Abstract
STUDY QUESTION
Are blood plasma trimethylamine N-oxide (TMAO) and related metabolites linked to the odds of asthenozoospermia?
SUMMARY ANSWER
Increased blood plasma TMAO levels were positively associated with the odds of asthenozoospermia, while elevated levels of choline and L-carnitine were related to reduced asthenozoospermia odds, implying that TMAO and its related metabolites might play an important role in the development of asthenozoospermia.
WHAT IS KNOWN ALREADY
Sperm motility and concentration are profoundly impaired by excessive reactive oxygen species (ROS). A positive correlation has been established between ROS levels and TMAO, which is regarded as a key regulatory factor for initiating mitochondrial ROS production. However, the precise interplay between TMAO and its metabolites and sperm quality remains inconclusive and insufficient.
STUDY DESIGN, SIZE, DURATION
This case–control study was conducted from June 2020 to December 2020. A total of 314 pairs of asthenozoospermia cases and normozoospermia controls, matched based on age, BMI, and smoking status, were included.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Blood plasma levels of TMAO and five related metabolites, such as choline, betaine, L-carnitine, methionine, and dimethylglycine, were measured using a liquid chromatography system coupled with tandem mass spectrometry. Multivariable conditional logistic regression models were used to estimate the odds ratios (ORs) and corresponding 95% CIs.
MAIN RESULTS AND THE ROLE OF CHANCE
Compared with the lowest quartile, a significant association was observed between blood plasma TMAO level (OR = 1.80, 95% CI = 1.16–2.81) and the odds of asthenozoospermia for the highest quartile. In contrast, choline (OR = 0.59, 95% CI = 0.37–0.92) and L-carnitine (OR = 0.58, 95% CI = 0.37–0.90) levels were significant inversely associated with the odds of asthenozoospermia. Additionally, for each per SD change, significant dose–response relationships were noted with increased odds of asthenozoospermia linked to elevated TMAO (OR = 1.31, 95% CI = 1.12–1.55), as well as L-carnitine (OR = 0.79, 95% CI = 0.67–0.93) and total methyl donors exposure (OR = 0.82, 95% CI = 0.70–0.96) levels.
LIMITATIONS, REASONS FOR CAUTION
We cannot infer causality from this study due to the case–control study. Since the current study was conducted on a population of Chinese men, the extrapolated results may not accurately reflect other regions or populations. As blood plasma TMAO and its metabolites were measured at a single time point and may not accurately represent long-term concentrations, the enduring effects on sperm quality may not be fully captured. Another limitation of the current study lies in its relatively modest sample size, which may have been insufficient to reach statistical power in subgroup analyses.
WIDER IMPLICATIONS OF THE FINDINGS
This study indicated that elevated blood plasma TMAO levels were associated with increased odds of asthenozoospermia, while higher concentrations of choline and L-carnitine decreased asthenozoospermia odds. Our results provide novel evidence that TMAO and its metabolites may serve as potential biomarkers for asthenozoospermia.
STUDY FUNDING/COMPETING INTEREST(S)
No funding was received for this study. All authors have no conflict of interest to declare.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: asthenozoospermia, case–control study, China, sperm quality, trimethylamine N-oxide
WHAT DOES THIS MEAN FOR PATIENTS?
Male infertility is a global public health issue, and reduced sperm motility, medically referred to as ‘asthenozoospermia’, is one of the main causes of male infertility, accounting for 20–40% of male infertility cases. The debate on why semen quality is diminishing has persisted for decades, with several physiological, environmental, and genetic factors all being considered. Oxidative stress, which can be thought of as an imbalance between damaging types of oxygen, referred to by scientists as reactive oxygen species, and a body’s ability to counteract the damage they inflict, is one of the primary mechanisms of male infertility.
Excessive reactive oxygen species can severely impair sperm motility and concentration, and a positive correlation was previously established between their levels and a substance investigated in this study, known as trimethylamine N-oxide (TMAO). TMAO is produced through the metabolism of substances such as choline, phosphatidylcholine, L-carnitine, and betaine in the diet by gut microbes. Subsequently, it is absorbed into the bloodstream and can be detected in blood plasma. However, regarding the odds of asthenozoospermia, the role of TMAO and its metabolites (related substances), including choline, betaine, L-carnitine, methionine, dimethylglycine, and total methyl donors (the sum of choline, betaine, and methionine), remains inconclusive and insufficient.
Hence, we performed a hospital-based matched case–control study involving 314 pairs of patients (cases) with asthenozoospermia matched with healthy study participants (controls) with normal sperm to make a thorough inquiry into this topic.
Our results showed that increased TMAO levels in blood were correlated with increased odds of asthenozoospermia, whereas increased choline and L-carnitine levels were linked to lower odds of asthenozoospermia. Further analyses suggested that eating more red meat may enhance the effects of TMAO-related substances on asthenozoospermia.
Therefore, for patients with asthenozoospermia, reducing the consumption of red meat may help mitigate the negative impacts associated with the combined action of red meat and these metabolites on sperm motility. If further studies, including those in non-Chinese populations, confirm our findings, there may be potential to improve sperm quality by focusing on blood plasma TMAO and its associated metabolites as key indicators.
Introduction
The global disease burden of infertility has continued to increase, progressively emerging as a significant health challenge (Sun et al., 2019b). Recognition of the critical significance of male reproductive function has increased in recent years (Levine et al., 2018). The causes of infertility include low sperm counts, poor sperm quality, endocrinopathies, lifestyle factors (such as smoking and obesity), congenital anatomical factors, gonadotoxic exposures, and aging (Eisenberg et al., 2023), with asthenozoospermia being one of the most common causes (Chemes, 2000; Huynh et al., 2002; Curi et al., 2003). A study conducted in Argentina revealed that asthenozoospermia was a prevalent cause of male infertility, with a prevalence of 18.71%. Furthermore, the combined prevalence of asthenozoospermia with oligospermia and/or teratozoospermia rose to 63.13% (Curi et al., 2003). An analysis of 38 905 infertile male patients in China found that 50.5% were diagnosed with asthenozoospermia, with the prevalence steadily rising from 2008 to 2016 (Wu et al., 2021). Research on the causes of asthenozoospermia and strategies to prevent further disruption of male reproductive health are urgently needed.
Choline, an essential nutrient, plays a crucial role in the proper functioning of the liver, muscles, and brain, as well as in lipid metabolism and the composition and repair of cell membranes (Zeisel and da Costa, 2009; Wallace, 2018). Gut microbiota is recognized as the second genome of the human body (Jiao et al., 2022), with previous studies highlighting its contribution to human metabolism and health (Koeth et al., 2013; Tang et al., 2013, 2019). The gut microbiota converts dietary precursors such as choline, phosphatidylcholine, L-carnitine, and betaine into trimethylamine (TMA), which is subsequently metabolized by the hepatic enzyme flavin monooxygenase-3 to produce TMAO (Li et al., 2017; Wang et al., 2023). Previous research indicates that diet is a key factor in regulating TMAO and its related metabolites. The Mediterranean diet, rich in cereals, fruits, vegetables, and legumes, has been linked to lower TMAO levels (De Filippis et al., 2016; Castañón-Apilánez et al., 2024; Latif et al., 2025; Theodoridis et al., 2025). Conversely, red meats like beef and pork are major dietary sources of L-carnitine and choline. Gut microbiota converts L-carnitine and choline into TMA, which is then oxidized in the liver and released as TMAO, thereby increasing blood plasma TMAO concentrations (Koeth et al., 2013; Guerreiro et al., 2025). Another diet, the very low-calorie ketogenic diet (VLCKD), which is known for its role in weight loss and the generation of anti-inflammatory ketone bodies, has drawn attention for its therapeutic effect in male sexual dysfunction (Condorelli et al., 2022). It has been reported that the levels of TMAO significantly decrease after intervention with the VLCKD (Verde et al., 2024a, 2024b; Theodoridis et al., 2025). Moreover, a plant-based diet and a high-dairy diet have been linked to a reduction in TMAO concentration, while a high-protein and high-fat diet is associated with an increase in TMAO concentration (Theodoridis et al., 2025). Notably, restricting total energy consumption has been shown to effectively reduce both TMAO and choline levels (Heianza et al., 2019; Battillo and Malin, 2023). Lifestyle interventions, such as a hypocaloric diet and regular physical activity, appear to be effective in reducing blood plasma TMAO (Erickson et al., 2019; Theodoridis et al., 2025). However, the effect of lifestyle interventions on TMAO is controversial. There are also studies indicating that dietary modifications and following exercise recommendations show no significant impact on TMAO levels (Randrianarisoa et al., 2016; Troseid et al., 2016). Previous studies have demonstrated that TMAO additionally stimulates reactive oxygen species (ROS) generation, particularly mitochondrial ROS (Chen et al., 2017), indicating a positive correlation between ROS levels and TMAO (Darbandi et al., 2019). Excessive ROS can significantly impair total sperm motility and sperm concentration (Darbandi et al., 2019). Additionally, Mateo-Otero et al. (2021) identified an inverse relationship between TMAO concentrations in pig seminal plasma and viable sperm with high membrane destabilization, suggesting TMAO could serve as a potential biomarker of sperm function. Increasing evidence links oxidative stress (OS) to the pathogenesis of male infertility (Bisht et al., 2017; Agarwal et al., 2021a, 2021b), and the term ‘Male Oxidative Stress Infertility’ has been coined to describe these male-specific effects (Agarwal et al., 2019). Choline, betaine, L-carnitine, methionine, and dimethylglycine (DMG) all contribute to combating OS (Zhao et al., 2018; Bai et al., 2019; Khedr and Werida, 2022; Dong et al., 2024), but their potential connection to asthenozoospermia remains unexplored.
In this study, we aimed to investigate the association between blood plasma TMAO and related metabolites, such as choline, betaine, L-carnitine, methionine, DMG, and total methyl donors (the sum of choline, betaine, and methionine), and asthenozoospermia odds in a case–control study.
Materials and methods
Study design and subjects
This case–control study was conducted from June 2020 to December 2020. Male patients attending the Center of Reproductive Medicine at Shengjing Hospital of China Medical University were included in the study. In total, 597 asthenozoospermia cases and 612 healthy controls were initially recruited, prior to evaluating all exclusion criteria and case–control matching (Fig. 1). All participants underwent sperm analysis and physical examinations and completed self-administered questionnaires. Trained nurses reviewed all the completed questionnaires and promptly contacted participants to correct any missing or unclear responses. All participants provided written informed consent upon enrollment in the study. This study was approved by the ethics committee of Shengjing Hospital of China Medical University and was conducted in accordance with the principles of the Declaration of Helsinki (Goodyear et al., 2007; World Medical Association, 2013) as well as the Strengthening the Reporting of Observational Studies in Epidemiology statement (von Elm et al., 2007).
Figure 1.
Flow diagram of the selection of men for the hospital-based matched case–control study to assess the relations of blood plasma trimethylamine N-oxide and related metabolites and asthenozoospermia odds.
We excluded participants who failed to provide complete basic and dietary information (e.g. age, height, weight, household income, physical activity level, smoking and drinking alcohol status, occupational status, education level, total energy intake, total red meat intake, and sexual abstinence time) (n = 10). Participants who provided blood samples with chylomicronemia were excluded (n = 11). Those with a history of varicocele, a known confounding factor, were excluded as well (n = 6) (Eslamian et al., 2012; Jensen et al., 2017; Liu et al., 2021; Lv et al., 2022). Additionally, individuals with implausible total energy intake values (>6000 or <800 kcal/day) were removed from the analysis (n = 15) (Shi et al., 2018). Each asthenozoospermia case was 1:1 matched with a healthy control based on age, BMI, and smoking status. In the end, a total of 314 cases and 314 matched controls were included in the final analysis (Fig. 1). This sample size of 628 men met the required number to achieve adequate statistical power, as determined through Power Analysis & Sample Size (PASS, NCSS, LLC; Kaysville, UT, USA) 11.0 software.
Semen collection and analysis
The methods for semen collection and analysis have been thoroughly described elsewhere (Liu et al., 2021, 2022; Wang et al., 2021a). All participants were instructed to abstain from ejaculation for 3–7 days prior to providing a semen sample. The samples were collected via masturbation into a sterile container, ensuring no contact with condoms or lubricants. After collection, the semen was allowed to liquefy for no more than 60 min before analysis. Sperm parameters were assessed using a WLJY9000 analysis instrument (Beijing Weili New Century Science & Tech. Dev. Co. Ltd, Beijing, China). Each semen sample was examined twice by two experienced technicians, and the reference values of normal sperm were identified according to the WHO criteria (World Health Organization, 2021).
Definition of asthenozoospermia
Asthenozoospermia was diagnosed based on the criteria outlined in the fifth edition of the World Health Organization laboratory manual for the examination and processing of human semen (World Health Organization, 2010). It was defined as having a total motility (progressive+non-progressive) of less than 40%, which includes rapidly progressive, slowly progressive, and non-progressive sperm, as well as progressive motility of less than 32%, encompassing both rapidly and slowly progressive motility, within 60 min of ejaculation over the past 3 months. The total sperm count and the percentage of morphologically normal spermatozoa were above the reference values for the lower limits. The control group consisted of men with normal sperm parameters, including ≥39 million sperm per ejaculate, ≥15 million sperm per milliliter, ≥40% total motility, ≥32% progressive motility, and ≥4% normal morphology.
Data collection
Demographic, socio-economic features, smoking status, alcohol intake, physical activity levels, lifestyle factors, dietary data, and sexual abstinence time were obtained at baseline through self-administered questionnaires. Anthropometric data were collected by trained investigators using standardized techniques and equipment. Moreover, clinical data were extracted from digital medical records, and blood plasma samples were collected from each participant at the time of recruitment. Body weight and height were measured during physical examinations. BMI was calculated by dividing weight (kg) by height squared (m2). Physical activity was expressed as metabolic equivalent (MET) hours per week, determined by multiplying the frequency of physical activity by its corresponding MET coefficient (Du et al., 2013).
In addition, dietary intake information was collected using a 110-item food frequency questionnaire (Cui et al., 2023; Huang et al., 2023; Zhao et al., 2023), and our previous studies have demonstrated its reliability and validity (Liu et al., 2021; Wang et al., 2021b; Cui et al., 2022). All patients reported how often they consumed each food in the year prior to their diagnosis of asthenozoospermia. Intake frequency was divided into seven categories: ‘almost never’, ‘2–3 times a month’, ‘once a week’, ‘2–3 times a week’, ‘4–6 times a week’, ‘once a day’, ‘≥2 times a day’. For most food groups, the reproducibility coefficients (Spearman and intraclass correlation coefficients) were above 0.5, and the Spearman correlation coefficients ranged between 0.3 and 0.7 for most food groups between the questionnaire and weighed diet records. Based on the China Food Composition Table, the frequency of consumption of each food was multiplied by the nutrient content of the designated portions to determine the daily intake of each food (Hu et al., 2018; Yang et al., 2018).
TMAO and its related metabolites measurement
At baseline, a venous blood sample was collected from each subject and centrifuged at 1800 g for 10 min. Blood plasma was separated and stored at −80°C for analysis. TMAO and its related metabolites were extracted from blood plasma by solid phase extraction and measured by a liquid chromatography system coupled with tandem mass spectrometry (LC-MS/MS) (Wang et al., 2014; Kalagi et al., 2022; Kijpaisalratana et al., 2023). Blood plasma samples were analyzed in batches, and samples from cases and controls were mixed and anonymized prior to delivery to the laboratory, ensuring a fully blinded assessment while accounting for any potential batch differences in the analysis process. As an additional quality control measure to ensure the accuracy, consistency, and repeatability of the method, all laboratory procedures were completed in 2024, and ∼10% of the blood plasma samples were subjected to repeat analysis. The coefficient of variation in the laboratory for the blood plasma TMAO measurements was consistently below 10%.
Statistical analyses
Categorical variables were expressed as numbers with percentages. Mean with SD was utilized for normally distributed continuous variables, and median with quartile range was used for non-normally distributed continuous variables. Student’s t-tests (normally distributed) or the Wilcoxon signed rank test (non-normally distributed) were used for continuous variables, and the chi-square test was used for categorical variables to compare the differences between the case and the control group. Spearman correlation analysis was employed to assess the associations between blood plasma levels of TMAO and its metabolites. Blood plasma TMAO and its metabolite levels were categorized into quartiles according to their distribution among the controls. Total methyl donors were the sum of choline, betaine, and methionine (Huang et al., 2020). A multivariable conditional logistic regression model was applied to estimate the odds ratios (ORs) and 95% CIs with the lowest quartile as the reference group. The selection of confounders was based on previous literature and guided by the directed acyclic graph (Supplementary Fig. S1) (Tennant et al., 2021). Accordingly, adjusted potential confounders included educational level (junior secondary or below, senior high school/technical secondary school, or junior college/university or above), total energy intake (kcal/d), alcohol intake (yes or no), sexual abstinence time (days), serum creatinine (µmol/l), and total red meat intake (g/day), with Model 1 adjusted for educational level, total energy, and alcohol intake and Model 2 further adjusted for serum creatinine, total red meat intake, and sexual abstinence time based on Model 1. The linear trend tests were examined by using the median value of each quartile. We also modeled each variable as a continuous variable and derived ORs and 95% CIs.
Subgroup analyses were carried out based on alcohol intake (yes and no), total energy intake (below median and above median), and total red meat intake (below median and above median). The potential interactions between TMAO and related metabolites and these stratified variables were analyzed by using cross-product terms in the multivariable models.
Sensitivity analyses were conducted to assess the robustness of the results. Drawing on clinical significance and published literature, two additional models were conducted based on the Model 2: (i) further adjusted for physical activity (Pingitore et al., 2015; Sun et al., 2019a); and (ii) further adjusted for occupation (Daoud et al., 2017; Wu et al., 2021). All analyses were performed by SAS 9.4 (SAS Institute, Cary, NC, USA). A two-sided alpha level of 0.05 was chosen for the statistical significance of all the analyses.
Results
Baseline characteristics of the population
Table 1 summarizes the general characteristics of the 314 pairs of subjects. When compared with the controls, participants with asthenozoospermia exhibited significantly lower sperm concentrations and total sperm counts, and lower percentages with progressive motility, total motility, and normal sperm morphology (all P < 0.05).
Table 1.
Characteristics of the participants.
| Characteristics | Control group (n = 314) | Case group (n = 314) | P-value |
|---|---|---|---|
| Age (years) | 32 (30–35) | 32 (30–36) | 0.57 |
| BMI (kg/m²) | 25.82 (23.36–28.73) | 26.12 (23.94–28.40) | 0.43 |
| Smoking status | 1.00 | ||
| No | 163 (51.91) | 163 (51.91) | |
| Yes | 151 (48.09) | 151 (48.09) | |
| Alcohol drinking | 0.25 | ||
| No | 182 (57.96) | 197 (62.74) | |
| Yes | 132 (42.04) | 117 (37.26) | |
| Occupation | 0.81 | ||
| Employed | 173 (55.10) | 177 (56.37) | |
| Unemployed | 141 (44.90) | 137 (43.63) | |
| Educational level | 0.48 | ||
| Junior secondary or below | 75 (23.89) | 65 (20.70) | |
| Senior high/technical secondary | 37 (11.78) | 45 (14.33) | |
| Junior college/university or above | 202 (64.33) | 204 (64.97) | |
| Serum creatinine (µmol/l) | 77.10 (62.51–93.32) | 76.67 (64.06–93.06) | 0.95 |
| Physical activity (MET*h/d) | 125.53 (95.55–223.87) | 131.33 (101.33–213.27) | 0.29 |
| Total energy intake (kcal/d) | 1685.05 (1402.45–2117.06) | 1756.55 (1412.89–2196.67) | 0.24 |
| Total red meat intake (g/day) | 82.87 (59.99–96.99) | 79.85 (49.24–95.37) | 0.05 |
| Sexual abstinence time (days) | 4 (3–5) | 4 (3–5) | 1.00 |
| Semen parameters | |||
| Ejaculate volume (ml) | 3.20 (2.50–4.20) | 3.50 (2.80–4.40) | 0.23 |
| Sperm concentration (106/ml) | 63.99 (42.87–85.23) | 48.93 (31.73–71.15) | <0.001 |
| Total sperm count (106/ml) | 214.94 (139.38–291.06) | 166.26 (106.64–249.85) | <0.001 |
| Progressive motility (%) | 43.03 (38.43–49.79) | 22.37 (15.27–28.21) | <0.001 |
| Total motility (%) | 53.61 (46.89–62.20) | 27.79 (19.82–35.12) | <0.001 |
| Normal sperm morphology (%) | 6.00 (4.00–8.00) | 5.00 (4.00–7.00) | <0.001 |
Values are means (SDs) for normally distributed continuous variables (age at diagnosis), medians (interquartile ranges) for skewed distributed continuous variables (BMI, physical activity, serum creatine, total energy intake, and total red meat intake), and values are numbers (percentages) for categorical variables. P-values <0.05 were considered statistically significant.
P-values were determined with Student’s t-tests (normally distributed) or Wilcoxon signed rank test (non-normally distributed) for continuous variables, and chi-square test for categorical variables to compare the differences between the case and the control group. All statistical tests are two sided.
MET, metabolic equivalent.
Concentrations of blood plasma TMAO and related metabolites
The distributions of blood plasma TMAO and related metabolites concentrations are shown in Table 2. The blood plasma TMAO concentration was elevated in the case group compared with the control group (P < 0.05). Conversely, the concentrations of choline, L-carnitine, methionine, and methyl donors in blood plasma were relatively higher in the control group than in the case group (all P < 0.05). Spearman correlation analysis identified associations between the concentrations of TMAO and its related metabolites (Fig. 2). Notably, the strongest correlation was observed between blood plasma choline and L-carnitine levels (Spearman’s rank correlation coefficients = 0.39).
Table 2.
Blood plasma TMAO and related metabolites in the study population.
| CV (%) | Median (IQR) (µmol/l) |
P-value | |||
|---|---|---|---|---|---|
| Total population | Case group | Control group | |||
| TMAO | 8.4 | 7.95 (4.50–11.50) | 8.49 (7.51–4.92) | 7.10 (6.62–4.18) | <0.01 |
| Choline | 5.7 | 14.68 (11.10–19.36) | 14.15 (8.55–10.66) | 15.09 (8.24–11.71) | 0.01 |
| Betaine | 6.9 | 39.12 (30.31–48.91) | 37.81 (20.12–28.89) | 39.91 (17.21–31.34) | 0.31 |
| L-carnitine | 4.3 | 43.75 (33.40–57.03) | 42.22 (24.69–30.73) | 45.97 (22.05–36.03) | <0.01 |
| Methionine | 8.8 | 28.56 (20.70–36.65) | 26.65 (16.06–19.78) | 29.63 (15.60–21.42) | 0.01 |
| DMG | 7.1 | 9.19 (5.97–12.51) | 8.69 (6.23–5.90) | 9.35 (6.54–6.08) | 0.29 |
| Methyl donors | – | 82.54 (67.89–97.45) | 79.40 (28.40–67.30) | 85.18 (29.80–69.79) | 0.01 |
CV, coefficient of variation; DMG, dimethylglycine; IQR, interquartile range; TMAO, trimethylamine-N-oxide.
P-values < 0.05 were considered statistically significant.
P-values were obtained from the Wilcoxon rank sum test between the case group and the control group.
Figure 2.
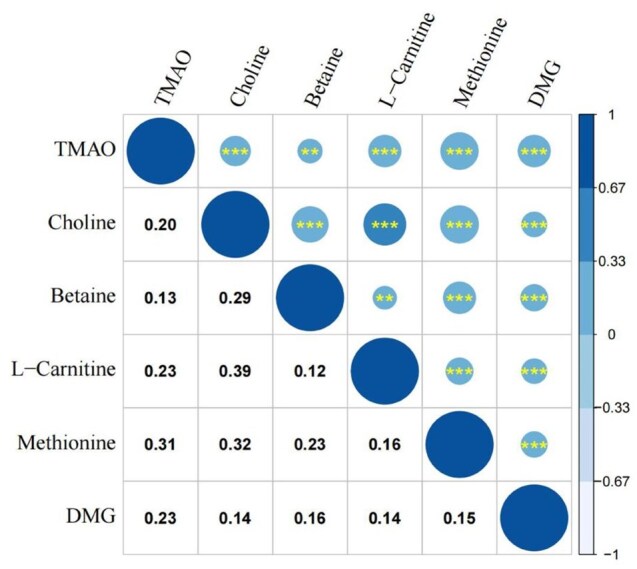
Spearman correlations among blood plasma trimethylamine N-oxide and related metabolites. The correlation among blood plasma TMAO and related metabolites is statistically significant (**P < 0.01; ***P < 0.001). The numbers below the navy-blue circles in the figure represent the magnitude of the Spearman’s rank correlation coefficients. The scale on the right side reflects the strength and direction of the linear relationship between variables. The closer the absolute value of the coefficient is to 1, the stronger the linear relationship is; a positive coefficient indicates a positive correlation; a negative coefficient indicates a negative correlation; a coefficient of 0 indicates the absence of a linear relationship. DMG, dimethylglycine; TMAO, trimethylamine N-oxide.
Associations of blood plasma TMAO and its related metabolites with the odds of asthenozoospermia
Table 3 shows the associations between blood plasma TMAO and related metabolites and the odds of asthenozoospermia. In multivariate-adjusted models, TMAO levels at the highest quartile were significantly associated with increased odds of developing asthenozoospermia compared with the lowest quartile (OR = 1.80, 95% CI = 1.16–2.81). In contrast, blood plasma concentrations of choline (OR = 0.59, 95% CI = 0.37–0.92) and L-carnitine (OR = 0.58, 95% CI = 0.37–0.90) were significantly inversely associated with the odds of asthenozoospermia, when comparing the highest quartile with the lowest quartile. Furthermore, significant dose–response relationships were observed, with increased odds of asthenozoospermia linked to higher TMAO levels (OR = 1.31, 95% CI = 1.12–1.55) for each per SD change. Additionally, decreased odds of asthenozoospermia were associated with lower levels of L-carnitine (OR = 0.79, 95% CI = 0.67–0.93) and total methyl donors exposure (OR = 0.82, 95% CI = 0.70–0.96).
Table 3.
Odds ratios and 95% CIs for the association between blood plasma TMAO and related metabolites with asthenozoospermia.
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P for trend | Per SD increment a | |
|---|---|---|---|---|---|---|
| TMAO (µmol/l) | ≤ 4.18 | 4.18–7.10 | 7.10–10.80 | >10.80 | ||
| Cases/controls | 66/79 | 58/78 | 77/79 | 113/78 | ||
| Crude model | 1.00 (Ref) | 0.89 (0.56–1.43) | 1.17 (0.74–1.84) | 1.73 (1.12–2.68) | <0.05 | 1.29 (1.10–1.52) |
| Model 1 | 1.00 (Ref) | 0.88 (0.55–1.41) | 1.15 (0.73–1.81) | 1.75 (1.13–2.71) | <0.05 | 1.30 (1.11–1.53) |
| Model 2 | 1.00 (Ref) | 0.88 (0.54–1.42) | 1.18 (0.74–1.86) | 1.80 (1.16–2.81) | <0.05 | 1.31 (1.12–1.55) |
| Choline (µmol/l) | ≤ 11.71 | 11.71–15.09 | 15.09–19.95 | >19.95 | ||
| Cases/controls | 101/79 | 68/78 | 86/79 | 59/78 | ||
| Crude model | 1.00 (Ref) | 0.68 (0.44–1.06) | 0.85 (0.56–1.30) | 0.59 (0.38–0.93) | 0.06 | 0.82 (0.70–0.96) |
| Model 1 | 0.67 (0.43–1.05) | 0.84 (0.55–1.29) | 0.58 (0.37–0.92) | 0.06 | 0.82 (0.70–0.96) | |
| Model 2 | 1.00 (Ref) | 0.68 (0.44–1.06) | 0.82 (0.53–1.26) | 0.59 (0.37–0.92) | 0.06 | 0.82 (0.70–0.96) |
| Betaine (µmol/l) | ≤ 31.34 | 31.34–39.91 | 39.91–48.55 | >48.55 | ||
| Cases/controls | 101/79 | 69/78 | 61/79 | 83/78 | ||
| Crude model | 1.00 (Ref) | 0.69 (0.45–1.07) | 0.60 (0.39–0.94) | 0.83 (0.54–1.28) | 0.32 | 0.93 (0.79–1.08) |
| Model 1 | 1.00 (Ref) | 0.69 (0.44–1.07) | 0.61 (0.39–0.95) | 0.83 (0.54–1.27) | 0.31 | 0.92 (0.79–1.08) |
| Model 2 | 1.00 (Ref) | 0.68 (0.44–1.05) | 0.60 (0.38–0.95) | 0.83 (0.54–1.29) | 0.33 | 0.92 (0.78–1.08) |
| L-carnitine (µmol/l) | ≤ 36.03 | 36.03–45.97 | 45.97–58.08 | >58.08 | ||
| Cases/controls | 118/79 | 66/78 | 65/79 | 65/78 | ||
| Crude model | 1.00 (Ref) | 0.57 (0.37–0.88) | 0.55 (0.36–0.85) | 0.56 (0.36–0.86) | <0.05 | 0.79 (0.67––0.92) |
| Model 1 | 1.00 (Ref) | 0.56 (0.36–0.87) | 0.56 (0.36–0.86) | 0.57 (0.37–0.89) | <0.05 | 0.79 (0.68–0.93) |
| Model 2 | 1.00 (Ref) | 0.56 (0.36–0.87) | 0.55 (0.35–0.85) | 0.58 (0.37–0.90) | <0.05 | 0.79 (0.67–0.93) |
| Methionine (µmol/l) | ≤ 21.42 | 21.42–29.63 | 29.63–37.02 | >37.02 | ||
| Cases/controls | 95/79 | 86/78 | 62/79 | 71/78 | ||
| Crude model | 1.00 (Ref) | 0.92 (0.60–1.41) | 0.65 (0.42–1.02) | 0.76 (0.49–1.17) | 0.11 | 0.82 (0.70–0.96) |
| Model 1 | 1.00 (Ref) | 0.92 (0.60–1.41) | 0.65 (0.42–1.02) | 0.76 (0.49–1.19) | 0.12 | 0.82 (0.70–0.97) |
| Model 2 | 1.00 (Ref) | 0.92 (0.59–1.41) | 0.66 (0.42–1.04) | 0.77 (0.49–1.20) | 0.13 | 0.83 (0.70–0.97) |
| DMG (µmol/l) | ≤ 6.08 | 6.08–9.35 | 9.35–12.62 | >12.62 | ||
| Cases/controls | 85/79 | 80/78 | 80/79 | 69/78 | ||
| Crude model | 1.00 (Ref) | 0.95 (0.62–1.48) | 0.94 (0.61–1.46) | 0.82 (0.53–1.28) | 0.62 | 0.92 (0.78–1.07) |
| Model 1 | 1.00 (Ref) | 0.96 (0.62–1.49) | 0.92 (0.60–1.43) | 0.82 (0.53–1.29) | 0.62 | 0.92 (0.78–1.07) |
| Model 2 | 1.00 (Ref) | 0.93 (0.60–1.46) | 0.93 (0.60–1.45) | 0.84 (0.53–1.31) | 0.60 | 0.92 (0.78–1.08) |
| Methyl donors (µmol/l) | ≤ 69.79 | 69.79–85.18 | 85.18–99.59 | >99.59 | ||
| Cases/controls | 95/79 | 91/78 | 62/79 | 66/78 | ||
| Crude model | 1.00 (Ref) | 0.97 (0.63–1.48) | 0.65 (0.42–1.02) | 0.70 (0.45–1.10) | <0.05 | 0.82 (0.70–0.96) |
| Model 1 | 1.00 (Ref) | 0.95 (0.62–1.46) | 0.63 (0.40–0.99) | 0.71 (0.45–1.10) | <0.05 | 0.82 (0.70–0.96) |
| Model 2 | 1.00 (Ref) | 0.92 (0.59–1.41) | 0.62 (0.39–0.97) | 0.70 (0.45–1.09) | <0.05 | 0.82 (0.70–0.96) |
Quartile values for each model represent OR (95% CI). P-values < 0.05 were considered statistically significant.
DMG, dimethylglycine; OR, odds ratios; Ref, reference; TMAO, trimethylamine-N-oxide.
Model 1: Adjusted for educational level, total energy, and alcohol intake.
Model 2: Further adjusted for serum creatinine, total red meat intake, and sexual abstinence time based on Model 1.
The SD of TMAO, choline, betaine, L-carnitine, methionine, DMG, and methyl donors were 4.23 µmol/l, 4.91 µmol/l, 11.69 µmol/l, 14.13 µmol/l, 10.69 µmol/l, 4.23 µmol/l, and 20.10 µmol/l, respectively.
Subgroup analysis, interaction, and sensitivity analysis
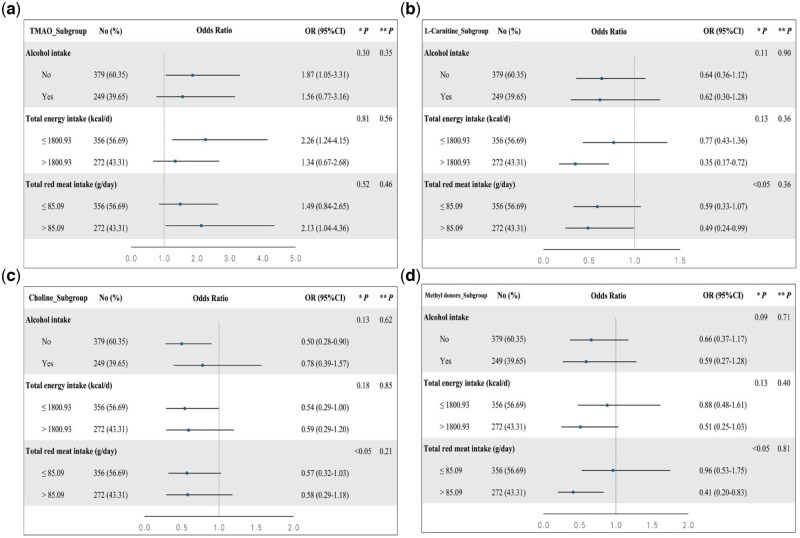
To further analyze the relationship between blood plasma TMAO and related metabolites and the odds of asthenozoospermia, subgroup analyses were performed based on demographic and clinical characteristics (Supplementary Table S1). In terms of the odds of asthenozoospermia, multiplicative interactions were observed between total red meat intake and choline, betaine, L-carnitine, methionine, DMG, as well as total methyl donors (all P < 0.05) (Supplementary Table S1). Regarding the relationship between TMAO and L-carnitine levels and the odds of asthenozoospermia, most subgroup analyses were oriented in the same direction as the main findings (Fig. 3). Among the other metabolites evaluated, choline and total methyl donors levels showed statistical significance across several specific subgroups (Fig. 3), while no subgroup analysis of betaine, methionine, and DMG exhibited statistically significant outcomes (Supplementary Fig. S2).
Figure 3.
Subgroup analyses of the associations between TMAO, L-carnitine, choline, and methyl donors and the odds of asthenozoospermia. (A) Subgroup analyses of the association between TMAO and the odds of asthenozoospermia. (B) Subgroup analyses of the association between L-carnitine and the odds of asthenozoospermia. (C) Subgroup analyses of the association between choline and the odds of asthenozoospermia. (D) Subgroup analyses of the association between methyl donors and the odds of asthenozoospermia. kcal/d, total energy intake of 1 day; OR, odds ratio; TMAO, trimethylamine N-oxide. * P for multiplicative interaction. ** P for additive interaction. P-values < 0.05 were considered statistically significant.
Sensitivity analyses were performed by using different models. We further adjusted separately for physical activity and occupation, and found that the estimated associations between blood plasma TMAO and related metabolites and the odds of asthenozoospermia were broadly consistent with the main analysis results (Supplementary Table S2).
Discussion
The present case–control study indicated that increased blood plasma TMAO levels were positively associated with the odds of asthenozoospermia, while elevated levels of choline and L-carnitine were linked to the reduced odds. To the best of our knowledge, we are the first to establish a connection between TMAO and its metabolites and asthenozoospermia odds.
Dietary habits can affect TMAO concentrations (Koeth et al., 2013; Janeiro et al., 2018), which results in significant differences in its concentrations across countries and regions. In our study, the median blood plasma concentration of TMAO was 7.95 µmol/l, relatively higher when compared with results from a cross-sectional study (median 3.00 µmol/l) (Wu et al., 2020) and a case–control study (median 1.47 µmol/l) (Shan et al., 2017). This may be attributable to the fact that our study exclusively included male patients, whereas previous studies have demonstrated that blood plasma TMAO levels are elevated in males compared with females (Obeid et al., 2017; Manor et al., 2018; Barrea et al., 2019). In contrast, the blood plasma TMAO level observed in our study was relatively lower than that found in a matched case–control study in Xinjiang, China (median: 8.59 µmol/l) (Ma et al., 2024). Previous research has shown that prolonged consumption of red meat increases TMAO levels (Wang et al., 2019), whereas the average red meat intake in Xinjiang is 133.0 g/day, substantially higher than the 81.0 g/day reported in our study (Zhang et al., 2021; Fang et al., 2023). Well-designed cohort studies have demonstrated that habitual intake of a relatively large amount of red meat is significantly associated with higher concentrations of TMAO (Buffa et al., 2022; Li et al., 2022). Long-term consumption of red meat can increase the levels of TMAO by three different mechanisms (Wang et al., 2019): increasing nutrient density of dietary TMA precursors; enhancing microbial TMA/TMAO production from carnitine, but not choline; and reducing renal TMAO excretion. Stopping the consumption of red meat has been proven to reduce the blood plasma TMAO within 4 weeks (Wang et al., 2019). Given that our study identified a multiplicative interaction between total red meat intake and all examined TMAO metabolites, it is crucial to focus on reducing red meat consumption, as it may act as a potential regulator of the relationship between TMAO metabolites and asthenozoospermia.
Our findings suggest that TMAO increases the odds of asthenozoospermia, while choline and L-carnitine reduce it. An analysis of 151 semen samples from normozoospermic men showed that TMAO may influence the methylation of the H19-Igf2 gene to increase ROS levels, while high ROS levels reduce total sperm motility and sperm concentration (Darbandi et al., 2019). An animal study using pigs has shown that TMAO is associated with sperm quality and function and could be used as a potential biomarker of sperm function (Mateo-Otero et al., 2021). However, the above study was done using pig seminal plasma, while our results came from blood plasma. In addition, for the related metabolites of TMAO, choline is a key factor in regulating the sperm membrane structure and fluidity, and this nutrient plays a crucial role in the maturation and fertilizing ability of sperm (Lazaros et al., 2012; Hanley, 2023). L-carnitine, as an antioxidant, has a protective effect on sperm motility in low temperature or low pressure and low oxygen environments (Chang et al., 2023; Kaltsas, 2023; Nateghian et al., 2023). Several animal studies have shown that betaine has a significant protective effect on sperm (Attia et al., 2019; Billah et al., 2022; Meng et al., 2022; Mori et al., 2022; Li et al., 2024) and an improving effect on oligospermia (Lin et al., 2024), but no correlation between betaine and asthenozoospermia was found in our study. Notably, different scholars hold opposite views on whether methionine damages or protects sperm. One study showed that methionine restriction mitigated the decline in sperm quality (Wu et al., 2023), but another study verified that methionine restriction induced a decline in sperm viability and sperm transferred during mating (Wei et al., 2024). Taken together, the relationship between these metabolites and asthenozoospermia remains inconsistent, and further studies are needed to evaluate this association.
TMAO and its metabolites may affect sperm function through a variety of mechanisms. TMAO can increase ROS production, which leads to lipid peroxidation of the sperm membrane, damages sperm DNA integrity, and induces OS (Darbandi et al., 2019). Excessive OS can lead to changes in the testicular microenvironment and fragmentation of sperm DNA, which further contributes to asthenozoospermia (Arya et al., 2022). Second, choline and other related metabolites of TMAO can reduce membrane stability and lead to abnormal membrane fluidity, thus affecting flagellar movement (Lazaros et al., 2012; Hanley, 2023). Third, choline, betaine, and methionine can be used as methyl donors for DNA methylation, which plays an important role in male germ cell development and spermatogenesis (Lundy et al., 2021). It has been confirmed that betaine can reverse the apoptosis of spermatogenic cells by increasing the methylation level of Spata, Specc, and Spag target genes through the PIWI/Pi-RNA pathway and up-regulation of methyltransferases, including DNA methyltransferases and histone methyltransferases (Lin et al., 2024). Finally, evidence suggests that TMAO promotes vascular inflammation in mice by activating nuclear factor kappa B and subsequently increasing the expression of pro-inflammatory mediators (Tacconi et al., 2023), and inflammatory processes can impair sperm maturation and migration, thereby impacting sperm function (Piomboni et al., 2008; Liu et al., 2021).
Our research has several strengths. First, to our knowledge, this is the first study to explore the relationship between blood plasma TMAO concentrations and its related metabolites and odds of asthenozoospermia. Second, while most previous studies have focused on measuring TMAO or just one of its metabolites, our study included TMAO along with several of its metabolites. By employing multiple indicators, we provided a more comprehensive perspective, thus enhancing the accuracy of the association between TMAO and its metabolites and asthenozoospermia odds. Third, we employed the LC-MS/MS method to quantify TMAO and its related metabolites in blood plasma samples. Due to its high accuracy and sensitivity, this technique provides reliable and valid data. Additionally, we matched each asthenozoospermia case with a healthy control by 1:1 to minimize the potential confounding effects.
There are still some limitations to our study. First, we cannot infer causality from this study due to the case–control study. Second, since the current study was conducted on a population of Chinese men, the extrapolated results may not accurately reflect other regions or populations. Third, a limitation of the current study lies in its relatively modest sample size. Although the statistical power of the primary analysis exceeded 90%, the sample size in the subgroup analysis may still have been insufficient. Fourth, our findings may have been influenced by other factors that can contribute to asthenozoospermia, including exposure to environmental pollutants (Szabo et al., 2023), cellular and molecular factors (Shahrokhi et al., 2020), as well as genetic causes (Heidary et al., 2020), all of which were not assessed in this study. Lastly, blood plasma TMAO and its metabolites were measured at a single time point and may not accurately represent long-term concentrations, which may not fully capture the enduring effects on sperm quality. Nevertheless, large well-designed cohort studies are warranted in the future to explore whether there is a key association between TMAO and its related metabolites and risk of asthenozoospermia.
Conclusion
In this case–control study, elevated blood plasma TMAO levels were associated with increased odds of asthenozoospermia, while higher concentrations of choline and L-carnitine decreased asthenozoospermia odds. These findings may serve as a foundation for future investigations aimed at exploring causality and developing novel metabolome-based diagnostics and therapeutics for men suffering from this complex and emotionally challenging condition.
Supplementary Material
Acknowledgements
We thank the research team for their daily efforts in material collection and article writing.
Contributor Information
Ze Xing, Center of Reproductive Medicine, Shengjing Hospital of China Medical University, Shenyang, P.R. China; Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, P.R. China.
Meng-Meng Xie, Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, P.R. China.
Hui-Han Wang, Department of Hematology, Shengjing Hospital of China Medical University, Shenyang, P.R. China.
Qi Cui, Department of Laboratory Medicine, Shengjing Hospital of China Medical University, Shenyang, P.R. China.
Xiao-Bin Wang, Center of Reproductive Medicine, Shengjing Hospital of China Medical University, Shenyang, P.R. China; Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, P.R. China.
Supplementary data
Supplementary data are available at Human Reproduction Open online.
Data availability
The data that support the findings of our study are available from the corresponding author upon reasonable request.
Authors’ roles
Z.X. and X.-B.W. conceived the study. Z.X., M.-M.X., Q.C., and X.-B.W. collected the data. Z.X., M.-M.X., Q.C., and X.-B.W. analyzed and interpreted the data. Z.X. and M.-M.X. drafted the article. Z.X., M.-M.X., H.-H.W., Q.C., and X.-B.W. revised the article critically for important intellectual content. All authors approved the final vision of manuscript, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
No funding was received for this study.
Conflict of interest
All authors have no conflict of interest to declare.
References
- Agarwal A, Baskaran S, Parekh N, Cho CL, Henkel R, Vij S, Arafa M, Panner Selvam MK, Shah R. Male infertility. Lancet 2021. a;397:319–333. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Leisegang K, Majzoub A, Henkel R, Finelli R, Panner Selvam MK, Tadros N, Parekh N, Ko EY, Cho CL et al. Utility of antioxidants in the treatment of male infertility: clinical guidelines based on a systematic review and analysis of evidence. World J Mens Health 2021. b;39:233–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Parekh N, Panner Selvam MK, Henkel R, Shah R, Homa ST, Ramasamy R, Ko E, Tremellen K, Esteves S et al. Male oxidative stress infertility (MOSI): proposed terminology and clinical practice guidelines for management of idiopathic male infertility. World J Mens Health 2019;37:296–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya D, Balasinor N, Singh D. Varicocoele-associated male infertility: cellular and molecular perspectives of pathophysiology. Andrology 2022;10:1463–1483. [DOI] [PubMed] [Google Scholar]
- Attia YA, El-Naggar AS, Abou-Shehema BM, Abdella AA. Effect of supplementation with trimethylglycine (betaine) and/or vitamins on semen quality, fertility, antioxidant status, DNA repair and welfare of roosters exposed to chronic heat stress. Animals (Basel) 2019;9:547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai K, Jiang L, Zhu S, Feng C, Zhao Y, Zhang L, Wang T. Dimethylglycine sodium salt protects against oxidative damage and mitochondrial dysfunction in the small intestines of mice. Int J Mol Med 2019;43:2199–2211. [DOI] [PubMed] [Google Scholar]
- Barrea L, Annunziata G, Muscogiuri G, Laudisio D, Di Somma C, Maisto M, Tenore GC, Colao A, Savastano S. Trimethylamine N-oxide, Mediterranean diet, and nutrition in healthy, normal-weight adults: also a matter of sex? Nutrition 2019;62:7–17. [DOI] [PubMed] [Google Scholar]
- Battillo DJ, Malin SK. Impact of caloric restriction and exercise on trimethylamine N-oxide metabolism in women with obesity. Nutrients 2023;15:1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah MM, Khatiwada S, Lecomte V, Morris MJ, Maloney CA. Ameliorating high-fat diet-induced sperm and testicular oxidative damage by micronutrient-based antioxidant intervention in rats. Eur J Nutr 2022;61:3741–3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht S, Faiq M, Tolahunase M, Dada R. Oxidative stress and male infertility. Nat Rev Urol 2017;14:470–485. [DOI] [PubMed] [Google Scholar]
- Buffa JA, Romano KA, Copeland MF, Cody DB, Zhu W, Galvez R, Fu X, Ward K, Ferrell M, Dai HJ et al. The microbial gbu gene cluster links cardiovascular disease risk associated with red meat consumption to microbiota L-carnitine catabolism. Nat Microbiol 2022;7:73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañón-Apilánez M, García-Cabo C, Martin-Martin C, Prieto B, Cernuda-Morollón E, Rodríguez-González P, Pineda-Cevallos D, Benavente L, Calleja S, López-Cancio E. Mediterranean diet prior to ischemic stroke and potential circulating mediators of favorable outcomes. Nutrients 2024;16:3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D, Kong F, Jiang W, Li F, Zhang C, Ding H, Kang Y, Li W, Huang C, Zhou X et al. Effects of L-carnitine administration on sperm and sex hormone levels in a male Wistar rat reproductive system injury model in a high-altitude hypobaric hypoxic environment. Reprod Sci 2023;30:2231–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemes HE. Phenotypes of sperm pathology: genetic and acquired forms in infertile men. J Androl 2000;21:799–808. [PubMed] [Google Scholar]
- Chen ML, Zhu XH, Ran L, Lang HD, Yi L, Mi MT. Trimethylamine-N-oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS signaling pathway. J Am Heart Assoc 2017;6:e006347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condorelli RA, Aversa A, Basile L, Cannarella R, Mongioi LM, Cimino L, Perelli S, Caprio M, Cimino S, Calogero AE et al. Beneficial effects of the very-low-calorie ketogenic diet on the symptoms of male accessory gland inflammation. Nutrients 2022;14:1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Q, Wang HH, Wu QJ, Wang XB, Guo RH, Leng X, Tan XL, Du Q, Pan BC. Diet quality scores and asthenoteratozoospermia risk: finding from a hospital-based case-control study in China. Front Nutr 2022;9:859143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Q, Xia Y, Liu Y, Sun Y, Ye K, Li W, Wu Q, Chang Q, Zhao Y. Validity and reproducibility of a FFQ for assessing dietary intake among residents of northeast China: northeast cohort study of China. Br J Nutr 2023;129:1252–1265. [DOI] [PubMed] [Google Scholar]
- Curi SM, Ariagno JI, Chenlo PH, Mendeluk GR, Pugliese MN, Segovia LMS, Repetto HEH, Blanco AM. Asthenozoospermia: analysis of a large population. Arch Androl 2003;49:343–349. [DOI] [PubMed] [Google Scholar]
- Daoud S, Sellami A, Bouassida M, Kebaili S, Ammar Keskes L, Rebai T, Chakroun Feki N. Routine assessment of occupational exposure and its relation to semen quality in infertile men: a cross-sectional study. Turk J Med Sci 2017;47:902–907. [DOI] [PubMed] [Google Scholar]
- Darbandi M, Darbandi S, Agarwal A, Baskaran S, Dutta S, Sengupta P, Khorram Khorshid HR, Esteves S, Gilany K, Hedayati M et al. Reactive oxygen species-induced alterations in H19-Igf2 methylation patterns, seminal plasma metabolites, and semen quality. J Assist Reprod Genet 2019;36:241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, Serrazanetti DI, Di Cagno R, Ferrocino I, Lazzi C et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016;65:1812–1821. [DOI] [PubMed] [Google Scholar]
- Dong B, Jiang Y, Shi B, Zhang Z, Zhang Z. Selenomethionine alleviates decabromodiphenyl ether-induced oxidative stress and ferroptosis via the NRF2/GPX4 pathway in the chicken brain. J Hazard Mater 2024;465:133307. [DOI] [PubMed] [Google Scholar]
- Du H, Bennett D, Li L, Whitlock G, Guo Y, Collins R, Chen J, Bian Z, Hong LS, Feng S et al. ; China Kadoorie Biobank Collaborative Group. Physical activity and sedentary leisure time and their associations with BMI, waist circumference, and percentage body fat in 0.5 million adults: the China Kadoorie Biobank study. Am J Clin Nutr 2013;97:487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg ML, Esteves SC, Lamb DJ, Hotaling JM, Giwercman A, Hwang K, Cheng YS. Male infertility. Nat Rev Dis Primers 2023;9:49. [DOI] [PubMed] [Google Scholar]
- Erickson ML, Malin SK, Wang Z, Brown JM, Hazen SL, Kirwan JP. Effects of lifestyle intervention on plasma trimethylamine N-oxide in obese adults. Nutrients 2019;11:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslamian G, Amirjannati N, Rashidkhani B, Sadeghi MR, Hekmatdoost A. Intake of food groups and idiopathic asthenozoospermia: a case-control study. Hum Reprod 2012;27:3328–3336. [DOI] [PubMed] [Google Scholar]
- Fang Y, Xia J, Lian Y, Zhang M, Kang Y, Zhao Z, Wang L, Yin P, Wang Z, Ye C et al. The burden of cardiovascular disease attributable to dietary risk factors in the provinces of China, 2002-2018: a nationwide population-based study. Lancet Reg Health West Pac 2023;37:100784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear MD, Krleza-Jeric K, Lemmens T. The Declaration of Helsinki. BMJ 2007;335:624–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro G, Deon M, Becker GS, Dos Reis BG, Wajner M, Vargas CR. Neuroprotective effects of L-carnitine towards oxidative stress and inflammatory processes: a review of its importance as a therapeutic drug in some disorders. Metab Brain Dis 2025;40:127. [DOI] [PubMed] [Google Scholar]
- Hanley PJ. Elusive physiological role of prostatic acid phosphatase (PAP): generation of choline for sperm motility via auto-and paracrine cholinergic signaling. Front Physiol 2023;14:1327769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heianza Y, Sun D, Li X, DiDonato JA, Bray GA, Sacks FM, Qi L. Gut microbiota metabolites, amino acid metabolites and improvements in insulin sensitivity and glucose metabolism: the POUNDS Lost trial. Gut 2019;68:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidary Z, Saliminejad K, Zaki-Dizaji M, Khorram Khorshid HR. Genetic aspects of idiopathic asthenozoospermia as a cause of male infertility. Hum Fertil (Camb) 2020;23:83–92. [DOI] [PubMed] [Google Scholar]
- Hu Y, Ding M, Yuan C, Wu K, Smith-Warner SA, Hu FB, Chan AT, Meyerhardt JA, Ogino S, Fuchs CS et al. Association between coffee intake after diagnosis of colorectal cancer and reduced mortality. Gastroenterology 2018;154:916–926. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Zhang Y, Wang X, Guo R, Leng X, Du Q, Wu Q, Pan B, Zhao Y. Dietary total antioxidant capacity and the risk of developing asthenozoospermia: a hospital-based case-control study in China. Hum Reprod 2023;38:537–548. [DOI] [PubMed] [Google Scholar]
- Huang JY, Luu HN, Butler LM, Midttun O, Ulvik A, Wang R, Jin A, Gao YT, Tan Y, Ueland PM et al. A prospective evaluation of serum methionine-related metabolites in relation to pancreatic cancer risk in two prospective cohort studies. Int J Cancer 2020;147:1917–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh T, Mollard R, Trounson A. Selected genetic factors associated with male infertility. Hum Reprod Update 2002;8:183–198. [DOI] [PubMed] [Google Scholar]
- Janeiro MH, Ramirez MJ, Milagro FI, Martinez JA, Solas M. Implication of trimethylamine N-oxide (TMAO) in disease: potential biomarker or new therapeutic target. Nutrients 2018;10:1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen CFS, Ostergren P, Dupree JM, Ohl DA, Sonksen J, Fode M. Varicocele and male infertility. Nat Rev Urol 2017;14:523–533. [DOI] [PubMed] [Google Scholar]
- Jiao J, Xu P, Wang X, Xing Z, Dong S, Li G, Yao X, Guo R, Feng T, Yao W et al. Enterotypes in asthenospermia patients with obesity. Sci Rep 2022;12:16993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalagi NA, Thota RN, Stojanovski E, Alburikan KA, Garg ML. Association between plasma trimethylamine N-oxide levels and type 2 diabetes: a case control study. Nutrients 2022;14:2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltsas A. Oxidative stress and male infertility: the protective role of antioxidants. Medicina (Kaunas) 2023;59:1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr NF, Werida RH. l-carnitine modulates autophagy, oxidative stress and inflammation in trazodone induced testicular toxicity. Life Sci 2022;290:120025. [DOI] [PubMed] [Google Scholar]
- Kijpaisalratana N, Ament Z, Bevers MB, Bhave VM, Garcia Guarniz AL, Couch CA, Irvin MR, Kimberly WT. Trimethylamine N-oxide and white matter hyperintensity volume among patients with acute ischemic stroke. JAMA Netw Open 2023;6:e2330446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latif F, Mubbashir A, Khan MS, Shaikh Z, Memon A, Alvares J, Azhar A, Jain H, Ahmed R, Kanagala SG. Trimethylamine N-oxide in cardiovascular disease: pathophysiology and the potential role of statins. Life Sci 2025;361:123304. [DOI] [PubMed] [Google Scholar]
- Lazaros L, Xita N, Hatzi E, Kaponis A, Makrydimas G, Takenaka A, Sofikitis N, Stefos T, Zikopoulos K, Georgiou I. Phosphatidylethanolamine N-methyltransferase and choline dehydrogenase gene polymorphisms are associated with human sperm concentration. Asian J Androl 2012;14:778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine H, Mohri H, Ekbom A, Ramos L, Parker G, Roldan E, Jovine L, Koelle S, Lindstrand A, Immler S et al. Male reproductive health statement (XIIIth international symposium on Spermatology, May 9th–12th 2018, Stockholm, Sweden. Basic Clin Androl 2018;28:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Liu C, Chen Y, Zhao Y, Tan M, He B. Protective effects of betaine on boar sperm quality during liquid storage and transport. Animals (Basel) 2024;14:2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Li Y, Ivey KL, Wang DD, Wilkinson JE, Franke A, Lee KH, Chan A, Huttenhower C, Hu FB et al. Interplay between diet and gut microbiome, and circulating concentrations of trimethylamine N-oxide: findings from a longitudinal cohort of US men. Gut 2022;71:724–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XS, Obeid S, Klingenberg R, Gencer B, Mach F, Raber L, Windecker S, Rodondi N, Nanchen D, Muller O et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J 2017;38:814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Ge X, Gao L, Chen Y, Su T, Ma M, Wang H, Chen C, Han B, Liu D. Betaine alleviates spermatogenic cells apoptosis of oligoasthenozoospermia rat model by up-regulating methyltransferases and affecting DNA methylation. Phytomedicine 2024;129:155713. [DOI] [PubMed] [Google Scholar]
- Liu FH, Wang XB, Wen ZY, Wang HY, Zhang M, Zhang S, Jiang YT, Zhang JY, Sun H, Pan BC et al. Dietary inflammatory index and risk of asthenozoospermia: a hospital-based case-controlled study in China. Front Nutr 2021;8:706869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YS, Zhang YX, Wang XB, Wu QJ, Liu FH, Pan BC, Zhao YH. Associations between meat and vegetable intake, cooking methods, and asthenozoospermia: a hospital-based case-control study in China. Nutrients 2022;14:1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy SD, Sangwan N, Parekh NV, Selvam MKP, Gupta S, McCaffrey P, Bessoff K, Vala A, Agarwal A, Sabanegh ES et al. Functional and taxonomic dysbiosis of the gut, urine, and semen microbiomes in male infertility. Eur Urol 2021;79:826–836. [DOI] [PubMed] [Google Scholar]
- Lv JL, Wu QJ, Wang XB, Du Q, Liu FH, Guo RH, Leng X, Pan BC, Zhao YH. Intake of ultra-processed foods and asthenozoospermia odds: a hospital-based case-control study. Front Nutr 2022;9:941745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R, Shi G, Li Y, Shi H. Trimethylamine N-oxide, choline and its metabolites are associated with the risk of non-alcoholic fatty liver disease. Br J Nutr 2024;131:1915–1923. [DOI] [PubMed] [Google Scholar]
- Manor O, Zubair N, Conomos MP, Xu X, Rohwer JE, Krafft CE, Lovejoy JC, Magis AT. A multi-omic association study of trimethylamine N-oxide. Cell Rep 2018;24:935–946. [DOI] [PubMed] [Google Scholar]
- Mateo-Otero Y, Fernandez-Lopez P, Ribas-Maynou J, Roca J, Miro J, Yeste M, Barranco I. Metabolite profiling of pig seminal plasma identifies potential biomarkers for sperm resilience to liquid preservation. Front Cell Dev Biol 2021;9:669974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Peng L, Xu J, Guo D, Cao W, Xu Y, Li S. Betaine attenuate chronic restraint stress-induced changes in testicular damage and oxidative stress in male mice. Reprod Biol Endocrinol 2022;20:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori N, Ishihara M, Tasaki H, Sankai T, Otsuki J. The effect of betaine for mouse sperm cryopreservation. Cryobiology 2022;106:157–159. [DOI] [PubMed] [Google Scholar]
- Nateghian Z, Nasr-Esfahani MH, Talaei-Khozani T, Tavalaee M, Aliabadi E. L-carnitine and pentoxifylline supplementation improves sperm viability and motility at low temperature. Int J Fertil Steril 2023;17:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeid R, Awwad HM, Keller M, Geisel J. Trimethylamine-N-oxide and its biological variations in vegetarians. Eur J Nutr 2017;56:2599–2609. [DOI] [PubMed] [Google Scholar]
- Pingitore A, Lima GP, Mastorci F, Quinones A, Iervasi G, Vassalle C. Exercise and oxidative stress: potential effects of antioxidant dietary strategies in sports. Nutrition 2015;31:916–922. [DOI] [PubMed] [Google Scholar]
- Piomboni P, Gambera L, Serafini F, Campanella G, Morgante G, De Leo V. Sperm quality improvement after natural anti-oxidant treatment of asthenoteratospermic men with leukocytospermia. Asian J Androl 2008;10:201–206. [DOI] [PubMed] [Google Scholar]
- Randrianarisoa E, Lehn-Stefan A, Wang X, Hoene M, Peter A, Heinzmann SS, Zhao X, Konigsrainer I, Konigsrainer A, Balletshofer B et al. Relationship of serum trimethylamine N-oxide (TMAO) levels with early atherosclerosis in humans. Sci Rep 2016;6:26745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrokhi SZ, Salehi P, Alyasin A, Taghiyar S, Deemeh MR. Asthenozoospermia: cellular and molecular contributing factors and treatment strategies. Andrologia 2020;52:e13463. [DOI] [PubMed] [Google Scholar]
- Shan Z, Sun T, Huang H, Chen S, Chen L, Luo C, Yang W, Yang X, Yao P, Cheng J et al. Association between microbiota-dependent metabolite trimethylamine-N-oxide and type 2 diabetes. Am J Clin Nutr 2017;106:888–894. [DOI] [PubMed] [Google Scholar]
- Shi Z, Taylor AW, Riley M, Byles J, Liu J, Noakes M. Association between dietary patterns, cadmium intake and chronic kidney disease among adults. Clin Nutr 2018;37:276–284. [DOI] [PubMed] [Google Scholar]
- Sun B, Messerlian C, Sun ZH, Duan P, Chen HG, Chen YJ, Wang P, Wang L, Meng TQ, Wang Q et al. Physical activity and sedentary time in relation to semen quality in healthy men screened as potential sperm donors. Hum Reprod 2019. a;34:2330–2339. [DOI] [PubMed] [Google Scholar]
- Sun H, Gong TT, Jiang YT, Zhang S, Zhao YH, Wu QJ. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990-2017: results from a global burden of disease study, 2017. Aging (Albany NY) 2019. b;11:10952–10991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo A, Vancsa S, Hegyi P, Varadi A, Forintos A, Filipov T, Acs J, Acs N, Szarvas T, Nyirady P et al. Lifestyle-, environmental-, and additional health factors associated with an increased sperm DNA fragmentation: a systematic review and meta-analysis. Reprod Biol Endocrinol 2023;21:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacconi E, Palma G, De Biase D, Luciano A, Barbieri M, de Nigris F, Bruzzese F. Microbiota effect on trimethylamine N-oxide production: from cancer to fitness – a practical preventing recommendation and therapies. Nutrients 2023;15:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013;368:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WHW, Backhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC State. J Am Coll Cardiol 2019;73:2089–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant PWG, Murray EJ, Arnold KF, Berrie L, Fox MP, Gadd SC, Harrison WJ, Keeble C, Ranker LR, Textor J et al. Use of directed acyclic graphs (DAGs) to identify confounders in applied health research: review and recommendations. Int J Epidemiol 2021;50:620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoridis X, Papaemmanouil A, Papageorgiou N, Savopoulos C, Chourdakis M, Triantafyllou A. The association between lifestyle interventions and trimethylamine N-oxide: a systematic-narrative hybrid literature review. Nutrients 2025;17:1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troseid M, Hov JR, Nestvold TK, Thoresen H, Berge RK, Svardal A, Lappegard KT. Major increase in microbiota-dependent proatherogenic metabolite TMAO one year after bariatric surgery. Metab Syndr Relat Disord 2016;14:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde L, Cacciapuoti S, Caiazzo G, Megna M, Martora F, Cavaliere A, Mattera M, Maisto M, Tenore GC, Colao A et al. Very low-calorie ketogenic diet (VLCKD) in the management of hidradenitis suppurativa (Acne Inversa): an effective and safe tool for improvement of the clinical severity of disease. Results of a pilot study. J Transl Med 2024. a;22:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde L, Frias-Toral E, Cacciapuoti S, Simancas-Racines D, Megna M, Caiazzo G, Potestio L, Maisto M, Tenore GC, Colao A et al. Very low-calorie ketogenic diet (VLCKD): a therapeutic nutritional tool for acne? J Transl Med 2024. b;22:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- Wallace TC. A comprehensive review of eggs, choline, and lutein on cognition across the life-span. J Am Coll Nutr 2018;37:269–285. [DOI] [PubMed] [Google Scholar]
- Wang M, Li XS, Wang Z, de Oliveira Otto MC, Lemaitre RN, Fretts A, Sotoodehnia N, Budoff M, Nemet I, DiDonato JA et al. Trimethylamine N-oxide is associated with long-term mortality risk: the multi-ethnic study of atherosclerosis. Eur Heart J 2023;44:1608–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XB, Wu QJ, Guo RH, Leng X, Du Q, Zhao YH, Pan BC. Dairy product consumption and oligo-astheno-teratozoospermia risk: a hospital-based case-control study in China. Front Nutr 2021. a;8:742375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XB, Wu QJ, Liu FH, Zhang S, Wang HY, Guo RH, Leng X, Du Q, Zhao YH, Pan BC. The association between dairy product consumption and asthenozoospermia risk: a hospital-based case-control study. Front Nutr 2021. b;8:714291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, Koeth RA, Li L, Wu Y, Tang WHW et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J 2019;40:583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Levison BS, Hazen JE, Donahue L, Li XM, Hazen SL. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem 2014;455:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Liu S, Liu J, Sun Y, Allen AE, Reid MA, Locasale JW. Separation of reproductive decline from lifespan extension during methionine restriction. Nat Aging 2024;4:1089–1101. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th edn. Geneva, Switzerland: World Health Organization, 2010. [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. Geneva: World Health Organization, 2021. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama 2013;310:2191–2194. [DOI] [PubMed] [Google Scholar]
- Wu C, Xue F, Lian Y, Zhang J, Wu D, Xie N, Chang W, Chen F, Wang L, Wei W et al. Relationship between elevated plasma trimethylamine N-oxide levels and increased stroke injury. Neurology 2020;94:e667–e677. [DOI] [PubMed] [Google Scholar]
- Wu Y, Li H, Miao Y, Peng J, Wei H. Effects of methionine restriction from different sources on sperm quality in aging mice. Nutrients 2023;15:4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZG, Chen WK, Fei QJ, Liu YL, Liu XD, Huang H, Shang XJ. Analysis of semen quality of 38 905 infertile male patients during 2008-2016 in Wenzhou, China. Asian J Androl 2021;23:314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YX, Wang YG, He M, Pan XC, Wang Z. China Food Composition, Standard Edition. Beijing: Peking University Medical Press, 2018. [Google Scholar]
- Zeisel SH, da Costa KA. Choline: an essential nutrient for public health. Nutr Rev 2009;67:615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lu J, Yan Y, Liu J, Wang M. Antibiotic residues in cattle and sheep meat and human exposure assessment in southern Xinjiang, China. Food Sci Nutr 2021;9:6152–6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, He F, Wu C, Li P, Li N, Deng J, Zhu G, Ren W, Peng Y. Betaine in inflammation: mechanistic aspects and applications. Front Immunol 2018;9:1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JQ, Wang XB, Leng X, Wei YF, Huang DH, Lv JL, Du Q, Guo RH, Pan BC, Wu QJ, Zhao YH. Dietary fat and fatty acid consumptions and the odds of asthenozoospermia: a case-control study in China. Hum Reprod Open 2023;2023:hoad030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of our study are available from the corresponding author upon reasonable request.