Abstract
The effect of decompression on the structure of Methanococcus jannaschii, an extremely thermophilic deep-sea methanogen, was studied in a novel high-pressure, high-temperature bioreactor. The cell envelope of M. jannaschii appeared to rupture upon rapid decompression (ca. 1 s) from 260 atm of hyperbaric pressure. When decompression from 260 atm was performed over 5 min, the proportion of ruptured cells decreased significantly. In contrast to the effect produced by decompression from hyperbaric pressure, decompression from a hydrostatic pressure of 260 atm did not induce cell lysis.
The deep sea below 1,000 m accounts for 89% of the earth's total ocean and is believed to harbor a rich variety of undiscovered organisms exhibiting genetic and metabolic diversity (5, 14, 18). Even though decompression is nearly unavoidable at some point during research on deep-sea microorganisms, data on microbial survival upon decompression are rare due to limitations in the techniques and facilities suitable for high-pressure cultivation (19, 20). The only report of bacterial death upon decompression concerned gas vacuolate bacteria (7). In contrast, bacteria without gas vacuoles, such as Escherichia coli and Corynebacterium xerosis, were reported to survive rapid decompression from extreme gas supersaturation at 300 atm (6, 7). Likewise, the deep-sea heterotrophic isolate MT-41 obtained from 10,476 m did not exhibit any morphological changes upon decompression from hydrostatic pressure but did undergo ultrastructural alterations with increasing time at atmospheric pressure (3).
Hyperbaric bioreactor systems containing both liquid and gas phases offer advantages for the cultivation of autotrophic microorganisms at high pressures, most notably, better control of gaseous substrates and products (2, 10, 12, 13, 16). Considering the fact that many deep-sea extremophiles isolated to date grow on a gaseous substrate and produce a gaseous product(s) (8), hyperbaric pressurization should be an effective tool for efficient cultivation.
In the present work, we developed a high-pressure, high-temperature culturing system featuring both hyperbaric and hydrostatic bioreactors. Preliminary growth studies were performed with Methanococcus jannaschii, an extremely thermophilic methanogen originally isolated from a depth of 2,600 m (9). Scanning electron microscopy (SEM) revealed that the cell envelope of M. jannaschii ruptured upon decompression from 260 atm of hyperbaric pressure, with the degree of the disruption depending on the rate of decompression. These results have important implications for the collection and retrieval of microorganisms from deep-sea habitats containing high concentrations of dissolved gases and for the sampling of organisms from high-pressure laboratory reactors.
M. jannaschii was grown in serum bottles (125 ml) by strict anaerobic techniques, as described by Balch and Wolfe (1). The composition of the growth medium was the same as that described previously (17). Before starting the cultivation at 80°C in a shaking incubator, the gas atmosphere was anaerobically adjusted to 30 lb/in2 with a gas mixture of H2-CO2 (4:1). Exponentially growing cells were used as the inoculum. The inoculum sizes in the hyperbaric and hydrostatic bioreactors were 10 and 30% of the working volumes, respectively.
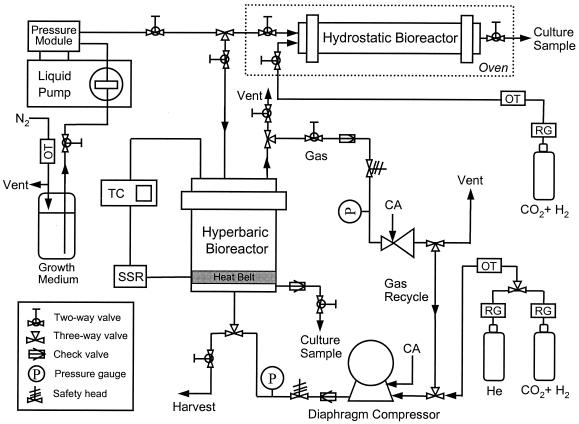
A schematic diagram of the high-pressure reactor system is shown in Fig. 1. All tubing and connectors used were obtained from High Pressure Equipment Co. (Erie, Pa.) and were designed to endure pressures up to 1,360 atm. A confined gasket-closure reactor (model GC-17; High Pressure Equipment Co.) was used as the hyperbaric bioreactor and was capable of operation at up to 200°C and 590 atm. The bioreactor had a total volume of 1.15 liters. A thermocouple (OMEGA Inc., Stamford, Conn.) inserted into a stainless steel 316 well extending three-fourths of the way into the bioreactor provided the signal to a proportional-integral-derivative controller (OMEGA Inc.) for precise control of the temperature through a heating belt (OMEGA Inc.). A diaphragm compressor (Newport Scientific Inc., Jessup, Md.) was used to supply H2, CO2, and He at high pressures. An oxygen trap (Alltech Inc., Deerfield, Ill.) was installed between the diaphragm compressor and the gas cylinder. The gas atmosphere in the hyperbaric bioreactor was H2-CO2 (4:1) up to 7.8 atm, and He was used for pressurization over 7.8 atm. The pressure of the hyperbaric bioreactor was precisely controlled with an air-actuated back-pressure regulator (Tescom, Elk River, Minn.) placed in the exit line of the gas loop.
FIG. 1.
Schematic diagram of high-pressure, high-temperature bioreactor system. The dotted lines signify an oven into which the pressurized hydrostatic bioreactor was placed after being disconnected to control the temperature. SSR, solid-state relay; TC, temperature controller; OT, oxygen trap; CA, compressed air; RG, regulator.
After 1 h of startup operation, gas flow was switched to the recycle mode in order to minimize the loss of gaseous substrates. Liquid medium and inoculum were supplied anaerobically via a liquid pump combined with a pressure module (Prostar 210 SDM; Varian, Santa Clara, Calif.). Culture samples were taken periodically from a liquid outlet line with a microcontrol metering valve (High Pressure Equipment Co.), during which the maximum flow rate was always maintained below 10 ml/min. Culture harvest was obtained through a port in the bottom of the bioreactor after gradual ventilation of gases to equilibrate the bioreactor at atmospheric pressure. A tubular reactor (model MS-18; High Pressure Equipment Co.) was used as the hydrostatic bioreactor and had a volume of 65 ml. The hydrostatic bioreactor was first pressurized with H2-CO2 (4:1) to 7.8 atm, and its pressure was increased to 260 atm hydrostatically by pumping in cells and growth medium with the liquid pump. Growth medium (45 ml) was anaerobically inoculated in a serum bottle with 20 ml of exponentially growing M. jannaschii culture, followed by transfer into the hydrostatic reactor through the liquid pump until the internal pressure reached 260 atm. Temperature control was achieved by incubating the pressurized hydrostatic bioreactor in an oven after it was disconnected, as indicated in Fig. 1. The reactor was reconnected to the system and repressurized to 260 atm every 2 h throughout the incubation period, during which time the pressure decreased by no more than 10%. After 10 h of cultivation, the hydrostatic bioreactor was depressurized in 1 s and the culture was withdrawn for further analysis. This experiment was performed twice with cultures of different initial cell densities.
Protein concentrations in each sample were measured by the Bio-Rad microassay technique (11). The turbidity of each culture sample was measured by the optical density at 660 nm (OD660). M. jannaschii cells were fixed with a solution of 1% (vol/vol) glutaraldehyde and 0.1% (vol/vol) osmium tetroxide for analysis by SEM. The fixed cells were deposited onto polycarbonate membrane filters (0.2-μm pore size; Millipore Corp., Bedford, Mass.) with a 10-ml syringe, followed by rinsing with 0.1 M sodium cacodylate buffer (pH 7.2) and dehydration with ethanol. SEM of the fixed M. jannaschii cells was performed on a model S-5000 scanning electron microscope (Hitachi, Tokyo, Japan) after critical point drying and sputter coating.
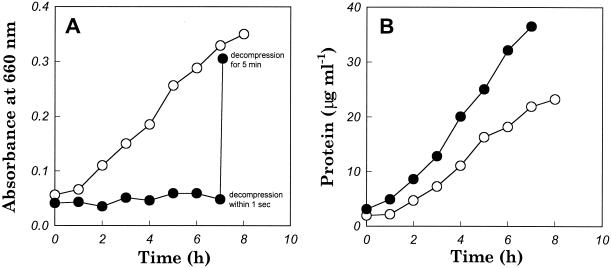
Growth of M. jannaschii in the hyperbaric bioreactor at 7.8 and 260 atm was analyzed by determining the OD660 (Fig. 2A) and the total protein content (Fig. 2B), for which culture samples were withdrawn through a microcontrol metering valve with a low flow rate (<10 ml/min) but a rapid decompression time (<1 s). M. jannaschii exhibited barophilic growth according to the change in protein content, consistent with the results reported by Miller et al. (11). However, there was no substantial increase in the OD660 of the culture grown at 260 atm, which remained below 0.1. Similar results were obtained in repeated experiments carried out under hyperbaric conditions at 260 atm. After the same 260-atm culture was harvested with a longer decompression time (5 min), the OD660 had increased more than sixfold (Fig. 2A). However, assuming that the protein measurements shown in Fig. 2B reflect the true growth curves, the OD660 value from the 260-atm harvest was still low due to the artifact of cell rupture.
FIG. 2.
Growth of M. jannaschii at 7.8 atm (○) and 260 atm (•). Both cultivations were performed at 80°C under hyperbaric pressure. Every sample was withdrawn in 1 s except for the sample labeled “decompression for 5 min,” which was obtained following a gradual decompression and cell harvesting step. (A) Absorbance changes of culture sample; (B) protein content changes in culture sample.
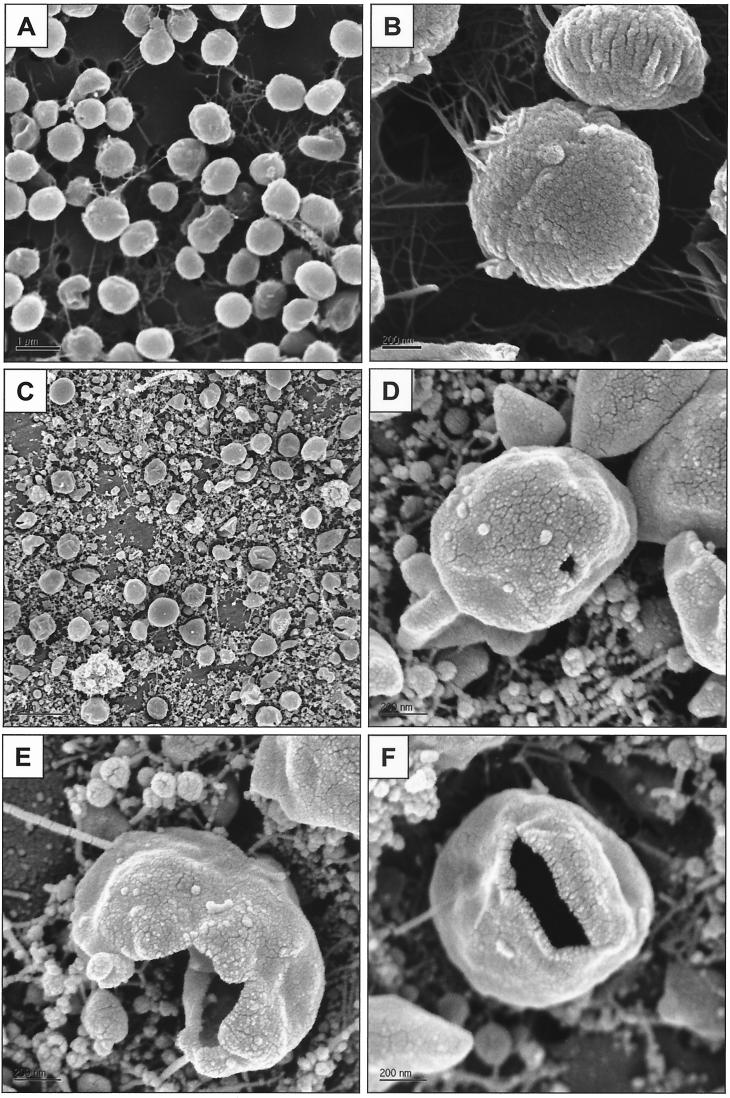
In order to investigate the effect of decompression on cell morphology, M. jannaschii grown at different pressures was analyzed by SEM. As shown in Fig. 3A and B, M. jannaschii grown at 7.8 atm maintained the same morphology as that previously reported (9). In contrast, the culture grown at 260 atm and decompressed in 1 s contained ruptured cells, widespread clusters of flagella, and debris that appeared to be from the cell envelopes (Fig. 3C through F).
FIG. 3.
Scanning electron micrographs of M. jannaschii at different pressure conditions. Culture samples were decompressed in 1 s through a microcontrol metering valve with a low flow rate (<10 ml/min). M. jannaschii was grown at 7.8 atm (A and B) and at 260 atm (C through F).
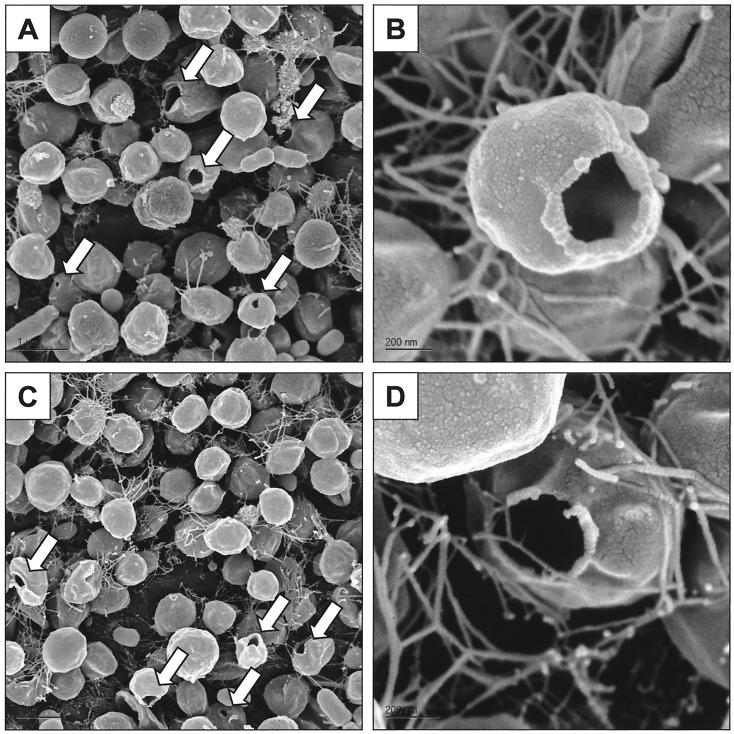
The SEM photographs of M. jannaschii harvested via slow decompression for 5 min revealed that most cells maintained their normal morphology, although some ruptured cells were still evident (Fig. 4A and B). Moreover, the amounts of cellular debris and clustered flagella were much smaller than they were after rapid decompression (Fig. 3C). We also examined the morphology of M. jannaschii after incubation for 24 h at 1 atm following a slow harvest from 260 atm. As shown in Fig. 4C and D, there was no significant change in the morphology of M. jannaschii after the 24-h incubation. This result is in contrast to the previously observed behavior of MT-41, a deep-sea heterotrophic bacterium isolated from 10,476 m, which did exhibit aberrations in morphology after a 24-h incubation at 1 atm (3).
FIG. 4.
Scanning electron micrographs of M. jannaschii harvested with slow decompression from 260 atm for 5 min. Shown are the morphologies of cells fixed right after decompression (A and B) and 24 h after decompression (C and D).
From these observations it became evident that decompression from the hyperbaric gas system led to the rupture of M. jannaschii and that decompression time is an important determinant of cell damage. Thus, the low OD660 observed after rapid decompression from 260 atm can be ascribed to the smaller absorptivity of cellular debris compared to that in intact cells. The higher absorbance of whole cells is to be expected, given that the absorptivity coefficient in the Beer-Lambert law is dependent on the cross-sectional area of the analyte (15).
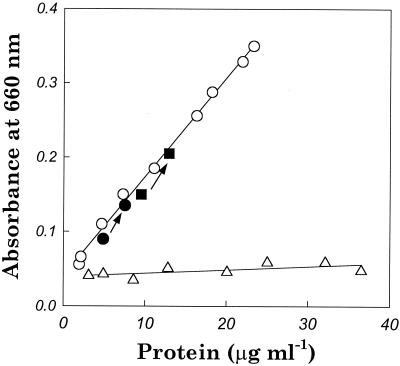
The effect of decompression from high hydrostatic pressure was investigated by cultivating M. jannaschii in the hydrostatic bioreactor at 260 atm without using He. In this experiment, decompression was completed in 1 s after cultivation. As shown in Fig. 5, M. jannaschii exhibited a lower growth rate at 260 atm in the hydrostatic bioreactor, but, unlike the results seen with the hyperbaric bioreactor, the OD660 remained above 0.09. Furthermore, the correlation between the OD660 and the protein content was close to that of the 7.8-atm culture in the hyperbaric bioreactor, implying that decompression from hydrostatic pressure did not cause cell lysis.
FIG. 5.
Growth of M. jannaschii in a hydrostatic bioreactor at 260 atm (•, ▪) and in the hyperbaric bioreactor at 7.8 atm (○) and 260 atm (▵). OD660s (after a 1-s decompression) and protein concentrations for growth in the hyperbaric bioreactor were correlated by linear regression. Arrows indicate cell growth for 10 h in the hydrostatic bioreactor starting from two different cell densities. The initial cell densities are indicated by the lower solid symbols.
The observed rupture by rapid decompression may have been promoted by the increased intracellular solubility of He at high pressures, in which cellular disruption was induced by the rapid expansion of concentrated He in the cytoplasm through the cell envelope during decompression of the sample. In this regard, it is noteworthy that the solubility of He in water increases ca. 31-fold from 7.8 to 260 atm (4). In contrast, a damaging effect caused by high shear during sampling could be ruled out for the following reasons: (1) the culture harvest remaining in the bioreactor following decompression also contained many ruptured cells, (2) the flow rate used for sampling from the 260 atm hyperbaric culture was fairly low (<10 ml/min), and (3) in some ruptured cells, only a small section of the cell envelope was missing, consistent with the presence of a blowout mechanism induced by rapid expansion.
In summary, the present work illustrates that rapid decompression from a hyperbaric environment can lead to cell rupture in deep-sea microorganisms regardless of whether a membrane-enclosed vacuole is present. This result should be borne in mind in laboratory studies of microbial growth at high pressures and in the sampling of deep-sea habitats containing high concentrations of dissolved gases.
Acknowledgments
This research was supported by the National Science Foundation (BES-9816490) and the Schlumberger Fellowship of D.S.C.
We thank Eric Grunland at the UC Berkeley Chemistry Machine Shop and Tracy Hsiao for their help in the design of the high-pressure, high-temperature bioreactor system.
REFERENCES
- 1.Balch, W. E., and R. S. Wolfe. 1976. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressurized atmosphere. Appl. Environ. Microbiol. 32:781-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernhardt, G., R. Jaenicke, H.-D. Lüdemann, H. König, and K. O. Stetter. 1988. High pressure enhances the growth rate of the thermophilic archaebacterium Methanococcus thermolithotrophicus without extending its temperature range. Appl. Environ. Microbiol. 54:1258-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chastain, R. A., and A. A. Yayanos. 1991. Ultrastructural changes in an obligately barophilic marine bacterium after decompression. Appl. Environ. Microbiol. 57:1489-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clever, H. L. 1979. Helium and neon—gas solubilities, p. 257. In A. S. Kertes (ed.), Solubility data series. IUPAC, Pergamon Press, Oxford, England.
- 5.Deming, J. W. 1998. Deep ocean environmental biotechnology. Curr. Opin. Biotechnol. 9:283-287. [DOI] [PubMed] [Google Scholar]
- 6.Hemmingsen, B. B., and E. A. Hemmingsen. 1978. Tolerance of bacteria to extreme gas supersaturations. Biochem. Biophys. Res. Commun. 85:1379-1384. [DOI] [PubMed] [Google Scholar]
- 7.Hemmingsen, B. B., and E. A. Hemmingsen. 1980. Rupture of the cell envelope by induced intracellular gas phase expansion in gas vacuolate bacteria. J. Bacteriol. 143:841-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huber, H., and K. O. Stetter. 1998. Hyperthermophiles and their possible potential in biotechnology. J. Biotechnol. 64:39-52. [Google Scholar]
- 9.Jones, W. J., J. A. Leigh, F. Mayer, C. R. Woese, and R. S. Wolfe. 1983. Methanococcus jannaschii sp. nov., an extremely thermophilic methanogen from a submarine hydrothermal vent. Arch. Microbiol. 136:254-261. [Google Scholar]
- 10.Miller, J. F., E. L. Almond, N. N. Shah, J. M. Ludlow, J. A. Zollweg, W. B. Streett, S. H. Zinder, and D. S. Clark. 1988. High-pressure-temperature bioreactor for studying pressure-temperature relationships in bacterial growth and productivity. Biotechnol. Bioeng. 31:407-413. [DOI] [PubMed] [Google Scholar]
- 11.Miller, J. F., N. N. Shah, C. M. Nelson, J. M. Ludlow, and D. S. Clark. 1988. Pressure and temperature effects on growth and methane production of the extreme thermophile Methanococcus jannaschii. Appl. Environ. Microbiol. 54:3039-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson, C. M., M. R. Schuppenhauer, and D. S. Clark. 1991. Effects of hyperbaric pressure on a deep-sea archaebacterium in stainless steel and glass-lined vessels. Appl. Environ. Microbiol. 57:3576-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson, C. M., M. R. Schuppenhauer, and D. S. Clark. 1992. High-pressure, high-temperature bioreactor for comparing effects of hyperbaric and hydrostatic pressure on bacterial growth. Appl. Environ. Microbiol. 58:1789-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prieur, D., and V. T. Marteinsson. 1997. Prokaryotes living under elevated hydrostatic pressure. Adv. Biochem. Eng. 61:23-35. [Google Scholar]
- 15.Sommer, L. 1989. Analytical absorption spectrophotometry in the visible and ultraviolet: the principles. Elsevier Science Publishers, Amsterdam, The Netherlands.
- 16.Sturm, F. J., S. A. Hurwitz, J. W. Deming, and R. M. Kelly. 1987. Growth of the extreme thermophile Sulfolobus acidocaldarius in a hyperbaric helium bioreactor. Biotechnol. Bioeng. 29:1066-1074. [DOI] [PubMed] [Google Scholar]
- 17.Tsao, J.-H., S. M. Kaneshiro, S. Yu, and D. S. Clark. 1994. Continuous culture of Methanococcus jannaschii, an extremely thermophilic methanogen. Biotechnol. Bioeng. 43:258-261. [DOI] [PubMed] [Google Scholar]
- 18.Yayanos, A. A. 1986. Evolutional and ecological implications of the properties of deep-sea barophilic bacteria. Proc. Natl. Acad. Sci. USA 83:9542-9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yayanos, A. A. 1995. Microbiology to 10,500 meters in the deep sea. Annu. Rev. Microbiol. 49:777-805. [DOI] [PubMed] [Google Scholar]
- 20.Yayanos, A. A., and A. S. Dietz. 1983. Death of a hadal deep-sea bacterium after decompression. Science 220:497-498. [DOI] [PubMed] [Google Scholar]