Abstract
Background:
Increasing antibiotic resistance compromises therapeutic options for Helicobacter pylori (H. pylori) infection, especially in penicillin-allergic individuals.
Objectives:
This trial aimed to assess the efficacy and safety of 14-day vonoprazan–minocycline (VM) dual therapy against bismuth-containing quadruple therapy (B-quadruple therapy), as initial treatment for H. pylori infection.
Design:
This study was a single-center, open-label, and non-inferiority randomized controlled trial.
Methods:
In this study, 240 individuals with H. pylori infection who have not received therapy were randomly assigned 1:1 to either the VM dual therapy group (vonoprazan 20 mg plus minocycline 100 mg, administered twice daily) or the B-quadruple therapy group (rabeprazole 10 mg, amoxicillin 1000 mg, clarithromycin 500 mg, and bismuth potassium citrate 220 mg, all administered twice daily). The primary outcome was to evaluate the non-inferiority of eradication rates between the two groups. Secondary outcomes included assessments of AEs and compliance.
Results:
The eradication rates of VM dual group and B-quadruple therapy group were 87.5% and 88.3%, respectively, by intention-to-treat (ITT) analysis; 92.1% and 94.6% by modified ITT (mITT) analysis; and 92.0% and 95.5% by per-protocol (PP) analysis. The eradication rates of the VM group were non-inferior to those of the B-quadruple therapy group in ITT, mITT, and PP analyses (one-sided p-values were 0.02, 0.01, and 0.02). The incidence of AEs was higher in the B-quadruple therapy group (28.3%) than in the VM group (16.7%, p = 0.03). Good compliance was achieved in both groups (p = 0.60).
Conclusion:
The VM dual therapy was not inferior to the B-quadruple therapy in the initial treatment of H. pylori infection, and the incidence of AEs was lower compared to B-quadruple therapy.
Trial registration:
This trial was registered on the Chinese Clinical Trial Registry with the registration number ChiCTR2400081461.
Keywords: bismuth-containing quadruple therapy, dual therapy, Helicobacter pylori, minocycline
Plain language summary
Fourteen-day vonoprazan-minocycline dual therapy versus bismuth-containing quadruple therapy for first-line helicobacter pylori eradication: a single-center randomized clinical trial
Increasing antibiotic resistance compromises therapeutic options for Helicobacter pylori (H. pylori) infection, especially in penicillin-allergic individuals. This trial aimed to assess the efficacy and safety of 14-day vonoprazan-minocycline (VM) dual therapy against bismuth-containing quadruple therapy (B-quadruple therapy), as initial treatment for H. pylori infection. The VM dual therapy was not inferior to B-quadruple therapy in the initial treatment of H. pylori infection, and the incidence of AEs was lower compared to B-quadruple therapy.
Introduction
Helicobacter pylori (H. pylori) affects nearly half of the global population, representing a major public health concern. 1 With the drastically increased antibiotic resistance, the eradication rate of triple therapy has decreased. 2 Current expert consensus and international guidelines advocate bismuth-containing quadruple therapy (B-quadruple therapy) as the primary eradication therapy.1,3,4 The standard triple regimen plus bismuth is the most commonly used B-quadruple therapy in China, 5 but the eradication rate in the real world is less than 85%. 6 In addition, B-quadruple therapy faces constraints due to high side effects and poor compliance. 7 The use of broad-spectrum antibiotics may cause secondary antibiotic resistance, constraining the choice of rescue strategies for those who have failed in first-line eradication. Exploring a simplified eradication program with a high eradication rate and fewer adverse reactions (AEs) is of great significance.
Amoxicillin is a commonly used antibiotic to eradicate H. pylori, and its resistance rate is rare worldwide (less than 4% on average). 8 As a time-dependent antibiotic, the optimal bactericidal effect can be achieved by increasing the dosage and frequency of amoxicillin to maintain plasma concentration above its minimum inhibitory concentration (MIC). 9 Meanwhile, the enhanced inhibition of gastric acid improves the stability of amoxicillin and keeps H. pylori in a growth state and more sensitive to antibiotics. 10 The newly developed potassium-competitive acid blocker (P-CAB) vonoprazan shows superior acid-inhibiting effects with prolonged duration compared to conventional proton pump inhibitors (PPIs). 11 Recent clinical trials have confirmed the efficacy and safety of either high-dose PPI or standard-dose vonoprazan–amoxicillin dual therapy in the H. pylori management of Asia.12,13
Unfortunately, dual therapy involving amoxicillin remains contraindicated for penicillin-allergic patients. A recent study substituted amoxicillin with tetracycline in patients allergic to penicillin, indicating comparable efficacy between vonoprazan–tetracycline dual therapy and B-quadruple therapy. 14 Nonetheless, tetracycline faces practical limitations due to regional unavailability and frequent adverse events that often compromise compliance. As a second-generation semi-synthetic tetracycline, minocycline exhibits a lower resistance rate against H. pylori comparable to or possibly exceeding amoxicillin.15,16 Numerous studies have confirmed that quadruple regimens containing minocycline have good eradication rates for both initial and refractory H. pylori infection.16,17 However, no prospective studies have investigated a minocycline-based dual regimen for eradicating H. pylori infection. We previously administered vonoprazan–minocycline (VM) dual regimen in penicillin-allergic patients with repeated antibiotic exposure, or patients with multiple treatment failures using amoxicillin-containing regimens, and found that the VM regimen’s eradication rate exceeded 90% across patients with treatment-naive and multiple failures. 18
Consequently, we designed this prospective randomized controlled trial (RCT) to assess the effectiveness and safety of 14-day VM dual therapy when contrasted with B-quadruple therapy as the primary therapy for H. pylori infection.
Methods
Study design and ethical issues
This RCT was conducted as a prospective, single-center, open-label, non-inferior study and was registered on the Chinese Clinical Trial Registry (identifier: ChiCTR2400081461, accessible at www.chictr.org.cn). The reporting of this study conformed to the Consolidated Standards of Reporting Trials (CONSORT) statement (Supplemental Material). 19 The investigation followed the Declaration of Helsinki and was approved by the Clinical Research Ethics Committee of Peking University First Hospital (Scientific Research No. 2024-034). All participants provided written informed consent before enrollment, and we anonymized all the relevant information of the patients in our study.
Study population
Initial screening was performed at Peking University First Hospital’s Gastroenterology Division from March 2024 to October 2024. Inclusion criteria included (1) aged 18–70 years; (2) confirmed H. pylori infection through 13 C urea breath test (UBT); and (3) no prior treatment for H. pylori infection. Exclusion criteria included (1) current pregnancy or breastfeeding status; (2) hypersensitivity to protocol medications; (3) recent use of antibiotics, bismuth preparations, or acid-suppressing medications (including H2 receptor antagonists, PPIs, or P-CABs) within the 4 weeks preceding enrollment; (4) history of subtotal or total gastrectomy; and (5) severe concurrent diseases or malignancies.
Randomization and blinding
The randomization sequence was generated using SPSS statistical software (v26.0; IBM Corp., Armonk, NY, USA) through simple randomization without blocking. Participants were randomly assigned in a 1:1 proportion to either the 14-day VM dual therapy cohort or the 14-day B-quadruple therapy. Allocation concealment was ensured by sealing the computer-generated sequence in sequentially numbered, opaque envelopes. These were securely stored by an independent administrative coordinator uninvolved in recruitment. Following consent acquisition, the researchers phoned this independent coordinator to receive encrypted group assignments, ensuring proper concealment of treatment distribution. The sequence remained undisclosed to all investigators until the intervention was assigned. Both investigators and patients maintained awareness of the treatment regimens.
Interventions and follow-up
The VM dual group involved administering 20 mg of vonoprazan and 100 mg of minocycline twice daily for 14 days. Participants were directed to take these medications 30 min after breakfast and dinner. The B-quadruple therapy involved administering 10 mg of rabeprazole, 1000 mg of amoxicillin, 500 mg of clarithromycin, and 220 mg of bismuth potassium citrate twice daily for an equivalent duration. Administration instructions specified taking rabeprazole and bismuth half an hour before meals, while amoxicillin and clarithromycin were administered 30 min after meals. Manufacturers of drugs are shown in Table S1. Prior to initiating treatment, all participants received health guidance on medication management and potential AEs. Throughout treatment and during 2–4 weeks of treatment, patients were monitored through phone calls to review their medication use and report any AEs. Participants were asked to return for 13 C-UBT at least 4 weeks after concluding the prescribed medication course to accurately determine H. pylori status.
Antimicrobial susceptibility tests of H. pylori under different pH conditions
To clarify how an acidic environment influences the minocycline’s antibacterial activity, we used eight clinical H. pylori strains with previously determined MICs ranging from 0.06 to 2 mg/L from the Institute for Infectious Disease Control and Prevention of the Chinese Center for Disease Control and Prevention (CDC). H. pylori 26,695 served as the quality control strain. MICs were conducted through agar dilution methods in keeping with the guidelines of the National Committee for Clinical Laboratory Standards (NCCLS).
Strains were cultured on agar medium supplemented with 5% defibrinated sheep blood and serially diluted minocycline, and the MICs were determined after 72-h incubation. The pH values of the culture medium were adjusted using hydrochloric acid to 7.3 and 6.0.
Outcomes
The primary endpoint was the eradication rate in each group, defined as a successful 13 C-UBT (Fisher Analysen instruments GmbH, Leipzig, Germany) test at least 4 weeks post-treatment, with a cutoff value of 4‰. The secondary outcomes included compliance and AEs. Medication adherence was assessed by measuring the proportion of days with correct medication intake during the 14-day treatment period, with good compliance strictly defined as taking ⩾80% of the prescribed tablets and achieving a proportion of days with correct intake ⩾80%. AEs included any newly reported symptoms during intervention, with severe events categorized as those significantly affecting daily functioning or necessitating hospital care. The influence of different pH conditions on the MICs of minocycline against H. pylori in vitro was also reported.
Sample size and statistical analysis
The calculation of required participants centered on the primary endpoint. We hypothesized a success rate of 88% in the control cohort 20 and an anticipated success rate of 90% in the VM dual group. 18 The non-inferiority margin was selected as −10%, with an 80% power (1 − β), a 1:1 randomization ratio, and a one-sided alpha (α) value of 0.025. The sample size targeted for each group was determined to be at least 118, considering a 10% loss of follow-up.
The analysis was conducted by intention-to-treat (ITT, encompassing all subjects administered at least one medication dose), modified ITT (mITT, participants completing 13 C-UBT after receiving at least one dose of medication), and per-protocol (PP, participants who consumed ⩾80% of the prescribed drugs and achieved correct intake on ⩾80% of days, with available 13 C-UBT results). Patients with poor compliance were excluded from the PP analyses. Analyses were performed utilizing SPSS statistical software (v26.0; IBM Corp., Armonk, NY, USA) and SAS platform (v9.4; SAS Institute, Cary, NC, USA). Normally distributed continuous data were analyzed using Student’s t-test, whereas non-parametric comparisons employed the Mann–Whitney U test. Fisher’s exact test or Pearson’s chi-square test, as applicable, was used to assess categorical variables. Continuous data were supplied as mean ± standard deviation (SD), and categorical data were expressed as numbers and percentages (%). All statistical tests employed two-tailed p-values, with the exception of the inferiority analysis, maintaining a significance threshold of p < 0.05.
Results
Patients enrolled and baseline characteristics
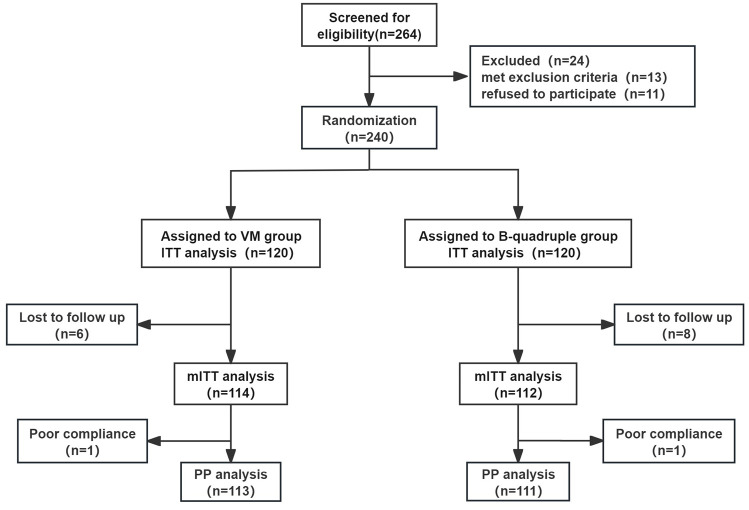
As shown in Figure 1, 264 patients underwent eligibility screening from March 2024 to October 2024, with 240 participants subsequently randomized into either the VM dual group or the B-quadruple therapy group. Among these, six patients in the VM dual group and eight patients in the B-quadruple therapy group discontinued participation without completing 13 C-UBT assessments. In addition, one patient in each of the two groups discontinued treatment (took less than 80% of tablets) but received 13 C-UBT follow-up. Comparative analysis of demographic profiles and clinical parameters, as shown in Table 1, revealed no statistically significant variations among the baseline characteristics of both therapeutic groups.
Figure 1.
Enrollment flowchart.
B-quadruple, rabeprazole, amoxicillin, clarithromycin, and bismuth potassium citrate; ITT, intention-to-treat; mITT, modified intention-to-treat; PP, per protocol; VM, vonoprazan and minocycline dual therapy.
Table 1.
Participants’ baseline and clinical features.
| Variables | VM dual | B-quadruple | p Value |
|---|---|---|---|
| No. subjects | 120 | 120 | — |
| Age (year, mean ± SD) | 43.4 ± 11.6 | 42.4 ± 11.4 | 0.55 |
| Gender (male/female) | 49/71 | 57/63 | 0.30 |
| BMI (kg/m2, mean ± SD) | 23.6 ± 3.7 | 23.7 ± 3.5 | 0.85 |
| Smoking (%) | 12 (10.0) | 14 (11.7) | 0.68 |
| Alcohol consumption (%) | 23 (19.2) | 26 (21.7) | 0.63 |
| Family history of gastric malignancy (%) | 5 (4.2) | 6 (5.0) | 0.76 |
| Clinical conditions a (%) | |||
| Chronic gastritis | 51 (42.5) | 48 (40.0) | 0.69 |
| Peptic ulcer | 4 (3.3) | 9 (7.5) | 0.17 |
| Dyspepsia | 29 (24.2) | 30 (25.0) | 0.88 |
| Health examination | 36 (30.0) | 33 (27.5) | 0.67 |
Patients who underwent endoscopy before treatment were diagnosed with chronic gastritis or peptic ulcer according to endoscopic findings; those who had symptoms but did not undergo endoscopy were diagnosed as dyspepsia; those whose H. pylori infection was confirmed by physical examination, had no symptoms, and did not undergo gastroscopy were diagnosed as healthy.
B-quadruple, rabeprazole, amoxicillin, clarithromycin, and bismuth potassium citrate; BMI, body mass index; SD, standard deviation; VM, vonoprazan and minocycline dual therapy.
H. pylori eradication rates
As shown in Table 2, the ITT analysis revealed eradication rates of 87.5% (105/120, 95% CI: 80.2–92.8) for the VM group and 88.3% (106/120, 95% CI: 81.2–93.5) for the B-quadruple therapy group. The eradication rates were 94.6% (106/112, 95% CI: 88.7–98.0) for the B-quadruple therapy group and 92.1% (105/114, 95% CI: 85.5–96.3) for the VM dual group in the mITT analysis. The PP analysis demonstrated eradication rates of 92.0% (104/113, 95% CI: 85.4–96.3) for the VM group and 95.5% (106/111, 95% CI: 89.8–98.5) for the B-quadruple therapy group. The overall eradication rates did not show any significant differences between the two therapies (PP: p = 0.28; mITT: p = 0.44; ITT: p = 0.84).
Table 2.
H. pylori eradication rates of the two groups.
| Analysis | VM group | B-quadruple group | Difference | p Value for difference a | p Value for non-inferiority b |
|---|---|---|---|---|---|
| ITT | 87.5% (105/120) | 88.3% (106/120) | −0.8% | 0.84 | 0.02 |
| 95% CI | 80.2–92.8 | 81.2–93.5 | −9.1 to 7.4 | — | — |
| mITT | 92.1% (105/114) | 94.6% (106/112) | −2.5% | 0.44 | 0.01 |
| 95% CI | 85.5–96.3 | 88.7–98.0 | −9.0 to 3.9 | — | — |
| PP | 92.0% (104/113) | 95.5% (106/111) | −3.5% | 0.28 | 0.02 |
| 95% CI | 85.4–96.3 | 89.8–98.5 | −9.8 to 2.9 | — | — |
The p values were acquired from two-sided comparisons of the difference between the two groups.
The p values were calculated using one-sided test comparisons of non-inferiority between the two groups.
B-quadruple, rabeprazole, amoxicillin, clarithromycin, and bismuth potassium citrate; CI, confidence interval; ITT, intention-to treat; mITT, modified intention-to treat; PP, per-protocol; VM, vonoprazan and minocycline dual therapy.
In the ITT, mITT, and PP analyses, the VM dual therapy demonstrated non-inferiority to the B-quadruple therapy group. The null hypothesis positing that the VM dual group was inferior to the B-quadruple therapy was rejected (one-sided p-values of 0.02 for ITT, 0.01 for mITT, and 0.02 for PP analysis).
Safety and compliance
The AEs of all participants are shown in Table 3. The VM therapy demonstrated substantially fewer AEs than the B-quadruple therapy group, showing statistically significant differences (16.7% vs 28.3%, p = 0.03). In the VM group, abnormal taste, dizziness, and abdominal distension were the most common AEs, while in the B-quadruple therapy group, the top three frequent AEs were abnormal taste, nausea or vomiting, and diarrhea. In the VM group, three patients developed skin rash during treatment, suspected to be allergic to minocycline. All AEs were mild, allowing patients to continue with their prescribed medication, and resolved spontaneously after treatment cessation. Good compliance was achieved in both groups, with 94.2% of patients in the VM group and 92.5% in the B-quadruple therapy group, showing no significant difference (p = 0.60).
Table 3.
Safety and compliance in each group.
| Adverse effects | VM n (%) |
B-quadruple n (%) |
p Value |
|---|---|---|---|
| Total events | 20 (16.7) | 34 (28.3) | 0.03 |
| Diarrhea | 2 (1.6) | 6 (5.0) | 0.17 |
| Nausea/vomiting | 3 (2.5) | 7 (5.8) | 0.21 |
| Skin rash | 3 (2.5) | 0 (0) | 0.19 |
| Abnormal taste | 5 (4.2) | 19 (15.8) | 0.01 |
| Dizziness | 4 (3.3) | 0 (0) | 0.14 |
| Anorexia | 2 (1.6) | 2 (1.6) | 1.00 |
| Abdominal distension | 3 (2.5) | 4 (3.3) | 0.70 |
| Abdominal pain | 1 (0.8) | 3 (0.25) | 0.34 |
| Constipation | 1 (0.8) | 1 (0.8) | 1.00 |
| Regurgitation | 1 (0.8) | 5 (4.1) | 0.14 |
| Others | 1 (0.8) | 1 (0.8) | 1.00 |
| Compliance | 113 (94.2) | 111 (92.5) | 0.60 |
The p values were acquired from two-sided comparisons of the difference between the VM dual group and B-quadruple group. Compliance indicated subjects who took at least 80% of the study drugs.
B-quadruple, rabeprazole, amoxicillin, clarithromycin, and bismuth potassium citrate; VM, vonoprazan and minocycline dual therapy.
Impact of varying pH levels on the susceptibility of H. pylori to minocycline
The MICs of minocycline detected at different pH levels are shown in Table S2.
As the pH of the agar medium dropped from 7.3 to 6.0, the MICs of most H. pylori isolates to minocycline increased. Two of the strains tested exhibited a significant increase in MICs (from 0.06 to 0.25 mg/L and 0.5 to 2.0 mg/L) as the pH decreased from 7.3 to 6.0.
Discussion
This RCT represents the inaugural investigation assessing the 14-day VM dual regimen’s effectiveness and safety as initial treatment for H. pylori infection. And it is also the first trial to evaluate the influence of different pH conditions on the antibacterial activity of minocycline. Our findings demonstrated that the VM dual therapy was non-inferior to the B-quadruple therapy for the primary eradication of H. pylori infection. Furthermore, the VM dual group reported superior tolerability with significantly fewer AEs compared to the B-quadruple therapy group.
With the increase in antimicrobial resistance, traditional triple eradication regimens containing clarithromycin are no longer considered suitable for the empiric therapy of H. pylori in regions with high resistance levels.1,3 Notably, tetracycline and amoxicillin have consistently maintained relatively low resistance rates (<5.0%), making them the primary candidates for treating H. pylori infection. 8 However, the reported eradication rate of amoxicillin-containing dual therapy in European and American countries was relatively low. 21 And this regimen is not suitable for individuals with penicillin allergy. Tetracycline has been recommended for first-line or rescue treatment by many guidelines and consensus1,3,4; nevertheless, frequent AEs and limited accessibility restrict its widespread application.
Minocycline, a semi-synthetic tetracycline antibiotic, exhibits superior bactericidal activity against many pathogenic bacteria compared to tetracycline. 22 Previous studies indicated that the eradication rate of minocycline-based bismuth quadruple therapy was more than 90% in both first-line and rescue treatment; however, the incidence of AEs was about 50%.16,23 Although the minocycline-based quadruple therapy has good efficacy, there are still concerns about its complexity and the high incidence of AEs. Notably, in our recent retrospective study, we used a simplified regimen in which minocycline replaced amoxicillin or tetracycline in the previously reported dual regimens. The eradication rates in ITT analysis were 90.7% for treatment-naive patients, 84.6% for those with one failure, and 95.2% for patients with multiple failures. The overall AEs rate was only 23.0%. 18 Similarly, in our present study, the eradication rate of VM dual therapy was comparable to that of the B-quadruple therapy, 92.1% in the mITT analysis and 92.0% in the PP analysis, respectively. The incidence of AEs in the VM dual group was as low as 16.7%.
The high eradication rate achieved with dual therapy depends on two important factors. First, the dual regimen involves only one antibiotic. This necessitates that H. pylori strains should be highly sensitive to this antibiotic. The success of dual regimens based on P-CAB or high-dose PPI combined with amoxicillin, tetracycline, and sitafloxacin confirms this premise.13,14,24 Yet, current investigations demonstrated that the MIC and amoxicillin’s resistance rate dramatically increased along with the increase in the failure rate of amoxicillin-based eradication treatments. 25 Notably, the reported primary resistance rate of minocycline (less than 1%) was comparable to or even lower than that of amoxicillin.15,16 Previous studies reported a peak MIC of 0.125 µg/mL for minocycline against H. pylori and showed a rare rate of secondary resistance, making it a potential candidate antibiotic for dual therapy.15,26 On the other hand, the antimicrobial performance significantly correlates with gastric pH conditions. The Maastricht VI/Florence Consensus Report emphasizes persistent acid suppression to maintain a gastric pH > 6 for at least 75% of the day to achieve optimal eradication of H. pylori. 1 Currently available PPIs typically cannot achieve this goal. The oral administration of vonoprazan 20 mg twice daily has been reported to maintain gastric pH above 6 for up to 85% of the day. 27
There are no reports on the minocycline’s bactericidal activity under different pH environments yet. An experiment indicated that increased acidity significantly reduced antimicrobial sensitivity to fluoroquinolones such as ciprofloxacin and moxifloxacin. But the impact of pH on the MIC of amoxicillin and tetracycline did not seem to be significant. 28 Similar results were obtained in our in vitro test for the antibacterial activity of minocycline against H. pylori. Among the strains tested, as the pH of the culture medium dropped from 7.3 to 6, the MICs of two strains increased significantly from 0.06 to 0.25 mg/L and from 0.5 to 2.0 mg/L, respectively. The MICs of four strains increased within twofold dilutions, while the MICs of the remaining three strains were unaffected by pH fluctuations. Taken together, the effect of pH environment on the MIC of minocycline is similar to that of amoxicillin, and further studies are needed to verify this.
Compared with tetracycline, minocycline demonstrates a longer half-life and a higher lipid affinity, allowing it to be administered only twice a day. In our study, patients only took three tablets once after breakfast and once after dinner, which greatly simplified the medication process. In comparison with amoxicillin or tetracycline dual therapy, triple therapy, or B-quadruple therapy regimen, VM dual therapy greatly simplifies the medication process, which helps to improve the compliance of patients and overcomes the problem of eradication failure due to poor compliance.
Adherence and side effects are also important factors affecting the outcome of H. pylori eradication. In our study, both groups achieved adherence of more than 90%, which might be attributed to careful monitoring during medication and health education prior to medication. Despite the high eradication rates of bismuth-containing quadruple therapy, the high incidence of AEs was relatively unsatisfactory. One study using a quadruple regimen with tetracycline plus metronidazole reported an incidence of AEs as high as 71%, with nausea, bitter taste, and dyspepsia being the most prevalent. 5 This could be attributed to the use of two antibiotics in the treatment regimen. In our VM dual therapy, the rate of AEs was obviously lower than that in the control group (16.7% vs 28.3%, p = 0.03). In previous studies containing minocycline, dizziness was one of the most common and obvious side effects.16–18,23 Consistent with previous studies, dizziness ranked among the top three AEs in our study, with a relatively low incidence of 3.3%. Moreover, in the VM group, three patients developed a rash during treatment, suspected to be minocycline allergy. All clinical symptoms were mild, and no patient discontinued treatment due to side effects. Nevertheless, it is still necessary to optimize the dosage of minocycline to ensure efficacy and reduce the occurrence of AEs. A recent study investigated the optimal dose of minocycline in a bismuth-containing quadruple regimen and demonstrated that even 100 mg/day of minocycline could achieve efficacy comparable to tetracycline (400 mg QID) with lower AEs. 29 Whether the dosage of minocycline in the dual regimen can be further optimized needs to be studied.
There are several limitations in this study. First, we were unable to assess minocycline resistance in participants due to the difficulty of performing gastroscopic biopsies in every enrolled patient. In a previous investigation on the MIC distribution of minocycline to H. pylori, 131 clinical isolates were included for susceptibility testing. Among them, only two strains (1.5%) were low resistant to minocycline with the MIC of 2 mg/L (breakpoint > 1 mg/L) (unpublished data from CDC), suggesting that the resistance rate of H. pylori to minocycline in the Chinese population is extremely low. Second, our study did not assess the resistance rate of H. pylori to clarithromycin. Recent meta-analyses indicate that the primary clarithromycin resistance rates exceed 30% in mainland China.30,31 The higher resistance rate of clarithromycin could potentially compromise the efficacy of the clarithromycin-based B-quadruple therapy used as the control regimen, thereby affecting the judgment of the non-inferiority assessment for the VM dual therapy. Notably, the clarithromycin-based B-quadruple therapy remains a recommended first-line therapy in current Chinese guidelines, 3 and even in areas with high clarithromycin resistance, the eradication rate of B-quadruple therapy can still reach 90%.32,33 Similarly, in our study, the B-quadruple therapy containing clarithromycin also achieved an eradication rate of over 90%. This might, to some extent, alleviate the impact of the lack of clarithromycin resistance data in this study. Future studies should incorporate stratified analysis based on the antibiotic resistance of H. pylori. Third, considering the significant differences in the composition and administration methods between VM dual therapy and B-quadruple therapy, we chose an open-label trial rather than a double-blind trial, which could have affected the reporting of AEs and led to selection bias. There is an urgent need for multi-center and large-sample studies to further confirm the efficacy of the VM dual regimen in the first-line and rescue treatment of H. pylori infection.
Conclusion
Our study indicated that VM dual therapy was not inferior to B-quadruple therapy in the initial treatment of H. pylori infection, and the incidence of AEs was lower compared to B-quadruple therapy. This novel dual therapy has a high eradication rate, good compliance, and is friendly to patients allergic to penicillin, and may become a promising new option for H. pylori infection. Meanwhile, the promotion of this protocol still needs further verification through multi-center randomized controlled studies.
Supplemental Material
Supplemental material, sj-doc-1-tag-10.1177_17562848251366156 for Vonoprazan–minocycline dual therapy as a first-line treatment of Helicobacter pylori infection compared with empirical bismuth-containing quadruple therapy by Meng Li, Xiaolei Wang, Xinhong Dong, Guigen Teng, Yun Dai and Weihong Wang in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-2-tag-10.1177_17562848251366156 for Vonoprazan–minocycline dual therapy as a first-line treatment of Helicobacter pylori infection compared with empirical bismuth-containing quadruple therapy by Meng Li, Xiaolei Wang, Xinhong Dong, Guigen Teng, Yun Dai and Weihong Wang in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-3-tag-10.1177_17562848251366156 for Vonoprazan–minocycline dual therapy as a first-line treatment of Helicobacter pylori infection compared with empirical bismuth-containing quadruple therapy by Meng Li, Xiaolei Wang, Xinhong Dong, Guigen Teng, Yun Dai and Weihong Wang in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-4-tag-10.1177_17562848251366156 for Vonoprazan–minocycline dual therapy as a first-line treatment of Helicobacter pylori infection compared with empirical bismuth-containing quadruple therapy by Meng Li, Xiaolei Wang, Xinhong Dong, Guigen Teng, Yun Dai and Weihong Wang in Therapeutic Advances in Gastroenterology
Acknowledgments
We are particularly grateful to Professor Jianzhong Zhang and Ms. Lihua He (CDC) for providing H. pylori strains and sharing data on minocycline resistance.
Footnotes
ORCID iDs: Meng Li  https://orcid.org/0009-0001-0280-0111
https://orcid.org/0009-0001-0280-0111
Yun Dai  https://orcid.org/0000-0002-8854-631X
https://orcid.org/0000-0002-8854-631X
Weihong Wang  https://orcid.org/0000-0003-4740-7388
https://orcid.org/0000-0003-4740-7388
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Meng Li, Department of Gastroenterology, Peking University First Hospital, Beijing, China.
Xiaolei Wang, Department of Gastroenterology, Peking University First Hospital, Beijing, China.
Xinhong Dong, Department of Gastroenterology, Peking University First Hospital, Beijing, China.
Guigen Teng, Department of Gastroenterology, Peking University First Hospital, Beijing, China.
Yun Dai, Department of Gastroenterology, Peking University First Hospital, Beijing, China.
Weihong Wang, Department of Gastroenterology, Peking University First Hospital, No. 8 Xishiku Street, Beijing 100034, China.
Declarations
Ethics approval and consent to participate: This study was authorized by the Peking University First Hospital Ethics Committee (Scientific Research No. 2024-034) and registered at the Chinese Clinical Trial Registry (ChiCTR2400081461). Written informed consent was obtained from all patients.
Consent for publication: Not applicable. All the relevant information of the patients in our study was anonymized.
Author contributions: Meng Li: Data curation; Formal analysis; Investigation; Methodology; Software; Writing – original draft.
Xiaolei Wang: Conceptualization; Data curation; Investigation; Project administration; Resources; Supervision; Visualization.
Xinhong Dong: Conceptualization; Data curation; Supervision; Visualization.
Guigen Teng: Conceptualization; Resources; Software; Supervision; Validation.
Yun Dai: Methodology; Project administration; Resources; Supervision; Validation.
Weihong Wang: Conceptualization; Methodology; Project administration; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: All data relevant to the study were included in the article. Further inquiries can be directed to the corresponding author.
References
- 1. Malfertheiner P, Megraud F, Rokkas T, et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut 2022; 71: 1724–1762. [Google Scholar]
- 2. Yu Y, Xue J, Lin F, et al. Global primary antibiotic resistance rate of Helicobacter pylori in recent 10 years: a systematic review and meta-analysis. Helicobacter 2024; 29(3): e13103. [DOI] [PubMed] [Google Scholar]
- 3. Zhou L, Lu H, Song Z, et al. 2022 Chinese national clinical practice guideline on Helicobacter pylori eradication treatment. Chin Med J (Engl) 2022; 135(24): 2899–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chey WD, Howden CW, Moss SF, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol 2024; 119: 1730–1753. [DOI] [PubMed] [Google Scholar]
- 5. Song Z, Chen Y, Lu H, et al. Diagnosis and treatment of Helicobacter pylori infection by physicians in China: a nationwide cross-sectional study. Helicobacter 2022; 27(3): e12889. [DOI] [PubMed] [Google Scholar]
- 6. Yan TL, Gao JG, Wang JH, et al. Current status of Helicobacter pylori eradication and risk factors for eradication failure. World J Gastroenterol 2020; 26(32): 4846–4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheung KS, Lyu T, Deng Z, et al. Vonoprazan dual or triple therapy versus bismuth-quadruple therapy as first-line therapy for Helicobacter pylori infection: a three-arm, randomized clinical trial. Helicobacter 2024; 29(5): e13133. [DOI] [PubMed] [Google Scholar]
- 8. Bujanda L, Nyssen OP, Vaira D, et al. Antibiotic resistance prevalence and trends in patients infected with Helicobacter pylori in the period 2013-2020: results of the European Registry on H. pylori management (Hp-EuReg). Antibiotics (Basel) 2021; 10(9): 1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li C, Shi Y, Suo B, et al. PPI-amoxicillin dual therapy four times daily is superior to guidelines recommended regimens in the Helicobacter pylori eradication therapy within Asia: a systematic review and meta-analysis. Helicobacter 2021; 26(4): e12816. [DOI] [PubMed] [Google Scholar]
- 10. Sjöström JE, Larsson H. Factors affecting growth and antibiotic susceptibility of Helicobacter pylori: effect of pH and urea on the survival of a wild-type strain and a urease-deficient mutant. J Med Microbiol 1996; 44(6): 425–433. [DOI] [PubMed] [Google Scholar]
- 11. Otake K, Sakurai Y, Nishida H, et al. Characteristics of the novel potassium-competitive acid blocker vonoprazan fumarate (TAK-438). Adv Ther 2016; 33(7): 1140–1157. [DOI] [PubMed] [Google Scholar]
- 12. Li J, Shi H, Zhou F, et al. The efficacy and safety of regimens for Helicobacter pylori eradication treatment in China: a systemic review and network meta-analysis. J Clin Gastroenterol 2024; 58(1): 12–23. [DOI] [PubMed] [Google Scholar]
- 13. Rokkas T, Ekmektzoglou K, Niv Y, et al. Comparative efficacy and safety of potassium-competitive acid blocker-based dual, triple, and quadruple regimens for first-line Helicobacter pylori infection treatment: a systematic review and network meta-analysis. Am J Gastroenterol 2025; 120(4): 787–798. [DOI] [PubMed] [Google Scholar]
- 14. Gao W, Liu J, Wang X, et al. Simplified Helicobacter pylori therapy for patients with penicillin allergy: a randomised controlled trial of vonoprazan-tetracycline dual therapy. Gut 2024; 73(9): 1414–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Azzaya D, Gantuya B, Oyuntsetseg K, et al. High antibiotic resistance of Helicobacter pylori and its associated novel gene mutations among the Mongolian Population. Microorganisms 2020; 8(7): 1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang Y, Chen J, Ding Z, et al. Minocycline vs. Tetracycline in bismuth-containing quadruple therapy for Helicobacter pylori rescue treatment: a multicentre, randomized controlled trial. J Gastroenterol 2023; 58(7): 633–641. [DOI] [PubMed] [Google Scholar]
- 17. Suo B, Tian X, Zhang H, et al. Bismuth, esomeprazole, metronidazole, and minocycline or tetracycline as a first-line regimen for Helicobacter pylori eradication: a randomized controlled trial. Chin Med J (Engl) 2023; 136(8): 933–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang X, Teng G, Dong X, et al. The efficacy and safety of a simple 14-day vonoprazan-minocycline dual therapy for Helicobacter pylori eradication: a retrospective pilot study. Therap Adv Gastroenterol 2024; 17: 17562848241299734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010; 8: 18. [DOI] [PubMed] [Google Scholar]
- 20. Zamani M, Alizadeh-Tabari S, Zamani V, et al. Worldwide and regional efficacy estimates of first-line Helicobacter pylori treatments: a systematic review and network meta-analysis. J Clin Gastroenterol 2022; 56(2): 114–124. [DOI] [PubMed] [Google Scholar]
- 21. Chey WD, Mégraud F, Laine L, et al. Vonoprazan triple and dual therapy for Helicobacter pylori infection in the United States and Europe: randomized clinical trial. Gastroenterology 2022; 163(3): 608–619. [DOI] [PubMed] [Google Scholar]
- 22. Asadi A, Abdi M, Kouhsari E, et al. Minocycline, focus on mechanisms of resistance, antibacterial activity, and clinical effectiveness: back to the future. J Glob Antimicrob Resist 2020; 22: 161–174. [DOI] [PubMed] [Google Scholar]
- 23. You S, Tang X, Zhou J, et al. Minocycline/amoxicillin-based bismuth quadruple therapy for Helicobacter pylori eradication: a pilot study. Microorganisms 2024; 12(3): 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sugimoto M, Sahara S, Ichikawa H, et al. Four-times-daily dosing of rabeprazole with sitafloxacin, high-dose amoxicillin, or both for metronidazole-resistant infection with Helicobacter pylori in Japan. Helicobacter 2017; 22(1). [DOI] [PubMed] [Google Scholar]
- 25. Xie J, Peng J, Liu D, et al. Treatment failure is a key factor in the development of Helicobacter pylori resistance. Helicobacter 2024; 29(3): e13091. [DOI] [PubMed] [Google Scholar]
- 26. Murakami K, Sato R, Okimoto T, et al. Effectiveness of minocycline-based triple therapy for eradication of Helicobacter pylori infection. J Gastroenterol Hepatol 2006; 21(1 Pt 2): 262–267. [DOI] [PubMed] [Google Scholar]
- 27. Kagami T, Furuta T. Letter: probing the consequences of potent acid inhibition by vonoprazan - authors’ reply. Aliment Pharmacol Ther 2016; 44(3): 305. [DOI] [PubMed] [Google Scholar]
- 28. Cheng A, Sheng WH, Liou JM, et al. Comparative in vitro antimicrobial susceptibility and synergistic activity of antimicrobial combinations against Helicobacter pylori isolates in Taiwan. J Microbiol Immunol Infect 2015; 48(1): 72–79. [DOI] [PubMed] [Google Scholar]
- 29. Huang Y, Qiu S, Guo Y, et al. Optimization of minocycline-containing bismuth quadruple therapy for Helicobacter pylori rescue treatment: a real-world evidence study. Helicobacter 2024; 29(5): e13138. [DOI] [PubMed] [Google Scholar]
- 30. Zeng S, Kong Q, Wu X, et al. Antibiotic resistance of Helicobacter pylori in Mainland China: a focus on geographic differences through systematic review and meta-analysis. Int J Antimicrob Agents 2024; 64(5): 107325. [DOI] [PubMed] [Google Scholar]
- 31. Chen J, Li P, Huang Y, et al. Primary antibiotic resistance of Helicobacter pylori in different regions of china: a systematic review and meta-analysis. Pathogens 2022; 11(7): 786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Long X, Chen Q, Yu L, et al. Bismuth improves efficacy of proton-pump inhibitor clarithromycin, metronidazole triple Helicobacter pylori therapy despite a high prevalence of antimicrobial resistance. Helicobacter 2018; 23(3): e12485. [DOI] [PubMed] [Google Scholar]
- 33. Xie Y, Zhu Z, Wang J, et al. Ten-day quadruple therapy comprising low-dose rabeprazole, bismuth, amoxicillin, and tetracycline is an effective and safe first-line treatment for Helicobacter pylori infection in a population with high antibiotic resistance: a prospective, multicenter, randomized, parallel-controlled clinical trial in China. Antimicrob Agents Chemother 2018; 62(9): e00432-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-1-tag-10.1177_17562848251366156 for Vonoprazan–minocycline dual therapy as a first-line treatment of Helicobacter pylori infection compared with empirical bismuth-containing quadruple therapy by Meng Li, Xiaolei Wang, Xinhong Dong, Guigen Teng, Yun Dai and Weihong Wang in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-2-tag-10.1177_17562848251366156 for Vonoprazan–minocycline dual therapy as a first-line treatment of Helicobacter pylori infection compared with empirical bismuth-containing quadruple therapy by Meng Li, Xiaolei Wang, Xinhong Dong, Guigen Teng, Yun Dai and Weihong Wang in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-3-tag-10.1177_17562848251366156 for Vonoprazan–minocycline dual therapy as a first-line treatment of Helicobacter pylori infection compared with empirical bismuth-containing quadruple therapy by Meng Li, Xiaolei Wang, Xinhong Dong, Guigen Teng, Yun Dai and Weihong Wang in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-4-tag-10.1177_17562848251366156 for Vonoprazan–minocycline dual therapy as a first-line treatment of Helicobacter pylori infection compared with empirical bismuth-containing quadruple therapy by Meng Li, Xiaolei Wang, Xinhong Dong, Guigen Teng, Yun Dai and Weihong Wang in Therapeutic Advances in Gastroenterology