Abstract
Background
Efferocytosis, the process by which apoptotic cells are recognized and removed by phagocytes, plays a critical role in maintaining tissue homeostasis and modulating inflammatory responses. Over recent decades, an increasing number of studies have investigated the molecular mechanisms and clinical implications of efferocytosis. This bibliometric analysis aims to map the evolving trends, identify key contributors, and outline emerging research themes in this field.
Methods
A comprehensive search was conducted in Web of Science database, to collect literature related to efferocytosis from 2006 to 2024. The dataset was analyzed using several tools such as CiteSpace and VOSviewer. Analyses included evaluation of publication trends, citation networks, keyword co-occurrence, and co-cited references. Key metrics such as the most prolific authors, top contributing countries, and major research clusters were identified to understand the field’s evolution and interdisciplinary collaborations.
Results
The final dataset comprised 1549 scholarly works, consisting of 1166 original research articles and 383 review papers. The analysis revealed a steady increase in the number of publications concerning efferocytosis, particularly in the past decade. Geographically, China and the United States emerged as dominant contributors, representing over 64.4% of total publications. Among institutions, Harvard University demonstrated the highest research output in this field. Keyword analysis demonstrated the current research focus including molecular mechanisms and signaling regulation of efferocytosis, macrophage polarization and inflammatory modulation, pathological implications and therapeutic potential of efferocytosis in diseases. Inflammation, atherosclerosis, cardiovascular disease, myocardial infarction, and COPD are diseases that has received the most attention in this field. Several research topics including nanoparticle, neuroinflammation, fibrosis, immunometabolism, exosomes, apoptotic bodies, mesenchymal stem cells, aging, microglia, reactive oxygen species, CD47, lipid metabolism, immunotherapy, mitochondria, ferroptosis, may have great potential to be hot topics in the near future. Gene-focused investigations identified TNF, MERTK, IL10, LI6, and IL1b as the most extensively studied genetic elements in efferocytosis research.
Conclusions
This bibliometric study provides a comprehensive overview of the evolving research landscape in efferocytosis. These insights not only highlight the current milestones but also serve as a valuable guide for future research and policy-making aimed at harnessing efferocytosis for therapeutic innovations.
Keywords: Data mining, Research status, Research hotspots, Efferocytosis
Introduction
Programmed cell death is a fundamental biological process essential for maintaining homeostasis, regulating tissue development, and eliminating aberrant cells in multicellular organisms [1–2]. This process not only determines the fate of cellular self-destruction through intrinsic signaling mechanisms but also ensures the safe removal of deceased cells from tissues via specialized clearance mechanisms, thereby preventing the induction of local inflammation or autoimmune responses by cellular remnants. Among these mechanisms, efferocytosis, a critical step in the clearance of apoptotic cells, has been widely recognized as indispensable for maintaining tissue homeostasis [3–4]. During efferocytosis, professional phagocytes such as macrophages and dendritic cells, as well as non-professional phagocytes (e.g., epithelial cells and fibroblasts) in proximity to apoptotic cells, recognize and engulf these cells, thereby preventing the leakage of harmful intracellular substances and promoting the resolution of inflammation and tissue repair [5].
The molecular mechanisms underlying efferocytosis involve multiple signaling pathways and cytokine regulation. For instance, signaling molecules on the surface of phagocytes can recognize “eat-me” signals released by apoptotic cells, initiating a cascade of phagocytic and internalization processes [6]. Recent studies have further elucidated that the internalization of apoptotic cells relies on calcium influx and the activation of regulatory proteins, such as Mertk-mediated calcium influx, which plays a pivotal role in the formation of phagocytic cups and intracellular signal transduction [7–8]. Dysregulation of efferocytosis has been implicated in various pathological conditions, including inflammatory diseases [9–10], immune dysregulation [11–12], and even cancer [13–14]. For example, the failure to promptly clear apoptotic cells can lead to uncontrolled secondary necrosis, releasing intracellular contents that activate inflammatory responses and cause tissue damage. Conversely, efficient efferocytosis not only eliminates potentially pathogenic cells but also maintains local immune tolerance by suppressing inflammatory cascades. Resolvin E1, for instance, has been shown to significantly enhance the clearance efficiency of apoptotic cells, thereby alleviating acute or chronic inflammatory responses and demonstrating therapeutic efficacy in experimental models of severe aplastic anemia [15].
Over the past two decades, advancements in molecular biology techniques and the application of high-throughput technologies such as advanced imaging and single-cell sequencing have shifted the focus of efferocytosis research from descriptive studies to multifaceted mechanistic investigations [16]. The literature on efferocytosis has experienced exponential growth, reflecting its critical role in regulating cellular homeostasis, immune responses, and disease pathogenesis [17]. Given the centrality of efferocytosis in these processes, a systematic exploration of its research landscape and trends holds significant implications for the broader life sciences. However, current studies predominantly concentrate on elucidating biomolecular mechanisms and applying these insights to specific disease models, with relatively few bibliometric analyses of the global literature in this field [17–18]. For example, Cao et al., conducted a bibliometric analysis of efferocytosis in inflammation, and Hu et al., analyzed the trends and hotspots of efferocytosis in cardiovascular diseases from 2001 to 2022. However, these two studies only analyzed partial data of efferocytosis in the field of inflammation or cardiovascular diseases, and no study has yet analyzed data on the entire field of efferocytosis. At the same time, the above study did not involve some key indicators in this field, such as genetic data. Bibliometrics, as a tool for comprehensively analyzing research output, academic impact, and international collaboration patterns, has gained increasing traction in biomedical research [19, 20, 21]. Through bibliometric analysis, we can not only delineate the evolution and hotspots of a research field but also provide robust evidence to inform future research strategies and resource allocation.
Against this backdrop, this study aims to conduct a comprehensive and systematic bibliometric analysis of global research on efferocytosis. Specifically, we seek to address the following key questions: (1) What is the publication trend of efferocytosis research on a global scale? (2) Who are the prolific authors and high-impact journals in this field, and how have research foci evolved over time? (3) What are the potential breakthrough directions for future research? To answer these questions, this study will employ bibliometric methods to construct a spatiotemporal academic knowledge network, offering a macro-level yet in-depth perspective.
Methods
Data collection
Various academic databases exhibit distinct strengths and weaknesses in document retrieval, including platforms like PubMed, Google Scholar, and Web of Science (WoS). For this investigation, we employed the SCI-Expanded module within the WoS Core Collection (WoSCC), a product of Clarivate Analytics, to perform comprehensive literature searches, data collection, and bibliometric evaluations. Recognized as a premier resource, WoSCC stands out for its exceptional standardization, thoroughness, reliability, and practical utility in scientific research [22–23]. Its multifaceted nature, combined with the ability to assess output across various dimensions such as academic journals, geographical locations, research institutions, and individual scholars, along with seamless integration with essential analytical software, significantly enhances its utility.
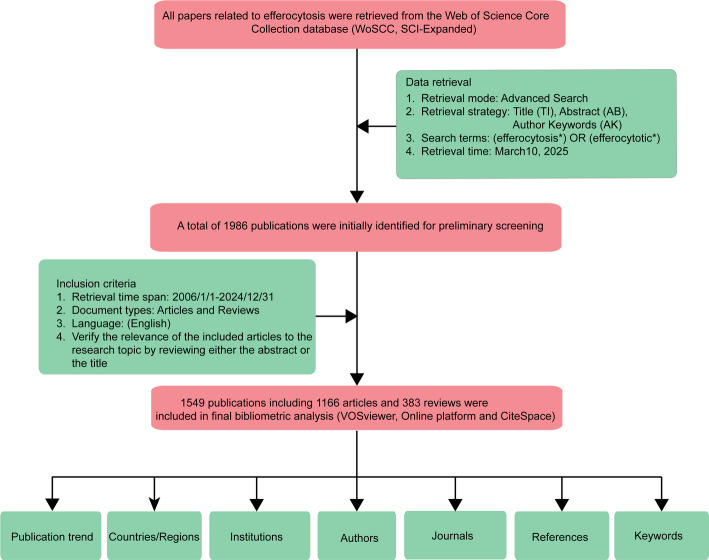
Our search parameters were configured with the following criteria: TI/AK/AB=((efferocytosis*) OR (efferocytotic*)), restricted to the Science Citation Index Expanded repository, without constraints on publication formats or temporal/linguistic boundaries. Document acquisition from WoSCC was completed within a 24-hour window on March 10, 2025, to prevent potential chronological biases, with records exported in “Full Record and Cited References” format and stored as tab-delimited text files labeled “download_.txt.” This process yielded 1986 documents spanning seven distinct publication categories. The collected references were subsequently processed through CiteSpace for data refinement. Our inclusion criteria mandated that only English-language articles and review papers be considered, resulting in 1549 documents after eliminating redundant entries. Two investigators independently examined the initial dataset, subsequently convening to resolve any discrepancies through discussion. The final corpus comprised 1549 papers, with the complete selection process illustrated in Fig. 1.
Fig. 1.
Schematic representation of the data collection and processing
Data analysis
To investigate scholarly outputs on efferocytosis, we performed a comprehensive bibliometric evaluation, employing diverse analytical tools to examine multiple dimensions such as contributing authors, publishing venues, cited works, geographical origins, and thematic keywords. Our methodological approach incorporated several computational resources: VOSviewer (version 1.6.20), CiteSpace (6.3.R1), SPSS (IBM SPSS Statistics, V.21), Microsoft Excel, and web-based platforms (bibliometric.com and citexs.com/Summary). Each application served distinct functions within our analytical framework.
VOSviewer, specifically developed for mapping and representing scholarly networks, facilitated the construction of various bibliometric visualizations [24]. Within our research framework, this tool enabled the generation of multiple network representations, including citation patterns, collaborative authorship, and keywords co-occurrences. Specific thresholds were established for different entities. Within these visual representations, node dimensions correspond to quantitative metrics such as publication volume, citation counts, or term frequencies. Color coding reflects cluster membership, with identical hues indicating shared thematic groupings. Spatial relationships between nodes denote conceptual proximity, while connecting lines represent relational ties, quantified through total link strength (TLS) measurements that assess the intensity of collaborative or citation-based connections [24].
For institutional analysis, journal dual-mapping, and reference co-citation examination, we utilized CiteSpace, configuring the temporal scope from January 2006 through December 2024, divided into annual segments. The analytical framework incorporated keyword and reference nodes, with selection criteria limited to the fifty most prominent items per temporal segment. Network optimization incorporated pathfinding methodologies with both segmented and consolidated pruning approaches, while maintaining standard configurations for remaining parameters [25]. In the resultant visualizations, node dimensions reflect parameter significance, with chromatic differentiation indicating cluster membership. Betweenness centrality (BC) metrics were employed to assess node influence within networks, while burst detection identified temporal citation patterns, with stronger values indicating heightened research interest during specific periods [26]. Structural integrity was evaluated through modularity (Q) scores, with values exceeding 0.3 indicating robust clustering. Cluster quality was assessed using silhouette (S) coefficients, where values surpassing 0.3, 0.5, and 0.7 denote progressively stronger cluster homogeneity [27]. Cluster identification employed keyword-based noun phrase extraction through likelihood ratio testing (p < 0.001), with subsequent expert refinement for precision. The dual-mapping functionality within CiteSpace, developed through Chen and Leydesdorff’s contributions, enabled cross-disciplinary citation pattern analysis [28]. This approach utilized a foundational map of 10,000 journals, generating citation trajectories that illustrate interdisciplinary research dynamics across scientific domains.
Temporal publication patterns were analyzed using Microsoft Excel, incorporating both annual and cumulative distribution metrics. Growth trends were evaluated through exponential regression analysis, with determination coefficients (R2) serving as goodness-of-fit indicators. Pearson correlation analysis was used to examine the relationship between publication volume and citation frequency. Furthermore, our investigation extended to efferocytosis-associated genetic elements through the citexs.com/Summary platform, facilitating comprehensive gene data aggregation. Protein interaction networks and functional enrichment analyses were conducted using the STRING database (string-db.org), focusing on extensively studied genetic markers within the research domain [29].
Results
Annual publication and citation trends
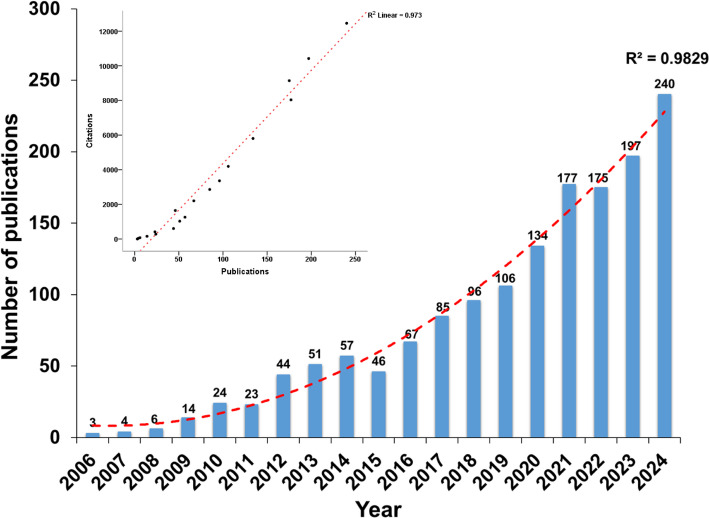
Between 2006 and 2024, the WoSCC database recorded 1549 scholarly works addressing efferocytosis. This corpus included 1166 primary research articles and 383 review articles, representing 75.27% and 24.73% of the total output, respectively (Fig. 2). Publication volumes demonstrated variable growth throughout the examined timeframe, surpassing 100 documents since 2019. The most productive year occurred in 2024, yielding 240 scientific contributions. The statistical evaluation demonstrates a remarkably growing trend in annual research output, as evidenced by a R2 of 0.9829. Analysis of citation patterns reveals that the 1549 documents collectively accumulated 66,324 citations, yielding an average citation rate of 42.82 per article. A strong positive association (r = 0.987, p < 0.001) was observed between annual publication volume and corresponding citation counts.
Fig. 2.
Annual publication volume of efferocytosis-related research. The red dashed line represents the fitted curve. The graph in the upper-left corner illustrates the correlation analysis between annual publication output and citation frequency
Visual analysis of high-yielding journals
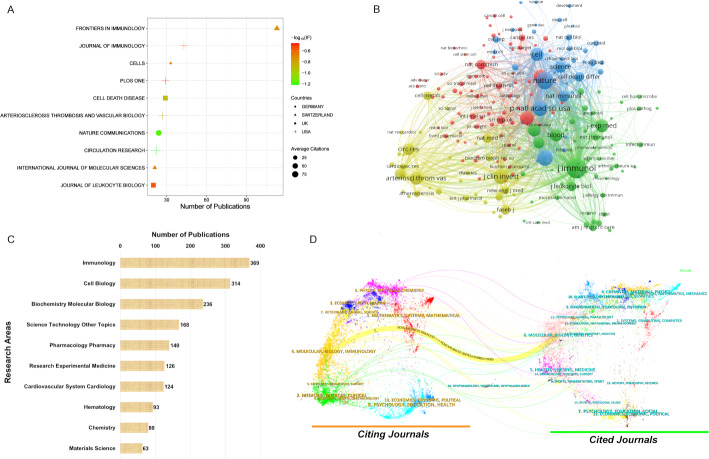
The comprehensive analysis identified 1549 relevant articles distributed across 497 academic journals, among which 73 journals published at least 5 papers each. Figure 3A presents detailed metrics for the ten most prolific journals. These leading journals collectively accounted for 361 articles, representing approximately one-quarter (23.3%) of the total scholarly output in this domain. Frontiers in Immunology ranked first with 113 papers, followed by Journal of Immunology (43 papers) and Cells (33 papers). The geographical distribution of these journals reveals a concentration in specific regions: four are based in the United States, three in Switzerland, two in United Kingdom, and one in Germany. Current JCR classifications indicate that 90% of these publications maintain Q1 or Q2 status, with Circulation Research leading in impact factor at 16.5. Citation analysis demonstrates that Circulation Research achieves the highest mean citation rate per article (95.36), followed by Arteriosclerosis Thrombosis and Vascular Biology (71.56) and Journal of Immunology (64.12). When two documents are simultaneously referenced by a third publication, they establish a co-citation relationship. The network visualization diagram in Fig. 3B illustrates 201 frequently co-cited journals, all exceeding 100 citations. Among these, The Journal of Immunology, Nature, and Journal of Biological Chemistry demonstrated the top 3 journals with the strongest TLS. The WoSCC platform facilitates categorization of publications into specific research categories, with Fig. 3C detailing the ten most productive subject areas. Immunology, Cell Biology, and Biochemistry Molecular Biology emerged as dominant disciplines within the efferocytosis research domain. Analysis of the dual-map visualization presented in Fig. 3D identified a primary citation trajectory. Journals referencing efferocytosis research predominantly originated from Molecular/Biology/Immunology categories, whereas the referenced publications were primarily concentrated within Molecular/Biology/Genetics domains.
Fig. 3.
(A) Detailed metrics for the top ten most prolific journals. The X-axis represents the number of publications, with values increasing toward the right. The size, color, and shape of the nodes denote the average citation count per journal, journal impact factor, and country of origin, respectively. (B) The network visualization diagram of co-cited journals. Each node represents a distinct journal, with its size proportional to the publication volume, larger nodes indicate greater numbers of publications. (C) Top ten most productive subject areas. (D) Analysis of the dual-map visualization. The left-side map represents citing journals (knowledge origins), while the right-side map shows cited journals (knowledge dissemination). Colored curves depict citation trajectories, with width indicating frequency and color corresponding to disciplinary clusters. The base maps organize journals by bibliographic coupling, with labels reflecting broad disciplines. The most statistically significant path (z-score > 3.0) reveals dominant knowledge diffusion
Visual analysis of main contributors
Contributions of countries/regions
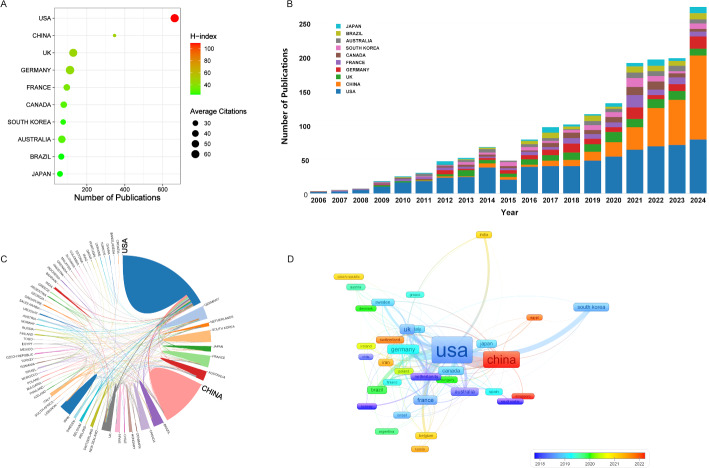
Research contributions spanned 67 distinct geographical areas, with Fig. 4A illustrating the ten most productive nations. The United States emerged as the leading contributor with 663 publications, followed by China (346) and Germany (111). When examining citation impact, the Germany dominated with 65.98 average citations per paper, trailed by the United States (61.51) and United Kingdom (59.08). Temporal analysis revealed consistent annual growth across these nations, with China demonstrating the most substantial increase in research output (Fig. 4B). Figure 4C and D illustrate international research cooperation through network diagrams, where connecting lines denote collaborative relationships, with thickness reflecting partnership strength. The United States maintains its most substantial research connections with China, France, and Germany. The VOSviewer-generated Fig. 4D utilizes a color-coded system to represent nations’ Average Appearance Year (AAY), where blue shades indicate early entrants and red tones represent recent participants in this scientific domain.
Fig. 4.
(A) Publication distribution by countries. The X-axis represents the number of publications, with values increasing toward the right. The size and color of the nodes denote the average citation count per country, and H-index, respectively. (B) Annual publication counts for the top 10 countries. Each color block represents a country. (C) Network map of collaborations among countries. The area of each country corresponds to its publication output, with larger areas indicating greater publication volumes. Connections between countries represent collaborative relationships, where thicker lines denote stronger cooperation. (D) Network visualization map of country co-authorship analysis by VOSviewer. Each node represents an individual country, while the connecting lines between nodes indicate the strength of collaboration. The node color corresponds to the average publication year, with reference to the color bar in the lower-right corner for specific year values
Contributions of institutions
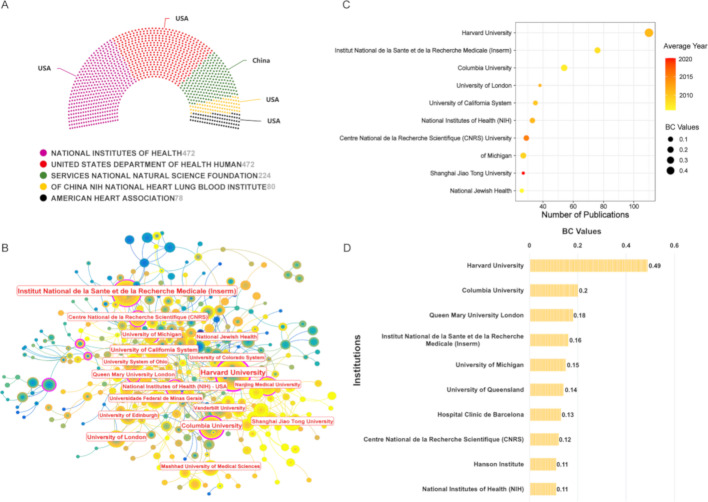
Figure 5A identifies the principal funding organizations supporting efferocytosis-related research, with most originating from the United States. The collaborative landscape among academic institutions is presented in Fig. 5B, where node size corresponds to research productivity. Entities demonstrating substantial influence, marked by BC values exceeding 0.1, are distinguished by purple rings. Regarding publication volume, Fig. 5C ranks Harvard University as the most productive institution, followed by the Institut National de la Sante et de la Recherche Medicale (Inserm) and Columbia University. When assessing network influence through BC metrics, Fig. 5D reveals Harvard University (BC = 0.49) as the most central entity, with Columbia University (BC = 0.2) and Queen Mary University London (BC = 0.18) completing the top three.
Fig. 5.
(A) The top 5 principal funding organizations supporting efferocytosis-related research. (B) The collaborative landscape among academic institutions using CiteSpace. Each node represents an individual institution, with its size being proportional to the publication count. Nodes with a betweenness centrality (BC) value exceeding 0.1 are highlighted with purple rings. (C) Visualization of high-yielding institutions. The X-axis represents the number of publications, with values increasing toward the right. The size and color of the nodes denote the BC values, and average year, respectively. (D) The top 10 institutions with the highest BC values
Contributions of authors
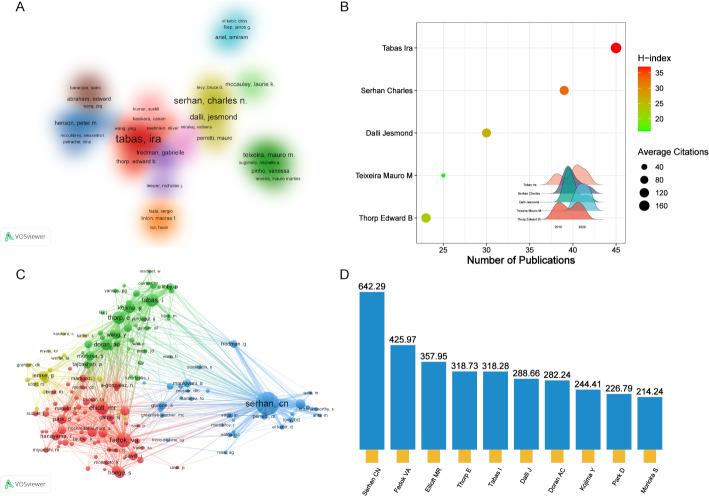
Figure 6A presents a co-authorship network comprising 92 researchers, each having published at least five papers. Authors sharing similar colors exhibit comparable collaborative patterns, forming 17 distinct research clusters. Columbia University’s Tabas Ira leads in publication output, followed by Harvard University’s Serhan Charles and Dalli Jesmond, as shown in Fig. 6B. The co-citation network among authors, visualized in Fig. 6C, was constructed using VOSviewer. Figure 6D ranks contributors by TLS, with Serhan CN, Fadok VA, and Elliott MR occupying the top three positions.
Fig. 6.
(A) Network visualization map of co-authorship of authors. The font size of author names corresponds to their publication output, with larger fonts indicating greater productivity. Distinct colors are assigned to different author clusters, where co-colored clusters represent tightly collaborative research groups. (B) Visualization of high-yielding authors. The X-axis represents the number of publications, with values increasing toward the right. The size and color of the nodes denote the average citations, and H-index, respectively. The graph in the lower-right corner illustrates the temporal variation in publication output by authors across different years. (C) Network visualization map of co-citation of authors. Each node represents an individual author, with node size proportional to citation frequency, larger nodes indicate higher citation counts. The inter-node connections represent co-citation relationships. (D) The top 10 authors with the highest TLS values
Visual analysis of highly-cited studies
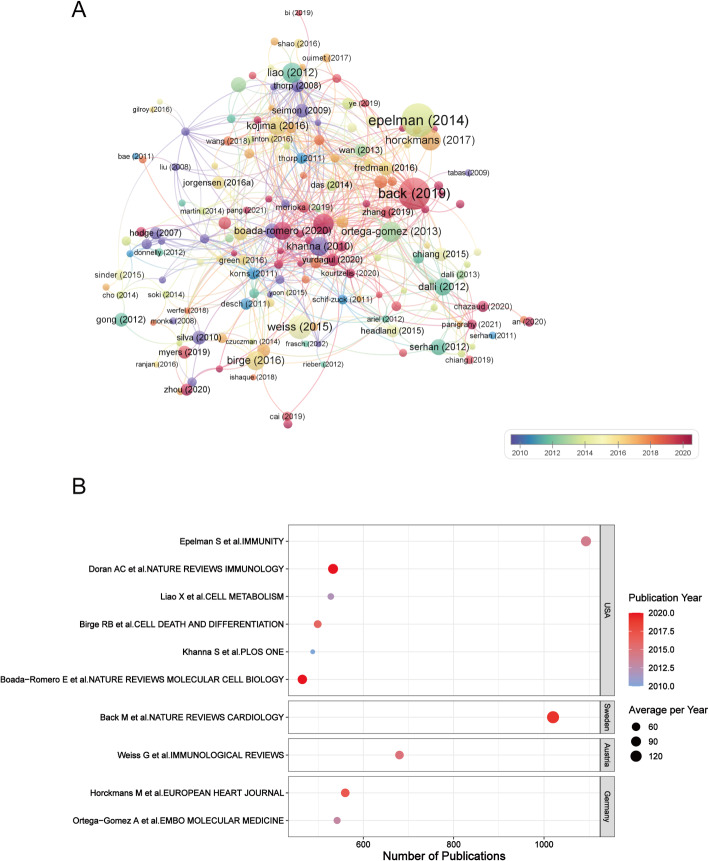
Figure 7A displays a citation network constructed through VOSviewer, where node dimensions reflect citation counts, allowing researchers to quickly identify seminal works through visual analysis. Figure 7B provides essential details regarding the ten most frequently referenced works. These influential publications appeared between 2010 and 2020. Among these, two groundbreaking studies have each exceeded 1000 citations, while all top-ranked publications have accumulated at least 465 citations.
Fig. 7.
(A) Paper citation analysis. Each node represents an individual publication, with its size proportional to the citation count. (B) Visualization of the top 10 highly cited studies. The X-axis represents the number of publications, with values increasing toward the right. The size and color of the nodes denote the average citations per year, and publication year, respectively
Visual analysis of co-cited references
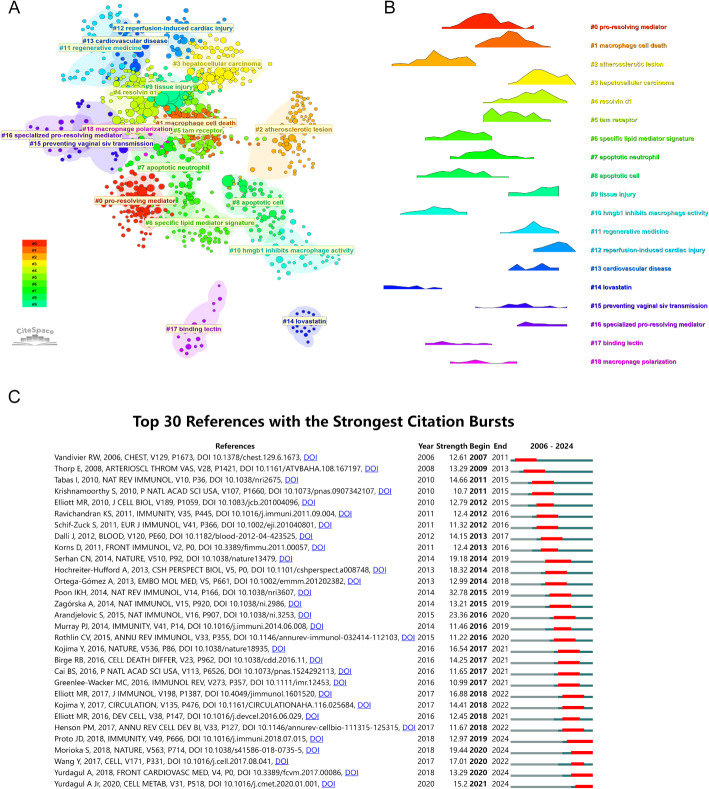
The examination of co-cited references provides a quantitative methodology for investigating scholarly connections through simultaneous citation patterns in subsequent publications. Network visualization (Fig. 8A) and chronological mapping (Fig. 8B) demonstrate the results of this analytical approach. Thematic research areas are represented by clustered nodes, each labeled with numerical identifiers derived from source materials. Utilizing the log-likelihood ratio (LLR) algorithm, the analysis identified 19 distinct thematic clusters. These categories encompass #0 pro-resolving mediator, #1 macrophage cell death, #2 atherosclerotic lesion, #3 hepatocellular carcinoma, #4 resolvin d1, #5 tam receptor, #6 specific lipid mediator signature, #7 apoptotic neutrophil, #8 apoptotic cell, #9 tissue injury, #10 hmgb1 inhibits macrophage activity, #11 regenerative medicine, #12 reperfusion-induced cardiac injury, #13 cardiovascular disease, #14 lovastatin, #15 preventing vaginal siv transmission, #16 specialized pro-resolving mediator, #17 binding lectin, and #18 macrophage polarization. Cluster validity was confirmed through statistical measures, with Q values above 0.3 and S scores exceeding 0.7. The chronological visualization (Fig. 8B) demonstrates particularly robust clustering, achieving an S value of 0.91 and Q coefficient of 0.76. Additionally, citation burst analysis highlights publications that experienced significant scholarly attention within specific periods. Figure 8C identifies the 30 most impactful references demonstrating notable citation surges across defined time intervals.
Fig. 8.
(A) Network visualization map of co-citation of references. Each node represents a single reference, with all citations algorithmically clustered into distinct groups. References belonging to the same cluster are labeled identically. (B) Chronological mapping of co-citation of references. (C) Citation burst analysis of references. Burst Strength (Strength) indicates the magnitude of a sudden increase in the citation frequency of a reference, with higher values signifying more significant influence. Burst Period, marked by red segments, represents the specific time interval during which the reference was intensively cited. Timeline reflects the start and end years of the burst, with background color blocks distinguishing different periods
Visual analysis of keywords analysis
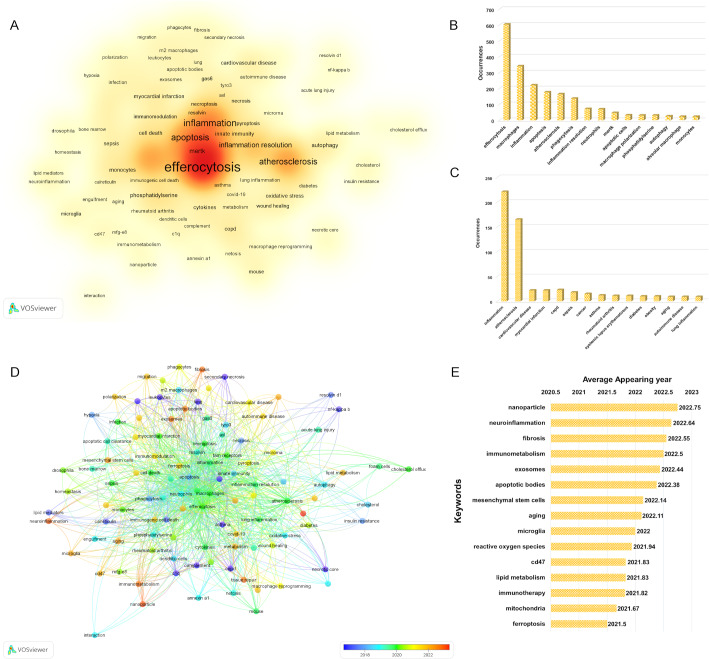
Examining key terms in academic publications offers valuable insights into research focus areas. Using VOSviewer, we conducted a keyword co-occurrence analysis, setting a minimum threshold of six appearances per term. This process generated a network where node size reflects term frequency, resulting in a density visualization incorporating 108 unique keywords from 1549 documents (Fig. 9A). The frequency distribution of the 15 most prevalent terms is shown in Fig. 9B, with notable mentions including efferocytosis, macrophages, inflammation, apoptosis, atherosclerosis, phagocytosis, inflammation resolution, neutrophils, Mertk, apoptotic cells, macrophage polarization, phosphatidylserine, autophagy, alveolar macrophage, monocytes. Disease-specific research keywords are outlined in Fig. 9C, revealing inflammation as the most studied condition, followed by atherosclerosis, cardiovascular disease, myocardial infarction, and COPD. The overlay visualization in Fig. 9D employs a color gradient based on AAY, with blue representing earlier terms and red indicating more recent ones. Figure 9E highlights the 15 terms with the most recent AAY, featuring emerging concepts such as nanoparticle, neuroinflammation, fibrosis, immunometabolism, exosomes, apoptotic bodies, mesenchymal stem cells, aging, microglia, reactive oxygen species, CD47, lipid metabolism, immunotherapy, mitochondria, ferroptosis, reflecting current research directions in the field.
Fig. 9.
(A) Density visualization map of co-occurrence of keywords. (B) The top 15 most prevalent terms. (C) The top 15 most prevalent disease related terms. (D) Overlay visualization map of co-occurrence of keywords. Node size reflects keyword frequency, color indicates clusters of semantically related terms, and connecting lines represent co-occurrence strength, with thicker lines denoting stronger associations. The overlay highlights temporal trends, with warmer hues (e.g., red) indicating emerging keywords and cooler tones (e.g., blue) representing established research foci. The average appearance year (AAY) of each node can be found according to the color bar in the lower right corner. (E) Top 15 keywords with the highest AAY
Visual analysis of hotspot genes
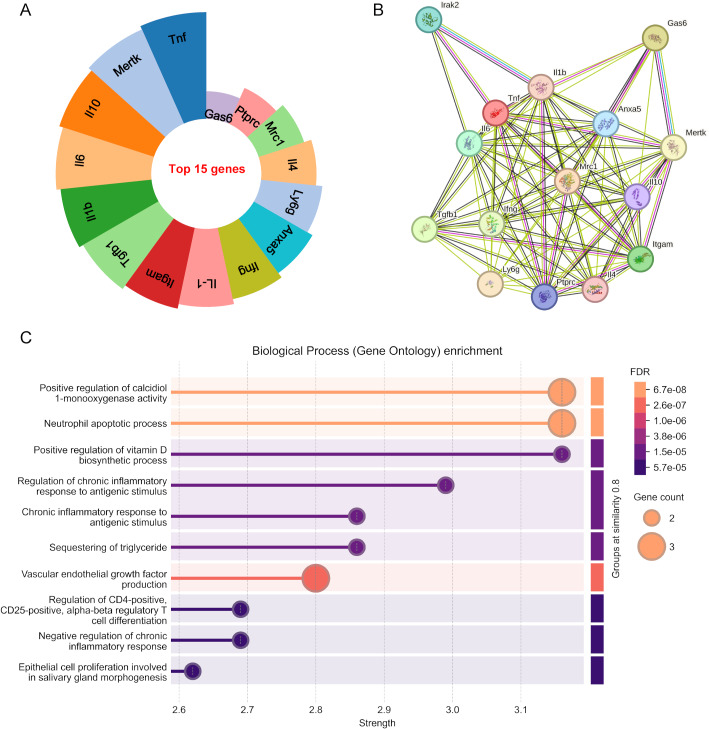
Utilizing online tools, we conducted an extensive analysis of highly studied genetic markers in efferocytosis. Figure 10A demonstrates the top 15 hotspot genes in this field. The following genes including TNF, MERTK, IL10, LI6, and IL1b emerged as the top 5 most intensively investigated genetic elements in efferocytosis research. Through the STRING platform, we established a comprehensive protein-protein interaction network to elucidate their molecular functions (Fig. 10B). Furthermore, we performed Gene Ontology (GO) pathway analysis on the twenty most prominent genes, presenting the results through bubble plot visualizations. The GO analysis, depicted in Fig. 10C, identified significant associations with Positive regulation of calcidiol1-monooxygenase activity, Neutrophil apoptotic process, Positive regulation of vitamin D biosynthetic process, and so on.
Fig. 10.
(A) Top 15 hotspot genes in efferocytosis research. (B) Protein-protein interaction network. (C) Gene Ontology pathway analysis. The X-axis represents the strength, with values increasing toward the right. The size and color of the nodes denote the gene count, and FDR, respectively
Discussion
Researchers investigating efferocytosis, ranging from emerging scholars to established experts, must systematically examine scientific literature to understand historical progress, current advancements, and future directions. This investigation addresses these needs by analyzing collaborative patterns among contributors, highly cited papers, co-cited references, as well as high-frequency keywords. Over past two decades, publication rates in this domain have shown exponential growth, reflecting its status as a prominent scientific focus area. Geographical analysis reveals the United States as the most prolific contributor based on publication volume, with prominent researchers including Tabas Ira from Columbia University [30], Serhan Charles [31] and Dalli Jesmond [32] from Harvard University. Meanwhile, the United States also maintains the highest citation impact and H-index metrics, likely due to its early research initiation and substantial funding commitments. Among the principal funding organizations supporting efferocytosis-related research, with most originating from the United States. Collaborative networks demonstrate strong research partnerships between American and Chinese investigators. European and Asian researchers are increasingly contributing to this field, though American scholars continue to exert substantial influence.
From the perspective of journal distribution, research outputs are highly concentrated in a limited number of high-impact journals, with those in the field of immunology being particularly prominent. This phenomenon reflects the strong connection between efferocytosis research and immunology, while also indicating that the research achievements in this field have gained recognition from high-level academic platforms. Notably, 90% of the core journals are ranked in Q1 or Q2, and their high average citation rates suggest that the overall research quality in this field is robust. In terms of research direction distribution, immunology, cell biology, and biochemistry and molecular biology constitute the three pillar disciplines of this field. This interdisciplinary characteristic highlights the broad application value and theoretical significance of efferocytosis research. Particularly noteworthy is the dual-map overlay analysis, which reveals that literature citing efferocytosis research primarily originates from the molecular/biology/immunology domains, while the cited literature is predominantly concentrated in the molecular/biology/genetics domains. This citation trajectory suggests that efferocytosis research may be evolving from its traditional immunological scope toward more fundamental molecular mechanisms and genetic regulation [33–34]. These findings indicate that future efferocytosis research may follow two important trends: first, continued deepening of interdisciplinary integration with immunology to explore novel mechanisms in immune regulation; and second, strengthening foundational research at the molecular and genetic levels to provide a more solid theoretical basis for clinical applications.
Through an analysis of the top ten most highly cited publications in the field of efferocytosis, it is evident that research in this area primarily focuses on the function and regulation of macrophages, the molecular mechanisms of inflammation resolution, the pathophysiological significance of efferocytosis in diseases, and the development of related therapeutic strategies [35, 36, 37, 38, 39, 40, 41, 42, 43, 44].
Firstly, the central role of macrophages in efferocytosis has garnered significant attention. Studies have shown that macrophages participate in tissue repair and inflammation resolution through local proliferation and phenotypic polarization. For instance, research by Epelman et al. [35] revealed distinct maintenance mechanisms of cardiac macrophages under steady-state and inflammatory conditions, demonstrating that embryonic-derived macrophages persist in the adult heart and maintain their population through local proliferation. Additionally, Horckmans et al. [38] further demonstrated that neutrophils, following myocardial infarction, regulate the polarization of macrophages toward a reparative phenotype by secreting neutrophil gelatinase-associated lipocalin (NGAL), thereby promoting cardiac repair. These studies underscore the critical role of macrophages in tissue homeostasis and repair, providing new insights for therapeutic strategies targeting macrophages. Secondly, the molecular mechanisms of inflammation resolution represent another key focus in efferocytosis research. One review by Bäck et al. [36] detailed the regulatory mechanisms of inflammation resolution in atherosclerosis, highlighting the important role of specialized pro-resolving lipid mediators in inhibiting plaque progression. Another study further integrated the key molecular and cellular mechanisms of inflammation resolution, proposing that the clearance of apoptotic neutrophils and the reprogramming of macrophages are central events in restoring tissue homeostasis [39].
Moreover, the pathophysiological significance of efferocytosis in diseases has been extensively investigated. For example, Liao et al. [41] found that macrophage autophagy plays a protective role in advanced atherosclerosis, and inhibiting autophagy exacerbates macrophage apoptosis and plaque necrosis. Khanna et al. [43] revealed that macrophage dysfunction in diabetic wounds impairs efferocytosis, thereby delaying inflammation resolution and wound healing. The immunoregulatory mechanisms of efferocytosis and its applications in infections and cancer have also attracted considerable attention. Birge et al. [42] demonstrated that phosphatidylserine (PS) on the surface of apoptotic cells acts as a global immunosuppressive signal, exploited by pathogens and cancer cells to evade immune surveillance in infectious and tumor microenvironments. This discovery provides a theoretical basis for developing PS-targeted therapeutic strategies. Furthermore, studies by Doran et al. [40] and Boada-Romero et al. [44] emphasized the broad role of efferocytosis in maintaining tissue homeostasis and host defense, exploring its pathophysiological significance in various diseases.
While the field has made significant progress in elucidating the molecular mechanisms of efferocytosis, clinical translation remains in its early stages, with most studies still at the preclinical level [35, 36, 37, 38, 39, 40, 41, 42, 43, 44]. However, several promising therapeutic strategies have emerged that target efferocytosis pathways, offering potential avenues for treating inflammatory diseases. For instance, modulation of “eat-me” signals, such as PS exposure and its receptors (TAM family receptors), has shown therapeutic potential [42]. Antibodies targeting the CD47-SIRPα axis are being evaluated in cancers and atherosclerosis, where impaired efferocytosis contributes to disease progression [45–46]. Similarly, small molecules that enhance MerTK signaling or inhibit its cleavage (ADAM17 inhibitors) are under investigation for rheumatoid arthritis and other chronic inflammatory conditions [47–48]. Metabolic interventions, such as PPARγ agonists (pioglitazone), have demonstrated the ability to boost efferocytosis in preclinical models of diabetes and atherosclerosis by modulating macrophage metabolism [49]. Additionally, biomimetic drug delivery systems, like erythrocyte-mimetic liposomes (Effero-RLP), have been designed to exploit efferocytosis pathways for targeted anti-inflammatory therapy in colitis models [50].
Through the network visualization and time series analysis of co-cited literature, this study has identified 19 major thematic clusters within the research domain of efferocytosis. These clusters not only reflect the current hotspots in the field but also reveal the knowledge structure and evolutionary trends of the discipline [51]. Cluster #0 (pro-resolving mediator) and Cluster #16 (specialized pro-resolving mediator) focus on the role of specialized pro-resolving mediators (SPMs) in inflammation resolution and tissue repair. These mediators play a crucial role in terminating inflammatory responses by regulating macrophage phagocytic functions and the clearance of apoptotic cells. Research indicates that SPMs such as Resolvin D1 (Cluster #4) not only promote inflammation resolution but also exhibit potential therapeutic value in cardiovascular diseases (Cluster #13) and regenerative medicine (Cluster #11) [52–53]. Cluster #1 (macrophage cell death) and Cluster #8 (apoptotic cell) highlight the central role of macrophage apoptosis in efferocytosis. As the primary phagocytic cells, the apoptosis of macrophages not only affects the dynamic balance of inflammatory responses but is also closely related to tissue damage (Cluster #9) and regenerative repair [54–55]. Additionally, Cluster #10 (hmgb1 inhibits macrophage activity) further suggests that high mobility group box 1 (HMGB1) may play a significant role in the pathological processes of inflammatory diseases by inhibiting macrophage activity [56–57].
In terms of disease-related research, Cluster #2 (atherosclerotic lesion) and Cluster #13 (cardiovascular disease) emphasize the importance of efferocytosis in atherosclerosis and cardiovascular diseases [58]. The impaired clearance of apoptotic cells may lead to the formation of necrotic cores within plaques, thereby exacerbating the progression of atherosclerosis. Furthermore, Cluster #12 (reperfusion-induced cardiac injury) reveals a potential link between reperfusion-induced myocardial injury and efferocytosis [59]. Disease-specific research keywords are outlined in Fig. 9C, revealing inflammation as the most studied condition, followed by atherosclerosis, cardiovascular disease, myocardial infarction, and COPD. In the context of cancer research, Cluster #3 (hepatocellular carcinoma) and Cluster #7 (apoptotic neutrophil) suggest that abnormalities in the clearance of apoptotic cells may be closely related to the development of hepatocellular carcinoma [60–61]. The role of neutrophil apoptosis and its clearance process in the tumor microenvironment warrants further exploration. Additionally, Cluster #5 (tam receptor) and Cluster #17 (binding lectin) elucidate the molecular mechanisms of TAM receptors and lectins in the recognition and phagocytosis of apoptotic cells [62]. Regarding pharmacological and interventional strategies, Cluster #14 (lovastatin) and Cluster #6 (specific lipid mediator signature) suggest that statins and specific lipid mediators may exert therapeutic effects by modulating efferocytosis [63–64]. For instance, lovastatin may influence macrophage function and the clearance of apoptotic cells by regulating lipid metabolism and inflammatory responses.
Keyword co-occurrence analysis serves as a pivotal bibliometric tool, enabling intuitive elucidation of core themes, knowledge structures, and evolutionary trends within a research field. By examining high-frequency keywords and their co-occurrence relationships, this approach facilitates the identification of research hotspots, interdisciplinary intersections, and potential emerging directions. In the present study, the visualization of keyword networks (Fig. 9A) and the distribution of high-frequency terms (Fig. 9B) delineate the primary research foci in efferocytosis studies, further mapping the conceptual framework of this domain. Based on the keyword analysis, efferocytosis research can be categorized into three principal directions: (i) Molecular Mechanisms and Signaling Regulation of Efferocytosis. High-frequency terms such as phagocytosis [65], phosphatidylserine [66], mertk (a TAM receptor family member) [62], and apoptotic cells underscore the current emphasis on the molecular recognition mechanisms and signaling pathways governing efferocytosis. Phosphatidylserine, functioning as an “eat-me” signal on apoptotic cell surfaces, engages with TAM receptors to form a central regulatory axis in efferocytosis. Furthermore, the inclusion of autophagy suggests potential crosstalk between autophagy and efferocytosis, warranting future investigations into their synergistic roles in inflammation and disease pathogenesis. (ii) Macrophage Polarization and Inflammatory Modulation. Keywords including macrophages, macrophage polarization, inflammation resolution, and alveolar macrophages highlight the pivotal role of macrophages in efferocytosis [54–55, 58]. The polarization states of macrophages (e.g., pro-inflammatory M1 versus anti-inflammatory M2 phenotypes) critically influence the efficiency of apoptotic cell clearance and the equilibrium of inflammatory microenvironments. Future studies should further dissect the functional heterogeneity of macrophage subsets (e.g., tissue-resident versus monocyte-derived populations) in disease-specific efferocytosis and explore therapeutic strategies targeting macrophage polarization. (iii) Pathological Implications and Therapeutic Potential of Efferocytosis in Diseases. The prominence of terms such as atherosclerosis, inflammation, and neutrophils underscore the association of efferocytosis with diverse pathological conditions [58].
In addition, these keywords with relatively latest AAY, such as nanoparticle, neuroinflammation, fibrosis, immunometabolism, exosomes, apoptotic bodies, mesenchymal stem cells, aging, microglia, reactive oxygen species, cd47, lipid metabolism, immunotherapy, mitochondria, ferroptosis, may have great potential to be hot topics in the near future. For example, nanoparticles offer an innovative strategy to enhance the immunogenicity of cancer therapy by precisely inhibiting the efferocytosis of tumor-associated macrophages [67, 68, 69]. In one study, PLGA-based nanocarriers encapsulating BMS777607-a hydrophobic inhibitor of the phosphatidylserine-dependent efferocytosis receptor in macrophages-effectively blocked the clearance of apoptotic cancer cells, redirecting them toward immunogenic secondary necrosis [70]. This process promotes the release of pro-inflammatory signals and activates antitumor immune responses. When combined with non-immunogenic chemotherapy (cisplatin), immunogenic chemotherapy (oxaliplatin), or radiotherapy, this nanoformulated efferocytosis inhibitor not only reverses tumor immunosuppression but also converts conventionally non-immunogenic treatments into systemically immunostimulatory therapies, significantly improving therapeutic outcomes. Further optimization of targeted delivery and exploration of combination regimens could establish this approach as a versatile platform to amplify the effects of existing cancer therapies, highlighting its broad clinical potential [67, 68, 69].
Limitations
This investigation employed bibliometric methods to examine structural and temporal patterns within the research domain, yet several constraints should be acknowledged. The analysis exclusively utilized data from the WoSCC repository, potentially omitting relevant studies indexed in alternative databases such as PubMed or Google Scholar. Nevertheless, WoSCC remains a predominant source for bibliometric investigations [71], offering extensive reference details and representing a substantial portion of scholarly outputs. A second limitation stems from the quantitative nature of our methodology, which emphasizes metrics like citation frequencies and network co-occurrences. Such approaches may not adequately reflect the clinical significance or translational potential of specific investigations. It is particularly noteworthy that foundational research often dominates citation metrics, potentially underrepresenting recent developments in efferocytosis research due to temporal citation lag. Furthermore, our selection criteria were restricted to English-language publications, consequently excluding non-English research, including contributions from Chinese academic platforms like CNKI. This linguistic limitation may have resulted in the oversight of potentially valuable studies published in other languages. While these constraints exist, the current analysis provides valuable insights into the field’s development and highlights key research trends and patterns.
Conclusion
This study not only maps the historical progression of efferocytosis research but also identifies gaps and opportunities for future investigations. While significant progress has been made in elucidating the role of efferocytosis in disease pathogenesis, further studies are needed to explore its therapeutic modulation in different pathological contexts. Additionally, the geographic disparity in research output calls for greater international collaboration to ensure a more inclusive and globally representative advancement of knowledge. By synthesizing these insights, this bibliometric analysis serves as a valuable resource for researchers, funding agencies, and policymakers, guiding strategic decisions to harness the full potential of efferocytosis in biomedical innovation and therapeutic development.
Acknowledgements
The authors express their gratitude to “Home-for-Researchers” (www.home-for-researchers.com) for their assistance in refining the English language in this manuscript.
Author contributions
Yanpeng Jian, Shijia Dong, Weijie Liu, Genfeng Li, Xiaoyu Lian, Yigong Wang designed the study. Yanpeng Jian, Shijia Dong contributed to data collection. Yanpeng Jian, Weijie Liu, Genfeng Li, Xiaoyu Lian, analyzed the data and drafted the manuscript. Yanpeng Jian, Yigong Wang revised and approved the final version of the manuscript. All authors read and approved the submitted version.
Funding
Not applicable.
Data availability
Data is available upon request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Clinical trial number
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yanpeng Jian, Email: jianyanpeng0721@126.com.
Yigong Wang, Email: 617693188@qq.com.
References
- 1.Karaji N, Sattentau QJ. Efferocytosis of Pathogen-Infected cells. Front Immunol. 2017;8:1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehrotra P, Ravichandran KS. Drugging the efferocytosis process: concepts and opportunities. Nat Rev Drug Discov. 2022;21(8):601–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y, Yao Y, Deng Y, et al. Regulation of efferocytosis as a novel cancer therapy. Cell Commun Signal. 2020;18(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trzeciak A, Wang YT, Perry JSA. First we eat, then we do everything else: the dynamic metabolic regulation of efferocytosis. Cell Metab. 2021;33(11):2126–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poon IKH, Ravichandran KS. Targeting efferocytosis in inflammaging. Annu Rev Pharmacol Toxicol. 2024;64:339–57. [DOI] [PubMed] [Google Scholar]
- 6.Hou G, Wang X, Wang A, et al. The role of secreted proteins in efferocytosis. Front Cell Dev Biol. 2024;11:1332482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moon H, Min C, Kim G, et al. Crbn modulates calcium influx by regulating Orai1 during efferocytosis. Nat Commun. 2020;11(1):5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang S, Min C, Moon H, et al. Internalization of apoptotic cells during efferocytosis requires Mertk-mediated calcium influx. Cell Death Dis. 2023;14(6):391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu YQ, Li ZZ, Han YL, et al. The role of efferocytosis in inflammatory bowel disease. Front Immunol. 2025;16:1524058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu MY, Wang EJ, Ye RD, et al. Enhancement of LC3-associated efferocytosis for the alleviation of intestinal inflammation. Autophagy. 2024;20(6):1442–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q, Liu H, Yin G, et al. Efferocytosis: current status and future prospects in the treatment of autoimmune diseases. Heliyon. 2024;10(7):e28399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdolmaleki F, Farahani N, Gheibi Hayat SM, et al. The role of efferocytosis in autoimmune diseases. Front Immunol. 2018;9:1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He X, Cheng X, Zhang Z, et al. Efferocytosis-related gene IL33 predicts prognosis and immune response and mediates proliferation and migration in vitro and in vivo of breast cancer. Front Pharmacol. 2025;16:1533571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng M, Chen S, Li K, et al. CD276-dependent efferocytosis by tumor-associated macrophages promotes immune evasion in bladder cancer. Nat Commun. 2024;15(1):2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grazda R, Seyfried AN, Maddipati KR, et al. Resolvin E1 improves efferocytosis and rescues severe aplastic anemia in mice. Cell Death Dis. 2024;15(5):324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan J, Liao YS, Zhang TC, et al. Integrating bulk RNA and Single-Cell sequencing data unveils efferocytosis patterns and CeRNA network in ischemic stroke. Transl Stroke Res. 2025;16(3):733–46. [DOI] [PubMed] [Google Scholar]
- 17.Cao X, Li F, Xie X, et al. Efferocytosis and inflammation: a bibliometric and systematic analysis. Front Med (Lausanne). 2025;12:1498503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu L, Lv Z, Gu Y, et al. A bibliometric analysis of efferocytosis in cardiovascular diseases from 2001 to 2022. Med (Baltim). 2023;102(39):e34366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyu Y, Zhao H, Zeng G, et al. Mapping the evolving trend of research on leukocyte telomere length: a text-mining study. Hum Genomics. 2024;18(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu H, Cheng K, Sun Z, et al. Trends of mapping knowledge structure and themes of cancer sonodynamic therapy: a text-mining study. Quant Imaging Med Surg. 2024;14(12):8734–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng K, Li Z, Sun Z, et al. The rapid growth of bibliometric studies: a call for international guidelines. Int J Surg. 2024;110(4):2446–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng K, Li W, Wu H, et al. Mapping knowledge structure and themes trends of cancer-associated fibroblasts: a text-mining study. Front Mol Biosci. 2023;10:1302016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Z, Cheng K, Shen Z, et al. Mapping knowledge landscapes and emerging trends of sonodynamic therapy: A bibliometric and visualized study. Front Pharmacol. 2023;13:1048211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Eck NJ, Waltman L. Software survey: vosviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Synnestvedt MB, Chen C, Holmes JH. CiteSpace II: visualization and knowledge discovery in bibliographic databases. AMIA Annu Symp Proc 2005:724–8. [PMC free article] [PubMed]
- 26.Liu Z, Li Z, Wang L, et al. Bibliometric analysis of the knowledge landscape of periodontal disease in pregnancy: A noteworthy multidisciplinary issue. J Multidiscip Healthc. 2023;16:3941–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen Z, Hu J, Wu H, et al. Global research trends and foci of artificial intelligence-based tumor pathology: a scientometric study. J Transl Med. 2022;20(1):409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen C, Leydesdorff L. Patterns of connections and movements in dual-map overlays: A new method of publication portfolio analysis. J Association Inform Sci Technol. 2014;65(2):334–51. [Google Scholar]
- 29.Wu H, Sun Z, Zeng G et al. Mapping knowledge structure and themes trends of ewing sarcoma: A Text-Mining study. Ann Surg Oncol. 2025. [DOI] [PubMed]
- 30.Tabas I. Apoptosis and efferocytosis in mouse models of atherosclerosis. Curr Drug Targets. 2007;8(12):1288–96. [DOI] [PubMed] [Google Scholar]
- 31.McCauley LK, Dalli J, Koh AJ, et al. Cutting edge: parathyroid hormone facilitates macrophage efferocytosis in bone marrow via proresolving mediators resolvin D1 and resolvin D2. J Immunol. 2014;193(1):26–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120(15):e60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romero-Molina C, Garretti F, Andrews SJ, et al. Microglial efferocytosis: diving into the alzheimer’s disease gene pool. Neuron. 2022;110(21):3513–33. [DOI] [PubMed] [Google Scholar]
- 34.Zang H, Ji X, Yao W, et al. Role of efferocytosis in chronic pain -- from molecular perspective. Neurobiol Dis. 2025;207:106857. [DOI] [PubMed] [Google Scholar]
- 35.Epelman S, Lavine KJ, Beaudin AE, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40(1):91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bäck M, Yurdagul A Jr, Tabas I, et al. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol. 2019;16(7):389–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss G, Schaible UE. Macrophage defense mechanisms against intracellular bacteria. Immunol Rev. 2015;264(1):182–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horckmans M, Ring L, Duchene J, et al. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J. 2017;38(3):187–97. [DOI] [PubMed] [Google Scholar]
- 39.Ortega-Gómez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO Mol Med. 2013;5(5):661–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doran AC, Yurdagul A Jr, Tabas I. Efferocytosis in health and disease. Nat Rev Immunol. 2020;20(4):254–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liao X, Sluimer JC, Wang Y, et al. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 2012;15(4):545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Birge RB, Boeltz S, Kumar S, et al. Phosphatidylserine is a global immunosuppressive signal in efferocytosis, infectious disease, and cancer. Cell Death Differ. 2016;23(6):962–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khanna S, Biswas S, Shang Y, et al. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS ONE. 2010;5(3):e9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boada-Romero E, Martinez J, Heckmann BL, et al. The clearance of dead cells by efferocytosis. Nat Rev Mol Cell Biol. 2020;21(7):398–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia Q, Liu F, Zhou Y, et al. CD47-SIRPα signaling-inspired engineered monocytes for preventing the progression of atherosclerotic plaques. Mater Today Bio. 2024;28:101178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ooki A, Osumi H, Shimozaki K, et al. Novel immunotherapy for gastric cancer: targeting the CD47-SIRPα axis. Cancer Metastasis Rev. 2025;44(2):52. [DOI] [PubMed] [Google Scholar]
- 47.Kim HJ, Trinh NT, Choi Y, et al. ADAM17 genetic variants and the response of TNF-α inhibitor in rheumatoid arthritis patients. Pharmgenomics Pers Med. 2020;13:81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan I, Schwarz J, Lücke K, et al. ADAM17 controls IL-6 signaling by cleavage of the murine IL-6Rα from the cell surface of leukocytes during inflammatory responses. J Leukoc Biol. 2016;99(5):749–60. [DOI] [PubMed] [Google Scholar]
- 49.Fernandez-Boyanapalli RF, Falcone EL, Zerbe CS, et al. Impaired efferocytosis in human chronic granulomatous disease is reversed by Pioglitazone treatment. J Allergy Clin Immunol. 2015;136(5):1399–e14013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han R, Ren Z, Wang Q, et al. Synthetic biomimetic liposomes Harness efferocytosis machinery for highly efficient Macrophages-Targeted drug delivery to alleviate inflammation. Adv Sci (Weinh). 2024;11(29):e2308325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ngai D, Schilperoort M, Tabas I. Efferocytosis-induced lactate enables the proliferation of pro-resolving macrophages to mediate tissue repair. Nat Metab. 2023;5(12):2206–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee HN, Surh YJ. Resolvin D1-mediated NOX2 inactivation rescues macrophages undertaking efferocytosis from oxidative stress-induced apoptosis. Biochem Pharmacol. 2013;86(6):759–69. [DOI] [PubMed] [Google Scholar]
- 53.Lee HN, Kundu JK, Cha YN, et al. Resolvin D1 stimulates efferocytosis through p50/p50-mediated suppression of tumor necrosis factor-α expression. J Cell Sci. 2013;126(Pt 17):4037–47. [DOI] [PubMed] [Google Scholar]
- 54.Sheng YR, Hu WT, Chen S, et al. Efferocytosis by macrophages in physiological and pathological conditions: regulatory pathways and molecular mechanisms. Front Immunol. 2024;15:1275203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin CJ, Peters KN, Behar SM. Macrophages clean up: efferocytosis and microbial control. Curr Opin Microbiol. 2014;17:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Zhang W, Xu Y, et al. Extracellular HMGB1 impairs Macrophage-Mediated efferocytosis by suppressing the Rab43-Controlled cell surface transport of CD91. Front Immunol. 2022;13:767630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Banerjee S, de Freitas A, Friggeri A, et al. Intracellular HMGB1 negatively regulates efferocytosis. J Immunol. 2011;187(9):4686–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He X, Li Z, Li S, et al. Huoxue Tongluo tablet enhances atherosclerosis efferocytosis by promoting the differentiation of Trem2 + macrophages via PPARγ signaling pathway. Phytomedicine. 2025;140:156579. [DOI] [PubMed] [Google Scholar]
- 59.Wang X, Du W, Li Y, et al. Macrophage-enriched Sectm1a promotes efficient efferocytosis to attenuate ischemia/reperfusion-induced cardiac injury. JCI Insight. 2024;9(5):e173832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li L, Li G, Zhai W. Single-cell transcriptomic analysis reveals efferocytosis signature predicting immunotherapy response in hepatocellular carcinoma. Dig Liver Dis. 2025. [DOI] [PubMed]
- 61.Xu K, Liu Y, Luo H, et al. Efferocytosis signatures as prognostic markers for revealing immune landscape and predicting immunotherapy response in hepatocellular carcinoma. Front Pharmacol. 2023;14:1218244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou L, Matsushima GK. Tyro3, axl, Mertk receptor-mediated efferocytosis and immune regulation in the tumor environment. Int Rev Cell Mol Biol. 2021;361:165–210. [DOI] [PubMed] [Google Scholar]
- 63.Morimoto K, Janssen WJ, Fessler MB, et al. Lovastatin enhances clearance of apoptotic cells (efferocytosis) with implications for chronic obstructive pulmonary disease. J Immunol. 2006;176(12):7657–65. [DOI] [PubMed] [Google Scholar]
- 64.Lee YJ, Kim MJ, Yoon YS, et al. Simvastatin treatment boosts benefits of apoptotic cell infusion in murine lung fibrosis. Cell Death Dis. 2017;8(6):e2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tajbakhsh A, Gheibihayat SM, Askari H, et al. Statin-regulated phagocytosis and efferocytosis in physiological and pathological conditions. Pharmacol Ther. 2022;238:108282. [DOI] [PubMed] [Google Scholar]
- 66.Zhang L, Richard AS, Jackson CB, et al. Phosphatidylethanolamine and phosphatidylserine synergize to enhance GAS6/AXL-Mediated virus infection and efferocytosis. J Virol. 2020;95(2):e02079–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Y, Zhang LF, Zhang JJ, et al. Spontaneous inflammation resolution inspired nanoparticles promote neutrophil apoptosis and macrophage efferocytosis for acute respiratory distress syndrome treatment. Adv Healthc Mater. 2025;14(6):e2402421. [DOI] [PubMed] [Google Scholar]
- 68.Chuang ST, Stein JB, Nevins S, et al. Enhancing CAR macrophage efferocytosis via surface engineered lipid nanoparticles targeting LXR signaling. Adv Mater. 2024;36(19):e2308377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu X, Ou X, Zhang T, et al. In situ neutrophil apoptosis and macrophage efferocytosis mediated by glycyrrhiza protein nanoparticles for acute inflammation therapy. J Control Release. 2024;369:215–30. [DOI] [PubMed] [Google Scholar]
- 70.Wu Y, Wang C, Yan Y, et al. Efferocytosis nanoinhibitors to promote secondary necrosis and potentiate the immunogenicity of conventional cancer therapies for improved therapeutic benefits. ACS Nano. 2023;17(18):18089–102. [DOI] [PubMed] [Google Scholar]
- 71.Wu H, Cheng K, Guo Q, et al. Mapping knowledge structure and themes trends of osteoporosis in rheumatoid arthritis: A bibliometric analysis. Front Med (Lausanne). 2021;8:787228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available upon request.