Abstract
Timely entry into mitosis requires activation of Polo-like kinase 1 (Plk1) by Aurora kinase A (Aurora A), but the upstream signaling trigger remains unclear. Here, we show that cyclic AMP (cAMP) signaling serves as a critical initiator of mitosis in mammalian cells. Specifically, the cAMP-dependent protein kinase (PKA) phosphorylates Bora, enabling it to bind Aurora A and recruit it to the Bora-Plk1 complex during G2 phase, thereby facilitating Aurora A-dependent activation of Plk1. Disruption of PKA-mediated Bora phosphorylation or the Bora-Aurora A interaction impairs Plk1 activation and delays the G2-to-mitosis (G2/M) transition. Conversely, a phospho-mimetic Bora mutant bypasses the requirement for PKA in promoting Bora-Aurora A interaction, Plk1 activation, and mitotic entry. Furthermore, PKA-mediated Bora phosphorylation and the resulting Bora-Aurora A interaction are essential for mitotic entry during DNA damage checkpoint recovery. Together, these findings identify the cAMP-PKA-Bora-Aurora A-Plk1 signaling cascade as a previously unrecognized and critical trigger for mitotic commitment.
Subject terms: Mitosis, Checkpoint signalling, Phosphorylation
Zhu et al. show that cAMP-dependent PKA phosphorylates Bora, enabling Aurora A binding and Plk1 activation. This modification ensures timely mitotic entry, unveiling a previously unrecognized interface between PKA signaling and cell cycle regulation.
Introduction
In the eukaryotic cell cycle, the genome is duplicated during S phase and partitioned in M phase. These two phases are separated by the gap phases G1 and G2, which regulate entry into S phase and mitosis, respectively1. A sophisticated regulatory network ensures that these events occur in a precise order and with proper timing. Disruption of this regulation can lead to severe consequences, including cancer and developmental disorders2.
Cyclins and their catalytic partners, cyclin-dependent kinases (Cdks), are central regulators of cell-cycle progression3. In higher eukaryotes, oscillations in cyclin levels govern Cdk activity. For example, during G1, mitogenic signals induce the transcription of G1-cyclin (cyclin D) and G1/S-cyclin (cyclin E), thereby promoting progression through the G1-to-S (G1/S) transition (Fig. 1A)4. After passing the G1/S boundary, cells replicate their DNA in S phase and enter G2 in preparation for mitosis. The G2-to-mitosis (G2/M) transition is driven by Cdk1 bound to M-cyclin (cyclin B), and the resulting cyclin B-Cdk1 complex phosphorylates numerous substrates required for mitotic progression5. However, cyclin B-Cdk1 remains inactive during early G2 due to inhibitory phosphorylation of Cdk1 by the Wee1 kinase6–8. This inhibition is relieved at the end of G2 when Cdc25 phosphatases remove the inhibitory phosphate group9–12. Both Wee1 and Cdc25 are themselves regulated by feedback loops involving cyclin B-Cdk1 activity13–16.
Fig. 1. PKA ensures timely progression through the G2/M transition.
A Schematic illustration of cell-cycle regulation at the G1/S and G2/M transitions. The initiating signal for the G2/M transition remains unknown. B HEK-293T cells transiently expressing Flag-Bora were subjected to immunoprecipitation (IP) using Flag-tag antibody-conjugated beads, followed by mass spectrometry. Kinases co-immunoprecipitated with Flag-Bora are listed, along with sequence coverage, number of matched peptides, and identification scores. C HEK-293T cells transiently expressing Flag-Bora were immunoprecipitated using Flag-tag antibody-conjugated or control protein A/G beads, followed by immunoblotting for PKA-Cα, Plk1, and Flag-tag. S. exp. short exposure, L. exp. long exposure. D, E Schematic of the experimental setup for double-thymidine block and release (DTR) combined with siRNA transfection (D). HeLa cells were transfected with control or PKA-Cα siRNA and subjected to double-thymidine treatment. Two days after transfection, cells were released into fresh medium containing nocodazole. Lysates were collected at the indicated time points post-DTR, and immunoblotted for H3S10ph, H3, PKA-Cα, and GAPDH (E). F, G HeLa cells were transfected with control, PKA-Cα, Bora, or Aurora A siRNA and synchronized by double-thymidine treatment. Two days after transfection, cells were released into fresh medium containing nocodazole and collected at the indicated time points post-DTR for flow cytometric analysis of DNA content. The percentage of cells in G1 and G2/M phases was quantified based on Supplementary Fig. 1G (F). Lysates collected at 8 h post-DTR were immunoblotted for PKA-Cα, Bora, Aurora A, and α-Tubulin (G). H–J Schematic of the experimental setup for RO-3306 treatment combined with siRNA transfection and mitotic index analysis (H). HeLa cells were transfected with control, Bora, or PKA-Cα siRNA and treated with RO-3306 for 24 h. Two days after transfection, cells were released into fresh medium containing nocodazole and analyzed for mitotic index at the indicated time points. The mitotic index was determined in approximately 3000 cells per condition across three independent experiments (I). Lysates collected 60 min after RO-3306 release were immunoblotted for PKA-Cα, Bora, and α-Tubulin (J). K, L Schematic of the DTR experiment combined with H-89 or DMSO treatment. HeLa cells were synchronized using a double-thymidine block and released into a nocodazole-containing medium with H-89 or DMSO (K). Lysates collected at the indicated time points post-DTR were immunoblotted for H3S10ph, H3, and GAPDH (L). M HeLa cells were subjected to double-thymidine treatment and released into fresh medium containing nocodazole with either H-89 or DMSO. Cells were collected at the indicated time points post-DTR for flow cytometric analysis of DNA content. The percentage of cells in G1 and G2/M phases was quantified based on Supplementary Fig. 1J. N, O Schematic of the experimental design for RO-3306 and H-89 treatment followed by mitotic index analysis (N). HeLa cells were treated with RO-3306 for 24 h. One hour prior to release from RO-3306, cells were pretreated with H-89 or DMSO for 1 h. Cells were then released into fresh medium containing nocodazole along with H-89 or DMSO and analyzed for mitotic index at the indicated time points. The mitotic index was determined in around 3000 cells per condition across three independent experiments (O). P, Q RPE-1 cells were transfected with control, Bora, or PKA-Cα siRNA and treated with RO-3306 for 16 h. Two days after transfection, cells were released into fresh medium containing nocodazole, and the mitotic index was assessed at the indicated time points. The mitotic index was determined in around 3000 cells per condition across three independent experiments (P). Lysates collected at 60 min after RO-3306 release were immunoblotted for PKA-Cα, Bora, and α-Tubulin (Q). R RPE-1 cells were treated with RO-3306 for 16 h. One hour prior to release, cells were pretreated with H-89 or DMSO. Cells were then released into fresh medium containing nocodazole and either H-89 or DMSO, and analyzed for mitotic index at the indicated time points. The mitotic index was assessed in approximately 2100 cells per condition across three independent experiments. Data are representative of two independent experiments in (C, E, G, L) and three independent experiments in (J, Q). Means and standard deviations (SDs) are shown in (I, O, P, R). Symbols in (F), (M): Triangles, G1; Squares, G2/M. Source data are provided as a Source data file.
Polo-like kinase 1 (Plk1) is a key activator of cyclin B-Cdk1. Plk1 promotes Wee1 degradation and Cdc25 activation, thereby enabling rapid activation of cyclin B-Cdk117–19. It also facilitates nuclear translocation of Cdc25C, one of the three Cdc25 phosphatases, and has been implicated, though controversially, in regulating cyclin B localization during prophase20–22. In human cells, Plk1-mediated phosphorylation of Cdc25C alone can be sufficient to initiate mitosis23. Although Plk1 is a well-established trigger of cyclin B-Cdk1 activation24, the upstream intracellular signal responsible for Plk1 activation remains unidentified16,25–35.
Plk1 activation requires phosphorylation of a conserved threonine residue (Thr210 in human Plk1) within its activation loop36. During G2, this phosphorylation is catalyzed by Aurora kinase A (Aurora A) in a reaction that critically depends on Bora37,38. Bora, initially identified in Drosophila as an Aurora A co-factor39, accumulates along with Aurora A during the G2 phase38,40,41. However, it remains unclear whether, and how, the Bora-Aurora A interaction is regulated to promote Plk1 activation.
In this study, we identify cyclic AMP (cAMP) as an upstream signal that initiates mitotic entry. We show that the cAMP-dependent protein kinase (PKA) phosphorylates Bora, enabling its interaction with Aurora A and subsequent activation of Plk1 during G2 phase. Disruption of this cAMP-PKA-Bora-Aurora A-Plk1 axis delays the G2/M transition, while a phospho-mimetic Bora mutant bypasses the requirement for PKA. These findings define this signaling cascade as a previously unrecognized and critical trigger for mitotic commitment.
Results
PKA ensures timely progression through the G2/M transition
To better understand Bora’s role in Plk1 activation, we used mass spectrometry-based proteomics to identify its phosphorylation sites and interacting proteins. Flag-tagged Bora (Flag-Bora) was immunoprecipitated from asynchronously growing HEK-293T cells and subjected to mass spectrometry analysis. We identified 40 serine/threonine phosphorylation sites in Bora, including 20 not previously reported in the PhosphoSitePlus database (Supplementary Fig. 1A and Supplementary Data 1). These findings prompted us to identify Bora-associated kinases in cells.
Mass spectrometry of Flag-Bora immunoprecipitates identified six protein kinases (IRAK1, RIOK1, MAP3K7, Plk1, PKA-Cα, and RIPK1), each represented by at least two detected peptides (Fig. 1B and Supplementary Data 2). Co-immunoprecipitation confirmed the interaction between Flag-Bora and Plk1, consistent with previous reports38,40,41. To assess the ability of these kinases to phosphorylate Bora, we excluded IRAK1 and RIPK1 as candidates due to the absence of canonical phosphorylation motifs (R/K/H-R/K/H-S/T-R-R) in Bora42. HEK-293T cells were then transfected with Bora-Myc or co-transfected with Bora-Myc and SFB-tagged RIOK1, MAP3K7, or PKA-Cα. Immunoprecipitation of Bora-Myc followed by immunoblotting with pan-phospho-Ser/Thr polyclonal antibodies revealed robust phosphorylation of Bora upon co-expression with PKA-Cα, but not with RIOK1 or MAP3K7 (Supplementary Fig. 1B), suggesting a potential role for PKA-Cα in Bora regulation. Control experiments confirmed that SFB-RIOK1 and SFB-MAP3K7 effectively phosphorylated their known targets, G3BP2 and ATAT1, respectively (Supplementary Fig. 1C, D).
PKA-Cα is the ubiquitously expressed catalytic subunit of PKA43. Immunoblot analysis following co-immunoprecipitation showed a specific interaction between Flag-Bora and endogenous PKA-Cα, although weaker than the interaction between Bora and Plk1 (Fig. 1C). Immunofluorescence microscopy in HeLa cells further showed that PKA-Cα localized to both the cytoplasm and centrosomes in G2 phase (Supplementary Fig. 1E). This is consistent with the reported cytoplasmic localization of Bora44–46, and supports our observation of Bora-PKA-Cα interaction.
We then investigated whether PKA plays a role in cell-cycle control. We first employed double-thymidine release (DTR) synchronization to assess the effect of PKA-Cα knockdown on cell-cycle progression from the G1/S boundary to mitosis. HeLa cells were transfected with control siRNA or PKA-Cα siRNA, then subjected to two rounds of thymidine block (Fig. 1D). After release into fresh medium containing nocodazole, a spindle poison used to block mitotic exit, cells were collected at various time points to assess the cumulative mitotic index using histone H3 Ser10 phosphorylation (H3S10ph) as a mitotic marker. PKA-Cα knockdown strongly reduced H3S10ph accumulation compared to control cells (Fig. 1E). We confirmed that PKA-Cα knockdown did not affect Aurora B-mediated H3S10ph in nocodazole-arrested mitotic cells (Supplementary Fig. 1F), suggesting that PKA-Cα depletion delays but does not block mitotic entry. Fluorescence-activated cell sorting (FACS) analysis showed that PKA-Cα knockdown did not affect cell-cycle progression from early S phase to G2 phase in DTR-synchronized HeLa cells (Fig. 1F, G and Supplementary Fig. 1G; see also Supplementary Fig. 8). Additionally, our observation of normal S-phase progression in Bora-depleted cells is consistent with previous findings38. These results indicate that PKA-Cα knockdown delays progression from the G1/S boundary into mitosis without impairing S-phase progression.
Next, we assessed the impact of PKA-Cα knockdown on mitotic entry following release from late-G2 arrest induced by the Cdk1 inhibitor RO-330647. HeLa cells transfected with control, PKA-Cα, or Bora siRNAs were treated with RO-3306 for 24 h, then released into nocodazole-containing medium and monitored by live imaging to determine the cumulative mitotic index (Fig. 1H). At 30 min and 60 min after RO-3306 removal, 27.9% and 34.8% of control siRNA-transfected cells, respectively, were in mitosis (Fig. 1I, J and Supplementary Fig. 1H), indicating rapid mitotic entry. In contrast, mitotic entry was observed in only 11.7% and 15.9% of PKA-Cα-depleted cells at the corresponding time points, similar to the results observed in cells transfected with Bora siRNA. These results indicate that PKA-Cα depletion delays progression from the late-G2 phase into mitosis, akin to Bora depletion38.
To further evaluate if PKA activity contributes to cell-cycle progression, we treated HeLa cells released from DTR with nocodazole and H-89, a small-molecule inhibitor of PKA (Fig. 1K)48, and found that H-89 treatment significantly reduced H3S10ph accumulation (Fig. 1L). Additionally, the H3S10ph signal remained unaffected when nocodazole-arrested mitotic cells were treated with H-89, while phosphorylation of CREB at Ser133 (CREB-S133ph), a known PKA target, was dramatically diminished (Supplementary Fig. 1I). Thus, once cells are arrested in mitosis by nocodazole, PKA activity is not required to maintain H3S10 phosphorylation. FACS analysis confirmed that H-89 did not affect S-phase progression in DTR-synchronized HeLa cells (Fig. 1M and Supplementary Fig. 1J). Thus, H-89 treatment delays mitotic entry without perturbing S-phase progression.
Next, we evaluated the impact of transiently inhibiting PKA activity during the late-G2 phase on the G2/M transition. When HeLa cells were released from RO-3306-induced late-G2 phase arrest, H-89 treatment in the presence of nocodazole markedly reduced mitotic cell accumulation in a dose-dependent manner (Fig. 1N, O and Supplementary Fig. 1K). Hence, acute inhibition of PKA kinase activity in HeLa cells during the late-G2 phase results in a delayed transition to mitosis. Similarly, mitotic entry after release from RO-3306 was delayed in non-transformed RPE-1 cells following either PKA-Cα depletion or H-89 treatment (Fig. 1P–R).
Together, these results establish PKA as a Bora-interacting kinase essential for timely G2/M transition.
PKA phosphorylates Bora at Ser59 (S59)
Next, we sought to identify the specific phosphorylation site(s) on Bora targeted by PKA. Co-expression of PKA-Cα-GFP with various fragments of Bora-Myc in HEK-293T cells allowed the assessment of Bora phosphorylation using the pan-phospho-Ser/Thr antibody. Our results revealed that overexpression of PKA-Cα-GFP resulted in robust phosphorylation of the Bora-Myc fragment spanning amino acids 1-100, while no detectable phosphorylation was observed in fragments 101-200, 201-310, or 311-559 (Supplementary Fig. 2A). Furthermore, co-expression with PKA-Cα-GFP led to strong phosphorylation of Bora (41-100)-Myc, but not Bora (1-40)-Myc (Supplementary Fig. 2B), suggesting that residues 41-100 of Bora contain serine/threonine residue(s) susceptible to phosphorylation by PKA-Cα.
In the human Bora (41-100) fragment, three serine/threonine residues were unambiguously identified as phosphorylated sites (see Supplementary Fig. 1A), among which S59 is conserved across vertebrates and lies within a sequence resembling the PKA consensus motif, [R/K/x] - [R/K] - x - [S/T] - Φ (Fig. 2A)42, where “x” denotes any amino acid and “Φ” represents a hydrophobic residue. Immunoblot analysis using the pan-phospho-Ser/Thr antibody revealed that overexpression of PKA-Cα-GFP substantially phosphorylated full-length Bora-Myc, but not the Bora-S59A-Myc mutant in which S59 was replaced with alanine (Supplementary Fig. 2C), indicating that S59 is a likely PKA-Cα phosphorylation site in cells.
Fig. 2. PKA phosphorylates Bora at S59.
A Multiple sequence alignment of Bora proteins surrounding the conserved residue S59. S59 is highlighted in red, and the surrounding conserved residues are highlighted in gray. B HeLa cells stably expressing Bora-Myc (WT or S59A) were immunoblotted for Bora-S59ph, Myc-tag, and α-Tubulin. C HeLa cells stably expressing Bora-Myc were synchronized using a double-thymidine block. After the second thymidine treatment, cells were released into fresh medium containing nocodazole. Lysates were collected at the indicated time points post-DTR and immunoblotted for Bora-S59ph, Myc-tag, H3S10ph, H3, Plk1, Aurora A, and α-Tubulin. D HeLa cells stably expressing Bora-Myc were transiently transfected with PKA-Cα-GFP. At 24 h post-transfection, cells were immunoblotted for Bora-S59ph, Myc-tag, GFP, and α-Tubulin. E HeLa cells stably expressing Bora-Myc were transfected with control or PKA-Cα siRNA. At 48 h post-transfection, cells were immunoblotted for Bora-S59ph, Myc-tag, PKA-Cα, and α-Tubulin. F HeLa cells stably expressing Bora-Myc were treated with H-89 or DMSO for 4 h and then subjected to immunoblotting for Bora-S59ph, Myc-tag, and α-Tubulin. G HeLa cells stably expressing Bora-Myc were treated with 8-Br-cAMP or DMSO for 30 min, followed by immunoblotting with antibodies for Bora-S59ph, Myc-tag, and α-Tubulin. H HeLa cells stably expressing Bora-Myc were treated with Forskolin or DMSO for 30 min and then subjected to immunoblotting for Bora-S59ph, Myc-tag, and α-Tubulin. I MBP-Bora was subjected to an in vitro kinase reaction with GST-PKA-Cα, followed by immunoblotting for Bora-S59ph, MBP, and GST. Data are representative of three independent experiments in (B, D–I) and two independent experiments in (C). Source data are provided as a Source data file.
We developed rabbit polyclonal antibodies that specifically recognize Bora phosphorylated at S59. We stably expressed wild-type (referred to as WT where necessary) Bora-Myc and the Bora-S59A-Myc mutant, engineered with silent mutations in the siRNA target region, in HeLa cells (Fig. 2B). We noticed that these proteins were expressed at levels much higher than that of endogenous Bora (Supplementary Fig. 2D). Regardless, stably overexpressed Bora-Myc co-immunoprecipitated endogenous PKA-Cα and Plk1 (Supplementary Fig. 2E), as observed with transiently expressed Flag-Bora (see Fig. 1C). Immunoblot analysis using these antibodies confirmed phosphorylation of Bora at S59 (Bora-S59ph) in asynchronous HeLa cells stably expressing Bora-Myc (Fig. 2B). The absence of signal in cells expressing the Bora-S59A-Myc mutant validated antibody specificity and confirmed that the detected signal represents authentic Bora-S59 phosphorylation in cells. Immunoblot analysis of Bora-Myc-expressing HeLa cells released from the second round of thymidine treatment showed that Bora-S59 phosphorylation mirrored the kinetics of Bora-Myc accumulation: it began in early S phase, peaked in late G2, and declined in mitosis (Fig. 2C).
We then examined the role of PKA-Cα in Bora-S59 phosphorylation. Bora-S59 phosphorylation was strongly enhanced in cells overexpressing PKA-Cα-GFP (Fig. 2D). Moreover, knockdown of PKA-Cα markedly reduced Bora-S59 phosphorylation (Fig. 2E). Similarly, pharmacological inhibition of PKA activity with H-89 substantially decreased the level of Bora-S59ph (Fig. 2F). Consistent with the regulation of PKA activity by cellular cAMP levels49, Bora-S59 phosphorylation was dramatically elevated following brief treatment with 8-Br-cAMP (Fig. 2G), a non-degradable cAMP analog. Furthermore, Bora-S59 phosphorylation was strongly increased following Forskolin treatment (Fig. 2H), a diterpenoid that activates adenylate cyclase and elevates intracellular cAMP. These results indicate that Bora-S59 phosphorylation in cells primarily depends on PKA activity.
To determine whether PKA can directly phosphorylate Bora at S59, we performed in vitro kinase reactions using bacterially expressed recombinant proteins. Glutathione S-transferase (GST)-fused PKA-Cα (GST-PKA-Cα) efficiently phosphorylated maltose-binding protein (MBP)-tagged Bora (MBP-Bora) at S59 in the presence of ATP (Fig. 2I). Similar results were obtained using immunopurified PKA-Cα-GFP from HEK-293T cells (Supplementary Fig. 2F).
HeLa cells, like many somatic cell types, express two major catalytic isoforms of PKA: PKA-Cα and PKA-Cβ49. In vitro kinase assays showed that GST-PKA-Cβ could also phosphorylate MBP-Bora at S59 (Supplementary Fig. 2G). However, overexpression or knockdown of PKA-Cβ had no detectable effect on Bora-S59ph levels in HeLa cells (Supplementary Fig. 2H, I).
Taken together, these findings demonstrate that PKA-Cα is the physiologically relevant kinase responsible for Bora-S59 phosphorylation in cells.
PKA ensures timely G2/M transition by phosphorylating Bora-S59
We next investigated the effect of S59 phosphorylation on Bora’s function in facilitating mitotic entry. Following double-thymidine block and release into nocodazole, control siRNA-transfected cells stably expressing the phosphorylation-deficient Bora-S59A-Myc mutant entered mitosis at rates comparable to those expressing wild-type Bora-Myc (Fig. 3A). In contrast, when endogenous Bora was depleted, cells expressing the Bora-S59A-Myc mutant exhibited a marked delay in mitotic entry compared to those expressing Bora-Myc (Fig. 3B). These results indicate that Bora-S59A is defective in supporting timely progression from the G1/S boundary into mitosis.
Fig. 3. PKA ensures timely G2/M transition by phosphorylating Bora-S59.
A, B HeLa cells stably expressing siRNA-resistant Bora-Myc (WT or S59A) were transfected with control siRNA (A) or Bora siRNA (B) and subjected to double-thymidine treatment. Two days after transfection, cells were released into fresh medium containing nocodazole. Lysates were collected at the indicated time points post-DTR and immunoblotted for H3S10ph, H3, and GAPDH. C Parental HeLa cells and HeLa cells stably expressing siRNA-resistant Bora-Myc (WT, S59A, or S59D) were immunoblotted for Myc-tag and α-Tubulin. D, E HeLa cells stably expressing siRNA-resistant Bora-Myc (WT or S59D) were transfected with control siRNA (D) or Bora siRNA (E) and subjected to double-thymidine treatment. Two days after transfection, cells were released into fresh medium containing nocodazole. Lysates were collected at the indicated time points post-DTR and immunoblotted for H3S10ph, H3, and GAPDH. F Parental HeLa cells and HeLa cells stably expressing siRNA-resistant Bora-Myc (WT, S59A, or S59D) were transfected with control or Bora siRNA and treated with RO-3306 for 24 h. Two days after transfection, cells were released into fresh medium containing nocodazole and analyzed for mitotic index at the indicated time points. The mitotic index was determined in around 2100 cells per condition across three independent experiments. G, H HeLa cells stably expressing Bora-Myc (G) or Bora-S59D-Myc (H) were transfected with control or PKA-Cα siRNA and subjected to double-thymidine treatment. Two days after transfection, cells were released into fresh medium containing nocodazole. Lysates were collected at the indicated time points post-DTR and immunoblotted for H3S10ph, H3, and PKA-Cα. I, J HeLa cells stably expressing Bora-Myc (WT or S59D) were subjected to double-thymidine treatment and released into fresh medium containing nocodazole supplemented with DMSO (I) or H-89 (J). Lysates were collected at the indicated time points post-DTR and immunoblotted for H3S10ph, H3, and GAPDH. K, L HeLa cells stably expressing Bora-Myc (WT or S59D) were transfected with control or PKA-Cα siRNA and treated with RO-3306 for 24 h. Two days after transfection, cells were released into fresh medium containing nocodazole and subjected to mitotic index analysis at the indicated time points (K). Lysates collected at 60 min after RO-3306 release were immunoblotted for PKA-Cα, α-Tubulin, and Myc-tag (L). The mitotic index was determined in around 2100 cells per condition across three independent experiments. M HeLa cells stably expressing Bora-Myc (WT or S59D) were treated with RO-3306 for 24 h. Before release from RO-3306, cells were pretreated with H-89 or DMSO for 1 h. Cells were then released into fresh medium containing nocodazole and either H-89 or DMSO, followed by mitotic index analysis at the indicated time points. The mitotic index was determined in around 2100 cells per condition from three independent experiments. Data are representative of two independent experiments in (A–E, G–J) and three independent experiments in (L). Means and SDs are shown in (F, K, M). Source data are provided as a Source data file.
We further examined cell-cycle progression in HeLa cells expressing a phospho-mimetic Bora-S59D mutant, in which S59 was substituted with aspartate. To this end, we generated stable cell lines expressing siRNA-resistant Bora-S59D-Myc at levels comparable to those of Bora-Myc and Bora-S59A-Myc (Fig. 3C). Cells expressing Bora-S59D-Myc progressed into mitosis at rates similar to those expressing Bora-Myc, regardless of whether endogenous Bora was depleted (Fig. 3D, E). Moreover, following depletion of endogenous Bora, Bora-S59D-Myc-expressing cells entered mitosis faster than those expressing Bora-S59A-Myc (Supplementary Fig. 3A, B). These findings indicate that, like wild-type Bora, Bora-S59D is sufficient to drive timely progression from early S phase to mitosis.
Next, we assessed the impact of Bora-S59 mutations on mitotic entry upon release from RO-3306-induced late-G2 arrest. In the presence of endogenous Bora, cells stably expressing Bora-Myc, Bora-S59A-Myc, or Bora-S59D-Myc progressed into mitosis at rates similar to parental HeLa cells (Fig. 3F and Supplementary Fig. 3C). Notably, upon endogenous Bora depletion, cells expressing Bora-Myc or Bora-S59D-Myc progressed into mitosis faster than HeLa cells and those expressing Bora-S59A-Myc. Thus, the Bora-S59D mutant supports timely G2/M transition.
To validate these findings, we generated HeLa-derived stable cell lines with inducible expression of Bora-GFP variants (WT, S59A, and S59D) at levels comparable to endogenous Bora (Supplementary Fig. 3D). Co-immunoprecipitation confirmed that Bora-GFP interacted with endogenous PKA-Cα and Plk1 (Supplementary Fig. 3E). Following endogenous Bora depletion, mitotic entry after RO-3306 release was severely delayed in cells expressing Bora-S59A-GFP, but not in those expressing Bora-GFP or Bora-S59D-GFP (Supplementary Fig. 3F). Moreover, similar results were obtained in endogenous Bora-depleted RPE-1 cells expressing the Bora-GFP variants (WT, S59A, or S59D) (Supplementary Fig. 3G, H), supporting the role of Bora-S59 phosphorylation in promoting G2/M transition in non-transformed cells.
To determine whether PKA promotes mitotic entry solely through Bora-S59 phosphorylation, we examined the effect of PKA-Cα knockdown on mitotic entry in cells expressing Bora-Myc or Bora-S59D-Myc. In the DTR assay, PKA-Cα depletion delayed mitotic entry in cells expressing Bora-Myc but not Bora-S59D-Myc (Fig. 3G, H and Supplementary Fig. 3I, J). Similarly, following PKA inhibition by H-89, Bora-S59D-Myc-expressing cells entered mitosis faster than Bora-Myc-expressing cells (Fig. 3I, J). Furthermore, in the RO-3306 washout assay, both PKA-Cα knockdown and H-89 treatment delayed the G2/M transition in Bora-Myc-expressing cells, but not in Bora-S59D-Myc-expressing cells (Fig. 3K–M). Moreover, Bora-S59D-expressing cells progress into mitosis with similar kinetics regardless of PKA-Cα depletion or H-89 treatment (Fig. 3K, M). These results indicate that the phospho-mimetic Bora-S59D mutant bypasses the requirement for PKA activity in promoting the G2/M transition.
Together, these results demonstrate that S59 phosphorylation is essential for Bora’s role in mitotic entry, and that PKA promotes the G2/M transition primarily through this modification.
Bora-S59 phosphorylation enables Aurora A binding and enhances Plk1 activation
To elucidate the role of Bora-S59 phosphorylation in the G2/M transition, we investigated its effect on Bora’s interaction with Plk1 and Aurora A. Using HeLa cells stably expressing full-length Bora-Myc variants, we performed co-immunoprecipitation assays to assess these interactions. Plk1 was co-immunoprecipitated by Bora-Myc, Bora-S59A-Myc, and Bora-S59D-Myc with comparable efficiency (Fig. 4A), indicating that S59 is dispensable for the interaction between Bora and Plk1. Interestingly, Aurora A was efficiently co-immunoprecipitated with Bora-Myc and Bora-S59D-Myc, but only weakly with Bora-S59A-Myc, suggesting that S59 phosphorylation is crucial for Bora binding to Aurora A. Additionally, Bora-Myc immunoprecipitated Plk1 much more efficiently than Aurora A, indicating a higher intrinsic affinity between Bora and Plk1 than between Bora and Aurora A. Similar results were observed in cells with induced expression of Bora-GFP (WT, S59A, or S59D) variants (Supplementary Fig. 4A).
Fig. 4. Bora-S59 phosphorylation enables Aurora A binding and enhances Plk1 activation.
A Parental HeLa cells and HeLa cells stably expressing Bora-Myc (WT, S59A, or S59D) were subjected to immunoprecipitation using Myc-tag antibody-conjugated beads, followed by immunoblotting for Aurora A, Plk1, and Myc-tag. B, C U2OS-LacO cells transiently expressing the indicated proteins were immunostained for Aurora A and DAPI. Representative images are shown, with arrows pointing to the LacO repeats and arrowheads to centrosome-localized Aurora A (B). Scale bars represent 10 µm. The relative fluorescence intensity ratio of Aurora A to EGFP at the LacO repeats was quantified in 30 cells per condition (C). Data are presented as Means and SDs. Statistical analysis was performed using a two-tailed unpaired Student’s t-test. D HeLa cell lysates were subjected to pull-down using MBP or MBP-Bora (WT or S59D), followed by immunoblotting for Aurora A and Coomassie brilliant blue (CBB) staining. E Recombinant MBP-Bora (WT or S59ph) was subjected to immunoblotting for MBP and Bora-S59ph, followed by CBB staining. F MBP-Bora (WT or S59ph) were subjected to pull-down using GST or GST-Aurora A, followed by immunoblotting for MBP and CBB staining. G 6xHis-Aurora A was subjected to pull-down using Bora 49-69 peptides (WT or S59ph) immobilized on beads, followed by immunoblotting for Aurora A and streptavidin-HRP. H GST-Plk1 and GST-CENP-A were phosphorylated in vitro by 6xHis-Aurora A, with or without MBP-Bora (S59A or S59ph), followed by immunoblotting for GST, Plk1-T210ph, CENP-A-S7ph, Aurora A, Bora-S59ph, and MBP. I Parental HeLa cells and HeLa cells stably expressing siRNA-resistant Bora-Myc (WT, S59A, or S59D) were transfected with control or Bora siRNA. At 48 h after transfection, cells were subjected to immunoprecipitation using the Plk1 antibody, followed by immunoblotting for Plk1-T210ph, Plk1, Myc-tag, and GAPDH. Data are representative of three independent experiments in (A, D–I). In A, H: S. exp. short exposure, L. exp. long exposure. Source data are provided as a Source data file.
We next examined whether Bora-S59 phosphorylation promotes the recruitment of Aurora A and Plk1 in cells. For this purpose, we utilized a U2OS-derived cell line with multiple Lac operator (LacO) repeats integrated into a defined chromosomal locus (hereafter referred to as U2OS-LacO cells)50. As a negative control, tethering EGFP-fused Lac repressor (EGFP-LacI), which contains a nuclear localization signal, to the LacO repeats did not recruit endogenous Aurora A (Fig. 4B, C). In contrast, tethering wild-type Bora or the Bora-S59D mutant to LacO repeats led to robust recruitment of endogenous Aurora A, whereas the Bora-S59A mutant did not. All forms of EGFP-LacI-Bora fusion proteins (WT, S59A, and S59D) efficiently recruited endogenous Plk1 (Supplementary Fig. 4B, C). These data indicate that the phospho-mimetic S59D mutation enables Bora binding to Aurora A in cells.
To confirm this interaction biochemically, we performed pull-down assays using recombinant MBP-tagged Bora proteins. The MBP-Bora-S59D mutant efficiently pulled down endogenous Aurora A from asynchronous HeLa cell lysates, while wild-type unphosphorylated MBP-Bora and MBP alone did not (Fig. 4D), indicating that the phospho-mimetic S59D mutation enables Bora to bind Aurora A.
To assess whether S59 phosphorylation directly enhances Bora’s interaction with Aurora A in vitro, we expressed MBP-Bora-S59ph, where S59 was phosphorylated in Escherichia coli, using an expanded genetic code to incorporate phosphoserine (Fig. 4E and Supplementary Fig. 4D)51,52. Pull-down assays revealed that GST-tagged Aurora A bound efficiently to MBP-Bora-S59ph, but only weakly to unphosphorylated MBP-Bora (Fig. 4F). Similarly, an S59-phosphorylated Bora peptide (residues 49-69) efficiently pulled down 6xHis-tagged Aurora A (6xHis-Aurora A), whereas the non-phosphorylated peptide did not (Fig. 4G). Therefore, phosphorylation at S59 enables the direct interaction of Bora with Aurora A in vitro.
Phosphorylation of Plk1 at Thr210 (Plk1-T210ph) by Aurora A is critical for timely G2/M transition37,38. We therefore examined whether Bora-S59 phosphorylation affects this process. In vitro kinase assays using recombinant 6xHis-Aurora A and GST-fused Plk1 (GST-Plk1) were performed in the presence or absence of MBP-Bora-S59ph or MBP-Bora-S59A. As shown in Fig. 4H, Aurora A alone or with MBP-Bora-S59A weakly phosphorylated Thr210 of GST-Plk1. In contrast, the presence of MBP-Bora-S59ph strongly enhanced Thr210 phosphorylation. As a control, Aurora A efficiently phosphorylated Ser7 of CENP-A, a known substrate53,54, regardless of whether Bora proteins were present. Thus, Bora-S59 phosphorylation specifically enhances Aurora A-mediated Plk1 activation in vitro.
To validate these findings in cells, we analyzed Thr210 phosphorylation of Plk1 in HeLa cells expressing siRNA-resistant Bora-Myc, Bora-S59A-Myc, or Bora-S59D-Myc following depletion of endogenous Bora. Plk1 was immunopurified from asynchronous cells, and Thr210 phosphorylation was analyzed by immunoblotting. Plk1-T210ph levels were comparable in cells expressing Bora-Myc and Bora-S59D-Myc, but significantly lower in Bora-S59A-Myc-expressing cells and parental HeLa cells (Fig. 4I). Similar results were observed in HeLa cells with induced expression of Bora-GFP variants (Supplementary Fig. 4E). Thus, wild-type and phospho-mimetic Bora-S59D support proper Plk1-T210 phosphorylation, whereas the phosphorylation-deficient Bora-S59A does not.
Although Bora is largely degraded during mitosis (see Fig. 2C)38,40,46, a fraction persists and continues to support Plk1 activation55. To assess the role of Bora-S59 phosphorylation in mitosis, we examined chromosome segregation and nuclear morphology in cells expressing Bora-Myc variants following endogenous Bora depletion. Bora knockdown increased chromosome mis-segregation, including lagging chromosomes (predominantly) and occasional chromosome bridges (Supplementary Fig. 4F, G), as well as abnormal nuclear morphologies such as multinucleation and micronucleation (Supplementary Fig. 4H, I). These phenotypes are consistent with the known role of Bora in chromosome segregation and cell division55, as well as the role for Plk1 in mitotic spindle assembly and cytokinesis56,57. Importantly, these defects were largely rescued by exogenous expression of wild-type Bora-Myc but not the S59A mutant. These findings underscore the critical role of Bora-S59 phosphorylation in ensuring Plk1 activation, and consequently proper chromosome segregation and cytokinesis.
In summary, these data demonstrate that phosphorylation at Bora-S59 facilitates its binding to Aurora A and promotes Aurora A-mediated activation of Plk1. This S59 phosphorylation-dependent enhancement of Bora-Aurora A interaction and subsequent Plk1 activation underlies the essential role of Bora-S59 phosphorylation in promoting the G2/M transition.
An Aurora A mutant defective in binding S59-phosphorylated Bora fails to activate Plk1 and promote G2/M transition
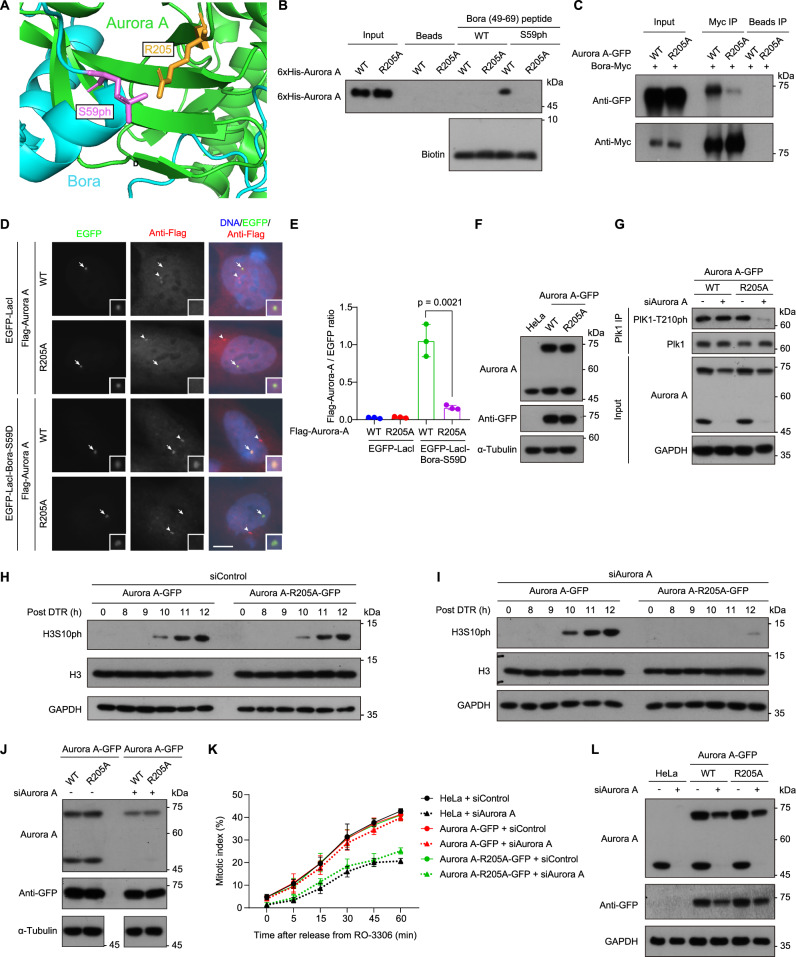
To further investigate the functional significance of the interaction between Aurora A and S59-phosphorylated Bora, we used AlphaFold3 to predict the structure of full-length Aurora A in complex with S59-phosphorylated Bora (Supplementary Fig. 5A). The model suggested an interaction between Arg205 of Aurora A and the phosphate group at Bora-S59 (Fig. 5A). This interaction was confirmed by peptide pull-down assays, which showed binding of the Bora-S59ph peptide to 6xHis-Aurora A, but not to the R205A mutant in which Arg205 was replaced by alanine (Fig. 5B). Co-immunoprecipitation assays also revealed much weaker binding between Aurora A-R205A-GFP and Bora-Myc than between Aurora A-GFP and Bora-Myc (Fig. 5C). The R205A mutation did not affect co-immunoprecipitation with Flag-tagged TPX2 (Flag-TPX2), a well-characterized Aurora A interactor (Supplementary Fig. 5B)58. Moreover, tethering EGFP-LacI-Bora-S59D to the LacO repeats in U2OS-LacO cells recruited Flag-tagged Aurora A (Flag-Aurora A) efficiently but failed to recruit the R205A mutant (Fig. 5D, E and Supplementary Fig. 5C–E). Collectively, these data highlight the critical role of Aurora A residue R205 in binding S59-phosphorylated Bora, both in vitro and in cellular contexts.
Fig. 5. An Aurora A mutant defective in binding S59-phosphorylated Bora fails to activate Plk1 and promote G2/M transition.
A Zoomed-in cartoon presentation of the Aurora A-Bora complex, with Aurora A in green and Bora in cyan. Key residues involved in the interaction between Aurora A and Bora are labeled and depicted as sticks. B 6xHis-Aurora A (WT or R205A) was subjected to pull-down with Bora 49-69 peptides (WT or S59ph) immobilized on beads, followed by immunoblotting for Aurora A and streptavidin-HRP. C HEK-293T cells transiently co-expressing Bora-Myc and Aurora A-GFP (WT or R205A) were subjected to immunoprecipitation with the Myc-tag antibody-conjugated beads or control protein A/G beads, followed by immunoblotting for the Myc-tag and GFP. D, E U2OS-LacO cells transiently expressing the indicated proteins were immunostained with the Flag-tag antibody and DAPI. Example images are shown, with arrows pointing to the LacO repeats and arrowheads indicating centrosome-localized Flag-Aurora A (D). Scale bars, 10 µm. The relative fluorescence intensity ratio of Flag-Aurora A to EGFP at the LacO repeats was quantified in 90 cells per condition from three independent experiments (E). Statistical analysis was performed using a two-tailed unpaired Student’s t-test. F Parental HeLa cells and HeLa cells stably expressing siRNA-resistant Aurora A-GFP (WT or R205A) were immunoblotted for Aurora A, GFP, and α-Tubulin. G HeLa cells stably expressing siRNA-resistant Aurora A-GFP (WT or R205A) were transfected with control or Aurora A siRNA. At 48 h post-transfection, cells were subjected to immunoprecipitation with the Plk1 antibody, followed by immunoblotting for Plk1-T210ph, Plk1, Aurora A, and GAPDH. H–J HeLa cells stably expressing siRNA-resistant Aurora A-GFP (WT or R205A) were transfected with control (H) or Aurora A siRNA (I) and subjected to double-thymidine treatment. Two days after transfection, cells were released into fresh medium containing nocodazole. Cells were collected at the indicated time points post-DTR and immunoblotted for H3S10ph, H3, and GAPDH. Lysates collected at 12 h post-DTR were immunoblotted for Aurora A, GFP, and α-Tubulin (J). K, L Parental HeLa cells and HeLa cells stably expressing siRNA-resistant Aurora A-GFP (WT or R205A) were transfected with control or Aurora A siRNA, then treated with RO-3306 for 24 h. Two days after transfection, cells were released into fresh medium containing nocodazole and subjected to mitotic index analysis at the indicated time points (K). Lysates collected 60 min after release from RO-3306 were immunoblotted for Aurora A, GFP, and GAPDH (L). The mitotic index was determined in around 2100 cells per condition from three independent experiments. Data are representative of two independent experiments in (B, C, F–J) and three independent experiments in (L). Means and SDs are shown in (E) and (K). Source data are provided as a Source data file.
To evaluate whether the Aurora A-Bora interaction is required for Plk1 activation, we stably expressed siRNA-resistant Aurora A-GFP (WT or R205A) in HeLa cells (Fig. 5F). Consistent with Aurora A’s known role in Plk1 activation and G2/M transition37,38, knockdown of endogenous Aurora A in HeLa cells substantially reduced Plk1-T210 phosphorylation and delayed mitotic entry after DTR (Supplementary Fig. 5F, G). FACS analysis showed that Aurora A depletion in DTR-synchronized cells did not affect S-phase progression (see Fig. 1F, G and Supplementary Fig. 1G). Notably, endogenous Aurora A depletion strongly reduced Plk1-T210 phosphorylation in asynchronous cells expressing Aurora A-R205A-GFP but not in those expressing Aurora A-GFP (Fig. 5G), indicating that the R205A mutation impairs Aurora A’s ability to activate Plk1.
Analysis of cell-cycle progression following DTR showed that knockdown of endogenous Aurora A strongly delayed mitotic entry in cells expressing Aurora A-R205A-GFP, but not in those expressing Aurora A-GFP (Fig. 5H–J). Likewise, following release from RO-3306-induced late-G2 arrest, endogenous Aurora A depletion delayed G2/M transition in both parental HeLa cells and Aurora A-R205A-GFP-expressing cells, but not in Aurora A-GFP-expressing cells (Fig. 5K, L). Thus, the R205A mutation substantially impairs Aurora A’s function in promoting G2/M transition.
We further examined whether the Aurora A-R205A mutation affects M phase progression. As expected59, Aurora A knockdown caused chromosome segregation and cytokinesis defects (Supplementary Fig. 5H-K), which were substantially rescued by expression of Aurora A-GFP, but not the R205A mutant. The M phase defects observed in Aurora A-R205A-expressing cells are consistent with those in Bora-S59A-expressing cells (see Supplementary Fig. 4F-I).
Given that R205 lies within the kinase domain of Aurora A, we next asked whether the R205A mutation affects kinase activity. In vitro kinase assays using 6xHis-Aurora A (WT or R205A) and GST-CENP-A showed that both wild-type Aurora A and its R205A mutant phosphorylated CENP-A at Ser7 with comparable efficiency, at least in a single-time-point assay (Supplementary Fig. 5L). Thus, the R205A mutation does not appear to compromise the intrinsic kinase activity of Aurora A, suggesting that the defects observed in Aurora A-R205A-expressing cells result from impaired interaction with Bora rather than a loss of catalytic activity.
Together, these findings underscore the critical role of the interaction between Aurora A and S59-phosphorylated Bora in mediating Plk1 activation and ensuring timely G2/M transition.
PKA promotes the Bora-Aurora A interaction and Plk1 activation by phosphorylating Bora-S59
We next investigated whether PKA regulates the Bora-Aurora A interaction and Plk1 activation solely or in part through phosphorylation of Bora at S59. To test this, we performed two-step phosphorylation and pull-down assays to evaluate the effect of PKA-mediated phosphorylation on the Bora-Aurora A interaction in vitro. First, MBP-Bora was phosphorylated in vitro using PKA-Cα-GFP immunopurified from HEK-293T cells. After removing the kinase, the phosphorylated MBP-Bora was used in pull-down assays with GST-Aurora A. As shown in Fig. 6A, GST-Aurora A efficiently pulled down MBP-Bora phosphorylated at S59 by PKA-Cα-GFP, but not MBP-Bora incubated with the kinase-dead mutant PKA-Cα-K73A-GFP, indicating that phosphorylation is necessary for binding. Moreover, GST-Aurora A failed to bind MBP-Bora-S59A even after incubation with PKA-Cα-GFP (Fig. 6B), confirming that phosphorylation at S59 is essential for the Bora-Aurora A interaction. These results demonstrate that PKA-mediated phosphorylation of Bora at S59 enables its direct binding to Aurora A in vitro.
Fig. 6. PKA promotes the Bora-Aurora A interaction and Plk1 activation by phosphorylating Bora-S59.
A MBP-Bora was subjected to in vitro kinase reactions using PKA-Cα-GFP (WT or K73A) immunoprecipitated from HEK-293T cells. After removal of the GFP antibody-conjugated beads by centrifugation, GST-Aurora A was used to pull down MBP-Bora. Samples were then immunoblotted for Bora-S59ph and MBP, and CBB staining. B MBP-Bora (WT or S59A) was subjected to in vitro kinase reactions using PKA-Cα-GFP immunoprecipitated from HEK-293T cells. After removal of the GFP antibody-conjugated beads by centrifugation, GST-Aurora A was used to pull down MBP-Bora, followed by immunoblotting for Bora-S59ph and MBP, and CBB staining. C HeLa cells stably expressing Bora-Myc (WT or S59D) were transfected with control or PKA-Cα siRNA. At 48 h post-transfection, cells were subjected to immunoprecipitation using the Myc-tag antibody-conjugated beads, followed by immunoblotting for Aurora A, Plk1, Myc-tag, and PKA-Cα. D HeLa cells stably expressing Bora-Myc (WT or S59D) were treated with H-89 or DMSO for 4 h, then subjected to immunoprecipitation using the Myc-tag antibody-conjugated beads, followed by immunoblotting for Aurora A, Plk1, Myc-tag, CREB, and CREB-S133ph. S. exp., short exposure; L. exp., long exposure. E, F Parental HeLa cells (E) and HeLa cells stably expressing Bora-Myc (WT or S59D) (F) were transfected with control or PKA-Cα siRNA. At 48 h post-transfection, cells were subjected to immunoprecipitation with the Plk1 antibody, followed by immunoblotting for Plk1-T210ph, Plk1, and PKA-Cα. G, H Parental HeLa cells (G) and HeLa cells stably expressing Bora-Myc (WT or S59D) (H) were treated with H-89 or DMSO for 4 h, then subjected to immunoprecipitation with the Plk1 antibody, followed by immunoblotting for Plk1-T210ph and Plk1. Data are representative of two independent experiments in (A–H). Source data are provided as a Source data file.
We next examined whether PKA-Cα is required for the Bora-Aurora A interaction in cells. Co-immunoprecipitation assays in HeLa cells stably expressing Bora-Myc or Bora-S59D-Myc showed that knockdown of PKA-Cα substantially reduced the interaction between Bora-Myc and Aurora A, but had no detectable effect on the interaction between Bora-S59D-Myc and Aurora A (Fig. 6C). Similar results were observed upon inhibition of PKA activity with H-89 (Fig. 6D). In contrast, neither PKA-Cα knockdown nor H-89 treatment affected the interaction of Bora-Myc or Bora-S59D-Myc with Plk1. These results indicate that PKA activity is essential for the Bora-Aurora A interaction, but not for the Bora-Plk1 interaction. Furthermore, the insensitivity of Bora-S59D to PKA inhibition suggests that PKA promotes Aurora A binding specifically through phosphorylation of Bora at S59.
To determine whether PKA promotes Plk1 activation solely through Bora-S59 phosphorylation, we analyzed Plk1-T210 phosphorylation in parental HeLa cells and in cells stably expressing either Bora-Myc or Bora-S59D-Myc. Consistent with PKA’s role in the G2/M transition, PKA-Cα knockdown strongly reduced Plk1-T210 phosphorylation in asynchronous HeLa cells and Bora-Myc-expressing cells (Fig. 6E, F). By contrast, Plk1-T210 phosphorylation was largely unaffected by PKA-Cα depletion in cells expressing Bora-S59D-Myc, indicating that Plk1 activation in these cells does not depend on PKA-Cα.
We further assessed the effect of pharmacological PKA inhibition on Plk1 activation. Treatment with H-89 strongly diminished Plk1-T210 phosphorylation in parental HeLa cells and Bora-Myc-expressing cells (Fig. 6G, H). However, Plk1-T210 phosphorylation remained largely unaffected in Bora-S59D-Myc-expressing cells treated with H-89 (Fig. 6H), further confirming that PKA activity is dispensable for Plk1 activation in the presence of the phospho-mimetic Bora mutant.
Taken together, these results demonstrate that PKA promotes Plk1 activation, and thereby the G2/M transition, exclusively through phosphorylation of Bora at S59.
PKA-mediated Bora-Aurora A interaction is essential for mitotic entry after DNA damage checkpoint recovery
Previous studies in mammalian cells have shown that Plk1 is required for mitotic entry following recovery from DNA damage-induced G2-phase arrest60, and that Aurora A-mediated Plk1 activation promotes this checkpoint recovery37. To investigate this further, we examined whether PKA-mediated Bora-S59 phosphorylation is required for mitotic entry following recovery from doxorubicin-induced DNA damage.
Cells were treated with doxorubicin 6 h after release from the second thymidine block, a time point at which most cells were in G2 phase (Fig. 7A). One hour later, cells were transferred to fresh medium containing nocodazole, with or without the ATM/ATR kinase inhibitor caffeine, and imaged hourly for up to 5 h. The addition of caffeine enabled control siRNA-transfected HeLa cells to enter mitosis, indicative of checkpoint recovery. Knockdown of endogenous Bora in HeLa cells markedly impaired caffeine-induced checkpoint recovery. This defect was largely rescued by expression of Bora-Myc or Bora-S59D-Myc but not Bora-S59A-Myc (Fig. 7B and Supplementary Fig. 6A).
Fig. 7. PKA-mediated Bora-Aurora A interaction is essential for mitotic entry after DNA damage checkpoint recovery.
A, B Schematic of the experimental setup (A). Parental HeLa cells and HeLa cells stably expressing siRNA-resistant Bora-Myc (WT, S59A, or S59D) were transfected with control or Bora siRNA, followed by double-thymidine treatment. At 6 h after release from the second thymidine block, cells were treated with doxorubicin for 1 h, then released into fresh medium containing nocodazole with or without caffeine. Cells were subsequently analyzed for mitotic index at the indicated time points. The mitotic index was determined in around 2700 cells per condition from three independent experiments (B). C, D Parental HeLa cells and HeLa cells stably expressing siRNA-resistant Aurora A-GFP (WT or R205A) were transfected with control or Aurora A siRNA. Cell synchronization and drug treatment were performed as described in (A). Mitotic index analysis was conducted at the indicated time points in around 2100 cells per condition from three independent experiments (C). Lysates collected at 5 h after doxorubicin release were immunoblotted for Aurora A and GAPDH (D). Data are representative of three independent experiments in (D). Means and SDs are shown in (B, C). Source data are provided as a Source data file.
Similarly, depletion of endogenous Aurora A in HeLa cells strongly inhibited caffeine-induced checkpoint recovery (Fig. 7C, D and Supplementary Fig. 6B). Notably, checkpoint recovery was rescued by exogenous expression of Aurora A-GFP, but not by the R205 A mutant, consistent with its inability to bind S59-phosphorylated Bora (Fig. 5A–E).
Together, these data highlight the essential role of Bora-S59 phosphorylation, and the resulting Bora-Aurora A interaction, in promoting mitotic entry during DNA damage checkpoint recovery.
Cyclin A-Cdk1-mediated Bora-S112 phosphorylation promotes Plk1 activation but not Bora-Aurora A interaction
Previous work showed that cyclin A-Cdk1 phosphorylates frog Bora at Ser110 (equivalent to Ser112 in human Bora) to promote Plk1 activation during G2 phase29. This activation is thought to occur by promoting Aurora A autophosphorylation in trans61. Consistent with this, cyclin A-Cdk1 activity accumulates during G2, and cyclin A depletion by a genetic degron prevents mitotic entry30. We next investigated whether this phosphorylation affects Bora’s interaction with Aurora A or Plk1, and whether it contributes to Plk1 activation and G2/M transition in human cells.
To this end, we established a HeLa-derived cell line stably expressing the siRNA-resistant Bora-S112A-GFP mutant in which S112 was substituted with alanine (Fig. 8A). Immunoblotting with S59ph-specific antibodies confirmed that the S112A mutation does not affect Bora-S59 phosphorylation (Fig. 8B), indicating that PKA-mediated Bora-S59 phosphorylation occurs independently of Bora-S112 phosphorylation.
Fig. 8. Cyclin A-Cdk1-mediated Bora-S112 phosphorylation promotes Plk1 activation but not Bora-Aurora A interaction.
A Parental HeLa cells and HeLa cells stably expressing siRNA-resistant Bora-GFP (WT, S59A, or S112A) were immunoblotted for GFP and α-Tubulin. B HeLa cells stably expressing siRNA-resistant Bora-GFP (WT, S59A, or S112A) were immunoblotted for GFP and Bora-S59ph. C Parental HeLa cells and HeLa cells stably expressing siRNA-resistant Bora-GFP (WT, S59A, or S112A) were subjected to immunoprecipitation using GFP antibody-conjugated beads, followed by immunoblotting for Aurora A, Plk1, and GFP. D Parental HeLa cells and HeLa cells stably expressing siRNA-resistant Bora-GFP (WT, S59A, or S112A) were transfected with control or Bora siRNA. At 48 h after transfection, cells were subjected to immunoprecipitation with the Plk1 antibody, followed by immunoblotting for Plk1-T210ph, Plk1, GFP, and α-Tubulin. E Parental HeLa cells and HeLa cells stably expressing siRNA-resistant Bora-GFP (WT, S59A, or S112A) were transfected with control or Bora siRNA, followed by treatment with RO-3306 for 24 h. Two days after transfection, cells were released into fresh medium containing nocodazole and subjected to mitotic index analysis at the indicated time points. The mitotic index was determined in around 2100 cells per condition from three independent experiments. F Parental HeLa cells and HeLa cells stably expressing siRNA-resistant Bora-GFP (WT, S59A, or S112A) were transfected with control or Bora siRNA. Cell synchronization and drug treatment were performed as described in Fig. 7A. Cells were subjected to mitotic index analysis at the indicated time points, with around 2100 cells analyzed per condition from three independent experiments. G A schematic model illustrating how PKA-mediated phosphorylation of Bora at S59 ensures the timely onset of mitosis. Note that Cyclin A-Cdk1-phosphorylation of Bora at S112 can also promote Plk1 activation, likely by physically and/or functionally substituting for the phosphorylated activation segment on Aurora A (not depicted)61,67. Data are representative of two independent experiments in (A–D). Means and SDs are shown in (E, F). Source data are provided as a Source data file.
We next assessed whether the S112A mutation alters Bora’s ability to bind Aurora A or Plk1. Both Bora-GFP and Bora-S112A-GFP co-immunoprecipitated endogenous Aurora A with similar efficiency, in sharp contrast to the Aurora A-binding-deficient mutant Bora-S59A-GFP (Fig. 8C). Furthermore, all Bora-GFP variants (WT, S112A, and S59A) co-immunoprecipitated endogenous Plk1 with comparable efficiency. Thus, Bora-S112 phosphorylation is not required for its interaction with Aurora A or Plk1.
To test whether the S112A mutation affects Plk1 activation, we immunoprecipitated Plk1 from asynchronous cells depleted of endogenous Bora and expressing Bora-GFP, Bora-S112A-GFP, or Bora-S59A-GFP. Immunoblotting with the Plk1-T210ph-specific antibody showed that Plk1-T210ph levels were substantially lower in cells expressing Bora-S112A-GFP or Bora-S59A-GFP compared to those expressing Bora-GFP (Fig. 8D), indicating that Bora-S112 phosphorylation promotes Plk1 activation in cells.
Finally, we assessed the role of Bora-S112 phosphorylation in the G2/M transition. Upon release from RO-3306-induced G2 arrest, the G2/M transition was similarly delayed in endogenous Bora-depleted cells expressing Bora-S112A-GFP or Bora-S59A-GFP (Fig. 8E and Supplementary Fig. 7A). Thus, S112 phosphorylation is required for efficient G2/M transition. Furthermore, after doxorubicin-induced DNA damage, caffeine addition led to mitotic entry in endogenous Bora-depleted cells expressing Bora-GFP but not in those expressing Bora-S112A-GFP (Fig. 8F and Supplementary Fig. 7B), suggesting that Bora-S112 phosphorylation also contributes to mitotic entry during checkpoint recovery.
Collectively, these findings demonstrate that cyclin A-Cdk1-mediated Bora-S112 phosphorylation promotes Plk1 activation and G2/M transition without affecting Bora-S59 phosphorylation and Bora-Aurora A interaction.
Discussion
Entry into mitosis is driven by activation of the cyclin B-Cdk1 complex, which is governed by tightly regulated feedback loops. However, the upstream signal that initiates this activation has remained elusive16,25–29,62–64. In this study, we demonstrate that cAMP-PKA signaling serves as a critical upstream input governing the G2/M transition in mammalian cells. Specifically, PKA, activated by cAMP, employs its ubiquitously expressed catalytic subunit PKA-Cα to phosphorylate Bora at S59. This modification enables Bora to bind Aurora A and recruit it to the Bora-Plk1 complex, thereby facilitating Aurora A-dependent activation of Plk1. Our findings identify PKA-mediated phosphorylation of Bora as a key upstream event that initiates Plk1 activation and drives mitotic entry after an unperturbed G2 phase. Moreover, we show that PKA-primed Plk1 activation is also essential for mitotic entry during recovery from the DNA damage checkpoint (Fig. 8G).
Bora is widely recognized as a co-factor of Aurora A39. Unexpectedly, we found that Bora requires prior phosphorylation by PKA at S59 to bind Aurora A effectively. This PKA-dependent event is necessary for subsequent Plk1 activation. These results position cAMP-PKA signaling as an initiating signal for the G2/M transition. They further suggest that passive removal of negative regulators in G2 is insufficient to trigger mitotic entry; instead, a positive input from cAMP-PKA signaling is required. Our data also show that Bora-S59 phosphorylation is already detectable in early S phase, and the phospho-mimetic Bora-S59D mutant does not accelerate mitotic entry. Thus, we propose that the gradual accumulation of the complex formed between S59-phosphorylated Bora, Aurora A, and Plk1 during G2 phase may constitute the rate-limiting step for triggering mitotic entry.
Bora is also phosphorylated by Cdk140,41. In C. elegans, cyclin B-Cdk1 phosphorylation of Bora stimulates Aurora A-mediated Plk1 activation65, possibly by enhancing Bora-Plk1 interaction66. In human cells, cyclin A-Cdk1/2 activity is required for Plk1 activation in G2 phase, with an unknown mechanism23. In Xenopus egg extracts, cyclin A-Cdk1 phosphorylation of Bora at Ser110 (corresponding to S112 in human Bora) promotes Plk1 activation29, likely by facilitating Aurora A autophosphorylation in trans61. However, it remains unclear how Bora-S110 phosphorylation persists under conditions that inhibit Aurora A autophosphorylation67. Cyclin A-Cdk1-mediated Bora phosphorylation has been proposed as a mitotic trigger28,29, consistent with cyclin A-Cdk1’s ability to escape Wee1-mediated inhibitory phosphorylation29,68. Our data show that both cyclin A-Cdk1-mediated Bora-S112 phosphorylation and PKA-mediated Bora-S59 phosphorylation promote Plk1 activation and mitotic entry, but only S59 phosphorylation is required for Aurora A binding. We propose a sequential model in which PKA-mediated Bora-S59 phosphorylation first enables assembly of the Bora-Aurora A complex, and cyclin A-Cdk1-mediated Bora-S112 phosphorylation subsequently licenses Aurora A autophosphorylation in trans within this complex, ultimately driving Plk1 activation (Fig. 8G).
Although cAMP signaling is well known for regulating diverse cellular processes, its involvement in mitotic entry has not been defined49. In most animal cells, cAMP signals act primarily through PKA49, which is best known for phosphorylating transcription regulators such as CREB to drive sustained changes in gene expression. In contrast, our data reveal that PKA ensures timely G2/M transition through a transcription-independent mechanism. First, acute inhibition of PKA activity in late G2 delays mitotic entry. Second, cells expressing the phosphorylation-deficient Bora-S59A mutant exhibit delayed mitotic entry after release from late-G2 arrest. Third, the phospho-mimetic Bora-S59D mutant bypasses PKA dependence for G2/M transition. Fourth, an Aurora A-R205 A mutant unable to bind S59-phosphorylated Bora fails to support timely G2/M transition. Together, these results indicate that PKA acts through direct phosphorylation of Bora-S59 to trigger mitotic commitment.
PKA is a tetrameric holoenzyme composed of a regulatory subunit dimer and two catalytic subunits43. Upon stimulation of G-protein-coupled receptors (GPCRs), adenylyl cyclase is activated and intracellular cAMP rises69, releasing catalytic subunits that phosphorylate targets involved in metabolism, growth, and differentiation49. Given our discovery of PKA’s role in Plk1 activation, future studies should identify extracellular cues that engage the GPCR-adenylyl cyclase-cAMP-PKA-Bora axis to promote mitotic entry. Elucidating these signals will link G2/M transition to the extracellular environment. It is also possible that constitutive GPCR-adenylyl cyclase activity, which elevates basal PKA signaling, may serve as a permissive cue for G2/M progression. Indeed, tonic cAMP/PKA signaling has been discussed in other contexts70,71.
The spatiotemporal regulation of cAMP-PKA signaling is crucial for proper cellular responses72. The PKA regulatory subunit R1α (PKA-R1α) undergoes liquid-liquid phase separation (LLPS) to form biomolecular condensates that compartmentalize cAMP and locally regulate PKA activity73,74. It will be important to determine whether LLPS of PKA-R1α influences Bora phosphorylation, particularly since Bora contains a large intrinsically disordered region (see Supplementary Fig. 5A)61.
Aurora A and Plk1 are frequently overexpressed in human cancers and often correlate with poor prognosis59. Small-molecule inhibitors targeting these kinases are in clinical trials. Aberrant cAMP-PKA signaling has also been implicated in tumorigenesis. Our finding that PKA promotes G2/M transition is consistent with reports of oncogenic PKA activity in various cancers, including its role in supporting small-cell lung cancer (SCLC) stem cells75,76. Identification of the cAMP-PKA-Bora-Aurora A-Plk1 signaling axis as a key mitotic trigger offers a mechanistic explanation for how elevated PKA, Aurora A, or Plk1 activity can drive oncogenesis. Disrupting this axis, for example, by targeting the PKA-Bora or Bora-Aurora A interfaces, could provide potential therapeutic strategies.
In conclusion, this study identifies a previously unrecognized intracellular signaling pathway that initiates mitotic commitment, revealing an important layer of cell-cycle control and highlighting PKA, Aurora A, and Plk1 as promising targets for cancer therapy.
Methods
Cell lines, plasmids, siRNAs, transfection, and drug treatments
All cells were from American Type Culture Collection (ATCC) and were cultured in DMEM supplemented with 10% FBS (Gibco) and 1% penicillin/streptomycin, and maintained at 37 °C in a humidified incubator with 5% CO2. U2OS-LacO cells, kindly provided by Dr. David Spector (Cold Spring Harbor Laboratory, USA), were maintained in the presence of 100 μg/ml hygromycin (Sigma). HeLa cells stably expressing Bora-Myc (WT, S59A, or S59D), Bora-GFP (WT, S59A, or S112A), inducible Bora-GFP (WT, S59A, or S59D), and Aurora A-GFP (WT or R205A) were isolated and maintained in 1.5-2 μg/ml blasticidin (Sigma). RPE-1 cells stably expressing Bora-GFP (WT, S59A, or S59D) were isolated and maintained in 5 μg/ml blasticidin (Sigma).
To generate Flag-Bora, Flag-Aurora A, Flag-TPX2, SFB-PKA-Cα, SFB-RIOK1, and SFB-MAP3K7 constructs, the corresponding cDNAs in entry vectors were transferred into Gateway-compatible destination vectors containing either an N-terminal Flag-tag or an N-terminal SFB-tag, which is a triple tag composed of an S-tag, a Flag-tag, and a streptavidin-binding peptide. For the pEF6-Bora-Myc construct series, PCR-amplified full-length Bora or its variant fragments (1-40, 41-100, 1-100, 101-200, 201-310, 311-559) were cloned into the pEF6-Myc vector using the KpnI/EcoRV restriction sites. Plasmids encoding GST-fused PKA-Cα, Plk1, CENP-A, and Aurora A were generated by subcloning the corresponding PCR-amplified fragments into pGEX-4T1 (GE Healthcare) using the BamHI restriction site. The pBos-Bora-GFP, pBos-Aurora A-GFP, pBos-G3BP2-GFP, pBos-ATAT1-GFP, and pBos-PKA-Cα-GFP constructs were generated by replacing the H2B coding sequence in pBos-H2B-GFP (BD PharMingen) with the corresponding PCR-amplified fragments using the KpnI/BamHI restriction sites. To generate the pBos-Tet-on-Bora-GFP construct, the PCR-amplified rtTA-Advanced fragment, the tetracycline response element with a minimal CMV promoter, and Bora were subcloned into the KpnI/BamHI sites of pBos-H2B-GFP (BD PharMingen) using the MultiF Seamless Assembly Mix Kit (ABclonal Biotechnology). The MBP-Bora-10× His plasmid was generated by subcloning PCR-amplified Bora and the 10xHis-tag into the BamHI/EcoRI sites of pGEX-4T1 (GE Healthcare), followed by replacement of the GST-tag with an MBP-tag. Plasmid expressing 6xHis-Aurora A was constructed by subcloning the PCR-amplified products encoding Aurora A into the pET45b vector. To generate the EGFP-LacI fusion construct, the PCR-amplified fragments encoding Bora were inserted into the BamHI site of pSV2-EGFP-LacI.
All point mutations were introduced using either the QuikChange II XL Site-Directed Mutagenesis Kit (Agilent Technologies) or the MultiF Seamless Assembly Mix Kit (ABclonal Biotechnology). All constructs were verified by DNA sequencing to confirm the presence of desired mutations and the absence of unwanted sequence alterations.
siRNA duplexes targeting Bora, Aurora A, PKA-Cα, and PKA-Cβ were purchased from Integrated DNA Technologies (IDT) or RiboBio: siBora (5’-CUAUGAGACUUCAGAUGUAdTdT-3’), siAurora A (5’-CCCUCAAUCUAGAACGCUAdTdT-3’), siPKA-Cα (5’-CAGCCCACUUGGAUCAGUUUGdTdT-3’), siPKA-Cβ (5’-CAAUAUUGUUGUUGGUCAAdTdT-3’). siRNA transfections were carried out twice at a 24-h interval using Oligofectamine (Invitrogen) or Lipofectamine RNAiMAX (Thermo Fisher Scientific). Plasmid transfections were performed using FuGENE 6 (Promega) or Lipofectamine 2000 (Thermo Fisher Scientific).
Cell cycle synchronization was achieved as follows: cells were arrested in late-G2 phase using RO-3306 (9 µM for HeLa cells and 6 µM for RPE-1 cells; Selleck), or at the G1/S boundary using a double-thymidine block (2 mM, Sigma). To induce DNA damage, cells were treated with doxorubicin (0.5 µM, Selleck), and DNA-damage-signaling was subsequently silenced by the addition of caffeine (2 mg/ml). Expression of Bora-GFP variants was induced by treatment with 0.1 µg/ml Doxycycline (Selleck) for 24 h. Other drugs used in this study included H-89 (20 µM), nocodazole (100 ng/ml), 8-Br-cAmp (0.1 mM), and Forskolin (1 µM), all obtained from Sigma or Selleck Chemicals.
Antibodies
The following rabbit polyclonal antibodies were used: GFP (A11122, Invitrogen), GAPDH (14C10, Cell Signaling Technology/CST), Flag-tag (A01868, GenScript), PKA-Cα (4782, CST), PKA-Cβ (12232-1-AP, Proteintech), histone H3 (ab1791, Abcam), pan-phospho-Ser/Thr (AP0893, ABclonal), MBP (E8032, New England BioLabs), CREB (9197, CST), CREB-S133ph (9198, CST), Aurora A (14475, CST), GST (G7781, Sigma), CENP-A-S7ph (2187, CST). Rabbit polyclonal antibodies against Bora phosphorylated at S59 (Bora-S59ph) were generated by immunization with a synthetic phosphopeptide, KFRW(pS)IDQLA, where pS indicates phosphoserine. Mouse monoclonal antibodies included: anti-H3S10ph (9706, CST), α-Tubulin (T6074, Sigma), γ-Tubulin (T6557, Sigma), Myc-tag (05-724, Millipore), Flag-tag (clone M2, F-3165, Sigma), GFP (M20004, Abmart), Plk1 (ab17057, Abcam), Plk1-T210ph (558400, BD Transduction).
Secondary antibodies for immunoblotting included: goat anti-rabbit IgG-HRP (7074, CST) and horse anti-mouse IgG-HRP (7076, CST). Secondary antibodies for immunofluorescence staining included: donkey anti-rabbit IgG conjugated to Alexa Fluor Cy3 (711-165-152, Jackson ImmunoResearch); anti-mouse IgG conjugated to Alexa Fluor Cy3 (715-165-150, Jackson ImmunoResearch).
Fluorescence microscopy and quantification
HeLa and U2OS-LacO cells were fixed with 2% paraformaldehyde (PFA) in PBS for 10 min at room temperature, followed by permeabilization with 0.5% Triton X-100 in PBS for 5 min. For Plk1 staining, cells were fixed with 1% PFA in PBS for 5 min, quenched with 0.1 M glycine in PBS for 1 h, and then permeabilized with 0.1% Triton X-100 in PBS for 3 min. Fixed cells were blocked with 3% bovine serum albumin (BSA) in PBS and incubated with primary antibodies for 1-2 h, followed by secondary antibodies for 1 h, all at room temperature in 3% BSA/PBS. DNA was counterstained with DAPI (4′,6-diamidino-2-phenylindole) for 5 min. Images were acquired at room temperature using a Nikon ECLIPSE Ni microscope equipped with a Plan Apo Fluor 60x oil immersion objective (NA 1.4) and a Clara CCD camera (Andor Technology).
To quantify the relative enrichment of proteins at the LacO transgene array in U2OS-LacO cells, the average fluorescence intensity of antibody staining was measured within regions encompassing the EGFP-LacI signal and adjacent nuclear areas. After background subtraction, the ratio of the average immunostaining intensity of the protein of interest to the EGFP signal intensity at the LacO array was calculated.
Flow cytometry
Cells were harvested, washed with PBS, and fixed with ice-cold 70% ethanol overnight. Following fixation, cells were washed with PBS and treated with RNase A (200 µg/ml) at 37 °C for 30 min, then stained with propidium iodide (20 µg/ml). DNA content was analyzed using a FACScan flow cytometer (Beckman), and data were processed with FlowJo software.
Recombinant protein expression and purification
Plasmids encoding GST-, MBP-, or 6xHis-tagged proteins were transformed into E. coli BL21 (DE3) competent cells. Transformed cells were grown in LB medium with appropriate antibiotics at 37 °C until optical density at 600 nm (OD600) reached 0.6-0.8. Protein expression was then induced with 0.4 mM IPTG at 16 °C for 16 h. Cells were lysed by sonication in one of the following buffers: buffer A (50 mM Tris-HCl, pH 8.0, 300 mM NaCl, 1 mM EDTA, 1% Triton X-100; for GST fusion proteins); buffer B (50 mM Tris-HCl, pH 7.5, 300 mM NaCl, 1 mM EDTA, 0.5% Triton X-100; for MBP fusion proteins); or buffer C (50 mM Tris-HCl, pH 7.5, 300 mM NaCl, 0.5% Triton X-100; for 6×His fusion proteins). Lysates were clarified by centrifugation and incubated with Glutathione Sepharose 4B beads (GE Healthcare), Amylose Resin (New England Biolabs), or Ni NTA beads (Smart-Lifesciences), depending on the fusion tag. The resins were washed with the corresponding lysis buffer and eluted with 100 mM glutathione, 10 mM maltose, or 300 mM imidazole, respectively.
To construct the plasmid encoding MBP-Bora-S59ph-10×His, a point mutation was introduced at S59, converting its codon to “TAG”. The pEVOL-SEP plasmid, which encodes SEP-tRNA and SEP synthetase under control of the E. coli proK promoter and arabinose-inducible promoter, respectively, was used to facilitate site-specific incorporation of phosphoserine. C321ΔSerB cells were generated from C321.ΔA.exp (Addgene #49018) using lambda Red-mediated recombination, replacing the serB gene with a kanamycin resistance cassette. The MBP-Bora-S59ph-10×His and pEVOL-SEP plasmids were co-transformed into C321ΔSerB cells. Cultures were grown in LB medium under antibiotic selection at 37 °C until OD600 reached 0.6-0.8. Protein expression was induced with 0.4 mM IPTG at 16 °C for 16 h in the presence of 0.08% glucose and 0.2% arabinose. Cells were lysed by sonication in lysis buffer (50 mM Tris-HCl, pH 8.0, 300 mM NaCl, 20 mM imidazole), and MBP-Bora-S59ph-10×His was first purified using a Ni-NTA column (Smart-Lifesciences) according to the manufacturer’s instructions and eluted with 300 mM imidazole. The eluate was then incubated with Amylose Resin (New England Biolabs) at 4 °C for 8 h. The resin was washed with lysis buffer, and the protein was eluted with 10 mM maltose.
Pull-down assays, immunoprecipitation, and immunoblotting
For MBP fusion protein pull-down assays with cell lysates, HeLa cells were lysed in P150 buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 10 mM MgCl2, 5 mM EDTA) supplemented with 1 mM dithiothreitol (DTT), protease inhibitor cocktail (P8340, Sigma), 1 mM phenylmethylsulfonyl fluoride (PMSF), 0.1 μM okadaic acid (Calbiochem), 10 mM NaF and 20 mM β-glycerophosphate, and Benzonase (GenScript). After removing insoluble materials by high-speed centrifugation, lysates were precleared with Amylose Resin (New England Biolabs) and then incubated with MBP fusion proteins immobilized on beads for 4 h at 4 °C. For GST-Aurora A pull-down of MBP-Bora, recombinant proteins were incubated for 2 h at 4 °C with bead-immobilized GST fusion proteins in P150 buffer. For peptide pull-down of 6xHis-Aurora A, 1 μg Bora (49-69) peptides (WT or S59ph) were incubated with 4 μg of recombinant proteins in 500 μl P150 buffer for 1 h at 4 °C, followed by incubation with NeutrAvidin Plus Ultralink Resin (Thermo Fisher Scientific) for 1 h. The Bora peptide (residues 49-69, 95% purity), with a C-terminal lysine-biotin modification, was synthesized by GL Biochem (Shanghai). After pull-down assays, beads were washed 3-5 times with the same buffer, boiled in SDS sample buffer, and subjected to analysis by immunoblotting or CBB staining.
For immunoprecipitation, cells were lysed in P50 or P150 buffer (50 mM Tris-HCl, pH 7.5, 50 mM or 150 mM NaCl, 1% Triton X-100, 10 mM MgCl2, 5 mM EDTA) supplemented with 1 mM DTT, protease inhibitor cocktail (P8340, Sigma), 1 mM PMSF, 0.1 μM okadaic acid (Calbiochem), 10 mM NaF, 20 mM β-glycerophosphate, and Benzonase (GenScript). After removal of insoluble materials by high-speed centrifugation, lysates were precleared with rProtein A/G beads (Smart-Lifesciences, SA032100). Precleared lysates were incubated with Anti-Flag M2 Affinity gel (Sigma, A2220), Anti-Myc Affinity gel (GNI, GNI4510-MC), Anti-GFP Affinity beads (Smart-Lifesciences, SA070005), or control rProtein A/G beads for 4 h at 4 °C. For endogenous Plk1 immunoprecipitation, lysates were incubated with Plk1 antibodies for 3 h at 4 °C before addition of rProtein A/G beads for a further 1 h. Beads were washed 3-5 times with the same buffer, boiled in standard SDS sample buffer, and subjected to immunoblotting. SDS-PAGE and immunoblotting were carried out with standard procedures. Uncropped and unprocessed scans of the blots are provided in the Source data file.
n vitro kinase reaction
For in vitro phosphorylation assays of MBP-Bora, GST-Plk1, or GST-CENP-A, 0.5 μg of substrate was incubated with 0.2 μg of recombinant GST-PKA-Cα or 6×His-Aurora A. For kinase reactions using immunoprecipitated kinase, asynchronous HEK-293T cells transiently expressing PKA-Cα-GFP were lysed in buffer containing 50 mM Tris-HCl (pH 7.5), 500 mM NaCl, 1% Triton X-100, and 10 mM MgCl2, supplemented with protease and phosphatase inhibitors as in P500 buffer; Anti-GFP immunoprecipitates were then incubated with 0.5 μg of MBP-Bora. All kinase reactions were carried out in 50 μl of kinase reaction buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 100 mM DTT, and 10 mM MgCl2) supplemented with 0.3 mM ATP, and incubated at 30 °C for 30 min to 60 min. Reactions were terminated by the addition of SDS sample buffer, boiled, and analyzed by immunoblotting.
Mass spectrometry
For the data presented in Fig. 1B, Supplementary Fig. 1A, and Supplementary Data 1 and 2, immunoprecipitated Flag-Bora and its binding proteins were separated by SDS-PAGE. Gels were lightly stained with Coomassie brilliant blue, and then protein gel bands were cut into small pieces and de-stained with buffer (25 mM NH4HCO3/25% methanol, pH 8.0). Proteins were reduced with 10 mM DTT for 60 min at 56 °C and alkylated with 55 mM iodoacetamide for 45 min. For digestion, 0.5 µg of sequencing-grade modified trypsin (Promega, USA) was added, followed by a 37 °C overnight incubation. Prior to mass spectrometry analysis, the peptide preparations were desalted using StageTip and reconstituted with 0.1% formic acid (FA). Mass spectrometry was performed using a Thermo Scientific Q Exactive HF-X Hybrid Quadrupole-Orbitrap mass spectrometer instrument coupled with an Easy-nLC 1200 system. Mobile phases A and B were water and 80% acetonitrile, respectively, with 0.1% FA. Protein digests were loaded directly onto an analytical column (75 µm × 15 cm, 1.9 µm C18, 1 µm tip) at a flow rate of 450 nl/min. Samples were separated using a linear gradient of 6-8% B for 2 min, 8-27% B for 79 min, 27-40% B for 31 min, 40-90% B for 5 min, 90% B for 3 min. MS/MS data were collected in a data-dependent manner using a top 20 method with a full MS mass range from 400 to 1400 m/z, 60,000 resolutions, and an AGC target of 3 × 106. MS2 scans were triggered when an ion intensity threshold of 4.5 × 105 was reached. A dynamic exclusion time of 30 s was used. Ions with charge states 1, 6, 8, and more than 8 were excluded. The resulting raw data were uploaded into Proteome Discoverer (PD) and were searched against the UniProtKB protein sequence database.
Structure prediction
The structure of the complex formed between the full-length human Aurora A sequence (Uniprot: O14965) and the full-length human Bora sequence (Uniprot: Q6PGQ7-1; phosphorylated at S59) was predicted using AlphaFold3. The parameters used for prediction were the default settings of AlphaFold3, with no constraints applied. Predictions were performed via the public AlphaFold3 web server (https://www.alphafoldserver.com), which generated five models ranked all pointing to the importance of Aurora A residue Arg205 for interacting with phosphorylated S59 of Bora. A representative complex was visualized in PyMOL.
Statistical analysis and sequence alignment
Statistical analyses were performed using a two-tailed unpaired Student’s t-test in GraphPad Prism 8. P < 0.05 was considered statistically significant. Multiple sequence alignment of Bora orthologs was performed using UniProt (https://www.uniprot.org/), and the resulting figures were formatted in Microsoft Word.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
We thank members of our labs for helpful discussions, and Lei Zhao and Xiaoqing Zhang for contributions to the early stages of this work. This work was supported by the National Outstanding Youth Science Fund Project of National Natural Science Foundation of China (32025011 to F.W.), the National Key R&D Program of China (2022YFA1303102 and 2022YFA1105203 to F.W.), the National Natural Science Foundation of China (32270772 to H.Y., 32061160470 to F.W., and 32100583 to Q.C.), the Natural Science Foundation of Zhejiang Province (LZ24C070001 to F.W.), the China Postdoctoral Science Foundation (2020M681838 to Q.C., 2025T180763 and GZC20251022 to X.Y.), and the Central Guidance for Local Scientific and Technological Development Funding Project (2025ZY01106 to F.W.). We also acknowledge the Life Sciences Institute Core Facility and Dr. Cheng Ma at the Core Facility of Zhejiang University School of Medicine.
Author contributions
H.Y. and F.W. designed the research. M.Z., S.Z., and Y.Z. performed research. M.Z., S.Z., Y.Z., H.Y., and F.W. analyzed data. Q.C., X.Y., and L.Z. contributed new reagents and analytic tools. Q.C., X.Y., H.Y., and F.W. acquired funding. H.Y. and F.W. wrote the manuscript with the input from M.Z., S.Z., Y.Z., Q.C., and X.Y.
Peer review
Peer review information
Nature Communications thanks Olivier Gavet and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The mass spectrometry data generated in this study have been deposited in the ProteomeXchange Consortium under accession codes PXD066562 and PXD066630, respectively. All data supporting the findings of this study are available within the paper and its Supplementary Information. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Min Zhu, Shinan Zhou, Yingqi Zhang.
Contributor Information
Haiyan Yan, Email: yanhy@hzcu.edu.cn.
Fangwei Wang, Email: fwwang@zju.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-63352-y.
References
- 1.Nurse, P. A long twentieth century of the cell cycle and beyond. Cell100, 71–78 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Matthews, H. K., Bertoli, C. & de Bruin, R. A. M. Cell cycle control in cancer. Nat. Rev. Mol. Cell. Bio.23, 74–88 (2022). [DOI] [PubMed] [Google Scholar]
- 3.Hochegger, H., Takeda, S. & Hunt, T. Cyclin-dependent kinases and cell-cycle transitions: Does one fit all? Nat. Rev. Mol. Cell. Bio.9, 910–916 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Bertoli, C., Skotheim, J. M. & de Bruin, R. A. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell. Biol.14, 518–528 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan, D. O. Principles of CDK regulation. Nature374, 131–134 (1995). [DOI] [PubMed] [Google Scholar]
- 6.Mueller, P. R., Coleman, T. R., Kumagai, A. & Dunphy, W. G. Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science270, 86–90 (1995). [DOI] [PubMed] [Google Scholar]
- 7.Parker, L. L. & Piwnicaworms, H. Inactivation of the p34cdc2–cyclin B complex by the human Wee1 tyrosine kinase. Science257, 1955–1957 (1992). [DOI] [PubMed] [Google Scholar]
- 8.Norbury, C., Blow, J. & Nurse, P. Regulatory phosphorylation of the p34cdc2 Protein kinase in vertebrates. EMBO J.10, 3321–3329 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunphy, W. G. & Kumagai, A. The cdc25 protein contains an intrinsic phosphatase activity. Cell67, 189–196 (1991). [DOI] [PubMed] [Google Scholar]
- 10.Kumagai, A. & Dunphy, W. G. The cdc25 protein controls tyrosine dephosphorylation of the cdc2 protein in a cell-free system. Cell64, 903–914 (1991). [DOI] [PubMed] [Google Scholar]
- 11.Strausfeld, U. et al. Dephosphorylation and activation of a p34cdc2/cyclin B complex in vitro by human CDC25 protein. Nature351, 242–245 (1991). [DOI] [PubMed] [Google Scholar]
- 12.Gautier, J., Solomon, M. J., Booher, R. N., Bazan, J. F. & Kirschner, M. W. Cdc25 is a specific tyrosine phosphatase that directly activates P34cdc2. Cell67, 197–211 (1991). [DOI] [PubMed] [Google Scholar]
- 13.Galaktionov, K. & Beach, D. Specific activation of cdc25 tyrosine phosphatases by B-type cyclins: evidence for multiple roles of mitotic cyclins. Cell67, 1181–1194 (1991). [DOI] [PubMed] [Google Scholar]
- 14.Watanabe, N. et al. Cyclin-dependent kinase (CDK) phosphorylation destabilizes somatic Wee1 via multiple pathways. Proc. Natl. Acad. Sci. USA102, 11663–11668 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann, I., Clarke, P. R., Marcote, M. J., Karsenti, E. & Draetta, G. Phosphorylation and activation of human cdc25-C by cdc2-cyclin B and its involvement in the self-amplification of MPF at mitosis. EMBO J.12, 53–63 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindqvist, A., Rodríguez-Bravo, V. & Medema, R. H. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J. Cell Biol.185, 193–202 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roshak, A. K. et al. The human-like kinase, PLK, regulates cdc2/cyclin B through phosphorylation and activation of the cdc25C phosphatase. Cell Signal12, 405–411 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Kumagai, A. & Dunphy, W. G. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science273, 1377–1380 (1996). [DOI] [PubMed] [Google Scholar]
- 19.Watanabe, N. et al. M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFbeta-TrCP. Proc. Natl. Acad. Sci. USA101, 4419–4424 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toyoshima-Morimoto, F., Taniguchi, E. & Nishida, E. Plk1 promotes nuclear translocation of human cdc25C during prophase. EMBO Rep.3, 341–348 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gavet, O. & Pines, J. Activation of cyclin B1-Cdk1 synchronizes events in the nucleus and the cytoplasm at mitosis. J. Cell Biol.189, 247–259 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toyoshima-Morimoti, F., Taniguchi, E., Shinya, N., Iwamatsu, A. & Nishida, E. Polo-like kinase 1 phosphorylates cyclin B1 and targets it to the nucleus during prophase. Nature410, 215–220 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Gheghiani, L., Loew, D., Lombard, B., Mansfeld, J. & Gavet, O. PLK1 activation in late G2 sets up commitment to mitosis. Cell Rep.19, 2060–2073 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Pintard, L. & Archambault, V. A unified view of spatio-temporal control of mitotic entry: Polo kinase as the key. Open Biol.8, 180114 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hégarat, N., Rata, S. & Hochegger, H. Bistability of mitotic entry and exit switches during open mitosis in mammalian cells. Bioessays38, 627–643 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Rhind, N. & Russell, P. Signaling pathways that regulate cell division. Cold Spring Harb. Perspect. Biol.4, a005942 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nussinov, R., Zhang, W. A., Liu, Y. L. & Jang, H. Mitogen signaling strength and duration can control cell cycle decisions. Sci. Adv.10, eadm9211 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng, G. & Yu, H. T. Cyclin A turns on Bora to light the path to mitosis. Dev. Cell45, 542–543 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Vigneron, S. et al. Cyclin A-cdk1-dependent phosphorylation of Bora is the triggering factor promoting mitotic entry. Dev. Cell45, 637–650 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Hégarat, N. et al. Cyclin A triggers mitosis either via the Greatwall kinase pathway or cyclin B. EMBO J.39, e104419 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Farrell, P. H. Triggering the all-or-nothing switch into mitosis. Trends Cell Biol.11, 512–519 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joukov, V. & De Nicolo, A. Aurora-PLK1 cascades as key signaling modules in the regulation of mitosis. Sci. Signal.11, eaar4195 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Bruinsma, W., Raaijmakers, J. A. & Medema, R. H. Switching Polo-like kinase-1 on and off in time and space. Trends Biochem. Sci.37, 534–542 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Zitouni, S., Nabais, C., Jana, S. C., Guerrero, A. & Bettencourt-Dias, M. Polo-like kinases: structural variations lead to multiple functions. Nat. Rev. Mol. Cell Biol.15, 433–452 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Eckerdt, F. & Mailer, J. L. Kicking off the polo game. Trends Bioch. Sci.33, 511–513 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Jang, Y. J., Ma, S., Terada, Y. & Erikson, R. L. Phosphorylation of threonine 210 and the role of serine 137 in the regulation of mammalian polo-like kinase. J. Biol. Chem.277, 44115–44120 (2002). [DOI] [PubMed] [Google Scholar]
- 37.Macurek, L. et al. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature455, 119–123 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Seki, A., Coppinger, J. A., Jang, C. Y., Yates, J. R. & Fang, G. Bora and the kinase Aurora a cooperatively activate the kinase Plk1 and control mitotic entry. Science320, 1655–1658 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hutterer, A. et al. Mitotic activation of the kinase Aurora-A requires its binding partner Bora. Dev. Cell11, 147–157 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Seki, A. et al. Plk1- and beta-TrCP-dependent degradation of Bora controls mitotic progression. J. Cell Biol.181, 65–78 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan, E. H., Santamaria, A., Sillje, H. H. & Nigg, E. A. Plk1 regulates mitotic Aurora A function through betaTrCP-dependent degradation of hBora. Chromosoma117, 457–469 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson, J. L. et al. An atlas of substrate specificities for the human serine/threonine kinome. Nature613, 759–766 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor, S. S., Ilouz, R., Zhang, P. & Kornev, A. P. Assembly of allosteric macromolecular switches: lessons from PKA. Nat. Rev. Mol. Cell Biol.13, 646–658 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruinsma, W. et al. Spatial separation of Plk1 phosphorylation and activity. Front. Oncol.5, 132 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cascales, H. S. et al. Cyclin A2 localises in the cytoplasm at the S/G2 transition to activate PLK1. Life Sci. Alliance4, e202000980 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan, E. H. Y., Santamaria, A., Silljé, H. H. W. & Nigg, E. A. Plk1 regulates mitotic Aurora A function through βTrCP-dependent degradation of hBora. Chromosoma117, 457–469 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vassilev, L. T. et al. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc. Natl. Acad. Sci. USA103, 10660–10665 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watson, N. A. et al. Kinase inhibition profiles as a tool to identify kinases for specific phosphorylation sites. Nat. Commun.11, 1684 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sassone-Corsi, P. The cyclic AMP pathway. Cold Spring Harb. Perspect. Biol.4, a011148 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Janicki, S. M. et al. From silencing to gene expression: real-time analysis in single cells. Cell116, 683–698 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park, H. S. et al. Expanding the genetic code of Escherichia coli with phosphoserine. Science333, 1151–1154 (2011). [DOI] [PMC free article] [PubMed]
- 52.Pirman, N. L. et al. A flexible codon in genomically recoded Escherichiacoli permits programmable protein phosphorylation. Nat. Commun.6, 8130 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eot-Houllier, G. et al. Aurora A-dependent CENP-A phosphorylation at inner centromeres protects bioriented chromosomes against cohesion fatigue. Nat. Commun.9, 1888 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kunitoku, N. et al. CENP-A phosphorylation by Aurora-A in prophase is required for enrichment of Aurora-B inner centromeres and for kinetochore function. Dev. Cell5, 853–864 (2003). [DOI] [PubMed] [Google Scholar]
- 55.Bruinsma, W., Macurek, L., Freire, R., Lindqvist, A. & Medema, R. H. Bora and Aurora-A continue to activate Plk1 in mitosis. J. Cell Sci.127, 801–811 (2014). [DOI] [PubMed] [Google Scholar]
- 56.Lénárt, P. et al. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr. Biol.17, 304–315 (2007). [DOI] [PubMed] [Google Scholar]
- 57.Petronczki, M., Glotzer, M., Kraut, N. & Peters, J. M. Polo-like kinase 1 triggers the initiation of cytokinesis in human cells by promoting recruitment of the RhoGEF Ect2 to the central spindle. Dev. Cell12, 713–725 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Kufer, T. A. et al. Human TPX2 is required for targeting Aurora-A kinase to the spindle. J. Cell Biol.158, 617–623 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lens, S. M. A., Voest, E. E. & Medema, R. H. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nat. Rev. Cancer10, 825–841 (2010). [DOI] [PubMed] [Google Scholar]
- 60.van Vugt, M. A., Bras, A. & Medema, R. H. Polo-like kinase-1 controls recovery from a G2 DNA damage-induced arrest in mammalian cells. Mol. Cell15, 799–811 (2004). [DOI] [PubMed] [Google Scholar]
- 61.Tavernier, N. et al. Bora phosphorylation substitutes in trans for T-loop phosphorylation in Aurora A to promote mitotic entry. Nat. Commun.12, 1899 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Foijer, F., Wolthuis, R. M. F., Doodeman, V., Medema, R. H. & te Riele, H. Mitogen requirement for cell cycle progression in the absence of pocket protein activity. Cancer Cell8, 455–466 (2005). [DOI] [PubMed] [Google Scholar]
- 63.Hehnly, H. et al. A mitotic kinase scaffold depleted in testicular seminomas impacts spindle orientation in germ line stem cells. Elife4, e09384 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wieser, S. & Pines, J. The biochemistry of mitosis. Cold Spring Harb. Perspect. Biol.7, a015776 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tavernier, N. et al. Cdk1 phosphorylates SPAT-1/Bora to trigger PLK-1 activation and drive mitotic entry in embryos. J. Cell Biol.208, 661–669 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thomas, Y. et al. Cdk1 phosphorylates SPAT-1/Bora to promote Plk1 activation in and human Cells. Cell Rep.15, 510–518 (2016). [DOI] [PubMed] [Google Scholar]
- 67.Tavernier, N., Sicheri, F. & Pintard, L. Aurora A kinase activation: different means to different ends. J. Cell Biol.220, e202106128 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clarke, P. R., Leiss, D., Pagano, M. & Karsenti, E. Cyclin A- and cyclin B-dependent protein-kinases are regulated by different mechanisms in Xenopus egg extracts. EMBO J.11, 1751–1761 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pierce, K. L., Premont, R. T. & Lefkowitz, R. J. Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol.3, 639–650 (2002). [DOI] [PubMed] [Google Scholar]
- 70.Seifert, R. & Wenzel-Seifert, K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch. Pharm.366, 381–416 (2002). [DOI] [PubMed] [Google Scholar]
- 71.Leurs, R., Smit, M. J., Alewijnse, A. E. & Timmerman, H. Agonist-independent regulation of constitutively active G-protein-coupled receptors. Trends Biochem. Sci.23, 418–422 (1998). [DOI] [PubMed] [Google Scholar]
- 72.Zaccolo, M., Zerio, A. & Lobo, M. J. Subcellular organization of the cAMP signaling pathway. Pharm. Rev.73, 278–309 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang, J. Z. et al. Phase separation of a PKA regulatory subunit controls cAMP compartmentation and oncogenic signaling. Cell182, 1531–1544 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hardy, J. C. et al. Molecular determinants and signaling effects of PKA RIα phase separation. Mol. Cell84, 1570–1584 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chan, G. K. et al. Oncogenic PKA signaling increases c-MYC protein expression through multiple targetable mechanisms. Elife12, e69521 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coles, G. L. et al. Unbiased proteomic profiling uncovers a targetable GNAS/PKA/PP2A axis in small cell lung cancer stem cells. Cancer Cell38, 129–143 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The mass spectrometry data generated in this study have been deposited in the ProteomeXchange Consortium under accession codes PXD066562 and PXD066630, respectively. All data supporting the findings of this study are available within the paper and its Supplementary Information. Source data are provided with this paper.