Abstract
Blastobacter spp. are freshwater bacteria that form rosette structures by cellular attachment to a common base. Comparative analyses of ribosomal 16S rRNA gene and internally transcribed spacer region sequences indicated that B. denitrificans is a member of the α-subdivision of Proteobacteria. Among the α-Proteobacteria, B. denitrificans was related to a cluster of genera, including Rhodopseudomonas palustris, Afipia felis, Nitrobacter hamburgensis, and Bradyrhizobium spp. Although the precise phylogenetic relationships among these genera could not be established with a high degree of confidence, the sequences of B. denitrificans and several bradyrhizobial isolates from nodules of Aeschynomene indica were almost identical. Bradyrhizobia are bacteria that form nitrogen-fixing symbioses with legumes, including soybeans (Glycine max) and members of the genus Aeschynomene. From symbiotic infectiveness tests we demonstrated that the type strain for B. denitrificans, IFAM 1005, was capable of forming an effective nitrogen-fixing symbiosis with A. indica. Not only do these results reveal a previously unknown ecological adaptation of a relatively obscure aquatic bacterium, but they also demonstrate how evidence gathered from molecular systematic analyses can sometimes provide clues for predicting ecological behavior.
Comparative sequence analyses of the 16S rRNA genes are widely used to reconstruct phylogenetic relationships among bacteria (15). A remarkable degree of ecological diversity exists within subgroup 2b of the α-Proteobacteria (9) because members include nitrogen-fixing symbionts of leguminous plants (Bradyrhizobium spp.), mammalian pathogens (Afipia spp.), nitrifying soil bacteria (Nitrobacter spp.), phototrophic bacteria (Rhodopseudomonas palustris), and the aquatic bud- and cluster-forming freshwater bacterium Blastobacter denitrificans (25).
Bradyrhizobium japonicum and Bradyrhizobium elkanii are most frequently associated with the formation of a nitrogen-fixing symbiosis with soybean. Perhaps it is no surprise that the stem-nodulating symbiotic bacteria of the flood-tolerant legume Aeschynomene indica (2, 29) are closely related to Bradyrhizobium japonicum, as estimated from 16S rRNA gene sequence divergence (17, 33, 34). However, from these reconstructions it also has been shown that Bradyrhizobium japonicum appears to be more closely related to the other genera of subgroup 2b than it is to Bradyrhizobium elkanii (25).
Not only does this indicate that the ecological adaptations within this group are poor indicators of phylogenetic relatedness, but it also implies that the genus Bradyrhizobium is polyphyletic.
The central focus of this study initially was to confirm the phylogenetic placement of bradyrhizobial isolates of A. indica since they reportedly are more closely related to Bradyrhizobium japonicum and other nonbradyrhizobial members of subgroup 2b than they are to Bradyrhizobium elkanii (17). From our analysis, which included type strains representing the named genera in the α-subdivision subgroup 2b, we concluded that some isolates were closely related to B. denitrificans. Therefore, we examined the possibility that the type strain of B. denitrificans (IFAM 1005) would form a symbiotic relationship with A. indica.
MATERIALS AND METHODS
Bacterial strains.
The strains used in this study, obtained from the U.S. Department of Agriculture Agricultural Research Service National Rhizobium Germplasm Collection, were originally isolated by van Berkum et al. (29) and are described in Table 1. B. denitrificans type strain IFAM 1005 (LMG 8443) and Afipia felis type strain ATCC 49715 were kindly provided by the Belgian Culture Collection of Microorganisms and the American Type Culture Collection, respectively. The sources of A. indica strain BTAi1, A. americana strain USDA 3177, Methylobacterium extorquens ATCC 8457 and ATCC 14718, M. organophylum ATCC 27886, M. rhodinum ATCC 14821, Rhodobacter sphaeroides 2.4.1, and Rhodopseudomonas palustris GH were as described previously (29). Sequencing results with Rhodobacter sphaeroides served as an outgroup for reconstruction of phylogenies from 16S rRNA gene and internally transcribed spacer (ITS) region sequence divergence.
TABLE 1.
Bradyrhizobial strains originating from A. indica used in this study
| USDA strain | Origin | Bchl phenotypea |
|---|---|---|
| USDA 4363 | New Delhi, India | 2 |
| USDA 4366 | New Delhi, India | 3 |
| USDA 4371 | Maryland, United States | 2 |
| USDA 4379 | Amazonas, Brazil | 3 |
| USDA 4393 | Babungo, Cameroon | 1 |
| USDA 4399 | Maryland, United States | 2 |
| USDA 4406 | France | 1 |
| USDA 4409 | Ibadan, Nigeria | 1 |
| USDA 4410 | Ibadan, Nigeria | 3 |
| USDA 4415 | Lampung, Indonesia | 1 |
| USDA 4421 | Tamil Nadu, India | 1 |
| USDA 4424 | Louisiana, United States | 2 |
| USDA 4430 | Ibadan, Nigeria | - |
| USDA 4435 | Maryland, United States | 1 |
| USDA 4440 | Marondera, Zimbabwe | 1 |
Phenotypes: 1, no pigment formation; 2, pigment formation during day-night cycle; 3, pigment formation in the dark (29). -, Unknown.
Growth of the bacteria and DNA isolation.
The phototrophic bradyrhizobia were grown in 50 ml of modified arabinose gluconate (MAG) broth (24) for the large-scale isolation of DNA purified by CsCl density centrifugation (19). MAG or charcoal-yeast extract broth (10 ml) was used to grow B. denitrificans or Afipia felis, respectively, for small-scale DNA preparations by using a Tissue and Blood DNA Extraction kit (Qiagen, Inc., Chatsworth, Calif.). Purification of DNA from the other cultures used in this study was as described by van Berkum et al. (29). Concentrations of DNA in solution were measured spectrophotometrically at 260 nm by using a Gilford Response Spectrophotometer (Gilford Instrument Laboratories, Oberlin, Ohio).
PCR amplification and sequencing analysis.
Primers 16Sa and 16Sb (26) were used for amplification of the 16S rRNA gene locus because PCR with primers fD1 and rD1 (31) sometimes failed or produced multiple bands. We determined by computer analysis by using Oligo (National Biosciences, Inc., Plymouth, Minn.) that the unsatisfactory amplification of the 16S rRNA gene with primers fD1 and rD1 probably resulted from the poor internal stability of these primers that may lead to false priming. The 16S rRNA genes were amplified in 120-μl volumes as described before (28) except for the primers and the PCR buffer [60 mM Tris-HCl, 15 mM (NH4)2SO4, and 3.5 mM MgCl2 at pH 9.0]. Primers 450 and 1440 (26) were used to amplify the ITS region and are located in conserved regions of the 3′ end of the 16S rRNA gene and the 5′ end of the 23S rRNA gene. The PCR products generated with this primer pair also contained the intervening region (7, 10, 13, 14, 16, 20, 21, 32) of the 23S rRNA gene. PCR conditions were those described for the 16S rRNA gene. The PCR products were purified by using QIAquick Spin columns (Qiagen). A Perkin-Elmer 377 DNA Sequencer in combination with a DyeDeoxy Terminator Cycle Sequencing Kit (Perkin-Elmer, Foster City, Calif.) was used for sequencing the purified PCR products as described previously (26, 28). The sequences for USDA 4415, USDA 4409, USDA 4435, USDA 4440, USDA 4393, USDA 4363, USDA 4366, USDA 4410, USDA 4421, USDA 4424, BTAi1 (USDA 4362), USDA 4430, USDA 4371, USDA 4399, USDA 4406, USDA 4379, USDA 3177, B. denitrificans (LMG 8443), Afipia felis (ATCC 49715), M. rhodinum (ATCC 14821), M. extorquens (ATCC 8457), M. organophylum (ATCC 27886), and Rhodobacter sphaeroides (2.4.1) have been deposited in the GenBank database under accession numbers AF338159 through AF338182, respectively.
Analysis of the sequence data.
The sequences were aligned by using the PILEUP program in the Wisconsin package of the Genetics Computer Group (Madison, Wis.). Aligned sequences were analyzed by using the Molecular Evolutionary Genetics Analysis (MEGA) package, version 1.01 (11), which was also used to generate bootstrap confidence values with 500 permutations of the data sets.
Southern hybridization analysis for detecting nifHDK.
DNAs of B. denitrificans LMG 8443, the A. indica isolate USDA 4424, and Bradyrhizobium japonicum USDA 110 were restricted with EcoRI (Promega, Madison, Wis.) according to the manufacturer's protocol, and ca. 1 μg of digested DNA was separated according to molecular size in 0.7% agarose gels as described previously (29). Southern transfer and Southern hybridization with pDC4 (the nifHDK probe was kindly provided by Gary Ditta, University of California, San Diego) also were as described previously (29), except that the Gene Images random prime labeling module in conjunction with Gene Images CDP-Star detection module (Amersham Pharmacia Biotech, Piscataway, N.J.) was used according to the manufacturer's specifications to detect the nifHDK genes in the target DNAs.
Electron microscopy.
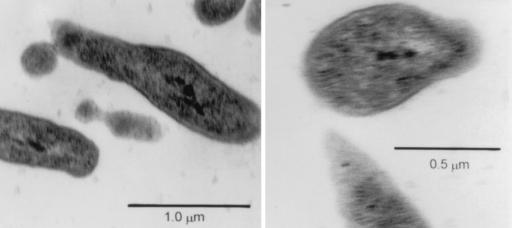
Cultures of B. denitrificans recovered from nodules of A. indica were prepared for electron microscopy to examine cells for budding according to protocols used previously (30).
Plant tests.
Seeds of A. indica were surface sterilized with concentrated H2SO4 for 3 min and then washed five times with sterile distilled water. The treated seeds were sown in sterile sand-filled Leonard jars (12), and 10 ml of MAG-grown late-log-phase 200-ml broth cultures was used to inoculate each jar. The cultures tested for symbiosis were BTAi1, USDA 4424, and B. denitrificans IFAM 1005 (LMG 8443). Each treatment was prepared in five replications, and five jars without inoculated bacteria served as controls. The plants were grown in a greenhouse without supplemental lighting for 34 days during July and August. The plants were uprooted, and the tops were cut off to determine nitrogenase activities as described by van Berkum et al. (29). Determinations for the concentration of ethylene in each chamber were as described by van Berkum and Sloger (27). The plant tops were dried at 60°C for 2 days to determine dry matter and total nitrogen contents as described previously (24). Nodules were used to isolate bacterial occupants in culture as described previously (29). Surface-sterilized seeds also were germinated on water agar plates at 30°C for 24 h to evaluate BTAi1 and B. denitrificans IFAM 1005 (LMG 8443) for nodulation of A. indica in growth pouches in triplicate on two separate occasions as described by van Berkum et al. (29).
RESULTS
Sequencing results.
The molecular sizes of the PCR products obtained with amplification reactions with primers for the 16S rRNA gene were relatively uniform, varying in size only by ca. 100 bp. The products with Afipia felis strain ATCC 49715 and B. denitrificans strain IFAM 1005 (LMG 8443) were ca. 1,480 bp. Our 16S rRNA gene sequences for B. denitrificans and for Afipia felis were consistent with accessions S46917 and AF003937 for these two taxa in the GenBank database.
The molecular sizes of the PCR products obtained with amplification reactions with primers for the ITS region were variable. Among the 16 strains of A. indica, the products ranged from ca. 1,380 to 1,710 bp, and among the four methylotrophs, they ranged from ca. 1,085 to 1,165 bp. The products with Bradyrhizobium strain USDA 3177, Rhodobacter sphaeroides 2.4.1, Afipia felis strain ATCC 49715, Rhodopseudomonas palustris strain GH, and B. denitrificans strain IFAM 1005 (LMG 8443) were ca. 1,420, 1,165, 1,350, 1,400, and 1,670 bp, respectively.
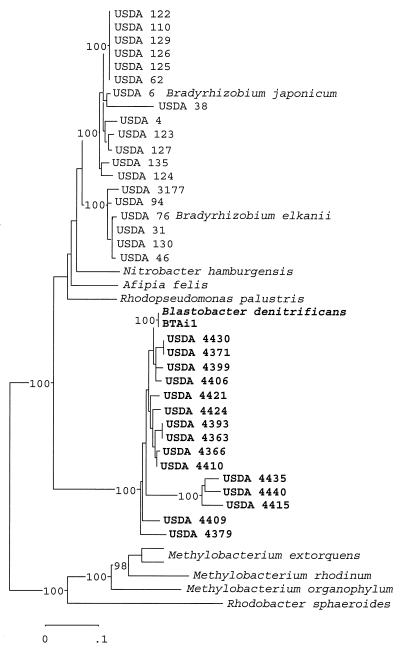
Forty-four ITS region sequences were aligned and resulted in an alignment length of 1,650 bases. In a neighbor-joining tree generated from the aligned sequences, we placed the serotype strains representing Bradyrhizobium elkanii with strains representing Bradyrhizobium japonicum (Fig. 1). Placement of N. hamburgensis, Afipia felis, Rhodopseudomonas palustris, and B. denitrificans was proximal to the genus Bradyrhizobium. However, B. denitrificans IFAM 1005 (LMG 8443) and the A. indica isolates formed a distinct group separated from the other subgroup 2b genera.
FIG. 1.
Evolutionary relationship reconstructed from sequence divergence of the ITS region. Jukes-Cantor distances were derived from the aligned sequences to construct an unrooted tree by using the neighbor-joining method. The sequences were aligned by using the PILEUP program in the Wisconsin package of the Genetics Computer Group, and the aligned sequences were analyzed by using the MEGA package, version 1.01 (11). Five hundred permutations of the data set were generated in a bootstrap analysis to derive a majority rule consensus tree. The levels of support for the presence of nodes above a value of 90% are indicated in the neighbor-joining tree. The sequences for Bradyrhizobium japonicum USDA 122, USDA 123, USDA 124, USDA 125, USDA 126, USDA 127, USDA 129, USDA 135, USDA 38, USDA 4, USDA 62, and USDA 110; for Bradyrhizobium elkanii USDA 130, USDA 31, USDA 46, USDA 76, and USDA 94; and for M. extorquens ATCC 14718 were obtained from GenBank accession numbers AF208503, AF208504, AF208505, AF208506, AF208507, AF208508, AF208509, AF208511, AF208514, AF208515, AF208517, Z35330, AF208510, AF208512, AF208516, U35000, AF208518, and AF293375, respectively.
Nodulation tests.
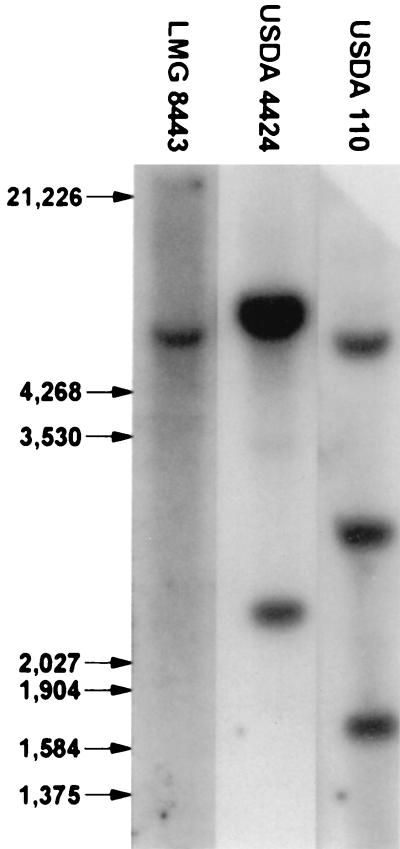
Plant tests were done to evaluate whether B. denitrificans was able to form a symbiosis with A. indica since, on the basis of rRNA sequence similarities, it is closely related to isolates originating from nodules of this legume host plant. All three (LMG 8443, USDA 4424, and BTAi1) nodulated A. indica, whereas uninoculated control plants formed no nodules. Nitrogen fixation in the inoculated plants was evident by their increased growth and by the presence of acetylene reduction activity in their roots (Table 2). The symbiotic response of B. denitrificans was similar to that of USDA 4424 and was superior to that of BTAi1. The ability of B. denitrificans to express nitrogenase activity probably was due to the presence of a DNA region with homology to nifHDK of Sinorhizobium meliloti cloned in pDC4 (Fig. 2). The B. denitrificans plant test was repeated two additional times. In each case B. denitrificans nodulated A. indica, whereas the uninoculated plants developed no nodules. Isolations were subsequently made from nodules of the plants inoculated with B. denitrificans, and the identity of the nodule occupants was confirmed because the ITS region sequences of B. denitrificans and the nodule isolates were identical (data not shown) and because these isolates had the ability to propagate by budding (Fig. 3).
TABLE 2.
Nitrogen fixation by symbioses of A. indica and B. denitrificans (LMG 8443) compared to two other strains isolated from nodules of this host legumea
| Strain | Plant dry wt (mg/plant) | Total plant nitrogen (mg/plant) | Acetylene reductionb (μmol of CH2H4/plant) |
|---|---|---|---|
| LMG 8443 | 302 A | 11.7 A | 11.5 A |
| USDA 4424 | 299 A | 11.1 A | 4.7 B |
| BTAi1 | 241 B | 7.3 B | 6.5 B |
| Control | 44 C | 0.4 C | 0 C |
Values in the same column flanked by the same capital letter are not significantly different at the 5% level of probability as determined by Duncan's new multiple range test.
That is, nitrogenase activity.
FIG. 2.
Southern hybridization analysis with nifHDK genes of S. meliloti cloned in pDC4 as probe targeting B. denitrificans (LMG 8443), A. indica isolate USDA 4424, and Bradyrhizobium japonicum USDA 110 DNA. Molecular sizes in base pairs are indicated in the left margin.
FIG. 3.
Electron micrographs of B. denitrificans LMG 8443 after isolation in culture from nodules of A. indica.
DISCUSSION
In this study we estimated the phylogenetic relationships among Bradyrhizobium strains originating from either soybeans or Aeschynomene and compared their evolutionary relationships with several closely related nonrhizobial genera. Strains representing 17 different serogroups of the soybean bradyrhizobia were included (26). We also examined sequence divergence among the ITS regions in these strains because these loci appear to evolve more rapidly than the 16S genes in the same operons (3). The ITS region was previously used to demonstrate considerable genetic variation among a collection of bradyrhizobial isolates of soybean (26). The significance of our results is that the relative placements of members within subgroup 2b of the α-Proteobacteria differed between reconstructions derived from 16S and ITS region sequence divergence. Additional loci will need to be examined to provide a more informed decision about the phylogenetic placements of B. denitrificans and the bradyrhizobial isolates of A. indica.
We predicted from our results that B. denitrificans could well be a symbiotic bacterium of A. indica, since it was placed very close to several isolates of A. indica in our estimates of phylogenies based on 16S rRNA gene and ITS region sequence divergence. We confirmed this hypothesis through several plant tests in which the type strain of B. denitrificans, IFAM 1005 (LMG 8443), nodulated and formed nitrogen-fixing symbioses with A. indica. Since this strain of B. denitrificans was originally isolated from lake water in Germany (8) and not from a plant nodule, there was no reason (besides the rRNA data) to suspect that it might also be a nitrogen-fixing symbiont of legume plants. These results demonstrate how evidence gathered from the study of molecular systematics can sometimes provide clues for predicting ecological characteristics of poorly understood species. Even though our prediction was accurate in this case, it is important to recognize the need for caution when associating a metabolic function with an organism from molecular systematic data alone (1).
From our result the natural tendency might be to view the bradyrhizobia of A. indica as Blastobacter species or to consider changing the genus Blastobacter to Bradyrhizobium. However, before such changes in nomenclature can be proposed it is important to examine both the taxonomic status of Blastobacter and the bradyrhizobia that include the phototrophs.
Zavarzin (35) originally proposed the genus Blastobacter and described the type species, B. henricii, from cells observed in lake water without isolating it in pure culture. Four additional isolates were examined and each was proposed as a separate species, B. aggregatus, B. capsulatus, B. denitrificans, and B. natatorius (23), each listed on the Approved Lists of Bacterial Names (18). However, the genus Blastobacter is highly heterogeneous since these described species fall into at least three distinct branches of the α-subdivision of the Proteobacteria (9). Therefore, Hugenholtz et al. (9) suggested that, with the exception of B. aggregatus and B. capsulatus, the different species should not be incorporated into the same genus. As a solution to the problems in the taxonomy in Blastobacter, Sly and Cahill (22) proposed that new genera be created for the validated species and transferred the type strain for B. natatorius to the new genus Blastomonas as the type strain Blastomonas natatoria. Therefore, from our results it would seem justified that the type strain for B. denitrificans (IFAM 1005, LMG 8443) either be transferred to an existing genus or become the type strain for a new genus.
The symbiotic bacteria of A. indica are unique because they nodulate the plant stems, branches, and roots; some produce photosynthetic pigments (2, 4, 5, 6). Based on these and other phenotypic characters, Eaglesham et al. (5) proposed that these photosynthetic symbiotic bacteria be classified as Photorhizobium thompsonianum with BTAi1 (USDA 4362) as the type strain. However, this proposal was not validated and, on the basis of 16S rRNA gene sequence comparisons, it was concluded that these bacteria would be more appropriately classified as Bradyrhizobium (18, 33, 34).
Except for our ITS sequence data, it would seem warranted to suggest that B. denitrificans be reclassified as a Bradyrhizobium, perhaps as a separate species from Bradyrhizobium japonicum, Bradyrhizobium elkanii, and Bradyrhizobium liaoningense. However, we propose that the taxonomy of B. denitrificans and the symbionts of the A. indica cross-inoculation group (2) be left unchanged until their phylogenetic placement in relationship with the genus Bradyrhizobium can be confirmed by using sequence divergence of other gene loci in addition to the 16S rRNA gene and the ITS region.
Acknowledgments
We thank Kenneth Lee Nash, Patrick Elia, and Charlie Murphy for excellent technical assistance.
REFERENCES
- 1.Achenbach, L. A., and J. D. Coates. 2000. Disparity between bacterial phylogeny and physiology. ASM News 66:814-815. [Google Scholar]
- 2.Alazard, D. 1985. Stem and root nodulation in Aeschynomene spp. Appl. Environ. Microbiol. 50:832-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barry, T., G. Colleran, M. Glennon, L. K. Dunican, and F. Gannon. 1991. The 16S/23S ribosomal spacer region as a target for DNA probes to identify eubacteria. PCR Methods Appl. 1:51-56. [DOI] [PubMed] [Google Scholar]
- 4.Eaglesham, A. R. J., and A. A. Szalay. 1983. Aerial stem nodules on Aeschynomene spp. Plant Sci. Lett. 29:265-282. [Google Scholar]
- 5.Eaglesham, A. R. J., J. M. Ellis, W. R. Evans, D. E. Fleischmann, M. Hungria, and R. W. F. Hardy. 1990. The first photosynthetic N2-fixing Rhizobium: characteristics, p. 805-811. In P. M. Gresshoff, L. E. Roth, G. Stacey, and W. E. Newton (ed.), Nitrogen fixation: achievements and objectives. Chapman & Hall, New York, N.Y.
- 6.Evans, W. R., D. E. Fleischmann, H. E. Calvert, P. V. Pyati, G. M. Alter, and N. S. Subba Rao. 1990. Bacteriochlorophyll and photosynthetic reaction centers in Rhizobium strain BTAi1. Appl. Environ. Microbiol. 56:3445-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evguenieva-Hackenberg, E., and S. Selenska-Pobell. 1995. Variability of the 5′-end of the large subunit rDNA and the presence of a new short class of rRNA in Rhizobiaceae. Lett. Appl. Microbiol. 21:402-405. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch, P., and M. Müller. 1985. Blastobacter aggregatus sp. nov., Blastobacter capsulatus sp. nov., and Blastobacter denitrificans sp. nov., new budding bacteria from fresh water habitats. Syst. Appl. Microbiol. 6:281-286. [Google Scholar]
- 9.Hugenholtz, P., E. Stackebrandt, and J. A. Fuerst. 1994. A phylogenetic analysis of the genus Blastobacter with a view to its future reclassification. Syst. Appl. Microbiol. 17:51-57. [Google Scholar]
- 10.Kordes, E., S. Jock, J. Fritsch, F. Bosch, and G. Klug. 1994. Cloning of a gene involved in rRNA precursor processing and 23S rRNA cleavage in Rhodobacter capsulatus. J. Bacteriol. 176:1121-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar, S., Tamura, K., and M. Nei. 1993. MEGA: molecular evolutionary genetics analysis, version 1.01. Pennsylvania State University, University Park.
- 12.Leonard, L. T. 1943. A simple assembly for use in the testing for cultures of rhizobia. J. Bacteriol. 45:523-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lessie, T. G. 1965. The atypical ribosomal RNA complement of Rhodopseudomonas sphaeroides. J. Gen. Microbiol. 39:311-320. [DOI] [PubMed] [Google Scholar]
- 14.Mackay, M. L., B. Zablen, C. R. Woese, and W. F. Doolittle. 1979. Homologies in processing and sequence between the 23S ribosomal ribonucleic acids of Paracoccus denitrificans and Rhodopseudomonas sphaeroides. Arch. Microbiol. 123:165-172. [Google Scholar]
- 15.Maidak, B. L., N. Larsen, M. J. McCaughey, R. Overbeek, G. J. Olsen, K. Fogel, J. Blandy, and C. R. Woese. 1994. The ribosomal database project. Nucleic Acids Res. 22:3485-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marrs, B., and S. Kaplan. 1970. 23S precursor ribosomal RNA of Rhodopseudomonas sphaeroides. J. Mol. Biol. 49:297-317. [DOI] [PubMed] [Google Scholar]
- 17.Molouba, F., J. Lorquin, A. Willems, B. Hoste, E. Giraud, B. Druyfus, M. Gillis, P. de Lajudie, and C. Masson-Boivin. 1999. Photosynthetic bradyrhizobia from Aeschynomene spp. are specific to stem-nodulated species and form a separate 16S ribosomal DNA restriction fragment length group. Appl. Environ. Microbiol. 65:3084-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore, W. E. C., and L. V. H. Moore. 1992. Index of the bacterial and yeast nomenclatural changes. American Society for Microbiology, Washington, D.C.
- 19.Navarro, R. B., A. A. T. Vargas, E. C. Schröder, and P. van Berkum. 1993. Uptake hydrogenase (Hup) in common bean (Phaseolus vulgaris) symbioses. Appl. Environ. Microbiol. 59:4161-4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuch, W., and U. E. Loening. 1975. The ribosomal ribonucleic acid of Agrobacterium tumefaciens. Biochem. J. 149:17-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selenska-Pobell, S., and E. Evguenieva-Hackenberg. 1995. Fragmentations of the large-subunit rRNA in the family Rhizobiaceae. J. Bacteriol. 177:6993-6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sly, L. I., and M. M. Cahill. 1997. Transfer of Blastobacter natatorius (Sly 1985) to the genus Blastomonas gen. nov. as Blastomonas natatoria comb. nov. Int. J. Syst. Bacteriol. 47:566-568. [DOI] [PubMed] [Google Scholar]
- 23.Trotensko, Y. A., N. V. Doronina, and P. Hirsch. 1989. Genus Blastobacter, p. 1963-1968. In J. T. Staley, M. P. Bryant, N. Pfennig, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology. The Williams and Wilkins Co., Baltimore, Md.
- 24.van Berkum, P. 1990. Evidence for a third uptake hydrogenase phenotype among the soybean bradyrhizobia. Appl. Environ. Microbiol. 56:3835-3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Berkum, P., and B. D. Eardly. 1998. Molecular evolutionary systematics of the Rhizobiaceae, p. 1-24. In H. Spaink, A. Kondorosi, and P. Hooykaas (ed.), The Rhizobiaceae. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 26.van Berkum, P., and J. J. Fuhrmann. 2000. Evolutionary relationships among the soybean bradyrhizobia reconstructed from 16S rRNA gene and internally transcribed spacer region sequence divergence. Int. J. Syst. Evol. Microbiol. 50:2165-2172. [DOI] [PubMed] [Google Scholar]
- 27.van Berkum, P., and C. Sloger. 1979. Immediate acetylene reduction by excised grass roots not previously preincubated at low oxugen tensions. Plant Physiol. 64:739-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Berkum, P., D. Beyene, and B. D. Eardly. 1996. Phylogenetic relationships among Rhizobium species nodulating the common bean Phaseolus vulgaris L. Int. J. Syst. Bacteriol. 46:240-244. [DOI] [PubMed] [Google Scholar]
- 29.van Berkum, P., R. E. Tully, and D. L. Keister. 1995. Nonpigmented and bacteriochlorophyll-containing bradyrhizobia isolated from Aeschynomene indica. Appl. Environ. Microbiol. 61:623-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Berkum, P., D. Beyene, G. Bao, T. S. Campbell, and B. D. Eardly. 1998. Rhizobium mongolense sp. nov. is one of three rhizobial genotypes identified which nodulate and form nitrogen-fixing symbioses with Medicago ruthenica [(L.)Ledebout]. Int. J. Syst. Bacteriol. 48:13-22. [DOI] [PubMed] [Google Scholar]
- 31.Weisburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winkler, M. E. 1979. Ribosomal ribonucleic acid isolated from Salmonella typhimurium: absence of the intact 23S species. J. Bacteriol. 139:842-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong, F. Y. K., E. Stackebrandt, J. K. Ladha, D. E. Fleischman, R. A. Date, and J. A. Fuerst. 1994. Phylogenetic analysis of Bradyrhizobium japonicum and photosynthetic stem-nodulating bacteria from Aeschynomene species grown in separated geographical regions. Appl. Environ. Microbiol. 60:940-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young, J. P. W., H. L. Downer, and B. D. Eardly. 1991. Phylogeny of the phototrophic Rhizobium strain BTAi1 by polymerase chain reaction-based sequencing of a 16S rRNA gene segment. J. Bacteriol. 173:2271-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zavarzin, G. A. 1961. Budding bacteria. Mikrobiologiya 30:952-975. [PubMed] [Google Scholar]