Abstract
Background
Tree rejuvenation is the transition from adult state to juvenile state. Successive grafting can result in tree rejuvenation. Flavonoids constitute a prominent class of secondary metabolites that play critical roles in regulating cellular physiology, signaling, and the transduction between plant and environment interactions. Meanwhile, endogenous phytohormones are instrumental in restoring the juvenile features of trees. However, little is known about the flavonoid and hormone biosynthesis in rejuvenated Ginkgo biloba L.
Results
Here we rejuvenated the surviving 4,000-year-old Ginkgo through successive grafting. The results revealed leaf cleavage, leaf width, leaf length, and leaf area increased significantly in S1 (first-step graft). Additionally, the total flavonoid content of S2 (second-step graft) was the highest. Indole acetic acid (IAA), gibberellins (GA), and zeatin-riboside (ZR) increased, and abscisic acid (ABA) decreased in each successive generation. Transcriptome analysis of gene expression fragments per kilobase of exon model per million mapped fragments (FPKM) revealed 1756–5689 significantly differentially expressed genes (DEGs) that clustered into nine distinct expression modules. The weighted gene co-expression network analysis (WGCNA) revealed bisque4 and palevioletred3 had the highest correlation coefficient with the phenotype and physiology of rejuvenated Ginkgo and identified only eight genes were annotated in top hub ten. The expression profiles corresponding DEGs in flavonoid biosynthesis (25 DEGs) and plant hormone signal transduction (36 DEGs) were revealed. Most of these DEGs were up-regulated from S1 to S2, while down-regulated in S3 (third-step graft). Small RNA sequencing revealed 3281 miRNAs including 621 novel miRNAs. Combined analysis both of transcriptome and small RNA, miR395 was the core miRNA and targeted to iron-containing protein (FAO1) and pentatricopeptide repeat-containing protein At1g11290 (PCMP-H40).
Conclusions
The comprehensive analysis of the RNA-seq, small RNA, and physiological data in this study provided candidate genes and clarified the regulatory mechanism of successive grafting in rejuvenated Ginkgo, suggesting that the number of successive generations of grafted rejuvenation should not exceed the third generation (S₃).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12864-025-11935-w.
Keywords: Endogenous phytohormones, Flavonoid content, Rejuvenated Ginkgo, RNA-seq, Small RNA
Introduction
Rejuvenation in plants is the process of reverting to a previous developmental stage, thereby regaining some or all of the growth characteristics of the juvenile stage [1, 2]. Forest trees, which are larger and have longer life cycles, experience a gradual decline in characteristics, such as resistance, growth capacity, and rooting capacity, as they age. Ginkgo, an economically significant tree species native to China [3], has limited wild resources and is a crucial germplasm resource. Ancient trees like the 4,000-year-old specimen studied here face progressive vitality loss, including reduced photosynthetic capacity, hormonal dysregulation and, declining flavonoid synthesis—key traits underpinning their utility. These declines threaten the preservation of elite germplasm and necessitate interventions like grafting-induced rejuvenation. By restoring juvenile traits, such as enhanced leaf morphology and secondary metabolism, rejuvenation could mitigate senescence-driven losses [4].
Forest rejuvenation methods include stem cutting, continuous cutting, serial grafting, and succession of tissue culture [4]. Rejuvenation trees exhibit tenderer terminal buds than mature trees and surpass the initial individuals in terms of their capacity for growth and reproduction. Regenerating buds also demonstrate a higher photosynthetic rate and chlorophyll content than did adult buds [5]. Stemming and rejuvenation have been found to increase the medicinal constituent contents of Ginkgo biloba leaves [6]. Serial grafts involve grafting new annual shoots of mature trees onto young rootstocks, followed by successive grafts of young spikes and young rootstocks, ultimately resulting in a significant increase in the rooting capacity of mature trees. Rejuvenation has been shown to reduce the effect of maturity on morphological characteristics, particularly in the leaf and root systems. Juvenile characteristics, such as deep leaf cleavage and a high number of leaves, tended to reappear after rejuvenation treatments of Pyrus betulae folia and Malus xiaojinensis [3, 7].
Recent studies indicate that key genes significantly increased after rejuvenation, including chalcone synthase (CHS), phenylalanine ammonia-lyase (PAL), flavonol synthase (FLS), geranylgeranyl pyrophosphate (GGP), WUSCHEL-related homeobox (WOX), apetala2 response factor (AP2), cytokinin-independent 1 (CKI1), and auxin response factor (ARF), and the key genes significantly decrease, including alpha-glucosidase-like (AGL) and suppressor of overexpression of constans (SOC) [8]. In Ginkgo, homologs of these genes (GbCHS, GbPAL, GbFLS) have been identified and linked to flavonoid biosynthesis and stress responses [4, 6], but their roles in rejuvenation remain poorly understood. For instance, GbWOX and GbARF are hypothesized to regulate meristem activity during grafting, though functional validation is lacking [8]. Rejuvenation treatments, such as successive grafting and rooting of yellowed cuttings, have led to increased expression of almost all JrWOX genes in 23-year-old walnuts, as identified through genome-wide analyses [9]. In Arabidopsis thaliana, miR156 is strongly expressed in the juvenile stage and its expression decreases with plant aging [10–14]. While miR156 is conserved in Ginkgo, its expression dynamics during rejuvenation are undocumented. Similarly, miR172, which promotes adult phase transitions in angiosperms, has not been characterized rejuvenation context in Ginkgo. Successive graft rejuvenation in adult Sequoia can result in increased miR156 expression and decreased miRNA172 expression. Enrichment analyses suggested that the target genes of these benefited from the restoration of photosynthesis and improved the rooting capacity [15]. Integrated transcriptome and small RNA analyses can reveal gene expression changes before and after transcription in plants, uncovering new insights that conventional methods might miss. This approach is particularly valuable for identifying post-transcriptional regulatory mechanisms, such as miRNA-mediated gene silencing, which play critical roles in developmental transitions like rejuvenation [15]. By combining these datasets, we aimed to elucidate transcriptional networks underlying the rejuvenation process in Ginkgo.
In this study, we collected the branches from ancient 4000-years-old Ginkgo biloba tree and rejuvenated them through serial grafts. We assessed the growth parameters, leaf morphology, and endogenous hormone changes in successive generations after rejuvenation. Additionally, we screened for molecular mechanisms related to rejuvenation by integrating high-throughput transcriptome and small RNA analyses. This research aimed to enhance the theory and practice of tree rejuvenation, mitigate the effects of age on trees, improve the efficiency and sustainability of Ginkgo growth, and hold significant theoretical and practical implications for revitalizing the Ginkgo industry and cultivating of high-quality resources.
Materials and methods
Plant materials
The spikes for continuous grafting of Ginkgo biloba were sourced from the oldest 4,000-year-old female Ginkgo ancient trees at Dinglin Temple in Juxian County, Shandong Province (35°35′49″N, 118°44′0″E). The stocks were live seedlings planted in 2015 in the Ginkgo germplasm resource nursery at the Baima base of Nanjing Forestry University. Ancient Ginkgo leaves were designated as S0. Scions were derived from ancient Ginkgo and grafted onto 10–20 trees, classified as S1 (first-step graft). Similarly, S1 spikes were grafted to produce the S2 (second-step graft). S2 spikes were grafted to generate the S3 (third-step graft). Detailed information on sampling and grafting timelines is provided in Supplementary Table S1. Additionally, leaves from 5-year-old female (F) and male (M) Ginkgo plants, bred from seed sowing (Supplementary Table S1), were randomly collected for comparative analysis of juvenile characteristics. All materials were randomly collected from branches with normal growth and free of pests and diseases.
Morphological and physiological assessments in rejuvenated leaves
Cleavage length and width were measured using Vernier calipers, with cleavage length longer than 1.0 cm considered as one cleavage, and the number of cleavages per leaf was averaged. Leaf length was determined through the base of its petiole to the tip, and leaf width was measured as the maximum transverse width. Leaf area was determined by photographing and measuring using the ImageJ (v1.53). All measurements were repeated ten times.
The Ginkgo flavonoid content of entirely unfolded mature leaves from different generations of rejuvenation were collected for determination of flavonoid content, which was repeated six times. The flavonoid concentration of Ginkgo was determined using high-performance liquid chromatography (HPLC) [16]. The ground blade leaf sample (0.5 g) was refluxed for two hours with 50 mL of chloroform in a Soxhlet extractor and then evaporated to dryness. The dried residue was subsequently extracted with 50 mL of methanol for four hours at 80 °C. After cooling to room temperature, the eluent was diluted to 50 mL with methanol, and the concentrations of isorhamnetin, kaempferol, and quercetin were measured by HPLC. The HPLC system (Waters 1525, Canby, OR, USA) was operated under the following conditions: the mobile phase consisted of a methanol and 0.4% H3PO4 solution (56:44, v/v) at a flow rate of 1.0 mL/min; the column temperature was maintained at 30 °C; and the detection wavelength was set at 360 nm. Total flavonoid content was calculated according Chinese Pharmacopoeia Commission (2015) as: Total flavonoid content = (quercetin + kaempferol + isorhamnetin content) × 2.51.
The concentrations and dynamic changes of four endogenous hormones, auxin (IAA), abscisic acid (ABA), gibberellins (GA) and zeatin riboside (ZR), were measured using enzyme-linked immunosorbent assay (ELISA, Shanghai Enzymatic Biotechnology Company Ltd., Shanghai, China). Over 0.5 g of each sample was collected and determined using the kit provided by the college of agronomy and biotechnology, China Agricultural University. The data were analyzed using IBM SPSS 26 software to determine differences, with different letters in the bar chart indicating significant differences (P < 0.05).
RNA sequencing and data analysis
The total RNA was extracted using TRIzol reagent kit (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. The RNA sample quality was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). The RNA library sequencing was conducted using Illumina HiseqTM 2500 (Gene Denovo Biotechnology Co.Ltd., Guangzhou, China), with a total 16 libraries sequenced. Sequencing data in this paper can be found in the National Center for Biotechnology Information under accession number PRJNA1104754. The clean reads were mapped to the latest Ginkgo reference genome using HISAT2.4. The ribosomal RNA (rRNA) database was mapped using the short-read alignment program Bowtie2 (v2.2.8). The mapped reads from every sample were assembled in StringTie (v1.3.1) through an approach based on references. RNA differential expression between the rejuvenated groups was analyzed using DESeq2 [17] and edgeR [18] between the two samples.
Weighted gene co-expression network analysis (WGCNA)
Co-expression networks were constructed using WGCNA package in R (v1.47). To find out biologically significant modules, module eigengenes were used to calculate the correlation coefficient with rejuvenated Ginkgo. Subsequently, correlation analysis was performed to determine the correlation between each co-expression module and the collected continuous rejuvenated data of Ginkgo, enabling the identification of hub genes associated with rejuvenation. All 16 sets of transcriptome expression data of rejuvenation-related genes in three generations Ginkgo leaves were then utilized to construct a co-expression network module. A standard process was used to minimize the noise. An adjacency matrix was constructed using a soft threshold power (β = 10). Networks were identified using a dynamic tree-cut algorithm with a minimum cluster size of 30 and merging threshold of 0.25. Hub genes were identified based on their eigengene connectivity (KME > 0.7) [19]. Finally, the gene regulatory networks were visualized using Cytoscape (v3.3.0).
Small RNA library construction and data processing
Sixteen small RNA sequencing libraries were constructed following the recommended protocol and sequenced using Illumina HiseqTM 2500. Sequencing data in this paper can be found in the National Center for Biotechnology Information under accession number PRJNA1104759. After sequencing, clean tags from various generations of rejuvenated materials were aligned with small RNAs in the GeneBank database (Release 209.0) and the Rfam database (Release 11.0) to identify and eliminate rRNA, scRNA, snoRNA, snRNA, and tRNA. Subsequently, the clean tags were compared against the miRBase database (Release 22) to identify known miRNAs in the studied species. And novel miRNA candidates were identified based on their genome positions and hairpin structures predicted by mirdeep2. The total miRNA population comprises known, existing, and novel miRNAs. Differential miRNAs were identified based on a twofold or greater change in expression with a significance threshold of P < 0.05, using edgeR software [18].
Association analysis
We analyzed negatively regulated miRNA-target gene pairs in different generations of Ginkgo samples that were grafted successively. This analysis led to the construction of a miRNA-target gene regulatory network. Additionally, we enriched and analyzed the target genes of the miRNAs, providing clues for the subsequent selection of target genes for rejuvenation. To analyze miRNA differential expression, screening parameters were set at P < 0.05 and |log2 FC|> 1, whereas for the corresponding target genes, we used FDR < 0.05 and |log2FC|> 1. We employed patmatch software (v1.2) to analyze miRNA target genes through the complementary matching of small RNAs with target genes.
qRT-PCR validation mRNAs and miRNAs
Quantitative real-time PCR (qRT-PCR) was conducted using MonAmp™ RapidStart Universal SYBR® Green qPCR Mix (Monad, China). GbCHS (F: CAAGCGCATGTGCGACAAGT, R: CACCTCCACCACCATGT) [20] and U6 (CCCTGCGCAAGGATGACACGC) [21] were used as endogenous control. The primers information in this study were provided in Supplementary Table S2. The 2−∆∆Ct method was used to calculate relative expression levels. Each reaction in every experiment was repeated three times.
Statistical analysis
The phenotypic (leaf cleavage, leaf width, leaf length, and leaf area) and physiological data (flavonoid and hormone measurements) and qRT-PCR data are presented as the means ± standard deviations (SDs) of at least three independent experiments. The statistical significance of differences between the treatment and control conditions was assessed by two-sided Student’s t-tests and ANOVA with post hoc tests. P values < 0.05 and < 0.01 were regarded as statistically significant and highly significant, respectively.
Results
Morphological and physiological changes in rejuvenated leaves
In rejuvenated plants, the number of leaf cleavages, cleavage depth, cleavage length, and cleavage width were significantly increased in S1 compared with S0, resulting in the largest leaf length, width and area among the samples, indicating notable changes in leaf morphology (Fig. 1A). Subsequent grafts (S2 and S3) showed a decline in leaf cleavages, cleavage length, and cleavage width (Supplementary Table S3). The most significant decrease in leaf length observed in S3 (Fig. 1B), and leaf width in S2 (Fig. 1C). The leaf area of S1 (average leaf area ± SD = 15.4 ± 0.28 cm2) was significantly different from that of S2 and S3, while no significant difference was observed between S2 and S3 in terms of leaf area (Fig. 1D).
Fig. 1.
Morphological changes in rejuvenated leaves. A Changes in leaf morphology. B Leaf length. C Leaf width. D Laef area. S0, aged tree S0; S1, rejuvenation S1; S2, rejuvenation S2; S3, rejuvenation S3; F, juvenile female plant; M, juvenile male plant
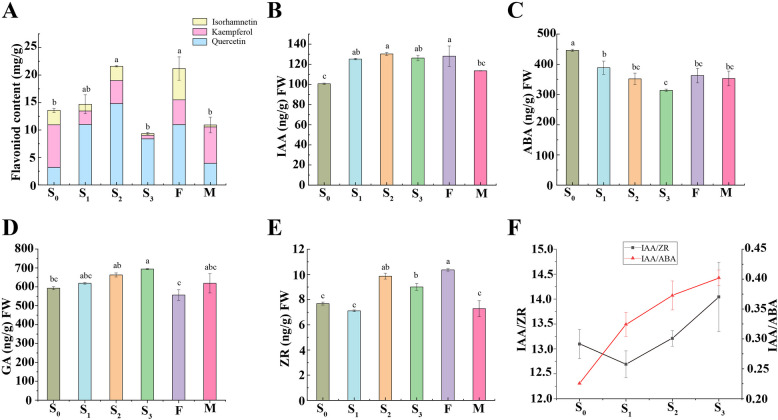
Total flavonoid content in S2 (average content ± SD = 21.69 ± 0.20 mg/g) increased by 59.04% compared to S0. It was approached the content of juvenile of male Ginkgo, while in S3 decreased to an average content ± SD of 9.40 ± 0.32 mg/g. Total flavonoid content fluctuated after various grafting and rejuvenation treatments, with quercetin > kaempferol > isorhamnetin in terms of content levels (Fig. 2A). The IAA content increased after grafting from S0, reaching an average content ± SD of 130.20 ng/g S2 (Fig. 2B). The ABA content in S3 significantly decreased by 30% (P < 0.05) compared to S0 (Fig. 2C). The GA content steadily increased with successive grafting (Fig. 2D). The ZR content initially increased in S2 exhibiting a 28.30% than S0, and S3 showed a significant 17.2% increase compared to S0 (Fig. 2E). Hormone content was ranked from high to low as follows: GA > ABA > IAA > ZR. The ratios between the two endogenous hormone contents exhibited more consistent variations among the different successional grafts than the individual hormone contents. Specifically, IAA/ZR and IAA/ABA ratios varied with the number of successional grafts (Fig. 2F). These findings suggested that grafting can alter the content and proportion of endogenous hormones in Ginkgo leaves, thereby influencing their physiological status. The results of correlation analysis revealed that the flavonoids in rejuvenated grafts primarily influence the changes in endogenous hormones (Supplementary Table S4).
Fig. 2.
Physiological changes in rejuvenated leaves. A Changes in total flavonoid content and its components. B IAA production changes. C Changes in ABA levels. D Changes in GA. E ZR changes. F The ratio changes of endogenous hormones
The transcriptomic analysis of rejuvenated Ginkgo
To elucidate the differences in transcriptional regulation expression among different successive grafts of rejuvenated Ginkgo, we selected 16 samples (F1, F2, M1, M2, S0-1, S0-2, S0-3, S1-1, S1-2, S1-3, S2-1, S2-2, S2-3, S3-1, S3-2, and S3-3) with varying macromolecule contents for transcriptome analyses. Sixteen RNA libraries were created and analyzed, generating 932.51 GB of clean data (Q30 > 92%), averaging 53.45–75.86 million reads per sample (Supplementary Table S5). Principal component analysis (PCA) and correlation analysis plots showed clear clustering among biological repetitions and clear separation among different successional stages (S0, S1, S2, S3) and juvenile control samples (F, M) of Ginkgo biloba (Fig. 3A). Pearson correlation coefficient analysis of the replicates within groups showed good reproducibility (Fig. 3B). Additionally, the global trends of the expression levels of the genes obtained significantly differed (Fig. 3C). Across the 16 groups, 38,103 differentially expressed genes (DEGs) were identified, with 18,227 upregulated and 19,867 downregulated genes (Fig. 3D). Venn diagrams revealed that 773–4328 genes were expressed in the different successional stages and juvenile control samples, with the highest gene count observed in S3. Additionally, 31–111 genes were co-expressed across different successions, with the number of co-expressed genes increasing at each successional stage from S1 to S3 (Supplementary Fig. 1).
Fig. 3.
Transcriptomic analysis of rejuvenated leaves at three generations with biological replicates. A Principal component analysis (PCA) of the samples. B Heatmap of the correlation matrix between samples. The numbers indicate the Pearson correlation coefficients. C Samples expression violin plot. D The upregulated and downregulated DEGs in different comparisons
Among the genes expressed across different successional stages (S0 v 1, S0 v 2, S0 v 3, S1 v 2, S1 v 3, S2 v 3) in Ginkgo, the number of co-expressed genes varied from 3 to 6 (Supplementary Table S6). The highest number of co-expressed genes unique to S3 compared to S2 (S2 v 3) was predicted to be sphingomyelin synthase family proteins, hypothetical proteins, structural domain proteins, and membrane-associated kinase regulatory factors. GO enrichment analysis revealed an enrichment of DEGs in five biological processes (BP) and two cellular components (CC), with no enrichment in molecular function (MF). Further analyses indicated that the main enriched components were cell–cell junction, the synthesis of cellular amino acids, and the sulfur amino acid synthesis process, suggesting that the co-expressed DEGs are in S0v2 were primarily engaged in the biological functions related to growth and development (Supplementary Fig. 2).
A total of 1303 genes were annotated for 64 KEGG metabolic pathways. The 105, 220, 105, 190, 288, and 395 genes were annotated to KEGG pathways in the S0 vs. S1, S0 vs. S2, S0 vs. S3, S1 vs. S2, S1 vs. S3, and S2 vs. S3 groups, respectively. 12 and 14 significantly enriched metabolic pathways were obtained in the S0 vs. S2 and S0 vs. S3 groups (Q value < 0.05). Alternatively, the pathways with the highest number of annotated genes were related to the metabolism, photosynthesis, and ribosome protein processing in endoplasmic reticulum. To investigate genes associated with inter-successional (S1, S2, S3, recorded as Sir) rejuvenation, DEGs screened for all expressed genes were enriched. KEGG pathway enrichment analysis revealed that the 410 DEGs were enriched in synthesis and degradation of ketone bodies, protein processing in endoplasmic reticulum, and terpenoid backbone biosynthesis. And then we compared the male and female between inter-successional (Sir vs. F) to identify the genes of rejuvenation. There are 1104 DEGs enriched in the pathways of plant-pathogen interaction and synthesis and degradation of ketone bodies (Supplementary Fig. 3).
To better understanding of the dynamics of differential gene expression in the grafted rejuvenation material, we performed the trend analyses on the four successive generations of S0, S1, S2, and S3. The results indicated that the expression dynamics of all differential genes could be categorized into 20 expression profiles, with nine colors profiles being the most significant (Supplementary Fig. 4 A). The GO and KEGG enrichment analyses of these genes showed that the up-regulated genes in Profile 19 were enriched in kinase activity, protein kinase activity, phosphorus metabolic process, and mitogen-activated protein kinase (MAPK) signaling pathway-plant. Conversely, the downregulated genes in Profile 0 were enriched for oxidoreductase activity, single-organism metabolic process, myofibril, and biosynthesis of secondary metabolites (Supplementary Fig. 4B).
Fig. 4.
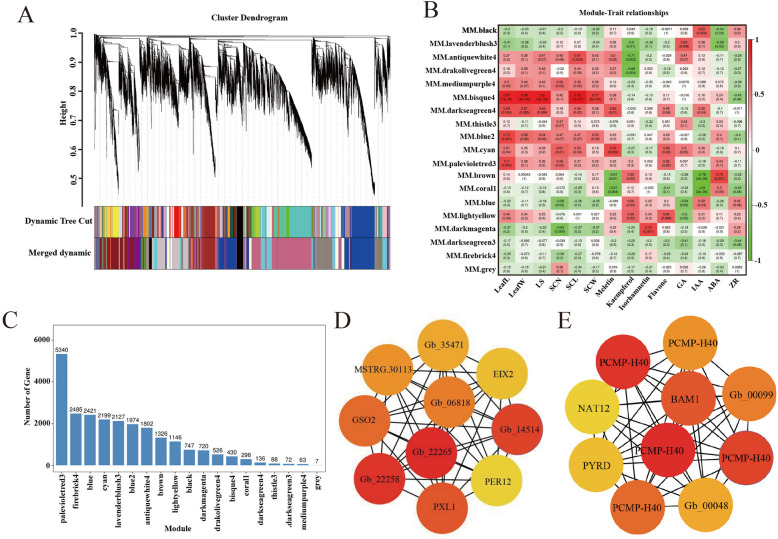
Modules and regulation networks identified by weighted gene co-expression network analysis (WGCNA) in this study. A Co-expression modules for hierarchical clustering tree. B Correlation indices and correlations between traits and modules. C Number of genes in the module. D and E Top 10 hub genes. The top 10 hub genes ranged from red to yellow according to rank color
Network analysis of rejuvenation Ginkgo based on WGCNA
To further identify the hub genes involved in rejuvenation during Ginkgo grafting, genes related to rejuvenation were identified by WGCNA. Using the genes obtained in the transcriptome data (Fig S5A), construct a gene clustering tree based on the correlation of gene expression, and classify gene modules according to the clustering relationship between genes. A total of 19 different co-expression modules were identified in this study (Fig. 4A). The module-trait correlation results showed that the bisque4 module had the highest correlation coefficient with the leaves traits, including leaf length (LeafL), leaf width (LeafW), number of leaf cleavages (SCL), and cleavage width (SCW) (Fig. 4B). The palevioletred3 module positively correlated with physiological and biochemical parameters, which is the highest number of unigenes among the 19 modules (Fig. 4C). This suggests that bisque4 and palevioletred3 are key modules in determining Ginkgo rejuvenation (Supplementary Fig. 5 A). The expression level of bisque4 module was significantly increased in S1(Supplementary Fig. 5B), and the palevioletred3 module was significantly decreased in S3(Supplementary Fig. 5 C), so these two modules may contain the key genes of successional grafting rejuvenated Ginkgo. Furthermore, the gene co-expression network was also constructed by Cytoscape software, and top ten hub genes were identified according to connections (Fig. 4D, E). The results showed that the hub genes related to leaf phenotype in bisque4 module including MSTRG.15343, Gb_06818, and Gb_32905. These genes were annotated as serine/threonine protein kinase, monogalactosyldiacylglycerol (MGDG), and receptor-like serine/threonine-protein kinase (RCH1). The hub genes in palevioletred3 module, such as Gb_00194, Gb_00201, Gb_00204, Gb_00054 and Gb_00195 were annotated as a member of the pentatricopeptide repeat-containing protein At1g11290 (PCMP-H40) family, Gb_00189 was annotated as leucinerich repeat receptor-like serine/threonine-protein kinase (BAM1), Gb_00090 was annotated as riboflavin biosynthesis protein (PYRD), and Gb_00197 was annotated as nucleobase-ascorbate transporter 12. These genes may regulate the physiological and biochemical changes in the process of Ginkgo rejuvenating.
Fig. 5.
Regulation of structural genes involved in the biosynthetic pathways of flavonoid during Ginkgo rejuvenation (S0, S1, S2, and S3). CHS, chalcone synthase; F3H, flavanone 3-hydroxylase; FLS, flavonol synthase; CYP73A, cytochrome P450 73 A; CCOAOMT, caffeoyl-CoA O-methyltransferase; DFR, dihydroflavonol 4-reductase; CYP75B1, cytochrome P450 75B1; ANS, anthocyanidin reductase; ANR, anthocyanidin reductase
Analysis of DEGs related to flavonoid biosynthesis
The flavonoid biosynthesis initiated from the phenylalanine metabolic pathway, which was a highly conserved pathway in plants and the Ginkgo’s flavonoid biosynthesis regulatory network remained incompletely understood. Through the transcriptome analysis, we identified 25 DEGs pertained to the flavonoid biosynthesis during the Ginkgo rejuvenation. Among these, the expression of genes CHS, lavanone 3-hydroxylase (F3H), flavonol synthase (FLS), cytochrome P450 75B1 (CYP75B1), and anthocyanidin reductase (ANR) were significantly increased (Fig. 5). Additionally, the expression patterns of dihydroflavonol 4-reductase (DFR) and Gb_38699 showed an increase and then decrease (Supplementary Fig. 6 A), consistent with the trend in total flavonoid content. Key genes involved in the pathway of flavonoid biosynthesis (Fig. 6), like caffeoyl-CoA O-methyltransferase (CCOAOMT) and anthocyanidin reductase (ANS) were upregulated during the rejuvenation, indicating their crucial regulatory roles in flavonoid biosynthesis.
Fig. 6.
Expression level of DEGs in plant hormone signal transduction and concentration of DEGs in Zeatin biosynthesis for Ginkgo rejuvenation at different stages (S0, S1, S2, and S3). TIR1, transport inhibitor response 1; AUX/IAA, auxin/indole-3-acetic acid; ARF, auxin response factor; GH3, gretchen hagen 3; SAUR, small auxin-up RNA; CRE1, cytokinin response 1; AHP, arabidopsis histidine phosphotransfer protein; B-ARR, type-B arabidopsis response regulator; CYP735A, cytochrome P450 735 A; CKX, cytokinin oxidase/dehydrogenase; miaA, tRNA isopentenyltransferase; CISZOG, cis-zeatin o-glucosyltransferase; GID1, gibberellin insensitive dwarf 1; GID2, gibberellin insensitive dwarf 2; PYR/PYL, pyrabactin resistance/PYR1-like; PP2C, protein phosphatase 2 C; SnRK2, sucrose non-fermenting 1-related protein kinase 2; ABF, ABRE-binding factor; ABRE, ABA-responsive element. Dotted arrows represent indirect effects
DEGs related to plant hormone signaling and biosynthesis
A total of 36 DEGs were enriched into plant hormone signal transduction and zeatin biosynthesis for rejuvenated Ginkgo. These genes were located in detailed metabolic pathway to understand their roles (Fig. 6). Differences in endogenous hormone content were observed in the successive grafted rejuvenated Ginkgo. We identified 21 DEGs related to IAA biosynthesis and signaling. Based on their expression trend during rejuvenation, these genes can be divided into two groups. One group including At1g31550, IAMT1, AO1, BNA2, TDC2, AAO4, Os07g0282300, gretchen hagen 3.5 (GH3.5), and ELI5, showed a significant decreasing trend, eventually reaching levels similar to those of juvenile Ginkgo. The second group showed no significant changes in the synthesis and signaling processes of the IAA hormone (Supplementary Fig. 6B).
The DEGs related to the ABA synthesis and signaling were identified seventeen. The expression of pyrabactin resistance-like 8 (PYL8) and the RZ-1C significantly increased during rejuvenation. PYL family, particularly PYL5, PYL4, and PYL2, showed the highest expression in S1 (Supplementary Fig. 6 C). We identified five differential genes related to GA synthesis and signaling, including gibberellic acid-stimulated arabidopsis 10 (GASA10), gibberellin 20-oxidase 2 (GA20OX2), gibberellin insensitive dwarf 1 C (GID1C), and gibberellin 2-oxidase 8 (GA2OX8). The expression of these genes showed an increasing trend, similar to that of the childhood Ginkgo expression levels, which was consistent with the trends in GA content determination. This indicates that grafting treatment significantly effects GA levels (Supplementary Fig. 6D).
We identified ten DEGs related to ZR biosynthesis (Fig. 6). The expression of cytokinin oxidase/dehydrogenases (CKX), zeaxanthin epoxide cyclase (ZOG), and miaA significantly increased, mirroring the pattern observed in juvenile Ginkgo and aligned with the trend in ZR content determination. The highest expression of CKX5 and isopentenyltransferase 9 (IPT9) observed in S2 (Supplementary Fig. 6E). This suggests that these genes, which are involved in ZR hormone synthesis and signaling, play crucial roles in the grafting and rejuvenation of Ginkgo.
Overview of the small RNAs analysis
To assess small RNAs changes during the process of grafting rejuvenated Ginkgo, small RNAs derived from leaves were sequenced. Sixteen libraries were created and analyzed, generating 439 Gb of clean data was obtained, averaging 24.60–33.59 million reads per group (Supplementary Table S7). The analysis of miRNA sequence lengths showed that known miRNAs ranged from 18 to 35 nt, whereas new miRNAs ranged from 20 to 24 nt, with 21 nt and 22 nt miRNAs being the most prevalent among the new ones (Fig. 7A). Furthermore, the analysis of the first base preference of new miRNAs revealed that 72.2% of mature miRNA sequences started with adenine (A), followed by guanine (G) at 15.3%, cytosine (C) at 5.4%, and uracil (U) at 7.1%. Thus, adenine was the most common first base in the mature miRNA sequences (Supplementary Fig. 7 and Supplementary Fig. 8). Additionally, the global trends of the expression level of the miRNAs obtained following generation significantly differed (Fig. 7B). In total, 475 differentially expressed miRNAs (DEMs) were identified. Additionally, 269 miRNAs were upregulated, while 206 miRNAs were downregulated in the 16 groups. The number of upregulated differentially expressed miRNAs in different grafted successions and in the control of the upper stages was significantly higher than the number of downregulated miRNAs (Fig. 7C). In the classification annotation, the number of unannotated small RNAs in the 16 samples was significantly higher than that of the annotated small RNAs. The proportion of miRNAs was 15.71% ~ 17.47%, which was greater than other types of non-coding RNAs (rRNA, snRNA, snoRNA, tRNA). It indicates that many new small non-coding RNAs have not been discovered (Supplementary Table S8).
Fig. 7.
A The distribution of sRNA sequences identified in the sequencing libraries. The X-axis represents sRNAs of different lengths, while the Y-axis shows the percent frequency of sRNAs of a define length. B Heatmap of miRNAs from rejuvenation samples. C The upregulated and downregulated differentially expressed miRNAs (DEMs) in different comparisons
Fig. 8.
Interaction network diagram of miRNA targeted genes involved in the regulation of Ginkgo rejuvenation process
The attribution analysis of miRNAs revealed 3281 miRNAs in the miRNA database of the Ginkgo succeeding grafting rejuvenation process, including 2660 known miRNAs and 621 new miRNAs (Supplementary Table S9). The known miRNAs belong to 393 preserved miRNA families, like miR156, miR169, miR166, miR171, and miR395. MiR169 was the most abundant with 408 members, whereas miR156 emerged as the most preserved, existing across 56 species (Supplementary Table S10). The expression patterns of 3281 miRNAs were analyzed by performing trend analysis, and 3 color profiles had a significant number of assigned miRNAs. The expression of miRNAs increased with an increase in the number of grafted successions (Supplementary Fig. 9). Additionally, there were duplications of differential genes among grafted generations, with only miR395 being co-expressed among them, suggesting that miR395 played a key role in Ginkgo rejuvenation (Supplementary Fig. 10).
Combined transcriptome and miRNA analysis
To investigate the rejuvenation response mechanism of Ginkgo under successive grafting treatments and to study the regulatory relationship between miRNAs and their target genes (Supplementary Table S11), we analyzed miRNAs along with transcriptome data. The results revealed that all groups had miRNAs corresponding to the target genes, resulting in varying numbers of miRNA-target gene pairs. The analysis of miRNA-target gene pairs showed that multiple miRNAs can target a single target gene, and different members of the same miRNA family can target the same gene or different members of the same gene family. For example, miR530 and miR1030 target the same transcript (Gb_21532), miR1551 targets 34 genes, miR3951 targets 7 genes, and miR169 targets two genes. This suggested that miRNAs were regulated by multiple genes during Ginkgo rejuvenation.
Functional annotation of the candidate target genes of differentially expressed miRNAs by GO and KEGG enrichment. The data analysis revealed that S2 v 3 had the highest number of target genes of differentially expressed miRNAs, and S0 v 1 had the lowest. The functional classification patterns of the target genes among the grafted generations were similar in the GO enrichment (Supplementary Fig. 11). In the classification of enriched biological processes, the target genes of differentially expressed miRNAs in the comparison group were mainly distributed in BP and MF. The target genes in BP were mainly concentrated in the regulation of cellular processes and biological regulation, whereas those in MF were mainly distributed in ADP binding and nucleotide binding. The result differed slightly from the annotated classification results of differential genes obtained by transcriptome sequencing, while all were mainly enriched in plant growth and development and biological regulation pathways. The KEGG enrichment analysis (Supplementary Fig. 12) revealed a consistent pattern among the grafted successions, with the number of target genes of differentially expressed miRNAs mainly enriched in plant-pathogen interactions and monoterpenoid biosynthesis pathway. Although slightly different from the classification of differential genes in the transcriptome analysis, both sets were predominantly enriched in plant physiological metabolic pathways, indicating their crucial roles in rejuvenation.
In order to reveal the coregulated miRNAs, the negatively correlated miRNA-target gene pairs were analyzed by interaction network analysis. The results showed that the miRNA-target gene interaction network involved 399 interactions. The cytocluster plug-in combined miRNA-target genes into ten significant clusters using functional module identification (P = 0.03, Quality = 1). Among them, miR395 targeted PCMP-H40 gene and iron-containing protein (FAO1) were the key clusters, which was generally consistent with the results of transcriptome and miRNA screening that miR395 and PCMP-H40 gene family were the key factors for rejuvenation process of Ginkgo (Fig. 8).
To validate the identified expression patterns of miRNAs and corresponding target genes involved in the process of Ginkgo rejuvenation, 16 genes and 10 miRNAs were randomly selected for qRT-PCR analysis (Supplementary Fig. 13 and Supplementary Fig. 14). The expression changes trend of genes and miRNAs observed from qRT-PCR analysis was similar to that of high-throughput sequencing. To sum up, these data revealed that there was good consistency between the high-throughput sequencing and qRT-PCR, indicating the reliability and accuracy of small RNA and mRNA transcriptome sequencing.
Discussion
Changes of Ginkgo leaves morphology in the process of succeeding grafting rejuvenation
Changes in leaf blade morphology signify the transition of a tree from juvenile to adult stages and can serve as an indicator for evaluating rejuvenation. Leaf lobing is a juvenile characteristic of Ginkgo trees. Throughout the evolution of Ginkgo, the lobes of the leaf blade have transitioned from the deep and numerous to the shallow and few [22]. Fossilized Ginkgo leaves often exhibit more lobes [23, 24]. The leaf blades of young Ginkgo trees display a deeply lobed leaf phenotype [25], which is also observed in grafted Ginkgo trees and possibly reflects ancestral juvenile morphological traits. Our research revealed a significant increase in S1 leaf lobe following graft rejuvenation, indicating changes in leaf morphology after grafting (Fig. 1A). However, this feature may not consistently occur simultaneously, as the S2 and S3 leaf lobes did not show a consistent increasing trend.
The flavonoids and endogenous hormones changes in rejuvenated Ginkgo
During forest tree rejuvenation, phenotypic changes are primarily regulated by endogenous plant factors that induce the production of secondary metabolites to adapt to growth conditions [26, 27]. Flavonoids, among the most important secondary metabolites, accumulate in plants, and their levels are influenced by age and developmental stage. In Arabidopsis thaliana and Phyllostachys edulis, the total flavonol glycoside content in the leaves typically decreases with leaf maturation [28, 29]. In our study, total flavonoid content exhibited an increasing and then decreasing trend after successive graft rejuvenation treatments (Fig. 2A). The decrease in S3 may be attributed to S2 being the optimal number of successions for Ginkgo flavonoid rejuvenation. It has been demonstrated that rejuvenation is highly effective after more than two successions. But as successive juvenile generations increased, negative rejuvenation may occur [30, 31], which was consistent with our findings. However, the underlying cause has remained unclear.
IAA, CK, GA, and ZR are the major regulatory hormones involved in plant growth, development, and rejuvenation [32–35]. Researchers have closely monitored changes in these hormones during the plant rejuvenation [36–38]. IAA content significantly increased, while ABA content decreased in walnut scions after rejuvenation compared to that in adult scions. However, the differences in GA and ZR content changes were not significant [38]. The IAA content in apple M9 and M26 leaves increased significantly with each rejuvenation succession, whereas the ABA content showed a gradual decline [39, 40]. Our study also observed a significant increase in the IAA content and a decrease in the ABA content in Ginkgo leaves after rejuvenation (Fig. 2 B and C), consistent with the findings in walnut and apple.
IAA, CK, and GA can regulate the synthesis of secondary metabolites, such as flavonoids, terpenoids, and alkaloids in plants [41–45]. We found a significant negative correlation between total flavonoid content and IAA/ZR (Fig. 2F), indicating a close relationship between endogenous hormones and total flavonoids. This suggested that flavonoids and endogenous hormones interact to regulate Ginkgo rejuvenation.
Molecular mechanisms of Ginkgo successional graft rejuvenation
Flavonoid biosynthesis is a complex process, and recent research on Ginkgo has identified genes related to this pathway, including GbCHS, GbF3H, GbANS, GbCHI, GbFLS and GbDFR [46–48]. In our study, the transcriptome sequencing of Ginkgo leaves after serial graft and rejuvenation treatment revealed 25 genes related to flavonoid biosynthesis. The expression of key genes, such as CHS, DFR, CHI, and ANR, correlated with changes in total flavonoid content, suggesting their importance in the flavonoid biosynthesis pathway during Ginkgo rejuvenation. The IAA is a crucial regulator of plant growth and development, and the polar auxin transporter PIN-formed protein (PIN), ARF, small auxin-upregulated RNA (SAUR), and GH3 gene families respond to these changes [49–51]. We observed a significant decrease in GH3.5, suggesting its role in reducing IAA content during Ginkgo rejuvenation (Supplementary Fig. 6B). The PYL (PYR/PYL/RCAR) family is the largest known group of ABA phytohormone receptors. Its primary role is to recognize ABA signaling and initiate the signaling process [52]. In this study, the PYL gene family, which was part of the ABA biosynthesis pathway, showed abundant enrichment in S1, indicating significantly higher expression (Supplementary Fig. 6 C). This suggested activation of the ABA signaling pathway in S1 in response to rejuvenation. The GA biosynthesis and metabolism involves multiple enzymatic processes. GA20-oxidase is a key enzyme in the final step of the GA biosynthetic pathway [53, 54], while GA2-oxidase is crucial for GA degradation [55]. This study observed an increasing trend in the expression of GA20OX2, GA2OX8, and GA2OX1, indicating that upregulation of these key genes in young compounds promoted a significant increase in GA content (Supplementary Fig. 6D). ZR is a major component of CK [56], and cytokinin oxidase (CKX) catalyzes the cytokinin degradation, thereby inhibiting the CK signaling pathway [57]. This study found a significant increase in the expression of CKX9, CKX3, and CKX7 (Supplementary Fig. 6E), suggesting that CKX regulates ZR changes during Ginkgo rejuvenation. Plant miRNAs play diverse regulatory roles in biological and metabolic activities, including developmental regulation, cellular differentiation, signal transmission, growth control, and responses to biotic and abiotic stressors.
This study identified the miR395 as a key regulator of Ginkgo rejuvenation, showing peak expression during S₂ and targeting PCMP-H40 and FAO1. While traditionally linked to sulfur metabolism, it was recognized its broader role through: (1) Senescence suppression via PCMP-H40 downregulation, enhancing photosynthetic efficiency in juvenile-like leaves; (2) Hormone modulation, with miR395 levels correlating with IAA/GA increases and ABA decreases, mirroring its known ABA-sensitivity reduction in other species [58, 59]; and (3) Stress resilience via FAO1-mediated iron regulation, indirectly influencing GA biosynthesis. The miR395 decline in S₃ aligns with reduced rejuvenation efficacy, suggesting its role as a molecular switch. This work expands the function of miR395 beyond nutrient stress, positioning it as a potential biomarker for graft success. Future studies should validate these mechanisms through functional assays.
While this study advances our understanding of Ginkgo rejuvenation, some limitations warrant discussion. First, key hub genes (PCMP-H40, FAO1, BAM1) and miR395-target interactions were identified computationally but lack experimental validation. Future work should employ CRISPR editing and luciferase assays to confirm their functional roles. The analysis included only three graft generations (S₁-S₃), expanding to additional generations would strengthen conclusions about optimal rejuvenation stages. Third, while transcriptomic and small RNA data were integrated, incorporating proteomics could provide deeper mechanistic insights into post-translational regulation. Finally, the practical implications for Ginkgo cultivation require validation through field trials to assess long-term stability of rejuvenation effects. Addressing these gaps will enhance both fundamental knowledge and applied outcomes in tree biotechnology. Actually, plant rejuvenation is a complex process influenced by various factors. Studies on telomere length, epigenetics, proteomics, and metabolomics have demonstrated that restoring epigenomic integrity can reverse senescence and achieve rejuvenation [60]. Understanding these aspects is crucial for revealing Ginkgo rejuvenation and warrants attention in the future research.
Conclusion
This study revealed for the first time to investigate the leaf morphology, physiological and molecular mechanisms of leaf changes during rejuvenation after three years of continuous succeeding grafting in Ginkgo. The novel miR395-PCMP-H40/FAO1 regulatory network as a key mechanism driving Ginkgo rejuvenation through successive grafting. We demonstrated that miR395, the only consistently differentially expressed miRNA across three graft generations, targets PCMP-H40 and FAO1 to coordinate rejuvenation processes. Transcriptomic analysis identified two critical modules (bisque4 and palevioletred3), with PCMP-H40 emerging as a central hub gene. Physiological data showed optimal rejuvenation in S2 grafts, characterized by peaked flavonoid content and coordinated hormonal changes (increased IAA/GA/ZR, decreased ABA). These findings provide both fundamental insights into perennial plant rejuvenation and practical targets for improving ginkgo cultivation through molecular breeding approaches.
Supplementary Information
Acknowledgements
The authors are grateful to all members of the laboratory for their technical assistance and stimulating discussions.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Authors’ contributions
QG, FL and RW designed the experiments; FL, TZ, PJ, RW and XG performed the experiments; FL, TZ, PJ, and RW analyzed the data; FL and QG wrote the article. All the authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by project for National Natural Science Foundation of China (31971648), the Talent Introduction Project Study of Nanjing Forestry University (GXL2018001) on Ginkgo biloba and other important tree germplasm resources, and Jiangsu Province Graduate Scientific Research and Innovation Plan Project (KYCX23_1250).
Data availability
The raw data in transcriptome and Small RNA for our analysis have been submitted to the National Center for Biotechnology Information (NCBI https://www.ncbi.nlm.nih.gov/) database Sequence Read Archive (SRA https://www.ncbi.nlm.nih.gov/sra/) with PRJNA1104754 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1104754) and PRJNA1104759 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1104759) as the SRA number.
Declarations
Ethics approval and consent to participate
Our study was conducted in full compliance with local regulations. This article did not involve any studies with human, animals, or endangered species. The collection of plant materials and the experimental procedures conducted in this study adhered to institutional, national and international guidelines and legal requirements.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Martínková J, Šmilauer P, Mihulka S, Latzel V, Klimešová J. The effect of injury on whole-plant senescence: an experiment with two root-sprouting Barbarea species. Ann Bot. 2016;117:667–79. 10.1093/aob/mcw010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Z, Sun Y, Li Y. Plant rejuvenation: from phenotypes to mechanisms. Plant Cell Rep. 2020;39:1249–62. 10.1007/s00299-020-02577-1. [DOI] [PubMed] [Google Scholar]
- 3.Zhao Y, Paule J, Fu C, Koch MA. Out of China: distribution history of Ginkgo biloba L. Taxon. 2010;59:495–504. 10.1002/tax.592014. [Google Scholar]
- 4.Lu Z, Zhu L, Lu J, Shen N, Wang L, Liu S, Wang Q, Yu W, Kato-N H, Li W, Jin B, Wang L, Lin J. Rejuvenation increases leaf biomass and flavonoid accumulation in Ginkgo biloba. Hortic Res. 2022;9:uhab018. 10.1093/hr/uhab018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang L, Suwenza L, Huang B, Murashige T, Mahdi E, Gundy R. Rejuvenation of Sequoia sempervirens by repeated grafting of shoot tips onto juvenile rootstocks in vitro 1: model for phase reversal of trees. Plant Physiol. 1992;98:166–73. 10.1104/pp.98.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao M, Gan Q, Xu Y, Lu J, Zhong L, Wang M, Liu S, Wang L. Pruning improves seedling development and bioactive secondary metabolite accumulation in the leaves of Ginkgo biloba. Trees. 2022;36:953–66. 10.1007/s00468-021-02260-2. [Google Scholar]
- 7.Xiao Z, Ji N, Zhang X, Zhang Y, Wang Y, Wu T, Han Z. The lose of juvenility elicits adventitious rooting recalcitrance in apple rootstocks. Plant Cell Tissue Organ Cult. 2014;119:51–63. 10.1007/s11240-014-0513-5. [Google Scholar]
- 8.Yan J, Zhang S, Tong M, Lu J, Wang T, Xu Y, Li W, Wang L. Physiological and genetic analysis of leaves from the resprouters of an old Ginkgo biloba tree. Forests. 2021;12: 1255. 10.3390/f12091255. [Google Scholar]
- 9.Chang Y, Song X, Zhang Q, Liu H, Bai Y, Lei X, Pei D. Genome-wide identification of WOX gene family and expression analysis during rejuvenational rhizogenesis in walnut (Juglans regia L.). Forests. 2020;11:16. 10.3390/f11010016. [Google Scholar]
- 10.Schwab R, Palatnik JF, Riester M, Schommer C, Schmid M, Weigel D. Specific effects of microRNAs on the plant transcriptome. Dev Cell. 2005;8:517–27. 10.1016/j.devcel.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 11.Chuck G, Cigan A, Saeteurn K. The heterochronic maize mutant Corngrass1 results from overexpression of a tandem microRNA. Nat Genet. 2007;39:544–9. 10.1038/ng2001. [DOI] [PubMed] [Google Scholar]
- 12.Wu G, Park MY, Conway SR, Wang J, Weigel D, Poethig RS. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell. 2009;138:750–9. 10.1016/j.cell.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massoumi M, Krens FA, Visser RGF, De Klerk GM. Azacytidine and miR156 promote rooting in adult but not in juvenile Arabidopsis tissues. J Plant Physiol. 2017;208:52–60. 10.1016/j.jplph.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Ye B, Zhang K, Wang J. The role of miR156 in rejuvenation in Arabidopsis thaliana. J Integr Plant Biol. 2020;62:550–5. 10.1111/jipb.12855. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Shen C, Lin W, Chu H, Huang B, Kuo C, Yeh K, Huang L, Chang I. Small RNAs of sequoia sempervirens during rejuvenation and phase change. Plant Biol. 2013;15:27–36. 10.1111/j.1438-8677.2012.00622.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Q, Mu K, Xu M, Ma X, Ni Z, Wang J, Xu L. Variation in the concentrations of major secondary metabolites in ginkgo leaves from different geographical populations. Forests. 2017;8:266. 10.3390/f8080266. [Google Scholar]
- 17.Love M, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15: 550. 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson MD, McCarthy DJ, Smyth GK. EdgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–40. 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langfelder P, Mischel P, Horvath S. When is hub gene selection better than standard meta-analysis? PLoS One. 2011;8: e61505. 10.1371/journal.pone.0061505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu Y, Zhang Y, Smarda P, Bures P, Guo Q. Transcriptome and proteome associated analysis of flavonoid metabolism in haploid Ginkgo biloba. Int J Biol Macromol. 2023;224:306–18. 10.1016/j.ijbiomac.2022.10.125. [DOI] [PubMed] [Google Scholar]
- 21.Li W, He Z, Yang S, Ye Y, Jiang H, Wang L. Construction and analysis of a library of miRNA in gold-coloured mutant leaves of Ginkgo biloba L. Folia Hortic. 2019;31:81–92. [Google Scholar]
- 22.Hang H, Bauer M, Mio W, Mander L. Geometric and topological approaches to shape variation in Ginkgo leaves. R Soc Open Sci. 2021;8: 210978. 10.1098/rsos.210978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Z, Zheng S. The missing link in Ginkgo evolution. Nature. 2003;423:821–2. 10.1038/423821a. [DOI] [PubMed] [Google Scholar]
- 24.Leigh A, Zwieniecki MA, Rockwell FE, Boyce CK, Nicotra AB, Holbrook NM. Structural and hydraulic correlates of heterophylly in Ginkgo biloba. New Phytol. 2011;459–70. 10.1111/j.1469-8137.2010.03476.x. [DOI] [PubMed] [Google Scholar]
- 25.Bauer K, Grauvogel-Stamm L, Kustatcher E, Krings M. Fossil ginkgophyte seedlings from the triassic of France resemble modern Ginkgo biloba. BMC Ecology and Evolution. 2013;13:177. 10.1186/1471-2148-13-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye J, Zhang X, Tan J, Xu F, Cheng S, Chen Z, Zhang W, Liao Y. Global identification of Ginkgo biloba microRNAs and insight into their role in metabolism regulatory network of terpene trilactones by high-throughput sequencing and degradome analysis. Ind Crops Prod. 2020;148: 112289. 10.1016/j.indcrop.2020.112289. [Google Scholar]
- 27.Rachappanavar V, Padiyal A, Sharma J, Gupta S. Plant hormone-mediated stress regulation responses in fruit crops- a review. Sci Hortic. 2022;304: 111302. 10.1016/j.scienta.2022.111302. [Google Scholar]
- 28.Peer KR, Greenwood MS. Maturation, topophysis and other factors in relation to rooting in Larix. Tree Physiol. 2001;21(4):267–72. 10.1093/treephys/21.4.267. [DOI] [PubMed] [Google Scholar]
- 29.Yao X, Shang E, Zhou G, Tang Y, Guo S, Su S, Jin C, Qian D, Qin Y, Duan J. Comparative characterization of total flavonol glycosides and terpene lactones at different ages, from different cultivation sources and genders of Ginkgo biloba leaves. Int J Mol Sci. 2012;13:10305–15. 10.3390/ijms130810305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heide OM. Juvenility, maturation and rejuvenation in plants: adventitious bud formation as a novel rejuvenation process. J Hortic Sci Biotechnol. 2019;94:2–11. 10.1080/14620316.2018.1482795. [Google Scholar]
- 31.Vidoy-Mercado I, Narváez I, Palomo-Ríos E, Litz RE, Barceló-Muñoz A, Pliego-Alfaro F. Reinvigoration/rejuvenation induced through micrografting of tree species: signaling through graft union. Plants. 2021;10: 1197. 10.3390/plants10061197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu F, Xue S, Deng L, Zhang S, Li Y, Zhao X. The piperazine compound ASP activates an auxin response in Arabidopsis thaliana. BMC Genomics. 2020;21: 788. 10.1186/s12864-020-07203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wojciechowska N, Sobieszczuk-Nowicka E, Bagniewska-Zadworna A. Plant organ senescence-regulation by manifold pathways. Plant Biol. 2018;20:167–81. 10.1111/plb.12672. [DOI] [PubMed] [Google Scholar]
- 34.Brookbank BP, Patel J, Gazzarrini S, Nambara E. Role of basal ABA in plant growth and development. Genes. 2021;12: 1936. 10.3390/genes12121936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peter H. The current status of research on gibberellin biosynthesis. Plant Cell Physiol. 2022;61:1832–49. 10.1093/pcp/pcaa092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang T, Davies PJ, Reid JB. Genetic dissection of the relative roles of auxin and gibberellin in the regulation of stem elongation in intact klight-grown peas. Plant Physiol. 1996;110:1029–34. 10.1104/pp.110.3.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dodd IC, Puértolas J, Huber K, Pérez-Pérez JG, Wright HR, Blackwell MSA. The importance of soil drying and re-wetting in crop phytohormonal and nutritional responses to deficit irrigation. J Exp Bot. 2015;66:2239–52. 10.1093/jxb/eru532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Z, Shi Q, Chen P, Sun F, Creech D, Lu Z, Yin Y, Yu C. Grafting causes physiological changes and promotes adventitious root formation in rejuvenated soft shoots of taxodium hybrid “Zhongshanshan.” Plants. 2023;12(1): 201. 10.3390/plants12010201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lavee S, Haskal A. Partial fruiting regulation of olive trees (Olea europaea L.) with paclobutrazol and gibberellic acid in the orchard. Adv Hortic Sci. 1993;1993(7):83–6 (https://www.jstor.org/stable/42881682). [Google Scholar]
- 40.Loreti E, Povero G, Novi G, Solfanelli C, Alpi A, Perata P. Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytol. 2008;179:1004–16. 10.1111/j.1469-8137.2008.02511.x. [DOI] [PubMed] [Google Scholar]
- 41.Ji X, Wang Y, Zhang R, Wu S, An M, Li M, Wang C, Chen X, Zhang Y, Chen X. Effect of auxin, cytokinin and nitrogen on anthocyanin biosynthesis in callus cultures of red-fleshed apple (Malus sieversii f.niedzwetzkyana). Plant Cell Tissue Organ Cult. 2015;120:325–37. 10.1007/s11240-014-0609-y. [Google Scholar]
- 42.Kuhn B, Geisler M, Bigler L, Ringli C. Flavonols accumulate asymmetrically and affect auxin transport in Arabidopsis. Plant Physiol. 2011;156:585–95. 10.1104/pp.111.175976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pop T, Pamfil D, Bellini C. Auxin control in the formation of adventitious roots. Not Bot Horti Agrobot Cluj-Napoca. 2011;39:307–16. 10.15835/nbha3916101. [Google Scholar]
- 44.Bari R, Jones J. Role of plant hormones in plant defence responses. Plant Mol Biol. 2009;69:473–88. 10.1007/s11103-008-9435-0. [DOI] [PubMed] [Google Scholar]
- 45.Pang Y, Shen G, Liu C, Liu X, Tan F, Sun X, Tang K. Molecular cloning and sequence analysis of a novel chalcone synthase cDNA from Ginkgo biloba. DNA Seq. 2004;15:283–90. 10.1080/10425170412331283814. [DOI] [PubMed] [Google Scholar]
- 46.Shen G, Pang Y, Wu W, Deng Z, Zhao L, Cao Y, Sun X, Tang K. Cloning and characterization of a flavanone 3-hydroxylase gene from Ginkgo biloba. Biosci Rep. 2006;26:19–29. 10.1007/s10540-006-9007-y. [DOI] [PubMed] [Google Scholar]
- 47.Xu F, Cheng H, Cai R, Li L, Chang J, Zhu J, Zhang F, Chen L, Wang Y, Cheng S, Cheng S. Molecular cloning and function analysis of an anthocyanidin synthase gene from Ginkgo biloba, and its expression in abiotic stress responses. Mol Cells. 2008;26:536–47. 10.1016/S1016-8478(23)14034-9. [PubMed] [Google Scholar]
- 48.Luo J, Zhou J, Zhang J. Aux/IAA gene family in plants: molecular structure, regulation, and function. Int J Mol Sci. 2018;19: 259. 10.3390/ijms19010259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinto R, Freitas N, Máximo W, Cardoso T, Prudente D, Paiva L. Genome-wide analysis, transcription factor network approach and gene expression profile of GH3 genes over early somatic embryogenesis in Coffea spp. BMC Genomics. 2019;20:812. 10.1186/s12864-019-6176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feng S, Li N, Chen H, Liu Z, Li C, Zhou R, Zhang Y, Cao R, Ma X, Song X. Large-scale analysis of the ARF and Aux/IAA gene families in 406 horticultural and other plants. Mol Hortic. 2024;4: 13. 10.1186/s43897-024-00090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng K, Lu J, He X, Lan S, Zhai T, Cao S, Lin Y. Genome-wide identification and expression analysis of GATA family genes in Dimocarpus longan Lour. Int J Mol Sci. 2024;25: 731. 10.3390/ijms25020731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park S, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow T, Alfred S, Bonetta D, Finkelstein R, Provart N, Desveaux D, Rodriguez P, McCourt P, Zhu J, Schroeder J, Volkman B, Cutler S. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324:1068–71. 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hedden P, Phillips A. Gibberellin metabolism: new insights revealed by the genes. Trends Plant Sci. 2000;5:523–30. 10.1016/S1360-1385(00)01790-8. [DOI] [PubMed] [Google Scholar]
- 54.Zhao H, Dong J, Wang T. Function and expression analysis of gibberellin oxidases in apple. Plant Mol Biol Rep. 2010;28:231–8. 10.1007/s11105-009-0146-8. [Google Scholar]
- 55.Wuddineh W, Mazarei M, Zhang J, Poovaiah C, Mann D, Ziebell A, Sykes R, Davis M, Udvardi M, Stewart C. Identification and overexpression of gibberellin 2-oxidase (GA2ox) in switchgrass (Panicum virgatum L.) for improved plant architecture and reduced biomass recalcitrance. Plant Biotechnol J. 2015;13:636–47. 10.1111/pbi.12287. [DOI] [PubMed] [Google Scholar]
- 56.Ruzicka K, Simásková M, Duclercq J, Petrásek J, Zazímalová E, Simon S, Friml J, Montagu M, Benková E. Cytokinin regulates root meristem activity via modulation of the polar auxin transport. PNAS. 2009;106:4284–9. 10.1073/pnas.0900060106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmülling T, Werner T, Riefler M, Krupková E, Manns I. Structure and function of cytokinin oxidase/dehydrogenase genes of maize, rice, Arabidopsis and other species. J Plant Res. 2003;116:241–52. 10.1007/s10265-003-0096-4. [DOI] [PubMed] [Google Scholar]
- 58.Rajab H, Khan M, Malagoli M, Hell R, Wirtz M. Sulfate-induced stomata closure requires the canonical ABA signal transduction machinery. Plants-Basel. 2019;8:21. 10.3390/plants8010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Batool S, Uslu V, Rajab H, Ahmad N, Waadt R, Geiger D, Malagoli M, Xiang C, Hedrich R, Rennenberg H, Herschbach C, Hell R, Wirtz M. Sulfate is incorporated into cysteine to trigger ABA production and stomatal closure. Plant Cell. 2018;30:2973–87. 10.1105/tpc.18.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang J, Hayano M, Griffin P, Amorim J, Bonkowski M, Apostolides J, Salfati E, Blanchette M, Munding E, Bhakta M, Chew Y, Guo W, Yang X, Maybury-Lewis S, Tian X, Ross J, Coppotelli G, Meer M, Rogers-Hammond R, Vera D, Lu YR, Pippin J, Creswell M, Dou Z, Xu C, Mitchell S, Das A, O’Connell B, Thakur S, Kane A, Su Q, Mohri Y, Nishimura E, Schaevitz L, Garg N, Balta A, Rego M, Ksander M, Jakobs T, Zhong L, Wakimoto H, Andari J, Grimm D, Mostoslavsky R, Wagers A, Tsubota K, Bonasera S, Palmeira C, Seidman JG, Seidman C, Wolf N, Kreiling J, Sedivy J, Murphy G, Green R, Garcia B, Berger S, Oberdoerffer P, Shankland S, Gladyshev V, Ksander B, Pfenning A, Rajman L, Sinclair D. Loss of epigenetic information as a cause of mammalian aging. Cell. 2023;186:305–26. 10.1016/j.cell.2022.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data in transcriptome and Small RNA for our analysis have been submitted to the National Center for Biotechnology Information (NCBI https://www.ncbi.nlm.nih.gov/) database Sequence Read Archive (SRA https://www.ncbi.nlm.nih.gov/sra/) with PRJNA1104754 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1104754) and PRJNA1104759 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1104759) as the SRA number.