Abstract
Background
Ischemic stroke (IS) is a major cause of disability and death worldwide. However, previous observational studies failed to establish the causality between various exposures and IS occurrence. Currently, an increasing number of Mendelian randomization (MR) studies have been performed to investigate the causal effects of exposure factors on IS risk, but the results remain inconsistent and inconclusive. Thus, our systematic review summarized all published MR studies focusing on IS and its subtypes, and further identified causal relationships with robust evidence.
Methods
We retrieved PubMed and Embase databases for MR analyses exploring the correlation of genetically determined exposures with the risk of IS and its subtypes. For publications selected in the main analysis, we summed up these MR results and classified the strength of evidence into Grade 1–4 based on the significance and concordance of the findings from the primary and complementary MR methods, as well as the robustness of sensitivity analyses.
Results
After conducting a comprehensive search, a total of 207 published studies with 1199 MR associations were included. Among these causal correlations, 8 (Grade 1) and 81 (Grade 2) were graded as robust evidence, while 226 (Grade 3) and 884 (Grade 4) causal relationships were considered to have insufficient reliability. Specifically, with regard to IS susceptibility, genetically predicted diastolic blood pressure, type 2 diabetes, Parkinson’s disease, and primary aldosteronism were evaluated as the prominent robust causal determinants. As for IS subtypes, genetically determined higher vitamin K1 levels and primary aldosteronism were related to greater occurrence of large artery stroke and small vessel stroke, respectively, whereas increased adult height was associated with a lower risk of small vessel stroke but a higher risk of cardioembolic stroke.
Conclusions
Our field synopsis systematically summarized and evaluated the evidence strength for causality between various exposures and the occurrence of IS and its subtypes, and ultimately identified 89 causal relationships as convincing findings. We hope that our results can provide constructive and impressive insights for developing effective IS prevention strategies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12967-025-06992-4.
Keywords: Mendelian randomization, Ischemic stroke, Systematic review, Evidence grading
Introduction
Stroke is one of the main causes of long-term disability and mortality worldwide. In accordance with the global burden of disease (GBD) study 2019, the number of patients with stroke reached 12.22 million, and deaths caused by stroke rose to 6.55 million [1]. In addition, stroke also brings a huge economic burden to patients, families, and society, with global costs of stroke reaching $2059 billion in 2019 [2]. As the most common subtype of stroke, ischemic stroke (IS) accounts for approximately 80% of stroke cases [3]. Thus, it is necessary to have an in-depth insight into various risk factors to decrease the morbidity and mortality of IS. Previous observational studies have assessed the relationships of numerous environmental factors on the risk of IS and its subtypes, including large artery stroke (LAS), small vessel stroke (SVS), and cardioembolic stroke (CES), and have identified dozens of potential risk factors for IS susceptibility [4–6]. However, the direction and magnitude of these associations are sometimes inconsistent and controversial, which reflects the limitations of observational studies, including confounding, reverse causality and small sample size [7, 8]. Therefore, effective analysis methods for evaluating causal associations are urgently needed.
Mendelian randomization (MR) analysis utilizes genetic variants, usually single nucleotide polymorphisms (SNPs), as instrumental variables (IVs) for exposure to assess the causal relationship between exposure and clinical outcome of interest. Since genetic variants are randomly assigned at conception and not disturbed by environmental factors, the results of MR analyses are less susceptible to confounding factors [9, 10]. Additionally, MR studies can effectively avoid the possibility of reverse causality, as genetic variants are not affected by disease development [11]. Furthermore, two-sample MR, the most commonly used study design in MR analysis, typically identifies genetic variants associated with exposures from large-scale genome-wide association studies (GWAS) and then applies these genetic variants to independent outcome datasets, instead of obtaining exposure and outcome data from each patient, overcoming the problem of small sample size in observational studies [12]. Although MR studies have leveraged inherent methodological strengths to investigate the potential causal effects of various risk factors on IS and its subtypes, the findings have not always been consistent. For instance, Deng et al. performed an MR analysis by using summary-level genetic data from the UK Biobank and the MEGASTROKE consortium and found no causal association between tea consumption and IS occurrence [13]. However, a more recent MR study by Gao et al., despite utilizing the same GWAS statistics, reported a protective causal role of tea consumption in IS [14]. This discrepancy could be attributed to specific differences in the employed methods, further underscoring the necessity of evaluating the robustness of MR analyses based on standardized design. Consequently, in this field synopsis, we systematically reviewed all published MR studies exploring the risk of IS and its subtypes and assessed the evidence strength from these MR analyses to gain novel insights into IS prevention strategies.
Methods
Due to the fact that the data were extracted from previously published MR studies, this systematic review was exempt from institutional review board approval. Moreover, the authors declare that all supporting data are available within the article and its online additional files.
Search strategy
To comprehensively review MR studies of IS and its subtypes, we performed a systematic literature search of Embase and PubMed databases as of April 1, 2025, using the following search strategies: “mendelian randomization/mendelian randomisation” and “stroke/cerebrovascular disease/IS/cerebral infarction/brain infarction”. In addition, we screened the references of the retrieved articles for any potentially eligible articles. The entire search strategy for PubMed and Embase databases is displayed in Additional file 1.
Study selection
For articles identified using the above search strategy, we carefully examined their titles, abstracts and full texts. Studies met the following criteria: (1) with an MR design; (2) relevant to the risk of IS or its subtypes; (3) associated with various exposures, including anthropometric traits, socio-economic and lifestyle factors, dietary factors, various diseases and associated traits, as well as drug targets; and (4) published in English. In contrast, the following articles were excluded: (1) case reports, reviews, letters, editorials, commentaries, communications, or conference abstracts; (2) studies without full text available for further analysis; (3) duplicated studies. The screening process of eligible MR studies is depicted in Fig. 1.
Fig. 1.
Flowchart of MR studies selection process
In addition, when more than one study was available to assess the causal effect of the same exposure factor on IS risk, the main analysis would be selected according to the following three steps: (1) we first prioritized analyses with rigorous study designs that used SNP selection criteria including a genome-wide significance threshold of P < 5 × 10⁻⁸, a stringent linkage disequilibrium cutoff (r2 < 0.001), and F-statistic threshold above 10 to avoid weak instruments; (2) MR studies with the largest sample sizes were favored; (3) lastly, we tended to choose studies with exposure and outcome populations from the same ethnicity. The above screening process was completed independently by two authors, and any disagreements were resolved by discussion with a third author.
Data extraction
Two authors independently extracted the following data from eligible studies: first author’s name, year of publication, exposure and outcome of interest, sample size and ethnicity of exposure and outcome populations, number of genetic variants, P-value threshold, linkage disequilibrium (r2), instrument strength (F-statistic), variance explained (R2), effect estimates with 95% confidence intervals, P-value of effect estimates, study design (one-sample or two-sample MR), primary MR method and supplementary analysis methods. Specifically, when the exposure in MR studies was analyzed using multiple instrumental variables, we extracted the causal estimates from the inverse-variance weighted (IVW) method as the primary findings. Meanwhile, results from complementary methods, such as the weighted median, weighted mode, MR-Egger, and Mendelian Randomization robust adjusted profile score (MR-RAPS), were retrieved to assess consistency in the direction of effect and statistical significance. In contrast, when only a single genetic instrument was available for an exposure, causal estimates were sourced exclusively from the Wald ratio method, without the support of supplementary MR approaches. Lastly, due to the presence of heterogeneity and pleiotropy in MR studies, we extracted information on sensitivity analyses including leave-one-out analysis, MR Pleiotropy Residual Sum and Outlier (MR-PRESSO), MR Egger intercept, Cochran’s Q statistic, and the MR Steiger test to evaluate the credibility of the reported causal relationships.
Evaluation of robustness of associations identified by MR studies
The flowchart of the robustness assessment of the MR studies included in the main analysis is displayed in Fig. 2. The detailed grading criteria are presented as follows. Grade 1: All MR methods (IVW and supplementary) showed significant causal effects with consistent directions, and sensitivity analyses confirmed robustness. Grade 2: Primary IVW was significant, supplementary methods showed consistent directions, and sensitivity analyses confirmed robustness. Grade 3: At least one MR method was significant (with potential directionality inconsistencies). Grade 4: All MR methods were non-significant. Notably, statistical significance was determined using corrected P-values when multiple testing was reported; otherwise, we applied the nominal significance threshold of P < 0.05. Lastly, based on the abovementioned grading framework, Grade 1 and Grade 2 results were considered to provide robust evidence for the causal associations between various exposure factors and IS as well as its subtypes in our systematic review.
Fig. 2.
Flowchart of robustness assessment of MR studies included in main analysis
Results
Study identification
As depicted in Fig. 1, 2347 articles were identified from the PubMed and Embase databases. After removing 847 duplicated publications, we further excluded 773 irrelevant studies based on titles and abstracts. Next, through full-text screening, 382 studies were deleted and the specific reasons for the exclusion of full-text articles are displayed in Additional file 1. Ultimately, 345 articles were included in the present systematic review, and the detailed characteristics of all studies enrolled are presented in Additional file 2.
Characteristics of studies included in main analysis
Out of the 345 eligible articles, 207 MR studies were included in the main analysis (Fig. 1), and details of these MR analyses are provided in Additional file 3 [13–219]. Overall, we identified 1199 causal estimates from these 207 MR studies, which investigated the causal effects of 433 different exposures on IS and its subtypes. Of these 207 articles, the median number of SNPs used as instrumental variants was 13, ranging from 1 to 2,270. In addition, the median number of exposure sample size in MR studies was 166,161 (range 338 to 3,037,499). Regarding the number of outcome sample size, the median was 440,328 (range 11,312 to 1,614,080). Most of the data of SNPs for exposure were obtained from populations of European ancestry (n = 506; 98.4%), followed by Asian (n = 4; 0.8%) and multi-ancestry (n = 4; 0.8%), and the populations of outcome were derived from European (n = 490; 95.3%), multi-ancestry (n = 22; 4.4%) and Asian (n = 2; 0.3%). Moreover, 1,189 (99.9%) MR correlations used a two-sample MR design and 1 (0.1%) utilized a one-sample design.
Robustness of the evidence
Anthropometric traits
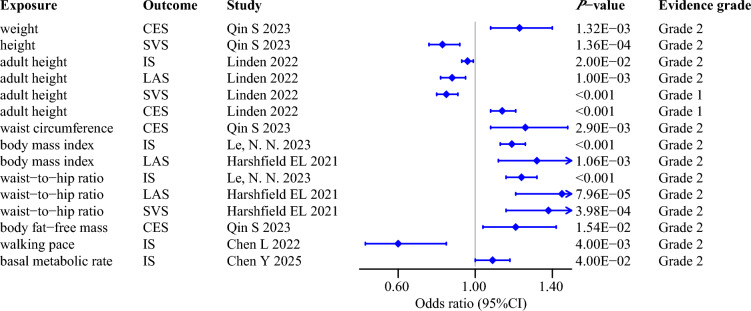
Following rigorous evaluation of 11 MR studies included in the main analysis, we ultimately identified 15 robust causal associations of anthropometric traits with the risk of IS and its subtypes (Fig. 3). Specifically, as comprehensive indicators reflecting body fat distribution and overall body size, robust MR results demonstrated that genetically predicted higher waist-to-hip ratio (WHR) and body mass index (BMI) were causally associated with an increased risk of IS and LAS [60, 92]. In addition, basic anthropometric traits such as adult height, waist circumference, and weight exhibited robust causal effects on specific stroke subtypes in MR analyses [110, 131]. For example, genetically predicted adult height, waist circumference, and weight causally increased the risk of CES [110, 131], however, per standard deviation (SD) increment in adult height was reported to significantly reduce the incidence of SVS by 15% in the MR analysis conducted by Linden et al. [110].
Fig. 3.
Robust evidence for the causal effects of anthropometric traits on ischemic stroke and its subtypes
Socio-economic and lifestyle factors
Socio-economic and lifestyle factors represented an important area in MR studies of IS, particularly given the limitations or infeasibility of conducting randomized controlled trials (RCTs). Within this category of exposures, 3 causal associations were assessed as grade 2 at the robust level (Fig. 4). Among these, the findings of the MR study indicated that genetically predicted educational attainment was associated with a significantly reduced risk of SVS [173]. As for sleep-related traits, Titova et al. found that genetic predisposition to short sleep duration was specifically associated with LAS, whereas their MR analyses yielded no causal relationship for other stroke subtypes [148]. Additionally, with respect to smoking, convincing MR evidence supported the idea that genetically determined higher lifetime smoking index causally elevated the risk of IS by 34% [92].
Fig. 4.
Robust evidence for the causal effects of socio-economic and lifestyle factors on ischemic stroke and its subtypes
Dietary factors
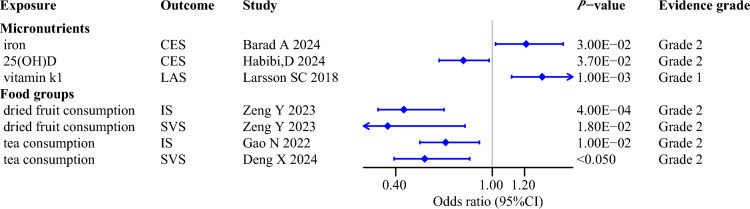
Among the dietary factors included in our systematic review, only 5 exposures were found to exert robust causal influence on IS and its subtypes, based on stringent selection criteria and comprehensive evaluation (Fig. 5). Of these associations, only the positive causal relationship between genetically predicted vitamin K1 concentrations and LAS was assessed as grade 1 [90], while the remaining dietary factors were assessed as grade 2. Specifically, MR studies focusing on various micronutrients clarified that genetic predisposition to per SD increase in circulating levels of iron elevated a 21% risk of CES [19]. In addition, MR analyses on the daily intake of common foods and beverages revealed robust causal links indicating that genetically determined higher consumption of dried fruit and tea protected against both IS and SVS [13, 14, 189].
Fig. 5.
Robust evidence for the causal effects of dietary factors on ischemic stroke and its subtypes
Cardiometabolic diseases and associated indices
As shown in Fig. 6, a total of 19 robust causal correlations were obtained from 29 MR analyses included in the main analysis, which investigated the associations of cardiometabolic diseases and related indices with the susceptibility to IS and its subtypes. Regarding blood pressure, the MR findings by Le et al. provided solid evidence that genetic liability to higher diastolic blood pressure (DBP) was causally related to the increased incidence of IS (Grade 1) [92]. However, although significant causal associations were observed between DBP and specific stroke subtypes, these findings failed to pass sensitivity analyses of pleiotropy or directional tests, thereby limiting the evidence strength of causal inference for specific IS subtypes [93, 139]. Similarly, in terms of blood lipid traits, although multiple MR studies reported significant causal relationships between low-density lipoprotein cholesterol (LDL-C) and the risk of IS as well as its subtypes, only the causal influence on overall IS development was graded as robust evidence (Grade 1) [92]. Moreover, genetically predicted higher levels of lipoprotein(a) elevated LAS occurrence, implying that lipoprotein(a) exerted its pathogenic influence primarily via atherosclerotic mechanisms, rather than uniformly affecting all stroke subtypes [71]. In addition, recently published MR studies also provided compelling insights that genetic predisposition to type 1 and type 2 diabetes (T2D), including various novel subtypes of type 2 diabetes, such as mild age-related diabetes and severe insulin-deficient diabetes, were causally linked to SVS incidence [134, 196].
Fig. 6.
Robust evidence for the causal effects of cardiometabolic diseases and associated indices on ischemic stroke and its subtypes
Non-cardiometabolic diseases and related indices
Beyond traditional vascular risk factors, a series of MR studies focusing on non-cardiovascular diseases identified 20 causal relationships classified as robust evidence (Fig. 7). Specifically, in the domain of neurological disorders, genetically predicted Parkinson's disease (PD) was found to be causally linked with the elevated occurrence of IS (Grade 1) [97]. Moreover, credible MR findings indicated that a greater genetic predisposition to primary aldosteronism (PA) had a detrimental causal influence on the risk of IS and SVS (Grade 1) [136]. Regarding autoimmune and inflammatory diseases, ankylosing spondylitis [122], systemic lupus erythematosus [49], and psoriasis [50] were identified to causally increase the risk of IS and/or its various subtypes, underscoring the potential critical role of chronic systemic inflammation in IS pathogenesis. As for the non-cardiometabolic biomarkers, two-sample MR studies evidently indicated that genetically determined forced vital capacity (FVC) was inversely related to SVS incidence [16], whereas higher levels of gamma-glutamyl transferase and estimated glomerular filtration rate were deemed causal determinants for CES and LAS development, respectively [81, 95].
Fig. 7.
Robust evidence for the causal effects of non-cardiometabolic diseases and associated indices on ischemic stroke and its subtypes
Drug targets
As displayed in Fig. 8, after systematically evaluating 33 MR analyses in main analysis, we identified 25 robust causal associations that shed light on potential pharmacological targets for the IS and its subtypes (Grade 2). As for the MR analyses investigating the causal effects of existing drug targets, a previous study leveraging genetic variants located within the genes encoding the lipid-lowering drugs unveiled that the reduced LDL-C concentration mediated by the 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) gene and Niemann-Pick C1-like 1 (NPC1L1) gene decreased the risk of SVS [170], which supported a possible protective role of HMGCR and NPC1L1 inhibitors against SVS. Similarly, Chen et al. observed strong evidence for the inverse causal relationship between the genetic prediction of sodium-glucose cotransporter protein 2 inhibitors, a novel class of orally administered antidiabetic agents, and the susceptibility to IS and CES [35]. In addition, robust MR findings were able to identify a wide range of undiscovered drug targets based on large-scale genome-wide datasets. For instance, in the MR analysis evaluating the causal impacts of 653 plasma proteins on the incidence of IS as well as its three major subtypes, several circulating biomarkers were recognized as robust candidate therapeutic targets. In detail, histo-blood group ABO system transferase (ABO) was found to be the potential target for LAS and CES, whereas scavenger receptor class A5 (SCARA5) and tumor necrosis factor-like weak inducer of apoptosis (TNFSF12) were suggested to be associated with lower occurrence of CES [36].
Fig. 8.
Robust evidence for the causal effects of drug targets on ischemic stroke and its subtypes
Discussion
Our field synopsis and systematic review summarized and assessed the robustness of the evidence for 1,199 causal estimates between a series of risk factors and the susceptibility to IS as well as its subtypes. Based on the rigorous evaluation framework, a total of 89 causal associations fulfilled the standard of robust evidence, including 8 classified as Grade 1 and 81 as Grade 2 (Additional file 4). In addition, 226 (Grade 3) and 884 (Grade 4) relationships were considered to have insufficient reliability (Additional file 5 and 6).
With respect to anthropometric traits, our field synopsis identified robust evidence that genetically determined adult height was causally associated with declined SVS risk, but elevated CES incidence [110]. Specifically, A large-scale observational study involving 33,197 participants found an inverse correlation between height and blood pressure [220]. Given that blood pressure is a recognized risk factor for SVS [221], it is plausible that the causality between adult height and reduced SVS risk may be partially mediated through blood pressure. On the contrary, the positive causal relationship between adult height and CES risk may be attributable to underlying structural cardiac changes. It was reported that taller people tended to have a larger left atrial diameter, which might increase the susceptibility to atrial fibrillation, thereby elevating the occurrence of cardioembolic stroke [222]. Additionally, robust evidence also supported the causal role of increased BMI and WHR in IS occurrence [92]. This finding was strongly supported by a prospective observational study involving 13,549 adults aged 45–64 years followed over a median of 16.9 years, which reported that higher BMI and WHR were linearly associated with increased risk of IS [223].
Regarding various lifestyle factors, the robust causal link between genetically determined short sleep duration and LAS occurrence may be explained by the following mechanism: chronic sleep loss could contribute to increased secretion of cortisol and specific pro-inflammatory factors such as TNF-α and interleukin-6, which in turn result in dyslipidemia, elevated arterial blood pressure and atherosclerosis, and ultimately lead to the development of IS [224–226]. As for smoking, credible evidence from MR analyses demonstrated a causal effect of higher lifetime smoking index on the risk of IS [92], which was aligned with the prospective observational studies conducted in substantial sample sizes [227, 228]. Furthermore, in mice undergoing transient middle cerebral artery occlusion, Li et al. found that cigarette smoke exposure could trigger the mobilization of peripheral monocytes and neutrophils, which may contribute to the exacerbation of neurological deficits and brain infarction [229].
For dietary factors, the recent MR analysis with robust evidence identified that higher tea consumption protected against SVS occurrence [13]. This association might be attributed to the antioxidative and anti-inflammatory properties of its components, such as caffeine, polyphenols, and flavonoids [230], which have been shown to mitigate ischemia/reperfusion injury and ameliorate endothelial dysfunction [231–234]. Moreover, robust MR findings suggested that genetically determined higher vitamin K1 levels could causally increase LAS risk [90], potentially due to the role of vitamin K1 in promoting atherogenesis, vascular calcification, and a prothrombotic state via activation of coagulation factors II, VII, IX, and X [235].
As for cardiometabolic diseases and associated indices, previous large-scale observational studies have proved that several well-known risk factors, including DBP [236, 237], type 1 and type 2 diabetes [238, 239], circulating levels of LDL-C [240, 241], and lipoprotein(a) [242] were closely related to the risk of IS and its subtypes. Moreover, a recent GWAS involving 42,393 Korean participants identified shared genetic susceptibility loci between diabetes and dyslipidemia and IS, suggesting a common genetic architecture underlying these diseases [243]. More importantly, compelling MR findings further supported the potential causal roles of these cardiometabolic disorders and related traits in the pathogenesis of IS [71, 92, 196]. Additionally, MR studies yielded valuable insights for evaluating the comparative effects of closely associated risk factors, for instance, multivariable MR analysis suggested that compared to other glycemic traits, 2 h glucose after an oral glucose challenge underscored the pivotal role in the development of IS and SVS [185]. Similarly, the MR framework proposed the independent causal impact of type 1 diabetes on SVS incidence, even after adjusting for various common cerebrovascular disease risk factors such as BMI, smoking, LDL, and triglycerides [196].
With regard to non-cardiometabolic diseases, two-sample MR analysis revealed a robust causal association between primary aldosteronism and the occurrence of IS and SVS [136], corroborated by a case–control study reporting a stroke incidence rate of 35 cases per 1000 person-years in patients with PA [244], nearly ten times higher than that observed in the general population [245]. Besides, in male mice, aldosterone was found to enhance superoxide production in cerebral arteries and to upregulate mRNA expression levels of pro-inflammatory cytokines in the brain, potentially impairing endothelial function and contributing to small vessel injury [246, 247]. Moreover, credible findings from MR studies supported the notion that PD was a causal risk factor for the development of IS [97], which may plausibly be attributed to the pathological accumulation of α-synuclein within the brain. In vivo experiments illustrated that transgenic mice with α-synuclein overexpression exhibited heightened susceptibility to ischemic brain injury compared to controls [248], while significant shrinkage in infarction volume was detected following knockdown of α-synuclein in rodents [249].
MR analyses were also applied to provide powerful support in the identification of drug targets. In our systematic review, compelling MR evidence from Xie et al. demonstrated that genetically proxied inhibition of NPC1L1 and HMGCR—the molecular targets of ezetimibe and statins, respectively—was causally associated with a lower risk of SVS [170]. Consistent with these genetic findings, a retrospective cohort study involving 11,009 participants showed that statin therapy targeting HMGCR could decrease IS risk by 24% [250]. Furthermore, a large-scale, double-blind, randomized trial found that combining ezetimibe with simvastatin resulted in a greater reduction in IS incidence compared with statin monotherapy [251, 252]. Moreover, robust MR results unveiled the protective role of SCARA5 for CES. A plausible explanation was the involvement of SCARA5 in iron homeostasis through ferritin-bound iron transport to organs such as the brain and heart [253], thus potentially reducing oxidative stress and attenuating atrial remodeling [254–256].
As the first field synopsis and systematic review focusing on MR associations with IS susceptibility, our study presented several major strengths. Firstly, we summarized the most updated and extensive evidence of MR studies so far to elucidate the causality of various exposures with IS as well as its subtypes. Additionally, for the assessment of evidence robustness, the present rigorous grading criteria we applied were on the basis of primary and complementary MR methods, as well as various sensitivity analyses, which enabled our results to be verified transparently and repetitively. However, the limitations of our study should also be acknowledged. First of all, although our systematic review covered a comprehensive set of risk and protective factors for IS, including anthropometric traits, socio-economic and lifestyle factors, dietary factors, various diseases and associated traits, as well as drug targets, other MR analyses investigating exposures beyond the predefined exposure range, such as brain imaging-derived phenotypes, were not included and evaluated. Secondly, details such as power calculations or the proportion of variance explained by selected genetic variants were often missing in most of the MR studies assessed in our review, making it challenging to distinguish whether non-significant findings truly reflected a lack of causal relationship or were simply due to insufficient statistical power. Thus, the introduction of MR reporting guidelines, such as the STROBE-MR checklist, is expected to improve the quality and transparency of future MR studies, thereby enhancing the robustness and interpretability of MR results. Lastly, since our genetic data were primarily based on individuals of European ancestry, the mentioned viewpoints revealing causal risk factors for IS and its subtypes should be applied to other ethnic groups with caution.
Conclusion
In summary, our systematic review comprehensively assessed the causal relationships of genetically predicted exposures, including anthropometric traits, socio-economic and lifestyle factors, dietary factors, various diseases and associated indices as well as drug targets, with the risk of IS and its major subtypes. After employing strict criteria, a total of 89 causal associations were identified robust evidence, among which the most compelling MR results included causal effects of genetically determined DBP, T2D, PD, and PA on overall IS risk, as well as correlations between vitamin K1 concentrations, PA, and adult height and occurrence of specific IS subtypes. We hope that the reliable MR findings obtained through robustness assessment in this systematic review could offer valuable insights into the etiology of ischemic stroke, and support clinicians in implementing more targeted prevention and management strategies for IS and its subtypes.
Supplementary Information
Acknowledgements
None.
Abbreviations
- GBD
Global burden of disease
- IS
Ischemic stroke
- LAS
Large artery stroke
- SVS
Small vessel stroke
- CES
Cardioembolic stroke
- MR
Mendelian randomization
- SNP
Single nucleotide polymorphism
- IV
Instrumental variables
- GWAS
Genome-wide association studies
- IVW
Inverse-variance weighted
- MR-RAPS
Mendelian randomization robust adjusted profile score
- MR PRESSO
MR pleiotropy residual sum and outlier
- WHR
Waist-to-hip ratio
- BMI
Body mass index
- SD
Standard deviation
- RCTs
Randomized controlled trials
- DBP
Diastolic blood pressure
- LDL-C
Low-density lipoprotein cholesterol
- T2D
Type 2 diabetes
- PD
Parkinson's disease
- PA
Primary aldosteronism
- FVC
Forced vital capacity
- HMGCR
3-Hydroxy-3-methylglutaryl-coenzyme A reductase
- NPC1L1
GeneandNiemann-Pick C1-like 1
- ABO
Histo-blood group ABO system transferase
- SCARA5
Scavenger receptor class A5
- TNFSF12
Tumor necrosis factor-like weak inducer of apoptosis
Author contributions
Conceptualization: Junyi Yang, Chen Han, Yue Zhang, Shutong Tan, and Xu Liu; Data curation: Qian Wu, Yumei Ma, Yuanjie Duan; Methodology: Yaxin Wang, Jinke Wang; Investigation: Binhui Liu, and Changqing Mu; Formal analysis: Junyi Yang, Chen Han, Yue Zhang and Shutong Tan; Project administration: Junyi Yang and Chen Han; Supervision: Jian Zhang and Xu Liu; Writing—original draft: Junyi Yang, Chen Han, Yue Zhang and Shutong Tan; Writing—review & editing: Xiaoqian Zhang, Jian Zhang and Xu Liu; Funding acquisition: Ruixia Zhu, Xiaoqian Zhang, Jian Zhang and Xu Liu. All authors read, critically revised, and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81400950), Natural Science Foundation of Liaoning Province (Grant No. 2019-MS-365, 2023-MSLH-406), Joint Program of the Science and Technology Plan of Liaoning Province (Grant No. 2023JH2/101700076).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The current study is based on the summary results from the published studies. Ethical approval for each of the included studies can be found in the original publications.
Competing interests
The authors have declared no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Junyi Yang, Chen Han, Yue Zhang and Shutong Tan have contributed equally to this work and share first authorship.
Contributor Information
Jian Zhang, Email: jzhang@cmu.edu.cn.
Xu Liu, Email: valentine1120@126.com.
References
- 1.Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795–820. [DOI] [PMC free article] [PubMed]
- 2.Gerstl JVE, Blitz SE, Qu QR, Yearley AG, Lassarén P, Lindberg R, et al. Global, regional, and national economic consequences of stroke. Stroke. 2023;54(9):2380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan J, Li Y, Fu X, Li L, Hao X, Li S. Nonhuman primate models of focal cerebral ischemia. Neural Regen Res. 2017;12(2):321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi Y, Guo L, Chen Y, Xie Q, Yan Z, Liu Y, et al. Risk factors for ischemic stroke: differences between cerebral small vessel and large artery atherosclerosis aetiologies. Folia Neuropathol. 2021;59(4):378–85. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Z, Zhao L, Lu Y, Meng X, Zhou X. Association between Chinese visceral adiposity index and risk of stroke incidence in middle-aged and elderly Chinese population: evidence from a large national cohort study. J Transl Med. 2023;21(1): 518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadeghi R, Norouzzadeh M, HasanRashedi M, Jamshidi S, Ahmadirad H, Alemrajabi M, et al. Dietary and circulating omega-6 fatty acids and their impact on cardiovascular disease, cancer risk, and mortality: a global meta-analysis of 150 cohorts and meta-regression. J Transl Med. 2025;23(1):314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colditz GA. Overview of the epidemiology methods and applications: strengths and limitations of observational study designs. Crit Rev Food Sci Nutr. 2010;50(Suppl 1(s1)):10–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang MT, Bolland MJ, Grey A. Reporting of limitations of observational research. JAMA Intern Med. 2015;175(9):1571–2. [DOI] [PubMed] [Google Scholar]
- 9.Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. 2017;36(11):1783–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C, Zhang H, Lan Y, Yan W, Liu S, Chen Y, et al. Statins as a risk factor for diabetic retinopathy: a Mendelian randomization and cross-sectional observational study. J Transl Med. 2024;22(1):298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Z, Yun Z, Lin J, Sun X, Wang Q, Duan J, et al. Comprehensive mendelian randomization analysis of plasma proteomics to identify new therapeutic targets for the treatment of coronary heart disease and myocardial infarction. J Transl Med. 2024;22(1):404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng J, Baird D, Borges MC, Bowden J, Hemani G, Haycock P, et al. Recent developments in Mendelian Randomization studies. Curr Epidemiol Rep. 2017;4(4):330–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng X, Lai R, Zhu J, Liang J, Chang W, Lv X, et al. Causal association between tea intake and acute cerebrovascular events: a multivariate mendelian randomized study in European populations. J Nutr. 2024;154(1):79–86. [DOI] [PubMed] [Google Scholar]
- 14.Gao N, Ni M, Song J, Kong M, Wei D, Dong A. Causal relationship between tea intake and cardiovascular diseases: a Mendelian randomization study. Front Nutr. 2022;9: 938201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alton P, Hughes DM, Zhao SS. Cardiovascular safety of genetically proxied interleukin-5 inhibition: a mendelian randomization study. Respir Investig. 2023;61(2):149–52. [DOI] [PubMed] [Google Scholar]
- 16.Au Yeung SL, Borges MC, Lawlor DA, Schooling CM. Impact of lung function on cardiovascular diseases and cardiovascular risk factors: a two sample bidirectional Mendelian randomisation study. Thorax. 2022;77(2):164–71. [DOI] [PubMed] [Google Scholar]
- 17.Au Yeung SL, Schooling CM. Impact of urinary sodium on cardiovascular disease and risk factors: a 2 sample Mendelian randomization study. Clin Nutr. 2021;40(4):1990–6. [DOI] [PubMed] [Google Scholar]
- 18.Bahls M, Leitzmann MF, Karch A, Teumer A, Dörr M, Felix SB, et al. Physical activity, sedentary behavior and risk of coronary artery disease, myocardial infarction and ischemic stroke: a two-sample Mendelian randomization study. Clin Res Cardiol. 2021;110(10):1564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barad A, Clark AG, Pressman EK, O’Brien KO. Associations between genetically predicted iron status and cardiovascular disease risk: a Mendelian randomization study. J Am Heart Assoc. 2024;13(11): e034991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bell S, Gibson JT, Harshfield EL, Markus HS. Is periodontitis a risk factor for ischaemic stroke, coronary artery disease and subclinical atherosclerosis? A Mendelian randomization study. Atherosclerosis. 2020;313:111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benn M, Emanuelsson F, Tybjærg-Hansen A, Nordestgaard BG. Impact of high glucose levels and glucose lowering on risk of ischaemic stroke: a Mendelian randomisation study and meta-analysis. Diabetologia. 2021;64(7):1492–503. [DOI] [PubMed] [Google Scholar]
- 22.Borges MC, Haycock PC, Zheng J, Hemani G, Holmes MV, Davey Smith G, et al. Role of circulating polyunsaturated fatty acids on cardiovascular diseases risk: analysis using Mendelian randomization and fatty acid genetic association data from over 114,000 UK Biobank participants. BMC Med. 2022;20(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai D, Fu Y, Song Y, Lin H, Ba Y, Lian J. A causal relationship between irritability and cardiovascular diseases: a Mendelian randomization study. Front Cardiovasc Med. 2023;10:1174329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen G, Lin T, Wu M, Cai G, Wu C, Ding Q, et al. Causal association of cytokines and growth factors with stroke and its subtypes: a Mendelian randomization study. Mol Neurobiol. 2024;61(6):3212–22. [DOI] [PubMed] [Google Scholar]
- 25.Chen H, Chen W, Zheng L. Genetic liability to asthma and risk of cardiovascular diseases: a Mendelian randomization study. Front Genet. 2022;13: 879468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen H, Sun X, Zhuo C, Zhao J, Zu A, Wang Q, et al. Cardiac Troponin I and risk of stroke: a Mendelian randomization Study. Int J General Med. 2022;15:1575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen H, Zhuo C, Zheng L. Assessing causal associations of atopic dermatitis with heart failure and other cardiovascular outcomes: a Mendelian randomization study. Front Cardiovasc Med. 2022;9: 868850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L, Sun X, He Y, Zheng L. Self-reported walking pace and risk of cardiovascular diseases: a two-sample Mendelian randomization study. Front Genet. 2022;13: 871302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen R, Liu H, Zhang G, Zhang Q, Hua W, Zhang L, et al. Antioxidants and the risk of stroke: results from NHANES and two-sample Mendelian randomization study. Eur J Med Res. 2024;29(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen S, Chen Z, Jiang X, Lin C, Ji J. Modifiable risk factors mediate the effect of gastroesophageal reflux disease on stroke and subtypes: a Mendelian randomization study. J Stroke Cerebrovasc Dis. 2024;33(4): 107612. [DOI] [PubMed] [Google Scholar]
- 31.Chen S, Chen Z, Xu Q, Jiang X, Lin C, Ji J. Dual effects of serum urate on stroke risk and prognosis: insights from Mendelian randomization. Front Neurol. 2024;15: 1359292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen W, Wang S, Lv W, Pan Y. Causal associations of insulin resistance with coronary artery disease and ischemic stroke: a Mendelian randomization analysis. BMJ Open Diabetes Res Care. 2020. 10.1136/bmjdrc-2020-001217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, Li A, Zhou W, Yao L. No genetic association between iron deficiency anemia and ischemic stroke and its subtypes: a bidirectional two-sample Mendelian randomization study. Front Neurol. 2024;15: 1408758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Zhang X, Liu M, Zhang Y, Li S, Zhou L, et al. The association between basal metabolic rate and ischemic stroke: a Mendelian randomization study. Front Neurol. 2025;16: 1434740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Z, Meng H, Guo Y, Sun H, Zhang W, Guo Y, et al. Sodium-glucose cotransporter protein 2 inhibition, plasma proteins, and ischemic stroke: a mediation Mendelian randomization and colocalization study. J Stroke Cerebrovasc Dis. 2025;34(1): 108136. [DOI] [PubMed] [Google Scholar]
- 36.Chong M, Sjaarda J, Pigeyre M, Mohammadi-Shemirani P, Lali R, Shoamanesh A, et al. Novel drug targets for ischemic stroke identified through Mendelian randomization analysis of the blood proteome. Circulation. 2019;140(10):819–30. [DOI] [PubMed] [Google Scholar]
- 37.Ciofani JL, Han D, Allahwala UK, Woolf B, Gill D, Bhindi R. Lipids, blood pressure, and diabetes mellitus on risk of cardiovascular diseases in East Asians: a Mendelian randomization study. Am J Cardiol. 2023;205:329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cupido AJ, Asselbergs FW, Natarajan P, Ridker PM, Hovingh GK, Schmidt AF. Dissecting the IL-6 pathway in cardiometabolic disease: a Mendelian randomization study on both IL6 and IL6R. Br J Clin Pharmacol. 2022;88(6):2875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daghlas I, Gill D. Mechanisms of hypercoagulability driving stroke risk in obesity: a mendelian randomization study. Neurology. 2024;103(1): e209431. [DOI] [PubMed] [Google Scholar]
- 40.de La Harpe R, Schoeler T, Thorball CW, Thomas A, Kutalik Z, Vaucher J. Cannabis use and atherosclerotic cardiovascular disease: a Mendelian randomization study. BMC Cardiovasc Disord. 2023;23(1):611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng X, Chang W, Zhu J, Lv X, Lai R, Cai Y, et al. Hypothyroidism’s effect on stroke limited to specific subtypes: a Mendelian randomization study. J Stroke Cerebrovasc Dis. 2024;33(7): 107737. [DOI] [PubMed] [Google Scholar]
- 42.Du W, Yang S, Zhou H, Wu Y, Cai Y, Meng H, et al. The association between constipation and stroke based on the NHANES and Mendelian randomization study. Front Neurosci. 2023;17:1276032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duan L, Xiao R, Liu S, Shi Y, Feng Y. Causality between cognitive performance and cardiovascular disease: a bidirectional Mendelian randomization study. Gene. 2024;891: 147822. [DOI] [PubMed] [Google Scholar]
- 44.Duan X, Du X, Zheng G, Zhou X, Tan N, Li G, et al. Causality between migraine and cardiovascular disease: a bidirectional mendelian randomization study. J Headache Pain. 2024;25(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan M, Li N, Huang L, Chen C, Dong X, Gao W. Exploring potential drug targets in multiple cardiovascular diseases: a study based on proteome-wide Mendelian randomization and colocalization analysis. Cardiovasc Ther. 2025;2025:5711316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan Z, Zhao J, Chen J, Hu W, Ma J, Ma X. Causal associations of osteoporosis with stroke: a bidirectional Mendelian randomization study. Osteoporos Int. 2024;35(12):2127–35. [DOI] [PubMed] [Google Scholar]
- 47.Fang H, Liu W, Zhang L, Pei L, Gao Y, Zhao L, et al. A bidirectional Mendelian randomization study of selenium levels and ischemic stroke. Front Genet. 2022;13: 782691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gan T, Hu J, Liu W, Li C, Xu Q, Wang Y, et al. Causal association between anemia and cardiovascular disease: a 2-sample bidirectional Mendelian randomization study. J Am Heart Assoc. 2023;12(12): e029689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao N, Kong M, Li X, Wei D, Zhu X, Hong Z, et al. Systemic lupus erythematosus and cardiovascular disease: a Mendelian randomization study. Front Immunol. 2022;13: 908831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao N, Kong M, Li X, Zhu X, Wei D, Ni M, et al. The association between psoriasis and risk of cardiovascular disease: a mendelian randomization analysis. Front Immunol. 2022;13: 918224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao Y, Wang Z, Yu J, Chen L. Thyroid cancer and cardiovascular diseases: a Mendelian randomization study. Front Cardiovasc Med. 2024;11: 1344515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Georgakis MK, Gill D, Rannikmäe K, Traylor M, Anderson CD, Lee JM, et al. Genetically determined levels of circulating cytokines and risk of stroke. Circulation. 2019;139(2):256–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Georgakis MK, Gill D, Webb AJS, Evangelou E, Elliott P, Sudlow CLM, et al. Genetically determined blood pressure, antihypertensive drug classes, and risk of stroke subtypes. Neurology. 2020;95(4):e353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Georgakis MK, Harshfield EL, Malik R, Franceschini N, Langenberg C, Wareham NJ, et al. Diabetes mellitus, glycemic traits, and cerebrovascular disease: a Mendelian randomization study. Neurology. 2021;96(13):e1732–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gill D, Efstathiadou A, Cawood K, Tzoulaki I, Dehghan A. Education protects against coronary heart disease and stroke independently of cognitive function: evidence from Mendelian randomization. Int J Epidemiol. 2019;48(5):1468–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gu J, Wang Q, Wu X, Zhang H, Wu C, Qiu W. Causal paradigm between common comorbidities of cardiovascular and metabolism-related diseases in elderly: evidence from cross-sectional and Mendelian randomization studies. Diabet Metabol Syndrome Obes Target Ther. 2023;16:2953–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo C, Harshfield EL, Markus HS. Sleep characteristics and risk of stroke and dementia: an observational and mendelian randomization study. Neurology. 2024;102(5): e209141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo Y, Sun H, Hou S, Zhang W, Liu H, Zhu L, et al. Inflammatory cytokines and stroke and its subtypes: a genetic correlation and two-sample Mendelian randomization study. Front Mol Neurosci. 2023;16:1294450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Habibi D, Teymoori F, Ebrahimi N, Fateh ST, Najd-Hassan-Bonab L, Saeidian AH, et al. Causal effect of serum 25 hydroxyvitamin D concentration on cardioembolic stroke: evidence from two-sample Mendelian randomization. Nutr Metab Cardiovasc Dis. 2024;34(5):1305–13. [DOI] [PubMed] [Google Scholar]
- 60.Harshfield EL, Georgakis MK, Malik R, Dichgans M, Markus HS. Modifiable lifestyle factors and risk of stroke: a mendelian randomization analysis. Stroke. 2021;52(3):931–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harshfield EL, Sims MC, Traylor M, Ouwehand WH, Markus HS. The role of haematological traits in risk of ischaemic stroke and its subtypes. Brain. 2020;143(1):210–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.He Y, Liu Y, Meng H, Sun J, Rui Y, Tian X, et al. Genetically elevated Selenoprotein S levels and risk of stroke: a two-sample Mendelian randomization analysis. Int J Mol Sci. 2025. 10.3390/ijms26041652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu C, Li Y, Qian Y, Wu Z, Hu B, Peng Z. Kidney function and cardiovascular diseases: a large-scale observational and Mendelian randomization study. Front Immunol. 2023;14:1190938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu J, Wang H, Zhou Y. Genetically predicted chronic rhinosinusitis and the risk of stroke: a two-sample mendelian randomization study. Front Neurol. 2023;14: 1294321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu MJ, Hu S, Tan JS, Yang YJ. Individual or familial diabetes in relation to eight cardiovascular diseases: a two-sample Mendelian randomization study. Nutr Metab Cardiovasc Dis. 2023;33(4):883–91. [DOI] [PubMed] [Google Scholar]
- 66.Hu MJ, Tan JS, Gao XJ, Yang JG, Yang YJ. Effect of cheese intake on cardiovascular diseases and cardiovascular biomarkers. Nutrients. 2022. 10.3390/nu14142936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang J. Assessment of the causal association between celiac disease and cardiovascular diseases. Front Cardiovasc Med. 2022;9:1017209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang J, He Z, Xu M, Du J, Zhao YT. Socioeconomic status may affect association of vegetable intake with risk of ischemic cardio-cerebral vascular disease: a Mendelian randomization study. Front Nutr. 2023;10:1161175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang S, Huang F, Mei C, Tian F, Fan Y, Bao J. Systemic lupus erythematosus and the risk of cardiovascular diseases: a two-sample Mendelian randomization study. Front Cardiovasc Med. 2022;9: 896499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang Y, Liu Y, Ma Y, Tu T, Liu N, Bai F, et al. Associations of visceral adipose tissue, circulating protein biomarkers, and risk of cardiovascular diseases: a mendelian randomization analysis. Front Cell Dev Biol. 2022;10: 840866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang Y, Zhang R, Han L, Wu Y, Deng X, Xu T, et al. Lipoprotein(a) and stroke: a two-sample Mendelian randomization study. Front Aging Neurosci. 2023;15:1178079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ji Y, Du Z, Zheng K, Jiang Y, Ren C, Zhu H, et al. Bidirectional causal association between ischemic stroke and five mental disorders. Acta Psychiatr Scand. 2023;148(4):359–67. [DOI] [PubMed] [Google Scholar]
- 73.Jia Y, Guo D, Sun L, Shi M, Zhang K, Yang P, et al. Self-reported daytime napping, daytime sleepiness, and other sleep phenotypes in the development of cardiometabolic diseases: a mendelian randomization study. Eur J Prev Cardiol. 2022;29(15):1982–91. [DOI] [PubMed] [Google Scholar]
- 74.Jia Y, Wang R, Guo D, Sun L, Shi M, Zhang K, et al. Contribution of metabolic risk factors and lifestyle behaviors to cardiovascular disease: a mendelian randomization study. Nutr Metab Cardiovasc Dis. 2022;32(8):1972–81. [DOI] [PubMed] [Google Scholar]
- 75.Jian Z, Yuan C, Ma Y. Blood pressure mediated the effects of urinary uromodulin levels on myocardial infarction: a Mendelian randomization study. Hypertension. 2022;79(11):2430–8. [DOI] [PubMed] [Google Scholar]
- 76.Jiang Y, Liu Q, Wang C, Zhao Y, Jin C, Sun M, et al. The interplay between cytokines and stroke: a bi-directional Mendelian randomization study. Sci Rep. 2024;14(1): 17657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiang Z, Sun Y, Wang Z, Liu S. Causal relations between ischemic stroke and epilepsy: a bidirectional Mendelian randomization study. Heliyon. 2024;10(11): e32532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jin A, Wang M, Chen W, Yan H, Xiang X, Pan Y. Differential effects of genetically determined cholesterol efflux capacity on coronary artery disease and ischemic stroke. Front Cardiovasc Med. 2022;9: 891148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jin T, Huang W, Pang Q, Cao Z, Xing D, Guo S, et al. Genetically identified mediators associated with increased risk of stroke and cardiovascular disease in individuals with autism spectrum disorder. J Psychiatr Res. 2024;174:172–80. [DOI] [PubMed] [Google Scholar]
- 80.Kang X, Jiao T, Wang H, Pernow J, Wirdefeldt K. Mendelian randomization study on the causal effects of tumor necrosis factor inhibition on coronary artery disease and ischemic stroke among the general population. EBioMedicine. 2022;76: 103824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kelly DM, Georgakis MK, Franceschini N, Blacker D, Viswanathan A, Anderson CD. Interplay between chronic kidney disease, hypertension, and stroke: insights from a multivariable Mendelian randomization analysis. Neurology. 2023;101(20):e1960–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koohi F, Harshfield EL, Shatunov A, Markus HS. Does thrombosis play a causal role in lacunar stroke and cerebral small vessel disease? Stroke. 2024;55(4):934–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kwok MK, Kawachi I, Rehkopf D, Schooling CM. The role of cortisol in ischemic heart disease, ischemic stroke, type 2 diabetes, and cardiovascular disease risk factors: a bi-directional Mendelian randomization study. BMC Med. 2020;18(1):363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kwok MK, Schooling CM. Mendelian randomization study on atrial fibrillation and cardiovascular disease subtypes. Sci Rep. 2021;11(1):18682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lai S, Jin Q, Wang D, Li T, Wang X. Effects of menstrual disorders and dysmenorrhea on cardiovascular disease: a Mendelian randomization study. Front Endocrinol. 2024;15: 1302312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Larsson SC, Allara E, Mason AM, Michaëlsson K, Burgess S. Thyroid function and dysfunction in relation to 16 cardiovascular diseases. Circ Genom Precis Med. 2019;12(3): e002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Larsson SC, Bäck M, Rees JMB, Mason AM, Burgess S. Body mass index and body composition in relation to 14 cardiovascular conditions in UK Biobank: a Mendelian randomization study. Eur Heart J. 2020;41(2):221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Larsson SC, Burgess S, Michaëlsson K. Smoking and stroke: a mendelian randomization study. Ann Neurol. 2019;86(3):468–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Larsson SC, Traylor M, Burgess S, Boncoraglio GB, Jern C, Michaëlsson K, et al. Serum magnesium and calcium levels in relation to ischemic stroke: Mendelian randomization study. Neurology. 2019;92(9):e944–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Larsson SC, Traylor M, Markus HS. Circulating vitamin K₁ levels in relation to ischemic stroke and its subtypes: a mendelian randomization study. Nutrients. 2018. 10.3390/nu10111575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Larsson SC, Traylor M, Markus HS. Homocysteine and small vessel stroke: a mendelian randomization analysis. Ann Neurol. 2019;85(4):495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Le NN, Tran TQB, du Toit C, Gill D, Padmanabhan S. Establishing plausibility of cardiovascular adverse effects of immunotherapies using Mendelian randomisation. Front Cardiovasc Med. 2023;10: 1116799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Le NN, Tran TQB, Lip S, McCallum L, McClure J, Dominiczak AF, et al. Unravelling the distinct effects of systolic and diastolic blood pressure using Mendelian randomisation. Genes. 2022. 10.3390/genes13071226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee KJ, Lee SJ, Bae HJ, Sung J. Exploring the causal inference of migraine on stroke: a Mendelian randomization study. Eur J Neurol. 2022;29(1):335–8. [DOI] [PubMed] [Google Scholar]
- 95.Lee Y, Seo JH. Potential causal association between elevated gamma-glutamyl transferase level and stroke: a two-sample Mendelian randomization study. Biomolecules. 2023. 10.3390/biom13111592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leng Y, Li Y, Wang J, Deng P, Wang W, Wu J, et al. Sepsis as an independent risk factor in atrial fibrillation and cardioembolic stroke. Front Endocrinol. 2023;14: 1056274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li F, Wang Y, Hou X, Cao L, Zhou X, Yuan W, et al. Genetic predisposition to neurodegenerative diseases and risk of stroke: a Mendelian randomization study. Front Neurosci. 2022;16: 995045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li G, Zhang H, Jiang J. Genetic associations of childhood and adult BMI on chronic heart failure and ischemic stroke: a Mendelian randomization. IJC Heart Vasc. 2024;52: 101425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li HQ, Feng YW, Yang YX, Leng XY, Zhang PC, Chen SD, et al. Causal relations between exposome and stroke: a mendelian randomization study. J Stroke. 2022;24(2):236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li J, Li J, Shen L, Wang H, Zheng T, Hui Y, et al. Investigating the causal association of postpartum depression with cerebrovascular diseases and cognitive impairment: a Mendelian randomization study. Front Psychiatry. 2023;14: 1196055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li J, Shen S, Yu C, Sun S, Zheng P. Integrated single cell-RNA sequencing and Mendelian randomization for ischemic stroke and metabolic syndrome. iScience. 2024;27(7): 110240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li M, Xu Y, Wu J, Wu C, Li A, Ji X. Circulating N-terminal probrain natriuretic peptide levels in relation to ischemic stroke and its subtypes: a mendelian randomization study. Front Genet. 2022;13: 795479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li P, Dong Z, Chen W, Yang G. Causal relations between obstructive sleep apnea and stroke: a mendelian randomization study. Nat Sci Sleep. 2023;15:257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li Y, Xiang W, Xue H, Meng T, Zhang T, Zhang J, et al. The impact of platelet indices on ischemic stroke: a Mendelian randomization study and mediation analysis. Front Neurol. 2023;14:1302008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li Z, Liu X, Wen J, Wang Z, Xie Y, Zhu L, et al. Genetically proxied appendicular lean mass and stroke risk: a two-step mendelian randomization study. J Stroke Cerebrovasc Dis. 2024;33(10): 107915. [DOI] [PubMed] [Google Scholar]
- 106.Liang C, Chen Q, Zhang Y. Association of thrombopoietin-related drugs with thromboembolic events: Mendelian randomization and a real-world study. Ther Adv Drug Saf. 2024;15:20420986231224236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liang Q, Peng Z. Evaluating the effect of green tea intake on cardiovascular diseases: a Mendelian randomization study in European and East Asian populations. Medicine. 2024;103(29): e38977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin L, Lin J, Qiu J, Liufu N, Lin S, Wei F, et al. Genetic liability to multi-site chronic pain increases the risk of cardiovascular disease. Br J Anaesth. 2023;131(2):373–84. [DOI] [PubMed] [Google Scholar]
- 109.Lin S, Li YE, Wang Y. Multi-cohort analysis reveals genetic predispositions to clonal hematopoiesis as mutation-specific risk factors for stroke. Adv Genet. 2025;6(1): 2400047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Linden AB, Clarke R, Hammami I, Hopewell JC, Guo Y, Whiteley WN, et al. Genetic associations of adult height with risk of cardioembolic and other subtypes of ischemic stroke: a mendelian randomization study in multiple ancestries. PLoS Med. 2022;19(4): e1003967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu B, Qin Z, Cai Z, Liu Z, Chen YL, Yin X, et al. Evaluating the causal association between inflammatory bowel disease and risk of atherosclerotic cardiovascular disease: univariable and multivariable Mendelian randomization study. Biomedicines. 2023. 10.3390/biomedicines11092543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu C, Mao C, Li S, Su Y, Liu H, Wang X, et al. Myasthenia gravis and ischemic stroke: a bidirectional Mendelian randomization study. Curr Neurovasc Res. 2023;20(2):270–9. [DOI] [PubMed] [Google Scholar]
- 113.Liu J, Cai D. Causal relationship of cereal intake and type with cardiovascular disease: a Mendelian randomization study. Front Nutr. 2023;10: 1320120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu R, Shi X, Feng J, Piao J, Yang Z, Zhao Y, et al. Ischemic stroke and cerebral microbleeds: a two-sample bidirectional Mendelian randomization study. Neurol Ther. 2023;12(4):1299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu Y, Clarke R, Bennett DA, Zong G, Gan W. Iron status and risk of heart disease, stroke, and diabetes: a Mendelian randomization study in European adults. J Am Heart Assoc. 2024;13(6): e031732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lu W, Pu B, Wang S, Li M, An Y, Lian J, et al. Bidirectional two-sample Mendelian randomization analysis identifies causal associations between cardiovascular diseases and frozen shoulder. J Orthop Surg Res. 2024;19(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lv Y, Cheng X, Dong Q. SGLT1 and SGLT2 inhibition, circulating metabolites, and cerebral small vessel disease: a mediation Mendelian Randomization study. Cardiovasc Diabetol. 2024;23(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ma C, Wu M, Gao J, Liu C, Xie Y, Lv Q, et al. Periodontitis and stroke: a Mendelian randomization study. Brain Behav. 2023;13(2): e2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ma Y, Wang M, Chen X, Ruan W, Yao J, Lian X. Daytime sleepiness and risk of stroke: a Mendelian randomization analysis. Clin Neurol Neurosurg. 2021;208: 106857. [DOI] [PubMed] [Google Scholar]
- 120.Marini S, Georgakis MK, Chung J, Henry JQA, Dichgans M, Rosand J, et al. Genetic overlap and causal inferences between kidney function and cerebrovascular disease. Neurology. 2020;94(24):e2581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martens LG, Luo J, Willems van Dijk K, Jukema JW, Noordam R, van Heemst D. Diet-derived antioxidants do not decrease risk of ischemic stroke: a Mendelian randomization study in 1 million people. J Am Heart Assoc. 2021;10(23):22567. [DOI] [PMC free article] [PubMed]
- 122.Mei J, Wei P, Zhang L, Ding H, Zhang W, Tang Y, et al. Impact of ankylosing spondylitis on stroke limited to specific subtypes: Evidence from Mendelian randomization study. Front Immunol. 2022;13:1095622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Myserlis EP, Georgakis MK, Parodi L, Mayerhofer E, Rosand J, Banerjee C, et al. The role of the gluteofemoral adipose tissue in cerebrovascular disease risk: evidence from a mendelian randomization and mediation analysis. medRxiv : the preprint server for health sciences. 2024.
- 124.Nethander M, Quester J, Vandenput L, Ohlsson C. Association of genetically predicted serum estradiol with risk of thromboembolism in men: a Mendelian randomization study. J Clin Endocrinol Metab. 2021;106(8):e3078–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Niu YY, Aierken A, Feng L. Unraveling the link between dietary factors and cardiovascular metabolic diseases: Insights from a two-sample Mendelian Randomization investigation. Heart Lung. 2024;63:72–7. [DOI] [PubMed] [Google Scholar]
- 126.Qi Y, Shang X, Han T, Han N, Jiang Z, Yan H, et al. Serum cystatin C and stroke risk: a national cohort and Mendelian randomization study. Front Endocrinol. 2024;15: 1355948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Qian F, Du X, He Y. Causal association of inflammation with ischemic stroke and its subtypes: a bidirectional Mendelian randomization study. J Stroke Cerebrovasc Dis. 2025;34(2): 108190. [DOI] [PubMed] [Google Scholar]
- 128.Qian Y, Ye D, Huang H, Wu DJH, Zhuang Y, Jiang X, et al. Coffee consumption and risk of stroke: a mendelian randomization study. Ann Neurol. 2020;87(4):525–32. [DOI] [PubMed] [Google Scholar]
- 129.Qin H, Suo S, Yang F, Hao P, Zhang X. The role of digestive system diseases in cerebrovascular disease: a comprehensive mendelian randomization study. Front Neurol. 2024;15:1389352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Qin H, Yang F, Zhao H, Zhao J, Lin S, Shang Y, et al. Associations of lipids and lipid-lowering drugs with risk of stroke: a Mendelian randomization study. Front Neurol. 2023;14:1185986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Qin S, Wang M, Gill D, Zhang Z, Liu X. The mediating role of atrial fibrillation in causal associations between risk factors and stroke: a Mendelian randomization study. Epidemiol Health. 2024;46: e2024005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Qu H, He C, Xu H, Sun X. Investigating the association of breast cancer and stroke: a two-sample Mendelian randomization study. Medicine. 2023;102(38): e35037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Qu K, Li M, Yu P, Jiang W, Dong M. Hypertensive disorders of pregnancy and stroke: a univariate and multivariate Mendelian randomization study. Front Endocrinol. 2024;15: 1366023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ruan Z, Zhao J. Differential ischemic stroke risk linked to novel subtypes of type 2 diabetes: insights from a Mendelian randomization analysis. Endocrine. 2024;84(3):980–8. [DOI] [PubMed] [Google Scholar]
- 135.Schmidt AF, Hunt NB, Gordillo-Marañón M, Charoen P, Drenos F, Kivimaki M, et al. Cholesteryl ester transfer protein (CETP) as a drug target for cardiovascular disease. Nat Commun. 2021;12(1):5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shen R, Pan C, Yu J, Dong C, Li Z, Zhang J, et al. Primary aldosteronism influences cardiac structure, function, and disease risk: evidence from Mendelian randomization analysis. J Clin Hypertens. 2024;26(11):1301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shi F, Zhang G, Li J, Shu L, Yu C, Ren D, et al. Integrated analysis of single cell-RNA sequencing and Mendelian randomization identifies lactate dehydrogenase B as a target of melatonin in ischemic stroke. CNS Neurosci Ther. 2024;30(5): e14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shi Y, Bao L, Li Y, Ou D, Li J, Liu X, et al. Multi-omics combined to investigate potential druggable therapeutic targets for stroke: a systematic Mendelian randomization study and transcriptome verification. J Affect Disord. 2024;366:196–209. [DOI] [PubMed] [Google Scholar]
- 139.Si S, Tewara MA, Li Y, Li W, Chen X, Yuan T, et al. Causal pathways from body components and regional fat to extensive metabolic phenotypes: a Mendelian randomization study. Obesity. 2020;28(8):1536–49. [DOI] [PubMed] [Google Scholar]
- 140.Song J, Gao N, Chen Z, Xu G, Kong M, Wei D, et al. Shared genetic etiology of vessel diseases: a genome-wide multi-traits association analysis. Thromb Res. 2024;241: 109102. [DOI] [PubMed] [Google Scholar]
- 141.Song L, Sun J, Söderholm M, Melander O, Orho-Melander M, Nilsson J, et al. Association of TIM-1 (T-cell immunoglobulin and mucin domain 1) with incidence of stroke. Arterioscler Thromb Vasc Biol. 2020;40(7):1777–86. [DOI] [PubMed] [Google Scholar]
- 142.Soremekun O, Musanabaganwa C, Uwineza A, Ardissino M, Rajasundaram S, Wani AH, et al. A mendelian randomization study of genetic liability to post-traumatic stress disorder and risk of ischemic stroke. Transl Psychiatry. 2023;13(1):237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sun H, Tang Q, Yan X, Xie W, Xu Y, Zhang W. Cathepsins and neurological diseases: a Mendelian randomization study. Front Neurosci. 2024;18:1454369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sun J, Borné Y, Edsfeldt A, Wang Y, Pan M, Melander O, et al. Genetic susceptibility to mood disorders and risk of stroke: a polygenic risk score and Mendelian randomization study. Stroke. 2023;54(5):1340–6. [DOI] [PubMed] [Google Scholar]
- 145.Sun W, Zhang L, Liu W, Tian M, Wang X, Liang J, et al. Stroke and myocardial infarction: a bidirectional Mendelian randomization study. Int J General Med. 2021;14:9537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tao Y, Luo J, Xu Y, Wang H, Tian J, Yang S, et al. Narcolepsy and cardiovascular disease: a two-sample Mendelian randomization study. Sleep Med. 2024;113:6–12. [DOI] [PubMed] [Google Scholar]
- 147.Thériault S, Sjaarda J, Chong M, Hess S, Gerstein H, Paré G. Identification of circulating proteins associated with blood pressure using Mendelian randomization. Circ Genom Precis Med. 2020;13(1): e002605. [DOI] [PubMed] [Google Scholar]
- 148.Titova OE, Michaëlsson K, Larsson SC. Sleep duration and stroke: prospective cohort study and Mendelian randomization analysis. Stroke. 2020;51(11):3279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Titova OE, Yuan S, Baron JA, Lindberg E, Michaëlsson K, Larsson SC. Sleep-disordered breathing-related symptoms and risk of stroke: cohort study and Mendelian randomization analysis. J Neurol. 2022;269(5):2460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tschiderer L, Peters SAE, van der Schouw YT, van Westing AC, Tong TYN, Willeit P, et al. Age at menopause and the risk of stroke: observational and Mendelian randomization analysis in 204 244 postmenopausal women. J Am Heart Assoc. 2023;12(18): e030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tschiderer L, van der Schouw YT, Burgess S, Bloemenkamp KW, Seekircher L, Willeit P, et al. Hypertensive disorders of pregnancy and cardiovascular disease risk: a Mendelian randomisation study. Eur Heart J. 2023. 10.1093/eurheartj/ehad655.2726 [DOI] [PubMed] [Google Scholar]
- 152.Verdiesen RMG, von Berg J, Said MA, van der Harst P, Mahajan A, van Gils CH, et al. Anti-Müllerian hormone and cardiometabolic disease in women: a two-sample Mendelian randomization study. Rev Cardiovasc Med. 2022;23(8):269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vissers LET, Sluijs I, Burgess S, Forouhi NG, Freisling H, Imamura F, et al. Milk intake and incident stroke and CHD in populations of European descent: a Mendelian randomisation study. Br J Nutr. 2022;128(9):1789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang K, Lin X, Sheng S, Chen D, Liu X, Yao K. Association between glaucoma and stroke: a bidirectional mendelian randomization study. Adv Ophthalmol Pract Res. 2024;4(3):147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang M, Daghlas I, Zhang Z, Gill D, Liu D. MTHFR polymorphisms, homocysteine elevation, and ischemic stroke susceptibility in East Asian and European populations. Neurology. 2025;104(3): e210245. [DOI] [PubMed] [Google Scholar]
- 156.Wang M, Zhang Z, Liu D, Ji L, Huang S, Cao L, et al. Genetic predisposition to Parkinson’s disease and risk of cardio and cerebrovascular disease: a Mendelian randomization study. Parkinsonism Relat Disord. 2022;94:49–53. [DOI] [PubMed] [Google Scholar]
- 157.Wang S, Liu Y, Wu K, Xia D, Dong X. Osteoarthritis and risk of cardiovascular diseases: a Mendelian randomization study. Injury. 2023. [DOI] [PubMed]
- 158.Wang S, Zha L, Chen J, Du D, Liu D, Zhong M, et al. The relationship between lipoprotein(a) and risk of cardiovascular disease: a Mendelian randomization analysis. Eur J Med Res. 2022;27(1):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang W, Liu M, Wang Z, Ma L, Zhao Y, Ye W, et al. A bidirectional Mendelian randomization study of the causal association between ischemic stroke, coronary heart disease, and hydrocephalus. Brain Behav. 2024;14(10): e70090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang X, Pang W, Hu X, Shu T, Luo Y, Li J, et al. Conventional and genetic association between migraine and stroke with druggable genome-wide Mendelian randomization. Hum Genet. 2025;144(4):391–404. [DOI] [PubMed] [Google Scholar]
- 161.Wang Y, Liu Y, Xia M, Cao S. A mendelian randomization study about causal associations between tofu consumption and stroke as well as related subtypes. J Integr Neurosci. 2024;23(11):198. [DOI] [PubMed] [Google Scholar]
- 162.Wang Z, Yang F, Ma M, Bao Q, Shen J, Ye F, et al. The impact of growth differentiation factor 15 on the risk of cardiovascular diseases: two-sample Mendelian randomization study. BMC Cardiovasc Disord. 2020;20(1):462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wen Z, Zhu L, He W, Liang T, Zhong Q, Long J, et al. Exploring the causal inference of inflammatory bowel disease and ischemic stroke: a bidirectional two-sample Mendelian randomization study. J Thromb Thrombolysis. 2025;58(2):340–8. [DOI] [PubMed] [Google Scholar]
- 164.Wu BS, Chen SF, Huang SY, Ou YN, Deng YT, Chen SD, et al. Identifying causal genes for stroke via integrating the proteome and transcriptome from brain and blood. J Transl Med. 2022;20(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wu D, Xiong F, Ran Q, Liu J, Wu Q, Wang L, et al. Mendelian randomization of chronic hepatitis B and cardiovascular disease. Front Cardiovasc Med. 2024;11:1332557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wu S, Meena D, Yarmolinsky J, Gill D, Smith A, Dib MJ, et al. Mendelian randomization and Bayesian colocalization analysis implicate glycoprotein VI as a potential drug target for cardioembolic stroke in South Asian populations. J Am Heart Assoc. 2024;13(16): e035008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wu W, Fan D, Que B, Chen Y, Qiu R. Investigation on the relationship between hemoglobin concentration and stroke risk: a bidirectional Mendelian randomization study. Front Neurol. 2024;15:1327873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xiang W, Shen Y, Li Y, Chen S, Cao Q, Xu L. Causal association between mental disorders and cerebrovascular diseases: evidence from Mendelian randomization study. J Affect Disord. 2025;368:461–70. [DOI] [PubMed] [Google Scholar]
- 169.Xie Y, Chen H, Xu J, Qu P, Zhu L, Tan Y, et al. Cheese consumption on atherosclerosis, atherosclerotic cardiovascular diseases and its complications: a two-sample Mendelian randomization study. Nutr Metab Cardiovasc Dis. 2024;34(3):691–8. [DOI] [PubMed] [Google Scholar]
- 170.Xie Y, Liu S, Wang X, Huang H, Wang M, Qu W, et al. Lipids, apolipoproteins, lipid-lowering drugs, and the risk of cerebral small vessel disease: a mendelian randomization study. J Am Heart Assoc. 2024;13(16): e032409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Xu H, Wang X, Geng G, Xu X, Liu L, Zhang Y, et al. Association of circulating branched-chain amino acids with cardiovascular diseases: a mendelian randomization study. Nutrients. 2023. 10.3390/nu15071580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Xu L, Zhang R, Zhang X, Liu B, Huang D, Liu Y, et al. Plasma proteomes and genome-wide association data for causal protein identification in stroke. Mol Neurobiol. 2025;62(2):2450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xu N, Qiu Y, Ainiwan D, Wang B, Alifu X, Zhou H, et al. Mediating factors in the association between educational attainment and stroke: a mendelian randomization study. SSM. 2025;29: 101766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xu P, Wei Y, Wu H, Zhang L. Genetic associations between rapid eye movement (REM) sleep behavior disorder and cardiovascular diseases. PLoS ONE. 2024;19(5): e0301112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yang F, Chen S, Qu Z, Wang K, Xie X, Cui H. Genetic liability to sedentary behavior in relation to stroke, its subtypes and neurodegenerative diseases: a Mendelian randomization study. Front Aging Neurosci. 2021;13: 757388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yang F, Hu T, He K, Ying J, Cui H. Multiple sclerosis and the risk of cardiovascular diseases: a mendelian randomization study. Front Immunol. 2022;13: 861885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ye C, Kong L, Wang Y, Zheng J, Xu M, Xu Y, et al. Causal associations of sarcopenia-related traits with cardiometabolic disease and Alzheimer’s disease and the mediating role of insulin resistance: a Mendelian randomization study. Aging Cell. 2023;22(9): e13923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ye D, Huang H, Wu DJH, Zhang W, Zhou F, Qian Y, et al. Association between circulating linoleic acid and risk of ischemic stroke. Front Genet. 2020;11: 582623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ye M, Chen J, Ma J, Wang J, Zhang C, Chen B, et al. Causal association of cardiovascular disease with erectile dysfunction: a two-sample bidirectional Mendelian randomization analysis. Andrology. 2023;11(7):1368–76. [DOI] [PubMed] [Google Scholar]
- 180.Yi X, Zhu J, Zhang X, Huang N, Cheng Y. Leukemia and risk of stroke: a Mendelian randomization analysis. BMC Neurol. 2025;25(1): 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yu Z, Liu X, Feng X, Zhang X, Gao R. Causal relationship between novel antidiabetic drugs and ischemic stroke: a drug-targeted Mendelian randomization study. Front Cardiovasc Med. 2024;11:1449185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yuan S, Bäck M, Bruzelius M, Mason AM, Burgess S, Larsson S. Plasma phospholipid fatty acids, FADS1 and risk of 15 cardiovascular diseases: a Mendelian randomisation study. Nutrients. 2019. 10.3390/nu11123001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yuan S, Burgess S, Laffan M, Mason AM, Dichgans M, Gill D, et al. Genetically proxied inhibition of coagulation factors and risk of cardiovascular disease: a mendelian randomization study. J Am Heart Assoc. 2021;10(8): e019644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yuan S, Carter P, Mason AM, Yang F, Burgess S, Larsson SC. Genetic liability to rheumatoid arthritis in relation to coronary artery disease and stroke risk. Arthritis Rheumatol. 2022;74(10):1638–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yuan S, Mason AM, Burgess S, Larsson SC. Differentiating associations of glycemic traits with atherosclerotic and thrombotic outcomes: Mendelian randomization investigation. Diabetes. 2022;71(10):2222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yuan S, Mason AM, Carter P, Burgess S, Larsson SC. Homocysteine, B vitamins, and cardiovascular disease: a Mendelian randomization study. BMC Med. 2021;19(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yuan S, Zheng JS, Mason AM, Burgess S, Larsson SC. Genetically predicted circulating vitamin C in relation to cardiovascular disease. Eur J Prev Cardiol. 2022;28(16):1829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yuan T, Si S, Li Y, Li W, Chen X, Liu C, et al. Roles for circulating polyunsaturated fatty acids in ischemic stroke and modifiable factors: a Mendelian randomization study. Nutr J. 2020;19(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zeng Y, Cao S, Yang H. Causal associations between dried fruit intake and cardiovascular disease: a Mendelian randomization study. Front Cardiovasc Med. 2023;10:1080252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhang F, Xian D, Feng J, Ning L, Jiang T, Xu W, et al. Causal relationship between Alzheimer’s disease and cardiovascular disease: a bidirectional Mendelian randomization analysis. Aging (Albany NY). 2023;15(17):9022–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhang J, Lu H, Cao M, Zhang J, Liu D, Meng X, et al. Metabolic traits and risk of ischemic stroke in Japanese and European populations: a two-sample Mendelian randomization study. Metabolites. 2024. 10.3390/metabo14050255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhang J, Wu F, Chen S, Zhu Y, Luo X, Qiu X. Genetic predisposition to severe COVID-19 might increase the risk of stroke: a two-sample Mendelian randomization study. Front Genet. 2022;13: 895211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang K, Jia Y, Wang R, Guo D, Yang P, Sun L, et al. Rheumatoid arthritis and the risk of major cardiometabolic diseases: a mendelian randomization study. Scand J Rheumatol. 2023;52(4):335–41. [DOI] [PubMed] [Google Scholar]
- 194.Zhang L, Liu W, Sun W, Wang X, Tian M, Pei LL, et al. Heart failure and ischemic stroke: a bidirectional and multivariable mendelian randomization study. Front Genet. 2021;12: 771044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhang L, Yu K, Huo J, Mei S, Zhao Z, Zhu B. Causal relationship between spondylarthritis and stroke in a European population: a two sample Mendelian randomization study. Front Immunol. 2023;14: 1253986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang P, Zhang Z, Zhong J, Zheng X, Zhou J, Sun W. Cardiovascular diseases consequences of type 1, type 2 diabetes mellitus and glycemic traits: a Mendelian randomization study. Diabetes Res Clin Pract. 2024;208: 111094. [DOI] [PubMed] [Google Scholar]
- 197.Zhang Q, Shen C, Zhang L, Wang M. Causal relationship between chronic hepatitis B and stroke in East Asians: a Mendelian randomization study. J Cardiovasc Dev Dis. 2024. 10.3390/jcdd11080247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhang T, Au Yeung SL, Schooling CM. Associations of arachidonic acid synthesis with cardiovascular risk factors and relation to ischemic heart disease and stroke: a univariable and multivariable mendelian randomization study. Nutrients. 2021. 10.3390/nu13051489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhang T, Zhao JV, Schooling CM. The associations of plasma phospholipid arachidonic acid with cardiovascular diseases: a Mendelian randomization study. EBioMedicine. 2021;63: 103189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang W, Li Y, Pang M, Yue X. Causal relationship between hypertension and ischemic stroke: a two-sample Mendelian randomization study. Brain Circ. 2024;10(3):257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang Y, Jiang M, Wu D, Li M, Ji X. The causal relationship between steroid hormones and risk of stroke: evidence from a two-sample Mendelian randomization study. Mol Brain. 2025;18(1): 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhang Y, Liu H, Dai Y, Ye F, Luo W, Tu S, et al. Evaluation of bidirectional causal association between cardiovascular diseases and pneumonia: A Mendelian randomization study. Eur J Prev Cardiol. 2024. [DOI] [PubMed]
- 203.Zhao H, Zhu J, Ju L, Sun L, Tse LA, Kinra S, et al. Osteoarthritis & stroke: a bidirectional mendelian randomization study. Osteoarthritis Cartilage. 2022;30(10):1390–7. [DOI] [PubMed] [Google Scholar]
- 204.Zhao J, Chen H, Zhuo C, Xia S. Cannabis use and the risk of cardiovascular diseases: a mendelian randomization study. Front Cardiovasc Med. 2021;8: 676850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhao JV, Burgess S, Fan B, Schooling CM. L-carnitine, a friend or foe for cardiovascular disease? A Mendelian randomization study. BMC Med. 2022;20(1): 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zheng H, Wang W, Qiu W, Feng Y. Association of antihypertensive drug target genes with stroke subtypes: a Mendelian randomization study. J Stroke Cerebrovasc Dis. 2025;34(3): 108244. [DOI] [PubMed] [Google Scholar]
- 207.Zheng X, Yang Y, Chen J, Lu B. Dissecting the causal relationship between household income status and genetic susceptibility to cardiovascular-related diseases: insights from bidirectional mendelian randomization study. BMC Public Health. 2023;23(1):749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhou M, Dong J, Zha L, Liao Y. Causal association between periodontal diseases and cardiovascular diseases. Genes. 2021;13(1): 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhou P, Jiang X, Wang D. Hidradenitis suppurativa and cardiovascular diseases: a bidirectional Mendelian randomization study. Skin Res Technol. 2024;30(7): e13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhu T, Cui J, Goodarzi MO. Polycystic ovary syndrome and risk of type 2 diabetes, coronary heart disease, and stroke. Diabetes. 2021;70(2):627–37. [DOI] [PubMed] [Google Scholar]
- 211.Zhu Y, Li M, Wang H, Yang F, Pang X, Du R, et al. Genetically proxied antidiabetic drugs targets and stroke risk. J Transl Med. 2023;21(1): 681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhu Y, Wang Y, Cui Z, Liu F, Hu J. Identification of pleiotropic and specific therapeutic targets for cardio-cerebral diseases: a large-scale proteome-wide mendelian randomization and colocalization study. PLoS ONE. 2024;19(5): e0300500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhu Y, Xin X, Yu Z, Guan S, Wang J, Liu Q, et al. Causal associations of male infertility with stroke: a two-sample Mendelian randomization study. Front Endocrinol. 2024;15: 1338077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhuo C, Chen L, Wang Q, Cai H, Lin Z, Pan H, et al. Association of age at first sexual intercourse and lifetime number of sexual partners with cardiovascular diseases: a bi-directional Mendelian randomization study. Front Cardiovasc Med. 2023;10: 1267906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zou X, Wang L, Wang S, Zhang Y, Ma J, Chen L, et al. Promising therapeutic targets for ischemic stroke identified from plasma and cerebrospinal fluid proteomes: a multicenter Mendelian randomization study. Int J Surg. 2024;110(2):766–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zou X, Wang L, Xiao L, Xu Z, Yao T, Shen M, et al. Deciphering the irregular risk of stroke increased by obesity classes: a stratified Mendelian randomization study. Front Endocrinol. 2021;12: 750999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zou XL, Wang S, Wang LY, Xiao LX, Yao TX, Zeng Y, et al. Childhood obesity and risk of stroke: a Mendelian randomisation analysis. Front Genet. 2021;12: 727475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zuber V, Cameron A, Myserlis EP, Bottolo L, Fernandez-Cadenas I, Burgess S, et al. Leveraging genetic data to elucidate the relationship between COVID-19 and ischemic stroke. J Am Heart Assoc. 2021;10(22): e022433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zuo Z, Tong Y, Li M, Wang Z, Wang X, Guo X, et al. Effect of genetically determined BCAA levels on cardiovascular diseases and their risk factors: a Mendelian randomization study. Nutr Metab Cardiovasc Dis. 2023;33(12):2406–12. [DOI] [PubMed] [Google Scholar]
- 220.Song L, Shen L, Li H, Liu B, Zheng X, Liang Y, et al. Height and prevalence of hypertension in a middle-aged and older Chinese population. Sci Rep. 2016;6: 39480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Webb AJS, Werring DJ. New insights into cerebrovascular pathophysiology and hypertension. Stroke. 2022;53(4):1054–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tsang TS, Barnes ME, Bailey KR, Leibson CL, Montgomery SC, Takemoto Y, et al. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc. 2001;76(5):467–75. [DOI] [PubMed] [Google Scholar]
- 223.Abete I, Arriola L, Etxezarreta N, Mozo I, Moreno-Iribas C, Amiano P, et al. Association between different obesity measures and the risk of stroke in the EPIC Spanish cohort. Eur J Nutr. 2015;54(3):365–75. [DOI] [PubMed] [Google Scholar]
- 224.Miller MA, Cappuccio FP. Inflammation, sleep, obesity and cardiovascular disease. Curr Vasc Pharmacol. 2007;5(2):93–102. [DOI] [PubMed] [Google Scholar]
- 225.Zimberg IZ, Dâmaso A, Del Re M, Carneiro AM, de Sá Souza H, de Lira FS, et al. Short sleep duration and obesity: mechanisms and future perspectives. Cell Biochem Funct. 2012;30(6):524–9. [DOI] [PubMed] [Google Scholar]
- 226.Grandner MA, Sands-Lincoln MR, Pak VM, Garland SN. Sleep duration, cardiovascular disease, and proinflammatory biomarkers. Nat Sci Sleep. 2013;5:93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Robbins AS, Manson JE, Lee IM, Satterfield S, Hennekens CH. Cigarette smoking and stroke in a cohort of U.S. male physicians. Ann Intern Med. 1994;120(6):458–62. [DOI] [PubMed] [Google Scholar]
- 228.Rissanen I, Oura P, Paananen M, Miettunen J, Geerlings MI. Smoking trajectories and risk of stroke until age of 50 years - the Northern Finland Birth Cohort 1966. PLoS ONE. 2019;14(12): e0225909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Li H, Li X, Gao S, Wang D, Gao X, Li Y, et al. Exposure to cigarette smoke augments post-ischemic brain injury and inflammation via mobilization of neutrophils and monocytes. Front Immunol. 2019;10:2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Braidy N, Jugder BE, Poljak A, Jayasena T, Mansour H, Nabavi SM, et al. Resveratrol as a potential therapeutic candidate for the treatment and management of alzheimer’s disease. Curr Top Med Chem. 2016;16(17):1951–60. [DOI] [PubMed] [Google Scholar]
- 231.Khan N, Mukhtar H. Tea polyphenols for health promotion. Life Sci. 2007;81(7):519–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Lee W, Min WK, Chun S, Lee YW, Park H, Lee DH, et al. Long-term effects of green tea ingestion on atherosclerotic biological markers in smokers. Clin Biochem. 2005;38(1):84–7. [DOI] [PubMed] [Google Scholar]
- 233.Wang W, Zhang ZZ, Wu Y, Wang RQ, Chen JW, Chen J, et al. (-)-Epigallocatechin-3-gallate ameliorates atherosclerosis and modulates hepatic lipid metabolic gene expression in apolipoprotein E knockout mice: involvement of TTC39B. Front Pharmacol. 2018;9: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhan XL, Yang XH, Gu YH, Guo LL, Jin HM. Epigallocatechin gallate protects against homocysteine-induced vascular smooth muscle cell proliferation. Mol Cell Biochem. 2018;439(1–2):131–40. [DOI] [PubMed] [Google Scholar]
- 235.Dalmeijer GW, van der Schouw YT, Booth SL, de Jong PA, Beulens JW. Phylloquinone concentrations and the risk of vascular calcification in healthy women. Arterioscler Thromb Vasc Biol. 2014;34(7):1587–90. [DOI] [PubMed] [Google Scholar]
- 236.Chen CL, Huang JY, Liu L, Yu YL, Shen G, Lo K, et al. Relationship between diastolic blood pressure and the first ischaemic stroke in elderly patients with hypertension. Postgrad Med J. 2020;96(1139):525–9. [DOI] [PubMed] [Google Scholar]
- 237.Song MS, Choi YJ, Kim H, Nam MJ, Lee CW, Han K, et al. Relationship between blood pressure levels and ischemic stroke, myocardial infarction, and mortality in very elderly patients taking antihypertensives: a nationwide population-based cohort study. BMC Geriatr. 2021;21(1):620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Hägg-Holmberg S, Dahlström EH, Forsblom CM, Harjutsalo V, Liebkind R, Putaala J, et al. The role of blood pressure in risk of ischemic and hemorrhagic stroke in type 1 diabetes. Cardiovasc Diabetol. 2019;18(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Mavridis A, Viktorisson A, Eliasson B, von Euler M, Sunnerhagen KS. Risk of ischemic and hemorrhagic stroke in individuals with type 1 and type 2 diabetes: a nationwide cohort study in Sweden. Neurology. 2025;104(7): e213480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lv P, Jin H, Liu Y, Cui W, Peng Q, Liu R, et al. Comparison of risk factor between lacunar stroke and large artery atherosclerosis stroke: a cross-sectional study in China. PLoS ONE. 2016;11(3): e0149605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.O’Donnell MJ, McQueen M, Sniderman A, Pare G, Wang X, Hankey GJ, et al. Association of lipids, lipoproteins, and apolipoproteins with stroke subtypes in an international case control study (INTERSTROKE). J stroke. 2022;24(2):224–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Petersen NH, Schmied AB, Zeller JA, Plendl H, Deuschl G, Zunker P. Lp(a) lipoprotein and plasminogen activity in patients with different etiology of ischemic stroke. Cerebrovasc Dis. 2007;23(2–3):188–93. [DOI] [PubMed] [Google Scholar]
- 243.Song Y, Choi JE, Kwon YJ, Chang HJ, Kim JO, Park DH, et al. Identification of susceptibility loci for cardiovascular disease in adults with hypertension, diabetes, and dyslipidemia. J Transl Med. 2021;19(1): 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kao CC, Wu CH, Lin YH, Chang CC, Chen HH, Wu MS, et al. Risk of ischemic stroke in primary aldosteronism patients. Clin Chim Acta. 2015;438:86–9. [DOI] [PubMed] [Google Scholar]
- 245.Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2(1):43–53. [DOI] [PubMed] [Google Scholar]
- 246.Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Dinh QN, Young MJ, Evans MA, Drummond GR, Sobey CG, Chrissobolis S. Aldosterone-induced oxidative stress and inflammation in the brain are mediated by the endothelial cell mineralocorticoid receptor. Brain Res. 2016;1637:146–53. [DOI] [PubMed] [Google Scholar]
- 248.Unal-Cevik I, Gursoy-Ozdemir Y, Yemisci M, Lule S, Gurer G, Can A, et al. Alpha-synuclein aggregation induced by brief ischemia negatively impacts neuronal survival in vivo: a study in [A30P]alpha-synuclein transgenic mouse. J Cereb Blood Flow Metab. 2011;31(3):913–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kim T, Mehta SL, Kaimal B, Lyons K, Dempsey RJ, Vemuganti R. Poststroke induction of α-synuclein mediates ischemic brain damage. J Neurosci. 2016;36(26):7055–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Yoo J, Jeon J, Baek M, Song SO, Kim J. Impact of statin treatment on cardiovascular risk in patients with type 1 diabetes: a population-based cohort study. J Transl Med. 2023;21(1):806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387–97. [DOI] [PubMed] [Google Scholar]
- 252.Hackam DG, Hegele RA. Lipid-modifying therapies and stroke prevention. Curr Neurol Neurosci Rep. 2022;22(7):375–82. [DOI] [PubMed] [Google Scholar]
- 253.Li JY, Paragas N, Ned RM, Qiu A, Viltard M, Leete T, et al. Scara5 is a ferritin receptor mediating non-transferrin iron delivery. Dev Cell. 2009;16(1):35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Franchini M, Targher G, Montagnana M, Lippi G. Iron and thrombosis. Ann Hematol. 2008;87(3):167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Galaris D, Barbouti A, Pantopoulos K. Iron homeostasis and oxidative stress: an intimate relationship. Biochimica et Biophysica Acta (BBA). 2019;1866(12): 118535. [DOI] [PubMed] [Google Scholar]
- 256.Yang HJ, Kong B, Shuai W, Zhang JJ, Huang H. Shensong yangxin attenuates metabolic syndrome-induced atrial fibrillation via inhibition of ferroportin-mediated intracellular iron overload. Phytomedicine. 2022;101: 154086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.