Abstract
An indwelling pleural catheter (IPC) is a valuable tool in the management of pleural effusions, allowing drainage strategies to be tailored to match patient-centred goals. Previously, IPCs were primarily utilised in malignant pleural effusion (MPE) in the presence of non-expandable lung (NEL) or after the failure of chemical pleurodesis. Several studies have compared IPC to intercostal chest drain (ICD) with talc pleurodesis (TP), as well as different drainage regimens, resulting in a transition of practice. Continued developments have led to novel adjuncts, such as digital drainage, which allow controlled flow rates. The emerging field of intrapleural therapy in MPE is gaining attention as a potential new treatment modality, possibly increasing the scope of IPCs further. This article will provide a narrative review of the role of IPCs and will be based on published evidence to date and highlight the importance of an individualised, patient-centred care approach.
Keywords: Indwelling pleural catheter, IPC, Pleural effusion, Malignant pleural effusion, Benign pleural effusion
Key Summary Points
| Indwelling pleural catheters (IPC) are as effective as talc pleurodesis at improving breathlessness. |
| Patients with IPCs will spend less time in the hospital immediately and over the next 12 months. |
| More frequent drainage improves the rate of pleurodesis in patients with IPCs. |
| Talc via IPC in patients without non-expandable lung (NEL) is safe and increases the likelihood of pleurodesis. |
Introduction
What is an Indwelling Pleural Catheter (IPC)?
An IPC is a flexible, multi-fenestrated, silicone-based catheter with a polyester cuff. The catheter is tunnelled through around 5 cm of subcutaneous tissue before a tract is made for the distal part of the drain to enter the pleural space. The polyester cuff is the main site for fibrous attachment, holding the drain in situ. The proximal end has a one-way valve that can then attach to a vacuum drainage bottle when required. IPCs were first introduced in 1997 after approval from the US Food and Drug Administration (FDA) for the palliation of dyspnoea due to malignant pleural effusion (MPE) and for providing pleurodesis prior to approving their usage in all recurrent effusions in 2001 [1, 2] (Fig. 1).
Fig. 1.
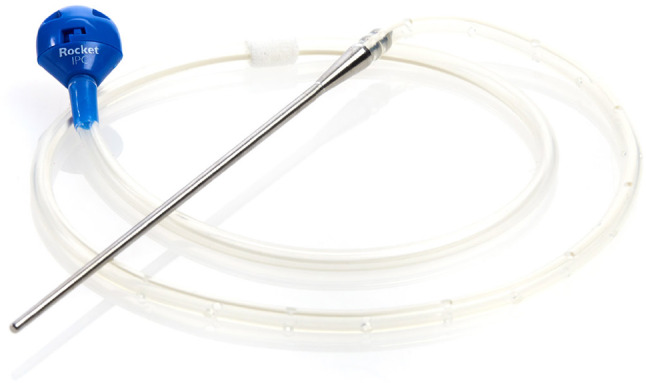
An IPC (image reproduced with permission from Rocket Medical plc). IPC indwelling pleural catheters
Technique for Placement of IPC
The technique for inserting an IPC is relatively standard. The description below is based on the expert opinion of the authors and will be for a patient undergoing IPC placement for a pleural effusion.
Through shared decision-making, an IPC will have been selected as the modality for management and preprocedural checks will have been performed in line with relevant local or national guidance, for example, the British Thoracic Society Clinical Statement on Pleural Procedures [3].
In general, the patient is positioned in the lateral decubitus position, with the hemithorax containing the pleural effusion facing upward. The procedure is often performed in a dedicated space. Monitoring is not required unless using sedation, but some centres will be doing these procedures in a theatre environment where monitoring is de rigeur’. A thoracic ultrasound will be performed to ascertain the presence of sufficient fluid and to determine a safe space for the pleural entry point. Within the same rib space, a tunnel exit site will be marked roughly 5–6 cm anterior to the pleural entry site.
Once an appropriate pleural space is selected, the area is cleaned using an aseptic technique and local anaesthetic (a dosage of 3 mg/kg is suggested for 1% lignocaine) is infiltrated subcutaneously and then deeper into the subcutaneous layers until the pleural space is reached. Pleural fluid should be aspirated as a confirmatory sign of being in the correct space. The needle is then retracted and further anaesthetic is infiltrated along a subcutaneous tract in the same intercostal space for approximately 5–6 cm proximally. A 1-cm incision is then made at the initial pleural puncture site and at the end of the anaesthetised tract. Using either a metal tunneller or small curved forceps, the tract is enlarged.
A needle is then passed directly into the pleural effusion at the initial puncture site. Using the Seldinger technique, a wire is passed into the pleural space and left in place. A dilator/trocar is passed over the wire and widens the hole in the parietal pleura. The wire and dilator are now removed, leaving a tear-away sheath in place. The drain tubing is now passed through the subcutaneous tract, taking care that the end that will attach to a vacuum bottle is front-facing and that the fibrotic cuff of the drain lies midway under the tract. The distal end of the drain is now passed through the tear-away sheath, and when all the drain fenestrations are intrathoracic, the sheath is peeled away with gentle pressure applied simultaneously over the entry point to make sure the drain stays in place.
The proximal end of the tract can be closed with glue or a suture and the drain secured at the other end with two sutures on either side of the drain. The sutures need to be removed in 7–10 days. A training video from the American Association for Bronchology and Interventional Pulmonology (AABIP) depicts the above [4].
Indications for Insertion
Since 2001, IPCs have been approved for any cause of recurrent pleural effusion resistant to treatment, though the most common indication remains MPE [2]. Benign causes of treatment-refractory pleural effusions include effusions secondary to cardiac failure, hepatic hydrothorax, inflammatory pleuritis, chylothorax, yellow nail syndrome, and empyema [5, 6] (Fig. 2).
Fig. 2.
Summary of the utility of IPCs. Images of IPC reproduced with permission from Rocket Medical plc. Images of Passio™ reproduced with permission from Bearpac Medical (Moultonborough, NH, USA). IPC indwelling pleural catheters
IPCs in Non-Expandable Lung (NEL)
In cases of malignancy, the lung may be unable to expand fully to occupy the thoracic cavity due to disease-related factors, such as thickening of the visceral pleura leading to adherence to the lung, or the presence of a centrally obstructing tumour that prevents complete re-expansion. This leads to a lack of apposition between the parietal and visceral pleura. The term NEL is defined in British Thoracic Society (BTS) guidelines as when > 25% of lung is not in apposition with the chest wall. Whilst surgical decortication can help, patients are often frail and unable to undergo such extensive operations: guidelines thus advocate for the insertion of IPC for symptom control, likely due to the failure of chemical pleurodesis [7, 8].
Malignant Pleural Effusion (MPE)
The role of IPCs in MPE has evolved over time from initially only indicated in patients who had failed chemical pleurodesis or those with NEL to now being a first-line intervention [7, 8]. Whilst practice patterns may differ globally, in the UK this transition in practice was recommended during the COVID-19 (C-19) pandemic where initial intentions were to minimise admissions for intercostal chest drains (ICD) and talc pleurodesis (TP) and to offer alternative strategies such as IPCs or large volume thoracentesis. The C-19 pandemic also promoted training of family members to drain IPCs to reduce contact with services [9].
Current guidelines advocate for patient choice when it comes to pleural intervention in MPE [8]. The TIME-2 study was the first randomised controlled trial (RCT) to directly compare IPC versus TP via ICD [10]. Using visual analogue score (VAS) for dyspnoea, this demonstrated that there was no significant difference in breathlessness, chest pain or quality of life at 42 days between these two interventions. Secondary outcomes did illustrate a shorter hospital stay; however, the study was not powered to focus on this outcome.
These findings were mirrored by the more recent NVALT-14 RCT, which showed no significant difference in dyspnoea using modified Borg score (MBS) for both IPC and TP at rest and during exercise [11]. The study similarly showed shorter hospital stays and fewer re-interventions in the IPC arm of the study.
The AMPLE-1 study focussed on length of hospital stay, showing that IPCs reduced effusion-related hospital stay to 1 day (interquartile range (IQR) 1–3 days) compared to 4 days (IQR 3–6 days) for TP and all-cause hospital stay to 10 days (IQR 3–17 days) compared to 12 days (IQR 7–21 days) [12]. Additionally, the number of additional ipsilateral procedures was reduced to 4.1% in the IPC arm compared to 22.5% in the TP arm, with no difference in mortality or complications.
These studies support the rationale for an informed patient choice when it comes to pleural intervention in MPE, which is reflected by guidelines. The website mypleuraleffusionjourney.com is a valuable resource aimed at supporting patients and caregivers in their decision-making for pleural interventions [13].
In MPE, patients may be on or due to commence systemic anticancer treatment (SACT). There have been no RCTs looking at the optimal timing of IPC (i.e., before/during/after SACT) but Porcel et al. have critically reviewed ten studies in this area looking at the safety and efficacy of IPC [14]. This study found that the timing of SACT had no effect on the IPC infection rate, including if patients were neutropenic. Patients with an IPC who had an IPC whilst undergoing SACT had an improved quality-adjusted survival. Overall, the study concluded that efficacy and safety are not affected by the timing of IPC insertion and that IPC insertion should not be delayed pending SACT.
Benign Pleural Effusion (BPE)
Bhatnagar et al. performed a multicentre review of IPC insertions for non-malignant effusions between 2007 and 2013 reviewing a total of 57 IPC insertions [5]. The most common indication was a hepatic hydrothorax [HH] (33%), followed by inflammatory pleuritis (26%), empyema (16%), cardiac failure (16%), yellow nail syndrome (5%), and chylothorax (4%). The majority of patients had no complications (84%), with only 3.5% of patients developing a suspected pleural infection. The IPC was removed in 49% of cases with 33% of all patients achieving pleurodesis at a median time of 71 days. The rate of pleurodesis varied depending on the initial indication with HH showing the least likely to achieve pleurodesis (11%). Patil et al. did a systematic review of the use of IPCs in BPEs including pleural effusions secondary to cardiac disease (49.8%), hepatic disease (12.3%), chylothorax (3.4%), empyema (2.8%), inflammatory pleuritis (6.5%), yellow nail syndrome (1.5%), renal disease (4.0%), and other non-malignant diseases (19.7%) [6]. Their review showed that 51.3% (95% CI, 37.1–65.6%) achieved pleurodesis with an overall complication rate of 17.2% (95% CI, 9.8–24.5%) with an empyema rate of 2.3% (95% CI, 0.0–4.7%), again showing that IPCs are a practical solution for refractory BPE.
The main body of evidence for IPCs in congestive heart failure were based on single-centre, non-randomised studies, looking at symptoms and pleurodesis. These studies showed IPC improved dyspnoea and that auto pleurodesis rates ranged between 24 and 41% within 66–150 days (see Table 1) [15–20].
Table 1.
Studies evaluating the use of IPCs in congestive cardiac failure (CCF) related pleural effusions; copied with permission [21]
| Study | Study design | Sample size (n) | Primary outcome | Pleurodesis success (%) | Time to pleurodesis (days) | Complications |
|---|---|---|---|---|---|---|
| Borgeson et al. (2009) [15] | Prospective, single centre | 22 | Rate of pleurodesis | 41 | 109 (median) | Infection, catheter occlusion |
| Srour et al. (2013) [16] | Prospective, single centre | 43 | Improvement in breathlessness | 29% | 66 (median) | None |
| Freeman et al. (2014) [17] | Retrospective, single centre | 40 | Palliation of effusion | 35% | 150 (mean) | None |
| Majid et al. (2016), Group 1a [18] | Retrospective, single centre |
15 (TTP + IPC) |
Improvement in breathlessness and pleurodesis | 80% | 11 (median) | Cellulitis |
| Majid et al. (2016) Group 2b [18] | Retrospective, single centre | 28 (IPC only) | Improvement in breathlessness and pleurodesis | 25% | 66 (median) | Empyema (2/28), cellulitis |
| Frost et al. (2020) [19] | Retrospective, single centre | 30 | Symptom control | 24% | NR | Cellulitis, IPC malfunction |
| Walker et al. (2022) [20] | Randomised control, multicentre | 21 | Mean daily dyspnoea score over 12 weeks from randomisation | Disease-specific data not recorded | Disease-specific data not recorded | Cellulitis, empyema |
NR not recorded. TTP thoracoscopy with talc poudrage, IPC indwelling pleural catheters
Patil et al. performed a systematic review and meta-analysis of BPE [6]. When looking at the subgroup analysis for congestive cardiac failure, the study demonstrated successful pleurodesis in 42.1% with a 4-day-shorter hospital stay (including re-admission) for those patients treated with IPC compared to those who were not. Wijayaratne et al. completed a narrative review on cardiac-related pleural effusions, concluding that although TT is a viable option, there can be challenges in repeatedly interrupting anticoagulation or antiplatelet therapy [21]. With this in mind, the evidence would suggest there is a role for IPC in those patients with refractory congestive cardiac failure.
It is worth expanding on HH. HH can present in 5–15% of patients with advanced liver disease as a result of cirrhosis and portal hypertension [22]. HH refractory to salt restriction and diuretics is a common clinical problem and can be present in up to 25% of patients [23]. Management of such cases brings challenges, with the definitive management being liver transplantation; though due to comorbidities and other considerations, many patients are not eligible. A trans-jugular intrahepatic portosystemic shunt (TIPSS) can be considered with success rates up to 80%, but the risk of hepatic encephalopathy is increased. Frequently, these patients require repeated TT; the role of IPC has been debated, particularly with the concern regarding infection in this cohort. IPCs have been used as a bridge to transplantation and as a palliative measure. Prior to the REDUCE study, there were no RCTs looking at IPC in HH. There was significant variability in pleurodesis success and pleural infection rates, as shown in Table 2 [5, 20, 24–27].
Table 2.
Studies evaluating the use of IPCs in hepatic hydrothorax
| Study | Study design | Sample size (n) | Pleurodesis success (%) | Mean time to pleurodesis (days) | Pleural infection rate (%) |
|---|---|---|---|---|---|
| Bhatnagar et al. (2014) [5] | Prospective, multicentre | 19 | 10 | 222 | 5.3 |
| Chen et al. (2016) [24] | Prospective, single centre | 24 | 33 | 131.8 | 16.7 |
| Kinese et al. (2019) [25] | Retrospective, single centre | 62 | 14.5 | 118 | 16 |
| Shojaee et al. (2019) [26] | Retrospective, single centre | 79 | 27.8 | 55 | 10 |
| Bartnicki-Navarette et al. (2020) [27] | Retrospective | 13 | 69 | 34 | 7.6 |
| Walker et al. (2022) [20] | Multicentre RCT | 8 | NR | NR | 0 |
NR not recorded, IPC indwelling pleural catheters
Avula et al. completed a meta-analysis to look at whether IPC is an effective and less invasive management for refractory HH and its possible use as a bridge to transplantation [28]. A total of 269 patients from ten studies were included. The primary outcome looked at spontaneous pleurodesis, whilst secondary outcomes included time to pleurodesis and complication rates. Importantly, the analysis did not include dyspnoea or quality of life scores. It demonstrated the success rate for pleurodesis was 47%, with an average time of 104.3 days. Complication rate was high at 30.36% with pleural infection present 12.4%, and an IPC-related mortality rate of 3.35%.
The evidence suggests that infection is a significant and relatively common complication with IPC in hepatic hydrothorax, yet mortality directly attributed to IPC-related complications is low.
The REDUCE trial was the first RCT looking at refractory transudative pleural effusions where malignancy and infection had been ruled out [20]. Patients were randomised to either insertion of an IPC or to therapeutic thoracentesis (TT). Thirty-three patients were randomised to IPC and 35 patients to TT. The most common underlying aetiology was heart failure (67.6%), followed by liver failure (23.5%), and renal failure (8.8%). The primary outcome looked at mean breathlessness scores using a visual analogue score (VAS) score over a 12-week period. This showed no significant difference in breathlessness between the two arms of the study (39.7 ± 29.44 mm in IPC arm versus 45.0 ± 26.1 mm in the TT arm). This was despite a significant difference in the volume of fluid drained during the study period (17,412 ± 17,936 ml in the IPC arm and 2901 ± 2416 ml in the TT arm; difference: 13,892 ml). There were more adverse events in the IPC arm (59% vs. 37%), the majority of which had little impact on the patient and reassuringly only one IPC-related infection, with none in the HH group in contrast to previous studies. Patients in the IPC arm required fewer pleural procedures. Although IPCs were not shown to offer superior control of breathlessness, Walker et al. postulated there could be a role for IPC in those patients who do not tolerate repeat TT, those whose preference is an IPC in order to minimise hospital attendance and those where repeatedly stopping anticoagulation is not desired. Following this, the REDUCE- 2 feasibility trial focussed specifically on those patients with heart failure, randomising patients to either TT or IPC insertion, talc injection, and daily drainage [29]. The study has concluded, and the results are awaited at the time of writing this manuscript.
The REDUCE trial showed no significant difference in daily breathless scores between IPC and TT, where the other studies focus on pleurodesis. There is no consensus on the optimal management; nevertheless, a role exists for this approach in patients with refractory disease, especially when TIPSS has been considered and the treatment is intended for palliative purposes.
Drainage Strategies and Pleurodesis
There are recent studies that have looked into drainage strategies in terms of both symptom control and pleurodesis. The AMPLE-2 RCT randomised patients to aggressive drainage (daily for 60 days or auto pleurodesis) versus symptom-guided drainage (unless auto pleurodesis occurred) [30]. The primary outcome of breathlessness showed no significant difference between the two arms with geometric mean VAS scores 13.1 mm (95% CI 9.8–17.4) for aggressive drainage versus 17.3 mm (95% CI 13.0–22.0) for symptom-guided drainage. It did demonstrate increased auto pleurodesis rates in the aggressive drainage arm 37.2% vs. 11.4% at 60 days and 44.2% vs. 15.9% at 6 months, respectively. Thus the drainage strategy could be chosen depending on the patients’ wishes; if pleurodesis was desired, then an aggressive drainage strategy could be employed, whereas if only palliation of symptoms was desired, then a symptom-guided drainage approach could be adopted. The premise that aggressive drainage promotes pleurodesis is supported by the ASAP trial [31]. Patients were randomised to daily drainage via the IPC versus alternate-day drainage. This showed a 47% success rate of auto pleurodesis in the daily drainage arm versus 24% in the alternate day arm (p = 0.03) and that the median time to achieve pleurodesis was 54 days (95% CI 34–83) compared to 90 days (95% CI 70 to non-estimable).
The exact mechanism of auto pleurodesis is unclear. There is no evidence at present suggesting a chemical or an immune-mediated reactivity to the silicone. It has been suggested that keeping the pleural space dry by frequent drainage leads to more overall contact between the parietal and visceral pleura thus increasing the chances of pleurodesis [32].
Warren et al. also hypothesised that there could be an inflammatory aspect to auto pleurodesis. It is common for fluid appearances to change from serous to serosanguinous and for patients to have pleuritic chest pain, often described as a “tug” on completion of drainage. It is possible this causes an inflammatory reaction that promotes pleurodesis. This could support the idea that the frequency of drainage leads to an increasing inflammatory response, improving auto pleurodesis rates [32].
Combined Approaches
There have been several studies that have looked at combining IPC with alternate pleurodesis methods, predominantly TP either via IPC or a talc poudrage at thoracoscopy. The IPC Plus study randomised patients with MPE to either 4 g of sterile talc or placebo (50 ml saline) after 10 days of daily drainage, comparing pleurodesis rates at day 35 [33]. Importantly, patients who had appearances of NEL were excluded. This showed improved pleurodesis rates with TP compared to the placebo arm with rates of 43% and 23%, respectively (hazard ratio, 2.20; 95% confidence interval, 1.23 to 3.92; p = 0.008). Important secondary outcomes looking at quality of life (QLQ-C30 scores) and chest pain (VAS score) showed improved outcomes in the TP group at all time points during their study, showing added benefits other than purely pleurodesis success. This is an important study, combining both aggressive early drainage and TP to maximise pleurodesis success, which doubles when compared to auto pleurodesis rates.
The EPIToME study was an observational study that included 102 consecutive patients [34]. They used the results from AMPLE-1, AMPLE-2, TIME-2, ASAP, and IPC-Plus to create a treatment algorithm looking at pleurodesis success. All patients had an IPC inserted as the first-line treatment and had their fluid drained to dryness. Patients were then assessed for lung expansion to determine whether pleurodesis would be suitable. Fifty-five (53.9%) patients were deemed unsuitable for pleurodesis, 31 due to NEL, with the remaining unsuitable due to patient or oncologist preference. Forty-seven (46%) patients were deemed suitable for pleurodesis and a talc slurry was instilled via the IPC followed by daily drainages for 14 days or until pleurodesis was achieved; 74% of these patients had a successful pleurodesis with a median time of 20 days. They inferred that using real-world data, TP via IPC would not be suitable for about half the population of patients with MPE; however, there were good success rates using TP and daily drainages, which is consistent with the previous studies looking at aggressive drainage and use of talc as a sclerosing agent.
The OPTIMUM study compared a combined approach of IPC with TP versus chest drain and TP, importantly looking at global health status at 30 days using the EORTC QLQ-C30 questionnaire [35]. Seventy patients were randomised to the intended outpatient IPC arm and 72 patients to the inpatient (median stay 4 days) chest drain arm. Both showed improvement in global health status with a mean difference of 13.11 (p < 0.001) in the IPC arm and 10.11 (p < 0.001) in the chest drain arm, with no significant difference between these two groups. This supports the importance of an informed patient choice when deciding fluid-management strategies.
Krochmal et al. retrospectively evaluated a rapid pleurodesis protocol that combined thoracoscopy with talc poudrage (TTP) with insertion of an IPC with the aim of pleurodesis and early removal of IPC [36]. Twenty-nine patients had undergone the rapid pleurodesis protocol, showing a median inpatient stay of 2 days (IQR 1–3 days) and median time to removal of IPC of 10 days (IQR 7–14 days) with an overall pleurodesis success rate of 93%.
The TACTIC RCT evaluated the role of combined approaches, with the hypothesis being that adding talc would allow for an earlier IPC removal and avoid further attendance for administration of talc slurry via the IPC at a later date [37]. This RCT directly compared TTP combined with IPC (TTP + IPC) to standard care, being TTP alone. Publication of the results is awaited.
Other methods have been used. The SWIFT trial looked at whether a silver nitrate-coated IPC (SNCIPC) improved pleurodesis rates [38]. Silver nitrate has been shown to be an effective chemical pleurodesis agent comparable to talc [39]. A total of 119 patients were randomised either to SNCIPC or a standard IPC. The time to pleurodesis was faster in the SNCIPC arm with a median time of 4 days (IQR 2–15 days) versus 11 days (IQR 9–23 days), but pleurodesis was only achieved in 22% in the SNCIPC arm compared to 32% in the standard IPC arm, thus not showing any benefit of using a SNCIPC.
A small cohort of 13 patients had iodopovidone instilled via IPC looking at pleurodesis [40]. 10 (77%) patients achieved pleurodesis with a median time of 5 days and median time for IPC removal of 16 days, which may suggest iodopovidone could allow for early drain removal (Fig. 3).
Fig. 3.
Timeline of IPC trials. TP talc pleurodesis, ICD intercostal chest drain, TTP thoracoscopy with talc poudrage, SN silver nitrate, TT therapeutic thoracentesis, IPC indwelling pleural catheters
Guidelines
The overall evidence for IPC versus ICD and TP for MPE show equivalence when looking at breathlessness and quality of life scores, supporting the importance of informed patient choice when it comes to management strategies. This has been reflected in the updated British Thoracic Society, European Respiratory Society (ERS) and American Thoracic Society (ATS) guidelines, including importantly considering IPC as a first-line management and not only in patients with NEL or those who have failed chemical pleurodesis [8, 41–43]. This highlights how the role of IPC has changed in the last 20 years, particularly with respect to MPE.
Digital IPC Drainage Devices
Occasionally, patients may experience pain on IPC drainage, particularly those with NEL due to the negative intrapleural pressure. IPCs use a vacuum-based drainage system using pressures as high as – 995 cmH20, which may limit volumes drained due to tolerance [44]. Novel digital drainage devices have been developed that allow for lower pressures with variable flow rates. The Passio™ (Bearpac Medical) pump drainage system comprises of a digital handheld control unit, allowing the flow rate to be controlled with four differing speeds that can be selected based on the patient’s level of comfort. The device initially induces an initial peak of around – 115 cmH20 during start-up and quickly reduces to a vacuum level of approximately – 45 to – 70 cmH20 during drainage. An evaluation looking at the Passio™ system showed promising results [45, 46]. Patients who experienced drainage-related pain (assessed using VAS scores) at their 2-week post-IPC insertion review, were switched to a Passio™ system and pain on drainage was re-assessed. This demonstrated that patients had lower VAS pain scores mid-drainage (20.15 mm vs. 51.68 mm), end of drainage (27.28 mm vs. 46.68 mm) and 10-min post-drainage (14.81 mm vs. 61.38 mm) compared to vacuum bottle drainage. The AESOP trial will be the first RCT to directly compare Passio™ and standard IPC and is recruiting at the time of writing [47].
The Geyser™ (Tintron Labs) digital drainage system provides drainage over a 4-min pre-programmed cycle, which gradually ramps up the pressure to allow for 250-ml drainage (can drain a total of 750 ml in one procedure). A small study compared the Geyser system to standard vacuum bottles with VAS pain scores showing there was reduced pain using the Geyser system (9.1 mm vs. 21.9 mm, respectively, p = 0.027) [48]. These small studies both demonstrated no complications and that there may be a role for digital drainage in those patients who struggle with pain during drainage.
Intrapleural Treatment
The IPC provides a conduit into the pleural space and can be used to direct treatment locally, particularly in mesothelioma, where the only detectable disease may be on the pleura [49]. Some early studies in the 1990s showed potential advantages to intrapleural treatment, mainly in stage 1 disease, although studies into systemic treatment took precedence at that time [50–53].
With the increasing number of IPCs, it is felt that studies looking at intrapleural treatment should be expanded, particularly in those with diseases limited to the pleura, leading to an increase in the number of trials in this area. Intrapleural therapy has been shown to be safe in phase 1 studies looking at gene-mediated cytotoxic immunotherapy and HSV1716 (a replication-restricted herpes simplex virus) [54, 55]. Intrapleural bevacizumab provided higher response rates than when administered intravenously and, importantly, also demonstrating lower toxicities; possibly due to lower blood concentrations when delivered intrapleurally [56].
The MITOPE study is an ongoing study looking at instilling RSO-021 (cyclic oligopeptide of the thiopeptide class) into the pleural space via an IPC [57]. In phase 1 of the trial, intrapleural RSO-021 was instilled weekly with three dosing regimens (90, 120, and 180 mg) with a 3 + 3 dose-escalation design; 12 of 15 patients recruited had mesothelioma. Three (20%) patients had drug-limiting toxicities at 120 mg and 180 mg. The maximum tolerated dose was 90 mg per week. At this dose, there was a partial response (59%) in mesothelioma target lesions. Further evaluation during phase 2 of this study is ongoing at time of writing, looking at weekly instillation of either 45 mg or 90 mg of RSO-021 either as monotherapy or in combination with IV paclitaxel [58].
Cost-Effectiveness
IPCs require ongoing outpatient medical and nursing support. The first cost-effectiveness analysis compared IPC versus TP [59]. This was based on the TIME-2 trial cost-model analysis, where UK patients and caregivers drained their IPCs rather than nursing staff. When nursing input was greater than 2 h per week, IPCs were deemed more costly. Length of time for ongoing support also had a role in determining cost-effectiveness, and it was found that a survival of less than 14 weeks would make an IPC cost effective.
A USA cost-effectiveness analysis comparing different drainage strategies as per ASAP, AMPLE-2 and IPC-Plus regimens suggested that daily drainage as per the ASAP trial was never cost-effective [60]. However, if the patient has MPE with an expandable lung, the most cost-effective treatment was IPC and TP as per the IPC-plus pathway, however, if a patient had MPE with NEL, a symptom-guided drainage strategy as per AMPLE-2 would be cost-effective.
A systematic review of costs associated with IPC in MPE showed average cost ranged from < €1100 if survival was less than 6 weeks to €4028 in those with mesothelioma. If complications arise requiring extra investigations, appointments or admission, costs can escalate to > €5000 [61].
Ongoing and Future Research
In the field of MPE, AMPLE-3 has completed and aims to compare IPC insertion versus video-assisted thoracoscopic surgery (VATS) pleurodesis, primarily looking at re-intervention required post-procedure [62]. AMPLE-4 is solely looking at whether applying topical mupirocin at the exit site during IPC insertion will reduce IPC-related infections in MPE [63]. STREAMLINE is a feasibility study recruiting to see whether performing a pleural biopsy and IPC insertion as first diagnostic procedure in place of solely a pleural aspiration in order to accelerate the patient pathway [64]. For benign effusions, the REDUCE-2 feasibility study has completed recruitment looking at IPCs in refractory heart failure comparing TT versus IPC, talc injection, and daily drainage [29]. Finally, for drainage strategies, AESOP will be the first RCT and is currently recruiting to look at the efficacy and safety of Passio™ drainage system compared to standard vacuum drainage [47]. The NEWTON study is in the recruitment phase, comparing quality of life scores between gravity drainage against standard vacuum drainage [65]. PACMAN is a study looking at supporting patients with self-drainage of their IPCs by developing a self-management intervention and is recruiting patients and carers for semi-structured interviews [66]. ASAP 2 has completed recruitment, and results are awaited [67]. This study investigates whether combining aggressive daily drainage with talc instillation at baseline stimulates pleurodesis quicker than daily drainage alone (Fig. 4).
Fig. 4.
Algorithm for IPC usage using current evidence. *e.g., renal failure, chylothorax, inflammatory pleuritis. MPE malignant pleural effusion, BPE benign pleural effusion, ICD intercostal chest drain, TP talc pleurodesis, AP antiplatelet, AC anticoagulation, IPC indwelling pleural catheters
Conclusions
The role of the IPC has evolved, and it is no longer only considered as a last resort for palliation in MPE. It has become an essential tool in our arsenal for managing all-cause pleural effusion in appropriately selected patients. Its ability to provide ongoing access to the pleural cavity may offer future opportunities for targeted therapies, potentially transforming the management landscape of malignant pleural effusion.
Author Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. Abdulla Baguneid performed a literature review and designed and wrote the original draft. Thisarana Wijayratne wrote and reviewed the manuscript. Avinash Aujayeb conceived the idea of the article and revised the manuscript for content. Rakesh Panchal conceived the idea of the article, designed, wrote, reviewed and supervised the manuscript.
Funding
No funding or sponsorship was received for this study or publication of this article.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Declarations
Conflict of Interest
Avinash Aujayeb is an Editorial Board member of Pulmonary Therapy. Avinash Aujayeb was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Abdulla Baguneid, Thisarana Wijayaratne, and Rakesh Panchal have no conflicts of interest.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Summary of Safety and Effectives Denver PleurX Pleural Catheter Kit Denver Home Drainage Kit. Rockville, Food and Drug Administration 1997.
- 2.PleurX Pleural Catheter and Drainage Kits. Rockville, Food and Drug Administration, 2001
- 3.Asciak R, Bedawi EO, Bhatnagar R, Clive AO, Hassan M, Lloyd H, et al. British Thoracic Society clinical statement on pleural procedures. Thorax. 2023;78(Suppl 3): s43. [DOI] [PubMed] [Google Scholar]
- 4.https://www.youtube.com/watch?v=dJW2tM1RPcY accessed on 26th March 2025
- 5.Bhatnagar R, Reid ED, Corcoran JP, et al. Indwelling pleural catheters for non-malignant effusions: a multicentre review of practice. Thorax. 2014;69:959–61. [DOI] [PubMed] [Google Scholar]
- 6.Patil M, Dhillon SS, Attwood K, Saoud M, Alraiyes AH, Harris K. Management of benign pleural effusions using indwelling pleural catheters: a systematic review and meta-analysis. Chest. 2017;151(3):626–35. [DOI] [PubMed] [Google Scholar]
- 7.Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010. 10.1136/thx.2010.136994. [DOI] [PubMed] [Google Scholar]
- 8.Roberts ME, Rahman NM, Maskell NA, et al. British Thoracic Society guideline for pleural disease. Thorax. 2023;78:1143–56. [DOI] [PubMed] [Google Scholar]
- 9.Hallifax R, Wrightson JM, Bibby A, et al. Pleural Services during the COVID-19 Pandemic. British Thoracic Society. https://www.brit-thoracic.org.uk/document-library/qualityimprovement/covid-19/pleural-services-during-covid-19- pandemic/ (accessed on 17th October 2024)
- 10.Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA. 2012;307(22):2383–9. 10.1001/jama.2012.5535. [DOI] [PubMed] [Google Scholar]
- 11.Boshuizen RC, Noort V, Burgers JA, Herder GJM, Hashemi SMS, Hiltermann TJN, et al. A randomized controlled trial comparing indwelling pleural catheters with talc pleurodesis (NVALT-14). Lung Cancer. 2017;108:9–14. [DOI] [PubMed] [Google Scholar]
- 12.Thomas R, Fysh ETH, Smith NA, Lee P, Kwan BCH, Yap E, Horwood FC, Piccolo F, Lam DCL, Garske LA, Shrestha R, Kosky C, Read CA, Murray K, Lee YCG. Effect of an indwelling pleural catheter vs talc pleurodesis on hospitalization days in patients with malignant pleural effusion: the AMPLE randomized clinical trial. JAMA. 2017;318(19):1903–12. 10.1001/jama.2017.17426.PMID:29164255;PMCID:PMC5820726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.My pleural effusion journey. https://mypleuraleffusionjourney.com/ (accessed on 20 March 2025)
- 14.Porcel JM, Cordovilla R, Tazi-Mezalek R, Barrios-Barreto D, Pérez-Pallarés J, Novais e Bastos H, et al. Efficacy and safety of indwelling catheter for malignant pleural effusions related to timing of cancer therapy: a systematic review. Arch Bronconeumol. 2023;59(9):566–74. [DOI] [PubMed] [Google Scholar]
- 15.Borgeson D, Defranchi S, Lam C, Lin G, Iii F. Chronic indwelling pleural catheters reduce hospitalizations in advanced heart failure with refractory pleural effusions. J Card Fail. 2009. 10.1016/j.cardfail.2009.06.133. [Google Scholar]
- 16.Srour N, Potechin R, Amjadi K. Use of indwelling pleural catheters for cardiogenic pleural effusions. Chest. 2013;144(5):1603–8. [DOI] [PubMed] [Google Scholar]
- 17.Freeman RK, Ascioti AJ, Dake M, Mahidhara RS. A propensity-matched comparison of pleurodesis or tunneled pleural catheter for heart failure patients with recurrent pleural effusion. Ann Thorac Surg. 2014. 10.1016/j.athoracsur.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 18.Majid A, Kheir F, Fashjian M, Chatterji S, Fernandez-Bussy S, Ochoa S, et al. Tunneled pleural catheter placement with and without talc poudrage for treatment of pleural effusions due to congestive heart failure. Ann Am Thorac Soc. 2016;13(2):212–6. [DOI] [PubMed] [Google Scholar]
- 19.Frost N, Ruwwe-Glösenkamp C, Raspe M, Brünger M, Temmesfeld-Wollbrück B, Suttorp N, et al. Indwelling pleural catheters for non-malignant pleural effusions: report on a single centre’s 10 years of experience. Open Respir Res. 2020. 10.1136/bmjresp-2019-000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker SP, Bintcliffe O, Keenan E, Stadon L, Evison M, Haris M, et al. Randomised trial of indwelling pleural catheters for refractory transudative pleural effusions. Eur Respir J. 2022;59(2):2101362. [DOI] [PubMed] [Google Scholar]
- 21.Wijayaratne T, Yousuf A, Panchal R. Cardiac related pleural effusions: a narrative review. J Thorac Dis. 2024;16(2):1674–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilbert CR, Shojaee S, Maldonado F, Yarmus LB, Bedawi E, Feller-Kopman D, Rahman NM, Akulian JA, Gorden JA. Pleural interventions in the management of hepatic hydrothorax. Chest. 2022;161(1):276–83. [DOI] [PubMed] [Google Scholar]
- 23.Porcel JM. Management of refractory hepatic hydrothorax. Curr Opin Pulmonary Med. 2014. 10.1097/MCP.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 24.Chen A, Massoni J, Jung D, Crippin J. Indwelling tunneled pleural catheters for the management of hepatic hydrothorax. A pilot study. Ann Am Thorac Soc. 2016;13(6):862–6. [DOI] [PubMed] [Google Scholar]
- 25.Kniese C, Diab K, Ghabril M, Bosslet G. Indwelling pleural catheters in hepatic hydrothorax. Chest. 2019. 10.1016/j.chest.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shojaee S, Rahman N, Haas K, Kern R, Leise M, Alnijoumi M, et al. Indwelling tunneled pleural catheters for refractory hepatic hydrothorax in patients with cirrhosis: a multicenter study. Chest. 2019;155(3):546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartnicki-Navarrete I, Valladares A, Bolaños-Morales F, Torre G, Páez-Zayas V, García-Juárez I. Tu1683 indwelling pleural catheters versus serial thoracentesis in the treatment of refractory hepatic hydrothorax: a multicenter cohort study. Gastroenterology. 2020. 10.1016/S0016-5085(20)34296-7. [Google Scholar]
- 28.Avula A, Acharya S, Anwar S, Narula N, Chalhoub M, Maroun R, et al. Indwelling pleural catheter (IPC) for the management of hepatic hydrothorax: the known and the unknown. J Bronchology Interv Pulmonol. 2022;29(3):179–85. [DOI] [PubMed] [Google Scholar]
- 29.www.isrctn.com/ISRCTN10499680 accessed 20th March 2025
- 30.Muruganandan S, Azzopardi M, Fitzgerald DB, Shrestha R, Kwan BCH, Lam DCL, et al. Aggressive versus symptom-guided drainage of malignant pleural effusion via indwelling pleural catheters (AMPLE-2): an open-label randomised trial. Lancet Respir Med. 2018;6(9):671–80. [DOI] [PubMed] [Google Scholar]
- 31.Wahidi MM, Reddy C, Yarmus L, Feller-Kopman D, Musani A, Shepherd RW, et al. Randomized trial of pleural fluid drainage frequency in patients with malignant pleural effusions. The ASAP trial. Am J Respir Crit Care Med. 2017;195(8):1050–7. [DOI] [PubMed] [Google Scholar]
- 32.Warren WH, Kim AW, Liptay MJ. Identification of clinical factors predicting Pleurx® catheter removal in patients treated for malignant pleural effusion. Eur J Cardiothorac Surg. 2008;33(1):89–94. [DOI] [PubMed] [Google Scholar]
- 33.Bhatnagar R, Keenan Emma K, Morley Anna J, Kahan Brennan C, Stanton Andrew E, Haris M, et al. Outpatient talc administration by indwelling pleural catheter for malignant effusion. N Engl J Med. 2018;378(14):1313–22. [DOI] [PubMed] [Google Scholar]
- 34.Fitzgerald DB, Muruganandan S, Stanley C, Badiei A, Murray K, Read CA, et al. EPIToME (Early Pleurodesis via IPC with Talc for Malignant Effusion): Evaluation of a new management algorithm. Europ Resp J. 2019. 10.1183/13993003.congress-2019.OA493. [Google Scholar]
- 35.Sivakumar P, Fitzgerald DB, Ip H, Rao D, West A, Noorzad F, et al. The impact of outpatient versus inpatient management on health-related quality of life outcomes for patients with malignant pleural effusion: the OPTIMUM randomised clinical trial. Eur Respir J. 2024. 10.1183/13993003.01215-2022. [DOI] [PubMed] [Google Scholar]
- 36.Krochmal R, Reddy C, Yarmus L, Desai NR, Feller-Kopman D, Lee HJ. Patient evaluation for rapid pleurodesis of malignant pleural effusions. J Thorac Dis. 2016;8(9):2538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dipper A, Sundaralingam A, Hedley E, Tucker E, White P, Bhatnagar R, et al. The randomised thoracoscopic talc poudrage+indwelling pleural catheters versus thoracoscopic talc poudrage only in malignant pleural effusion trial (TACTIC): study protocol for a randomised controlled trial. Open Respir Res. 2023. 10.1136/bmjresp-2023-001682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shrager JB, Bhatnagar R, Kearney CT, Retzlaff NP, Cohen E, Stanton AE, et al. Silver nitrate-coated versus standard indwelling pleural catheter for malignant effusions: the swift randomized trial. Ann Am Thorac Soc. 2022;19(10):1722–9. [DOI] [PubMed] [Google Scholar]
- 39.Paschoalini Mda S, Vargas FS, Marchi E, Pereira JR, Jatene FB, Antonangelo L, et al. Prospective randomized trial of silver nitrate vs talc slurry in pleurodesis for symptomatic malignant pleural effusions. Chest. 2005;128(2):684–9. [DOI] [PubMed] [Google Scholar]
- 40.Matus I, Ho P. Ambulatory iodopovidone instillation via indwelling pleural catheters for malignant pleural effusions. J Bronchol Intervent Pulmonol. 2019. 10.1097/LBR.0000000000000599. [DOI] [PubMed] [Google Scholar]
- 41.Piggott LM, Hayes C, Greene J, Fitzgerald DB. Malignant pleural disease. Breathe. 2024;19(4): 230145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feller-Kopman DJ, Reddy CB, DeCamp MM, Diekemper RL, Gould MK, Henry T, et al. Management of malignant pleural effusions an Official ATS/STS/STR Clinical practice guideline. Am J Respirat Critical Care Med. 2018;198(7):839–49. [DOI] [PubMed] [Google Scholar]
- 43.Management of malignant pleural effusions. Am J Respir Crit Care Med. 2000;162(5):1987–2001. [DOI] [PubMed] [Google Scholar]
- 44.Thakkar D, Lamb CR, Quadri SM. Using a Novel Digital Pleural Drainage Device: A Proof of Concept. C23 Pleural Disease, Cf, And Other Magical Creatures. American Thoracic Society International Conference Abstracts: American Thoracic Society; 2019. p. A4338-A
- 45.Yousuf A, Hinchcliffe F, Johnstone S, Mohammad S, Sudhir R, Panchal R. S139 Experience of using a novel digital drainage system via an indwelling pleural catheter (IPC): a case series. Thorax. 2023;78(Suppl 4):A101-A2
- 46.Wijayaratne T, Hinchcliffe F, Rizvi S, Mavilakandy A, Johnstone S, Sudhir R, et al. S26 Feasibility and effectiveness of the Passio™ digital drainage system in reducing chest pain during IPC pleural drainage. Thorax. 2024;79(Suppl 2):A24-A5
- 47.www.isrctn.com/ISRCTN16390322 accessed 20th March 2025
- 48.Welch H, Barton E, Beech E, Patole S, Stadon L, Maskell N. Does a novel Indwelling Pleural Catheter drainage system improve patient experience? European Respiratory Journal.62(suppl 67):OA1563
- 49.Blyth KG, Adusumilli PS, Astoul P, Darlison L, Lee YCG, Mansfield AS, et al. Leveraging the pleural space for anticancer therapies in pleural mesothelioma. Lancet Respir Med. 2024;12(6):476–83. [DOI] [PubMed] [Google Scholar]
- 50.Boutin C, Viallat JR, van Zandwijk N, et al. Activity of intrapleural recombinant gamma-interferon in malignant mesothelioma. Cancer. 1991;67:2033–7. [DOI] [PubMed] [Google Scholar]
- 51.Astoul P, Viallat J-R, Laurent JC, Brandely M, Boutin C. Intrapleural recombinant IL-2 in passive immunotherapy for malignant pleural effusion. Chest. 1993;103:209–13. [DOI] [PubMed] [Google Scholar]
- 52.Boutin C, Nussbaum E, Monnet I, et al. Intrapleural treatment with recombinant gamma-interferon in early stage malignant pleural mesothelioma. Cancer. 1994;74:2460–7. [DOI] [PubMed] [Google Scholar]
- 53.Astoul P, Picat-Joossen D, Viallat J-R, Boutin C. Intrapleural administration of interleukin-2 for the treatment of patients with malignant pleural mesothelioma. Cancer. 1998;83:2099–104. [DOI] [PubMed] [Google Scholar]
- 54.Aggarwal C, Haas AR, Metzger S, Aguilar LK, Aguilar-Cordova E, Manzanera AG, et al. Phase I study of intrapleural gene-mediated cytotoxic immunotherapy in patients with malignant pleural effusion. Mol Ther. 2018;26(5):1198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Danson SJ, Conner J, Edwards JG, Blyth KG, Fisher PM, Muthana M, et al. Oncolytic herpesvirus therapy for mesothelioma - A phase I/IIa trial of intrapleural administration of HSV1716. Lung Cancer. 2020;150:145–51. [DOI] [PubMed] [Google Scholar]
- 56.Nie K, Zhang Z, You Y, Zhuang X, Zhang C, Ji Y. A randomized clinical study to compare intrapleural infusion with intravenous infusion of bevacizumab in the management of malignant pleural effusion in patients with non-small-cell lung cancer. Thorac Cancer. 2020;11(1):8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fennell DA, Dulloo S, Lord S, Thistlethwaite F, Blyth KG, Szlosarek PW, et al. First-in-human phase I clinical trial of RSO-021, a first-in-class covalent inhibitor of mitochondrial peroxiredoxin 3 (PRX3), in patients with malignant pleural effusion due to mesothelioma and other advanced solid tumors (MITOPE). J Clin Oncol. 2024. 10.1200/JCO.2024.42.16_suppl.3019. [Google Scholar]
- 58.Dulloo S, Lord S, Fennell DA, Thistlethwaite F, Maskell N, Popat S, et al. Phase 2 study to evaluate the novel mitochondrial PRX3 inhibitor, RSO-021, as an intrapleural monotherapy and in combination with IV paclitaxel in patients with malignant pleural effusion due to mesothelioma or another advanced solid tumor. J Clin Oncol. 2024. 10.1200/JCO.2024.42.16_suppl.TPS8124. [Google Scholar]
- 59.Olfert JA, Penz ED, Manns BJ, Mishra EK, Davies HE, Miller RF, et al. Cost-effectiveness of indwelling pleural catheter compared with talc in malignant pleural effusion. Respirology. 2017;22(4):764–70. [DOI] [PubMed] [Google Scholar]
- 60.Shafiq M, Simkovich S, Hossen S, Feller-Kopman DJ. Indwelling pleural catheter drainage strategy for malignant effusion: a cost-effectiveness analysis. Ann Am Thorac Soc. 2020;17(6):746–53. [DOI] [PubMed] [Google Scholar]
- 61.Botana-Rial M, Ramos-Hernández C, Lojo-Rodríguez I, Represas-Represas C, Ruano-Raviña A, Leiro-Fernández V, et al. Cost-effectiveness of malignant pleural effusion with indwelling catheter: systematic review. J Palliat Med. 2020;24(8):1206–12. [DOI] [PubMed] [Google Scholar]
- 62.Fitzgerald DB, Sidhu C, Budgeon C, Tan AL, Read CA, Kwan BCH, et al. Australasian Malignant PLeural Effusion (AMPLE)-3 trial: study protocol for a multi-centre randomised study comparing indwelling pleural catheter (±talc pleurodesis) versus video-assisted thoracoscopic surgery for management of malignant pleural effusion. Trials. 2022;23(1):530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lau EPM, Ing M, Vekaria S, Tan AL, Charlesworth C, Fysh E, et al. Australasian Malignant PLeural Effusion (AMPLE)-4 trial: study protocol for a multi-centre randomised trial of topical antibiotics prophylaxis for infections of indwelling pleural catheters. Trials. 2024;25(1):249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.www.isrctn.com/ISRCTN12371566 accessed 20th March 2025
- 65.https://clinicaltrials.gov/study/NCT03831386 accessed 20th March 2025
- 66.https://arc-eoe.nihr.ac.uk/research-implementation/research-themes/palliative-and-end-life-care/pleural-catheters-co accessed 20th March 2025
- 67.www.cancer.gov/research/participate/clinical-trials-search/v?id=NCI-2022-01561 accessed 20th March 2025
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.





