Abstract
Lipids were analyzed by gas chromatography-mass spectrometry for a 7-week in vitro decay of eucalypt wood by four ligninolytic basidiomycetes. The sound wood contained up to 75 mg of lipophilic compounds per 100 g of wood. Hydrolysis of sterol esters, which represented 38% of total wood lipids, occurred during the fungal decay. The initial increase of linoleic and other free unsaturated fatty acids paralleled the decrease of sterol esters. Moreover, new lipid compounds were found at advanced stages of wood decay that were identified from their mass spectra as unsaturated dicarboxylic acids consisting of a long aliphatic chain attached to the C-3 position of itaconic acid. These dicarboxylic acids were especially abundant in the wood treated with Ceriporiopsis subvermispora (up to 24 mg per 100 g of wood) but also were produced by Phlebia radiata, Pleurotus pulmonarius, and Bjerkandera adusta. We hypothesize that three main alkylitaconic acids (tetradecylitaconic, cis-7-hexadecenylitaconic, and hexadecylitaconic acids) are synthesized by fungi in condensation reactions involving palmitic, oleic, and stearic acids. We suggest that both wood unsaturated fatty acids (present in free form or released from esters during natural decay) and unsaturated metabolites synthesized by fungi could serve as a source for peroxidizable lipids in lignin degradation by white rot basidiomycetes.
Fungi produce a variety of lipids including fatty acids in free or esterified form, e.g., glycerides, phospholipids, glycolipids, sterol esters, sphingolipids, or simple esters, as well as other lipids, e.g., free sterols, sterol glycosides, and hydrocarbons (46, 54, 56). Vegetative mycelium (from pure cultures) and fruit bodies of basidiomycetes differ in both lipid content and fatty acid profiles. Linoleic (18:29c,12c), oleic (18:19c), and palmitic (16:0) acids are the main fatty acids in most cases. Linoleic acid is very abundant in fruit bodies and may account for as much as 70 to 80% of the lipids present (1, 42, 48, 49). Other saturated and unsaturated fatty acids are present in basidiomycetes at lower levels. As reported for other filamentous fungi, unsaturated fatty acids could help basidiomycetes adapt to low growth temperatures (11, 12). These fatty acids also may have a role in lignin degradation.
Lipid peroxidation reactions could be a part of the lignin degradation process by white rot basidiomycetes. When unsaturated fatty acids are oxidized in the presence of manganese peroxidase (MnP), a ligninolytic peroxidase that oxidizes Mn2+ to Mn3+, lipid hydroperoxides and free radicals are formed. This reaction is considered to be sufficient for lignin degradation by some ligninolytic fungi, e.g., Ceriporiopsis (syn. Poria) subvermispora, that lack lignin peroxidase (LiP) (4, 31-33). LiP, which has a high redox potential that enables direct oxidation of nonphenolic lignin units, was described in the white rot fungus Phanerochaete chrysosporium together with MnP (37). However, LiP has not been detected in some efficient lignin-degrading fungi (27, 45), and unlike MnP, LiP is rarely found during fungal decay of lignocellulosic substrates.
Recent studies of MnP-mediated peroxidation of lipids have shown that a free carboxyl group is required for linoleic acid peroxidation in a reaction that is initiated by the Mn3+ generated by MnP (55). The acyl radical formed reacts with oxygen to generate a lipid peroxyl radical (LOO·) that can react with other fatty acid molecules, thereby propagating the reaction. The resulting lipid hydroperoxide (LOOH) acts as an MnP-oxidizing substrate in the place of H2O2 and can also react with metals. Among the products of metal reactions are lipid alkoxyl radicals (LO·) that are stronger oxidants than the peroxyl radicals. The role of these lipid-derived radicals in lignin degradation is not well understood; however, one hypothesis is that MnP-mediated lipid peroxidation is a common mechanism used for lignin degradation by many basidiomycetes (32, 33).
Fungal metabolism is one source of unsaturated lipids for peroxidation reactions (7, 34), but peroxidizable plant lipids also could be used. In previous studies, we have analyzed eucalypt wood lipids involved in pitch deposit formation during the manufacturing of chlorine-free eucalypt pulp (14, 15, 17, 23). Simultaneously, a large number of fungi were screened for the biotechnological control of pitch (43), and four basidiomycetes were selected because of their ability to efficiently remove these compounds from eucalypt wood, resulting in reduced levels in pulp (24, 26). When the removal of wood lipophilic compounds by these fungi was analyzed, several new lipids were detected and were especially abundant in the C. subvermispora cultures. These compounds were identified by mass spectrometry (MS) as a novel series of alkylitaconic acids synthesized by the fungi. These and other unsaturated lipids could have a role in wood lignin degradation by C. subvermispora and other white rot fungi via lipid radicals generated by peroxidation reactions.
MATERIALS AND METHODS
Fungi.
We used four strains from the Centraalbureau voor Schimmelcultures (CBS) (Baarn, The Netherlands): Bjerkandera adusta CBS 230.93, Phlebia radiata CBS 184.83, Pleurotus pulmonarius CBS 507.85, and C. subvermispora CBS 347.63. C. subvermispora IJFM A716 (FP90031) and IJFM A718 (CZ-3) were received from K.-E. Eriksson.
Wood decay.
We evaluated in vitro decay of Eucalyptus globulus wood (obtained from the ENCE mill in Pontevedra, Spain) under solid-state fermentation conditions. Two grams (dry weight) of small chips (1 to 2 by 10 to 20 mm) and 4 ml of water were placed in 100-ml Erlenmeyer flasks, sterilized for 20 min at 120°C, and then inoculated with pellets from a fungal culture; the culture was grown in glucose-peptone medium (36) and had been washed and resuspended in 1.5 ml of sterile water (the dry weight of inoculum was 0.5 mg per g of wood). The cotton-plugged flasks were covered with aluminum foil to maintain the humidity and incubated (static) at 28°C for 7 weeks. The time course of lipophilic compounds in the decaying wood samples was followed by analyzing the contents of three complete flasks per fungus after 0, 1, 2, 4, and 7 weeks of incubation. The basidiomycete biomass in the decayed wood was calculated from the total content of ergosterol. An average ergosterol content of 0.4 mg g of mycelium−1 was used, as reported for the related white rot fungus P. chrysosporium (13).
Lipid analysis.
The wood samples were dried in a forced-air oven at 60°C, milled using a knife mill (Janke and Kunkel, Staufen, Germany), and extracted with acetone in a Soxhlet extraction apparatus for 8 h (51). The extracts were evaporated to dryness and resuspended in chloroform for chromatographic analysis of the lipophilic fraction. The gas chromatography (GC) analyses were performed on a model GC 8000 Top gas chromatograph (Thermo Finnigan, San Jose, Calif.) coupled to a quadruple mass spectrometer detector (model Voyager; ThermoQuest Finnigan) equipped with a fused silica capillary column (model DB-5HT; 15 m by 0.25 mm [inner diameter]; film thickness, 0.1 μm; J&W). The oven was heated from 120°C (1 min) to 380°C (5 min) at 10°C min−1. The injector and transfer line temperatures were set at 300 and 350°C, respectively. Helium was used as the carrier gas, and the injection was performed in splitless mode. Peaks were quantified by area, and a mixture of standards was used to elaborate calibration curves (the fatty acid calibration curve was used for alkylitaconic acids). Trimethylsilyldiazomethane methylation and bis(trimethylsilyl)trifluoroacetamide (BSTFA) silylation, alone or subsequently applied, were used to elucidate the molecular structure of new fungal metabolites. Double-bond position in the alkyl chain of alkylitaconic acids was determined by hydroxylation with osmium tetroxide followed by silylation of the diol derivative using BSTFA (16). Compounds were identified by comparing their mass spectra with mass spectra in the Wiley and Nist libraries, by mass “fragmentography” and, when possible, by comparison with standards.
RESULTS
Lipid analysis during eucalypt wood decay by white rot fungi.
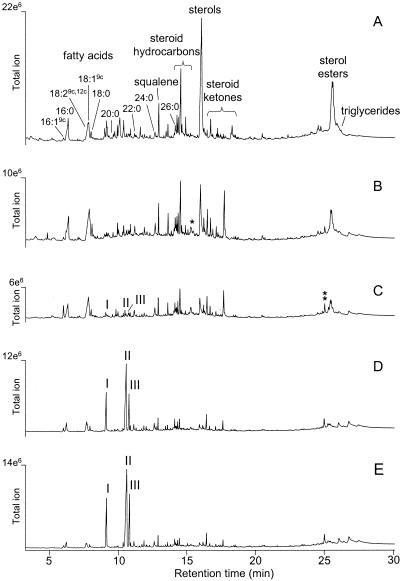
The major lipids (Fig. 1A) in the sound eucalypt wood were (i) sitosterol and other minor sterols, e.g., stigmastanol, citrostadienol, fucosterol, cycloartenol, and 24-methylenecycloartanol, either in free form or as esters with unsaturated and saturated fatty acids, e.g., linoleic, oleic, palmitic, and palmitoleic acids (16:19c), or glucosides; (ii) free fatty acids, the most abundant being palmitic, oleic, linoleic, and stearic (18:0) acids (minor peaks of other free fatty acids are indicated on the chromatogram); (iii) various steroid hydrocarbons, e.g., stigmasta-3,5-diene, and ketones, e.g., stigmast-4-en-3-one and stigmasta-3,5-dien-7-one; and (iv) squalene. The amount of these compounds in the wood changed dramatically during fungal growth. As decay progressed (the highest wood degradation rate occurred between 2 and 4 weeks of incubation), wood lipophilic compounds were degraded and new lipid compounds were identified. These compounds were especially common in the wood degraded by C. subvermispora (Fig. 1B to E), where three new lipids (peaks I to III) were the major compounds at advanced stages of decay.
FIG. 1.
Total-ion GC-MS chromatograms of the lipophilic fraction of acetone extract from eucalypt wood decayed by C. subvermispora. (A) Control wood; (B) 1-week decay (1% weight loss); (C) 2-week decay (2% weight loss); (D) 4-week decay (10% weight loss); (E) 7-week decay (14% weight loss). Sterol glucosides are not shown because they appear only in silylated samples. Linoleic and oleic acids coeluted in the same peak (their respective abundances were obtained from single-ion chromatographic profiles). The free ergosterol and ergosterol ester peaks in the decayed wood are indicated by one and two asterisks, respectively.
The most extensive degradation of wood lipids was achieved with P. radiata and C. subvermispora (Table 1), which also caused the highest loss of wood weight. The lowest lipid and wood degradation occurred with P. pulmonarius. Among the different lipid classes, free and esterified sterols were the most extensively degraded, while significant amounts of triglycerides, steroid ketones, and hydrocarbons remained after the fungal decay of wood. The new lipids (peaks I to III) were not found in sound eucalypt wood but were found in the samples treated with each of the four fungi.
TABLE 1.
Composition of lipophilic extracts including three alkylitaconic-type metabolites in sound eucalypt wood and after 7-week decay with four basidiomycetes
| Basidiomycete | Fungal biomass (%) | Wt loss (%) | Total lipids | Composition (mg per 100 g of wood)a
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Free fatty acids
|
Alkylitaconic acids
|
Free terpenoids
|
Esters
|
|||||||||||||||||
| 16:0 | 18:29c,12c | 18:19c | 18:0 | I | II | III | SQ | SH | S | SK | SE | TG | ||||||||
| None (sound wood) | 0 | 0 | 79 | 4.9 ± 0.3 | 1.5 ± 0.2 | 2.7 ± 0.9 | 0.80 ± 0.05 | 0 | 0 | 0 | 2.4 ± 0.42 | 9.2 ± 0.5 | 23 ± 3 | 2.3 ± 0.1 | 31 ± 1 | 0.81 ± 0.02 | ||||
| C. subvermispora | 0.37 | 14 | 35 | 0.76 ± 0.01 | 0.31 ± 0.05 | 0.71 ± 0.02 | 0.29 ± 0.05 | 5.6 ± 0.7 | 13 ± 1.7 | 4.6 ± 0.5 | 0.32 ± 0.24 | 2.1 ± 0.3 | 0.44 ± 0.09 | 1.8 ± 0.2 | 1.6 ± 0.2 | 2.7 ± 0.3 | ||||
| P. radiata | 0.09 | 12 | 8.0 | 0.56 ± 0.16 | 0.32 ± 0.10 | 0.37 ± 0.10 | 0.14 ± 0.03 | 0.05 ± 0.05 | 0.12 ± 0.11 | 0.04 ± 0.03 | 0.19 ± 0.12 | 1.3 ± 0.2 | 0.76 ± 0.18 | 1.4 ± 0.2 | 0.78 ± 0.12 | 1.9 ± 0.3 | ||||
| B. adusta | 0.29 | 12 | 25 | 1.1 ± 0.3 | 0.68 ± 0.24 | 1.2 ± 0.3 | 0.44 ± 0.08 | 0.19 ± 0.20 | 0.43 ± 0.45 | 0.14 ± 0.16 | 0.78 ± 0.45 | 5.2 ± 0.8 | 3.4 ± 0.7 | 3.0 ± 0.4 | 5.2 ± 0.3 | 3.1 ± 3.1 | ||||
| P. pulmonarius | 0.03 | 3.3 | 21 | 1.2 ± 0.2 | 0.37 ± 0.17 | 0.92 ± 0.10 | 0.42 ± 0.07 | 0.05 ± 0.02 | 0.14 ± 0.05 | 0.04 ± 0.02 | 0.44 ± 0.23 | 3.4 ± 0.2 | 8.0 ± 0.4 | 2.6 ± 0.1 | 2.6 ± 0.2 | 0.93 ± 0.27 | ||||
Values are means ± 95% confidence intervals (three replicates). Abbreviations: 16:0, palmitic acid; 18:29c,12c, linoleic acid; 18:19c, oleic acid; 18:0, stearic acid; I to III, lipid fungal metabolites (see Fig. 2); SQ, squalene; SH, steroid hydrocarbons; S, sterols; SK, steroid ketones; SE, sterol esters; TG, triglycerides.
Chemical identification of new lipids in the decayed wood.
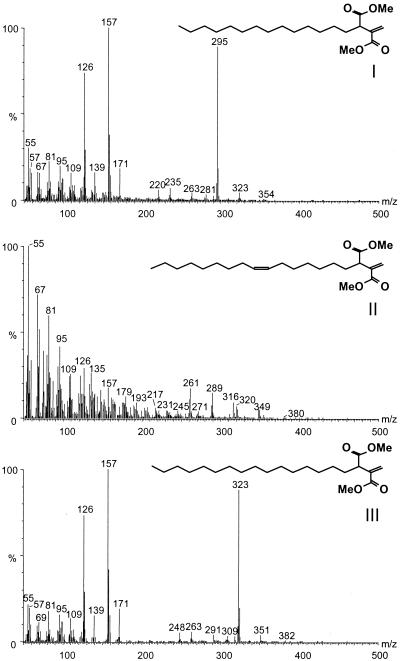
The chemical structure of the new lipids was proposed from the mass spectra of underivatized, methylated, and silylated extracts from the wood decayed by C. subvermispora for 4 and 7 weeks. These lipids are unsaturated dicarboxylic acids consisting of a long aliphatic chain attached to the C-3 position of itaconic acid (methylenebutanedioic acid). The three major peaks, I, II, and III, were identified as 2-methylene-3-carboxyheptadecanoic (tetradecylitaconic) acid, cis-2-methylene-3-carboxy-10-nonadecenoic (cis-7-hexadecenylitaconic) acid, and 2-methylene-3-carboxynonadecanoic (hexadecylitaconic) acid, respectively (Fig. 2).
FIG. 2.
Mass spectra and formulae of the three lipid metabolites present in eucalypt wood decayed by different basidiomycetes as the corresponding methyl esters: I, methyl 2-methylene-3-carboxyheptadecanoate (methyl tetradecylitaconate); II, methyl cis-2-methylene-3-carboxy-10-nonadecenoate (methyl cis-7-hexadecenylitaconate); III, methyl 2-methylene-3-carboxynonadecanoate (methyl hexadecylitaconate).
The formation of the methyl ester derivatives of these lipids introduced two methyl groups, indicating that these compounds are dicarboxylic acids. Subsequent silylation of the methyl esters did not introduce any trimethylsilyl group, confirming the absence of further free hydroxyl groups after methylation. The mass spectra of the methyl derivatives of compounds I and III are very similar, with similar fragmentation patterns and a difference of two methylene groups in the molecular ion. These mass spectra have as a base peak intense fragments at m/z 157 from the itaconate group that was formed by cleavage of the allylic C-C. The fragment at m/z 171 was formed in a similar manner from the citraconic isomer. A McLafferty rearrangement of the methyl ester produced the fragment at m/z 158 (35). The fragment at m/z 126 may arise from a loss of methoxyl from the fragment at m/z 157. The molecular ions, at m/z 354 and 382 for compounds I and III, respectively, are clearly present in the mass spectra. Loss of a methoxyl group (M-OCH3) or a carboxylate group (M-COOCH3) gives rise to the fragments at m/z 323 and 295 for peak I and m/z 351 and 323 for peak III, which are prominent in the mass spectra. On the other hand, the mass spectrum of the methyl derivative of peak II is identical to that reported previously (19) for the methyl derivative of a compound isolated from a culture of C. subvermispora grown on beech wood and identified as (Z)-1,7-nonadecadien-2,3-dicarboxylic acid (which also may be termed cis-4-hexadecenylitaconic acid). The molecular ion at m/z 380 is very weak, but the fragments arising from losses of methoxy and carboxylate groups at m/z 349 and m/z 321, respectively, are clearly present. The itaconate fragment at m/z 157 also is clearly visible in the mass spectrum of the methyl derivative of peak II. The chemical structures deduced from mass spectra of methylated derivatives of the three fungal lipids were confirmed by the mass spectra of the underivatized and silylated compounds.
Hydroxylation of the double bond of hexadecenylitaconic acid followed by silylation and GC-MS analysis yielded peaks with an intense m/z 215 fragment corresponding to the cleavage of a diol located at C-7 of the alkyl chain and indicated that compound II is cis-7-hexadecenylitaconic acid.
In addition to these three major compounds (with retention times of 9.1, 10.5 and 10.8 min after GC-MS of underivatized extracts), other alkylitaconic acids, present as minor peaks or traces, were tentatively identified from their mass spectra as 2-methylene-3-carboxypentadecanoic (dodecylitaconic) acid at 7.2 min, 2-methylene-3-carboxyheptadecenoic (tetradecenylitaconic) acid at 8.8 min, 2-methylene-3-carboxyeneicosenoic (octadecenylitaconic) acid at 12.2 min, and 2-methylene-3-carboxyeneicosanoic (octadecylitaconic) acid at 12.3 min.
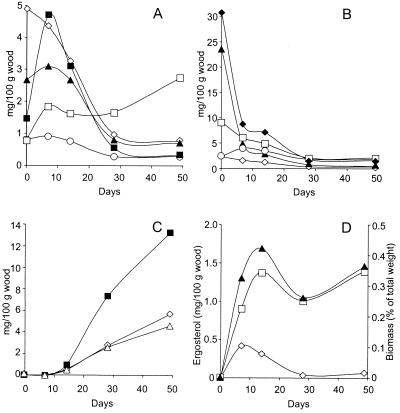
Time course of lipids in the wood cultures of C. subvermispora.
Free unsaturated linoleic and oleic acids (Fig. 3A), which are major constituents of wood sterol esters, increased during the first week of decay, paralleling the decrease in sterol esters (Fig. 3B). Saturated palmitic and stearic acids progressively decreased and triglycerides increased as decay progressed (Fig. 3A), while the amount of free sterols decreased (Fig. 3B). Less pronounced decreases occurred in the amount of steroid hydrocarbons, even though the level of steroid ketones increased during the first week, and comparatively high levels of these compounds remained at the end of the 7-week period (Fig. 3B).
FIG. 3.
Lipids and fungal biomass during 7-week decay of eucalypt wood by C. subvermispora. (A) Palmitic (◊), stearic (○), oleic (▴), and linoleic (▪) acids and total triglycerides (□). (B) Free (▴) and esterified (⧫) sterols, steroid ketones (○), and hydrocarbons (□). (C) Alkylitaconic acids I (◊), II (▪), and III (▵). (D) Free (◊) and esterified (□) ergosterol and fungal biomass (▴). The 95% confidence limits for all values are below 20% of the means (three replicates).
In contrast to most wood lipids, which strongly decreased during fungal decay, the main alkylitaconic acids (Fig. 3C) increased after the second week, attaining total amounts similar to those of the major lipid classes in the sound eucalypt wood, i.e., free and esterified sterols. The three alkylitaconic acids were also found in C. subvermispora CZ-3 (IJFM A718) and FP90031 (IJFM A716) cultures grown under the same conditions (data not shown). Free and esterified ergosterol were not detectable in the sound eucalypt wood and were relatively minor peaks in the decayed wood (asterisks in Fig. 1) but were used to estimate basidiomycete biomass. Free ergosterol was detected only in the 1 or 2 week samples, while the ester peaked after 3 weeks and remained stable for the remainder of the 7-week period (Fig. 3D). Since the free form was a low percentage of the total ergosterol in the decayed wood, the time course of fungal biomass paralleled that of esterified ergosterol.
DISCUSSION
Biodegradation of wood lipids.
Extractives, i.e., the compounds extractable from wood with organic solvents, consist of both polar and lipophilic molecules, with the amount and composition of the two fractions varying by plant species (21, 28). The accumulation of lipophilic extractives in pulp and paper mills (forming the so-called pitch deposits) results in low-quality pulp and blockages that can halt operations and lead to economic losses (29). In the present study, as before (23, 50), sterol esters (consisting mainly of sitosterol esterified with unsaturated fatty acids) and free sitosterol were the main extractable lipids from E. globulus wood. Higher amounts of triglycerides characterize the lipid fractions from other hardwoods, while softwoods commonly contain triglycerides and resin acids (21).
Wood triglycerides can be hydrolyzed by various microorganisms, including both ascomycete- and basidiomycete-type fungi (43). The released fatty acids can be metabolized or used to synthesize microbial lipids. Steroids, however, are more recalcitrant towards microbial degradation. Esterases that hydrolyze sterol esters are much rarer than are microbial lipases (25). In the present study, we found that the four basidiomycetes efficiently degraded common eucalypt wood lipids, including free and esterified sitosterol, the highest degradation rates being caused by P. radiata and C. subvermispora. C. subvermispora produced more biomass than did the other three basidiomycetes. P. radiata efficiently removed extractives despite its low biomass production on the eucalypt wood.
The time course of changes in lipid classes during wood decay showed an initial increase in unsaturated free fatty acids. This was mainly related to sterol ester hydrolysis, since triglycerides were minor lipid components in the eucalypt wood. The amount of triglycerides increased during the studied decay period, suggesting fungal esterification of fatty acids. The steroid ketones also increased during wood decay, probably because they are among the first products of oxidative degradation of sitosterol and other eucalypt sterols (18, 47). The constant value for biomass of C. subvermispora (0.25 to 0.45%) throughout wood decay suggests continued fungal activity even after a colonization phase, as reported for other wood-colonizing fungi (22).
Fungal production of alkylitaconic acids.
The new lipid compounds we found in the decayed wood were first evaluated as possible degradation products of the main wood lipids. However, we concluded that they result from fungal biosynthetic activity. We identified three main alkylitaconic acids in the wood decayed by all four basidiomycetes studied, but the amount of these compounds was 1 to 2 orders of magnitude higher in the C. subvermispora wood cultures. These compounds were not detected in sound eucalypt wood.
Hexadecenylitaconic acid (peak II) has also been isolated from C. subvermispora grown on beech wood and identified using MS, and 1H, 13C, and bidimensional nuclear magnetic resonance (NMR) (19). The amount of hexadecenylitaconic acid we obtained from eucalypt wood (approximately 7 mg 100 g of wood−1) was similar to that reported on beech wood preextracted with benzene-ethanol (2:1) and supplemented with glucose and ammonium (19). An itaconic acid-type microbial metabolite with a long alkyl chain, 15-hydroxyhexadecylitaconic acid (a hydroxylated form of compound III), also has been isolated from the lichen Usnea aliphatica (35).
The mass spectrum of methylated peak II was nearly identical to that previously reported as corresponding to (Z)-1,7-nonadecadiene-2,3-dicarboxylic acid (cis-4-hexadecenylitaconic acid) (19). However, the results obtained here suggest that the location of the double bond in the unsaturated alkyl chain of this hexadecenylitaconic acid from C. subvermispora could be erroneously assigned by Enoki et al. (19). The m/z 215 fragment in the mass spectra of products obtained after double-bond hydroxylation and silylation suggests a double bond at C-7. An m/z 257 fragment should be obtained from hydroxylation and silylation of cis-4-hexadecenylitaconic acid, which was not observed. As discussed by del Río et al. (16), this conclusion is also supported by the 13C NMR spectrum of this compound (19) that shows a shift difference between the two olefinic carbons of the alkyl chain (0.3 ppm) that is smaller than expected if the double bond was located at C-4, closer to the carboxylates. Similar differences have been reported between olefinic carbon signals in the 13C NMR spectra of oleic (18:19c) and petroselinic (18:16c) acids (38).
The two other alkylitaconic acids (peaks I and III) have not been previously reported. We deduced their chemical structures, and those of the four minor compounds (dodecylitaconic, tetradecenylitaconic, octadecenylitaconic, and octadecylitaconic acids), from the mass spectra of underivatized, methylated, and silylated derivatives (16). The three dominant alkylitaconic acids also were found in cultures of C. subvermispora CZ-3 and FP90031. The diunsaturated one (peak II) had been described previously from FP90031 (19).
Proposed biosynthetic pathways.
This series of fungal alkylitaconic acids could be synthesized by carbonyl-methylene condensation as reported for related long-chain aliphatic di- or tricarboxylic acids produced by lichens and fungi (52, 53). The seven alkylitaconic acids can be derived formally by condensation of the carbonyl group of oxaloacetate with the α-methylene groups of myristic (14:0), palmitic, palmitoleic, stearic, oleic, arachidic (20:0), and eicosenoic (20:1) acids. Various 14- to 20-C fatty acids were found in the sound eucalypt wood but also can be synthesized by white rot basidiomycetes, although the 20-C fatty acids are rare (1, 54). When free fatty acids were analyzed in cultures of C. subvermispora grown on beech wood, palmitic, linoleic, stearic, and two octadecenoic isomers were reported (20).
The proposed reaction is similar to oxaloacetate and acetyl-S-coenzyme A (-CoA) condensation for citric acid formation in the tricarboxylic-acid cycle. Therefore, fatty acids probably will be activated to their CoA-SH thioesters (a requisite for most fatty acid reactions) before reacting with oxaloacetate. Citrate synthases have been characterized from a variety of organisms, but synthases catalyzing the formation of alkylcitric acids are relatively rare, although two of them, producing decylcitric and decylhomocitric acids, have been purified from Penicillium lehmanii (syn. Penicillium spiculisporum) (39, 40). Formation of alkylitaconic acids is probably regulated by the substrate specificity of the alkylcitrate synthases. Although nothing is known about these enzymes in basidiomycetes, the P. lehmanii decylcitrate synthase exhibits preference for CoA thioesters of 12 C or shorter saturated fatty acids (39, 41).
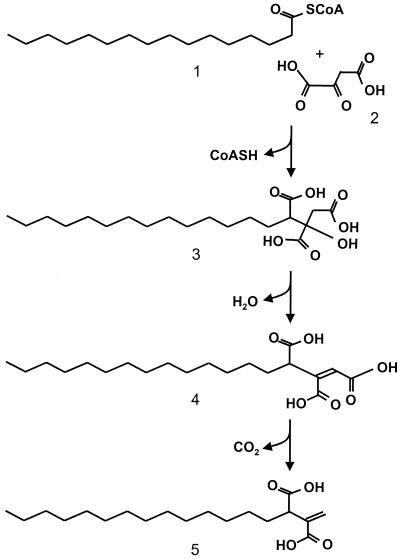
The product of condensation of oxaloacetate with the α-methylene group of the fatty acids is exemplified by agaricic (hexadecylcitric) acid, a long-chain aliphatic tricarboxylic metabolite first described in 1907 from Laricifomes officinalis (syn. Polyporus officinalis), norcaperatic (tetradecylcitric) acid, or the two P. lehmanii metabolites (10). Removal of the hydroxyl group from alkylcitric acids would result in alkyl-cis-aconitic acid intermediates. Their decarboxylation, leaving a methylene group as proposed for itaconic acid synthesis (8), would yield the series of alkylitaconic acids described from the wood cultures of C. subvermispora.
For example (Fig. 4), tetradecylitaconic acid (peak I) could be synthesized from palmitic acid via three steps: (i) condensation of oxaloacetic acid and palmitoyl-S-CoA; (ii) hydroxyl removal and double-bond formation; and (iii) decarboxylation yielding tetradecylitaconic acid. Similar biosynthetic pathways could be postulated for the other lipid metabolites described. This biosynthetic scheme is also compatible with the structure that we propose for compound II, cis-7-hexadecenylitaconic acid instead of cis-4-hexadecenylitaconic acid as previously proposed (19). The cis-7-hexadecenylitaconic acid would be formed from oleic acid, which is present in high amounts in the lipophilic extracts of both eucalypt wood and C. subvermispora biomass, whereas the cis-4-hexadecenylitaconic acid probably would be formed from petroselinic acid, which is not common in nature and has not been reported in either eucalypt wood or solid state cultures of C. subvermispora. In lichens and some other fungi a related biosynthetic pathway leads to a series of lactone-type metabolites (52, 53).
FIG. 4.
Proposed biosynthetic scheme for tetradecylitaconic acid including: condensation of the oxaloacetic acid (step 2) carbonyl group with the α-methylene group of palmitoyl-S-CoA (step 1), hydroxyl removal from the resulting tetradecylcitric acid (step 3) and double bond formation, and decarboxylation of resulting tetradecyl-cis-aconitic acid (step 4), forming tetradecylitaconic acid (step 5).
In conclusion, we found that esters of the unsaturated sterol sitosterol with linoleic and oleic acids are the main lipids in eucalypt wood and that their biodegradation results in a transient increase of free unsaturated fatty acids that are consumed in biosynthetic or degradative reactions. The role of sterol radicals in wood decay remains to be determined. In parallel to lipid degradation, white rot fungi synthesize a novel series of unsaturated alkyldicarboxylic acids derived from fatty acids. These acids are the major lipids at the advanced stages of wood decay by C. subvermispora. The presence of lipid hydroperoxides and thiobarbituric acid reactive substances, an indicator of lipid peroxidation, is characteristic of these stages (20). The involvement of alkylitaconic acids in peroxidation reactions, together with unsaturated fatty acids, is suggested by both the presence of one or two double bonds and their dicarboxylic nature, but this involvement remains to be confirmed (9, 30). It has been proposed that lipid radicals from MnP-mediated lipid peroxidation are involved in lignin (4, 31-33) and xenobiotic degradation (5-7, 44) by C. subvermispora and other fungi. Moreover, the ability of white rot fungi to degrade lignin has been related to higher production of unsaturated lipids compared with the brown rot species (34). However, more studies are required to identify the lipid peroxidation mechanisms operating in nature, and to establish a relationship between lipid metabolism and the ability of C. subvermispora to selectively remove lignin from lignocellulosic materials (2, 3, 20).
Acknowledgments
We thank K.-E. Eriksson (STFI, Stockholm, Sweden) for C. subvermispora strains CZ-3 and FP90031, J. Romero (ENCE, Pontevedra, Spain) for providing E. globulus wood chips, and F. Guillén (CIB, CSIC, Madrid) for useful discussions on the role of free radicals in lignin degradation.
This study was supported by the European Commission (contract QLK5-99-1357) and the Spanish CICYT (FEDER project 1FD97-0742). A.G. is thankful for a “Ramòn y Cajal” contract of the Spanish MCYT.
REFERENCES
- 1.Aho, L., and R. Kurkela. 1978. Free fatty acids of wood mushrooms. Nahrung 22:603-607. [PubMed] [Google Scholar]
- 2.Akhtar, M. 1994. Biomechanical pulping of aspen wood chips with 3 strains of Ceriporiopsis subvermispora. Holzforschung 48:199-202. [Google Scholar]
- 3.Akin, D. E., W. H. Morrison, L. L. Rigsby, G. R. Gamble, A. Sethuraman, and K. E. L. Eriksson. 1996. Biological delignification of plant components by the white rot fungi Ceriporiopsis subvermispora and Cyathus stercoreus. Anim. Feed Sci. Tech. 63:305-321. [Google Scholar]
- 4.Bao, W. L., Y. Fukushima, K. A. Jensen, M. A. Moen, and K. E. Hammel. 1994. Oxidative degradation of non-phenolic lignin during lipid peroxidation by fungal manganese peroxidase. FEBS Lett. 354:297-300. [DOI] [PubMed] [Google Scholar]
- 5.Bogan, B. W., and R. T. Lamar. 1995. One-electron oxidation in the degradation of creosote polycyclic aromatic hydrocarbons by Phanerochaete chrysosporium. Appl. Environ. Microbiol. 61:2631-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogan, B. W., and R. T. Lamar. 1996. Polycyclic aromatic hydrocarbon-degrading capabilities of Phanerochaete laevis HHB-1625 and its extracellular ligninolytic enzymes. Appl. Environ. Microbiol. 62:1597-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogan, B. W., R. T. Lamar, and K. E. Hammel. 1996. Fluorene oxidation in vivo by Phanerochaete chrysosporium and in vitro during manganese-dependent lipid peroxidation. Appl. Environ. Microbiol. 62:1788-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnarme, P., B. Gillet, A. M. Sepulchre, C. Role, and C. Ducrocq. 1995. Itaconate biosynthesis in Aspergillus terreus. J. Bacteriol. 177:3573-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bors, W., M. Erben-Russ, C. Michel, and M. Saran. 1990. Radical mechanisms in fatty acid and lipid peroxidation, p. 1-16. In A. Crastes de Paulet, L. Douste-Blazy, and R. Paoletti (ed.), Free radicals, lipoproteins and membrane lipids. Plenum Press, New York, N.Y.
- 10.Brandänge, S., S. Josephson, L. Mörch, and S. Vallén. 1977. Absolute configurations of alkylcitric acids. Acta Chem. Scand. B 31:307-312. [Google Scholar]
- 11.Dart, R. K. 1976. Effect of temperature on the fatty-acid composition of Sporotrichum thermophile. Trans. Br. Mycol. Soc. 66:532-533. [Google Scholar]
- 12.Dart, R. K., and R. J. Stretton. 1976. Fatty acid composition of Sporotrichum species. Trans. Br. Mycol. Soc. 66:529-532. [Google Scholar]
- 13.Davis, M. W., and R. T. Lamar. 1992. Evaluation of methods to extract ergosterol for quantitation of soil fungal biomass. Soil Biol. Biochem. 24:189-198. [Google Scholar]
- 14.del Río, J. C., A. Gutiérrez, and F. J. González-Vila. 1999. Analysis of impurities occurring in a totally chlorine free-bleached Kraft pulp. J. Chromatogr. 830:227-232. [Google Scholar]
- 15.del Río, J. C., A. Gutiérrez, F. J. González-Vila, F. Martín, and J. Romero. 1998. Characterization of organic deposits produced in the Kraft pulping of Eucalyptus globulus wood. J. Chromatogr. 823:457-465. [Google Scholar]
- 16.del Río, J. C., A. Gutiérrez, M. J. Martínez, and A. T. Martínez. 2002. Identification of a novel series of alkylitaconic acids in wood cultures of Ceriporiopsis subvermispora by gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 16:62-68. [DOI] [PubMed] [Google Scholar]
- 17.del Río, J. C., J. Romero, and A. Gutiérrez. 2000. Analysis of pitch deposits produced in Kraft pulp mills using a totally chlorine free bleaching sequence. J. Chromatogr. A 874:235-245. [DOI] [PubMed] [Google Scholar]
- 18.Dutta, R. K., M. K. Roy, and H. D. Singh. 1992. Metabolic blocks in the degradation of β-sitosterol by a plasmid-cured strain of Arthrobacter oxydans. J. Basic Microbiol. 32:167-176. [DOI] [PubMed] [Google Scholar]
- 19.Enoki, M., T. Watanabe, Y. Honda, and M. Kuwahara. 2000. A novel fluorescent dicarboxylic acid, (Z)-1,7-nonadecadiene-2,3-dicarboxylic acid, produced by white-rot fungus Ceriporiopsis subvermispora. Chem. Lett. 2000:54-55. [Google Scholar]
- 20.Enoki, M., T. Watanabe, S. Nakagame, K. Koller, K. Messner, Y. Honda, and M. Kuwahara. 1999. Extracellular lipid peroxidation of selective white-rot fungus. Ceriporiopsis subvermispora. FEMS Microbiol. Lett. 180:205-211. [DOI] [PubMed] [Google Scholar]
- 21.Fengel, D., and G. Wegener. 1984. Wood: chemistry, ultrastructure, reactions. De Gruyter, Berlin, Germany.
- 22.Gao, Y., T. Chen, and C. Breuil. 1993. Ergosterol: a measure of fungal growth in wood for staining and pitch control fungi. Biotechnol. Tech. 7:621-626. [Google Scholar]
- 23.Gutiérrez, A., J. C. del Río, F. J. González-Vila, and F. Martín. 1999. Chemical composition of lipophilic extractives from Eucalyptus globulus Labill. wood. Holzforschung 53:481-486. [Google Scholar]
- 24.Gutiérrez, A., J. C. del Río, M. J. Martínez, and A. T. Martínez. 1999. Fungal degradation of lipophilic extractives in Eucalyptus globulus wood. Appl. Environ. Microbiol. 65:1367-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gutiérrez, A., J. C. del Río, M. J. Martínez, and A. T. Martínez. 2001. The biotechnological control of pitch in paper pulp manufacturing. Trends Biotechnol. 19:340-348. [DOI] [PubMed] [Google Scholar]
- 26.Gutiérrez, A., M. J. Martínez, J. C. del Río, J. Romero, J. Canaval, G. Lenon, and A. T. Martínez. 2000. Fungal pretreatment of Eucalyptus wood can strongly decrease the amount of lipophilic extractives during chlorine-free kraft pulping. Environ. Sci. Technol. 34:3705-3709. [Google Scholar]
- 27.Hatakka, A. 1994. Lignin-modifying enzymes from selected white-rot fungi: production and role in lignin degradation. FEMS Microbiol. Rev. 13:125-135. [Google Scholar]
- 28.Hillis, W. E. 1962. Wood extractives. Academic Press, London, United Kingdom.
- 29.Hillis, W. E., and M. Sumimoto. 1989. Effect of extractives on pulping, p. 880-920. In J. W. Rowe (ed.), Natural products of woody plants. II. Springer-Verlag, Berlin, Germany.
- 30.Hofrichter, M., D. Ziegenhagen, T. Vares, M. Friedrich, M. G. Jager, W. Fritsche, and A. Hatakka. 1998. Oxidative decomposition of malonic acid as basis for the action of manganese peroxidase in the absence of hydrogen peroxide. FEBS Lett. 434:362-366. [DOI] [PubMed] [Google Scholar]
- 31.Jensen, K. A. Jr., W. Bao, S. Kawai, E. Srebotnik, and K. E. Hammel. 1996. Manganese-dependent cleavage of nonphenolic lignin structures by Ceriporiopsis subvermispora in the absence of lignin peroxidase. Appl. Environ. Microbiol. 62:3679-3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kapich, A., M. Hofrichter, T. Vares, and A. Hatakka. 1999. Coupling of manganese peroxidase-mediated lipid peroxidation with destruction of nonphenolic lignin model compounds and 14C-labeled lignins. Biochem. Biophys. Res. Commun. 259:212-219. [DOI] [PubMed] [Google Scholar]
- 33.Kapich, A. N., K. A. Jensen, and K. E. Hammel. 1999. Peroxyl radicals are potential agents of lignin biodegradation. FEBS Lett. 461:115-119. [DOI] [PubMed] [Google Scholar]
- 34.Kapich, A. N., and L. N. Shishbina. 1995. Lipid peroxidation and its regulation in the mycelium of xylotrophic basidiomycetes. Microbiology 64:266-271. [Google Scholar]
- 35.Keogh, M. F., and M. E. Zurita. 1977. α-(15-Hydroxyhexadecyl)itaconic acid from Usnea aliphatica. Phytochemistry 16:134-135. [Google Scholar]
- 36.Kimura, Y., Y. Asada, and M. Kuwahara. 1990. Screening of basidiomycetes for lignin peroxidase genes using a DNA probe. Appl. Microbiol. Biotechnol. 32:436-442. [DOI] [PubMed] [Google Scholar]
- 37.Kirk, T. K., and R. L. Farrell. 1987. Enzymatic “combustion”: the microbial degradation of lignin. Annu. Rev. Microbiol. 41:465-505. [DOI] [PubMed] [Google Scholar]
- 38.Lie Ken Jie, M. S. F., and J. Mustafa. 1997. High-resolution nuclear magnetic resonance spectroscopy: applications to fatty acids and triacylglycerols. Lipids 32:1019-1034. [DOI] [PubMed] [Google Scholar]
- 39.Måhlén, A. 1971. Properties of 2-decylcitrate synthase from Penicillium spiculisporum Lehman. Eur. J. Biochem. 22:104-114. [DOI] [PubMed] [Google Scholar]
- 40.Måhlén, A. 1973. Purification and some properties of 2-decylhomocitrate synthase from Penicillium spiculisporum. Eur. J. Biochem. 38:32-39. [DOI] [PubMed] [Google Scholar]
- 41.Måhlén, A., and S. Gatenbeck. 1968. A metabolic variation in Penicillium spiculisporum Lehman. II. Purification and some properties of the enzyme synthesizing (−)-decylcitric acid. Acta Chem. Scand. 22:2617-2623. [DOI] [PubMed] [Google Scholar]
- 42.Martínez, A. T., J. M. Barrasa, A. Prieto, and M. N. Blanco. 1991. Fatty acid composition and taxonomic status of Ganoderma australe from southern Chile. Mycol. Res. 95:782-784. [Google Scholar]
- 43.Martínez, M. J., J. M. Barrasa, A. Gutiérrez, J. C. del Río, and A. T. Martínez. 1999. Fungal screening for biological removal of extractives from Eucalyptus globulus Labill. wood. Can. J. Bot. 77:1513-1522. [Google Scholar]
- 44.Moen, M. A., and K. E. Hammel. 1994. Lipid peroxidation by the manganese peroxidase of Phanerochaete chrysosporium is the basis for phenanthrene oxidation by the intact fungus. Appl. Environ. Microbiol. 60:1956-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peláez, F., M. J. Martínez, and A. T. Martínez. 1995. Screening of 68 species of basidiomycetes for enzymes involved in lignin degradation. Mycol. Res. 99:37-42. [Google Scholar]
- 46.Sancholle, M., and D. Lösel. 1996. Lipids in fungal biotechnology, p. 339-367. In U. Kück (ed.), The mycota. II. Genetics and bio/technology. Springer-Verlag, Berlin, Germany.
- 47.Satyanarayana, T., and L. Chavant. 1987. Bioconversion and binding of sterols by thermophilic moulds. Folia Microbiol. 32:354-359. [DOI] [PubMed] [Google Scholar]
- 48.Shaw, R. 1966. The polyunsaturated fatty acids of microorganisms. Adv. Lipid Res. 4:107-174. [DOI] [PubMed] [Google Scholar]
- 49.Shaw, R. 1967. Fatty acids of fruiting bodies of basidiomycetes. Nature 213:86-87. [Google Scholar]
- 50.Swan, B., and I. S. Åkerblom. 1967. Wood extractives from Eucalyptus globulus Labill. Sven. Papperstidn. 70:239-244. [Google Scholar]
- 51.TAPPI. 1996. Test methods 1996-1997. TAPPI, Atlanta, Ga.
- 52.Turner, W. B. 1971. Fungal metabolites. Academic Press, London, United Kingdom.
- 53.Turner, W. B., and D. C. Aldridge. 1983. Fungal metabolites II. Academic Press, London, United Kingdom.
- 54.Wassef, M. K. 1977. Fungal lipids. Adv. Lipid Res. 15:159-232. [Google Scholar]
- 55.Watanabe, T., S. Katayama, M. Enoki, Y. H. Honda, and M. Kuwahara. 2000. Formation of acyl radical in lipid peroxidation of linoleic acid by manganese-dependent peroxidase from Ceriporiopsis subvermispora and Bjerkandera adusta. Eur. J. Biochem. 267:4222-4231. [DOI] [PubMed] [Google Scholar]
- 56.Weete, J. D. 1974. Fungal lipid biochemistry. Plenum Press, New York, N.Y.