Abstract
Seventy-eight Enterococcus faecium strains from various sources were characterized by random amplified polymorphic DNA (RAPD)-PCR, amplified fragment length polymorphism (AFLP), and pulsed-field gel electrophoresis (PFGE) analysis of SmaI restriction patterns. Two main genomic groups (I and II) were obtained in both RAPD-PCR and AFLP analyses. DNA-DNA hybridization values between representative strains of both groups demonstrated a mean DNA-DNA reassociation level of 71%. PFGE analysis revealed high genetic strain diversity within the two genomic groups. Only group I contained strains originating from human clinical samples or strains that were vancomycin-resistant or beta-hemolytic. No differentiating phenotypic features between groups I and II were found using the rapid ID 32 STREP system. The two groups could be further subdivided into, respectively, four and three subclusters in both RAPD-PCR and AFLP analyses, and a high correlation was seen between the subclusters generated by these two methods. Subclusters of group I were to some extent correlated with origin, pathogenicity, and bacteriocinogeny of the strains. Host specificity of E. faecium strains was not confirmed.
Enterococcus faecium and Enterococcus faecalis are the most frequently detected enterococcal species in the human gastrointestinal tract (9, 15, 46, 52). In poultry and cattle, the incidence of E. faecium is less prevalent and decreases with age. Various frequencies have been found in the feces of preruminant calves and ruminating young cattle. E. faecium was reported to predominate in porcine fecal samples, and it has also been associated with dogs and cats (11, 12, 13, 14, 15, 37).
Since the late 1970s, E. faecium and E. faecalis have been identified as causes of nosocomial infections, causing an increasing incidence of endocarditis, bacteremia, urinary tract, and neonatal infections (19, 42, 53, 54). Other enterococci have rarely been reported to be pathogenic to humans (15, 33, 35, 39, 44). The ratio of infections due to E. faecalis to those due to all other Enterococcus species was previously approximately 10:1. In recent years, E. faecalis has still dominated as a causative agent in enterococcal bacteremia, but there is a shift which is likely due to the emergence of vancomycin-resistant enterococci, with the predominance of the species E. faecium among this subset of enterococcal isolates (45). It has been demonstrated that enterococci show the ability to take up (27, 28) and transfer antibiotic resistance genes, both vertically (42) and horizontally (36, 48).
On the other hand, these two enterococcal species occur in raw and processed meats, as well as in fermented meat and dairy products, in particular, traditional cheeses in which they may contribute to ripening and product flavor (8, 21, 23, 38, 40, 58). Considering the clinical situation, the safety of E. faecium and E. faecalis strains associated with food fermentations and also their use as probiotics are nowadays questioned (21).
Numerous epidemiological studies involving strain level characterization of enterococci have been done. Many of these studies deal with nosocomial infections and the prevalence of vancomycin-resistant E. faecium in individual or in several hospitals (4, 16, 17, 24, 29, 41, 43) or with the assumed transmission of vancomycin-resistant enterococci from animals to humans (5, 6). However, very few studies have been done to reveal the genomic relationships between vancomycin-resistant and susceptible E. faecium isolates of human, food, and animal origin. Willems et al. (62) and Quednau et al. (51) studied the genomic relationships of antibiotic-resistant E. faecium strains from different sources using amplified fragment length polymorphism (AFLP) analysis and restriction endonuclease analysis (REA) of total chromosomal DNA, respectively. In both studies, host specificity was proposed for strains from, e.g., chicken, pig, and human origin.
In the present study, the intraspecies strain relationships of a large set of susceptible and vancomycin-resistant E. faecium strains from humans, animals, and foods, collected from different European countries, were investigated by different typing methodologies. In total 78 E. faecium isolates were characterized using three genomic typing techniques, random amplified polymorphic DNA (RAPD)-PCR (four different primers), AFLP (two different primer combinations), and pulsed-field gel electrophoresis (PFGE) analysis of SmaI patterns. It is demonstrated that the species E. faecium is composed of two genomic groups which were further divided into, respectively, four and three subclusters. The typing results were evaluated with regard to the origin of the strains, safety aspects such as beta-hemolysis and glycopeptide antibiotic resistance, and bacteriocinogeny.
MATERIALS AND METHODS
Bacterial strains.
The E. faecium strains examined in this study are listed in Table 1. Additional strain information is available in the catalogue of enterococci of the FAIR-E collection (59). The catalogue and strains are available at the BCCM/LMG Bacteria Collection (http://www.belspo.be/bccm/lmg.htm). Strains were grown on MRS agar (Oxoid) at 37°C for 24 h, unless indicated otherwise.
TABLE 1.
E. faecium strains studied
| Genomic group | Subclustera
|
Strainb | Origin | van genec | Hemolysisd | Bacteriocin gene(s)e | |
|---|---|---|---|---|---|---|---|
| RAPD | AFLP | ||||||
| I | R1 | A1 | FAIR-E 21 (=LMG 20636) | Ostrich, cecum; South Africa; 1996 | ND | γ | |
| R1 | S | FAIR-E 23 (=LMG 20638) | Ostrich, cecum; South Africa; 1996 | ND | β | A | |
| R1 | A1 | FAIR-E 25 | Pharmaceutical product; Italy; 1992 | ND | γ | ||
| R1 | A1 | FAIR-E 26 | Black olives (Greek); Germany | ND | γ | A, B | |
| R1 | A1 | FAIR-E 31 (=LMG 20640) | Minipig, feces; Germany | ND | γ | A | |
| R1 | A1 | FAIR-E 34 (=LMG 20643) | Sausage; Germany; 1997 | ND | γ | A | |
| R1 | A1 | FAIR-E 38 | Human, wound pus; Italy; 1994 | vanA | γ | ||
| R1 | S | FAIR-E 41 | Human, clinical; Italy; 1997 | vanA | γ | ||
| R1 | S | FAIR-E 102 (=LMG 20705) | Pig; The Netherlands; 1994 | vanA | γ | ||
| R1 | A3 | FAIR-E 120 (=LMG 20722) | Human, feces; Belgium; 1997 | ND | γ | ||
| R1 | S | FAIR-E 121 (=LMG 20723) | Human, feces; Belgium; 1997 | ND | γ | A, P | |
| R1 | A1 | FAIR-E 128 (=LMG 20730) | Human, wound; The Netherlands; 1995 | vanA | γ | ||
| R1 | A1 | FAIR-E 130 (=LMG 20732) | Human, blood; The Netherlands; 1995 | vanA | γ | ||
| R1 | A1 | FAIR-E 131 (=LMG 20733) | Human, urine; The Netherlands; 1995 | vanA | γ | A, B | |
| R1 | A1 | FAIR-E 132 (=LMG 20734) | Human, rectum; The Netherlands; 1995 | vanA | γ | A | |
| R1 | A1 | FAIR-E 133 (=LMG 20735) | Human, rectum; The Netherlands; 1995 | vanA | γ | ||
| R1 | A1 | FAIR-E 134 (=LMG 20736) | Human, bile; The Netherlands; 1996 | vanA | γ | A, B | |
| R1 | A1 | FAIR-E 135 (=LMG 20737) | Human, blood; The Netherlands; 1996 | vanA | γ | A, B | |
| R1 | A1 | FAIR-E 137 (=LMG 20739) | Human, bile; The Netherlands; 1996 | vanA | γ | A, B | |
| R1 | A1 | FAIR-E 151 (=LMG 14207) | Mozzarella; Belgium; 1993 | ND | γ | ||
| R1 | A1 | FAIR-E 152 (=LMG 15077) | Japanese quail | ND | γ | A, P | |
| R1 | A1 | FAIR-E 153 (=LMG 15080) | European bison, rumen | ND | γ | A | |
| R1 | A1 | FAIR-E 155 (=LMG 16170) | Duck, intestine; Belgium | vanA | γ | ||
| R1 | A1 | FAIR-E 156 (=LMG 16270) | Dog; Belgium | vanA | γ | ||
| R1 | S | FAIR-E 157 (=LMG 16271) | Chicken; Belgium | vanA | γ | ||
| R1 | A1 | FAIR-E 159 (=LMG 16299) | Horse; Belgium; 1990-1991 | ND | γ | ||
| R1 | A1 | FAIR-E 170 | Silage (farm); Belgium | ND | γ | A, B, P | |
| R1 | A1 | FAIR-E 171 | Gouda cheese; Belgium | ND | γ | A, B, P | |
| R1 | A1 | FAIR-E 172 | Calf, rumen; Slovakia | ND | γ | A | |
| R1 | A1 | FAIR-E 178 | Minipig, feces; Germany; 1995 | ND | γ | A, B, P | |
| R1 | A1 | FAIR-E 189 (=LMG 20751) | Human, urine; Germany | vanA | γ | ||
| R1 | A3 | FAIR-E 215 (=LMG 20777) | Feta cheese; Greece; 1995 | ND | γ | ND | |
| R1 | A1 | LMG 11423 T | |||||
| R2 | A2 | FAIR-E 9 (=LMG 20624) | Brine of feta cheese; Greece; 1991 | ND | γ | ||
| R2 | A2 | FAIR-E 50 (=LMG 20655) | Fontina cheese; Italy; 1980 | ND | γ | ||
| R2 | A2 | FAIR-E 83 (=LMG 20687) | Semihard cheese; Italy; 1997 | ND | γ | ||
| R2 | A2 | FAIR-E 119 (=LMG 20721) | Human, feces; Belgium; 1997 | ND | γ | A, B, P | |
| R2 | A2 | FAIR-E 365 (=LMG 20908) | Scamorza cheese; Italy; 1997 | ND | γ | ||
| R3 | A3 | FAIR-E 3 (=LMG 20618) | Feta cheese; Greece; 1988 | ND | γ | A, 31 | |
| R3 | A3 | FAIR-E 6 (=LMG 20621) | Kasseri cheese; Greece; 1994 | ND | γ | ||
| R3 | A4 | FAIR-E 13 (=LMG 20628) | Brine of feta cheese; Greece; 1992 | ND | γ | A, 31 | |
| R3 | A3 | FAIR-E 80 (=LMG 20684) | Asiago cheese; Italy; 1997 | ND | γ | ||
| R3 | A1 | FAIR-E 84 | Soft cheese; Italy; 1997 | vanA | γ | A, P | |
| R3 | A3 | FAIR-E 118 (=LMG 20720) | Human, feces; Belgium; 1997 | ND | γ | A, P | |
| R3 | A3 | FAIR-E 154 (=LMG 15709) | Cheese | ND | γ | ||
| R3 | A4 | FAIR-E 196 (=LMG 20758) | Feta cheese; Greece; 1995 | ND | βhf | A, P | |
| R3 | A4 | FAIR-E 198 (=LMG 20760) | Feta cheese; Greece; 1995 | ND | γ | A, P | |
| R3 | A4 | FAIR-E 201 (=LMG 20763) | Feta cheese; Greece; 1995 | ND | βh | A, P | |
| R3 | A4 | FAIR-E 202 (=LMG 20764) | Feta cheese; Greece; 1995 | ND | βh | A, P | |
| R3 | A4 | FAIR-E 206 (=LMG 20768) | Feta cheese; Greece; 1995 | ND | βh | A, P | |
| R3 | A3 | FAIR-E 207 (=LMG 20769) | Feta cheese; Greece; 1995 | ND | γ | A, P | |
| R3 | A3 | FAIR-E 212 (=LMG 20774) | Feta cheese; Greece; 1995 | ND | γ | A, P | |
| R3 | A4 | FAIR-E 218 (=LMG 20780) | Feta cheese; Greece; 1995 | ND | γ | A, 31 | |
| R3 | S | FAIR-E 225 (=LMG 20787) | Cheddar-type cheese; Ireland; 1994 | ND | γ | ||
| R3 | A3 | FAIR-E 227 (=LMG 20789) | Cheddar cheese; Ireland; 1994 | ND | γ | ND | |
| R3 | A3 | FAIR-E 243 (=LMG 20804) | Casu-Axedu cheese (Sardinia); 1992-1993 | ND | γ | ||
| R3 | A2 | FAIR-E 254 (=LMG 20814) | Manchego cheese; Spain; 1992-1993 | ND | γ | ||
| R3 | A3 | FAIR-E 383 (=LMG 20926) | Raw milk; Italy; 1969 | ND | γ | ||
| R4 | A3 | FAIR-E 150t1 (=LMG 14204t1) | Goat cheese with pork meat; Belgium; 1993 | ND | γ | A, P | |
| R4 | A3 | FAIR-E 338 (=LMG 20890) | Asiago cheese; Italy; 1997 | ND | γ | ||
| R4 | A3 | FAIR-E 345 (=LMG 20897) | Asiago cheese; Italy; 1997 | ND | γ | ||
| S | A1 | FAIR-E 158 (=LMG 16297) | Pig; Belgium; 1989 | vanA | γ | ||
| II | R5 | A5 | FAIR-E 15 (=LMG 20630) | Dawadawa (soumbala); Burkina Faso; 1995 | ND | γ | |
| R5 | A5 | FAIR-E 149 (=LMG 14203) | Pig, heart meat; Belgium; 1993 | ND | γ | ND | |
| R5 | A5 | FAIR-E 160t1 (=LMG 13597t1) | Vegetables; Belgium | ND | γ | ||
| R5 | A5 | FAIR-E 394 (=LMG 20937) | Human, feces; Ireland; 1997 | ND | γ | ||
| R5 | A5 | FAIR-E 396 (=LMG 20939) | Human, feces; Ireland; 1997 | ND | γ | ||
| R5 | A5 | FAIR-E 400 (=LMG 20943) | Human, feces; Ireland; 1997 | ND | γ | ||
| R5 | A5 | FAIR-E 403 (=LMG 20946) | Human, feces; Ireland; 1997 | ND | γ | ||
| R6 | A6 | FAIR-E 20 (=LMG 20635) | Swiss cheese salad; Germany; 1990 | ND | γ | ND | |
| R6 | A6 | FAIR-E 24 | Pharmaceutical product; Italy; 1992 | ND | γ | ND | |
| R6 | A6 | FAIR-E 27 (=LMG 20639) | Minipig, feces; Germany | ND | γ | P | |
| R6 | A6 | FAIR-E 210 (=LMG 20772) | Feta cheese; Greece; 1995 | ND | γ | P | |
| R6 | A6 | FAIR-E 266 (=LMG 20824) | Monte Veronese cheese; Italy; 1997 | ND | γ | ND | |
| R6 | A6 | FAIR-E 280 (=LMG 20836) | Monte Veronese cheese; Italy; 1997 | ND | γ | ||
| R6 | A6 | FAIR-E 349 (=LMG 20901) | Montasio cheese; Italy; 1997 | ND | γ | ||
| R7 | A7 | FAIR-E 362 (=LMG 20905) | Ricotta cheese; Italy; 1997 | ND | γ | A, P | |
| R7 | A7 | FAIR-E 366 (=LMG 20909) | Scamorza cheese; Italy; 1997 | ND | γ | A, P, 31 | |
Groups as delineated in Fig. 1; S, separate position.
FAIR-E, collection of the EU-project FAIR-CT-3078 (57); LMG, BCCM/LMG Bacteria Collection, Laboratorium voor Microbiologie, Universiteit Gent, Ghent Belgium.
Presence of the vanA gene was confirmed for all strains showing resistance to vancomycin and teicoplanin using the broth dilution technique. ND, no resistance observed using the broth dilution technique and van genes were not detected.
Beta (β)- and gamma (γ)-hemolytic activity was determined on human blood and sheep blood.
Bacteriocin gene determination in strains showing inhibitory activity on plate toward one or more indicator strains tested. ND, strain showing inhibitory activity on plate, but none of the tested genes was detected.
βh, beta-hemolysis only on human blood and not on sheep blood.
PAGE of whole-cell proteins.
Whole-cell protein extracts were prepared, and one-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE) analysis was performed as described by Pot et al. (50). A densitometric analysis, normalization and interpolation of the protein profiles, and a numerical analysis were performed by using the GelCompar software package (version 3.1 and 4.2, respectively; Applied Maths).
RAPD-PCR analysis.
DNA was extracted according to the method of Pitcher et al. (49). The following four random primers were used for DNA amplification: M13 (31), AP4 (4), 1253 (1), and D8635 (1). Amplification, electrophoresis, pattern recognition, and normalization were performed as described previously (2). As in reference 2, two different reaction conditions were applied for primer D8635.
For each strain, the normalized profiles obtained with the four different primers were assembled one after the other into a combined profile using GelCompar version 4.1 software. These combined patterns were imported into the Bionumerics version 1.5 software (Applied Maths) and were analyzed by using the Pearson product moment correlation coefficient and the unweighted pair group with mathematical average clustering algorithm (UPGMA).
AFLP analysis.
Genomic DNA was isolated by using the DNA isolation kit Puregene (Gentra Systems), according to the protocol supplied by the manufacturer. The preparation of templates (using a combination of the restriction enzymes HindIII and MseI) and a preselective PCR amplification was performed as described by Gancheva et al. (25). For selective PCR amplification the following primers were used: M01-ABI (5′GATGAGTCCTGAGTAAA3′), M02-ABI (5′GATGAGTCCTGAGTAAC3′), and the fluorescently labeled primer H01-6FAM (5′GACTGCGTACCAGCTTA3′) (Pharmacia Biotech) (selective bases at the 3′ end are underlined). PCRs with two primer combinations were performed: H01-6FAM and M01-ABI as well as H01-6FAM and M02-ABI. The selective PCR amplification, sample preparation and separation on a denaturing polyacrylamide gel on an ABI Prism 377 DNA sequencer was as described previously (25).
The data were registered during the electrophoresis run by the ABI Prism 377 data collection software and analyzed with Gene Scan 2.1 software (Applied Biosystems). ABICON software (Applied Maths) was used to import the patterns in GelCompar version 4.2 for normalization. These profiles were transferred to Bionumerics version 1.5 software where patterns generated with both primer combinations were analyzed separately using the Pearson product moment correlation coefficient. The UPGMA cluster analysis was performed using the “composite data set” comparison tool in which the similarity matrices of both individual experiments are averaged.
PFGE analysis.
Cells from 5 ml of an overnight-grown tryptone soy broth (Oxoid) culture were used for DNA isolation. The in situ DNA isolation protocol and digestion of the agarose-embedded DNA were performed as described by Björkroth et al. (7). SmaI restriction endonuclease was selected (Promega) due to its good ability to characterize Enterococcus DNA in PFGE experiments (41, 47). The samples were electrophoresed through 1.0% (wt/vol) agarose gel (SeaKem Gold; FMC BioProducts) in 0.5× TBE (45 mM Tris, 4.5 mM boric acid [pH 8.3], and 1 mM sodium EDTA) at 14°C in a CHEF-DR III system (Bio-Rad). Interpolation ramping from an initial pulse time of 0.1 s to a final 25 s for 18 h at 6 V/cm with a 120° angle was used.
Photographs of the PFGE banding patterns were scanned by Hewlett-Packard ScanJet 4c/T scanner. Numerical analysis of macrorestriction patterns was performed using the GelCompar system version 4.2 (Applied Maths). Based on the banding patterns of internal controls, 1% position tolerance was allowed between the strains. The similarity between all pairs was expressed by a Dice coefficient correlation, and UPGMA clustering was used for the construction of the dendrogram.
DNA-DNA hybridization experiments.
DNA extraction, determination of the G+C content, and DNA-DNA hybridizations were performed as described previously (60).
Phenotypic identification.
The isolates were phenotypically identified with the rapid ID 32 STREP system according to the protocol specified by the manufacturer (bioMérieux).
Hemolysis activity.
Production of hemolysin was determined by streaking the Enterococcus cultures (grown in MRS broth for 18 h) on both human and sheep blood agar plates. Human blood agar was prepared using Columbia blood agar base (BBL) with 5% defibrinated human blood (containing all four blood types) added according to the manufacturer's instructions. Sheep blood agar was commercially available (BBL). Blood agar plates were incubated at 37°C for 24 h, after which plates were examined for hemolysis. Presence and absence of zones of clearing around the colonies were interpreted as beta-hemolysis and gamma-hemolysis activity, respectively.
Detection of glycopeptide resistance.
Susceptibilities to vancomycin and teicoplanin were checked using the broth dilution technique. Strains were inoculated in Trypticase soy broth (Oxoid) with 3 g of yeast extract liter−1 and with either vancomycin or teicoplanin at a concentration ranging from 2 to 64 μg ml−1. After an overnight incubation at 37°C, growth was noted and the MIC (the first dilution at which no growth was observed) was determined. The presence of van genes was checked using the PCR as described by Dutka-Malen et al. (19). Four different primer pairs (vanA, vanB, vanC1, and vanC2) were used in the assay.
Antimicrobial testing and detection of bacteriocin structural genes.
Sixteen-hour cultures of enterococci were checked for inhibitory activity by the agar well diffusion assay towards Clostridium tyrobutyricum LMG 1285T or the agar spot test towards Listeria innocua LMG 11387T and LMG 13568 and Propionibacterium freudenreichii subsp. shermanii LMG 16424T as described in detail by Andrighetto et al. (2). Strains showing a positive reaction in one of the above-mentioned tests were further genotypically investigated by PCR for the presence of structural genes for the bacteriocins enterocin A (3), enterocin B (3), enterocin P (10), and bacteriocin 31 (57). The specific primers used for PCR amplification of these genes are listed in Table 2. PCR amplification was done using total DNA isolated by the rapid alkaline lysis method as described in detail by Dutka-Malen et al. (18). In a first series of PCRs, 2 μl of total DNA was used as a template. Two microliters of the resulting PCR amplifications were used as a template in a second series of nested PCRs. PCR was performed on a DNA thermal cycler (Biometra Thermocycler T3) in a final volume of 50 μl containing 1× PCR buffer (20 mM Tris-HCl [pH 8.4], 50 mM KCl), 1.5 mM MgCl2, a 200 μM concentration of each of the four deoxynucleoside triphosphates, a 0.5 μM concentration of each of the primers, and 1.25 U of Taq DNA polymerase (GibcoBRL). The cycles used were 95°C for 5 min for the first cycle; 95°C for 30 s, 58 or 56°C for 30 s, and 72°C for 30 s for the next 30 cycles; and 72°C for 5 min for the last cycle. PCR products were resolved by electrophoresis on 8% polyacrylamide-Tris-acetate-EDTA gel. The total DNA of E. faecium CTC 492 (encoding the enterocins A and B [3]) and plasmid DNA of E. faecalis FA2-2 carrying the plasmid pYI171 (encoding bacteriocin 31 [57]) was used as a positive control for the corresponding primers. No control was available for enterocin P; therefore, PCR amplicons were sequenced with an automated DNA sequencer (ALF DNA sequencer; Amersham) to confirm specific enterocin P sequences.
TABLE 2.
Specific terminal and nested primers for the PCR detection of the enterocin structural genes
| Enterocin | Forward primer | Nested primer | Reverse primer |
|---|---|---|---|
| A | 5′-GGT ACC ACT CAT AGT GGA AA-3′ | 5′-AAT GTA CGG TCG ATT GGG CCA-3′ | 5′-CCC TGG AAT TGC TCC ACC TAA-3′ |
| B | 5′-CAA AAT GTA AAA GAA TTA AGT ACG-3′ | 5′-AAC TTA TCT AAA GGT GGA GCA-3′ | 5′-AGA GTA TAC ATT TGC TAA CCC-3′ |
| P | 5′-GCT ACG CGT TCA TAT GGT AAT-3′ | 5′-GCT AAA GAG AAT ATT GCA GGA-3′ | |
| 31 | 5′-CCT ACG TAT TAC GGA AAT GGT-3′ | 5′-TGG GTA GAC TGG AAT AAA GCT-3′ | 5′-AAT GGT TGG GTA CAA CAT GGC-3′ |
RESULTS
PAGE of whole-cell proteins.
The level of reproducibility was investigated by the inclusion of duplicate protein extracts of several strains, as well as repetitions of the electrophoretic runs, and an average correlation of 0.94 was obtained. All isolates were identified to the species level as E. faecium by pairwise numerical comparison with an extensive in-house database, present at BCCM/LMG, which comprises multiple well-characterized reference strains of all validly described Enterococcus species (data not shown). Also, after the construction of a dendrogram, the strains grouped with other E. faecium strains in a single cluster clearly differentiated from other enterococcal species (data not shown).
RAPD-PCR analysis.
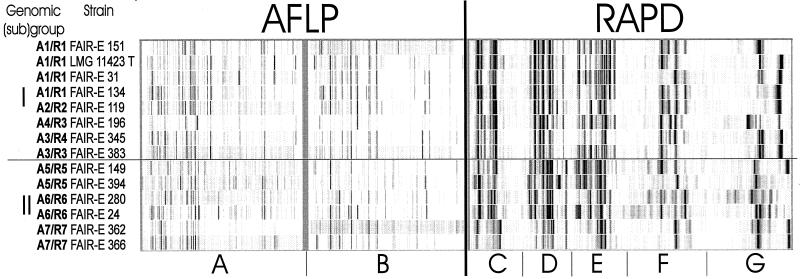
The reproducibility of PCR assays and running conditions estimated by analysis of duplicate DNA extracts of several strains was 0.91. Purified DNA of all E. faecium strains was used as template in five amplification reactions with single primers. To increase the accuracy and discriminative power of the method, the electrophoretic patterns were combined for numerical analysis. Figure 1 (left dendrogram) shows the UPGMA tree. At a correlation level of 0.34, two main groups were distinguished, indicated as group I and II. Each group was further subdivided into, respectively, four (R1 to R4) and three (R5 to R7) subclusters. A photograph of the digitized band patterns obtained with the different PCR assays for representative strains of each of the subclusters is given in Fig. 2. The differences in the band patterns of the two main groups (I and II) were substantial.
FIG. 1.
Dendrograms derived from UPGMA linkage of r values (expressed as percentages) of RAPD-PCR (left side) and AFLP (right side) profiles. Strains in boldface type group in the corresponding subcluster in both analyses.
FIG. 2.
Digitized and normalized patterns of selected strains obtained with AFLP fingerprinting with two primer combinations: H01-6FAM and M01-ABI (A) and H01-6FAM and M02-ABI (B) and RAPD-PCR fingerprinting using four primers—M13 (C), D8635 (D), AP4 (E), and 1253 (F)—and primer D8635 (G) (primer used with two different reaction conditions).
AFLP analysis.
The reproducibility of the method was evaluated by analysis of duplicates of strains with independently prepared templates, different preselective PCR experiments, different selective PCR experiments, and different electrophoretic runs. The correlation levels varied between 0.84 and 0.94. AFLP analysis, performed with two primer combinations (H01-6FAM and M01-ABI; H01-6FAM and M02-ABI), resulted in 30 to 40 bands per combination which varied from 50 to 500 bp. By averaging the similarity matrices of both experiments, a resulting dendrogram was obtained which clearly divided the species into two main groups, groups I and II (Fig. 1; right dendrogram). As demonstrated in Fig. 2, significant differences were visually observed between the two groups. Each group was further subdivided into, respectively, four (A1 to A4) and three (A5 to A7) subclusters.
PFGE analysis.
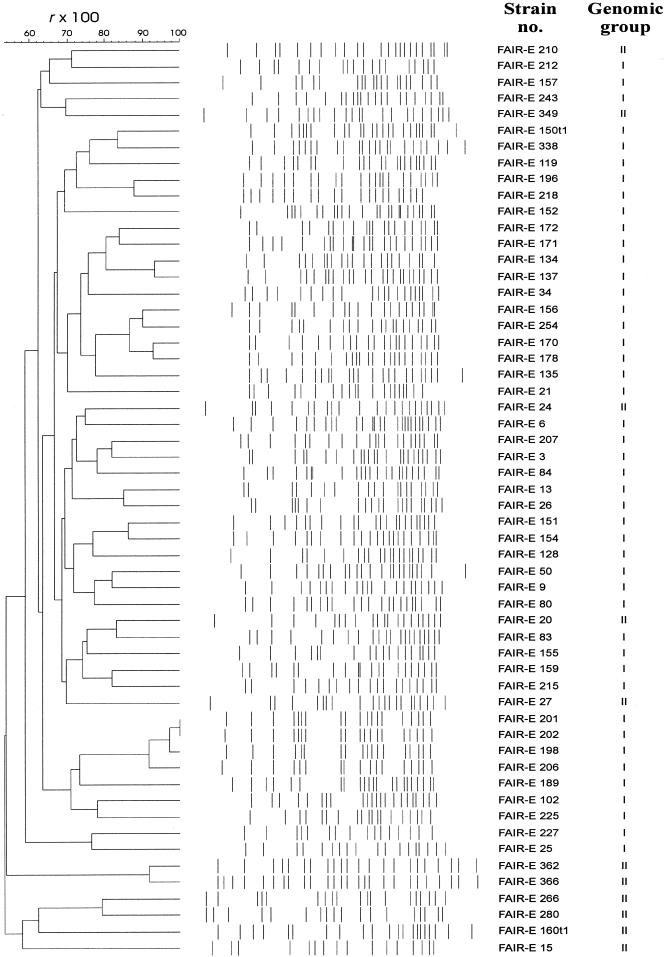
The reproducibility between different restriction digests and electrophoretic runs for genomes of individual strains was 0.93. The number of bands per strain varied between 16 and 26. Cluster analysis and visual inspection of the patterns (Fig. 3) showed a high variability among the patterns obtained. High similarity was observed only between a few pairs of isolates (see discussion).
FIG. 3.
Dendrogram derived from UPGMA linkage of r values (expressed as percentages) and normalized banding patterns of PFGE fingerprints.
DNA-DNA hybridization experiments.
Three representative isolates from group I and II and the type strain of E. faecium were selected for DNA-DNA hybridizations. Standard deviations of 7% were observed between duplicate analyses. The data demonstrated that within each main group (groups I and II) a mean homology value of 82% was found, whereas a lower value of 71% was calculated between the main groups (Table 3).
TABLE 3.
Percentage DNA-DNA binding by reassociation studies and G+C mol% of E. faecium strains
| Genomic groupa | Strain | G+C (mol %) | % DNA-DNA binding with strain:
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| LMG 11324 T | FAIR-E 128 | FAIR-E 365 | FAIR-E 206 | FAIR-E 400 | FAIR-E 210 | FAIR-E 366 | |||
| I | LMG 11324 T | 38.3 | 100 | ||||||
| FAIR-E 128 | 38.5 | 89 | 100 | ||||||
| FAIR-E 365 | 38.5 | 90 | 84 | 100 | |||||
| FAIR-E 206 | 37.4 | 74 | 76 | 79 | 100 | ||||
| II | FAIR-E 400 | 38.1 | 76 | 75 | 65 | 67 | 100 | ||
| FAIR-E 210 | 38.9 | 78 | 71 | 71 | 72 | 83 | 100 | ||
| FAIR-E 366 | 38.7 | 75 | 71 | 69 | 67 | 77 | 85 | 100 | |
See Fig. 1.
Phenotypic identification.
The isolates were phenotypically identified with the rapid ID 32 STREP system. The following features are characteristic of all of the strains studied: arginine dihydrolase enzyme activity and acid production from maltose and ribose. None of the strains showed β-glucuronidase, alkaline phosphatase, or urease enzyme activity or acidified pullulan. All other characteristics tested and listed in Table 4 were strain dependent. No features were found that differentiate genomic groups I and II.
TABLE 4.
Phenotypic characterization of the E. faecium strains studieda
| Characteristic | % of strains showing characteristic in genomic group:
|
|
|---|---|---|
| I (n = 62) | II (n = 16) | |
| Acetoin production | 97 | 94 |
| Alanine-phenylalanine-proline arylamidase | 2 | 0 |
| Pyroglutamic acid arylamidase | 98 | 100 |
| Glycyl-tryptophan arylamidase | 59 | 50 |
| α-Galactosidase | 34 | 63 |
| β-Galactosidase | 10 | 0 |
| β-Galactosidaseb | 84 | 81 |
| β-Glucosidase | 82 | 100 |
| N-Acetyl-β-glucosaminidase | 49 | 69 |
| Hydrolysis of hippurate | 51 | 44 |
| β-Mannosidase | 75 | 87 |
| Acidification of: | ||
| l-Arabinose | 92 | 81 |
| d-Arabitol | 2 | 6 |
| Cyclodextrin | 75 | 100 |
| Methyl-β-d-glucopyranoside | 80 | 81 |
| Glycogen | 0 | 19 |
| Lactose | 98 | 88 |
| Mannitol | 80 | 100 |
| Melezitose | 0 | 19 |
| Melibiose | 28 | 60 |
| Raffinose | 2 | 31 |
| Saccharose | 62 | 88 |
| Sorbitol | 0 | 19 |
| Tagatose | 13 | 38 |
| Trehalose | 97 | 100 |
All strains were positive for the arginine dihydrolase enzyme activity and acidification of maltose and ribose. None of the strains showed β-glucuronidase, alkaline phosphatase, or urease enzyme activity or acidified pullulane.
Measured by addition of Fast Blue BB reagent.
Hemolysis activity.
Beta-hemolytic activity on both human and sheep blood was observed for only one strain: FAIR-E 23 (Table 1). Four strains (FAIR-E 196, FAIR-E 201, FAIR-E 202, and FAIR-E 206) showed beta-hemolysis on human blood, but not on sheep blood. No hemolytic activity was observed for any of the other strains studied.
Detection of glycopeptide resistance.
Using the broth dilution technique, MICs of vancomycin and teicoplanin were determined. Subsequently, a screening was performed for the presence of van genes and confirmed that all strains for which the MIC of vancomycin was in a range of 32 to >64 μg ml−1 and the MIC of teicoplanin was in a range of 4 to 64 μg ml−1 contain the vanA gene (Table 1). For all other strains, the MIC of both antibiotics was 2 μg ml−1 and no van genes were detected.
Antimicrobial testing.
The presence of one or more enterocin genes (A, B, P, and 31) in strains showing antimicrobial activity towards one of the four indicator strains used (see Materials and Methods) is shown in Table 1. For six strains showing inhibition on plates, none of the above-mentioned genes were detected and for 34 strains one or more of the enterocins A, B, P, and 31 were present.
DISCUSSION
To our knowledge, only a few studies have revealed correlations between strain relatedness and origin or pathogenicity of E. faecium strains. Devriese and Pot (15) demonstrated host species-associated biochemical characters for (e.g.) poultry isolates which were described as usually raffinose positive and for canine strains which were described as sorbitol positive, and most bovine and canine strains produced acid from d-xylose, while strains from other origins were d-xylose negative. Teixeira et al. (55) also observed a considerable phenotypic heterogeneity among human strains (10 phenotypes were recognized), and it was suggested that some of these phenotypic markers might be useful in the detection of antibiotic-resistant strains and to follow their transmission. Willems et al. (62) and Quednau et al. (51) used genotypic results for determining relatedness of antibiotic-resistant E. faecium strains and concluded that strains from chicken, pork, and human origin were host specific. In these two studies, conclusions were based on the interpretation of AFLP and REA groupings, respectively. In these typing studies mentioned (15, 51, 55, 62), strain relatedness was investigated using a single methodology. We emphasize, however, that there are no objective means for validating the genomic relationships at the intraspecies level unless evidence of congruency is provided using different typing approaches.
Diversity of the strain collection.
Strains originated from Belgium, Germany, Greece, Ireland, Italy, and The Netherlands, and a few strains were from other European and non-European countries. The study not only included human (clin-ical and nonclinical isolates) and animal (isolates from at least 10 different animals) strains, but also strains originating from food (mainly isolates from cheese) (Table 1). PFGE (using SmaI), which is currently considered to be the “gold standard” for strain differentiation of enterococci (4, 26, 30, 34, 56), was applied on the majority of the strains to verify whether the isolates represent different strains or may be considered clones of the same parent strain. Although some uncertainty remains as to how much similarity isolates should demonstrate in order to be called clonally related (56), we observed a significant diversity among most isolates in order to consider them to originate from different clonal lineages (Fig. 3). Considering the very diverse origin and habitat of the selected cultures, this strain diversity was expected and also confirmed. Numerical and visual comparison of PFGE patterns (Fig. 3) suggests that only the following isolates are possibly clonal lineages: two isolates from human bile in The Netherlands (FAIR-E 134 and FAIR-E 137), four isolates from feta cheese in Greece (FAIR-E 198, FAIR-E 201, FAIR-E 202, and FAIR-E 206), and two isolates from Italian cheeses (FAIR-E 362 and FAIR-E 366).
Delineation of intraspecies genomic groups.
For detecting the intraspecies strain relationships, we applied RAPD-PCR and AFLP, both with multiple primers or primer combinations in order to enlarge the number of bands and to avoid biasing of the numerical analysis due to the presence of too few bands. Although both approaches analyze entire genomes, a crucial difference between the two techniques is that RAPD-PCR is prone to variations in amplification efficiency due to small variations in experimental conditions or the quality of chemicals which may affect the reproducibility of the method, whereas AFLP primers perfectly match their target site and mismatches are not expected to participate in the amplification process (32).
The very high congruency between the cluster analyses obtained by both methods is remarkable (Fig. 1). In both analyses, two main groups, groups I and II, were delineated, comprising 62 and 16 strains, respectively. Within genomic group I, four subclusters were recognized (R1 to R4 and A1 to A4, respectively). A high correlation was found in subclusters R1 and A1 and in R2 and A2, respectively. Most of the strains from the clusters R3 and R4 were found in the AFLP clusters A3 and A4, but not necessarily in the corresponding cluster number. Only six strains assigned to a particular subcluster in the RAPD-PCR dendrogram occupied separate positions in the AFLP analysis. In group II, three identical subclusters with exactly the same set of strains were found in the RAPD-PCR and AFLP cluster analysis (R5 to R7 and A5 to A7, respectively).
No literature data have thus far suggested the presence of two genomic groups in E. faecium, which were also easily recognized after analyses with single primers or primer combinations and not only after combined numerical analyses (Fig. 2; data not shown). Therefore, in a first approach, we investigated whether these two groups represent different genomic species or not. Respectively, four and three strains were selected from different subclusters of both groups (including the type strain), and DNA-DNA hybridizations were performed. A mean DNA homology of 71% was observed between the main groups I and II, whereas in both groups the level of DNA relatedness was significantly higher and showed a mean value of 82% (Table 3). Although there is a clear indication of a lower overall DNA homology between the two groups than within the individual groups, the data confirm that all strains constitute a single genomic species on the basis of the recommendations proposed by Wayne et al. (61). With the rapid ID 32 STREP strip, no phenotypic features were found which clearly differentiate the two genomic groups. Among the many variable features (Table 4), we only noticed different percentages of strains that are positive for particular tests.
Correlation with origin, pathogenicity, and bacteriocinogeny.
Interestingly, comparison of the origin of the strains of the different groups and subclusters revealed that the main groups I and II both comprise strains from (healthy) humans, animals, and food and that no correlation is found with the country of isolation. However, all human clinical strains clustered in group I. Regarding the source of the strains per subcluster in group I, we noticed that all animal isolates and human clinical strains grouped in a single subcluster (R1 or A1), whereas nearly all the food strains grouped in three clusters (R2 to R4 or A2 to A4). Isolates from healthy humans are spread over the different subclusters. In group II, subclusters R5 and R6 contain strains from different countries from food, animal, and human (only R5) origin. Subcluster R7 contains only two Italian food isolates, for which a clonal relationship is suggested (Fig. 3). Considering the low number of strains in group II, conclusions concerning origin in the delineated subclusters are, however, premature.
The genotypic grouping was also compared with possible pathogenic traits of the strains. All resistant strains (containing the vanA gene) clustered in group I, and the majority of them belonged to subcluster R1 and/or A1 (a separate position in one of the analyses was observed for strains FAIR-E 41, FAIR-E 102, FAIR-E 157, and FAIR-E 158). FAIR-E 84 is the only vanA strain that grouped inconsistently by RAPD-PCR and AFLP with regard to its position in subclusters R3 and A1, respectively. All strains were also tested for their hemolytic activity on human and sheep blood. Only five strains showed beta-hemolytic activity on at least one of the blood types and all clustered in genomic group I. One strain, FAIR-E 23, isolated from ostrich in South Africa, was beta-hemolytic on both blood types and grouped in subcluster R1 or occupied a separate position as seen by AFLP analysis. Four strains, all isolated from feta cheese in Greece, were hemolytic only on human blood. They all grouped in subclusters R3 and A4, respectively. The isolation of hemolytic enterococci from cheese may have sanitary implications and also illustrates the notion that virulence factors may occur in enterococci originating from food, as has previously been demonstrated (20, 22).
About half of the strains displayed antagonistic activity towards food-spoiling bacteria or related taxa (C. tyrobutyricum, L. innocua, or P. freudenreichii), and for most of them the presence of one or more of the enterocin genes A, B, P, and 31 was confirmed. All enterocin B-containing strains are found in group I, and with the exception of one strain (FAIR-E 119), all belonged to subcluster R1. Six of the eight strains for which inhibitory activity was seen on plates and for which no known genes were detected belonged to genomic group II. In the latter group, only the two strains of subcluster R7 (A7) contain known but different bacteriocin genes.
When comparing data from the present study with those presented by Willems et al. (62) and Quednau et al. (51), host specificity for human and animal strains (although for most animals only one or a few strains were investigated) is not confirmed by our results. In the study of Willems et al. (62) host specificity of vancomycin-resistant E. faecium isolates was suggested for pigs, chickens, calves, and humans. For the humans, the authors furthermore distinguished between strains from nonhospitalized and hospitalized individuals. Although there is a major difference to our study, in that the majority of the strains are isolated from food and are susceptible to vancomycin, the use of the term “host specificity” in the study of Willems et al. (62) may be questioned, given that farmers and slaughterers (also nonhospitalized individuals) contain the same flora as the animals they are working with. Furthermore, the restricted geographic origin of most of their isolates (mostly from The Netherlands) and presumed epidemiological relatedness among isolates (demonstrated for part of the hospitalized persons; other hosts not studied) are points of discussion. In this context, and as suggested in the discussion of Willems et al. (62), the term “ecovar specificity” would be more appropriate until a more diverse set of isolates is studied. The separate cluster obtained for strains from hospitalized persons nevertheless remains remarkable, although we should bear in mind that these vancomycin-resistant strains may be endemic in the hospital(s) and should not necessarily be seen as part of the natural flora of the patients as suggested in the work of Willems et al. (62).
Host specificity of E. faecium isolates from chicken, pork, and humans is also suggested by Quednau et al. (51), but contrary to the study of Willems et al. (62) no subdivision was obtained according to clinical or healthy-subject strains. The authors delineate multiple clusters for each host, often consisting of only a few isolates from the same and restricted geographic origin. This could be an indication that REA, used in the study, is measuring clonal relationships and not relatedness between epidemiologically unrelated strains. We presume that, as for PFGE analysis, the deeper branches in the REA cluster analysis might not provide valuable information on the genomic relationships among epidemiologically nonrelated enterococcal strains. This cannot, however, be verified as no second typing approach (using an alternative technique) was applied by Quednau et al. (51) on the same set of strains to confirm the REA grouping.
Conclusion.
Data from the present study demonstrate that two main genomic groups, groups I and II, are recognized in the species E. faecium. No host specificity or correlation with the country of isolation is found, but the investigated human clinical strains, antibiotic-resistant strains, and beta-hemolytic strains are found only in genomic group I. In this genomic group, animal and human clinical strains grouped in a single subcluster (including the majority of vancomycin-resistant and enterocin B-producing strains), while nearly all strains originating from food grouped in the other subclusters. Human strains from healthy individuals are found in genomic groups I and II.
Should a separate grouping of more extended sets of potentially pathogenic strains be confirmed in future studies, the observed genomic heterogeneity within the species E. faecium may be useful for the selection of safe strains for starter cultures in food or probiotic preparations.
Acknowledgments
This work was supported by the European Communities EC project “Enterococci in food fermentations. Functional and safety aspects” (program FAIR-CT97-3078). The work of K. J. Björkroth was financially supported by a grant from the National Academy of Finland. E. Knijff was a recipient of Marie Curie TMR grant FAIR-98-5034. M. R. Foulquié Moreno was a recipient of a Marie Curie Fellowship (grant FAIR-CT97-5013).
We thank Karen Lefebvre for her skilled technical assistance.
REFERENCES
- 1.Akopyanz, N., N. O. Bukanov, T. U. Westblom, S. Kresovich, and D. E. Berg. 1992. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acid Res. 20:5137-5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrighetto, C., E. Knijff, A. Lombardi, S. Torriani, M. Vancanneyt, K. Kersters, J. Swings, and F. Dellaglio. 2001. Phenotypic and genetic diversity of enterococci isolated from Italian cheeses. J. Dairy Res. 68:303-316. [DOI] [PubMed] [Google Scholar]
- 3.Aymerich, T., H. Holo, L. S. Havarstein, M. Hugas, M. Garriga, and I. F. Nes. 1996. Biochemical and genetic characterization of enterocin A from Enterococcus faecium, a new antilisterial bacteriocin in the pediocin family of bacteriocins. Appl. Environ. Microbiol. 62:1676-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbier, N., P. Saulnier, E. Chachaty, S. Dumontier, and A. Andremont. 1996. Random amplified polymorphic DNA typing versus pulsed-field gel electrophoresis for epidemiological typing of vancomycin-resistant enterococci. J. Clin. Microbiol. 34:1096-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bates, J., Z. Jordens, and J. B. Selkon. 1993. Evidence for an animal origin of vancomycin-resistant enterococci. Lancet 342:490-491. [DOI] [PubMed] [Google Scholar]
- 6.Bates, J., J. Z. Jordens, and D. T. Griffiths. 1994. Farm animals as a putative reservoir for vancomycin-resistant enterococcal infection in man. J. Antimicrob. Chemother. 34:507-516. [DOI] [PubMed] [Google Scholar]
- 7.Björkroth, J., J. Ridell, and H. Korkeala. 1996. Characterization of Lactobacillus sake strains associated with production of ropy slime by randomly amplified polymorphic DNA (RAPD) and pulsed-field gel electrophoresis (PFGE) patterns. Int. J. Food Microbiol. 31:59-68. [DOI] [PubMed] [Google Scholar]
- 8.Centeno, J. A., S. Menéndez, and J. L. Rodríguez-Otero. 1996. Main microbial flora present as natural starters in Cebreiro raw cow's-milk cheese (Northwest Spain). Int. J. Food Microbiol. 33:307-313. [DOI] [PubMed] [Google Scholar]
- 9.Chenoweth, C., and D. Schaberg. 1990. The epidemiology of enterococci. Eur. J. Clin. Microbiol. Infect. Dis. 9:80-89. [DOI] [PubMed] [Google Scholar]
- 10.Cintas, L. M., P. Casaus, L. S. Havarstein, P. E. Hernandez, and I. F. Nes. 1997. Biochemical and genetic characterization of enterocin P, a novel sec-dependent bacteriocin from Enterococcus faecium with a broad antimicrobial spectrum. Appl. Environ. Microbiol. 63:4321-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devriese, L. A., J. L. Cruz Colque, F. Haesebrouck, M. Desmidt, E. Uyttebroek, and R. Ducatelle. 1992. Identification and composition of the tonsillar and anal enterococcal and streptococcal flora of dogs and cats. J. Appl. Bacteriol. 73:421-425. [DOI] [PubMed] [Google Scholar]
- 12.Devriese, L. A., J. Hommez, B. Pot, and F. Haesebrouck. 1994. Identification and composition of the streptococcal and enterococcal flora of tonsils, intestines and faeces of pigs. J. Appl. Bacteriol. 77:31-36. [DOI] [PubMed] [Google Scholar]
- 13.Devriese, L. A., J. Hommez, R. Wijfels, and F. Haesebrouck. 1991. Composition of the enterococcal and streptococcal intestinal flora of poultry. J. Appl. Bacteriol. 71:46-50. [PubMed] [Google Scholar]
- 14.Devriese, L. A., L. Laurier, P. De Herdt, and F. Haesebrouck. 1992. Enterococcal and streptococcal species isolated from faeces of calves, young cattle and dairy cows. J. Appl. Bacteriol. 72:29-31. [DOI] [PubMed] [Google Scholar]
- 15.Devriese, L. A., and B. Pot. 1995. The genus Enterococcus, p. 327-367. In B. J. B. Wood and W. H. Holzapfel (ed.), The lactic acid bacteria, vol. 2. The genera of lactic acid bacteria. Blackie Academic, London, United Kingdom. [Google Scholar]
- 16.Donabedian, S. M., J. W. Chow, J. M. Boyce, R. E. McCabe, S. M. Markowitz, P. E. Coudron, A. Kuritza, C. L. Pierson, and M. J. Zervos. 1992. Molecular typing of ampicillin-resistant, non-β-lactamase-producing Enterococcus faecium isolates from diverse geographic areas. J. Clin. Microbiol. 30:2757-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunne, W. M., and W. Wang. 1997. Clonal dissemination and colony morphotype variation of vancomycin-resistant Enterococcus faecium isolates in metropolitan Detroit, Michigan. J. Clin. Microbiol. 35:388-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dutka-Malen, S., R. Leclercq, V. Coutant, J. Duval, and P. Courvalin. 1990. Phenotypic and genotypic heterogeneity of glycopeptide resistance determinants in gram-positive bacteria. Antimicrob. Agents Chemother. 34:1875-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutka-Malen, S., S. Evers, and P. Courvalin. 1995. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J. Clin. Microbiol. 33:24-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eaton, T. J., and M. J. Gasson. 2001. Molecular screening of Enterococcus virulence determinants and potential for genetic exchange between food and medical isolates. Appl. Environ. Microbiol. 67:1628-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franz, C. M. A. P., W. H. Holzapfel, and M. E. Stiles. 1999. Enterococci at the crossroads of food safety? Int. J. Food Microbiol. 47:1-24. [DOI] [PubMed] [Google Scholar]
- 22.Franz, C. M. A. P., A. B. Muscholl-Silberhorn, N. M. K. Yousif, M. Vancanneyt, J. Swings, and W. H. Holzapfel. 2001. Incidence of virulence factors and antibiotic resistance among enterococci isolated from food. Appl. Environ. Microbiol. 67:4385-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freitas, A. C., C. Pais, F. X. Malcata, and T. A. Hogg. 1995. Microbiological characterization of Picante de Beira Baixa cheese. J. Food Prot. 59:155-160. [DOI] [PubMed] [Google Scholar]
- 24.Fridkin, S. K., D. S. Yokoe, C. G. Whitney, A. Onderdonk, and D. C. Hooper. 1998. Epidemiology of a dominant clonal strain of vancomycin-resistant Enterococcus faecium at separate hospitals in Boston, Massachusetts. J. Clin. Microbiol. 36:965-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gancheva, A., B. Pot, K. Vanhonacker, B. Hoste, and K. Kersters. 1999. A polyphasic approach towards the identification of strains belonging to Lactobacillus acidophilus and related species. Syst. Appl. Microbiol. 22:573-585. [DOI] [PubMed] [Google Scholar]
- 26.Gordillo, M. E., K. V. Singh, and B. E. Murray. 1993. Comparison of ribotyping and pulsed-field gel electrophoresis for subspecies differentiation of strains of Enterococcus faecalis. J. Clin. Microbiol. 31:1570-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon, S., J. M. Swensson, B. C. Hill, N. E. Pigott, R. R. Facklam, R. Cooksey, and C. Thornsberry. 1992. Antimicrobial susceptibility patterns of common and unusual species of enterococci causing infections in the United States. J. Clin. Microbiol. 30:2373-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray, J., P. J. Marsh, D. Stewart, and S. J. Pedler. 1994. Enterococcus bacteraemia: a prospective study of 125 episodes. J. Hosp. Infect. 27:179-186. [DOI] [PubMed] [Google Scholar]
- 29.Green, M., K. Barbadora, S. Donabedian, and M. J. Zervos. 1995. Comparison of field inversion gel electrophoresis with contour-clamped homogeneous electric field electrophoresis as a typing method for Enterococcus faecium. J. Clin. Microbiol. 33:1554-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall, L. M. C., B. Duke, M. Guiney, and R. Williams. 1992. Typing of Enterococcus species by DNA restriction fragment analysis. J. Clin. Microbiol. 30:915-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huey, B., and J. Hall. 1989. Hypervariable DNA fingerprinting in Escherichia coli. Minisatellite probe from bacteriophage M13. J. Bacteriol. 171:2528-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janssen, P., R. Coopman, G. Huys, J. Swings, M. Bleeker, P. Vos, M. Zabeau, and K. Kersters. 1996. Evaluation of the DNA fingerprinting method AFLP as a new tool in bacterial taxonomy. Microbiology 142:1881-1893. [DOI] [PubMed] [Google Scholar]
- 33.Jett, B. D., M. M. Huyke, and M. S. Gilmore. 1994. Virulence of enterococci. Clin. Microbiol. Rev. 7:462-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kühn, I., L. G. Burman, S. Haeggman, K. Tullus, and B. E. Murray. 1995. Biochemical fingerprinting compared with ribotyping and pulsed-field gel electrophoresis of DNA for epidemiological typing of enterococci. J. Clin. Microbiol. 33:2812-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leclercq, R. 1997. Enterococci acquire new kinds of resistance. Clin. Infect. Dis. 24(Suppl. 1):S80-S84. [DOI] [PubMed] [Google Scholar]
- 36.Leclercq, R., E. Derlot, M. Weber, J. Duval, and P. Courvalin. 1989. Transferable vancomycin and teicoplanin resistance in Enterococcus faecium. Antimicrob. Agents Chemother. 33:10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leclercq, H., L. A. Devriese, and D. A. A. Mossel. 1996. Taxonomical changes in intestinal (faecal) enterococci and streptococci: consequences on their use as indicators of faecal contamination in drinking water. J. Appl. Bacteriol. 81:459-466. [DOI] [PubMed] [Google Scholar]
- 38.Litopoulou-Tzanetaki, E., and N. Tzanetakis. 1992. Microbiology of white brined cheese made from raw goat milk. Food Microbiol. 9:13-19. [Google Scholar]
- 39.Low, D. E., B. M. Willey, S. Betschel, and B. Kreiswirth. 1994. Enterococci: pathogens of the 90s. Eur. J. Surg. Suppl. 573:19-24. [PubMed] [Google Scholar]
- 40.Macedo, A. C., F. X. Malcata, and T. A. Hogg. 1995. Microbiological profile in Serra ewe's cheese during ripening. J. Appl. Bacteriol. 79:1-11. [Google Scholar]
- 41.Miranda, A. G., K. V. Singh, and B. E. Murray. 1991. DNA fingerprinting of Enterococcus faecium by pulsed-field gel electrophoresis may be a useful epidemiologic tool. J. Clin. Microbiol. 29:2752-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moellering, R. C. 1992. Emergence of Enterococcus as a significant pathogen. Clin. Infect. Dis. 14:1173-1178. [DOI] [PubMed] [Google Scholar]
- 43.Morrison, D., N. Woodford, S. P. Barrett, P. Sisson, and B. D. Cookson. 1999. DNA banding pattern polymorphism in vancomycin-resistant Enterococcus faecium and criteria for defining strains. J. Clin. Microbiol. 37:1084-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morrison, D., N. Woodford, and B. Cookson. 1997. Enterococci as emerging pathogens of humans. J. Appl. Microbiol. Symp. Suppl. 83:89S-99S. [DOI] [PubMed] [Google Scholar]
- 45.Mundy, L. M., D. F. Sahm, and M. Gilmore. 2000. Relationships between enterococcal virulence and antimicrobial resistance. Clin. Microbiol. Rev. 13:513-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murray, B. E. 1990. The life and times of the Enterococcus. Clin. Microbiol. Rev. 3:46-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murray, B. E., K. V. Singh, D. J. Heath, B. R. Sharma, and G. M. Weinstock. 1990. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J. Clin. Microbiol. 28:2059-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noble, W. C., Z. Virani, and R. G. A. Cree. 1992. Co-transfer of vancomycin and other resistance genes from Enterococcus faecalis NCTC 12201 to Staphylococcus aureus. FEMS Microbiol. Lett. 93:195-198. [DOI] [PubMed] [Google Scholar]
- 49.Pitcher, D. G., N. A. Saunders, and R. J. Owen. 1989. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett. Appl. Microbiol. 8:151-156. [Google Scholar]
- 50.Pot, B., P. Vandamme, and K. Kersters. 1994. Analysis of electrophoretic whole-organism protein fingerprints, p. 493-521. In M. Goodfellow and A. G. O'Donnell (ed.), Chemical methods in prokaryotic systematics. J. Wiley and Sons, Chichester, United Kingdom.
- 51.Quednau, M., S. Ahrne, and G. Molin. 1999. Genomic relationships between Enterococcus faecium strains from different sources and with different antibiotic resistance profiles evaluated by restriction endonuclease analysis of total chromosomal DNA using EcoRI and PvuII. Appl. Environ. Microbiol. 65:1777-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruoff, K. L. 1990. Recent taxonomic changes in the genus Enterococcus. Eur. J. Clin. Microbiol. Infect. Dis. 9:75-79. [DOI] [PubMed] [Google Scholar]
- 53.Schaberg, D. R., D. H. Culver, and R. P. Gaynes. 1991. Major trends in the microbial etiology of nosocomial infections. Am. J. Med. 912(Suppl. 3B):72S-75S. [DOI] [PubMed] [Google Scholar]
- 54.Tailor, S. A. N., E. M. Bailey, and M. J. Rybak. 1993. Enterococcus, an emerging pathogen. Ann. Pharmacother. 27:1231-1242. [DOI] [PubMed] [Google Scholar]
- 55.Teixeira, L. M., R. R. Facklam, A. G. Steigerwalt, N. E. Pigott, V. L. C. Merquior, and D. J. Brenner. 1995. Correlation between phenotypic characteristics and DNA relatedness within Enterococcus faecium strains. J. Clin. Microbiol. 33:1520-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tomayko, J. F., and B. E. Murray. 1995. Analysis of Enterococcus faecalis isolates from intercontinental sources by multilocus enzyme electrophoresis and pulsed-field gel electrophoresis. J. Clin. Microbiol. 33:2903-2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tomita, H., S. Fujimoto, K. Tanimoto, and Y. Ike. 1997. Cloning and genetic sequence analysis of the bacteriocin 31 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pPD1. J. Bacteriol. 179:7843-7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tzanetakis, N., and E. Litopoulou-Tzanetaki. 1992. Changes in numbers and kinds of lactic acid bacteria in feta and teleme, two Greek cheeses from ewes' milk. J. Dairy Sci. 75:1389-1393. [Google Scholar]
- 59.Vancanneyt, M., K. Kersters, and J. Swings. 1999. Catalogue of enterococci of the FAIR-E collection. BCCM/LMG Bacteria Collection, Ghent, Belgium.
- 60.Vancanneyt, M., C. Snauwaert, I. Cleenwerck, M. Baele, P. Descheemaeker, H. Goossens, B. Pot, P. Vandamme, J. Swings, F. Haesebrouck, and L. A. Devriese. 2001. Enterococcus villorum sp. nov., an enteroadherent bacterium associated with diarrhea in piglets. Int. J. Syst. Evol. Bacteriol. 51:393-400. [DOI] [PubMed] [Google Scholar]
- 61.Wayne, L. G., D. J. Brenner, R. R. Colwell, P. A. D. Grimont, O. Kandler, M. I. Krichevsky, L. H. Moore, W. E. C. Moore, R. G. E. Murray, E. Stackebrandt, M. P. Starr, and H. G. Trüper. 1987. Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. Int. J. Syst. Bacteriol. 37:463-464. [Google Scholar]
- 62.Willems, R. J. L., J. Top, N. van den Braak, A. van Belkum, H. Endtz, D. Mevius, E. Stobberingh, A. van den Bogaard, and J. D. A. van Embden. 2000. Host specificity of vancomycin-resistant Enterococcus faecium. J. Infect. Dis. 182:816-823. [DOI] [PubMed] [Google Scholar]