Abstract
Sunlight inactivation in fresh (river) water of fecal coliforms, enterococci, Escherichia coli, somatic coliphages, and F-RNA phages from waste stabilization pond (WSP) effluent was compared. Ten experiments were conducted outdoors in 300-liter chambers, held at 14°C (mean river water temperature). Sunlight inactivation (kS) rates, as a function of cumulative global solar radiation (insolation), were all more than 10 times higher than the corresponding dark inactivation (kD) rates in enclosed (control) chambers. The overall kS ranking (from greatest to least inactivation) was as follows: enterococci > fecal coliforms ≥ E. coli > somatic coliphages > F-RNA phages. In winter, fecal coliform and enterococci inactivation rates were similar but, in summer, enterococci were inactivated far more rapidly. In four experiments that included freshwater-raw sewage mixtures, enterococci survived longer than fecal coliforms (a pattern opposite to that observed with the WSP effluent), but there was little difference in phage inactivation between effluents. In two experiments which included simulated estuarine water and seawater, sunlight inactivation of all of the indicators increased with increasing salinity. Inactivation rates in freshwater, as seen under different optical filters, decreased with the increase in the spectral cutoff (50% light transmission) wavelength. The enterococci and F-RNA phages were inactivated by a wide range of wavelengths, suggesting photooxidative damage. Inactivation of fecal coliforms and somatic coliphages was mainly by shorter (UV-B) wavelengths, a result consistent with photobiological damage. Fecal coliform repair mechanisms appear to be activated in WSPs, and the surviving cells exhibit greater sunlight resistance in natural waters than those from raw sewage. In contrast, enterococci appear to suffer photooxidative damage in WSPs, rendering them susceptible to further photooxidative damage after discharge. This suggests that they are unsuitable as indicators of WSP effluent discharges to natural waters. Although somatic coliphages are more sunlight resistant than the other indicators in seawater, F-RNA phages are the most resistant in freshwater, where they may thus better represent enteric virus survival.
Sunlight appears to be the most important mechanism inactivating sewage microorganisms in shallow seawater, although it has been reported to exert different rates of inactivation on various fecal indicator bacteria and bacteriophages (6, 12, 31, 33, 39). These studies indicated a ranking of sunlight inactivation rates (kSs) (from the greatest to the least) as follows: fecal coliforms > enterococci > F-RNA phages > somatic coliphages. This ranking could be largely explained by the relationship between the intrinsic susceptibility of each indicator to different solar wavelengths and the differential penetration of these wavelengths in seawater.
There appears to be little equivalent information available for freshwaters, for which most of the published results are derived either from laboratory microcosms (11, 25, 29) or from field studies in which solar radiation was not measured (8, 17, 35). The applicability of seawater studies to fresh and estuarine waters is doubtful, because of the likely effects on microbial inactivation of differences in optical characteristics (7, 20), salinity (10, 34), and autochthonous biota (13, 23, 24, 30, 36).
The seawater studies described above largely involved fecal indicators from raw sewage, whereas most effluents discharged to natural waters receive some form of treatment. This is often in waste stabilization ponds (WSPs) which, in New Zealand, are used in ca. 70% of plants treating domestic sewage (unpublished database [New Zealand Water and Wastes Association]). In contrast to indicators from raw sewage, those from WSPs have already been exposed to sunlight, which suggests that they may respond differently to further sunlight exposure. Evidence that this occurs with some indicators was found in one of the seawater experiments described by Sinton et al. (33), wherein fecal coliforms from WSP effluent were found to be more sunlight resistant than those from raw sewage.
In the present study, we describe a series of 10 outdoor experiments, conducted over a 2-year period, designed primarily to determine the rates of sunlight inactivation in fresh river water of fecal indicators from WSP effluent. However, four experiments included comparisons of WSP effluent with raw sewage, and two included saline waters. The following five indicators were selected for the study: (i) fecal coliforms are useful “baseline” organisms, because their sunlight inactivation in seawater is well characterized (6, 12, 31, 33); (ii) Escherichia coli and (iii) enterococci have been adopted in Canada, New Zealand, Australia, and some of the United States as indicators in fresh and marine recreational waters, respectively; (iv) F-RNA phages have been suggested as enteric virus models and have been correlated with enterovirus counts in freshwaters (16); and (v) somatic coliphages have been shown to be more sunlight resistant than F-RNA phages in seawater (33, 39).
MATERIALS AND METHODS
Experimental facilities.
The outdoor experimental facilities—located 10 km south of Christchurch (latitude 43°S), New Zealand—were described in detail by Sinton et al. (33). Briefly, the water-effluent mixtures were held in foil-lined, white, plastic, open-top chambers, filled to a depth of 560 mm (volume, 300 liters). Experimental procedures were designed to minimize between-chamber differences. To provide a thermal water jacket, the chambers were placed in a 13,000-liter swimming pool filled to a level 100 mm below that inside the chambers. The chamber water temperature was maintained near 14°C (mean temperature of the river water collection site [see below]) by cooling the pool water with ice. A submersible pump was used to stir the water in each chamber. A sampling tube from each chamber was connected to a seal on a manifold beside the pool. Samples were collected by applying a vacuum to a sterile bottle attached to the appropriate seal.
Experimental procedures.
Ten experiments—seven in summer and three in winter—were conducted over 2 years (Table 1). Each sunlight-exposed chamber was paired with a dark control (fitted with an aluminum lid and a foil skirt to exclude light), except for the salinity experiments (in which there was no room for dark chambers). In experiment 6, four additional chambers, each covered with a different long-pass optical filter, were used to gauge the contributions of different regions of the UV-visible solar spectrum to inactivation. The λ50 value for each filter (the “spectral cutoff” as a 50% transmission wavelength) is given in Table 1 (experiment 6); the spectral transmission curves are presented in Sinton et al. (33).
TABLE 1.
Summary of experimental program
| Expt no. | Season, weather | Effluent and water type | Comment(s) |
|---|---|---|---|
| 1 | Summer, clear sky | WSP effluent in river water | |
| 2 | Winter, cloudy | WSP effluent in river water; raw sewage in river water | Effluent comparison |
| 3 | Winter, clear sky | WSP effluent in river water | Two-day experiment |
| 4 | Summer, cloudy | WSP effluent in river water; raw sewage in river water | Effluent comparison |
| 5 | Summer, heavy cloud | WSP effluent or raw sewage in river water, simulated estuarine watera, or seawater | Salinity effects on two effluents |
| 6 | Summer, clear sky | WSP effluent in river water | Optical filter experiment: full sun, 318 nm, 342 nm; 396 nm, 556 nm, dark |
| 7 | Summer, clear sky | WSP effluent or raw sewage in river water, simulated estuarine watera, or seawater | Salinity effects on two effluents |
| 8 | Winter, clear sky | WSP effluent in river water | |
| 9 | Summer, some cloud | WSP effluent in river water | Variability experiment: 10 samples after 3 h of sunlight exposure |
| 10 | Summer, clear sky | WSP effluent in river water | Two-day experiment |
That is, a 50:50 seawater-river water mixture.
Freshwater was collected from the nearby LII River. This is a spring-fed stream with a mean flow of 0.9 m3 s−1, low counts of all of the indicators used, and a small temperature range: 12°C (winter) to 16°C (summer) and ca. 14°C for most of the year. Seawater was collected from the shoreline at Lyttelton Harbor, from a site which also has low indicator counts. Estuarine water was simulated with a 50:50 mixture of seawater and river water. Effluent samples were collected from the Christchurch sewage treatment plant, in which the sewage passes through primary sedimentation tanks, trickling filters, aeration basins, clarifiers, and a WSP system. Raw sewage was collected as it entered the plant, and sieved (1-mm mesh) to remove coarse solids. WSP effluent was collected at the outlet of the first of a series of three WSPs. Effluents were stored overnight at 5 to 6°C (tests showed no significant overnight change in indicator counts).
In each experiment, 300 liters of water was pumped into each chamber and held overnight. The following morning, effluent was injected on the hour after the water was first exposed to direct sunlight to give a 10% (vol/vol) mixture of WSP effluent or a 3% (vol/vol) mixture of raw sewage (Table 1). The greater WSP effluent concentration was required to ensure adequate initial indicator counts, particularly of enterococci. The chamber contents were stirred for 3 min, and the first sample was collected. Subsequent samples were collected on the hour for 8 h. In experiments 3 and 10 (Table 1), sampling continued overnight (every 3 h), followed by hourly sampling for 8 daylight hours on the second day.
Experiment 9 (Table 1) was designed to gauge the overall variability associated with sample collection, transport and assay. After 3 h of sunlight exposure, 10 samples were collected in quick succession (over 6 min) from both a sunlight-exposed and a dark (control) chamber. Sample transport and assay was as described below.
Samples were collected in sterile, foil-wrapped, glass bottles. The first 100 ml was discarded, and then a 1,000-ml sample was collected. Sample bottles were held in the dark at 6 to 8°C for transfer to the laboratory.
Laboratory assays.
Samples were assayed for indicator bacteria by membrane filtration (Millipore EZ-Pak; 0.45-μm pore size). Fecal coliforms were incubated on mFC agar (BBL) at 44.5 ± 0.2°C for 24 ± 2 h (1). After the coliforms were counted, the membranes were transferred to nutrient agar containing 4-methylumbelliferyl-β-d-glucuronide (MUG; Difco) and incubated at 35°C for 4 h. Fecal coliform colonies exhibiting a ring of fluorescence under a UV-A lamp were counted as E. coli (37). Enterococci were incubated on mE agar (Difco) at 41 ± 0.5°C for 48 h, followed by transfer to EIA Agar (Difco) for a further 20 min at 41 ± 0.5°C (1). Bacterial counts were expressed as CFU/100 ml. Toward the end of some experiments, filtration of 500-ml samples was required to obtain counts of enterococci.
The bacterial indicators and F-RNA phages were assayed within 60 min of collection, but it was not possible to process both phages on the same day. Accordingly, a subsample was held in the dark at 4°C for assay of somatic coliphages on the following day (tests indicated a titer reduction of only 3% if the samples were assayed within 24 h). Somatic coliphages and F-RNA phages were assayed by using either replicate 2-ml overlay pour plates (1) for the chambers seeded with raw sewage (high phage concentrations) or the membrane filtration-swirling elution method (100-ml aliquots) of Sinton et al. (32) for the chambers seeded with WSP effluent (low phage concentrations). Counts were expressed as PFU per 100 ml. Because the data were normalized (as percent survival curves), neither the delayed assay nor different assay procedures were considered likely to have influenced the results. The somatic coliphage host was E. coli 13706/60 (32), and the F-RNA phage host was Salmonella enterica serovar Typhimurium WG49 (18).
Solar radiation and temperature measurements.
Global (i.e., diffuse plus direct) solar radiation was measured on site with a LI-COR LI-200SA pyranometer connected to a LI-COR LI-1000 Datalogger. The LI-200SA has an arbitrary response to radiation in the 400- to 1,100-nm range (short-visible to near-infrared wavelengths) and is calibrated for broad-band solar energy measurement. Chamber water temperatures were monitored hourly. Tests in two experiments showed no change in dissolved oxygen or pH over time.
Calculation of inactivation parameters.
A linear regression line was fitted to the (loge-transformed) counts from the dark chambers in each experiment to derive the dark inactivation rate coefficient (kD) in loge units per hour.
In chambers exposed to sunlight, the percent survival (p) at exposure time t was defined as p = 100 N/No, where N is the CFU or PFU count and No is the initial count. Each p value was corrected for dark inactivation by using the data from the dark control chamber for the particular experiment and the equation pS = p · exp(−kDt), where pS is the corrected sunlight value.
A linear regression line was fitted to each set of (loge-transformed) bacteriophage sunlight inactivation pS values. Sunlight inactivation parameters for the phages were obtained from plots of loge pS versus insolation (cumulative global solar radiation [units are given in megajoules per square meter, i.e., insolation is a measure of sunlight exposure or “dose”]).
The bacterial sunlight inactivation curves usually displayed a recognizable shoulder, so the approach described in Sinton et al. (31) was adopted. A two-parameter, multitarget kinetic expression (14) was fitted to the data as follows: pS = 100 {1 − [1 − exp(−ksS)]ns}, where S is insolation and kS is the sunlight inactivation coefficient (in square meters per megajoule). The exponent, nS, is a dimensionless parameter (the “shoulder constant”) and was evaluated as nS = po/100, where loge (po) is the y-axis intercept of the regression line. The bacterial inactivation coefficient was obtained from the final slope of the inactivation curve (kS = −Δ loge p/ΔS) by using the linear regression of loge pS versus S, omitting points in the shoulder region where present.
For comparison with other studies, the S90 and T90 values (respectively, the insolation and time taken to achieve a 90% reduction in the CFU or PFU count) were also calculated. The sunlight T90 was derived from the mean kT value as 2.303/kT, where kT is the slope of the regression line fitted to the loge-transformed counts in the sunlight chambers plotted against time. The dark T90 was derived directly from the mean kD value, as 2.303/kD, and S90 was derived directly from the mean kS value, as 2.303/kS.
Because four experiments (Table 1) involved comparisons of two different effluent types and concentrations, and/or three different water types, the possibility of differences in light attenuation between the mixtures (and consequent differences in depth-averaged light exposure) was investigated. For each mixture, spectral light absorption and scattering was measured on a Jasco 7850 UV-Visible spectrophotometer. The spectral irradiance attenuation coefficient was calculated by using the equation given by Kirk (22). The average irradiance over the 560-mm mixed depth of the chambers was then calculated as a proportion of the incident spectral irradiance by using the equation of Morowitz (27). These calculations showed that, despite different appearances, the experimental mixtures had very similar spectral light attenuation in the most highly biocidal (UV-visible) region. For example, the depth-averaged irradiance at 340 nm—used as the wavelength of comparison, based on the results of Davies-Colley et al. (4)—ranged only from 33 to 35% of the incident radiation in the different mixtures. Accordingly, the survival curves in all of the experiments are presented as a function of incident insolation, uncorrected for attenuation.
RESULTS
Data variability.
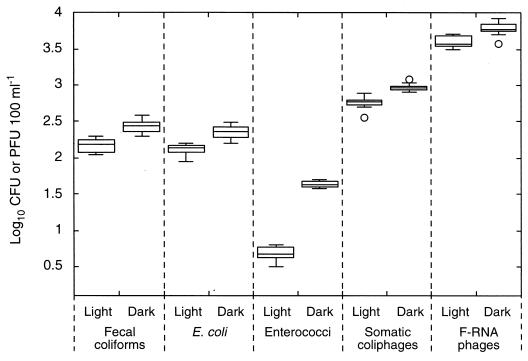
The results of the variability experiment are presented as box plots in Fig. 1. Fecal coliforms exhibited the greatest variability, and somatic coliphages exhibited the least variability (although both somatic phage plots contained an outlier). Figure 1 also shows close similarity between the fecal coliform and E. coli data. Up to 78% of the fecal coliforms in the initial samples were recorded as E. coli, and these proportions did not change over time because inactivation of E. coli closely followed that of fecal coliforms in all experiments. Accordingly, for clarity, the E. coli data are included in the tables but not in the figures.
FIG. 1.
Variability associated with sample collection, transport, and assay. Each box plot indicates the counts obtained from 10 samples, collected over 6 min from each chamber, after 3 h of sunlight or dark inactivation. The cross-pieces of each box plot represent (from top to bottom) maximum, upper-quartile, median, lower-quartile, and minimum values. An outlier (○) is defined as a point whose value is either above the upper quartile or below the lower quartile by 1.5 times the interquartile distance.
Dark inactivation.
Dark inactivation rates are presented in Table 2. In the chambers containing WSP effluent in river water (Table 2), the mean kD ranking (from the greatest to the least inactivation) was E. coli ≥ enterococci ≥ fecal coliforms > F-RNA phages > somatic coliphages. Dark inactivation was slower in raw sewage-river water than in WSP effluent-river water (except for an anomalously high E. coli value), and the phages were again inactivated more slowly than the bacteria (dark inactivation of F-RNA phages was undetectable).
TABLE 2.
Dark inactivation parameters for WSP effluent and raw sewage in fresh (river) watera
| Indicator | Effluent | kD (h−1) (n; CV%)b | T90 (h) |
|---|---|---|---|
| Fecal coliforms | WSP | 0.0162 (60; 14) | 144 |
| RS | 0.007 (17; 18) | 329 | |
| E. coli | WSP | 0.0171 (59; 18) | 135 |
| RS | 0.023 (17; 10) | 100 | |
| Enterococci | WSP | 0.0168 (60; 26) | 135 |
| RS | 0.012 (17; 18) | 192 | |
| Somatic coliphages | WSP | 0.008 (60; 26) | 288 |
| RS | 0.001 (17; 17) | 2,303 | |
| F-RNA phages | WSP | 0.014 (43; 12) | 165 |
| RS | ≈0 (17) | -c |
The kD values were calculated from the combined percent survival plots loge for all available experiments and are presented at up to 4 decimal places for comparison of the small differences. No dark controls were possible in experiments 5 and 7.
For each kD value, the number of samples (n) and the coefficient of variation (CV%) are given in parentheses as indicated.
Not calculated (near zero inactivation).
Sunlight inactivation.
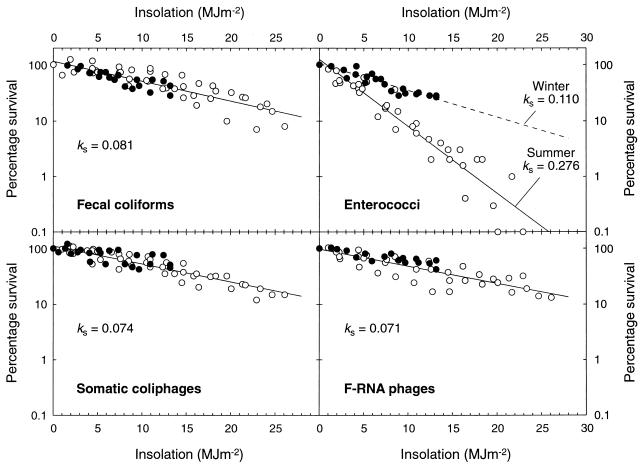
The mean sunlight inactivation parameters (both effluents) are presented in Table 3, and the log-linear data (i.e., shoulder points removed) from all of the WSP effluent-river water survival experiments are combined as log-linear regression lines in Fig. 2. For WSP fecal coliforms, the data were well fitted to single regression lines. All of the phage inactivation curves were log linear, with no shoulders evident. Both phages exhibited slightly higher sunlight inactivation (kS) rates in summer (Table 3), but the data were reasonably well fitted to single regression lines (Fig. 2). However, the enterococci exhibited marked seasonal differences in sunlight inactivation rates (Table 3), and thus separate summer and winter regression lines (and the kS values from Table 3) are presented in Fig. 2 (single kS values are given for the other indicators).
TABLE 3.
Mean sunlight inactivation parameters for indicators from WSP effluent and raw sewage in fresh (river) watera
| Indicator | Effluent |
kS (m2 MJ−1) (n; CV%)b
|
nS
|
S90 (MJ m−2)
|
T90 (h)
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Summer | Winter | Summer | Winter | Summer | Winter | Summer | Winter | ||
| Fecal coliforms | WSP | 0.086 (42; 30) | 0.084 (20; 15) | 1.3 | 1.1 | 26.9 | 27.4 | 8.8 | 16.8 |
| RS | 0.275 (22; 23) | 0.216 (7; 20) | 3.8 | 1.4 | 8.4 | 10.7 | 3.3 | 7.7 | |
| E. coli | WSP | 0.078 (41; 44) | 0.073 (22; 14) | 1.1 | 1.0 | 29.4 | 31.6 | 9.2 | 20.3 |
| RS | 0.287 (22; 38) | 0.237 (7; 19) | 4.8 | 2.0 | 8.0 | 9.7 | 3.3 | 6.9 | |
| Enterococci | WSP | 0.276 (39; 30) | 0.110 (21; 19) | 1.3 | 1.0 | 8.4 | 21.0 | 3.0 | 14.5 |
| RS | 0.137 (21; 35) | 0.138 (7; 13) | 2.4 | 1.6 | 16.8 | 16.7 | 6.4 | 12.6 | |
| Somatic coliphages | WSP | 0.077 (50; 19) | 0.049 (25; 20) | NA | NA | 30.0 | 46.7 | 11.6 | 30.6 |
| RS | 0.098 (27; 29) | 0.087 (9; 20) | NA | NA | 23.4 | 26.4 | 8.3 | 15.9 | |
| F-RNA phages | WSP | 0.070 (43; 25) | 0.050 (18; 11) | NA | NA | 32.8 | 45.7 | 13.3 | 27.5 |
| RS | 0.075 (26; 18) | 0.074 (9; 10) | NA | NA | 30.9 | 30.8 | 12.5 | 19.1 | |
The insolation received during the 8-h experiments ranged from 7 to 15 MJ m−2 in winter and from 17 to 26 MJ m−2 in summer. WSP, WSP effluent; RS, raw sewage; NA, not applicable.
The kS values were calculated from the combined loge percent survival plots and are divided into summer and winter values (the combined values, where applicable, are given in Fig. 2). For each kS value, the number of samples (n) and the coefficient of variation (CV%) are given in parentheses.
nS is the shoulder size.
FIG. 2.
Inactivation in river water, as a function of insolation, of fecal coliforms, enterococci, somatic coliphages, and F-RNA phages from WSP effluent. The data are from all summer (○) and winter (•) survival experiments. The bacterial results are the linear portions of the inactivation curves (i.e., the shoulder points have been removed). The enterococci data are divided into winter and summer curves, with separate kS values. Combined kS values are presented for the other indicators.
Comparison of the Fig. 2 regression lines according to the method of Zar (40) showed that summer enterococcus inactivation was significantly faster than in winter (P < 0.1) and was also significantly faster than that of the other indicators (P < 0.001). However, there were no significant differences (P > 0.1) between the summer and winter curves for the fecal coliforms and phages (thus, single regression lines are presented) or between winter inactivation rates of enterococci and those of the other indicators.
Table 3 shows that the phage and winter enterococcus inactivation rates were broadly similar between effluents. However, fecal coliform and E. coli inactivation rates were much lower for WSP effluent-river water than for raw sewage-river water. In contrast, WSP enterococci were inactivated markedly more rapidly in summer than raw sewage enterococci. Shoulder sizes (a measure of initial resistance to sunlight inactivation) were all higher for the bacteria from raw sewage (compared to WSP bacteria), but no other consistent shoulder patterns were evident.
From Fig. 2 and Table 3, a kS ranking for indicators in WSP effluent-river water (from the greatest to the least inactivation) may be derived as follows: enterococci > fecal coliforms ≥ E. coli > somatic coliphages > F-RNA phages. The equivalent ranking (Table 3) for raw sewage-river water is as follows: fecal coliforms ≥ E. coli > enterococci > somatic coliphages > F-RNA phages (i.e., a reversal of the ranking of fecal coliforms [including E. coli] and enterococci between effluents).
Effects of salinity and effluent type.
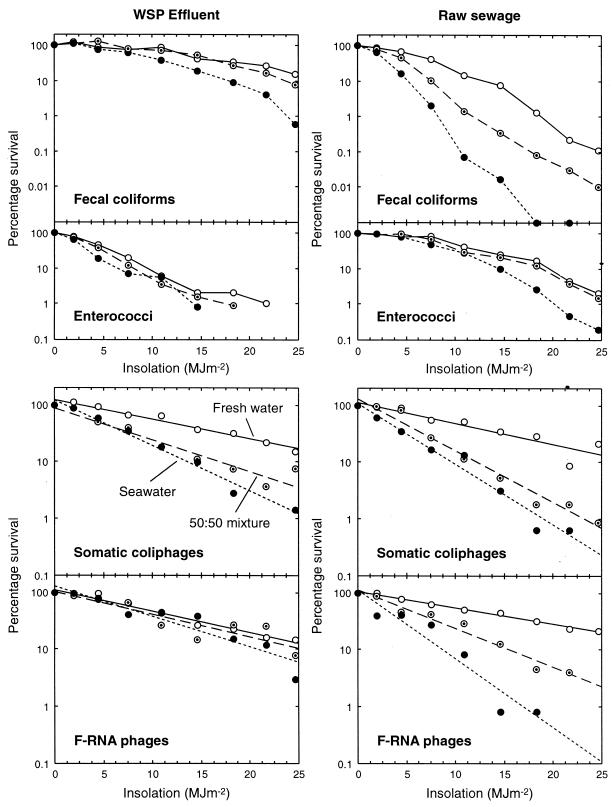
A heavy cloud cover slowed inactivation in the first salinity experiment (experiment 5; Table 1), whereas clear skies prevailed in the repeat experiment (experiment 7). The kS values for both experiments are presented in Table 4, and the data from experiment 7 are plotted in Fig. 3. The experiment 5 plots (not presented) were similar (suggesting reasonable repeatability) but with poorer curve definition due to the lower overall insolation.
TABLE 4.
Sunlight inactivation coefficients (kS) for fecal indicators from raw sewage and WSP effluent in freshwater, simulated estuarine water (50:50 mixture), and seawatera
| Indicator | Expt no. |
kS for indicator from:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Raw sewage in:
|
WSP effluent in:
|
||||||||
| Freshwater | 50:50 mixture | Seawater | Freshwater | 50:50 mixture | Seawater | ||||
| Fecal coliforms | 5 | 0.281 | 0.452 | 0.622 | 0.095 | 0.149 | 0.231 | ||
| 7 | 0.361 | 0.421 | 0.593 | 0.085 | 0.122 | 0.205 | |||
| E. coli | 5 | 0.277 | 0.429 | 0.626 | 0.086 | 0.142 | 0.216 | ||
| 7 | 0.395 | 0.421 | 0.580 | 0.080 | 0.119 | 0.196 | |||
| Enterococci | 5 | 0.131 | 0.155 | 0.197 | 0.224 | 0.233 | 0.306 | ||
| 7 | 0.209 | 0.189 | 0.338 | 0.227 | 0.279 | 0.314 | |||
| Somatic coliphages | 5 | 0.141 | 0.164 | 0.199 | 0.074 | 0.362 | 0.180 | ||
| 7 | 0.084 | 0.209 | 0.246 | 0.079 | 0.129 | 0.184 | |||
| F-RNA phages | 5 | 0.103 | 0.143 | 0.132 | |||||
| 7 | 0.065 | 0.187 | 0.278 | 0.086 | 0.092 | 0.123 | |||
In experiments 5 and 7, bacterial shoulder points were removed for slope calculation.
FIG. 3.
Sunlight inactivation in river water (○), simulated estuarine water (⊙), and seawater (•) of fecal coliforms, enterococci, somatic coliphages, and F-RNA phages from WSP effluent and raw sewage. For clarity, the phage data are presented as regression lines. Note the differences in the y-axis scales.
The key features of Table 4 and Fig. 3 are as follows. (i) Sunlight inactivation increased with increasing salinity for all of the indicators, from both effluents, but salinity had the least influence on enterococci and F-RNA phages from WSP effluent. (ii) In freshwater, inactivation rates were markedly lower for fecal coliforms from WSP effluent than for those from raw sewage, markedly higher for enterococci from WSP effluent than for those from raw sewage, and broadly similar between effluents for both phages. (iii) Inactivation of sewage F-RNA phages increased noticeably with increasing salinity, but this trend was less marked for those from WSP effluent. (iv) For each combination of effluent and salinity, phage inactivation rates were mostly lower than for the bacteria.
Two-day experiments.
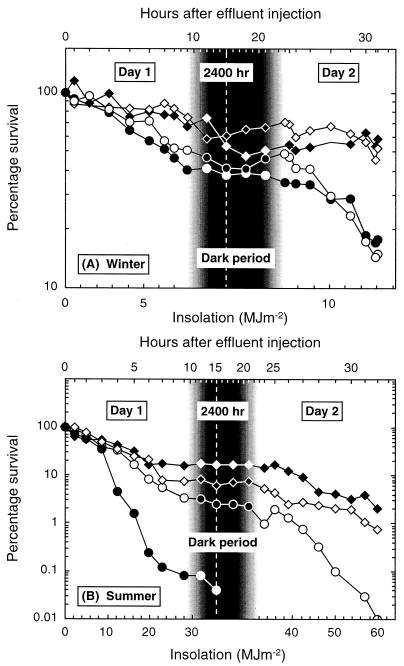
Both the winter and summer 2-day experiments (Fig. 4) were conducted over 32 h under clear-sky conditions. However, the summer dark period was ca. 3 h shorter than in winter, and there was 4.6 times more insolation. Consistent with this difference, the final percent reduction in fecal coliforms was 4 orders of magnitude in summer compared to <1 in winter. The lower winter insolation also resulted in poorer curve definition and no marked inactivation rate differences between the fecal coliforms and the enterococci or between the phages. However, bacterial inactivation was more rapid than for the phages. All indicators were present at the end of day 2 in winter; the final counts (per 100 ml) were as follows: fecal coliforms, 1,980; enterococci, 500; somatic coliphages, 75; and F-RNA phages, 3,980.
FIG. 4.
Results of winter (A) and summer (B) 2-day experiments. Inactivation in river water of fecal coliforms (○), enterococci (•), somatic coliphages (⋄), and F-RNA phages (♦) from WSP effluent, as a function of insolation and time. During daylight hours, the insolation scale is linear and the time scale is nonlinear. During the overnight period, the insolation scale is nonlinear, the time scale is linear, and the symbol shades are inverted.
Under the higher summer insolation, clear inactivation rate differences emerged. There was an early reduction in the counts of enterococci, which disappeared (<1 CFU 500 ml−1) on day 1. Fecal coliforms exhibited far greater sunlight resistance and did not fall to 1 CFU 100 ml−1 until the late afternoon of day 2. The phages again exhibited greater sunlight resistance than the bacteria, with F-RNA phages more resistant than the somatic coliphages. The final counts (per 100 ml) were as follows: fecal coliforms, 1; somatic coliphages, 9; and F-RNA phages, 1,000.
Contribution of different spectral regions to inactivation.
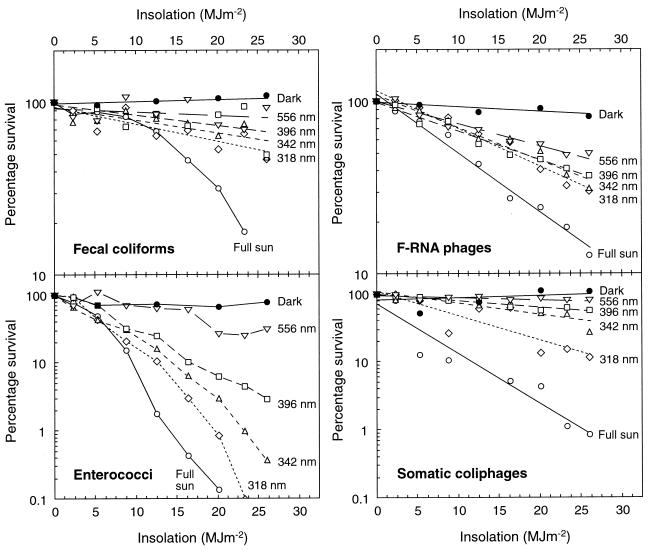
In the optical filter experiment (Fig. 5), inactivation rates of all of the indicators decreased with the increase in the 50% spectral cutoff wavelength (λ50) of the filters. The inactivation of enterococci was more rapid than for fecal coliforms beneath all of the filters (and in full sun). Enterococci also tended to be inactivated by a wide range of wavelengths, whereas the fecal coliforms were mainly susceptible to wavelengths of <318 nm. F-RNA phages were inactivated by a wide range of wavelengths, whereas somatic coliphages were mainly inactivated by UV-B wavelengths (<318 nm), with a secondary contribution from short UV-A wavelengths (318 to 342 nm).
FIG. 5.
Inactivation in river water of fecal coliforms, enterococci, F-RNA phages, and somatic coliphages from WSP effluent in the dark (•), under full sun (○), and under 556-nm (orange) (▿), 396-nm (polycarbonate, □), 342-nm (acrylic, ▵), and 318-nm (polyester, ⋄) optical filters. For clarity, the phage data and most of the fecal coliform data are presented as regression lines. Note the difference in y-axis scales.
DISCUSSION
Data variability.
There was less variability in indicator counts in freshwater (Fig. 1) than in a previous study (33), in which seawater probably had a more detrimental effect on indicator assays. F-RNA phage variability was greater than for the somatic coliphages, probably because of variable F-pilus production on the serovar Typhimurium WG49 host (33, 39).
Dark inactivation.
Dark inactivation rates were low for all of the indicators (Table 2) and thus made little contribution to overall inactivation in the sunlight-exposed chambers. There were no seasonal differences, probably because the chambers were always maintained close to the mean river temperature of 14°C. For the (larger) WSP effluent data set, the kD differences were small, but the bacteria were generally inactivated more rapidly were than the phages. Similar results have been reported for groundwater microcosms (21).
Dark inactivation of bacteria in freshwater may be attributed to inhibitory substances (23) and to the activities of predatory and lytic organisms (11, 13, 26). The antiviral compounds exuded by marine microbes (36) are probably also produced in freshwaters. The higher dark inactivation rates for indicators in the WSP mixtures compared to raw sewage suggest higher levels of protozoan activity and, possibly, microbial exudates.
Sunlight inactivation. (i) General.
Sunlight inactivation in freshwater was ca. 10 times greater than dark inactivation for all of the indicators (calculated from the kD and kT values in Tables 2 and 3) and was directly related to the amount of solar radiation received. Insolation-dependent parameters (e.g., S90 values) were broadly similar between summer and winter (except for WSP enterococci, which were inactivated far more rapidly in summer), but the time-dependent parameters (T90s) were higher in winter than in summer. There were marked differences in sunlight inactivation rates, depending on the indicator, effluent type, and salinity of the water (discussed below). Although our data are derived from well-mixed, shallow microcosms, extrapolation of inactivation rates to poorly mixed waters should be possible as a function of the irradiance and, thus, the depth in the water column.
(ii) Effects of salinity.
There was a consistent pattern of increasing sunlight inactivation with increasing salinity for all of the indicators (Table 4 and Fig. 3). This is in agreement with the results of Solić and Krstulović (34), who showed that higher salinities increased rates of sunlight inactivation of laboratory-cultured fecal coliforms. Evison (10) also showed that dark inactivation of E. coli and fecal streptococci increased at higher salinities.
A 3% raw sewage mixture was used to enable comparisons with the results in Sinton et al. (31, 33), whereas a 10% WSP effluent mixture was required to provide adequate initial indicator counts (particularly for enterococci). Thus, higher salinities in the sewage-saline water chambers may have increased apparent inactivation rate differences between effluents in the saline waters (and reduced the validity of these comparisons). However, between-effluent comparisons for the freshwaters are valid and are consistent with the results from the other experiments, i.e., the sunlight inactivation rates were lower for WSP fecal coliforms than for raw sewage fecal coliforms, were just the opposite for enterococci, and were broadly similar between effluents for both phages. Overall, our results suggest that fecal coliforms (including E. coli) and F-RNA phages are the most susceptible to sunlight inactivation at higher salinities, and enterococci and somatic coliphages are the least susceptible.
(iii) Fecal coliforms and E. coli.
The fecal coliform and E. coli assay procedures were not completely independent (i.e., the latter was a subset of the former). Nevertheless, the strong similarity between the inactivation parameters (Table 3) suggests that these two indicators will provide a similar indication of the level of fecal pollution in sunlight-exposed natural waters.
Our study consistently confirmed the results of the single experiment of Sinton et al. (33), which indicated that fecal coliforms from WSP effluent are more sunlight resistant than those from raw sewage. An explanation for this phenomenon may be found in the processes involved in sunlight damage to bacterial cells, and their subsequent repair, as described by Calkins and Barcelo (3), Eisenstark (9), Harm (14), and Jagger (19).
Briefly, the UV-B portion of the solar spectrum is the most bactericidal, causing direct (photobiological) DNA damage. At wavelengths of >329 nm, photochemical mechanisms (particularly photooxidation) become more important, acting through photosensitizers to damage organelles, principally the cytoplasmic membrane. Because irradiance at the earth's surface falls sharply below ca. 400 nm, longer solar wavelengths may account for a significant proportion of bacterial inactivation. This effect is probably magnified in natural waters by the increase in sunlight attenuation that occurs with decreasing wavelength (7, 20).
The results of the optical filter experiment (Fig. 5) show that about half the inactivation of WSP fecal coliforms in freshwater was caused by wavelengths below 318 nm, i.e., in the UV-B range. This is consistent with a predominantly photobiological mechanism of inactivation. Davies-Colley et al. (4, 5) also found that E. coli was inactivated in WSP effluent primarily by the UV-B component of sunlight. However, this process was partly oxygen dependent, suggesting that some UV-B inactivation may be photooxidative, mediated by endogenous photosensitizers.
Although some photobiological damage is lethal (19), some bacteria (including E. coli) can utilize a complex combination of processes—excision (dark) repair, photoreactivation, and postreplicative repair (part of the “SOS” response)—to repair DNA. Although longer UV-A wavelengths appear to inhibit some of these processes (9), the overall effect of exposure to longer sunlight wavelengths is the repair of photobiological damage.
Thus, the results reported here and by Davies-Colley et al. (4) and Sinton et al. (33) suggest that, because a significant proportion of the damage to fecal coliforms in WSPs is photobiological, much of it is repairable, and the surviving cells emerge with their various repair mechanisms activated. These repair processes appear to continue in natural waters. In contrast, because it appears to take up to 24 h for repair mechanisms to be fully activated, raw sewage fecal coliforms are initially inactivated faster than those from WSPs. Figure 3 also shows that raw sewage fecal coliforms are more strongly affected by increasing salinities than the other indicators, presumably because of the loss of ionic integrity of the cell (28), arising from photooxidative damage to the cell wall (as noted above, the apparent lesser effect of salinity on the WSP fecal coliforms was probably partly a function of the lower salinities of the WSP mixtures).
(iv) Enterococcci.
The faster inactivation of WSP enterococci compared to raw sewage enterococci (Tables 3 and 4; Fig. 2 and 3) suggests that (in contrast to fecal coliforms) they were rendered sunlight sensitive through damage accumulated in the WSP. Because more damage was received in the WSP in summer than in winter, they were inactivated at a similar rate to fecal coliforms in winter, but far more rapidly in summer (Fig. 2 and 4). Davies-Colley et al. (4, 5) also demonstrated a more rapid sunlight inactivation of enterococci in WSP effluent compared to E. coli, F-RNA phages, and F-DNA phages.
Our results (Tables 3 and 4; Fig. 2 to 4) indicate that sunlight damage to WSP enterococci continues in fresh and saline waters. The broad spread of inactivating wavelengths (Fig. 5) suggests that the principal inactivating mechanism in freshwater is photooxidation. Similar results have been obtained from optical filter experiments in seawater (31) and WSP effluent (4). It is not clear why enterococci suffer more photooxidative damage than fecal coliforms in a WSP, but they would appear to possess a narrower range of biochemical defense mechanisms (2).
Raw sewage enterococci discharged into seawater appear to be initially more sunlight resistant than fecal coliforms (31, 33) (Table 4 and Fig. 3), possibly because of greater cell wall resistance to the effects of salinity. However, once sunlight damage to enterococcal cells has occurred, it appears to be irreversible. This suggests a lack of repair mechanisms, although laboratory studies have produced conflicting evidence both for (15) and against (38) enterococcal photoreactivation.
Our enterococcus results support and extend the findings of Davies-Colley et al. (4, 5), who concluded that enterococci are less suitable than E. coli as indicators within WSPs because of their rapid inactivation. We conclude that enterococci are also unsuitable as indicators in natural waters receiving WSP effluents. Not only are enterococcus counts likely to be lower than for fecal coliforms in WSP discharges, but enterococci will also be more rapidly inactivated in the receiving waters. We therefore consider that enterococci are only suitable as bacterial indicators for sewage discharges to marine waters on the first day of sunlight exposure, when survival rates tend to exceed those of fecal coliforms.
(v) Bacteriophages.
There was no evidence of phage replication in the effluent-freshwater mixtures. Hernández-Delgado and Toranzos (17) also found that neither F-RNA phages nor somatic coliphages replicated in river waters. Thus, as in seawater (33), we have assumed that sunlight inactivation mechanisms in freshwater can be interpreted in terms of their effects on the phage virions themselves rather than on complex phage-host interactions.
Somatic coliphage and F-RNA phage inactivation rates appeared to be largely unaffected by effluent type, and both were more sunlight resistant than were fecal coliforms and enterococci over a range of salinities (Table 4 and Fig. 3). F-RNA phages appear to be slightly more sunlight resistant than somatic coliphages in freshwater (Table 3 and Fig. 2) but are less sunlight resistant in seawater (Fig. 3) (33, 39). The salinity and optical filter experiments (Fig. 3 and 5) and the results of Davies-Colley et al. (4, 5) and Sinton et al. (33) suggest that F-RNA phages are less susceptible than somatic coliphages to photobiological damage by UV-B wavelengths and more susceptible to photooxidative damage (by a wide range of inactivating wavelengths), particularly in seawater. In contrast, somatic coliphages are highly susceptible to wavelengths below 342 nm (UV-B and short UV-A). This is consistent with laboratory studies showing that that the inactivating spectrum for somatic coliphage T7 peaks at ca. 334 nm (9).
If we assume that enteric viruses exhibit similar sunlight inactivation rates to enteric phages, then our results suggest that both somatic coliphages and F-RNA phages will be better models of enteric virus survival in natural waters than fecal coliforms or enterococci. An earlier study (33) suggested that somatic coliphages are better virus models in seawater. In the present study, the slight difference between the phage inactivation rates in river water (Fig. 2) became more significant over 2 days (Fig. 4), when final F-RNA phage counts were 50 to 100 times higher than those of somatic coliphages. This suggests that F-RNA phages are potentially more useful for virological water quality monitoring in freshwaters.
Acknowledgments
We thank the staff of the Christchurch City Council for access to the sewage treatment plant; B. Gilpin of the Institute of Environmental Science and Research, M. J. Noonan of Lincoln University, A. M. Donnison of AgResearch, and the anonymous reviewers for valuable review comments; and G. Devane and G. Stanton for assistance with the field work and sample transport to the laboratory.
This research was funded from the New Zealand Public Good Science Fund, administered by the New Zealand Foundation for Research, Science, and Technology.
REFERENCES
- 1.American Public Health Association. 1998. Standard methods for the examination of water and wastewater, 20th ed. American Public Health Association, AWWA, WPCF, Washington, D.C.
- 2.Britton, L., D. P. Malinowski, and I. Fridovich. 1978. Superoxide dismutase and oxygen metabolism in Streptococcus faecalis and comparisons with other organisms. J. Bacteriol. 134:229-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calkins, J., and J. A. Barcelo. 1982. Action spectra, p. 143-150. In J. Calkins (ed.), The role of solar ultraviolet radiation in marine ecosystems. Plenum Press, New York, N.Y.
- 4.Davies-Colley, R. J., A. M. Donnison, and D. J. Speed. 1997. Sunlight wavelengths inactivating faecal indicator microorganisms in waste stabilisation ponds. Water Sci. Technol. 35:219-255. [Google Scholar]
- 5.Davies-Colley, R. J., A. M. Donnison, D. J. Speed, C. M. Ross, and J. W. Nagels. 1999. Inactivation of faecal indicator microorganisms in waste stabilisation ponds: interaction of environmental factors with sunlight. Water Res. 33:1220-1230. [Google Scholar]
- 6.Davies-Colley, R. J., R. G. Bell, and A. M. Donnison. 1994. Sunlight inactivation of enterococci and fecal coliforms within sewage effluent diluted in seawater. Appl. Environ. Microbiol. 60:2049-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies-Colley, R. J., W. N. Vant, and D. G. Smith. 1993. Colour and clarity of natural waters. Science and management of optical water quality. Ellis-Horwood, New York, N.Y.
- 8.de Wet, C. M. E., S. N. Venter, N. Rodda, R. Kfir, M. C. Steynberg, D. Hohls, and G. du Plessis. 1995. A survival study of Escherichia coli in a South Africa river using membrane diffusion chambers. Water Sci. Technol. 31:185-188. [Google Scholar]
- 9.Eisenstark, A. 1989. Bacterial genes involved in response to near-ultraviolet radiation. Adv. Genet. 26:99-147. [DOI] [PubMed] [Google Scholar]
- 10.Evison, L. M. 1988. Comparative studies on the survival of indicator organisms and pathogens in fresh and sea water. Water Sci. Technol. 20:309-315. [Google Scholar]
- 11.Flint, K. P. 1987. The long-term survival of Escherichia coli in river water. J. Appl. Bacteriol. 63:261-270. [DOI] [PubMed] [Google Scholar]
- 12.Gameson, A. L. H. 1986. Bacterial mortality, part 3, p. 1-74. In Investigations of sewage discharges to some British coastal waters. WRc Technical Report TR 239. WRc Environment, Medmenham, United Kingdom.
- 13.González, J. M., J. Irriberri, L. Egea, and I. Barcina. 1990. Differential rates of digestion of bacteria by freshwater and marine phagotrophic protozoa. Appl. Environ. Microbiol. 56:1851-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harm, W. 1980. Biological effects of ultraviolet radiation. Cambridge University Press, London, England.
- 15.Harris, G. D., V. D. Adams, D. L. Sorensen, and M. S. Curtis. 1987. Ultraviolet inactivation of selected bacteria and viruses with photoreactivation of the bacteria. Water Res. 21:687-692. [Google Scholar]
- 16.Havelaar, A. H., M. van Olphen, and Y. Drost. 1993. F-specific RNA bacteriophages are adequate model organisms for enteric viruses in fresh water. Appl. Environ. Microbiol. 59:2956-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernández-Delgado, E. A., and G. A. Toranzos. 1995. In situ replication studies of somatic and male-specific coliphages in a tropical pristine river. Water Sci. Technol. 31:247-250. [Google Scholar]
- 18.International Organization for Standardization. 1995. Water quality--detection and enumeration of bacteriophages, part I. Enumeration of F-specific RNA bacteriophages. ISO 10705-1. International Organisation for Standardization, Geneva, Switzerland.
- 19.Jagger, J. 1985. Solar-UV actions on living cells. Praeger, New York, N.Y.
- 20.Jerlov, N. G. 1976. Marine optics. Elsevier Oceanography Series, vol. 14. Elsevier Scientific Publishing Co., Amsterdam, The Netherlands.
- 21.Keswick, B. H., C. P. Gerba, S. L. Secor, and I. Cech. 1982. Survival of enteric viruses and indicator bacteria in groundwater. J. Environ. Sci. Health Part A 17:902-912. [Google Scholar]
- 22.Kirk, J. T. O. 1994. Light and photosynthesis in aquatic ecosystems, 2nd ed. Cambridge University Press, Cambridge.
- 23.Klein, T. M., and M. Alexander. 1986. Bacterial inhibitors in lake water. Appl. Environ. Microbiol. 52:114-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCambridge, J., and T. A. McMeekin. 1981. Effect of solar radiation and predacious micro-organisms on survival of fecal and other bacteria. Appl. Environ. Microbiol. 41:1083-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McFeters, G. A., and D. G. Stuart. 1972. Survival of coliform bacteria in natural waters: field and laboratory studies with membrane filter chambers. Appl. Microbiol. 24:805-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell, G. C., J. H. Baker, and H. A. Sleigh. 1988. Feeding of a freshwater flagellate Bodo saltans on diverse bacteria. J. Protozool. 35:219-222. [Google Scholar]
- 27.Morowitz, H. J. 1950. Absorption effects in volume irradiation of microorganisms. Science 111:229-230. [DOI] [PubMed] [Google Scholar]
- 28.Moss, S. H., and K. C. Smith. 1981. Membrane damage can be a significant factor in the inactivation of Escherichia coli by near-ultraviolet radiation. Photochem. Photobiol. 33:203-210. [DOI] [PubMed] [Google Scholar]
- 29.Niemi, M. 1976. Survival of Escherichia coli phage T7 in different water types. Water Res. 10:751-755. [Google Scholar]
- 30.Rhodes, M. W., and H. I. Kator. 1990. Effects of sunlight and autochthonous microbiota on Escherichia coli survival in an estuarine environment. Curr. Microbiol. 21:65-73. [Google Scholar]
- 31.Sinton, L. W., R. J. Davies-Colley, and R. G. Bell. 1994. Inactivation of enterococci and fecal coliforms from sewage and meatworks effluents in seawater chambers. Appl. Environ. Microbiol. 60:2040-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinton, L. W., R. K. Finlay, and A. J. Reid. 1996. A simple membrane filtration-elution method for the enumeration of F-RNA, F-DNA and somatic coliphages in 100-ml water samples. J. Microbiol. Methods 25:257-269. [Google Scholar]
- 33.Sinton, L. W., R. K. Finlay, and P. A. Lynch. 1999. Sunlight inactivation of fecal bacteriophages and bacteria in sewage-polluted seawater. Appl. Environ. Microbiol. 65:3605-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solić, M., and N. Krstulović. 1992. Separate and combined effects of solar radiation, temperature, salinity, and pH on the survival of faecal coliforms in seawater. Mar. Pollut. Bull. 24:411-416. [Google Scholar]
- 35.Springthorpe, V. S., C. L. Loh, W. J. Robertson, and S. A. Sattar. 1993. In situ survival of indicator bacteria, MS-2 phage and human pathogenic viruses in river water. Water Sci. Technol. 27:413-420. [Google Scholar]
- 36.Toranzo, A. E., J. L. Barja, and F. M. Hetrick. 1982. Antiviral activity of antibiotic-producing marine bacteria. Can. J. Microbiol. 28:231-238. [DOI] [PubMed] [Google Scholar]
- 37.U.S. Environmental Protection Agency. 1991. Test methods for Escherichia coli in drinking waters. EPA-600/4-91/016. U.S. Environmental Protection Agency, Washington, D.C.
- 38.Whitby, G. E., G. Palmateer, W. G. Cook, J. Maarschalkerweerd, D. Huber, and K. Flood. 1984. Ultraviolet disinfection of secondary effluent. J. Water Pollut. Control Fed. 56:844-850. [Google Scholar]
- 39.WRc. 1991. Surrogate viral indicators. Water Research Centre, Medmenham, United Kingdom.
- 40.Zar, J. 1996. Biostatistical analysis, 3rd ed. Prentice-Hall, Inc., Upper Saddle River, N.J.