To the Editor,
Antisynthetase syndrome (ASyS) belongs to the group of idiopathic inflammatory myopathies, manifesting principally with inflammatory myositis, arthritis, interstitial lung disease, Raynaud’s phenomenon and mechanics hands. The physiopathology of the disease remains poorly understood, however different autoantibodies targeting an aminoacyl-tRNA synthetase have been identified [1].
Over the past few years, some cases of new onset of ASyS developing during anti-tumor necrosis factor (TNF)-α therapies have been reported, thereby questioning its possible causal role in these patients. However, these observations remain extremely rare. Herein, we describe two cases of patients developing ASyS during anti-TNF-alpha therapies.
Case 1: Anti-PL7 Antisynthetase Syndrome During Adalimumab Treatment in a Patient with Ankylosing Spondylitis
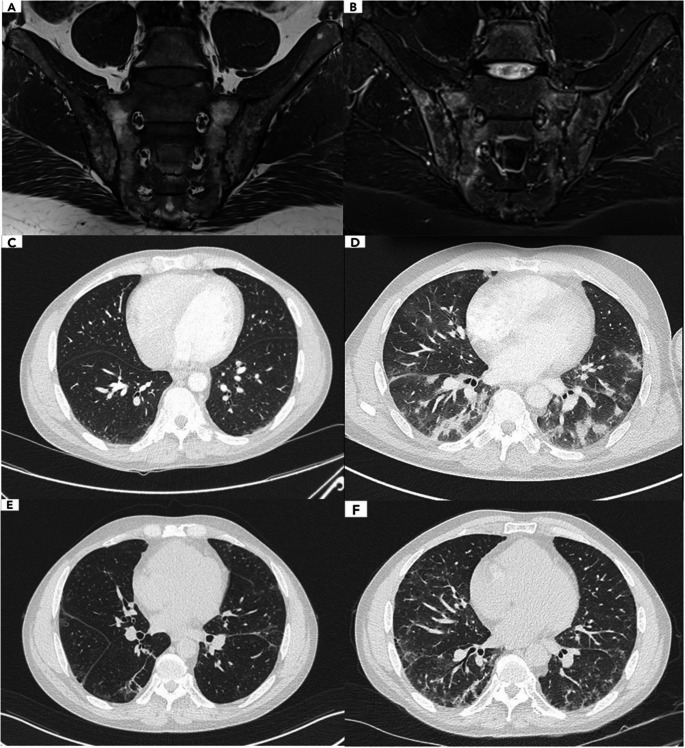
Our first patient is a 59-year-old HLA-B27 positive male, diagnosed with axial ankylosing spondylitis in 2016, in a context of cervical, lumbar and hip pain, with bilateral sacroiliitis, syndesmophytes and Romanus lesions on his initial MRI (Fig. 1A-B). He was well controlled with non-steroidal anti-inflammatory (NSAI) drugs until September 2019, when pain reoccurred; adalimumab 40 mg bimonthly was introduced. His chest-CT scan showed no special feature at that time (Fig. 1C).
Fig. 1.
Sacroiliac MRI at diagnosis and chest CT scan evolution over time (Patient 1) (A) Sacroiliac MRI showing subchondral bone erosion and fatty conversion, reflecting chronic structural damage (T1). B Peripheral subchondral bone edema and anterior capsule infiltration, reflecting active inflammation (STIR). C Chest CT scan: before adalimumab introduction, showing no interstitial lung disease. D Eleven months after adalimumab introduction, showing diffuse interstitial pneumopathy with bilateral ground glass opacities. E Regression of the lung infiltrates, six months after oral methylprednisolone. F Worsening of the pulmonary lesions with progression to pulmonary fibrosis, one year after diagnosis
In August 2020 he was hospitalized in pneumology for a suspected post-COVID pneumonia in a context of fatigue and growing dyspnea. Arterial blood gases at admission revealed hypoxemia with pH 7.45, PCO2 36 mmHg, PO2 63 mmHg, SaO2 92%. His biology showed total leucocytes of 9.240/uL, neutrophiles 6.480/uL, CRP 44 mg/L. Chest-CT scan revealed diffuse interstitial pneumopathy with bilateral ground glass opacities and an estimated 25–50% damage (Fig. 1D). Pulmonary artery thrombosis was ruled out. Cardiac assessment was unremarkable. SARS-CoV-2 serology was not tested, however all the SARS-CoV-2 PCR were negative during the hospitalization. Bronchoalveolar lavage disclosed 1300 leucocytes/uL with 54% of macrophages, 31% of lymphocytes, 13% of neutrophiles. Bacterial, mycologic and mycobacterial cultures came back negative. Invasive aspergillosis, Pneumocystis jirovecii, Chlamydia pneumoniae, Mycoplasma pneumoniae and other viral agents including SARS-CoV-2 were ruled out by PCR. Adalimumab was discontinued. Oral methylprednisolone 1 mg/kg was introduced. The patient evolved well and a reevaluation at six months showed a regression of the lung infiltrates (Fig. 1E). His pulmonary function tests (PFT) and arterial blood gases showed a positive evolution.
In October 2021, corticoids were discontinued and adalimumab was reintroduced as joint pain reoccurred. He was later hospitalized in our department for insufficient pain control and his lung condition was reassessed. Chest-CT scan showed stable lung damage, however his PFT worsened. Immunological tests revealed positive antinuclear antibody (ANA) at 1:80, detected by indirect immunofluorescence assay (IFI Hep-2000, EuroBio Scientific, France), with a cytoplasmic dense fine speckled pattern (ICAP AC-19), together with positive anti-PL7 antibodies (immunoblot, EuroImmun, Germany).
Looking back at the patient’s chest-CT history to determine the onset of his interstitial lung disease (ILD), we understood that it appeared in August 2020 when he was misdiagnosed as having COVID pneumonia. We concluded in an anti-PL7 ASyS with isolated lung involvement, which first manifestations occurred eleven months after adalimumab’s introduction. Interestingly, we were able to test ANA and anti-PL7 antibodies on the patient’s serum before adalimumab’s introduction: they were negative, showing a seroconversion during adalimumab treatment. Adalimumab was discontinued and a treatment with oral methylprednisolone 0.5 mg/kg was reintroduced. On his latest evaluation, his PFT showed no amelioration, with a progression to pulmonary fibrosis (Fig. 1F): rituximab was recently introduced.
Case 2: Anti-EJ Antisynthetase Syndrome in a Patient with Ankylosing Spondylitis Treated with Adalimumab
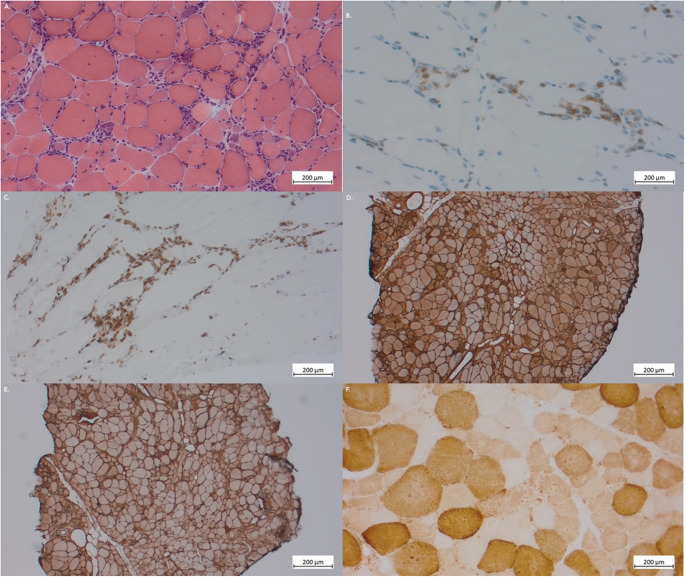
A 56-year-old patient was diagnosed with axial and peripheric ankylosing spondylitis in March 2010 with inflammatory low back and buttock pain, peripheral arthralgias (ankles, heels, wrists), dactylitis, cutaneous psoriasis and HLA-B27 positivity. She tried different drugs including NSAI and tramadol, then methotrexate, with insufficient pain control. Adalimumab 40 mg bimonthly was introduced in June 2011. At this time, her biology showed negative ANA (IFI HEp-2) and normal CPK dosage (148 UI/L, N < 170). She evolved well until May 2017, when she reported sudden dysphagia and acute proximal muscle weakness. Physical examination revealed lowered muscle strength in the psoas (MRC-5 scale 3/5). Her biology revealed elevated CPK (2.366 UI/L). Muscle MRI of the lower limbs showed T2/STIR hypersignals consistent with active myositis. Electromyography showed a myogenic pattern and acute inflammatory necrosis. Muscle biopsy was consistent with polymyositis (Fig. 2). Autoimmune tests revealed positive ANA 1:320 (IFI HEp-2, cytoplasmic fine speckled ICAP AC-20) with positive anti-EJ antibodies (immunoblot, EuroImmun). Thoraco-abdominal-pelvic scan showed no ILD and no lesion suspected of malignancy. Adalimumab was discontinued. A treatment with corticosteroids 1 mg/kg, methotrexate 25 mg weekly and monthly IgIV 2 g/kg was introduced. She responded well and fully recovered. Prednisone was tapered at 5 mg daily.
Fig. 2.
Muscle biopsy at the time of diagnosis (Patient 2). A Diffuse lymphocytic infiltrates in muscle fibers and endomysium. Hematoxylin-eosin stain, x10. B T CD8+ lymphocytes marking, invading muscle fibers, x20. C T CD3+ lymphocytes marking, invading muscle fibers, x10. D Diffuse class-1 HLA expression, x10. E Diffuse HLA-DR expression, x10. F Multiple negative COX marking fibers, x10
We concluded in an anti-EJ ASyS which first manifestations occurred seventy months after adalimumab’s introduction. For this reason, we were unable to test the presence of anti-EJ antibodies on the patient’s serum before adalimumab’s introduction, however her ANA were negative.
Discussion
Anti-TNF-alpha therapies are known to cause various autoimmune diseases, such as drug-induced lupus erythematosus, psoriasis, demyelinating neurologic diseases or interstitial lung diseases [2]. Antisynthetase syndromes occurring during anti-TNF-alpha therapies have also been described and raise growing attention.
Zengin et al. [3] described three cases of anti-JO1 ASyS, including two patients with rheumatoid arthritis (RA) during adalimumab therapy. Clinical manifestations occurred 6 months after adalimumab’s introduction in the first case, and more than 3 years in their second case. The third patient with ankylosing spondylitis reported the first manifestations 2,5 months after etanercept’s introduction. Anti-TNF-alpha agents were discontinued and the three patients evolved well with the addition of other immunosuppressive agents such as pulse methylprednisolone 1 g x3 days, methotrexate, cyclophosphamide or rituximab. Hall et al. [4] described a case of seronegative RA who developed anti-JO1 ASyS 6 months after the onset of etanercept. Ishiguro et al. reported a case of anti-PL7 ASyS manifesting 17 months after the initiation of etanercept in the context of RA [5]. Ishikawa et al. reported a case of anti-PL12 ASyS developing two months after the initiation of etanercept in a patient with seropositive RA [6]. Unfortunately, the presence or absence of these autoantibodies before anti-TNF-alpha’s introductions were not specified. A frequent scenario is that patients with ASyS can initially have isolated joint manifestations which can be misinterpreted as RA or other rheumatic inflammatory diseases, which in facts were the first signs of a misdiagnosed ASyS. Another explanation is that some patients may share genetic predispositions, which could act as polygenic risk factors for autoimmunity and explain why they develop multiple autoimmune diseases over time, as it has been reported in large cohorts of patients with idiopathic inflammatory myopathies [7].
Patients with previously known ASyS autoantibodies, with new clinical manifestations occurring during anti-TNF-alpha therapies, were described [8]. These cases suggest that anti-TNF-alpha agents may play a ‘triggering’ role rather than an inducing role. We previously reported a case of anti-PL7 ASyS with severe ILD during golimumab treatment in a patient with psoriatic arthritis, which first manifestations occurred 18 months after golimumab’s introduction [9]. The patient evolved well with high-dose methylprednisolone and mycophenolate mofetil. Interestingly, anti-PL7 antibodies tested negative on the patient’s serum before anti-TNF-alpha introduction, thereby suggesting an inducing role.
Because the mechanisms and physiopathology of ASyS remain poorly elucidated, it is difficult to understand how anti-TNF-alpha can be involved in the development of the disease. A first hypothesis is called the ‘cytokine shift’ induced by anti-TNF-alpha agents. A rapid increase of pro-inflammatory cytokines such as IFN-ɣ after TNF-α blockage could trigger autoimmune processes through an induced Th1 lymphocytic response [2, 8]. Another plausible explanation could be that anti-TNF-alpha agents repress cytotoxic T cell response, which role is to control B cell auto-immunity, thereby promoting autoimmune reactions such as ASyS [8].
In conclusion, we report two cases of new onset of antisynthetase syndrome during adalimumab treatment in patients with ankylosing spondylitis. Our first patient developed interstitial lung disease with the presence of anti-PL7 autoantibodies, eleven months after adalimumab’s introduction. To our knowledge, this is the first documented case of adalimumab-induced anti-PL7 ASyS. Anti-PL7 serum negativity before adalimumab’s introduction, together with the clear improvement after withdrawal of adalimumab, followed by a positive rechallenge, suggest that the ASyS occurred because of adalimumab. Moreover, his chest-CT scan was free of lung infiltrate before adalimumab’s introduction. Our second patient developed anti-EJ myositis during adalimumab therapy, which is the first documented case of anti-EJ ASyS associated with anti-TNF-alpha.
Our data and the previously reported cases suggest that antisynthetase syndrome may be induced or exacerbated during anti-TNF-alpha therapy.
Clinicians should be aware of this association, particularly with patients showing a deterioration of respiratory functions during anti-TNF-alpha therapy, as interstitial lung disease is the most severe and potentially life-threatening complication of antisynthetase syndrome.
Acknowledgements
None.
Author Contributions
ARW collected the data and wrote the text. HB, SLL and YA reviewed the manuscript and approved the final version. SLL provided the muscle biopsy images and prepared figure 2. HB and YA were involved in the patient’s care. OB supervised the work, reviewed the manuscript and approved the final version. All authors reviewed and approved the manuscript.
Funding
No funding was received for this work.
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Conflicts of interest
The authors report no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cavagna L, Trallero-Araguás E, Meloni F, Gonzalez-Gay MA, et al. Influence of antisynthetase antibodies specificities on antisynthetase syndrome clinical spectrum time course. J Clin Med. 2019;8(11):2013. 10.3390/jcm8112013. PMID: 31752231; PMCID: PMC6912490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopetuso LR, Cuomo C, Mignini I, Gasbarrini A, Papa A. Focus on Anti-Tumour necrosis factor (TNF)-α-Related autoimmune diseases. Int J Mol Sci. 2023;24(9):8187. 10.3390/ijms24098187. PMID: 37175894; PMCID: PMC10179362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zengin O, Onder ME, Alkan S, Kimyon G, Hüseynova N, Demir ZH, Kısacık B, Onat AM. Three cases of anti-TNF induced myositis and literature review. Rev Bras Reumatol Engl Ed. 2017;57(6):590–5. 10.1016/j.rbre.2016.05.003. English, Portuguese. [DOI] [PubMed] [Google Scholar]
- 4.Hall HA, Zimmermann B. Evolution of dermatomyositis during therapy with a tumor necrosis factor alpha inhibitor. Arthritis Rheum. 2006;55(6):982–4. 10.1002/art.22358. PMID: 17139649. [DOI] [PubMed] [Google Scholar]
- 5.Ishiguro T, Takayanagi N, Miyahara Y, Yanagisawa T, Sugita Y. [Antisynthetase (anti PL-7 antibody) syndrome presenting as a skin rash and exacerbation of interstitial pneumonia during treatment for rheumatoid arthritis]. Nihon Kokyuki Gakkai Zasshi. 2010;48(3):240–6. Japanese. PMID: 20387531. [PubMed] [Google Scholar]
- 6.Ishikawa Y, Yukawa N, Kawabata D, Ohmura K, Fujii T, Usui T, Mimori T. A case of antisynthetase syndrome in a rheumatoid arthritis patient with anti-PL-12 antibody following treatment with etanercept. Clin Rheumatol. 2011;30(3):429–32. 10.1007/s10067-010-1666-1. Epub 2011 Jan 11 PMID: 21221686. [DOI] [PubMed] [Google Scholar]
- 7.Che WI, Westerlind H, Lundberg IE, Hellgren K, Kuja-Halkola R, Holmqvist ME. Familial autoimmunity in patients with idiopathic inflammatory myopathies. J Intern Med. 2023;293(2):200–11. 10.1111/joim.13573. Epub 2022 Oct 10. PMID: 36165332; PMCID: PMC10092836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunasso AM, Aberer W, Massone C. New onset of dermatomyositis/polymyositis during anti-TNF-α therapies: a systematic literature review. Sci World J. 2014;2014:179180. 10.1155/2014/179180. PMID: 24600322; PMCID: PMC3926249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wery AR, Bruyneel M, Alcan I, Semeu PK. Case report: Golimumab-induced anti-PL7 antisynthetase syndrome. Int J Rheum Dis. 2024;27(1):e15039. 10.1111/1756-185X.15039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.