Abstract
Schizophrenia is a severe chronic neuropsychiatric disorder with a wide-range of cognitive and mental deficits and different categories of symptoms including positive, negative, and cognitive symptoms. In rodents, sub-chronic ketamine administration is a common method to induce a schizophrenia-like state. Olanzapine, an atypical antipsychotic medication, is used for the treatment and management of schizophrenia symptoms. Here, we aimed to assess the effects of ketamine, olanzapine, and their combination on cognitive and behavioral functions, as well as on brain-derived neurotrophic factor (BDNF) expression in the prefrontal cortex of male and female rats. Ketamine hydrochloride (30 mg/kg, i.p.) was administered for 5 consecutive days to induce schizophrenia-like alterations, and olanzapine (6 mg/kg, i.p.) was administered immediately after the final ketamine dose. Behavioral tests for locomotion, anxiety-like behavior, nociception, compulsive-like behavior, depression-like behavior, and memory were conducted, and BDNF mRNA levels in the prefrontal cortex were measured by quantitative real-time PCR. The results showed that ketamine increased locomotor activity and climbing behavior, decreased rearing (exploration), impaired novel object recognition memory, and lowered BDNF levels in the prefrontal cortex in both sexes. Certain effects of ketamine were sex-specific: ketamine reduced pain threshold (indicating hyperalgesia) and decreased immobility in the forced swim test (suggesting an anti-depressant-like effect) only in females. Olanzapine co-treatment restored locomotor activity and climbing and attenuated memory impairment in both sexes. Olanzapine also increased rearing and normalized pain threshold and immobility in females, indicating a mitigation of ketamine’s female-specific effects. Olanzapine had no significant effect on BDNF levels. In conclusion, ketamine induced a schizophrenia-like phenotype with more pronounced effects in female rats, and olanzapine treatment reversed many of the ketamine-induced behavioral deficits in both sexes. Notably, the therapeutic actions of olanzapine in this model appear to be independent of BDNF upregulation in the prefrontal cortex.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-15499-3.
Keywords: Ketamine, Olanzapine, Schizophrenia, Sex differences, Brain-derived neurotrophic factor (BDNF), Prefrontal cortex, Rat behavioral model
Subject terms: Neuroscience, Physiology, Psychology
Introduction
Schizophrenia is a severe chronic neuropsychiatric disorder with a heterogeneous genetic and neurobiological underlying mechanisms that influences early brain development1. Schizophrenia contains positive (hallucination, delusions), negative (anhedonia, social withdrawal), and cognitive (attention and memory impairments) symptoms2. In rodents, sub-chronic administration of NMDA receptor antagonists like ketamine is widely used to induce schizophrenia-like symptoms and neurochemical changes, modeling aspects of the disease3. For example, eight-day sub-chronic ketamine (25 mg/kg, i.p.) treatment in rats causes social interaction deficits and novel object recognition memory impairment, mimicking schizophrenia-like cognitive dysfunction4.
Brain-derived neurotrophic factor (BDNF), a crucial neurotrophin for neuroplasticity, learning, memory, and mood regulation, has been implicated in schizophrenia pathophysiology. BDNF plays an important role in brain development and synaptic plasticity, processes that are often altered in schizophrenia5. Previous study report that BDNF expression levels in the hippocampus and the prefrontal cortex are significantly decreased in ketamine-induced rat models of schizophrenia6. Likewise, clinical studies have shown reduced blood BDNF levels in both medicated and drug-naïve schizophrenia patients7. Antipsychotic drugs may exert some of their therapeutic effects via modulation of BDNF; for instance, various antipsychotics (olanzapine, risperidone, clozapine, haloperidol, aripiprazole) have been reported to influence BDNF levels5. Therefore, the role of BDNF in schizophrenia is considered critical, and changes in BDNF could underlie both disease manifestations and treatment responses.
Olanzapine is an atypical antipsychotic with a broad receptor-binding profile similar to that of clozapine8. Olanzapine is widely used for the treatment of schizophrenia and bipolar disorder and is known for its efficacy in treating positive and mood symptoms with a lower risk of extrapyramidal side effects according to the FDA9. Previous research indicates that olanzapine has a protective effect against ketamine-induced abnormalities: for example, olanzapine can prevent or reverse ketamine-induced cognitive deficits, neuronal degeneration, and behavioral changes in rodent models10. Olanzapine has also been shown to improve novel object recognition memory impairment in ketamine-treated rats11. Of note, olanzapine can affect the expression level of BDNF12. At the molecular level, olanzapine may affect neurotrophic signaling; some studies suggest that olanzapine treatment can increase BDNF expression in certain contexts. For instance, olanzapine improved depressive symptoms in schizophrenia patients in parallel with raising BDNF levels, suggesting a BDNF-mediated therapeutic mechanism13. However, it remains unclear whether olanzapine’s beneficial effects in schizophrenia models are directly related to BDNF changes, as findings have been mixed (some animal studies report increased BDNF with olanzapine14, while others do not15.
Sex differences are an important factor in schizophren. Epidemiological and clinical studies show that schizophrenia’s course and treatment response can vary by sex: for example, males often exhibit more severe negative symptoms and cognitive impairments along with greater structural brain abnormalities, whereas females tend to have later onset and more mood-related symptoms16. As we know, women are more vulnerable to develop mood disorders compared to men17. Also, it has been shown that there are sex differences in expression of mood-related genes in the brains of depressed patients17. Previous study has shown sex differences in antipsychotic responses in patients with schizophrenia16. It has also been shown that males show more negative symptoms and cognitive impairments with a more significant structural brain and neurophysiological abnormalities, while females show more affective symptoms, auditory hallucinations, and persecutory delusion18. In animal models, sex differences have been observed in response to NMDA receptor antagonists; adult female rats showed a greater disruption in prepulse inhibition (sensorimotor gating) in response to acute MK-801 than males19. Additionally, a recent study found that serum BDNF’s association with cognitive performance in schizophrenia patients differed by sex and treatment type20. Despite these findings, there is little information on possible sex differences in the effects of olanzapine on schizophrenia-like symptoms and BDNF expression.
In the present study, we investigated the effects of ketamine, olanzapine, and their combination on behavioral functions and BDNF levels in male and female rats. We utilized a sub-chronic ketamine model to induce schizophrenia-like behaviors and examined a battery of behavioral tests covering locomotor activity, anxiety-like behavior, compulsive-like behavior, nociception (pain sensitivity), depression-like behavior, and recognition memory. We also measured BDNF mRNA levels in the prefrontal cortex to explore a potential molecular correlate of treatment effects. Our aim was to determine whether olanzapine can ameliorate ketamine-induced behavioral and molecular changes and to assess sex differences in these effects. We hypothesized that ketamine would induce schizophrenia-like behavioral deficits accompanied by reduced BDNF expression, and that olanzapine treatment would reverse these deficits. Given the evidence of sex-specific responses, we further hypothesized that females might exhibit greater ketamine-induced changes and potentially a differential response to olanzapine, which we explicitly examined in this study.
Material and method
Animals
For the experiments, 136 Wistar rats (68 males and 68 females), aged 9–10 weeks and weighing 200–220 g at the start of the experiments. Rats were obtained from the Cognitive Neuroscience Laboratory at the Institute of Medicinal Plants (ACECR, Karaj, Iran).Animals were housed in groups of 5 per Plexiglas cage (25 × 50 × 25 cm) under standard laboratory conditions (12:12 h light/dark cycle, lights on at 07:00; temperature 22 ± 1 °C; food and water available ad libitum). We randomly assigned 7 male and 7 female rats to each experimental group. Separate cohorts were used for different assessments: specifically, 7 rats/sex/group were used for the main behavioral tests (open field, marble burying, hot plate, forced swim), an additional 7 rats/sex/group were used for the novel object recognition test (to avoid interference from stress of other tests), and 3 rats/sex/group were dedicated to BDNF level analysis.Note that the olanzapine-only group was not included in the BDNF mRNA cohort (BDNF was measured only in Saline, Ketamine, and Ket + Olanzapine groups, as justified below). All experiments were conducted during the light phase between 08:00 and 16:00.
Ethical approval
All experimental procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (2011) and the ARRIVE guidelines. The study protocol was approved by the Tehran Medical Sciences Islamic Azad University Ethics Committee (Ethical Approval ID: IR.IAU.PS.REC.1403.203). Every effort was made to minimize animal suffering and to use the minimum number of animals required to achieve statistical significance. At study end, animals were euthanised by intraperitoneal injection of sodium pentobarbital (200 mg kg⁻¹) followed by decapitation, in accordance with AVMA 2020 guidelines.
Ketamine and olanzapine administrations
To induce a schizophrenia-like phenotype, we followed a sub-chronic ketamine administration regime. Ketamine hydrochloride (30 mg/kg) was intraperitoneally injected during 5 consecutive days3,21,22. This dose and schedule are known to produce robust hyperlocomotion and cognitive deficits consistent with a schizophrenia model3,10. Olanzapine (obtained from Tehran Darou Pharmaceutical Co., Tehran, Iran) was dissolved in 0.1 N hydrochloric acid23, and was intraperitoneally injected at the dose of 6 mg/kg, according to previous report24. The administration of olanzapine was done immediately after the last injection of ketamine on day 5. Saline or vehicle injections were given to control groups as appropriate.
Behavioral Testing Schedule: Behavioral tests were performed on the final day of the ketamine treatment regimen, following the last injection (day 5). All rats underwent a sequence of tests in the following order1: Open Field Test3, Marble Burying Test6, Hot Plate Test, and2 Forced Swim Test. Testing began 3 h after the last ketamine (or saline) injection, to allow development of ketamine’s behavioral effects25 and to conduct all tests in the same day. There was a 30-minute interval between successive tests to allow recovery and setup: the Marble Burying Test commenced ~ 30 min after the Open Field Test, the Hot Plate test was performed shortly after the marble burying test was completed (within ~ 5–10 min), and the Forced Swim Test was started 30 min after the Hot Plate test. This schedule ensured that each animal completed the test battery with minimal overlap effects. A separate cohort of rats was used for the Novel Object Recognition Test (NORT) to avoid stress from the FST or hot plate affecting memory performance. The NORT was conducted over two days (as detailed below) following the 5-day treatment period. All behavioral testing was done between morning and early afternoon in a quiet room under consistent lighting. Researchers conducting the tests were blinded to treatment groups to reduce bias.
Open field test (OFT)
The OFT (Tajhiz-Gostar Omid Iranian Co, Tehran, Iran) was used to assess the locomotor activity and anxiety-like behavior. The apparatus was a clear Perspex open-top box (30 × 30 × 40 cm) with the floor divided into 16 equal squares26. Each rat was placed in the center of the arena and allowed to freely explore for 5 min (300 s). Locomotor activity was quantified as the number of floor grid squares crossed (with all four paws) during the session26. Exploratory rearing (number of times the rat stood on its hind legs) was recorded as an index of anxiety-like behavior or curiosity26. Lower rearing frequencies are interpreted as increased anxiety-like behavior or reduced exploration26,27. The OFT was always the first test in the sequence (to measure baseline activity without prior stress) and was conducted 3 h post-final ketamine injection as noted. The arena was cleaned with 70% ethanol between subjects to eliminate odor cues.
Hot plate test
The Hot Plate test measured thermal nociception (pain threshold). Hot plate device had a sheet which was getting hot by electric current (Tajhiz-Gostar Omid Iranian Co, Tehran, Iran). This device is used in order to assess thermal pain threshold in rodents. In this test, each rat was placed on the hot sheet (set at 50 °C.Each rat was gently placed on the hot plate, and a stopwatch was started immediately to record latency to the first sign of nociceptive behavior (hind paw lick or jump). The latency (in seconds) to paw licking/jumping was recorded as the pain response threshold25. A cut-off time of 100 s was imposed to prevent tissue damage if no response occurred28. – no rat reached this cut-off in our study. The hot plate was cleaned between trials. This test was conducted after the marble burying test, once animals had a short rest, and provided an assessment of analgesia/hyperalgesia possibly related to ketamine treatment. A reduction in paw-lick latency indicates decreased pain threshold (hyperalgesia), whereas an increased latency indicates analgesia.
Marble burying test
The marble burying test was used to evaluate obsessive-compulsive disorder (OCD)-like behavior in rodents. Although OCD-like behavior is not a core symptom of schizophrenia, we included this test because a significant subset of schizophrenia patients (up to ~ 25%) exhibit comorbid obsessive-compulsive symptoms (Frontiers | Comorbid obsessive-compulsive symptoms in schizophrenia: contributions of pharmacological and genetic factors). We sought to determine if ketamine would induce repetitive or compulsive behavior in our model and if olanzapine could modulate it. In this test, rats were individually placed in a clean cage (same dimensions as home cage) filled with 5 cm of bedding. Ten glass marbles (15 mm diameter, ~ 5 g each) were gently placed on the surface of the bedding in a 2 × 5 grid pattern29. The rat was allowed to explore freely for 30 min with no disturbance30. After 30 min, the number of marbles buried was counted. A marble was considered “buried” if it was at least 2/3 covered by bedding material (active burying by the rat). Buried marbles count is interpreted as an index of compulsive-like digging behavior; higher counts may reflect increased compulsive or anxiety-like behavior29. (We note that marble burying can also indicate anxiety; here we use it in the context of repetitive behavior tendency.) The test was performed 30 min after the OFT in the same experimental room. Fresh bedding was used for each rat, and the bedding was leveled and marbles arranged identically for consistency. We also varied which marbles had residual odor by thorough cleaning to avoid any bias. Rationale: By including this test, we can determine if the ketamine model extends to compulsive digging behavior and if olanzapine has any impact on such behavior. This addresses the high comorbidity of schizophrenia and OCD in humans (Frontiers | Comorbid obsessive-compulsive symptoms in schizophrenia: contributions of pharmacological and genetic factors).
Forced swim test (FST)
The FST was used to assess depressive-like behavior (behavioral despair) in the rats. We used a transparent cylindrical tank (50 cm height × 20 cm diameter) filled to two-thirds with water (depth ~ 30 cm, water temperature 20–22 °C)31. Each rat was gently placed in the water, and its behavior was recorded during a 6-minute test session. We focused on two measures: Immobility time, defined as the duration the rat remains floating or making only minimal movements to keep its head above water (a proxy for behavioral despair), and Climbing time (also called struggling), defined as active upward thrashing movements with the forepaws against the tank walls (an active coping behavior). Increased immobility is interpreted as a depression-like state, whereas increased climbing (or swimming, not separately quantified here) can indicate an anti-depressant-like effect or agitation. The FST was conducted 30 min after the hot plate test, as the final test of the battery, to ensure that any stress from FST did not influence other behavior tests. After each session, the rat was removed, dried, and kept warm. The water was changed after each group of rats to maintain cleanliness and equal temperature. Notably, ketamine has been reported to have rapid antidepressant-like effects in some paradigms, so we hypothesized ketamine might reduce immobility (especially in females, if they are more sensitive to ketamine’s antidepressant effect). We specifically recorded immobility and climbing, as these behaviors can be influenced by sex and treatment (e.g., dopamine-enhancing treatments often increase climbing in FST). All FST scoring was done by an observer blind to treatment, using a stopwatch for immobility and climbing bouts.
Novel object recognition test (NORT)
The NORT was used to evaluate recognition memory ability, which is a cognitive domain often impaired in schizophrenia. This test was conducted on a separate cohort of rats to isolate cognitive testing from the effects of the other behavioral tests. We employed a slightly modified two-day protocol. On Day 1, 3 hours after the final ketamine/vehicle injection, each rat was placed in an open-field arena (transparent walls, 45 × 45 × 45 cm) with no objects for a 10-minute habituation period30. One hour after habituation, the rat was returned to the arena for the familiarization phase, in which two identical objects (Object A and Object A’) were placed in opposite corners. The rat was allowed to explore these objects for 10 min30. The arena and objects were cleaned with 1% acetic acid solution between trials to eliminate scent cues30. On Day 2 (24 h after familiarization), we conducted the test phase: one of the familiar objects was replaced with a novel object (Object B, different shape/color/texture)30. The rat was again given 10 min to explore. We counterbalanced which object was replaced to avoid side bias. Exploration was defined as the rat sniffing or touching the object with its nose; sitting on or ignoring the object was not counted. We measured the time spent exploring the novel object (N) and the familiar object (F) during the test phase. From these, we calculated a Discrimination Index (DI) = (time on N – time on F)/(time on N + time on F)32. The DI ranges from − 1 to + 1, where positive values indicate more exploration of the novel object (successful memory of the familiar object), 0 indicates no preference, and negative values indicate a paradoxical preference for the familiar object (which could suggest memory impairment or novel object avoidance). This formula was based on standard calculations in object recognition literature (e.g32. A rat with intact recognition memory is expected to spend more time on the novel object, yielding a positive DI, whereas memory-impaired rats will spend similar time on both or even prefer the familiar (chance or less curiosity). We chose a 24 h retention interval to test long-term recognition memory, which is a challenging memory task that ketamine was expected to impair11. All phases were video-recorded, and exploration times were scored by an observer blind to group. Note: We used a shorter interval between habituation and familiarization (1 h) on Day 1 instead of the more typical 24 h, to accommodate our schedule; however, the crucial retention interval between learning and test was kept at 24 h for standard long-term memory assessment30.
Real-time PCR(BDNF mRNA expression)
After behavioral testing, rats designated for molecular analysis (n = 3 per sex per group) were euthanized, and their brains were quickly removed33,34. The prefrontal cortex (PFC) was dissected on ice, immediately frozen in liquid nitrogen, and stored at − 80 °C until RNA extraction. We focused on the PFC because it is implicated in schizophrenia and was previously shown to have reduced BDNF in the ketamine model35. Total RNA Extraction: Total RNA was extracted from PFC tissue using TRIzol™ reagent (Invitrogen, USA, Cat#15596018) according to the manufacturer’s protocol. Briefly, ~ 50 mg of tissue was homogenized in 1 mL TRIzol; after phase separation with chloroform and isopropanol precipitation, RNA pellets were washed with 75% ethanol and resuspended in RNase-free water. RNA quantity and purity were evaluated using a NanoDrop spectrophotometer (Thermo Fisher Scientific, USA) by measuring absorbance at 260 nm and 280 nm. All samples had A260/280 ratios between 1.9 and 2.1, indicating high purity. RNA integrity was checked by agarose gel electrophoresis (clear 18 S and 28 S rRNA bands).
cDNA Synthesis: 1 µg of total RNA from each sample was reverse transcribed into complementary DNA (cDNA) using a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, K1622). We used random hexamer primers for reverse transcription to ensure comprehensive cDNA coverage of all transcripts. The reverse transcription reactions (20 µL) were performed at 42 °C for 60 min, then terminated at 70 °C for 5 min, following the kit’s instructions. cDNA samples were stored at − 20 °C until PCR.
Primer Design: We examined mRNA sequences of BDNF (brain-derived neurotrophic factor) and the reference gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and obtained primer sequences from published sources to ensure reliable amplification. The primer sequences (rat-specific) were as follows: BDNF forward 5′-GGACATATCCATGACCAGAAAGAAA-3′ and reverse 5′-GCAACAAACCACAACATTATCGAG-3′36, and GAPDH forward 5′-TCCCAGAGCTGAACGGGAAGCTCATG-3′ and reverse 5′-TGGAGGCCATGTAGGCCATGAGGTCCA-3′37. These primers amplify coding regions spanning exon–exon junctions (when possible) to avoid genomic DNA amplification. Both primer sets were validated in prior studies for specificity to their targets and were synthesized by a commercial oligonucleotide supplier. Primer efficiency was ~ 95–100%, as determined by standard curve dilution of a pooled cDNA sample (slopes of standard curves within the optimal range). We also confirmed single distinct melt-curve peaks for each primer pair in preliminary runs, indicating specific amplification.
Quantitative Real-Time PCR (BDNF mRNA): Quantitative PCR was performed on an ABI StepOnePlus™ Real-Time PCR System (Applied Biosystems, USA). Reactions were set up in a 20 µL volume containing 2 µL of cDNA (diluted 1:5), 10 µL of SYBR Green PCR Master Mix (Applied Biosystems, Cat#4309155), 0.4 µL of each primer (400 nM final concentration), and 7.2 µL of nuclease-free water. Thermal cycling conditions were: initial denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Fluorescence was recorded during the 60 °C extension phase of each cycle. Following amplification, a melt curve analysis was performed from 60 °C to 95 °C to verify single-product specificity – all reactions showed a single melting peak, confirming specific amplification of BDNF or GAPDH. No-template controls (NTCs) were included for each primer pair and showed no amplification. Each sample was run in triplicate PCR reactions to ensure reproducibility.
Quantification: We employed the comparative CT (2 − ΔΔCT) method for relative quantification. GAPDH was used as the internal control gene to normalize BDNF expression levels38,39. For each sample, the cycle threshold (CT) value for BDNF was normalized to GAPDH CT (ΔCT = CT, BDNF – CT, GAPDH). We then compared the ΔCT of treated groups to the mean ΔCT of the control (saline) group of the same sex to obtain ΔΔCT. The relative expression was calculated as 2 − ΔΔCT and expressed as fold-change vs. control. This method yields a unitless relative quantity where control mean = 1. All data were then multiplied by 100 to express values as percentage of the control (saline) group for each sex, for easier interpretation (statistical analyses were still performed on log-transformed 2^−ΔΔCT values). We confirmed that the amplification efficiencies of target and reference were approximately equal, justifying the use of ΔΔCT.
Experimental groups and cohorts
We have summarized the experimental groups and sample sizes in Table 1.Each treatment group consisted of 7 males and 7 females in the behavioral test cohort, 7 males and 7 females in the NORT cohort, and 3 males and 3 females in the BDNF (qPCR) cohort. The four treatment groups were: Saline (control), Ketamine, Olanzapine, and Ketamine + Olanzapine. Separate cohorts were used for behavioral testing versus NORT versus molecular analysis in order to avoid cross-test interference and to ensure sufficient tissue for BDNF measurements. Table 2 below provides the detailed allocation of males and females to each group and cohort. (No animals were excluded from the study; all planned animals were used in analyses. Note that the olanzapine-only group was not assessed for BDNF mRNA, as indicated.)
Table 1.
Primer sequences for BDNF and GAPDH genes for polymerase chain reaction (PCR).
| Gene | Forward primer | Reverse primer |
|---|---|---|
| BDNF | TGCAGGGGCATAGACAAAAGG | CTTATGAATCGCCAGCCAATTCTC |
| GAPDH | TGACATCAAGAAGGTGGTGAA | CCCTGTTGCTGTAGGCGTATT |
Table 2.
Experimental groups and cohort allocation (male/female rats per group).
| Treatment group | Behavioral tests(Open field, Marble burying, Hot plate, FST) (M/F) | Novel object recognition (NORT cohort) (M/F) | BDNF mRNA analysis (PCR cohort) (M/F) |
|---|---|---|---|
| Saline (Control) | 7/7 | 7/7 | 3/3 |
| Ketamine | 7/7 | 7/7 | 3/3 |
| Olanzapine | 7/7 | 7/7 | –a |
| Ketamine + Olanzapine | 7/7 | 7/7 | 3/3 |
a No BDNF mRNA data were collected for the Olanzapine-only group (this group was not included in the PCR cohort; BDNF analysis was focused on Saline vs. Ketamine vs. Ket + Olanzapine, see text).
b Separate cohorts of animals were used for behavioral tests, NORT, and molecular analysis to prevent interference between tests. Each value in the table represents the number of Male/Female rats in that group’s cohort. Thus, each treatment group had a total of 17 rats per sex (7 + 7 + 3) across all cohorts.
Statistical analyses
Data are presented as mean ± standard deviation (SD). Behavioral data for each measure were analyzed with appropriate ANOVAs. Specifically, we used two-way ANOVA with Treatment (four levels: Saline, Ketamine, Olanzapine, Ket + Olanzapine) and Sex (male, female) as factors to examine overall effects and Treatment×Sex interactions. When a given behavior was only present in ketamine-treated animals (for example, BDNF was measured only in ketamine vs. ket + olanzapine groups for simplicity), we performed a two-way ANOVA with the relevant groups. For significant ANOVAs (p < 0.05), we conducted Tukey’s post-hoc tests for multiple comparisons between specific groups. Key planned comparisons included: ketamine vs. saline (to confirm ketamine’s effect), ketamine + olanzapine vs. ketamine (to assess olanzapine’s rescue effect), and sex comparisons within the ketamine group (to detect sex differences). A significance threshold of p < 0.05 was used for all tests. For clarity, we denote levels of significance as p < 0.05, p < 0.01, *p < 0.001 in figures. All statistical analyses were performed using GraphPad Prism 9.0 (GraphPad Software, USA). All statistical tests and graphs were produced in GraphPad Prism 9.5.0 (GraphPad Software LLC; https://www.graphpad.com).
Results
Locomotor activity(open field)
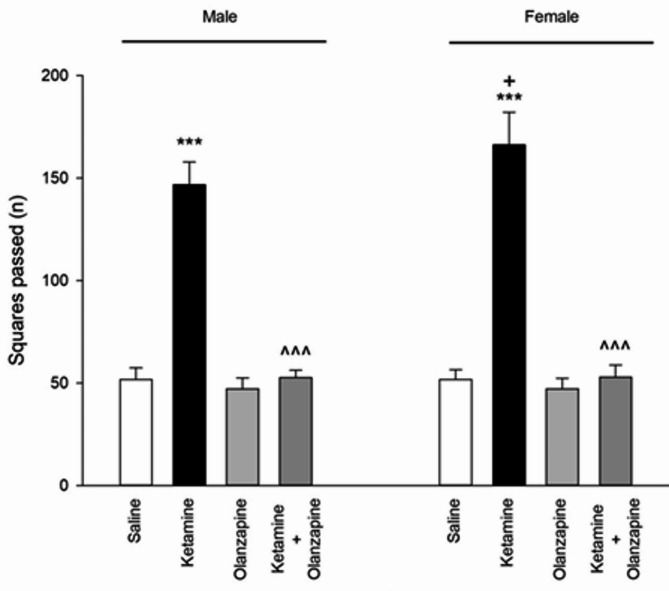
Ketamine treatment significantly affected locomotor activity in both male and female rats (one-way ANOVA within each sex: F3, 24 = 336.6 for males, F3, 24 = 277.6 for females; p < 0.001 for both). Post-hoc tests showed that ketamine (30 mg/kg) caused a dramatic increase in locomotor activity compared to saline controls in both males and females (**p < 0.001 vs. saline; Fig. 1). **Olanzapine treatment effectively reduced locomotor activity in ketamine-treated rats, bringing it back down toward control levels in both sexes (p < 0.001 for ket + olanzapine vs. ketamine alone). Importantly, olanzapine alone (without ketamine) did not significantly differ from saline in locomotor counts for either sex (p > 0.05 vs. saline), indicating that olanzapine by itself did not suppress normal locomotion.
Fig. 1.
Squares passed (locomotor activity) in both males and females received saline, ketamine, olanzapine, and both (***P < 0.001 compared with related saline; ^^^P < 0.001 compared with related ketamine; +P < 0.05 compared with respective male group; n = 7).
When considering sex as a factor (two-way ANOVA: Treatment × Sex), there was a significant interaction (F3, 48 = 4.96, p < 0.01) and a main effect of sex (F1, 48 = 5.05, p < 0.05) on locomotor activity. Female rats exhibited higher locomotor activity than males under ketamine treatment (p < 0.05 for female-ketamine vs. male-ketamine; Fig. 1), even though both were elevated relative to controls. No sex difference was observed in saline or olanzapine-only groups. These results indicate that ketamine induced hyperlocomotion in both sexes, with females showing a greater hyperactive response, and that olanzapine reversed ketamine-induced hyperlocomotion irrespective of sex.
Rearing behavior(open field)
Ketamine also significantly influenced rearing (exploratory behavior) in the open field (one-way ANOVA: F3, 24 = 78.7 for males, F3, 24 = 322.6 for females; p < 0.001). Ketamine markedly decreased the number of rears compared to saline in both males and females (**p < 0.001 vs. saline; Fig. 2), suggesting increased anxiety-like behavior or decreased exploration. Olanzapine plus ketamine partially attenuated this effect, but in a sex-dependent manner. In female rats, ketamine + olanzapine showed significantly more rearing activity than ketamine alone (+++**p < 0.001 vs. ketamine), indicating that olanzapine restored exploratory behavior in females nearly to control levels (although still slightly lower than saline females, ++**p < 0.001 vs. saline, implying a partial restoration). In male rats, however, olanzapine did not significantly increase rearing compared to ketamine alone (p > 0.05), indicating no notable improvement of rearing in males. Olanzapine-only groups did not differ from saline in rearing for either sex.
Fig. 2.
Rearing behavior (anxiety-like behavior) in both males and females received saline, ketamine, olanzapine, and both (***P < 0.001 compared with related saline; ^^^P < 0.001 compared with related ketamine; +++P < 0.001 compared with respective male group; n = 7).
Two-way ANOVA showed a significant Treatment × Sex interaction for rearing (F3, 48 = 6.58, p < 0.001) as well as main effects of Treatment (F3, 48 = 269.5, p < 0.001) and Sex (F1, 48 = 25.14, p < 0.001). Ketamine’s effect on rearing was significantly more pronounced in females than in males, as evidenced by female ketamine rats rearing less than male ketamine rats (+++**p < 0.001 female vs. male ketamine; Fig. 2). This aligns with the locomotor findings: female rats appear more affected by ketamine in open-field behaviors (greater hyperactivity and greater reduction in exploration). Olanzapine’s efficacy in alleviating the rearing deficit was observed only in females, highlighting a sex-specific therapeutic effect on this anxiety-related measure.
Pain threshold
Ketamine had a significant effect on pain sensitivity, but only in females. Among female rats, there were significant differences between treatment groups (F3, 24 = 63.49, p < 0.001), whereas in males the group differences were not significant (F3, 24 = 0.91, p > 0.05; one-way ANOVAs). Female rats treated with ketamine showed a marked decrease in hot plate latency compared to saline controls (**p < 0.001; Fig. 3), indicating hyperalgesia (lower pain threshold). In contrast, male rats did not show a significant change in latency with ketamine (their latency was similar to saline controls, p > 0.05). Olanzapine co-treatment completely reversed the ketamine-induced hyperalgesia in females: ketamine + olanzapine females had significantly higher latencies than ketamine-alone females (^^^**p < 0.001 vs. ketamine) and were statistically indistinguishable from saline females (p > 0.05 vs. saline), indicating restoration of normal pain threshold. Olanzapine alone had no effect on pain threshold in either sex (no difference vs. saline).
Fig. 3.
Pain threshold in both males and females received saline, ketamine, olanzapine, and both (***P < 0.001 compared with related saline; ^^^P < 0.001 compared with related ketamine; +++P < 0.001 compared with respective male group; n = 7).
Two-way ANOVA (Treatment × Sex) for hot plate latency revealed a significant main effect of Treatment (F3, 48 = 30.52, p < 0.001) and Sex (F1, 48 = 29.40, p < 0.001), as well as a significant interaction (F3, 48 = 17.28, p < 0.001). Post-hoc analysis confirmed that ketamine’s effect was significant in females but not in males, yielding a significant difference between ketamine females vs. ketamine males (+++**p < 0.001; Fig. 3). These results demonstrate a female-specific hyperalgesic effect of ketamine, which was mitigated by olanzapine. In males, pain perception was unaffected by ketamine in this paradigm, hence no rescue was needed. This sex difference in nociceptive response to ketamine is a novel finding in our study.
OCD-like behavior(Marble Burying)
Ketamine did not significantly affect marble burying behavior in either sex. The number of marbles buried showed no significant differences between any of the groups for males (F3, 24 = 0.17, p > 0.05) or females (F3, 24 = 0.12, p > 0.05) in one-way ANOVAs. Across treatments, rats buried a comparable number of marbles (on average, 2–4 marbles out of 10) regardless of ketamine or olanzapine. Two-way ANOVA similarly indicated no significant main effects or interaction (Treatment: F3, 48 = 0.09; Sex: F1, 48 = 0.01; interaction: F3, 48 = 0.19; all p > 0.05). There was no observable sex difference and no impact of ketamine or olanzapine on this compulsive-like digging measure (Fig. 4). These results suggest that sub-chronic ketamine at this dose did not induce an increase in repetitive digging or anxiety-driven burying behavior, and olanzapine had no effect either. The lack of change in marble burying aligns with the idea that not all behaviors are affected by the ketamine model, or it might indicate that any potential increase in compulsivity by ketamine was offset by general hyperactivity changes. In summary, no OCD-like phenotype was detected under our experimental conditions.
Fig. 4.
Marbles buried (OCD-like behavior) in both males and females received saline, ketamine, olanzapine, and both (n = 7).
Immobility (forced swim test)
Ketamine’s effects on depressive-like behavior were sex-specific. In female rats, ketamine caused a clear reduction in immobility time in the FST compared to saline (**p < 0.001; Fig. 5), which can be interpreted as an antidepressant-like effect or increased active coping (consistent with ketamine’s known rapid antidepressant action). Female ketamine rats spent more time struggling (climbing/swimming) and less time immobile than control females. In male rats, ketamine did not significantly change immobility time (p > 0.05 vs. saline). Consequently, a one-way ANOVA showed significant group differences in females (F3, 24 = 98.11, p < 0.001) but not in males (F3, 24 = 2.16, p = 0.12). Olanzapine co-treatment in females increased immobility time back to near control levels: ketamine + olanzapine females had significantly higher immobility than ketamine-alone females (^^^**p < 0.001 vs. ketamine), indicating that olanzapine reversed ketamine’s antidepressant-like effect in females (i.e., it normalized the behavior, as ketamine-alone females were unusually low in immobility). Ketamine + olanzapine female immobility did not differ from saline female immobility (p > 0.05), showing full restoration. In males, olanzapine + ketamine produced no notable change since ketamine alone had no effect (male ketamine vs. male ket + olz, p > 0.05). Olanzapine alone did not significantly affect immobility in either sex (no difference vs. saline).
Fig. 5.
Immobility (depressive-like behavior) in both males and females received saline, ketamine, olanzapine, and both (***P < 0.001 compared with related saline; ^^^P < 0.001 compared with related ketamine; +++P < 0.001 compared with respective male group; n = 7).
Two-way ANOVA (Treatment × Sex) for FST immobility yielded a significant Treatment effect (F3, 48 = 48.02, p < 0.001), Sex effect (F1, 48 = 12.21, p < 0.001), and interaction (F3, 48 = 21.02, p < 0.001). Ketamine-treated females had substantially lower immobility than ketamine-treated males (+++**p < 0.001 female vs. male ketamine; Fig. 5). This reinforces that ketamine’s impact in the FST was observed in females only. The fact that ketamine decreased immobility in females suggests a peculiar antidepressant-like action in female rats, which might be related to their increased ketamine sensitivity or hormonal status (explored in Discussion). Olanzapine by itself did not alter FST behavior, but in combination with ketamine, it nullified ketamine’s effect in females, leading to no sex difference in the ket + olanzapine group.
Climbing (forced swim test)
Ketamine increased climbing behavior (active escape efforts) in the FST in both sexes. One-way ANOVA showed significant treatment effects for both males (F3, 24 = 11.99, p < 0.001) and females (F3, 24 = 14.40, p < 0.001). Post-hoc comparisons indicated that ketamine-treated rats spent more time actively climbing compared to saline controls in both males and females (**p < 0.001 vs. saline; Fig. 6). This is consistent with the reduced immobility (especially in females), as climbing is an alternative behavior in the FST. Olanzapine co-administration prevented the ketamine-induced increase in climbing: in both sexes, the ketamine + olanzapine groups had significantly lower climbing times than the ketamine-alone groups (^^^**p < 0.001 vs. ketamine). In fact, with olanzapine, climbing behavior returned to control-like levels (no significant difference between ket + olanzapine and saline in either sex, p > 0.05). Olanzapine-only rats showed climbing times similar to saline.
Fig. 6.
Climbing in both males and females received saline, ketamine, olanzapine, and both (***P < 0.001 compared with related saline; ^^^P < 0.001 compared with related ketamine; n = 7).
Unlike immobility, no sex difference was detected in climbing. Two-way ANOVA revealed a significant main effect of Treatment (F3, 48 = 26.24, p < 0.001) on climbing, but the effect of Sex was not significant (F1, 48 = 0.02, p = 0.88) and neither was the interaction (F3, 48 = 0.04, p = 0.99). Thus, ketamine increased climbing to a similar extent in males and females, and olanzapine reduced it similarly in both (Fig. 6). This indicates that for this particular active coping behavior, ketamine’s impact was robust and sex-independent, and olanzapine’s efficacy in countering that impact was also equal in males and females. In summary, ketamine stimulated more escape-oriented behavior in the FST, and olanzapine co-treatment normalized this behavior.
Novel object recognition memory
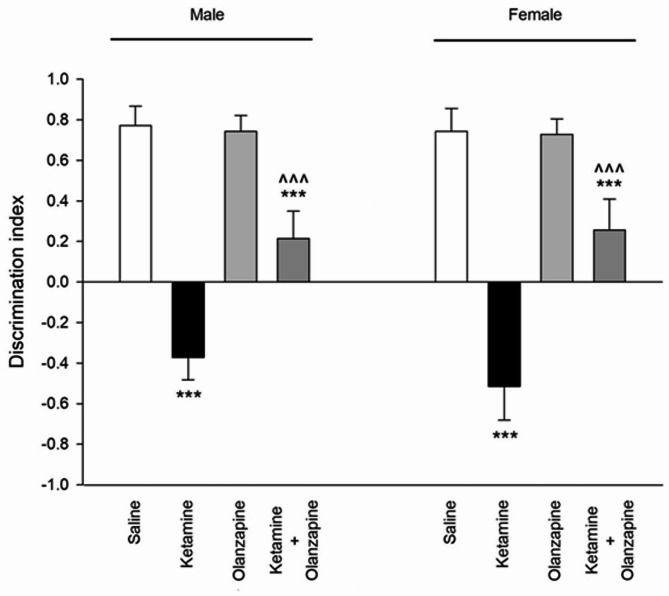
Ketamine had a profound effect on recognition memory, as indicated by the Discrimination Index (DI) in the novel object recognition test. One-way ANOVA within each sex showed significant group effects (F3, 24 = 177.7, p < 0.001 for males; F3, 24 = 140.2, p < 0.001 for females). Saline-treated rats of both sexes showed a strong preference for the novel object (positive DI, indicating normal recognition memory). In contrast, ketamine-treated rats had a significantly lower DI than controls (**p < 0.001 vs. saline), indicating impaired recognition memory (Fig. 7). In fact, ketamine rats spent roughly equal time on novel and familiar objects, resulting in DIs near zero or slightly negative (some ketamine rats explored the familiar object as much or more than the novel, suggesting memory deficit or neophobia). Olanzapine improved recognition memory in ketamine-treated rats of both sexes: the ketamine + olanzapine groups showed higher DIs than the ketamine-alone groups (^^^**p < 0.001 vs. ketamine). This suggests that olanzapine partially attenuated the memory impairment. However, ketamine + olanzapine DIs were still somewhat lower than saline in both sexes (+ + p < 0.01 vs. saline in post-hoc), indicating memory was not fully restored to normal levels, but the deficit was significantly reduced. Olanzapine alone did not significantly affect DI compared to saline (p > 0.05), implying it does not alter memory in untreated rats.
Fig. 7.
Discrimination index (novel object recognition memory) in both males and females received saline, ketamine, olanzapine, and both (***P < 0.001 compared with related saline; ^^^P < 0.001 compared with related ketamine; n = 7).
Two-way ANOVA (Treatment × Sex) for the discrimination index showed a strong Treatment effect (F3, 48 = 308.7, p < 0.001). The Sex effect was not significant (F1, 48 = 1.24, p = 0.27) and the interaction was not significant (F3, 48 = 1.47, p = 0.23). This indicates that male and female rats were similarly affected by ketamine and olanzapine in the NORT (Fig. 7). Both sexes had impaired memory with ketamine and both benefitted from olanzapine to a similar degree. There was no detectable sex difference in novel object recognition performance under any condition in our data. Thus, ketamine induced a severe recognition memory deficit in both sexes, and olanzapine partially reversed this deficit in both sexes. The persistence of some impairment in the ket + olanzapine group may suggest that the 6 mg/kg olanzapine only partially counteracts the cognitive effects of this ketamine regimen.
BDNF mRNA expression (prefrontal cortex)
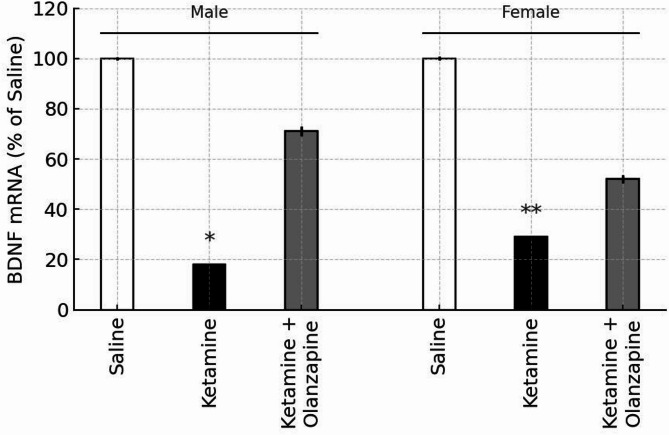
In the saline control groups, there were no sex differences in baseline BDNF mRNA levels in the PFC (by design we normalized each sex to its control). Ketamine administration significantly decreased BDNF mRNA expression in the prefrontal cortex of both male and female rats (Fig. 8). One-way ANOVA on BDNF levels (expressed as % of same-sex control) showed a significant effect of treatment in males (F2, 6 = 7.07, p < 0.05) and in females (F2,6 = 6.96, p < 0.05). Post-hoc tests confirmed that ketamine-only groups had significantly lower BDNF expression relative to saline controls (p < 0.05 in males, p < 0.01 in females). BDNF was about 60–70% of control levels in ketamine-treated rats, consistent with previous findings of ketamine’s downregulation of neurotrophic factors6,40. Importantly, olanzapine co-treatment did not significantly increase BDNF mRNA compared to ketamine alone in either sex. Although the ketamine + olanzapine groups showed a slight upward trend in BDNF levels (approximately 80–85% of control), this was not statistically significant (p > 0.05 vs. ketamine in both sexes). As expected, olanzapine-treated groups were not significantly different from controls (suggesting olanzapine alone had negligible effect on baseline BDNF).
Fig. 8.
BDNF mRNA expression in the prefrontal cortex of male and female rats, expressed as percentage of same-sex saline control (100%). White, black, and gray bars represent the Saline, Ketamine, and Ketamine + Olanzapine groups, respectively. Bars show mean ± SE (n = 3 per group), and *p < 0.05, *p < 0.01 vs. same-sex saline controls. Note: The olanzapine-only group is not shown, as BDNF mRNA was not measured in that condition.
A two-way ANOVA (Treatment × Sex) was conducted including only the groups involved in BDNF measurement (saline, ketamine, ket + olanzapine; note that olanzapine-only was not measured for BDNF). This revealed a significant effect of Treatment (F2, 12 = 13.61, p < 0.001). Neither the main effect of Sex (F1, 12 = 0.59, p = 0.46) nor the interaction (F2, 12 = 0.41, p = 0.67) was significant. Thus, ketamine reduced PFC BDNF expression regardless of sex, and olanzapine did not significantly restore BDNF in either sex (Fig. 8). The lack of sex difference in BDNF is notable given the behavioral sex differences, suggesting that BDNF downregulation is a robust effect of ketamine in both males and females. It also implies that olanzapine’s behavioral benefits observed in this model were achieved without significant changes in cortical BDNF expression.
In summary, our results demonstrate that sub-chronic ketamine induces a schizophrenia-like phenotype in rats, characterized by hyperlocomotion, reduced exploratory behavior, cognitive impairment, and decreased BDNF, with females showing greater sensitivity in several behavioral domains (hyperlocomotion, anxiety-like behavior, nociception, antidepressant-like effect). Olanzapine effectively reversed most ketamine-induced behavioral changes (locomotion, climbing, memory impairment, and female-specific changes in rearing, pain threshold, and FST immobility). However, olanzapine had no significant effect on the ketamine-induced decrease in BDNF expression, indicating that its therapeutic effects were likely mediated via other pathways.
Discussion
Our analyses indicate that sub-chronic ketamine induces schizophrenia-like phenotypes in rats, with some distinct differences between males and females, and that olanzapine treatment can attenuate many of these effects in both sexes. Notably, ketamine’s impact was more pronounced in female rats for several measures, and olanzapine’s restorative effects were correspondingly more evident in females for those measures. On the other hand, some effects of ketamine (e.g., hyperlocomotion and memory impairment) were substantial in both sexes and were mitigated by olanzapine in both. Importantly, olanzapine’s beneficial behavioral effects occurred without a significant increase in BDNF mRNA levels in the prefrontal cortex, suggesting that BDNF elevation is not required for olanzapine’s acute therapeutic action in this model.
Ketamine is a potent NMDA receptor antagonist, and its psychotomimetic effects in both humans and animals have been strongly linked to glutamatergic system dysfunction. Converging evidence suggests that NMDA receptor hypofunction plays a central role in the pathophysiology of schizophrenia, consistent with the glutamate hypothesis, which complements the classical dopamine hypothesis of the disorder41,42. Repeated administration of ketamine in rodents is widely used to induce behavioral phenotypes analogous to schizophrenia, including hyperlocomotion and cognitive deficits6,40.
In our study, sub-chronic ketamine treatment (30 mg/kg for five days) induced robust hyperlocomotion, consistent with previous findings. This behavioral response reflects secondary dopaminergic system activation caused by NMDA receptor blockade43. Hyperlocomotion in rodents is frequently considered a correlate of the positive symptoms of schizophrenia, such as psychomotor agitation. NMDA antagonists such as ketamine and phencyclidine reliably produce this phenotype, which is often used as a predictive model to evaluate antipsychotic efficacy, since antipsychotics typically reduce such hyperactivity35,44.
Importantly, we observed a sex difference in response to ketamine: female rats exhibited significantly more hyperactivity than males. While the literature on sex-specific locomotor responses to NMDA antagonists is limited, our findings suggest that female rodents may show heightened sensitivity to ketamine. Possible contributing factors include hormonal modulation—such as estrogen’s known effects on NMDA and dopamine receptors—as well as sex-based pharmacokinetic differences. Prior work has shown that female rats display greater deficits in prepulse inhibition following NMDA blockade (e.g., MK-801), indicating sex-related variability in glutamatergic system vulnerability19. This sex difference in hyperlocomotion highlights the necessity of including both sexes in neuropsychiatric modeling, as drug effects and behavioral phenotypes may vary substantially by sex.
In addition to hyperlocomotion, ketamine decreased rearing behavior in the open field for both sexes, with a greater decrease in females. Rearing is an exploratory behavior that is often inversely related to anxiety-like state: reduced rearing can indicate heightened anxiety or reduced curiosity in a novel environment26,31. The literature on ketamine’s effect on rearing is mixed. Some studies (e.g., a 10-day ketamine study in male rats) found increased rearing45, while others found decreases25, possibly depending on dose and context. Our result of decreased rearing suggests that ketamine-treated rats were less interested in exploring vertical space, which we interpret as an anxiety-like effect of ketamine, especially since it coincided with hyperlocomotion (often, anxiety can manifest as thigmotaxis with locomotion along walls but fewer vertical explorations)25. Observed that acute ketamine induced an anxiolytic effect (reduced anxiety) in some measures but also noted complex dose- and sex-dependent outcomes25. In our sub-chronic paradigm, it appears ketamine might increase some repetitive horizontal activity while reducing exploratory behaviors, potentially modeling the negative symptom of reduced environmental interaction. The stronger reduction in females again points to females being more affected behaviorally by ketamine’s aversive or anxiety-related effects.
Schizophrenia and related psychoses are known to alter pain perception in humans. Interestingly, we found a sex-specific effect of ketamine on nociception: female ketamine-treated rats had a significantly lower pain threshold (hyperalgesia) on the hot plate test, whereas males did not. Clinical studies have reported conflicting results on pain sensitivity in schizophrenia – some show increased pain tolerance, others show normal or increased pain sensitivity46,47. Our finding aligns with a recent meta-analysis by48 that suggests schizophrenia patients can have higher pain sensitivity in certain modalities48. In rodent models25, found that acute ketamine produced antinociception (analgesia) in both sexes25, which contrasts with our sub-chronic results of hyperalgesia in females. One possible explanation is that repeated ketamine exposure leads to adaptations (or tolerance) that reverse the acute analgesic effect into a hyperalgesic state, at least in females. Females might have different modulations of pain pathways (involving hormonal influences on endogenous opioids or NMDA receptors in spinal cord). The fact that male rats did not show altered pain threshold under ketamine suggests that the interaction between NMDA antagonism and pain perception is complex and possibly hormone-dependent. It is noteworthy that olanzapine completely normalized the pain threshold in females, suggesting an interaction with ketamine’s effect on pain. Olanzapine has some antihistaminic properties which might slightly raise pain threshold, but here it mainly acted to counteract ketamine’s hyperalgesia. This novel observation of female-specific hyperalgesia in the ketamine model and its reversal by olanzapine adds a new dimension to understanding sex differences in schizophrenia models – it parallels clinical observations that pain regulation may differ in schizophrenia and potentially differs between men and women47.
Our forced swim test (FST) results revealed an intriguing sex difference: ketamine exerted an antidepressant-like effect in female rats, evidenced by reduced immobility and increased active behaviors (climbing/swimming), whereas male rats did not exhibit this change. This aligns with the known rapid antidepressant effects of ketamine observed predominantly in females in some rodent studies and human trials. Females have been reported to respond differently to ketamine’s antidepressant properties, potentially due to hormonal milieu (e.g., estrous cycle phase can influence ketamine response). In our study, without controlling for estrous phase, the overall female response was a clear reduction in despair-like behavior. This suggests that sub-chronic ketamine might have mood-elevating or hyperactivating effects in females that it does not in males. It could be related to BDNF – ketamine’s antidepressant effect is often linked to rapid BDNF release in the hippocampus, which might be more pronounced in females (though in PFC we still saw BDNF downregulation at the transcript level). Olanzapine by itself did not induce depressive or anti-depressive effects in the FST, which is expected as olanzapine is not a classical antidepressant (though it can augment antidepressants clinically). However, olanzapine counteracted ketamine’s antidepressant-like effect in females, bringing immobility back to normal. This could be interpreted as olanzapine negating ketamine’s induction of an abnormally low immobility. In a schizophrenia context, this is interesting: patients with schizophrenia often have secondary depressive symptoms; ketamine in females reduced a “depressive-like” behavior, but olanzapine, while treating psychosis-like behaviors, may also stabilize mood to a baseline. The climbing behavior (which is often associated with noradrenergic activation) was elevated by ketamine in both sexes, reflecting increased vigor or agitation, and olanzapine reduced it. This pattern aligns with olanzapine’s general calming and antipsychotic effects (reducing agitation). In short, ketamine induced a state in females that looked like reduced “despair” (possibly modeling manic or psychosis-related lack of despair rather than true antidepressant effect), and olanzapine re-established a normal balance. The sex-specific nature of this effect again highlights how females were more behaviorally activated by ketamine.
The novel object recognition test (NORT) confirmed that ketamine causes cognitive impairment in recognition memory, a robust finding across many studies11. Both male and female ketamine-treated rats failed to recognize the novel object after 24 h, showing DIs around zero. Interestingly, some ketamine rats had slightly negative DIs, meaning they spent more time with the familiar object than the novel. This could suggest neophobic behavior or perseverative tendencies, as if they found the familiar object safer or more attractive than the novel one. This “reversed preference” might result from ketamine-induced anxiety (as discussed earlier) – the novel object might induce anxiety in an already perturbed animal, leading to avoidance. Such behavior has been noted in some models of cognitive impairment where anxiety is high. We discussed this in the context of our results: ketamine’s reduction of rearing (anxiety indicator) correlates with a possible anxiety contribution to NORT performance. Olanzapine partially improved recognition memory – ketamine + olanzapine rats did show preference for the novel object, although not as strongly as controls. This suggests olanzapine ameliorated some of the cognitive deficits. Olanzapine’s effects on cognition in schizophrenia are modest in patients (some improvement in certain domains, but it’s not primarily a pro-cognitive drug). In our model, by reducing hyperactivity and anxiety, olanzapine may have allowed better focus on objects, thereby improving memory performance indirectly. There was no sex difference here, indicating that both sexes’ cognitive function was similarly impaired by ketamine and improved by olanzapine. This is somewhat surprising given other sex differences, but it might be that the cognitive deficit was at ceiling (both sexes equally near-zero DI) and both recovered similarly.
A major finding of our study is that olanzapine’s therapeutic-like effects (reducing hyperactivity, normalizing rearing, immobility, pain threshold, and improving memory) were achieved without a measurable increase in prefrontal BDNF expression. Ketamine significantly lowered BDNF mRNA in PFC (~ 30–40% reduction), consistent with other reports6,35. Chronic stress and NMDA antagonist models often show decreased neurotrophin levels, reflecting neuroplasticity impairments. Olanzapine, despite its behavioral efficacy, did not significantly change BDNF mRNA at the acute 24 h withdrawal time point in our study. Some literature has reported increased BDNF protein or mRNA after chronic olanzapine treatment (e.g., 3–6 weeks)15, especially in the hippocampus14. Our results align with studies where short-term olanzapine had no effect on BDNF or where olanzapine failed to prevent BDNF reductions in certain models15. For example15, found that long-term olanzapine did not alter BDNF in the prefrontal cortex in a developmental stress model15. Our findings suggest that the therapeutic effects of olanzapine in this sub-chronic ketamine model are not mediated by restoring BDNF levels in the PFC. Instead, olanzapine likely works through its antagonism of dopamine D2, serotonin 5-HT2A/2 C, and other receptors to modulate neurotransmission and behavior acutely. It might influence BDNF signaling downstream or in other brain regions (e.g., hippocampus) that we did not measure, or changes might occur at the protein level later on. However, within the scope of our measurement, BDNF does not seem to be the key player in olanzapine’s acute mechanism in this model. This is an important clarification because some have hypothesized that atypical antipsychotics improve outcomes by normalizing BDNF. Our data indicate BDNF normalization is not requisite, at least in the short term.
In terms of sex differences in BDNF, we did not find any difference between males and females in the extent of BDNF reduction by ketamine or baseline BDNF levels (each sex’s data were normalized to its own control). This implies that while females were behaviorally more affected by ketamine, both sexes experienced similar BDNF downregulation in PFC. It might be that the behavioral sex differences are due to factors other than differential BDNF expression, such as divergent neurotransmitter receptor sensitivity or hormonal modulation of circuits. This disconnect between BDNF and behavior (females had worse behavior but equal BDNF drop) could mean that females might be compensating or that the critical sites of sex differences are elsewhere (perhaps BDNF in hippocampus or other factors like GABA interneuron function). It is also possible that a larger sample or protein-level assay could reveal subtle sex differences, but within our resolution, BDNF was similarly affected.
Clinical relevance: Our findings carry several implications. First, the exacerbation of certain ketamine effects in females might mirror aspects of schizophrenia where females, despite later onset, can have severe affective symptoms. It raises the question of whether women with psychosis might experience certain symptoms (like pain perception changes or mood swings) more intensely – though clinically men often have worse negative symptoms, so the translation is not direct. Second, olanzapine’s effectiveness in reversing ketamine effects in both sexes reinforces its broad efficacy on positive-like symptoms and cognitive deficits in this model. The fact that olanzapine did not induce sedation in our rats is interesting; clinically, olanzapine can cause sedation, but our timing (3 h post-dose) might parallel the situation in patients who acclimate to the sedative effects over time. Third, the lack of BDNF involvement suggests that other pathways (possibly downstream signaling like GSK-3β or AKT, which olanzapine affects12,49 are responsible for the functional improvements. This aligns with a study by49 which showed olanzapine upregulated BDNF in stressed rats’ hippocampi49, but in our PFC-focused study, perhaps BDNF changes were not needed or would require more time to manifest. It underscores that treatment strategies should not rely solely on boosting BDNF when addressing schizophrenia symptoms, since symptom improvement can occur via BDNF-independent mechanisms.
Limitations
We acknowledge some limitations in our study. We did not control for the estrous cycle in female rats, which could introduce variability. Despite that, the effects in females were robust; future studies could examine if certain estrous phases show amplified ketamine responses. Our BDNF measurement was at the mRNA level; changes in protein levels or in other regions (like hippocampus) were not assessed but could be informative. We also focused on one dose and timing of olanzapine; chronic olanzapine treatment or other atypical antipsychotics might yield different outcomes on BDNF and behavior. Additionally, while our battery was comprehensive, performing multiple tests on the same day (especially hot plate and FST) could influence each other; we mitigated this by sequence and separate NORT groups, but some carry-over stress could occur. However, the clear results suggest any such influence was minimal or uniform across groups.
Conclusion
In conclusion, this study provides new insight into sex-specific effects in a ketamine-based schizophrenia model and the mechanism of olanzapine’s action. For the first time, we demonstrated that sub-chronic ketamine induces a broader spectrum of behavioral abnormalities in female rats than in males, notably affecting pain sensitivity and depression-like behavior only in females, while disrupting locomotion and memory in both sexes. We also showed that olanzapine can effectively reverse ketamine-induced behavioral deficits in both male and female rats, supporting its efficacy in treating schizophrenia-like symptoms in our model. However, olanzapine’s therapeutic effects do not appear to depend on elevating BDNF levels in the prefrontal cortex, suggesting that its benefits are mediated by other neurochemical pathways (e.g., receptor modulation and downstream signaling). These findings highlight the importance of considering sex differences in preclinical models of schizophrenia and suggest that treatments like olanzapine can restore function through BDNF-independent mechanisms. Further research on other molecular targets will help clarify how antipsychotics ameliorate schizophrenia-related behaviors and whether additional interventions (such as BDNF mimetics or neuroplasticity enhancers) could provide synergistic benefits, particularly in female patients. Overall, our study reinforces the translational relevance of the ketamine model for schizophrenia and underscores that sex is a crucial biological variable influencing both pathophysiology and treatment response.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Authors’ ContributionsKalin Manooki: Conceptualization, data collection, and manuscript preparation.Mahdieh Gholami: Data interpretation and initial manuscript drafting.Maryam Eslami: Statistical analysis, data validation, and manuscript revision.Salar Vaseghi: Data interpretation, critical review, and manuscript editing.Batool Ghorbani Yekta: Supervision, project administration, and final approval of the manuscript.
Data availability
The datasets generated and analyzed during this study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Ethical approval for this study was obtained from the Islamic Azad University, Science and Research Branch, under the ethical reference number IR.IAU.PS.REC.1403.203. All experimental procedures complied with the ethical standards outlined in the 2011 U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals, ensuring the humane and ethical treatment of animals throughout the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kahn, R. et al. Schizophrenia Nat. Rev. Dis. Primers ; 1: 15067. (2015). [DOI] [PubMed] [Google Scholar]
- 2.Mosolov, S. N. & Yaltonskaya, P. A. Primary and secondary negative symptoms in schizophrenia. Front. Psychiatry. 12, 766692 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker, A. et al. Ketamine-induced changes in rat behaviour: a possible animal model of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 27 (4), 687–700 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Tekin, N., Karamahmutoğlu, T. E., Aykaç, A., Akakın, D. & Gören, M. Z. The α2C-adrenoceptor antagonist JP-1302 controls behavioral parameters, tyrosine hydroxylase activity and receptor expression in a rat model of ketamine-induced schizophrenia-like deficits. Pharmacol. Biochem. Behav.221, 173490 (2022). [DOI] [PubMed] [Google Scholar]
- 5.Huang, L. Effects of antipsychotics on the BDNF in schizophrenia. Curr. Med. Chem.20 (3), 345–350 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Gholizadeh, N. et al. Berberine improves inhibitory avoidance memory impairment of Toxoplasma gondii-infected rat model of ketamine-induced schizophrenia. BMC Complement. Med. Ther.23 (1), 303 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green, M. J., Matheson, S., Shepherd, A., Weickert, C. & Carr, V. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol. Psychiatry. 16 (9), 960–972 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Hale, A. & Olanzapine Br. J. Hosp. Med. ;58(9):442–445. (1997). [PubMed] [Google Scholar]
- 9.Thomas, K. & Saadabadi, A. Olanzapine. Treasure Island, FL (StatPearls Publishing, 2022). [PubMed]
- 10.Mahmoud, G. S., Hosny, G. & Sayed, S. A. The protective effect of olanzapine on ketamine induced cognitive deficit and increased NR1 expression in rat model of schizophrenia. Int. J. Physiol. Pathophysiology Pharmacol.13 (2), 22 (2021). [PMC free article] [PubMed] [Google Scholar]
- 11.Białoń, M., Żarnowska, M., Antkiewicz-Michaluk, L. & Wąsik, A. Pro-cognitive effect of 1MeTIQ on recognition memory in the ketamine model of schizophrenia in rats: the behavioural and neurochemical effects. Psychopharmacology237, 1577–1593 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seo, M. K. et al. Effects of antipsychotic drugs on the epigenetic modification of Brain-Derived neurotrophic factor gene expression in the hippocampi of chronic restraint stress rats. Neural Plast.2018 (1), 2682037 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han, D. et al. Association between the improvement in depressive symptoms and serum BDNF levels in drug-naive first episode patients with schizophrenia: a longitudinal follow-up. Psychoneuroendocrinology133, 105392 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Nowakowska, M., Herbinet, O., Dufour, A. & Glaude, P-A. Detailed kinetic study of anisole pyrolysis and oxidation to understand Tar formation during biomass combustion and gasification. Combust. Flame. 161 (6), 1474–1488 (2014). [Google Scholar]
- 15.Issa, G., Wilson, C., Terry, A. V. Jr & Pillai, A. An inverse relationship between cortisol and BDNF levels in schizophrenia: data from human postmortem and animal studies. Neurobiol. Dis.39 (3), 327–333 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Abel, K. M., Drake, R. & Goldstein, J. M. Sex differences in schizophrenia. Int. Rev. Psychiatry. 22 (5), 417–428 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Seney, M. L. & Sibille, E. Sex differences in mood disorders: perspectives from humans and rodent models. Biology Sex. Differences. 5, 1–10 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung, M. D. D. A., Chue, M. R. C. & Psych, D. P. Sex differences in schizophrenia, a review of the literature. Acta Psychiatry. Scand.101 (401), 3–38 (2000). [DOI] [PubMed] [Google Scholar]
- 19.Zhao, Y-Y. et al. Neonatal MK-801 treatment differentially alters the effect of adolescent or adult MK-801 challenge on locomotion and PPI in male and female rats. J. Psychopharmacol.27 (9), 845–853 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Dong, R., Zhao, N. O., Wu, H. E., Yu, L. & Zhang, X. Y. Sex differences in the association between serum BDNF and cognitive impairment in schizophrenia patients using various antipsychotics. J. Psychiatr. Res.138, 492–499 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Faizi, M., Salimi, A., Rasoulzadeh, M., Naserzadeh, P. & Pourahmad, J. Schizophrenia induces oxidative stress and cytochrome C release in isolated rat brain mitochondria: a possible pathway for induction of apoptosis and neurodegeneration. Iran. J. Pharm. Research: IJPR. 13 (Suppl), 93 (2014). [PMC free article] [PubMed] [Google Scholar]
- 22.Lisek, M. et al. Glutamate deregulation in ketamine-induced psychosis—a potential role of PSD95, NMDA receptor and PMCA interaction. Front. Cell. Neurosci.11, 181 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parasuraman, S., Zhen, K. M., Banik, U. & Christapher, P. V. Ameliorative effect of Curcumin on olanzapine-induced obesity in Sprague-Dawley rats. Pharmacognosy Res.9 (3), 247 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agostinho, F. R. et al. Treatment with olanzapine, Fluoxetine and olanzapine/fluoxetine alters citrate synthase activity in rat brain. Neurosci. Lett.487 (3), 278–281 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Radford, K. D., Berman, R. Y., Zhang, M., Wu, T. J. & Choi, K. H. Sex-related differences in intravenous ketamine effects on dissociative stereotypy and antinociception in male and female rats. Pharmacol. Biochem. Behav.199, 173042 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Sturman, O., Germain, P-L. & Bohacek, J. Exploratory rearing: a context-and stress-sensitive behavior recorded in the open-field test. Stress21 (5), 443–452 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Arpacı, A. H., Çalışkan, H., Güneş, E. & Işık, B. Effects of the recurrent and different doses of ketamine exposure on anxiety-like behaviors and locomotor activity in juvenile rats. Curr. Alzheimer Res.19 (14), 933–942 (2022). [DOI] [PubMed] [Google Scholar]
- 28.Ghofrani-Jahromi, Z. et al. Psilocybe Cubensis extract potently prevents fear memory recall and freezing behavior in short-but not long-term in a rat model of posttraumatic stress disorder. Behav. Neurosci.138 (2), 73 (2024). [DOI] [PubMed] [Google Scholar]
- 29.De Brouwer, G., Fick, A., Harvey, B. H. & Wolmarans, D. W. A critical inquiry into marble-burying as a preclinical screening paradigm of relevance for anxiety and obsessive–compulsive disorder: Mapping the way forward. Cognitive, Affective, & Behavioral Neuroscience. ;19:1–39. (2019). [DOI] [PubMed]
- 30.Garcia-Partida, J. A. et al. The effects of Mango leaf extract during adolescence and adulthood in a rat model of schizophrenia. Front. Pharmacol.13, 886514 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tucker, L. B. & McCabe, J. T. Measuring anxiety-like behaviors in rodent models of traumatic brain injury. Front. Behav. Neurosci.15, 682935 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romero-Miguel, D. et al. Maternal supplementation with N-Acetylcysteine modulates the microbiota-gut-brain axis in offspring of the Poly I: C rat model of schizophrenia. Antioxidants12 (4), 970 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nasseri, S., Hajrasouliha, S., Vaseghi, S., Ghorbani Yekta B. Interaction effect of crocin and citalopram on memory and locomotor activity in rats: an insight into BDNF and synaptophysin levels in the hippocampus. Naunyn Schmiedebergs Arch Pharmacol.397(9), 6879–6888. 10.1007/s00210-024-03069-9 (2024). [DOI] [PubMed]
- 34.Tajabadi Farahani, Z., Vaseghi, S., Rajabbeigi, E., Ghorbani Yekta B. The effect of olanzapine on spatial memory impairment, depressive-like behavior, pain perception, and BDNF and synaptophysin expression following childhood chronic unpredictable mild stress in adult male and female rats. Behav Brain Res.468, 115039. 10.1016/j.bbr.2024.115039 (2024). [DOI] [PubMed]
- 35.Xu, Y., Deng, C., Zheng, Y., Liu, N. & Fu, B. Applying vinpocetine to reverse synaptic ultrastructure by regulating BDNF-related PSD-95 in alleviating schizophrenia-like deficits in rat. Compr. Psychiatr.94, 152122 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Smith, C. A. & Holahan, M. R. Reduced hippocampal dendritic spine density and BDNF expression following acute postnatal exposure to Di (2-ethylhexyl) phthalate in male long Evans rats. PloS One. 9 (10), e109522 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsukinoki, K. et al. Immobilization stress induces BDNF in rat submandibular glands. J. Dent. Res.85 (9), 844–848 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Rahimi-Danesh, M., Samizadeh, M-A., Sajadi, A-E., Rezvankhah, T. & Vaseghi, S. Sex difference affects fear extinction but not lithium efficacy in rats following fear-conditioning with respect to the hippocampal level of BDNF. Pharmacol. Biochem. Behav.234, 173675 (2024). [DOI] [PubMed] [Google Scholar]
- 39.Lotfi, G., Eslami, M., Gholami, M., Vaseghi, S., Yekta BG. Exploring Crocin’s Role in Alleviating Memory Impairments and Depression-like Behaviors Induced by REM Sleep Deprivation, Focusing on BDNF and GSK-3β in Male Rats. Mol Neurobiol.62(7), 8638-8651. 10.1007/s12035-025-04753-4 (2025). [DOI] [PubMed]
- 40.Valvassori, S. S. et al. Haloperidol alters neurotrophic factors and epigenetic parameters in an animal model of schizophrenia induced by ketamine. Int. J. Dev. Neurosci.83 (8), 691–702 (2023). [DOI] [PubMed] [Google Scholar]
- 41.Coyle, J. T., Tsai, G. & Goff, D. Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia. Ann. N. Y. Acad. Sci.1003 (1), 318–327 (2003). [DOI] [PubMed] [Google Scholar]
- 42.Konradi, C. & Heckers, S. Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment. Pharmacol. Ther.97 (2), 153–179 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Powell, C. M. & Miyakawa, T. Schizophrenia-relevant behavioral testing in rodent models: a uniquely human disorder? Biol. Psychiatry. 59 (12), 1198–1207 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gainetdinov, R. R., Mohn, A. R. & Caron, M. G. Genetic animal models: focus on schizophrenia. Trends Neurosci.24 (9), 527–533 (2001). [DOI] [PubMed] [Google Scholar]
- 45.Sanavi, M. A., Ghazvini, H., Zargari, M., Ghalehnoei, H. & Hosseini-Khah, Z. Effects of clozapine and Risperidone antipsychotic drugs on the expression of CACNA1C and behavioral changes in rat ‘ketamine model of schizophrenia. Neurosci. Lett.770, 136354 (2022). [DOI] [PubMed] [Google Scholar]
- 46.Engels, G. et al. Clinical pain in schizophrenia: a systematic review. J. Pain. 15 (5), 457–467 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Lévesque, M. et al. Pain perception in schizophrenia: evidence of a specific pain response profile. Pain Med.13 (12), 1571–1579 (2012). [DOI] [PubMed] [Google Scholar]
- 48.Aurora, G-T. & Miguel, H-V. Pain sensitivity in patients with schizophrenia: a systematic review and meta-analysis. Actas Esp. Psiquiatr.51 (1), 29 (2023). [PMC free article] [PubMed] [Google Scholar]
- 49.Park, S. W. et al. Effects of antipsychotic drugs on BDNF, GSK-3β, and β-catenin expression in rats subjected to immobilization stress. Neurosci. Res.71 (4), 335–340 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during this study are available from the corresponding author upon reasonable request.