Abstract
Background
Persistent postoperative low back pain, including persistent spinal pain syndrome type 2 (PSPS-T2), is a global healthcare challenge that lacks objective phenotypic diagnostic criteria or validated biomarkers. Molecular imaging techniques (nuclear medicine) could aid in establishing more objective phenotypical parameters as they are able to visualize biological processes underlying several diseases even before anatomical changes are present. This review aims to provide an overview of the status quo of molecular imaging for the diagnosis of PSPS-T2.
Method and results
An extensive search of PubMed and Embase was conducted to identify relevant studies that comprise imaging techniques using a radiopharmaceutical substance. Evidence reveals that these techniques can provide valuable insights into the underlying pathologies and mechanisms of PSPS-T2 by detecting pain generators that would have otherwise gone unnoticed. Moreover, the meta-analysis showed a pooled sensitivity of 90.3% (95% CI: 53–100%) and a specificity of 89.1% (95% CI: 31–100%) for [18F]NaF PET-CT, and a pooled sensitivity of 61.5% (95% CI: 7–93%) and specificity of 96% (95% CI: 21–100%) for diphosphonates SPECT-CT.
Conclusion
These findings suggest a potential utility in identifying those who are likely to benefit from surgical re-intervention. This illustrates the potential of molecular imaging in establishing a personalized-medicine approach. However, the retrospective design and the limited sample sizes are among the limitations of the included studies and further research is needed to unravel the potential of molecular imaging techniques as a tool to detect phenotypical biomarkers and to optimize patient care in patients with persistent postoperative low back pain, including PSPS-T2.
Keywords: Persistent spinal pain syndrome type 2, Molecular imaging, Chronic pain, Back pain, post-surgical pain, Nuclear imaging
Introduction
Persistent spinal pain syndrome type 2 (PSPS-T2) is a collective term for conditions with persistent or emerging back or lower extremities pain after lumbar spine surgery (Christelis et al. 2021). Patients suffering from PSPS-T2 disclose severe pain intensities, mostly preeminent in the legs, as well as low health-related quality of life ratings, indicating a heavy strain on the patient (Thomson and Jacques 2009). Furthermore, they place a greater financial burden on society in terms of increased rates of work disability and higher medical costs (Thomson and Jacques 2009). According to a study conducted in the United Kingdom, approximately one in five patients undergoing lumbar spine surgery will develop persistent postoperative pain within a period of two years (Weir et al. 2017). Furthermore, a 10-year follow-up study of lumbar surgery patients revealed that 20.6% of them reported ongoing pain (Inoue et al. 2017). Although PSPS-T2 concerns a global healthcare challenge, it remains a clinical diagnosis without objectifiable phenotypical diagnostic criteria or validated biomarkers. Even more, the role of medical imaging, either as an objective diagnostic tool or as a companion diagnostic tool, remains limited as imaging-derived anatomical data poorly correlate with clinical symptomatology (Witkam et al. 2022). This explains why there are no established phenotypical clinical correlates with different imaging techniques for PSPS-T2 so far. However, there is evidence that alterations in the metabolism of bone and soft tissue precede structural changes in low back pain prior to surgery. A recent review shows that anatomical CT and MRI findings often correlate poorly with clinical symptoms and responses to treatment such as surgery. Molecular imaging techniques (e.g., positron-emission tomography (PET) and single-photon emission tomography (SPECT) offer a promising alternative, identifying areas of remodeling, metabolic changes, inflammation and other physiological processes not previously seen on structural imaging. These findings highlight the potential of molecular imaging to provide deeper insights into the underlying biological mechanisms of disease which could have implications for the improvement of diagnosis and treatment (Sweetwood et al. 2023).
For that reason, molecular imaging techniques, could be more precise in delineating objective imaging phenotypical parameters. To date, there is no specific overview of the opportunities and challenges of these functional imaging techniques in patients with persistent low back pain after surgery, including PSPS-T2 patients. The current systematic literature review is therefore aimed to provide an overview of the status quo regarding molecular imaging techniques for diagnosing PSPS-T2.
Methods
Search strategy
To ensure a comprehensive and rigorous analysis of the available literature, this systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al. 2021). A systematic literature search was conducted using the PubMed and Embase databases. The search string is listed below. All searches were conducted until July 2025. The execution and corresponding protocol of this systematic review have not been published.
Specifically, the following concepts were covered using both Medical Subject Headings (MeSH) and free-text terms: failed back surgery syndrome (FBSS), sciatica, chronic postsurgical pain, post-discectomy syndrome, chronic pain after spinal surgery, low back pain, leg pain, neuropathic pain, and persistent spinal pain syndrome (PSPS). These were combined with terms related to nuclear medicine diagnostics, including positron emission tomography (PET), fluorodeoxyglucose PET (FDG-PET), PET-CT, single photon emission computed tomography (SPECT), SPET, scintigraphy, bone scans, and general radionuclide imaging. Appropriate synonyms and variations in terminology were included to ensure comprehensive retrieval.
Assessment of the retrieved literature
Criteria for articles to be included in this study comprised: (1) The studied population should include human subjects suffering from PSPS-T2; (2) The investigation method should comprise any imaging technique which uses a radiopharmaceutical substance; (3) The aim of the study was to elucidate mechanisms which might play a role in the pathophysiological processes which occur in PSPS-T2. Further, to be included, articles should present original information and have to be written in either English, German, or Dutch. Case studies, letters to the editor, reviews and conference abstracts were not considered eligible and were therefore excluded from further assessment.
Using the aforementioned inclusion and exclusion criteria, all articles underwent a first round of reviewing. During this round, the title and abstract were screened for relevance. The full text was retrieved for articles considered potentially eligible or when the information from the title and abstract did not provide sufficient information. The second review round comprised a full-text assessment. Two independent reviewers (L.B. and D.H.) screened the retrieved studies for eligibility, and any discrepancies were resolved through consensus. The data were extracted using a standardized form. The quality of the studies was assessed using the Cochrane Risk of Bias tool.
A narrative synthesis of the results was conducted to present an overview of the retrieved evidence.
Meta-analysis
A meta-analysis with continuous random effects was carried out using R (https://www.r-project.org). For the meta-analysis of diphosphonate SPECT-CT studies, we grouped studies that used different diphosphonate tracers together. This approach is supported by a comprehensive study that found no significant qualitative or quantitative differences among the different diphosphonates used (MDP, HDP, DPD) (Frühling et al. 1986). Forest-plots were created to graphically present the pooled sensitivity and specificity for each molecular imaging technique. Heterogeneity of the analysis was calculated with R. Heterogeneity in meta-analyses serves as an indication of the variation between included studies. To measure heterogeneity, Cochran’s Q was calculated as the weighted sum of squared differences between individual study effects and the pooled effect across studies. The heterogeneity index (I2) is a proportion of total variability explained by heterogeneity and is independent from the number of included studies. I2 refers to the percentage of variation across studies and highlights the inconsistency across studies and ranges from 0% (i.e., no heterogeneity) to 100% (i.e., the highest heterogeneity) (Higgins et al. 2003).
Results
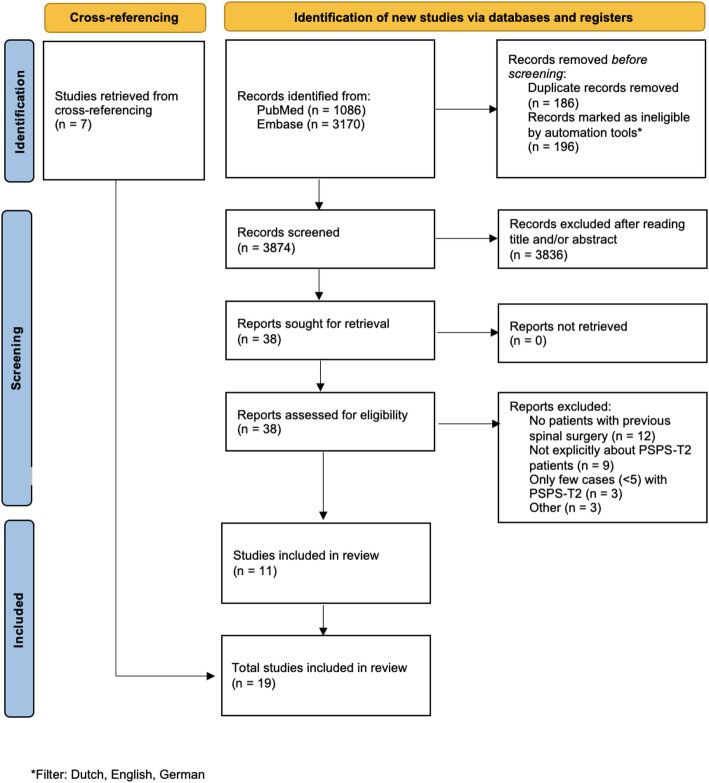
A total of 4256 articles were obtained from the literature searches in PubMed and EMBASE. After applying filters to include only articles in Dutch, English, or German, 196 articles were excluded from further analysis. Duplicate removal led to the exclusion of an additional 186 papers, after which 3874 unique articles remained. Following title and abstract screening, 38 articles were selected for full-text assessment. Ultimately, 19 articles were included in the review, of which seven were identified through cross-referencing. The article selection process is illustrated in Fig. 1, according to the PRISMA guidelines.
Fig. 1.
Flowchart illustrating the selection process
Narrative synthesis of literature
Bone scintigraphy and SPECT-CT
Older studies using planar scintigraphy imaging showed limited sensitivity and diagnostic value. In light of this reviews scope, the technique is now considered obsolete due to poor anatomical resolution and high rates of false negatives (Even-Sapir et al. 1994; Lusins et al. 1989; Gates and McDonald 1999; Larsen et al. 1996).
SPECT-CT, combining functional imaging with anatomical detail, has shown clinical utility in patients with PSPS-T2, particularly when conventional imaging (e.g., MRI or CT) is inconclusive. Various studies have used [99mTc]Tc-labeled diphosphonates, such as [99mTc]Tc-HDP, [99mTc]Tc-DPD, and [99mTc]Tc-MDP, which accumulate in areas of increased osteoblastic activity. It has previously been shown that SPECT can be useful in obtaining diagnostic information in patients with low back pain in general (i.e., not specifically PSPS-T2), that was not previously detected with radiography or planar imaging (Ryan et al. 1992). This was also found in 37 patients with persistent pain after lumbar fusion in whom [99mTc]Tc-DPD SPECT-CT outperformed planar/SPECT imaging in diagnostic accuracy. Nearly half of the cases were misclassified using planar/SPECT alone. SPECT-CT enabled more precise identification of pseudoarthrosis and pain generators (Sumer et al. 2013). Also, in a retrospective analysis of 10 patients with suspected pseudoarthrosis, [99mTc]Tc-HDP SPECT-CT was superior to CT in detecting facet joint degeneration and nonunion, but not screw loosening (Rager et al. 2012). Another study showed that SPECT-CT using [99mTc]Tc-HDP was able to detect hardware-related complications with an overall diagnostic accuracy of 92%. Sensitivity and specificity for screw loosening were 75% and 97.4% at the patient level. Findings were confirmed with surgical or clinical follow-up (Hudyana et al. 2016). In another study, [99mTc]Tc-MDP SPECT/CT correctly predicted surgical findings regarding screw stability in 6 out of 9 patients. Two scans were inconclusive, and one case was mislocalized. Differences in timing between surgery and imaging were suggested to explain incongruent findings 6 of 9 patients (Damgaard et al. 2010). Among patients with PSPS-T2, [99mTc]Tc-MDP SPECT-CT helped identify disk inflammation and pseudoarthrosis. Surgical outcomes were more favorable in patients with positive SPECT-CT findings than those with abnormalities only on MRI or radiography, supporting its value in guiding reintervention (Huang et al. 2019). The influence of [99mTc]Tc-MDP SPECT-CT on the clinical decision-making process was also demonstrated in a study by Acosta et al. (2021). Of 18 patients with persistent pain post-lumbar fusion and inconclusive standard imaging, SPECT-CT revealed focal degenerative pathologies in nine patients, and in five patients, failed fusions were detected. SPECT-CT influenced treatment in one-third of cases. Surgery based on SPECT-CT findings led to reduced pain scores and decreased analgesic use, again showing its practical relevance in therapy planning (Acosta et al. 2021). In a large, retrospective study of PSPS-T2 patients with inconclusive CT/MRI findings (n = 187), it was found that 85.6% of patients had a region with abnormal uptake on [99mTc]Tc-HDP SPECT-CT. Uptake patterns changed over time post-surgery, with decreased activity in fusion segments, but increased activity in adjacent segments, suggesting a combination of changes in biomechanical stress distribution and degenerative progression. In this retrospective study, it was furthermore found that SPECT-CT guided treatment in 64% of cases and prevented unnecessary interventions in patients with negative findings (Al-Riyami et al. 2019).
An overview of these studies can be found in Table 1.
Table 1.
Overview of the SPECT-CT and bone SPECT studies
| Participants | Goal | Procedure | Image assessment | Results | Limitations | |
|---|---|---|---|---|---|---|
| Acosta et al. (2021) | N = 18 with at least 1 year of persistent post-surgical back pain after lumbar fusion | Evaluate [99mTc]Tc-MDP SPECT-CT as an adjunct to conventional imaging for identifying spinal pain generators. | Injection of 24 mCi technetium-99m methylene diphosphonate, short [99mTc]Tc-MDP | Not mentioned | SPECT-CT successfully identified requiring reintervention, which led to a 75% improvement in narcotic use and a 44.1% reduction in VAS scores. The most frequent area of tracer uptake was at the site of the prior fusion (27.7%). | Small sample size; Retrospective design; Limited generalizability due to single-center setting |
| Al-Riyami et al. (2019) | N = 187 with persistent/recurrent lumbar pain after lumbar spinal surgery with stabilization (LSSS) and inconclusive radiography, CT, and/or MRI | Assess [99mTc]Tc-HDP bone SPECT-CT for detecting pain generators and evaluate uptake patterns in post-LSS patients. | Scan took place 4 h after injection of 800 MBq technetium-99m hydroxymethylene diphosphonate, short [99mTc]Tc-HDP | Images were assessed in consensus by a team consisting of a radiologist/nuclear medicine physician and a nuclear medicine physician- | 85.6% presented with positive imaging finding, most commonly in stabilized segments. High uptake was more common in patients with recent surgery or with at least 3 stabilized segments but decreased over time. The proportion of patients with increased activity in adjacent segments increased over time. | Retrospective design; Referral bias; No control over interval between previous imaging and SPECT-CT; Lack of comparison with surgical exploration as gold standard; Incomplete assessment of SPECT-CT’s predictive value for therapy success. |
| Damgaard et al. (2010) | N = 9 with persistent back pain after lumbosacral surgery | Determine whether fusion stability could be predicted from SPECT-CT findings by correlating imaging findings with surgical reexploration results | Planar whole-body scan and/or static scan performed 3 h after injection of 500–600 MBq [99mTc]Tc-MDP, followed by combined SPECT-CT with low dose CT. | SPECT/CT images were evaluated by a single expert to assess the stability of pedicle screws and fused lumbar segments. | In 6 of 9 cases, SPECT-CT fully or partially identified the level of pedicle screw loosening; 2 cases were inconclusive, and 1 case showed incorrect localization. | Small sample size and retrospective design; uncertain whether increased tracer uptake reflects normal remodeling or pathology; potential low specificity of bone scintigraphy may apply to SPECT/CT as well. |
| Even-Sapir et al. (1994) |
N = 33 with back pain after lumbar fusion surgery Two groups: N = 9 (surgery less than 4 years ago) N = 24 (surgery more than 4 years ago) |
Assess the utility of SPECT in evaluating painful late effects following spinal fusion surgery | Planar images (anterior and posterior) were acquired 3–4 h after intravenous injection of 740–925 MBq of technetium-99m imidodiphosphonate, short [99mTc]Tc-IDP | Three nuclear medicine physicians independently and blindly assessed the fusion status and determined potential abnormalities in the spine and sacroiliac joints. Consensus agreement was used for the final evaluation. | Failed fusion was associated with abnormalities detected by SPECT early after surgery. As more time passed since surgery, the abnormalities observed were related to late adverse events in apparently solid fusions. | Bias might have led to higher incidence of lesions in free segments adjacent to the fusion |
| Gates & McDonald (1999) | N = 63 with back pain who had lumbar spinal surgery |
Differentiate patients with skeletal lesions from those without Correlate abnormal uptake patterns and time since surgery with the type of surgery performed |
Administration of [99mTc]Tc-MDP | Correlation with surgery and other imaging modalities reported, but image reviewers not specified. | Bone SPECT helped exclude bony abnormalities in some patients and identified lesions such as pseudarthrosis, facet and disc space abnormalities, sacroiliitis, and vertebral body lesions. Most abnormalities were within the operative field. | Possible selection bias of individual physicians when referring patients for bone scan |
| Huang et al. (2019) |
N = 16 diagnosed with failed back surgery syndrome (FBSS) N = 7 underwent additional lumbar fusion surgery based on imaging findings N = 9 received only conservative treatment (painkillers and rehabilitation) due to negative findings on X-ray, MRI, and SPECT/CT |
Assess the potential application of SPECT-CT in defining FBSS-related lesions | Whole-body planar scintigraphy was performed 3–4 h after intravenous injection of 740 MBq [99mTc]Tc-MDP, followed by SPECT-CT | Two blinded nuclear medicine physicians independently assessed the images | SPECT-CT may aid in identifying FBSS-related lesions and guide treatment management. | Small sample size |
| Hudyana et al. (2016) | N = 48 who were referred for bone SPECT-CT due to persistent or recurrent pain after lumbar spinal fusion | Investigate usefulness of SPECT-CT in detecting pedicle screw loosening, using surgical findings and clinical follow-up as reference standards. | Whole-body planar scan and SPECT-CT were performed 3 h after intravenous injection of 740 MBq [99mTc]Tc-HDP. | Two experienced nuclear medicine specialists evaluated the SPECT-CT scans. Misalignments between SPECT and CT were corrected as needed. Lesions indicating possible hardware loosening (defined as focal areas of markedly increased uptake) were assessed. Findings were validated against surgical results when available, or clinical follow-up otherwise. | SPECT-CT showed 100% sensitivity and 89.7% specificity for detecting hardware loosening after lumbar fusion | Unclear cause of false-positive findings in 3 patients; Retrospective design of the study. |
| Larsen et al. (1996) |
N = 25 with previous lumbar fusion and persistent severe pain who were scheduled for hardware removal and fusion inspection Out of this group, 20 underwent preoperative bone scintigraphy |
Compare findings of preoperative plain radiography, flexion and extension radiography, CT and bone scintigraphy to surgical exploration. | [99mTc] was injected intravenously. No further details provided. | A single blinded radiologist interpreted each study for signs of solid fusion of pseudoarthrosis. An absence of increased uptake on scintigraphy was interpreted as solid fusion. The definite fusion status was visually inspected during surgery. |
Increased uptake was observed in 4 out of 20 cases, with only 2 having confirmed solid fusion during surgery. Overall, none of the preoperative imaging modalities—plain radiographs, flexion-extension views, CT, or bone scintigraphy—showed a statistically significant correlation with surgical findings. |
Not explicitly stated. |
| Rager et al. (2012) | N = 10 patients with recurrent back and/or leg pain after anterior and/or posterior lumbar fusion, all suspected of pseudarthrosis based on conventional radiologic imaging | Compare findings of SPECT-CT with CT alone. | Planar whole-body scans and static lumbar spine scans were performed 3 h after injecting [99mTc]Tc-HDP (10 MBq/Kg). | One musculoskeletal radiologist and one nuclear medicine physician jointly analyzed images in consensus, evaluating screw loosening, nonunion through or around cages, and facet joint degeneration. | Compared with CT alone, SPECT-CT appears to improve specificity for detecting nonunion of interbody devices and increase sensitivity for identifying symptomatic degenerative disease. All cases of screw loosening on CT showed abnormal uptake on SPECT-CT, while SPECT-CT detected increased uptake in cases missed by CT alone, particularly for facet joint degeneration. | Not explicitly stated. |
| Sumer et al. (2013) | N = 37 with lower back pain of the spine after lumbar fusion surgery | Investigate the added diagnostic value of skeletal SPECT-CT compared to planar-SPECT by assessing changes in diagnostic classification | Planar whole-body scan was performed 3 h after intravenous injection of 383–724 MBq [99mTc]Tc-DPD | One nuclear medicine specialist analyzed the imaging data, to identify pathological tracer uptake and classify lesions into diagnostic categories. | SPECT-CT led to a change in diagnostic category in 45.2% of lesions detected by planar/SPECT, improving localization and morphological characterization of pathological uptake. | No independent gold standard reference; surgical re-exploration was limited and unsystematic; arbitrary reference for tracer uptake may cause bias; analysis performed by a single observer, limiting reproducibility. |
[18F]NaF PET-CT
Several studies highlighted the added diagnostic value of [18F]NaF PET-CT in patients with PSPS-T2, particularly when conventional imaging fails to identify the underlying cause. Pouldar et al. (2016) retrospectively studied 25 patients with persistent pain after spinal fusion. While MRI was inconclusive in all cases, [18F]NaF PET-CT revealed increased osteoblastic activity in 88% of patients, identifying potential pain generators and leading to treatment changes in 72% (Pouldar et al. 2017). Another study involving 67 patients (25 post-fusion, 42 post-laminectomy) showed that [18F]NaF PET-CT could help localize abnormal bone metabolism more reliably in fusion patients (100%) than in those with laminectomy (65%). Facet joint involvement differed between groups, with more isolated facet uptake in the laminectomy group (Gamie and El-Maghraby 2008). Further research in 36 post-fusion patients demonstrated a correlation between PET uptake at the vertebral endplates and clinical outcomes (e.g., Oswestry Disability Index), as well as with CT-confirmed pseudoarthrosis, suggesting a link between increased tracer uptake and mechanical stress at the fusion site (Peters et al. 2015a, b). In a cohort of 15 patients with persistent or recurrent low back pain without a clear clinical explanation, increased [18F]NaF uptake at the vertebral endplates was observed. This uptake strongly correlated with the degree of cage subsidence, as assessed on CT, regardless of follow-up duration. Fusion status was evaluated based on intervertebral bone bridging, and cage subsidence was scored separately for upper and lower vertebrae. These findings suggest that [18F]NaF PET-CT may detect biomechanical stress or nonunion in the fusion region and help identify instability as a potential pain generator in PSPS-T2 (Brans et al. 2012). In another study, [18F]NaF PET-CT identified regions of failed fusion in 18 patients who subsequently underwent revision surgery, with intraoperative findings confirming implant failure in all cases. Interestingly, this group also had a high prevalence (38.9%) of surgical site infections caused by Cutibacterium acnes. However, this had no influence on the distribution of increased tracer uptake around the cages and it was not possible to distinguish between C. acnes positive patients and C. acnes negative patients based on visual assessment of the [18F]NaF PET. A control group with complete fusion on CT showed no increased tracer uptake (Yaagoubi et al. 2022). A prospective trial in 22 PSPS-T2 patients with inconclusive clinical and CT findings reported that [18F]NaF PET-CT correctly identified surgical targets in 14 out of 15 operated patients. One false-positive result was attributed to early postoperative reactive changes (Quon et al. 2012). The study of Seifen et al. (2014) compared [18F]NaF-PET-CT to dynamic radiography for detecting screw loosening in 59 patients. PET-CT showed high diagnostic accuracy (89.8%), with excellent specificity (97.4%) and moderate sensitivity (75%) at the patient level (Seifen et al. 2015). Expanding on this diagnostic potential, a recent study has shown that 30 min of dynamic [18F]F PET-CT can provide clinically relevant kinetic parameters that allow differentiation between pseudoarthrosis and successful spinal fusion, even when static SUV values cannot. In this context, the dynamic approach may be able to account for the proposed patient-specific blood clearance rates and may therefore offer improved diagnostic value in cases where standard imaging is inconclusive (Peters et al. 2015a, b).
An overview of these studies can be found in Table 2.
Table 2.
Overview of the PET-CT studies
| Participants | Goal | Procedure | Image assessment | Results | Limitations | |
|---|---|---|---|---|---|---|
| Brans et al. (2012) | N = 15 with persisting symptoms after posterior lumbar interbody fusion | Investigate how vertebral [18F]F uptake correlates with high-resolution CT findings related to interbody fusion | Scan was performed 60 min after intravenous injection of 156–263 MBq of [18F]Fluoride | PET and CT images were independently assessed by two observers blinded from previous imaging results and clinical details. Interbody fusion was scored based on the presence of bony bridging, and cage subsidence was graded separately for the upper and lower vertebrae. | Cage subsidence was the most common CT abnormality (59%) and correlated with higher SUVmax values at vertebral endplates and pedicle screw entry points. No link was found between fusion and SUVmax. | Small and clinically heterogeneous patient sample; variable post-surgery intervals; lack of systematic surgical re-exploration as a gold standard; only one revision surgery performed |
| El Yaagoubiet al. (2022) |
N = 18 with presumed pseudoarthrosis N = 5 control group with clear fusion status on CT |
Assess the use of [18F]NaF PET-CT in diagnosing pseudoarthrosis after spinal fusion and identifying those that may benefit from revision surgery | Scan was performed 60 min after injection of 2.2 MBq/kg [18F]NaF | Two nuclear medicine physicians assessed increased [¹⁸F]NaF uptake visually and quantitatively, blinded only to revision surgery outcomes. CT images were independently reviewed by a radiologist blinded to PET-CT results. | [¹⁸F]NaF PET/CT correctly identified all cases of pseudarthrosis confirmed at revision surgery. Increased uptake correlated with interbody fusion mobility. In 38.9% of revision cases, Cutibacterium acnes infection was found, but it did not affect tracer uptake patterns. | Retrospective design; small sample size; not a pure control study; control group had heterogeneous surgical hardware; physicians were not fully blinded; potential selection bias; PET-CT value in other implant types remains unclear. |
| Gamie & El-Maghraby (2008) |
N = 67 of which N = 25 had previous spinal surgery N = 17 laminectomy or discectomy N = 8 lumbar fusion |
Determine the role of [18F]NaF PET-CT in evaluating disc and facet abnormalities | Scan was performed 45–60 min after injecting 444–555 MBq of [18F]NaF | Not mentioned | PET-CT showed positive uptake in 76% of the FBSS patients (100% in lumbar fusion subgroup and 65% in laminectomy subgroup) | Not mentioned |
| Peters, Willems et al. (2015) |
N = 36 N = 18 with recurrent or persistent low back pain and no apparent cause, assessed 9–76 months after posterior lumbar interbody fusion (PLIF) N = 18 with low back pain undergoing routine post-operative investigation between 11 and 14 months after posterior lumbar interbody fusion |
Investigate the relationship between findings on PET-CT and clinical symptoms after PLIF | Scan was performed 60 min after intravenous injection of 156–263 MBq of [18F]NaF | Two independent blinded observers determined volumes of interest (VOIs) for radionuclide uptake and scored interbody fusion based on bony bridging on CT scans. Discrepancies were resolved by consensus and verified by specialists. | No correlation was observed between fusion status and symptom severity. Significantly higher radiotracer uptake in vertebral endplates in patients with the worst clinical performance as indicated by the Oswestry Disability Index score. PET findings correlated better with symptom severity than those of CT. | No comparison to surgical exploration as gold standard; wide variability in follow-up times; small sample size. |
| Peters, Wierts et al. (2015) | N = 16 with persistent or recurrent low back pain after posterior lumbar interbody fusion surgery with no obvious clinical or radiological explanation | To compare dynamic 18 F-fluoride PE-/CT with static PET-CT in detecting lumbar pseudarthrosis. | Dynamic scanning was done immediately after injection of 156–214 MBq of [18F]NaF. Static scanning took place 60 min after injection. | Quantitative analysis of static and dynamic PET-CT scans. | Dynamic PET-CT can aid in differentiating pseudarthrosis from fusion after spinal surgery. Significant differences were observed in bone incorporation parameters and blood clearance rates, highlighting the potential of this method over traditional SUV measurements for diagnosing pseudarthrosis. | Possible shorter dynamic scan duration compared to other studies; Potential misalignment between dynamic and static scans due to patient movement; Variability in time interval between surgery and scan; Use of image-derived input function instead of use of arterial sampling as gold standard. |
| Pouldar et al. (2017) | N = 25 with persistent or recurrent back pain within 15 months after spinal fusion surgery and inconclusive prior MRI scans | Compare clinical accuracy of [18F]NaF PET-CT vs. CT in detecting pain generators and in influencing the course of treatment | Scan was performed 45 min after intravenous injection of 185–370 MBq of [18F]NaF | Three experienced radiologists independently and blindly reviewed CT and fused PET/CT images to evaluate screw loosening and incomplete union, assessing both structural changes and increased radiotracer uptake. | In 25 patients, 17 showed increased uptake on PET-CT, correlating strongly with pain sources In 88% PET-CT proved to be beneficial for decision making | Non-randomized, retrospective double-blind study design; Small sample size; Sampling bias |
| Quon et al. (2012) | N = 22 with recurrent post-surgical pain and inconclusive clinical and physical findings | Evaluate the utility of [18F]NaF PET-CT to identify lesions that require surgical intervention | Scan was performed 45 min after injection of 222–370 MBq [18F]NaF | A nuclear medicine physician and a musculoskeletal radiologist jointly reviewed PET/CT images to identify lesions suggestive of surgical intervention. Focal high-uptake areas correlating with typical hardware failure or pseudoarthrosis sites on CT were flagged. Findings were correlated with clinical history and prior CT. Results were validated surgically by an orthopedic surgeon assessing for hardware loosening or failure. | Sensitivity to detect the presence of lesion requiring surgery was 100% and specificity was 85.7%. One false-positive result was obtained | Effect of timing was not assessed; Small sample size |
| Seifen et al. (2015) | N = 59 with suspected screw loosening and pain in the area of previous intervertebral fusion stabilization | Evaluate the diagnostic value of [18F]F PET-CT for detecting pedicle screw loosening compared to functional radiology | Scan was performed 90 min after injection of 335–338 MBq of [18F]Fluoride | Two experienced nuclear medicine physicians interpreted the images and scanned for signs of screw loosening according to defined criteria. The final diagnosis was based on interoperative exploration and clinical follow-up. | PET-CT outperformed functional radiography in detecting screw loosening, showing higher accuracy, sensitivity, specificity, positive predictive value and negative predictive value. | Not explicitly stated. |
[18F]FDG PET-MRI
A recent study by Weng et al. (2025) compared the performance of conventional MRI versus [18F]FDG-PET-MRI in identifying pain generators in 30 PSPS-T2 patients (Weng et al. 2025). Pain sources were verified either through nerve blocks or by findings from revision lumbar spine surgery that took place within 1 months after scanning. The source of pain was considered accurately identified if the correct diagnosis, affected spinal level and side of pain were pinpointed. In order to assess intra-observer agreement, each scan was interpreted twice, with a one-month interval between readings. It was found that [18F]FDG-PET-MRI possesses higher diagnostic accuracy in detecting both spinal segment as well as non-spinal segment sources of pain compared to MRI alone. Furthermore, [18F]FDG-PET-MRI showed a greater intra-observer diagnostic agreement and lower SUVmax values between the two image interpretation sessions. To minimize patient-to-patient variability, the standardized uptake value ratio (SUVR) was used which normalizes data within the same scan. It was revealed that an SUVR greater than 1.3–1.5 can be considered as a reliable threshold for localizing the pain source. Even though, these first results seem promising, more research on the usefulness of [18F]FDG-PET-MRI is required in order to build up stronger evidence for its value for PSPS-T2 patients (Table 3).
Table 3.
Overview of the PET-MRI studies
| Participants | Goal | Procedure | Image assessment | Results | Limitations | |
|---|---|---|---|---|---|---|
| Weng et al. (2025) | N = 30 with chronic back pain with radicular leg pain lasting for over 6 months post-surgery, with no effect of conservative treatments or nerve blocks who underwent [18F]FDG PET-MRI | Assess the accuracy and effectiveness of [18F]FDG PET-MRI compared to MRI alone in diagnosing the cause of pain after lumbar spine surgery | Scan was performed 60 min after injection of 370 MBq of [18F]FDG | Two experienced readers, blinded to the clinical data, interpreted the scans twice with one month between rounds, jointly assessing MRI structural abnormalities and PET hot spots to determine the most likely chronic pain diagnosis and recording the affected level and side of the abnormalities. | [18F]FDG PET-MRI showed higher diagnostic accuracy and better intra-observer agreement than MRI alone in identifying both spinal and non-spinal pain sources. | Retrospective design; Small sample size; Lack of comparison between contrast agent MRI and PET/MRI. Certain MRI sequences were excluded due to potential artifacts, patient discomfort, and technical limitations. |
Meta-analysis
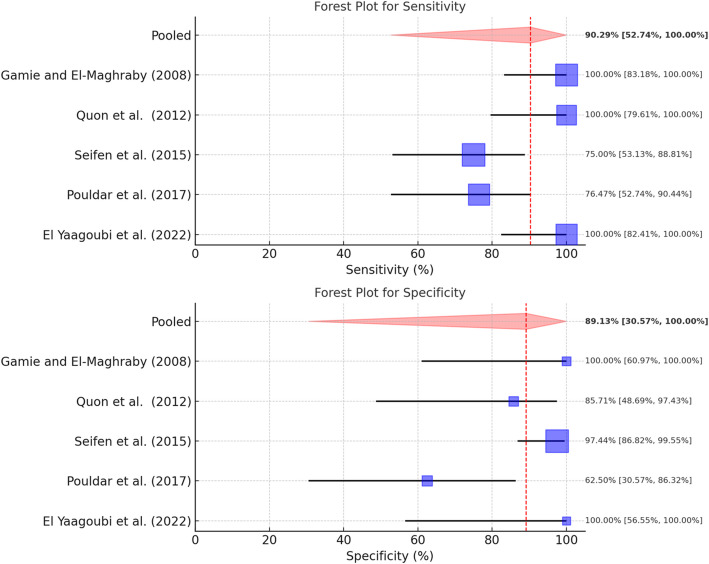
A total of five studies could be included in the meta-analysis of the [18F]NaF-PET-CT studies (Pouldar et al. 2017; Gamie and El-Maghraby 2008; Yaagoubi et al. 2022; Quon et al. 2012; Seifen et al. 2015). All five studies focused on the potential utility of [18F]NaF-PET-CT for the evaluation of recurrent symptoms after spinal (fusion) surgery and potentially identifying those patients requiring further surgical management. In total, 154 patients were included in this meta-analysis. A pooled sensitivity of 90.3% (95%-Confidence Interval 53–100%) was found, with substantial heterogeneity (I2 of 65.6%). Pooled specificity was found to be 89.1% (95%-Confidence Interval 31–100%), with moderate heterogeneity (I2 of 40.5%) (Fig. 2).
Fig. 2.
Forest plots for sensitivity and specificity of [18F]NaF PET-CT
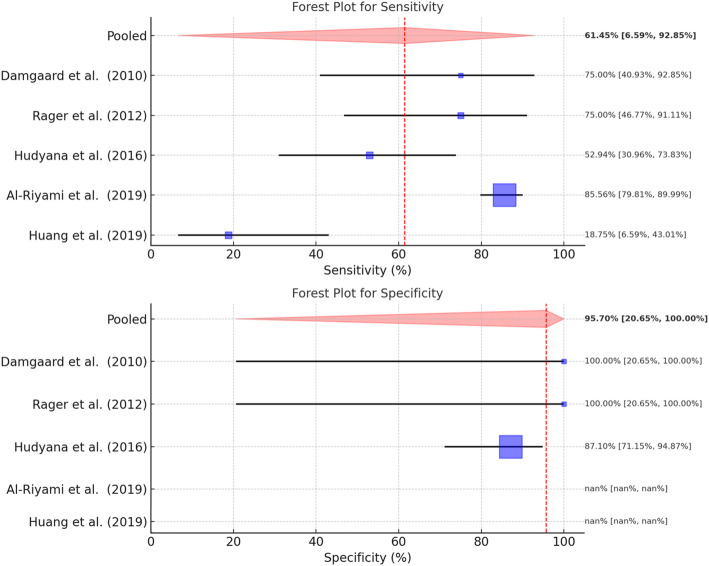
A total of five studies could be included in the meta-analysis of the diphosphonate SPECT-CT studies (Rager et al. 2012; Hudyana et al. 2016; Damgaard et al. 2010; Huang et al. 2019; Al-Riyami et al. 2019). In total, 273 patients were included in this meta-analysis on the diagnostic accuracy of diphosphonate SPECT-CT to identify the pain generator, including lack of fixation of metallic implants. A pooled sensitivity and specificity of 61.5% (95%-Confidence Interval: 7–93%) with considerable heterogeneity (I2 of 92.6%) and 96% (95%-Confidence Interval: 21–100%) was found, respectively (Fig. 3). Heterogeneity of the specificity analysis was considered non-influential with an I2 of 0%.
Fig. 3.
Forest plots for sensitivity and specificity of diphosphonate SPECT-CT. In cases where nan (“not a number”) is listed, it was not possible to extract any sensitivity and/or specificity data from the respective articles
Quality of the retrieved literature
Two researchers (L.B. and D.H.) independently assessed the quality of the retrieved literature using the Cochrane Risk of Bias tool. Any discrepancies were resolved by consensus. Overall, the studies presented a low risk of bias with regard to the index test and the reference standard. However, information on the flow and timing was often lacking, resulting in an unclear risk of bias for this item on the QUADAS-C checklist. Additionally, several studies presented a risk of bias with regard to patient selection. This was primarily due to the retrospective, non-randomized nature of the studies. Referral bias was suspected in some cases, where patients were selected based on the clinician’s decision for further assessment. In other cases, only patients with the most severe pain were chosen, indicating a high risk of selection bias. An overview of the assessment can be found in Table 4.
Table 4.
Quality assessment
| Study | Test | Risk of bias | ||||
|---|---|---|---|---|---|---|
| P | I | R | FT | |||
| Brans et al., 2012 | PET-CT | ✗ | ✓ | ✗ | ? | |
| El Yaagoubi et al., 2022 | PET-CT | ✗ | ✓ | ✓ | ? | |
| Gamie et al., 2008 | PET-CT | ✓ | ✓ | ✓ | ✓ | |
| Peters, Willems et al. 2015 | PET-CT | ✓ | ✓ | ✓ | ? | |
| Peters, Wierts et al. 2015 | PET-CT | ✓ | ✓ | ✗ | ? | |
| Pouldar et al., 2017 | PET-CT | ✗ | ✓ | ✓ | ✓ | |
| Quon et al., 2012 | PET-CT | ✓ | ? | ✓ | ✓ | |
| Seifen et al., 2015 | PET-CT | ✓ | ✓ | ? | ? | |
| Weng et al., 2025 | PET-MRI | ? | ✓ | ✓ | ? | |
| Acosta et al., 2021 | SPECT-CT | ✓ | ✗ | ✓ | ? | |
| Al-Riyami et al., 2019 | SPECT-CT | ✓ | ✓ | ✓ | ✓ | |
| Damgaard et al., 2010 | SPECT-CT | ✗ | ✗ | ✗ | ? | |
| Huang et al., 2019 | SPECT-CT | ✓ | ✓ | ✓ | ? | |
| Hudyana et al., 2016 | SPECT-CT | ✗ | ✓ | ✓ | ? | |
| Rager et al., 2012 | SPECT-CT | ✓ | ✓ | ✓ | ? | |
| Even-Sapir et al., 1994 | SPECT | ✗ | ✓ | ? | ? | |
| Gates et al., 1999 | SPECT | ✗ | ? | ✓ | ? | |
| Larsen et al., 1996 | SPECT | ✓ | ✓ | ✓ | ✗ | |
| Sumer et al., 2013 |
Planar/SPECT SPECT-CT |
✓ | ✗ | ✗ | ? | |
P = patient selection; I = index test; R = reference standard; FT = flow and timing
✓ indicates low risk; ✗ indicates high risk;? indicates unclear risk
Discussion
This systematic review provides an overview of the current evidence for the use of different molecular imaging techniques in PSPS-T2 patients. Our findings suggest that molecular imaging can detect pain generators that would have otherwise remained unnoticed. Furthermore, molecular imaging could help identifying patients who might benefit from surgical re-intervention. By that, the focus of these techniques lies in establishing a plausible cause for the recurrent pain. Three-dimensional molecular imaging techniques (i.e., PET-CT and SPECT-CT) focusing on bone metabolism are superior to planar imaging techniques regarding accurate anatomical localization. Specifically, our meta-analysis indicates that [18F]NaF-PET-CT appears to outperform diphosphonate SPECT-CT in terms of diagnostic accuracy for PSPS-T2 patients. However, substantial heterogeneity was noted in the [18F]NaF-PET-CT studies, suggesting variability in study design or patient populations. In contrast, the diphosphonate SPECT-CT studies exhibited consistent findings, though with lower diagnostic accuracy.
It is yet unclear whether increased tracer uptake reflects instability in the region where lumbar fusion was performed or whether this uptake is indicative of the physiological remodeling changes resulting from surgery or degenerative changes. In the study by Rager et al. (2012), the time interval between surgery and scan was less than one year in about half of the patients (Rager et al. 2012). Previous studies have shown that there can be increased activity at the operation site up to one year following surgery (Berquist 2006). Therefore, a false-positive SPECT investigation might be present when the scan is conducted too early after fusion surgery. Quon et al. (2012) discovered a false-positive finding in a patient who underwent SPECT-CT four months after fusion surgery (Quon et al. 2012). The findings by Even-Sapir et al. (1994) further strengthened this hypothesis, as four out of five patients who received surgery more than four years ago presented with either nonsolid or nonstabilizing fusions but had no focal abnormalities on their SPECT images. Contrarily, all patients from the group that underwent surgery less than four years ago and presented with failed fusions showed focal abnormalities on SPECT imaging. This difference suggests a potential natural progression of nonsolid fusions over time. Metabolic activity associated with failed fusions appears to be high in the early years after surgery but gradually decreases as time passes. The one patient from the late group that still presented with lesions on SPECT was, furthermore, the one with the shortest time interval between the fusion surgery and the scan (Even-Sapir et al. 1994). This might suggest that the time between surgery and scan has to be taken into account when evaluating fusion status and the presence of possible abnormalities and their correlating pain presentation within PSPS-T2. A similar follow-up period for all patients may nonetheless be difficult to achieve as patients can present with symptoms of pain at any given time in clinical practice.
Also, the optimal radiotracer for each patient or clinical examination is still a topic of debate. For example, 18F-fluorodeoxyglucose ([18F]FDG) has been found to be particularly useful for the evaluation and detection of infection and inflammation (Vaidyanathan et al. 2015), while [18F]NaF has been proven to be advantageous regarding its capacity to visualize bone metabolism specifically (Park et al. 2021). Also, [18F]NaF showed a higher bone uptake rate and faster blood clearance rate than technetium-99-labeled diphosphonates. Additionally, these technetium-99-labeled tracers have also been criticized for their lack of specificity (Segall et al. 2010; Adams and Banks 2022). Moreover, scans with 18F-fluoride can be conducted much faster, given the significantly shorter time between injection and imaging as well as the shorter imaging time itself (Grant et al. 2008). By this, [18F]NaF PET-CT could be particularly useful for patients with conditions requiring more rapid imaging or patients with difficulties tolerating longer imaging sessions in a supine position for a prolonged period of time due to their medical condition. PET-CT scans using [18F]NaF may, therefore, be the preferred method of choice in patients suffering from PSPS-T2 to visualize the pain generator. However, this technique may not be as widely available as SPECT, and it also comes with higher costs (Pouldar et al. 2017; Grant et al. 2008). From a broader diagnostic perspective, white blood cell (WBC) scintigraphy may represent a future potential imaging tool for detecting infection-related causes of postoperative spinal pain, given its use in orthopedic settings, although its specific role in PSPS-T2 remains to be clarified (Acker et al. 2001; Thélu-Vanysacker et al. 2018).
It is important to note that despite recent advances in the management of chronic pain, not all patients receive adequate pain relief from treatment. One of the reasons for this difference may be the great diversity in age, sex, and ethnicity and thus pathophysiology among the general population of patients suffering from chronic pain (Soliman et al. 2023). Regarding PSPS type 2, the complexity of the underlying pathophysiology is further demonstrated by the multifactorial etiology of pain, as indicated by a review that identified 22 different etiologies, with pathoanatomical factors such as stenosis or fibrosis being the most common (Clancy et al. 2017). As a result, additional research is needed to further explore the phenotypic characteristics of PSPS-T2. In this light, nuclear imaging techniques offer molecular insights that can contribute to the understanding of the underlying pathologies that are driving someone’s pain. They thus have the potential to pave the way for a more personalized medicine approach.
However, based on the current evidence and the quality of the scientific evidence, it is too early to incorporate [18F]NaF PET-CT and diphosphonate SPECT-CT into the diagnostic algorithm of patients suffering from PSPS-T2. Although some suggestions are made within the literature (e.g., PET-CT is suggested superior in detecting pseudoarthrosis or implant-related problems), sufficient comparative evidence is lacking. Furthermore, the timing of imaging the postoperative spine is still a topic of debate, as changes in bone metabolism might be influenced by time. Some papers suggested that [18F]NaF PET-CT could be helpful in determining whether a patient would benefit from surgical re-intervention, though the current evidence is too preliminary to deduce such a conclusion. Our meta-analysis supports these observations. Although [18F]NaF PET-CT demonstrated high diagnostic accuracy, the substantial heterogeneity between studies, indicates the need for more standardized protocols and larger studies. On the other hand, diphosphonate SPECT-CT showed lower diagnostic accuracy, which may limit its clinical utility. These findings highlight the necessity for further research to establish the definitive role of these imaging techniques in diagnosing and surveilling PSPS-T2 patients.
Limitations
Some limitations of the included studies should be noted. First, the limited sizes are a major drawback for this field of research. Second, many studies held a retrospective design, with possible biases as a result. For example, the study by El Yaagoubi et al. (2022) is at risk for selection bias as only patients with any kind of pain presentation that resulted in diagnostic evaluation resulting in a high suspicion of pseudoarthrosis were included (Yaagoubi et al. 2022). Similarly, Even-Sapir et al. (1994) merely included patients with the most severe pain (Even-Sapir et al. 1994). Thereby, it remains unknown whether an activation pattern observed on molecular imaging is the result of the persistent pain a PSPS-T2 patient experiences as a consequence of local inflammation or local osteoblastic reaction, as the described patterns could also be observed in post-surgical patients without persistent pain. Therefore, the current literature is lacking evidence of imaging findings in patients who underwent spinal surgery resulting in a complete reduction of their pain. This limitation, however, was overcome by the study of Peters et al. (2015), in which both patients with PSPS-T2 and successful operated patients were included, showing that increased accumulation of [18F]NaF was observed at the vertebral endplates was correlated with clinical performance (Peters et al. 2015a, b). Furthermore, they concluded that this activation pattern was the result of pseudoarthrosis occurring at the side of the unstable lumbar fusion.
Only limited studies tried to verify their imaging findings with other techniques, such as surgical exploration, which limits the potential of assessing the usefulness of nuclear imaging for predicting therapy management. In the case of Sumer et al. (2013), the surgical exploration was not done systematically and only in a small number of patients. Moreover, the reference region that was chosen to assess whether the tracer uptake threshold was significantly elevated in their study was chosen arbitrarily for some patients. This was the case in patients where the usual reference region had indications of degeneration (Sumer et al. 2013). As a result, this could have led to either over- or underestimation of the number of pathological areas present, and the question whether PSPS-T2 presentation was directly correlated to these findings remains unanswered.
Furthermore, there was substantial heterogeneity across studies with regards to clinical characteristics, imaging protocols, and methodological approaches. This heterogeneity significantly limits the interpretation of the pooled meta-analysis results. Although a random-effects model was used to account for variability between studies, the wide confidence intervals imply that the pooled estimates should be interpreted with caution. Nevertheless, this reflects the current state of evidence and emphasizes the need for more standardized protocols as well as larger, prospective studies to enhance comparability and reduce heterogeneity in the future.
Moreover, molecular imaging techniques in themselves possess certain limitations that should be noted, including the radiation exposure (Grant et al. 2008; Soliman et al. 2023). The current literature does not provide sufficient evidence that we can improve the healthcare for post-operative patients with persistent low back pain, including PSPS-T2 patients, by use of molecular imaging. Therefore, the radiation exposure involved with the standard application of molecular imaging investigations in this patient category seems unjustified.
Conclusion
This systematic review shows the potential that nuclear imaging techniques holds for patients with persistent postoperative low back pain, including spinal pain syndrome type 2. These imaging techniques are suggested valuable in providing insights into the underlying pathologies and mechanism driving persistent pain and in identifying patients who are likely to benefit from surgical reintervention. By detecting specific pain generators, nuclear imaging could serve as valuable phenotyping tool for stratifying patients within this population. However, there are still debates about the place of different radiotracers in the diagnostic and companion imaging setting. Future research can contribute to a deeper understanding of the potential of nuclear imaging, specifically in its role to find phenotypical biomarkers for the optimization of PSPS-T2 patient management and treatment.
Acknowledgements
Not applicable.
Abbreviations
- [18F]NaF
Sodium fluoride labeled with fluorine-18
- 99mTc
Technetium-99 m (radioisotope)
- CT
Computed tomography
- C. acnes
Cutibacterium acnes
- DPD
3,3-Diphosphono-1, 2-propanodicarboxylic acid (diphosphonate)
- FBSS
Failed back surgery syndrome
- FDG-PET
Fluorodeoxyglucose positron emission tomography
- HDP
Hydroxymethylene diphosphonate
- MDP
Methylene diphosphonate
- MRI
Magnetic resonance imaging
- PET-CT
Positron emission tomography-computed tomography
- PSPS-T2
Persistent spinal pain syndrome type 2
- SPECT-CT
Single-photon emission computed tomography-computed tomography
- SUV
Standardized uptake value
- SUVR
Standardized uptake value ratio
Author contributions
L.B. and D.H. conducted the electronic database search, assessed the retrieved literature, and evaluated the quality of the included studies. R.W. contributed to the search strategy development and aided in the retrieval of relevant literature. L.B. was responsible for writing the initial and subsequent drafts with support by the other authors. D.H. conducted the meta-analysis. All authors contributed to writing and editing the manuscript, discussed the results and commented on the manuscript.
Funding
Not applicable.
Data availability
Data used during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Acosta FL, Shapovalov VS, Lobo BM, Liker MA (2021) Radiographic evaluation of low back pain after lumbar fusion using Technetium-99 single-photon emission computed tomography: impact on clinical decision-making and outcome. Interdisciplinary Neurosurg 24:101089 [Google Scholar]
- Adams C, Banks KP (2022) Bone scan. StatPearls. Treasure Island (FL): StatPearls publishing copyright © 2022. StatPearls Publishing LLC.
- Al-Riyami K, Vöö S, Gnanasegaran G, Pressney I, Meir A, Casey A et al (2019) The role of bone SPECT/CT in patients with persistent or recurrent lumbar pain following lumbar spine stabilization surgery. Eur J Nucl Med Mol Imaging 46(4):989–998 [DOI] [PubMed] [Google Scholar]
- Berquist TH (2006) Imaging of the postoperative spine. Radiol Clin North Am 44(3):407–418 [DOI] [PubMed] [Google Scholar]
- Brans B, Weijers R, Halders S, Wierts R, Peters M, Punt I et al (2012) Assessment of bone graft incorporation by 18 F-fluoride positron-emission tomography/computed tomography in patients with persisting symptoms after posterior lumbar interbody fusion. EJNMMI Res 2(1):42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christelis N, Simpson B, Russo M, Stanton-Hicks M, Barolat G, Thomson S et al (2021) Persistent spinal pain syndrome: A proposal for failed back surgery syndrome and ICD-11. Pain Med 22(4):807–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy C, Quinn A, Wilson F (2017) The aetiologies of failed back surgery syndrome: A systematic review. J Back Musculoskelet Rehabil 30(3):395–402 [DOI] [PubMed] [Google Scholar]
- Damgaard M, Nimb L, Madsen JL (2010) The role of bone SPECT/CT in the evaluation of lumbar spinal fusion with metallic fixation devices. Clin Nucl Med 35(4):234–236 [DOI] [PubMed] [Google Scholar]
- El Yaagoubi Y, Loret JE, Lioret E, Thomas C, Simonneau A, Vinikoff L et al (2022) (18) F-NaF PET/CT in presumed aseptic pseudarthrosis after spinal fusion: correlation with findings at revision surgery and intraoperative cultures. World J Nucl Med 21(4):302–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Even-Sapir E, Martin RH, Mitchell MJ, Iles SE, Barnes DC, Clark AJ (1994) Assessment of painful late effects of lumbar spinal fusion with SPECT. J Nucl Med 35(3):416–422 [PubMed] [Google Scholar]
- Frühling J, Verbist A, Balikdjian D (1986) Which diphosphonate for routine bone scintigraphy (MDP, HDP or DPD)? Nucl Med Commun 7(6):415–425 [DOI] [PubMed] [Google Scholar]
- Gamie S, El-Maghraby T (2008) The role of PET/CT in evaluation of facet and disc abnormalities in patients with low back pain using (18)F-Fluoride. Nucl Med Rev Cent East Eur 11(1):17–21 [PubMed] [Google Scholar]
- Gates GF, McDonald RJ (1999) Bone SPECT of the back after lumbar surgery. Clin Nucl Med 24(6):395–403 [DOI] [PubMed] [Google Scholar]
- Grant FD, Fahey FH, Packard AB, Davis RT, Alavi A, Treves ST (2008) Skeletal PET with 18F-fluoride: applying new technology to an old tracer. J Nucl Med 49(1):68–78 [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B-R, Peng B-R, Pan L-K, Chen C-Y (2019) Potential usefulness of single photon emission computed tomography/computed tomography in management of patients with failed back surgery syndrome. J Med Imaging Health Inf 9:1–7 [Google Scholar]
- Hudyana H, Maes A, Vandenberghe T, Fidlers L, Sathekge M, Nicolai D et al (2016) Accuracy of bone SPECT/CT for identifying hardware loosening in patients who underwent lumbar fusion with pedicle screws. Eur J Nucl Med Mol Imaging 43(2):349–354 [DOI] [PubMed] [Google Scholar]
- Inoue S, Kamiya M, Nishihara M, Arai YP, Ikemoto T, Ushida T (2017) Prevalence, characteristics, and burden of failed back surgery syndrome: the influence of various residual symptoms on patient satisfaction and quality of life as assessed by a nationwide internet survey in Japan. J Pain Res 10:811–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen JM, Rimoldi RL, Capen DA, Nelson RW, Nagelberg S, Thomas JC (1996) Jr. Assessment of pseudarthrosis in pedicle screw fusion: a prospective study comparing plain radiographs, flexion/extension radiographs, CT scanning, and bone scintigraphy with operative findings. J Spinal Disord 9(2):117–120 [PubMed] [Google Scholar]
- Lusins JO, Danielski EF, Goldsmith SJ (1989) Bone SPECT in patients with persistent back pain after lumbar spine surgery. J Nucl Med 30(4):490–496 [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park PSU, Raynor WY, Sun Y, Werner TJ, Rajapakse CS, Alavi A (2021) (18)F-Sodium fluoride PET as a diagnostic modality for metabolic, autoimmune, and osteogenic bone disorders: cellular mechanisms and clinical applications. Int J Mol Sci. 22(12) [DOI] [PMC free article] [PubMed]
- Peters M, Willems P, Weijers R, Wierts R, Jutten L, Urbach C et al (2015a) Pseudarthrosis after lumbar spinal fusion: the role of 18F-fluoride PET/CT. Eur J Nucl Med Mol Imaging 42(12):1891–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters MJ, Wierts R, Jutten EM, Halders SG, Willems PC, Brans B (2015b) Evaluation of a short dynamic 18F-fluoride PET/CT scanning method to assess bone metabolic activity in spinal orthopedics. Ann Nucl Med 29(9):799–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouldar D, Bakshian S, Matthews R, Rao V, Manzano M, Dardashti S (2017) Utility of 18F sodium fluoride PET/CT imaging in the evaluation of postoperative pain following surgical spine fusion. Musculoskelet Surg 101(2):159–166 [DOI] [PubMed] [Google Scholar]
- Quon A, Dodd R, Iagaru A, de Abreu MR, Hennemann S, Alves Neto JM et al (2012) Initial investigation of ¹⁸F-NaF PET/CT for identification of vertebral sites amenable to surgical revision after spinal fusion surgery. Eur J Nucl Med Mol Imaging 39(11):1737–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rager O, Schaller K, Payer M, Tchernin D, Ratib O, Tessitore E (2012) SPECT/CT in differentiation of pseudarthrosis from other causes of back pain in lumbar spinal fusion: report on 10 consecutive cases. Clin Nucl Med 37(4):339–343 [DOI] [PubMed] [Google Scholar]
- Ryan PJ, Evans PA, Gibson T, Fogelman I (1992) Chronic low back pain: comparison of bone SPECT with radiography and CT. Radiology 182(3):849–854 [DOI] [PubMed] [Google Scholar]
- Segall G, Delbeke D, Stabin MG, Even-Sapir E, Fair J, Sajdak R et al (2010) SNM practice guideline for sodium 18F-fluoride PET/CT bone scans 1.0. J Nucl Med 51(11):1813–1820 [DOI] [PubMed] [Google Scholar]
- Seifen T, Rodrigues M, Rettenbacher L, Piotrowski W, Holzmannhofer J, Mc Coy M et al (2015) The value of (18)F-fluoride PET/CT in the assessment of screw loosening in patients after intervertebral fusion stabilization. Eur J Nucl Med Mol Imaging 42(2):272–277 [DOI] [PubMed] [Google Scholar]
- Soliman N, Kersebaum D, Lawn T, Sachau J, Sendel M, Vollert J (2023) Improving neuropathic pain treatment - by rigorous stratification from bench to bedside. J Neurochem [DOI] [PubMed]
- Sumer J, Schmidt D, Ritt P, Lell M, Forst R, Kuwert T et al (2013) SPECT/CT in patients with lower back pain after lumbar fusion surgery. Nucl Med Commun 34(10):964–970 [DOI] [PubMed] [Google Scholar]
- Sweetwood K, Junn JC, Qiao Y, Berven S, Shah V, Pampaloni MH et al (2023) Beyond anatomy: the role of molecular imaging in the evaluation of low back pain. Curr Radiol Rep 11(10):142–152 [Google Scholar]
- Thélu-Vanysacker M, Frédéric P, Charles-Edouard T, Alban B, Nicolas B, Tanguy B (2018) SPECT/CT in postoperative shoulder pain. Semin Nucl Med 48(5):469–482 [DOI] [PubMed] [Google Scholar]
- Thomson S, Jacques L (2009) Demographic characteristics of patients with severe neuropathic pain secondary to failed back surgery syndrome. Pain Pract 9(3):206–215 [DOI] [PubMed] [Google Scholar]
- Vaidyanathan S, Patel CN, Scarsbrook AF, Chowdhury FU (2015) FDG PET/CT in infection and inflammation–current and emerging clinical applications. Clin Radiol 70(7):787–800 [DOI] [PubMed] [Google Scholar]
- Van Acker F, Nuyts J, Maes A, Vanquickenborne B, Stuyck J, Bellemans J et al (2001) FDG-PET, 99mtc-HMPAO white blood cell SPET and bone scintigraphy in the evaluation of painful total knee arthroplasties. Eur J Nucl Med 28(10):1496–1504 [DOI] [PubMed] [Google Scholar]
- Weir S, Samnaliev M, Kuo T-C, Ni Choitir C, Tierney TS, Cumming D et al (2017) The incidence and healthcare costs of persistent postoperative pain following lumbar spine surgery in the UK: a cohort study using the clinical practice research datalink (CPRD) and hospital episode statistics (HES). BMJ Open 7(9):e017585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng Y-S, Tang C-T, Chang W-C, Huang G-S, Chiu C-H, Chiang S-W et al (2025) Added value of 18Fluorine-fluorodeoxyglucose (18F-FDG) PET/MRI for evaluation of failed back surgery syndrome: comparison with non-contrast MRI. Japanese J Radiol 43(3):509–519 [DOI] [PubMed] [Google Scholar]
- Witkam RL, Buckens CF, van Goethem JWM, Vissers KCP, Henssen D (2022) The current role and future directions of imaging in failed back surgery syndrome patients: an educational review. Insights Imaging 13(1):117 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used during the current study are available from the corresponding author on reasonable request.