Abstract
Background
Menstrual symptoms are a prevalent and frequently encountered woman's health condition. This study aimed to examine the effect of exercise on menstrual symptoms, sleep quality, fatigue, and physical activity levels.
Methods
The study was designed as a randomized controlled trial between September 2023 and December 2023. The study included 54 women aged 18 to 45 years. The participants were allocated to the exercise or the control group using simple randomization with a sealed envelope method. All participants were evaluated with the Menstrual Symptom Questionnaire (MSQ), the Menstrual Distress Questionnaire (MDQ), the Fatigue Severity Scale (FSS), the Pittsburgh Sleep Quality Index (PSQI), and the International Physical Activity Questionnaire-Short Form (IPAQ) before and after treatment. The control group did not receive an exercise program. The exercise group received strengthening, flexibility, and balance exercises. A moderate-intensity aerobic exercise and walking program was implemented at least three days per week. The study spanned three menstrual cycles for each woman and lasted an average of 12 weeks.
Results
Within-group analysis showed a significant decrease in MSQ (p=0.001), MDQ (menstrual) (p=0.002), FSS (p=0.003), and PSQI (p=0.001) scores after exercise. In contrast, the IPAQ score increased significantly in the exercise group p=0.001). In the control group, a significant decrease was observed only in the MDQ (intermenstrual) score (p=0.915). A comparison of the pre-treatment and post-treatment changes in the exercise and control groups revealed a significant decrease in MSQ (p=0.001), MDQ (menstrual) (p=0.023), and PSQI scores (p=0.001) and an increase in IPAQ scores (p=0.001) in the exercise group compared to the control group. However, the decrease in MDQ (pre-menstrual and intermenstrual) (p=0.626, p=0.348) and FSS scores (p=0.102) were not statistically different between the groups.
Conclusions
In conclusion, exercise can decrease the menstrual symptoms in women with menstrual symptoms as a primary outcome. Second, exercise can also increase their sleep quality. Therefore, it can be employed as a non-pharmacological adjuvant method to help women manage their symptoms.
Trial registration
The protocol is registered with http://clinicaltrials.gov/ (17/August/2023, Clinical Trial, NCT06006507).
Keywords: Menstrual symptoms, Physical activity, Sleep, Fatigue, Menstrual pain
Introduction
The menstrual cycle is characterized by a series of physiological changes initiated by the onset of menstruation and continuing until the preceding day of the next menstrual period. These changes are driven by significant hormonal fluctuations, which can give rise to a range of menstrual-related symptoms [1]. These symptoms are experienced by 90% of women worldwide, representing a significant public health concern [2]. Menstruation-related symptoms include dysmenorrhea, migraines, and a variety of somatic (e.g., breast tenderness, abdominal bloating, swelling) and affective symptoms (e.g., depression, irritability, anxiety, confusion) [2].
The most prevalent menstruation-related symptoms are premenstrual syndrome (PMS) and dysmenorrhea, which is defined as painful menstruation [3]. Dysmenorrhea is characterized by the presence of cramps in the back and lower abdomen, as well as nausea, diarrhea, fatigue, and headache [4]. PMS is a multifaceted condition characterized by the occurrence of emotional and physical symptoms, which typically manifest during the luteal phase of the menstrual cycle, approximately five days before menstruation [5]. The most severe manifestation of PMS is designated as premenstrual dysphoric disorder (PMDD), a condition that is associated with an elevated prevalence of cognitive disorders [6]. It has been reported that women suffering from PMS, PMDD, dysmenorrhea, or abnormal uterine bleeding have experienced suboptimal sleep quality [7, 8]. Also, in individuals with PMDD, the onset of menstruation is accompanied by a worsening of symptoms such as insomnia, fatigue, and memory impairment [9].
Given that menstrual symptoms persist until menopause, menstrual periods have a significant financial burden, including reduced work productivity, hospital visits, and medication costs [10]. Despite the several public health implications of menstrual symptoms, there is still a paucity of knowledge regarding effective management strategies [10]. The treatment of menstrual symptoms may be accomplished through the utilization of pharmacologic or nonpharmacologic methodologies. In consideration of the potential adverse effects and financial implications of pharmacological approaches, non-pharmacological methods have been proposed as an alternative [11]. These include healthy nutrition, psychosocial approaches, massage, meditation, heat application, psychotherapy, mind-body techniques, herbal supplements, acupuncture, and dance therapy [12].
A substantial amount of research has indicated that elevated levels of physical activity and modifications to lifestyle habits may serve to mitigate the symptoms commonly encountered by women, including fatigue and depression. Moreover, consistent exercise regimens have been demonstrated to alleviate menstrual symptoms [13]. It was observed that high physical activity levels were associated with a shorter duration of menstruation, a lower dysmenorrhea and polymenorrhea incidence, and a lower PMS prevalence [14]. Furthermore, physical exercise has been suggested to decrease the severity of PMS [12]. However, further research is needed to confirm these potential benefits [6, 7, 9]. It is evident that individuals experiencing excessive fatigue, suboptimal sleep quality, and menstrual symptoms encounter adverse health consequences [15]. Nevertheless, a consensus remains elusive regarding the optimal type, duration, and frequency of exercise for these individuals [15].
Therefore, the current study aimed to investigate the effect of the treatment of physical exercise on menstrual symptoms, sleep quality, fatigue, and physical activity level.
Methods
Study design
The research was carried out as a randomized controlled study, adhering to the ethical principles outlined in the Declaration of Helsinki and approved by the ethics committee. This study was conducted and reported following the Consolidated Standards of Reporting Trials (CONSORT) statement. This study was conducted between September 2023 and December 2023 after obtaining ethical approval from the Uskudar University Non-Interventional Research Ethics Committee with reference number 61351342/December 2022-67. The protocol is registered with http://clinicaltrials.gov/ (17/August/2023, Clinical Trial, NCT06006507).
Determination of sample size
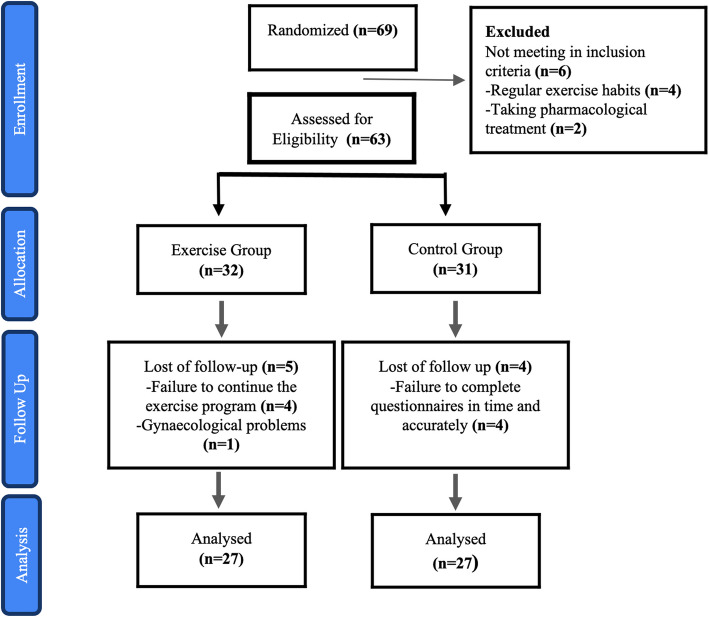
The sample size was calculated using the G*Power Version 3.1.6 program. A total of 54 participants were required to determine the medium effect size (f=0.25 effect) at 95% power and a 0.05 alpha significance level (one-sided) [16]. The population of the study consists of women between the ages of 18-45. The number of patients participating in the study was initially 63. However, 9 participants were excluded from the study for various reasons. Consequently, 54 participants completed the study (Figure 1).
Fig 1.
CONSORT flowchart
Randomization and blinding
A total of 69 participants initially participated in the study. Then, after assessing for eligibility, a total of 63 participants were included in the study and were randomly assigned to the exercise group (n:32) and the control group (n:31) by simple randomization. The concealment and randomization were carried out using the sealed envelope method. The names of all participants were recorded on individual pieces of paper and placed in separate envelopes by the physiotherapist. These were then randomly selected, and the participants were assigned to either the exercise or control group following the order of selection by the physiotherapist. The inclusion criteria were as follows: signing the voluntary consent form, being between the ages of 18 and 45, having a menstrual cycle, having emotional or physical problems during menstruation, and having a menstrual cycle between 21 and 35 days on average. Exclusion criteria were as follows: having any chronic disease or diagnosed gynecologic disease, receiving pharmacologic treatment for menstrual symptoms, having amenorrhea, exercising regularly, and having any orthopedic or neurologic problem preventing exercise. The study was finally finalized with 54 participants (n=27 for each group). Figure 1 shows the flow of participants throughout the study.
Exercise procedure
The participants were randomly allocated to two groups: an exercise group and a control group. The participants were contacted individually, and their menstrual patterns were documented. The Personal Information Form, Menstrual Symptom Questionnaire (MSQ), Menstrual Distress Questionnaire (MDQ), Fatigue Severity Scale (FSS), Pittsburgh Sleep Quality Index (PSQI), International Physical Activity Questionnaire-Short From (IPAQ) were prepared in Google Forms and sent via WhatsApp on the first day of menstruation before the study commenced. The exercise group received exercise programs via the phone application Fiziu on the first day of their menstrual periods. The application provided visual and audio support for the exercises, which were defined in personal accounts. The participants'email addresses and phone numbers were utilized to access the application, and a code was provided to the participants for the exercise program created through the application. The initial exercises were performed with the physiotherapist, and the subsequent exercises were assigned as an individual home program. A follow-up form was created to monitor the progress of the exercise group. The practicing physiotherapist met with the participants on two days per week and recorded each meeting on this form. No application was undertaken with the control group. Throughout the study, the research team contacted the participants regularly to ascertain whether the subjects in the control group were not engaging in any physical activity and whether the subjects in the exercise group were adhering to the recommended exercise program. After approximately 12 weeks (on the first day of their fourth menstrual period), all questionnaires were completed, and the study was concluded.
Exercise program
The exercise group engaged in an exercise program that included 150 minutes of moderate-intensity walking per week and half an hour of strengthening, flexibility, and balance exercises two days per week for three months (with an average duration of 12 weeks). This program was designed following the World Health Organization's recommendations. A speech test was administered to enable individuals to self-determine the intensity of their exercise. Mild intensity is defined as a pace at which the individual can both speak and sing. Moderate intensity is defined as a pace at which the individual is unable to sing but can still speak. The most severe level of exertion is indicated by the individual being unable to speak or sing and experiencing respiratory distress [17]. The walking program was completed for a total of 150 minutes per week at a moderate intensity, distributed over at least three days of the week, with a minimum of 10 minutes per session. A program comprising whole-body strengthening, flexibility, and balance exercises was developed. The exercise program comprised a series of strengthening, flexibility, and balance exercises. The following exercises were prescribed for strengthening the muscles: squats, planks, bridges, sit-ups, scissors, prone back extensions, and modified push-ups. The following flexibility exercises were employed: shoulder roll, child's pose, adductor stretch, hamstring stretch, gastroc-soleus stretch, and cat camel exercise. A variety of postures on one leg were demonstrated as examples of balance exercises. The strengthening and flexibility exercises were planned as three sets of eight repetitions, while the balance exercises were planned for one minute.
Outcome measurements
The following demographic information was recorded in the demographic information form: age, height, weight, body mass index (BMI), employment status, education level, exercise habits, marital status, pregnancy status, number of pregnancies, smoking and alcohol habits. Furthermore, the age at menarche, menstrual pattern (regular-irregular), cycle duration, number of days of the menstrual period, and use of painkillers during menstruation were also recorded to ascertain the menstrual history and menstrual period characteristics. The primary outcome measure of this study was to examine the impact of physical exercise on the menstrual symptoms of women. The secondary outcome measures were to assess the effect of this treatment on sleep quality, fatigue, and physical activity levels. These outcomes were measured at the outset and conclusion of the study using the following questionnaires.
Menstrual Symptom Questionnaire (MSQ)
It is a 5-point Likert-type scale and consists of 24 items with three sub-parameters:'negative effects/somatic effects,''menstrual pain,'and'coping methods.'Respondents select one of the following options for the symptoms they experience:"never, rarely, sometimes, often, and always."Never: 1, Rarely: 2, Sometimes: 3, Frequently: 4, Always: 5 points. The MSQ score is obtained by averaging the points given. A higher score indicates more severe menstrual symptoms. Turkish validity and reliability were developed by Güvenç et al. [18].
Menstrual Distress Questionnaire (MDQ)
The Menstrual Distress Questionnaire is comprised of eight subgroups, namely pain, water retention, autonomic reaction, negative affect, impaired concentration, behavioral changes, revitalization, and control, in addition to increased appetite. The scale comprises 47 items and is of the Likert-type. It evaluates PMS symptoms separately for the menstrual, premenstrual, and intermenstrual periods. Each item is scored from 0 to 4, with the maximum score that can be obtained from the scale being 184 for each period. The Menstrual Distress Complaint List was translated into Turkish, and its validity and reliability were evaluated by Kızılkaya [19].
Fatigue Severity Scale (FSS)
The FSS is comprised of nine items in total. Each item on the scale is evaluated according to a Likert-type scale, with scores ranging from 1 to 7. The scale score is calculated by taking the mean of the nine items. A higher score on the scale indicates a greater degree of fatigue. A score of 4 or above is indicative of severe fatigue [20]. The Turkish validity and reliability study was conducted by Armutlu et al. [21].
Pittsburgh Sleep Quality Index (PSQI)
The Pittsburgh Sleep Quality Index (PSQI) was developed as a means of assessing sleep quality. The test comprises 24 questions distributed across ten main headings and is designed to assess sleep quality over the past month. The PSQI comprises seven sub-headings, as follows: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, sleep medication use, and daytime dysfunction. Each component is evaluated on a 0–3-point scale, with a total of 21 points. A score greater than 5 indicates poor sleep quality [22]. Ağargün et al. conducted Turkish validity and reliability studies [23].
International Physical Activity Questionnaire-Short Form (IPAQ)
The International Physical Activity Questionnaire (IPAQ) was developed to assess the duration of various forms of physical activity, including vigorous exercise (e.g., soccer, volleyball, high-speed running, weight lifting), moderate exercise (e.g., light weight-bearing, cycling at moderate speed, dancing, table tennis), walking, and time spent sitting during the day. The short version of the questionnaire assesses the subject's activities over the past seven days. The validity and reliability study of the questionnaire in our country was conducted by Öztürk et al. [24]. To calculate the total score from the questionnaire, the total days and minutes of walking, moderate physical activity, and vigorous physical activity are calculated. The questions in the questionnaire cover a minimum of 10 minutes of activity. The duration of walking, moderate-intensity physical activity, and vigorous physical activity are converted into METs (metabolic equivalents) corresponding to basal metabolic rate to calculate the total physical activity score (MET-min/week). According to the questionnaire, the physical activity score in a category is obtained by multiplying the total minutes and days of activity by the MET equivalent of that activity. These MET values are based on the metabolic rate of a 60-kilogram individual. One MET equals approximately 3.5 O2/kg/min [24].
Data analysis
The data were analyzed using SPSS 15.0 (IBM SPSS Statistics for Windows, Version 15.0. Armonk, NY: IBM Corp) software. To compare the scale scores, parametric methods were employed. Normality was assessed using the Kolmogorov-Smirnov normality test. Independent t-tests were employed for normally distributed measurements, while paired t-tests were utilized to compare pre-and post-treatment measurements within each group. The MSQ, MDQ (premenstrual), MDQ (menstrual), FSS, and IPAQ were analyzed with an Independent T-test due to their normal distribution. As the MSQ, MDQ (premenstrual, menstrual), FSS, PSQI, and IPAQ questionnaires exhibited a normal distribution, an Independent t-test was employed for their analysis. As the data for MDQ (pre-menstrual) post-treatment, MDQ (menstrual) pre-treatment, MDQ (intermenstrual) before and after treatment, and PSQI before and after treatment exhibited a normal distribution, the independent t-test was employed. A Mann-Whitney U test was employed to facilitate comparison between groups, given that the MDQ (intermenstrual) scores exhibited a non-normal distribution. A paired t-test was employed for the intra-group comparison of MDQ, FSS, and PSQI in the exercise group, as well as for the intra-group comparison of MSQ, FSS, PSQI, and IPAQ in the control group, given that the observed differences were normally distributed. The Wilcoxon test was employed for the intragroup comparison of MSQ and IPAQ in the exercise group and the intragroup comparison of MDQ in the control group, given that the differences were normally distributed. The proportions in the groups were compared with the chi-square test, with a statistical significance level of p < 0.05.
Results
This was a randomized clinical study with 54 participants with menstrual symptoms conducted between September 2023 and December 2023. After the group allocation, five people in the exercise group and 4 participants in the control were excluded from the study because they did not complete the exercises or were unwilling to participate. At the end of the study, demographic data of the exercise (n=27) and control (n=27) groups are shown in Table 1. The sociodemographic information indicates that the groups exhibited statistically similar characteristics. Except for the number of pregnancies and age at menarche (p=0.016), no statistically significant differences were observed in women's health-related data, socio-demographic characteristics, menstruation, and lifestyle patterns (p >0.05).
Table 1.
Demographic characteristics of the participants
| Variable |
Exercise (n:27) |
Control (n:27) |
p* |
|---|---|---|---|
| X ±SD | X ±SD | ||
| Age | 30.3±6.3 | 32.7±6.6 | 0.100a |
| Height (cm) | 164.3±5.0 | 163.7±5.5 | 0.761a |
| Weight (kg) | 60.1±6.3 | 59.3±8.1 | 0.843a |
| BMI (kg/m2) | 22.2±2.3 | 22.1±2.4 | 0.749a |
| Number of Birth | 0.3±0.8 | 0.6±0.8 | 0.060a |
| Number of Pregnancy | 0.4±1.2 | 0.7±1.1 | 0.049a |
| Age at first period | 13.4±1.0 | 12.6±1.1 | 0.016a |
| Duration of menstrual period (days) | 6.6±1.5 | 6.3±1.6 | 0.460a |
| Duration of the menstrual cycle (days) | 28.5±4.5 | 29.0±1.9 | 0.380a |
| Employment Status | n (%) | n (%) | |
| Working | 24 (88.9) | 23 (85.2) | 1.000b |
| Not working | 3 (11.1) | 4 (14.8) | |
| Education Status | |||
| Primary school | 0 (0.0) | 1 (3.7) | 0.105b |
| High school | 0 (0.0) | 3 (11.1) | |
| University | 27 (100) | 23 (85.2) | |
| Marital Status | |||
| Single | 18 (66.7) | 12 (44.4) | 0.100b |
| Married | 9 (33.3) | 15 (55.6) | |
| Use of Painkillers During Period | |||
| Sometimes | 14 (51.9) | 7 (25.9) | 0.145b |
| Every time | 5 (18.5) | 7 (25.9) | |
| No | 8 (29.6) | 13 (48.1) | |
| Exercise Habits (previous last 1 year) | |||
| Yes | 13 (48.1) | 7 (25.9) | 0.091b |
| No | 14 (51.9) | 20 (74.1) | |
| Smoking | |||
| No | 20 (74.1) | 21 (77.8) | 0.750b |
| Yes | 7 (25.9) | 6 (22.2) | |
| Alcohol Use | |||
| No | 20 (74.1) | 20 (74.1) | 1.000b |
| Yes | 7 (25.9) | 7 (25.9) | |
X Mean, SD Standard Deviation, BMI Body Mass Index
*p<0.05
aMann Whitney U Test
bChi-Square Test
The comparison differences of the assessments among the groups are presented in Table 2. Within-group analysis showed a significant decrease in MSQ (p=0.001), MDQ (menstrual) (p=0.002), FSS (p=0.003), and PSQI (p=0.001) scores after exercise. In contrast, the IPAQ score increased significantly in the exercise group (p=0.001). In the control group, a significant decrease was observed only in the MDQ (intermenstrual) score (p=0.915).
Table 2.
Comparison difference of MSQ, MDQ, FSS, PSQI, and IPAQ measures before and after the exercise treatment therapy. The change in the scores was calculated by subtracting post-treatment scores from pre-treatment scores
| Rating Scale |
Exercise Group (n:27) X ± SD |
Control Group (n:27) X ± SD |
P1 value |
Exercise Group Change X ± SD |
Control Group Change X ± SD |
P2 value |
|---|---|---|---|---|---|---|
| MSQ | ||||||
| Pre-treatment | 3.0±0.6 | 2.8±0.8 | 0.189a | 0.35±0.30 | −0.06±0.46 | 0.001a |
| Post-treatment | 2.7±0.5 | 2.8±0.8 | 0.437a | |||
| ptime | 0.001c | 0.532d | ||||
| MDQ (pre-menstrual) | ||||||
| Pre-treatment | 40.3±23.5 | 44.2±31.9 | 0.615a | 5.19±20.72 | 2.63±17.28 | 0.626a |
| Post-treatment | 36.0±28.4 | 41.6±33.1 | 0.593b | |||
| ptime | 0.213d | 0.475c | ||||
| MDQ (menstrual) | ||||||
| Pre-treatment | 53.4±28.0 | 48.6±32.2 | 0.320b | 11.88±17.81 | 0.63±17.19 | 0.023a |
| Post-treatment | 41.5±27.0 | 48.0±29.5 | 0.408a | |||
| ptime | 0.002d | 0.976c | ||||
| MDQ (inter-menstrual) | ||||||
| Pre-treatment | 14.9±13.1 | 26.1±25.0 | 0.169b | −0.24±11.08 | 6.33±16.29 | 0.348b |
| Post-treatment | 16.1±12.9 | 19.8±22.0 | 0.927b | |||
| ptime | 0.915d | 0.026c | ||||
| FSS | ||||||
| Pre-treatment | 4.6±1.2 | 4.0±1.5 | 0.134 | 0.55±0.89 | −0.01±1.50 | 0.102a |
| Post-treatment | 4.1±1.3 | 4.1±1.3 | 0.980a | |||
| ptime | 0.003d | 0.986d | ||||
| PSQI | ||||||
| Pre-treatment | 8.1±3.9 | 5.6±2.7 | 0.014b | 2.56±2.26 | 0.22±2.39 | 0.001a |
| Post-treatment | 5.6±3.4 | 5.4±3.1 | 0.701b | |||
| ptime | 0.001d | 0.633d | ||||
| IPAQ-Short form | ||||||
| Pre-treatment | 1192.8±549.2 | 814.5±442.0 | 0.007a | −571.8±435.4 | −35.4±404.3 | 0.001a |
| Post-treatment | 1764.6±685.1 | 849.9±509.1 | 0.001a | |||
| ptime | 0.001c | 0.653d | ||||
X Mean, SD Standard Deviation, ptime Within-group treatment difference (p), P1 value Inter-group comparison of post and pre-treatment values (p), P2 value Inter-group treatment difference (p). MSQ Menstrual Symptom Questionnaire, MDQ Menstrual Distress Questionnaire, FSS Fatigue Severity Scale, PSQI Pittsburgh Sleep Quality Index, IPAQ International Physical Activity Questionnaire-Short From.
*p<0.05
aIndependent T-test
bMann Whitney U Test
cWilcoxon Test
dDependent T-test
No significant difference was observed between the exercise and the control group in the pre-treatment MSQ (p=0.189), MDQ (pre-menstrual, menstrual, inter-menstrual) (p=0.615, 0.320, 0.169), and FSS scores (p=0.134). However, the exercise group exhibited statistically higher values than the control group in the PSQI (p=0.014) and IPAQ scores (p=0.007). There was no significant difference between the exercise group and the control group in terms of MSQ (p=0.437), MDQ (pre-menstrual, menstrual, inter-menstrual) (p=0.593, p=0.408, p=0.927), and PSQI (p=0.701) scores after treatment, whereas the values of the exercise group were statistically higher than the control group in the IPAQ score (p=0.001).
A comparison of the pre-treatment and post-treatment changes in the exercise and control groups revealed a significant decrease in MSQ (p=0.001), MDQ (menstrual) (p=0.023), and PSQI (p=0.001) scores and an increase in IPAQ scores in the exercise group compared to the control group (p=0.001). However, the decrease in MDQ (pre-menstrual and intermenstrual) (p=0.626, p=0.348) and FSS (p=0.102) scores was not statistically different between the groups.
Discussion
Our study was the first to investigate the effects of whole-body strengthening, flexibility, balance, and aerobic exercises on menstrual symptom severity, sleep quality, fatigue severity, and physical activity level in people with menstrual symptoms. The primary outcome was menstrual symptom severity. The secondary outcomes included sleep quality, fatigue severity, and physical activity level. The main results of the present study were: (i) The exercise group exhibited a marked reduction in MSQ, MDQ (menstrual), FSS, and PSQI scores, accompanied by an increase in IPAQ scores in post-treatment; (ii) Compared with the control group, decrease in MSQ, MDQ (menstrual), and PSQI scores were significantly higher in exercise group; (ii) The participants receiving exercise treatment showed a significant increase in IPAQ score in comparison to non-exercising women with the menstrual syndrome.
Early age of menarche is associated with the early onset of ovarian function and the fluctuation of steroid hormones in individuals who lack physical and psychological maturity [10]. In another study, it was stated that the early age of menarche increased the complaints of dysmenorrhea [25]. Furthermore, a reduction in the number of pregnancies has been linked to an elevated risk of developing more severe PMS symptoms [26]. In our study, the exercise and control groups were distributed in a heterogeneous population concerning age at menarche and the number of pregnancies. The mean age at menarche was lower, and the mean number of pregnancies was higher in the control group. Given that no differences were observed between the groups in the pre-treatment evaluation of the MSQ and MDQ questionnaires, it can be concluded that the heterogeneity of the number of pregnancies and menarche age did not exert any influence on the course of the study.
It has been demonstrated that aerobic exercise is effective in alleviating physical symptoms associated with PMS and dysmenorrhea, including bloating, vomiting, hot flashes, and increased appetite [27]. Another study demonstrated that aerobic exercise is an effective intervention for reducing both physical and psychological symptoms in individuals with PMS and PMDD [5]. Vaghela et al. demonstrated that yoga and physical exercise can alleviate the pain severity and PMS after four weeks however, the yoga group exhibited greater improvements than the aerobic exercise group [28]. In women with menstrual abnormalities, regular exercise has been demonstrated to reduce menstrual irregularities [29]. In another study, functional exercise programs have been shown to be effective on the MSQ, PSQI, and low back and abdominal pain of dysmenorrhea patients [30]. Core exercise programs have been shown to improve the pain level and quality of life of people with dysmenorrhea [31]. Another meta-analysis study stated that menstrual pain can be reduced with aerobic exercise, strengthening, and flexibility exercises [32]. In this study, whole-body strengthening, flexibility, balance, and aerobic exercises were applied in combination and alleviated symptoms such as menstrual pain and negative somatic effects. Therefore, the notable decline in MSQ scores among the exercise group is consistent with the findings of previous studies.
Menstruation-related symptoms, including bleeding, pain, fatigue, and mood changes, collectively referred to as menstrual distress, have a significant impact on a woman's physical, social, and emotional well-being [33]. Previous research has demonstrated that menstruation affects mood and cognitive function, potentially resulting in negative experiences that give rise to menstrual-related concerns and difficulties in coping with them [34]. PMS and PMDD are thought to occur during the luteal phase of the menstrual cycle, approximately one week before menstruation. It is reported that these conditions resolve with the onset of menstruation [7, 9]. In this study, the data related to the pre-menstrual period were based on 1 week before menstruation. There were no significant changes in the MDQ (pre-menstrual) scores before and after treatment in the distress scores of the exercise and control groups. Accordingly, the absence of a reduction in the MDQ score for the menstrual period is inconsistent with the existing literature. This may be related to psychosocial factors or life stressors beyond the control of the current study. The prevalence of symptoms among women with PMS and PMDD is higher during the luteal phase. However, these women also report experiencing insomnia, inattention, fatigue, and memory problems during the follicular phase. The existing literature emphasizes the importance of physical activity in managing PMS and PMDD [9]. The findings of this study support the association between exercise and menstrual symptoms. Many factors can affect women's menstrual symptoms, such as poor eating habits, inactive lifestyles, alcohol and smoking habits, anxiety, and stress [9, 35]. In this study, the MDQ score of the inter-menstrual period decreased significantly in the control group despite the absence of any physical exercise. However, the control group's change did not lead to a significant difference in between-group comparisons, indicating possible lifestyle changes of participants.
Fatigue is one of the most common menstrual symptoms [36]. In women with heavy menstrual bleeding, it was found that ferritin levels and physical functions decreased, and fatigue increased in parallel with the increase in the duration of menstruation [37]. People with moderate levels of physical activity showed an effective reduction in PMS symptoms such as sleepiness, fatigue, pain, and increased appetite [35] Although there was no significance between the groups, the decrease in fatigue severity in the exercise group is in parallel with the literature.
In our study, although the exercise group had significantly worse sleep quality than the control group before treatment, the improvement in sleep quality after exercise was significant compared to the control group. This may be supported by studies suggesting that exercise improves sleep quality [38, 39]. Given that circadian rhythms are irregular in individuals with sleep disorders, the release of gonadotropin-releasing hormone from the pituitary gland is inhibited, which in turn increases the severity of menstrual symptoms [7]. A correlation has been identified between increased sleepiness, decreased sleep quality, and short sleep duration with the onset of dysmenorrhea, PMS, and irregular menstruation [7]. It has been reported that 12-week Pilates exercises improved sleep quality in women with dysmenorrhea [40]. In particular, studies have demonstrated that moderate-intensity exercise programs conducted three days a week for 12 weeks to six months have the greatest impact on sleep quality [41].
The existing literature indicates that high levels of physical activity are associated with a reduction in the average duration of menstruation, the incidence of dysmenorrhea and polymenorrhea, and PMS [14]. Another study found a significant increase in dysmenorrhea complaints due to decreased physical activity levels during the pandemic period [42]. Therefore, the increased level of physical activity in the exercise group in this study may also be associated with reduced symptoms in this group.
The precise mechanism through which exercise can alleviate menstrual symptoms remains uncertain. However, it is postulated that this may be due to an increase in blood flow, the production of anti-inflammatory compounds, and a reduction in aldosterone levels as a consequence of exercise. An increase in blood flow fastens the transfer of wastes and prostaglandins, which are the main cause of menstrual pain, from the body [27]. In addition, prior studies have demonstrated that engaging in physical activity is advantageous in regulating hippocampal hormonal levels, consequently aiding in the maintenance of hippocampal plasticity, cognition, and emotional stability [43, 44]. Consequently, these mechanisms could potentially result in a decrease in the prevalence of menstrual symptoms, as demonstrated by our study.
This research is the first to evaluate the efficacy of whole-body strengthening, flexibility, balance, and aerobic exercises in women with menstrual syndromes and to examine their functional and patient-reported outcomes. The exercise program of this study differs from others in the literature in several key ways. Primarily, it integrates aerobic, strengthening, flexibility, and balance exercises, adopting a more holistic approach compared to single-type exercise strategies [45, 46] Furthermore, the intensity of exercise is determined by a talk test, following the recommendations of the World Health Organization, thus offering a more objective approach [17]. Whereas other studies tend to concentrate on a single type of exercise or shorter-term programs, our program incorporates a total of 150 minutes of moderate-intensity walking weekly, in addition to strengthening, flexibility, and balance exercises on two days per week, to achieve a more consistent and comprehensive impact. However, it is important to consider the limitations. Firstly, the study relied on self-report data, which may have resulted in social acceptability and recall biases. Secondly, the assessment of heart rate and other objective indicators of exercise, in addition to the use of standard instruments for self-reported physical activity, were not included in the study design. Thirdly, considering the nature of our study, it was not feasible to blind the participants and the evaluator concerning group allocation. In addition, the investigation failed to explore the lasting effects of the exercise regarding the sustainability generalizability of the outcomes. It is, therefore essential to implement an extended period of physical exercise and prolonged surveillance to ascertain whether the benefits of the exercise program are sustained in the long term without the need for sustained intervention. Lastly, the study population was selected from individuals presenting with any one or more of the symptoms associated with the menstrual cycle. Therefore, more comprehensive and evidence-based data-driven studies are needed to analyze the specific effect of exercise on menstruation-related symptoms with larger samples sizes.
Conclusions
In the present study, we have demonstrated that enhanced muscle awareness, correct muscle usage, and strengthening through prescribed exercise programs may be effective in relieving menstrual symptoms. The study indicated that these approaches can be safely self-administered and serve as a convenient, cost-effective, and non-invasive adjuvant therapy that does not require any intrusive procedures. The whole-body strengthening, flexibility, balance, and aerobic exercises have been demonstrated to be effective in reducing menstrual symptoms in terms of both functional outcomes and sleep quality.
Acknowledgments
Not applicable.
Abbreviations
- MSQ
Menstrual Symptom Questionnaire
- MDQ
Menstrual Distress Questionnaire
- FSS
Fatigue Severity Scale
- PSQI
Pittsburgh Sleep Quality Index
- IPAQ
International Physical Activity Questionnaire
Authors’ contributions
MK and ÖŞ analyzed and interpreted the patient data. ÖŞ was a major contributor to writing the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Data availability
Data sets generated during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted after obtaining ethical approval from the Uskudar University Non-Interventional Research Ethics Committee with reference number 61351342/December 2022-67. Informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mitsuhashi R, Sawai A, Kiyohara K, Shiraki H, Nakata Y. Factors associated with the prevalence and severity of menstrual-related symptoms: a systematic review and meta-analysis. Int J Environ Res Public Health. 2023;20:569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrero S, Abbamonte LH, Giordano M, Alessandri F, Anserini P, Remorgida V, et al. What is the desired menstrual frequency of women without menstruation-related symptoms? Contracep. 2006;73(5):537–41. [DOI] [PubMed] [Google Scholar]
- 3.Arafa AE, Senosy SA, Helmy HK, Moamed AA. Prevalence and patterns of dysmenorrhea and premenstrual syndrome among Egyptian girls (12–25 years). Middle East Fertil Soc J. 2018;23 (4):486-490.
- 4.Ferries-Rowe E, Corey E, Archer JS. Primary dysmenorrhea: diagnosis and therapy. Obst Gynecol. 2020;136(5):1047–58. [DOI] [PubMed] [Google Scholar]
- 5.Liguori F, Saraiello E, Calella P. Premenstrual syndrome and premenstrual dysphoric disorder’s impact on quality of life, and the role of physical activity. Medicina (Lithuania). 2023;59(11):2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gudipally PR, Sharma GK. Premenstrual Syndrome .- StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. [PubMed]
- 7.Jeon B, Baek J. Menstrual disturbances and its association with sleep disturbances: a systematic review. BMC Womens Health. 2023;23(1):470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Kang B, Zhao X, Cui X, Chen J, Wang L. Association between depression and dysmenorrhea among adolescent girls: multiple mediating effects of binge eating and sleep quality. BMC Womens Health. 2023;23(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin PC, Ko CH, Lin YJ, Yen JY. Insomnia, inattention and fatigue symptoms of women with premenstrual dysphoric disorder. Int J Environ Res Public Health. 2021;18(12):6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eshetu N, Abebe H, Fikadu E, Getaye S, Jemal S, Geze S, et al. Premenstrual syndrome, coping mechanisms and associated factors among Wolkite university female regular students, Ethiopia, 2021. BMC Womens Health. 2022;22(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarı Çetin H, Erbil N. Premenstrual Sendromda Ağrı Yönetimi. Ordu Üniversitesi Hemşirelik Çalışmaları Dergisi. 2020;3 (2): 202-210.
- 12.Mizuta R, Maeda N, Komiya M, Suzuki Y, Tashiro T, Kaneda K, et al. The relationship between the severity of perimenstrual symptoms and a regular exercise habit in Japanese young women: a cross-sectional online survey. BMC Womens Health. 2022;22(1):200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selvi Öztürk, Anita Karaca. The role of midwife and nurse in relation to premenstrual symptoms and healthy lifestyle behaviors. .2019 8(2):105-10
- 14.Güney E, Ünver H, Aksoy Derya Y, Uçar T. Fiziksel Egzersiz Düzeylerinin Menstrual Siklusa Etkileri. DÜ Sağlık Bil Enst Derg. 2017;7 (3):137-142.
- 15.Türkan A, Kaya S.. Kadın Sağlığında Fizyoterapi ve Rehabilitasyon. Kalkan Matbaacılık, Ankara; 2016.
- 16.Park YJ, Shin H, Jeon S, Cho I, Park HJ. Development and effects of college-based lifestyle modification program for menstrual health of young adult women with irregular menses: A randomized controlled trial. Int J Environ Res Public Health. 2021;18(1):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster C, Porcari JP, Anderson J, Paulson M, Smaczny D, Webber H, et al. The talk test as a marker of exercise training intensity. J Cardiopulm Rehabil Prev. 2008;28(1):24–30; quiz 31-2. [DOI] [PubMed] [Google Scholar]
- 18.Güvenç G, Seven M, Akyüz A. Adaptation of the menstrual symptom questionnaire into Turkish. TAF Preventive Medicine Bulletin. 2014;13 :367-374.
- 19.Kizilkaya N, Tuncel N. Perimenstrual Şikayetlerin Hafifletilmesinde Hemşirelik Girişimlerinin Etkinliği. Florence Nightingale Hemşirelik Dergisi. 2015;8 (32):66-79.
- 20.Krupp LB, Larocca NG, Muir Nash J, Steinberg AD. The fatigue severity scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121–3. [DOI] [PubMed] [Google Scholar]
- 21.Armutlu K, Cetisli Korkmaz N, Keser I, Sumbuloglu V, Irem Akbiyik D, Guney Z, et al. The validity and reliability of the Fatigue Severity Scale in Turkish multiple sclerosis patients. Int J Rehab Res. 2007;30(1):81–5. [DOI] [PubMed] [Google Scholar]
- 22.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 23.Ağargün MY, Kara H AÖ. The validity and reliability of the Pittsburgh Sleep Quality Index. Turkish journal of psychiatry. 1996;7 (2):107-115.
- 24.Öztürk M. Üniversitede Eğitim-Öğretim Gören Öğrencilerde Uluslararası Fiziksel Aktivite Anketinin Geçerliliği Ve Güvenirliği ve Fiziksel Aktivite Düzeylerinin Belirlenmesi. Yüksek Lisans Tezi, HAcettepe ÜNİVERSİTESİ SAĞLIK BİLİMLERİ ENSTİTÜSÜ, Ankara. 2005.
- 25.Sönmez T, Çapik A, Akkaş M. Evaluation of symptoms related to menstruation period in midwifery students. Anadolu Hemşirelik ve Sağlık Bilim Dergisi. 2019;22:25–32. [Google Scholar]
- 26.Burcu Önal. Premenstrual Sendromda Risk Faktörleri ve Tedavi Arama Davranışının Araştırılması. Dokuz Eylül Üniversitesi Tıp Fakültesi Aile Hekimliği Anabilim Dalı; 2011.
- 27.Dehnavi Z, Jafarnejad F, Kamali Z. The Effect of aerobic exercise on primary dysmenorrhea: A clinical trial study. J Educ Health Promot. 2018;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaghela N, Mishra D, Sheth M, Dani VB. To compare the effects of aerobic exercise and yoga on Premenstrual syndrome. J Educ Health Promot. 2019;8:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.İmamoğlu PA-O. Kardi̇yo bosu egzersi̇zi̇ni̇n sedanter kadinlarda menstruasyon, stres ve depresyon üzeri̇ne etki̇leri̇. Journal of Turkish Studies. 2019;14(6) :2939-2948.
- 30.Kirmizigil B, Demiralp C. Effectiveness of functional exercises on pain and sleep quality in patients with primary dysmenorrhea: a randomized clinical trial. Arch Gynecol Obstet. 2020;302(1):153–63. [DOI] [PubMed] [Google Scholar]
- 31.Sinem Bağcı. The effect of core exercises on pain and quality of life in young people with primary dysmenorrhea: a non-randomized study. PhD Thesis. Necmettin Erbakan Üniversitesi; 2021.
- 32.Carroquino-Garcia P, Jiménez-Rejano JJ, Medrano-Sanchez E, De La Casa-Almeida M, Diaz-Mohedo E, Suarez-Serrano C. Therapeutic exercise in the treatment of primary dysmenorrhea: a systematic review and meta-analysis. Physical Therapy. 2019;99(10):1371–80. [DOI] [PubMed] [Google Scholar]
- 33.Matteson KA, Zaluski KM. Menstrual health as a part of preventive health care. Obst Gynecol Clin North Am. 2019;46(3):441–53. [DOI] [PubMed] [Google Scholar]
- 34.Vannuccini S, Rossi E, Cassioli E, Cirone D, Castellini G, Ricca V, et al. Menstrual Distress Questionnaire (MEDI-Q): a new tool to assess menstruation-related distress. Reprod Biomed Online. 2021;43(6):1107–16. [DOI] [PubMed] [Google Scholar]
- 35.Kawabe R, Chen CY, Morino S, Mukaiyama K, Shinohara Y, Kato M, et al. The relationship between high physical activity and premenstrual syndrome in Japanese female college students. BMC Sports Sci Med Rehabil. 2022;14(1):175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernández-Martínez E, Onieva-Zafra MD, Abreu-Sánchez A, Fernández-Muñóz JJ, Parra-Fernández ML. Absenteeism during menstruation among nursing students in Spain. Int J Environ Res Public Health. 2020;17 (1):53. [DOI] [PMC free article] [PubMed]
- 37.Kocaoz S, Cirpan R, Degirmencioglu AZ. The prevalence and impacts heavy menstrual bleeding on anemia, fatigue and quality of life in women of reproductive age. Pak J Med Sci. 2019;35(2):365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ayşenur Tuncer, Fatih Enzin, Sevgi Gamze Felek İri, Elif Dinler, Zerrin Pelin, Kezban Bayramlar. The importance of exercise therapy in disorders. Zeugma Sağlık Araştırmaları Dergisi. 2020;2 (2):89-97.
- 39.Vanderlinden J, Boen F, Van Uffelen JGZ. Effects of physical activity programs on sleep outcomes in older adults: A systematic review. International Journal of Behavioral Nutrition and Physical Activity. 2020;17(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song BH, Kim J. Effects of Pilates on Pain, Physical Function, Sleep Quality, and Psychological Factors in Young Women with Dysmenorrhea: A Preliminary Randomized Controlled Study. Healthcare (Switzerland). 2023;11(14):2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sullivan Bisson AN, Robinson SA, Lachman ME. Walk to a better night of sleep: testing the relationship between physical activity and sleep. Sleep Health. 2019;5(5):487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ayhan T, Yildiz A, Bektaş G, Büyükturan B, Büyükturan Ö, Varol S. Pandeminin Fiziksel Aktivite ve Dismenoreye Etkisinin Birlikte İncelenmesi. Turk J Health Sport (TJHS). 2023;TJHS Vol:4 Issue:1 TJHS Vol:4 Issue:1 :17-21.
- 43.Tsai SY. Effect of yoga exercise on premenstrual symptoms among female employees in Taiwan. Int J Environ Res Public Health. 2016;13(7):721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dehnavi ZM, Zadeh MN, Sadeghi S, Jafanejad F, Mojahedy M, Shakeri Mohammad Taghi and Sardar MA, et al. Study of the effects of 8 weeks of regular aerobic exercise on the intensity of premenstrual syndrome symptoms. Pharmacophore. 2017;8 (6).
- 45.Ravichandran H, Janakiraman B. Effect of Aerobic Exercises in Improving Premenstrual Symptoms Among Healthy Women: A Systematic Review of Randomized Controlled Trials. Int J Women’s Health. 2022;14:1105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maged AM, Abbassy AH, Sakr HRS, Elsawah H, Wagih H, Ogila AI, et al. Effect of swimming exercise on premenstrual syndrome. Arch Gynecol Obstet. 2018;297(4):951–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sets generated during the current study are available from the corresponding author upon reasonable request.