Abstract
Symbioses with gut microorganisms provides a means by which terrestrial herbivores are able to obtain energy. These microorganisms ferment cell wall materials of plants to short-chain fatty acids (SCFA), which are then absorbed and used by the host animal. Many marine herbivorous fishes contain SCFA (predominantly acetate) in their hindgut, indicative of gut microbial activity, but rates of SCFA production have not been measured. Such information is an important prerequisite to understanding the contribution that gut microorganisms make in satisfying the energy needs of the fish. We have estimated the rates of acetate production in the gut of three species of temperate marine herbivorous fish from northeastern New Zealand: Kyphosus sydneyanus (family Kyphosidae), Odax pullus (family Odacidae), and Aplodactylus arctidens (family Aplodactylidae). Ex vivo preparations of freshly caught fish were maintained with their respiratory and circulatory systems intact, radiolabeled acetate was injected into ligated hindgut sections, and gut fluid was sampled at 20-min intervals for 2 h. Ranges for acetate turnover in the hindguts of the studied species were determined from the slope of plots as the log of the specific radioactivity of acetate versus time and pool size, expressed on a nanomole per milliliter per minute basis. Values were 450 to 570 (K. sydneyanus), 373 to 551 (O. pullus), and 130 to 312 (A. arctidens). These rates are comparable to those found in the guts of herbivorous reptiles and mammals. To determine the contribution of metabolic pathways to the fate of acetate, rates of sulfate reduction and methanogenesis were measured in the fore-, mid-, and hindgut sections of the three fish species. Both rates increased from the distal to proximal end of the hindgut, where sulfate reduction accounted for only a small proportion (<5%) of acetate methyl group transformed to CO2, and exceeded methanogenesis from acetate by >50-fold. When gut size was taken into account, acetate uptake from the hindgut of the fish species, determined on a millimole per day per kilogram of body weight basis, was 70 (K. sydneyanus), 18 (O. pullus), and 10 (A. arctidens).
Marine herbivorous fish harbor a great diversity of gut microorganisms (1, 9-11, 15, 23, 33, 40, 43, 45). Despite their importance in terms of microbial biodiversity, the role of these microorganisms in the digestive processes of the host fish is not well understood. In contrast, it is well known that most terrestrial vertebrate herbivores contain populations of symbiotic organisms that play a key role in digestion by breaking down plant cell walls (cellulose and hemicellulose) to simple compounds such as short-chain fatty acids (SCFA). The SCFA are then taken up by the host and are used for energy generation and biosynthesis (44).
The algae eaten by marine herbivorous fishes are phylogenetically diverse. Unlike terrestrial plants, algae are supported by water and have a lower proportion of structural elements to cell contents (8). Consequently, it is not surprising that algae differ considerably from terrestrial plants in their chemical composition (38). Despite this, studies on digestion and uptake in marine herbivorous fishes have tended to extrapolate from analogies with terrestrial herbivores without taking into account the differences in alimentary structure, the biochemical composition of the diet, and the inorganic composition of the gut contents (8).
Presently our knowledge of the digestive processes in herbivorous fish is based mainly on studies of gut anatomy and pH (24), descriptions of SCFA-producing microbial isolates and their fermentations (33-35), and measurements of SCFA in the gut contents of various species (13-16). There have also been limited studies on SCFA uptake from fish gut lumen (46-48), in which net movement of acetate into the blood was demonstrated, and a single study of the metabolism of whole assemblages of the gut microbiota (42). The ability of fishes to utilize acetate as an energy source via acetyl-coenzyme A synthetase was demonstrated by Clements et al. (13). The data on uptake, combined with estimates of SCFA concentrations in the gut contents of different species of herbivorous fish, indicate that SCFA could be an important source of energy to marine herbivorous fishes (15). However, to date only a single estimate of SCFA production in herbivorous fish is available, and this involved indirect extrapolation from measurements of the enzymatic activities of gut endosymbionts (42). Indeed, the only direct measurements of SCFA contribution to total energy intake in ectothermic vertebrates as a whole are on herbivorous reptiles, where values range from 30% (iguana) to 100% (turtle) (5).
In this paper we identify and quantify the contribution of gut microbiota to energy metabolism by determining the kinetics of production and utilization of SCFA in the gut and the rates of uptake of SCFA across the gut wall in three species of temperate marine herbivorous fishes from New Zealand. This has been achieved through studies on turnover of SCFA and by measuring rates of terminal anaerobic processes in the gut. The results are discussed in relation to the contribution of gut microbiota to the energy metabolism of herbivorous fishes.
MATERIALS AND METHODS
Species and sites.
The study species were the butterfish Odax pullus (family Odacidae), the marblefish Aplodactylus arctidens (family Aplodactylidae), and the silver drummer Kyphosus sydneyanus (family Kyphosidae). All three species are exclusively herbivorous as adults and feed on a range of phaeophyte, chlorophyte, and rhodophyte algae (7, 12, 16). Specimens were collected from coastal reefs in the Hauraki Gulf, situated in the vicinity of Leigh (174°48′E, 36°20′S), on the northeastern coast of New Zealand. Fish were speared in close proximity to the research vessel so that the time from capture to start of experiment was minimized.
Experimental gut preparations.
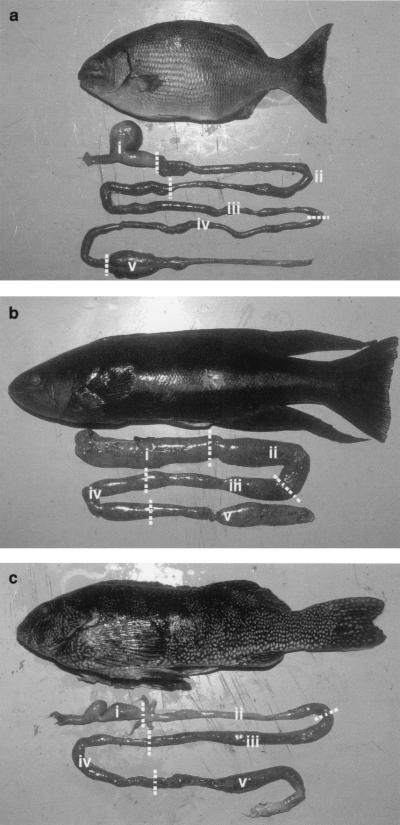
To determine biochemical activities of different regions, the gut was allocated into five sections, following the guidelines of Clements and Choat (16) (Fig. 1). For O. pullus, which lacks a stomach (10), the gut was divided into five sections of equal length. For A. arctidens, the stomach was defined as segment I and the intestine was divided equally into segments II through V. For K. sydneyanus, the stomach was defined as segment I and the hindgut chamber, a swelling at the distal end of the intestine separated from the proximal intestine by a sphincter (16, 40), was defined as segment V. The intestine proximal to segment V in K. sydneyanus was split into three sections of equal length, which were designated segments II to IV.
FIG. 1.
Photographs of K. sydneyanus (a), O. pullus (b), and A. arctidens (c) showing gut anatomy and designation of gut segments.
For SCFA turnover measurements, wild, free-feeding fish were speared through the dorsal musculature or the caudal peduncle with fine-gauge spears to minimize damage. Fish were brought aboard the research vessel and immediately euthanatized by pithing. Only fish in which the circulatory system remained largely intact and functional following spearing and pithing were used for experiments. The fish was then placed upside down and secured in a cut sponge immersed in a seawater bath, with the underbelly exposed. A seawater hose was inserted into the mouth to allow irrigation of the gills throughout the experiment so that oxygen supply to the blood was maintained. The belly was rinsed with distilled water and the gastrointestinal tract was exposed, enabling the posterior gut sections (IV or V) to be tied off without damaging the vascular system. For studies on terminal anaerobic processes, guts were removed intact from freshly speared specimens and placed on a plastic tray for sampling.
Incubation and sampling techniques.
For studies on SCFA turnover in the gut, labeled [2-14C]acetate (0.2 ml, 55 mCi mmol−1, 25 mCi ml−1) was injected into the tied off section of the gut and the contents carefully massaged to ensure even distribution of label throughout the entire gut section. Gut fluid (duplicate 1-ml samples) was removed at 20-min intervals over a 2-h time period with a syringe with a 16-gauge needle. Samples were transferred to 1-ml plastic centrifuge tubes, sealed, and then quick frozen in liquid nitrogen. For studies on methanogenesis, gut content samples (4 ml) were removed from sections of gut with a sawed-off 4-ml plastic syringe and were transferred to Balch tubes (26.5 ml) under a 70% N2-30% CO2 gas stream. Tubes were sealed with butyl septum stoppers and were incubated at ambient seawater temperature (17 to 23°C). Studies on sulfate reduction were carried out by incubating 2 ml of gut contents in sawed-off plastic syringes (3.0 ml) sealed with butyl septum stoppers. Two microcuries of Na235SO4 (100 mCi mmol −1, 10 μCi ml−1) was injected into each sample, shaken to evenly distribute the label, and incubated under the same conditions used for measuring methanogenesis.
For incubations in which metabolism of acetate into methane and CO2 was measured, [2-14C]acetate (0.2 ml, 51 mCi mmol−1, 25 μCi ml−1) was added via syringe to butyl septum-stoppered 10-ml flat-bottomed glass vials, each containing 4 ml of gut contents under a 70% N2-30% CO2 gas mixture. Samples were incubated at ambient seawater temperature. The vials contained a glass center tube for CO2 capture in NaOH. Incubations were terminated by adding 2.5 ml of 3 N NaOH to the center tubes, immediately followed by addition of 0.2 ml of 50% H2SO4 to the contents. Vials were stored for 2 h before analysis to enable complete absorption of CO2.
Analysis of radioactive incubations.
Analysis of the radiolabeled gases methane and carbon dioxide from [2-14C]acetate incubations was carried out as previously described (36). This entailed removal of gas from terminated incubations after gas equilibration with a gas-tight syringe and counting of 14CH4 in sealed scintillation vials, followed by removal of the center tube and counting of an aliquot of NaOH containing trapped 14CO2 in toluene-based scintillant. Analysis of sulfate reduction and determination of rates was carried out by measuring H235S produced from 35SO42− as previously described (36). For analysis of incubations carried out to measure turnover rate, frozen gut samples were thawed and centrifuged (10,000 × g at 4°C for 12 min), and the supernatants were passed through a filter (0.45 μm) into high-performance liquid chromatography vials. SCFA were separated on a Shimadzu high-performance liquid chromatography system on an Alltech C-18 column at 28°C with a flow rate of 0.5 ml min−1 and were detected at 210 nm. The eluate peak corresponding to acetate was collected. Standards showed that >90% of the label could be recovered. Radioactivity of collected acetate was determined by liquid scintillation counting (36).
Analysis of nonradioactive incubations.
Methane and hydrogen were detected by gas chromatographic procedures as previously described (36). SCFA were detected by gas chromatographic procedures following centrifugation of gut contents (6,000 × g for 20 min at 2°C) and acidification of the supernatant. Separation was achieved on a Scientific Glass Engineering Ltd. BP-21 column (0.53 mm internal diameter, 2 m length) at 80°C with a nitrogen carrier gas flow of 3 ml min−1 in a Hewlett Packard (model 5890) gas chromatograph equipped with a flame-ionization detector.
Experimental design and data analysis.
SCFA production, methane production, sulfate reduction, and the contribution of acetate methyl carbon to methane and CO2 were determined with single-factor randomization block experiments with replicate fish. Turnover rate constants (k) were determined from the slope (m) of semilogarithmic plots of the decrease in specific radioactivity of the SCFA versus time (30). Rates of SCFA production were determined as [SCFA]av (mean concentration of SCFA × k, expressed as the mean ± one standard deviation in nanomoles per milliliter per minute).
Chemicals.
All chemicals were of reagent grade and were obtained from commercial sources. The radioisotopes [2-14C]acetate (51 mCi mmol−1) and Na235SO4 (100 mCi mmol −1) were obtained from the Radiochemical Center, Amersham, England.
RESULTS
Composition of gut contents.
The dry-weight range for digesta in the proximal and posterior gut contents of the three fish species is shown in Table 1. In the foregut (section I), the dry weight of gut contents ranged from 9.5% (O. pullus) to 20% (K. sydneyanus) of the total weight, and visual observations showed that undigested seaweed was the major component. With increasing gut length the dry-weight composition decreased so that in the penultimate and distal gut sections it amounted to between 7 and 9% of the total gut content weight.
TABLE 1.
Percentage of dry weight of gut contents in anterior and posterior gut sections of the three fish species
| Gut section | % Dry weighta
|
||
|---|---|---|---|
| K. sydneyanus | O. pullus | A. arctidens | |
| I | 20.0 ± 4.4 | 9.5 ± 1.2 | 13.8 ± 1.7 |
| IV | 6.7 ± 0.7 | 7.7 ± 1.1 | 9.2 ± 2.8 |
| V | 7.5 ± 0.9 | 7.5 ± 0.9 | 7.7 ± 1.2 |
For the various gut sections of each study species, n = 3.
Methane production and sulfate reduction.
Methanogenesis and sulfate reduction were determined in each gut section in the various study species. With few exceptions methanogenesis and sulfate reduction increased in rate from the proximal to distal gut sections. Highest rates were observed in the penultimate and distal gut portions but did not exceed 7.8 pmol ml−1 min−1 (sulfate reduction) or 0.7 pmol ml−1 min−1 (methanogenesis). With few exceptions rates of sulfate reduction exceeded those of methanogenesis, although the extent of this depended upon the fish species and gut section. There was considerable variation in rates of sulfate reduction between individuals of the same species.
SCFA levels in the gut.
Levels of the SCFAs acetate, propionate, and butyrate increased with gut section from the proximal to the distal end (Table 2). Levels of individual SCFAs were highest to lowest in the order acetate > propionate > butyrate for all species and for all gut sections. This was represented by the ratio acetate:propionate:butyrate in sections I to V of 37:13:2 (K. sydneyanus), 20:4:1 (O. pullus), and 8:3:1 in A. arctidens. Between gut sections there was little change in SCFA ratios; however, the elevated values in the hindgut sections were consistent with more elevated microbial activities compared to those for foregut sections.
TABLE 2.
Levels of SCFA in various sections of the gut of the three study species
| Species | Gut section | Level of SCFA (mM)a
|
||
|---|---|---|---|---|
| Acetate | Propionate | Butyrate | ||
| K. sydneyanus | I | 1.1 | 0.0 | 0.0 |
| II | 1.2 ± 0.4 | 0.3 ± 0.1 | 0.2 ± 0.1 | |
| III | 11.2 ± 0.7 | 2.7 ± 0.2 | 0.5 ± 0.1 | |
| IV | 37.4 ± 5.6 | 13.9 ± 0.3 | 2.1 ± 0.3 | |
| V | 37.5 ± 7.5 | 12.8 ± 0.8 | 1.3 ± 0.3 | |
| O. pullus | I | 4.9 ± 0.3 | 0.3 ± 0.1 | 0.2 ± 0.1 |
| II | 7.6 ± 1.6 | 0.2 ± 0.1 | 0.1 | |
| III | 20.3 ± 6.0 | 4.8 ± 2.3 | 1.6 ± 0.1 | |
| IV | 18.5 ± 0.9 | 4.3 ± 0.9 | 0.6 ± 0.1 | |
| V | 20.8 ± 0.5 | 4.7 ± 1.1 | 1.0 ± 0.1 | |
| A. arctidens | I | 1.4 ± 0.5 | 0.3 ± 0.1 | 0.1 |
| II | 1.7 ± 0.4 | 0.3 ± 0.1 | 0.1 | |
| III | 3.3 ± 0.8 | 0.7 ± 0.2 | 0.4 ± 0.1 | |
| IV | 7.6 ± 0.9 | 2.3 ± 0.4 | 1.3 ± 0.5 | |
| V | 8.3 ± 0.6 | 4.1 ± 0.7 | 1.3 ± 0.2 | |
n ≥ 3. Values are means ± one standard deviation (where the error is presented). No error is presented for values that are ≤0.1 mM.
Turnover rate.
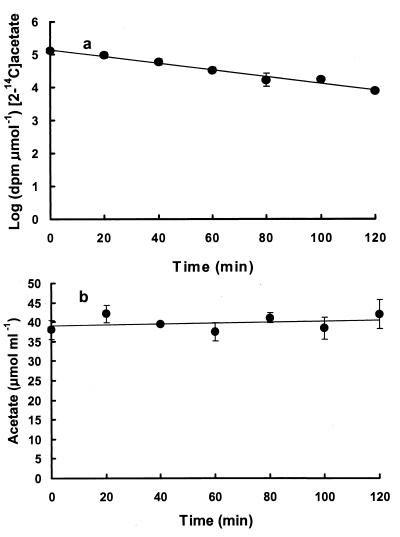
Since SCFA measurements suggested microbial activity was highest in gut sections IV and V, determinations on turnover of acetate were carried out on these sections. Furthermore, there were difficulties with preparations of anterior gut sections for turnover studies because of physical problems of access (i.e., damaging the gut vascular system by exposing the less superficial gut sections). For sections IV and V of all three fish species semilogarithmic plots of specific radioactivity of acetate versus time were linear, such as is shown in Fig. 2 for K. sydneyanus. Under conditions of constant pool size, the turnover rate constant (k) is equal to 2.303 times the slope (m) of the plots. For all three species of fish, the pool size of acetate did not vary appreciably during the time course of experiments (Table 3 and Fig. 2b), enabling calculations of the turnover rate constant. The rates of acetate turnover were determined by multiplying the turnover rate constants by pool sizes. Mean turnover of acetate (nanomole per milliliter per minute) in sections IV and V were 569 and 448 (K. sydneyanus), 551 and 373 (O. pullus), and 312 and 130 (A. arctidens). Thus, for gut sections IV and V turnover decreased in the order K. sydneyanus > O. pullus > A. arctidens, and in all three species rates for section IV exceeded those for section V.
FIG. 2.
Plots of (a) log of decrease in specific activity of [2-14C]acetate versus time in gut section V of K. sydneyanus and (b) mean concentrations of acetate over the same time intervals at ambient seawater temperature (19°C). Values are means of duplicate determinations ± one standard deviation.
TABLE 3.
Determination of turnover rates for acetate in hindmost gut sections IV and V of the three fish species
| Species | Gut section | Expta | Temp (°C) | Turnover constantb (min−1) | Mean pool sizec (μmol ml−1) | Acetate turnoverd (nmol ml−1 min−1) |
|---|---|---|---|---|---|---|
| K. sydneyanus | IV | 1 | 19 | 0.0051 | 55.2 ± 7.6 | 281 |
| 2 | 22.5 | 0.015 | 29.2 ± 4.1 | 438 | ||
| 3 | 16 | 0.02 | 32.2 ± 4.6 | 655 | ||
| 4 | 18.5 | 0.019 | 38.9 ± 8.6 | 747 | ||
| 5 | 18.5 | 0.027 | 26.9 ± 7.6 | 724 | ||
| V | 1 | 19 | 0.0145 | 42.7 ± 3.9 | 619 | |
| 2 | 22.5 | 0.0063 | 44.3 ± 7.4 | 274 | ||
| 3 | 16 | 0.0162 | 27.6 ± 1.5 | 450 | ||
| 4 | 18.5 | 0.0107 | 42.0 ± 4.2 | 452 | ||
| O. pullus | IV | 1 | 20 | 0.04 | 18.6 ± 3.8 | 749 |
| 2 | 20 | 0.036 | 14.5 ± 3.4 | 525 | ||
| 3 | 16 | 0.028 | 13.3 ± 2.3 | 381 | ||
| V | 1 | 20 | 0.021 | 5.9 ± 1.1 | 130 | |
| 2 | 20 | 0.046 | 10.8 ± 2.5 | 497 | ||
| 3 | 18 | 0.025 | 19.7 ± 8.5 | 492 | ||
| A. arctidens | IV | 1 | 19 | 0.0256 | 10.1 ± 4.2 | 259 |
| 2 | 16 | 0.0246 | 14.9 ± 7.6 | 365 | ||
| V | 1 | 22 | 0.0064 | 18.1 ± 4.9 | 116 | |
| 2 | 19 | 0.0069 | 20.3 ± 4.9 | 140 | ||
| 3 | 16 | 0.0126 | 10.6 ± 4.8 | 134 |
In each experiment samples were removed from the gut section in duplicate at various intervals over 120 min (n = 5 and 4 for sections IV and V of K. sydneyanus and 3 for the same gut sections in O. pullus and A. arctidens, respectively).
Determined as the slope of plots of log specific activity [2-14C]acetate versus time (see Fig. 2) × 2.303.
Means are for samples taken in duplicate over the time course of the experiment (120 min) ± one standard deviation.
Determined as the pool size versus turnover rate constant.
Carbon partitioning between methane and CO2 from [2-14C]acetate metabolism and contribution of sulfate reduction and methanogenesis to acetate turnover.
Incubation of [2-14C]acetate with gut contents obtained from sections IV and V of all three fish species resulted in the incorporation of label mainly into CO2, with only a small proportion to methane (Table 4). The total amount of CO2 and methane produced from labeled acetate was calculated from the disintegrations per minute of the gases produced linearly over time (usually 20 to 30 h) on the assumption that the specific activity was the same as that of starting acetate. For all three fish species the rate of CO2 production from the methyl group of acetate exceeded methanogenesis by a factor of ≥50 and contributed to <1% of acetate turnover. Sulfate reduction never exceeded 10 pmol ml−1 min−1, and assuming coupling as in the equation CH3COO− + SO42− → 2HCO3− + HS−, it would have accounted for only a small proportion (<5%) of CO2 produced from acetate. Thus, acetate was mainly oxidized via alternative pathways. Since these account for only a small proportion of the acetate turned over, it can be concluded that most of the acetate produced was not degraded in the gut system.
TABLE 4.
Proportions of acetate methyl group converted to methane and CO2 and comparison of acetate oxidation with turnover
| Species | Gut section | Methane production from acetate (pmol ml−1 min−1)a | CO2 from acetate oxidation (pmol ml−1min−1)a | Mean turnover of acetate (nmol ml−1 min−1)b | % Acetate methyl oxidation of turnoverc |
|---|---|---|---|---|---|
| K. sydneyanus | IV | 3.10 ± 0.40 | 3,883 ± 634 | 569 | 0.68 |
| V | 1.10 ± 0.47 | 2,826 ± 635 | 448 | 0.63 | |
| O. pullus | IV | 0.92 ± 0.50 | 1,675 ± 252 | 551 | 0.30 |
| V | 4.10 ± 0.15 | 198 ± 17 | 373 | 0.05 | |
| A. arctidens | IV | 1.37 ± 0.50 | 2,425 ± 154 | 312 | 0.77 |
| V | 0.14 ± 0.03 | 903 ± 383 | 130 | 0.69 |
Determined after 21 to 48 h of incubation from disintegrations per minute and assuming the specific radioactivity was the same as that for acetate at the beginning of the experiment.
Values are means obtained from Table 3.
Rate of acetate methyl carbon oxidation/turnover × 100.
Estimation of acetate uptake rate from the hindgut of study species.
Table 5 shows the weight range for the fish species examined together with the measured weight range of hindgut sections. Weight was found to be equivalent to volume for both hindgut sections for the three study species (slope, 0.96 to 1.06; intercept, ∼0; correlation coefficient, r ≥ 0.98). Since microbial degradation of acetate in the gut sections accounted for <1% of the acetate produced, the rate of acetate uptake across the gut was assumed to be equivalent to the turnover rate and was calculated by multiplying the gut volume by the turnover rate. On this basis, rates of absorption expressed as millimoles per day were substantially higher for K. sydneyanus than for the other two species, even when body weight is taken into account. With the exception of K. sydneyanus, uptake in section IV exceeded that of section V. Uptake from the hindgut (sections IV and V) on a millimole per day per kilogram of body weight basis decreased in the order K. sydneyanus, 70; O. pullus, 18; A. arctidens, 10.
TABLE 5.
Estimation of acetate uptake in hindgut sections of the fish species
| Species | Weight range (g)a | Gut section | Weight range of gut section (g)a | Acetate turnover (nmol ml−1 min−1)b | Rate of absorption (mmol day−1)c | Acetate available for energy (mmol day−1 kg−1) |
|---|---|---|---|---|---|---|
| K. sydneyanus | 2,050-4,200 | IV | 78-160 | 570 | 64-131 | 32 |
| V | 117-238 | 448 | 75-153 | 37 | ||
| O. pullus | 800-1,500 | IV | 11-21 | 551 | 9-17 | 11 |
| V | 11-21 | 373 | 6-11 | 7 | ||
| A. arctidens | 741-2,880 | IV | 10-37 | 312 | 5-17 | 7 |
| V | 10-37 | 130 | 2-7 | 3 |
n = 16 (K. sydneyanus), 43 (O. pullus), or 38 (A. arctidens).
Values are means of turnovers determined for the relevant gut sections in Table 3.
Determined from gut weight × turnover rate, in which the assumption is that 1 g of weight of gut is equivalent to 1 cm3.
Levels of radioactive acetate in the blood.
At the conclusion of some turnover experiments, levels of radioactivity in acetate were measured in the blood by sampling the caudal vein and the heart. The results (Table 6) compare the specific radioactivities of acetate in the blood with that for acetate in the gut at the beginning of the experiments. They show that the specific radioactivities were roughly comparable, indicating that absorption had taken place. Determination of total recovery of label in host tissues was beyond the design of the experiment.
TABLE 6.
Comparison of specific radioactivity of acetate incorporated into the vascular system of host fish during turnover experiments with that in the gut lumen at the start of incubation
| Species | Gut fraction | Host system | Acetatea (μmol ml−1) | Labela ([dpm ml−1] × 104) | Specific activity ([dpm μmol−1] × 104) |
|---|---|---|---|---|---|
| K. sydneyanus | IV | Gut lumen | 32.0 | 204.12 | 6.4 |
| Blood | 1.9 | 3.04 | 1.6 | ||
| V | Gut lumen | 40.2 | 86.84 | 2.1 | |
| Blood | 1.7 | 0.99 | 0.6 | ||
| O. pullus | IV | Gut lumen | 10.7 | 63.40 | 5.9 |
| Blood | 1.5 (1.1) | 0.45 (2.17) | 0.3 (1.9) | ||
| A. arctidens | V | Gut lumen | 20.2 | 148.65 | 7.4 |
| Blood | 0.7 | 1.47 | 2.1 |
Values for the gut were determined at the beginning of the turnover experiments. Those for the blood were determined at the end of turnover experiments (2 h) for samples taken from the caudal vein (no parentheses) or from the heart (parentheses). The corresponding turnover experiment results depicted in Table 3 are 3 (section IV) and 2 (section V) (K. sydneyanus), 3 (O. pullus), and 2 (A. arctidens).
DISCUSSION
The present study is the first to quantify fermentation rates in the hindgut of marine herbivorous fishes. In all three of our study fish species acetate was turned over in the gut at rates comparable to those found in terrestrial vertebrates and amphibians (Table 7), despite the much lower body temperature of these fishes (17 to 23°C). This contradicts suggestions (27) that elevated temperatures are required for well-developed fermentation systems in marine herbivorous fishes. The high fermentation rates measured in this study were unexpected in fishes living in temperate waters, and they suggest that symbiotic microbial processes in these fishes are more important than we previously thought.
TABLE 7.
Comparison of rates for acetate turnover in hindguts of marine herbivorous fin fish and those of other vertebrates
| Animal | Gut section | Acetate turnover (nmol ml−1 min−1) | % Maintenance energy requirementa | Reference |
|---|---|---|---|---|
| Sheep | Rumen | 383 | 23 | 25 |
| Pig | Cecum | 483-550 | 12 | 26 |
| Wombat | Hindgut | 100-267 | 30 | 2 |
| Possum | Hindgut | 316 | 16 | 21 |
| Green iguana | Hindgut | 41 | ND | 28 |
| Lizard | Hindgut | 407 | 47 | 22 |
| Marine turtle | Cecum | 150 | ND | 4 |
| Freshwater turtle | Small intestine | 132 | ND | 6 |
| K. sydneyanus | Hindgut | 450-570 | ND | This paper |
| O. pullus | Hindgut | 373-551 | ND | This paper |
| A. arctidens | Hindgut | 130-312 | ND | This paper |
ND, not determined.
The ex vivo preparations used for the acetate turnover experiments in this study were a compromise between in vitro experiments and in vivo experiments. Although in vitro experiments have been widely used to estimate fermentation rates in gut systems (for examples see reference 22), they do not allow the estimation of SCFA uptake from the gut. Experiments on free-swimming fish are impractical due to the difficulties associated with capture and postoperative stress in large, active, aquatic organisms. Furthermore, the complex gut coiling characteristic of marine herbivorous fishes (see reference 15) makes it very difficult to accurately insert a cannula through the body wall without first opening the gut cavity (i.e., in a living fish). Our ex vivo preparations had the advantage of maintaining vascular circulation to the gut throughout, as evidenced by the disappearance of labeled acetate from the lumen and the constant acetate pool size. As a check on the viability of our preparations we monitored the color of the gills and the mesenteric circulation for the duration of the experiments. Both of these indicated the presence of oxygenated blood. Furthermore, when we collected arterial blood from the heart at the conclusion of some experiments the heart was still beating.
The substrates for acetate production in our study fish species are likely to be seaweed carbohydrates that are not assimilated in the anterior intestine. In O. pullus, which as an adult feeds mainly on phaeophytes (12), the predominant substrates in the gut will be laminarin, mannitol, and alginic acid (38). In A. arctidens, which feeds on rhodophytes and chlorophytes (7), the main polysaccharides present in the gut will be starch, floridean starch, carrageenan, agar, and a variety of other complex polymers (38). K. sydneyanus feeds on a wide variety of phaeophyte, chlorophyte, and rhodophyte species (16, 40), and so will contain a combination of the seaweed constituents present in the other two fish species. At present almost nothing is known about the fate of algal substrates along the gut of marine herbivorous fishes and their use by gastrointestinal microbes. Vertebrates are thought to lack an active transport mechanism for mannitol (29, 41), and since this sugar alcohol is the primary photosynthate of phaeophytes, it is present in large quantities in these algae (38). Seeto et al. (42) demonstrated that the gut flora in the Australian herbivorous fish species Odax cyanomelas had a high capacity for the utilization of mannitol, and given the above concluded that mannitol was an important substrate for fermentation. Algal cell wall polysaccharides such as alginic acid and carrageenan are likely to be resistant to endogenous fish enzymes, and even algal storage compounds such as starch and floridean starch can be highly resistant to fish amylase (51). Thus, there is potentially a huge range of algal substrates which could be fermented to acetate in the gut of these fishes.
Previous workers have suggested that specialized intestinal morphology is required for efficient hindgut fermentation (for examples see reference 27). Of our study species only K. sydneyanus could be said to fulfill this requirement. The hindgut chamber (i.e., segment V in our study) in this species is separated from the proximal intestine by a muscular sphincter (40), which presumably regulates the flow of digesta. It is interesting that fermentation rates were as high or higher in the segment of intestine anterior to the hindgut chamber (i.e., our segment IV), showing that fermentation reactions are by no means limited to this structure. Furthermore, the rates of fermentation estimated in our other two study species, both of which have simple, undifferentiated hindguts, argue against the requirement for specialized alimentary morphology. Given this, the function of the hindgut chamber in kyphosids remains unclear. The higher daily yield of acetate to K. sydneyanus compared to that of the other two fish species is largely the result of hindgut volume relative to body size, not differences in fermentation rates or gut architecture per se.
While rates of sulfate reduction and methanogenesis increased along the gut from the distal to proximal ends, these were very low in comparison with acetate turnover, indicating that neither process played a significant role in the gut metabolism of acetate. Among the factors which could have limited these processes are redox, pH, sulfate concentration, and gut throughput time. However, low redox levels which would be conducive to methanogenesis have been reported in several species closely related to those in this study (13), and with the possible exception of O. pullus the gut pH of these species (50) would be unlikely to preclude the growth of methanogens. Levels of sulfate in the gastrointestinal tract (≥20 mM) are unlikely to be limiting to sulfate reduction, as rates much higher than those reported here have been achieved in other anaerobic environments at much lower levels of the acceptor and at similar temperatures (31, 32, 49). One possibility that could explain the low rates is gut throughput time. Mesophilic sulfate reducers and methanogens have doubling times for growth ranging from 3 to 4 h for hydrogen utilizers to near 20 h for SCFA utilizers (49). With few exceptions temperate marine herbivorous fishes are thought to have gut retention times of 20 to 50 h (3, 17, 40). This may be too rapid to allow the establishment and growth of these microbial species during passage through the gut and may explain why sulfate reduction and methanogenesis accounted for only a small proportion of the acetate utilized.
Other than sulfate reduction and methanogenesis, a process which could have contributed to acetate oxidation is the production of hydrogen and carbon dioxide by acetogens (19). However, since total acetate oxidizing and cleavage processes accounted for <1% of acetate turnover, these processes were not further investigated.
The appearance of labeled acetate in the vascular system in which specific radioactivities were of the same order as those of the fatty acid in the gut lumen at the beginning of the turnover experiments demonstrated that acetate was absorbed across the gut wall. A precise determination of the recovery of label was not possible, however, because of the likely incorporation into various tissues from the bloodstream and metabolism to CO2. The findings that only a small amount of label was lost through microbial activity in the gut (e.g., sulfate reduction and methanogenesis) and that the pool size was relatively constant is also consistent with acetate being mainly absorbed across the gut wall. Thus, fermentation reactions in the hindgut of these fishes appear to be efficient in terms of conversion of seaweed substrates to energy in a form that can be used by the host fish.
The energy made available through acetate uptake would almost certainly represent only part of the total energy for the host from SCFA, since it is likely that other SCFA, such as propionate and butyrate, also present in the gut of these fishes (13, 16, and this study) are also absorbed. There is also the possibility for uptake of SCFA in the fore- and midgut sections, although on the basis of SCFA profiles (Table 2) it would seem unlikely that these would be major sites for acetate turnover.
It is not yet possible to determine the contribution of acetate to the total energy needs of host fish. To do so would require accurate information on gut throughput times and the calorific value of food ingested per day, both of which are very difficult to estimate for wild fish. One possibility is to raise fish in captivity, but this can cause fish stress and loss of gut flora (20, 39), which may compromise gut function and lead to difficulties in the interpretation of results. Another problem with experiments in captivity is replicating the wild diets of fishes that feed on a wide variety of dietary algal species.
In conclusion, there is little doubt from our results that acetate produced by microbial fermentation constitutes an important source of energy to the host, and while similarities can be drawn with terrestrial herbivores, some important differences are apparent. Among the more obvious are diet and temperature. In terms of temperature it is of interest that acetate turnover in the hindgut of the study fish species is similar to or higher than the turnover found in the guts of endothermic mammals (Table 7). This suggests that these temperate herbivorous fishes have efficient mechanisms for fermentation coupled to seaweed digestion. In terms of diet the differences with terrestrial herbivores may be even more apparent. In the better-studied terrestrial plant/herbivore relationships plant composition can be discussed in terms of fiber, where fiber may be defined as material resistant to endogenous digestion (18). In contrast, marine algae contain a complex array of cell wall and storage polysaccharides, and the relationship of these to fish digestive processes is poorly understood (8). Herbivorous fish may have adapted to these complex algal diets in several ways, including high food intake and gut throughput rates, optimization of key digestive enzymes, and various degrees of reliance on microbial processes in the gut. At present we are unable to resolve these possibilities for any given fish species, but these aspects are the subject of ongoing research.
Acknowledgments
We gratefully acknowledge Garth Cooper, Tony Roberton, and Henry Kaspar for helpful advice and comments; Brady Doak, Murray Birch, and Isabel Pasch for help on board the research vessel Proteus; Damian Moran and Lindsey Zemke-White for help with fish collection; and John Montgomery for helping develop the ex vivo technique. Thanks are also expressed to Henry Kaspar and Howard Choat for their help in preparing the grant with which this work was funded.
The work was supported by a Marsden grant from the Royal Society of New Zealand.
REFERENCES
- 1.Angert, E. R., K. D. Clements, and N. R. Pace. 1993. The largest bacterium. Nature 362:239-241. [DOI] [PubMed] [Google Scholar]
- 2.Barboza, P. S., and I. D. Hume. 1992. Hindgut fermentation in wombats: two marsupial grazers. J. Comp. Physiol. Biochem. 162B:561-566. [DOI] [PubMed] [Google Scholar]
- 3.Benavides, A. G., J. M. Cancino, and F. P. Ojeda. 1994. Ontogenetic changes in gut dimensions and macroalgal digestibility in the marine herbivorous fish Aplodactylus punctatus. Funct. Ecol. 8:46-51. [Google Scholar]
- 4.Bjorndal, K. A. 1979. Cellulose digestion and volatile fatty acid production in the green turtle Chelonia mydas. Comp. Biochem. Physiol. 63A:127-133. [Google Scholar]
- 5.Bjorndal, K. A. 1997. Fermentation in reptiles and amphibians, p. 199-228. In R. I. Mackie and B. A. White (ed.), Gastrointestinal microbiology, vol. 1. Gastrointestinal ecosystems and fermentations. Chapman and Hall, New York, N.Y. [Google Scholar]
- 6.Bjorndal, K. A., and A. B. Bolten. 1990. Digestive processing in a herbivorous freshwater turtle: consequences of small intestine fermentation. Physiol. Zool. 63:1232-1247. [Google Scholar]
- 7.Choat, J. H., and K. D. Clements. 1992. Diet in odacid and aplodactylid fishes from Australia and New Zealand. Aust. J. Mar. Freshwater Res. 43:1451-1459. [Google Scholar]
- 8.Choat, J. H., and K. D. Clements. 1998. Vertebrate herbivores in marine and terrestrial environments: a nutritional ecology perspective. Annu. Rev. Ecol. Syst. 29:375-403. [Google Scholar]
- 9.Clements, K. D., D. C. Sutton, and J. H. Choat. 1989. Occurrence and characteristics of unusual protistan symbionts from surgeonfishes (Acanthurideae) of the Great Barrier Reef, Australia. Mar. Biol. 102:403-412. [Google Scholar]
- 10.Clements, K. D. 1991. Endosymbiotic communities of two herbivorous labroid fishes Odax cyanomelas and Odax pullus. Mar. Biol. 106:223-229. [Google Scholar]
- 11.Clements, K., and S. Bullivant. 1991. An unusual symbiont from the gut of surgeonfishes may be the largest known procaryote. J. Bacteriol. 173:5359-5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clements, K. D., and J. H. Choat. 1993. Influence of season, ontogeny and tide on the diet of the temperate marine herbivorous fish Odax pullus (Odacidae). Mar. Biol. 117:213-220. [Google Scholar]
- 13.Clements, K. D., V. P. Gleeson, and M. Slaytor. 1994. Short chain fatty acid metabolism in temperate marine herbivorous fish. J. Comp. Physiol. B 164:372-377. [Google Scholar]
- 14.Clements, K., and H. Choat. 1995. Fermentation in tropical marine herbivorous fishes. Physiol. Zool. 68:355-378. [Google Scholar]
- 15.Clements, K. D. 1997. Fermentation and gastrointestinal microorganisms in fishes, p. 156-198. In R. I. Mackie and B. A. White (ed.), Gastrointestinal microbiology, vol. 1. Gastrointestinal ecosystems and fermentations. Chapman and Hall, New York, N.Y. [Google Scholar]
- 16.Clements, K. D., and J. H. Choat. 1997. Comparison of herbivory in the closely related marine fish genera Girella and Kyphosus. Mar. Biol. 127:579-586. [Google Scholar]
- 17.Clements, K. D., and D. Rees. 1998. Preservation of inherent contractility in isolated gut segments from herbivorous and carnivorous marine fish. J. Comp. Physiol. B 168:61-72. [Google Scholar]
- 18.Cummings, J. H., and G. T. Macfarlane. 1991. The control and consequences of bacterial fermentation in the human colon. J. Appl. Bacteriol. 70:443-459. [DOI] [PubMed] [Google Scholar]
- 19.Dolfing, J. 1988. Acetogenesis, p. 417-468. In A. J. B. Zehnder (ed.), Biology of anaerobic organisms. John Wiley and Sons, New York, N.Y.
- 20.Fishelson, L., W. L. Montgomery, and A. A. Myrberg. 1985. A unique symbiosis in the gut of tropical herbivorous surgeonfish (Acanthuridae: teleostei) from the Red Sea. Science 229:49-51. [DOI] [PubMed] [Google Scholar]
- 21.Foley, W. J., I. D. Hume, and S. J. Cork. 1989. Fermentation in the hindgut of the greater glider (Petauroides volans) and the brushtail possum (Trichosurus vulpecula), two arboreal folivores. Physiol. Zool. 62:1126-1143. [Google Scholar]
- 22.Foley, W. J., A. Bouskila, A. Shkolnik, and I. Chosniak. 1992. Microbial digestion in the herbivorous lizard Uromastyx aegyptius (Agamidae). J. Zool. (Lond.) 226:387-398. [Google Scholar]
- 23.Grim, J. N. 1993. Description of the somatic kinetics and vestibular organisation of Balantidium jocularum sp. nov. and possible taxonomic implications for the class Litostomatea and the genus Balantium. Acta Protozool. 32:37-41. [Google Scholar]
- 24.Horn, M. H. 1989. Biology of marine herbivorous fishes. Oceanogr. Mar. Biol. Annu. Rev. 27:167-272. [Google Scholar]
- 25.Hume, I. D. 1977. Production of volatile fatty acids in two species of wallaby and in sheep. Comp. Biochem. Physiol. 56A:299-304. [Google Scholar]
- 26.Imoto, S., and S. Namioka. 1978. VFA metabolism in the pig. J. Anim. Sci. 47:479-487. [DOI] [PubMed] [Google Scholar]
- 27.Kandel, J. S., M. H. Horn, and W. Van Antwerp. 1995. Volatile fatty acids in the hindguts of herbivorous fishes from temperate and tropical marine waters. J. Fish Biol. 45:527-529. [Google Scholar]
- 28.McBee, R. H., and V. H. McBee. 1982. The hindgut fermentation of the green iguana, Iguana iguana, p. 77-83. In G. M. Burghardt and A. S. Rand (ed.), Iguanas of the world: their behavior, ecology and conservation. Noyes, Parke Ridge, N.Y.
- 29.Moroshita, Y. 1994. The effect of dietary mannitol on the caecal microflora and short-chain fatty acids in rats. Lett. Appl. Microbiol. 18:27-29. [Google Scholar]
- 30.Mountfort, D. O., and R. A. Asher. 1978. Changes in the proportions of acetate and carbon dioxide used as methane precursors during the anaerobic digestion of bovine waste. Appl. Environ. Microbiol. 35:648-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mountfort, D. O., R. A. Asher, E. L. Mays, and J. M. Tiedje. 1980. Carbon and electron flow in mud and sandflat intertidal sediments at Delaware Inlet, Nelson, New Zealand. Appl. Environ. Microbiol. 39:686-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mountfort, D. O., and R. A. Asher. 1981. Role of sulfate reduction versus methanogenesis in terminal carbon flow in polluted intertidal sediment of Waimea Inlet, Nelson, New Zealand. Appl. Environ. Microbiol. 42:252-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mountfort, D. O., and L. L. Rhodes. 1991. Anaerobic growth and fermentation characteristics of Paecilomyces lilacinus isolated from mullet gut. Appl. Environ. Microbiol. 57:1963-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mountfort, D. O., W. D. Grant, H. Morgan, F. Rainey, and E. Stackebrandt. 1993. Isolation and characterization of an obligately anaerobic, pectinolytic member of the genus Eubacterium from mullet gut. Arch. Microbiol. 159:289-295. [Google Scholar]
- 35.Mountfort, D. O., F. A. Rainey, J. Burghardt, and E. Stackebrandt. 1994. Clostridium grantii sp. nov., a new obligately anaerobic alginolytic bacterium isolated from mullet gut. Arch. Microbiol. 162:173-179. [DOI] [PubMed] [Google Scholar]
- 36.Mountfort, D. O., H. F. Kaspar, M. Downes, and R. A. Asher. 1999. Partitioning during carbon and electron flow in sediments of a low salinity meltwater pond near Bratina Island, McMurdo Ice Shelf, Antarctica. Appl. Environ. Microbiol. 65:5493-5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oremland, R. S. 1988. Biogeochemistry of methanogenic bacteria, p. 641-706. In A. J. B. Zehnder (ed.), Biology of anaerobic microorganisms. John Wiley and Sons Inc., New York, N.Y.
- 38.Painter, T. J. 1983. Algal polysaccharides, p. 196-285. In G. O. Aspinall (ed.), The polysaccharides, vol. 2. Academic Press, London, United Kingdom. [Google Scholar]
- 39.Pankhurst, N. W., and G. Van der Kraak. 1997. Effects of stress on reproduction and growth of fish, p. 73-93. In G. K. Iwama, A. D. Pickering, J. P. Sumpter, and C. B. Schreck (ed.), Fish stress and health in aquaculture. Cambridge University Press, Cambridge, United Kingdom.
- 40.Rimmer, D. W., and W. J. Wiebe. 1987. Fermentative microbial digestion in herbivorous fishes. J. Fish Biol. 31:229-236. [Google Scholar]
- 41.Saunders, D. R., and H. S. Wiggins. 1981. Conservation of mannitol, lactulose and raffinose by the human colon. Am. J. Physiol. 241:G397-G402. [DOI] [PubMed] [Google Scholar]
- 42.Seeto, G. S., P. C. Veivers, K. D. Clements, and M. Slaytor. 1996. Carbohydrate utilisation by microbial symbionts in the marine herbivorous fishes Odax cyanomelas and Crinodus lophodon. J. Comp. Physiol. B 165:571-579. [Google Scholar]
- 43.Sogin, M. L. 1993. Giants among procaryotes. Nature 362:207. [DOI] [PubMed] [Google Scholar]
- 44.Stevens, C. E., and I. D. Hume. 1995. Comparative physiology of the vertebrate digestive system, 2nd ed. Cambridge University Press, Cambridge, United Kingdom.
- 45.Sutton, D. C., and K. D. Clements. 1988. Aerobic heterotrophic gastrointestinal microflora of tropical marine fishes, p. 185-190. In J. H. Choat et al. (ed.), Proceedings of the 6th International Coral Reef Symposium, vol. 3. International Coral Reef Committee, Townsville, Australia. [Google Scholar]
- 46.Titus, E., and G. A. Ahearn. 1988. Short-chain fatty acid transport in the intestine of a herbivorous teleost. J. Exp. Biol. 135:77-94. [DOI] [PubMed] [Google Scholar]
- 47.Titus, E., and G. A. Ahearn. 1991. Transintestinal acetate transport in a herbivorous teleost: anion exchange at the basolateral membrane. J. Exp. Biol. 156:41-61. [Google Scholar]
- 48.Titus, E., and G. A. Ahearn. 1992. Vertebrate gastro-intestinal fermentation: transport mechanisms for volatile fatty acids. Am. J. Physiol. 262:R574.. [DOI] [PubMed] [Google Scholar]
- 49.Widdel, F. 1988. Microbiology and ecology of sulphate and sulfur-reducing bacteria, p. 469-585. In A. J. B Zehnder (ed.), Biology of anaerobic microorganisms. John Wiley and Sons, New York, N.Y.
- 50.Zemke-White, W. L., K. D. Clements, and P. J. Harris. 1999. Acid lysis of macro-algae by marine herbivorous fishes: myth or digestive mechanism. J. Exp. Mar. Biol. Ecol. 233:95-113. [Google Scholar]
- 51.Zemke-White, W. L., and K. D. Clements. 1999. Chlorophyte and rhodophyte starches as factors in diet choice by marine herbivorous fish. J. Exp. Mar. Biol. Ecol. 240:137-149. [Google Scholar]