Abstract
Potato tubers collected from different areas showed the prevalence of dry rot with characteristic white mycelia like symptoms of Fusarium pathogen. Fusarium species from diseased tubers were isolated based on morphological features. 18 S-Internal Transcribed Spacer (ITS) and Translation Elongation factor 1-α (TEF1-α) based analysis followed by phylogenetic tree constructed using ITS, TEF1-α and RNA polymerase II subunit B (RPB2) identified Fusarium isolates as Fusarium verticilloides, Fusarium soloni, Fusarium falciforme and Fusarium oxysporum. According to our knowledge, this study is the first report of the occurrence of Fusarium falciforme causing dry rot on potato tubers in Pakistan. The pathogenicity test confirmed the re-appearance of dry rot symptoms with Fusarium falciforme on potato tuber. The formation of Fusarium falciforme Ag NPs was confirmed using UV-Visible spectroscopy, X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR) and Field-emission scanning electron microscopy (FE-SEM) analysis. The synthesized Ag NPs showed color change with Fusarium falciforme and accordingly, UV peak was observed at 431 nm. The XRD revealed Ag based specific angles at 36.14°, 44.26°, 64.42° and 77.44° corresponding to face cubic structure. Similarly, FTIR band absorption at 3297 cm−1, 1626 cm−1 and 1057 cm−1 ascribed the presence of various biomolecules with O-H, N-H and C-N stretching vibrations based on Ag NPs synthesis. SEM indicated 29 nm synthesized mainly spherical Ag NPs and EDS analysis showed the presence of Ag in NPs. Maximum zone of inhibition with Fusarium falciforme Ag NPs was observed against Pseudomonas aeruginosa, Escherichia coli, Pseudomonas syringae and Staphylococcus aureus at 0.05 LSD significance.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-025-07124-2.
Keywords: Fusarium falciforme, Silver nanoparticles, Potato, Antibacterial
Introduction
Potato (Solanum tuberosum L.) is an important crop plant grown around the world for food and nutrition. In Pakistan, potato production averaged a yield of 8 million tons in 2023 [1]. Potato is affected by many fungal pathogens that cause major economic losses. Potato dry rot is caused by Fusarium that is a devastating fungal disease [2]. Fusaium equiseti (F. equiseti), Fusaium sambucinum (F. sambucinum), Fusaium manal (F. solani) complex, Fusaium oxysporum (F. oxysporum), Fusaium semitectum (F. semitectum) Fusaium graminearum (F. graminearum) and Fusaium venenatum (F. venenatum) are pathogenic species of potato worldwide [3, 4]. Symptoms of dry rot in potato include sunken and wrinkled brown to black patches on tubers, which exhibit reduced dry matter content and shriveled flesh. As storage duration increases, these wrinkled patches may develop cottony white, purple, pink, or brick orange spores and mycelia masses [4]. Consequently, internal tissues undergo black or brown rot. The pathogen gains entry through wounds, which serve as primary infection site, entering xylem vessels causing leaf chlorosis followed by necrosis, stunting, wilting and eventually plant death [2]. Fusarium species can survive for many years as fungal propagules in the soil, colonize living plants or plant debris, as saprophytes, endophytes or heterophytes. Of different species, F. oxysporum produces white color colony with a pink or violet center when grown on potato dextrose agar (PDA) media. F. solani also produces white color colony on PDA but it becomes blue-green or bluish brown. On the underside, this may be pale, tea-with-milk-brown, or reddish brown. F. solani grows rapidly, but not as rapidly as F. oxysporum [2, 5]. F. solani species complex (FSSC) is one of the prevalent pathogens of dry rot of potato including F. keratoplasticum, F. falciforme, and F. solani [6]. Nearly all species are able to produce mycotoxins that are toxic [2, 4]. Fusarium species mycotoxins are aflatoxins, fumonisins, zearalenone, deoxynivalenol, cyclopiazonic acid and trichothecenes. Trichothecenes possess sesquiterpene isoprenoids that are produced by F. culmorum, F. graminearum and F. sambucinum. Trichothecenes inhibit protein synthesis in the mitochondria that allows reactive oxygen species build up in the cell resulting in oxidative stress and induction of programmed cell death or apoptosis [2, 4]. These species also contains beauvericins and enniatins which are cyclic depsipeptides [7, 8]. Zralenone is another secondary metabolite produced by F. culmorum, F. graminearum, F. cerealis, F. semitectum, F. verticillioides and F. equiseti. Zralenone is thus a potent estrogenic metabolite produced by many Fusarium species. Similarly, Deoxynivalenol is the most widely distributed mycotoxin produced by F. graminearum and F. culmorum [4, 8, 9]. Fumonisins are produced by F. verticillioides and closely related F. proliferatum and F. subglutinans. The fumonisins have a structure similar to sphingolipids. Since Fusarium is of wide occurrence and distribution, a sensitive and rapid surface plasmon resonance imaging assay has detected these mycotoxin such as cyclopiazonic acid in maize and cheese [10]. Similarly, nanoparticles-based lateral flow immunoassay is used for secondary metabolites detection. The rapidly evolving field of nanotechnology has recently gained significant importance due to its management of various medical aliments and agricultural diseases made possible by their strong antimicrobial activity. Nanoparticles typically range from 1 to 100 nm in size and have been implicated into a wide range of profound applications. Nanoparticles, particular silver nanoparticles (Ag NPs) have gained attention due to their unique small size, large surface area to mass ratio that confer nanoparticles distinct physical and chemical properties and rendering them cost-efficient, highly stability and less toxic [11, 12]. Biologically synthesized Ag NPs are preferred as compared to their counter-parts physical and chemical methods because they encounter problems with scalability and hazardable toxins during mass production. Biologically synthesized Ag NPs are made using plants, fungi and bacteria [11, 12]. Different fungi-based Ag NPs are reported including Aspergillus, Penicillium and Fusarium species [13, 14]. These are preferred due to their eco-friendly, bulk production and reproducibility [15]. F. oxysporum base Ag NPs were produced with antibacterial activity against Escherichia coli, Staphylococcus aureus and that showed cytotoxic activity against human breast carcinoma MCF-7 cell line [16]. F. scirpi Ag NPs had antimicrobial potential against uropathogenic Escherichia coli (E. coli) biofilms [17]. Similarly, F. mangiferae Ag NPs also showed antibiofilm potential [18]. Ag NPs and Au NPs of F. pseudonygama gave antibiofilm, antioxidant activity and anticancer activity against cancer cell lines [19]. Ag NPs were synthesized by using F. pallidoroseum biomass and they showed significant antilarval activity against white grub larvae [20]. Extracellular synthesis of Ag NPs by F. acuminatum from ginger showed resistance to Staphylococcus aureus (S. aureus), Salmonella typhi (S. typhi), and E. coli showing simple, eco-friendly method for their production [21]. Hydrogel with PEG-coated F. verticillioides Ag NPs showed effective wound healing ability with low toxicity and high tolerability with MRSA-infected wounded mice as compared to commercially available silver sulfadiazine cream showing F. coated Ag NPs potential for their wound healing ability [22]. F. chlamydosporum and Penicillium chrysogenum (P. chrysogenum) Ag NPs showed cytotoxic effect against cancer cells rendering them as potential therapeutic agents [23]. F. solani based Ag NPs and F. solani gold (Au) nanoparticles showed significant impact on grain borne fungi and cancer cell lines respectively [24, 25]. Thus, Fusarium Ag NPs can be regarded as a source of novel bioactive secondary metabolites having a wide range of biological activities. The objective of this research was first to identify Fusarium species causing potato dry rot using ITS, TEF1-α and RPB2 sequencing followed by construction of phylogenetic tree based on the unique identified sequence. This study is also aimed at investigating Fusarium Ag NPs role against pathogenic bacteria. The synthesized Ag NPs were characterized using UV, SEM, FTIR, XRD and EDS. To the best of our knowledge, this is the first report of fungi that is isolated and used to synthesize and characterize Ag NPs and used against bacterial pathogens.
Materials and methods
Sample collection
Potato plants were collected from potato growing areas of Khyber Pakhtunkhwa (KP). The potato plant was identified by Dr. Abdul Nazir, plant taxonomist at Department of Environmental Sciences, COMSATS University Islamabad (CUI) Abbottabad Campus, Pakistan. The voucher specimen for potato plant bearing tubers (CUHA-453) was submitted in the herbarium of COMSATS University Islamabad, Abbottabad Campus, Pakistan. Potato tubers with symptoms of dry rot like pits, patches, rough dried surfaces with white, pink or black mycelia in these pits were collected from northern areas of Pakistan including Batakundi, Mansehra, Abbottabad and Haripur.
Morphological identification
Fungal isolation from the infected tubers was conducted. The tubers were surface sterilized using 70% ethanol and rinsed with distilled water. The infected part of the tuber was cut and homogenized in distilled water using pestle and motel. Serial dilutions were made from homogenized mixture and 500 uL from 10−9 dilution was spread on the PDA media plates. The plates were sealed, labeled and incubated for 7 days at 28 °C [12]. For morphological identification, the fungal colonies grown on the PDA plates were subjected to fungus purification using hyphal tip incision. The fungal hyphae from serial dilutions grown on PDA media plates were further cultured on new media. The procedure was repeatedly done for fungal isolates collected from different regions to get pure colonies. These cultures were incubated for 7 days at 28 °C to examine fungus morphological features like appearance, colony color, pigmentation and texture. After morphological identification, fungal colonies were subjected to DNA extraction.
DNA extraction, PCR and species identification
For DNA extraction, each fungal colony was cultured on PDB (Potato Dextrose Broth) media and kept on shaking incubation for 3–4 days at 28˚C. Centrifugation was done at 10,000 rpm for 5 minutes to collect fungal pellets. These pellets were dissolved in 10% SDS, 2% CTAB, 5 M NaCl and TE buffer (50 mM Tris, 10 mM of EDTA) and incubated for 15 minutes at 65 °C followed by 24:1 chloroform: isoamyl alcohol treatment. The contents were mixed and centrifuged for 7000 rpm for 7 minutes. The supernatant was treated with isopropanol and 70% ethanol for DNA precipitation [26]. The DNA extracted from 15 samples was suspended in the TE buffer and stored at 4 °C for PCR identification. TEF1-α primers EF1 5’ATGGGTAAGGARGACAAGAC’3; EF2 5’ GGARGTACCAGTSATCATGTT’3) were used for identification of Fusarium species. Initial denaturation at 94 °C for 3 min, 35 cycles of denaturation at 94 °C for 30 s, annealing at 56 °C for 30 s, and extension at 72 °C for 1 min, followed by a final extension at 72 °C for 10 min [3, 27, 28]. 18 S ITS primers ITS1 (5’TCCGTAGGTGAACCTGCGG’3) and ITS4 (5’TCCTCCGCTTATTGATATGC’3) were also applied to fungal isolate under similar PCR conditions with 58 °C annealing temperature. The ITS and TEF1-α PCR products were run on gel electrophoresis (1% Agarose and 0.5 mg/mL Ethidium bromide in TE buffer) at 120 V for 30 min and visualized using transilluminator. Four isolates based on morphological differences and PCR confirmation were chosen for DNA sequencing using ITS, TEF1-α primers (mentioned above) and RPB2 primers RPB2 forward (5’GGGGAGAACAGAAGAAGGC3’) and RPB2 reverse (5’CCCATAGCTTGATTACCAT’3). Four different Fusarium species uniquely identified in this study were submitted to NCBI GenBank https://www.ncbi.nlm.nih.gov/ and accession numbers were obtained for phylogenetic tree construction.
Phylogenetic tree construction
A multigene phylogenetic tree was constructed using NCBI BLASTn based on the concatenated sequences of ITS, TEF1-α and RPB2 from the four different isolates and the selected reference strains [29]. Neonectria ditissima (CBS100316) was included as the outgroup. The phylogenetic analysis was performed using the PhyloSuite integrated toolkit, which incorporates programs such as MAFFT, Gblocks, and IQ-TREE2 [30]. The individual gene sequences (24 ingroup sequences: three loci for each of the four isolates, four selected reference Fusarium species and the three loci for the outgroup, total 27 sequences; Supplementary Table 1) were aligned using MAFFT [31], potentially trimmed and then concatenated per taxon. Maximum Likelihood (ML) analysis was conducted using IQ-TREE v2.2.0 [32]. The substitution model was selected using Partition Finder [33], with HKY + I + F identified as the best-fit model for the concatenated dataset. Branch support was assessed using the Shimodaira–Hasegawa-like approximate likelihood-ratio test (SH-aLRT) [34].
Pathogenicity test
Pathogenicity confirmation of the uniquely identified fungal strain was achieved by re-creating the symptoms for pathogenicity test. Certified potato tubers were surface sterilized with 70% ethanol and washed with distilled water. After air drying, the tubers were syringe inoculated with 50 uL of 3 × 104/mL spore suspension in sterile distilled water and stored at 28 °C for 7 days. After 7 days, the tubers were checked for symptoms development similar to infected tubers from which fungus was initially isolated [12].
Ag NPs synthesis
Fusarium biomass was prepared as reported Husseiny et al. [11]. F. falciforme (Accession nos. ITS PV211459, TEF1-α PQ775194, RPB2 PV626798) identified in this research was used for Ag NPs synthesis. Briefly, the fungal culture was incubated at 28 ℃ for 7 days at 200 rpm. After 7 days, biomass was collected by centrifugation at 8000 rpm for 20 min and was added to dd H2O followed by 3 days of incubation at 200 rpm at 28 ℃. The solution was filtered, and cell free extract was used for fungal Ag NPs synthesis. 1:1 cell-free extract to silver nitrate (1 mg/mL AgNO3 solution) was used with incubation at 1500 rpm, 28 ℃ for 72 h. pH was maintained at 9.0. Visible color change was observed after incubation time from almost opaque to black which indicates formation of Ag NPs [11].
UV-Visible spectral analysis
Cell free extract and Ag NPs synthesized from F. falciforme cell free extract was monitored by UV-vis Spectrophotometer (UV-1602, BMS) with 200–800 nm range [35].
FTIR spectral analysis
FTIR (Thermo Scientific, USA) was used to measure the Bio reduction of Ag+ by cell-free extract of F. falciforme. Spectra was recorded between 4000 and 400 cm−1 [17].
FE-SEM analysis
Dried Ag NPs was attached to the specimen stub for obtaining the SEM image. The micrograph was obtained using 10 kV under 5–10 torr vacuum pressure. The presence of metallic Ag and organic substrates were measured by EDS. MIRA3 TESCAN electron microscope was used for the process [36].
XRD analysis
For examination of crystalline structure of F. falciforme Ag NPs, Cu radiation recorded on an X-ray diffractometer (BRUKER D8) in continuous scanning 2θ mode at 40 kV/20 mA. Scherrer’s formula was used to compute the mean crystallite dimensions of the specimen based on the formula:
D = β1/2 cosθ/Kλ.
D denotes the particle’s size, K denotes a constant, λ denotes the wavelength, β1/2 denotes the full width at half maximum, and θ denotes the Bragg’s angle. By applying Origin software, the obtained diffraction patterns were shown for F. falciforme Ag NPs structural characterization [37].
Antimicrobial activity
The Agar well diffusion method was used for antimicrobial activity [11]. Four bacterial pathogens including E. coli (ATCC 10536), Pseudomonas syringe (P. syringe; DC 3000), S. aureus (KX262679) and Pseudomonas aeruginosa (P. aeruginosa; ATCC 9027) were cultured in Nutrient broth [12]. These cultures were spread on Nutrient agar plates. Six wells were used along with positive control as Kanamycin and negative as dd H2O. Three wells were loaded with 30 µg/µL, 60 µg/µL and 90 µg/µL concentrations of Ag NPs along with 60 µL cell-free extract of F. falciforme in one well. The experiment was done in triplicate. Plates containing P. syringe (DC 3000) was incubate at 28 ℃ while E. coli, S. aureus and P. aeruginosa were incubated at 37 ℃ for 24 h [11, 12].
Statistical analysis
R software was used to analyze data for antibacterial activity (R Core Team 2023). The standard error (± SE) was used to average the data readings. The least significant difference (LSD) test was used to do a one-way analysis of variance (ANOVA) at p < 0.05 [38].
Results
Dry rot of potato tubers
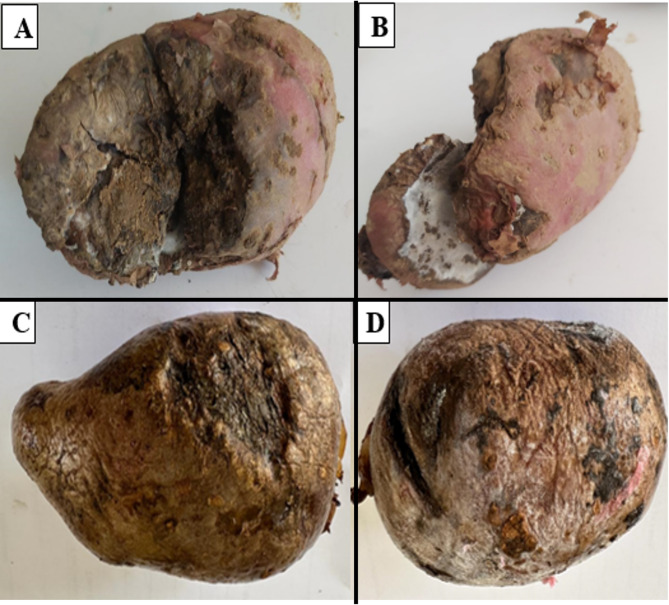
Potato tubers with symptoms of dry rot like pits and patches with white or black mycelia in the pits with shriveled skin were collected from different fields of potato producing northern regions of KP, Pakistan. These include 3 fields of Batakundi, 9 fields of Mansehra, 2 fields of Abbottabad and 4 Haripur (Fig. 1). The tubers were collected during the potato growing seasons from these areas in October-November 2022 and April-May 2023. These tubers were brought to Biotechnology lab at CUI Abbottabad and stored at 4 °C for fungus identification.
Fig. 1.
Dry rot diseased tubers from KP Pakistan; (A) Batakundi and (B) Mansehra (C) Haripur and (D) Abbottabad showing clear symptoms of dry rot of potato
Morphology of different Fusarium species isolated from North-Pakistan
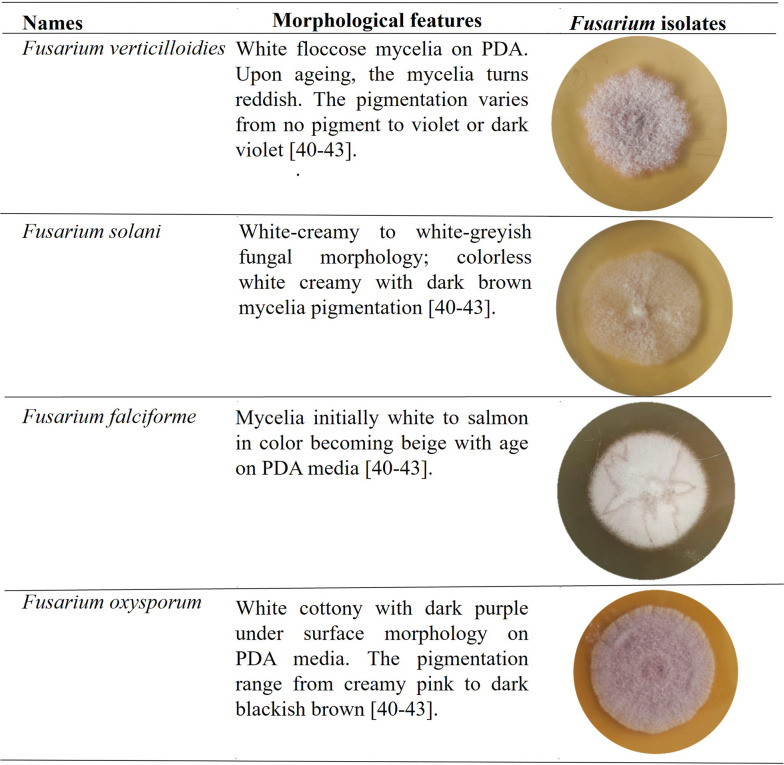
Fungal isolates showing Fusarium dry rot symptoms were identified from potato tubers grown in the north-region [39]. Morphological features on the PDA media included white, brown to dark pink and blackish brown colonies [2, 3, 39]. Fusarium species identified in this study exhibited diverse appearances from initial culture to 7 days after incubation at 28 °C. The morphological features of Fusarium species identified in this study are listed in Table 1 [40–42].
Table 1.
Morphology of different Fusarium species
PCR identification and sequencing of Fusarium species
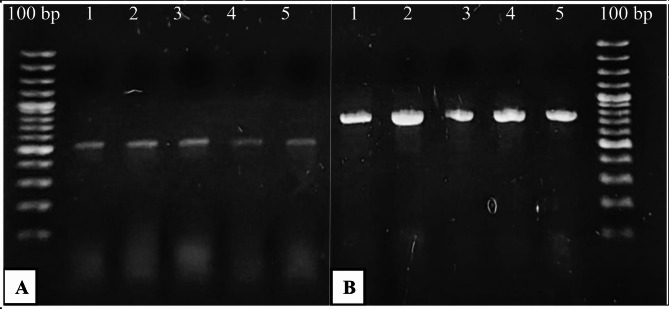
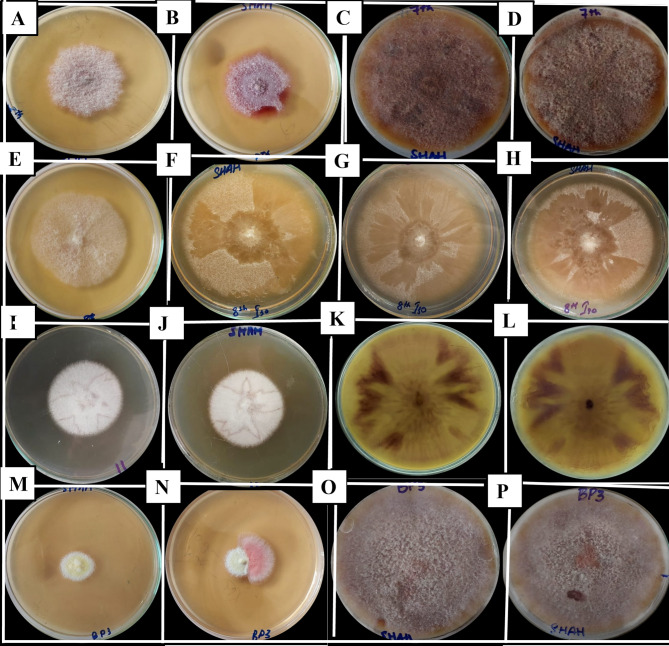
Fungal isolates/colonies were subjected to PCR with TEF1-α and ITS primers. A PCR band of approximately 550 bp appeared at 56 °C annealing temperature with TEF1-α and 780 bp band with ITS at 58 °C annealing temperature. This indicated the presence of Fusarium in dry rot infected tubers with TEF1-α and ITS (Fig. 2; Supplementary Fig. 1). Further molecular characterization was achieved using ITS, TEF1-α and RPB2 sequencing. Four different Fusarium species were identified based on ITS, TEF1‐α and RPB2 sequencing in this study. The isolates ZHW3, ZHW4, ZHW5, and ZHW6 were identified as F. verticilloides, F. solani, F. falciforme and F. oxysporum respectively using Illumina Sanger sequencing (Fig. 3).
Fig. 2.
Fusarium species with (A) TEF1-α and (B) ITS PCR applied to dry rot samples isolated from diseased potato tubers
Fig. 3.
Fusarium cultures isolated from Batakundi, Manshera and Haripur, KP, Pakistan (A–D) ZHW3 Fusarium verticilliodies (NCBI accession number ITS PV211456, TEF1-α PQ775192, RPB2 PV606467); (E–H) ZHW4 Fusarium solani (NCBI accession numbers ITS PV211459, TEF1‐α PQ775194, RPB2 PV626798); (I–L) ZHW5; Fusarium falciforme (NCBI under accession numbers ITS PV211464, TEF1‐α PQ775196, RPB2 PV626797); (M–P) ZHW6 Fusarium oxysporum (NCBI accession numbers ITS PV211468, TEF1‐α PQ775198, RPB2 PV606468)
Phylogenetic tree
The phylogenetic tree was constructed using ITS TEF-1α and RPB2 sequences from four different isolates. The phylogeny identified three main clades based on fungal isolates. Clade 1 consisted of F. oxysporum (HSGF03) closely related to strain ZHW6 identified as F. oxysporum from Batakundi region. Clade 2 included F. verticillioides (NRRL20958) closely related to strain ZHW3 identified as F. verticilloides. Clade 3 comprised of F. solani species complex (FSSC) of F. solani (F238) and F. falciforme (NCS238) with strains ZHW4 from Batakundi and ZHW5 from Manshera region identified as F. solani and F. falciforme respectively. The identified four strains showed a very close phylogenetic relationship in their respective clades, indicating minimal divergence within clades. It is also noteworthy that both ZHW4 (F. solani) and ZHW5 (F. falciforme) identified in this study, belonged to the FSSC complex of Clade 3 and shared a common ancestor, underscoring their close relationship. Similarly, Clade 1 and Clade 2 shared their respective common ancestry and exhibited a small degree of divergence. The high bootstrap values are built on robustness of our findings based on the phylogenetic tree (Fig. 4).
Fig. 4.
Maximum likelihood phylogenetic tree based on concatenated sequences using ITS, TEF1-α and RPB2 show the phylogenetic relationships of Fusarium isolates ZHW3, ZHW4, ZHW5 and ZHW6 with selected reference strains. The tree was inferred using IQ-TREE v2.2.0 under the HKY + I + F model, which was selected by Partition Finder as the best-fit model. Support values from the Shimodaira-Hasegawa-like approximate likelihood-ratio test (SH-aLRT) are shown at the nodes (values ≥ 80% are displayed). Neonectria ditissima (CBS100316) was used as the outgroup. Isolates ZHW5 (marked with a red star) and F. falciforme (NCS54) are highlighted in red. The scale bar indicates 0.1 nucleotide substitutions per site. Clade 1, Clade 2, and Clade 3 represent major phylogenetic groupings
Pathogenicity confirmation
To regenerate potato dry rot symptoms, certified potato tubers were inoculated with F. falciforme identified in this study. After 7 days of incubation at 28 ℃, fungal symptoms appeared on the infected tubers which resulted in re-appearance and confirmation of potato dry rot (Fig. 5).
Fig. 5.

Pathogenicity test to confirm Fusarium symptoms on potato tubers
Ag NPs synthesis and UV-Vis spectroscopy
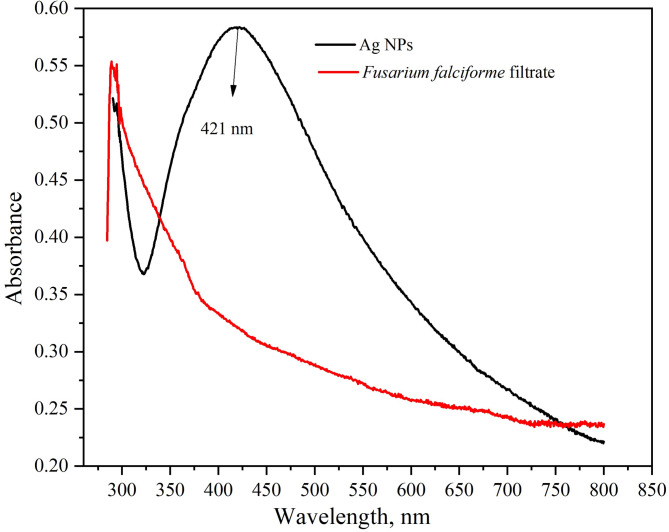
Ag NPs were synthesized by mixing silver nitrate solution (1 mg/mL) and F. falciforme extract in 1:1 ratio at 28 °C and 9 pH [11]. After a while, the reaction mixture gradually changed into a dark-brown suspension indicating the formation of Ag NPs (Fig. 6). The primary characterization method for Ag NPs synthesis is UV-vis spectroscopy that shows Ag NPs formation that depends upon their shape, size, and distribution based on surface plasmon resonance (SPR). A peak of 421 nm confirmed the formation of Ag NPs in this study (Fig. 7) according to previous report [33]. Ag NPs synthesized using F. oxysporum showed maximum absorbance peak at 420 nm [16]. Absorbance spectrum by UV spectrometer showed a plasmon resonance absorbance around 420–440 nm for Ag/AgCl NPs synthesized from F. oxysporum [40].
Fig. 6.
Synthesis of Ag NPs. (A) Fungal Biomass (B) Fungal Filtrate (C) Fusarium Ag NPs
Fig. 7.
UV-Visible spectrum of Fusarium Ag NPs
XRD
The diffractogram of Ag NPs is represented in Fig. 7. The XRD pattern of biosynthesized Ag NPs using fungal filtrate of F. falciforme was carried out with 2θ values ranging from 20°–80°. Indexing of synthesized Ag NP crystal peaks was carried out by Ag crystal card (ICSD No.00.03–0291) of face center cubic. The diffraction pattern of F. falciforme Ag NPs displayed four strong peaks at 2θ values the characteristic peaks at 36.14°, 44.26°, 64.42° and 77.44° correspond to the (111), (200), (220) and (311) structure. Four distinct diffraction peaks of silver were observed at Bragg’s planes (111, 200, 220, 311) for the face-centered cubic structure of synthesized Ag NPs (Fig. 8).
Fig. 8.
XRD pattern of Ag NPs
FTIR
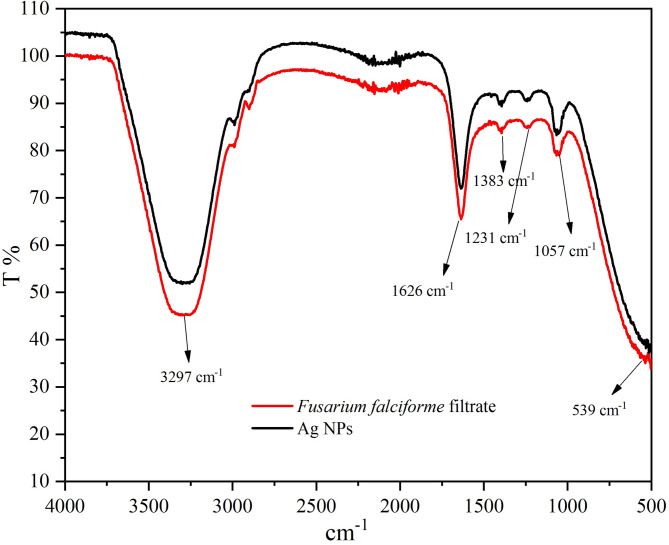
FTIR analysis showed the chemical composition of synthesized Ag NPs. The occurrence of several functional groups/metabolites in F. pseudonygama are responsible for the reduction of silver ions into silver that act as capping and stabilizing agents [19]. Peak at 1626 cm−1 corresponds to the alkenes stretch and a broad peak at 3297 cm−1 assigned to O–H and N–H stretching vibrations of amide (Fig. 9). Absorption spectra showed similar band pattern with 1644 and 3282 cm−1 for C = O and N-H group respectively for Ag NPs [20]. A 539 cm−1 band in FTIR spectrum indicated fungal extract alkyl halides [20].
Fig. 9.
FTIR pattern of synthesized Ag NPs
FESEM and EDS
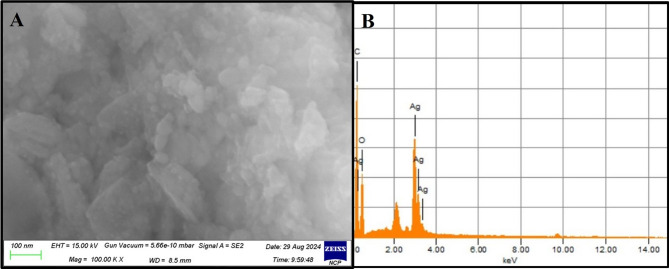
SEM provided a high-resolution image of synthesized F. falciforme Ag NPs based on their morphology, size and distribution. The micrograph showed the synthesized silver nanoparticles (Fig. 10A). Synthesized Ag NPs were found to be 29 nm based on Bragg’s equation. SEM micrograph recorded for Ag NPs showed spherical nanoparticles [33]. The elemental composition present in the sample was presented with the help of EDS analysis showed Ag to be % present by weight in synthesized Ag NPs. This indicated a high amount of silver with an optical absorption band at ~ 3 eV in Ag NPs (Fig. 10B). EDS showed a convincing peak for oxygen with a relative mass percentage which confirmed its presence as a major constituent of nanoparticles. The presence of oxygen could be assigned to emission of X-rays from free amino groups from F. falciforme. Similarly, carbon presence in EDS indicated the presence of sugars in the fungal mass extract (Fig. 10B).
Fig. 10.
SEM images of (A) Ag NPs and (B) EDS of Ag NPs
Antimicrobial activity
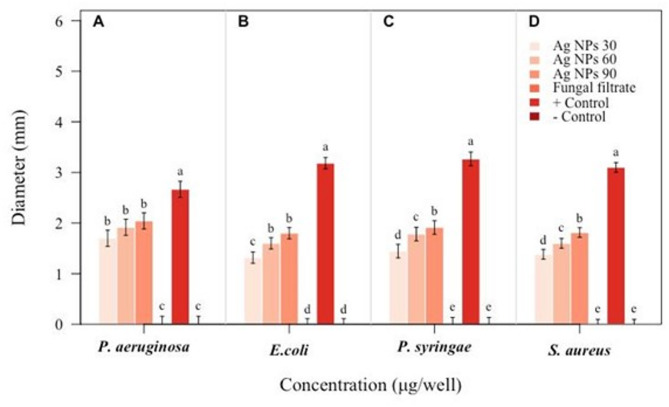
Antibacterial assay of biosynthesized Ag NPs was studied against pathogenic strains of P. aeruginosa, E. coli, P. syringae and S. aureus using agar well diffusion method (Fig. 11). The results based on zone of inhibition (Fig. 12) showed that Ag NPs were effective against all bacteria strains including P. aeruginosa, E. coli, P. syringae and S. aureus [45, 46]. Both gram positive and gram-negative bacteria showed zone of inhibition, with maximum zone of inhibition of 2.0, 1.8, 1.9 and 1.8-mm for P. aeruginosa, E. coli, P. syringae and S. aureus respectively with 90 ug/uL Ag NPs with LSD at 0.05 SD (Fig. 12).
Fig. 11.

Fusarium falciforme Ag NPs antibacterial activity at 30, 60 and 90 ug/uL concentration (A) Pseudomonas aeruginosa (B) Escherichia coli (C) Pseudomonas syringae (D) Staphylococcus aureus. Ampicillin is used as positive control and Fusarium filtrate (Ff) at 60 ug/uL
Fig. 12.
Antibacterial activity of Ag NPs. Zone of inhibition with Ag NPs at 30, 60 and 90 ug/uL Ag NPs (A) Pseudomonas aeruginosa (B) Escherichia coli (C) Pseudomonas syringae DC3000 (D) Staphylococcus aureus. Data is represented as the means ± SD from 3 replicates. Different letters represent significant differences at p ≤ 0.05 Fisher LSD one way ANOVA
Discussion
Potato is a major crop grown in Pakistan and worldwide. However, it is affected by various fungal diseases including dry rot [2]. Symptoms of whitish brown to black tissue patches on potato tubers with wrinkled skin indicated the presence of dry rot on potato tubers in the north-Pakistan (Figs. 1 and 2). Morphological features of fungal isolates on PDA media from Batakundi area, indicated purple or very bright red color colonies. These colonies turn dark purple after 7 days of incubation at 28 °C (Fig. 3A-D). Fungal colonies from potato tubers of Batakundi region appeared white with a very thin layer of mycelia on the surface media (Fig. 3E-H). Similarly, Fusarium species isolated from infected tubers of Mansehra region were white in color (Fig. 3I-L). These fungal cultures later changed color to brownish white, however the colonies remained the same with star like pattern (Fig. 3I-L). Other fungal colonies appeared maroon or purple (Fig. 3M-P). Similar observations were made by Gangaraj et al. in 2021 that indicated white, brown and purple-maroon color colonies with guava wilt [41]. Our results with F. falciforme produced floccose mycelia while F. solani produced sparse mycelia arranged in concentric rings on PDA media (Table 1); similar to results by Chehri et al. [6]. After morphological identification, PCR identification showed approximately 550 and 780 bp band size with TEF1-α and ITS primers indicating the possible presence of Fusarium species in dry rot infected tubers [3]. The sequencing of fungal isolates based on ITS, RPB2 and TEF1-α identified ZHW5 as Fusarium from FSSC. This report is similar to Gherbawy et al. 2021 identification of Fusarium pathogens from FSSC [43]. Phylogenetic tree construction of Fusarium species based on TEF1-α, RPB2 and ITS identified unique Fusarium isolate from F. solani complex as F. falciforme causing dry rot on potato tubers [43]. According to our knowledge, this is the first report of the identification of tuber dry rot caused by F. falciforme in Pakistan based on sequencing followed by concatenated phylogenetic tree (Figs. 4 and 5). Report by Chehri et al. in 2014 similarly confirmed F. falciforme based on ITS and TEF1-α from FSSC causing dry rot on potato [6]. After the successful molecular and phylogenetic identification of F. falicforme, Ag NPs were synthesized using F. falciforme filtrate (Fig. 6). For Ag NPs synthesis, 1:1 cell-free extract to AgNO3 was used at pH 9.0 at 28 °C. The solution turned from cream to dark brown, indicating the formation of Ag NPs. F. solani Ag NPs were also reported to change from yellow to dark brown after synthesis with equal amount of fungal filtrate and AgNO3 [47]. Fungal nitrate reductase is a critical enzyme for the formation of Ag NPs with the reduction of Ag+2 to Ag [33]. Biomolecules and metabolites such as sugars and amides also play an essential role in the formation of Ag NPs. A UV- Vis peak of 421 nm was obtained in this study (Fig. 7). A similar peak showed the formation of F. pallidoroseum Ag NPs [20]. F. nygamai Ag NPs produced an absorbance peak in the UV–visible spectrum at 420 nm [44]. After initial characterization, a diffraction pattern indexed at (111), (200), (220) and (311) lattice planes for face centered cubic was obtained for F. falciforme Ag NPs (Fig. 8) similarly corresponding to 38.15°, 44.18°, 64.63° and 77.50° for F. oxysporum Ag NPs [16]. Another study also indicated 2θ values corresponded to silver crystalline based F. pseudonygamai Ag NPs with face-centered cubic structure [19]. F. falciforme filtrate containing biomolecules including enzymes, proteins and polysaccharides showed a strong affinity for Ag NPs synthesis. F. falciforme Ag NPs FTIR analysis demonstrated stretching vibration of 3297 cm−1 as amide, the band stretching at 1626 cm−1 represented amide I and 1644 cm−1 showed carbonyl band stretching (Fig. 9). Similar reports are available where amino acids, sugars and other biomolecules from fungal filtrates resulted in Ag+2 reduction [14, 18]. Additionally, electrostatic attraction between the nanoparticles and fungus-based biomolecules is involved in capping and enhanced stable formation of Ag NPs [17, 18]. Soliman et al. in 2022 showed F. pseudonygam Ag NPs with Ag as predominant element and spherical shape biosynthesized nanoparticles through SEM and EDS analysis similar to our study (Fig. 10) [19]. After successful characterization, 2.0, 1.8, 1.9 and 1.8-mm zones of inhibition (Figs. 11 and 12) were recorded for P. aeruginosa, E. coli, P. syringae and S. aureus respectively with F. falciforme Ag NPs [16]. F. oxysporum Ag NPs agar well diffusion method with E. coli and S. aureus showed 2.0- and 1.6-mm zone of inhibition. Antibacterial activity of F. acuminatum Ag NPs was recorded against S. aureus followed by Staphylococcus epidermidis (S. epidermidis), S. typhi and E. coli [48]. F. semitectum Ag NPs showed significant antibacterial activity against K. pneumonia and P. aeruginosa [49]. Both gram-positive and gram-negative bacteria including Bacillus subtilis (B. subtilis), S. aureus, P. aeruginosa, E. coli and fungal pathogens Candida albicans (C. albicans), Aspergillus niger (A. niger) were impacted by Fusarium Ag NPs penetrating through cell wall and causing cell damage [44]. Inside the cell, these Ag NPs disrupt metabolic and cellular processes and cause DNA degradation. Reactive oxygen species (ROS) are the key players responsible for protein denaturation, lipid peroxidation and DNA damage. Finally, these processes lead to the cell death and apoptosis of such important biological entities [50, 51].
Conclusion
This is the first report of the identification of Fusarium falicforme and its Ag NPs synthesis. Fusarium falicforme was identified based on the phylogenetic tree using ITS, TEF1-a and RPB2. Ag NPs synthesized were characterized through UV, FTIR, SEM and XRD showed their successful use against P. aeruginosa, E. coli, P. syringae and S. aureus.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors like to thank IST Islamabad Abbottabad for technical assistance in data generation. The authors are thankful to Dr. Rafiq Ahmed CUI Abbottabad Pakistan for providing bacterial strains to carry out this research work. We also like to thank Dr. Zhao lab at Nanjing China for helping us with the sequencing results and Dr. Abdul Nazir at CUI Abbottabad for providing us with potato (Solanum tuberosum L.) voucher specimen ID CUHA 453.
Abbreviations
- Ag
Silver
- AgNO3
Silver nitrate
- ANOVA
Analysis of Variance
- Ag NPs
Silver Nano particles
- BLASTn
Basic Local Alignment Search Tool, Nucleotide BLAST
- cm
centimeter
- CTAB
Cetyltrimethylammonium bromide
- Cu
Copper
- oC
degree Celsius
- DNA
Deoxyribonucleic acid
- EDS
Energy Dispersive X-ray Spectroscopy
- EDTA
Ethylenediaminetetraacetic acid
- eV
Electron volts
- FSSC
Fusarium solani species complex
- FTIR
Fourier Transform Infrared Spectroscopy
- ICSD
Inorganic Crystal Structure Database
- ITS
Internal Transcribed Spacer
- KP
Khyber Pakhtunkhwa
- kV
Kilovolt
- LSD
Least Significant Difference
- mA
Milliampere
- µg/µL
Micrograms per microliter
- µL
Microliter
- mL
Milliliter
- NCBI
National Center for Biotechnology Information
- nm
Nanometer
- PDA
Potato dextrose agar
- PDB
Potato dextrose broth
- ROS
Reactive oxygen species
- RPB2
RNA polymerase II subunit B
- rpm
Revolutions per minute
- SD
Standard deviation
- SE
Standard error
- SEM
Scanning Electron Microscopy
- TEF1-α
Translation Elongation factor 1-α
- Tris
Tris (hydroxymethyl) aminomethane
- UV
Ultraviolet
- UV-Vis
Ultraviolet-Visible
- XRD
X-ray Diffraction
Author contributions
S. H. S collected the data and wrote the manuscript. X. S helped with the Phylogenetic tree. S. B applied microbial statistics and reviewed the manuscript. H. Z, M.S and M. S. B critically analyzed the manuscript. B. I analyzed the FTIR and XRD data. I. S, S N and I N provided critical input, reviewed and helped write the manuscript. A B designed the study. All authors read and approved the manuscript.
Funding
No funding was requested for this work.
Data availability
Fusarium verticilliodies (NCBI accession number ITS PV211456, TEF1‐α PQ775192, RPB2 PV606467); Fusarium solani (NCBI accession numbers ITS PV211464, TEF1-a PQ775196, RPB2 PV626797); Fusarium falciforme (NCBI under accession numbers ITS PV211459, TEF1-a PQ775194, RPB2 PV626798); Fusarium oxysporum (NCBI accession numbers ITS PV211468, TEF1-a PQ775198, RPB2 PV606468); available at https://www.ncbi.nlm.nih.gov/.
Declarations
Competing interests
The authors declare no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All authors have read and approved the manuscript. The tuber samples used in study were obtained with the permission of landowners at KP Pakistan and are used for research purpose only.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Food and Agriculture Organization of the United Nations. FAOSTAT Statistical Database. [Rome]; 2023: FAO.
- 2.Tiwari RK, Kumar R, Sharma S, Sagar V, Aggarwal R, Naga KC, Lal MK, Chourasia KN, Kumar D, Kumar M. Potato dry rot disease: current status, pathogenomics and management. 3 Biotech. 2020;10:503. 10.1007/s13205-020-02496-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azil N, Stefańczyk E, Sobkowiak S, Chihat S, Boureghda H, Sliwka S. Identification and pathogenicity of F. spp. associated with tuber dry rot and wilt of potato in Algeria. Eur J Plant Pathol. 2021;159:495–509. 10.1007/s10658-020-02177-5. [Google Scholar]
- 4.Xue H, Liu Q, Yang Z, Pathogenicity. Mycotoxin production, and control of potato dry rot caused by Fusarium spp. Rev J Fungi. 2023;9:1–17. 10.3390/jof9080843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harish J, Jambhulkar PP, Bajpai R, Arya M, Babele PK, Chaturvedi SK, Kumar A, Lakshman DK. Morphological characterization, pathogenicity screening, and molecular identification of Fusarium spp. isolates causing post-flowering stalk rot in maize. Front Microbiol. 2023;14:1121781. 10.3389/fmicb.2023.1121781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chehri K, Ghasempour HR, Karimi N. Molecular phylogenetic and pathogenetic characterization of F. solani species complex (FSSC), the cause of dry rot on potato in Iran. Microb Pathog. 2014;67:14–9. 10.1016/j.micpath.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Chulze SN, Tittlemier S, Torres AM, Editorial. Fusarium species as plant and human pathogens, Mycotoxin producers, and biotechnological importance. Front Fungal Biol. 2023;4:1320198. 10.3389/ffunb.2023.1320198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryła M, Pierzgalski A, Zapaśnik A, Uwineza PA, Ksieniewicz-Woźniak E, Modrzewska M, Waśkiewicz A. Recent Research on F. Mycotoxins in Maize—A. Rev Foods. 2022;11:3465. 10.3390/foods11213465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ropejko K, Twarużek M. Zearalenone and its metabolites-general overview, occurrence, and toxicity. Toxins (Basel). 2021;13: 1–12. 10.3390/toxins13010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hossain Z, Busman M, Maragos CM. Immunoassay utilizing imaging surface plasmon resonance for the detection of Cyclopiazonic acid (CPA) in maize and cheese. Anal Bioanal Chem. 2019;411:3543–52. 10.1007/s00216-019-01835-w. [DOI] [PubMed] [Google Scholar]
- 11.Husseiny SM, Salah TA, Anter HA. Biosynthesis of size-controlled silver nanoparticles by F. oxysporum, their antibacterial and antitumor activities. Beni-Suef Univ J Basic Appl Sci. 2015;4(3):225–31. 10.1016/j.bjbas.2016.04.001. [Google Scholar]
- 12.Shah SH, Shan X, Baig S, Zhao H, Ismail B, Shahzadi I, Baig A. First identification of potato tuber rot caused by penicillium solitum, its silver nanoparticles synthesis, characterization and use against harmful pathogens. Front Plant Sci. 2023; 1255480. 10.3389/fpls.2023.1255480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yousaf A, Waseem M, Javed A, Baig S, Ismail B, Baig A, Shahzadi I, Nawazish S, Zaman I. Augmented anticancer effect and antibacterial activity of silver nanoparticles synthesized by using Taxus Wallichiana leaf extract. PeerJ. 2022;10: e14391. 10.7717/peerj.14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balakumaran MD, Ramachandran R, Balashanmugam P, Mukeshkumar DJ, Kalaichelvan PT. Mycosynthesis of silver and gold nanoparticles: optimization, characterization and antimicrobial activity against human pathogens. Microbiol Res. 2016;182:8–20. 10.1016/j.micres.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Singh D, Rathod V, Ninganagouda S, Herimath J, Kulkarni P. Biosynthesis of silver nanoparticle by endophytic fungi pencillium sp. isolated from curcuma longa (turmeric) and its antibacterial activity against pathogenic gram-negative bacteria. J Pharm Res. 2013;7:448–53. 10.1016/j.jopr.2013.06.003. [Google Scholar]
- 16.Guilger-Casagrande M, de Lima R. Synthesis of silver nanoparticles mediated by fungi: a review. Front Bioeng Biotechnol. 2019;7:1–16. 10.3389/fbioe.2019.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodríguez-Serrano C, Guzmán-Moreno J, Ángeles-Chávez C, Rodríguez-González V, Ortega-Sigala JJ, Ramírez-Santoyo RM, Vidales-Rodríguez LE. Biosynthesis of silver nanoparticles by F. scirpi and its potential as antimicrobial agent against uropathogenic Escherichia coli biofilms. PLoS ONE. 2021;15:e0230275. 10.1371/journal.pone.0230275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamzah HM, Salah RF, Maroof MN. F. mangiferae as new cell factories for producing silver nanoparticles. J Microbiol Biotechnol. 2018;28:1654–63. 10.4014/jmb.1806.06023. [DOI] [PubMed] [Google Scholar]
- 19.Soliman MKY, Abu-Elghait M, Salem SS, Azab SM. Multifunctional properties of silver and gold nanoparticles synthesis by F. pseudonygamai. Biomass Conv. Bioref. 2022. 10.1007/s13399-022-03507-9. [Google Scholar]
- 20.Shukla G, Gaurav SS, Singh A, Rani P. Synthesis of mycogenic silver nanoparticles by F. pallidoroseum and evaluation of its larvicidal effect against white Grubs (Holotrichia sp). Mater Today: Proc. 2022;49:3517–27. 10.1016/j.matpr.2021.07.238. [Google Scholar]
- 21.Ingle A, Gade A, Pierrat S, Sonnichsen C, Rai M. Mycosynthesis of silver nanoparticles using the fungus F. acuminatum and its activity against some human pathogenic bacteria. Curr Nanosci. 2008;4:141144. 10.2174/157341308784340804. [Google Scholar]
- 22.Mekkawy AI, El-Mokhtar MA, Nafady NA, Yousef N, Hamad MA, El-Shanawany SM, Ibrahim EH, Elsabahy M. In vitro and in vivo evaluation of biologically synthesized silver nanoparticles for topical applications: effect of surface coating and loading into hydrogels. Int J Nanomed. 2017;12:759–77. 10.2147/IJN.S124294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalil NM, Abd El-Ghany MN, Rodríguez-Couto S. Antifungal and anti-mycotoxin efficacy of biogenic silver nanoparticles produced by F. chlamydosporum and penicillium chrysogenum at non-cytotoxic doses. Chemosphere. 2019;218:477–86. 10.1016/j.chemosphere.2018.11.129. [DOI] [PubMed] [Google Scholar]
- 24.Abd El-Aziz ARM, Al-Othman MR, Mahmoud MA, Metwaly HA. Biosynthesis of silver nanoparticles using F. solani and its impact on grain borne fungi. Dig J Nanomater Biostructures. 2015;10:655–62. [Google Scholar]
- 25.Clarance P, Luvankar B, Sales J, Khusro A, Agastian P, Tack JC, Al Khulaifi MM, AL-Shwaiman HA, Elgorban AM, Syed A, Kim HJ. Green synthesis and characterization of gold nanoparticles using endophytic fungi Fusarium solani and its in-vitro anticancer and biomedical applications. Saudi J Biol Sci. 2020;27:706–12. 10.1016/j.sjbs.2019.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang YJ, Zhang S, Liu XZ, Wen HA, Wang M. A simple method of genomic DNA extraction suitable for analysis of bulk fungal strains. Lett Appl Microbiol. 2010;51:114–8. 10.1111/j.1472-765X.2010.02867.x. [DOI] [PubMed] [Google Scholar]
- 27.Long H, Yin X, Zhao Z, Long Y, Fan J, Shu R, Gu G. First report of fruit blotch on plum caused by F. fujikuroi in China. Plant Dis. 2021;105:2256. 10.1094/PDIS-08-20-1784-PDN.33565889 [Google Scholar]
- 28.Wang RY, Gao B, Li XH, Ma J, Chen SL. First report of F. solani causing Fusarium root rot and stem canker on storage roots of sweet potato in China. Plant Dis. 2014;98:160–160. 10.1094/PDIS-06-13-0651-PDN. [DOI] [PubMed] [Google Scholar]
- 29.Torres-Cruz TJ, Whitaker BK, Proctor RH, Broders K, Laraba I, Kim HS, Brown DW, O’Donnell K, Estrada-Rodríguez TL, Lee YH, Cheong K, Wallace EC, McGee CT, Kang S, Geiser DMF. -ID v.3.0: an updated, downloadable resource for Fusarium species identification. Plant Dis. 2022;106:1610–6. 10.1094/PDIS-09-21-2105-SR/. [DOI] [PubMed] [Google Scholar]
- 30.Zhang D, Gao F, Jakovlić I, Hong Z, Zhnag J, Li WX, Wang GT. Mol Ecol Resour. 2020;20(1):348–55. 10.1111/1755-0998.13096. PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. [DOI] [PubMed]
- 31.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–80. 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodshams MD, Haeseler AV, Lanfear R. 2020. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Molecular biology and evol. 2020; 37(5): 1530–1534. 10.1093/molbev/msaa015 [DOI] [PMC free article] [PubMed]
- 33.Talavera G, Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol. 2007;56(4):564–77. 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
- 34.Hoang DT, Chernomor O, Von Haeseler A, Minh BQ, Vinh LS. UFboot2: improving the ultrafast bootstrap approximation. Mol Biol Evol. 2018;35(2):518–22. 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Durán N, Marcato PD, Alves OL, De Souza GI, Esposito E. Mechanistic aspects of biosynthesis of silver nanoparticles by several F. oxysporum strains. J Nanobiotechnol. 2005;3:1–7. 10.1186/1477-3155-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fathima BS, Balakrishnan RM. Biosynthesis and optimization of silver nanoparticles by endophytic fungus F. solani. Mater Lett. 2014;132:428–31. 10.1016/j.matlet.2014.06.143. [Google Scholar]
- 37.Aboody A MS. Silver/silver chloride (Ag/AgCl) nanoparticles synthesized from Azadirachta indica Lalex and its antibiofilm activity against fluconazole resistant Candida tropicalis. Artif Cells Nanomed Biotechnol. 2019;47:2107–13. 10.1080/21691401.2019.1620257. [DOI] [PubMed] [Google Scholar]
- 38.R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2023. [Google Scholar]
- 39.Vatankhah M, Saberi-Riseh R, Eskandari MM, Afzali H. Evaluation of some fungicides for the control of Fusarium dry rot of potato. J Crop Prot. 2019;8:275–85. [Google Scholar]
- 40.Gavrilova O, Orina A, Trubin I, Gagkaeva T. Identification and pathogenicity of Fusarium fungi associated with dry rot of potato tubers. Microorganisms. 2024;12:598. 10.3390/microorganisms12030598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gangaraj R, Nagaraja A, Gaba S, Das A, Prameeladevi T, Debbarma R, Choudhary SP, Kamil A, Kamil D. Occurrence, identification and pathogenicity of fusarium species associated with guava wilt disease in India. Archives Phytopathol Plant Prot. 2022;55(2):175–97. 10.1080/03235408.2021.2005368. [Google Scholar]
- 42.Xi K, Shan L, Yang Y, Zhang G, Zhang J, Guo W. Species Diversity and Chemotypes of F. Species Associated with Maize Stalk Rot in Yunnan Province of Southwest China. Front Microbiol. 2021;12:652062. 10.3389/fmicb.2021.652062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gherbawy YA, Hussein MA, Hassany NA, Shebany YM, Hassan S, El-Dawy EG. Phylogeny and pathogenicity of F. solani species complex (FSSC) associated with potato tubers. J Basic Microbiol. 2021;61:1133–44. 10.1002/jobm.202100393. [DOI] [PubMed] [Google Scholar]
- 44.Picoli SU, Durán M, Andrade PF, Duran N. Silver nanoparticles/silver chloride (Ag/AgCl) synthesized from Fusarium oxysporum acting against Klebsiella pneumouniae carbapenemase (KPC) and extended spectrum beta-lactamase (ESBL). Front Nanosci Nanotech. 2016;2:107–10. 10.15761/FNN.1000117. [Google Scholar]
- 45.El-Ansary AE, Omran AAA, Mohamed HI, El-Mahdy OM. Green synthesized silver nanoparticles mediated by F. nygamai isolate AJTYC1: characterizations, antioxidant, antimicrobial, anticancer, and photocatalytic activities and cytogenetic effects. Environ Sci Pollution Res Intel. 2023;30:100477–99. 10.1007/s11356-023-29414-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El Domany EB, Essam TM, Ahmed AE, Farghli AA. Biosynthesis, characterization, antibacterial and synergistic effect of silver nanoparticles using F. oxysporum. J Pure Appl Microbiol. 2017;11:1441–6. 10.22207/JPAM.11.3.27. [Google Scholar]
- 47.El Sayed MT, El-Sayed ASA. Biocidal activity of metal nanoparticles synthesized by Fusarium solani against multidrug-resistant bacteria and mycotoxigenic fungi. J Microbiol Biotechnol. 2020;30(2):226–36. 10.4014/jmb.1906.06070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trzcińska-Wencel J, Wypij M, Terzyk AP, Rai M, Golińska P. Biofabrication of novel silver and zinc oxide nanoparticles from F. solani IOR 825 and their potential application in agriculture as biocontrol agents of phytopathogens, and seed germination and seedling growth promoters. Front Chem. 2023;11:1235437. 10.3389/fchem.2023.1235437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ingle A, Rai M, Gade AK, Bawaskar M. F. solani: a novel biological agent for the extracellular synthesis of silver nanoparticles. J Nanopart Res. 2009;11:2079–85. 10.1007/s11051-008-9573-y. [Google Scholar]
- 50.Shelar G, Chavan. AF. semitectum mediated extracellular synthesis of silver nanoparticles and their antibacterial activity. Int J Biomed Adv Res. 2014;5:348–51. [Google Scholar]
- 51.Rudrappa M, Kumar RS, Nagaraja SK, Hiremath H, Gunagambhire PV, Almansour AI, Perumal K, Nayaka S. Myco-nanofabrication of silver nanoparticles by Penicillium Brasilianum NP5 and their antimicrobial, photoprotective and anticancer effect on MDA-MB-231 breast cancer cell line. Antibiotics. 2023;12:567. 10.3390/antibiotics12030567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
Supplementary Materials
Data Availability Statement
Fusarium verticilliodies (NCBI accession number ITS PV211456, TEF1‐α PQ775192, RPB2 PV606467); Fusarium solani (NCBI accession numbers ITS PV211464, TEF1-a PQ775196, RPB2 PV626797); Fusarium falciforme (NCBI under accession numbers ITS PV211459, TEF1-a PQ775194, RPB2 PV626798); Fusarium oxysporum (NCBI accession numbers ITS PV211468, TEF1-a PQ775198, RPB2 PV606468); available at https://www.ncbi.nlm.nih.gov/.