Abstract
We studied the resistance of various mycobacteria isolated from a water distribution system to chlorine. Chlorine disinfection efficiency is expressed as the coefficient of lethality (liters per minute per milligram) as follows: Mycobacterium fortuitum (0.02) > M. chelonae (0.03) > M. gordonae (0.09) > M. aurum (0.19). For a C · t value (product of the disinfectant concentration and contact time) of 60 mg · min · liter−1, frequently used in water treatment lines, chlorine disinfection inactivates over 4 log units of M. gordonae and 1.5 log units of M. fortuitum or M. chelonae. C · t values determined under similar conditions show that even the most susceptible species, M. aurum and M. gordonae, are 100 and 330 times more resistant to chlorine than Escherichia coli. We also investigated the effects of different parameters (medium, pH, and temperature) on chlorine disinfection in a chlorine-resistant M. gordonae model. Our experimental results follow the Arrhenius equation, allowing the inactivation rate to be predicted at different temperatures. Our results show that M. gordonae is more resistant to chlorine in low-nutrient media, such as those encountered in water, and that an increase in temperature (from 4°C to 25°C) and a decrease in pH result in better inactivation.
Mycobacteria, except the tubercle bacilli, responsible for tuberculosis, are usually referred as atypical or nontuberculous mycobacteria (NTM). These mycobacteria were originally considered to be unusual Mycobacterium tuberculosis strains. Now, more than 90 NTM species have been described. Unlike tubercle bacilli, which are obligate pathogens, NTM are ubiquitous (8, 10, 12, 15, 17, 26, 35, 40). They have been recovered from a wide variety of environmental sources, including water, soil, dust, and aerosols. Most of them are saprophytic, although some are potential pathogens and may be involved in pulmonary or cutaneous diseases or in lymphadenitis (15, 24, 46). Pulmonary infections occur in immunocompetent patients with predisposing lung conditions, such as smoking, chronic obstructive pulmonary diseases, pneumoconiosis, and silicosis. Disseminated infections may occur in immunocompromised patients. Before the introduction of protease inhibitors for antiretroviral therapy, disseminated infections due to NTM, especially M. avium, were frequent in AIDS patients. NTM infection is now one of the criteria used to diagnose AIDS in human immunodeficiency virus-positive patients (20).
Patient-to-patient transmission of mycobacterial infections has not be demonstrated even in severely immunocompromised patients with advanced AIDS and hospitalized in the same wards as patients with severe M. avium infections. Infection is thought to be acquired from the environment by ingestion, inhalation, or inoculation. Recently, there has been increasing evidence that water may be the vehicle by which the mycobacteria infect or colonize the human body (4, 5, 17, 25, 30, 39, 43-45, 48). Documented evidence has been provided from hospitals and from cases of nosocomial infections. In a dialysis-related outbreak of M. abscessus, a rapidly growing mycobacterial species, the causative organism was recovered from hospital tap water. The strains isolated from patients and from tap water were identified by molecular typing (50). Similarly, molecular analyses were used to identify M. avium strains recovered from a hospital hot water system and from cultures of blood from patients treated at that hospital. These data showed that hospital hot water supplies can be a source of nosocomial outbreaks of disseminated M. avium disease (43). A recent outbreak of spinal infections due to M. xenopi in patients who had undergone discovertebral surgery was shown to be related to the presence of M. xenopi in the tap water distribution network of a French surgical center (3). The occurrence of mycobacteria in tap water raises the possibility that aerosols, which are typically generated in showers, carrying mycobacteria constitute a route for pulmonary infections. This hypothesis was suggested for clustered cases of pulmonary infections due to M. xenopi in a hospital in which the water network was heavily contaminated with M. xenopi (9). However, hospitals are not the only places where mycobacterial contamination of tap water systems may be a major health issue. The sources of pulmonary infections due to M. kansasii in coal miners (28) and in the residents in a city apartment complex (36) were traced to tap water used for showers.
Mycobacteria have been isolated from public water distribution systems and from various other sites, including hot and cold water taps, ice machines, heated nebulizers, and shower heads sprays (8, 12, 15, 17, 21, 23, 33, 44). NTM found in drinking water distribution systems are residents able to colonize, to survive, to persist, and to grow in tap water and are not contaminants from another source (6). The resistance of mycobacteria to common disinfectants and their tolerance of a wide range of pHs and temperatures allow them to persist in drinking water systems (6, 8, 15, 29). The mechanisms responsible for the survival of mycobacteria in drinking water are not well understood. A recent European directive addressed water intended for human consumption, i.e., potable water, including drinking water, water for food preparation, and water for other domestic uses (European Union Council Directive 98/83/EC). Therefore, water used for personal hygiene is included in this definition. Thus, skin contact with contaminated water and the inhalation of aerosols generated from contaminated water may be risk factors legally covered by the directive. The European Union Council directive states, according to World Health Organization guidelines, that drinking water should not contain pathogenic microorganisms in such quantity or concentration able to deteriorate human health. This regulation means that water must be carefully treated and that the response of pathogens to treatment procedures must be carefully evaluated.
To evaluate the efficiency of drinking water treatment against mycobacteria, it is necessary in particular to evaluate the adequacy of disinfection conditions, such as chlorination. We investigated the chlorination efficiency for several species isolated from the water distribution system in Paris, France. In a preliminary study, water samples were collected in 2000 at 12 points along the treatment lines at two treatment plants and in parts of the distribution system. A wide range of species was identified and included M. fortuitum, M. chelonae, M. aurum, M. peregrinum, and M. gordonae. The chlorination efficiency was estimated for various species grown in standard culture medium. M. gordonae was used as a model to test the different parameters affecting chlorination efficiency, including pH, temperature, and the composition of the medium.
(This work is part of a doctoral thesis by C. Le Dantec.)
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The M. gordonae strain used in this study was isolated from drinking water sampled at the Laboratoire de Référence des Mycobactéries at the Pasteur Institute. The other mycobacterial strains used were isolated from cold public water supplies in Paris. All strains were identified by phenotypic and genotypic methods, including 16S rRNA and/or hsp65 gene analysis by current techniques (13, 31, 41).
Cells were grown in Middlebrook 7H9 liquid medium (Difco Laboratories, West Molesley, Surrey, United Kingdom) containing 10% (vol/vol) oleic acid-albumin enrichment and 0.05% (vol/vol) Tween 80 at their optimal growth temperature (30 or 37°C, depending on the strain) in a rotary shaker (120 rpm). At the end of the exponential phase (optical density at 600 nm, 0.8), the cells were centrifuged at 3,260 × g and washed twice in an equal volume of 0.05 M chlorine demand-free phosphate buffer at pH 7. This buffer was prepared by mixing 420 ml of 0.05 M KH2PO4 with 580 ml of 0.05 M Na2HPO4. The cell pellet was resuspended in 1,500 ml of the same buffer at a density of 105 bacterial cells per ml, and this suspension was used for the washes.
Hypochlorous acid challenge conditions.
A freshly prepared free chlorine stock solution (150 mg/liter) was added to the bacterial suspension at a final concentration of 0.5 mg/liter. After 0, 10, 20, 30, 40, 60, and 120 min of reaction with chlorine at room temperature with gentle shaking, samples (100 ml) were removed and quenched with 100 μl of sterile 0.5 mM sodium thiosulfate at pH 7.0 to stop the action of chlorine. Cultivable bacteria were assayed by spreading on Middlebrook 7H9 solid medium plates after serial dilution in Middlebrook 7H11 liquid medium. Free chlorine (hypochlorous acid and hypochlorite ions) was measured by the N,N-diethyl-p-phenylenediamine colorimetric method at each point (18). Colonies were counted after 5 days at 37 or 30°C. The data presented are the averages of a minimum of two replicates.
Influence of temperature, pH, and medium composition on M. gordonae inactivation rates.
M. gordonae was used as a model to test the different parameters affecting chlorination efficiency for mycobacteria.
To test the effect of the medium composition on inactivation rates, M. gordonae was grown in tap water previously filtered through a 0.45-μm-pore-size filter and supplemented with 10% Middlebrook 7H9 liquid medium. At the end of exponential phase, the cells were centrifuged, washed, and resuspended as described above.
To test the effect of temperature on inactivation rates, M. gordonae was inactivated with 0.5 mg of chlorine/liter at 4°C (in ice), at 16°C (in a water bath), and at room temperature (25°C). These experiments were carried out at pH 7.0.
The effect of pH on inactivation rates was studied with sterile chlorine demand-free phosphate buffer at pH 6 and pH 8. The pH 6 buffer contained 889 ml of 0.06 M KH2PO4 and 111 ml of 0.06 M Na2HPO4; the pH 8 buffer contained 37 ml of 0.06 M KH2PO4 and 963 ml of 0.06 M Na2HPO4. These experiments were conducted at 25°C.
Reagents.
All chemicals used were analytical grade. N,N-Diethyl-p-phenylenediamine and sodium thiosulfate were purchased from Sigma Chemical Co.
Disinfection kinetics.
The Chick-Watson law was used for defining the rate of inactivation of mycobacteria according to Chick (7) as modified by Watson (47): log10 (N0/N) = k · C · t. In this equation, N0 is the initial concentration of microorganisms, N is the concentration remaining at time t, C is the concentration of disinfectant, and k is the susceptibility or lethality coefficient of the microorganism.
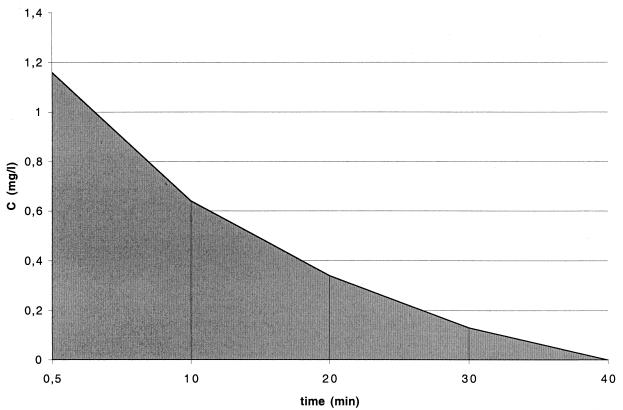
Due to reactions with residues of organic compounds, the concentration of free chlorine decreased as the experiments proceeded (Fig. 1). The curve represents free chlorine concentration measured at different times during inactivation, with an initial free chlorine concentration of 1.16 mgliter−1.
FIG. 1.
Schematic representation of the integration calculation of C · t values. The shaded area was used to estimate the integral term equation (equation 1) as described in Materials and Methods. The initial chlorine concentration was 1.16 mg/liter.
In order to accurately evaluate C · t values, chlorine decay was integrated as a function of time:
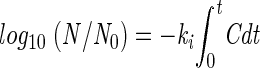 |
(1) |
In this equation, ki is the inactivation rate constant and Cdt is chlorine concentration decay integrated as a function of time.
The C · t values are represented by the areas under the curve presented in Fig. 1. The C · t values were calculated as follows: Cn · tn = (Cn−1 · tn − 1) + [(Cn + Cn+1)/2] · (tn+1 − tn). Linear regressions based on the decimal logarithm of the proportion of the initial concentration of mycobacteria remaining at time t (in minutes) for each strain were calculated and used to calculate the k values.
RESULTS
In this study, mycobacteria were inactivated with free chlorine in a phosphate buffer of a constant ionic strength. This procedure created well-controlled and reproducible experimental conditions.
The results are expressed as C · t values and k values. C · t values are the product of the disinfectant concentration in milligrams per liter and the contact time in minutes required to inactivate target microorganisms at a given temperature and pH. C · t values are usually expressed in log units. Different levels of inactivation (50, 90, and 99.9%, etc.) can be calculated from C · t and k values.
Effects of chlorine on the growth of atypical mycobacteria.
In a preliminary study, the occurrence and distribution of mycobacteria at 12 points along the treatment lines at two treatment plants and in parts of the distribution system related to these plants were investigated. An additional point consisted of the tap in our laboratory in Institut Pasteur. The majority of the samples were positive for mycobacteria. The most frequently isolated species were M. aurum, M. chelonae, M. fortuitum, and M. peregrinum among the rapidly growing species and M. gordonae and M. nonchromogenicum among the slowly growing species. However, 28% of the isolated cultures could not be assigned to any described species. M. aurum, M. chelonae, and M. fortuitum were selected as representatives of rapidly growing species, and M. gordonae was selected as a representative of slowly growing species to test the susceptibility of mycobacteria to chlorine.
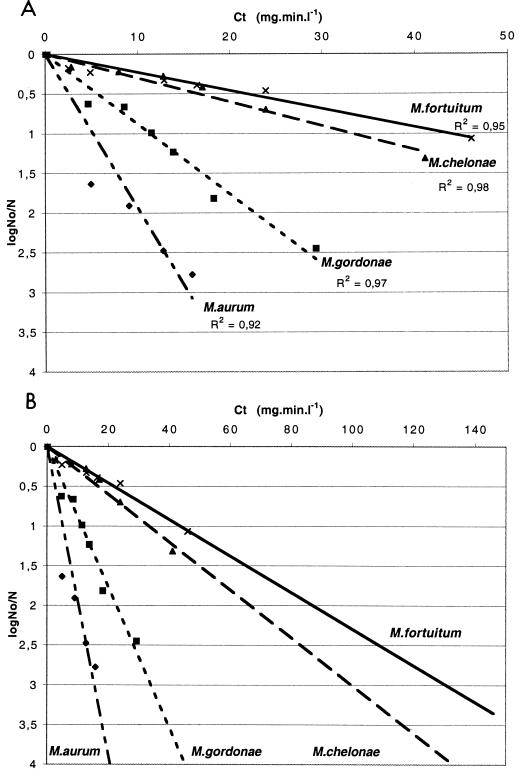
The resistance of mycobacteria to chlorine, expressed as the k value (liters per minute per milligram), was as follows: M. fortuitum (0.02) > M. chelonae (0.03) > M. gordonae (0.09) > M. aurum (0.19). Thus, M. chelonae and M. fortuitum are the most resistant whereas M. aurum appears to be the most susceptible mycobacterial species to chlorine (Fig. 2A). To reduce the number of cells by 2 log units (99%), the C · t value for M. aurum (15 mg · min · liter−1) had to be 9.5-fold lower than the C · t value for M. fortuitum; in other words, M. fortuitum was 9.5-fold more resistant than M. aurum.
FIG. 2.
Inactivation of various mycobacterial species with chlorine. (A) The data presented are the averages of a minimum of two replicates. Linear regressions based on the logarithm of the fraction of the original number of mycobacteria remaining at time t (in minutes) for each strain were calculated as shown in Fig. 1 and used to calculate C · t values. For each species tested, the experimental conditions were pH 7, a temperature of 25°C, and an initial chlorine concentration of 0.5 mg/liter. Cells were grown in Middlebrook 7H9-Tween medium. No, initial number of CFU; N, number of CFU at the time of the assays. (B) Extrapolation of experimental curves to determine C · t values for 3 log units of cell death. Slopes were calculated as follows: M. aurum, y = 0.19 x; M. gordonae, y = 0.09 x; M. chelonae, y = 0.03 x; and M. fortuitum, y = 0.04 x. R2, correlation coefficient values.
In water treatment lines, chorination conditions are very often 0.5 mg of chlorineliter−1 for 2 h, providing a C · t value of 60 mg · min · liter−1. From k values, it can be calculated that chlorination could eliminate over 5 log units of M. aurum, 4 log units of M. gordonae, but only 1.5 log units of M. fortuitum or M. chelonae (Fig. 2B).
Effects of medium composition on the susceptibility of M. gordonae to free chlorine.
M. gordonae, a frequent contaminant of tap water systems with an intermediate inactivation rate constant (k) among the mycobacterial species tested, was selected as a model to test the various chlorination conditions of mycobacterial inactivation.
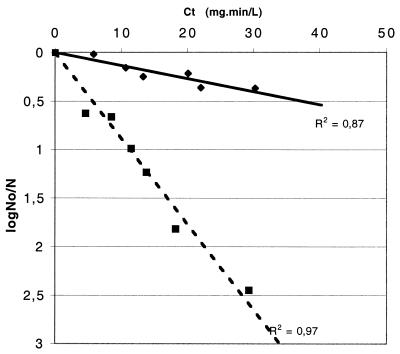
To examine the impact of growth conditions on mycobacteria, the chlorine susceptibilities of M. gordonae grown in 7H9-Tween medium and in filtered tap water supplemented with 10% 7H9-Tween medium were compared (Fig. 3). It was not possible to test M. gordonae in 100% tap water because it grew extremely poorly and did not yield enough CFU for a valid analysis.
FIG. 3.
Effects of culture medium on the susceptibility of M. gordonae to free chlorine. C · t values were calculated as described in the legend to Fig. 1. Experimental conditions were pH 7, a temperature of 25°C, and an initial chlorine concentration of 0.5 mg/liter. No, initial number of CFU; N, number of CFU at the time of the assays. Symbols: ⧫, water plus 10% Middlebrook 7H9-Tween medium (y = 0.01 x); ▪, 100% Middlebrook 7H9-Tween medium (y = 0.09 x). R2, correlation coefficient values.
Mycobacteria grown in tap water were more resistant to chlorine than cells grown in culture medium. The results show that the k value for M. gordonae grown in water supplemented with 10% 7H9-Tween medium was 0.01 liter · min · mg−1; that for M. gordonae grown in 7H9-Tween medium was 0.09 liter · min · mg−1. The growth of M. gordonae in the low-nutrient solution significantly increased the resistance of the microorganism to free chlorine.
Effects of temperature on inactivation rates.
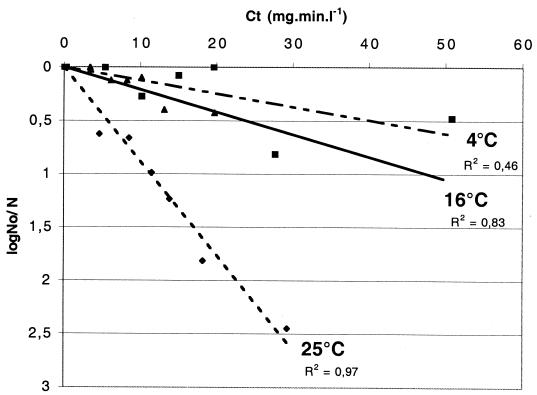
The effect of temperature on the bactericidal activity of chlorine was tested with M. gordonae (Fig. 4). For a C · t value of 60 mg · min · liter−1, M. gordonae showed less than 1 log10 unit or less than 1.5 log10 unit of inactivation at 4°C or at 16°C, respectively, whereas the number of CFU decreased by 5.5 log units at 25°C (Fig. 3). Therefore, chlorination was more efficient at higher temperatures.
FIG. 4.
Effects of temperature on chlorine inactivation of M. gordonae. Experimental conditions were pH 7 and an initial chlorine concentration of 0.5 mg/liter. Cells were grown in Middlebrook 7H9-Tween medium. The chlorine susceptibility of M. gordonae was analyzed at 4, 16, and 25°C. No, initial number of CFU; N, number of CFU at each time point. Symbols: ▪, 4°C (y = 0.01 x); ▴, 16°C (y = 0.02 x); ⧫, 25°C (y = 0.09 x). R2, correlation coefficient values.
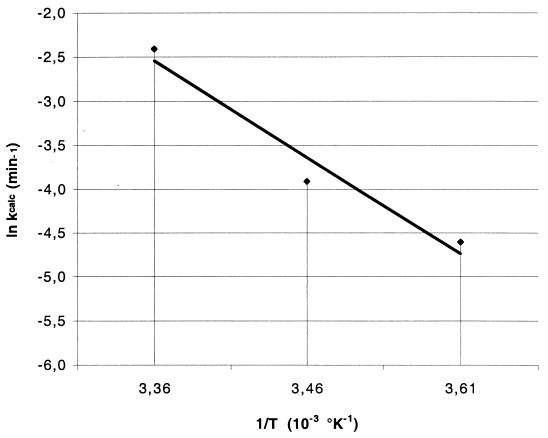
To determine whether the decrease in inactivation varied linearly with temperature, the validity of the Arrhenius expression was checked. For a simple chemical reaction, the dependence of ki on temperature is expressed by the classical Arrhenius equation:
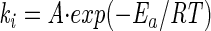 |
(2) |
where A is the frequency factor in 1/(moles per minute), Ea is the reaction activation energy in Joules per mole, R (8.314 J per mol per degree kelvin) is the ideal gas constant, and T is the absolute temperature in degrees kelvin. The temperature dependence of ki was consistent with the Arrhenius equation (Fig. 5). Linear regression of the data presented in Fig. 5 yielded an activation energy of 9.14 J/mol. In conclusion, these experimental conditions allowed the determination of the M. gordonae inactivation rate at different temperatures according to the Arrhenius equation.
FIG. 5.
Arrhenius plot of k values versus temperature. The abscissa values correspond to the temperatures tested in Fig. 4; 3.36 corresponds to 25°C, 3.46 corresponds to 16°C, and 3.61 corresponds to 4°C. Linear regression of the data yielded an activation energy of 9.14 J/mol and a log frequency factor of 1.10 as defined by the Arrhenius equation (equation 2).
Effects of pH on inactivation rates.
Free chlorine exists mainly in two different forms in aqueous solutions, HOCl and OCl−. The concentration of each form varies in a nonlinear manner according to the pH. For Escherichia coli, HOCl is more than 50-fold more effective than OCl− as a disinfectant (32). At pH 6.0, HOCl accounts for 98% of free chlorine, whereas at pH 10.0, OCl− accounts for over 99%. Between pHs 7.0 and 8.5, the proportions vary rapidly but not linearly; HOCl decreasing from 83% at pH 7.0 to 14% at pH 8.5. The equilibrium is temperature dependent, with lower temperatures resulting in slightly higher proportions of HOCl.
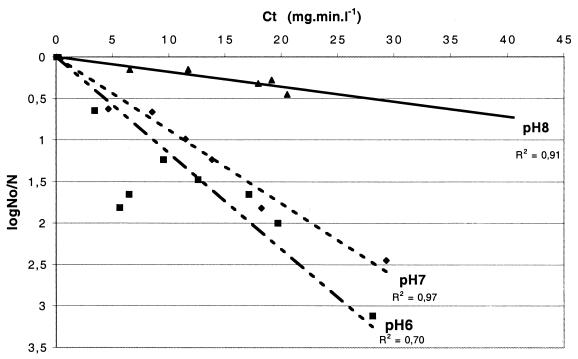
The results showed that chlorine disinfection for M. gordonae was more rapid at pH 6 than at pH 7 or pH 8 (Fig. 6). These results reflect the fact that more HOCl is present at a lower pH. After 10 min in the presence of chlorine at pH 6, the numbers of M. gordonae cells decreased by 0.64 log10 units (C · t, 3.4 mg · min · liter−1), compared to 0.15 log10 units (C · t, 6.5 mg · min · liter−1) at pH 8 for the same exposure (Fig. 6). The k value was sixfold higher at pH 6 and pH 7 than at pH 8. In conclusion, increasing pH decreases mycobacterial inactivation rates, highlighting the susceptibility of M. gordonae to HOCl.
FIG. 6.
Effects of pH on the rate of inactivation of M. gordonae. Experimental conditions were a temperature of 25°C and an initial chlorine concentration of 0.5 mg/liter. Cells were grown in Middlebrook 7H9-Tween medium. No, initial number of CFU; N, number of CFU at each time point. Experiments were conducted with different phosphate (0.05 M) buffers within the pH range of 6.0 to 8.0. Slopes were calculated as follows: pH 6, y = 0.11 x; pH 7, y = 0.09 x; and pH 8, y = 0.02 x. R2 , correlation coefficient values.
DISCUSSION
The chlorine resistance of mycobacteria isolated from the Parisian water distribution network and upstream internal networks is reported. Various species were identified, including M. fortuitum, M. chelonae, M. aurum, M. peregrinum, and M. gordonae. Interestingly, M. avium was not recovered. Most of the available data on chlorine disinfection of mycobacteria in the literature are based on the susceptibility of M. avium, a focus of interest of numerous studies due to its high clinical impact (14, 16, 17, 43). However, recent extensive studies of widely dispersed drinking water utilities in the United States showed that the frequencies of recovery and the numbers of M. avium in drinking water samples are low (10, 16). In Europe, M. avium is not frequently found in tap water, as shown by a German study in which 1.7% of the samples were positive for M. avium (30). A recent study in Greece also failed to detect M. avium in drinking water distribution systems (42). However, in these studies, other mycobacterial species, including M. chelonae, M. gordonae, and M. fortuitum, were frequently isolated.
Uncombined chlorine, in the form of hypochlorous acid (HOCl), is an extremely potent bactericidal agent, active even at concentrations of less than 0.1 mg · liter−1 on most bacteria and viruses (23). As a direct consequence, chlorination is one of the most widely used methods for the disinfection of water. Chlorination is comparatively inexpensive and easy to use, and chlorine remains active within the system for a considerable length of time. Chlorination conditions used in water distribution systems are based on the inactivation of several viruses and bacteria but not mycobacterial species or other pathogens, such as parasites. Information pertaining to the effect of hypochlorous acid on atypical mycobacteria is rather limited.
The results of this study are consistent with those of the study by Carson et al. (6), who found that M. chelonae and M. fortuitum are highly resistant to chlorine. Recently, Taylor et al. showed that M. avium strains have C · t values of 51 to 204 mg · min · liter−1 for 3 log units (99.9%) of cell death at pH 7 and 23°C (40). Stewart and Olson stressed the importance of the conditions in which organisms are grown before disinfection experiments (37). In this study, conditions similar to those used by Taylor et al. (40) were chosen: tests were performed at pH 7 and 23°C with strains grown in 7H9 Middlebrook broth medium. In this study, for 3 log units (99.9%) of inactivation, C · t values of 100 and 135 mg · min · liter−1 were calculated for M. chelonae and M. fortuitum, respectively (Fig. 2B). The similar high C · t values shown for M. avium by Taylor et al. (51 to 204 mg · min · liter−1) are 580 to 2,300 times higher than those for E. coli (40). The comparison of the C · t values showed that the most susceptible species in this study, M. aurum and M. gordonae, were still 100 and 330 times more resistant to chlorine than E. coli, respectively (40).
A C · t value of 60 mg · min · liter−1 maintained in a chlorination tank is not efficient against all mycobacterial species, specially M. chelonae and M. fortuitum, as shown in this study, or M. avium, as shown by others (29, 40). High chlorine concentrations can be used for disinfection purposes in a private distribution system, for example, in a hospital in which the water supply is highly contaminated with mycobacteria. As the resistance of mycobacteria to chlorine is species specific, it is difficult to establish standard chlorine concentrations and times (C · t) to reduce or eliminate waterborne mycobacteria. It is therefore important to consider the level of chlorine resistance of the mycobacterial species responsible for the contamination when establishing chlorination conditions for disinfection.
All the strains used in this study were waterborne strains because previous studies showed that clinical and environmental strains display different levels of chlorine resistance (29). Different parameters (medium, pH, and temperature) relating to chlorine resistance for waterborne M. gordonae were studied. The results showed that inactivation of atypical mycobacteria was more efficient at low temperatures and high pHs. Moreover, when M. gordonae was grown in filtered water supplemented with 10% 7H9 Middlebrook liquid medium, the inactivation rates decreased by a factor 10. In distribution networks, mycobacteria live at average temperatures (16°C) in neutral pHs (7 to 7.5). This information suggests that the chlorine resistance of mycobacteria isolated from fresh tap water was underestimated in this study.
Hypochlorous acid and hypochlorite ions are present simultaneously in water. Hypochlorous acid is the more active of the two components as a disinfectant. The disinfection efficiency increases with temperature, as the reaction rate of hypochlorous acid with bacterial components increases. Haas proposed a model for the inactivation of microorganisms assuming the existence of an intermediate disinfectant-organism complex that governs the rate of microbial inactivation (19).
Moreover, the lower the pH, the greater the proportion of hypochlorous acid. Once again, these experiments with mycobacteria were in agreement with results obtained for Yersinia enterocolitica (27). Hypochlorous acid is a highly destructive oxidant that reacts with various cellular compounds and affects metabolic processes. It alters membrane permeability, inhibits transport, cleaves proteins, and reacts with nucleotides. Hypochlorous acid reacts with unsaturated fatty acids, modifying membrane fluidity and permeability. The peculiar structure of the mycobacterial cell wall skeleton partly explains the high resistance of mycobacteria to chlorination. In mycobacteria, the peptidoglycan is covalently linked to mycolic acids, consisting of long fatty acids up to 90 carbon atoms, through an arabinogalactan bridge. Mycolic acids confer acid fastness to bacilli and represent a thick, hydrophobic barrier preventing diffusion and lowering permeability (1). The current techniques for isolating pure mycobacterial cultures from specimens that contain other microorganisms rely on the greater resistance of mycobacteria to acids, alkalis, or quaternary ammonium ions. Moreover, the external layer is composed of unique constituents noncovalently linked to the cell wall and consisting of peptidoglycolipids, glycolipids, lipopolysaccharides, phospholipids, sulfolipids, and nonlipidic molecules, proteins, and polysaccharides (1, 11). The composition of the outer layer is species specific, a fact which may explain the differential resistance of mycobacterial species to chlorination.
The effect of nutrients on disinfection resistance is the least well understood concept. In various organisms, especially Legionella pneumophila, Flavobacterium, and Klebsiella pneumoniae (22, 38), growth in low-nutrient conditions leads to higher chlorine resistance. Similarly, Taylor et al. showed that water-grown M. avium cells were 10-fold more resistant than medium-grown cells (40). These results are consistent with our results for other mycobacterial species. A tentative explanation could be related to information from previous studies which demonstrated that the total lipid content is doubled when M. phlei is grown in the presence of 1.4% sodium acetate (2). The difference in the resistance of mycobacterial species to chlorine could be related to the composition of the cell wall, especially the outer layer, which varies from species to species and according to growth conditions. Further studies are still necessary to identify the molecules responsible for resistance to chlorine.
Elimination of Mycobacterium species in a real water distribution system.
Our results confirm that mycobacteria are highly resistant to chlorine disinfection and document the optimal parameters for the inactivation of mycobacterial species. However, the test conditions are not necessarily those encountered in distribution systems. They are valid for free mycobacteria in the water distribution system. Mycobacteria have been shown to be able to replicate in biofilms, and solid-liquid interfaces may be regarded as sites of selective enrichment for these bacteria (34, 35). The high degree of cell wall hydrophobicity probably accounts for the strong adhesive properties displayed by mycobacterial factors and influences the numbers of mycobacteria in biofilms (16). Additional studies must be performed to test the efficiency of disinfection procedures for mycobacteria in biofilms.
The high C · t values necessary for inactivating mycobacteria to a great extent (at least 99.9%) are not always available in the final chlorination tank and in the distribution system. Careful calculation of real C · t values in global water treatment lines and distribution systems is necessary for evaluating the level of inactivation of mycobacteria.
Nevertheless, chlorination is not the sole efficient process. Filtration, clarification, and ozonation permit the efficient removal of microorganisms and must be investigated in order to evaluate the efficiencies of different treatments. Copper and silver ions are attractive candidates, as this disinfection technique requires little maintenance and results in residual disinfection throughout the distribution system. Ozone disinfection also has advantages, as it reduces taste, odor, and color and oxidizes organic substances. The main drawback of ozone is that chlorine disinfection still remains necessary at low concentrations to maintain the quality of water in distribution systems. Previous studies showed that C · t values required for a 99.9% reduction (3 log units of killing) in M. avium viability were 1.4 mg · min · liter−1 with copper and silver ions and 0.1 to 0.7 mg · min · liter−1 with ozone (40, 49). These C · t values are low compared to those for chlorine and highlight interest in these alternative or complementary disinfection methods for mycobacteria. The effects of copper ions and ozone are currently being tested on a range of waterborne isolates from various mycobacterial species in our laboratory.
Acknowledgments
This work received support from Lyonnaise des Eaux and Société Anonyme de Gestion des Eaux de Paris.
REFERENCES
- 1.Asselineau, C., and J. Asselineau. 1978. Lipides spécifiques des mycobactéries. Ann. Microbiol. 129:46-69.J. D. [PubMed] [Google Scholar]
- 2.Asselineau, J., and E. Lederer. 1953. Chimie des lipides bactériens. Prog. Chem. Nat. Org. Subst. 10:170-273. [Google Scholar]
- 3.Astagneau, P., N. Desplaces, V. Vincent, V. Chicheportiche, A. H. Botherel, S. Maugat, K. Lebascle, P. Leonard, J. C. Desenclos, J. Grosset, J. M. Ziza, and G. Brücker. 2001. Mycobacterium xenopi spinal infections after disco-vertebral surgery: investigation and screening of a large outbreak. Lancet 358:747-751. [DOI] [PubMed] [Google Scholar]
- 4.Bolan, G., A. L. Reingold, L. A. Carson, V. A. Silcox, C. L. Woodley, P. S. Hayes, A. W. Hightower, L. McFarland, J. W. d. Brown, and N. J. Petersen. 1985. Infections with Mycobacterium chelonei in patients receiving dialysis and using processed hemodialyzers. J. Infect. Dis. 152:1013-1019. [DOI] [PubMed] [Google Scholar]
- 5.Campagnaro, R. L., H. Teichtahl, and B. Dwyer. 1994. A pseudoepidemic of Mycobacterium chelonae: contamination of a bronchoscope and autocleaner. Aust. N. Z. J. Med. 24:693-695. [DOI] [PubMed] [Google Scholar]
- 6.Carson, L. A., N. J. Petersen, M. S. Favero, and S. M. Aguero. 1978. Growth characteristics of atypical mycobacteria in water and their comparative resistance to disinfectants. Appl. Environ. Microbiol. 36:839-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chick, H. 1908. An investigation of the laws of disinfection. J. Hyg. 8:92-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins, C. H., J. M. Grange, and M. D. Yates. 1984. Mycobacteria in water. J. Appl. Bacteriol. 57:193-211. [DOI] [PubMed] [Google Scholar]
- 9.Costrini, A. M., D. A. Mahler, W. M. Gross, J. E. Hawkins, R. Yesner, and N. D. D'Esopo. 1981. Clinical and roentgenographic features of nosocomial pulmonary disease due to Mycobacterium xenopi. Am. Rev. Respir. Dis. 123:104-109. [DOI] [PubMed] [Google Scholar]
- 10.Covert, T. C., M. R. Rodgers, A. L. Reyes, and G. N. Stelma, Jr. 1999. Occurrence of nontuberculous mycobacteria in environmental samples. Appl. Environ. Microbiol. 65:2492-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daffe, M., and G. Etienne. 1999. The capsule of Mycobacterium tuberculosis and its implications for pathogenicity. Tuber. Lung Dis. 79:153-169. [DOI] [PubMed] [Google Scholar]
- 12.Dailloux, M., C. Laurain, M. Weber, and P. Hartemann. 1999. Water and nontuberculous mycobacteria. Water Res. 33:2219-2228. [Google Scholar]
- 13.David, L. H., V. Levy-Frebault, and F. Papa. 1986. Méthodes de laboratoire pour Mycobacteriologie clinique. Unité de la Tuberculose et des Mycobactéries, Institut Pasteur, Paris, France.
- 14.du Moulin, G. C., K. D. Stottmeier, P. A. Pelletier, A. Y. Tsang, and J. Hedley-Whyte. 1988. Concentration of Mycobacterium avium by hospital hot water systems. JAMA 260:1599-1601. [DOI] [PubMed] [Google Scholar]
- 15.Falkinham, J. O., III. 1996. Epidemiology of infection by nontuberculous mycobacteria. Clin. Microbiol. Rev. 9:177-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falkinham, J. O., III, C. D. Norton, and M. W. LeChevallier. 2001. Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other mycobacteria in drinking water distribution systems. Appl. Environ. Microbiol. 67:1225-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goslee, S., and E. Wolinsky. 1976. Water as a source of potentially pathogenic mycobacteria. Am. Rev. Respir. Dis. 113:287-292. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg, A. E., L. S. Clesceri, and A. D. Eaton. 1992. Standard methods for the examination of water and wastewater, 18th ed. American Public Health Association, Washington, D.C.
- 19.Haas, C. N. 1980. A mechanistic kinetic model for chlorine disinfection. Env. Sci. Technol. 14:339-340. [DOI] [PubMed] [Google Scholar]
- 20.Inderlied, C. B., C. A. Kemper, and L. E. Bermudez. 1993. The Mycobacterium avium complex. Clin. Microbiol. Rev. 6:266-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laussucq, S., A. L. Baltch, R. P. Smith, R. W. Smithwick, B. J. Davis, E. K. Desjardin, V. A. Silcox, A. B. Spellacy, R. T. Zeimis, H. M. Gruft, et al. 1988. Nosocomial Mycobacterium fortuitum colonization from a contaminated ice machine. Am. Rev. Respir. Dis. 138:891-894. [DOI] [PubMed] [Google Scholar]
- 22.LeChevallier, M. W., C. D. Cawthon, and R. G. Lee. 1988. Factors promoting survival of bacteria in chlorinated water supplies. Appl. Environ. Microbiol. 54:649-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludovici, P. P., R. A. Phillips, and W. S. Jeter. 1977. Comparative inactivation of bacteria and viruses in tertiary-treated wastewater by chlorination, p. 359-390. In J. D. Johnson (ed.), Disinfection: water and wastewater. Ann Arbor Science Publishers, Ann Arbor, Mich.
- 24.Metchock, B. G., F. S. Nolte, and R. J. Wallace, Jr. 1999. Mycobacterium, p.399-437. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 25.Nolan, C. M., P. A. Hashisaki, and D. F. Dundas. 1991. An outbreak of soft-tissue infections due to Mycobacterium fortuitum associated with electromyography. J. Infect. Dis. 163:1150-1153. [DOI] [PubMed] [Google Scholar]
- 26.Pankhurst, C. L., N. W. Johnson, and R. G. Woods. 1998. Microbial contamination of dental unit waterlines: the scientific argument. Int. Dent. J. 48:359-368. [DOI] [PubMed] [Google Scholar]
- 27.Paz, M. L., M. V. Duaigues, A. Hanashiro, M. D'Aquino, and P. Santini. 1993. Antimicrobial effect of chlorine on Yersinia enterocolitica. J. Appl. Bacteriol. 75:220-225. [DOI] [PubMed] [Google Scholar]
- 28.Pelikan, M., Z. Mikova, J. Kaustova, and M. Kubin. 1973. Supply water as a probable cause of transfer of air-borne infections by atypical mycobacteria. Ceskoslovenska Hygiena 18:316-323. [Google Scholar]
- 29.Pelletier, P. A., G. C. du Moulin, and K. D. Stottmeier. 1988. Mycobacteria in public water supplies: comparative resistance to chlorine. Microbiol. Sci. 5:147-148. [PubMed] [Google Scholar]
- 30.Peters, M., C. Muller, S. Rusch-Gerdes, C. Seidel, U. Gobel, H. D. Pohle, and B. Ruf. 1995. Isolation of atypical mycobacteria from tap water in hospitals and homes: is this a possible source of disseminated MAC infection in AIDS patients? J. Infect. 31:39-44. [DOI] [PubMed] [Google Scholar]
- 31.Rogall, T., T. Flohr, and E. C. Bottger. 1990. Differentiation of Mycobacterium species by direct sequencing of amplified DNA. J. Gen. Microbiol. 136:1915-1920. [DOI] [PubMed] [Google Scholar]
- 32.Scarpino, P. V., G. Berg, L. S. Chang, D. Dahling, and M. Lucas. 1972. A comparative study of the inactivation of viruses in water by chlorine. Water Res. 6:959-965. [Google Scholar]
- 33.Schulze-Robbecke, R., C. Feldmann, R. Fischeder, B. Janning, M. Exner, and G. Wahl. 1995. Dental units: an environmental study of sources of potentially pathogenic mycobacteria. Tuber. Lung Dis. 76:318-323. [DOI] [PubMed] [Google Scholar]
- 34.Schulze-Robbecke, R., and R. Fischeder. 1989. Mycobacteria in biofilms. Zentbl. Hyg. Umweltmed. 188:385-390. [PubMed] [Google Scholar]
- 35.Schulze-Robbecke, R., B. Janning, and R. Fischeder. 1992. Occurrence of mycobacteria in biofilm samples. Tuber. Lung Dis. 73:141-144. [DOI] [PubMed] [Google Scholar]
- 36.Slosarek, M., M. Kubin, and M. Jaresova. 1993. Water-borne household infections due to Mycobacterium xenopi. Cent. Eur. J. Public Health 1:78-80. [PubMed] [Google Scholar]
- 37.Stewart, M. H., and B. H. Olson. 1986. Mechanisms of bacterial resistance to inorganic chloramines. Water Qual. Technol. Conf. Proc. 14:577-590. [Google Scholar]
- 38.Stewart, M. H., and B. H. Olson. 1992. Physiological studies of chloramine resistance developed by Klebsiella pneumoniae under low-nutrient growth conditions. Appl. Environ. Microbiol. 58:2918-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stine, T. M., A. A. Harris, S. Levin, N. Rivera, and R. L. Kaplan. 1987. A pseudoepidemic due to atypical mycobacteria in a hospital water supply. JAMA 258:809-811. [PubMed] [Google Scholar]
- 40.Taylor, R. H., J. O. Falkinham III, C. D. Norton, and M. W. LeChevallier. 2000. Chlorine, chloramine, chlorine dioxide, and ozone susceptibility of Mycobacterium avium. Appl. Environ. Microbiol. 66:1702-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Telenti, A., F. Marchesi, M. Balz, F. Bally, E. C. Bottger, and T. Bodmer. 1993. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 31:175-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsintzou, A., A. Vantarakis, O. Pagonopoulou, A. Athanassiadou, and M. Papapetropoulou. 2000. Environmental mycobacteria in drinking water before and after replacement of the water distribution network. Water Air Soil Pollut. 120:273-282. [Google Scholar]
- 43.Von Reyn, C. F., J. N. Maslow, T. W. Barber, J. O. Falkinham III, and R. D. Arbeit. 1994. Persistent colonisation of potable water as a source of Mycobacterium avium infection in AIDS. Lancet 343:1137-1141. [DOI] [PubMed] [Google Scholar]
- 44.Wallace, R. J., Jr., B. A. Brown, and D. E. Griffith. 1998. Nosocomial outbreaks/pseudo-outbreaks caused by nontuberculous mycobacteria. Annu. Rev. Microbiol. 52:453-490. [DOI] [PubMed] [Google Scholar]
- 45.Wallace, R. J., Jr., J. M. Musser, S. I. Hull, V. A. Silcox, L. C. Steele, G. D. Forrester, A. Labidi, and R. K. Selander. 1989. Diversity and sources of rapidly growing mycobacteria associated with infections following cardiac surgery. J. Infect. Dis. 159:708-716. [DOI] [PubMed] [Google Scholar]
- 46.Wallace, R. J., Jr., J. M. Swenson, V. A. Silcox, R. C. Good, J. A. Tschen, and M. S. Stone. 1983. Spectrum of disease due to rapidly growing mycobacteria. Rev. Infect. Dis. 5:657-679. [DOI] [PubMed] [Google Scholar]
- 47.Watson, H. E. 1908. A note on the variation of the rate of disinfection with change in the concentration of the disinfectant. J. Hyg. 8:536.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wenger, J. D., J. S. Spika, R. W. Smithwick, V. Pryor, D. W. Dodson, G. A. Carden, and K. C. Klontz. 1990. Outbreak of Mycobacterium chelonae infection associated with use of jet injectors. JAMA 264:373-376. [PubMed] [Google Scholar]
- 49.Yu-Sen E. Lin, R. D. V., Janet E. Stout, Christine A. McCartney and Victor L. Yu. 1998. Inactivation of Mycobacterium avium by copper and silver ions. Water Res. 32:1997-2000. [Google Scholar]
- 50.Zhang, Y., M. Rajagopalan, B. A. Brown, and R. J. Wallace, Jr. 1997. Randomly amplified polymorphic DNA PCR for comparison of Mycobacterium abscessus strains from nosocomial outbreaks. J. Clin. Microbiol. 35:3132-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]