Abstract
Objective:
Recent advances in Rosai-Dorfman-Destombes disease (RDD), notably molecular testing, targeted therapy, and PET-CT imaging, hold promise for better recognition and improved outcomes. This study presents patients diagnosed and treated in a “real world” setting, where navigating limited resources must be considered.
Methods:
This retrospective single-center review includes 15 adult patients diagnosed with RDD at Vancouver General Hospital between November 2015 and October 2023.
Results:
The cohort comprised five males and ten females with a median age 53 years (range 19–80 years). All 15 patients had extra-nodal disease; 11 patients exclusively had extra-nodal disease, and four patients also had lymph node involvement. Seven patients had tissue next-generation sequencing, identifying MAP2K1 mutations in four cases and a KRAS p.K117N mutation in one case that was treated with targeted therapy using trametinib. PET-CT was used for disease staging in four cases. Six patients with refractory disease tolerated lenalidomide and dexamethasone without significant toxicity; three patients achieved complete response, and three had partial response.
Conclusion:
This study highlights RDD’s diverse extra-nodal manifestations. Lenalidomide combined with dexamethasone is an effective and well-tolerated treatment option for select patients, especially those with refractory disease. Broad utilization of NGS and PET-CT can positively influence management decisions.
Keywords: Rosai-Dorfman disease, histiocytosis, cereblon, lenalidomide, next-generation sequencing, KRAS
INTRODUCTION
Rosai-Dorfman-Destombes disease (RDD) is a rare histiocyte disorder in the “R” group of the revised 2016 histiocyte classification [1]. It is characterized by large histiocytes exhibiting pale cytoplasm and frequent emperipolesis of lymphocytes and plasma cells, most reliably observed in nodal tissue [2–4]. Destombes initially described the disease in four African children in 1965, and subsequently Rosai and Dorfman published four patient cases with massive cervical lymphadenopathy and sinus histiocytosis in 1969 [2, 3]. The estimated prevalence is 1:200,000, with a median age of diagnosis at 20 years old, given the higher incidence among children and adolescents [1, 3].
RDD can pose a diagnostic challenge both in the clinical and laboratory setting. Although the classical presentation entails bilateral cervical lymphadenopathy, RDD can manifest heterogeneously in various extra-nodal sites including the skin, bone, kidneys, pancreas, central nervous system, and other organs [5]. Diagnosing RDD is complicated by the challenges of identifying characteristic histiocytes. RDD histiocytes stain CD1a negative and CD68, S100, and CD163 positive, but they are often sparse and difficult to locate amidst a nonspecific inflammatory background, especially when RDD is not initially considered in the differential diagnosis [5].
Over the past decade, several significant developments have profoundly impacted the diagnosis and management of RDD. The emergence of molecular testing represents the first significant advancement. RDD has historically been considered non-neoplastic with proposed autoimmune or infectious etiologies; however, recent molecular data have confirmed its clonal nature. Specifically, next-generation sequencing (NGS) can identify clonal mutations, typically in the mitogen-activated extracellular signal-regulated kinase (MAPK-ERK) pathway in about 40% of RDD patients [6–9]. Patients with confirmed MAPK-ERK mutations exhibit high response rates to targeted therapies, and even those lacking a known mutation may still respond [6, 10]. With the identification of mutations in RDD using NGS, there is potential to employ targeted therapy in patients to improve outcomes, especially in cases refractory to conventional therapies.
The second development is the increased utilization of positron emission tomography-computed tomography (PET-CT), which can improve disease staging in RDD. Notably, PET-CT can play a crucial role in revealing subclinical FDG-avid bone disease of RDD which can often be missed on plain computed tomography (CT) imaging [11, 12]. PET-CT imaging can also be useful in monitoring disease response over time, as it allows for tracking not only size changes of RDD lesions but also FDG activity, providing a comprehensive assessment of disease activity and treatment effectiveness.
Another notable advancement is the enhanced understanding of the spectrum of IgG4-related disease (IgG4-RD), which shares clinical and pathological overlaps with RDD. Both conditions can present with polyclonal hypergammaglobinemia (PHGG), lymphadenopathy, pachymeningitis, and increased IgG4-positive plasma cells with fibrosis in affected tissues [13, 14]. At our institution, this improved understanding has led to the development and application of a rational approach to managing patients with PHGG, enhancing our ability to provide comprehensive care and distinguish between overlapping disease entities [15, 16].
Most of the current research on RDD stems from specialized centers that have routinely utilized advanced diagnostic and therapeutic methods. Current guidelines on the diagnosis and treatment of RDD assume universal availability of novel interventions such as NGS, biologically targeted therapies, and PET-CT imaging, and recommend the liberal use of these modalities in RDD management [5, 17]. Vancouver General Hospital is a quaternary care hospital operating within a publicly funded healthcare system, where the adoption of novel interventions may progress more gradually compared to larger American centers due to resource limitations. Considering the rapidly evolving landscape of RDD, this study offers unique insights from a cohort of patients diagnosed and treated at a center where advanced interventions have only recently been more commonly utilized. While expertise in these novel interventions is present, their application at our center is selective, reflecting the real-world challenges and nuances in diagnosing and managing RDD. Our experience underscores the importance of judiciously integrating advanced interventions with established approaches, adapting to the unique context and resources of our healthcare setting.
MATERIALS & METHODS
Ethics approval was obtained from the University of British Columbia Clinical Research Ethics Board (H23–01853). This is a retrospective study that includes 15 adult patients (age >17 years) with a confirmed histopathological diagnosis of RDD between November 1, 2015 and October 31, 2023 at Vancouver General Hospital, a tertiary referral center in British Columbia, Canada. Demographic, clinical, histopathological, radiographic, and outcome data were collected from medical records. The data cut-off date was October 31, 2023. Definitive histopathological diagnosis by tissue biopsy were completed and reviewed by pathologists from the British Columbia Cancer Agency (BCCA). The IgG4/IgG ratio was determined by methods described by Deshpande, V. et al [18]. Follow-up duration was calculated from the date of disease diagnosis to the date of the last follow-up by their primary RDD clinician.
NGS was performed on DNA/RNA obtained from formalin-fixed paraffin-embedded tissue samples within an accredited clinical molecular testing laboratory using the Illumina Ampliseq Focus panel paired with an Illumina Miniseq [19]. Target space included but was not limited to the following: BRAF, RAF1, MAP2K1, MAP2K2, NRAS, KRAS, PIK3CA, and IDH2 [17, 20]. Variants were classified using AMP/ASCO/CAP guidelines, from Tier I (indicating strong clinical significance) to Tier IV (suggesting likely benign variants) [21]. Tier IV variants were not reported.
Treatment response was evaluated by clinical findings and/or radiographic imaging using PET-CT, CT, or magnetic resonance imaging (MRI). Treatment response was classified based on the patient’s best response, categorized as stable disease, progressive disease, partial response to treatment, or complete response to treatment. Complete response (CR) was defined by complete clinical or radiographic evidence demonstrating disease resolution. Partial response (PR) was defined by clinical or radiographic evidence showing reduction in lesion size or decreased enhancement.
RESULTS
Patient characteristics, presenting features, and organ involvement
This study comprises 15 patients with histologically confirmed RDD disease. The median age at diagnosis was 53 years (range 19–80 years), with five male patients and ten female patients. Other baseline features are characterized in Table 1. All 15 patients had extra-nodal disease (100%) and four of these patients also had nodal disease (27%). The presenting complaints are summarized in Table 2. Eight patients presented with dermatological findings (53%), including brown-pigmented spots, pain/tenderness, and bruising. Among them, four patients had localized skin involvement while the remaining four had diffuse skin lesions. Four patients had asymptomatic nodal involvement and no systemic symptoms. Three patients presented with bone involvement, including disease affecting the right tibia, parietal bone, and cerebellopontine angle. One patient (case 2) presented with severe thrombocytopenia responsive to corticosteroids and intravenous immunoglobulin, so it was presumed to be immune thrombocytopenia related to RDD. Another patient (case 3) had two periorbital masses causing right eye ptosis, increased tearing, irritation, swelling, and decreased vision (Figure 1a). For RDD in the abdomen, two patients developed pancreatic lesions that caused abdominal pain, jaundice, and pruritis. One of these patients (case 7) had CT findings of a bulky pancreatic head without evidence of pancreatitis (Figure 1b). One patient (case 13) with complaints of abdominal swelling and shortness of breath had RDD involving the omentum and bilateral nephromegaly (Figure 1c). This patient underwent an abdominal paracentesis that drained 6 litres of fluid. Their initial biopsy showed features consistent with IgG4-related sclerosing mesenteritis, but subsequent development of pleural effusions led to a pleural biopsy that confirmed RDD. On retrospective review, both the mesentery and pleural biopsy were found to be consistent with RDD. Additionally, one patient (case 12) presented with paroxysmal atrial flutter and was found to have an isolated, enlarging atrial mass on CT and PET-CT, with biopsy confirming RDD.
Table 1.
Summary of patient and disease features.
| Total patients | 15 |
| Median age at diagnosis (years) | 53 (range 19–80) |
| Gender (female:male) | 10:5 |
| Ethnicity South Asian White Indigenous Middle Eastern |
11 (73%) 2 (13%) 1 (7%) 1 (7%) |
| Median time from symptom onset to diagnosis (months) | 19.0 (range 3.0–60.0) |
| Median number of biopsies for diagnosis | 2 (range 1–3) |
| Median duration of follow-up (months) | 31.7 (range 0.4–88.9) |
| Deaths | 0 |
| Organ involvement Skin Lymph node Bone Head and neck Gastrointestinal Orbits Heart |
8 (53%) 4 (27%) 3 (20%) 3 (20%) 2 (13%) 1 (7%) 1 (7%) |
Table 2.
Individual patient characteristics, presenting features of RDD, and organ involvement.
| Case | Age at Diagnosis | Gender | Ethnicity | Clinical Problem at Presentation | Organs Involved | Nodal | Extra-nodal |
|---|---|---|---|---|---|---|---|
| 1 | 30 | M | Indigenous | Skin lesion on face | Skin (right cheek) | No | Yes |
| 2 | 80 | F | White | Immune thrombocytopenia | Lymph nodes (axillary, celiac, periesophageal), pancreas | Yes | Yes |
| 3 | 55 | F | South Asian | Diffuse skin lesions | Skin (face, trunk, palms, soles) | No | Yes |
| 4 | 80 | M | White | Unilateral proptosis, decreased vision | Orbit (right) | No | Yes |
| 5 | 40 | F | South Asian | Skin lesions on leg | Skin (left leg), bone, lymph nodes (cervical) | Yes | Yes |
| 6 | 61 | F | White | Skin lesions on leg | Skin (right and left legs, umbilicus) | No | Yes |
| 7 | 53 | M | South Asian | Abdominal pain, jaundice, pruritis | Lymph nodes (diffuse), pancreas, kidneys | Yes | Yes |
| 8 | 39 | F | South Asian | Progressive unilateral hearing loss | Central nervous system (cerebellopontine angle), skull | No | Yes |
| 9 | 19 | F | South Asian | Discomfort over parietal bone | Skull | No | Yes |
| 10 | 41 | F | South Asian | Bruising over arm | Skin (right arm) | No | Yes |
| 11 | 74 | M | White | Skin lesions on chest | Skin (chest) | No | Yes |
| 12 | 63 | F | South Asian | Paroxysmal atrial flutter | Heart (Left atrium) | No | Yes |
| 13 | 66 | M | Middle Eastern | Shortness of breath and abdominal distention | Omentum, pleura | No | Yes |
| 14 | 42 | F | South Asian | Skin lesion on leg | Skin (right leg, right breast, left flank) | No | Yes |
| 15 | 48 | F | South Asian | Diffuse skin lesions | Skin, lymph nodes, parotid glands, sinonasal system | Yes | Yes |
Figure 1: CT findings in Rosai-Dorfman-Destombes disease.
1a) Right orbital masses. 1b) Bulky pancreatic head without evidence of pancreatitis (coronal view of pancreatic head). 1c) Diffuse heterogeneous renal parenchymal enhancement with loss of normal corticomedullary differentiation; no focal lesion demonstrated.
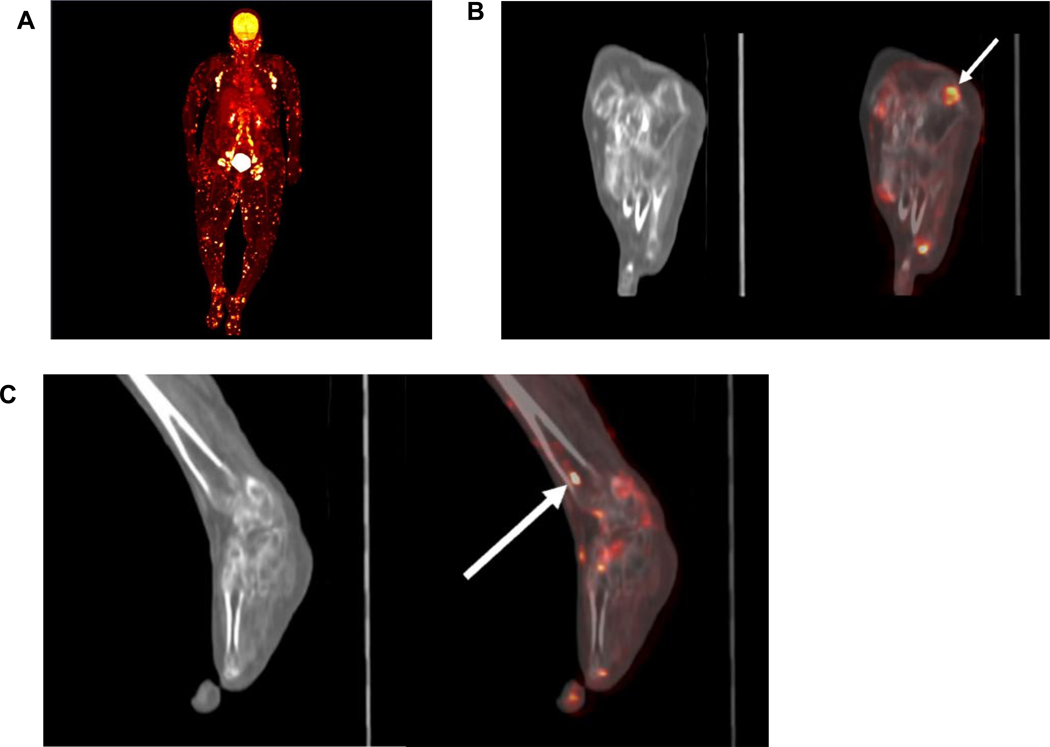
In this study, four patients underwent PET-CT imaging, and in all four cases PET-CT provided additional information for staging or disease relapse (Supplementary Table 1). Whole-body PET-CT can detect extensive multi-organ FDG-avid disease including nodal, cutaneous, subcutaneous, and parotid (Figure 2a). In addition, PET-CT can reveal subclinical RDD that appear subtle on plain CT, particularly in glands, skin, and bone (Figure 2b, 2c).
Figure 2: PET-CT images of Case 15.
2a) Maximum Intensity Pixel (MIP) view from baseline PET-CT demonstrating extensive multi-organ FDG-avid disease including nodal, cutaneous, subcutaneous, and parotid. 2b) Subtle lytic lesion in calcaneus on plain CT (left) better seen on fused PET-CT as an FDG-avid lesion (right). 2c) Tibial involvement not apparent on plain CT (left) but readily seen as an FDG-avid lesion on fused PET-CT (right).
Histopathologic features and laboratory findings
The mean number of biopsies to achieve diagnosis was 2 (range 1–3), with a mean interval of 19.0 months (range 3.0–60.0 months) between symptom onset and diagnosis (Table 1). Histopathology findings from all 15 patients were consistent with the classic morphologic and immunophenotypic features of RDD including CD68+, S100+, and CD1a- histiocytes with prominent nucleoli and at least focal emperipolesis (Table 3). The cases with nodal pathology showed classic expansion of the sinuses with easily identified histiocytes containing ample eosinophilic cytoplasm and engulfed inflammatory cells (Figure 3a). Extra-nodal cases were markedly fibrotic, with eosinophilic histiocytes distributed geographically mixed with areas of chronic inflammation including plasma cells (Figure 3b). Notably, many extra-nodal cases showed only focal emperipolesis and increased IgG4 positive plasma cells, with two cases reaching an IgG4/IgG ratio exceeding 40%, the threshold noted for IgG4-related disease (Supplementary Figure 1). Select laboratory findings captured at the time of RDD diagnosis are summarized in Table 3. Fourteen patients had an elevated C-reactive protein (CRP; median 16.7 mg/L, range 0.9–62.9). Two patients had positive antinuclear antibodies (ANA). Six patients had PHGG on serum protein electrophoresis with elevated IgG levels, and three of them had elevated IgG4 levels contributing to their PHGG.
Table 3.
Immunostaining of tissue biopsies (left) for CD68, S100, CD1a and serologic values (right) of eosinophils, IgG, IgG4, CRP, and ANA at time of RDD diagnosis.
| Immunostaining of tissue biopsies | Serologic findings at time of RDD Diagnosis | Next-generation sequencing | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Case | CD68 | S100 | CD1a | PHGG | IgG (6.7–15.2 g/L) |
IgG4 (0.052–1.25 g/L) |
CRP (<3.1 mg/L) |
Autoantibodies | |
| 1 | ND | + | - | No | 14.1 | 0.3 | 41.9 | Negative | ND |
| 2 | + | + | - | Yes | 24.0 | 5.7 | 23.0 | Negative | ND |
| 3 | + | + | - | Yes | 25.2 | 2.36 | 51.8 | ANA positive | Tier I: none Tier II: none Tier III: MAP2K1:c.361T>A, p.C121S (VAF 5.8%) |
| 4 | + | + | - | Yes | 14.4 | 1.12 | 6.3 | Negative | Tier I: none Tier II: none Tier III: none |
| 5 | + | + | - | No | 12.5 | 0.63 | 16.7 | Negative | ND |
| 6 | + | + | - | No | 7.9 | 0.066 | 4.3 | ND | Tier I: none Tier II: none Tier III: MAP2K1:c607G>A, p.E203K (VAF 3.5%) |
| 7 | + | + | - | Yes | 19.3 | 2.05 | 45.0 | ND | ND |
| 8 | + | + | - | No | 12.5 | 0.434 | 7.4 | Negative | Tier I: KRAS:c.351A>T, p.K117N (VAF 12%) Tier II: none Tier III: FUBP1:c1327A>G, p.I443V (VAF 51%); ERBB4:c2845G>A, p.V949I (VAF 49%) |
| 9 | + | + | - | No | 12.8 | 0.885 | 0.9 | Negative | Tier I: none Tier II: none Tier III: MAP2K1:c364A>G, p.N122D (VAF 2.0%); MAP2K1:c362G>C, p.C121S (VAF 3.1%) |
| 10 | + | + | - | Yes | 16.4 | 0.354 | 3.7 | RF positive, ANA neg | ND |
| 11 | + | + | - | No | 16.7 | 1.905 | 8.0 | Negative | ND |
| 12 | + | + | - | No | 6.2 | 0.322 | 62.9 | Negative | Tier I: none Tier II: none Tier III: none |
| 13 | + | + | - | No | 11.7 | 2.1 | 37.8 | Negative | ND |
| 14 | + | + | - | No | 11.5 | ND | 8.6 | ND | ND |
| 15 | + | + | - | Yes | 23.0 | 1.562 | 75.0 | ANA positive | Tier I: none Tier II: none Tier III: MAP2K1:c159T>A, p.F53L (VAF 2.9%) |
ANA: antinuclear antibody; ND: not done; PHGG: polyclonal hypergammaglobulinemia on serum protein electrophoresis; VAF: variant allele frequency
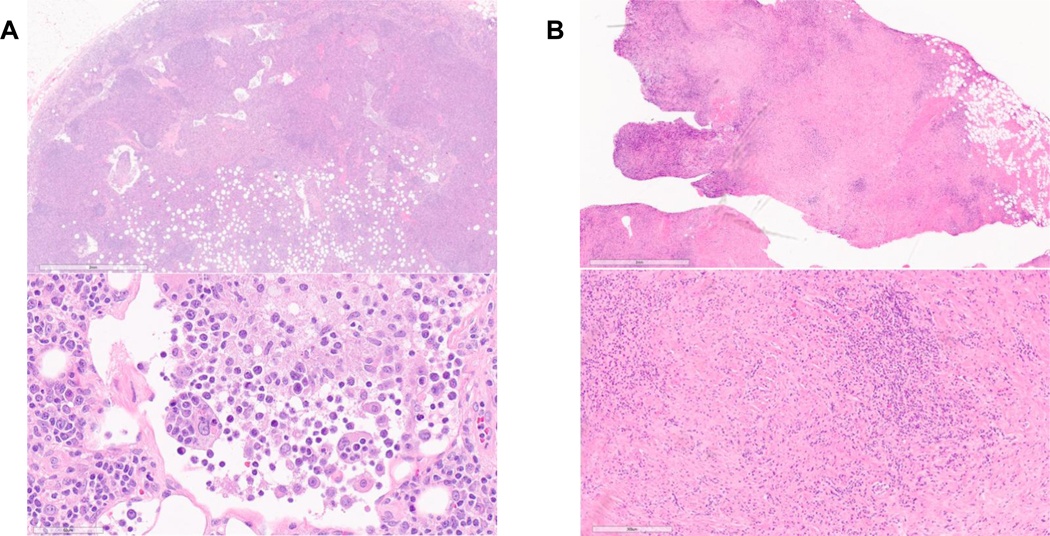
Figure 3:
H&E-stained sections of 3a) lymph node and 3b) cardiac mass involved by RDD at 2X (top) and 40X magnification (bottom). The lymph node shows classic expansion of the sinuses with easily identified histiocytes containing engulfed inflammatory cells (emperipolesis). The cardiac mass shows dense fibrosis, including areas suggestive of storiform fibrosis, and chronic inflammatory cells including plasma cells.
NGS was performed on the tissue samples of seven patients (Table 3). These patients had NGS completed because their disease was refractory to first line therapy. One patient (case 8) had a Tier I mutation of KRAS p.K117N and subsequently received targeted therapy with the kinase inhibitor trametinib. The same patient also had next-generation sequencing performed on their peripheral blood, however no mutations were detected in the MAP2K, KRAS, or PIK3C pathways. Among the six remaining patients who had sequenced tumours, four patients had MAP2K1 mutations identified. However, no Tier I or Tier II mutations were identified, and these patients did not receive any targeted therapies.
Treatments and outcomes
Patients were followed up for a mean duration of 31.7 months (range 0.4–88.9 months). Treatments used in each case and the responses are summarized in Supplementary Table 2. The most common first-line treatment was corticosteroids, either administered locally via intralesional injections (one patient) or systemically (eight patients). All nine patients who received steroids as the first-line treatment either had no response (six patients) or partial response (three patients). The systemic steroid agent that was used was prednisone at the dose of 1 mg/kg. While steroid therapy was generally well-tolerated, one patient developed steroid-induced psychosis and required immediate tapering of therapy. Surgical resection was performed as first- or second-line treatment in three patients, two of whom had skull involvement. Two of these patients achieved complete response while one patient developed localized disease relapse post-surgery. Radiation therapy was used in three patients with localized disease, and all three patients had partial response that required further management with alternative modalities. Other commonly used agents include rituximab, sirolimus, and cladribine. Two patients without any clinical or radiographic signs of H syndrome had partial response to tocilizumab, an interleukin-6 receptor inhibitor, as a second-line agent. One patient with a KRAS p.K117N mutation identified in tissue NGS showed partial response to trametinib, a mitogen-activated extracellular signal regulated kinase (MEK) inhibitor. The combination of lenalidomide and dexamethasone was used in six patients who showed either partial or no response to previous treatments. Among these patients, three achieved complete response while the remaining three demonstrated partial response.
DISCUSSION
RDD is a challenging disease to recognize and treat due to its heterogenous clinical presentation. Presenting complaints in our study varied widely, ranging from asymptomatic skin nodules to thrombocytopenia. While RDD is generally considered a self-limited disorder with an indolent clinical course, our series highlighted patients experiencing significant symptom burdens, such as an intracardiac mass causing arrhythmia, potential superior vena cava obstruction, or a periorbital tumor leading to vision impairment. Furthermore, while RDD is traditionally known to present with massive cervical lymphadenopathy [3], less than one-third of cases in our series presented with nodal involvement.
From a clinical standpoint, clues that may raise suspicion for RDD, especially the extra-nodal presentation, include cutaneous involvement with elevated IgG and IgG4 levels in the serum. All patients in our cohort had extra-nodal involvement, with eight patients demonstrating cutaneous or subcutaneous manifestations of RDD, which is the most prevalent among extra-nodal sites [5, 12, 22]. Additionally, CRP, even as a nonspecific inflammatory marker, can serve as a useful clue to raise suspicion for a systemic inflammatory process, even when a patient presents with an isolated, localized lesion. In our study, 14 out of 15 patients had an elevated CRP (>10 mg/L) at the time of RDD diagnosis, despite many having isolated cutaneous lesions affecting a localized region of the body.
In our study, six RDD patients exhibited PHGG, including elevated levels of IgG4. The overlapping clinical and pathological features of RDD with IgG4-RD can complicate the definitive diagnosis of RDD, especially by the histopathologic findings in the extra-nodal cases. Extra-nodal disease is known to be particularly challenging to diagnose, as the fibrosis and inflammation may obscure the characteristic histiocytes, which are more common in lymph nodes than extra-nodal tissue [5]. In this study, most non-nodal sites showed increased fibrosis and plasma cells. Two cases exhibited an IgG4/IgG ratio of 40% or more, with only rare histiocytes demonstrating emperipolesis, bringing IgG4-RD into the differential diagnosis. Clinicopathologic correlation and access to subspecialty pathology services were crucial in confirming the diagnosis of RDD in these challenging cases.
Most RDD patients do not harbor identifiable pathogenic mutations; however the detection of a mutation of significance can profoundly impact treatment strategies [17]. At our centre, seven patients were selected for NGS either because they had refractory disease following trials of multiple therapies or because they were diagnosed with RDD later when NGS became more accessible. The NGS panel included genes from the MAPK-ERK pathway, in which mutated genes were identified in patients with RDD [7, 8, 23, 24]. There is growing research showing that histiocytic neoplasms have notable dependence on the MAPK-ERK signaling pathway [6]. Among the six patients who had NGS, missense mutations in the MAP2K gene were identified in four cases, including a tier III MAP2K:c159T>A mutation that was previously reported in RDD [25]. Although many of these mutations were classified as Tier III variants, in silico predictors suggest their effect may be damaging and they have been classified as likely pathogenic or pathogenic in ClinVar for other disease states (e.g., lung adenocarcinoma). This suggests these variants may be damaging and contributing to the pathogenesis of RDD in these patients; one MAP2K1 variant (c.362G>C, p.C121S) was seen in two patients and is predicted to be damaging. As more data on the mutational landscape in RDD emerges, many of these mutations may be upgraded in the future. In addition, immunohistochemical stains such as OCT2 and cyclin D1 are becoming increasingly used for the characterization of RDD and may reflect upregulation of the phospho-ERK pathway [26, 27], however these stains are not routinely used at our center.
In one patient (case 8), a KRAS p.K117N mutation of strong clinical significance (Tier I) previously reported in other RDD cases was identified [12, 28]. This patient received trametinib, an oral small molecule MEK 1/2 inhibitor that is typically used in unresectable or metastatic melanoma [29]. Despite refractory disease to previous treatments, including surgical resection, systemic steroids, rituximab, and cladribine, this patient demonstrated partial response with trametinib, as evidenced by MRI findings indicating size reduction of their RDD lesions. This case underscores the efficacy of targeted therapy, particularly in cases of refractory disease. Future studies are warranted to fully delineate the genomic and transcriptomic landscape of RDD.
Four patients in this study underwent PET-CT imaging, which is increasing significant for RDD disease staging, particularly for detecting bone involvement [11]. At our centre, four patients were selected for PET-CT either due to concerns about subclinical bone lesions or because they were diagnosed later when RDD became accepted as an indication for PET-CT imaging. In RDD, bone involvement in the appendicular skeleton is often subclinical, whereas involvement of spine or pelvis often manifests with pain and discomfort [12]. Radiographically, RDD lesions may appear subtle on plain CT but demonstrate PET avidity due to their higher metabolic activity, making them more readily discernible on PET-CT imaging. Additionally, some RDD patients may present with lytic or mixed lytic/sclerotic lesions, in contrast to the symmetric osteosclerosis of the long bones, typically seen in Erdheim-Chester Disease, which usually spares the axial skeleton, hands, and feet [25].
For management of RDD, surgical resection is typically considered the treatment of choice for localized disease [5, 30, 31]; however, local recurrences are not uncommon [30, 32]. In our cohort, two patients initially underwent surgical resection, but both cases developed disease recurrence. For initial systemic therapy, corticosteroids were used for nine cases. However, eight patients had only partial response or experienced disease recurrence, requiring subsequent treatment with agents such as rituximab or methotrexate [33–36]. Despite the use of rituximab and methotrexate, which are both typically used in refractory disease, six of these patients developed further refractory disease and required subsequent trial of other treatment agents. Six patients with refractory RDD received a combination of lenalidomide and dexamethasone, and all patients had at least a partial response with no toxicity, and three of them achieved a complete response and are currently in disease remission. Notably, five of the six patients who received this combination of lenalidomide and dexamethasone had cutaneous disease, which allowed their treatment response to be easily monitored through clinical examination. These findings suggest that lenalidomide in combination with dexamethasone could be a valuable therapeutic option for refractory RDD, particular in cases with cutaneous involvement.
CONCLUSION
In this case series, we delved into the multifaceted challenges of diagnosing and managing RDD, highlighting its clinical heterogeneity, overlapping features with IgG4-RD, and the importance of evolving diagnostic and therapeutic strategies including NGS and PET-CT imaging. Notably, our experience from a setting where advanced interventions are not readily accessible provides unique insights into the real-world challenges faced in diagnosing and managing RDD. NGS emerges as a valuable tool especially if a targetable gene mutation in the MAPK-ERK pathway is identified, while PET-CT can substantially improve the detection of subclinical disease, particularly bone lesions. As RDD continues to be a diagnostic and therapeutic challenge, collaborative efforts between clinicians, pathologists, and researchers are crucial for improving outcomes and advancing our understanding of this rare disease.
Supplementary Material
Acknowledgements:
Luke Y.C. Chen’s research is supported by a philanthropic gift from the Hsu & Taylor Family through the UBC & VGH Foundation.
Funding:
No funding was received for conducting this study or to assist with the preparation of this manuscript.
Footnotes
STATEMENT AND DECLARATIONS
Ethics statement: This study was approved by the University of British Columbia Clinical Research Ethics Board; individual consent to participate and consent was waived for this minimal risk retrospective study.
Competing interests not specific to this manuscript:
LYCC: Honoraria – Glaxo-Smith-Kline, Recordati Rare Diseases
RJS: Honoraria – AbbVie, Jazz Pharmaceuticals, Takeda, Astellas, Pfizer; Research Funding – Jazz Pharmaceuticals
Conflicts of interests: No conflicts of interests to declare.
Availability of data and materials:
Data are available upon request from the corresponding authors.
REFERENCES
- 1.Emile J-F, Abla O, Fraitag S, et al. (2016) Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood 127:2672–2681. 10.1182/blood-2016-01-690636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Destombes P (1965) [Adenitis with lipid excess, in children or young adults, seen in the Antilles and in Mali. (4 cases)]. Bull Soc Pathol Exot Filiales 58:1169–1175 [PubMed] [Google Scholar]
- 3.Rosai J, Dorfman RF (1969) Sinus histiocytosis with massive lymphadenopathy. A newly recognized benign clinicopathological entity. Arch Pathol 87:63–70 [PubMed] [Google Scholar]
- 4.Favara BE, Feller AC, Pauli M, et al. (1997) Contemporary classification of histiocytic disorders. The WHO Committee On Histiocytic/Reticulum Cell Proliferations. Reclassification Working Group of the Histiocyte Society. Med Pediatr Oncol 29:157–166. [DOI] [PubMed] [Google Scholar]
- 5.Abla O, Jacobsen E, Picarsic J, et al. (2018) Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood 131:2877–2890. 10.1182/blood-2018-03-839753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diamond EL, Durham BH, Ulaner GA, et al. (2019) Efficacy of MEK inhibition in patients with histiocytic neoplasms. Nature 567:521–524. 10.1038/s41586-019-1012-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garces S, Medeiros LJ, Patel KP, et al. (2017) Mutually exclusive recurrent KRAS and MAP2K1 mutations in Rosai–Dorfman disease. Mod Pathol 30:1367–1377. 10.1038/modpathol.2017.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobsen E, Shanmugam V, Jagannathan J (2017) Rosai–Dorfman Disease with Activating KRAS Mutation — Response to Cobimetinib. N Engl J Med 377:2398–2399. 10.1056/NEJMc1713676 [DOI] [PubMed] [Google Scholar]
- 9.Diamond EL, Durham BH, Haroche J, et al. (2016) Diverse and Targetable Kinase Alterations Drive Histiocytic Neoplasms. Cancer Discov 6:154–165. 10.1158/2159-8290.CD-15-0913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abeykoon JP, Rech KL, Young JR, et al. (2022) Outcomes After Treatment With Cobimetinib in Patients With Rosai-Dorfman Disease Based on KRAS and MEK Alteration Status. JAMA Oncol 8:1816–1820. 10.1001/jamaoncol.2022.4432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahajan S, Nakajima R, Yabe M, et al. (2020) Rosai-Dorfman Disease—Utility of 18F-FDG PET/CT for Initial Evaluation and Follow-up. Clin Nucl Med 45:e260–e266. 10.1097/RLU.0000000000003014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goyal G, Ravindran A, Young JR, et al. (2020) Clinicopathological features, treatment approaches, and outcomes in Rosai-Dorfman disease. Haematologica 105:348–357. 10.3324/haematol.2019.219626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen LYC, Mattman A, Seidman MA, Carruthers MN (2019) IgG4-related disease: what a hematologist needs to know. Haematologica 104:444–455. 10.3324/haematol.2018.205526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akhtar D, Owen DR, Murphy SF, et al. (2023) Rosai-Dorfman-Destombes disease of the pancreas: the great masquerader. The Lancet 401:1115. 10.1016/S0140-6736(22)02604-6 [DOI] [PubMed] [Google Scholar]
- 15.Zhao EJ, Cheng CV, Mattman A, Chen LYC (2021) Polyclonal hypergammaglobulinaemia: assessment, clinical interpretation, and management. Lancet Haematol 8:e365–e375. 10.1016/S2352-3026(21)00056-9 [DOI] [PubMed] [Google Scholar]
- 16.Zhao EJ, Carruthers MN, Li CH, et al. (2020) Conditions associated with polyclonal hypergammaglobulinemia in the IgG4-related disease era: a retrospective study from a hematology tertiary care center. Haematologica 105:e121–e123. 10.3324/haematol.2019.219725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Go RS, Jacobsen E, Baiocchi R, et al. (2021) Histiocytic Neoplasms, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw JNCCN 19:1277–1303. 10.6004/jnccn.2021.0053 [DOI] [PubMed] [Google Scholar]
- 18.Deshpande V, Zen Y, Chan JK, et al. (2012) Consensus statement on the pathology of IgG4-related disease. Mod Pathol 25:1181–1192. 10.1038/modpathol.2012.72 [DOI] [PubMed] [Google Scholar]
- 19.Focus Panel – Cancer Genetics and Genomics Laboratory. http://cancergeneticslab.ca/genes/focus-panel/. Accessed 27 Aug 2023
- 20.Ruan G, Abeykoon JP, Acosta Medina AA, et al. (2022) Mutational Profiling of Rosai-Dorfman Disease and Association with Phenotype: A Multi-Institutional Study. Blood 140:3995–3996. 10.1182/blood-2022-159714 [DOI] [Google Scholar]
- 21.Li MM, Datto M, Duncavage EJ, et al. (2017) Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn JMD 19:4–23. 10.1016/j.jmoldx.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goyal G, Young JR, Koster MJ, et al. (2019) The Mayo Clinic Histiocytosis Working Group Consensus Statement for the Diagnosis and Evaluation of Adult Patients With Histiocytic Neoplasms: Erdheim-Chester Disease, Langerhans Cell Histiocytosis, and Rosai-Dorfman Disease. Mayo Clin Proc 94:2054–2071. 10.1016/j.mayocp.2019.02.023 [DOI] [PubMed] [Google Scholar]
- 23.Shanmugam V, Margolskee E, Kluk M, et al. (2016) Rosai-Dorfman Disease Harboring an Activating KRAS K117N Missense Mutation. Head Neck Pathol 10:394–399. 10.1007/s12105-016-0709-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matter MS, Bihl M, Juskevicius D, Tzankov A (2017) Is Rosai-Dorfman disease a reactve process? Detection of a MAP2K1 L115V mutation in a case of Rosai-Dorfman disease. Virchows Arch Int J Pathol 471:545–547. 10.1007/s00428-017-2173-4 [DOI] [PubMed] [Google Scholar]
- 25.Razanamahery J, Diamond EL, Cohen-Aubart F, et al. (2020) Erdheim-Chester disease with concomitant Rosai-Dorfman like lesions: a distinct entity mainly driven by MAP2K1. Haematologica 105:e5–e8. 10.3324/haematol.2019.216937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravindran A, Goyal G, Go RS, et al. (2021) Rosai-Dorfman Disease Displays a Unique Monocyte-Macrophage Phenotype Characterized by Expression of OCT2. Am J Surg Pathol 45:35–44. 10.1097/PAS.0000000000001617 [DOI] [PubMed] [Google Scholar]
- 27.Garces S, Medeiros LJ, Marques-Piubelli ML, et al. (2022) Cyclin D1 expression in Rosai-Dorfman disease: a near-constant finding that is not invariably associated with mitogen-activated protein kinase/extracellular signal-regulated kinase pathway activation. Hum Pathol 121:36–45. 10.1016/j.humpath.2021.12.013 [DOI] [PubMed] [Google Scholar]
- 28.Goyal G, Lau D, Nagle AM, et al. (2019) Tumor mutational burden and other predictive immunotherapy markers in histiocytic neoplasms. Blood 133:1607–1610. 10.1182/blood-2018-12-893917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flaherty KT, Robert C, Hersey P, et al. (2012) Improved Survival with MEK Inhibition in BRAF-Mutated Melanoma. N Engl J Med 367:107–114. 10.1056/NEJMoa1203421 [DOI] [PubMed] [Google Scholar]
- 30.Al-Khateeb THH (2016) Cutaneous Rosai-Dorfman Disease of the Face: A Comprehensive Literature Review and Case Report. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg 74:528–540. 10.1016/j.joms.2015.09.017 [DOI] [PubMed] [Google Scholar]
- 31.Forest F, N’guyen AT, Fesselet J, et al. (2014) Meningeal Rosai-Dorfman disease mimicking meningioma. Ann Hematol 93:937–940. 10.1007/s00277-013-1994-8 [DOI] [PubMed] [Google Scholar]
- 32.Chen H-H, Zhou S-H, Wang S-Q, et al. (2012) Factors associated with recurrence and therapeutic strategies for sinonasal Rosai-Dorfman disease. Head Neck 34:1504–1513. 10.1002/hed.21832 [DOI] [PubMed] [Google Scholar]
- 33.Horneff G, Jürgens H, Hort W, et al. (1996) Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): response to methotrexate and mercaptopurine. Med Pediatr Oncol 27:187–192. [DOI] [PubMed] [Google Scholar]
- 34.Inoue S, Onwuzurike N (2005) Venorelbine and methotrexate for the treatment of Rosai-Dorfman disease. Pediatr Blood Cancer 45:84–85; author reply 86. 10.1002/pbc.20361 [DOI] [PubMed] [Google Scholar]
- 35.Jabali Y, Smrcka V, Pradna J (2005) Rosai-Dorfman disease: successful long-term results by combination chemotherapy with prednisone, 6-mercaptopurine, methotrexate, and vinblastine: a case report. Int J Surg Pathol 13:285–289. 10.1177/106689690501300311 [DOI] [PubMed] [Google Scholar]
- 36.Petschner F, Walker UA, Schmitt-Gräff A, et al. (2001) [“Catastrophic systemic lupus erythematosus” with Rosai-Dorfman sinus histiocytosis. Successful treatment with anti-CD20/rutuximab]. Dtsch Med Wochenschr 1946 126:998–1001. 10.1055/s-2001-17109 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request from the corresponding authors.