemmas in diagnosis and the conundrum of treatment. Chest. 2020;158(4):1651–64. 10.1016/j.chest.2020.04.046. [DOI] [PubMed] [Google Scholar]
- 9.Waxman A, Restrepo-Jaramillo R, Thenappan T, et al. Inhaled treprostinil in pulmonary hypertension due to interstitial lung disease. N Engl J Med. 2021;384(4):325–34. 10.1056/NEJMoa2008470. [DOI] [PubMed] [Google Scholar]
- 10.Zisman DA, Karlamangla AS, Kawut SM, et al. Validation of a method to screen for pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2008;133(3):640–5. 10.1378/chest.07-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zisman DA, Ross DJ, Belperio JA, et al. Prediction of pulmonary hypertension in idiopathic pulmonary fibrosis. Respir Med. 2007;101(10):2153–9. 10.1016/j.rmed.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coghlan JG, Denton CP, Grunig E, et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis. 2014;73(7):1340–9. 10.1136/annrheumdis-2013-203301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parikh R, Konstantinidis I, O’Sullivan DM, Farber HW. Pulmonary hypertension in patients with interstitial lung disease: a tool for early detection. Pulm Circ. 2022;12(4): e12141. 10.1002/pul2.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parikh R, O’Sullivan DM, Farber HW. The PH-ILD Detection tool: external validation and use in patients with ILD. Pulm Circ. 2023;13(3): e12273. 10.1002/pul2.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nathan SD, Chandel A, Wang Y, et al. Derivation and validation of a noninvasive prediction tool to identify pulmonary hypertension in patients with IPF: evolution of the model FORD. J Heart Lung Transplant. 2024;43(4):547–53. 10.1016/j.healun.2023.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Rahaghi FF, Kolaitis NA, Adegunsoye A, et al. Screening strategies for pulmonary hypertension in patients with interstitial lung disease: a multidisciplinary Delphi Study. Chest. 2022;162(1):145–55. 10.1016/j.chest.2022.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43(38):3618–731. 10.1093/eurheartj/ehac237. [DOI] [PubMed] [Google Scholar]
- 18.Khan SL, Danoff SK, Kulkarni T, et al. Practice patterns for screening and treating interstitial lung disease-related pulmonary hypertension at specialty care centers in the United States. Ann Am Thorac Soc. 2024;21(7):1103–6. 10.1513/AnnalsATS.202402-141RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikkho SM, Richter MJ, Shen E, et al. Clinical significance of pulmonary hypertension in interstitial lung disease: a consensus statement from the Pulmonary Vascular Research Institute’s innovative drug development initiative-Group 3 pulmonary hypertension. Pulm Circ. 2022;12(3): e12127. 10.1002/pul2.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Case AN, SD, Abrencillo R, Bemiss B, Collins B, et al. Pulmonary hypertension related to ILD (for health care providers). Pulmonary Fibrosis Foundation & Pulmonary Hypertension Association; 2023.
- 21.Shlobin OA, Shen E, Wort SJ, et al. Pulmonary hypertension in the setting of interstitial lung disease: approach to management and treatment. A consensus statement from the Pulmonary Vascular Research Institute’s Innovative Drug Development Initiative-Group 3 Pulmonary Hypertension. Pulm Circ. 2024;14(1):e12310. 10.1002/pul2.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alhamad EH, Cal JG, Alrajhi NN, Alharbi WM. Predictors of mortality in patients with interstitial lung disease-associated pulmonary hypertension. J Clin Med. 2020. 10.3390/jcm9123828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King CS, Nathan SD. Pulmonary hypertension due to interstitial lung disease. Curr Opin Pulm Med. 2019;25(5):459–67. 10.1097/MCP.0000000000000599. [DOI] [PubMed] [Google Scholar]
- 24.Leuchte HH, Baumgartner RA, Nounou ME, et al. Brain natriuretic peptide is a prognostic parameter in chronic lung disease. Am J Respir Crit Care Med. 2006;173(7):744–50. 10.1164/rccm.200510-1545OC. [DOI] [PubMed] [Google Scholar]
- 25.Piccari L, Kovacs G, Jones S, et al. The European Voice of the Patient living with pulmonary hypertension associated with interstitial lung disease: diagnosis, symptoms, impacts, and treatments. Pulm Circ. 2024;14(2): e12405. 10.1002/pul2.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


