Abstract
Background
Small bowel obstruction (SBO) is a prevalent gastrointestinal disorder that consists primarily of two types: simple bowel obstruction (SiBO) and strangulated bowel obstruction (StBO). Due to life-threatening complications such as septic shock and multiple organ dysfunction syndrome, there is an urgent need for an easy-to-acquire predictive model for StBO based on clinical symptoms and laboratory.
Methods
A total of 453 patients diagnosed with SBO were randomly divided into training and validation datasets at a ratio of 7:3. The demographic, clinical, and laboratory data were collected. Least absolute shrinkage and selection operator (LASSO) regression was employed to identify relevant variables, and a multivariable logistic regression (LR) model was subsequently developed. The performance of the model was evaluated using receiver operating characteristic (ROC) curve analysis, and diagnostic metrics, including accuracy, sensitivity, specificity, and area under the curve (AUC), were calculated.
Results
Of the 453 patients diagnosed with SBO, 62 (13.7%) had StBO, and 391 (86.3%) had SiBO. Univariate analysis revealed significant associations between bowel ischemia and the following variables: body mass index (BMI, p = 0.027), neutrophil percentage (N, p = 0.002), aspartate aminotransferase (AST, p = 0.024), serum creatinine (p = 0.030), serum urea (p = 0.019), glucose (p = 0.029), prothrombin time (PT, p = 0.043), cessation of defecation and flatus (p = 0.013), tenderness (p = 0.004), and rebound tenderness (p < 0.001). A LASSO regression model with optimized regularization parameters (α = 0.3, λ = 0.0202; log[λ] = − 3.902) was used to select 10 predictors. Rebound tenderness (OR, 6.64; 95% CI, 2.97–15.48; p < 0.001), BMI (OR, 0.02; 95% CI, 0.00–0.37; p = 0.010), N (OR, 1.05; 95% CI,1.01–1.09; p = 0.009), and AST (OR, 1.97; 95% CI, 1.01–4.06, p = 0.055) were significantly associated with intestinal ischemia via multivariable LR. The final predictive model (BAR-N) had a strong performance, with an AUC of 0.784 in the training cohort and 0.750 in the validation cohort. Additionally, the model exhibited high specificity (90.3%) and accuracy (80.7%), although its sensitivity remained relatively low at 31.8%.
Conclusions
We developed an easy-to-acquire predictive model (BAR-N) for the diagnosis of StBO that incorporates both clinical and laboratory data. This model shows promise as an adjunctive decision-support tool, particularly in resource-limited or high-acuity settings. However, its generalizability is limited by the absence of external validation, underscoring the need for future multicenter studies to confirm its broader applicability.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12893-025-03045-x.
Keywords: Strangulated bowel obstruction, Predictive model, LASSO regression, Risk factors.
Introduction
Small bowel obstruction (SBO) is a prevalent gastrointestinal disorder that contributes significantly to morbidity and accounts for 12-16% of all surgical admissions worldwide, including those in the United States [1, 2]. SBOs are classified into two primary categories: simple bowel obstruction (SiBO) and strangulated bowel obstruction (StBO). Although SiBO typically resolves with conservative management such as bowel rest and nasogastric decompression, StBO requires urgent surgical intervention because of its potential to cause life-threatening complications, including bowel ischemia, necrosis, and shock [3–6]. Early and accurate diagnosis of StBO is critical because delayed intervention substantially increases mortality rates, which can be as high as 35% [3, 7].
Differentiating between SiBO and StBO remains a clinical challenge despite advancements in imaging techniques. Traditional diagnostic methods (including clinical examination and radiological imaging, such as computed tomography (CT)) have limited sensitivity and specificity for the detection of the early stages of bowel ischemia [8–10]. CT findings, such as mesenteric fluid, bowel wall edema, and the whirl sign, are commonly used to diagnose bowel ischemia. The diagnostic performance varies, with sensitivity ranging from 73 to 100% but lower specificity [8, 11, 12]. Furthermore, computed tomography angiography (CTA), although considered the gold standard for diagnosing bowel ischemia, is seldom used in emergency settings because of its high cost, limited utilization of contrast-enhanced CT in primary care hospital emergency departments, and the risk of contrast-induced nephropathy [1, 13].
In recent years, interest in developing easily accessible model predictive models based on clinical features and laboratory tests has increased. Several studies have identified biomarkers, such as white blood cell (WBC) count, blood urea nitrogen (BUN), sodium, and neutrophil percentage (NE%), that may serve as predictors of StBO [1, 14]. When incorporated into multidimensional models, these biomarkers offer the potential for a more effective and cost-efficient diagnostic approach, which could reduce unnecessary surgeries and improve patient outcomes [14–20].
This study aims to develop an easy-to-acquire predictive model that integrates clinical symptoms and laboratory results to distinguish StBO from SiBO. We hypothesize that this multidimensional approach will increase diagnostic accuracy and guide timely intervention.
Methods
Study population
This retrospective cohort study included 481 patients initially diagnosed with SBO between November 2016 and March 2024 at the Department of Emergency Surgery, Union Hospital, Fujian Medical University. Patients with a confirmed diagnosis of non-SBO (n = 10) and those with incomplete clinical data (n = 18) were excluded. StBO was diagnosed based on contrast-enhanced abdominal CT findings suggestive of bowel ischemia—such as reduced or absent bowel wall enhancement, mesenteric swirl sign, pneumatosis intestinalis, portal venous gas, or mesenteric edema—and confirmed intraoperatively and/or by postoperative histopathology demonstrating ischemia, necrosis, or infarction. Ultimately, 453 patients were enrolled: 391 patients with SiBO and 62 patients with StBO (Fig. 1). The study protocol was approved by the Institutional Review Board of Fujian Medical University Union Hospital, and all patients provided written informed consent.
Fig. 1.
Flowchart for patients enrollment
Data collection
Demographic and clinical data, including age, sex, body mass index (BMI), and comorbidities such as hypertension (HTN) and diabetes mellitus (DM), were collected. Symptoms, including vomiting, abdominal distension, cessation of defecation and flatus, tenderness, rebound tenderness, and a history of abdominal surgery, were recorded. Vital signs, including temperature, were also documented. The laboratory data included WBC count, monocyte absolute value (Monocyte), neutrophil percentage (N), lymphocyte absolute value (LC), platelet count (PLT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), hemoglobin (HB), albumin (ALB), calcium (CA), chloride (CL), creatinine (CR), urea (Urea), potassium (K), sodium (NA), glucose (Glucose), bicarbonate (HCO3), D-dimer (DDI), fibrinogen (FIB), activated partial thromboplastin time (APTT), international normalized ratio (INR), prothrombin time (PT), alpha-fetoprotein (AFP), cancer antigen 125 (CA125), cancer antigen 19 − 9 (CA199), and carcinoembryonic antigen (CEA). All the data were extracted from the hospital’s electronic medical records.
Statistical analysis
All the statistical analyses were performed using R software (version 4.3.3; R Foundation for Statistical Computing, Vienna, Austria).
Data preprocessing
Variables with more than 20% missing values (e.g., lactate, base excess [BE], and C-reactive protein [CRP]) were excluded from the analysis. For the remaining variables, missing data were handled using multiple imputation by chained equations (MICE). Five imputed datasets were generated and pooled using Rubin’s rules to account for between-imputation variability. Sensitivity analyses comparing imputed and complete-case datasets were planned to assess the robustness of the model to missing data assumptions.
All continuous variables were assessed for skewness. Variables with right-skewed or non-normal distributions were log-transformed using the natural logarithm to improve normality and linearity. For variables with zero or negative values, a constant (0.001) was added prior to transformation to ensure computational validity. Notably, neutrophil percentage (N) and body temperature were retained in their original form, as log transformation did not normalize their distributions and resulted in overly wide confidence intervals.
To address class imbalance—specifically the relatively small number of patients with StBO—and to prevent information leakage, the dataset was randomly divided into training (70%) and validation (30%) subsets using stratified sampling via the createDataPartition() function in the caret package, ensuring balanced outcome distribution across subsets. Multicollinearity was evaluated using the variance inflation factor (VIF), and variables with VIF > 10 were excluded from further analysis. Baseline characteristics between the ischemia and non-ischemia groups were compared using the scitb1 function in R (Table 1).
Table 1.
Baseline clinical characteristics of patients with SiBO and StBO
| Characteristic | SiBO(n=391) | StBO(n=62) | p-value |
|---|---|---|---|
| BMI_log | 3.03±0.14 | 2.99±0.14 | 0.027 |
| age_log | 4.02±0.32 | 4.08±0.27 | 0.191 |
| HB_log | 4.78±0.19 | 4.82±0.21 | 0.195 |
| N_raw | 73.53±12.57 | 78.99±12.30 | 0.002 |
| PLT_log | 5.33±0.39 | 5.34±0.36 | 0.861 |
| ALT_log | 2.73±0.65 | 2.85±0.66 | 0.157 |
| AST_log | 3.04±0.51 | 3.20±0.69 | 0.024 |
| ALB_log | 3.55±0.17 | 3.53±0.21 | 0.387 |
| CA_log | 0.75±0.09 | 0.76±0.09 | 0.417 |
| CL_log | 4.62±0.05 | 4.62±0.04 | 0.872 |
| CR_log | 4.26±0.39 | 4.38±0.41 | 0.030 |
| K_log | 1.39±0.13 | 1.41±0.17 | 0.210 |
| NA_log | 4.93±0.03 | 4.93±0.03 | 0.983 |
| Urea_log | 1.72±0.53 | 1.89±0.54 | 0.019 |
| Glucose_log | 1.93±0.40 | 2.05±0.36 | 0.029 |
| HCO3_log | 3.14±0.14 | 3.11±0.21 | 0.114 |
| APTT_log | 3.63±0.16 | 3.63±0.19 | 0.860 |
| DDI_log | 0.53±0.97 | 0.76±1.04 | 0.084 |
| FIB_log | 1.32±0.37 | 1.32±0.38 | 0.908 |
| INR_log | 0.07±0.12 | 0.10±0.17 | 0.203 |
| PT_log | 2.64±0.10 | 2.67±0.14 | 0.043 |
| AFP_log | 0.62±0.59 | 0.65±0.53 | 0.729 |
| CA125_log | 3.05±1.13 | 3.06±1.10 | 0.944 |
| CA199_log | 2.12±1.18 | 2.21±1.09 | 0.560 |
| CEA_log | 0.69±1.04 | 0.58±1.16 | 0.460 |
| Temperature_log | 36.67±0.47 | 36.61±0.39 | 0.328 |
| gender | 0.293 | ||
| 0 | 137 (35.04%) | 26 (41.94%) | |
| 1 | 254 (64.96%) | 36 (58.06%) | |
| HTN | 0.562 | ||
| 0 | 321 (82.10%) | 49 (79.03%) | |
| 1 | 70 (17.90%) | 13 (20.97%) | |
| DM | 0.588 | ||
| 0 | 365 (93.35%) | 59 (95.16%) | |
| 1 | 26 (6.65%) | 3 (4.84%) | |
| vomiting | 0.376 | ||
| 0 | 162 (41.43%) | 22 (35.48%) | |
| 1 | 229 (58.57%) | 40 (64.52%) | |
| abdominal_distention | 0.463 | ||
| 0 | 145 (37.08%) | 20 (32.26%) | |
| 1 | 246 (62.92%) | 42 (67.74%) | |
| cessation_of_defecation_and_flatus | 0.013 | ||
| 0 | 173 (44.25%) | 17 (27.42%) | |
| 1 | 218 (55.75%) | 45 (72.58%) | |
| tenderness | 0.004 | ||
| 0 | 87 (22.25%) | 4 (6.45%) | |
| 1 | 304 (77.75%) | 58 (93.55%) | |
| rebound_tenderness | <0.001 | ||
| 0 | 332 (84.91%) | 31 (50.00%) | |
| 1 | 59 (15.09%) | 31 (50.00%) | |
| abdominal_surgery | 0.909 | ||
| 0 | 129 (32.99%) | 20 (32.26%) | |
| 1 | 262 (67.01%) | 42 (67.74%) |
SiBO simple bowel obstruction, StBO strangulated bowel obstruction, BMI body mass index, HB hemoglobin, N neutrophil percentage, PLT platelet count, ALT alanine aminotransferase, AST aspartate aminotransferase, ALB albumin, CA calcium, CL chloride, CR creatinine, K potassium, NA sodium, HCO3 bicarbonate, APTT activated partial thromboplastin time, DDI D-dimer, FIB fibrinogen, INR international normalized ratio, PT prothrombin time, AFP alpha-fetoprotein, CA125 cancer antigen 125, CA199 cancer antigen 19-9, CEA carcinoembryonic antigen
Feature selection using LASSO regression
Feature selection was performed using the least absolute shrinkage and selection operator (LASSO) regression with the glmnet package in R. To optimize model sparsity and prevent overfitting, a series of elastic net mixing parameters (alpha values ranging from 0 to 1 in 0.1 increments) were systematically evaluated (Table 2; Fig. 2). For each alpha, 10-fold cross-validation was applied within the training cohort to identify the optimal regularization parameter (lambda) that minimized binomial deviance. The alpha value corresponding to the lowest mean cross-validation error (α = 0.3) was selected. Variables with non-zero coefficients at the optimal lambda were retained for subsequent model development.
Table 2.
Cross-validation (CV) errors for different alpha values in LASSO regression model tuning
| Alpha | CV Error |
|---|---|
| 0.0 | 0.7168408 |
| 0.1 | 0.7004462 |
| 0.2 | 0.7012206 |
| 0.3 | 0.6847877 (lowest error) |
| 0.4 | 0.7090998 |
| 0.5 | 0.6959095 |
| 0.6 | 0.6930266 |
| 0.7 | 0.7006909 |
| 0.8 | 0.6944237 |
| 0.9 | 0.6997921 |
| 1.0 | 0.6871141 |
Fig. 2.

Optimization of alpha parameter in elastic net regression. The red dashed line represents the optimal alpha value (α = 0.3), selected based on the minimum mean cross-validation error during model tuning
To prevent information leakage, all feature selection steps—including hyperparameter tuning and model fitting—were strictly limited to the training dataset. The finalized set of selected variables was then applied to an independent validation cohort for external performance evaluation.
Model development and evaluation
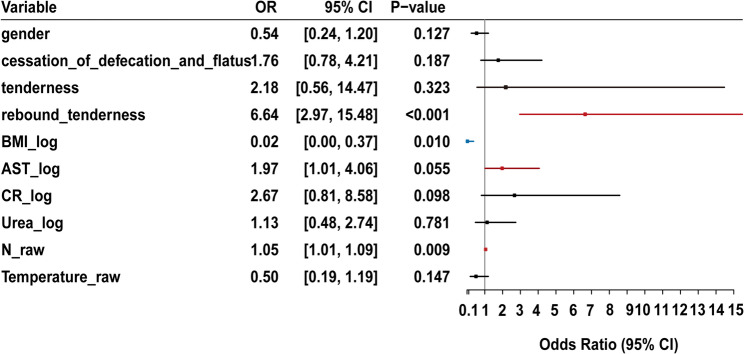
Following variable selection via LASSO regression, a multivariable logistic regression model was developed using the selected predictors. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to assess the strength and direction of association between each predictor and the outcome. A forest plot was generated to visualize effect sizes and statistical significance. Predictors with statistically significant associations (p < 0.05) were retained in the final model.
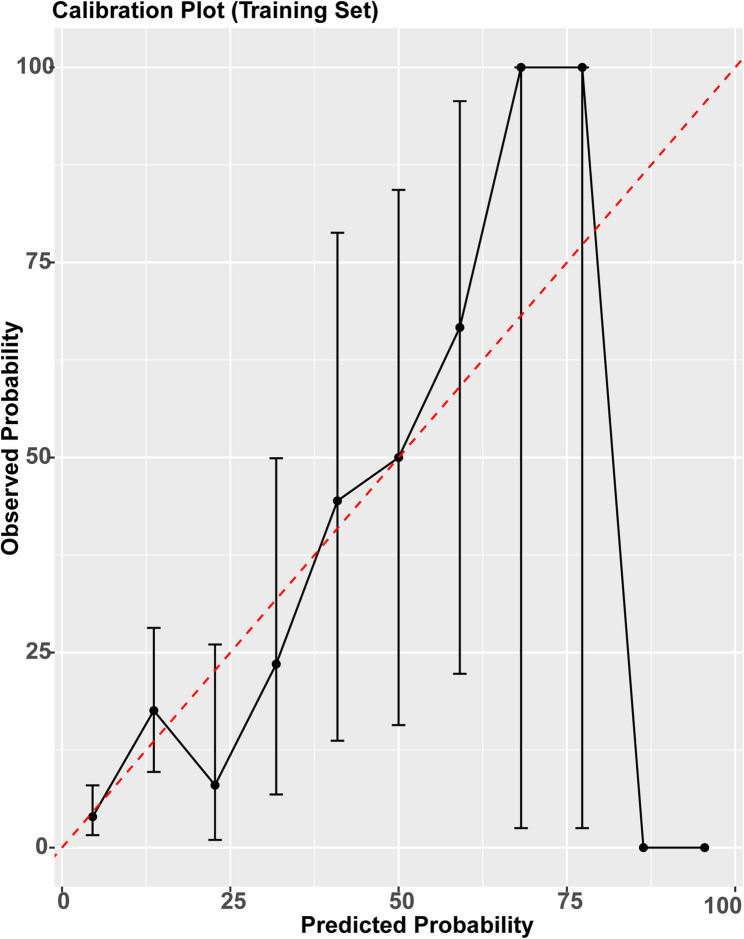
Model discrimination was evaluated using receiver operating characteristic (ROC) curve analysis. The area under the ROC curve (AUC) was computed for both the training and validation cohorts to assess the model’s ability to distinguish between patients with and without strangulated bowel obstruction (StBO). In addition, secondary performance metrics—including sensitivity, specificity, positive predictive value (precision), negative predictive value (NPV), accuracy, and F1-score—were calculated based on the validation set to provide a comprehensive evaluation of model performance. Model calibration was assessed using calibration curves and Brier scores. Calibration plots were generated for both the training and validation sets using bootstrapped resampling (1,000 iterations), and Brier scores were calculated to quantify the overall prediction error (Fig. 7).
Fig. 7.
Calibration curve of the model in the training set. The x-axis shows the predicted probability of StBO, while the y-axis shows the observed frequency. The dashed diagonal line represents perfect calibration. The solid curve demonstrates that the model's predicted risks closely correspond to actual outcomes, indicating good calibration.
To enhance clinical applicability, a nomogram was constructed using the rms package to provide an individualized, graphical risk prediction tool.
All statistical tests were two-tailed, and a p-value < 0.05 was considered statistically significant.
Results
Study population and data collection
To develop a predictive model, the 453 patients were randomly divided into a training set (70%) and a validation set (30%). In the training set, 278 patients (87.4%) were classified as nonischemic (SiBO), while 40 patients (12.6%) were diagnosed with intestinal ischemia (StBO). In the validation set, 113 patients (83.7%) were classified as nonischemic (SiBO), and 22 patients (16.3%) were diagnosed with intestinal ischemia (StBO). Significant differences in several variables, including BMI (BMI_log, p = 0.027), N (N_raw, p = 0.002), AST (AST_log, p = 0.024), CR (CR_log, p = 0.030), urea (Urea_log, p = 0.019), Glucose (Glucose_log, p = 0.029), PT (PT_log, p = 0.043), cessation of defecation and flatus (cessation_of_defecation_and_flatus, p = 0.013), tenderness (p = 0.004), and rebound tenderness (p < 0.001), were detected between the ischemic and nonischemic groups. These variables were selected as candidates for model development. In contrast, factors such as gender (p = 0.293) and age (age_log, p = 0.191) did not significantly differ between groups (Table 1).
Statistical analysis
Data preprocessing and feature selection
Because of the large number of variables and relatively small sample size, LASSO regression was employed to select the most relevant variables distinguishing SiBO from StBO in the training dataset. LASSO regression was combined with 10-fold cross-validation, and the optimal alpha value was defined as 0.3 for the final model (Fig. 2; Table 2). The optimal penalty parameter, log(λ) = − 3.902 (λ = 0.0202), was chosen, which resulted in 10 variables being retained as significant predictors (Fig. 3): gender, cessation of defecation and flatus, tenderness, rebound tenderness, BMI_log, N_raw, AST_log, CR_log, Urea_log, and Temperature_raw, all of which were ultimately incorporated into the predictive model.
Fig. 3.
(A,B)LASSO regression showed log(λ) = -3.902 when the error of the model is minimized, and 10 variables were selected for further logistic regression analysis.
Forest plot for the final logistic regression model
The final multivariable logistic regression model included 10 predictors selected through LASSO regression. To visually represent the associations between these predictors and the outcome variable, a forest plot was created that illustrated the magnitude and direction of each predictor’s association (Fig. 4). Significant predictors, with confidence intervals (CIs) that do not include the null value (OR = 1), are highlighted to emphasize their relevance in the model. Rebound tenderness (OR, 6.64; 95% CI, 2.97–15.48; p < 0.001), BMI_log (OR, 0.02; 95% CI, 0.00–0.37; p = 0.010), N_raw (OR, 1.05; 95% CI, 1.01–1.09; p = 0.009), and AST (OR, 1.97; 95% CI, 1.01–4.06, p = 0.055) were significantly associated with intestinal ischemia. In contrast, variables such as cessation of defecation and flatus (OR, 1.76; 95% CI, 0.78–4.21; p = 0.187) and Urea_log (OR, 1.13; 95% CI, 0.48–2.74; p = 0.781) were not statistically significant.
Fig. 4.
Forest plot of logistic regression analysis.
Nomogram for the final logistic regression model
To enhance the clinical utility of the model, a nomogram was developed using only predictors that remained statistically significant in the final logistic regression analysis. The nomogram provides an intuitive, user-friendly graphical tool for individualized risk estimation of intestinal ischemia (Fig. 5), highlighting the contribution of each predictor to the overall risk score.
Fig. 5.
The nomogram of the prediction model.
To facilitate interpretation and practical application, we addressed the issue of log-transformed variables (e.g., BMI_log, AST_log) by providing a supplementary conversion table (Table 3), which maps the transformed values to their corresponding raw clinical measurements. This allows clinicians to apply the nomogram without the need for logarithmic calculations, thereby improving its usability in routine clinical settings.
Table 3.
Previous studies on bowel ischemia
| Sample | Methods | Radiomics | Predictors | AUC | Specificity | |
|---|---|---|---|---|---|---|
| Xu WX, 2022 [1] | 281 | UA + LR | Y | pain duration≤3days, rebound tenderness, bowel sounds, potassium, sodium, BUN, radiomics score | 0.86 | 40.0-83.6% |
| Millet, I, 2017 [8] | 256 | UA + MA | Y | bowel wall enhancement, diffuse mesenteric haziness | 0.91 | 27.0-99.0% |
| Zhuang X, 2021 [14] | 132 | UA + LR | N | WBC, BUN, Ln D-dimer | 0.89 | 78.2% |
| Zhu Y, 2024 [21] | 560 | LR | Y | NE%, peritoneal irritation sign, and abdominal fluid, albumin emerged | 0.83 | / |
| Haridimos Markogiannakis, 2011 [22] | 100 | MA | N | PCT | 0.77 | / |
| This study | 481 | Lasso +LR | N | BMI, AST, rebound tenderness, NE% | 0.77 | 97.3% |
BUN blood urea nitrogen, WBC white blood cell count, NE% neutrophil percentage, LR logistic regression, UA univariate analyses, MA multivariate analyses, PCT procalcitonin, BMI body mass index, AST aspartate aminotransferase
Final logistic regression formula
A simplified logistic regression model was developed based on four key predictors: rebound tenderness, BMI_log, N_raw, and AST_log. The final equation is:
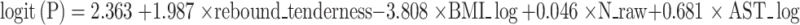 |
where P is the predicted probability of intestinal ischemia. This formula can be easily applied in clinical systems for risk calculation.
Model performance and validation
The final logistic regression model demonstrated good discriminatory ability in identifying patients with intestinal ischemia. With the optimal alpha value of 0.3 selected via LASSO, the area under the ROC curve (AUC) was 0.784 in the training cohort and 0.750 in the validation cohort (Fig. 6), indicating consistent performance across datasets.
Fig. 6.
The ROC of the prediction model. ROC curve for the training cohort (blue curve); ROC curve for the validation cohort (red curve), The model achieved an AUC of 0.784 in the training set and 0.750 in the validation set, indicating consistent discriminatory performance.
In the validation cohort, the model achieved a specificity of 0.903, an accuracy of 0.807, a negative predictive value (NPV) of 0.872, and a precision (positive predictive value, PPV) of 0.389. The Akaike Information Criterion (AIC) for the final model was 208.15. Sensitivity (recall) was relatively low at 0.318, and the F1-score was 0.350, reflecting the trade-off between positive case detection and prediction precision. Calibration was evaluated using Brier scores and calibration plots (Fig. 7). The Brier score was 0.091 in the training set and 0.123 in the validation set. Calibration curves demonstrated good agreement between predicted and observed probabilities, particularly in the low-to-intermediate risk range.
Discussion
This study developed an accessible predictive model for StBO using clinical characteristics and routine laboratory parameters selected through LASSO regression. In this study, LASSO regression with optimal regularization (α = 0.3, log[λ] = − 3.902) effectively reduced the influence of low-contributing variables and minimized the overfitting risks associated with stepwise regression. These four variables—rebound tenderness, BMI, neutrophil percentage, and AST—are rapidly obtainable in emergency settings, typically within 1–2 h. This enables early bedside risk stratification before imaging is available and may assist in preventing serious complications such as bowel necrosis and perforation.
The nomogram then transformed these complex model outputs into an intuitive scoring system, named BAR-N (BMI, AST, rebound tenderness, neutrophil percentage) for clarity and memorability. BAR-N enables emergency clinicians to estimate the probability of StBO by summing the points of these key predictors. It is suitable for both paper-based and digital deployment, supporting real-time decision-making. Its visual design supports real-time application, especially in emergency or rural care. A reference table (Table 4) is provided to assist in interpreting log-transformed variables.
Table 4.
Estimated costs of BAR-N model variables and abdominal CT examinations based on institutional billing data
| Item | Estimated Cost (RMB) |
|---|---|
| BAR-N Model Components | 71 |
| AST | 46 |
| N (neutrophil percentage) | 25 |
| Rebound tenderness | 0 |
| BMI | 0 |
| Imaging Examinations | |
| Non-contrast abdominal CT | 690 |
| Contrast-enhanced abdominal CT | 1250 |
All costs are based on standard hospital billing at our institution (RMB, 2024 rates). The BAR-N model relies on simple clinical and laboratory parameters with substantially lower direct costs compared to abdominal imaging
Rebound tenderness was a strong predictor (OR = 6.64), reinforcing its role as a critical clinical indicator of peritoneal irritation, which is consistent with the findings of previous studies [1, 23]. Similarly, neutrophil percentage (OR = 1.05) was positively associated with intestinal ischemia, reflecting the systemic inflammatory response triggered by ischemic injury [8, 24]. Ischemia-induced bacterial translocation and endotoxin release activate neutrophils, leading to leukocytosis and neutrophilia—a hallmark of advanced StBO [25]. Elevated AST (OR = 1.97) further supports the validity of the model, as intestinal ischemia disrupts barrier function and leads to endotoxin-driven hepatic injury and subsequent AST release [25, 26]. However, AST is a late-stage marker that requires cautious clinical interpretation [26, 27]. Notably, BMI demonstrated a strong protective effect against intestinal ischemia (OR = 0.02), although its underlying mechanism in the context of StBO remains unclear. This finding is somewhat counterintuitive, as elevated BMI is typically associated with increased risk for various adverse health outcomes. However, several plausible explanations exist. A higher BMI may serve as a metabolic reserve during periods of acute illness or physiological stress, potentially supporting recovery. Moreover, prior studies have reported U-shaped or J-shaped associations between BMI and clinical outcomes, in which both low and high BMI values are linked to poorer prognosis, whereas moderate BMI may confer protective effects [28, 29]. In addition, potential confounding factors—such as comorbidities, physical activity, muscle mass, and nutritional status—may influence the observed relationship between BMI and ischemia risk [30]. Further investigation is needed to clarify the impact of BMI on ischemia risk.
Our multidimensional model demonstrated good discriminatory performance, with an AUC of 0.784 in the training cohort and 0.750 in the validation cohort. It achieved high specificity (90.3%) and overall accuracy (80.7%), and a NPV of 0.872. The Brier scores were 0.091 for the training set (Fig. 7) and 0.123 for the validation set (Fig. 8), indicating acceptable calibration. Sensitivity was moderate (recall = 0.318). As with any predictive model, BAR-N entails a risk of misclassification: false positives may lead to unnecessary surgical interventions, while false negatives could delay treatment and increase the likelihood of bowel necrosis or mortality. Given these trade-offs, the BAR-N model is best utilized as a clinical decision support tool in frontline and primary care settings to assist with early screening, triage, and referral. Its primary value is in stratifying low-risk patients for safe monitoring or referral, supporting stepwise care. Importantly, the model is intended to complement—not replace—clinical judgment.
Fig. 8.
Calibration curve of the model in the validation set. The x-axis shows the predicted probability of StBO, while the y-axis shows the observed frequency. The dashed diagonal line represents perfect calibration. The solid curve demonstrates that the model’s predicted risks closely correspond to actual outcomes, indicating good calibration.
Notably, the model outperforms strategies based on WBC count, BE, and ascites (82.3% specificity) [31]. The AUC was comparable to that of CTA (AUC 0.807–0.814) (Table 4) [21, 22, 28, 29, 32]. However, it demonstrated a substantially lower risk of contrast-induced nephropathy [1] and incurs substantially lower procedural costs—approximately CNY 71 compared to CNY 690 for non-contrast CT or CNY 1,250 for CTA—based on institutional billing data (Table 5). Although a formal cost-effectiveness analysis was not conducted, this notable cost difference suggests a potential economic advantage, particularly in resource-limited settings. This cost-effectiveness aligns with WHO benchmarks for essential diagnostic packages in low-resource settings [33]. Positioning BAR-N as a practical adjunct to imaging-heavy diagnostic pathways, particularly in settings where imaging is delayed, unavailable, or cost-prohibitive.
Table 5.
Correspondence between log-transformed values and raw clinical values for BMI and AST
| Parameter | Raw Value | Log Value (ln) |
|---|---|---|
| BMI (kg/m2) | 15.0 | 2.71 |
| 15.5 | 2.74 | |
| 16.0 | 2.77 | |
| 16.5 | 2.80 | |
| 17.0 | 2.83 | |
| 17.5 | 2.86 | |
| 18.0 | 2.89 | |
| 18.5 | 2.92 | |
| 19.0 | 2.94 | |
| 19.5 | 2.97 | |
| 20.0 | 3.00 | |
| 20.5 | 3.02 | |
| 21.0 | 3.04 | |
| 21.5 | 3.07 | |
| 22.0 | 3.09 | |
| 22.5 | 3.11 | |
| 23.0 | 3.14 | |
| 23.5 | 3.16 | |
| 24.0 | 3.18 | |
| 24.5 | 3.20 | |
| 25.0 | 3.22 | |
| 25.5 | 3.24 | |
| 26.0 | 3.26 | |
| 26.5 | 3.28 | |
| 27.0 | 3.30 | |
| 27.5 | 3.31 | |
| 28.0 | 3.33 | |
| 28.5 | 3.35 | |
| 29.0 | 3.37 | |
| 29.5 | 3.38 | |
| 30.0 | 3.40 | |
| 30.5 | 3.42 | |
| 31.0 | 3.43 | |
| 31.5 | 3.45 | |
| 32.0 | 3.47 | |
| 32.5 | 3.48 | |
| 33.0 | 3.50 | |
| 33.5 | 3.51 | |
| 34.0 | 3.53 | |
| 34.5 | 3.54 | |
| 35.0 | 3.56 | |
| 35.5 | 3.57 | |
| 36.0 | 3.58 | |
| 36.5 | 3.60 | |
| 37.0 | 3.61 | |
| 37.5 | 3.62 | |
| 38.0 | 3.64 | |
| 38.5 | 3.65 | |
| 39.0 | 3.66 | |
| 39.5 | 3.68 | |
| 40.0 | 3.69 | |
| 44.0 | 3.78 | |
| AST (U/L) | 1.4 | 3.78 |
| 2.4 | 0.34 | |
| 3.4 | 0.88 | |
| 4.4 | 1.22 | |
| 5.4 | 1.48 | |
| 6.4 | 1.69 | |
| 7.4 | 1.86 | |
| 10.4 | 2.00 | |
| 15.4 | 2.34 | |
| 25.4 | 2.74 | |
| 35.4 | 3.23 | |
| 45.4 | 3.57 | |
| 60.4 | 3.82 | |
| 80.4 | 4.10 | |
| 120.4 | 4.39 | |
| 200.4 | 4.79 | |
| 300.4 | 5.30 | |
| 400.4 | 5.71 | |
| 438.0 | 6.00 |
BMI body mass index, AST aspartate aminotransferase
This study has several limitations. Its retrospective, single-center design without external validation may introduce selection bias and limit generalizability. The small number of StBO cases could reduce statistical power and increase overfitting risk. Advanced biomarkers and imaging data were not included, which may affect predictive accuracy [3, 14]. Additionally, the model was not compared with real-time physician judgment, limiting evaluation of its added clinical value.
Prospective, multicenter studies across diverse populations and healthcare settings are warranted to validate the model’s generalizability, optimize threshold selection, and support broader clinical adoption. Future research should also include head-to-head comparisons between the BAR-N model and real-time clinical judgment by experienced emergency physicians.
Conclusion
By integrating clinical signs and laboratory parameters, the model demonstrates strong performance, particularly in terms of high specificity. However, its moderate sensitivity highlights the need for it to be used as a supplementary aid in the early diagnosis of StBO, rather than as a replacement for clinical judgment and imaging. The inclusion of a nomogram further enhances its clinical applicability by enabling rapid risk stratification and more informed bedside decision-making. Future multicenter prospective validation and mechanistic studies—such as investigating the potential protective role of BMI—are warranted to support broader clinical adoption and promote more personalized management of SBO [34–36].
Supplementary Information
Acknowledgements
Not applicable.
Clinical trial number
Not applicable.
Authors’ contributions
Cuifeng Zheng, BaoWei Xu, and Pingxia Lu wrote the main manuscript text. Cuifeng Zheng, BaoWei Xu, and Pingxia Lu were responsible for conceptualization, formal analysis, investigation, methodology, validation, and visualization. Cuifeng Zheng, BaoWei Xu, Weixuan Xu, and Shenhui Lin contributed to the data curation and methodology. Zhengyuan Huang, Junrong Zhang, Xianqiang Chen, and Pingxia Lu acquired funding, while Zhengyuan Huang, Junrong Zhang, and Xianqiang Chen managed the project administration and provided resources. Cuifeng Zheng and Junrong Zhang worked on the software. Junrong Zhang and Zhengyuan Huang supervised the research. All authors reviewed and edited the manuscript.
Funding
This work was supported by the Fujian provincial health technology project for Young and Middle-aged Core Talents [grant number: 2021GGA015 to Zheng-yuan Huang]; the Joint Funds for the Innovation of Science and Technology, Fujian province [grant numbers: 2018Y9203 to Jun-rong Zhang, 2018Y92042 to Xian-qiang Chen]; and the Fujian Provincial Natural Science Foundation of China [grant number 2024J01596 to Ping-xia Lu].
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Board of Fujian Medical University Union Hospital. Written informed consent was obtained from all individual participants prior to their inclusion in the study. All procedures performed in studies involving human participants were conducted in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Cuifeng Zheng, BaoWei Xu and Pingxia Lu contributed equally to this work.
References
- 1.Xu WX, et al. Prediction and management of strangulated bowel obstruction: a multi-dimensional model analysis. BMC Gastroenterol. 2022;22:304. 10.1186/s12876-022-02363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maung AA, Piper JD, Barbosa GL, Rowell RR, Bokhari SE. F Evaluation and management of small-bowel obstruction: an Eastern association for the surgery of trauma practice management guideline. J Trauma Acute Care Surg. 2012. [DOI] [PubMed]
- 3.Grootjans J, Lenaerts K, Buurman WA, Dejong CHC, Derikx JPM. Life and death at the mucosal-luminal interface: new perspectives on human intestinal ischemia-reperfusion. World J Gastroenterol. 2016;22:2760–70. 10.3748/wjg.v22.i9.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.10784595. GM. -d. s.-.-x. E. I. G. J.-P. American gastroenterological association medical position statement: guidelines on I.testinal I.chemia. Gastroenterology. 2000. 10.1016/S0016-5085(00)70182-X.
- 5.Oldenburg WA, Rodenberg LL, Edmonds TJ, Burger HJ. Acute mesenteric ischemia: a clinical review. Arch Intern Med. 2004. 10.1001/archinte.164.10.1054. [DOI] [PubMed] [Google Scholar]
- 6.Kozuch PL, Brandt LJ. Review article: diagnosis and management of mesenteric ischaemia with an emphasis on pharmacotherapy. Aliment Pharm Ther. 2005;21:201–15. 10.1111/j.1365-2036.2005.02269.x. [DOI] [PubMed] [Google Scholar]
- 7.Pothiawala S, Gogna A. Early diagnosis of bowel obstruction and strangulation by computed tomography in emergency department. World J Emerg Med. 2012;3:227–31. 10.5847/wjem.j.issn.1920-8642.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Millet I, et al. Assessment of strangulation in adhesive small bowel obstruction on the basis of combined CT findings: implications for clinical care. Radiology. 2017;285:798–808. 10.1148/radiol.2017162352. [DOI] [PubMed] [Google Scholar]
- 9.Zielinski MD, et al. Small bowel obstruction-who needs an operation? A multivariate prediction model. World J Surg. 2010;34:910–9. 10.1007/s00268-010-0479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jancelewicz T, et al. Predicting strangulated small bowel obstruction: an old problem revisited. J Gastrointest Surg. 2009;13:93–9. 10.1007/s11605-008-0610-z. [DOI] [PubMed] [Google Scholar]
- 11.Millet I, Taourel P, Ruyer A, Molinari N. Value of CT findings to predict surgical ischemia in small bowel obstruction: A systematic review and meta-analysis. Eur Radiol. 2015;25:1823–35. 10.1007/s00330-014-3440-2. [DOI] [PubMed] [Google Scholar]
- 12.Zielinski MD, et al. Prospective, observational validation of a multivariate small-bowel obstruction model to predict the need for operative intervention. J Am Coll Surg. 2011;212:1068–76. 10.1016/j.jamcollsurg.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 13.Nijssen EC, et al. Prophylactic hydration to protect renal function from intravascular iodinated contrast material in patients at high risk of contrast-induced nephropathy (AMACING): a prospective, randomised, phase 3, controlled, open-label, non-inferiority trial. Lancet. 2017;389:1312–22. 10.1016/S0140-6736(17)30057-0. [DOI] [PubMed] [Google Scholar]
- 14.Zhuang X, Chen F, Zhou Q, Zhu Y, Yang X. A rapid preliminary prediction model for intestinal necrosis in acute mesenteric ischemia: a retrospective study. BMC Gastroenterol. 2021;21:154. 10.1186/s12876-021-01746-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zettervall SL, et al. Trends in treatment and mortality for mesenteric ischemia in the united States from 2000 to 2012. Ann Vasc Surg. 2017;42:111–9. 10.1016/j.avsg.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matray L, Roisin S, Sabbagh C. Arterial revascularization following acute mesenteric ischemia. J Visc Surg. 2020;157:53–8. 10.1016/j.jviscsurg.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Kim HK, Chun JM, Huh S. Anticoagulation and delayed bowel resection in the management of mesenteric venous thrombosis. World J Gastroenterol. 2013;19:5025–8. 10.3748/wjg.v19.i30.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang S, et al. Multidisciplinary Stepwise management strategy for acute superior mesenteric venous thrombosis: an intestinal stroke center experience. Thromb Res. 2015;135:36–45. 10.1016/j.thromres.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Nuzzo A, Huguet A, Corcos O. [Modern treatment of mesenteric ischemia]. Presse Med. 2018;47:519–30. 10.1016/j.lpm.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Nuzzo A, et al. Oral antibiotics reduce intestinal necrosis in acute mesenteric ischemia: A prospective cohort study. Am J Gastroenterol. 2019;114:348–51. 10.1038/s41395-018-0389-9. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Y, et al. Development and validation of a nomogram model to predict the risk of strangulated intestinal obstruction. Sci Rep. 2024;14:31049. 10.1038/s41598-024-82131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markogiannakis H, et al. Predictive value of procalcitonin for bowel ischemia and necrosis in bowel obstruction. Surgery. 2011;149:394–403. 10.1016/j.surg.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Graber SD, Sinz S, Turina M, Alkadhi H. Pneumatosis intestinalis in abdominal CT: predictors of short-term mortality in patients with clinical suspicion of mesenteric ischemia. Abdom Radiol (NY). 2022;47:1625–35. 10.1007/s00261-022-03410-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishikawa E, et al. Cecal intussusception in an adult with Cronkhite-Canada syndrome relieved by colonoscopy. Intern Med. 2010;49:1123–6. 10.2169/internalmedicine.49.2813. [DOI] [PubMed] [Google Scholar]
- 25.Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. Am J Transpl. 2011;11:1563–9. 10.1111/j.1600-6143.2011.03579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu B et al. Butyrate protects rat liver against total hepatic ischemia reperfusion injury with bowel congestion. PLoS ONE. 2014;9. https://doi.org/ARTNe10618410.1371/journal.pone.0106184 [DOI] [PMC free article] [PubMed]
- 27.Thompson JS, Bragg LE, West WW. Serum enzyme levels during intestinal ischemia. Ann Surg. 1990;211:369–73. 10.1097/00000658-199003000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee DH et al. Predicted lean body mass, fat mass, and all cause and cause specific mortality in men: prospective US cohort study. BMJ 362, k2575. 2018. 10.1136/bmj.k2575 [DOI] [PMC free article] [PubMed]
- 29.Aune D, et al. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156. 10.1136/bmj.i2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Pessot J, Pellegrin A, Regimbeau JM, Sabbagh C. Risk factors for ischemia/necrosis of the colonic stump after proctectomy and delayed coloanal anastomosis. Langenbecks Arch Surg. 2023;408:424. 10.1007/s00423-023-03157-z. [DOI] [PubMed] [Google Scholar]
- 31.Murao S et al. Ischemia prediction score (IsPS) in patients with strangulated small bowel obstruction: a retrospective cohort study. Bmc Gastroenterology. 2023;23.https://doi.org/ARTN13310.1186/s12876-023-02761-z [DOI] [PMC free article] [PubMed]
- 32.Kobayashi T et al. Prediction model for irreversible intestinal ischemia in strangulated bowel obstruction. Bmc Surgery. 2022;22. https://doi.org/ARTN32110.1186/s12893-022-01769-8. [DOI] [PMC free article] [PubMed]
- 33.Zhang XH, et al. Implementation of world health organization package of essential noncommunicable disease interventions (WHO PEN) for primary health care in Low-Resource settings: A policy statement from the world hypertension league. J Clin Hypertens. 2016;18:5–6. 10.1111/jch.12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang HD, et al. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020-21. Lancet. 2022;399:1513–36. 10.1016/S0140-6736(21)02796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gafita A, et al. Nomograms to predict outcomes after (177)Lu-PSMA therapy in men with metastatic castration-resistant prostate cancer: an international, multicentre, retrospective study. Lancet Oncol. 2021;22:1115–25. 10.1016/S1470-2045(21)00274-6. [DOI] [PubMed] [Google Scholar]
- 36.Huang YQ, et al. Development and validation of a radiomics nomogram for preoperative prediction of lymph node metastasis in colorectal Cancer. J Clin Oncol. 2016;34:2157–64. 10.1200/JCO.2015.65.9128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.