Abstract
Suspected infection requiring hospitalisation has highly heterogenous presentation. Yet, variances in host response and its implications are largely unknown. In this multicentre cohort of 3802 individual patients presenting to the Emergency Department (ED) with suspected infection requiring hospitalisation, we apply uniform manifold approximation and projections and K-means clustering to 29 plasma proteins to identify biologically discrete host response clusters. In this work, we first describe two large clusters, called “Dysregulated” and “Undifferentiated”, with abnormal protein concentrations and adverse outcomes in the former. Through further clustering, we identify 4 sub-clusters in the Dysregulated cluster, each with discrete biological signatures, clinical correlates, and outcomes. Clusters 3 and 4 are characterised by renal impairment and viral infections respectively. Clusters 5 and 6 are associated with bacterial culture positivity, with the former consistent with an immunosuppressed signature and worse outcomes, and the latter with gram-negative bacteria, higher IL-6 and IL-8, and better outcomes despite higher vasopressor use. These clusters are a biologically driven approach to characterising acute suspected infection and may lead to more targeted therapeutics.
Subject terms: Predictive markers, Translational research, Molecular medicine
Authors measure plasma proteins in a cohort of hospitalised patients presenting to the emergency department with suspected infection, revealing six discrete host response clusters that were driven by pathogen exposure and organ dysfunction, and had distinct clinical characteristics, hospital courses, and responses to treatment.
Introduction
Infections are the most frequent reason for patients presenting to the emergency department (ED) who require hospitalisation1, and is a major source of morbidity and mortality globally2–7. Supportive treatment and antibiotics aside, no new effective therapies targeting the host response have been proven, including in patients who develop sepsis and organ dysfunction, despite more than 100 randomised controlled trials in sepsis8. Increasingly, heterogeneity due to the broad clinical definition of sepsis is being implicated as a principal factor for these negative trials8,9. Host response to infection is varied and likely leads to heterogeneity that is meaningful to treatment response8,10,11, particularly when testing disease-modulating therapies. Our current approaches to classifying infection severity, including the Sepsis-3 definition12, incorporate neither the host’s biological response nor specific information of the pathogen, representing a barrier to advancing care.
Accordingly, investigators are increasingly seeking precision medicine approaches that aim to capture underlying subgroups or phenotypes in patients with sepsis, with a view to identifying treatment-responsive subgroups. Novel approaches to such schemes leverage machine learning algorithms to identify phenotypes of sepsis based on multivariate modelling that either uses clinical data13,14, molecular data15,16, or a combination of the two17. Albeit in the constraints of secondary analyses of clinical trials, several studies have shown differential treatment responses in these phenotypes13,17,18. While these phenotyping schemas are promising, most have been limited to critically ill patients later in their disease course, where, due to the high incidence of organ dysfunction and “bottleneck” activation of pathways leading to cellular death, host response may be less heterogeneous and less amenable to therapies19–21. Therefore, an important knowledge gap that we sought to address in our study is to evaluate the breadth of host response in patients suspected of infection presenting early in the emergency department (ED) across a broad range of severity, including among patients who have not met the clinical criteria for sepsis. To this end, whether patients with a dysregulated host response all meet sepsis criteria or whether all patients with suspected infection have a “dysregulated” response is also not known.
While, most prior protein-based phenotyping schemas have relied on 6–10 analytes for discovery, in this work, we leverage a panel of 29 protein biomarkers to characterise the landscape of the host response to infection. Further, we use this landscape to identify distinct biological clusters and leveraging granular electronic health record (EHR) data to describe their clinical characteristic and course and evaluate heterogeneity of treatment effect in the identified clusters.
Results
Study population
In total, 3802 patients were included in this analysis (Supplementary Fig. S1). Baseline demographics and characteristics of the population including their microbiology and outcomes are presented in Tables 1–3. The median age of the population was 66 (IQR: 54–77). Female sex constituted 1813/3802 (48%) of the population, and most patients were white, 2915/3802 (76.7%). The median worst SOFA score in the first 24 h was 2 (IQR: 0–4), and 50% of patients met the sepsis-3 criteria. The primary outcome of hospital discharge ≥ 7 days and/or in-hospital mortality was observed in 1456/3802 (38.3%). Overall, 207/3802 (5.4%) patients died in hospital, the median length of stay was 5 days (IQR: 3–9). 313/3802, (8.2%) required mechanical ventilation, and 386/3802 (10.2%) required vasopressors.
Table 2.
Microbiological characteristics of the patients included in the analysis (N = 3802)
| Microbiology – n (%) | |
|---|---|
| Bacterial culture positive | |
| All sites | 986 (25.9%) |
| Blood culture | 603 (15.9%) |
| Gram negative | 334 (8.8%) |
| Gram positive | 305 (8%) |
| Urine culture | 550 (14.5%) |
| Gram negative | 504 (13.3%) |
| Gram positive | 77 (2%) |
| Non-bacterial pathogens | |
| Viral | 41 (1.1%) |
| Fungal | 36 (0.9%) |
| Site of infection | |
|---|---|
| Circulatory | 54 (1.4%) |
| Central nervous system | 38 (1%) |
| Gestational | 15 (0.4%) |
| Gastrointestinal | 765 (20.1%) |
| Genitourinary | 1026 (27%) |
| Musculoskeletal | 120 (3.2%) |
| Respiratory | 909 (23.9%) |
| Skin | 433 (11.4%) |
| Other | 496 (13%) |
| Unknown site | 1156 (30.4%) |
Table 1.
Baseline characteristics of the patients included in the analysis (N = 3802)
| Demographics – n (%) | |
|---|---|
| Age, yrs | 66 [54,77] |
| Sex: Female | 1813 (47.7%) |
| Race | |
| White | 2915 (76.7%) |
| Black | 662 (17.4%) |
| Other | 225 (5.9%) |
| Site | |
| Beth Israel | 428 (11.3%) |
| Beaumont | 1023 (26.9%) |
| Mercy | 1561 (41.1%) |
| OSF | 790 (20.8%) |
| Body Mass Index, kg/ | 27.7 [23.2, 33.7] |
| Sepsis-3 criteria met | 1907 (50.2%) |
| SOFA score | 2 [0,4] |
| Vital Signs – median [IQR] | |
|---|---|
| Heart Rate, bpm | 100 [86, 115] |
| Respiratory Rate, bpm | 20 [18,24] |
| Temperature, C | 37 [36.6, 37.9] |
| Systolic Blood Pressure, mmHg | 116 [101, 133] |
| Diastolic Blood Pressure, mmHg | 66 [56,78] |
| SpO2/FiO2 | 452 [395, 462] |
| Laboratory Values – median [IQR] | |
|---|---|
| Lactate, mmol/L | 1.6 [1.2, 2.4] |
| Blood Urea Nitrogen, mg/dL | 20 [13,31] |
| Bilirubin, mg/dL | 0.6 [0.4, 1.0] |
| Creatinine, mg/dL | 1.1 [0.8, 1.7] |
| White Blood Cell Count, x/L | 11.3 [7.8, 15.8] |
| Platelets, x/L | 241 [178, 323] |
| Haemoglobin, g/dL | 11.7 [9.9, 13.3] |
| Comorbidities - n (%) | |
|---|---|
| Arrhythmia | 1213 (31.9%) |
| Chronic pulmonary disease | 1027 (27%) |
| Congestive heart failure | 1067 (28.1%) |
| Diabetes, complicated | 1073 (28.2%) |
| Hypertension, complicated | 1470 (38.7%) |
| Hypertension, uncomplicated | 1201 (31.6%) |
| Obesity | 859 (22.6%) |
| Other neurological disorders | 1023 (26.9%) |
| Renal failure | 1241 (32.6%) |
SOFA Sequential organ failure assessment score, IQR Interquartile range.
Table 3.
Primary and secondary outcomes of patients included in the final analysis (N = 3802)
| Outcomes – n (%) | |
|---|---|
| Discharged alive on or after day 7 or died in-hospital | 1456 (38.3%) |
| Died in-hospital | 207 (5.4%) |
| Mechanical ventilation ever | 313 (8.2%) |
| Vasopressors ever | 386 (10.2%) |
| Length of Stay, days – median [IQR] | 5 [3,9] |
Uniform manifold approximation and projections (UMAP)
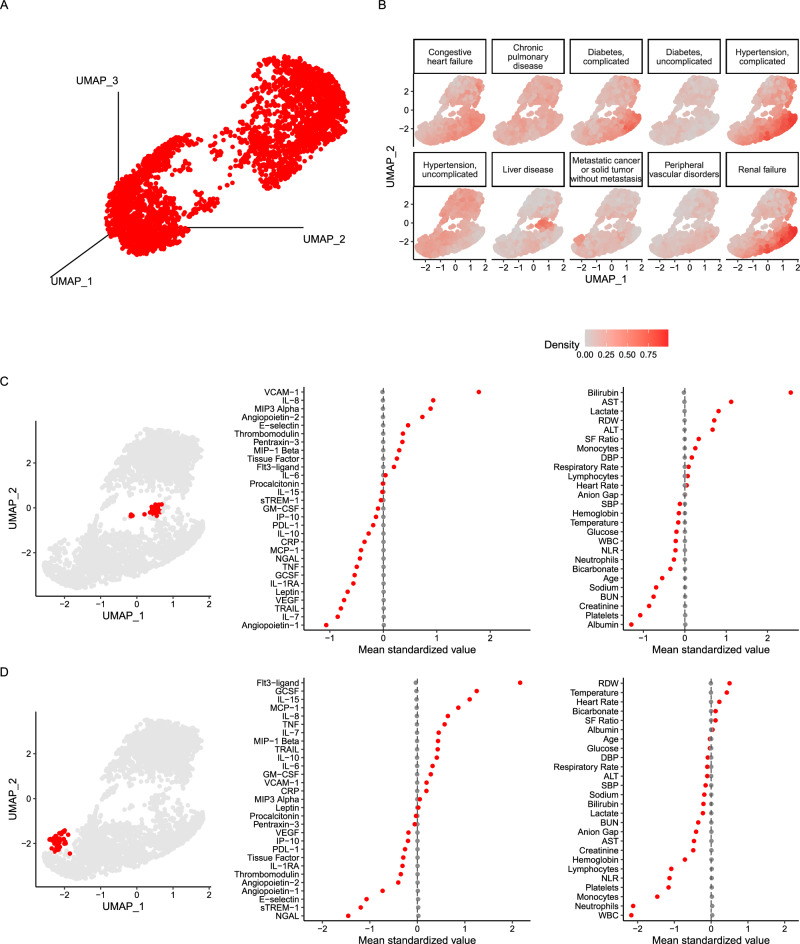
The baseline absolute values of the protein biomarkers are provided in Supplementary Table S1. Figure 1A shows the 3-D projection of the UMAP based on 29 biomarkers in the whole cohort, confirming that, according to plasma proteins, the studied population with suspected infection was highly heterogeneous. Projected positions on the UMAP did not differ according to study sites (Supplementary Fig. S2), but as anticipated, did differ according to recruitment volume, with greater density observed in the site with the highest number of patients (Mercy) and lowest in the site with the fewest patients (Beth Israel).
Fig. 1. UMAP of 29 plasma proteins in the population.
Panel (A): 3-dimensional representation of the UMAP of all 3802 patients included in the analysis. Panel (B): Distribution of frequently encountered comorbidities overlayed on a 2-dimensional representation of the UMAP. Panel (C): Highlighted area localised to past medical history of liver disease, alcohol abuse, and coagulopathy on a 2-dimensional representation of the UMAP (left panel; in red). Mean standardised values of protein biomarkers used in UMAP among patients in the area highlighted in the UMAP with a history of liver disease, alcohol abuse, and coagulopathy (centre panel). Mean standardised values of continuous clinical variables extracted from the electronic health record among patients in the area highlighted in the UMAP with a history of liver disease, alcohol abuse, and coagulopathy (right panel). Panel (D): Highlighted area localised to past medical history of solid tumour and or metastatic disease on a 2-dimensional representation of the UMAP (left panel; in red). Mean standardised values of protein biomarkers among patients in the area highlighted in the UMAP (centre panel). Mean standardised values of continuous clinical variables extracted from the electronic health record among patients in the area highlighted in the UMAP (right panel). Values were standardised to the mean values of the entire cohort for each variable in Panel (C and D). ALT Alanine Aminotransferase, AST Aspartate Aminotransferase, BUN Blood Urea Nitrogen, CRP C-reactive protein, DBP Diastolic blood pressure, FLT-3 ligand Fms-related tyrosine kinase 3 ligand, GCSF Granulocyte Colony-Stimulating Factor, GMCSF Granulocyte-Macrophage Colony-Stimulating Factor, IL Interleukin, IP-10 Interferon Gamma-Induced Protein 10, MCP Monocyte Chemoattractant Protein, MIP Macrophage Inflammatory Protein, NGAL Neutrophil Gelatinase-Associated Lipocalin, NLR Neutrophil:Lymphocyte, PDL-1 Programmed Death-Ligand 1, RDW Red Cell Distribution Width, SBP Systolic Blood Pressure, SF Ratio SpO2/FiO2, sTREM-1 Soluble Triggering Receptor Expressed on Myeloid Cells, TNF Tumour Necrosis Factor, TRAIL TNF-related apoptosis-inducing ligand, UMAPL Uniform Manifold Approximation and Projection, VCAM-1 Vascular Cell Adhesion Molecule-1, VEGF Vascular Endothelial Growth Factor, WBC White Blood Cell Count.
The distribution of frequently observed comorbidities is projected on UMAP in Fig. 1B. Notable discrete areas of projections were observed in patients with liver disease, renal failure, and tumours. Patients projected in the area associated with liver disease on the UMAP had higher levels of VCAM-1, IL-8, MIP-3α, ang-2, and lower ang-1, IL-7, TRAIL, and VEGF (Fig. 1C), with clinical features concordant with advanced liver disease with higher levels of bilirubin, liver enzymes, and lower platelet count and albumin (Fig. 1C). Patients projected in the area localised to tumour comorbidity had higher levels of FIT-3-ligand, GCSF, IL-15, MCP-1, and lower levels of NGAL and sTREM-1 (Fig. 1D). Clinically, the defining characteristics of patients projected in this area had lower white blood cell and platelet counts (Fig. 1D). The projection of the area with renal failure are discussed in the clustering portion of the analysis below.
Notably, demographics such as age categories, gender, and body mass index categories did not differ in their projection patterns beyond density of frequency (Supplementary Fig. S3). In race projections on the UMAP, the black race showed co-localisation to the UMAP area associated with renal failure comorbidity.
Phase 1 K-means clustering (Whole Cohort)
Consensus clustering metrics of the whole cohort UMAP suggested that the 2-cluster solution was optimal as it consistently had the lowest proportion of ambiguous clustering (PAC) across seeds (Supplementary Fig. S4). The 2-cluster solution was also congruous with the visual information observed from the UMAP, where two distinct regions of projections are easily identifiable (red/blue; Fig. 2A). The first cluster, referred herein as the “Dysregulated” cluster (N = 1973; 52%), had ubiquitously more deranged protein concentrations compared to the second cluster, herein referred to as the “Undifferentiated” cluster (N = 1829, 48%). The markers more elevated in the Dysregulated cluster included those associated with infection, inflammation, endothelial dysfunction, and coagulopathy (Fig. 2B; and Supplementary Table S2). Clinically, this group had higher values of markers of organ failure (e.g., creatinine and bilirubin), white blood cell count, and culture positivity (Fig. 2C and Supplementary Table S3). Primary outcomes and secondary outcomes were more frequently encountered in the Dysregulated cluster (Fig. 2D). Median SOFA scores (Dysregulated: 2 [1,5] vs Undifferentiated: 1 [0,2]; p < 0.001) and median length of stay (Dysregulated: 6 [4,10] vs Undifferentiated: 5 [3,8]; p < 0.001) were significantly higher in the Dysregulated cluster compared to the Undifferentiated cluster. Finally, we observed significant HTE (p = 0.032 interaction term of cluster and antibiotic delay), with increased risk for the primary outcome with delayed antibiotics in the Undifferentiated cluster but not in the Dysregulated cluster (Fig. 2E).
Fig. 2. Phase 1 K-means clustering of the UMAP.
Panel (A): The two clusters identified using K-means, the Dysregulated and Undifferentiated Clusters. Panel (B): Differences in mean standardised values of protein biomarkers used to create UMAP between patients in the Dysregulated and Undifferentiated Clusters. Panel (C): Differences in Mean standardised values of continuous clinical variables extracted from the electronic health record between patients in the Dysregulated and Undifferentiated Clusters. Values were standardised to the mean values of the entire cohort for each variable in Panels (B, C). Panel (D): Differences in primary and secondary outcomes between the Dysregulated and Undifferentiated Clusters. Panel (E): Odd ratio of developing the primary outcome (death or discharge > 7 days after admission) if antibiotics were delayed by more than three hours after order of blood cultures in the subset of patients who received antibiotics within 12 h after the time of the first vital sign (n = 2879). P-value is from the likelihood ratio test assessing the addition of the interaction term for cluster assignment and antibiotic delay status (yes/no) in a multivariable model with the primary outcome as the dependent variable. The odds ratios and corresponding 95% confidence intervals are shown with the point and error bars, respectively. ALT Alanine Aminotransferase; AST Aspartate Aminotransferase, BUN Blood Urea Nitrogen, CRP C-reactive protein, DBP: Diastolic blood pressure, FLT-3 ligand Fms-related tyrosine kinase 3 ligand, GCSF Granulocyte Colony-Stimulating Factor, GMCSF Granulocyte-Macrophage Colony-Stimulating Factor, IL Interleukin, IP-10 Interferon Gamma-Induced Protein 10, MCP Monocyte Chemoattractant Protein, MIP Macrophage Inflammatory Protein, NGAL Neutrophil Gelatinase-Associated Lipocalin, NLR Neutrophil:Lymphocyte, PDL-1 Programmed Death-Ligand 1, RDW ed Cell Distribution Width, SBP Systolic Blood Pressure, SF Ratio SpO2/FiO2, sTREM-1 Soluble Triggering Receptor Expressed on Myeloid Cells, TNF Tumour Necrosis Factor, TRAIL TNF-related apoptosis-inducing ligand, UMAPL Uniform Manifold Approximation and Projection, VCAM-1 Vascular Cell Adhesion Molecule-1, VEGF Vascular Endothelial Growth Factor, WBC White Blood Cell Count.
Phase 2: K-means clustering (Dysregulated/Undifferentiated)
Based on consensus clustering metrics, K-means clustering of the Undifferentiated cluster revealed two sub-clusters solution best fit this group of patients (Supplementary Fig. S5). The two clusters are represented in Fig. 3A. Cluster 1 (N = 1159; 30.5% total cohort) was defined by protein biomarker values among the lowest in the cohort and well below the cohort mean, and with the least abnormal clinical features (Fig. 3B and Supplementary Tables S4, 5). In Cluster 2 (N = 670; 17.6% total cohort), CRP values were higher than the cohort mean, and procalcitonin and IL-6 were at the mean values of the cohort. In contrast, means values of TRAIL, mip-1-β, and MCP-1 were lower in Cluster 2 compared to the rest of the cohort (Fig. 3C). Favourable outcomes were observed more frequently in Cluster 1 compared to Cluster 2, with fewer patients in hospital at day 7, fewer deaths, and lower rates of mechanical ventilation and vasopressor use (Fig. 3D); both of which were lower than the overall population rates in these two clusters.
Fig. 3. Phase 2 K-means re-clustering of the Undifferentiated cluster.
Panel (A): Depiction of the two subclusters identified in the Undifferentiated cluster, named Cluster 1 (light blue) and Cluster 2 (purple), on the 3-dimensional UMAP. Panel (B): Mean standardised values of protein biomarkers (left panel) and continuous clinical variables (right panel) in Cluster 1. Panel (C): Mean standardised values of protein biomarkers (left panel) and continuous clinical variables (right panel) in Cluster 2. Values were standardised to the mean values of the entire cohort for each variable in Panels (B and C). Panel (D): Differences in outcomes between Clusters 1 and 2. ALT Alanine Aminotransferase, AST Aspartate Aminotransferase, BUN Blood Urea Nitrogen, CRP C-reactive protein, DBP Diastolic blood pressure, FLT-3 ligand Fms-related tyrosine kinase 3 ligand; GCSF Granulocyte Colony-Stimulating Factor; GMCSF Granulocyte-Macrophage Colony-Stimulating Factor; IL Interleukin; IP-10 Interferon Gamma-Induced Protein 10; MCP Monocyte Chemoattractant Protein; MIP Macrophage Inflammatory Protein; NGAL Neutrophil Gelatinase-Associated Lipocalin; NLR Neutrophil:Lymphocyte; PDL-1 Programmed Death-Ligand 1; RDW Red Cell Distribution Width; SBP Systolic Blood Pressure; SF Ratio SpO2/FiO2; sTREM-1 Soluble Triggering Receptor Expressed on Myeloid Cells; TNF Tumour Necrosis Factor; TRAIL TNF-related apoptosis-inducing ligand; UMAPL Uniform Manifold Approximation and Projection; VCAM-1 Vascular Cell Adhesion Molecule-1; VEGF Vascular Endothelial Growth Factor; WBC White Blood Cell Count.
In the Dysregulated cluster, consensus clustering and PAC values showed that the 2-, 3-, or 4-cluster were all among the best fitting solutions (Supplementary Fig. S6). Subsequently, analysis of the merits of the biological characteristics of each clustering solution, we determined the 4-cluster solution provided the best biological discrimination for the dysregulated cohort and we selected this as the final sub-clustering solution (Fig. 4A). Differences in characteristics of these subclusters are presented in Supplementary Table S6, 7. Cluster 3 (N = 558; 14.7% total cohort) was characterised by higher concentrations of tissue factor, thrombomodulin, sTREM-1, NGAL, and angiopoietin-2. Notably, concentrations of proinflammatory cytokines such as IL-6, IL-8, and IL-1RA were close to the population mean (Fig. 4B, left panel). Clinically, this cluster was most notable for elevated creatinine, urea, and lower haemoglobin, suggesting that renal failure was an important driver of this cluster (Fig. 4B, right panel). Cluster 4 (N = 337; 8.9% total cohort) was associated with elevated concentrations of GMCSF, IP-10, TRAIL, IL-15, and FIT-3-ligand, and lower concentrations of NGAL and sTREM-1 (Fig. 4C, left panel). Notably, in this subcluster, white cell count, including neutrophil and lymphocyte counts, were lower (Fig. 4C, right panel). Cluster 5 (N = 663; 17.4% total cohort) was biologically characterised by higher concentrations of procalcitonin, IL-1RA, C-reactive protein, e-selectin, and IL-6, among several other biomarkers (Fig. 4D, left panel). Clinically, this cluster was notable for elevated white blood cell and neutrophil count and lower lymphocyte count relative to the rest of the population (Fig. 4D, right panel). Finally, Cluster 6 (N = 415; 10.9% total cohort) was biologically characterised by elevated MCP-1, GCSF, IL-10, IL-6, MIP-1-α, TNF-α, IL-1RA, and IL15 (Fig. 4E, left panel), with the most notable clinical features being the lowest lymphocyte counts among all the clusters and the highest temperature (Fig. 4E, right panel). The median SOFA scores in each cluster were: Cluster 3 (4 [2,5]), Cluster 4 (1 [0, 3]), Cluster 5 (2[1,5]), Cluster 6 (2 [1,5]).
Fig. 4. Phase 2 K-means re-clustering of the Dysregulated cluster.
Panel (A): Depiction of the four subclusters identified in the Dysregulated cluster named Cluster 3 (orange), Cluster 4 (brown), Cluster 5 (green), and Cluster 6 (yellow) on the 3-dimensional UMAP. Panel (B): Mean standardised values of protein biomarkers (left panel) and continuous clinical variables (right panel) in Cluster 3. Panel (C): Mean standardised values of protein biomarkers (left panel) and continuous clinical variables (right panel) in Cluster 4. Panel (D): Mean standardised values of protein biomarkers (left panel) and continuous clinical variables (right panel) in Cluster 5. Panel (E): Mean standardised values of protein biomarkers (left panel) and continuous clinical variables (right panel) in Cluster 4. Values were standardised to the mean values of the entire cohort for each variable in Panels B–E. ALT Alanine Aminotransferase, AST Aspartate Aminotransferase, BUN Blood Urea Nitrogen; CRP C-reactive protein; DBP Diastolic blood pressure; FLT-3 ligand Fms-related tyrosine kinase 3 ligand; GCSF Granulocyte Colony-Stimulating Factor; GMCSF Granulocyte-Macrophage Colony-Stimulating Factor; IL Interleukin; IP-10 Interferon Gamma-Induced Protein 10; MCP Monocyte Chemoattractant Protein; MIP Macrophage Inflammatory Protein; NGAL Neutrophil Gelatinase-Associated Lipocalin; NLR Neutrophil:Lymphocyte; PDL-1 Programmed Death-Ligand 1; RDW Red Cell Distribution Width; SBP Systolic Blood Pressure; SF Ratio SpO2/FiO2; sTREM-1 Soluble Triggering Receptor Expressed on Myeloid Cells; TNF Tumour Necrosis Factor; TRAIL TNF-related apoptosis-inducing ligand; UMAPL Uniform Manifold Approximation and Projection; VCAM-1 Vascular Cell Adhesion Molecule-1; VEGF Vascular Endothelial Growth Factor; WBC White Blood Cell Count.
Outcomes in clusters 3–6 are presented in the Fig. 5A. Adverse outcomes were least frequently observed in Cluster 4, followed by Cluster 6, then 5, and were worse in Cluster 3. An exception to this was vasopressor use, which was highest in Cluster 6, followed by Clusters 5 and 3, with the lowest use in Cluster 4. Notably, differences in the mean standardised values of protein biomarkers between those who experienced the primary outcome compared to those who did not were distinct in each cluster and tended to be proteins that were highest for that cluster (Supplementary Fig. S7 and S8).
Fig. 5. Clinical characteristics of Clusters 3–6.
Panel (A): Differences in outcomes between Cluster 3–6. Panel (B): Distribution of positive cultures stratified by Gram staining projected onto the 2-dimensional UMAP, with subcluster areas depicted by encircled areas. Panel (C): Distribution of viral pathogens identified projected onto the 2-dimensional UMAP. Panel (D): Odds ratio of clinical features associated with each cluster generated in logistic regression models looking at all patients in the dysregulated group (n = 1973). The odds ratios and corresponding 95% confidence intervals are shown with the point and surrounding error bars. Panel (E): Differences in selected protein biomarkers between patients with positive blood cultures with Gram-negative species in Clusters 5 and 6. The middle line in each violin plot represents the median. Statistical significance was determined by Wilcoxon rank sum tests. Exact p-values: CRP (9.86e-16), GCSF (2.99e-18), IL-10 (5.84e-20), IL-15 (1.94e-10), IL-6 (1.11e-17), IL-7 (5.30e-11), MCP-1(1.55e-26), MIP3 Alpha (3.79e-10). CRP C-reactive protein, GCSF Granulocyte Colony-Stimulating Factor, IL Interleukin, MCP-1 Monocyte Chemoattractant Protein-1, MIP-3 Macrophage Inflammatory Protein-3, SOFA Sequential organ failure assessment score. *p < 0.05; **p < 0.01; ***p < 0.001.
Given the protein signature characteristics of these four clusters, we sought to evaluate their association with the microbiological data. Both by UMAP projections (Supplementary Fig. S8) and prevalence (Supplementary Table S8), the site of infection (derived from ICD-10 codes following the categories defined by Chan et al.)22, did not localise to specific clusters. Evaluating culture and/or viral PCRs positive organism groups, Cluster 4 was notable for higher viral infection, gram-positive cultures localised to Cluster 5, and gram-negative cultures localised most frequently to Cluster 6 (Fig. 5B). Detailed microbiological data stratified by Clusters are presented in Supplementary Tables S8, 9. In an adjusted logistic regression model, among clinical variables, microbiological data were most strongly associated with the sub-clusters. Cluster 4 was most strongly associated with viral PCR positivity, Cluster 5 was most associated with positive gram-positive and gram-negative cultures, and Cluster 6 with gram-negative cultures (Fig. 5C). These findings were exaggerated when the models were restricted to blood culture positive organisms for Clusters 5 and 6 (Supplementary Fig. S10), rather than culture positive at any site. Finally, in direct comparison of differences in protein biomarkers in gram-negative bacteraemic patients in Cluster 5 versus Cluster 6, Cluster 5 was associated with higher CRP, whereas IL-10, IL-6, IL-15, IL-8, MCP-1, MIP-1α, and MIP-1-β were all lower (Fig. 5E and Supplementary Fig. S11). These patterns of differences were also similar among all-comers in Clusters 5 and 6 (Supplementary Fig. S12). Differences in major pathogens observed in bacteraemic patients between Clusters 5 and 6 are presented in Supplementary Table S9.
Finally, sensitivity analyses performed after splitting the cohorts into discovery and validation cohorts resulted in the emergence of UMAPs and clusters as described above. Overlap in cluster membership for Clusters 1–6 in the discovery and validation cohorts with the corresponding clusters in the whole cohort analysis was 81% (Supplementary Fig. S13).
Discussion
To the best of our knowledge, ours is the first study to comprehensively measure and characterise plasma proteins in patients with suspected infection presenting to the ED who required hospitalisation. Based on protein signatures, our findings suggest that this population is highly heterogeneous with several distinct circulating protein signature groups. Specifically, we observed distinct pathogen- and host-specific biological clusters that have differing implications in terms of hospital trajectories and outcomes, and potentially, treatment responses. Further, our findings suggest these biological clusters may not be identified using only clinical features. Taken together, the findings of this study highlight the value of the presented analytical pipeline of multiple proteins and machine learning approaches to visualise and parse out the heterogeneity of host response observed in patients presenting to the ED with infection.
Based on the UMAP, the data were compelling that the studied population could be split into two clusters—one with protein biomarker concentrations much higher that the cohort mean (Dysregulated) and a second cluster with concentrations much lower than the cohort mean (Undifferentiated). As anticipated, outcomes between these groups differed, with worse outcomes in the Dysregulated cluster. However, it is notable that within these clusters existed sub-clusters where the outcomes were worse in an Undifferentiated sub-cluster (Cluster 2) compared to a sub-cluster in the Dysregulated cluster (Cluster 4), and comparable to a sub-cluster with highly elevated protein biomarkers (Clusters 5), despite protein biomarkers being substantially lower in Cluster 2. These findings reiterate that heterogeneity in outcomes exists even after these populations are split into more biologically uniform groups and that host response studied at cross-sectional timepoints, while informative, do not convey complete information.
An unexpected finding in the Dysregulated and Undifferentiated clusters was the observed HTE with delayed antibiotics, where the worse primary outcome was only observed in the Undifferentiated cluster, and not the Dysregulated cluster. This finding was against our a priori hypothesis, and in contrast to other studies, where the benefit of earlier antibiotics was more frequently observed in patients with shock and comorbidities23,24. Several factors may explain this finding: (1) the population captured in our study may be substantially different to other reported studies, including a lower overall incidence of shock; (2) the stratification schema that we present is unique and not previously evaluated; and (3) all such studies, including our own, are analyses of observational data and capture many measured and unmeasured confounders, limiting the validity of the findings. Beyond these factors, we hypothesise a potential explanation for these finding may be that in the Undifferentiated cluster bacterial proliferation may be the main driver of morbidity, whereas in the Dysregulated cluster host factors may be a more critical determinant of morbidity, which are less directly under the influence of timely antibiotics. Nevertheless, along with other studies, our study emphasises the importance of timely antibiotics and extends the heterogeneity of response in lesser studied subgroups of patients with relatively normal host biological response.
Another key finding of our study was that among the clinical determinants of cluster membership in the Dysregulated sub-clusters, pathogens were among the most highly associated features in three subclusters. Notably, Cluster 4 was associated with viral infections, Cluster 6 was highly associated with gram-negative organisms; however, gram-negative organisms were also highly prevalent in Cluster 5, but not as strongly associated. These findings suggest that infection with the same pathogen can elicit heterogeneous responses in the host and that there may be several pathophysiological pathways leading to these divergent responses.
It is unclear from our study whether the observed difference in responses is due to host- or pathogen-specific factors or both. For example, the relatively higher levels of pro- and anti-inflammatory cytokines in Cluster 6, along with a more robust neutrophilia, temperature response and increased vasopressor use may be indicative of a robust immune system and a ‘balanced’ host response25. In contrast, Cluster 5, which was associated with low lymphocyte count and lower levels of most cytokines than Cluster 6, but elevated CRP and procalcitonin concentrations, may represent a relatively immunosuppressed state26. The differences in the immunocompetence between the two clusters may explain why outcomes in Cluster 6 were better despite higher vasopressor use. Importantly, the concepts of immunocompetence versus immunosuppressed/exhausted response to sepsis are well described, their validity in human studies, or the ability to discriminate them clinically has so far proved elusive27. Our phenotyping schema may offer such a solution; however, the validity of these findings in Clusters 5 and 6 warrants investigation with functional immune assays in future studies. Finally, while the biological characteristics of Cluster 6, including its strong association with gram-negative bacteraemia and interleukin concentrations, suggest that this Cluster may be analogous to the Hyperinflammatory molecular phenotype of critical illness17, given the low mortality in this cohort, it seems more likely that these subgroups are not the same, and their discrepancies may be a feature of differences in terms severity of infection and associated end-organ failure, which was lower in this study compared to prior studies.
Our findings also highlight the role of organ failure in the observed plasma biological signatures. Our large sample size and analytic approach allowed us to identify relatively small but biologically uniform groups that manifest largely due to organ failure. Cluster 3, which may have been incorporated into another cluster (likely Cluster 6) if the sample size was smaller, had some characteristics similar to Clusters 5 and 6 with higher pro-inflammatory cytokines. However, we hypothesise that in large part the cytokine concentrations in Cluster 3 are driven by the renal failure, either due to endothelial activation due to hyperureaemia, which can lead to an increase in tissue factor release28, or due to reduced cytokine clearance29. Notably, Cluster 3 had a negative association with culture positivity, suggesting that chronic, rather than acute, kidney disease may feature prominently in this population. Likewise, liver failure also had a distinct host protein signature projected on the UMAP, albeit the numbers were insufficient to emerge as a distinct cluster during analyses. The observed pattern of elevated markers of endothelial dysfunction among these patients in our study are consistent with previously described studies30,31. Together, these findings highlight that within a more simplistic phenotyping schema of sepsis, some variance of the host signature may be explained by organ failure as opposed to acute infection.
Given the retrospective nature of this study, its findings should not be practice-changing. However, there are several important implications for future clinical practice, particularly in the field of precision medicine in acute infection. First, our study highlights that in the context of suspected infection, dysregulated responses can be observed without organ failure. This has important implications for capturing patients at earlier phases of the host response and facilitating insights into pathways of recovery. Second, approximately half the hospitalised patients failed to elicit a notable biomarker response (Clusters 1 and 2), it may be that these patients are low yield and could be excluded from future studies that seek to investigate host-response targeted therapies. Third, Cluster 4, which was associated with viral infection, could be used as a tool in conjunction with rapid pathogen diagnostics for de-escalation of antibiotics and/or enriching patients for clinical trials evaluating anti-viral therapies. Fourth, our data would suggest that CRP may be a non-specific indicator of underlying host response in this population, for example, CRP levels in Cluster 2 were twice the levels of Cluster 6, yet proinflammatory and endothelial cytokines levels in Cluster 6 were greater than Cluster 2 by an order of magnitude. These findings are consistent with prior studies that have found CRP indifferent in biomarker-derived inflammatory phenotypes in critical illness32. Finally, Clusters 5 and 6 represent distinct responses to bacterial infection but with likely differing pathophysiological responses, which we believe may be high-yield targets for immunomodulatory therapies. Whilst compelling as hypotheses, all these potential applications will need validating in prospective studies.
Our study has several strengths. The sample size was large, and granular EHR data were available to complement the protein biomarker panels that were measured. Our sample and data acquisition were early in the course of hospital presentation. Our analytical pipelines were rigorous and reproducible, including sensitivity analyses, which added to the validity of the findings and identified clusters. Our study also has several limitations. First, adverse outcomes in terms of mechanical ventilation, vasopressor use, and in-hospital mortality were relatively low in this population, limiting analyses evaluating the association of the biomarker signatures with mortality. Second, the analyses were performed on cross-sectional data at a single time point, and the implication of changing signatures longitudinally over time was not evaluated. While our study design enabled us to capture data early in the emergency department, it is worth noting that despite a relatively uniform window of data collection, at presentation, patients were likely at differing stages of their illness due to variability in the time spent from the onset of symptoms to hospital presentation. This heterogeneity is another important limitation of our study. Third, the availability of baseline (pre-admission) SOFA score in the cohort were sparsely available, we used the approach suggested in the official Sepsis-3 guidelines, and assumed all patients baseline to be zero12. This may have overestimated the prevalence of sepsis. Fourth, the interpretation of our proposed cytokine signatures may be influenced by two unmeasured confounders, namely, our broad inclusion criteria may have included patients that have no true infection, and we did not have data on whether individuals had received antibiotics prior to their presentation to the ED. Fifth, the overlap of our biological phenotyping schema with other clinically derived schemas were not systematically evaluated and needs further investigation. Finally, the best approach to translating the UMAP and identified clusters into the clinical space is yet to be determined and represents important next steps in our future investigations.
In conclusion, we present a novel biomarker approach for evaluating and clustering patients with suspected infection that present to the ED and are later hospitalised. Our algorithms have identified distinct and discrete biological signatures with divergent outcomes and hospital trajectories, identifying an intuitive approach to patient classification that may inform distinct therapeutic options in future studies.
Methods
Study population
This was an analysis of a multicentre observational cohort of adult patients (age ≥ 18 years) presenting to the ED with suspected infection. The cohort was derived from four centres: Mercy Health (St. Louis, MO) between August 2020 and May 2021, Beaumont Hospital (Royal Oak, MI) between August 2020 and October 2021, OSF St Francis Medical Centre (Peoria, IL) between August 2018 and June 2021, and Beth Israel Deaconess Medical Centre (Boston, MA) between March 2022 and July 2022. If patients had an order of a blood culture in the ED, they were eligible for inclusion in the cohort. Research or hospital personnel then evaluated for the presence of remnant samples from a lithium heparin with gel separator tube in the clinical lab. Patients with a sample that was available and collected were included as part of the study. As the host response in COVID-19 is known to differ markedly from other causes of infection33,34, and our prior work has characterised their biological signatures previously35, consequently, COVID-19 patients were excluded from the analysis. As a main objective of the study was to evaluate host response early in the hospital course, we only included patients with plasma samples available within 6 hours of their first recorded vital sign in the ED and ± 3 h of their first blood culture. Finally, patients with more than 30% of biomarker data missing were also excluded from the analysis. The Institutional Review Board (IRB) at each site waived consent (IRB 967987, IRB 1597481, IRB 2018-459, IRB 33409).
Data and sample extraction
Clinical data were extracted retrospectively from the electronic health record (EHR). These included demographics, coded ICD-10 diagnoses, medications, vital signs, clinical laboratory test results (e.g., chemistry laboratory testing results, lactate), infection-related laboratory measurements (e.g., complete blood cell count with differential, C-reactive protein, procalcitonin), secondary outcomes metrics, and relevant data to conduct adjudication (e.g., microbiology results), and relevant orders (e.g., antibiotic administration). SOFA scores were calculated using data from the first 24 h after the first vital sign. The respiratory component of the SOFA score was determined using SpO2/FiO2 (S/F) due to the paucity of PaO2 measurements in the ED (0: S/F 301 + ; 1: S/F 220–300; 2: S/F 141–219; 3: S/F 67–140; 4: S/F 0–66). To account for extreme outliers in SpO2, changes in SOFA respiratory were ignored if they persisted for less than 30 minutes. For microbiological culture data, only data from the samples acquired within the first 6 h of biomarker sample acquisition were used for analysis. For microbiological results, blood cultures from the first 72 h of hospitalisation were included and classified into four categories: Gram-negative, Gram-positive, fungal, and negative cultures. Coagulase-negative staphylococci and Corynebacterium species were excluded as contaminants36. Viral diagnoses were extracted based on polymerase chain reaction viral tests and ICD-10 codes. For the site of infection, data were extracted using a previously described ICD-10 codes categorisation22.
Residual plasma samples leftover from routine clinical blood draws comprising four 175 µL aliquots were collected, frozen at − 80 °C, and shipped to a central laboratory. Plasma samples were collected from lithium heparin with gel separator (light green top) tubes. For the purposes of this study, residual blood from clinical draws made in the emergency department closest to the acquisition of the blood cultures were used to quantify protein biomarkers. Further details of data extraction procedures, cleaning, and sample handling and processing are provided in the supplementary information.
Assay procedures
Plasma samples for eligible patients were used to quantify 43 protein biomarkers. Protein concentrations were measured using multiplexed Luminex MagPix assays developed by R&D Systems. Laboratory quality control procedures adhered strictly to the FDA Bioanalytical Method Validation Guidance for Industry to ensure data quality (https://www.fda.gov/media/70858/download). All assays were measured in duplicates in the same plate. A full list of the proteins measured for this study are presented in the supplementary information.
Clustering of biomarkers
A priori, and in line with our prior procedures35, each biomarker’s distribution was systematically evaluated to decide whether it would be included for the clustering analysis based on missingness, assay quality, and/or inconsistencies in missingness rates across sites. As a result, 14 biomarkers were excluded from the analysis. Of the measured 43 biomarkers, for the primary analysis, 29 protein biomarkers (Supplementary Table S1) were used to create uniform manifold approximation and projections (UMAPs) and to identify clusters. These 29 biomarkers have putatively been categorised into the following broad physiological categories: (1) Pro-inflammatory cytokines: Interleukin (IL)-6, IL-8, Tumour Necrosis Factor TNF-α; (2) Anti-inflammatory / immunoregulatory Cytokines: IL-10, IL-1RA, Programmed Death-Ligand-1 (PD-L1); (3) Endothelial dysfunction and vascular integrity: angiopoietin (ang)-1, ang-2, E-selectin, Vascular Adhesion Molecule (VCAM)-1, Vascular Endothelial Growth Factor (VEGF), and thrombomodulin; (4) Acute phase reactants and systemic infection: C-Reactive Protein (CRP), procalcitonin, and pentraxin-3; (5) Myeloid cell activation and chemotaxis: Granulocyte Colony-Stimulating Factor (GCSF), Granulocyte-Macrophage Colony-Stimulating Factor (GMCSF), Interferon Gamma-Induced Protein (IP)-10, Monocyte Chemoattractant Protein (MCP), Macrophage Inflammatory Protein (MIP)-1β, MIP-3α, soluble Triggering Receptor Expressed on Myeloid cells (sTREM)-1, Tissue Factor; (6) Immune recovery / lymphocyte support: IL-7, IL-15, and Fms-related tyrosine kinase 3 (Fit-3)-ligand; (7) Other cytokines: Tumour necrosis factor-related apoptosis-inducing ligand (TRAIL), leptin, Neutrophil Gelatinase-Associated Lipocalin (NGAL).
As the protein biomarkers showed collinearity, first, principal component analysis (PCA) was used to minimise correlation across groups of protein biomarkers by creating uncorrelated linear combinations of the variables, and the dimension of the data was reduced to only include components that explained 95% of the total variation. Next, a UMAP was created to further reduce the selected PCA components into a 3-dimensional plot where each dot represented a single patient in the cohort. The rationale of the UMAP was to enable visualisation of the biological characteristics of the population from the standpoint of the biomarkers. Once the UMAP was constructed, demographics, comorbidities, and infection site and culture data were projected onto the UMAP. Finally, to determine underlying clusters, i.e., phenotypes with homogeneous biological characteristics, K-means consensus clustering was used in the low-dimensional UMAP space. Motivated by the findings of the initial K-means clustering solution, we performed a second phase of K-means analysis37,38. Phase 1 was K-means clustering on the UMAP of the whole population, and given that visually there appeared more unexplained heterogeneity within the identified clusters, in Phase 2, K-means clustering was performed independently on the clusters identified in Phase 1. Clinical data and outcomes were not used for the UMAP creation and consequently were not a factor for clustering either.
As a sensitivity analysis to evaluate the robustness of our clustering solutions, the whole cohort was split into discovery (Mercy and OSF) and replication cohorts (Beaumont and Beth Israel), and the above procedures (PCA, UMAP, and clustering) were performed separately in each cohort to compare agreement. Details of the procedures used for dimensionality reduction, UMAP, and clustering, including feature processing, transformation, and imputation, are provided in the supplementary information.
Outcomes
The primary adverse outcome for the analyses was hospital discharge on or after 7 days or in-hospital death. Secondary outcomes included vasopressor use, mechanical ventilation and in-hospital death.
Statistical analysis
Continuous variables are presented as median values with inter-quartile range (IQR), and categorical variables are presented as counts with percentages (%). The Kruskal-Wallis test was used for continuous variables and chi-squared for categorical variables. All hypothesis tests were two-sided unless otherwise specified; for tests based on chi-squared statistics, the rejection region was confined to the upper tail due to the non-negativity of the test statistic. To aide visualisation, the UMAP was plotted in 2 dimensions with points coloured based on k-nearest neighbours (knn)-based density in the 3-dimensional space. To assess for heterogeneity of treatment effect (HTE) with delayed antibiotics, defined as a delay in first sepsis antibiotics > 3 h after first vital sign, we created two groups. The first group were all patients that received delayed antibiotics, and the second was a corresponding group that comprised patients that received timely antibiotics, built using propensity matching, factoring in demographics, physiological abnormalities, and co-morbidities (details in the supplementary information). A logistic regression model was built to test for HTE with the primary outcome (death or hospital discharge ≥ 7 days) as the dependent variable and the interaction of the delayed antibiotics group and cluster allocation (undifferentiated vs dysregulated) as the independent variable.
All statistical analyses were performed using R version 4.2.3. Key packages are listed in the supplementary information.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
The study was funded by the following grants R35GM142992, R01HL173531 (P.S.), R35GM145330 (M.M.C.), K23GM144867 (S.B.).
Author contributions
P.S., A.B.S., M.M.C. and P.A.V. were responsible for study conception and design. P.S., A.B.S., M.M.C., S.B., G.L.W., C.L.E., A.B., L.S., B.R. and P.A.V. were responsible for the data cleaning and analysis. P.S., A.B.S., M.M.C., S.B., G.L.W., C.L.E., A.B., L.S., B.R., P.A.V., S.K., M.D.S., A.V.P., A.D., K.V.I., M.J.C., C.D., A.H., N.M., N.K., D.S., F.G., A.S., S.A., A.E., F.D., H.D., N.S.E. and S.S. were all responsible for data interpretation. P.S., A.S., M.M.C. and P.A.V. developed the first draft of the manuscript. All authors reviewed and edited the final version of the manuscript. P.S. and A.S. contributed equally.
Peer review
Peer review information
Nature Communications thanks Gustavo Sganzerla Martinez, Jesus F Bermejo-Martin, Willem Joost Wiersinga, Jean-Louis Vincent and the other anonymous reviewer for their contribution to the peer review of this work. A peer review file is available.
Data availability
The clinical and biomarker data are available under restricted access due to commercial confidentiality and data sharing legal agreements with Prenosis, Inc. Access can be obtained by submitting a request to Dr. Bobby Reddy Jr. Data access can be obtained for non-commercial, academic scientific research to be conducted on Prenosis’ servers. Data may be made available if the proposed use aligns with both the commercial and research objectives of Prenosis, subject to internal review and approval. Response to access requests will be made within 30 days of receipt, and the data will be made available for a period sufficient to complete the research investigation, typically 1 year.
Competing interests
The Authors declare the following competing interests: Drs. Sinha and Verhoef are consultants and have equity ownership in Prenosis Inc. Lopez-Espina, Bhargava, Watson, Schmalz, Khan, and Reddy. Jr are employed by Prenosis Inc. Dr. Sinha also consults for AstraZeneca. All other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Pratik Sinha, Alexandra B. Spicer.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-62848-x.
References
- 1.Salah, H. M. et al. Causes of hospitalization in the USA between 2005 and 2018. Eur. Heart J. Open1, oeab001 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy, J. L. et al. Infectious disease hospitalizations: United States, 2001 to 2014. Chest156, 255–268 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reinhart, K. et al. Recognizing sepsis as a global health priority - A WHO resolution. N. Engl. J. Med.377, 414–417 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Rhee, C. et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA318, 1241–1249 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudd, K. E. et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet395, 200–211 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torio, C. M. & Moore, B. J. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. (2006). [PubMed]
- 7.Wang, H. E. et al. Long-term mortality after community-acquired sepsis: a longitudinal population-based cohort study. BMJ Open4, e004283 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall, J. C. Why have clinical trials in sepsis failed? Trends Mol. Med.20, 195–203 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Bone, R. C. Why sepsis trials fail. JAMA276, 565–566 (1996). [PubMed] [Google Scholar]
- 10.Sinha, P., Meyer, N. J. & Calfee, C. S. Biological phenotyping in sepsis and acute respiratory distress syndrome. Annu. Rev. Med.74, 457–471 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavaillon, J. M., Singer, M. & Skirecki, T. Sepsis therapies: learning from 30 years of failure of translational research to propose new leads. EMBO Mol. Med.12, e10128 (2020). Apr 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singer, M. et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA315, 801–810 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seymour, C. W. et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA321, 2003–2017 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhavani, S. V. et al. Temperature trajectory subphenotypes correlate with immune responses in patients with sepsis. Crit. Care Med.48, 1645–1653 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong, H. R. et al. Identification of pediatric septic shock subclasses based on genome-wide expression profiling. BMC Med.7, 34 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burnham, K. L. et al. Shared and distinct aspects of the sepsis transcriptomic response to fecal peritonitis and pneumonia. Am. J. Respir. Crit. Care Med.196, 328–339 (2017). Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinha, P. et al. Identifying molecular phenotypes in sepsis: an analysis of two prospective observational cohorts and secondary analysis of two randomised controlled trials. Lancet Respir. Med.11, 965–974 (2023). Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antcliffe, D. B. et al. Transcriptomic signatures in sepsis and a differential response to steroids. from the VANISH randomized trial. Am. J. Respir. Crit. Care Med.199, 980–986 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mira, J. C. et al. Sepsis pathophysiology, chronic critical illness, and persistent inflammation-immunosuppression and catabolism syndrome. Crit. Care Med.45, 253–262 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daviaud, F. et al. Timing and causes of death in septic shock. Ann. Intensive Care5, 16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deutschman, C. S. & Tracey, K. J. Sepsis: current dogma and new perspectives. Immunity40, 463–475 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Chan, H. K., Khose, S., Chavez, S., Patel, B. & Wang, H. E. Updated estimates of sepsis hospitalizations at United States academic medical centers. J. Am. Coll. Emerg. Physicians Open3, e12782 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hechtman, R. K. et al. Heterogeneity of benefit from earlier time to antibiotics for sepsis. Am. J. Respir. Crit. Care Med.209, 852–860 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han, X. et al. Identifying high-risk subphenotypes and associated harms from delayed antibiotic orders and delivery. Crit. Care Med.49, 1694–1705 (2021). Oct 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Poll, T., van de Veerdonk, F. L., Scicluna, B. P. & Netea, M. G. The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol.17, 407–420 (2017). Jul. [DOI] [PubMed] [Google Scholar]
- 26.Hotchkiss, R. S., Monneret, G. & Payen, D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat. Rev. Immunol.13, 862–874 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Poll, T., Shankar-Hari, M. & Wiersinga, W. J. The immunology of sepsis. Immunity54, 2450–2464 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Gondouin, B. et al. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int.84, 733–744 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Carrero, J. J., Yilmaz, M. I., Lindholm, B. & Stenvinkel, P. Cytokine dysregulation in chronic kidney disease: how can we treat it? Blood Purif.26, 291–299 (2008). [DOI] [PubMed] [Google Scholar]
- 30.Farshidpour, M., Pace, S. & Volk, M. L. The clinical value of angiopoietin-2 in liver diseases. Clin. Liver Dis.19, 244–247 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruno, C. M. et al. Circulating adhesion molecules in patients with virus-related chronic diseases of the liver. World J. Gastroenterol.11, 4566–4569 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinha, P. et al. Latent class analysis of ARDS subphenotypes: a secondary analysis of the statins for acutely injured lungs from sepsis (SAILS) study. Intensive Care Med.44, 1859–1869 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinha, P., Matthay, M. A. & Calfee, C. S. Is a “Cytokine Storm” relevant to COVID-19? JAMA Intern Med.180, 1152–1154 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Leisman, D. E. et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med.8, 1233–1244 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verhoef, P. A. et al. Analysis of protein biomarkers From hospitalized COVID-19 patients reveals severity-specific signatures and two distinct latent profiles with differential responses to corticosteroids. Crit. Care Med.51, 1697–1705 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhavani, S. V. et al. The development and validation of a machine learning model to predict bacteremia and fungemia in hospitalised patients using electronic health record data. Crit. Care Med.48, e1020–e1028 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma, P. C., Chan, K. C. & Chiu, D. K. Clustering and re-clustering for pattern discovery in gene expression data. J. Bioinform. Comput. Biol.3, 281–301 (2005). Apr. [DOI] [PubMed] [Google Scholar]
- 38.Namvar M., Gholamian M. R. & KhakAbi S. A two phase clustering method for intelligent customer segmentation. In Proceedings of the International Conference on Intelligent Systems, Modelling and Simulation (ISMS) (2010).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The clinical and biomarker data are available under restricted access due to commercial confidentiality and data sharing legal agreements with Prenosis, Inc. Access can be obtained by submitting a request to Dr. Bobby Reddy Jr. Data access can be obtained for non-commercial, academic scientific research to be conducted on Prenosis’ servers. Data may be made available if the proposed use aligns with both the commercial and research objectives of Prenosis, subject to internal review and approval. Response to access requests will be made within 30 days of receipt, and the data will be made available for a period sufficient to complete the research investigation, typically 1 year.