Abstract
Nitrate and nitrite concentrations in the water and nitrous oxide and nitrite fluxes across the sediment-water interface were measured monthly in the River Colne estuary, England, from December 1996 to March 1998. Water column concentrations of N2O in the Colne were supersaturated with respect to air, indicating that the estuary was a source of N2O for the atmosphere. At the freshwater end of the estuary, nitrous oxide effluxes from the sediment were closely correlated with the nitrite concentrations in the overlying water and with the nitrite influx into the sediment. Increases in N2O production from sediments were about 10 times greater with the addition of nitrite than with the addition of nitrate. Rates of denitrification were stimulated to a larger extent by enhanced nitrite than by nitrate concentrations. At 550 μM nitrite or nitrate (the highest concentration used), the rates of denitrification were 600 μmol N · m−2 · h−1 with nitrite but only 180 μmol N · m−2 · h−1 with nitrate. The ratios of rates of nitrous oxide production and denitrification (N2O/N2 × 100) were significantly higher with the addition of nitrite (7 to 13% of denitrification) than with nitrate (2 to 4% of denitrification). The results suggested that in addition to anaerobic bacteria, which possess the complete denitrification pathway for N2 formation in the estuarine sediments, there may be two other groups of bacteria: nitrite denitrifiers, which reduce nitrite to N2 via N2O, and obligate nitrite-denitrifying bacteria, which reduce nitrite to N2O as the end product. Consideration of free-energy changes during N2O formation led to the conclusion that N2O formation using nitrite as the electron acceptor is favored in the Colne estuary and may be a critical factor regulating the formation of N2O in high-nutrient-load estuaries.
Nitrous oxide (N2O) is, after molecular nitrogen, the most abundant nitrogen compound in the atmosphere. Global long-term measurement series of tropospheric N2O show an annual growth rate of about 0.25 to 0.31% year−1 (18, 48), indicating that current global sources exceed sinks (4). Nitrous oxide has an atmospheric lifetime of about 150 years and a large greenhouse warming potential (36). When century-long effects are considered, the greenhouse warming potential of N2O is 310 times greater than that of CO2 (1).
According to Mathews (26), known global sinks exceed known sources by 40%, which implies either the presence of unknown sources of N2O or the underestimation of already-known sources. Generally, global budgets do not include estimates of sources of N2O in estuaries and coastal seas. A recent study (2), however, showed that when estuarine and coastal regions were included, a considerable portion (approximately 60%) of the global marine N2O flux was from estuarine and coastal regions, mainly due to high emission from estuaries. High N2O concentrations and high fluxes to the atmosphere have been described in some estuaries and coastal seas (2, 3, 6, 14, 19, 24, 27, 28, 35, 39, 40, 41, 42, 43), although their significance as a proportion of the estuarine N load usually has not been clear.
In the Colne estuary, England, Robinson et al. (35) showed that the formation of N2O occurred principally in the surface layer of the bottom sediments, where the sedimentary pool of nitrate was recharged rapidly by transport of nitrate into the sediment from the overlying water. N2O formation by water column nitrification was negligible. The rate of benthic denitrification, and formation of N2O, decreased rapidly after tidal exposure, as the sedimentary nitrate pool ran down when it was no longer being recharged from the water column. A small residual rate of N2O efflux from the sediment surface remained, presumably driven by nitrification within the sediment, but the high rate of N2O emission was only reinstated upon reestablishment of denitrification from high nitrate availability when the sediment was submerged in tidal water (35).
The concentration of N2O in the water column correlated with the nitrate concentration (35), and rates of denitrification from the sediment to the water increased by addition of nitrate to the water column (7). However, little is known about the effect of nitrite on N2O and N2 emissions from estuaries, although there are indications in freshwater that nitrite may be important to N2O formation (16). Thus, the present study was undertaken to measure the rates of N2O flux from the bottom sediments of the Colne estuary on the east coast of England throughout the annual cycle, under both illuminated and dark conditions, in order to examine the relationship between N2O emission and nitrite and nitrate concentrations in estuarine waters. Laboratory experiments were also carried out to examine the role of nitrite in N2O and N2 formation in the estuarine sediment.
MATERIALS AND METHODS
Sampling sites.
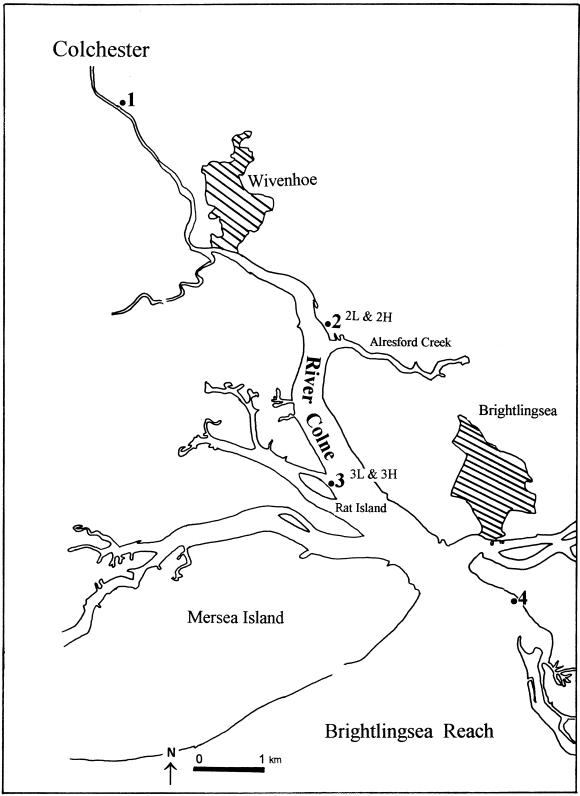
The Colne estuary is a small muddy mesotidal (3- to 5-m tidal range) estuary (Fig. 1) on the east coast of England, entering the North Sea at Brightlingsea (51°45′N, 1°3′E). The estuary catchment is 500 km2, of which the River Colne drains 300 km2, much of which is rich arable land. The estuary exhibits strong increasing gradients of both NO3− and NH4+ concentrations with distance upstream (20, 33) as a result of inputs from the River Colne and a major sewage treatment work at Colchester. Four benthic sampling sites were selected (Fig. 1), covering the full ranges of the estuarine nutrient gradients and sediment types along the estuary. At sites 2 and 3 in the central stretch of the estuary, where the largest areas of intertidal mud flat were found, both high-shore (mean high water) and low-shore (mean low water) stations (2H, 2L, 3H, and 3L) were sampled. The sediments from the high shore were tidally exposed to air periodically (about 10 h a day), and the sediments from the low shore were rarely exposed to air. The sediments at sites 1, 2, and 3 were fine silt, while at site 4, the sediments were clay with a very thin layer of fine muddy sand on the surface. The highest organic carbon content of the sediments was at site 1 (2 to 3% [dry weight]), and the lowest was at site 4 (0.4 to 0.5% [dry weight]) (for more detail, see reference 7).
FIG. 1.
Map of Colne estuary on the east coast of England showing positions of four sampling sites, with two stations (high shore and low shore) at sites 2 and 3 in the estuary.
In situ concentrations of N2O.
Samples of surface water (10 ml) were taken with a glass hypodermic syringe at low tide from four sites in the Colne estuary in February 1998 and run into an exetainer (Labco Ltd., High Wycombe, England) containing 100 μl of formaldehyde (38% [vol/vol]) to stop any further microbiological activity. Water temperature, atmospheric pressure, and salinity were also measured, as described previously (35). On our return to the laboratory, N2O concentrations in the water samples were measured in a gas chromatograph with an electron capture detector, as described by Robinson et al. (35). The dissolved N2O concentrations were calculated according to the method of Weiss and Price (49). Control samples of air were always taken when sampling so that the dissolved N2O concentrations could also be calculated as percentage saturation relative to the concentration of N2O in air.
In situ concentrations of nutrients.
Site water was collected at high tide at sites 1, 2, and 4 (at high tide, the nutrient concentrations and salinities at sites 3 and site 4 were the same). Samples of estuarine waters were filtered through glass fiber filter papers (GF/F; Whatman, Maidstone, United Kingdom) and frozen at −20°C for later analysis. The water samples were subsequently analyzed on a segmented flow autoanalyzer (Skalar Analytical B.V., Breda, The Netherlands) for nitrate, nitrite, ammonium, phosphate, and silicate (21).
Sediment-water fluxes of N2O and nutrients.
Monthly surveys were carried out to measure the rates of N2O flux and nitrate and nitrite flux across the sediment-water interface in the River Colne estuary from December 1996 to March 1998. Triplicate cores of sediment (approximately 30 cm long) were taken at low tide at each station with Perspex core tubes (8-cm internal diameter by 65-cm length). The cores were allowed to reequilibrate overnight immersed in a bath of site water taken at high tide. The water bath was maintained at in situ temperature by a thermocirculator circulating water through a heat exchanger coil in the bottom of the bath. The next day, the rates of N2O flux and nutrient flux were measured in the light (500 μmol · m−2 · s−1) and dark (7). The rationale for measuring fluxes in the light is that the photosynthesis and respiration of the microphytobenthos may change the redox in the surface layer of the sediment, and the competition of the microphytobenthos with nitrifiers for ammonium and denitrifiers for nitrate may directly or indirectly affect nutrient exchange and nitrous oxide fluxes across the sediment-water interface. At the start of the experiment (time zero), samples of water (10 ml) were taken from each core tube with a glass syringe and run into exetainers containing 100 μl of formaldehyde (38% [vol/vol]) for analyses of initial concentrations of N2O, and water samples (20 ml) for analysis of dissolved nutrients were also taken by filtration through glass fiber filters (GF/F) and frozen immediately at −20°C. Each completely water-filled core was then sealed with a butyl rubber bung from which a magnetic follower was suspended, extending into the water. The magnetic followers were driven (at 60 rpm) by a magnet on an external electric motor to maintain homogeneity and mixing in the water without resuspending the sediment surface. At the end of the incubation period (usually 2 h), each core tube was opened, and further samples of water were removed immediately from each tube for analyses of dissolved nutrient concentrations and N2O concentration and hence, by the difference, of the fluxes between sediment and water. Control cores with in situ water but no sediment were also incubated as described above. Their purpose was to check whether biological activity in the water column would cause any changes in the concentrations of N2O and nutrients in the water column.
Measurement of rates of denitrification and nitrous oxide production.
Multiple cores of sediment (≈10 cm deep) were taken in Perspex tubes (3.4-cm internal diameter by 22-cm length) from sites 1, 2, and 4 in the Colne estuary and were allowed to reequilibrate overnight immersed in a bath of N-free artificial seawater (Tropic Marin Centre Ltd., Rickmansworth, United Kingdom) with a salinity of 15‰. Nitrate or nitrite solution (10 mM) was added to every tube to give final concentrations in the water of 0, 50, 100, 200, 500, and 1,000 μM (three replicate cores at each concentration). For the cores from site 2, 15N-labeled nitrate or nitrite was also added to every core (final concentration, 110 μM 15NO3− or 15NO2−) so that denitrification could be measured by the paired-isotope technique (7, 32). The cores were allowed to equilibrate for 30 min at in situ temperature. Samples of water were then removed from above each core for analysis of the initial N2O and nutrient concentrations. The cores were then incubated at in situ temperature for 2 h. At the end of the incubation, the sediment and water in each core tube were rapidly but carefully mixed with a glass rod, without agitation of the liquid surface, to produce a slurry, and a sample of the slurry was removed with a glass hypodermic syringe into an exetainer containing 100 μl of formaldehyde (38% [vol/vol]) for subsequent measurement of dissolved N2O. (Preliminary experiments showed that there was no significant loss of dissolved N2O during this brief mixing and subsampling procedure.) The sample obtained after mixing showed the effect of either the nitrate or nitrite concentration on formation, rather than just emission to water, of N2O. For cores from site 2, a further sample of the slurry was removed with a glass hypodermic syringe into an exetainer containing 100 μl of ZnCl2 (50% [wt/vol]) for denitrification measurement.
Denitrification measured by isotope pairing.
The slurry samples were sent to the National Environmental Research Institute, Silkeborg, Denmark, for analysis of 28N2, 29N2, and 30N2 on a gas chromatograph coupled to a dual-inlet isotope ratio mass spectrometer (Europa Instruments, Crewe, United Kingdom). Denitrification rates were calculated according to the method of Nielsen 32; for more details, see reference 7).
Data analysis.
Multiple comparisons were made by analysis of variance using SYSTAT version 7 software (SPSS Inc., Chicago, Ill.).
RESULTS
In situ nitrate and nitrite concentrations.
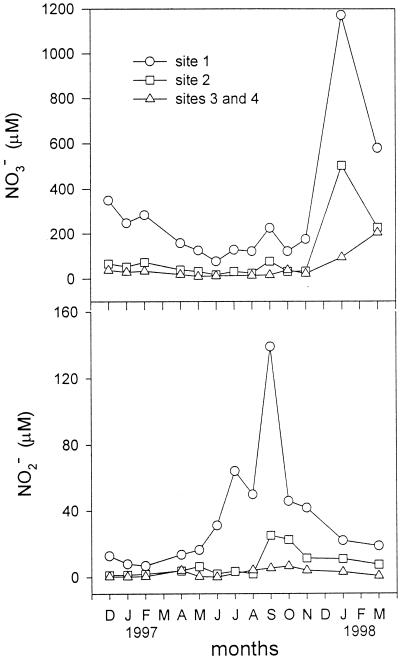
There was a decreasing gradient of nitrate and nitrite from the head to the mouth of the Colne estuary; nitrate was in the range of 76 to 1,176 μM at site 1, 16 to 504 μM at site 2, and 3 to 205 μM at sites 3 and 4 (Fig. 2). The nitrate concentration showed seasonal variation, with maximum concentration during early winter. The concentrations of nitrite at site 1 were high and exhibited distinct seasonal variation, with the maximum values in the summer. Some seasonal signal of nitrite concentration could still be seen at site 2 but was not detectable by site 4, where nitrite concentrations were generally <1 μM.
FIG. 2.
Nitrate and nitrite concentrations in water columns in Colne estuary. The data represent the means of three replicates.
In situ N2O concentrations.
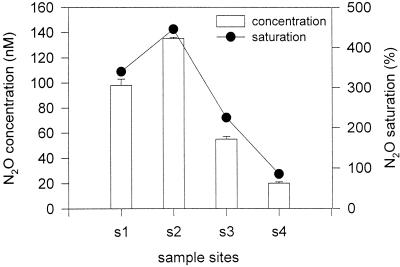
There was a pronounced N2O gradient in the water column along the Colne estuary. The concentrations were highest at site 2 and decreased further down the estuary (Fig. 3). Water column N2O concentrations were supersaturated at sites 1 to 3, and the saturations exhibited a gradient corresponding to the N2O concentrations in the water column, indicating these parts of the estuary were sources of atmospheric N2O.
FIG. 3.
Nitrous oxide concentrations in water columns and nitrous oxide saturation along Colne estuary. Water samples were collected in February 1998. The error bars indicate standard errors (n = 3).
Sediment-water fluxes of N2O.
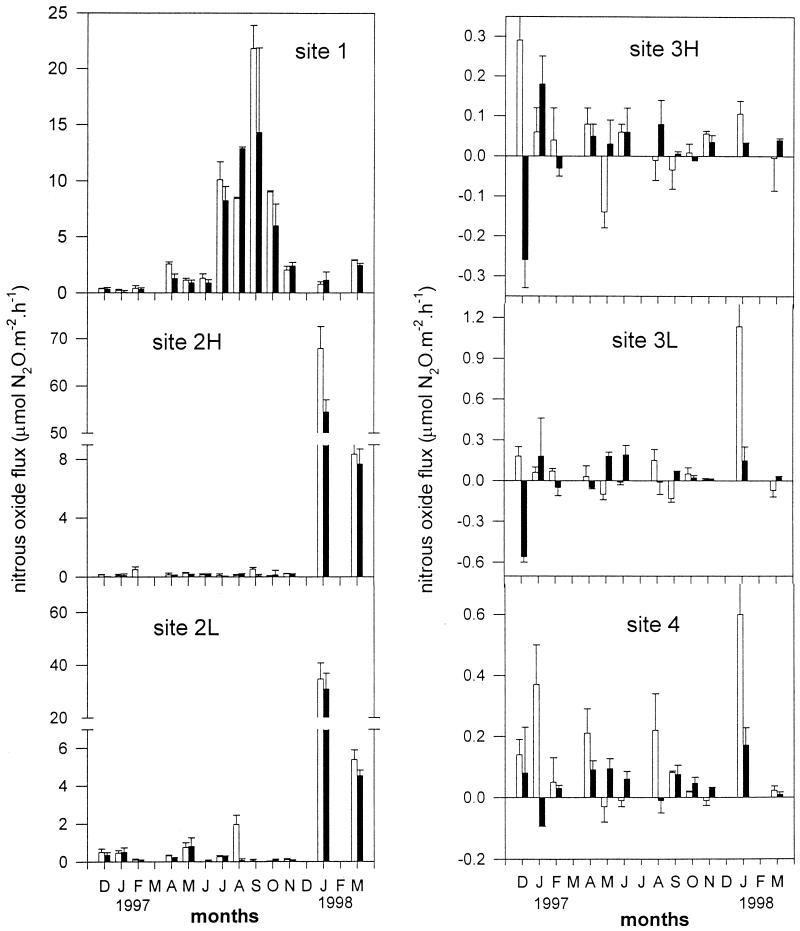
Concentrations of N2O in the control cores, with in situ water only, did not change during incubation. This means that any changes in N2O concentration in the cores with sediment were due to benthic activity. The highest exchange rates of N2O from the sediment to the water were at site 1 under both dark and light conditions during summer (Fig. 4). There were no significant (P < 0.05) differences between the rates under dark and light conditions at site 1. Distinct seasonal variation also occurred at site 1, with the highest rates of emission during the summer (July to October). At the other sites, N2O flux rates were much lower than at site 1 and decreased towards the seaward end of the estuary. Rates of N2O flux were similar in the low and high shore at sites 2 and 3. There was no consistent effect of illumination on N2O flux rates at these sites. There was also some evidence, particularly at sites 2 and 4, of large peaks of emission of N2O during the winter rather than the summer, coinciding with peak nitrate concentrations during the winter.
FIG. 4.
Rates of nitrous oxide flux across sediment-water interfaces at all sites in Colne estuary from December 1996 to March 1998. The error bars indicate standard errors (n = 3). The open and solid bars represent rates measured in the light and dark, respectively. H and L denote high-shore and low-shore stations.
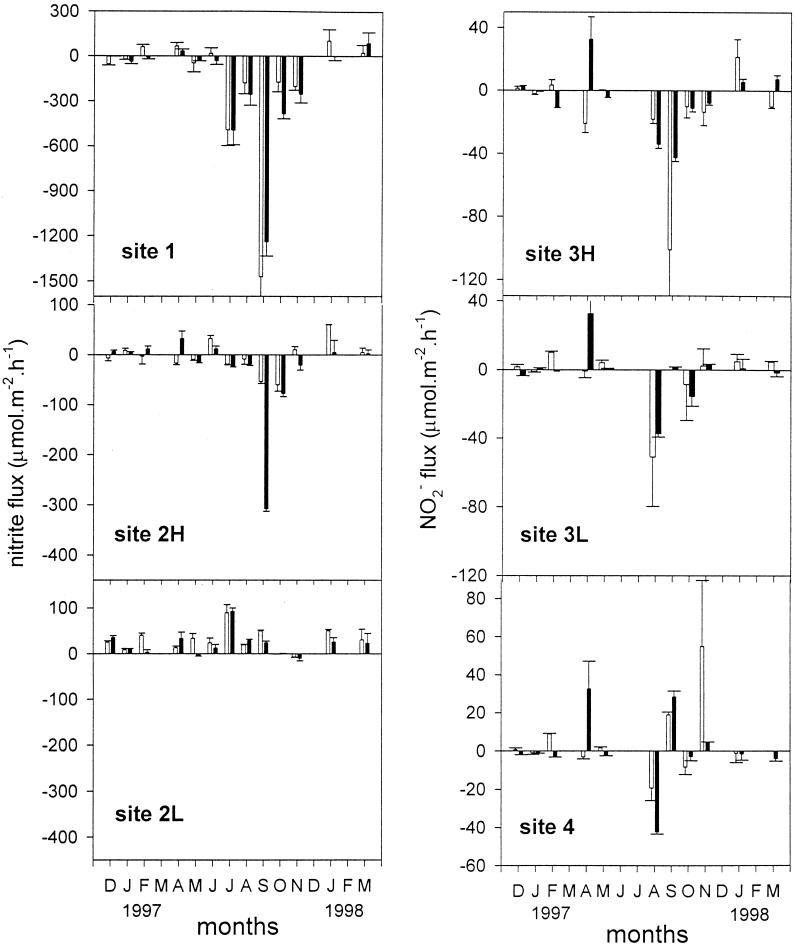
Nitrite exchange fluxes.
The sediment at site 1 in the Colne was predominantly a sink for nitrite from the water column, particularly during the summer (Fig. 5). The sediments at sites 2H and 3H were sinks for nitrite in most months, but at site 2L the sediments always emitted nitrite to the water column. At sites 3L and 4, the sediments were sinks in some months but sources in other months. Illumination had little effect on the rates of nitrite exchange. At site 1, higher rates of nitrite flux into the sediment in the summer corresponded to the higher concentrations of nitrite in the water column (Fig. 2 and 5).
FIG. 5.
Rates of nitrite flux across sediment-water interfaces at all sites in Colne estuary from December 1996 to March 1998. The error bars indicate standard errors (n = 3). The open and solid bars represent rates measured in the light and dark, respectively. H and L denote high-shore and low-shore stations.
Rates of denitrification with addition of nitrate and nitrite.
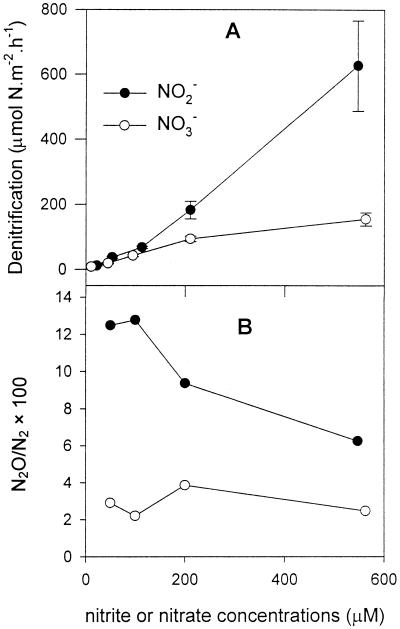
At site 2 in the Colne estuary, the addition of nitrate or nitrite to the water column stimulated the rates of denitrification, but the stimulation by nitrite was much greater than that by nitrate (Fig. 6A). The rates of denitrification responded to increases in nitrate concentration with Michaelis-Menten-type saturation kinetics, similar to our previous observation (7), but the rates increased almost linearly (r2 = 0.99) with nitrite concentrations up to the maximum concentration (550 μM) added.
FIG. 6.
(A) Rates of denitrification versus NO3− or NO2− concentrations with constant 110 μM 15NO3− (or 15NO2−) concentration in the overlying water column for sediment at site 2 of Colne estuary. Incubations were at 15°C in darkness using artificial seawater at a salinity of 15‰ in April 1998. The error bars indicate standard errors (n = 4). (B) Ratios of rates of nitrous oxide production and denitrification (N2O/N2 ×100) versus NO3− or NO2− concentrations in the water column.
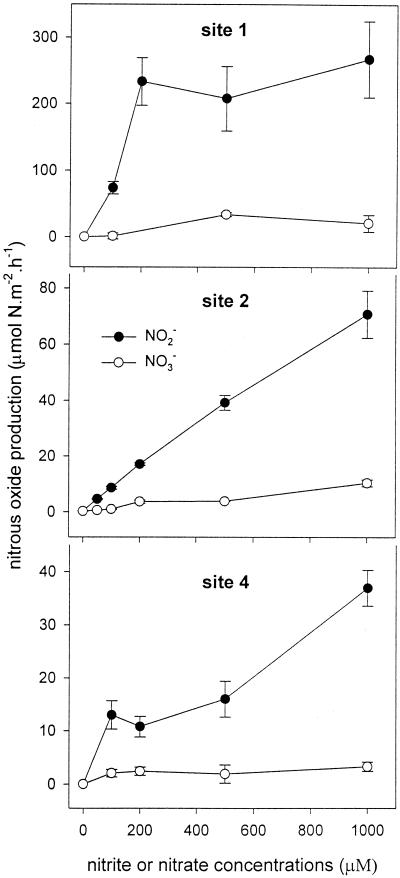
Stimulation of nitrous oxide flux by addition of nitrite or nitrate.
In the Colne estuary, the addition of either nitrite or nitrate increased the rate of N2O flux from the sediments at sites 1, 2, and 4 (Fig. 7; no data for site 3). The extents of the increase were about 10 times greater with nitrite than with nitrate. The highest rates of nitrous oxide flux were at site 1 (the head of the estuary), and the lowest rates were at site 4 (the mouth of the estuary). At site 1, the rates of nitrous oxide flux became saturated at about 200 μM nitrite, although at very high rates of N2O flux, while at sites 2 and 4, the rates increased almost linearly with nitrite concentration and no saturation was observed up to 1,000 μM nitrite.
FIG. 7.
Rates of nitrous oxide production across sediment-water interface versus NO3− or NO2− concentration in overlying water column for sediment at sites 1, 2, and 4 in Colne estuary. Incubations were at 15°C in darkness using artificial seawater at a salinity of 15‰ in April 1998. The error bars indicate standard errors (n = 3).
Ratios of nitrous oxide emission to dinitrogen gas emission.
The ratios of nitrous oxide emission to dinitrogen gas emission were calculated by dividing the rates of nitrous oxide production (Fig. 7, site 2) by the rates of denitrification (Fig. 6A). Increasing nitrate concentration did not vary the ratios (2 to 4%). With the addition of nitrite, the N2O/N2 ratios were significantly (P < 0.05) higher than with the addition of nitrate (Fig. 6B), and at lower nitrite concentrations (50 and 100 μM) the ratios were higher (12 to 13%) than those at higher nitrite concentrations (7 to 9%).
DISCUSSION
Annual survey.
In the estuarine sediments, N2O is produced from microbial nitrogen transformations. Generally, three microbial processes can lead to N2O production: denitrification (15, 22), nitrification (12, 15, 34, 50), and dissimilatory nitrate reduction to ammonium (44). Any factors which affect these processes would indirectly affect N2O production (9, 17). Denitrification is generally considered to be the primary estuarine source of N2O (13, 35). Ogilvie et al. (33) and Dong et al. (7) have shown that the relative importance of uncoupled denitrification (Dw), supported by nitrate transported into the sediments from the overlying water, and coupled nitrification-denitrification (Dn) in the sediments varied spatially from the head to the mouth of the Colne estuary, with 10 to 40% of the total denitrification (Dw + Dn) being from coupled nitrification-denitrification at the head of the estuary (site 1) and 25 to 80% of the total being from coupled nitrification-denitrification at the mouth of the estuary (site 4) (7). This indicates the greater importance of denitrification than of nitrification as a possible source for N2O at the head of the estuary, but at the mouth, nitrification may become more important as a possible source for N2O. Maximum rates of N2O emission at site 1 in the summer (Fig. 4) corresponded closely to the maximum rates of uncoupled denitrification (7), indicating that N2O was primarily derived from nitrate or nitrite reduction, not via nitrification. Moving seaward down the Colne estuary, nitrification may become more important as a source of N2O. It is probable that at site 4 the small net emission of N2O was from nitrification in the sandier sediments, but the rate of emission at site 4 was trivial compared with the high rates of N2O emission where uncoupled denitrification rates were high, as at site 1. However, accurate quantification of the portions of N2O from nitrification and from denitrification remains a topic for further study.
The concentrations and saturations of N2O in the overlying water column may indicate whether the sediments are sinks or sources of nitrous oxide. The supersaturations of N2O measured in most parts of the Colne estuary (Fig. 2) were consistent with the results of our previous study (35). Trimmer (unpublished data) found previously that supersaturations of N2O were also observed at all sites in the nearby Great Ouse estuary, England. The phenomenon of supersaturation of N2O has also been reported in other estuaries (2), up to 2,550% in the Potomac estuary (27) and 4,000% in the Humber estuary on the east coast of England (3). Generally, N2O saturations in estuarine waters were much higher than in the coastal waters and marginal seas (2, 3).
It was notable that the peak of the N2O flux rate in the Colne estuary occurred during the summer, coinciding with the seasonal peak of nitrite concentrations in the water column, not with nitrate concentrations, which peaked in early winter. Nitrite concentrations in estuarine waters are usually relatively small compared to those of nitrate or ammonium (33, 47) and have tended to be neglected in any considerations of the estuarine nitrogen budgets. Nitrite is often pooled together with nitrate as total oxidized nitrogen. Dissimilatory nitrate reduction in anaerobic sediments is believed to be responsible for nitrite accumulation in rivers (16). The degree to which nitrite is produced during nitrate reduction is influenced by the balance between electron donors and electron acceptors (17) and also by temperature (25; D. L. Lloyd and D. B. Nedwell, unpublished data). In the present study, we observed high nitrite concentrations in the water column during the summer of 1997 at site 1 in the Colne estuary. It is unlikely that high NO2− concentrations in the water column were due to export from the sediments, since there were net fluxes of nitrite from the water column into the sediment at site 1 in the summer (Fig. 5), but rather resulted from inputs of poorly nitrified effluent from Colchester Sewage Treatment Works.
The nitrite flux from the water column into the sediment (Fig. 5) and N2O flux out of the sediment (Fig. 4) were significantly (P < 0.05) correlated with the nitrite (but not nitrate) concentrations in the water column at site 1 (Fig. 2) and also with the rates of denitrification at site 1 (7). The sediments in the high intertidal of sites 2 and 3 were sinks for NO2− for most months, while sediments at other sites were relatively weak sources of nitrite efflux to the water (Fig. 5).
Experiments.
Addition of nitrite to the water column above the sediments greatly stimulated N2O production in the sediments at the three sites in the Colne estuary (Fig. 7). The extent of stimulation by enhanced nitrite was much greater than that by enhanced nitrate (Fig. 7). Sediment organic content and nitrate, nitrite, and ammonium concentrations in the overlying water column at site 1 were much higher than those at site 4 (7). Denitrifier and nitrifier communities in the sediment at site 1 would be expected to be adapted to such a high-nutrient environment and to show a much higher potential for N2O formation. This may be an explanation for the observation that the rates of N2O emission were much higher at the head than at the mouth of the Colne estuary (Fig. 7). Firestone and Tiedje (8) reported that a high concentration of nitrite inhibited the enzyme nitrous oxide reductase, leading to nitrous oxide accumulation in soil. However, no inhibitory effect of nitrite on nitrous oxide reductase could be inferred in the present study, since dinitrogen production during denitrification was also greatly stimulated by increased nitrite (Fig. 6A).
The nitrate concentration usually limits the rates of benthic denitrification (5, 7, 29, 30, 31, 33, 37). Thus, it is not surprising that addition of nitrate increased the rates of denitrification (Fig. 6A). However, the stimulation of denitrification by addition of nitrite was approximately 10 times higher than that by enhanced nitrate (Fig. 6A). There are two possible explanations for this observation. There may be a group of nitrite denitrifiers in the sediment which use only nitrite as their terminal electron acceptor and produce N2 as the end product and N2O as an intermediate. Tan and Rüger (45) found that in the Weser estuary and the German Bight, nitrate-dissimilating bacteria composed up to 0.8% of heterotrophic bacteria whereas up to 13% were nitrite-dissimilating bacteria. It is also possible that nitrate denitrifiers use nitrite more efficiently than nitrate. In the Colne estuary, since the potentials of both denitrification and N2O formation by these nitrite denitrifiers were very high (Fig. 6A and 7), these microorganisms must be in a state of nitrite limitation under the natural conditions because of the generally low concentrations of nitrite in the water columns and in sediment pore water. In this case, nitrate reduction to nitrite would be the limiting step in the process of denitrification in the sediments of the Colne estuary.
The ratios (N2O/N2 × 100) calculated from the annual survey results of N2O flux (Fig. 4) and rates of denitrification (7) were generally in the range of 0 to 10% in the Colne estuary, with an exceptionally high ratio of 100% at site 2 in January 1998 (data not shown). Such high ratios have also been found in other British rivers. In the sediments of the River Wiske, a nutrient-rich calcareous tributary of the River Swale in northeast England, nitrous oxide production accounted for 0 to 100% of total nitrogen gases (N2O + N2) produced by nitrate reduction, with an average value of 42% (10, 11).
The higher N2O/N2 ratios with added nitrite (7 to 13%) than with added nitrate (2 to 4%) (Fig. 6B) indicate that there exists a group of obligate nitrite-denitrifying bacteria which use only nitrite as an electron acceptor and produce N2O as the end product. Lloyd (25) and Lloyd and Nedwell (unpublished data) isolated from these sediments such obligate nitrite-denitrifying bacteria (Alcaligenes faecalis), which produced only N2O from nitrite. Some enteric bacteria are known to respire nitrite only to N2O (38), while other denitrifying bacteria, such as Aquaspirillum itersonii, also terminate anaerobic respiration at N2O (23). The present study showed that the role of nitrite in N2O formation was twofold. One was to stimulate the rate of N2O production (Fig. 7), and the other was to increase the N2O/N2 ratio in denitrification (Fig. 6B). In terms of the greenhouse effect, the higher N2O/N2 ratio is undesirable, and thus, control of nitrite input to estuaries would be an effective way of reducing the ratio.
Implications of free-energy changes during the processes of denitrification and nitrous oxide formation.
The reason why nitrite stimulated N2 and N2O production may also be related to the generation of energy. Anaerobic bacteria use nitrate or nitrite as electron acceptors to oxidize organic substrates in order to obtain energy. Under the limited availability of organic substrates and oxidizing agents in many sedimentary environments, and hence energy limitation, anaerobic bacteria must utilize these resources in an efficient way in order to acquire maximum energy. Where there is competition for limited resources, the organisms using them most efficiently are likely to outcompete those using them less efficiently.
The standard free-energy yields for the processes of denitrification are summarized in Table 1. Assuming H2 as a standard electron donor, the amount of standard free energy produced from a mole of H2 during N2O formation using nitrite as the electron acceptor (ΔG°1 = −226.5 KJ mol of H2−1 [reaction 1]) is higher than that using nitrate as the electron acceptor (ΔG°2 = −194.8 KJ mol of H2−1 [reaction 2]). Similarly, the amount of standard free energy produced per mole of H2 during N2 formation using nitrite as the electron acceptor (ΔG°3 = −265 KJ mol of H2−1 [reaction 3]) is higher than that using nitrate as the electron acceptor (ΔG°4 = −224.1 KJ mol of H2−1 [reaction 4]). This may explain to some extent why the stimulation of production of N2O and N2 by nitrite was much greater than the stimulation by nitrate, as it is energetically advantageous and would tend to select for bacteria using these reactions.
TABLE 1.
Standard free-energy changes for the reactions of denitrificationa
| Reaction | Substrates | Products | ΔG° (KJ per reaction) | ΔG° (KJ per mol of electron acceptor) | ΔG° (KJ per mol of H2) |
|---|---|---|---|---|---|
| 1 | 2H2 + 2H+ + 2NO2− | N2O + 3H2O | −454 | −226.5 | −226.5 |
| 2 | 4H2 + 2H+ + 2NO3− | N2O + 5H2O | −779.5 | −389.7 | −194.8 |
| 3 | 3H2 + 2H+ + 2NO2− | N2 + 4H2O | −795 | −397.5 | −265 |
| 4 | 5H2 + 2H+ + 2NO3− | N2 + 6 H2O | −1,120.5 | −560.3 | −224.1 |
| 5 | H2 + NO3− | NO2− + H2O | −163.2 | −163.2 | −163.2 |
| 6 | H2 + 2H+ + 2NO2− | 2NO + 2H2O | −147 | −73.5 | −147 |
| 7 | H2 + 2NO | N2O + H2O | −306.1 | −153 | −306.1 |
| 8 | H2 + N2O | N2 + H2O | −341.1 | −341.1 | −341.1 |
Data derived from reference 46.
Reactions 1 and 2 also show that 1 mol of nitrite can oxidize 1 mol of hydrogen, while 1 mol of nitrate can oxidize 2 mol of hydrogen. Calculating the energy per mole of electron acceptor, the amount of standard free energy produced during N2O formation with nitrite as the electron acceptor (ΔG°1 = −226.5 KJ/mol of NO2− [Table 1, reaction 1]) is lower than that with nitrate as the electron acceptor (ΔG°2 = −389.7 KJ/mol of NO3− [Table 1, reaction 2]). This implies that when the electron donor is abundant but the electron acceptor is limiting, reactions using nitrate as an electron acceptor should be favored.
The actual free-energy change (ΔG) is affected by the concentrations of the reactants and the products at a given temperature. Rearranging the formulas of reactions 1 and 2 gives the following formulas of reactions per mole of H2: H2 + H+ + NO2− = 0.5N2O + 1.5H2O (ΔG°a = −226.5 KJ mol of H2−1) (reaction a) and H2 + 0.5H+ + 0.5NO3− = 0.25N2O + 1.25H2O (ΔG°b = −194.8 KJ mol of H2−1) (reaction b).
The free-energy changes by reactions a and b (ΔGa and ΔGb) can be calculated {for the generalized reaction aA + bB = cC + dD, ΔG = ΔG° + RT ln([C]c[D]d/[A]a[B]b)} by the following equations: ΔGa= ΔG°a + RT ln([N2O]0.5/([H2][H+][NO2−])) and ΔGb = ΔG°b + RT ln([N2O]0.25/([H2][H+]0.5[NO3−]0.5)), in which the brackets denote molar concentrations, R is the gas constant (R = 8.31 · J · mol−1 · K−1), and T is the absolute temperature (in kelvins). When the free-energy change in reaction a equals that in reaction b, the following equation applies: ΔG°a + RT ln([N2O]0.5/([H2][H+][NO2−])) = ΔG°b + RT ln([N2O]0.25/([H2][H+]0.5[NO3−]0.5)). Rearranging this equation gives the equation [NO2−]/[NO3−]0.5 = [N2O]0.25/[H+]0.5 ex, where x = (ΔG°a − ΔG°b)/(RT), which shows that the concentration ratio [NO2−]/[NO3−]0.5 is a function of nitrous oxide concentration, proton concentration, and absolute temperature.
At 25°C (298 K) and pH = 8 (the normal pH value in estuarine water), the following equation applies: [NO2−]/[NO3−]0.5 = 0.02758[N2O]0.25.
In the Colne estuary, nitrous oxide concentrations in water or sediment were never <1 nM or >1,000 nM (35). Using these two extremes of nitrous oxide concentration as constraints, when [N2O] is 1 nM, [NO2−]/[NO3−]0.5 is 0.000155, and when [N2O] is 1,000 nM, [NO2−]/[NO3−]0.5 is 0.000871. This means that when [NO2−]/[NO3−]0.5 is 0.000155 or 0.000871 (at [N2O] = 1 or 1,000 nM, respectively), the free-energy changes in reaction a and reaction b should be the same. In other words, the same amounts of free energy should be produced in reaction a and reaction b, and thus the two reactions would proceed equally. In the case where [N2O] is 1 nM and when [NO2−]/[NO3−]0.5 is >0.000155, reaction a is favored. In the case where [N2O] is 1,000 nM and when [NO2−]/[NO3−]0.5 is >0.000871, reaction a is favored. In the Colne estuary, the [NO2−]/[NO3−]0.5 ratios in the water column were 2.756 ± 0.76 at site 1, 1.036 ± 0.34 at site 2, and 0.489 ± 0.15 at sites 3 and 4 from December 1996 to March 1998. These ratios are far more than 0.000871, assuming that the [NO2−]/[NO3−]0.5 ratios in the surface sediment are similar to those in the water column. Considering the fact that nitrifiers in the surface sediment produce nitrite and denitrifiers use nitrate, the [NO2−]/[NO3−]0.5 ratios in the surface sediment may be even higher than those in the water column, so the assumption is conservative. For example, measured [NO2−]/[NO3−]0.5 ratios in pore water in the top 0- to 1-cm layer of sediment gave values of 0.28 and 0.46 at sites 1 and 2, respectively. Reaction a with nitrite should be more favorable than reaction b with nitrate, which means that nitrite should be more favorable than nitrate as an electron acceptor in the process of N2O production.
Acknowledgments
This research was undertaken in the framework of the NICE project. We acknowledge the support from the European Commission's Marine Science and Technology Programme (MAST III) under contract MAS 3-CT96-0048.
Thanks are also due to M. Wilson (Department of Biological Sciences, University of Essex, Colchester, United Kingdom) for a helpful discussion about the calculation of free-energy changes by the reactions of denitrification and nitrous oxide formation.
REFERENCES
- 1.Albritton, D., R. Derwent, I. Isaksen, and L. M. Wuebbles. 1996. Radiative forcing of climate change, p. 118-131. In J. T. Houghton et al. (ed.), Climate change 1995, the science of climate change. Cambridge University Press, New York, N.Y.
- 2.Bange, H. W., S. Rapsomanikis, and M. O. Andrea. 1996. Nitrous oxide in coastal waters. Global Biogeochem. Cycles 10:197-207. [Google Scholar]
- 3.Barnes, J., and N. J. P. Owens. 1998. Denitrification and nitrous oxide concentrations in the Humber estuary, UK, and adjacent coastal zones. Mar. Pollut. Bull. 37:247-260. [Google Scholar]
- 4.Bouwman, A. F., K. M. Van Der Hoek, and L. G. C. Olivier. 1995. Uncertainties in the global source distribution of nitrous oxide. J. Geophys. Res. 100:2785-2800. [Google Scholar]
- 5.Christensen, P. B., L. P. Nielson, J. Sørensen, and N. V. Revsbech. 1990. Denitrification in nitrate-rich streams: diurnal and seasonal variation related to benthic oxygen metabolism. Limnol. Oceanogr. 35:640-652. [Google Scholar]
- 6.De Angelis, M. A., and L. I. Gordon. 1985. Upwelling and river run-off as sources of dissolved nitrous oxide to the Alsea estuary, Oregon. Estuarine Coastal Shelf Sci. 20:375-386. [Google Scholar]
- 7.Dong, L. F., D. O. C. Thornton, D. B. Nedwell, and G. J. C. Underwood. 2000. Denitrification in sediments of the River Colne estuary, England. Mar. Ecol. Prog. Ser. 203:109-122. [Google Scholar]
- 8.Firestone, M. K., and J. M. Tiedje. 1979. Temporal changes in nitrous oxide and dinitrogen from denitrification following onset of anaerobiosis. Appl. Environ. Microbiol. 38:673-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Focht, D. D. 1974. The effect of temperature, pH and aeration on the production of nitrous oxide and gaseous nitrogen--a zero-order kinetic model. Soil Sci. 118:173-179. [Google Scholar]
- 10.García-Ruiz, R., S. N. Pattinson, and B. A. Whitton. 1998. Denitrification and nitrous oxide production in sediments of Wiske, a lowland eutrophic river. Sci. Total Environ. 210-211:307-320. [Google Scholar]
- 11.García-Ruiz, R., S. N. Pattinson, and B. A. Whitton. 1998. Kinetic parameters of denitrification in a river continuum. Appl. Environ. Microbiol. 64:2533-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goreau, T. H. 1980. Production of NO2− and N2O by nitrifying bacteria at reduced concentrations of oxygen. Appl. Environ. Microbiol. 40:526-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahn, J. 1981. Nitrous oxide in the oceans, p. 191-241. In C. C. Delwiche (ed.), Denitrification, nitrification, and atmospheric nitrous oxide. John Wiley and Sons, Inc., New York, N.Y.
- 14.Jensen, H. B., K. S. Jørgensen, and J. Sørensen. 1984. Diurnal variation of nitrogen cycling in coastal, marine sediments. II. Nitrous oxide emission. Mar. Biol. 83:177-183. [Google Scholar]
- 15.Jørgensen, K. S., H. B. Jensen, and J. Sørensen. 1984. Nitrous oxide production from nitrification and denitrification in marine sediment at low oxygen concentrations. Can. J. Microbiol. 30:1073-1078. [Google Scholar]
- 16.Kelso, B. H. L., R. V. Smith, R. J. Laughlin, and D. Lennox. 1997. Dissimilatory nitrate reduction in anaerobic sediments leading to river nitrite accumulation. Appl. Environ. Microbiol. 63:4679-4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelso, B. H. L., R. V. Smith, and R. J. Laughlin. 1999. Effect of carbon substrates on nitrite accumulation in freshwater sediments. Appl. Environ. Microbiol. 65:61-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khalil, M. A. K., and R. A. Rasmussen. 1992. The global sources of nitrous oxide. J. Geophys. Res. 97:14651-14660. [Google Scholar]
- 19.Kieskamp, W. M., L. Lohse, E. Epping, and W. Helder. 1991. Seasonal variation in denitrification rates and nitrous oxide fluxes in intertidal sediments of the western Wadden Sea. Mar. Ecol. Prog. Ser. 72:145-151. [Google Scholar]
- 20.King, D., and D. B. Nedwell. 1987. The adaptation of nitrate-reducing bacterial communities in estuarine sediments in response to overlying nitrate load. FEMS Microbiol. Ecol. 45:15-20. [Google Scholar]
- 21.Kirkwood, D. S. 1996. Nutrients: a practical note on their determination in seawater. Techniques in marine environmental science no. 17. ICES, Copenhagen, Denmark.
- 22.Knowles, R. 1982. Denitrification. Microbiol. Rev. 46:43-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krieg, N. R. 1976. Biology of the chemoheterotrophic spirilla. Bacteriol. Rev. 40:55-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Law, C. S., A. P. Rees, and N. J. P. Owens. 1992. Nitrous oxide: estuarine sources and atmospheric fluxes. Estuarine Coastal Shelf Sci. 35:301-314. [Google Scholar]
- 25.Lloyd, D. L. 2000. Temperature effects on competition, selection and physiology of estuarine nitrate-respiring bacteria. Ph.D. thesis. University of Essex, Colchester, United Kingdom.
- 26.Mathews, E. 1994. Nitrogenous fertilisers: global distribution of consumption and associated emissions of nitrous oxide and ammonium. Global Biogeochem. Cycles 8:411-439. [Google Scholar]
- 27.McElroy, M. B., J. W. Elkins, S. C. Wofsy, C. E. Kolb, A. P. Duran, and W. A. Kaplan. 1978. Production and release of N2O from the Potomac estuary. Limnol. Oceanogr. 23:1168-1182. [Google Scholar]
- 28.Naqvi, S. W. A., D. A. Jayakumar, P. V. Narvekar, H. Naik, V. V. S. S. Sarma, W. D'Souza, S. Joseph, and M. D. George. 2000. Increased marine production of N2O due to intensifying anoxia on the Indian continental shelf. Nature 408:346-349. [DOI] [PubMed] [Google Scholar]
- 29.Nedwell, D. B., and M. Trimmer. 1996. Nitrogen fluxes through the upper estuary of the Great Ouse, England: the role of the bottom sediments. Mar. Ecol. Prog. Ser. 142:273-286. [Google Scholar]
- 30.Nedwell, D. B., T. D. Jickells, M. Trimmer, and R. Sanders. 1999. Nutrients in estuaries. Adv. Ecol. Res. 29:43-84. [Google Scholar]
- 31.Nielsen, K., L. P. Nielsen, and P. Rasmussen. 1995. Estuarine nitrogen retention independently estimated by the denitrification rate and mass balance methods: a study of Norsminde Fjord, Denmark. Mar. Ecol. Prog. Ser. 119:275-283. [Google Scholar]
- 32.Nielsen, L. P. 1992. Denitrification in sediment determined from nitrogen isotope pairing. FEMS Microbiol. Ecol. 86:357-362. [Google Scholar]
- 33.Ogilvie, B., D. B. Nedwell, R. M. Harrison, A. Robinson, and A. Sage. 1997. High nitrate, muddy estuaries as nitrogen sinks: the nitrogen budget of the River Colne estuary (United Kingdom). Mar. Ecol. Prog. Ser. 150:217-228. [Google Scholar]
- 34.Poth, M., and D. Focht. 1985. 15N kinetic analysis of N2O production by Nitrosomonas europaea: an examination of nitrifier denitrification. Appl. Environ. Microbiol. 49:1134-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson, A. D., D. B. Nedwell, R. M. Harrison, and B. D. Ogilvie. 1998. Hypernutrified estuaries as sources of N2O emission to the atmosphere: the estuary of the River Colne, Essex, UK. Mar. Ecol. Prog. Ser. 164:59-71. [Google Scholar]
- 36.Rodhe, H. 1990. A comparison of the contribution of various gases to the greenhouse effect. Science 248:1217-1219. [DOI] [PubMed] [Google Scholar]
- 37.Rysgaard, S., P. B. Christensen, and L. P. Nielsen. 1995. Seasonal variation in nitrification and denitrification in estuarine sediment colonised by benthic microalgae and bioturbating infauna. Mar. Ecol. Prog. Ser. 126:111-121. [Google Scholar]
- 38.Satoh, T., S. S. M. Hom, and K. T. Shanmugam. 1981. Production of nitrous oxide as a product of nitrite metabolism by enteric bacteria, p. 473-480. In J. M. Lyon (ed.), Nitrogen fixation. Plenum Press, New York, N.Y.
- 39.Seitzinger, S. P. 1988. Denitrification in freshwater and marine ecosystems: ecological and geochemical significance. Limnol. Oceanogr. 33:702-724. [Google Scholar]
- 40.Seitzinger, S. P., and S. W. Nixon. 1985. Eutrophication and the rate of denitrification and N2O production in coastal marine sediments. Limnol. Oceanogr. 30:1332-1339. [Google Scholar]
- 41.Seitzinger, S. P., S. W. Nixon, and M. E. Q. Pilson. 1984. Denitrification and nitrous oxide production in a coastal marine system. Limnol. Oceanogr. 29:78-83. [Google Scholar]
- 42.Seitzinger, S. P., M. E. Q. Pilson, and S. W. Nixon. 1983. Nitrous oxide production in nearshore marine sediments. Science 222:1244-1246. [DOI] [PubMed] [Google Scholar]
- 43.Sørensen, J. 1978. Occurrence of nitric and nitrous oxides in coastal marine sediments. Appl. Environ. Microbiol. 36:809-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith, M. S., and K. Zimmerman. 1981. Nitrous oxide production by nondenitrifying soil nitrate reducers. Soil Sci. Soc. Am. J. 45:865-871. [Google Scholar]
- 45.Tan, T. L., and H. J. Rüger. 1979. Denitrifiers in sediments of the Weser Estuary and the German Bight: densities of nitrate-dissimilating and nitrite-dissimilating bacteria. Veröff. Inst. Meeresforsch. Bremerh. 17:189-197. [Google Scholar]
- 46.Thauer, B. K., K. Jungermann, and K. Decher. 1977. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol. Rev. 41:100-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trimmer, M., D. B. Nedwell, D. B. Sivyer, and S. J. Malcolm. 1998. Nitrogen fluxes through the lower estuary of the river Great Ouse, England: the role of the bottom sediments. Mar. Ecol. Prog. Ser. 163:109-124. [Google Scholar]
- 48.Weiss, R. F. 1981. The temporal and spatial distribution of tropospheric nitrous oxide. J. Geophys. Res. 86:7185-7195. [Google Scholar]
- 49.Weiss, R. F., and B. A. Price. 1980. N2O solubility in water and seawater. Mar. Chem. 8:347-359. [Google Scholar]
- 50.Yoshida, T., and M. Alexander. 1970. Nitrous oxide formation by Nitrosomonas europaea and heterotrophic microorganisms. Soil Sci. Soc. Am. Proc. 34:880-882. [Google Scholar]