ABSTRACT
Objective
To present evidence‐ and consensus‐based guidelines for resuscitation of newborn puppies and kittens.
Design
Prioritized clinical questions pertaining to newborn resuscitation and in the Population–Intervention–Comparator–Outcome (PICO) format were used to inform systematic literature searches by information specialists, to extract research findings from relevant publications and synthesize them into evidence, to assess this evidence for quality, and, finally, to develop draft treatment recommendations. These steps were followed by a consensus process and a community commenting period prior to finalization of the project. These RECOVER Newborn Resuscitation Guidelines are a concise summary of the newborn resuscitation process to provide clear and actionable clinical instructions to veterinary professionals.
Setting
Transdisciplinary, international collaboration in university, specialty, and emergency practice.
Results
A total of 28 PICO questions pertaining to resuscitation of puppies and kittens at birth were addressed in this project. This resulted in 59 treatment recommendations that delineate an iterative approach to newborn resuscitation starting with airway clearance, tactile stimulation, and temperature control, as well as positive pressure ventilation, and instruct on more advanced measures such as CPR. An algorithm displays the flow of assessments and actions over the course of the resuscitation process.
Conclusions
These RECOVER Newborn Resuscitation Guidelines present a concise and comprehensive framework for resuscitation of puppies and kittens at birth. These works serve to support veterinary professionals and breeders, educational systems, and research initiatives in conducting, implementing, and advancing newborn resuscitation in puppies and kittens.
Keywords: birth, Cesarean section, cardiopulmonary resuscitation, consensus guidelines, evidence‐based veterinary medicine, neonatal resuscitation
Abbreviations
- CPA
cardiopulmonary arrest
- CRI
constant rate infusion
- C‐section
Cesarean section
- EE
Evidence Evaluator
- ET
endotracheal
- GRADE
Grading of Recommendations, Assessment, Development, and Evaluation
- HIE
hypoxic–ischemic encephalopathy
- HR
heart rate
- IO
intraosseous
- IQR
interquartile range
- PEEP
positive end‐expiratory pressure
- PICO
Population–Intervention–Comparator–Outcome
- PIP
peak inspiratory pressure
- PPV
positive pressure ventilation
- ROSC
return of spontaneous circulation
- RR
respiratory rate
- SI
sustained inflation
1. Introduction
At birth, newborn puppies and kittens undergo dramatic physiological changes that can lead to severe harm or even death if any of the involved critical processes fail [1]. Within minutes, the newborn must aerate its previously fluid‐filled lungs to transition gas exchange from the now‐removed placenta to the lungs, and simultaneously alter the way blood circulates through the heart and lungs in a fundamental way [1, 2, 3]. The goal of newborn resuscitation is to support animals at birth through their essential transition from intra‐ to extrauterine physiology. But how to do this best?
For several decades, the International Liaison Committee on Resuscitation (ILCOR) has provided evidence‐ and consensus‐based treatment recommendations for resuscitation of newborn infants, empowering medical personnel around the world to deliver resuscitation measures rooted in systematic appraisal of scientific knowledge [4, 5]. In veterinary medicine, RECOVER published evidence‐ and consensus‐based treatment recommendations for CPR in adult dogs and cats in 2012 and 2024, but these guidelines did not pertain to newborns [6, 7]. Also, while recommendations for the practical execution of CPR in neonates and newborns have been published previously, each has represented the expertise of individual subject matter experts in the field [8, 9, 10, 11]. No evidence‐ and consensus‐based guidelines have been available for resuscitation of newborn puppies and kittens in the first minutes after birth.
Physiology of the newborn is dynamic during transition and differs greatly from the adult. Given this difference, newborns require unique resuscitation methods that differ fundamentally from methods used to resuscitate post‐transitional dogs and cats in distress or in cardiopulmonary arrest (CPA).
The goal of the RECOVER Newborn Resuscitation Guidelines project was to generate evidence‐ and consensus‐based treatment recommendations for resuscitation of newborn puppies and kittens to arrive at a concise and actionable set of clinical instructions for veterinary professionals.
2. Methods
2.1. Definitions
“Newborn” herein is defined as a mammal such as a dog or cat from birth through the first few hours of life, spanning the transition from intrauterine, fetal life to extrauterine life. A “neonate” is a dog or cat from birth until initiation of weaning (approximately 4–5 weeks depending on species and breed), at which time it is referred to as “pediatric” until it reaches sexual maturity (e.g., approximately 6 months of age depending on species and breed).
“Newborn resuscitation” includes interventions delivered from birth through the first few hours after birth to support the establishment of breathing and postnatal circulation, and related emergency care. In most cases, these interventions will be applied in a measured fashion to support the newborn's independent efforts to transition from intrauterine to postnatal physiology. Thus, resuscitation in the newborn may be limited to single measures such as tactile stimulation, establishment of a patent airway (i.e., airway clearance), oxygen supplementation, or positive pressure ventilation (PPV), or may include all the above along with chest compressions (i.e., CPR).
“Cardiorespiratory transition at birth” describes the fundamental changes in respiratory and circulatory function that the newborn experiences at birth. In this process, fetal physiology, which entirely depends on placental gas exchange and has the right and left ventricles functioning in parallel, transitions to postnatal physiology where gas exchange is relegated entirely to the lungs and the right and left ventricles work in series to serve the pulmonary and systemic circulations, respectively (Figure 1). Supporting this transition from fetal to newborn life is the primary goal of newborn resuscitation.
FIGURE 1.

Transition of the cardiorespiratory system at birth. (A) The cardiorespiratory system prior to birth. Gas exchange occurs exclusively in the placenta. Its vascular bed is of low vascular resistance and receives the majority of the fetus's aortic blood flow. The lung is filled with fluid and has high vascular resistance. This leads to a relatively higher pressure on the right side of the heart than on the left side. This pressure gradient drives approximately 50% of oxygenated caudal vena cava blood to transit directly through the foramen ovale into the left atrium. This well‐oxygenated blood then perfuses the brain. The remaining 50% of caudal vena cava blood comingles with cranial vena cava blood that is of low oxygen saturation and leaves the right ventricle through the pulmonary artery, with most of it directly entering the descending aorta via the ductus arteriosus. As a consequence, the two ventricles function in parallel to generate aortic blood flow, with the preductal arterial oxygen saturation being markedly higher than the post‐ductal arterial oxygen saturation. (B) After the completion of cardiorespiratory transition, hours following birth. At birth, the placenta is removed, and the lungs are aerated. The removal of the placenta increases peripheral vascular resistance and consequently pressures in the aorta and the left ventricle. In contrast, the vascular resistance in the now air‐filled lungs decreases, pulmonary arterial blood flow through the lungs markedly increases, and blood flow through the ductus arteriosus ceases. This leads to an increase in left atrial pressure, to the functional closure of the foramen ovale, and to the cessation of the blood shunting from the right to the left atrium through it. Consequently, the two ventricles now function in series, with only the left ventricle generating aortic blood flow and with uniformly high oxygen saturation present throughout the arterial vascular system. PA, pulmonary artery; PV, pulmonary vein; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle. Artwork by Chrisoula Toupadakis Skouritakis, Ph.D., MediaLab Services Director, Department of Surgical and Radiological Sciences, School of Veterinary Medicine, University of California, Davis. Copyright: The RECOVER Initiative 2025. All rights reserved.
“Aeration of the lungs” is a critical process at birth [12]. Lung aeration reduces pulmonary vascular resistance and is the foundation for circulatory transition (Figure 1) [13]. A significant percentage of the fluid in airways and lungs is expelled during labor and delivery due to uterine contractions, posture changes, abdominal contraction of the dam, and the passage of the fetus through the birth canal [2]. In addition, the pulmonary alveolar epithelium changes from a fluid‐secreting (prenatal) to a fluid‐absorbing phenotype (postnatal), a transition orchestrated by a multitude of coinciding factors, including catecholamine release during birth, other endocrine factors, and an increase in alveolar PO2 with the first few breaths; these factors drive active sodium absorption through the alveolar membrane and thus clearance of alveolar fluid [14]. The very high transpulmonary pressure of 50 cm H2O or more during the first inspirations also plays a key role and can lead to fluid clearance of the lungs on its own with little contribution from the aforementioned mechanisms [15, 16]. High transpulmonary pressure also leads to aeration of the peripheral lungs during inspiration and helps to establish functional residual capacity; the adduction of the glottis during exhalation, as well as vocalization (an expiratory phenomenon), furthers the retention of end‐expiratory alveolar gas and supports the formation of functional residual capacity [16]. PPV and positive end‐expiratory pressure (PEEP) can support lung aeration if the newborn animal is not breathing within the first minute of birth on its own, or after tactile stimulation.
A “nonvigorous newborn puppy or kitten” is one that is not or is only weakly crying, has sluggish or no reflex irritability, and has low muscle tone or is flaccid. These newborns require resuscitative measures.
2.2. Evidence Evaluation
This guidelines project followed the 2024 RECOVER guidelines methodology published in detail elsewhere [17]. In short, these RECOVER Newborn Resuscitation Guidelines were generated using a modified version of the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system for guidelines generation in health care [18]. The process started with the generation of a list of prioritized clinical questions pertaining to newborn resuscitation in puppies and kittens, which was accomplished by the collaboration of newborn Domain Chairs and RECOVER Co‐Chairs. Twenty‐eight out of 40 initially proposed questions were included in the evidence evaluation.
These questions, written in the PICO (Population–Intervention–Comparator–Outcome) format, served as the foundation for information specialists to develop literature search strategiesa. These search strategies were applied to PubMed, CAB Direct, and Scopus, and the resulting articles were uploaded to a systematic review toolb for identification of relevant articles. Two Evidence Evaluators (EEs) were allocated to each PICO question and conducted an initial culling process based on title and abstract review. The full‐text version of articles was then uploaded to a purpose‐built RECOVER GRADE site, and EEs decided based on full‐text review whether to include an article for data extraction or to exclude it. The decision to include an article for full analysis was based on its relevance to components of the respective PICO question, including the study population, the intervention under evaluation, its comparator intervention, and the outcomes reported. Articles that were non‐peer reviewed, non‐English, reviews, meta‐analyses, case reports or case series, and observational or experimental studies without control groups were excluded from further review.
The EEs then extracted from each publication and for each outcome therein relevant quality metrics, such as study design and risk of bias, indirectness regarding population, intervention, comparator and outcome, imprecision, inconsistency, and other characteristics, following a questionnaire embedded in the RECOVER GRADE site. From these assessments, an Evidence Summary Table was generated for each outcome of every PICO question, and RECOVER Domain Chairs and Co‐Chairs used this information to further synthesize the evidence, to grade the overall quality of evidence for or against an intervention, and, ultimately, to draft treatment recommendations.
The quality of evidence was categorized as high, moderate, low, or very low, while evidence relevant to the most critical outcomes (e.g., survival to discharge) was prioritized over evidence found for lower priority outcomes (e.g., surrogate markers of perfusion). The quality of evidence was denoted as expert opinion if there was no evidence identified to develop a recommendation.
2.3. Drafting Treatment Recommendations
We then used the quality of evidence to determine how certain we can be that the desirable effects of an intervention outweigh the undesirable effects. Consequently, and in accordance with GRADE, we either recommended for an intervention if the certainty for benefit > risk was supported by high or moderate quality of evidence or suggested for an intervention if we were less certain (e.g., low or very low quality of evidence). To indicate that undesirable effects outweigh desirable effects, we recommended against or suggested against an intervention. In other words, a recommendation for or against an intervention indicates there is clear benefit or harm expected with that intervention, while a suggestion for or against an intervention indicates that there is probably overall benefit or harm, or that the effect might be small. Due to the importance of generating treatment recommendations capable of informing decision‐making in the clinical context, the committee further considered the feasibility (e.g., Can it be implemented?) and the ease or challenge (e.g., Is it worth the effort?) for implementation in a veterinary setting. Thus, the final strength of the recommendation for an intervention may be higher or lower than what the quality of evidence alone would indicate; detailed justification for each recommendation is provided in a companion article [19].
2.4. Consensus Process
A team of subject matter experts in the field of small animal theriogenology and reproduction (A.D., C.L., G.B., M.V., S.G.), RECOVER Domain Chairs (C.B., K.F.), and RECOVER Co‐Chairs (M.B., J.B., D.F.) then reviewed and commented on all draft treatment recommendations in a modified Delphi process for consensus finding [20]. After each round of commenting, treatment recommendations were refined as needed and were then voted on again, until the group reached consensus, defined a priori as at least 80% agreement with a recommendation. The Newborn Resuscitation Algorithm was included in the consensus process. The draft versions of newborn definitions, treatment recommendations, and the algorithm were then made accessiblec to the EEs, subject matter experts, and the veterinary community at large for a 2‐week commenting period. Comments were considered by the Co‐Chairs and Domain Chairs, and relevant treatment recommendations were refined to create a finalized set of consensus guidelines for resuscitation of newborn puppies and kittens, which appear in this publication. The most important recommendations are listed in Box 1. The structured summary for each PICO question can be found in an accompanying manuscript [19], and the complete Evidence Profile Worksheets and supportive files can be accessed in a public repositorya.
BOX 1 Most important treatment recommendations for resuscitation of newborn dogs and cats
-
Immediately after birth, these measures are provided to any newborn requiring resuscitation:
Drying and stimulating
Clearing the airway
Maintenance of normothermia
Apex beat palpation, auscultation, ECG, and Doppler ultrasound are suitable for heart rate (HR) determination, while pulse oximetry alone is not.
Positive pressure ventilation (PPV) is initiated in nonvigorous newborns with severe bradycardia (HR < 120/min).
PPV is initiated as early as possible in nonvigorous, apneic, or gasping newborns.
Appropriate reversal drugs are administered in newborns requiring resuscitation after a C‐section.
We did not reach consensus on the use of GV 26 acupoint stimulation in nonvigorous newborns.
Doxapram is not routinely administered in all newborns requiring resuscitation but is used in those that fail to respond to other supportive measures.
Chest compressions are started if there is no heartbeat at any time following birth.
Chest compressions are also started in newborns with very severe bradycardia (HR < 50/min) that does not improve after 30–60 seconds of PPV.
During CPR, four chest compressions are delivered at a rate of 150/min and alternate with one breath (compression:ventilation ratio = 4:1)
-
Drugs during CPR:
Administer epinephrine at 0.01–0.03 mg/kg IV or IO
No atropine should be administered
100% oxygen should be provided
3. Treatment Recommendations for Resuscitation of Newborn Puppies and Kittens
Table 1 lists all the treatment recommendations for resuscitation of newborn puppies and kittens. The consensus on science, justification for the treatment recommendations, knowledge gaps for each PICO, and full references associated with these treatment recommendations are contained within the accompanying paper on evidence and knowledge gap analysis for newborn resuscitation [19]. Additional information about the clinical application of select recommendations appears in the sections that follow. The sequence of newborn resuscitation measures and the decision points to direct interventions are presented in an algorithm (Figure 2). The RECOVER Newborn Resuscitation Algorithm was designed as a tool for prebriefing ahead of a birth to refresh the key decision points and interventions and to organize the resuscitation team. In contrast to CPA in adult dogs and cats, parturition is a more predictable event, which allows for preparation. Familiarization with an algorithm “just‐in‐time” and “just‐in‐place” before a delivery is a precondition for best use as a cognitive aid for real‐time guidance during the resuscitation process [21]. The explanations that follow are intended to guide the reader through the algorithm; the complete set of treatment recommendations appears in Table 1.
TABLE 1.
Treatment recommendations for resuscitation of newborn puppies and kittens. This table contains all RECOVER Newborn Resuscitation treatment recommendations, listed in the order of appearance in the algorithm.
| Treatment recommendation | Strength of recommendation | Quality of evidence | PICO |
|---|---|---|---|
| Airway clearance | |||
| For newborn puppies and kittens that require resuscitation at birth but are vigorous, we suggest using a clean, dry cloth to carefully remove fluid from around the nostrils and mouth. | Weak | Expert opinion | NB‐09 |
| For newborn puppies and kittens that are nonvigorous with excessive oropharyngeal fluid (clear or meconium‐stained), we suggest gentle nasal or oropharyngeal suctioning immediately followed by PPV. | Weak | Expert opinion | NB‐09 |
| For newborn puppies and kittens that are nonvigorous with excessive oropharyngeal fluid (clear or meconium‐stained), we suggest against the routine use of endotracheal suctioning. | Weak | Very low | NB‐09 |
| We suggest against interventions other than suctioning to clear excessive upper airway fluid in newborn puppies and kittens. | Weak | Expert opinion | NB‐10 |
| We recommend against the removal of upper airway fluid in newborn puppies and kittens by swinging. | Strong | Expert opinion | NB‐10 |
| Tactile stimulation | |||
| In newborn puppies and kittens that require resuscitation, we recommend the use of tactile stimulation (e.g., rubbing, drying) immediately after birth without delaying essential interventions such as PPV. | Strong | Very low | NB‐15 |
| Temperature control at birth | |||
| In newborn puppies and kittens that require resuscitation, we recommend maintenance of normothermia (95°F–99°F [35°C–37.2°C]) compared to no temperature control. | Strong | Moderate | NB‐11 |
| In newborn puppies and kittens that are hypothermic at birth without signs of HIE, we suggest actively rewarming newborns over 1–2 h to reach normothermia (35.0°C–37.2°C; 95°F–99°F), avoiding accidental hyperthermia. | Weak | Expert opinion | NB‐28 |
| Reversal drugs after C‐sections | |||
| In newborn puppies and kittens that require resuscitation after a C‐section for which the dam was administered an opioid, α2‐adrenoceptor agonist, or benzodiazepine, we recommend administration of the appropriate reversal drug. | Strong | Very low | NB‐20 |
| In newborn puppies and kittens that are vigorous at birth and in which the dam was administered an opioid, α2‐adrenoceptor agonist, or benzodiazepine, there is insufficient evidence to suggest for or against routine administration of reversal drugs. | Weak | Very low | NB‐20 |
| GV 26 acupoint stimulation | |||
| We did not reach consensus on whether to suggest for or against GV 26 stimulation in newborn puppies and kittens with inadequate spontaneous ventilation at birth. | NA | Expert opinion | NB‐08 |
| Doxapram | |||
| We recommend against the routine administration of doxapram in newborn puppies and kittens undergoing resuscitation. | Strong | Moderate | NB‐07 |
| In apneic or gasping newborn puppies and kittens that are not responding to PPV, we suggest doxapram administration. | Weak | Very low | NB‐07 |
| In newborn puppies and kittens that are bradypneic and that fail to respond to timely administration of other supportive measures, we suggest the use of doxapram. | Weak | Very low | NB‐07 |
| Atropine for the treatment of bradycardia | |||
| In newborn puppies and kittens that require resuscitation and that are bradycardic, we suggest against routine atropine administration. | Weak | Expert opinion | NB‐23 |
| Determination of HR | |||
| In newborn puppies and kittens requiring resuscitation, we suggest estimating the HR using any of the following techniques: apex beat palpation, cardiac auscultation, ECG, or Doppler ultrasound probe applied to the thorax. | Weak | Very low | NB‐04 |
| In newborn puppies and kittens requiring resuscitation, we recommend against the use of pulse oximetry as the only method to evaluate HR. | Strong | Very low | NB‐04 |
| Positive pressure ventilation in newborns not in CPA | |||
| We recommend initiating PPV in nonvigorous newborn puppies and kittens with cleared upper airways that are bradycardic. | Strong | Expert opinion | NB‐01 |
| We suggest using an HR below 120/min as the threshold for initiating PPV in nonvigorous newborn puppies and kittens with cleared upper airways. | Weak | Expert opinion | NB‐01 |
| We recommend starting PPV as early as possible in nonvigorous puppies and kittens that are gasping or are apneic, regardless of the HR. | Strong | Expert opinion | NB‐01 |
| We suggest the use of room air (21% oxygen) over 100% oxygen during early assisted ventilation of newborn puppies and kittens. | Weak | Very low | NB‐03 |
| We suggest the use of 100% oxygen in newborn puppies and kittens in which the HR fails to increase despite 1–2 min of PPV. | Weak | Expert opinion | NB‐03 |
| In newborn puppies and kittens without CPA that require PPV, we suggest the use of a tight‐fitting facemask attached to a self‐inflating resuscitator (i.e., “bag”) to deliver positive pressure breaths within 60 s of birth. | Weak | Very low | NB‐05 |
| In newborn puppies and kittens without CPA undergoing PPV by facemask and that fail to respond within 60 s (e.g., HR remains <120/min despite intervention), we suggest endotracheal intubation for continued PPV if feasible. | Weak | Expert opinion | NB‐05 |
| In newborn puppies and kittens that require resuscitation with PPV, we suggest administering PPV with an inspiratory time of 1 s and a peak inspiratory pressure of 20–25 cm H2O. | Weak | Very low | NB‐06 |
| In newborn puppies and kittens that require resuscitation with PPV, we suggest application of at least 4 and no more than 8 cm H2O positive end‐expiratory pressure. | Weak | Very low | NB‐06 |
| In newborn puppies and kittens with bradycardia, cyanosis, or inadequate breathing efforts that persist despite 30–60 s of standard PPV using 1 s inspiratory time at a peak inspiratory pressure of 20–25 cm H2O, we suggest giving a single 30‐s sustained inflation at 30–35 cm H2O, followed by continued standard PPV if indicated. | Weak | Low | NB‐06 |
| Initiation of chest compressions with very severe bradycardia | |||
| In very severely bradycardic newborn puppies and kittens that have received effective PPV and oxygen supplementation for at least 30 s, we recommend initiating chest compressions. | Strong | Expert opinion | NB‐02 |
| We suggest using an HR below 50/min as the threshold for initiating chest compressions in newborn puppies and kittens that have received effective PPV and oxygen supplementation for at least 30 s. | Weak | Expert opinion | NB‐02 |
| Chest compression point and animal position | |||
| In newborn puppies and kittens requiring chest compressions, we suggest application of chest compressions in the laterolateral direction. | Weak | Expert opinion | NB‐16 |
| In newborn puppies and kittens requiring chest compressions, we suggest ventrodorsal (sternal) chest compressions when additional resuscitation measures (e.g., umbilical cord cannulation, monitoring modalities) are facilitated by dorsal recumbency. | Weak | Expert opinion | NB‐16 |
| In newborn puppies and kittens requiring chest compressions in lateral recumbency, we recommend that one or two fingers be located over the heart to compress the chest toward the tabletop, or alternatively, that one or two fingers (index and middle finger) and the opposing thumb be used to compress the chest directly over the heart. | Strong | Expert | NB‐16 |
| In newborn puppies and kittens requiring chest compressions in which dorsal recumbency is preferred (e.g., for cannulation of the umbilical vein or pronounced wide‐chested thorax conformation), we recommend that one or two fingers (index and middle finger) compress the sternum toward the tabletop to achieve the targeted chest compression depth. | Strong | Expert opinion | NB‐16 |
| Chest compression rate and technique | |||
| We suggest delivering chest compressions in newborn puppies or kittens at 120–150 compressions per minute. | Weak | Very low | NB‐12 |
| In newborn puppies and kittens receiving chest compressions, we suggest a compression depth of one‐third to one‐half of the width of the chest for laterolateral compressions. | Weak | Very low | NB‐17 |
| In newborn puppies and kittens receiving chest compressions, we suggest a compression depth of one‐third the anterior–posterior (AP) diameter of the chest for ventrodorsal (i.e., sternal) compressions. | Weak | Very low | NB‐17 |
| Coordination of chest compressions and ventilation | |||
| In newborn puppies and kittens receiving chest compressions and PPV with a cuffed endotracheal tube in place, we suggest delivering breaths concurrently to chest compressions (asynchronized ventilation). | Weak | Very low | NB‐14 |
| In newborn puppies and kittens receiving chest compressions and PPV that are not intubated with a cuffed endotracheal tube, we recommend synchronized ventilation by pausing chest compressions to deliver breaths. | Strong | Expert opinion | NB‐14 |
| In newborn puppies and kittens receiving chest compressions and synchronized PPV, we suggest a compression:ventilation ratio of 4:1. | Weak | Expert opinion | NB‐13 |
| Oxygen supplementation during CPR | |||
| In newborn puppies and kittens receiving chest compressions, we suggest PPV with 100% oxygen. | Weak | Very low | NB‐18 |
| Epinephrine during CPR | |||
| In newborn puppies and kittens with a very low HR (i.e., HR < 50/min) despite 60 s of adequate PPV and chest compressions, we recommend administration of epinephrine. | Strong | Moderate | NB‐21 |
| In newborn puppies and kittens with very low HR (i.e., HR < 50/min) despite 60 s of adequate PPV and chest compressions, we recommend IV or intraosseous (IO) administration of epinephrine over intratracheal or intranasal administration. | Strong | Moderate | NB‐21 |
| In newborn puppies and kittens with very low HR (i.e., HR < 50/min ) despite adequate PPV and chest compressions, we suggest against IM administration of epinephrine (including intralingual IM administration). | Weak | Very low | NB‐21 |
| Epinephrine dose during CPR | |||
| In newborn puppies and kittens that remain very severely bradycardic (i.e., HR < 50/min) despite 60 s of PPV and chest compressions, we recommend administration of 0.01–0.03 mg/kg epinephrine IV/IO. | Strong | Very low | NB‐22 |
| In newborn puppies and kittens that remain very severely bradycardic (i.e., HR < 50/min) despite 60 s of PPV and chest compressions, we recommend against high‐dose (0.1 mg/kg) IV/IO epinephrine. | Strong | Very low | NB‐22 |
| In newborn puppies and kittens that remain very severely bradycardic (i.e., <50/min) despite 60 s of PPV and chest compressions, in which epinephrine is given endotracheally because IV/IO access is not possible or significantly delayed, we suggest 0.05–0.1 mg/kg epinephrine ET. | Weak | Very low | NB‐22 |
| Atropine during CPR | |||
| In newborn puppies and kittens that require resuscitation and are in CPA, we suggest against the routine administration of atropine during CPR. | Weak | Expert opinion | NB‐24 |
| Dextrose during resuscitation with or without CPA | |||
| In newborn puppies and kittens that require prolonged resuscitation, we suggest measuring the blood glucose concentration. | Weak | Expert opinion | NB‐19 |
| In hypoglycemic newborn puppies and kittens that require resuscitation (with or without CPA), we recommend dextrose supplementation with a slow bolus of 0.25 g/kg dextrose (e.g., 0.5 mL/100 g of 5% dextrose solution over 5 min) IV, IO, or IP. | Strong | Very low | NB‐19 |
| In hypoglycemic newborn puppies and kittens in which IV/IO or IP access is not possible, we suggest topical sublingual mucosal administration of dextrose at 0.5 g/kg (e.g., 0.1 mL/100 g of 50% dextrose solution). | Weak | Expert opinion | NB‐19 |
| In newborn puppies and kittens undergoing prolonged resuscitation (e.g., >10 min) that are not responding to standard measures (e.g., PPV, rewarming, stimulation) and in which BG concentrations cannot be measured, we suggest dextrose supplementation. | Weak | Expert opinion | NB‐19 |
| Termination of CPR | |||
| In newborn puppies and kittens receiving chest compressions, we suggest continuation of CPR for at least 15 min before abandoning resuscitation efforts as long as such efforts do not detract from the necessary care of littermates with a better prognosis. | Weak | Very low | NB‐25 |
| Temperature management with HIE | |||
| In newborn puppies and kittens with signs of acute HIE, we suggest permitting the animal to remain at a low normal temperature for newborn puppies and kittens (i.e., 35°C, 95°F) for 24 h after birth. | Weak | Expert opinion | NB‐27 |
| In newborn puppies and kittens that are hypothermic at birth and show evidence of HIE, we suggest rewarming at a rate no faster than 1°C/h to achieve normothermia (35.0°C–37.2°C; 95°F–99°F) while avoiding accidental hyperthermia. | Weak | Very low | NB‐28 |
| Blood glucose management after ROSC | |||
| In newborn puppies and kittens after ROSC that are nonvigorous and not nursing, we suggest measuring the blood glucose concentration. | Weak | Expert opinion | NB‐26 |
| In hypoglycemic newborn puppies and kittens after ROSC, we suggest supplementing dextrose by CRI (e.g., 2.5% dextrose in isotonic crystalloid fluids given at a physiologic rate IV or IO) rather than by bolus injection. | Weak | Very low | NB‐26 |
| In hypoglycemic newborn puppies and kittens after ROSC, we recommend dextrose supplementation with a slow bolus of 0.25 g/kg dextrose (e.g., 0.5 mL/100 g of 5% dextrose over 5 min) IV, IO, or IP, if an IV or IO CRI is not feasible. | Strong | Very low | NB‐26 |
| In hypoglycemic newborn puppies and kittens after ROSC in which IV, IO, or IP administrations are not possible, we suggest oral administration of dextrose at 0.5 g/kg (e.g., 0.1 mL/100 g of 50% dextrose). | Weak | Expert opinion | NB‐26 |
| In newborn puppies and kittens that are nonvigorous and not nursing after resuscitation and in which blood glucose concentrations cannot be measured, we suggest dextrose supplementation. | Weak | Expert opinion | NB‐26 |
Abbreviations: ALS, advanced life support; CPA, cardiopulmonary arrest; CRI, constant rate infusion; ET, endotracheal; HIE, hypoxic–ischemic encephalopathy; HR, heart rate; IO, intraosseous; IP, intraperitoneal; PPV, positive pressure ventilation; ROSC, return of spontaneous circulation; RR, respiratory rate; TV, tidal volume.
FIGURE 2.
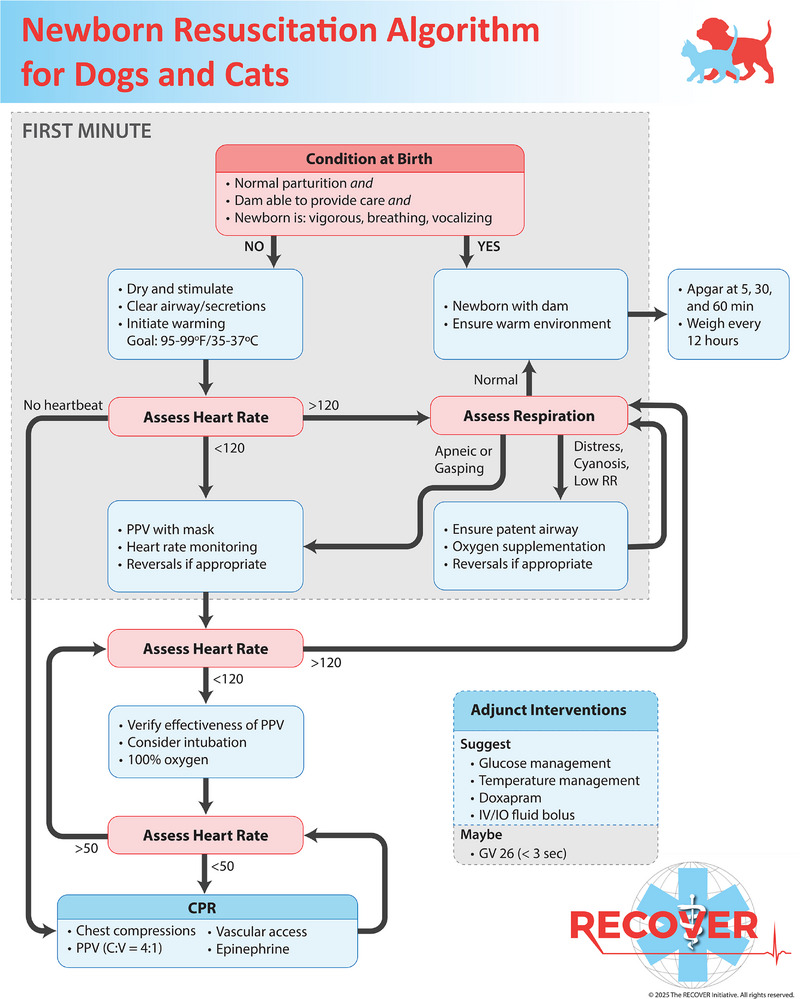
Newborn resuscitation algorithm for puppies and kittens. C:V, compression:ventilation ratio during bag–mask ventilation and chest compressions during CPR; IO, intraosseous; GV 26, needle stimulation of the Governing Vessel 26 acupoint; PPV, positive pressure ventilation; RR, respiratory rate. Artwork by Chrisoula Toupadakis Skouritakis, Ph.D., MediaLab Services Director, Department of Surgical and Radiological Sciences, School of Veterinary Medicine, University of California, Davis. Copyright: The RECOVER Initiative 2025. All rights reserved.
4. Newborns Requiring Resuscitation
Most newborn dogs and cats born by eutocia are not expected to require any human intervention at birth. Puppies and kittens born by normal parturition (e.g., vaginal eutocia) whose mother can provide immediate postpartum care and that are vigorous, breathing, and vocalizing immediately after birth do not require resuscitation. A vigorous newborn puppy or kitten is one that is breathing with a respiratory rate (RR) of at least 15/min, is vocalizing clearly, and displays adequate reflex irritability with strong spontaneous movements [22]. Newborns that do not require resuscitation should be left with, or immediately placed with, the dam and supervised to confirm she is aware, coordinated, and displaying adequate maternal behavior [23]. Routine postpartum management (i.e., umbilical care, weighing, physical examination for congenital defects) should be performed within 1 h of delivery (see below).
Apgar scoring as a method for newborn viability assessment was originally described for newborn infants and then modified for use in newborn puppies and kittens [24, 25, 26, 27, 28]. Scores of 0–2 are assigned to each parameter (heart rate [HR], respiratory effort, reflex irritability, motility, and mucous membrane color), with a lower score indicating more severe depression [26]. In newborn puppies, low modified Apgar scores obtained within 5 min were highly associated with early mortality and were significantly lower in puppies born by Cesarean section (C‐section) than in those born by eutocia [24, 25, 26]. However, as an Apgar score cutoff indicating the need for resuscitative measures at birth has not been established, we suggest against using a specific Apgar score to trigger resuscitation measures at birth. That said, once a newborn is identified as not requiring resuscitation or is recovering from initial resuscitation, it should be monitored using serial Apgar scoring (e.g., at 5, 30, and 60 min after birth) to identify those at risk of deterioration. Vigorous newborns should be weighed and examined for congenital conditions [29, 30], and their umbilical cord should be ligated, trimmed to 1 cm, and disinfected with a 2% tincture of iodine. The puppy or kitten can then be united with the dam in a warm environment (29°C–32°C [84°F–89°F]) that supports newborn normothermia (i.e., 35°C–37°C [95°F–99°F]). We suggest determining the body weight every 12 h, expecting a daily weight gain of 10%.
Nonvigorous newborn puppies and kittens require resuscitative measures. All canine and feline newborns delivered by C‐section, and especially those born by emergency C‐section, require support at birth as (1) these animals are generally of lower vitality than those born by vaginal eutocia [24, 25, 31, 32, 33] and (2) the dam is unable to provide care for the newborns during the initial period after birth. Thus, veterinary professionals will probably use these RECOVER Newborn Resuscitation Guidelines most commonly in the context of C‐section deliveries.
5. The First Minute
The first resuscitation measures immediately after birth include drying and stimulating the newborn, clearing its airway, and initiating measures to maintain normothermia. In practice, these measures are initiated concurrently and do not follow a strict order. Once these measures have succeeded, rescuers should determine the HR and assess the respiratory function within the first minute after delivery. The newborn puppy or kitten is expected to start vocalizing and moving within moments after birth. If not, information on HR and respiratory function is used to guide the initiation of appropriate resuscitative measures in support of the respiratory transition in that first minute.
5.1. Temperature Control at Birth
We recommend that all resuscitative measures be undertaken in a temperature‐controlled environment. The newborn's lack of thermoregulatory competency exposes it to the risk of hypothermia unless it is maintained in an environment that mitigates temperature loss. While we found only limited evidence in newborn puppies and kittens, multiple studies in human infants establish a strong relationship between hypothermia in newborns and morbidity (e.g., dysglycemia, sepsis) and mortality [34, 35, 36, 37, 38]. Newborn puppies born by C‐section were found to have a mean body temperature of 34.0°C (93.2°F) at birth, which further decreased to 32°C (89.6°F) after 20 min; hypothermia was mitigated but still significant in puppies born by vaginal eutocia (i.e., 36.2°C [97.2°C] at birth and 34.0°C [93.2°F] after 20 min) [32]. We therefore recommend undertaking measures to maintain normothermia (NB‐11). We consider a newborn rectal temperature between 35°C and 37°C (95°F and 99°F) to be normal. A suitable temperature control system should not interfere with other resuscitation efforts. Immediate drying of the newborn puppy or kitten with warm towels serves as an early measure to limit heat loss and also provides an element of tactile stimulation. Further, we suggest positioning the newborn on a dry, warm surface (e.g., warm towels) to shield it from conductive heat loss. Radiant heat loss can be reduced by placing the newborn beneath an overhead heat source (e.g., radiating heat warmer) while the rescuer administers necessary interventions. Care must be taken to avoid hyperthermia or skin burns, particularly because even vigorous newborns cannot reliably move away from too‐hot surfaces quickly enough to avoid hyperthermia or burns. High‐quality incubators generate a warm environment through circulating air and humidity; they can be suitable for post‐resuscitation maintenance of normothermia when set at 29°C–32°C (84°F–89°F). Adequate monitoring to avoid accidental hyperthermia is required to avoid harm [39].
When they have become hypothermic, we suggest actively rewarming newborn puppies and kittens relatively rapidly over 1–2 h (NB‐28) as long as they do not display signs of hypoxic–ischemic injury (e.g., mental obtundation). In this healthier population, rewarming rates of up to 5°C/h (8°F/h) were not associated with harm in infants [40, 41, 42, 43, 44], though a higher risk for temperature overshoot was noted in one study, and close monitoring is warranted to avoid hyperthermia [43].
5.2. Tactile Stimulation
We recommend the use of tactile stimulation, such as rubbing with a dry, clean cloth, in nonvigorous newborns immediately after birth, as long as this does not delay or interfere with time‐sensitive resuscitation measures such as PPV (NB‐15). Tactile stimulation is highly feasible and low‐risk when performed in a gentle, nontraumatic manner. Although tactile stimulation of newborns is a very common practice to stimulate breathing in veterinary species and infants, evidence to support the practice is very limited but generally neutral or in favor of the intervention [45, 46, 47]. One observational study in newborn infants found that tactile stimulation was associated with a relative risk reduction for the need of endotracheal (ET) intubation of nearly 60% [48].
5.3. Establishing a Patent Airway
The need to immediately remove fetal membranes to free the newborn's airways at birth is obvious, and accordingly, we did not evaluate any evidence to support this practice. Which measures to take, if any, to remove fluid from the newborn's airway, however, is less obvious. Fluid in the upper airways is typically cleared naturally and rapidly by the vigorous, vocalizing newborn without any external support required [12]. If the newborn is vigorous but needs support, for example, after a C‐section, we suggest using a clean, dry cloth to gently remove excess fluid from around the nostrils and muzzle (NB‐09). Suctioning exposes the airway to negative pressure and might counteract the goal of lung aeration, so it should not be done routinely. However, if the newborn puppy or kitten is nonvigorous, is not vocalizing loudly, and has excessive oropharyngeal fluid, whether clear or meconium stained, we suggest removal of that fluid by gentle, expedient nasal or oropharyngeal suctioning immediately followed by PPV (NB‐09). For removal of fluid from nostrils and rostral oral cavity, a suction bulb can be used, while a mucus aspirator (e.g., DeLee suction catheter with mucous trap) allows for deeper oropharyngeal suctioning. Importantly, undue aspiration should be avoided as this could impede vitally important lung aeration.
Multiple studies in newborn infants and experimental animals have evaluated the effect of ET suctioning in the presence of excessive amniotic fluid and have not identified any benefit regardless of whether said fluid is meconium stained or not [49, 50, 51, 52, 53]. Compared to newborn infants, ET suction in newborn puppies and kittens is technically difficult to accomplish and exposes the animal to harm, including the risk of laryngeal swelling, lung derecruitment (reduced lung aeration) with severe desaturation, and compromise of life‐saving measures such as PPV [53]. We therefore suggest against ET suctioning even if the oropharyngeal fluid is excessive and meconium stained (NB‐09). Any fluid removal methodology should be brief and interfere as little as possible with the timely initiation of PPV, which is a priority intervention in nonvigorous newborn puppies and kittens that are apneic or gasping, breathing inadequately, or severely bradycardic.
We suggest against interventions other than suctioning to clear excessive upper airway fluid in newborns (NB‐10). “Swinging” as a means to accelerate drainage of fluid from the newborn's airway by harnessing centrifugal force was historically advocated in human medicine over a century ago and has long been abandoned [54]. We recommend against this practice in newborn puppies and kittens due to the significant risk of intracranial hemorrhage, potential trauma, and aspiration of gastric contents (NB‐10) [55]. Additionally, swinging delays the initiation of more effective resuscitative measures such as PPV.
5.4. Heart Rate Assessment
The newborn's HR is a vital parameter used to guide resuscitation because it reflects the severity of hypoxemia; worsening bradycardia generally indicates the need for escalation of supportive measures, while an increase in HR suggests the possibility for de‐escalation of care.
Bradycardia in the newborn is a consequence of hypoxemia and is mediated through vagal and nonvagal mechanisms [56]. As the bradycardia itself is a surrogate measure for the severity of hypoxemia and other factors concerning pulmonary aeration, it reflects the progress of the newborn's cardiorespiratory transition.
In newborn puppies and kittens requiring resuscitation, the goal is to obtain the first HR measurement within the first 60 s after birth, although this is not always feasible [57]. In late pregnancy, one small study found that normal fetal HR by echocardiogram was 218 ± 7/min in puppies (n = 8) and 228 ± 35/min for kittens (n = 7) [58]. In puppies experiencing normal birth (vaginal eutocia), the mean HR immediately after delivery was approximately 180/min, whereas it was approximately 165/min after C‐section [32]. Puppies achieved a normal HR of 200–220/min after 5 min with eutocia and after 4 h following C‐section [32].
We suggest estimating the HR using apex beat palpation, cardiac auscultation, ECG, or Doppler ultrasound probe and suggest against the use of pulse oximetry as the only method (NB‐04). Although this has not been studied in newborn puppies and kittens, pulse oximetry was consistently inaccurate and slow at determining HR in newborn infants [59, 60, 61, 62]. While dry‐electrode ECG provided fast and accurate HR measurement in newborn infants, it is reasonable on practical grounds to obtain an initial HR by auscultation and only to escalate to continuous ECG monitoring when advanced resuscitation measures (e.g., prolonged PPV, CPR) are required.
We decided by consensus and based on expert opinion on specific, actionable HR cutoffs as decision markers to initiate certain resuscitative interventions. We suggest using an HR below 120/min as the threshold for initiating PPV when a newborn is also classified as nonvigorous, and the upper airway is clear (NB‐07). In addition, an HR <120/min warrants administration of appropriate reversal drugs (i.e., naloxone, atipamezole, or flumazenil) if exposure to the respective agonist is documented (NB‐20), such as during C‐section.
If there is no heartbeat identified in a nonvigorous newborn puppy or kitten that is apneic or gasping, the newborn is in CPA, and CPR should be started without delay.
If the HR is found to be >120/min, we next recommend clinical assessment of the newborn's respiratory function.
5.5. Respiratory Assessment
Normal RRs in newborns are generally >15/min, although RRs that are much higher have been reported with normal birth, such as > 40/min in puppies and >70/min in kittens [22, 28, 32]. If the RR is <15/min (including apnea or gasping), or if there is no clear vocalization, an overt increase in respiratory effort (i.e., labored breathing), or worsening cyanosis, then a specific set of resuscitative measures should be performed to support oxygenation.
5.5.1. Bradypnea, Respiratory Distress, or Cyanosis in the Newborn With an HR >120/min
The first step in all newborns is to ensure the airway is patent; if excessive upper airway fluid is present, repeated, short, and gentle suctioning with a bulb syringe or DeLee suction catheter should be considered, remembering the caveats mentioned previously to avoid removing air from the lung.
Additionally, we believe administration of supplemental oxygen can be considered in the presence of clear signs of respiratory distress or bradypnea (e.g., RR < 15/min) in conjunction with cyanosis in spontaneously breathing newborns with an HR >120/min. As oxygen saturations are very low even in normal newborn infants (e.g., SaO2 of 60%–65% is normal in the first minute after birth in newborn infants), low SpO2 values and thus cyanosis alone in the first minute after birth are not by themselves a concern and can represent “healthy” values [63]. Free‐flow oxygen can be administered, for example, by flow‐by method, which will typically lead to an inspiratory oxygen concentration of <50% or via a face mask that can lead to an inspired oxygen concentration of >80% depending on fresh gas flow and distance between mask and muzzle [64, 65]. However, we could not find any evidence for or against short‐term oxygen supplementation in spontaneously breathing newborns and only systematically reviewed scientific knowledge pertaining to oxygen supplementation in newborns undergoing PPV (see below, NB‐03). In that population, there is ample evidence that ventilatory support with pure oxygen is either of no benefit or harmful when compared to room air [66, 67, 68, 69]. In puppies and kittens receiving PPV, we therefore suggest the use of room air and to only increase to 100% oxygen if there is no response (e.g., no increase in HR) after 1–2 min of PPV alone (see NB‐03).
Finally, we suggest administration of the appropriate reversal drug (i.e., naloxone, atipamezole, flumazenil) in bradypneic or cyanotic newborn puppies and kittens after a C‐section if the bitch was premedicated with an opioid, α2‐adrenoceptor agonist, or benzodiazepine (Figure 3). While benzodiazepines are uncommonly used for premedication of bitches for C‐sections, benzodiazepine use in the mother during the perinatal period can cause profound obtundation and respiratory depression in newborn infants, puppies, and kittens and often requires repeated administration or constant rate infusion (CRI) of flumazenil [70, 71, 72]. However, if newborn puppies and kittens are vigorous at birth, we found insufficient evidence to suggest for or against routine administration of reversal drugs, even if the dam was administered an opioid, α2‐adrenoceptor agonist, or benzodiazepine in the perinatal period (NB‐20).
FIGURE 3.

Newborn resuscitation dosing chart. Drug volumes are provided by body weight in 100‐g increments to reduce the risk of calculation errors. Depending on the drug route, different drug doses and thus drug volumes are recommended. ET, endotracheal; IM, intramuscular; IN, intranasal; IO, intraosseous; IP, intraperitoneal; SC, subcutaneous; SL, topical sublingual. Copyright: The RECOVER Initiative 2025. All rights reserved.
5.5.2. Apnea, Gasping, or Severe Bradycardia in the Nonvigorous Newborn
In newborn puppies and kittens that are apneic or gasping, or are nonvigorous with a low HR (i.e., <120/min), we recommend active ventilatory support to aid in lung aeration by starting PPV once airway clearance (e.g., by oronasopharyngeal suctioning if required) has been accomplished (NB‐01). Administration of positive pressure breaths is the most important step to support lung aeration in newborns that are nonvigorous and apneic/gasping, even though the majority of newborn infants receiving PPV only require it for at most a few minutes (median 129 s; interquartile range [IQR] 157) [73]. Nevertheless, every 30‐s delay in starting PPV has been associated with an increase in mortality by 16% in newborn infants requiring PPV [74]. We therefore recommend starting PPV as early as possible and within the first minute of birth in nonvigorous puppies and kittens that are gasping or are apneic, regardless of the HR (NB‐01, NB‐05).
We suggest initiating PPV with a tight‐fitting face mask at a rate of 20–30/min, with an inspiratory time of 1 s and a peak inspiratory pressure (PIP) of 20–25 cm H2O (NB‐06). A target tidal volume is approximately 10–15 mL/kg, so only around 5 mL for a 300‐g puppy and an appropriately sized self‐inflating resuscitator bag should be used to deliver small tidal volumes (Figure 4). However, data from newborn infants suggest that a PIP of 30 cm H2O would produce a tidal volume of only 5 mL/kg initially due to the stiffness of the still fluid‐filled lungs early after birth, which would increase to 10 mL/kg after 30 s of PPV [75]. In the veterinary setting, peak pressure and tidal volume generally cannot be measured in newborns. It is therefore reasonable to use chest excursion and auscultation for breath sounds, as well as changes in HR, to monitor the efficacy of PPV. To support the establishment of functional residual capacity, we further provide a weak recommendation for a PEEP of 4–8 cm H2O, acknowledging that airway manometers and bag–mask systems with integrated PEEP valves are not widely available in the delivery areas of veterinary clinics (NB‐06). While there is evidence supporting the application of PEEP in animal studies and preterm newborn infants needing PPV at birth, a randomized controlled trial in term newborn infants did not show an effect of PEEP on clinically relevant outcomes [76, 77, 78].
FIGURE 4.

Positive pressure ventilation is a critically important intervention in newborn resuscitation and requires early initiation. We suggest the use of a tight‐fitting face mask and a suitable self‐inflating resuscitator bag to deliver effective breaths. Snout, neck, and back should be aligned, and dorsal recumbency should be avoided for easier air passage into the lungs. Signs of effective ventilation are a visible chest rise and an increase in HR. PPV should be initiated with room air, and 100% oxygen should be considered if there is no response after 1–2 min (e.g., no increase in HR observed). Artwork by Chrisoula Toupadakis Skouritakis, Ph.D., MediaLab Services Director, and Kailee Suess, Department of Surgical and Radiological Sciences, School of Veterinary Medicine, University of California, Davis. Copyright: The RECOVER Initiative 2025. All rights reserved.
As in adult dogs and cats undergoing bag–mask ventilation, the snout, neck, and thoracic spine should be aligned to minimize upper airway resistance and reduce the risk for gastric insufflation. Cricoid pressure or, more recently, ultrasound‐guided external compression of the esophagus has been used to prevent gastric gas distention during bag–mask ventilation in people [79]. Although untested, gentle digital pressure could be applied to the left side of the caudal third of the ventrolateral neck to occlude the esophagus and mitigate gastric insufflation during PPV.
In newborns that are nonvigorous, severely bradycardic, apneic, or gasping, we also recommend administering reversal drugs (i.e., naloxone, atipamezole, or flumazenil) if the dam received an opioid, α2‐adrenoceptor agonist, or benzodiazepine prepartum (NB‐20) (Figure 3).
However, we suggest against routine atropine administration in this population (NB‐23). Bradycardia can occur due to parasympathetic (i.e., “vagal”) activation even in the fetus and the newborn [80, 81], and bradycardia due to severe hypoxemia is at least in part vagally mediated, in addition to direct hypoxic myocardial depression [56, 82, 83]. However, newborn bradycardia in response to hypoxemia is considered an adaptive response in newborns to preserve myocardial function; additionally, despite the fact that atropine can increase the HR, it was shown to have little effect on indices of oxygen delivery, which is the primary objective of treatment [84, 85]. Rather than helping, atropine can worsen myocardial ischemia in the face of severe hypoxemia and thereby aggravate myocardial injury and circulatory compromise. The main treatment for severe bradycardia in transitional newborns is reversal of hypoxemia through respiratory support, foremost in the form of PPV.
If the newborn puppy or kitten is found to have no heartbeat, CPR should be initiated immediately. CPR involves initiating chest compressions in addition to PPV. CPR is discussed in detail later in the article.
6. Respiratory Support Beyond the First Minute
Results from research in newborn infants and experimental animals suggest that even when effective, it typically takes ≥30 s for PPV to lead to an increase in HR [86, 87, 88]. We therefore believe it is reasonable to reassess the HR after 30–60 s of PPV and only then modify resuscitative measures as directed. If the HR increases to >120/min, RR and effort should be assessed, and de‐escalation of respiratory support should be considered accordingly. However, if severe bradycardia persists (i.e., HR < 120/min) despite 1 min of PPV by bag–mask, the efficacy of this ventilation strategy should be questioned, and we suggest considering ET intubation when feasible (NB‐05), ventilation with 100% oxygen (NB‐03), and sustained inflation (SI) (NB‐06).
ET intubation should be considered, particularly in cases in which bag–mask ventilation overtly fails to generate adequate chest expansions and thoracic auscultation reveals absent breath sounds. In newborn puppies and kittens, ET intubation is an advanced and technically challenging procedure due to the small patient size. In many cases, the newborn's voluminous, fleshy tongue obscures clear identification of laryngeal landmarks critical to ET intubation, such as epiglottis, arytenoids, and vocal folds. The risks of traumatic, prolonged intubation attempts precluding concurrent PPV should be balanced against the expected benefit of improved lung aeration after successful ET intubation. The resultant risk–benefit ratio varies from case to case based on experience and number of resuscitation personnel, available equipment, and size and number of newborns; since these variables are often known antepartum, teams can discuss an airway management strategy during prebriefing.
While outside of the scope of these guidelines, we hereafter provide a short overview of the technique of ET intubation in newborns. ET intubation can be accomplished with small, uncuffed ET tubes (i.e., internal diameter 2.0 mm) in larger puppies, or venous catheters of appropriate size (e.g., 20, 18, 16, or 14 gauge) commensurate with the size of the animal. Venous catheter hubs will accept an ET tube connector with a 3.5‐mm internal diameter, which can thereby be connected to a resuscitator bag. A guidewire with a straight, blunt (atraumatic) end can be used to avoid exposure of delicate laryngeal tissue to the sharp end of the intravenous catheter. An otoscope speculum modified to form an open, illuminated track can assist ET intubation of very small newborns, while a human preterm laryngoscope blade (i.e., size‐0 Miller laryngoscope blade) suffices for larger newborns. To avoid bronchial insertion of the ET tube, its tip should reach no further than 1–2 mm beyond the thoracic inlet. Once in place, the ET tube is secured, and PPV is initiated targeting the same ventilation parameters as for bag–mask ventilation (i.e., inspiratory time, 1 s; PIP, 20–25 cm H2O; RR, 20–30/min). If available, a pressure safety (“pop‐off”) valve built into the manual resuscitator bag and limited to 25 cm H2O will alleviate the risk for excessive PIPs. As long as the newborn is correctly intubated, gastric insufflation should not occur. Indicators of effective ventilation/lung aeration include the presence of chest movements after each breath, breath sounds on auscultation, and signs of improvement in hypoxemia (e.g., increase in HR, resolution of cyanosis, increase in SpO2). Lack of chest movement and failure of the HR to increase warrant confirmation of correct intubation.
If the newborn puppy's or kitten's HR fails to increase to >120/min despite 1–2 min of PPV, we suggest switching the inspired gas from room air to 100% oxygen (NB‐03). This might or might not precede the decision for ET intubation. The desirable and undesirable effects of supplemental oxygen (i.e., 100% oxygen) compared to the use of room air during PPV have been extensively studied in newborn infants and in experimental studies in piglets and lambs, but not in newborn puppies and kittens. The combined evidence shows that PPV with room air is as effective as with 100% oxygen and that high inspiratory oxygen concentration has several undesirable effects, including increased mortality in newborn infants and oxidative injury across several organ systems documented in multiple species [66, 67, 68, 69, 89, 90, 91, 92]. It is possible that a more tailored increase in inspiratory oxygen concentration (e.g., 40% oxygen) would improve hypoxemia while avoiding the risks of pure oxygen, but this has not been well studied and is clinically difficult to implement. Thus, we recommend initiating PPV with room air but suggest the use of 100% oxygen if the HR fails to increase after 1–2 min of PPV (NB‐03). At this stage, especially if the HR continues to decrease, it is also reasonable to initiate monitoring of the HR several times a minute, such as by continuous ECG monitoring, to guide decision‐making as the resuscitation effort evolves.
An additional measure we suggest undertaking if the newborn puppy's or kitten's HR fails to increase to >120/min despite 1–2 min of PPV is to administer a single SI (i.e., 30‐s duration, 30–35 cm PIP) that is then followed by standard PPV (NB‐06). While there are no clinical or experimental studies documenting the benefit or harm of this technique for the most critical outcomes of favorable neurologic outcome and survival to discharge, data from one experimental study in transitional, asphyxiated newborn lambs showed an improvement in oxygenation and a marked reduction in the time to achieve HR >120/min with a single SI (median 8 s, IQR 6), compared to standard PPV (median 64 s, IQR 75). We believe this to be a feasible intervention if the animal is intubated with a cuffed ET tube or is ventilated with a tight‐fitting face mask. In the latter case, gentle digital pressure could be applied to the left side of the caudal third of the ventrolateral neck to occlude the esophagus and mitigate gastric insufflation during the maneuver.
7. Cardiopulmonary Resuscitation
Eighty‐five percent of term newborn infants start breathing within moments of birth, 10% will do so after drying and tactile stimulation, 5% will receive PPV, and only a small percentage (i.e., 0.1%–0.3%) undergo chest compressions, with even fewer receiving epinephrine [4, 93, 94, 95]. For newborn puppies and kittens, data on the prevalence of initial resuscitation measures are lacking, but we expect that, similar to people, CPR—the combined administration of chest compressions and PPV—will be required in a small subset of them.
7.1. When to Start Chest Compressions
Like in adult dogs and cats, we recommend initiation of CPR immediately once the rescuer recognizes its indication [6].
7.1.1. No Detectable Heartbeat
We suggest starting CPR (i.e., chest compressions in addition to PPV) in newborn puppies and kittens immediately if there is no heartbeat identified. As noted above, and in contrast to the recommendations for adult dogs and cats, the initial 30–60 s of nonresponsiveness and apnea in newborns should be treated with respiratory support maneuvers as long as the animal has a detectable heartbeat.
7.1.2. Persistent, Severe Bradycardia
In contrast to the recommendations for adult dogs and cats, we also suggest starting CPR in newborn puppies and kittens in which a heartbeat can be identified but that are found to be very severely bradycardic (i.e., HR < 50/min) for longer than 30 s despite PPV with oxygen supplementation (NB‐02). The rationale behind this recommendation is that the very poor cardiac output with extremely low HR can be augmented by external chest compressions. This specific HR cutoff, as well as the time interval of 30 s, has not been directly tested and is based on expert opinion, although findings in newborn lambs suggest that chest compressions in newborns with an HR <50/min improve blood flow [96, 97]. Due to the central importance of prioritizing lung aeration during newborn resuscitation and the fact that chest compressions may interfere with lung expansion, we suggest providing PPV first in newborns with very severe bradycardia (i.e., HR <50/min), and only starting CPR if the HR fails to increase. Experimental evidence in a newborn piglet asphyxiation model suggests that an HR increase with PPV alone can be expected after 15–30 s in animals with the capacity to respond [87]. In addition, one study showed that very severe bradycardia at birth quickly resolved in the majority of newborn infants with PPV alone; in 78% and 90% of the infants with an initial HR <60/min at birth, the HR increased to >60/min after 30 and 60 s, respectively [98].
7.2. Chest Compression Technique
While different chest compression techniques have not been studied in newborn puppies and kittens, the soft, malleable chest walls of newborns optimize the application of the cardiac pump mechanism for blood flow generation, where blood is expelled from the heart with pressure applied preferentially to the ventricles. We therefore suggest compressing the newborn chest in a laterolateral direction with the compression point located directly over the ventricles (NB‐16). With the newborn puppy or kitten positioned in lateral recumbency, chest compressions can be applied by placing one or two fingers (index and middle finger) over the ventricles and compressing toward the table surface (Figure 5A). Alternatively, rescuers can oppose one or two fingers (index and middle finger) and thumb on the chest over the ventricles and compress the chest to the targeted compression depth (Figure 5B). During laterolateral compressions, we suggest a compression depth of one‐third to one‐half of the chest width (NB‐17). Due to the very compliant chests, overcompression is easily possible in the newborn and must be avoided to prevent injury. Under specific circumstances (e.g., umbilical vein cannulation, pronounced wide‐chested thoracic conformation), we suggest sternal compression and recommend doing so by compressing the sternum with one or two fingers (index and middle finger) toward the table surface to reach the target depth (NB‐16) (Figure 4C). With sternal compression, a proportion of the external anterior–posterior chest diameter is internally occupied by the vertebral column, reducing the compressible portion of the chest cavity. We therefore suggest a compression depth of only one‐third the anterior–posterior chest diameter during sternal compressions in newborns (NB‐17). Considering the large fleshy tongues of newborn puppies and kittens, dorsal positioning carries the risk of significantly increasing upper airway resistance and thus may compromise PPV in nonintubated animals [99]. We therefore think it is reasonable to avoid routine dorsal positioning during newborn CPR in puppies and kittens.
FIGURE 5.

Chest compression techniques for newborn puppies and kittens. Compressions should be applied directly over the ventricles regardless of technique and animal position. The preferred animal position is lateral recumbency, but sternal compressions might be preferable in some instances (see text). (A) Compress the chest over the ventricles with one or two fingers toward the table surface (rate, 120–150/min; depth, 1/3–1/2 of chest width). (B) Compress the chest wall over the ventricles between one or two fingers and the opposing thumb (rate, 120–150/min; depth, 1/3–1/2 of chest width). (C) Compress the sternum with the newborn in dorsal recumbency (rate, 120–150/min; depth, 1/3 anterior–posterior chest diameter). Artwork by Chrisoula Toupadakis Skouritakis, Ph.D., MediaLab Services Director, and Kailee Suess, Department of Surgical and Radiological Sciences, School of Veterinary Medicine, University of California, Davis. Copyright: The RECOVER Initiative 2025. All rights reserved.
We suggest administering chest compressions in newborn puppies and kittens at a rate of 120–150/min, which is faster than what is recommended in adult dogs and cats (100–120/min) (NB‐12) [6]. We did not identify any experimental or clinical studies that delineate the optimal compression rate in small mammals with a comparable size to newborn puppies or kittens (i.e., 100–800 g body weight), nor any studies in transitional newborns. However, indirect evidence suggests a compression rate of at least 120/min should be targeted. In post‐transitional piglets (2 kg), compression rates of 150–180/min were associated with hemodynamic benefit over slower compression rates (i.e., 60/min to 120/min), with a compression rate of at least 120/min supported by other neonatal piglet studies [100, 101, 102]. Mathematical modeling furthermore suggests an optimal chest compression rate in animals <1 kg to be 150/min or more [103]. With very short compression distances of only 5–15 mm and the diminutive compression force required to achieve these distances, we consider a compression rate of 150/min feasible and of low probability to cause rescuer fatigue, unlike chest compressions in adult animals at higher rates [104].
7.3. Compressions and Ventilation Are Coordinated in Newborn CPR
Given the critical importance of effective ventilation in newborn puppies and kittens requiring resuscitation, administering chest compressions and positive pressure breaths as recommended in adult dogs and cats could impair lung aeration.
In newborn puppies and kittens without a cuffed ET tube in place, we recommended pausing chest compressions for the delivery of a breath (NB‐14). This applies to any newborns undergoing bag–mask ventilation. Chest compressions and ventilation are delivered in a coordinated or synchronized way where compressions alternate with ventilations at a predefined ratio (i.e., compression to ventilation ratio, or C:V ratio) of four chest compressions to one positive pressure breath (4:1) (NB‐13). This approach prioritizes ventilation compared to CPR in nonintubated adult dogs and cats in which a C:V ratio of 30 compressions to two positive pressure breaths is recommended [6]. The recommended C:V of 4:1 also recognizes the higher chest compression rate target in newborn puppies and kittens compared to newborn infants, in whom a C:V ratio of 3:1 is recommended [105]. In practice, around 15 rounds of four compressions to one ventilation are delivered every 30 s, with one 4:1 round administered every 2 s. This will amount to 120 compressions and 30 breaths per minute. We suggest delivering breaths with 100% oxygen during CPR (NB‐18). Patient response should be assessed every 30 s, and if the HR exceeds 50/min, chest compressions can be discontinued while continuing PPV and frequent/continuous HR monitoring.
In newborn puppies and kittens with a cuffed ET tube in place, we suggest delivering breaths concurrently to chest compressions (NB‐14). Breaths do not need to be coordinated or synchronized with compressions. This is the equivalent approach to adult dogs and cats when intubated, except for higher compression (i.e., 120–150/min) and ventilation rates (i.e., 20–30/min) in newborns. As for nonintubated animals, we suggest the use of 100% oxygen during CPR in intubated newborns.
7.4. Epinephrine and Vascular Access
If CPR lasting at least 1 min fails to lead to an increase in HR >50/min, we recommend parenteral administration of epinephrine (0.01–0.03 mg/kg) (NB‐21; NB‐22) (Figure 3). Multiple publications support the preferential use of IV or intraosseous (IO) routes over ET or intranasal (IN) routes [106, 107, 108, 109, 110, 111, 112, 113, 114]. Topical sublingual administration of epinephrine has not been tested, and it is plausible that it is not effective. If IV or IO access cannot be achieved in a timely manner, a single epinephrine dose by the ET route at a higher dose (0.05–0.1 mg/kg) was shown to have some effect, although with a delay [106]. However, ET administration also carries the same risk as for ET intubation, including prolonged interruption of PPV. ET drug administration, therefore, is most appropriate in newborns that are already intubated. As for the ET route, IN epinephrine at a higher dose showed lower plasma concentration and delayed but some effect when compared to IV administration [107, 115]. We suggest against the use of IM epinephrine, as data available from one small study did not identify an increase in epinephrine plasma concentrations until after return of spontaneous circulation (ROSC) [116]. We also advise against the practice of intralingual (IM) administration due to anecdotal reports of harm (i.e., tissue necrosis) associated with this practice. Although the effect of repeated administration of epinephrine has not been studied in newborn animals, we suggest administering IV/IO doses of epinephrine (0.01–0.03 mg/kg) every 3–5 min during ongoing CPR as recommended for adult dogs and cats [6].
In newborn puppies and kittens that require chest compressions, we suggest against the routine administration of atropine during CPR (NB‐24), which is the same recommendation as for newborns with bradycardia (NB‐23). We did not identify any evidence in newborn infants or experimental studies in newborn animals to support the use of atropine during CPR; instead, the primary goal of CPR is to reverse myocardial hypoxia by supporting lung aeration through PPV and coronary blood flow through high‐quality chest compressions and epinephrine administration. This recommendation, however, should not preclude the use of atropine in specific situations in post‐transitional neonatal puppies and kittens, such as severe bradycardia or CPA associated with ET intubation, gastrointestinal surgery, and other scenarios leading to vagal stimulation.
Vascular access (e.g., umbilical vein, jugular vein, IO cannula) should also be established to permit effective parenteral administration of other medications and IV fluids where the newborn is not responsive to prolonged resuscitation (see adjunct interventions). Which vascular access route to choose is contextually dependent on the size of the animal and the experience and preference of resuscitation team members [8].
7.5. Discontinuation of Resuscitation
At present, there are no clinical studies reporting survival rates of newborn puppies and kittens undergoing CPR, let alone how these survival rates are impacted by the duration of CPR. Nevertheless, durations of 15–30 min were proposed in the veterinary literature based on expert opinion [8, 11]. The most direct evidence is therefore drawn from research in newborn infants. In full‐term infants receiving CPR for a median duration of 6 min (IQR 13) in the delivery room (n = 439), 91% achieved ROSC and 83% survived to hospital discharge [117]. However, survival in newborn infants with a persistent Apgar score of 0 (i.e., absence of breathing, muscle tone, and consciousness), combined with an undetectable HR (indicating no detectable signs of life) despite 10 min of intensive resuscitation, is poor [118, 119]. On the other hand, multiple case series revealed that survival with a favorable neurologic outcome can occur even in infants with no signs of life after 10 min of CPR, that duration of CPR is only weakly associated with survival when adjusted for other risk factors, and that a definitive time point after which further resuscitation is considered futile has not been established [4, 117, 120, 121, 122, 123]. For newborn puppies and kittens undergoing CPR, we suggest continuing resuscitation efforts for at least 15 min before considering discontinuation (NB‐25). If there is no heartbeat identified after that duration of high‐quality CPR, having exhausted all recommended measures including epinephrine, chances for a favorable outcome are likely very low. Beyond the individual animal, rescuers should ensure that prolonged resource allocation to one animal does not critically compromise the care of littermates needing resuscitation but having a more favorable prognosis. Next to the duration of CPR, pet owner goals, the presence of significant congenital malformations (e.g., anasarca, gastroschisis, anencephaly, atresia ani), clear signs of death, and the ability to provide post‐cardiac arrest care will impact the decision whether to continue resuscitative efforts [29, 30].
8. Adjunct Interventions
Several interventions can be considered in addition to the primary resuscitative measures. These are treatments that might be used during prolonged resuscitation but that we have assessed as noncritical, and therefore, we did not systematically review the evidence pertaining to them (e.g., bicarbonate, fluid administration). Adjunct interventions are also those that can be considered in nonvigorous newborn puppies and kittens that fail to adequately respond to prolonged resuscitation efforts (e.g., dextrose during resuscitation; doxapram) or need additional support in the post‐resuscitative period (e.g., dextrose after resuscitation, temperature management). Finally, we also categorized GV 26 acupoint stimulation—an intervention that we did not reach consensus for or against its use—as an adjunctive measure until more evidence becomes available.
8.1. Blood Glucose (BG) Management
Hepatic glycogen stores are low and rapidly depleted, and the capacity for gluconeogenesis is limited in newborn puppies and kittens, such that they depend on oral intake of energy‐rich nutrients to maintain euglycemia after birth [124]. Nevertheless, hypoglycemia during the first few hours after birth, including during the initial resuscitation, is uncommon, and in newborn puppies, BG concentrations were similar if born by C‐section (4.9 ± 0.2 mmol/L) or by vaginal eutocia (4.5 ± 0.2 mmol/L, p = 0.31) [32]. If newborns fail to nurse, hypoglycemia can occur and has been associated with death in puppies and in neonates of other species [125, 126, 127, 128]. Indiscriminate glucose administration can be harmful, as not only hypoglycemia but also hyperglycemia has been shown to worsen neurologic outcome in newborns with hypoxic–ischemic brain injury [129, 130, 131, 132, 133]. We therefore suggest measuring BG concentration in newborn puppies and kittens that remain nonvigorous despite prolonged (i.e., >10 min) resuscitation (NB‐19). A drop of blood sufficient for a glucometer can be obtained by puncture of a paw pad [124]. If hypoglycemia (i.e., BG < 3 mmol/L [BG < 54 mg/dL]) is present during prolonged resuscitation, we recommend dextrose supplementation with a slow bolus of 0.25 g/kg dextrose (e.g., 0.5 mL/100 g of 5% dextrose solution over 5 min), administered IV, IO, or, less preferably, by intraperitoneal (IP) injection (NB‐19) (Figure 3). The intraperitoneal route is considered practical, effective, and safe if executed correctly, including maintenance of strict asepsis. Where parenteral dextrose administration cannot be accomplished, we suggest topical sublingual mucosal administration of dextrose (0.5 g/kg; 0.1 mL/100 of 50% dextrose solution) (NB‐19). Should it be impractical to determine the BG concentration in a newborn puppy or kitten that fails to respond to prolonged (i.e., >10 min) standard resuscitation measures (e.g., rewarming, stimulation, PPV), we suggest empirical administration of dextrose by one of the above‐cited routes (NB‐19).
In newborn puppies and kittens after resuscitation (i.e., after CPR and ROSC) that are nonvigorous and not nursing, we recommend confirming hypoglycemia before supplementing, if possible. If an IV or IO catheter is present, we suggest administering dextrose by CRI (e.g., 2.5% dextrose in isotonic crystalloid fluids given at a physiologic rate IV or IO) rather than bolus injection to avoid large fluctuations in BG concentration that can be harmful (NB‐26). After resuscitation, it may be more practical to establish IV or IO access and to initiate an infusion. A reasonable physiologic fluid rate is 80–120 mL/kg/day in neonatal puppies and 60–80 mL/kg/day in neonatal kittens. If an infusion is not feasible, we suggest proceeding as described above for dextrose supplementation in hypoglycemia during resuscitation (NB‐26). In addition, dextrose administration by orogastric tube is another possibility after ROSC as long as it is performed by experienced staff to avoid injury to the newborn [124]. Oral administration of dextrose by syringe can be considered in alert newborns.
8.2. Temperature Management
Targeted temperature management, a deliberate induction or maintenance of lower‐than‐normal body temperature within a certain range, is suggested in adult people, dogs, and cats that remain comatose after resuscitation from CPA, owing its benefits to multiple neuroprotective effects [7, 134, 135]. In term newborn infants with moderate to severe hypoxic–ischemic encephalopathy (HIE), therapeutic hypothermia (TH) (33°C–34°C for 72 h, started within 6 h of birth) has been recommended for over a decade [136]. However, TH or any temperature management has not previously been suggested in newborn puppies and kittens exposed to anoxic brain injury during birth. In fact, the clinical symptoms of HIE are not well delineated in this population, though they are well described in infants and foals [137, 138]. Clinical signs of HIE in these species include lethargy to stupor/coma, decreased to no activity, extensor rigidity to decerebrate posture, hypotonia to flaccidity, weak or absent sucking reflex, constricted or dilated pupils with absent pupillary light reflex, low HR, and intermittent breathing or apnea, depending on severity. Evidence suggests that the positive impacts of cooling on HIE outcomes in newborn animals depend on the severity of HIE, the time interval from injury to cooling, the degree of hypothermia, and the duration of TH [139]. Generally, a longer duration of TH is required for more severe injury [140, 141]; however, evidence from experimental animal studies suggests a neuroprotective effect can occur after cooling periods of only 24 h or less [142, 143, 144]. Given the restricted resources available in veterinary medicine, care of newborn puppies and kittens with moderate to severe HIE is generally out of reach. As only those newborns with milder signs of HIE have potential as viable animals, we considered a 24‐h duration of permissive hypothermia to be a pragmatic approach to balance risk, benefit, and feasibility of TH until more clinical veterinary data are available. Specifically, we suggest permitting newborn puppies and kittens with signs of acute HIE to remain at a low normal temperature for newborn puppies and kittens (i.e., 35°C [95°F]) for 24 h after birth (NB‐27). Since newborn puppies and kittens are relatively hypothermic at birth with rectal temperatures of 33.7 ± 1.4°C after C‐section and 33.1 ± 3.1°C after eutocia, which then increase to 35.1 ± 1.8°C and 33.2 ± 4.7°C after 1 h, respectively, active cooling is probably not required, and avoidance of active rewarming should suffice.
The speed of rewarming is another topic of concern in animals with HIE. A number of experimental animal studies of HIE in populations other than newborns demonstrated harm with fast (i.e., >1°C/h) rewarming rates [145, 146, 147, 148, 149, 150], and the RECOVER initiative therefore suggests to aim for slow rewarming (i.e., 0.25°C/h to 0.5°C/h) and recommends against rewarming rates that surpass 1°C/h in adult dogs and cats after ROSC [7, 151]. No clinical studies in newborn infants have been conducted, and in experimental animal studies including newborn transitional lambs and post‐transitional piglet models, no benefit of slow versus fast rewarming was found despite a 10‐ to 20‐fold difference in rewarming rates [152, 153, 154, 155, 156]. However, out of concern for rebound seizures, hypotension, and temperature overshoot (i.e., hyperthermia) with fast rewarming rates [157, 158], we suggest in newborns with clinical signs of HIE to rewarm at rates below 1°C/h to achieve normothermia (35.0°C–37.2°C [95°F–99°F]) while avoiding accidental hyperthermia (NB‐28). The rewarming rate is less of a concern in animals that are hypothermic at birth, as most newborn puppies and kittens are, as long as they have no clinical signs of HIE and did not undergo CPR.
8.3. Doxapram
The analeptic drug doxapram increases respiratory drive through effects on peripheral (i.e., carotid and aortic chemoreceptors) and central (i.e., brainstem respiratory control center) sites [159]. While doxapram was used for respiratory stimulation of newborn puppies and kittens with inadequate ventilatory function for more than 50 years, its routine use has been questioned more recently due to concerns about the drug's efficacy in severely hypoxic animals and the availability of other measures to support ventilation [9, 11, 160, 161]. In small animal medicine, it is effectively used as a respiratory stimulant to aid in the assessment of laryngeal dysfunction in dogs [162, 163]. There are currently no studies that support its use in newborn puppies and kittens with apnea/gasping or bradypnea. A recent randomized controlled trial studied the effect of intralingual injection of doxapram (approximately 10 mg/kg) versus saline injected within the first minute of birth in 171 puppies born by elective C‐section, regardless of the presence of respiratory compromise [164]. The study did not identify an effect on the primary outcome of 7‐day survival (92% with doxapram, 94% with saline, P = 0.63) or on Apgar scores over the first 20 min after birth. We therefore recommend against the routine administration of doxapram in newborn puppies and kittens undergoing resuscitation, such as after C‐section (NB‐07). Importantly, this study did not specifically evaluate the effect of doxapram in apneic/gasping newborns. Multiple clinical and experimental studies involving infants, calves, foals, and rats revealed a beneficial effect of doxapram on outcomes such as survival, oxygenation, and ventilation in neonates and newborns without apparent harm [165, 166, 167, 168, 169, 170]. However, due to very serious indirectness (e.g., species, life stage, and/or condition treated) and methodological shortcomings, significant uncertainty regarding the benefit of doxapram in newborn puppies and kittens remains. Importantly, a focus on doxapram administration and its expected response carries the risk of distraction from other more effective interventions in apneic/gasping newborns, foremost PPV. Thus, we suggest the use of doxapram in transitional puppies and kittens with respiratory compromise (i.e., apnea/gasping or bradypnea) only if they fail to respond after a period of time (e.g., ≥10 min) to other appropriate measures of support (e.g., PPV) (NB‐07). Doxapram is preferentially administered IV/IO, if this does not compromise other supportive measures, but intralingual IM, IN, or topical sublingual administration is also an acceptable route (Figure 3).
8.4. GV 26 Acupoint Stimulation
Stimulation of the GV 26 (Jen Chung; Rhenzong) acupoint has been recommended in newborn puppies and kittens with low RR or apnea that have a heartbeat [8, 11, 161, 171]. The acupoint is located where the philtrum joins the lower edge of the nostril and is stimulated by the insertion and twisting of a 25‐gauge needle at that point [172]. However, no controlled studies were conducted, and clinical reports are limited to case series, such that no conclusion regarding efficacy can be drawn [173, 174]. While these publications do not report overt harm due to GV 26 stimulation, the procedure may distract resuscitation team members from timely administration of interventions known to be effective, foremost PPV, particularly if the needle is applied for more than a few seconds. For these reasons, we initially formulated a recommendation against the use of GV 26, while a subset of subject matter experts strongly advocated for the use of GV 26 stimulation in nonvigorous, apneic newborn puppies and kittens. As no consensus could be achieved, we agreed to provide no recommendation for or against the use of GV 26 stimulation. We, however, also agreed that if resuscitation teams choose to use this technique in the absence of clear guidance, it should be brief (e.g., <2–3 seconds) and not interfere with initiation or quality of PPV.
8.5. Fluid Administration and Bicarbonate
The RECOVER Initiative did not systematically review the literature regarding fluid therapy and the administration of parenteral sodium bicarbonate during the resuscitation, including CPR, of newborn puppies and kittens. On a cursory review of the literature, we identified two experimental studies in post‐transitional piglets that received volume infusion during resuscitation from asphyxial CPA [175, 176]. In one study, normovolemic piglets (age 8 ± 4 days) were asphyxiated to an MAP of <20 mm Hg and then resuscitated according to the newborn algorithm (i.e., PPV ± CPR ± epinephrine); two sequential boluses of either 0.9% NaCl (10 mL/kg IV), 5% albumin (10 mL/kg IV), or sham (no fluid administration) were given over 5 min. Fluid administration led to no hemodynamic benefit but resulted in worse pulmonary edema and lung compliance 2 h after resuscitation. These results suggest against volume administration in euvolemic newborns undergoing resuscitation at birth [175]. Notably, this harmful effect might be further accentuated in transitional newborns with increased lung water and hypoxia‐related myocardial dysfunction [177]. A second study in neonatal piglets (age, 12–44 h), which combined asphyxiation with concurrent hemorrhagic arrest (blood loss, 30–34 mL/kg), tested the effect of standard resuscitation measures (i.e., PPV, chest compressions, and epinephrine) in combination with bolus administration of 0.9% saline or autologous whole blood on time to ROSC [176]. Volume expansion consisted of boluses of 10 mL/kg every 2 min until ROSC occurred. Time to ROSC was not different between animals receiving saline versus blood, although only a small volume of 3–4 mL/kg of either fluid needed to be administered until animals achieved ROSC. Based on the scarce evidence available, it is reasonable—just as during CPR in adult dogs and cats [6]—to reserve volume therapy for newborns with strong evidence of hypovolemia, such as overt blood loss (e.g., from umbilical cord hemorrhage, placental laceration), but to advise against its use in normovolemic newborns. Bolus administration of a balanced isotonic crystalloid at 1 mL/100 g over 5 min IV/IO is a reasonable starting point in newborn puppies and kittens with hypovolemia. It is often difficult to determine the volume status of newborn puppies and kittens and thus to ascertain ahead of time which animal will benefit from or be harmed by intravenous volume resuscitation. We therefore suggest a trial bolus of a balanced isotonic crystalloid (e.g., 1 mL/100 g IV or IO) if there is a suspicion of hypovolemia and the newborn has failed to respond to standard resuscitative measures.
Some degree of acidemia is always present at birth in both newborn puppies, kittens, and infants, and its severity has been associated with unfavorable outcomes, such as HIE, and might be of prognostic value [24, 32, 178, 179, 180]. However, the benefit of remediation of severe acidemia with buffer therapy, specifically the benefit of sodium bicarbonate administration, is uncertain. A single small randomized, controlled clinical trial, in which newborn infants requiring PPV for at least 5 min at birth received either sodium bicarbonate (1.8 mEq/kg IV) or an equivalent volume of 5% dextrose (4 mL/kg IV), showed no effect on the composite outcome of death or survival with abnormal neurological function at discharge (p = 0.88) [181]. We therefore consider it reasonable to follow the current recommendation for newborn infants when treating newborn puppies and kittens. We suggest against the administration of sodium bicarbonate to newborn puppies and kittens undergoing CPR unless all four of the following are true: resuscitation is prolonged (≥10 min), the animal fails to respond to standard measures (i.e., PPV, chest compressions, and epinephrine), effective ventilation is in place to avoid worsening of respiratory acidosis, and IV access is present [4].
9. Discussion
The 59 treatment recommendations (Table 1), prose explanations, dosage chart, images, and algorithm contained in this paper constitute the RECOVER Newborn Resuscitation Guidelines, a consensus guidelines paper synthesized from exhaustive evaluation and analysis of evidence relating to resuscitation of newborns across many species and honed through an iterative modified Delphi process considering input from subject matter experts and the international veterinary community. An international group of veterinary experts in small animal theriogenology and small animal emergency and critical care collaborated to tailor these recommendations to puppies and kittens based on a combination of this evidence evaluation and analysis, and expert opinion where inadequate evidence was available.
The primary goal of the RECOVER Newborn Resuscitation Guidelines is that they serve as foundational step‐by‐step instructions for the support (“resuscitation”) of puppies and kittens in the first minutes following birth in the clinical setting. All puppies and kittens delivered by C‐section, and many following medical dystocia, will require newborn resuscitation as presented here. Most will be delivered alive but in need of support, while some may be markedly distressed, and others may be “stillborn”. These guidelines contain treatment recommendations and an algorithm for prebriefing and use as a cognitive aid to optimize patient assessment and resultant treatments for these litters.
There are many differences between these Newborn Resuscitation Guidelines and the RECOVER CPR Guidelines for Dogs and Cats. The two main differences are the life stage addressed (newborn vs adult) and, notably, the fact that newborn resuscitation includes vital, life‐preserving treatments of animals that have not experienced CPA. The RECOVER CPR Guidelines focus almost entirely on dogs and cats that require CPR, whereas only a fraction of puppies and kittens for which these Newborn Resuscitation Guidelines are relevant will require CPR; in fact, while the majority will not require CPR, every puppy and kitten delivered by C‐section requires some resuscitative measures addressed in these guidelines to optimize their outcome. Additionally, while the focus of resuscitation in adult dogs and cats currently lies in delivering high‐quality chest compressions along with PPV, support of the newborn in transition focuses almost entirely on respiratory support in an effort to aerate the newborn lung. In addition to PPV, chest compressions are performed in newborns that have very severe bradycardia (i.e., HR < 50/min), and atropine is not recommended for such cases. These differences in treatment recommendations underscore the dissimilarity between newborn and adult physiology and, thus, the cause of instability: hypoxemia is nearly always the culprit in newborn distress, and thus, respiratory support is nearly always the answer.
Scarce evidence was available in newborn puppies and none in newborn kittens, and therefore, most of the evidence we used to develop these guidelines was highly indirect in species and setting. Much information was gleaned from studies out of human delivery rooms and from experimental studies in near‐term fetal lambs and other species. While modern veterinary medicine provides many advantages for animal health, our hospitals generally lack the material and personnel resources of human delivery rooms and bear little resemblance to the tightly controlled laboratory environments in which near‐term lamb fetuses are studied. Therefore, the recommendations contained herein are the best we could develop with the evidence available in combination with the expert opinions of a group of small animal theriogenologists, a group of small animal emergency and critical care specialists, and the international veterinary community. There are many knowledge gaps. We have outlined many of these knowledge gaps in the accompanying paper [19] and hope research will address these knowledge gaps so that future iterations of these guidelines will be built on species‐ and setting‐specific evidence.
While these RECOVER Newborn Resuscitation Guidelines are a first attempt at consensus guidelines for resuscitation of newborn puppies and kittens, we anticipate that the recommendations and algorithm contained here will enable practitioners to address C‐section and dystocia deliveries in a systematic way to enhance patient outcomes. We urge practitioners to prebrief the algorithm and dosage chart prior to deliveries and then to follow the recommendations. The RECOVER Initiative welcomes input regarding outcomes and future directions of inquiry, and we encourage clinical researchers in the small animal theriogenology and emergency spaces to perform clinical studies to address critical knowledge gaps regarding newborn resuscitation.
Conflicts of Interest
Dr. Burkitt‐Creedon is the Editor of the Journal but only participated in the peer review process as an author. The authors declare no other conflicts of interest.
Acknowledgments
The authors would like to acknowledge the following individuals and organizations without whom an endeavor of this size would have been impossible. Peter Morley, Vinay Nadkarni, and the International Liaison Committee on Resuscitation provided invaluable mentorship, guidance, and a wealth of experience to inform the RECOVER guidelines process. RECOVER as an organization grew out of the American College of Veterinary Emergency and Critical Care and the Veterinary Emergency and Critical Care Society, and we are grateful for the ongoing support of both organizations as we work to produce guidelines, educational content, and research to improve the care of critically ill and injured animals. We are particularly grateful to the Information Specialists who collaborated with Co‐Chairs and Domain Chairs to build processes and produce optimal search strategies that captured the most relevant evidence for us to evaluate. We thank the 56 veterinarians and veterinacy technicians for serving as Evidence Evaluators for the Newborn Domain reported in this manuscript. Finally, we are grateful to the members of the worldwide veterinary community who reviewed and commented on the draft treatment recommendations posted in May of 2025, whose comments and reflections helped improve the recommendations’ clarity and content.
Boller M., Burkitt‐Creedon J. M., Fletcher D. J., et al. “RECOVER Guidelines: Newborn Resuscitation in Dogs and Cats. Clinical Guidelines.” Journal of Veterinary Emergency and Critical Care 35, no. S1 (2025): 35, 60–85. 10.1111/vec.70013
Manuel Boller and Jamie M. Burkitt‐Creedon contributed equally to this work.
Funding: Project support was received from Boehringer Ingelheim Animal Health and Zoetis Animal Health, both of which helped to fund the purpose‐developed, web‐based system used for evidence evaluation.
Endnotes
Boller, Manuel; Burkitt‐Creedon, Jamie M; and Fletcher, Daniel J. 2025. “RECOVER Newborn Guidelines 2025.” OSF. June 14. https://osf.io/wxzqa/ [accessed on June 14, 2025].
Covidence, Melbourne, VIC, Australia.
NowComment, www.nowcomment.com (accessed on June 8, 2025).
References
- 1. Katheria A. and Finer N. N., “Newborn Resuscitation,” in Avery's Diseases of the Newborn, 10th ed., ed. Gleason C. A. and Juul S. E. (Elsevier, 2018), 273–288. [Google Scholar]
- 2. Hooper S. B., Kitchen M. J., Polglase G. R., et al., “The Physiology of Neonatal Resuscitation,” Current Opinion in Pediatrics 30, no. 2 (2018): 187–191. [DOI] [PubMed] [Google Scholar]
- 3. Finnemore A. and Groves A., “Physiology of the Fetal and Transitional Circulation,” Seminars in Fetal & Neonatal Medicine 20, no. 4 (2015): 210–216. [DOI] [PubMed] [Google Scholar]
- 4. Wyckoff M. H., Wyllie J., Aziz K., et al., “Neonatal Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations,” Circulation 142, no. 16_suppl_1 (2020): S185–S221. [DOI] [PubMed] [Google Scholar]
- 5. Yamada N. K., Szyld E., Strand M. L., et al., “2023 American Heart Association and American Academy of Pediatrics Focused Update on Neonatal Resuscitation: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care,” Circulation 149, no. 1 (2024): e157–e166. [DOI] [PubMed] [Google Scholar]
- 6. Burkitt‐Creedon J. M., Boller M., Fletcher D. J., et al., “2024 RECOVER Guidelines: Updated Treatment Recommendations for CPR in Dogs and Cats,” Journal of Veterinary Emergency and Critical Care 34, no. Suppl 1 (2024): 104–123. [DOI] [PubMed] [Google Scholar]
- 7. Fletcher D. J., Boller M., Brainard B. M., et al., “RECOVER Evidence and Knowledge Gap Analysis on Veterinary CPR. Part 7: Clinical Guidelines,” Journal of Veterinary Emergency and Critical Care 22, no. Suppl 1 (2012): S102–S131. [DOI] [PubMed] [Google Scholar]
- 8. Traas A. M., “Resuscitation of Canine and Feline Neonates,” Theriogenology 70, no. 3 (2008): 343–348. [DOI] [PubMed] [Google Scholar]
- 9. Moon P. F., Massat B. J., and Pascoe P. J., “Neonatal Critical Care,” Veterinary Clinics of North America: Small Animal Practice 31, no. 2 (2001): 343–367. [DOI] [PubMed] [Google Scholar]
- 10. Cavanagh A. A., “Neonatal Resuscitation,” Veterinary Team Brief 5, no. 5 (2017): 27–28. [Google Scholar]
- 11. Davidson A. P., “Neonatal Resuscitation: Improving the Outcome,” Veterinary Clinics of North America: Small Animal Practice 44, no. 2 (2014): 191–204. [DOI] [PubMed] [Google Scholar]
- 12. Bruckner M. and Schmölzer G. M., “Physiologic Changes During Neonatal Transition and the Influence of Respiratory Support,” Clinics in Perinatology 48, no. 4 (2021): 697–709. [DOI] [PubMed] [Google Scholar]
- 13. Hooper S. B., te Pas A. B., Lang J., et al., “Cardiovascular Transition at Birth: A Physiological Sequence,” Pediatric Research 77, no. 5 (2015): 608–614. [DOI] [PubMed] [Google Scholar]
- 14. Barker P. M. and Olver R. E., “Invited Review: Clearance of Lung Liquid During the Perinatal Period,” Journal of Applied Physiology 93, no. 4 (2002): 1542–1548. [DOI] [PubMed] [Google Scholar]
- 15. Hooper S. B., Kitchen M. J., Wallace M. J., et al., “Imaging Lung Aeration and Lung Liquid Clearance at Birth,” The FASEB Journal 21, no. 12 (2007): 3329–3337. [DOI] [PubMed] [Google Scholar]
- 16. te Pas A. B., Davis P. G., Hooper S. B., and Morley C. J., “From Liquid to Air: Breathing After Birth,” Journal of Pediatrics 152, no. 5 (2008): 607–611. [DOI] [PubMed] [Google Scholar]
- 17. Fletcher D. J., Boller M., Burkitt‐Creedon J. M., et al., “2024 RECOVER Guidelines: Methods, Evidence Identification, Evaluation, and Consensus Process for Development of Treatment Recommendations,” Journal of Veterinary Emergency and Critical Care 34, no. S1 (2024): 3–15. [DOI] [PubMed] [Google Scholar]
- 18. Guyatt G. H., Oxman A. D., Kunz R., et al., “GRADE: Going From Evidence to Recommendations,” BMJ 336, no. 7652 (2008): 1049–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boller M. B. J., “RECOVER Newborn Resuscitation Treatment Recommendations: Place Holder,” Journal of Veterinary Emergency and Critical Care (2025). [Google Scholar]
- 20. Boulkedid R., Abdoul H., Loustau M., et al., “Using and Reporting the Delphi Method for Selecting Healthcare Quality Indicators: A Systematic Review,” PLoS ONE 6, no. 6 (2011): e20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Greig P. R., Zolger D., Onwochei D. N., et al., “Cognitive Aids in the Management of Clinical Emergencies: A Systematic Review,” Anaesthesia 78, no. 3 (2023): 343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Veronesi M. C., “Assessment of Canine Neonatal Viability—The Apgar Score,” Reproduction in Domestic Animals = Zuchthygiene 51 (2016): 46–50. [DOI] [PubMed] [Google Scholar]
- 23. Santos N. R., Beck A., and Fontbonne A., “A Review of Maternal Behaviour in Dogs and Potential Areas for Further Research,” Journal of Small Animal Practice 61, no. 2 (2020): 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vassalo F. G., Simões C. R. B., Sudano M. J., et al., “Topics in the Routine Assessment of Newborn Puppy Viability,” Topics in Companion Animal Medicine 30, no. 1 (2015): 16–21. [DOI] [PubMed] [Google Scholar]
- 25. Titkova R., Fialkovicova M., Karasova M., and Hajurka J., “Puppy Apgar Scores After Vaginal Delivery and Caesarean Section,” Veterinarni Medicina 62, no. 9 (2017): 488–492. [Google Scholar]
- 26. Veronesi M. C., Panzani S., Faustini M., and Rota A., “An Apgar Scoring System for Routine Assessment of Newborn Puppy Viability and Short‐Term Survival Prognosis,” Theriogenology 72, no. 3 (2009): 401–407. [DOI] [PubMed] [Google Scholar]
- 27. Apgar V., “A Proposal for a New Method of Evaluation of the Newborn Infant,” Current Researches in Anesthesia and Analgesia 32, no. 4 (1953): 260–267. [PubMed] [Google Scholar]
- 28. Hibaru V. Y., Pereira K., Fuchs K. D. M., et al., “Topics in the Routine Assessment of Newborn Kitten Vitality: Apgar Score, Reflexes and Complementary Assessments,” Journal of Feline Medicine and Surgery 24, no. 6 (2022): e34–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Estevam M. V., Toniollo G. H., and Apparicio M., “The Most Common Congenital Malformations in Dogs: Literature Review and Practical Guide,” Research in Veterinary Science 171 (2024): 105230. [DOI] [PubMed] [Google Scholar]
- 30. Hoskins J. D., “Congenital Defects of Cats,” Compendium: Continuing Education for the Practicing Veterinarian 17, no. 3 (1995): 385–405. [Google Scholar]
- 31. Silva L. C. G., Lúcio C. F., Veiga G. A. L., et al., “Neonatal Clinical Evaluation, Blood Gas and Radiographic Assessment After Normal Birth, Vaginal Dystocia or Caesarean Section in Dogs,” Reproduction in Domestic Animals = Zuchthygiene 44, no. s2 (2009): 160–163. [DOI] [PubMed] [Google Scholar]
- 32. Abreu R. A., Almeida L. L., Rosa Filho R. R. D., et al., “Canine Pulmonary Clearance During Feto‐Neonatal Transition According to the Type of Delivery,” Theriogenology 224 (2024): 156–162. [DOI] [PubMed] [Google Scholar]
- 33. Adams D. J., Ellerbrock R. E., Wallace M. L., et al., “Risk Factors for Neonatal Mortality Prior to Hospital Discharge in Brachycephalic and Nonbrachycephalic Dogs Undergoing Cesarean Section,” Veterinary Surgery 51, no. 7 (2022): 1052–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abd‐El Hamid S. A., Badr‐El Din M. M., Dabous N. I., and Saad K. M., “Effect of the Use of a Polyethylene Wrap on the Morbidity and Mortality of Very Low Birth Weight Infants in Alexandria University Children's Hospital,” Journal of the Egyptian Public Health Association 87, no. 5‐6 (2012): 104–108. [DOI] [PubMed] [Google Scholar]
- 35. Lenclen R., Mazraani M., Jugie M., et al., “[Use of a Polyethylene Bag: A Way to Improve the Thermal Environment of the Premature Newborn at the Delivery Room],” Archives De Pediatrie 9, no. 3 (2002): 238–244. [DOI] [PubMed] [Google Scholar]
- 36. Meyer M. P., Payton M. J., Salmon A., et al., “A Clinical Comparison of Radiant Warmer and Incubator Care for Preterm Infants From Birth to 1800 Grams,” Pediatrics 108, no. 2 (2001): 395–401. [DOI] [PubMed] [Google Scholar]
- 37. Laptook A. R., Salhab W., and Bhaskar B., “Admission Temperature of Low Birth Weight Infants: Predictors and Associated Morbidities,” Pediatrics 119, no. 3 (2007): e643–e649. [DOI] [PubMed] [Google Scholar]
- 38. Mullany L. C., Katz J., Khatry S. K., et al., “Risk of Mortality Associated With Neonatal Hypothermia in Southern Nepal,” Archives of Pediatrics & Adolescent Medicine 164, no. 7 (2010): 650–656. [DOI] [PubMed] [Google Scholar]
- 39. Christensen B. W. and Erb H. N., “An Investigation of the Discrepancy Between Set and Actual Temperature of Neonatal Incubators: Concern for Hypothermia and Hyperthermia,” Journal of the American Veterinary Medical Association 262, no. 1 (2024): 68–71. [DOI] [PubMed] [Google Scholar]
- 40.Rech Morassutti F, Cavallin F., Zaramella P., et al., “Association of Rewarming Rate on Neonatal Outcomes in Extremely Low Birth Weight Infants With Hypothermia,” Journal of Pediatrics 167, no. 3 (2015): 557.e2–561.e2. [DOI] [PubMed] [Google Scholar]
- 41. Feldman A., De Benedictis B., Alpan G., et al., “Morbidity and Mortality Associated With Rewarming Hypothermic Very Low Birth Weight Infants,” Journal of Neonatal‐Perinatal Medicine 9, no. 3 (2016): 295–302. [DOI] [PubMed] [Google Scholar]
- 42. Jain P., Dalal J. S., and Gathwala G., “Rapid vs. Slow Rewarming for Management of Moderate to Severe Hypothermia in Low‐Birth‐Weight Pre‐Term Neonates—An Open Label Randomized Controlled Trial,” Journal of Tropical Pediatrics 67, no. 1 (2021): fmaa098. [DOI] [PubMed] [Google Scholar]
- 43. Rossi E., Maziku D. M., Leluko D. E., et al., “Rewarming Rate of Hypothermic Neonates in a Low‐Resource Setting: A Retrospective Single‐Center Study,” Frontiers in Pediatrics 11 (2023): 1113897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Motil K. J., Blackburn M. G., and Pleasure J. R., “The Effects of Four Different Radiant Warmer Temperature Set‐Points Used for Rewarming Neonates,” Journal of Pediatrics 85, no. 4 (1974): 546–550. [DOI] [PubMed] [Google Scholar]
- 45. Scarpelli E. M., Condorelli S., and Cosmi E. V., “Cutaneous Stimulation and Generation of Breathing in the Fetus,” Pediatric Research 11, no. 1 Pt 1 (1977): 24–28. [PubMed] [Google Scholar]
- 46. Faridy E. E., “Instinctive Resuscitation of the Newborn Rat,” Respiration Physiology 51, no. 1 (1983): 1–19. [DOI] [PubMed] [Google Scholar]
- 47. Kaufmann M., Mense L., Springer L., and Dekker J., “Tactile Stimulation in the Delivery Room: Past, Present, Future. A Systematic Review,” Pediatric Research 96, no. 3 (2024): 616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dekker J., Martherus T., Cramer S. J. E., et al., “Tactile Stimulation to Stimulate Spontaneous Breathing During Stabilization of Preterm Infants at Birth: A Retrospective Analysis,” Frontiers in Pediatrics 5 (2017): 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chettri S., Adhisivam B., and Bhat B. V., “Endotracheal Suction for Nonvigorous Neonates Born Through Meconium Stained Amniotic Fluid: A Randomized Controlled Trial,” Journal of Pediatrics 166, no. 5 (2015): 1208.e1–1213.e1. [DOI] [PubMed] [Google Scholar]
- 50. Nangia S., Sunder S., Biswas R., and Saili A., “Endotracheal Suction in Term Non Vigorous Meconium Stained Neonates—A Pilot Study,” Resuscitation 105 (2016): 79–84. [DOI] [PubMed] [Google Scholar]
- 51. Kumar A., Kumar P., and Basu S., “Endotracheal Suctioning for Prevention of Meconium Aspiration Syndrome: A Randomized Controlled Trial,” European Journal of Pediatrics 178, no. 12 (2019): 1825–1832. [DOI] [PubMed] [Google Scholar]
- 52. Viraraghavan V. R., Nangia S., Prathik B. H., et al., “Yield of Meconium in Non‐Vigorous Neonates Undergoing Endotracheal Suctioning and Profile of all Neonates Born Through Meconium‐Stained Amniotic Fluid: A Prospective Observational Study,” Paediatrics and International Child Health 38, no. 4 (2018): 266–270. [DOI] [PubMed] [Google Scholar]
- 53. Lakshminrusimha S., Mathew B., Nair J., et al., “Tracheal Suctioning Improves Gas Exchange but Not Hemodynamics in Asphyxiated Lambs With Meconium Aspiration,” Pediatric Research 77, no. 2 (2015): 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baskett T. F. and Nagele F., “Bernhard Schultze and the Swinging Neonate,” Resuscitation 51, no. 1 (2001): 3–6. [DOI] [PubMed] [Google Scholar]
- 55. Grundy S. A., Liu S. M., and Davidson A. P., “Intracranial Trauma in a Dog due to Being “Swung”,” Topics in Companion Animal Medicine 24, no. 2 (2009): 100–103. [DOI] [PubMed] [Google Scholar]
- 56. Giussani D. A., Spencer J. A., Moore P. J., et al., “Afferent and Efferent Components of the Cardiovascular Reflex Responses to Acute Hypoxia in Term Fetal Sheep,” The Journal of Physiology 461, no. 1 (1993): 431–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McCarthy L. K., Morley C. J., Davis P. G., et al., “Timing of Interventions in the Delivery Room: Does Reality Compare With Neonatal Resuscitation Guidelines?,” Journal of Pediatrics 163, no. 6 (2013): 1553.e1–1557.e1. [DOI] [PubMed] [Google Scholar]
- 58. Verstegen J. P., Silva L. D., Onclin K., and Donnay I., “Echocardiographic Study of Heart Rate in Dog and Cat Fetuses in Utero,” Journal of Reproduction and Fertility Supplement 47 (1993): 175–180. [PubMed] [Google Scholar]
- 59. Walter L. M., Ahmed B., Odoi A., et al., “Bradycardias Are Associated With More Severe Effects on Cerebral Oxygenation in Very Preterm Infants Than in Late Preterm Infants,” Early Human Development 127 (2018): 33–41. [DOI] [PubMed] [Google Scholar]
- 60. Iglesias B., Rodri Guez M. A. J., Aleo E., et al., “3‐Lead Electrocardiogram Is More Reliable Than Pulse Oximetry to Detect Bradycardia During Stabilisation at Birth of Very Preterm Infants,” Archives of Disease in Childhood: Fetal and Neonatal Edition 103, no. 3 (2018): F233–F237. [DOI] [PubMed] [Google Scholar]
- 61. Katheria A., Rich W., and Finer N., “Electrocardiogram Provides a Continuous Heart Rate Faster Than Oximetry During Neonatal Resuscitation,” Pediatrics 130, no. 5 (2012): e1177–e1181. [DOI] [PubMed] [Google Scholar]
- 62. Murphy M. C., De Angelis L., McCarthy L. K., and O'Donnell C. P. F., “Comparison of Infant Heart Rate Assessment by Auscultation, ECG and Oximetry in the Delivery Room,” Archives of Disease in Childhood: Fetal and Neonatal Edition 103, no. 5 (2018): F490–F492. [DOI] [PubMed] [Google Scholar]
- 63. Saugstad O. D., “Oxygen Saturations Immediately After Birth,” Journal of Pediatrics 148, no. 5 (2006): 569–570. [DOI] [PubMed] [Google Scholar]
- 64. Davies P., Cheng D., Fox A., and Lee L., “The Efficacy of Noncontact Oxygen Delivery Methods,” Pediatrics 110, no. 5 (2002): 964–967. [DOI] [PubMed] [Google Scholar]
- 65. Dawson J. A., Davis P. G., O'Donnell C. P., et al., “Free‐Flow Oxygen Delivery to Newly Born Infants,” Archives of Disease in Childhood: Fetal and Neonatal Edition 92, no. 2 (2007): F132–F134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Saugstad O. D., Rootwelt T., and Aalen O., “Resuscitation of Asphyxiated Newborn Infants With Room Air or Oxygen: An International Controlled Trial: The Resair 2 Study,” Pediatrics 102, no. 1 (1998): e1. [DOI] [PubMed] [Google Scholar]
- 67. Ramji S., Ahuja S., Thirupuram S., et al., “Resuscitation of Asphyxie Newborn Infants With Room Air or 100% Oxygen,” Pediatric Research 34, no. 6 (1993): 809–812. [DOI] [PubMed] [Google Scholar]
- 68. Ramji S., Rasaily R., Mishra P. K., et al., “Resuscitation of Asphyxiated Newborns With Room Air or 100% Oxygen at Birth: A Multicentric Clinical Trial,” Indian Pediatrics 40, no. 6 (2003): 510–517. [PubMed] [Google Scholar]
- 69. Bajaj N., Udani R. H., and Nanavati R. N., “Room Air vs. 100 Per Cent Oxygen for Neonatal Resuscitation: A Controlled Clinical Trial,” Journal of Tropical Pediatrics 51, no. 4 (2005): 206–211. [DOI] [PubMed] [Google Scholar]
- 70. Cone A. M., Nadel S., and Sweeney B., “Flumazenil Reverses Diazepam‐Induced Neonatal Apnoea and Hypotonia,” European Journal of Pediatrics 152, no. 5 (1993): 458–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Richard P., Autret E., Bardol J., et al., “The Use of Flumazenil in a Neonate,” Journal of Toxicology Clinical Toxicology 29, no. 1 (1991): 137–140. [DOI] [PubMed] [Google Scholar]
- 72. Dixon J. C., Speidel B. D., and Dixon J. J., “Neonatal Flumazenil Therapy Reverses Maternal Diazepam,” Acta Paediatrica 87, no. 2 (1998): 225–226. [DOI] [PubMed] [Google Scholar]
- 73. Pike H., Kolstad V., Eilevstjønn J., et al., “Newborn Resuscitation Timelines: Accurately Capturing Treatment in the Delivery Room,” Resuscitation 197 (2024): 110156. [DOI] [PubMed] [Google Scholar]
- 74. Ersdal H. L., Mduma E., Svensen E., and Perlman J. M., “Early Initiation of Basic Resuscitation Interventions Including Face Mask Ventilation May Reduce Birth Asphyxia Related Mortality in Low‐Income Countries: A Prospective Descriptive Observational Study,” Resuscitation 83, no. 7 (2012): 869–873. [DOI] [PubMed] [Google Scholar]
- 75. Boon A. W., Milner A. D., and Hopkin I. E., “Lung Expansion, Tidal Exchange, and Formation of the Functional Residual Capacity During Resuscitation of Asphyxiated Neonates,” Journal of Pediatrics 95, no. 6 (1979): 1031–1036. [DOI] [PubMed] [Google Scholar]
- 76. Holte K., Ersdal H., Eilevstjønn J., et al., “Positive End‐Expiratory Pressure in Newborn Resuscitation Around Term: A Randomized Controlled Trial,” Pediatrics 146, no. 4 (2020): e20200494. [DOI] [PubMed] [Google Scholar]
- 77. Probyn M. E., Hooper S. B., Dargaville P. A., et al., “Positive End Expiratory Pressure During Resuscitation of Premature Lambs Rapidly Improves Blood Gases Without Adversely Affecting Arterial Pressure,” Pediatric Research 56, no. 2 (2004): 198–204. [DOI] [PubMed] [Google Scholar]
- 78. Hooper S. B., Siew M. L., Kitchen M. J., and te Pas A. B., “Establishing Functional Residual Capacity in the Non‐Breathing Infant,” Seminars in Fetal & Neonatal Medicine 18, no. 6 (2013): 336–343. [DOI] [PubMed] [Google Scholar]
- 79. Kim E. H., Cho S. A., Kang P., et al., “Ultrasound‐Guided Esophageal Compression During Mask Ventilation in Small Children: A Prospective Observational Study,” BMC Anesthesiology 22, no. 1 (2022): 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Danilo P. Jr., Rosen M. R., and Hordof A. J., “Effects of Acetylcholine on the Ventricular Specialized Conducting System of Neonatal and Adult Dogs,” Circulation Research 43, no. 5 (1978): 777–784. [DOI] [PubMed] [Google Scholar]
- 81. Goodlin R. C. and Haesslein H. C., “Fetal Reacting Bradycardia,” American Journal of Obstetrics and Gynecology 129, no. 8 (1977): 845–856. [DOI] [PubMed] [Google Scholar]
- 82. Walker A. M., Cannata J. P., Dowling M. H., et al., “Age‐Dependent Pattern of Autonomic Heart Rate Control During Hypoxia in Fetal and Newborn Lambs,” Biology of the Neonate 35, no. 3/4 (1979): 198–208. [DOI] [PubMed] [Google Scholar]
- 83. Lear C. A., Beacom M. J., Dhillon S. K., et al., “Dissecting the Contributions of the Peripheral Chemoreflex and Myocardial Hypoxia to Fetal Heart Rate Decelerations in Near‐Term Fetal Sheep,” The Journal of Physiology 601, no. 10 (2023): 2017–2041. [DOI] [PubMed] [Google Scholar]
- 84. Parer J. T., “The Effect of Atropine on Heart Rate and Oxygen Consumption of the Hypoxic Fetus,” American Journal of Obstetrics and Gynecology 148, no. 8 (1984): 1118–1122. [DOI] [PubMed] [Google Scholar]
- 85. Joyce W. and Wang T., “Regulation of Heart Rate in Vertebrates During Hypoxia: A Comparative Overview,” Acta Physiologica 234, no. 3 (2022): e13779. [DOI] [PubMed] [Google Scholar]
- 86. Yam C. H., Dawson J. A., Schmolzer G. M., et al., “Heart Rate Changes During Resuscitation of Newly Born Infants <30 Weeks Gestation: An Observational Study,” Archives of Disease in Childhood: Fetal and Neonatal Edition 96, no. 2 (2011): F102–F107. [DOI] [PubMed] [Google Scholar]
- 87. Espinoza M. L., Cheung P.‐Y., Lee T.‐F., et al., “Heart Rate Changes During Positive Pressure Ventilation After Asphyxia‐Induced Bradycardia in a Porcine Model of Neonatal Resuscitation,” Archives of Disease in Childhood: Fetal and Neonatal Edition 104, no. 1 (2019): F98–F101. [DOI] [PubMed] [Google Scholar]
- 88. Kibsgaard A., Ersdal H., Kvaløy J. T., et al., “Newborns Requiring Resuscitation: Two Thirds Have Heart Rate ≥100 Beats/Minute in the First Minute After Birth,” Acta Paediatrica 112, no. 4 (2023): 697–705. [DOI] [PubMed] [Google Scholar]
- 89. Vento M., Asensi M., Sastre J., et al., “Six Years of Experience With the Use of Room Air for the Resuscitation of Asphyxiated Newly Born Term Infants,” Biology of the Neonate 79, no. 3‐4 (2001): 261–267. [DOI] [PubMed] [Google Scholar]
- 90. Vento M., Asensi M., Sastre J., et al., “Oxidative Stress in Asphyxiated Term Infants Resuscitated With 100% Oxygen,” Journal of Pediatrics 142, no. 3 (2003): 240–246. [DOI] [PubMed] [Google Scholar]
- 91. Vento M., Sastre J., Asensi M. A., and Viña J., “Room‐Air Resuscitation Causes Less Damage to Heart and Kidney Than 100% Oxygen,” American Journal of Respiratory and Critical Care Medicine 172, no. 11 (2005): 1393–1398. [DOI] [PubMed] [Google Scholar]
- 92. Lakshminrusimha S., Steinhorn R. H., Wedgwood S., et al., “Pulmonary Hemodynamics and Vascular Reactivity in Asphyxiated Term Lambs Resuscitated With 21 and 100% Oxygen,” Journal of Applied Physiology 111, no. 5 (2011): 1441–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Bjorland P. A., Oymar K., Ersdal H. L., and Rettedal S. I., “Incidence of Newborn Resuscitative Interventions at Birth and Short‐Term Outcomes: A Regional Population‐Based Study,” BMJ Paediatrics Open 3, no. 1 (2019): e000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Foglia E. E., Shah B. A., and Szyld E., “Positive Pressure Ventilation at Birth,” Seminars in Perinatology 46, no. 6 (2022): 151623. [DOI] [PubMed] [Google Scholar]
- 95. Kapadia P., Hurst C., Harley D., et al., “Trends in Neonatal Resuscitation Patterns in Queensland, Australia — A 10‐Year Retrospective Cohort Study,” Resuscitation 157 (2020): 126–132. [DOI] [PubMed] [Google Scholar]
- 96. Alhassen Z., Vali P., Lesneski A., et al., “What Is the Optimal Heart Rate Cut‐Off for Initiating Chest Compressions in Neonatal Bradycardia?,” Pediatrics 147 (2021): 753. [Google Scholar]
- 97. Chandrasekharan P., Gugino S., Koenigsknecht C., et al., “Chest Compressions During Neonatal Bradycardia Do Not Enhance Flow to Vital Organs but Reduce Inherent Cardiac Activity,” Pediatrics 149 (2022): 654. [Google Scholar]
- 98. Rettedal S., Kibsgaard A., Kvaløy J. T., et al., “Prevalence of Bradycardia in 4876 Newborns in the First Minute After Birth and Association With Positive Pressure Ventilation: A Population‐Based Cross‐Sectional Study,” Archives of Disease in Childhood: Fetal and Neonatal Edition 109, no. 4 (2024): 371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mortola J. P. and Fisher J. T., “Mouth and Nose Resistance in Newborn Kittens and Puppies,” Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology 51, no. 3 (1981): 641–645. [DOI] [PubMed] [Google Scholar]
- 100. Bruckner M., Neset M., O'Reilly M., et al., “Haemodynamic Changes With Varying Chest Compression Rates in Asphyxiated Piglets,” Archives of Disease in Childhood: Fetal and Neonatal Edition 108, no. 2 (2023): 200–203. [DOI] [PubMed] [Google Scholar]
- 101. Patel S., Cheung P.‐Y., Lee T.‐F., et al., “Asynchronous Ventilation at 120 Compared With 90 or 100 Compressions per Minute Improves Haemodynamic Recovery in Asphyxiated Newborn Piglets,” Archives of Disease in Childhood: Fetal and Neonatal Edition 105, no. 4 (2020): 357–363. [DOI] [PubMed] [Google Scholar]
- 102. Hamrick J. T., Hamrick J. L., Bhalala U., et al., “End‐Tidal CO2‐Guided Chest Compression Delivery Improves Survival in a Neonatal Asphyxial Cardiac Arrest Model,” Pediatric Critical Care Medicine 18, no. 11 (2017): e575–e584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Babbs C. F., Meyer A., and Nadkarni V., “Neonatal CPR: Room at the Top—A Mathematical Study of Optimal Chest Compression Frequency Versus Body Size,” Resuscitation 80, no. 11 (2009): 1280–1284. [DOI] [PubMed] [Google Scholar]
- 104. Reyes‐Martínez M. and Herrería‐Bustillo V. J., “Evaluation of Compressor Fatigue at 150 Compressions per Minute During Cardiopulmonary Resuscitation Using a Large Dog Manikin,” Journal of Veterinary Emergency and Critical Care 33, no. 5 (2023): 495–500. [DOI] [PubMed] [Google Scholar]
- 105. Madar J., Roehr C. C., Ainsworth S., et al., “European Resuscitation Council Guidelines,” Resuscitation 161 (2021): 291–326. [DOI] [PubMed] [Google Scholar]
- 106. Polglase G. R., Brian Y., Tantanis D., et al., “Endotracheal Epinephrine at Standard Versus High Dose for Resuscitation of Asystolic Newborn Lambs,” Resuscitation 198 (2024): 110191. [DOI] [PubMed] [Google Scholar]
- 107. de Jager J., Pothof R., Crossley K. J., et al., “Evaluating the Efficacy of Endotracheal and Intranasal Epinephrine Administration in Severely Asphyxic Bradycardic Newborn Lambs: A Randomised Preclinical Study,” Archives of Disease in Childhood: Fetal and Neonatal Edition 110, no. 2 (2025): 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Vali P., Chandrasekharan P., Rawat M., et al., “Evaluation of Timing and Route of Epinephrine in a Neonatal Model of Asphyxial Arrest,” Journal of the American Heart Association 6, no. 2 (2017): e004402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Barber C. A. and Wyckoff M. H., “Use and Efficacy of Endotracheal Versus Intravenous Epinephrine During Neonatal Cardiopulmonary Resuscitation in the Delivery Room,” Pediatrics 118, no. 3 (2006): 1028–1034. [DOI] [PubMed] [Google Scholar]
- 110. Halling C., Sparks J. E., Christie L., and Wyckoff M. H., “Efficacy of Intravenous and Endotracheal Epinephrine During Neonatal Cardiopulmonary Resuscitation in the Delivery Room,” Journal of Pediatrics 185 (2017): 232–236. [DOI] [PubMed] [Google Scholar]
- 111. Nair J., Vali P., Gugino S. F., et al., “Bioavailability of Endotracheal Epinephrine in an Ovine Model of Neonatal Resuscitation,” Early Human Development 130 (2019): 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Roberts J. R., Greenberg M. I., Knaub M. A., et al., “Blood Levels Following Intravenous and Endotracheal Epinephrine Administration,” Journal of the American College of Emergency Physicians 8, no. 2 (1979): 53–56. [DOI] [PubMed] [Google Scholar]
- 113. Roberts C. T., Klink S., Schmölzer G. M., et al., “Comparison of Intraosseous and Intravenous Epinephrine Administration During Resuscitation of Asphyxiated Newborn Lambs,” Archives of Disease in Childhood: Fetal and Neonatal Edition 107, no. 3 (2022): 311–316. [DOI] [PubMed] [Google Scholar]
- 114. Steele A. M. and Oram J. L., “Catheterization of the Venous Compartment,” in Advanced Monitoring and Procedures for Small Animal Emergency and Critical Care, 2nd ed., ed. Burkitt Creedon J. M. and Davis H. (Wiley Blackwell, 2023), 81–101. [Google Scholar]
- 115. Songstad N. T., Klingenberg C., McGillick E. V., et al., “Efficacy of Intravenous, Endotracheal, or Nasal Adrenaline Administration During Resuscitation of Near‐Term Asphyxiated Lambs,” Frontiers in Pediatrics 8 (2020): 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Berkelhamer S. K., Vali P., Nair J., et al., “Inadequate Bioavailability of Intramuscular Epinephrine in a Neonatal Asphyxia Model,” Frontiers in Pediatrics 10 (2022): 828130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Foglia E. E., Jensen E. A., Wyckoff M. H., et al., “Survival After Delivery Room Cardiopulmonary Resuscitation: A National Registry Study,” Resuscitation 152 (2020): 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Patel H. and Beeby P. J., “Resuscitation Beyond 10 Minutes of Term Babies Born Without Signs of Life,” Journal of Paediatrics and Child Health 40, no. 3 (2004): 136–138. [DOI] [PubMed] [Google Scholar]
- 119. Harrington D. J., Redman C. W., Moulden M., and Greenwood C. E., “The Long‐Term Outcome in Surviving Infants With Apgar Zero at 10 Minutes: A Systematic Review of the Literature and Hospital‐Based Cohort,” American Journal of Obstetrics and Gynecology 196, no. 5 (2007): 463.e1–463.e5. [DOI] [PubMed] [Google Scholar]
- 120. Steiner H. and Neligan G., “Perinatal Cardiac Arrest. Quality of the Survivors,” Archives of Disease in Childhood 50, no. 9 (1975): 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kasdorf E., Laptook A., Azzopardi D., et al., “Improving Infant Outcome With a 10 Min Apgar of 0,” Archives of Disease in Childhood: Fetal and Neonatal Edition 100, no. 2 (2015): F102–F105. [DOI] [PubMed] [Google Scholar]
- 122. Sproat T., Hearn R., and Harigopal S., “Outcome of Babies With no Detectable Heart Rate Before 10 Minutes of Age, and the Effect of Gestation,” Archives of Disease in Childhood: Fetal and Neonatal Edition 102, no. 3 (2017): F262–F265. [DOI] [PubMed] [Google Scholar]
- 123. Shibasaki J., Mukai T., Tsuda K., et al., “Outcomes Related to 10‐min Apgar Scores of Zero in Japan,” Archives of Disease in Childhood: Fetal and Neonatal Edition 105, no. 1 (2020): 64–68. [DOI] [PubMed] [Google Scholar]
- 124. da Mata Fuchs K., Pereira K., Xavier G. M., et al., “Neonatal Hypoglycemia in Dogs—Pathophysiology, Risk Factors, Diagnosis and Treatment,” Frontiers in Veterinary Science 11 (2024): 1345933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Grundy S. A., “Clinically Relevant Physiology of the Neonate,” Veterinary Clinics of North America: Small Animal Practice 36, no. 3 (2006): 443–459. [DOI] [PubMed] [Google Scholar]
- 126. Mila H., Grellet A., Delebarre M., et al., “Monitoring of the Newborn Dog and Prediction of Neonatal Mortality,” Preventive Veterinary Medicine 143 (2017): 11–20. [DOI] [PubMed] [Google Scholar]
- 127. Hollis A. R., Furr M. O., Magdesian K. G., et al., “Blood Glucose Concentrations in Critically Ill Neonatal Foals,” Journal of Veterinary Internal Medicine 22, no. 5 (2008): 1223–1227. [DOI] [PubMed] [Google Scholar]
- 128. Panzardi A., Bernardi M. L., Mellagi A. P., et al., “Newborn Piglet Traits Associated With Survival and Growth Performance Until Weaning,” Preventive Veterinary Medicine 110, no. 2 (2013): 206–213. [DOI] [PubMed] [Google Scholar]
- 129. Sheldon R. A., Partridge J. C., and Ferriero D. M., “Postischemic Hyperglycemia Is Not Protective to the Neonatal Rat Brain,” Pediatric Research 32, no. 4 (1992): 489–493. [DOI] [PubMed] [Google Scholar]
- 130. Vannucci R. C., Rossini A., and Towfighi J., “Effect of Hyperglycemia on Ischemic Brain Damage During Hypothermic Circulatory Arrest in Newborn Dogs,” Pediatric Research 40, no. 2 (1996): 177–184. [DOI] [PubMed] [Google Scholar]
- 131. Salhab W. A., Wyckoff M. H., Laptook A. R., and Perlman J. M., “Initial Hypoglycemia and Neonatal Brain Injury in Term Infants With Severe Fetal Acidemia,” Pediatrics 114, no. 2 (2004): 361–366. [DOI] [PubMed] [Google Scholar]
- 132. Nadeem M., Murray D. M., Boylan G. B., et al., “Early Blood Glucose Profile and Neurodevelopmental Outcome at Two Years in Neonatal Hypoxic‐Ischaemic Encephalopathy,” BMC Pediatrics 11 (2011): 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Basu S. K., Kaiser J. R., Guffey D., et al., “Hypoglycaemia and Hyperglycaemia Are Associated With Unfavourable Outcome in Infants With Hypoxic Ischaemic Encephalopathy: A Post Hoc Analysis of the CoolCap Study,” Archives of Disease in Childhood: Fetal and Neonatal Edition 101, no. 2 (2016): F149–F155. [DOI] [PubMed] [Google Scholar]
- 134. Greif R., Bray J. E., Djärv T., et al., “2024 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations: Summary From the Basic Life Support; Advanced Life Support; Pediatric Life Support; Neonatal Life Support; Education, Implementation, and Teams; and First Aid Task Forces,” Circulation 150, no. 24 (2024): e580–e687. [DOI] [PubMed] [Google Scholar]
- 135. Polderman K. H., “Mechanisms of Action, Physiological Effects, and Complications of Hypothermia,” Critical Care Medicine 37, no. 7 (2009): S186–S202. [DOI] [PubMed] [Google Scholar]
- 136. Perlman J. M., Wyllie J., Kattwinkel J., et al., “Part 11: Neonatal Resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations,” Circulation 122, no. 16 SUPPL. 2 (2010): S516–S538. [DOI] [PubMed] [Google Scholar]
- 137. Lemyre B. and Chau V., “Hypothermia for Newborns With Hypoxic‐Ischemic Encephalopathy,” Paediatrics & Child Health 23, no. 4 (2018): 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Toribio R. E., “Equine Neonatal Encephalopathy: Facts, Evidence, and Opinions,” Veterinary Clinics of North America: Small Animal Practice 35, no. 2 (2019): 363–378. [DOI] [PubMed] [Google Scholar]
- 139. Davidson J. O., Wassink G., van den Heuij L. G., et al., “Therapeutic Hypothermia for Neonatal Hypoxic‐Ischemic Encephalopathy—Where to From Here?,” Frontiers in Neurology 6 (2015): 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Colbourne F. and Corbett D., “Delayed and Prolonged Post‐Ischemic Hypothermia Is Neuroprotective in the Gerbil,” Brain Research 654, no. 2 (1994): 265–272. [DOI] [PubMed] [Google Scholar]
- 141. Clark D. L., Penner M., Orellana‐Jordan I. M., and Colbourne F., “Comparison of 12, 24 and 48 h of Systemic Hypothermia on Outcome After Permanent Focal Ischemia in Rat,” Experimental Neurology 212, no. 2 (2008): 386–392. [DOI] [PubMed] [Google Scholar]
- 142. Agnew D. M., Koehler R. C., Guerguerian A.‐M., et al., “Hypothermia for 24 Hours After Asphyxic Cardiac Arrest in Piglets Provides Striatal Neuroprotection That Is Sustained 10 Days After Rewarming,” Pediatric Research 54, no. 2 (2003): 253–262. [DOI] [PubMed] [Google Scholar]
- 143. Laptook A. R., Corbett R. J. T., Sterett R., et al., “Modest Hypothermia Provides Partial Neuroprotection When Used for Immediate Resuscitation After Brain Ischemia,” Pediatric Research 42, no. 1 (1997): 17–23. [DOI] [PubMed] [Google Scholar]
- 144. Thoresen M., Satas S., Løberg E. M., et al., “Twenty‐Four Hours of Mild Hypothermia in Unsedated Newborn Pigs Starting After a Severe Global Hypoxic‐Ischemic Insult Is Not Neuroprotective,” Pediatric Research 50, no. 3 (2001): 405–411. [DOI] [PubMed] [Google Scholar]
- 145. Lu X., Ma L., Sun S., et al., “The Effects of the Rate of Postresuscitation Rewarming Following Hypothermia on Outcomes of Cardiopulmonary Resuscitation in a Rat Model,” Critical Care Medicine 42, no. 2 (2014): e106–e113. [DOI] [PubMed] [Google Scholar]
- 146. Berger C., Xia F., Köhrmann M., and Schwab S., “Hypothermia in Acute Stroke‐Slow Versus Fast Rewarming. An Experimental Study in Rats,” Experimental Neurology 204, no. 1 (2007): 131–137. [DOI] [PubMed] [Google Scholar]
- 147. Suehiro E., Ueda Y., Wei E. P., et al., “Posttraumatic Hypothermia Followed by Slow Rewarming Protects the Cerebral Microcirculation,” Journal of Neurotrauma 20, no. 4 (2003): 381–390. [DOI] [PubMed] [Google Scholar]
- 148. Suehiro E. and Povlishock J. T., “Exacerbation of Traumatically Induced Axonal Injury by Rapid Posthypothermic Rewarming and Attenuation of Axonal Change by Cyclosporin A,” Journal of Neurosurgery 94, no. 3 (2001): 493–498. [DOI] [PubMed] [Google Scholar]
- 149. Bisschops L. L. A., Hoedemaekers C. W. E., Mollnes T. E., and van der Hoeven J. G., “Rewarming After Hypothermia After Cardiac Arrest Shifts the Inflammatory Balance,” Critical Care Medicine 40, no. 4 (2012): 1136–1142. [DOI] [PubMed] [Google Scholar]
- 150. Zhu S.‐Z., Gu Y., Wu Z., et al., “Hypothermia Followed by Rapid Rewarming Exacerbates Ischemia‐Induced Brain Injury and Augments Inflammatory Response in Rats,” Biochemical and Biophysical Research Communications 474, no. 1 (2016): 175–181. [DOI] [PubMed] [Google Scholar]
- 151. Smarick S. D., Haskins S. C., Boller M., et al., “RECOVER Evidence and Knowledge Gap Analysis on Veterinary CPR. Part 6: Post‐Cardiac Arrest Care,” Journal of Veterinary Emergency and Critical Care 22, no. Suppl 1 (2012): S85–S101. [DOI] [PubMed] [Google Scholar]
- 152. Davidson J. O., Wassink G., Draghi V., et al., “Limited Benefit of Slow Rewarming After Cerebral Hypothermia for Global Cerebral Ischemia in Near‐Term Fetal Sheep,” Journal of Cerebral Blood Flow and Metabolism 39, no. 11 (2019): 2246–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Wang B., Armstrong J. S., Lee J.‐H., et al., “Rewarming From Therapeutic Hypothermia Induces Cortical Neuron Apoptosis in a Swine Model of Neonatal Hypoxic‐Ischemic Encephalopathy,” Journal of Cerebral Blood Flow and Metabolism 35, no. 5 (2015): 781–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Wang B., Armstrong J. S., Reyes M., et al., “White Matter Apoptosis Is Increased by Delayed Hypothermia and Rewarming in a Neonatal Piglet Model of Hypoxic Ischemic Encephalopathy,” Neuroscience 316 (2016): 296–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Draghi V., Wassink G., Zhou K. Q., et al., “Differential Effects of Slow Rewarming After Cerebral Hypothermia on White Matter Recovery After Global Cerebral Ischemia in Near‐Term Fetal Sheep,” Scientific Reports 9, no. 1 (2019): 10142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. McDouall A., Zhou K. Q., Davies A., et al., “Slow Rewarming After Hypothermia Does Not Ameliorate White Matter Injury After Hypoxia‐Ischemia in Near‐Term Fetal Sheep,” Pediatric Research 97, no. 3 (2025): 1209–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Thoresen M. and Whitelaw A., “Cardiovascular Changes During Mild Therapeutic Hypothermia and Rewarming in Infants With Hypoxic‐Ischemic Encephalopathy,” Pediatrics 106, no. 1 Pt 1 (2000): 92–99. [DOI] [PubMed] [Google Scholar]
- 158. Battin M., Bennet L., and Gunn A. J., “Rebound Seizures During Rewarming,” Pediatrics 114, no. 5 (2004): 1369–1369. [DOI] [PubMed] [Google Scholar]
- 159. Yost C. S., “A New Look at the Respiratory Stimulant Doxapram,” CNS Drug Reviews 12, no. 3‐4 (2006): 236–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Holladay J. R., “Routine Use of Doxapram Hydrochloride in Neonatal Pups Delivered by Cesarean Section,” Veterinary Medicine, Small Animal Clinician 66, no. 1 (1971): 28. [PubMed] [Google Scholar]
- 161. Wilborn R. R., “Small Animal Neonatal Health,” Veterinary Clinics of North America: Small Animal Practice 48, no. 4 (2018): 683–699. [DOI] [PubMed] [Google Scholar]
- 162. Miller C. J., McKiernan B. C., Pace J., and Fettman M. J., “The Effects of Doxapram Hydrochloride (Dopram‐V) on Laryngeal Function in Healthy Dogs,” Journal of Veterinary Internal Medicine 16, no. 5 (2002): 524–528. [DOI] [PubMed] [Google Scholar]
- 163. Tobias K. M., Jackson A. M., and Harvey R. C., “Effects of Doxapram HCl on Laryngeal Function of Normal Dogs and Dogs With Naturally Occurring Laryngeal Paralysis,” Veterinary Anaesthesia and Analgesia 31, no. 4 (2004): 258–263. [DOI] [PubMed] [Google Scholar]
- 164. Hyndman T. H., Fretwell S., Bowden R. S., et al., “The Effect of Doxapram on Survival and APGAR Score in Newborn Puppies Delivered by Elective Caesarean: A Randomized Controlled Trial,” Journal of Veterinary Pharmacology and Therapeutics 46, no. 6 (2023): 353–364. [DOI] [PubMed] [Google Scholar]
- 165. Balıkcı E. and Yıldız A., “Effects on Arterial Blood Gases and Some Clinical Parameters of Caffeine, Atropine Sulphate or Doxapram Hydrochloride in Calves With Neonatal Asphyxia,” Revue de Medecine Veterinaire 160, no. 6 (2009): 282–287. [Google Scholar]
- 166. Yamazaki T., Kajiwara M., Itahashi K., and Fujimura M., “Low‐Dose Doxapram Therapy for Idiopathic Apnea of Prematurity,” Pediatrics International 43, no. 2 (2001): 124–127. [DOI] [PubMed] [Google Scholar]
- 167. Uehara H., Yoshioka H., Nagai H., et al., “Doxapram Accentuates White Matter Injury in Neonatal Rats Following Bilateral Carotid Artery Occlusion,” Neuroscience Letters 281, no. 2‐3 (2000): 191–194. [DOI] [PubMed] [Google Scholar]
- 168. Bleul U. and Bylang T., “Effects of Doxapram, Prethcamide and Lobeline on Spirometric, Blood Gas and Acid‐Base Variables in Healthy New‐Born Calves,” Veterinary Journal 194, no. 2 (2012): 240–246. [DOI] [PubMed] [Google Scholar]
- 169. Flint R., Halbmeijer N., Meesters N., et al., “Retrospective Study Shows That Doxapram Therapy Avoided the Need for Endotracheal Intubation in Most Premature Neonates,” Acta Paediatrica 106, no. 5 (2017): 733–739. [DOI] [PubMed] [Google Scholar]
- 170. Giguere S., Sanchez L. C., Shih A., et al., “Comparison of the Effects of Caffeine and Doxapram on Respiratory and Cardiovascular Function in Foals With Induced Respiratory Acidosis,” American Journal of Veterinary Research 68, no. 12 (2007): 1407–1416. [DOI] [PubMed] [Google Scholar]
- 171. Allweiler S., “Anesthesia for Small Animal Cesarean Surgery and Neonatal Resuscitation,” Clinical Theriogenology 14, no. 3 (2022): 170–173. [Google Scholar]
- 172. Chan W.‐W., Chen K.‐Y., Liu H., et al., “Acupuncture for General Veterinary Practice,” Journal of Veterinary Medical Science 63, no. 10 (2001): 1057–1062. [DOI] [PubMed] [Google Scholar]
- 173. Janssens L., Altman S., and Rogers P. A., “Respiratory and Cardiac Arrest Under General Anaesthesia: Treatment by Acupuncture of the Nasal Philtrum,” The Veterinary Record 105, no. 12 (1979): 273–276. [DOI] [PubMed] [Google Scholar]
- 174. Skarda R. T., “Anesthesia Case of the Month,” Journal of the American Veterinary Medical Association 214, no. 1 (1999): 37–39. [PubMed] [Google Scholar]
- 175. Wyckoff M., Garcia D., Margraf L., et al., “Randomized Trial of Volume Infusion During Resuscitation of Asphyxiated Neonatal Piglets,” Pediatric Research 61, no. 4 (2007): 415–420. [DOI] [PubMed] [Google Scholar]
- 176. Mendler M. R., Schwarz S., Hechenrieder L., et al., “Successful Resuscitation in a Model of Asphyxia and Hemorrhage to Test Different Volume Resuscitation Strategies. A Study in Newborn Piglets After Transition,” Frontiers in Pediatrics 6 (2018): 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Sankaran D., Lane E. C. A., Valdez R., et al., “Role of Volume Replacement During Neonatal Resuscitation in the Delivery Room,” Children 9, no. 10 (2022): 1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Antończyk A., Ochota M., and Niżański W., “Umbilical Cord Blood Gas Parameters and Apgar Scoring in Assessment of New‐Born Dogs Delivered by Cesarean Section,” Animals 11, no. 3 (2021): 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Yeh P., Emary K., and Impey L., “The Relationship Between Umbilical Cord Arterial pH and Serious Adverse Neonatal Outcome: Analysis of 51,519 Consecutive Validated Samples,” BJOG: An International Journal of Obstetrics & Gynaecology 119, no. 7 (2012): 824–831. [DOI] [PubMed] [Google Scholar]
- 180. Lorain P., Bower A., Gottardi E., et al., “Risk Factors for Hypoxic‐Ischemic Encephalopathy in Cases of Severe Acidosis: A Case‐Control Study,” Acta Obstetricia et Gynecologica Scandinavica 101, no. 4 (2022): 471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Lokesh L., Kumar P., Murki S., and Narang A., “A Randomized Controlled Trial of Sodium Bicarbonate in Neonatal Resuscitation—Effect on Immediate Outcome,” Resuscitation 60, no. 2 (2004): 219–223. [DOI] [PubMed] [Google Scholar]


