Abstract
Background
Despite high post-implementation adherence, clinicians may have unresolved questions or concerns regarding use of a protocol to standardize routine daily coordination of the spontaneous awakening trial (SAT) and spontaneous breathing trial (SBT) on ventilated patients. Unresolved questions or concerns may unwittingly curtail practice normalization, impacting practice sustainment when implementation support is withdrawn. The objective of this study was to identify unresolved questions or concerns that may persist following successful implementation of a coordinated SAT/SBT (C-SAT/SBT) protocol.
Methods
We used an attributed, cross-sectional survey of physicians, advanced practice providers, nurses and respiratory therapists likely to have participated in a C-SAT/SBT in 12 hospitals (15 intensive care units) in Utah and Idaho. We evaluated clinician perceptions of acceptability, including ease of use, usefulness and confidence, along with perceived practice normalization, six months post implementation of a protocol to routinize C-SAT/SBT use.
Results
C-SAT/SBT adherence was 83.1% at the 6th month post implementation. 606 clinicians completed the survey (response rate: 50.0%). Perceived individual usefulness, ease of use, and confidence using the C-SAT/SBT protocol were high [range: 72.1%-88.1% agree/strongly agree], though individuals not performing an SAT or SBT in more than six months and respiratory therapists scored lower. Perceived practice normalization was similar with 82.0% aggregate agreement [agree/strongly agree]. However, when stratifying respondents into four categories based upon respondent percentage agreement with all statements, 71% did not agree with at least one practice normalization statement and 27% agreed with less than 80% of statements, varying by role and site. Sets of observable characteristics or phenotypes regarding the degree of practice normalization begin to emerge by subgroup.
Conclusions
Unresolved questions or concerns may persist regarding implementation of a C-SAT/SBT protocol among certain population subgroups despite current high practice adherence and high levels of perceived acceptability, including ease of use, usefulness and confidence. It is not clear what impact these unresolved questions or concerns may have on practice normalization and multi-year practice sustainment systemwide, including whether targeted late post-implementation strategies are needed to mitigate concerns and promote sustainment when implementation support is withdrawn.
Supplementary Information
The online version contains supplementary material available at 10.1186/s43058-025-00770-5.
Keywords: Spontaneous awakening trial, Spontaneous breathing trial, Acceptability, Practice normalization, Practice sustainment, Normalization process theory, Normalization MeAsure Development (NoMAD) survey
Contributions to the literature.
• National guidelines for ventilated patients recommend routine daily coordination of spontaneous awakening and breathing trials, which remain underused.
• Although we found high post-implementation adherence and individual practice acceptance of a coordinated spontaneous awakening and breathing trial protocol, unresolved questions or concerns persisted among subgroups. This may unwittingly curtail practice normalization, impacting practice sustainment when implementation support is withdrawn.
• These findings contribute to recognized gaps in the literature, including use of theory-informed tools in the intensive care unit, such as the Normalization MeAsure Development (NOMAD) survey, to ascertain potential post-implementation threats to practice normalization impacting sustainment, allowing time for mitigation.
Background
Invasive mechanical ventilation (IMV) is a lifesaving treatment each year for over 400,000 US patients with acute respiratory failure [1]. Sedation and analgesia improve patient comfort and ventilator synchrony, reduce oxygen use, and decrease pain. However, the benefits of sedation and analgesia need to be balanced by the potential harm of prolonged sedation and IMV. Daily interruptions in sedation generally performed by a nurse [spontaneous awakening trials (SAT)] reduce IMV duration and intensive care unit (ICU) length of stay (LOS) while preserving patient comfort and safety [2, 3]. Daily spontaneous breathing trials (SBT), usually performed by a respiratory therapist (RT), decrease IMV duration and reduce ICU patient costs for all types of respiratory failure [4–9]. When SAT and SBT are paired – meaning the SAT is followed shortly by an SBT in a coordinated fashion (C-SAT/SBT) – this evidence-based practice increases ventilator free days, decreases ICU LOS, improves mortality [10], and reduces ventilator-associated events [11]. As part of a coordinated bundle, C-SAT/SBT reduces ICU costs and improves mortality [12–14].
Despite national guidelines recommending daily performance of C-SAT/SBTs [15–17], they remain underused [18–23]. Implementations of C-SAT/SBT have focused on multistep protocols led by nurses and respiratory therapists [2, 4, 10, 14, 24–27]. Implementation determinants include patient, clinician, intervention (protocol) and ICU system factors that require individual and group behavior change to increase use [28–32], including a clear understanding of protocol steps, role clarity, ability to predict team member behaviors and tight coordination of daily sequencing between RT and nurse dyads working as care teams [28–32]. Intermountain Health (Intermountain) recently implemented a C-SAT/SBT protocol using a supervisor-led audit and feedback strategy with remote monitoring and prompting at some sites as part of a clinical trial to improve adherence to C-SAT/SBT (NCT05141396) [33]. The objective of this study was to utilize an attributed cross-sectional survey using measures of both perceived individual and group behavior to evaluate acceptability and the degree of perceived practice normalization at a timepoint 6 months post implementation of a protocol to promote adherence to C-SAT/SBT, adjusting for pre-defined strata. The 6-month post implementation period was selected, absent an empirical rationale, based upon the adherence rate and our internal experience with the time required for an evidence-based intervention to fully diffuse within the organization. We hypothesized that the inclusion of a measure of practice normalization, associated with future practice sustainability, could assist in identifying subgroups for possible tailored late-stage implementation strategies.
Methods
This cross-sectional survey was conducted under a 5-year U-01 grant with the National Heart, Lung, and Blood Institute (U01HL159878) and in conjunction with an ongoing Type II hybrid-effectiveness implementation cluster-randomized trial studying the differential impact of incremental telehealth-enabled remote monitoring and prompting on adherence to coordinated awakening and breathing trials (TEACH). (NCT05141396) [33].
Implementation context and interventions
The site population includes 12 Intermountain hospitals (15 adult ICUs) in Utah and Idaho providing IMV care to over 3600 adult patients annually in diverse settings [32]. The 12 hospitals include one quaternary care and teaching hospital, four tertiary care regional hospitals and seven community hospitals. The quaternary care hospital includes separate medical/surgical, thoracic, neurologic and coronary units, with a general ICU at all remaining hospitals. The one quaternary care and four tertiary care regional hospital ICUs are staffed by intensivists, with the seven community hospital ICUs staffed by hospitalists. Intermountain operates a systemwide ICU telemedicine system (tele-critical care) in which ICU nurses, respiratory therapists (RTs), advanced practice providers (APPs) and critical care physicians assist with monitoring and quality improvement for all adult ICU patients. For patients admitted to community hospitals, a tele-critical care board-certified critical care physician provides proactive and on-call consultation with the hospitalist-staffed ICU.
The primary clinical intervention was the deployment of a standardized C-SAT/SBT screening and performance protocol algorithm at all study sites developed from literature review; input from subject matter experts; input from physicians and APPs, nurses and RTs; and qualitative investigation of bedside practices and barriers related to C-SAT/SBT [32, 33]. Supervisor-led audit and feedback was the principal implementation strategy deployed across all study sites to promote use of the C-SAT/SBT protocol by individual clinicians. To enable supervisor-led audit and feedback, supervisors received adherence reporting weekly from a systemwide electronic dashboard. Supporting strategies included use of an enterprise cross-disciplinary executive implementation team; selection and training of local site project leaders/champions given their defined site leadership/training role, experience using C-SAT/SBT and perceived local influence; use of an enterprise implementation advisor; and the development and distribution of an implementation toolkit to each site. Certain sites in the larger TEACH study were randomized to also receive incremental targeted C-SAT/SBT remote monitoring and prompting from a centralized tele-critical care team. A complete description of the implementation strategies along with the methodology for calculating C-SAT/SBT adherence are published elsewhere [33].
Subjects
We surveyed all physicians (critical care intensivists and hospitalists), APPs, nurses and RTs employed at the in-scope hospitals as of March 1, 2023 (6 months following implementation of the C-SAT/SBT protocol), who could treat an IMV patient in their role. All physicians and APPs caring for patients in the ICUs are employed by Intermountain. After drawing and validating the survey population, the survey was conducted from May–August 2023. Hospitalists at community hospitals who had not treated at least one IMV patient in the past year were excluded. The Intermountain Health Institutional Review Board reviewed this study and granted approval with a waiver of documentation of informed consent. (#1052246).
Survey instrument and conduct
We adhered to best practices in survey research [34]. To assess perceived individual behaviors 6 months post implementation, we included more classical individual survey questions adapted from Acceptability of the Intervention Model [35] and the Technology Acceptance Model [36, 37] regarding ease of use, usefulness and confidence using the C-SAT/SBT protocol. These models are influential extensions of the theory of reasoned action and commonly used for measuring individual behavioral intent to use an intervention [35–37].
To evaluate the level of perceived practice normalization of the C-SAT/SBT protocol at a timepoint 6 months post-implementation, we analyzed individual and group behavior constructs using normalization process theory (NPT). NPT is a middle-range sociologic theory [38] with roots in complex adaptive systems theory [39, 40]. NPT and its related framework focus on both the individual and collective behaviors shown to be important in empirical studies in routinization of implementation processes [41]. The framework has good face validity in designing and evaluating evidence-based practice implementations [42–47]; and is useful across a wide variety of clinical settings [48], but with limited applications to date in adult ICU settings [49, 50]. To measure practice normalization using NPT, we adapted the previously validated practice-agnostic Normalization MeAsure Development (NoMAD) survey for comparison of the relative level of normalization across sites or studies [51–53] using the normalization process scale of 1 to 5 (5 = strongly agree), with higher statement agreement suggesting higher potential for the practice to normalize [52]. Consistent with recommendations by the original NoMAD development team, we applied the theory constructs flexibly in developing the Likert-style statements to reflect local context and the nature of the intervention and to focus on what issues were deemed most important in this context to intervention stakeholders (physicians/APPs, nurses and RTs) and to minimize survey burden to busy front-line teams [52, 54]. (Participant demographic information was also captured (Table S1)). The reconciliation of the final NoMAD constructs used in the survey along with the final survey are included in the Supplemental Materials.
To minimize survey burden and administration, respondents received the appropriate statements based upon their professional role (intensivist/APP/hospitalist – 15 statements; nurse/RT – 21 statements). After optimization for brevity and content based on literature and subject matter expertise, questions were validated with nurse and RT subject matter experts using the think-aloud method of cognitive interviewing [55]. The final survey instrument implemented using Research Electronic Data Capture system (REDCap, Vanderbilt University, Nashville, TN, USA) [56] and employed branching logic and contingent prompts for missing responses.
The survey was attributed to the respondent to facilitate future linking of survey responses to actual C-SAT/SBT use. No incentives or compensation were given to respondents to complete the survey. All respondents were employees of Intermountain. Each respondent received an individual notification via email to participate. Survey links contained in the email were unique to individuals, preventing more than one response per participant. The survey interface did not require respondents to answer every question before submission. Individuals who did not respond to the survey received at least 3 reminder emails over a three-month period after which the survey was closed to further responses. Surveys were pre-specified for exclusion if the participant failed to respond to at least three of the Likert-style statements. Surveys were not excluded if the respondent failed to provide demographic or work-related descriptive information.
Statistical analysis
The primary pre-specified outcomes were the respondents’ overall perceptions of acceptability using classical measures of individual behavior change including ease of use, usefulness and overall confidence, along with respondents’ perceptions regarding perceived practice normalization at a timepoint of 6 months post-implementation. The NoMAD use guidance does not prescribe specific formulae for the aggregation of construct-level statements into NoMAD domain and overall aggregate normalization scores [51, 52]. As such, we counted the number of statement responses marked agree/strongly agree overall and by domain with Likert-style variables presented as both non-dichotomized and dichotomized results (agree/strongly agree vs all other). To address our hypothesis that measures would likely vary by pre-defined strata, we examined individual statement responses by professional role (intensivist, hospitalist or advanced practice provider; nurse; respiratory therapist); clinical practice experience (≤ 5 years, > 5 years); project role (not site project leader/champion, site project leader/champion); site ICU physician staffing model (intensivist, hospitalist); clinical experience at site (≤ 1 year, > 1 year) and when they last performed an SAT or SBT (≤ 6 months, > 6 months). Survey responses were collected over four months. To evaluate whether response patterns differed between earlier and later responders, we examined response differences based upon timing of participant response from the initial survey request date using pre-determined cut-points (≤ 2 months, > 2 months). To further understand patterns of normalization by individual respondent, absent an existing empirical rationale, we stratified the study population into four groups based upon individual respondent level of agreement with the normalization survey constructs: agree with 100% of constructs; 80–99%; 60–79%; and < 60% and assessed variability in results by the pre-defined strata noted above.
We utilized descriptive statistics to characterize the results. Testing of variation in pre-specified strata was done using Chi-square tests for categorized or dichotomized Likert-style variables and Wilcoxon rank sum or Kruskal Wallis tests, as appropriate, for non-dichotomized Likert-style variables. For the stratified analysis, we accounted for clustering by hospital—including site-level variation in implementation strategy, intensity, workflow and culture—using random effects regression. Survey responses and between group variation are depicted using diverging stacked bar charts. The level of normalization agreement by individual respondent was visualized using a grid or matrix heat map to facilitate inspection of row, column and joint data, using a diverging color scheme with mid-range values in white, and an increased intensity of green or red in the higher or lower values, respectively [57]. Response proportions are reported with binomial exact 95% CIs adjusted for clustering by hospital. Correlations between individual behavior change statement rank responses and perceived practice normalization statement rank responses were tested using Kendall’s tau-b rank correlation. The tau-b coefficient was computed using pairwise deletion with a Bonferroni correction and bias-corrected and accelerated (BCa) bootstrap confidence intervals. A two-tailed P-value of 0.05 or less was considered statistically significant for all tests. Analysis employed Stata version 13 (StataCorp LP).
Results
Targeted study population and response rates
From September 1, 2022, to February 28, 2023, the 15 ICUs had 1077 patients ≥ 16 years old, excluding solid organ donors, on IMV for ≥ 24 h. This population was 41% female with a mean age of 57 years. Median IMV duration was 4 days (interquartile range [IQR], 2–6 days). The median patient Acute Physiology and Chronic Evaluation Score IV was 90 (IQR, 70–111). The median Charlson comorbidity index count per patient was 4 (IQR, 2–6) (Table 1). The C-SAT/SBT adherence across study sites was 83.1% at the 6th month post implementation (February 2023).
Table 1.
Characteristics of patients receiving invasive mechanical ventilation for ≥ 24 h at study sites from September 1, 2022, to February 28, 2023
| Characteristics | Patients (N = 1077) |
|---|---|
| Age, mean, yr (SD) | 57 (18) |
| Age groups, n (%) | |
| 16–17 yr | 6 (< 1) |
| 18–54 yr | 422 (39) |
| 55–79 yr | 563 (52) |
| 80 + yr | 86 (8) |
| Female, n (%) | 445 (41) |
| Ethnicity, n (%) | |
| Hispanic | 124 (12) |
| Not Hispanic | 854 (79) |
| Unknown | 99 (9) |
| Race, n (%) | |
| Black | 14 (1) |
| White | 857 (80) |
| Other | 125 (12) |
| Unknown | 81 (8) |
| Duration of invasive mechanical ventilation | |
| Mean, d (range) | 7 (1–103) |
| Median, d (IQR) | 4 (2–6) |
| Charlson comorbidities, median (IQR) | 4 (2–6) |
| Acute Physiology and Chronic Health Evaluation Score IV, median (IQR) | 90 (70–111) |
Of 1211 clinicians eligible to receive and take the survey (Figure S1), 606 clinicians including 88 providers (physicians and APPs) (54.0%), 342 nurses (49.2%) and 176 RTs (49.9%) included survey responses for an overall response rate of 50.0% consistent across professional roles. Respondents completing had a median age of 37 years [IQR: 29–46 years]. Sixty-three percent (63%) of clinicians had clinical experience > 5 years with 62% having > 3 years of clinical experience at their current hospital ICU. Ninety percent (90%) of clinicians have participated in at least 1 SAT/SBT in the past 6 months, consistent across professional roles. An overview of the characteristics of the respondents is provided in Table 2.
Table 2.
Final respondent profile by professional role
| Survey Response | |||||
|---|---|---|---|---|---|
| Overall (n = 606) | Physicians/APPs (n = 88) | Nurses (n = 342) | Respiratory Therapists (n = 176) | ||
| Age, years, median (IQR) | All | 39 (29–46) | 43 (39–50) | 33 (27–43) | 39.5 (29–50) |
| Gender, n (%)* | Woman | 375 (62) | 27 (31) | 247 (72) | 101 (57) |
| Man | 204 (34) | 55 (63) | 86 (25) | 63 (36) | |
| Transgender | 1 (0) | 0 (0) | 0 (0) | 1 (1) | |
| Prefer not to reply | 8 (1) | 3 (3) | 2 (1) | 3 (2) | |
| No response | 18 (3) | 3 (3) | 7 (2) | 8 (5) | |
| Race, n (%) | White | 530 (87) | 76 (86) | 303 (89) | 151 (86) |
| Black or African American | 4 (1) | 0 (0) | 3 (1) | 1 (1) | |
| Native American, Native Indian, Alaskan Native | 6 (1) | 0 (0) | 4 (1) | 2 (1) | |
| Asian | 17 (3) | 4 (5) | 11 (3) | 2 (1) | |
| Native Hawaiian, Pacific Islander | 1 (0) | 0 (0) | 1 (0) | 0 (0) | |
| Bi-racial, multi-racial, mixed race | 20 (3) | 4 (5) | 8(2) | 8 (5) | |
| No response | 28 (5) | 4 (5) | 12 (4) | 12 (7) | |
| Ethnicity, n (%) | Non-Hispanic | 544 (90) | 82 (93) | 313 (92) | 149 (85) |
| Hispanic | 39 (6) | 2 (2) | 20 (6) | 17 (10) | |
| No response | 23 (4) | 4 (5) | 9 (3) | 10 (6) | |
| Project role, n (%)* | Site leader/champion (Nurse/RT) | 54 (9) | n/a | 29 (8) | 25 (14) |
| Hospital setting by staffing, n (%)* | Hospitalist | 115 (19) | 23 (26) | 47 (14) | 45 (26) |
| Intensivist | 491 (81) | 65 (74) | 295 (86) | 131 (74) | |
| Experience in clinical practice, years, n (%)* | < 1 year | 22 (4) | 1 (1) | 18 (5) | 3 (2) |
| 1–5 years | 196 (32) | 11 (13) | 128 (37) | 57 (32) | |
| 6–10 years | 119 (20) | 22 (25) | 69 (20) | 28 (16) | |
| > 10 years | 258 (43) | 52 (59) | 125 (37) | 81 (46) | |
| No response | 11 (2) | 2 (2) | 2 (1) | 7 (4) | |
| Experience at current hospital, years, n (%)* | < 1 year | 68 (11) | 11 (13) | 47 (14) | 10 (6) |
| 1–3 years | 155 (26) | 10 (11) | 88 (26) | 57 (32) | |
| 4–6 years | 125 (21) | 21 (24) | 74 (22) | 30 (17) | |
| > 6 years | 251 (41) | 46 (52) | 132 (39) | 73 (41) | |
| No Response | 7 (1) | 0 (0) | 1 (0) | 6 (3) | |
| Times participating in C-SAT/SBT in the past 6 months, n (%)* | Never | 61 (10) | 8 (9) | 28 (8) | 25 (14) |
| Once | 38 (6) | 5 (6) | 14 (4) | 19 (11) | |
| 2–5 times | 120 (20) | 18 (20) | 68 (20) | 34 (19) | |
| > 5 times | 380 (63) | 57 (65) | 230 (67) | 93 (53) | |
| No response | 7 (1) | 0 (0) | 2 (1) | 5 (3) | |
| Survey completion, n (%) | ≤ 2 months from receipt | 527 (87) | 78 (89) | 292 (85) | 157 (89) |
| > 2 months from receipt | 79 (13) | 10 (11) | 50 (15) | 19 (11) | |
Significant difference by professional role across two or more stratum at p < 0.05 Abbreviations: SAT – Spontaneous awakening trial; SBT – spontaneous breathing trial; C-SAT/SBT—coordinated SAT/SBT; y-years; IQR – interquartile range
Survey respondent demographics were representative of the target population by demographic characteristics (clinician age, gender and race) and ICU site staff model type. Response rates varied by age group, project role, clinician ethnicity and individual site (Table S1).
Main results
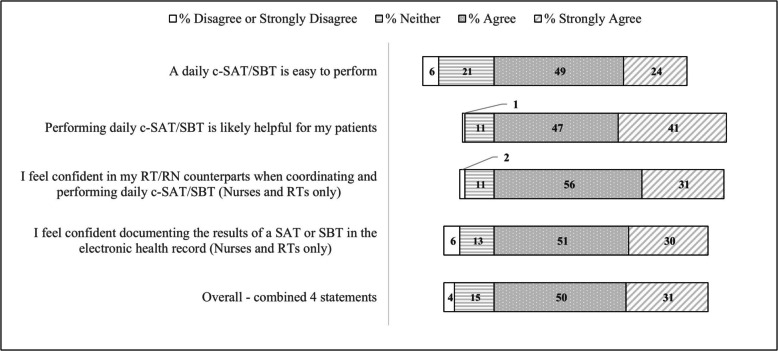
Classical measures of individual behavior change are shown in Fig. 1. Overall, 81.5% (95%CI: 79.1–83.7%) of the 4 statements were marked agree/strongly agree. RTs and those who had not performed a C-SAT/SBT in the past 6 months reported consistently lower agreement across most statements. (Table S2).
Fig. 1.
Clinicians’ opinions about the perceived ease of use, usefulness and confidence using and documenting the Intermountain Health coordinated spontaneous awakening and breathing trial protocol
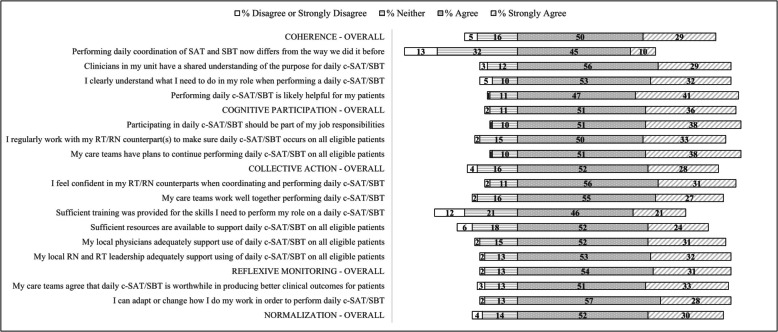
Perceived C-SAT/SBT practice normalization is shown in Fig. 2. Adjusting for site random effect, respondents agreed/strongly agreed with 82.0% (95%CI: 81.1–82.9%) of the practice normalization statements (Figure S2). RTs, those who had not performed a C-SAT/SBT in the past 6 months and (to a lesser extent) respondents from hospitalist-staffed ICUs and those that were not site project leaders/champions, reported consistently lower agreement across individual constructs. Hospitalist-staffed ICUs reported lower levels of shared team understanding of the purpose of daily C-SAT/SBT and their role in performing one. They also reported lower levels of training and physician support. (Table S3-S6).
Fig. 2.
Clinicians’ opinions regarding perceived practice normalization of coordinated spontaneous awakening and spontaneous breathing trials by individual construct statement
Nearly all comparisons between classical individual behavior change statements and practice normalization statements showed significant positive correlation (Table S7-S8), with an overall moderate to strong positive correlation τ = 0.64 (95% BCa CI: 0.60-0.68) when comparing combined results.
Stratifying individual respondent results into 4 groups based upon percentage agreement with all practice normalization statements, potential differences emerged (Fig. 3). Group 1 clinicians (n = 175, 29% of respondents) agreed with all and Group 2 clinicians (n = 265, 44%) agreed with 80–99% of perceived practice normalization statements, suggesting a higher level of practice normalization within these two groups. Group 3 clinicians (n = 88, 14.5%) agreed with 60–79% and Group 4 clinicians (n = 78, 12.9%) agreed with < 60% of practice normalization statements suggesting a lower level of perceived practice normalization in these two groups.
Fig. 3.
A grid heat map visualizing clinicians’ opinions regarding perceived practice normalization of coordinated awakening and spontaneous breath trials by individual respondent percentage agreement with practice normalization statements. Absent an existing empirical rationale, the percentage agreement was divided into four groups as follows: Group 1: 100% or complete agreement with all statements; Group 2: 80–99% agreement with statements; Group 3: 60–79% agreement with statements; Group 4: < 60% agreement with statements
In the Fig. 3 grid heat map, Group 2 clinicians who agreed with 80–99% of normalization statements stand out as having a markedly low 39% agreement with the statement “performing daily coordination of SAT and SBT now differs from the way they did it before”. Regarding group level practice normalization activities, respondents in Group 3 who agreed with 60–79% of normalization statements were: less likely than Group 1 and 2 combined to agree that “daily c-SAT/SBT is worthwhile in producing better clinical outcomes for patients” (70%); felt less “confident in their RN/RT counterparts when coordinating and performing daily c-SAT/SBT” (76%); and felt less likely that their “care teams work well together when performing a daily c-SAT/SBT” (69%). The level of agreement with sufficiency of resources and training and adequacy of local physician support were also lower than for Group 1 and 2 (25%, 55% and 70%, respectively). Group 4 respondents expressed majority agreement with only 2 of the statements, that “performing a C-SAT/SBT was part of their job responsibilities” (55%) and that it was “likely helpful for their patients” (60%) with less than half agreeing with each of the remaining practice normalization statements. Hospitalist-staffed ICUs, physicians and RTs, those who had not performed a C-SAT/SBT in the past 6 months, and those that were not site project leaders/champions were disproportionately represented in Group 3 or 4. (Table S9) Analyzing role by site type, physicians at hospitalist-led sites and RTs at intensivist-led sites had the highest unadjusted probability of being in Group 3 or 4 (Table 3).
Table 3.
Unadjusted probability of being included in a group that agrees with < 80% of all practice normalization statements (n = 606 respondents)
| Hospitalist-staffed sites 95% CI, binomial exact |
Intensivist-staffed sites 95% CI, binomial exact |
|
|---|---|---|
| Physician |
.57 (.35-.77) n = 23 |
.29 (.19-.42) n = 65 |
| Nurse |
.32 (.19-.47) n = 47 |
.20 (.16-.25) n = 295 |
| Respiratory Therapist |
.29 (.16-.44) n = 45 |
.35 (.27-.44) n = 131 |
Discussion
Our study found subjective individual reports of ease of use, usefulness and confidence were high following implementation of a C-SAT/SBT protocol with high adherence at 6 months post-implementation, though comparative studies are limited. One survey of a national teaching hospital ICU in Italy found that 71% of respondents judged the C-SAT/SBT protocols in their system easy to understand and use as part of an ABCDEF bundle [58]. A separate study in the United States implementing ABCDE bundle at 5 adult ICU, 1 step down unit, and a special care unit located in a 624-bed academic medical center that surveyed interprofessional ICU team members found a high level (83%) of individual understanding of bundle components (including SAT/SBT) 9 months post implementation, with 75% expressing confidence in their ability to use the bundle. However, only 50% felt the bundle improved patient outcomes. Post-implementation daily SAT rates for patients on a continuously infused opioid medication were 50% and post-implementation SBT rates were measured at 84% (measured as at least one SBT during the ICU stay) [24].
Physicians at hospitalist-led sites had lower agreement with practice normalization statements (Table 3). The study includes 7 smaller urban and rural hospital sites with hospitalist-staffed ICUs that handle meaningfully lower volumes of ventilated patients (< 10% of IMV patients impacted by the study). While implementation strategies were deployed across all sites, we may have been less successful including hospitalist-led sites in this implementation. Hospitalists at these sites are responsible for all inpatient care, including ICU care with intensivist support via tele-critical care. These sites act as receiving sites for patients needing intensive care when ventilation management may occur for a short duration, often resulting from acute circumstances, such as a drug overdose or postoperative care. More serious ICU cases involving longer duration ventilation management are usually transferred rapidly to one of the five intensivist-managed ICUs. Given the system-level organization of IMV care, hospitalist-led sites perform c-SAT/SBT far less frequently, providing them with fewer opportunities to mobilize collectively and build routines. At this stage of the clinical trial, overall aggregate adherence to C-SAT/SBT was similar at community hospitals and intensivist-staffed locations. The long-term impact of lower perceived practice normalization among these sites on practice sustainment is not known.
RTs at intensivist-led sites had lower agreement with practice normalization statements (Table 3).The 5 intensivist-staffed sites in this study are geographically distributed in the larger urban areas across Utah and account for almost 90% of IMV patient volume across the study sites. RTs at these sites have historically had a high degree of local autonomy making ventilator management decisions, under the direction of the local physician. Introduction of more standard approaches for ventilator management systemwide have met with some resistance at these larger sites [59]. Further, RTs at intensivist-led sites can be responsible for 6–8 IMV patients on any given day, sometimes across multiple ICUs with different physician/nurse care teams, while the nurse is responsible for 1–2 patients in close physical proximity. When initiating and performing a C-SAT/SBT, the nurse and RT perform non-exchangeable roles where one member of the dyad has some control over the actions of the other [60, 61]. C-SAT/SBT intervention elements that contribute to the asymmetric nature of this relationship include: (1) successful RT performance of the SBT is dependent on nurse performance of the SAT and not vice versa; (2) RN’s initiate the SAT first, giving the RN greater control than the RT over C-SAT/SBT timing; and (3) RNs perform fewer C-SAT/SBTs than RTs with RTs sharing a disproportionate daily time burden relative to the RN to ensure full adherence to C-SAT/SBT. RT frustration with a lack of control, identified as a potential challenge in pre-implementation interviews [32] along with the RTs downstream visibility to how each C-SAT/SBT concludes, may further explain disparities between RT and RN perceptions regarding the degree of practice normalization. Effective collaboration between the nurse and RT is not an automatic by-product of co-location [62]. While protocols, along with checklists and decision support tools, are designed to minimize perceived power disparities [63], additional strategies that target joint determinants may need to be considered to address remaining RT concerns, including ensuring that the desired workflow interaction is reasonably scripted and understood by both roles. It is unclear how RT perceptions regarding practice normalization may impact practice sustainment over time.
Identified correlations between classical measures of individual behavior change and practice normalization statements (designed to capture both individual and group behaviors) are noteworthy given practical considerations. While healthcare delivery systems want to understand whether an evidence-based practice will persist once implementation support is withdrawn by assessing clinician intent, the use of surveys to measure intent comes with a resource burden [54]. More research is needed to determine whether simpler survey instruments that are faster and less burdensome may be sufficient to support routine operational use in efforts to measure sustainment risk.
Strengths and limitations
This study has limitations. This cross-sectional study was not designed to establish a causal association between the defined implementation strategies and improvements in system adherence to C-SAT/SBT. The study is limited to a single measurement of perceived practice normalization at a timepoint 6 months post implementation and not a set of repeated measures of practice normalization over time. While respondents represent a diverse set of ICU practice settings, the generalizability of study results may be limited by our conducting the study within a single delivery system which has specific system and site level administrative polices, patient mix and volumes, geographic location and site distribution, degree of operational integration and available system infrastructure (including ICU telemedicine, electronic health record platform, et al.). Clinician staffing and patient characteristics may not be replicated in all settings. While clear communications were given regarding the intent and purpose of the survey, and the survey was conducted by researchers not directly affiliated with the department, the overall response rate and respondent answers may have been influenced by the researcher’s alignment with the institution as well as the attributed nature of the survey.
The study has several strengths, including an appreciable response rate (> 50%) from a large cross-disciplinary sample of physicians, nurses and respiratory therapists with diverse backgrounds and professional experience, and consistent response rates across professional roles. Respondents were drawn from a geographically diverse set of ICU types, hospital trauma center designations, physician staffing models, physical locations (urban, rural and frontier) and patient ICU volumes with a random effects model to adjust for between site variation. The study was conducted in conjunction with a larger effort to implement and sustain adherence to a C-SAT/SBT protocol in a delivery system. The results of this study suggest that despite current high practice levels and perceived acceptability, including ease of use, usefulness and confidence 6 months post-implementation, unresolved questions or concerns persist among certain respondent sub-groups. Currently it is not clear what impact these unresolved questions or concerns may have on multi-year practice sustainment systemwide, including whether targeted late post-implementation strategies are needed to address remaining unresolved concerns to promote sustainment once implementation support is withdrawn. These results are actionable and will inform further research associated with the sustainment phase of a type II hybrid implementation-effectiveness trial (NCT05141396) to evaluate the impact that these respondent sub-groups have over time on practice sustainment once implementation support is withdrawn.
Supplementary Information
Acknowledgements
We want to acknowledge the Intermountain Health caregivers that work tirelessly on behalf of their patients.
Abbreviations
- SAT
Spontaneous awakening trial
- SBT
Spontaneous breathing trial
- C-SAT/SBT
Coordinated spontaneous awakening and breathing trial
- IMV
Invasive mechanical ventilation
- TEACH
Telehealth-Enabled Real-Time Audit and Feedback for Clinician AdHerence type II hybrid effectiveness-implementation cluster randomized trial
- ICU
Intensive care unit
- LOS
Length of stay
- APP
Advanced practice provider
- RN
Registered nurse
- RT
Respiratory therapist
- NPT
Normalization Process Theory
- NoMAD
Normalization MeAsure Development survey
Authors’ contributions
AJK, JK, IDP, DL, DW, LL, RH, CKG and RS contributed to the conception and design of the work. AJK, DL, AK, CW, CS, CJ, LC and JRJ made specific contributions to data acquisition and analysis. All authors participated in the initial drafting and revisions of the work, approved the final revised version, and accepted accountability for the overall integrity of the research process and the article.
Funding
Provided by a grant from the National Heart Lung and Blood Institute of the National Institutes of Health (U01 HL159878). The National Heart Lung and Blood Institute had no specific role in conceptualization, design, data collection, analysis, decision to publish or preparation of the manuscript. The views in this article do not communicate an official position of Intermountain Health or the National Institutes of Health.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
The Intermountain Health Institutional Review Board reviewed this study and granted approval with a waiver of documentation of informed consent (#1052246).
Consent for publication
Not applicable.
Competing interests
AJK, JK, DL, AK, DW, CW, CS, CJ, LC, JRJ, LL and CG – the authors have no competing financial or non-financial interests.
IP—Dr. Peltan reports funding to his institution from Bluejay Diagnostics and Novartis, both unaffiliated with this research.
RH – Dr. Holubkov reports a consulting arrangement with Pfizer Inc (DSMB memberships) unaffiliated with this research.
RS—Dr. Srivastava is a physician founder of the I-PASS Patient Safety Institute. His employer, Intermountain Health, owns his equity in the I-PASS Patient Safety Institute. Dr. Srivastava is supported in part by grants from the NIH, CDC, AHRQ and PCORI. Dr. Srivastava has received monetary awards, honorariums, and travel reimbursement from multiple academic and professional organizations for talks about pediatric hospitalist research networks and quality of care.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wunsch H, Linde-Zwirble WT, Angus DC, Hartman ME, Milbrandt EB, Kahn JM. The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010;38(10):1947–53. [DOI] [PubMed] [Google Scholar]
- 2.Brook AD, Ahrens TS, Schaiff R, et al. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med. 1999;27(12):2609–15. [DOI] [PubMed] [Google Scholar]
- 3.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342(20):1471–7. [DOI] [PubMed] [Google Scholar]
- 4.Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996;335(25):1864–9. [DOI] [PubMed] [Google Scholar]
- 5.MacIntyre NR, Cook DJ, Ely EW Jr, et al. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest. 2001;120(6 Suppl):375s–95s. [DOI] [PubMed] [Google Scholar]
- 6.Esteban A, Frutos F, Tobin MJ, et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med. 1995;332(6):345–50. [DOI] [PubMed] [Google Scholar]
- 7.Kollef MH, Shapiro SD, Silver P, et al. A randomized, controlled trial of protocol-directed versus physician-directed weaning from mechanical ventilation. Crit Care Med. 1997;25(4):567–74. [DOI] [PubMed] [Google Scholar]
- 8.Marelich GP, Murin S, Battistella F, Inciardi J, Vierra T, Roby M. Protocol weaning of mechanical ventilation in medical and surgical patients by respiratory care practitioners and nurses: effect on weaning time and incidence of ventilator-associated pneumonia. Chest. 2000;118(2):459–67. [DOI] [PubMed] [Google Scholar]
- 9.Lellouche F, Mancebo J, Jolliet P, et al. A multicenter randomized trial of computer-driven protocolized weaning from mechanical ventilation. Am J Respir Crit Care Med. 2006;174(8):894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (awakening and breathing controlled trial): a randomised controlled trial. Lancet. 2008;371(9607):126–34. [DOI] [PubMed] [Google Scholar]
- 11.Klompas M, Anderson D, Trick W, et al. The preventability of ventilator-associated events. The CDC Prevention Epicenters Wake Up and Breathe Collaborative. Am J Respir Crit Care Med. 2015;191(3):292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marra A, Ely EW, Pandharipande PP, Patel MB. The ABCDEF Bundle in Critical Care. Crit Care Clin. 2017;33(2):225–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collinsworth AW, Priest EL, Masica AL. Evaluating the cost-effectiveness of the ABCDE bundle: impact of bundle adherence on inpatient and 1-year mortality and costs of care. Crit Care Med. 2020. 10.1097/CCM.0000000000004609. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh SJ, Otusanya O, Gershengorn HB, et al. Staged implementation of awakening and breathing, coordination, delirium monitoring and management, and early mobilization bundle improves patient outcomes and reduces hospital costs. Crit Care Med. 2019;47(7):885–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan E, Zakhary B, Amaral A, et al. Liberation from mechanical ventilation in critically ill adults. An official ATS/ACCP clinical practice guideline. Ann Am Thorac Soc. 2017;14(3):441–3. [DOI] [PubMed] [Google Scholar]
- 16.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. [DOI] [PubMed] [Google Scholar]
- 17.Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018. 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 18.Miller MA, Krein SL, George CT, Watson SR, Hyzy RC, Iwashyna TJ. Diverse attitudes to and understandings of spontaneous awakening trials: results from a statewide quality improvement collaborative*. Crit Care Med. 2013;41(8):1976–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kher S, Roberts RJ, Garpestad E, et al. Development, implementation, and evaluation of an institutional daily awakening and spontaneous breathing trial protocol: a quality improvement project. J Intensive Care Med. 2013;28(3):189–97. [DOI] [PubMed] [Google Scholar]
- 20.Morandi A, Piva S, Ely EW, et al. Worldwide Survey of the “Assessing Pain, Both Spontaneous Awakening and Breathing Trials, Choice of Drugs, Delirium Monitoring/Management, Early Exercise/Mobility, and Family Empowerment” (ABCDEF) Bundle. Crit Care Med. 2017;45(11):e1111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jansson MM, Syrjälä HP, Ala-Kokko TI. Implementation of strategies to liberate patients from mechanical ventilation in a tertiary-level medical center. Am J Infect Control. 2019;47(9):1065–70. [DOI] [PubMed] [Google Scholar]
- 22.Mendez MP, Lazar MH, Digiovine B, et al. Dedicated multidisciplinary ventilator bundle team and compliance with sedation vacation. Am J Crit Care. 2013;22(1):54–60. [DOI] [PubMed] [Google Scholar]
- 23.Stollings JL, Foss JJ, Ely EW, et al. Pharmacist leadership in ICU quality improvement: coordinating spontaneous awakening and breathing trials. Ann Pharmacother. 2015;49(8):883–91. [DOI] [PubMed] [Google Scholar]
- 24.Balas MC, Burke WJ, Gannon D, Cohen MZ, Colburn L, Bevil C, Franz D, Olsen KM, Ely EW, Vasilevskis EE. Implementing the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle into everyday care: opportunities, challenges, and lessons learned for implementing the ICU pain, agitation, and delirium guidelines. Crit Care Med. 2013;41(9 Suppl 1):S116–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balas MC, Vasilevskis EE, Olsen KM, et al. Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle. Crit Care Med. 2014;42(5):1024–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chumpia MM, Ganz DA, Chang ET, de Peralta SS. Reducing the rare event: lessons from the implementation of a ventilator bundle. BMJ Open Qual. 2019;8(2): e000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan BA, Fadel WF, Tricker JL, et al. Effectiveness of implementing a wake up and breathe program on sedation and delirium in the ICU. Crit Care Med. 2014;42(12):e791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balas MC, Pun BT, Pasero C, et al. Common challenges to effective ABCDEF bundle implementation: the ICU liberation campaign experience. Crit Care Nurse. 2019;39(1):46–60. [DOI] [PubMed] [Google Scholar]
- 29.Costa DK, White MR, Ginier E, et al. Identifying barriers to delivering the awakening and breathing coordination, delirium, and early exercise/mobility bundle to minimize adverse outcomes for mechanically ventilated patients: a systematic review. Chest. 2017;152(2):304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrothers KM, Barr J, Spurlock B, Ridgely MS, Damberg CL, Ely EW. Contextual issues influencing implementation and outcomes associated with an integrated approach to managing pain, agitation, and delirium in adult ICUs. Crit Care Med. 2013;41(9 Suppl 1):S128-135. [DOI] [PubMed] [Google Scholar]
- 31.Ely EW, Bennett PA, Bowton DL, Murphy SM, Florance AM, Haponik EF. Large scale implementation of a respiratory therapist-driven protocol for ventilator weaning. Am J Respir Crit Care Med. 1999;159(2):439–46. [DOI] [PubMed] [Google Scholar]
- 32.Olsen GH, Gee PM, Wolfe D, Winberg C, Carpenter L, Jones C, Jacobs JR, Leither L, Peltan ID, Singer SJ, Asch SM, Grissom CK, Srivastava R, Knighton AJ. Awakening and breathing coordination: a mixed-methods analysis of determinants of implementation. Ann Am Thorac Soc. 2023;20(10):1483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grissom CK, Holubkov R, Carpenter L, Hanna B, Jacobs JR, Jones C, Knighton AJ, Leither L, Lisonbee D, Peltan ID, Winberg C, Wolfe D, Srivastava R. Implementation of coordinated spontaneous awakening and breathing trials using telehealth-enabled, real-time audit and feedback for clinician adherence (TEACH): a type II hybrid effectiveness-implementation cluster-randomized trial. Implement Sci. 2023;18:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langbecker D, Caffery LJ, Gillespie N, Smith AC. Using survey methods in telehealth research: a practical guide. J Telemed Telecare. 2017;23:770–9. [DOI] [PubMed] [Google Scholar]
- 35.Weiner BJ, Lewis CC, Stanick C, Powell BJ, Dorsey CN, Clary AS, Boynton MH, Halko H. Psychometric assessment of three newly developed implementation outcome measures. Implement Sci. 2017;12(108):1–12. 10.1186/s13012-017-0635-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis FD. Perceived usefulness, perceived ease of use, and user acceptance of information technology. MIS Q. 1989;13(3):319–40. [Google Scholar]
- 37.Venkatesh V, Morris MG, Davis GB, Davis FD. “User acceptance of information technology: Toward a unified view” (PDF). MIS Q. 2003;27(3):425–78. [Google Scholar]
- 38.May CR, Mair F, Finch T, et al. Development of a theory of implementation and integration: Normalization process theory. Implement Sci. 2009;4(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.May C. Towards a general theory of implementation. Implementation Sci. 2013;8(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.May CR, Johnson M, Finch T. Implementation, context and complexity. Implementation Sci. 2016;11(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson MJ, Leaf AA, Pearson F, Clark HW, Dimitrov BD, Pope C, May CR. Successfully implementing and embedding guidelines to improve the nutrition and growth of preterm infants in neonatal intensive care: a prospective interventional study. BMJ Open. 2017;7(12): e017727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.May C, Finch T, Cornford J. Integrating telecare for chronic disease management in the community: what needs to be done? London: NIHR; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mair F, May C, Murray E. Understanding the implementation and integration of E-health services. London: National Co-ordinating Centre for the National Institute for Health Research Service Delivery and Organisation Programme (NCCSDO); 2009. [Google Scholar]
- 44.Mair F, May C, O’Donnell C. Factors that promote or inhibit the implementation of e-health systems: an explanatory systematic review. Bull World Health Organ. 2012;90(5):357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gunn J, Kokanovic R, Palmer V. Re-organising the care of depression and other related disorders in the Australian primary health care setting. Canberra: Australian Primary Health Care Research Institute; 2009.
- 46.Pencille LJ, Campbell ME, Van Houten HK. Protocol for the osteoporosis choice trial. A pilot randomized trial of a decision aid in primary care practice. Trials. 2009;10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murray E, Treweek S, Pope C. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med. 2010;8(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.May CR, Cummings A, Girling M, et al. Using normalization process theory in feasibility studies and process evaluations of complex healthcare interventions: a systematic review. Implement Sci. 2018;13:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oesch S, Verweij L, Clack L, Finch T, Riguzzi M, Naef R. Implementation of a multicomponent family support intervention in adult intensive care units: study protocol for an embedded mixed-methods multiple case study (FICUS implementation study). BMJ Open. 2023;13(8): e074142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roberti J, Jorro F, Rodríguez V, et al. Theory-driven, rapid formative research on quality improvement intervention for critical care of patients with COVID-19 in Argentina. Glob Qual Nurs Res. 2021. 10.1177/23333936211015660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rapley T, Girling M, Mair FS, et al. Improving the normalization of complex interventions: part 1 - development of the NoMAD instrument for assessing implementation work based on normalization process theory (NPT). BMC Med Res Methodol. 2018;18:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Finch TL, Girling M, May CR, et al. Improving the normalization of complex interventions: part 2 - validation of the NoMAD instrument for assessing implementation work based on normalization process theory (NPT). BMC Med Res Methodol. 2018;18:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lamarche L, Clark RE, Parascandalo F, Mangin D. The implementation and validation of the NoMAD during a complex primary care intervention. BMC Med Res Methodol. 2022Jun 19;22(1):175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glasgow RE, Riley WT. Pragmatic measures: what they are and why we need them. Am J Prev Med. 2013;45(2):237–43. [DOI] [PubMed] [Google Scholar]
- 55.Padilla J, Leighton J. Cognitive interviewing and think-aloud methods. In: Zumbo BD, Hubley AM, eds. Understanding and Investigating Response Processes in Validation Research. New York, NY, USA: Springer; 2017.
- 56.Harris PA, Taylor R, Minor BL. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95: 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilkinson L, Friendly M. The History of the Cluster Heat Map. American Stat. 63(2), 179–184.
- 58.Pinto F, Biancofiore G. The ABCDE Bundle: A Survey of Nurses Knowledge and Attitudes in the Intensive Care Units of a National Teaching Hospital in Italy. Dimens Crit Care Nurs. 2016;35(6):309–314. [DOI] [PubMed]
- 59.Knighton AJ, Kean J, Wolfe D, Allen L, Jacobs J, Carpenter L, Winberg C, Berry JG, Peltan ID, Grissom CK, Srivastava R. Multi-factorial barriers and facilitators to high adherence to lung-protective ventilation using a computerized protocol: a mixed methods study. Implement Sci Commun. 2020;1(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fiske AP. The four elementary forms of sociality: framework for a unified theory of social relations. Psychol Rev. 1992;99(4):689–723. [DOI] [PubMed] [Google Scholar]
- 61.Favre M, Sornette D. A generic model of dyadic social relationships. PLoS ONE. 2015;10(3): e0120882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hackman JR. Why teams don’t work. In: Tindale RS, Heath L, Edwards J, editors. Theory and research on small groups. New York, NY: Plenum; 1998. p. 245–67. [Google Scholar]
- 63.Costa DK, Barg FK, Asch DA, Kahn JM. Facilitators of an interprofessional approach to care in medical and mixed medical/surgical ICUs: a multicenter qualitative study. Res Nurs Health. 2014;37(4):326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.