Abstract
Tenax-TA, a solid-phase sorbent, was used as an alternative to hexadecane for continuous delivery of tetrachloroethene (PCE) to Desulfuromonas strain BB1, a chloro-respiring microorganism. In both batch and bioreactor configurations, Tenax not only maintained low, steady-state concentrations of PCE in an active culture for several months but also adsorbed the product of dechlorination, cis-1,2-dichloroethene, before it approached toxic levels.
Maintaining a constant concentration of tetrachloroethene (PCE) in a microbially active aqueous system is difficult due to its volatile and sorptive nature. If undiluted PCE is introduced into a system to maintain a saturating concentration, most microorganisms will suffer toxic effects. To avoid problems with toxicity, some researchers add small amounts of PCE intermittently to their cultures, making kinetic analysis difficult. Another widely used alternative for delivery of substrate is to dissolve the PCE into a relatively inert carrier, such as hexadecane (7, 9, 13, 15, 21). Although hexadecane has a high capacity for PCE, its capacity for cis-1,2-dichloroethene (cis-DCE) is much less, potentially resulting in a toxic buildup of DCE in actively dechlorinating cultures (F. E. Löffler, J. Li, J. W. Urbance, and J. M. Tiedje, Abstr. 98th Gen. Meet. Am. Soc. Microbiol., abstr. Q-77, p. 450, 1998). Also, by using hexadecane as a PCE carrier, researchers may be inadvertently providing an alternative carbon source to their cultures. The long list of bacteria that can utilize hexadecane as a carbon source (4, 11, 12, 18, 19) includes sulfate-reducing bacteria (1), many of which are known for their ability to reductively dechlorinate PCE (7, 13, 20, 22; Löffler et al., Abstr. 98th Gen. Meet. Am. Soc. Microbiol., 1998). Additionally, hydrophobic vitamins like B12 (octanol-water partitioning coefficient for vitamin B12 [log Kow-B12 = 3.57] [16]) and lipoic acid (log Kow-lipoic acid = 3.40 [6]) are likely to partition more favorably into hexadecane (log Kow-hexadecane = 8.25 [6]) than into water (log Kow-water = −1.38 [9]). Indeed, vitamin B12 partitions more strongly into hexadecane than does PCE (log Kow-PCE = 3.40 [8]). This is a significant concern, since several dehalogenases characterized from chloro-respiring bacteria require corrinoid cofactors, like vitamin B12, to function (10). Thus, although hexadecane can serve as a PCE carrier for some bacteria and as a carbon source for others, its presence can limit the availability of essential nutrients and also produce toxic effects in bacteria with low hydrocarbon tolerance (23). This combination of properties warrants the examination of an alternative PCE delivery system for the cultivation of pure and enrichment cultures.
As an alternative to hexadecane, the solid-phase sorbent Tenax-TA [poly(2,6-diphenyl-p-phenylene oxide)] may be useful for researchers requiring low, steady-state concentrations of volatile organic compound (VOC) substrates in their experiments. Tenax-TA is manufactured commercially for use in thermal desorption gas chromatograph systems. This lightweight, hydrophobic, porous polymer has an extremely high affinity and sorption capacity for VOCs like PCE (17). Using Tenax for VOC delivery to aqueous cultures has several advantages over the traditional hexadecane method. Unlike hexadecane, Tenax has similar sorption capacities for similar classes of VOCs (17), meaning that potentially toxic degradation products will be removed from solution. Also, unlike with hexadecane, it is not necessary for Tenax to be in direct contact with the culture medium: VOCs can be transferred through headspace in a variety of reactor configurations. This last point is especially relevant for lengthy experiments, in which hexadecane may strip essential organic nutrients out of solution and potentially fail to alleviate the toxic effects of metabolites accumulated over time.
To show that Tenax can be used as a solid-phase carrier for delivering PCE to a dechlorinating culture, a set of batch experiments was conducted. Further, to demonstrate that Tenax can be used to maintain low, steady-state concentrations of VOCs in an aqueous medium for extended periods, a bioreactor was developed in which the PCE-loaded Tenax was physically separated from the liquid medium and the PCE was transferred to the active culture via headspace circulation.
Medium preparation and source cultures.
A reduced anaerobic basal salts medium was used for all experiments (Löffler et al., Abstr. 98th Gen. Meet. Am. Soc. Microbiol., 1998). After the medium was autoclaved, Wolfe's vitamin solution (2) and 0.2 mM Na2S were added from sterilized anaerobic stock solutions. The acetate-oxidizing anaerobe Desulfuromonas strain BB1, which reductively dechlorinates PCE to cis-DCE, was used for all experiments (Löffler et al., Abstr. 98th Gen. Meet. Am. Soc. Microbiol., 1998). Strain BB1 was maintained in 160-ml serum bottles with 1 mM acetate, and PCE was added to give initial aqueous concentrations of 20 mg liter−1 (0.12 mM) and was replenished periodically after it was consumed.
Analytical methods.
Aqueous concentrations of PCE, trichloroethene (TCE), and cis-DCE were determined using a Hewlett-Packard 5890A gas chromatograph equipped with photoionization and electrolytic conductivity detectors and a DB-624 column. Acetate concentrations were determined using a Waters (Milford, Mass.) high-performance liquid chromatograph equipped with an HPX-87H column (Bio-Rad, Hercules, Calif.). Chloride ion concentrations were determined by the colorimetric assay described by Bergmann and Sanik (3).
Tenax preparation and isotherm analysis.
Tenax-TA was purchased from Alltech (Deerfield, Ill.). To ensure the uniformity of the Tenax beads, the fine particulates were removed by washing the material in a number 40 (0.425-mm-pore-size) sieve with 1 liter of deionized water per g of Tenax. To determine the Tenax loading capacity for PCE and the corresponding equilibrium aqueous PCE concentrations, isotherms were determined at temperatures ranging from 15 to 60°C. A mass of PCE ranging from 97 μg to 48.7 mg was added to 0.8 g of washed Tenax in 20 ml of phosphate-buffered deionized water in a closed tube. After at least 48 h at one of the designated temperatures, the equilibrium concentrations of PCE in the headspace were measured in duplicate tubes. The resulting isotherms fit the Freundlich equation log CT = log K + 1/n(log CL), where CT is the PCE concentration on the Tenax (in milligrams per gram), CL is the equilibrium PCE concentration in the liquid (in milligrams per liter), and K and n are constants at any temperature (Table 1). PCE concentrations were enriched on the Tenax by as much as 6,580-fold relative to the equilibrium aqueous concentration on a milligram-per-kilogram basis (Table 1). Thus, it is possible to maintain a nearly constant concentration of PCE for a long period of time, depending on the size of the Tenax bed and the utilization rate of the microorganisms.
TABLE 1.
Freundlich isotherm results, where equilibrium concentrations of PCE in aqueous solution are relative to the amount of PCE adsorbed to Tenax at different temperatures
| Temp (°C) | K | 1/n | CT (mg g−1) | CLa (mg liter−1) | Concn factorb |
|---|---|---|---|---|---|
| 15 | 14.6 | 0.606 | 50 | 7.6 | 6,580 |
| 25 | 10.9 | 0.631 | 50 | 11.2 | 4,460 |
| 45 | 8.25 | 0.702 | 50 | 13.0 | 3,850 |
| 60 | 8.02 | 0.725 | 50 | 12.5 | 4,000 |
CL predicted based on CT and the equation in the text.
Equal to CT/CL × 1,000 and gives a relative factor based on a milligram-per-kilogram Tenax or water basis.
Growth of strain BB1 using the Tenax PCE delivery system.
To demonstrate that Tenax would work as a VOC substrate carrier, we devised experiments in which PCE-loaded Tenax served as the only source of electron acceptor for strain BB1. Anaerobic-culture tubes containing 20 ml of medium, 1.3 g of washed Tenax, and 44 μl of undiluted PCE (nominally 3,500 mg liter−1) were allowed to equilibrate for 24 h at 30°C, at which time the aqueous PCE concentration was approximately 20 mg liter−1. This meant that the PCE concentration on Tenax was increased by a factor of about 2,750 relative to that of the aqueous medium on a milligram-per-kilogram basis. The vials were then inoculated with 1% (vol/vol) BB1 culture. Triplicate cultures and controls (without cells) were incubated at 30°C for 100 days. Based on measurements taken at the end of the experiment of the total quantity of chloride released, the total PCE dechlorinated in this experiment was 405 mg liter−1, 20 times more than could have been degraded with just one feeding at this aqueous concentration (20 mg liter−1).
To further evaluate Tenax as an ideal substrate delivery tool for VOCs, we designed a bioreactor that would deliver VOCs via the gas phase to study the growth of the PCE-dechlorinating culture. A gas-phase delivery system eliminates any contact of the Tenax with the culture medium, thus minimizing the possible sequestration of essential vitamins by the Tenax. The bioreactor, designated the VOC interface transfer apparatus (VITA), is composed of three main components: a culture vessel, the Tenax bed, and a gas pump (Fig. 1). All components are glass, stainless steel, Teflon, or Viton to minimize the loss of VOCs due to adsorption. This bioreactor provides the VOC substrate to microorganisms by circulating the gas phase through the Tenax bed, where the VOC, in this case PCE, is desorbed from the Tenax beads. The PCE-rich headspace is then cycled into the culture vessel and bubbled through the culture medium. As the bubbles rise through the liquid phase, the PCE is transferred into the medium to maintain the chosen equilibrium. The headspace, now with a reduced concentration of PCE, is then cycled back to the Tenax bed for replenishment.
FIG. 1.
VITA used to deliver PCE to the chloro-respiring strain BB1.
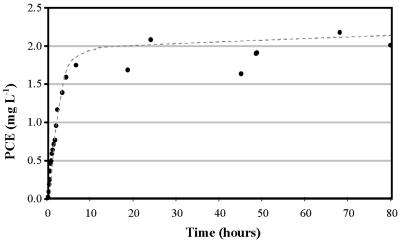
Figure 2 illustrates a typical desorption profile of a preloaded Tenax bed once it is connected in line with a sterile VITA. The PCE concentration in the reactor was monitored by taking headspace samples with a gastight syringe, and a Henry's law constant of 0.617 for PCE was used to calculate the concentration in the aqueous phase (5). Analysis of liquid samples taken at the end of the experiment verified the calculated aqueous-phase concentrations. The desorption profile shows that after approximately 24 h at a headspace circulation rate of 20 ml min−1, the PCE had reached equilibrium.
FIG. 2.
PCE desorption profile from a Tenax bed in a VITA. Aqueous-phase concentrations of PCE are shown (circles). The trend line is fitted to the PCE concentration data and indicates the rapid acquisition of equilibrium PCE concentrations with the Tenax bed.
Prior to use, the culture vessels were cleaned, sterilized, pressure tested, and filled with nitrogen gas. Undiluted PCE was added through the liquid sampling port of the bioreactor and allowed to equilibrate with 400 ml of aqueous medium and 0.8 g of Tenax by circulating the headspace gas at a rate of 20 ml min−1. The amount of PCE added to the Tenax bed was estimated from the isotherm data to target a specific aqueous concentration. The volume of PCE added to each VITA and the resulting aqueous equilibrium PCE concentrations at the time of inoculation were as follows: 45 μl and 4 mg liter−1, respectively, for reactor 1; 150 μl and 15 mg liter−1, respectively, for reactor 2; and 75 μl and 6 mg liter−1, respectively, for the control. Before inoculation, the medium in each reactor was amended with vitamins and 1.6 mM acetate. An inoculum (4% [vol/vol]) was added to the active reactors but not to the control. The reactors were incubated at 24°C (±1°C) for the duration of the experiment
At least every 5 days, triplicate liquid samples were taken and analyzed for acetate, chloride, and chloroethenes. By measuring the aqueous-phase PCE concentration, it was determined that the control reactor lost only 15% of its PCE over the course of the experiment (40 days), showing that a relatively constant concentration of PCE can be maintained with Tenax as a source (the lost PCE was probably absorbed into the Viton tubing in the pump). In both reactors 1 and 2, the acetate concentration decreased relative to an increase in chloride and biomass. For example, based on the decrease in PCE in reactor 1, the concentration of chloride produced was 3.03 mmol per mmol of acetate consumed by strain BB1. The growth rate of strain BB1 appeared to be significantly affected by the electron acceptor concentration. At a PCE concentration of 4 mg liter−1, the growth of strain BB1 was limited and appeared to follow zero-order kinetics, with a maximum growth rate and specific growth rate constant (μ) of 37 mg liter−1 day−1 and 0.166 day−1, respectively. When the PCE concentration was raised to 15 mg liter−1, the growth rate increased by an order of magnitude to 399 mg liter−1 day−1, with a μ of 0.526 day−1. The data show that the PCE concentration can be manipulated by changing the level of loading on the Tenax and that this can result in different growth rates. The volatile metabolite cis-DCE was also removed from the liquid medium by gas stripping and subsequent trapping on the Tenax, thus reducing the potential for toxicity. For example, in reactor 1, based on the mass of PCE degraded according to chloride analysis, 41% of the expected cis-DCE was found in the aqueous medium, with the balance likely adsorbed to the Tenax bed.
As a VOC delivery tool, the solid-phase sorbent Tenax-TA is an easy and useful alternative to conventional methods. Tenax was shown to maintain steady-state PCE concentrations for several weeks in chloro-respiring cultures and also to adsorb the product of dechlorination before it reached toxic levels. Although Tenax was the sorbent used in this study, it is quite likely that other solid-phase sorbents, like zeolites (14), may be equally effective and perhaps less costly as sources for feeding VOC-degrading cultures. Additional experiments using the VITA are planned using Tenax, although materials such as zeolites, which may be more suitable for different VOCs, will be evaluated.
The novel VITA bioreactor that utilizes both the sorptive and desorptive properties of Tenax proved to be a useful tool for studying the growth dynamics of Desulfuromonas strain BB1. At steady-state PCE concentrations of 4 and 15 mg liter−1, growth rates are quite different, as was expected. The VITA developed in this study might be used to determine kinetic parameters for a variety of VOC-degrading cultures and also for the enrichment and growth of such organisms when a continuous feed of substrate is desirable. This ability to feed continuously is particularly beneficial for VOCs with limited solubilities and for concentration-sensitive cultures. Indeed, this method may be useful for studying the unique biochemistry of VOC degradation under a variety of conditions.
Acknowledgments
The support of a National Science Foundation graduate fellowship, the University of Illinois Research Board, and the Environmental Engineering Department at the University of Illinois at Urbana-Champaign is gratefully acknowledged.
Gee-Liek Yeo is thanked for conducting the isotherm experiments, and Frank Löffler is thanked for his donation of strain BB1, which was so critical to this work.
REFERENCES
- 1.Aeckersberg, F., F. Bak, and F. Widdel. 1991. Anaerobic oxidation of saturated hydrocarbons to carbon dioxide by a new type of sulfate-reducing bacterium. Arch. Microbiol. 156:5-14. [Google Scholar]
- 2.Atlas, R. M. 1997. Handbook of microbiological media. CRC Press, New York, N.Y.
- 3.Bergmann, J. G., and J. Sanik, Jr. 1957. Determination of trace amounts of chlorine in napthta. Anal. Chem. 29:241-243. [Google Scholar]
- 4.Bouchez, N. M., H. Rakatozafy, R. Marchal, J. Y. Leveau, and J. P. Vandecasteele. 1999. Diversity of bacterial strains degrading hexadecane in relation to the mode of substrate uptake. J. Appl. Microbiol. 86:421-428. [DOI] [PubMed] [Google Scholar]
- 5.Brennan, R. A., N. Nirmalakhandan, and R. E. Speece. 1998. Comparison of predictive methods for Henrys law coefficients of organic chemicals. Water Res. 32:1901-1911. [Google Scholar]
- 6.Coates, M., D. W. Connell, and D. M. Barron. 1985. Aqueous solubility and octan-1-ol to water partition coefficients of aliphatic hydrocarbons. Environ. Sci. Technol. 19:628-632. [DOI] [PubMed] [Google Scholar]
- 7.Gerritse, J., V. Renard, T. M. Pedro Gomes, P. A. Lawson, M. D. Collins, and J. C. Gottschal. 1996. Desulfitobacterium sp. strain PCE1, an anaerobic bacterium that can grow by reductive dechlorination of tetrachloroethene or ortho-chlorinated phenols. Arch. Microbiol. 165:132-140. [DOI] [PubMed] [Google Scholar]
- 8.Hansch, C., D. Hoekman, A. Leo, L. T. Zhang, and P. Li. 1995. The expanding role of quantitative structure-activity-relationships (QSAR) in toxiology. Toxicol. Lett. 79:45-53. [DOI] [PubMed] [Google Scholar]
- 9.Holliger, C., G. Schraa, A. J. M. Stams, and A. J. B. Zehnder. 1993. A highly purified enrichment culture couples the reductive dechlorination of tetrachloroethene to growth. Appl. Environ. Microbiol. 59:2991-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holliger, C., G. Wohlfarth, and G. Diekert. 1999. Reductive dechlorination in the energy metabolism of anaerobic bacteria. FEMS Microbiol. Rev. 22:383-398. [Google Scholar]
- 11.Ishige, T., A. Tani, Y. Sakai, and N. Kato. 2000. Long-chain aldehyde dehydrogenase that participates in n-alkane utilization and wax ester synthesis in Acinetobacter sp. strain M-1. Appl. Environ. Microbiol. 66:3481-3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim, S. H., E. J. Lim, S. O. Lee, J. D. Lee, and T. H. Lee. 2000. Purification and characterization of biosurfactants from Nocardia sp. L-417. Biotechnol. Appl. Biochem. 31:249-253. [DOI] [PubMed] [Google Scholar]
- 13.Krumholz, L. R., R. Sharp, and S. S. Fishbain. 1996. A freshwater anaerobe coupling acetate oxidation to tetrachloroethylene dehalogenation. Appl. Environ. Microbiol. 62:4108-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li, J., and C. J. Werth. 2001. Evaluating competitive sorption mechanisms of volatile organic compounds in soils and sediments using polymers and zeolites. Environ. Sci. Technol. 35:568-574. [DOI] [PubMed] [Google Scholar]
- 15.Maymo-Gatell, X., I. Nijenhuis, and S. H. Zinder. 2001. Reductive dechlorination of cis-1,2-dichloroethene and vinyl chloride by “Dehalococoides ethenogenes.” Environ. Sci. Technol. 35:516-521. [DOI] [PubMed] [Google Scholar]
- 16.Meylan, W. M., and P. H. Howard. 1995. Atom fragment contribution method for estimating octanol-water partition coefficients. J. Pharm. Sci. 84:83-92. [DOI] [PubMed] [Google Scholar]
- 17.Pignatello, J. J. 1990. Slowly reversible sorption of aliphatic halocarbons in soils. I. Formation of residual fractions. Environ. Toxicol. Chem. 9:1107-1115. [Google Scholar]
- 18.Radwan, S. S., G. Barabas, N. A. Sorkhoh, S. Damjanovich, I. Szabo, J. Szollosi, J. Matko, A. Penyige, T. Hirano, and I. M. Szabo. 1998. Hydrocarbon uptake by Streptomyces. FEMS Microbiol. Lett. 169:87-94. [DOI] [PubMed] [Google Scholar]
- 19.Razak, C. N. A., W. F. Wang, S. H. S. A. Rahman, M. Basri, and A. B. Salleh. 1999. Isolation of the crude oil degrading marine Acinetobacter sp. E11. Acta Biotechnol. 19:213-223. [Google Scholar]
- 20.Sanford, R. A., J. R. Cole, F. E. Löffler, and J. M. Tiedje. 1996. Characterization of Desulfitobacterium chlororespirans sp. nov., which grows by coupling the oxidation of lactate to the reductive dechlorination of 3-chloro-4-hydroxybenzoate. Appl. Environ. Microbiol. 62:3800-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scholz-Muramatsu, H., A. Neumann, M. Meβmer, E. Moore, and G. Diekert. 1995. Isolation and characterization of Dehalospirillum multivorans gen. nov., sp. nov., a tetrachloroethene-utilizing, strictly anaerobic bacterium. Arch. Microbiol. 163:48-56. [Google Scholar]
- 22.Utkin, I., C. Woese, and J. Wiegel. 1994. Isolation and characterization of Desulfitobacterium dehalogenans gen. nov., an anaerobic bacterium which reductively dechlorinates chlorophenolic compounds. Int. J. Syst. Bacteriol. 44:612-619. [DOI] [PubMed] [Google Scholar]
- 23.Whyte, L. G., S. J. Slagman, F. Pietrantonio, L. Bourbonniere, S. F. Koval, J. R. Lawrence, W. E. Inniss, and C. W. Greer. 1999. Physiological adaptations involved in alkane assimilation at a low temperature by Rhodococcus sp. strain Q15. Appl. Environ. Microbiol. 65:2961-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]