Abstract
Metabolic dysfunction-associated steatotic liver disease (MASLD) is the hepatic manifestation of metabolic syndrome. Hepatic lipotoxicity and inflammation are two key factors driving progression of steatosis to metabolic dysfunction-associated steatohepatitis (MASH). The presence of MASH increases the risk of cardiovascular events, cirrhosis, hepatocellular carcinoma (HCC) and non-liver malignancies. Although MASLD and lipid species have been extensively examined in persons with type 2 diabetes, much less is known in type 1 diabetes. We examined the association of key lipid species with MASLD in individuals with type 1 diabetes. We designed a cross-sectional study of 30 participants with type 1 diabetes recr1uited from our institutional diabetes clinics. All participants had fasting blood drawn for targeted lipidomics and underwent a FibroScan. Those with steatosis score of ≥ 248 as determined by controlled attenuation parameter (CAP) were categorized as cases (n = 17); those with steatosis score < 248 were categorized as controls (n = 13). BMI was significantly higher in cases than controls (P = 0.0007) and used significantly higher 24-h insulin doses than controls (P = 0.004). Cases displayed significantly higher circulating levels of total ceramides (P = 0.02), diacylglycerols (P = 0.0009) and triacylglycerols (P = 0.0004). The two groups displayed similar levels of hexosylceramides, dihydrosphingomyelins, sphingomyelins, and phosphatidylcholines. Similar to previous findings, numerous sphingolipids species, diacylglycerols, and triacylglycerols were found to correlate positively with higher BMI and 24-h insulin dose. Total circulating dihydroceramides, ceramides, diacylglycerols, and triacylglycerols levels significantly correlated with steatosis score (P < 0.05). None of the lipid species correlated with fibrosis score. These results suggest that persons with type 1 diabetes and MASLD have a higher BMI, are likely to be insulin resistant, and display elevated circulating levels of dihydroceramides, ceramides, diacylglycerols, and triacylglycerols, which are strongly associated with the pathogenesis of steatotic liver disease.
Keywords: Fatty liver, Steatosis, Diabetes mellitus, Type 1, Cross-sectional study, Insulin resistance, Body mass index
Subject terms: Endocrinology, Endocrine system and metabolic diseases, Biochemistry, Lipidomics
Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a spectrum of chronic liver disorders comprising steatosis, metabolic dysfunction-associated steatohepatitis (MASH), and cirrhosis. MASH-related cirrhosis is the second leading cause of liver transplant in the U.S1.. The prevalence of MASLD in adults with type 1 diabetes (T1D) is estimated at 22%2. MASLD is due to multiple, complex metabolic processes in the liver, which are not completely understood. The combination of increased dietary intake of saturated fats, dysfunctional fatty acid metabolism in peripheral and visceral adipocytes and insufficient fatty acid oxidation in the liver, results in free fatty acids and sphingolipids accumulation in the liver3. The resultant lipotoxicity in the liver leads to insulin resistance, activation of sterol regulatory element binding protein-1c (SREBP-1c) transcription factor, which regulates lipogenic genes and promotes fatty acid synthesis in the liver4, hepatocellular injury in MASH5, production of reactive oxygen species (ROS), mitochondrial and lysosomal dysfunction, endoplasmic reticulum stress and apoptosis6,7. Some individuals with steatosis who do not develop MASH may have protective mechanisms against the effects of lipotoxicity8.
Alterations of hepatic sphingolipid metabolism leads to ceramides accumulation, which may play a role in the progression of steatosis to MASH, by inducing cell stress and cell death9. Ceramides have been linked to hepatic insulin resistance, and they inhibit several mediators of the insulin signaling pathway, such as insulin receptor substrate 1, phosphatidylinositol 3-kinase, and Akt10. Kartsoli et al. reported that lipid intermediates such as ceramides and diacylglycerols are more likely to cause hepatic insulin resistance than other lipid species, which contributes to increased occurrence of MASLD11. This study also showed that phosphatidylcholines, a major component of cell membranes, is reduced in MASLD, impairs the synthesis and secretion of very low-density lipoprotein (VLDL), and leads to increased triglycerides accumulation in the liver. Perakakis et al. reported that serum lipids such as phosphatidylcholines, sphingomyelins, ceramides, and diacylglycerols were predictive of the presence of liver steatosis, MASH, and significant fibrosis12.
We investigated the role of various lipid species such as ceramides, dihydroceramides, phosphatidylcholines, diacylglycerols, and triacylglycerols, in the development of insulin resistance and MASLD in persons with T1D. We expected to show that ceramides, dihydroceramides, diacylglycerols and triacylglycerols are elevated in participants with T1D and FibroScan evidence of MASLD.
Participants and study design
This study was approved by the University of Iowa Institutional Review Board (approval #: 202,405,130) and all participants provided informed consent. We conducted a cross-sectional study of 30 participants with T1D recruited from the University of Iowa diabetes and endocrine clinics, from 2021 to 2022. All methods were carried out in accordance with relevant guidelines and regulations. Study eligibility included age ≥ 18 and history of T1D with duration > 5 years. T1D was confirmed by evidence of either undetectable C-peptide or positive anti-glutamic acid decarboxylase antibody or a typical history of T1D determined by an experienced diabetologist/endocrinologist. Exclusion criteria included alcohol consumption > 20 g/day in women or 30 g/day in men, hepatitis B or C infection, autoimmune liver disorders or other known metabolic causes of chronic liver disease such as hemochromatosis and Wilson’s disease, current pregnancy or lactation, medications known to cause steatosis, or illicit drug use. Each participant attended a single visit after fasting a minimum of 8 h, in our Institutional Clinical Research Unit (CRU). The study visit included plasma drawn for targeted liquid chromatography-tandem mass spectrometry (LC–MS/MS) lipidomics analysis and FibroScan (transient elastography), to assess liver fat and fibrosis.
FibroScan (Echosens, Waltham, MA) was performed with either M or XL probes13. A liver stiffness score in kilopascal (kPa) was determined from the measurement of shear wave propagation and used as a quantitative index of liver fibrosis stage, ranging from absent (stage F0-1, score < 8.2 kPa) through severe fibrosis (stage F4, 13.6 kPa)14. A controlled attenuation parameter (CAP) score in decibels per meter (dB/m) was determined and used as a quantitative index of hepatic steatosis, ranging from absent (S0, < 248), mild (S1, 248–267), moderate (S2, 268–279) to severe (S3, > 280)15.
Fasting blood samples were processed by cold centrifuge for 15 min at a speed of 3,000xg. Extracted plasma was stored at − 80 °C, until LC–MS/MS lipidomics analysis was performed on all samples as one batch (Supplementary Data).
Statistical analyses were conducted using GraphPad Prism version 9. Data was checked for normality using the Shapiro–Wilk test, with p-value > 0.05 was considered normally distributed. Spearman correlation analysis was performed to analyze the correlation between lipid species and clinical parameters. The between-group difference for lipids was evaluated using Wilcoxon rank sum. Values for lipid species are presented as medians with inter-quartile ranges. P-values were adjusted using the Benjamini–Hochberg false discovery rate (FDR) correction method.
To evaluate the ability of BMI and selected lipid species to discriminate between case and control groups, receiver operating characteristic (ROC) curve analyses were conducted. The area under the ROC curve (AUC) was calculated for each individual variable, and pairwise comparisons between AUCs were performed using DeLong’s test for correlated ROC curves. All analyses were conducted using R (version 4.2.0) with the pROC package, and a two-sided p-value < 0.05 was considered statistically significant.
Given the exploratory nature of the study, a priori sample size calculation was performed. However, a post hoc power analysis based on the observed differences in triglycerides, diglycerides, and ceramide levels indicated power values ranging from 0.72 to 0.80 depending on the variable.
Results
Clinical characteristics of all participants are presented in Table 1. Among the 30 participants, 17 were categorized as cases (steatosis score ≥ 248) and 13 as controls (steatosis score < 248). Both groups had similar age distributions (P = 0.65). BMI was significantly higher in cases than controls (P = 0.0007). Cases used higher 24-h insulin doses than controls (P = 0.004). There was no difference in hemoglobin A1c (HbA1c) between groups (P = 0.094).
Table 1.
Participant characteristics. Data show 1median with interquartile range and Mean (SD). Values represent number of participants.
| Controls (n = 13)1 | Cases (n = 17)1 | P-value | |
|---|---|---|---|
| Age | 38 (33.0–49.0) | 41 (29–66.5) | 0.65 |
| Gender (M/F) | 6/7 | 9/8 | |
| BMI | 27.6 (5.0) | 36.5 (7.1) | 0.0007 |
| Fibrosis score | 4.1 (3.7–7.2) | 5.9 (5.5–9.1) | 0.0006 |
| Fibrosis stage | |||
|
F0-F1 F2 F3 F4 |
13 0 0 0 |
13 1 2 1 |
|
| Steatosis score | 211.5 (190.5–217.3) | 319.5 (277.5–355.0) | < 0.0001 |
| Steatosis stage | |||
|
S0 S1 S2 S3 |
13 0 0 0 |
0 4 3 10 |
|
| HbA1c | 7.1 (6.1–7.5) | 7.6 (6.8–7.9) | 0.094 |
| 24-h insulin dose | 45.5 (34.3–60) | 75.0 (54–127.8) | 0.004 |
P < 0.01.
Plasma sphingolipids levels
To determine the relationship between circulating sphingolipids and FibroScan findings, we compared plasma sphingolipid levels quantified with LC–MS/MS between cases and controls. Dihydroceramides (dhCer), ceramides (Cer), hexosylceramides (HexCer), dihydrosphingomyelins (dhSM), sphingomyelins (SM), phosphatidylcholines, (PC) diacylglycerols (DG), and triglycerides (TG) were assayed. Sphingomyelin species C16:0, C18:0, C20:0, C22:0, C24:0, and C24:1 were detected. Seven species of phosphatidylcholines were detected (C34:1, C34:2, C36:0, C36:1, C36:2, C36:3, C36:4). Cases showed significantly higher circulating Cer, DG, and TG levels (Table 2). DhCer and dhSM trended higher in cases but this trend was not statistically significant. No significant difference was detected between groups in SM or HexCer levels.
Table 2.
Comparison of lipid species between controls and cases.
| Lipid species (nmol/ml) | Control [Median (interquartile range)] (n = 13) | Cases [Median (interquartile range)] (n = 17) | p-value | FDR |
|---|---|---|---|---|
| Dihydroceramides | ||||
| dhCer 18:0;O2/16:0 | 0.030 (0.0026–0.031) | 0.032 (0.025–0.043) | 0.05 | 0.15 |
| dhCer 18:0;O2/18:0 | 0.013 (0.010–0.017) | 0.015 (0.011–0.024) | 0.14 | 0.23 |
| dhCer 18:0;O2/20:0 | 0.007 (0.006–0.010) | 0.010 (0.008–0.015) | 0.04 | 0.15 |
| dhCer 18:0;O2/22:0 | 0.032 (0.024–0.038) | 0.042 (0.031–0.073) | 0.05 | 0.15 |
| dhCer 18:0;O2/24:0 | 0.045 (0.036–0.054) | 0.059 (0.042–0.089) | 0.04 | 0.15 |
| dhCer 18:0;O2/24:1 | 0.029 (0.019–0.043) | 0.035 (0.027–0.064) | 0.11 | 0.21 |
| Total dihydro ceramides | 0.152 (0.127–0.198) | 0.202 (0.148–0.310) | 0.06 | 0.15 |
| Ceramides | ||||
| Cer 18:1;O2/16:0 | 0.25 (0.22–0.31) | 0.28 (0.26–0.34) | 0.10 | 0.21 |
| Cer 18:1;O2/18:0 | 0.07 (0.05–0.08) | 0.08 (0.07–0.11) | 0.02 | 0.12 |
| Cer 18:1;O2/20:0 | 0.07 (0.05–0.07) | 0.08 (0.07–0.10) | 0.01 | 0.12 |
| Cer 18:1;O2/22:0 | 0.82 (0.75–0.92) | 1.04 (0.78–1.34) | 0.06 | 0.15 |
| Cer 18:1;O2/24:0 | 2.2 (2.04–2.56) | 2.77 (2.40–3.56) | 0.02 | 0.12 |
| Cer 18:1;O2/24:1 | 0.83 (0.67–1.19) | 1.1 (0.84–1.15) | 0.14 | 0.23 |
| Total Ceramides | 4.54 (3.89–4.88) | 5.41 (4.57–6.81) | 0.02 | 0.12 |
| Dihydrosphingomyelins | ||||
| dhSM 18:0;O2/16:0 | 4.82(4.04–5.80) | 5.36 (4.61–6.26) | 0.28 | 0.41 |
| dhSM 18:0;O2/18:0 | 0.91 (0.57–1.46) | 1.37 (0.89–2.06) | 0.10 | 0.21 |
| dhSM 18:0;O2/20:0 | 0.35 (0.27–0.46) | 0.48 (0.39–0.83) | 0.02 | 0.12 |
| dhSM 18:0;O2/22:0 | 0.35 (0.29–0.50) | 0.54 (0.39–0.98) | 0.02 | 0.12 |
| dhSM 18:0;O2/24:0 | 0.09 (0.07–0.12) | 0.13 (0.10–0.21) | 0.04 | 0.15 |
| dhSM 18:0;O2/24:1 | 1.22 (0.94–1.50) | 1.47 (1.21–2.05) | 0.07 | 0.17 |
| Total Dihydrosphingomyelins | 7.7 (6.45–10.01) | 9.20 (7.52 −13.45) | 0.08 | 0.19 |
| Sphingomyelins | ||||
| SM 18:1;O2/16:0 | 172.3 (143.3 −184.50) | 171.60 (141.70 −181.50) | 0.87 | 0.87 |
| SM 18:1;O2/18:0 | 23.92 (17.71–25.45) | 23.92 (21.78–28.68) | 0.30 | 0.42 |
| SM 18:1;O2/20:0 | 23.40 (21.0–21.53) | 26.59 (21.44–32.66) | 0.13 | 0.23 |
| SM 18:1;O2/22:0 | 47.91 (41.54–58.87) | 52.59 (42.82–68.17) | 0.51 | 0.60 |
| SM 18:1;O2/24:0 | 32.81 (28.15–40.63) | 32.14 (27.01–54.72) | 0.68 | 0.72 |
| SM 18:1;O2/24:1 | 70.74 (54.69–74.96) | 69.87 (62.46–83.99) | 0.53 | 0.62 |
| Total Sphingomyelins | 374.90 (315.30–403.90) | 372.9 (325.20–440.60) | 0.48 | 0.59 |
| Hexosylceramides | ||||
| Hex-Cer 18:1;O2/16:0 | 0.67 (0.55–0.76) | 0.57 (0.44–0.80) | 0.62 | 0.69 |
| Hex-Cer 18:1;O2/18:0 | 0.07 (0.05–0.07) | 0.06 (0.04–0.07) | 0.43 | 0.57 |
| Hex-Cer 18:1;O2/20:0 | 0.06 (0.04–0.07) | 0.04 (0.03–0.06) | 0.36 | 0.49 |
| Hex-Cer 18:1;O2/22:0 | 0.50 (0.42–0.64) | 0.42 (0.31–0.59) | 0.18 | 0.28 |
| Hex-Cer 18:1;O2/24:0 | 0.62 (0.55–0.67) | 0.52 (0.34–0.78) | 0.48 | 0.59 |
| Hex-Cer 18:1;O2/24:1 | 0.38 (0.31–0.42) | 0.38 (0.22–0.43) | 0.80 | 0.83 |
| Total Hexosylceramides | 2.35 (2.07–2.60) | 2.00 (1.46–2.86) | 0.68 | 0.72 |
| Diacylglycerols | 33.20 (29.20–44.20) | 51.20 (43.60–83.38) | 0.0009 | 0.02 |
| Phosphatidylcholines | 1184 (1125–1324) | 1275 (1190—1548) | 0.13 | 0.23 |
| Triacylglycerols | 531.7 (380.7–658.9) | 789.6 (643.6–1695) | 0.0004 | 0.02 |
Cer, Ceramides; HexCer, Hexosylceramides; dhSM, dihydrosphingomyelins; SM, sphingomyelins. Data show median with interquartile range. Wilcoxon rank sum test was performed to estimate significance in difference in lipid species between two groups. Bold represents statistically significant difference between controls and cases.
We next analyzed the different molecular species of plasma dhCer, Cer, dhSM, SM, and HexCer between groups (Fig. 1). The dhCer species C20:0 and C24:0 levels were significantly higher in some cases. Cer species C18:0, C20:0, and C24:0 were significantly higher in the case group.
Fig. 1.
Bar charts of molecular lipid species in plasma in cases (n = 17) and controls (n = 13). (A) Dihydroceramides. (B) Ceramides. (C) Dihydrosphingomyelins. (D) Sphingomyelins. (E) Phosphatidylcholines. (F) Diacylglycerols. (G) Triacylglycerols. Data shown as median and interquartile range. *Statistical significance, P < 0.05; ***P > 0.001 by Wilcoxon rank sum test.
Among the detected dhSM species, only C20:0, C22:0, and C24:0 were significantly higher in cases compared to controls and no changes were observed in SM species. We detected no significant difference between groups in levels of HexCer species C16:0, C18:0, C20:0, C22:0, C24:0 and C24:1. DG and TG levels were significantly higher in cases, while no difference was observed in PC levels between groups.
Plasma sphingolipids and MASLD
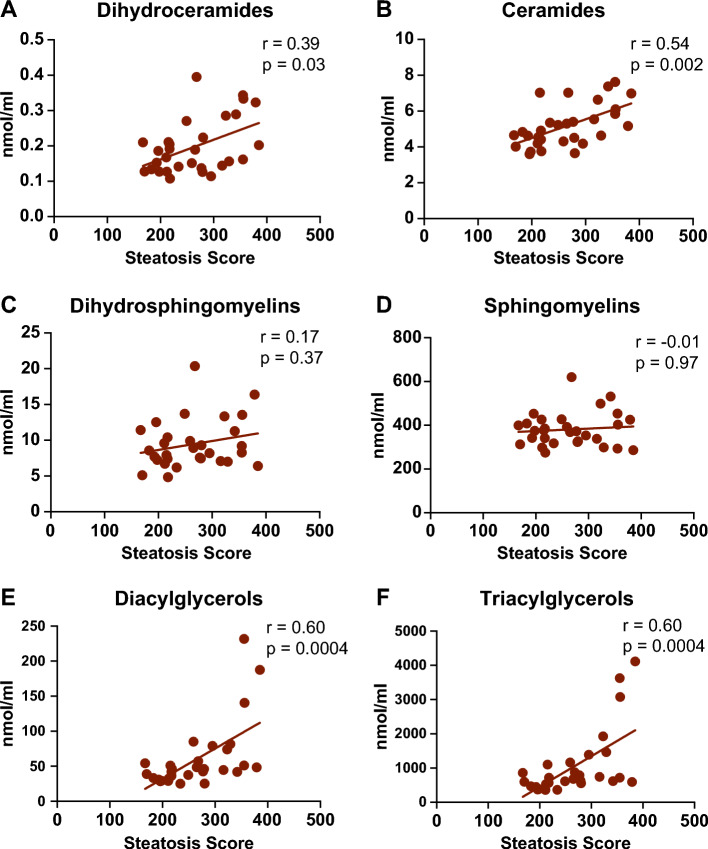
To investigate the association between plasma sphingolipids and MASLD in T1D, we examined the correlation between the levels of different lipid species and FibroScan parameters (fibrosis and steatosis score; Table 3). Sphingolipids positively correlated with steatosis score (Fig. 2) included dhCer species C20:0, C22:0, and C24:0, Cer C18:0, C20:0, C22:0, and C24:0, and dhSM C20:0 and C22:0. We observed that 3 participants with severe steatosis (S3) had the highest levels of DG and TG (Fig. 2E-F). Sphingolipids that positively correlated with fibrosis score included DhCer species C22:0 and C24:0, Cer C22:0 and C24:0, dhSM C20:0, C22:0, and C24:0, and sphingomyelins C20:0 and C22:0 (Fig. 3). Interestingly, a significant positive correlation was observed between DG and TG levels with steatosis score.
Table 3.
Correlation between lipid species with fibrosis and steatosis score.
| Lipid species (nmol/ml) | Fibrosis score | Steatosis score | ||
|---|---|---|---|---|
| r | p | r | p | |
| Dihydroceramides | ||||
| dhCer 18:0;O2/16:0 | 0.16 | 0.40 | 0.21 | 0.26 |
| dhCer 18:0;O2/18:0 | 0.12 | 0.52 | 0.33 | 0.07 |
| dhCer 18:0;O2/20:0 | 0.31 | 0.09 | 0.39 | 0.03 |
| dhCer 18:0;O2/22:0 | 0.40 | 0.03 | 0.42 | 0.02 |
| dhCer 18:0;O2/24:0 | 0.38 | 0.04 | 0.41 | 0.02 |
| dhCer 18:0;O2/24:1 | 0.11 | 0.56 | 0.26 | 0.16 |
| Total Dihydroceramides | 0.29 | 0.11 | 0.39 | 0.03 |
| Ceramides | ||||
| Cer 18:1;O2/16:0 | 0.31 | 0.09 | 0.31 | 0.09 |
| Cer 18:1;O2/18:0 | 0.18 | 0.34 | 0.47 | 0.007 |
| Cer 18:1;O2/20:0 | 0.27 | 0.15 | 0.51 | 0.004 |
| Cer 18:1;O2/22:0 | 0.41 | 0.02 | 0.49 | 0.005 |
| Cer 18:1;O2/24:0 | 0.36 | 0.04 | 0.48 | 0.007 |
| Cer 18:1;O2/24:1 | −0.09 | 0.64 | 0.33 | 0.07 |
| Total Ceramides | 0.34 | 0.07 | 0.54 | 0.002 |
| Dihydrosphingomyelins | ||||
| dhSM 18:0;O2/16:0 | 0.24 | 0.20 | 0.07 | 0.73 |
| dhSM 18:0;O2/18:0 | 0.22 | 0.25 | 0.24 | 0.21 |
| dhSM 18:0;O2/20:0 | 0.42 | 0.02 | 0.39 | 0.03 |
| dhSM 18:0;O2/22:0 | 0.48 | 0.007 | 0.39 | 0.03 |
| dhSM 18:0;O2/24:0 | 0.39 | 0.03 | 0.31 | 0.09 |
| dhSM 18:0;O2/24:1 | 0.27 | 0.16 | 0.18 | 0.33 |
| Total Dihydrosphingomyelins | 0.27 | 0.15 | 0.17 | 0.37 |
| Sphingomyelins | ||||
| SM 18:1;O2/16:0 | 0.09 | 0.62 | −0.15 | 0.41 |
| SM 18:1;O2/18:0 | 0.06 | 0.76 | 0.14 | 0.47 |
| SM 18:1;O2/20:0 | 0.42 | 0.02 | 0.28 | 0.14 |
| SM 18:1;O2/22:0 | 0.45 | 0.01 | 0.08 | 0.67 |
| SM 18:1;O2/24:0 | 0.29 | 0.12 | −0.06 | 0.77 |
| SM 18:1;O2/24:1 | 0.15 | 0.41 | −0.007 | 0.97 |
| Total Sphingomyelins | 0.24 | 0.20 | −0.01 | 0.97 |
| Hexosylceramides | ||||
| Hex-Cer 18:1;O2/16:0 | −0.10 | 0.60 | −0.21 | 0.25 |
| Hex-Cer 18:1;O2/18:0 | −0.14 | 0.45 | −0.31 | 0.09 |
| Hex-Cer 18:1;O2/20:0 | −0.17 | 0.38 | −0.31 | 0.09 |
| Hex-Cer 18:1;O2/22:0 | −0.03 | 0.87 | −0.35 | 0.05 |
| Hex-Cer 18:1;O2/24:0 | −0.10 | 0.59 | −0.35 | 0.06 |
| Hex-Cer 18:1;O2/24:1 | −0.12 | 0.53 | −0.24 | 0.20 |
| Total Hexosylceramides | −0.03 | 0.89 | −0.24 | 0.20 |
| Diacylglycerols | 0.28 | 0.13 | 0.60 | 0.004 |
| Phosphatidylcholines | 0.28 | 0.13 | 0.23 | 0.22 |
| Triacylglycerols | 0.28 | 0.13 | 0.61 | 0.0004 |
r = Spearman correlation coefficient; bold represents significant correlation.
Fig. 2.
Correlation between lipid species and steatosis score. r = Spearman correlation coefficient; statistical significance at P < 0.05.
Fig. 3.
Correlation between lipid species and fibrosis score. r = Spearman correlation coefficient; statistical significance at P < 0.05.
As obesity is an important risk factor for the pathogenesis of MASLD, we next investigated the correlation of BMI with lipid species and 24-h insulin dose (Table 4). Levels of dhCer species C20:0, C22:0 and C24:0, SM species C18:0, C20:0, and C24:1, and dhSM species C16:0, C18:0, C20:0, C22:0, C24:0, and C24:1 were positively correlated with BMI. A significant correlation was observed between DG and TG levels and BMI. We found a significant correlation between dhCer, Cer, dhSM, DG, and TG levels and 24-h insulin dose as a surrogate marker of insulin resistance. HexCer and PC levels were not significantly correlated with BMI or 24-h insulin dose.
Table 4.
Correlation between lipid species with BMI and 24-h insulin dose.
| Lipid species (nmol/ml) | BMI | Insulin dose | ||
|---|---|---|---|---|
| r | P-value | r | P-value | |
| Dihydroceramides | ||||
| dhCer 18:0;O2/16:0 | 0.29 | 0.12 | 0.26 | 0.16 |
| dhCer 18:0;O2/18:0 | 0.34 | 0.07 | 0.22 | 0.23 |
| dhCer 18:0;O2/20:0 | 0.54 | 0.002 | 0.38 | 0.03 |
| dhCer 18:0;O2/22:0 | 0.49 | 0.005 | 0.41 | 0.02 |
| dhCer 18:0;O2/24:0 | 0.46 | 0.01 | 0.52 | 0.003 |
| dhCer 18:0;O2/24:1 | 0.42 | 0.02 | 0.23 | 0.22 |
| Total Dihydroceramides | 0.47 | 0.008 | 0.41 | 0.02 |
| Ceramides | ||||
| Cer 18:1;O2/16:0 | 0.28 | 0.12 | 0.47 | 0.008 |
| Cer 18:1;O2/18:0 | 0.42 | 0.02 | 0.41 | 0.02 |
| Cer 18:1;O2/20:0 | 0.39 | 0.03 | 0.52 | 0.003 |
| Cer 18:1;O2/22:0 | 0.31 | 0.08 | 0.66 | < 0.0001 |
| Cer 18:1;O2/24:0 | 0.33 | 0.07 | 0.67 | < 0.0001 |
| Cer 18:1;O2/24:1 | 0.25 | 0.17 | 0.30 | 0.09 |
| Total Ceramides | 0.34 | 0.06 | 0.65 | < 0.0001 |
| Dihydrosphingomyelins | ||||
| dhSM 18:0;O2/16:0 | 0.46 | 0.01 | −0.04 | 0.82 |
| dhSM 18:0;O2/18:0 | 0.46 | 0.01 | 0.15 | 0.45 |
| dhSM 18:0;O2/20:0 | 0.58 | 0.008 | 0.36 | 0.04 |
| dhSM 18:0;O2/22:0 | 0.60 | 0.004 | 0.40 | 0.03 |
| dhSM 18:0;O2/24:0 | 0.54 | 0.002 | 0.40 | 0.03 |
| dhSM 18:0;O2/24:1 | 0.50 | 0.004 | 0.24 | 0.20 |
| Total Dihydrosphingomyelins | 0.51 | 0.004 | 0.08 | 0.67 |
| Sphingomyelins | ||||
| SM 18:1;O2/16:0 | 0.27 | 0.14 | 0.04 | 0.80 |
| SM 18:1;O2/18:0 | 0.38 | 0.04 | 0.04 | 0.81 |
| SM 18:1;O2/20:0 | 0.46 | 0.01 | 0.31 | 0.09 |
| SM 18:1;O2/22:0 | 0.33 | 0.07 | 0.18 | 0.32 |
| SM 18:1;O2/24:0 | 0.22 | 0.25 | 0.18 | 0.35 |
| SM 18:1;O2/24:1 | 0.40 | 0.02 | −0.04 | 0.86 |
| Total Sphingomyelins | 0.38 | 0.03 | 0.08 | 0.67 |
| Hexosylceramides | ||||
| Hex-Cer 18:1;O2/16:0 | 0.15 | 0.42 | −0.04 | 0.82 |
| Hex-Cer 18:1;O2/18:0 | −0.07 | 0.73 | −0.28 | 0.14 |
| Hex-Cer 18:1;O2/20:0 | −0.09 | 0.62 | −0.08 | 0.69 |
| Hex-Cer 18:1;O2/22:0 | −0.08 | 0.66 | −0.08 | 0.68 |
| Hex-Cer 18:1;O2/24:0 | −0.09 | 0.62 | −0.13 | 0.48 |
| Hex-Cer 18:1;O2/24:1 | 0.02 | 0.88 | −0.17 | 0.36 |
| Total Hexosylceramides | 0.05 | 0.79 | −0.04 | 0.82 |
| Diacylglycerols | 0.53 | 0.002 | 0.44 | 0.01 |
| Phosphatidylcholines | 0.24 | 0.20 | 0.23 | 0.21 |
| Triacylglycerols | 0.52 | 0.003 | 0.40 | 0.03 |
r = Spearman correlation coefficient; bold represents significant correlation.
We next examined whether glycemic control correlated with lipid species and found that only dhCer in the control group correlated with HbA1C (r = 0.67, P = 0.01; Fig. 4, Table 5).
Fig. 4.
Correlation between lipid species and HbA1C. r = Spearman correlation coefficient; statistical significance at P < 0.05.
Table 5.
Correlation between lipid species with HbA1c (%).
| Lipid species (nmol/ml) | HbA1c | |||
|---|---|---|---|---|
| Control | Cases | |||
| r | P-value | r | P-value | |
| Dihydroceramides | 0.67 | 0.01 | −0.41 | 0.09 |
| Ceramides | 0.27 | 0.37 | 0.003 | 0.99 |
| Dihydrosphingomyelins | 0.44 | 0.13 | −0.17 | 0.50 |
| Sphingomyelins | 0.21 | 0.49 | 0.10 | 0.68 |
| Hexosylceramides | −0.02 | 0.93 | 0.07 | 0.76 |
| Diacylglycerols | 0.24 | 0.41 | −0.01 | 0.95 |
| Phosphatidylcholines | 0.41 | 0.15 | −0.09 | 0.73 |
| Triacylglycerols | 0.28 | 0.35 | 0.02 | 0.93 |
r = Spearman correlation coefficient; bold represents significant correlation.
Due to the significant difference in BMI between the two groups, we analyzed the correlation of lipid species with fibrosis, steatosis score, 24-h insulin dose, and HbA1C, adjusted for BMI (Table 6). Total Cer, DH, and TG levels were found to have a significantly positive correlation with steatosis score (P < 0.05) and SM levels had a significantly negative correlation with steatosis score (P < 0.05). Ceramides positively correlated with 24-h insulin dose (P < 0.01). No lipid species correlated with fibrosis score or HbA1C.
Table 6.
Correlation between lipid species with fibrosis score, steatosis score, BMI and 24-h insulin dose and HbA1C adjusted with BMI.
| Lipid species (nmol/ml) | Fibrosis score | Steatosis score | Insulin dose | HbA1C |
|---|---|---|---|---|
| Dihydroceramides | 0.02 | 0.11 | 0.27 | −0.11 |
| Ceramides | 0.18 | 0.44* | 0.60** | 0.17 |
| Dihydrosphingomyelins | −0.05 | −0.29 | −0.16 | −0.17 |
| Sphingomyelins | 0.02 | −0.40* | −0.09 | −0.10 |
| Diacylglycerols | −0.04 | 0.38* | 0.29 | 0.14 |
| Triacylglycerols | −0.03 | 0.40* | 0.23 | 0.11 |
*P ≤ 0.05, **P ≤ 0.01. Data show correlation coefficient (r).
ROC curve analysis was performed to assess the discriminatory performance of BMI and selected lipid species—ceramides, triglycerides, and diglycerides, in differentiating between case and control groups. These lipid species were selected for ROC analysis based on their significantly elevated levels in case vs. control groups. BMI exhibited high discriminatory ability, yielding an area under the curve (AUC) of 0.891. Among the lipid species, TG and DG also had high discriminative performance with AUCs of 0.86 and 0.85 respectively, while ceramides showed fair discriminatory capacity (AUC = 0.71) [Fig. 5].
Fig. 5.

ROC Curves; BMI vs lipids.
Statistical comparisons of AUCs using DeLong’s test indicated no significant differences between BMI and any of the lipid markers. Specifically, the comparison between BMI and ceramide approached significance (Z = 1.87, p = 0.061), while comparisons with TG (Z = 0.31, p = 0.76) and DG (Z = 0.49, p = 0.62) were non-significant. These findings suggest that although BMI demonstrated the highest individual AUC, TG and DG possess comparable ability to distinguish cases from controls. Collectively, the results indicate that circulating lipid species, particularly TG and DG, may serve as informative biomarkers for case–control discrimination.
Discussion
To our knowledge, this is the first study of lipid subspecies in persons with T1D in the presence or absence of MASLD. We found that more than 50% of our participants with T1D had evidence of MASLD. Those that did had a mean BMI of 36.5 and displayed elevated circulating levels of dihydroceramides, ceramides, diacylglycerols, and triacylglycerols.
MASLD is the hepatic manifestation of metabolic syndrome16. The prevalence of MASLD increases concurrently with risk factors for obesity and type 2 diabetes (T2D)17. Although MASLD has been extensively studied in persons with T2D, little is known about risk factors for severity and progression of MASLD in persons with T1D. As the rate of obesity continues to increase globally and in persons with T1D18, the prevalence of MASLD and its complications in T1D should also increase, underscoring the need for better understanding of MASLD in T1D.
Our participants with MASLD compared to those without had features of insulin resistance. In addition to the higher BMI and lipids, our cases were taking a higher mean 24-h insulin dose, despite having higher HbA1C levels than controls, consistent with insulin resistance. Even higher insulin doses in the case participants would presumably be required if glycemia was lowered to the levels found in controls. Interestingly however, there is evidence that MASLD increases the risk for cardiovascular diseases, including subclinical atherosclerosis, independent from insulin resistance and other risk factors19. This implies an additional synergistic effect of MASLD and IR on cardiovascular disease.
Studies have shown that nutritional overload increases the accumulation of bioactive lipids such as DG and ceramides that disrupt the secondary signal transducers of insulin receptor and are mediators of hepatic insulin resistance20. Specifically, DG mediates hepatic insulin resistance by inhibiting protein kinase C which inactivates IRS-1 (insulin receptor substrate)21 while ceramides induce hepatic insulin resistance by inhibiting protein kinase B22.
Our participants with T1D and MASLD compared to those with T1D without MASLD had significantly elevated levels of DG and Cer, in line with findings by Luukkonen et al., which showed that patients with high liver fat content have higher hepatic Cer and dhCer concentrations23. Our study showed that the elevated levels of DG and Cer correlated with our FibroScan steatosis and fibrosis scores and with 24 h insulin dosing and BMI. Ceramides with short chains (< C18) exert more adverse hepatic effects, while the very long chain ceramides (> C22) have more protective effects24. Our data indicate significant elevation of different chain lengths ranging from small, medium, and large chain ceramides. Notably, although our study revealed a significant positive correlation of dhCer, Cer, DG, and TG with steatosis, none of these lipid subspecies correlated with fibrosis. While we did not measure lipid levels in persons without diabetes, it has been shown that ceramides and triacylglycerols are elevated in patients without diabetes who had evidence of visceral adiposity25.
There are emerging recommendations for the treatment of MASLD. Lifestyle intervention including a healthy diet and exercise counseling is the foundation of management of MASLD26. Pharmacological agents such as the Glucagon-like peptide 1 (GLP1) receptor agonists and pioglitazone have demonstrated efficacy in improving MASLD27,28, though their utility in T1D requires further assessment. In 2024, the FDA approved resmetirom for management of MASH. Resmetirom is a thyromimetic that selectively activates the thyroid hormone receptor beta (TRß) in the liver and increases hepatic fat oxidation29. However, efficacy and safety of resmetirom in T1D patients with MASH remains unknown.
Conclusions
Our study showed positive correlations of DG, TG, dhCer and Cer with steatosis, BMI, and 24-h insulin dose. The correlations noted with DG, TG, and Cer with steatosis persisted after controlling for BMI. These findings suggest that the changes seen on FibroScan occur as a direct toxic effect of lipids on hepatocytes. More research is needed to understand wwhich lipid species play a major role in liver toxicity in patients with TID and MASLD.
Limitations
We acknowledge several limitations of our study. Our sample size was small and this was a cross-sectional, cohort study. Our study was designed as an exploratory study, due to the paucity of data in T1D patients with MASLD. Also, we are aware that the positive correlations obtained do not equate to causality and a larger, prospective cohort study in T1D patients would be required to understand how various lipid species affect the various stages of MASLD. Our lipidomic analyses were limited and did not include subclasses of free fatty acids, phospholipids such as lysophosphatidic acid, phosphatidic acid, polyunsaturated fatty acids (PUFA), proinflammatory eicosanoids, and endocannabinoids, which may act as second messengers on key metabolic pathways in MASLD. These would be addressed in a follow up study.
Supplementary Information
Acknowledgements
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002537. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would also like to acknowledge the help of the following University of Iowa personnel: Kris Greiner, Teresa Ruggle, Jesse Espinoza, Kimberly Sprenger, Jessica Valestin, Mary McCormick and Darby Donovan.
Author contributions
AT, HT and BC contributed to the writing of the manuscript text; AT and HT prepared the tables and Figs. 1, 2, 3, 4. All Authors reviewed the manuscript.
Funding
The study was conducted using an Internal grant from the University of Iowa Division of Endocrinology and Metabolism. This study was also supported by the following grants: NIH1RO1HL134738. (D.J.); NIH1R01DK123043 (W.I.S.); NIH DK115824, DK116888, and DK116450 (S.A.S.); and DK124326 (B.C.); American Diabetes Association 7–21-JDF-033 (B.C.); Carver Charitable Trust 25–5906 (B.C); Diabetes Action Research and Education Foundation 522 (B.C.); and American Heart Foundation Postdoctoral Fellowship 25POST1374595 (H.T).
Data availability
Datasets generated during the current study are available in the following repository: 10.6084/m9.figshare.29218382.v1.
Declarations
Competing interests
S.A.S is a consultant and shareholder with Centaurus Therapeutics. All the remaining authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-15778-z.
References
- 1.Younossi, Z. M. et al. Nonalcoholic steatohepatitis is the most rapidly increasing indication for liver transplantation in the United States. Clin. Gastroenterol. Hepatol.10.1016/j.cgh.2020.05.064 (2021). [DOI] [PubMed] [Google Scholar]
- 2.de Vries, M., Westerink, J., Kaasjager, K. & de Valk, H. W. Prevalence of nonalcoholic fatty liver disease (NAFLD) in patients with type 1 diabetes mellitus: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab.105(12), 3842–3853. 10.1210/clinem/dgaa575 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carli, F., Della Pepa, G., Sabatini, S., Vidal Puig, A. & Gastaldelli, A. Lipid metabolism in MASLD and MASH: From mechanism to the clinic. JHEP Rep.6(12), 101185. 10.1016/j.jhepr.2024.101185 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foretz, M., Guichard, C., Ferre, P. & Foufelle, F. Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc. Natl. Acad. Sci. U S A96(22), 12737–12742. 10.1073/pnas.96.22.12737 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olzmann, J. A. & Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol.20(3), 137–155. 10.1038/s41580-018-0085-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pirola, C. J. et al. Epigenetic modification of liver mitochondrial DNA is associated with histological severity of nonalcoholic fatty liver disease. Gut62(9), 1356–1363. 10.1136/gutjnl-2012-302962 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Huang, J. et al. Applying lipidomics to non-alcoholic fatty liver disease: A clinical perspective. Nutrient15(8), 1992. 10.3390/nu15081992 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stefan, N., Staiger, H. & Haring, H. U. Dissociation between fatty liver and insulin resistance: The role of adipose triacylglycerol lipase. Diabetologia54(1), 7–9. 10.1007/s00125-010-1938-y (2011). [DOI] [PubMed] [Google Scholar]
- 9.Nikolova-Karakashian, M. Alcoholic and non-alcoholic fatty liver disease: Focus on ceramide. Adv. Biol. Regul.70, 40–50. 10.1016/j.jbior.2018.11.004 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Summers, S. A. Sphingolipids and insulin resistance: The five Ws. Curr. Opin. Lipidol.21(2), 128–135. 10.1097/MOL.0b013e3283373b66 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Kartsoli, S., Kostara, C. E., Tsimihodimos, V., Bairaktari, E. T. & Christodoulou, D. K. Lipidomics in non-alcoholic fatty liver disease. World J. Hepatol.12(8), 436–450. 10.4254/wjh.v12.i8.436 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perakakis, N. et al. Non-invasive diagnosis of non-alcoholic steatohepatitis and fibrosis with the use of omics and supervised learning: A proof of concept study. Metabolism101, 154005. 10.1016/j.metabol.2019.154005 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Myers, R. P. et al. Feasibility and diagnostic performance of the FibroScan XL probe for liver stiffness measurement in overweight and obese patients. Hepatology55(1), 199–208. 10.1002/hep.24624 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Eddowes, P. J. et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology156(6), 1717–1730. 10.1053/j.gastro.2019.01.042 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Karlas, T. et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J. Hepatol.66(5), 1022–1030. 10.1016/j.jhep.2016.12.022 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Polyzos, S. A., Bugianesi, E., Kountouras, J. & Mantzoros, C. S. Nonalcoholic fatty liver disease: Updates on associations with the metabolic syndrome and lipid profile and effects of treatment with PPAR-gamma agonists. Metabolism66, 64–68. 10.1016/j.metabol.2016.08.001 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Hazlehurst, J. M., Woods, C., Marjot, T., Cobbold, J. F. & Tomlinson, J. W. Non-alcoholic fatty liver disease and diabetes. Metabolism65(8), 1096–1108. 10.1016/j.metabol.2016.01.001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Purnell, J. Q. et al. Impact of excessive weight gain on cardiovascular outcomes in type 1 diabetes: Results from the diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) study. Diabet. Care40(12), 1756–1762. 10.2337/dc16-2523 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oni, E. T. et al. A systematic review: Burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care?. Atherosclerosis230(2), 258–267. 10.1016/j.atherosclerosis.2013.07.052 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Bo, T. et al. Hepatic selective insulin resistance at the intersection of insulin signaling and metabolic dysfunction-associated steatotic liver disease. Cell Metab.36(5), 947–968. 10.1016/j.cmet.2024.04.006 (2024). [DOI] [PubMed] [Google Scholar]
- 21.Lyu, K. et al. A membrane-bound diacylglycerol species induces PKCϵ-mediated hepatic insulin resistance. Cell Metab.10.1016/j.cmet.2020.08.001 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Memaj, P. & Jornayvaz, F. R. Non-alcoholic fatty liver disease in type 1 diabetes: Prevalence and pathophysiology. Front. Endocrinol. (Lausanne)13, 1031633. 10.3389/fendo.2022.1031633 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luukkonen, P. K. et al. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J. Hepatol.64(5), 1167–1175. 10.1016/j.jhep.2016.01.002 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Montgomery, M. K. et al. Regulation of glucose homeostasis and insulin action by ceramide acyl-chain length: A beneficial role for very long-chain sphingolipid species. Biochim. Biophys. Acta1861(11), 1828–1839. 10.1016/j.bbalip.2016.08.016 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Neeland, et al. Relation of plasma ceramides to visceral adiposity, insulin resistance and the development of type 2 diabetes. Dallas. Heart Stud. Diabetol.61, 2570–2579 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rinella, M. E. et al. AASLD Practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology77, 1797–1835 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newsome, P. N. et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N. Engl. J. Med.384, 1113–2428 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Cusi, K. et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: A randomized trial. Ann. Intern. Med.165(5), 305–315. 10.7326/M15-1774 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Harrison, S. A. et al. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N. Engl. J. Med.390(6), 497–509. 10.1056/NEJMoa2309000 (2024). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets generated during the current study are available in the following repository: 10.6084/m9.figshare.29218382.v1.